Research Progress on the Preparation, Physiological Activity and Action Mechanism of Plant Derived Bioactive Peptides
-
摘要: 近年来,随着消费者对功能性食品和特殊医学用途配方食品需求的增长,具有潜在食用及药用价值的生物活性肽(Bioactive Peptides,BAPs)受到了广泛关注。植物源BAPs来源广泛、安全无毒。目前,大量的植物源BAPs经分离纯化被鉴定出来,除了具有丰富的营养价值之外,还具有抗氧化、降糖、降血压和抑菌等多种生理活性。本文就植物源BAPs的制备方法、生理活性、作用机制等方面进行综述,并对目前研究中存在的不足和未来发展方向进行探讨,以期为植物源BAPs在后续食品和医药领域的进一步研究和工业应用提供有益的理论支撑。Abstract: In recent years, with the increasing demand of consumers for functional foods and formula foods for special medical purposes, bioactive peptides (BAPs) with potential edible and medicinal value have received extensive attention. Plant-derived BAPs come from a wide range of sources, are safe and non-toxic. At present, a large number of plant-derived BAPs have been identified by isolation and purification. In addition to rich nutritional value, they also have various physiological activities such as antioxidant, hypoglycemic, hypertensive and antibacterial. In this paper, the preparation methods, physiological activity and action mechanism of plant-derived BAPs are reviewed, and the existing shortcomings and future development direction are discussed, in order to provide beneficial theoretical support for further research and industrial application of plant-derived BAPs in the field of food and medicine.
-
Keywords:
- bioactive peptides /
- preparation methods /
- physiological activity /
- action mechanism
-
生物活性肽(Bioactive Peptides,BAPs)是由2~20个氨基酸残基组成的特异性蛋白质片段,一般通过体外水解、体内胃肠道消化或微生物发酵获得[1]。BAPs的生物学活性与其氨基酸组成、序列和结构相关,能够直接被人体肠道消化吸收,并以完整的形式进入循环系统,从而具有较高的生物利用度[2]。与传统化学药物相比,BAPs具有更大的组织亲和力和特异性,并且副作用更小[3],因此在预防和治疗许多疾病方面有很大的应用前景。
植物源BAPs的来源包括豆类、谷物、坚果、水果和蔬菜等[4],与动物来源相比,植物源BAPs来源丰富、制备简单且成本低,具有良好的发展趋势[5]。近年来,植物源BAPs的多种生物活性不断被挖掘,包括降血压、抗氧化、抗菌、降血糖和抗癌等[6]。相关研究指出,植物源BAPs可作为药物成分用于治疗糖尿病和高血压等慢性代谢疾病[7],具有很高的食用及药用价值,能够满足人体健康的多种需求。
随着现代科学技术的发展,植物源BAPs分离纯化体系逐渐成熟,研究人员对植物源BAPs的探究不断深入,植物源BAPs特有的结构特性和功能机制日益受到关注,然而,对植物源BAPs的作用机制及潜在应用等方面的研究较少。因此,本文基于近年来植物源BAPs的最新研究进展,重点对植物源BAPs的制备方法、生理活性和作用机理进行综述,为植物源BAPs构效-量效关系及营养健康食品的开发及产业化应用提供理论依据。
1. 植物源生物活性肽制备方法
植物源BAPs根据来源可分为天然BAPs和人工合成BAPs,其制备方法主要包括溶剂萃取、酶解、微生物发酵、酶-菌协同、化学合成和DNA重组技术等[8],各种制备方法的优缺点详见表1。
表 1 生物活性肽制备方法优缺点Table 1. Advantages and disadvantages of bioactive peptide preparation methods制备方法 优点 缺点 溶剂萃取 专一性强、操作简单、条件温和 提取率低、溶剂残留、成本较高 酶解 特异性强、反应时间短、操作可控 成本较高、难以纯化、水解酶类型较少 微生物发酵 安全无毒、成本较低、酶的多样性 生产周期长,效率较低、产物较复杂 酶-菌协同 成本效益、高效安全 技术复杂、纯化复杂、酶稳定性较低 化学合成 技术成熟、精确可控、可制定性强 成本昂贵、需要目标肽序列 1.1 溶剂萃取法
溶剂萃取法制备BAPs常利用相似相溶的原理,常见的溶剂主要是盐溶液,其对多肽具有良好的稳定性和溶解度,此外,有机溶剂因其与多肽结合牢固,具有更多的非极性侧链也可作为提取溶剂[9]。使用溶剂萃取法制备多肽时,制备条件(时间、温度、溶剂体积比、pH)的优化是关键的一步,通过优化这些参数,可以提高多肽得率。Mahatmanto等[10]采用乙腈/水/甲酸(25:24:1)、乙酸钠(20 mmol/L,pH5.0)、碳酸氢铵(5 mmol/L,pH8.0)和沸水4种方法,实现了从苦瓜种子中对9种已知胱氨酸结肽的最有效提取,这4种方法获得多肽产率分别为65.7%、22.9%、7.0%、2.7%。此外,可采用新型加工技术(微波、超声、亚临界水等)结合溶剂萃取制备BAPs,以提高多肽得率。Hernández-corroto等[11]使用由氯化胆碱和乙酸构成的天然深共晶溶剂(NADES)和加压液体萃取(PLE)法连续从石榴皮中提取活性肽,在最佳条件下(超声振幅80%、提取时间15 min、温度60 ℃,样品量27.8 mg),肽得率为13.3 g/100 g。但由于溶剂萃取法制备BAPs周期长、成本高、得率低、不易进行规模化生产而未被普遍应用。
1.2 酶解法
酶解法是制备BAPs最常用的方法,主要是通过微生物来源的碱性蛋白酶和中性蛋白酶[12]、动物来源的胰蛋白酶和胃蛋白酶[13]、植物来源的木瓜蛋白酶和菠萝蛋白酶[14]等不同蛋白酶对蛋白质进行水解,促使蛋白质内部释放出不同活性的肽段。由于蛋白酶对底物具有一定的特异性,并且水解条件(pH、温度、时间、料液比、底物浓度)和水解程度不同,会形成氨基酸组成和排列顺序不同的肽段,从而影响BAPs的功能活性。Liao等[15]采用木瓜蛋白酶水解小麦胚芽蛋白制备抗氧化肽,发现在酶添加量为8000 U/g、pH6、水解温度为55 ℃和底物浓度为2%时,小麦胚芽蛋白的水解度最高,可达到65%,并鉴定出分子量在3~5 kDa的小麦胚芽蛋白肽具有较高的抗氧化活性。Lu等[16]采用碱性蛋白酶和胰蛋白酶双酶体系在45 ℃、200 r/min的酶解条件下反应2 h制备了芝麻蛋白水解物,并通过质谱鉴定发现7种肽段,其中肽SYPTECRMR抗氧化活性最强,其1,1-二苯基-2-三硝基苯肼(DPPH)自由基清除率和2,2'-联氮-双-3-乙基苯并噻唑啉-6-磺酸(ABTS)阳离子自由基清除率的半抑制浓度(IC50)值分别为0.105 mg/mL和0.004 mg/mL。滕飞等[17]采用中性蛋白酶水解大豆制备降糖肽,结果表明,当酶添加量7000 U/g、底物浓度3%、酶解时间1 h时,大豆肽具有较高的降糖活性。和丽等[18]采用复合蛋白酶和木瓜蛋白酶进行复配制备青刺果抑菌肽,当酶添加量4.5%、酶解时间4.7 h、pH6.9时,水解度可达到73.92%,对金黄色葡萄球菌的抑菌率可达到31.62%。综上,选择最适酶或酶组合是获得高产率和不同活性肽段的重要条件。
1.3 微生物发酵法
微生物发酵法是利用微生物发酵过程中产生的复合酶系将大分子蛋白分解为具有特异活性的小分子肽[19],研究表明,枯草芽孢杆菌[20]、乳酸菌[21]、酵母菌[22]和曲霉[23]等菌种适用于多肽制备。一些细菌如枯草芽孢杆菌发酵后主要以产内切蛋白酶为主,通过水解切断蛋白质肽链中间以产生小分子多肽,而一些真菌如米曲霉主要以产端肽酶为主,通过切除末端氨基酸,达到酶解与脱苦一步到位的目的[24]。因此,由于不同微生物产生蛋白酶的底物特异性,所制备的BAPs功能活性也不同。尹乐斌等[25]利用枯草芽孢杆菌发酵豆渣制备大豆多肽,发现在接种量3%、发酵温度37 ℃、发酵时间60 h条件时大豆多肽的得率达到88.12%。龙久铃等[26]以苏麻饼粕为原料利用米曲霉发酵制备多肽,在发酵时间48 h、发酵温度28 ℃、接种量5.5%、料液比1:1条件下,多肽含量为59.97 mg/g,比发酵前增加了2.66倍,DPPH自由基清除率为83.65%,比发酵前增加了0.23倍。Xie等[27]以豆粕为原料利用枯草芽孢杆菌发酵制备抗氧化肽,在发酵时间40 h,发酵温度37 ℃,接种量1%条件下,肽含量最高可达12.36±0.02 mg/mL。Du等[28]采用植物乳杆菌发酵制备黑芝麻肽,结果表明,在发酵时间36 h,发酵温度30 ℃,接种量3%条件下,发酵液的ACE抑制活性最高。与其他方法相比,微生物发酵法降低了生产成本,但也具有一定的缺点如生产周期长,效率相对较低等问题,因此,其发酵技术应进一步完善,以提高肽得率和肽纯度。
1.4 酶-菌协同法
酶-菌协同法是指在制备过程中,添加益生菌和酶制剂,通过益生菌与酶制剂的协同作用,最大化将蛋白质水解成小分子多肽。酶解法的制备规模通常受到酶本身的产量和成本的制约,限制了大规模多肽的制备[29]。发酵是微生物驱动的过程,由于微生物蛋白酶的多样性,可大量水解底物蛋白富集BAPs,在酶解前使用菌株发酵可以增加BAPs的释放量,并能够通过减少抗营养因子的产生进而改善原料的功能特性,被广泛应用于多肽的制备[30]。Fan等[31]通过枯草芽孢杆菌、鼠李糖乳杆菌、保加利亚乳杆菌等16种菌株发酵并添加脂肪酶、淀粉酶酶解辅助发酵制备藜麦多肽,结果表明酶-菌协同法制备的多肽比单一酶解法制备的多肽具有更高的ACE抑制活性。同时,发酵后的藜麦肽对大肠杆菌有明显的抑制作用。Ma等[32]采用酶-菌协同法制备鹰嘴豆肽,通过添加0.5% α-淀粉酶和0.1%脂肪酶对鹰嘴豆进行酶解,协同嗜酸乳杆菌发酵,结果表明,此方法发酵后的肽产率最高,为52.99%±0.88%,并且对α-葡萄糖苷酶的抑制率最高。酶-菌协同法结合了发酵和酶解两个步骤,大大提高了多肽产率,并且适当的酶还可以实现对特定蛋白质的水解,减少后续纯化步骤的复杂性,提高多肽纯度。但酶-菌协同法操作较复杂,需要精确控制多个参数,以确保整个过程的成功。
1.5 化学合成法
化学合成法是指利用多肽合成器等设备,对已知氨基酸序列的多肽进行化学合成,能够大量获得高纯度的单肽,以进一步研究其功能特性,主要是通过氨基酸的脱水缩合完成,按合成介质可分为液相合成和固相合成,液相合成是通过从多肽链的C端逐步添加所需的氨基酸,或通过先合成多个片段,然后缩合各片段以合成靶多肽的方式完成[33]。固相合成法中普遍被人们采用的是由Merrifield于1963年创立的方法[34],其主要是将受保护氨基酸的C端重复偶联于不溶性树脂载体上,再将脱保护的氨基酸N端通过缩合反应与羧基已经活化的第二个氨基酸连接,重复上述操作,直至多肽链完整[35]。Shwaiki等[36]固相合成了从龙葵中提取的衍生肽Snakin-1,纯度高达80%。李婉秋[37]通过固相合成法合成九种葵花籽肽SFNVD、EADLYN、ALEPIER、AEDFQV、DFVNPQ、NQLDEYQ、ETPEE、FEGGSIE和GNIFNG,合成肽纯度均高于95%。Zhao等[38]固相合成了TWLPLPR、YVLLPSPK和KVPPLLY三种新型核桃肽,合成肽纯度均高于90%。化学合成方法有利于有效去除副产物,以获得高纯度的肽,随着合成技术和纯化技术的不断进步,其在未来的研究和应用中将发挥更大的潜力。
2. 植物源BAPs生理活性与作用机制
植物源BAPs是植物蛋白质的水解产物,可以通过微生物发酵、酶解和萃取等多种方法制备。植物源BAPs从前体蛋白释放,因其肽序列、氨基酸组成以及化学结构等方面均有差异而表现出不同的生理活性。近年来,大量植物源BAPs生理活性评价研究主要集中在抗氧化、降糖、降压、抗菌、抗癌、免疫活性、调节肠道菌群等方面。
2.1 抗氧化活性
氧化应激可导致机体细胞凋亡、组织损伤和疾病产生,如糖尿病、炎症、癌症和衰老等。植物源抗氧化肽是天然抗氧化剂的良好来源,可以增强机体的抗氧化能力,保护机体免受活性氧损伤,降低慢性疾病的风险[39]。抗氧化肽作用机制主要包括:通过提供质子或电子直接清除自由基、抑制脂质过氧化反应、螯合金属离子、激活机体抗氧化防御系统[40]。Lu等[16]从芝麻蛋白水解物中鉴定出具有较强抗氧化活性的肽SYPTECRMR,研究表明肽SYPTECRMR的抗氧化活性可能与C端氨基酸残基精氨酸、半胱氨酸和蛋氨酸周围负电荷基团的存在有关。Zheng等[41]通过水解藜麦蛋白分离出3种具有抗氧化活性的肽,其中肽QFLLAGR因其谷氨酰胺和精氨酸残基侧链氨基的存在,表现出较高的Fe2+螯合能力(82.48%)。Chen等[42]研究表明,从微藻蛋白水解产物中提取的肽NDAEYGICGF能够减少乙醇所诱导的人类肝细胞癌(HepG2)的氧化应激反应,推测可能是由于该肽对γ-谷氨酰胺转肽酶(GGT)蛋白表达的抑制作用。这些机制使抗氧化肽能够减轻自由基对细胞和组织的氧化损伤,保护细胞免受氧化应激的伤害,同时维护细胞的正常功能。抗氧化肽的作用机制可能因具体的抗氧化肽类型和细胞环境的不同而有所差异。
2.2 降糖活性
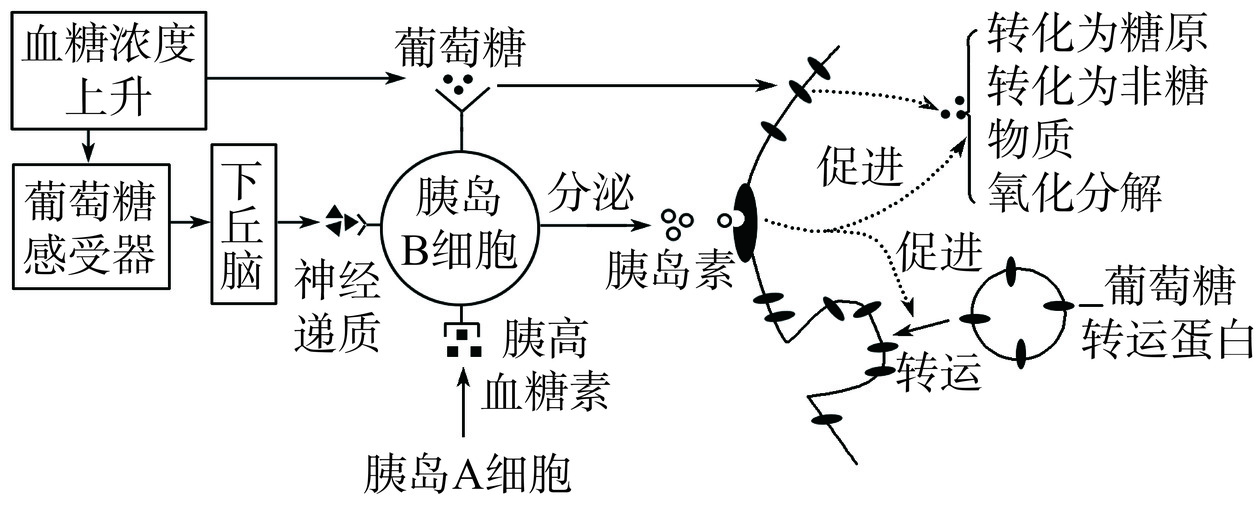
糖尿病是一种严重而复杂的慢性代谢疾病,其特征是由于胰岛素抵抗或胰岛素分泌不足而导致的血糖升高[43]。大量研究表明,α-淀粉酶、α-葡萄糖苷酶和二肽基肽酶是调节血糖的关键酶[44],抑制这些酶的活性被认为是控制糖尿病的有效策略。降糖肽的作用机制主要包括:促进胰岛素分泌、抑制肝糖原分解、抑制胃肠葡萄糖吸收(如图1)[45]。Wang等[46]从核桃蛋白水解物中鉴定出一种新型抗糖尿病肽(LPLLR),该肽对α-淀粉酶和α-葡萄糖苷酶具有抑制作用,并指出LPLLR的抗糖尿病机制是通过激活IRS-1/PI3K/Akt和AMPK胰岛素信号通路,从而改善葡萄糖摄取和糖原合成,减少糖异生。Xu等[47]通过5种酶水解大豆分离蛋白,结果表明,碱性蛋白酶水解产生的BAPs表现出最高的降糖活性,通过分析其分子量分布和氨基酸组成,发现碱性蛋白水解物中芳香族氨基酸和疏水氨基酸含量与降糖活性呈正相关。Wei等[48]以芍药凤丹籽粕蛋白为原料,采用连续酶解法制备多肽混合物,通过液相色谱-电喷雾电离源-质谱/质谱联用(LC-ESI-MS/MS)法鉴定出6种新型肽段,其中,两种新型肽PPPM和TTPM表现出较强的α-葡萄糖苷酶抑制活性。Vilcacundo等[49]通过胃肠道模拟体外消化评估了藜麦多肽对α-淀粉酶和α-葡萄糖苷酶的抑制活性,结果表明,在胃部消化释放的多肽具有较强的降糖活性。目前,已有大量研究证明植物源BAPs具有降糖活性,但缺乏细胞培养和体外动物模型实验进一步验证植物源BAPs的具体降糖效果。此外,其潜在的作用靶点与功能机制还需要进一步挖掘。
2.3 ACE抑制活性
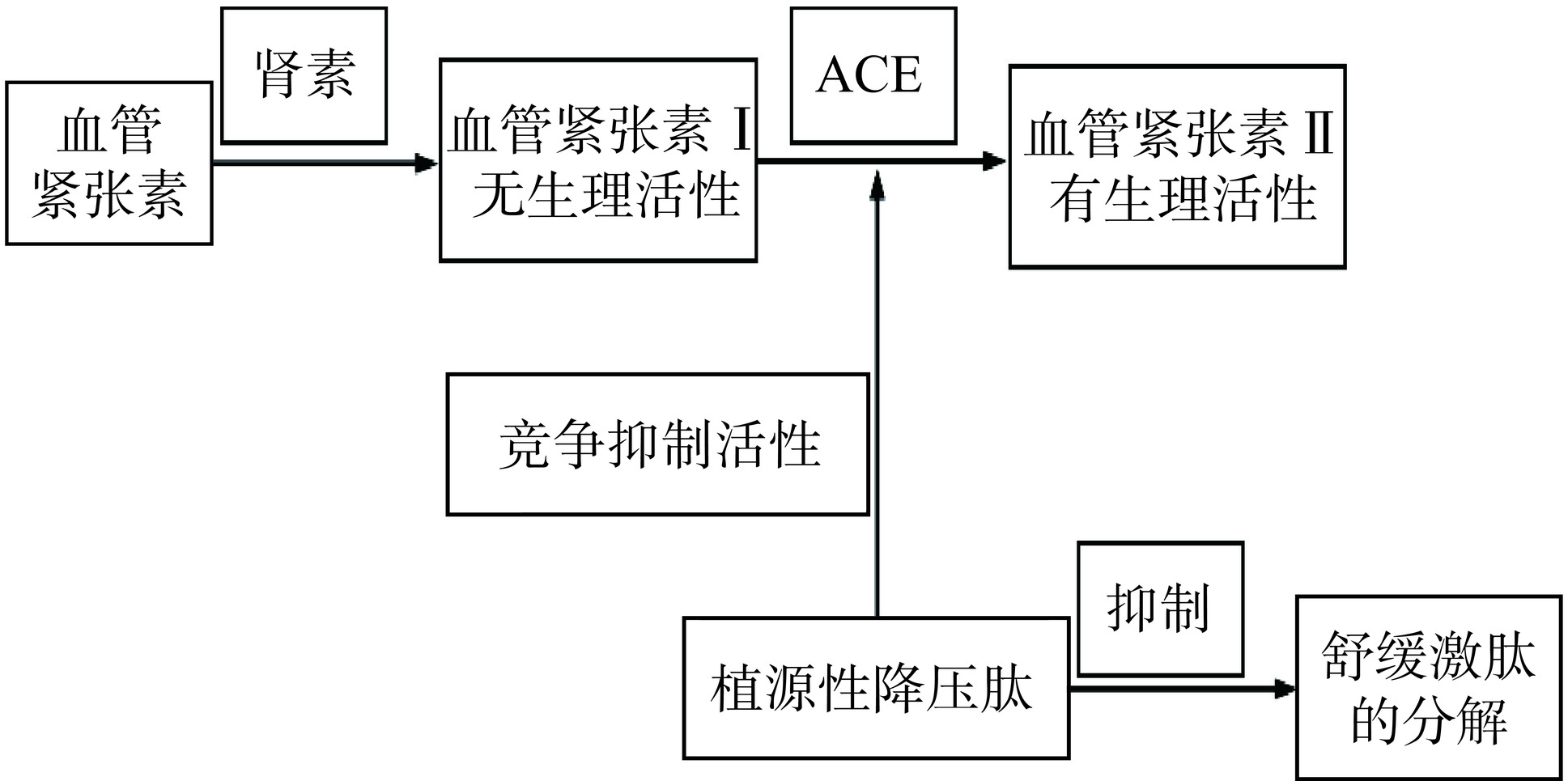
血管紧张素转换酶(ACE)在血压调节中发挥重要作用,主要作用于血管紧张素I,可通过肾素-血管紧张素系统(Renin-angiotensin system,RAS)产生血管紧张素II,进而使激肽释放酶-激肽系统(Kallikrein-kinin system,KKS)中的缓激肽失活,最终导致血压升高[50],通过抑制ACE调节RAS被认为是其降血压活性的主要机制(如图2)[51]。Duan等[52]从油菜籽中分离并鉴定出新型ACE抑制肽FQW、FRW和CPF,其在体外有较强的ACE抑制活性,IC50值分别为46.84、46.30和131.35 μmol/mL,分子对接表明,这3种肽段均能通过氢键和疏水作用与ACE活性位点相互作用。Shobako等[53]从米糠蛋白中分离鉴定了一种新的降压肽LRA,并对其降压作用机制和LRA的构效关系进行了探讨,结果表明,LRA可以通过一氧化氮(NO)介导的血管松弛通过内皮细胞释放NO,并作用于血管平滑肌细胞,诱导血管的舒张和松弛,从而引起血管扩张和降低血压。Zou等[54]在体外构建自发性高血压大鼠模型,评估麦麸蛋白水解产物的ACE抑制活性,结果表明,麦麸蛋白肽对大鼠体内的肾素和ACE有显著的抑制作用。肽的ACE抑制活性受肽链长度、组成和序列等结构特征的影响。这些新型抑制剂可能具有更好的生物利用度、抗酶解性和选择性,将为ACE抑制肽的临床应用带来更好的前景。
2.4 抑菌活性
抑菌肽是由多种生物产生的具有抗菌活性的小肽,因其高效、特异且副作用小并且不会引起微生物的抗生素耐药性而引起越来越多的人关注[55]。抑菌肽的作用机制主要涉及其与微生物细胞膜相互作用、破坏微生物膜、抑制蛋白质和核酸的合成等[56]。Hu等[57]用发酵核桃粉制备抑菌肽,鉴定出FGGDSTHP、ALGGGY、YVVPW、PLLRW 4种抑菌肽,分子对接验证了YVVPW通过氢键、疏水作用与水杨酸表现出协同作用,平均最小抑制浓度降低85.44%。Kong等[58]对棉籽蛋白进行水解,并通过分离纯化鉴定出3种新型抑菌肽HHRRFSLY、KFMPT、RRLFSDY,证明了3种肽通过破坏大肠杆菌的细胞膜从而达到抑菌效果。王丽芳等[59]以龙井茶叶为原料制备抗菌肽,结果表明,分子量<18.4 kDa的肽对金黄色葡萄球菌和大肠埃希菌均有明显的抑菌活性,可抑制冷却肉中微生物的生长,对冷却肉具有防腐保鲜作用。近年来,研究人员通过多种途径不断发现和设计新型抑菌肽,包括从天然抑菌肽中进行改造、合成分子肽段和激发宿主免疫系统等。这些方法为开发更具活性和稳定性的抑菌肽提供了新的思路。
2.5 抗癌活性
近年来,随着医学研究的进步和新型抗肿瘤药物的出现,多肽化合物在临床抗癌治疗中的作用受到了广泛关注。多肽化合物可直接或间接作用于肿瘤细胞,调节肿瘤细胞的生长和凋亡,促进与肿瘤凋亡相关的物质活性或信号[60]。Rasaratnam等[61]研究了从大蒜中分离的多肽对白血病细胞系的抗癌活性,鉴定出了一种新型抗癌肽VKLRSLLCS,该肽被证明可显著抑制MOLT-4和K562白血病细胞系的细胞增殖,通过抗凋亡Bcl-2蛋白家族对白血病细胞系具有凋亡诱导特性。Xie等[62]研究证明核桃蛋白水解物(WPH-Al)显著抑制结肠癌HCT116细胞的生长并诱导其凋亡,通过计算机模拟从分子量<1 kDa的肽中鉴定了三种具有抗癌活性的肽(PISLKSE、VSLP和SHTLP)。Li等[63]采用木瓜蛋白酶水解绿豆制备多肽,证实绿豆肽可以抑制小鼠肿瘤细胞的增殖,促进HepG2细胞凋亡,并鉴定出了2种可抑制HepG2细胞增殖的绿豆肽片段(VEG、PQG、LAF和EGA)。目前,抗癌肽的作用机制是一个重要的研究方向。通过研究抗癌肽与肿瘤细胞的相互作用,揭示其对细胞凋亡、细胞周期调控、血管生成等的影响,以促进其在功能食品或特殊医疗用途食品中发挥显著作用。
2.6 免疫活性
研究表明,机体失去对免疫调节系统的控制可能导致多种疾病发生,如肿瘤、脑损伤、肥胖和肾脏疾病[64]。据文献报道,植物源免疫调节肽对先天和适应性免疫反应均有影响,包括增加自然杀伤细胞(NK细胞)的细胞毒性,增强巨噬细胞的吞噬能力,促进淋巴细胞和免疫细胞的增殖和成熟,提高宿主对入侵病原体的防御能力,促进抗体、细胞因子和趋化因子等免疫调节剂的诱导。Ndiaye等[65]采用嗜热菌蛋白酶水解豌豆种子制备豌豆水解物(PPH),通过小鼠模型实验证明其免疫活性,PPH显著抑制了其促炎细胞因子TNF-α和IL-6的分泌,其抑制率分别高达35%和80%,小鼠口服PPH增强了其腹膜巨噬细胞的吞噬活性,刺激了肠道粘膜免疫反应,并提高了小肠中代表肠粘膜免疫的IgA+和细胞因子阳性细胞的数量。Wen等[66]采用碱性蛋白酶水解大豆制备多肽,通过对肽片段进行鉴定,筛选出51种肽序列,其中46种为免疫调节肽,通过评价其免疫活性和作用机制发现其可以促进巨噬细胞的增殖。Fang等[67]采用RAW264.7细胞模型从富硒大米蛋白水解物中筛选出免疫调节含硒肽,鉴定出25个含硒肽序列,其中人工合成的肽TSeMMM在80 μg/mL浓度下具有出色的免疫调节活性。目前,已从大豆、小麦、水稻、玉米、黄豆、苋菜等多种植物源中报道了免疫调节肽,这些研究表明,植物源的免疫调节肽具有广泛的生物活性,能够通过不同的机制调节免疫反应,为食品科学和营养学领域提供了重要的研究资源。
2.7 调节肠道菌群
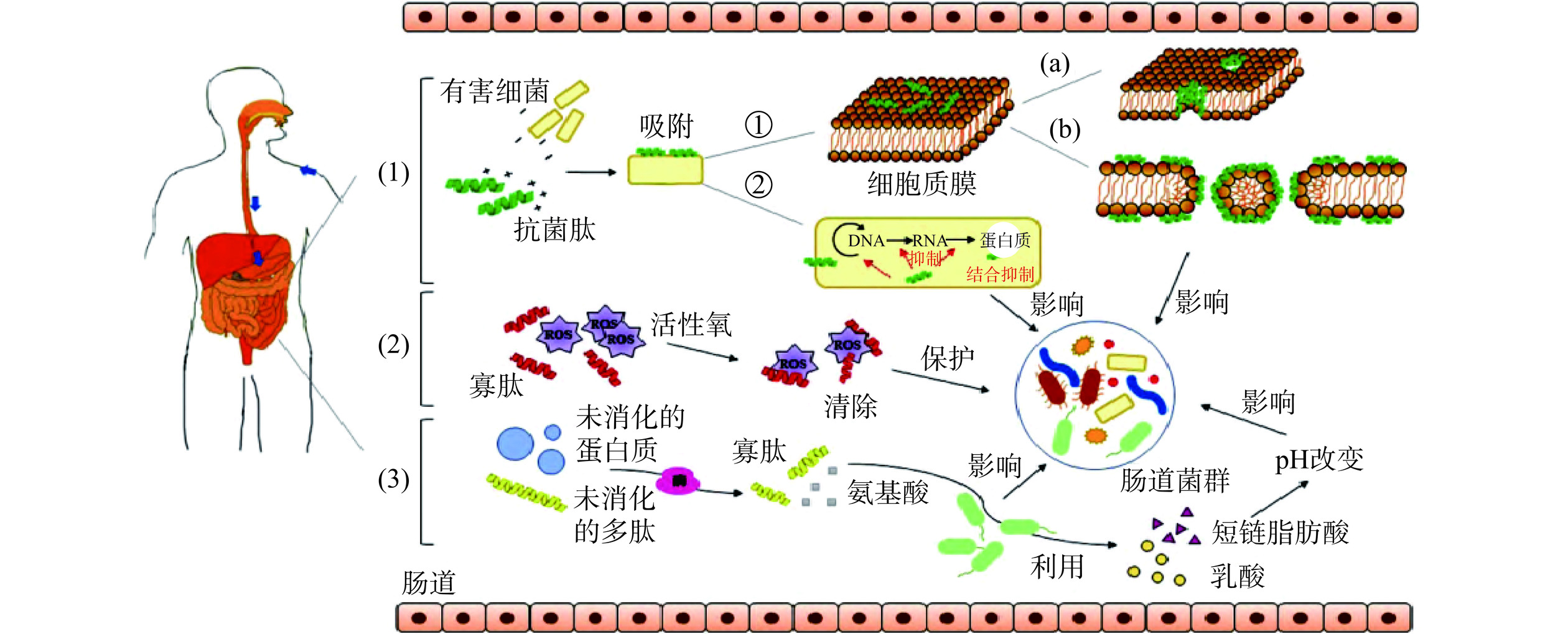
近年来人们普遍认为肠道微生物在人类各种疾病中发挥着重要作用,被认为是人体的“第二基因组”。研究指出,BAPs能够对肠道菌群起到调节作用,其机制主要包括破坏有害菌体的结构、清除肠道ROS、作为肠道菌群的底物被利用(如图3)[68]。陈元蓉[69]采用模拟胃肠道消化法和超滤膜分离技术对小麦胚芽抗炎肽进行分离纯化,并通过LC-MS/MS法对肽段进行鉴定,结果表明,小麦胚芽活性肽APEPAPAF显著提高了Simpson指数,并降低了Shannon指数,此外,小麦胚芽活性肽APEPAPAF显著降低了拟杆菌属的丰度,提高了杜氏杆菌和毛螺旋菌菌属的丰度。Zhang等[70]分析了小豆蔻含硒肽对肠道微生物菌群的调节及影响,在给予小豆蔻含硒肽的抗生素处理小鼠中发现阿克曼菌属、厚壁菌门和拟杆菌门的丰度较高,而变形菌门的丰度降低。Zhang等[71]研究表明,分子量<3 kDa的大豆肽能够抑制细胞内脂质的过氧化反应,保护细胞免受ROS的损害,此外,添加大豆肽可缓解机体氧化应激反应,并且有助于提高肠道微生物群落的多样性和丰富度。
2.8 改善记忆和认知
记忆和认知能力下降是各种神经退化性疾病的主要临床症状,如阿尔兹海默症和与年龄有关的痴呆症,神经退行性疾病与多种病理特征和机制有关,包括线粒体功能障碍、神经营养信号障碍、神经递质减少、胆碱能系统损伤以及神经元损伤等[72]。Chen等[73]研究表明核桃粕水解物具有很强的羟基清除能力,此外在Morris水迷宫试验和小鼠避光/避暗试验中核桃粕水解物能有效对抗D-半乳糖诱导的学习和记忆障碍,抑制H2O2诱导的PC12细胞凋亡。Zhao等[38]报道从核桃中分离的三种神经保护肽(KVPPLLY、YVLLPSPK和TWLPLPR)可通过提高相关基因水平调节PC12细胞自噬,纠正自噬反应对细胞存活和预防神经退行性疾病至关重要。Ju等[74]从大豆蛋白水解物中鉴定出一种生物活性肽VHVV,该肽具有血脑屏障渗透性和对胃蛋白酶和胰酶的抗性,口服VHVV可通过激活环磷腺苷效应元件结合蛋白(CREB)介导的下游蛋白和上调脑源性神经营养因子(BDNF)的分子水平表达,抑制自发性高血压大鼠神经元退化和记忆丧失。目前,对植物源性神经保护肽的研究主要集中在对其功效的评价上,口服给药的生物活性肽能否通过肠屏障和血脑屏障,以完整的形式到达脑组织,目前尚不完全清楚。
3. 结论与展望
植物源BAPs因其多样的生物活性和广泛的应用前景而受到营养和食品领域的广泛关注,目前的制备方法(酶解法和微生物发酵法),已成功应用于活性肽的提取,但仍需进一步优化技术以提高效率和降低成本。研究表明,这些活性肽通过多种机制发挥作用,包括抗氧化、抗菌和免疫调节等,为多种慢性疾病提供了潜在的预防和治疗策略,尽管有许多研究表明植物来源的多肽对健康有益,但其人体功效以及临床应用还需要更深入的研究,植物源BAPs及其功能片段如何通过机体代谢发挥其生物活性(肠道屏障、血脑屏障等)以及其潜在的作用靶点与功能机制还需要进一步挖掘。因此,未来研究更应该着重基于人体健康效应的BAPs“构效-量效”关系,这对深入了解肽的靶向作用机制、优化其生物活性以及精准设计功能性食品或药物具有重要意义。
-
表 1 生物活性肽制备方法优缺点
Table 1 Advantages and disadvantages of bioactive peptide preparation methods
制备方法 优点 缺点 溶剂萃取 专一性强、操作简单、条件温和 提取率低、溶剂残留、成本较高 酶解 特异性强、反应时间短、操作可控 成本较高、难以纯化、水解酶类型较少 微生物发酵 安全无毒、成本较低、酶的多样性 生产周期长,效率较低、产物较复杂 酶-菌协同 成本效益、高效安全 技术复杂、纯化复杂、酶稳定性较低 化学合成 技术成熟、精确可控、可制定性强 成本昂贵、需要目标肽序列 -
[1] ZHANG Y, LIU L, ZHANG M, et al. The research progress of bioactive peptides derived from traditional natural products in China[J]. Molecules,2023,28(17):6421. doi: 10.3390/molecules28176421
[2] ZOU T B, HE T P, LI H B, et al. The structure-activity relationship of the antioxidant peptides from natural proteins[J]. Molecules,2016,21(1):72. doi: 10.3390/molecules21010072
[3] FENG Y, YIN Z, ZHANG D, et al. Chinese medicine protein and peptide in gene and cell therapy[J]. Current Protein & Peptide Science,2019,20(3):251−264.
[4] HU Y, NI C, WANG Y, et al. Research progress on the preparation and function of antioxidant peptides from walnuts[J]. International Journal of Molecular Sciences,2023,24(19):14853. doi: 10.3390/ijms241914853
[5] SOSALAGERE C, ADESEGUN K B, SHARMA P. Isolation and functionalities of bioactive peptides from fruits and vegetables:A reviews[J]. Food Chemistry,2022,366:130494. doi: 10.1016/j.foodchem.2021.130494
[6] PIOVESANA S, CAPRIOTTI A L, CAVALIERE C, et al. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation[J]. Analytical and Bioanalytical Chemistry,2018,410(15):3425−3444. doi: 10.1007/s00216-018-0852-x
[7] XU Q, HONG H, WU J, et al. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane:A review[J]. Trends in Food Science and Technology,2019,86:399−411. doi: 10.1016/j.jpgs.2019.02.050
[8] ZHAO C, XIAO Y, LING S, et al. Recombination and purification of elastin-like polypeptides[J]. Methods in Molecular Biology,2021,2347:97−103.
[9] BAGHALABADI V, RAZMI H, DOUCETTE A. Salt-mediated organic solvent precipitation for enhanced recovery of peptides generated by pepsin digestion[J]. Proteomes,2021,9(4):44. doi: 10.3390/proteomes9040044
[10] MAHATMANTO T, POTH A G, MYLNE J S, et al. A comparative study of extraction methods reveals preferred solvents for cystine knot peptide isolation from Momordica cochinchinensis seeds[J]. Fitoterapia,2014,95:22−33. doi: 10.1016/j.fitote.2014.02.016
[11] HERNÁNDEZ-CORROTO E, PLAZA M, MARINA L M, et al. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents[J]. Innovative Food Science and Emerging Technologies, 2020, 60:102314-102314.
[12] ZHANG Q, TONG X, LI Y, et al. Purification and characterization of antioxidant peptides from alcalase-hydrolyzed soybean (Glycine max L.) hydrolysate and their cytoprotective effects in human intestinal Caco-2 cells[J]. Journal of Agriculture and Food Chemistry,2019,67(20):5772−5781. doi: 10.1021/acs.jafc.9b01235
[13] ONG J H, KOH J A, CAO H, et al. Purification, identification and characterization of antioxidant peptides from corn silk tryptic hydrolysate:An integrated in vitro-in silico approach[J]. Antioxidants,2021,10(11):1822. doi: 10.3390/antiox10111822
[14] TIAN S, DU K, YAN F, et al. Microwave-assisted enzymatic hydrolysis of wheat germ albumin to prepare polypeptides and influence on physical and chemical properties[J]. Food Chemistry,2022,374:131707. doi: 10.1016/j.foodchem.2021.131707
[15] LIAO A M, LI X X, GU Z, et al. Preparation and identification of an antioxidant peptide from wheat embryo albumin and characterization of its Maillard reaction products[J]. Journal of Food Science,2022,87(6):2549−2562. doi: 10.1111/1750-3841.16191
[16] LU X, ZHANG L, SUN Q, et al. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L. ) protein hydrolysate[J]. Food Research International,2019,116:707−716. doi: 10.1016/j.foodres.2018.09.001
[17] 滕飞, 李欣芯, 孟繁达, 等. 超声波-微波预处理辅助酶解制备大豆降糖肽及其稳定性分析[J]. 现代食品,2023,29(21):59−65. [TENG F, LI X X, MENG F D, et al. Preparation of soybean glucose-lowering peptide by ultrasound-microwave pretreatment-assisted enzymatic digestion and its stability analysis[J]. Modern Food,2023,29(21):59−65.] TENG F, LI X X, MENG F D, et al. Preparation of soybean glucose-lowering peptide by ultrasound-microwave pretreatment-assisted enzymatic digestion and its stability analysis[J]. Modern Food, 2023, 29(21): 59−65.
[18] 和丽, 熊海涛, 王雪峰, 等. 响应面试验优化复合酶法制备青刺果抗菌肽的工艺研究[J]. 中国油脂,2021,46(6):33−37. [HE L, XIONG H T, WANG X F, et al. Optimization of preparation of antimicrobial peptides from Prinsepia utilis Royle by compound enzymes using response surface methodology[J]. Chinese Grease,2021,46(6):33−37.] HE L, XIONG H T, WANG X F, et al. Optimization of preparation of antimicrobial peptides from Prinsepia utilis Royle by compound enzymes using response surface methodology[J]. Chinese Grease, 2021, 46(6): 33−37.
[19] 刘奇. 微生物发酵法制备植物基免疫功能肽及其活性研究[D]. 哈尔滨:东北林业大学, 2020. [LIU Q. Research on preparation and activity of plant-based immune function peptide through microbial fermentation method[D]. Harbin:Northeast Agricultural University, 2020.] LIU Q. Research on preparation and activity of plant-based immune function peptide through microbial fermentation method[D]. Harbin: Northeast Agricultural University, 2020.
[20] 王芬, 赵腊梅. 枯草芽孢杆菌固态发酵花生粕的条件优化[J]. 饲料研究,2022,45(2):75−78. [WANG F, ZHAO L M. Optimization of solid fermentation conditions of peanut meal by Bacillus subtilis[J]. Feed Study,2022,45(2):75−78.] WANG F, ZHAO L M. Optimization of solid fermentation conditions of peanut meal by Bacillus subtilis[J]. Feed Study, 2022, 45(2): 75−78.
[21] CHEN L, WANG L, LI J, et al. Antihypertensive potential of fermented milk:The contribution of lactic acid bacteria proteolysis system and the resultant angiotensin-converting enzyme inhibitory peptide[J]. Food Function,2021,12(22):11121−11131. doi: 10.1039/D1FO02435C
[22] OLIVEIRA A S, FERREIRA C, PEREIRA J O, et al. Spent brewer's yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides:An overview[J]. Journal of The Science of Food and Agriculture,2022,208:1116−1126.
[23] ZANUTTO-ELGUI M R, VIEIRA J C S, PRADO D Z D, et al. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases[J]. Food Chemistry,2019,278:823−831. doi: 10.1016/j.foodchem.2018.11.119
[24] SHIN H Y, KIM S M, LEE J H, et al. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae:Effects on phenolic acid composition and antioxidant activity of bran extracts[J]. Food Chemistry,2019,272:235−241. doi: 10.1016/j.foodchem.2018.07.174
[25] 尹乐斌, 李乐乐, 何平, 等. 枯草芽孢杆菌发酵豆渣制备多肽及其活性研究[J]. 中国酿造,2022,41(1):75−79. [YIN L B, LI L L, HE P, et al. Preparation and activity of peptides from soybean dregs by fermentation with Bacillus subtilis[J]. Chinese Brewing,2022,41(1):75−79.] YIN L B, LI L L, HE P, et al. Preparation and activity of peptides from soybean dregs by fermentation with Bacillus subtilis[J]. Chinese Brewing, 2022, 41(1): 75−79.
[26] 龙久铃, 朱秋劲, 白晶, 等. 米曲霉固态发酵苏麻饼粕产抗氧化肽工艺优化[J]. 轻工学报,2021,36(4):18−28. [LONG J L, ZHU Q J, BAI J, et al. Optimization of production of antioxidant peptide by solid state fermentation of Fructus oryzae cake by Aspergillus o-ryzae[J]. Journal of Light Industry,2021,36(4):18−28.] LONG J L, ZHU Q J, BAI J, et al. Optimization of production of antioxidant peptide by solid state fermentation of Fructus oryzae cake by Aspergillus o-ryzae[J]. Journal of Light Industry, 2021, 36(4): 18−28.
[27] XIE M, MA Y, AN F, et al. Ultrasound-assisted fermentation for antioxidant peptides preparation from okara:Optimization, stability, and functional analyses[J]. Food Chemistry,2024,439:138078. doi: 10.1016/j.foodchem.2023.138078
[28] DU T, HUANG J, XU X, et al. Effects of fermentation with Lactiplantibacillus plantarum NCU116 on the antihypertensive activity and protein structure of black sesame seed[J]. International Journal of Biological Macromolecules, 2024, 262(Pt 1):129811.
[29] 胡子聪, 于阿立, 刘香云, 等. 食物蛋白源生物活性肽的研究进展[J]. 食品工业,2022,43(6):271−276. [HU Z C, YU A L, LIU X Y, et al. Advances on bioactive peptides derived from food proteins[J]. Food Industry,2022,43(6):271−276.] HU Z C, YU A L, LIU X Y, et al. Advances on bioactive peptides derived from food proteins[J]. Food Industry, 2022, 43(6): 271−276.
[30] 张树华. 发酵法制备绿豆多肽[D]. 济南:齐鲁工业大学, 2014:25−56. [ZHANG S H. Mung bean polypeptides was prepared by fermentation[D]. Jinan:Qilu University of Technology, 2014:25−56.] ZHANG S H. Mung bean polypeptides was prepared by fermentation[D]. Jinan: Qilu University of Technology, 2014: 25−56.
[31] FAN X, MA X, MAIMAITIYIMING R, et al. Study on the preparation process of quinoa anti-hypertensive peptide and its stability[J]. Frontiers in Nutrition,2023,9:1119042. doi: 10.3389/fnut.2022.1119042
[32] MA X, FAN X, WANG D, et al. Study on preparation of chickpea peptide and its effect on blood glucose[J]. Frontiers in Nutrition,2022,9:988628. doi: 10.3389/fnut.2022.988628
[33] 王宇航, 陈学明, 刘俗生, 等. 一种多肽固相合成方法与纯化策略研究[J]. 中国生物工程杂志,2023,43(1):35−41. [WANG Y H, CHEN X M, LIU Y S, et al. Study on solid-phase synthesis and purification strategy of polypeptide[J]. China Biotechnology,2023,43(1):35−41.] WANG Y H, CHEN X M, LIU Y S, et al. Study on solid-phase synthesis and purification strategy of polypeptide[J]. China Biotechnology, 2023, 43(1): 35−41.
[34] CHEN C H, BEPLER T, PEPPER K, et al. Synthetic molecular evolution of antimicrobial peptides[J]. Current Opinion in Biotechnology,2022,75:102718. doi: 10.1016/j.copbio.2022.102718
[35] 张红玉. 紫苏粕多肽制备及抗氧化活性研究[D]. 太原:中北大学, 2023. [ZHANG H Y. Preparation and antioxidant activity of Perilla meal polypeptide[D]. Taiyuan:North University of China, 2023.] ZHANG H Y. Preparation and antioxidant activity of Perilla meal polypeptide[D]. Taiyuan: North University of China, 2023.
[36] SHWAIKI L N, ARENDT E K, LYNCH K M, Study on the characterisation and application of synthetic peptide Snakin-1 derived from potato tubers-action against food spoilage yeast[J]. Food Control, 2020, 118:107362.
[37] 李婉秋. 葵花籽肽呈鲜味特性及其稳定性的研究[D]. 呼和浩特:内蒙古农业大学, 2022. [LI W Q. Study on the umami characteristics and stability of sunflower seed peptides[D]. Hohhot:Inner Mongolia Agricultural University, 2022.] LI W Q. Study on the umami characteristics and stability of sunflower seed peptides[D]. Hohhot: Inner Mongolia Agricultural University, 2022.
[38] ZHAO F, WANG J, LU H, et al. Neuroprotection by walnut-derived peptides through autophagy promotion via Akt/mTOR signaling pathway against oxidative stress in PC12 cells[J]. Journal of Agricultural and Food Chemistry,2020,68(11):3638−3648. doi: 10.1021/acs.jafc.9b08252
[39] ZHANG F, QU J, THAKUR K, et al. Purification and identification of an antioxidative peptide from peony (Paeonia suffruticosa Andr. ) seed dreg[J]. Food Chemistry,2019,285:266−274. doi: 10.1016/j.foodchem.2019.01.168
[40] ZHU Y, LAO F, PAN X, et al. Food protein-derived antioxidant peptides:Molecular mechanism, stability and bioavailability[J]. Biomolecules,2022,12(11):1622. doi: 10.3390/biom12111622
[41] ZHENG Y, WANG X, ZHUANG Y, et al. Isolation of novel ACE-inhibitory and antioxidant peptides from quinoa bran albumin assisted with an in silico approach:Characterization, in vivo antihypertension, and molecular docking[J]. Molecules (Basel, Switzerland),2019,24(24):4562. doi: 10.3390/molecules24244562
[42] CHEN M F, ZHANG Y Y, DI H M, et al. Antioxidant peptide purified from enzymatic hydrolysates of Isochrysis zhanjiangensis and its protective effect against ethanol induced oxidative stress of HepG2 cells[J]. Biotechnol Bioproc E,2019,24(2):308−317. doi: 10.1007/s12257-018-0391-5
[43] VALENCIA-MEJÍA E, BATISTA K A, FERNÁNDEZ J J A, et al. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.)[J]. Food Research International (Ottawa, Ont. ),2019,121:238−246. doi: 10.1016/j.foodres.2019.03.043
[44] 吴彤. 核桃降血糖活性肽的分离纯化、结构鉴定及降血糖作用机理研究[D]. 长春:吉林农业大学, 2020. [WU T. Isolation, purification, structural identification and mechanism of hypoglycemic active peptide from walnut[D]. Changchun:Jilin Agricultural University, 2020.] WU T. Isolation, purification, structural identification and mechanism of hypoglycemic active peptide from walnut[D]. Changchun: Jilin Agricultural University, 2020.
[45] 张廷新, 李富强, 张楠, 等. 降糖肽的制备、生物学效应及其构效关系研究进展[J]. 食品工业科技,2022,43(8):433−442. [ZHANG T X, LI F Q, ZHANG N, et al. Advances in preparation, biological effect and structure-activity relationship of hypoglycemic peptides[J]. Science and Technology of Food Industry,2022,43(8):433−442.] ZHANG T X, LI F Q, ZHANG N, et al. Advances in preparation, biological effect and structure-activity relationship of hypoglycemic peptides[J]. Science and Technology of Food Industry, 2022, 43(8): 433−442.
[46] WANG J, WU T, FANG L, et al. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells[J]. Journal of Functional Foods,2020,69:103944. doi: 10.1016/j.jff.2020.103944
[47] XU Y, YANG Y, MA C M, et al. Characterization of the structure, antioxidant activity and hypoglycemic activity of soy (Glycine max L. ) protein hydrolysates[J]. Food Research International, 2023, 173(Pt 2):113473.
[48] WEI R, LIN L, LI T, et al. Separation, identification, and design of α-glucosidase inhibitory peptides based on the molecular mechanism from Paeonia ostii 'Feng Dan' seed protein[J]. Journal of Food Science,2022,87(11):4892−4904. doi: 10.1111/1750-3841.16340
[49] VILCACUNDO R, CRISTÍNA M V, HERNÁNDEZ-LEDESMA B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion[J]. Journal of Functional Foods,2017,35:531−539. doi: 10.1016/j.jff.2017.06.024
[50] XUE L, YIN R, HOWELL K, et al. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme-inhibitory peptides[J]. Comprehensive Reviews in Food Science and Food Safety,2021,20(2):1150−1187. doi: 10.1111/1541-4337.12711
[51] 朱泽洋, 李熔, 袁字怡, 等. 植物源性降压肽作用机制、制备与评价方式及相关研究进展[J]. 食品工业科技,2022,43(18):501−508. [ZHU Z Y, LI R, YUAN Z Y, et al. Mechanism, preparation, evaluation and related research progress of plant-derived antihypertensive peptides[J]. Science and Technology of Food Industry,2022,43(18):501−508.] ZHU Z Y, LI R, YUAN Z Y, et al. Mechanism, preparation, evaluation and related research progress of plant-derived antihypertensive peptides[J]. Science and Technology of Food Industry, 2022, 43(18): 501−508.
[52] DUAN X, DONG Y, ZHANG M, et al. Identification and molecular interactions of novel ACE inhibitory peptides from rapeseed protein[J]. Food Chemistry,2023,422:136085. doi: 10.1016/j.foodchem.2023.136085
[53] SHOBAKO N, ISHIKADO A, OGAWA Y, et al. Vasorelaxant and antihypertensive effects that are dependent on the endothelial NO system exhibited by rice bran-derived tripeptide[J]. Journal of Agricultural and Food Chemistry,2019,67(5):1437−1442. doi: 10.1021/acs.jafc.8b06341
[54] ZOU Z, WANG M, WANG Z, et al. Antihypertensive and antioxidant activities of enzymatic wheat bran protein hydrolysates[J]. J Food Biochem,2020(44):e13090.
[55] ZHANG R, XU L, DONG C. Antimicrobial peptides:An overview of their structure, function and mechanism of action[J]. Protein and Peptide Letters,2022,29(8):641−650. doi: 10.2174/0929866529666220613102145
[56] VALDEZ-MIRAMONTES C E, DE HARO-ACOSTA J, ARÉCHIGA-FLORES C F, et al. Antimicrobial peptides in domestic animals and their applications in veterinary medicine[J]. Peptides,2020,142:170576.
[57] HU Y, LING Y, QIN Z, et al. Isolation, identification, and synergistic mechanism of a novel antimicrobial peptide and phenolic compound from fermented walnut meal and their application in Rosa roxbughii Tratt spoilage fungus[J]. Food Chemistry,2024,433:137333. doi: 10.1016/j.foodchem.2023.137333
[58] KONG X, SONG W, HUA Y, et al. Insights into the antibacterial activity of cottonseed protein-derived peptide against Escherichia coli[J]. Food Function,2020,11(11):10047−10057. doi: 10.1039/D0FO01279C
[59] 王丽芳, 叶良, 谢忠稳, 等. 茶叶抗菌肽粗提物的抑菌活性及其对冷却肉保鲜的影响[J]. 浙江农业学报,2022,34(10):2268−2276. [WANG L F, YE L, XIE Z W, et al. Antibacterial activity of tea antimicrobial peptide extracts and its effect on the preservation of chilled meat[J]. Zhejiang Agricultural Journal,2022,34(10):2268−2276.] WANG L F, YE L, XIE Z W, et al. Antibacterial activity of tea antimicrobial peptide extracts and its effect on the preservation of chilled meat[J]. Zhejiang Agricultural Journal, 2022, 34(10): 2268−2276.
[60] ZHU Z, ZHOU H, CHEN F, et al. Sinomenine derivatives:Synthesis, antitumor activity, and apoptotic induction in MCF-7 Cells via IL-6/PI3K/Akt/NF-κB signaling pathway[J]. Chem Med Chem,2022,17(14):00234.
[61] RASARATNAM K, NANTASENAMAT C, PHAONAKROP N, et al. A novel peptide isolated from garlic shows anticancer effect against leukemic cell lines via interaction with Bcl-2 family proteins[J]. Chemical Biology & Drug Design,2021,97(5):1017−1028.
[62] XIE J, HONG Z S, DAI J J, et al. Isolation and identification of anti-colorectal cancer peptides from walnut proteins and associated in silico analysis[J]. Journal of Functional Foods,2024,112:105952. doi: 10.1016/j.jff.2023.105952
[63] LI M Q, ZHANG Y J, XIA S W, et al. Finding and isolation of novel peptides with anti-proliferation ability of hepatocellular carcinoma cells from mung bean protein hydrolysates[J]. Journal of Functional Foods,2019,62:103557. doi: 10.1016/j.jff.2019.103557
[64] LIU Y, LI L, LI Y, et al. Research progress on tumor-associated macrophages and inflammation in cervical cancer[J]. Biomed Research International, 2020, 6842963.
[65] NDIAYE F, VUONG T, DUARTE J, et al. Anti-oxidant, anti-inflammatory and immunomodulating properties of an enzymatic protein hydrolysate from yellow field pea seeds[J]. European Journal of Nutrition,2012,51(1):29−37. doi: 10.1007/s00394-011-0186-3
[66] WEN L, JIANG Y, ZHOU X, et al. Structure identification of soybean peptides and their immunomodulatory activity[J]. Food Chemistry,2021,359:129970. doi: 10.1016/j.foodchem.2021.129970
[67] FANG Y, PAN X, ZHAO E, et al. Isolation and identification of immunomodulatory selenium-containing peptides from selenium-enriched rice protein hydrolysates[J]. Food Chemistry,2019,275:696−702. doi: 10.1016/j.foodchem.2018.09.115
[68] 侯梦凡, 胡晓. 生物活性肽对肠道菌群调节作用研究进展[J]. 中国食品学报,2022,22(11):410−423. [HOU M F, HU X. Research progress on regulating effects of bioactive peptides on gut microbiota[J]. Journal of Chinese Institute of Food Science and Technology,2022,22(11):410−423.] HOU M F, HU X. Research progress on regulating effects of bioactive peptides on gut microbiota[J]. Journal of Chinese Institute of Food Science and Technology, 2022, 22(11): 410−423.
[69] 陈元蓉. 小麦胚芽活性肽对DSS诱导小鼠结肠炎的改善作用及机制研究[D]. 南京:南京财经大学, 2022. [CHEN Y R. Effect and mechanism of wheat germ active peptide on colitis induced by DSS in mice[D]. Nanjing:Nanjing University of Finance and Economics, 2022.] CHEN Y R. Effect and mechanism of wheat germ active peptide on colitis induced by DSS in mice[D]. Nanjing: Nanjing University of Finance and Economics, 2022.
[70] ZHANG X, JIA L, HE H, et al. Modulation of oxidative stress and gut microbiota by selenium-containing peptides from Cardamine enshiensis and structural-based characterization[J]. Food Chemistry,2022,395:133547. doi: 10.1016/j.foodchem.2022.133547
[71] ZHANG Q Z, TONG X H, SUI X N, et al. Antioxidant activity and protective effects of alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells[J]. Food Research International,2018,111:256−264. doi: 10.1016/j.foodres.2018.05.046
[72] ZHAO L, LI D, QI X, et al. Potential of food-derived bioactive peptides in alleviation and prevention of Alzheimer's disease[J]. Food Function,2022,13(21):10851−10869. doi: 10.1039/D2FO02278H
[73] CHEN H, ZHAO M, LIN L, et al. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory[J]. Food Research International,2015,78:216−223. doi: 10.1016/j.foodres.2015.10.008
[74] JU D T, K A K, KUO W W, et al. Bioactive peptide VHVV upregulates the long-term memory-related biomarkers in adult spontaneously hypertensive rats[J]. Nternational Journal of Molecular Sciences,2019,20(12):3069.





 下载:
下载:
 下载:
下载:



