Preparation, Modification, Characterization and Antioxidant Activities of Apigenin Liposomes
-
摘要: 目的:为提高芹菜素水溶性、稳定性,促进其充分发挥抗氧化生物活性,本研究拟制备芹菜素脂质体(Apigenin liposomes,AP-L),并用壳聚糖(Chitosan,CS)修饰脂质体表面。方法:比较低速离心法和过膜法两种包封率测定方法的适用性。以包封率为评价指标,比较薄膜分散-超声法和乙醇注入-超声法两种AP-L制备方法,并通过单因素实验和响应面试验优化AP-L制备工艺。通过静电吸附方式将壳聚糖吸附于脂质体表面,得到壳聚糖芹菜素脂质体(Chitosan apigenin liposomes,CS-AP-L)。考察修饰前后脂质体的外观、微观形态、粒径大小及分布、电位、包封率和稳定性的变化。通过DPPH和ABTS+自由基清除实验考察芹菜素水分散体(Apigenin dispersion,AP-D)、AP-L和CS-AP-L的抗氧化能力。结果:低速离心法测定脂质体包封率更准确。薄膜分散-超声法制备AP-L包封率更高。AP-L最佳制备工艺为:芹菜素6 mg,芹菜素磷脂质量比1:24,磷脂胆固醇质量比6:1,乙醇6.3 mL,磷酸盐缓冲液20 mL,水化温度50 ℃,超声时间20 min。AP-L粒径为157.34±1.87 nm,多分散系数(Polydispersity index,PDI)为0.240±0.025,包封率为66.50%±1.00%,CS-AP-L粒径为564.22±39.7 nm,PDI为0.292±0.022,包封率为60.17%±1.97%。稳定性实验显示常温保存30 d后,AP-L渗漏率为21.92%±4.84%,CS-AP-L渗漏率为10.64%±0.28%,CS-AP-L更稳定。DPPH和ABTS+自由基清除实验表明,AP-L和CS-AP-L均可以提高芹菜素的自由基清除能力,且CS-AP-L的自由基清除能力更强。结论:本研究制备的CS-AP-L粒径大小合适,分布均匀,包封率较高、稳定性较好,具有促进芹菜素抗氧化活性的潜力,可为开发芹菜素衍生产品提供参考依据。Abstract: Objective: To improve the water solubility and stability of apigenin and promote its antioxidant biological activity, apigenin liposomes (AP-L) were developed and subsequently surface-modified with chitosan (CS). Methods: The appropriateness of two methods, low-speed centrifugation and membrane filtration, for measuring encapsulation efficiency was evaluated. Encapsulation efficiency served as the evaluation criterion to compare the AP-L preparation methods employing the film dispersion-ultrasound and ethanol injection-ultrasound techniques. Additionally, the preparation process underwent optimization via both single-factor experiments and response surface tests. Chitosan was adsorbed onto the surface of liposomes through electrostatic adsorption to produce chitosan apigenin liposomes (CS-AP-L). The appearance, microstructure, particle size and distribution, potential, encapsulation efficiency and stability of the liposomes were observed before and after chitosan modification. The antioxidant capacity of apigenin dispersion (AP-D), AP-L and CS-AP-L was investigated by DPPH and ABTS+ free radical scavenging assays. Results: The low-speed centrifugation method was found to be more precise in determining the entrapment efficiency of liposomes, while the membrane dispersion-ultrasonic method demonstrated higher encapsulation efficiency. The optimal preparation process for AP-L included 6 mg of apigenin, an apigenin to phospholipid ratio of 1:24, a phospholipid to cholesterol ratio of 6:1, 6.3 mL of ethanol, 20 mL of phosphate buffer solution (PBS), hydration temperature 50 ℃ and ultrasonic time 20 min. The particle size of AP-L was 157.34±1.87 nm, the polydispersity index (PDI) was 0.240±0.025, and the encapsulation efficiency was 66.50%±1.00%. The particle size of CS-AP-L increased to 564.22±39.7 nm, the PDI was 0.292±0.022, and the encapsulation efficiency decreased to 60.17%±1.97%. After being stored at room temperature for 30 days, the leakage rate of AP-L was 21.92%±4.84%, compared to 10.64%±0.28% for CS-AP-L. This indicated that CS-AP-L demonstrated superior stability. Furthermore, the DPPH and ABTS+ free radical scavenging experiment demonstrated that both AP-L and CS-AP-L could enhance the free radical scavenging capacity of apigenin, with CS-AP-L showing even greater scavenging capability. Conclusion: The CS-AP-L prepared in this study exhibits appropriate particle size, uniform distribution, high encapsulation efficiency, good stability, and the capability to enhance the antioxidant activities of apigenin, thereby offering valuable insights for the development of apigenin derivative products.
-
Keywords:
- apigenin /
- liposome /
- encapsulation efficiency /
- stability /
- antioxidant activities /
- chitosan
-
芹菜素(Apigenin,AP)是一类具有代表性的天然黄酮类化合物,广泛存在于各种蔬菜、水果、豆科植物、茶叶等食源性植物食物中。芹菜素具有抗氧化[1]、抗炎[2]、抗癌[3]和提高自身免疫力[4]等多种生物活性,因而在食品、保健品、药品等领域受到广泛关注。然而,芹菜素在水中溶解度低,且稳定性差,导致体内吸收差,生物活性作用发挥受限等问题[5]。将芹菜素载入纳米载体中,不仅可以增加芹菜素与介质的有效接触面积,提高其在水中的溶解度[6],而且可以减缓芹菜素的降解速度,让芹菜素可以更充分地发挥包括抗氧化在内的多种生物活性作用。
常见的纳米载体有脂质体、聚合物胶束、纳米乳液等。脂质体是一种具有双分子层膜结构的囊泡,该囊泡具有独立的亲水相和亲脂相空间,水不溶性的活性物质可以分散在脂质层,从而提高其水溶性和稳定性[7]。脂质体的粒径在几十到几百纳米之间不等,因而更容易被体内吸收[8]。脂质体组成成分与细胞膜类似,具有高度的生物相容性和生物可降解性[9−10]。因此,脂质体被广泛用于各种生物活性化合物的封装。然而,脂质体的主要组成成分磷脂容易发生氧化断裂,导致脂质体破裂和活性物质的泄露[11]。将高分子聚合物吸附在脂质体的表面,在脂质体表面形成一层保护膜,有助于保持脂质体的形态结构并提高其动态和机械稳定性。
壳聚糖(Chitosan,CS)是一种天然的高分子多糖,其生物相容性好、可降解,被广泛应用于食品工业、日用化学、医药行业等方面[12]。壳聚糖的氨基可与磷脂分子的磷酸基和羰基相结合,桥连多个磷脂分子,增加局部磷脂分子密度,降低脂质体膜的流动性,减少磷脂分子氧化降解[13],适合用于脂质体表面修饰。Sebaaly等[14]用壳聚糖修饰丁香酚脂质体,结果表明,在4 ℃的储存条件下修饰后的丁香酚脂质体的包封率可以保持2个月不变,显著增加了丁香酚脂质体的稳定性。在另一项研究中显示,壳聚糖的修饰可以改善天花粉蛋白-3-O-葡萄糖苷脂质体的热稳定性和食品模拟稳定性,并提高其在体外消化过程中的保留率[15]。Wang等[16]制备了肉桂醛脂质体,并以不同浓度的壳聚糖为修饰剂修饰脂质体表面,结果表明壳聚糖修饰可以显著提高肉桂醛脂质体的抗菌活性。因此,用壳聚糖修饰脂质体表面,可以提高脂质体的稳定性,降低活性物质的泄露,减少活性物质的降解,从而促进活性物质充分发挥生物活性作用。
综上所述,为了提高芹菜素的水溶性、稳定性和抗氧化生物活性,本研究拟制备大小合适且分布均匀的芹菜素脂质体(Apigenin liposomes,AP-L),并用壳聚糖对芹菜素脂质体表面进行修饰。考察修饰前后脂质体的外观、微观形态、粒径大小及分布、电位、包封率和稳定性的变化。并通过DPPH和ABTS+自由清除实验考察芹菜素抗氧化活性的变化,为芹菜素衍生产品的开发提供参考依据。
1. 材料与方法
1.1 材料与仪器
芹菜素 含量>98%,西安金绿生物技术有限公司;蛋黄卵磷脂、胆固醇(分析纯)、壳聚糖(脱乙酰度≥95%)、无水乙醇(分析纯)、维生素C(含量≥99.7%)、DPPH(含量96%) 国药集团化学试剂有限公司;ABTS+自由基清除能力检测试剂盒(50T/24S) Solarbio公司。
UV-3100紫外可见分光光度计 上海美谱达仪器有限公司;ATY124电子天平 岛津中国;KDC-12低速离心机 安徽中科中佳科学仪器有限公司;RE-207BAT旋转蒸发仪 南京科尔仪器设备有限公司;DF-101S集热式恒温加热磁力搅拌器 巩义市予华仪器有限责任公司;KQ-500E型超声仪 昆山市超声仪器有限公司;E100电子显微镜 日本Nikon;NanoBrook Omni粒径仪 美国布鲁克海文仪器公司。
1.2 实验方法
1.2.1 AP-L不同制备方法比较
以包封率为指标,比较薄膜分散-超声法和乙醇注入-超声法两种芹菜素脂质体制备方法。
1.2.1.1 薄膜分散-超声法
根据Wu等[17]报道的方法并做适当调整,按照表1所示精密称取芹菜素、磷脂、胆固醇于茄形瓶中,加无水乙醇超声溶解完全。通过旋转蒸发仪减压加热去除无水乙醇,转速为190 r/min,温度为40 ℃。待无水乙醇完全去除,形成一层脂质薄膜后,加入PBS在常压条件下加热旋转进行水化,转速为190 r/min,水化至形成含有淡蓝乳光的均一溶液,将得到的脂质体混悬液置于水浴超声中,超声后离心去除未包封的芹菜素即得AP-L。
表 1 AP-L不同成分具体用量Table 1. Accurate amount of different ingredients in AP-L样品序号 芹菜素质量
(mg)芹菜素磷脂
质量比磷脂胆固醇
质量比无水乙醇
(mL)PBS(mL) 1 6 1:20 6:1 6 20 2 6 1:20 8:1 6 20 3 6 1:25 6:1 6 20 4 6 1:25 8:1 6 20 1.2.1.2 乙醇注入-超声法
根据Liang等[18]报道的方法并做适当调整,按照表1所示量取PBS置于磁力搅拌器上搅拌预热备用(55 ℃),并精密称取芹菜素、磷脂、胆固醇,加无水乙醇超声溶解完全。在高速搅拌下,用注射器将芹菜素脂质乙醇溶液逐滴滴入预热好的PBS中,继续加热搅拌直至乙醇挥发完全无醇味,然后将得到的脂质体混悬液置于水浴超声中,超声后离心去除未包封的芹菜素即得AP-L。
1.2.2 AP-L包封率测定方法比较
1.2.2.1 芹菜素标准曲线绘制
精密称取芹菜素120 mg于100 mL容量瓶中,无水乙醇超声溶解完全并定容至刻度线,从上述溶液中依次精密移取1、0.7、0.6、0.5、0.4 mL溶液至100 mL容量瓶中,无水乙醇稀释并定容至刻度线,于340 nm处测定溶液的紫外吸收值A。将紫外吸收值A和溶液浓度c(mg/mL)进行线性回归,计算标准曲线。
1.2.2.2 过膜法
根据赵茜茜等[19]报道的方法,将超声后的脂质体取0.08 mL于10 mL容量瓶中,无水乙醇稀释破乳并定容至刻度线,于340 nm处测定样品的紫外吸收值,记为A总,并将吸收值带入标准曲线中计算得到C总。然后将脂质体过0.22 μm滤膜,取滤液0.08 mL于10 mL容量瓶中,无水乙醇稀释破乳并定容至刻度线,于340 nm处测定样品的紫外吸收值,记为A包封,并将吸收值带入标准曲线中计算得到C包封。按公式(1)计算脂质体包封率。
E(%)=C包封C总×100 (1) 式中:E表示为脂质体包封率,%;C包封表示为包裹在脂质体中的芹菜素浓度,mg/mL;C总表示为脂质体中的总的芹菜素浓度,mg/mL。
1.2.2.3 过膜法对包封率测定影响
分别制备0.3 mg/mL AP-D 和 AP-L。取 AP-D 适量,采用 0.22 µm 滤膜过滤,滤液于 340 nm 处测定紫外吸收,并计算其中芹菜素含量,考察滤膜分离游离芹菜素的效果。另取经过超声的脂质体适量,经0.22 µm滤膜过滤一次后收集滤液(获得含芹菜素的脂质体),取适量滤液经0.22 µm滤膜再次过滤,并收集滤液,按“1.2.2.2”项所述方法分别测定两次过滤后的芹菜素脂质体的包封率,考察滤膜是否吸附脂质体包载的芹菜素。
1.2.2.4 低速离心法
根据郭丽姗等[20]报道的方法并做适当调整,首先,将超声好的脂质体取0.08 mL于10 mL容量瓶中,无水乙醇稀释破乳并定容至刻度线,于340 nm处测定样品的紫外吸收值,记为A总,并将吸收值带入标准曲线中计算得到C总。然后将适量脂质体装于离心管中,在一定转速下离心适当时间,取上清液0.08 mL于10 mL容量瓶中,无水乙醇稀释破乳并定容至刻度线,于340 nm处测定样品的紫外吸收值,记为A包封,并将吸收值带入标准曲线中计算得到C包封。按公式(1)计算脂质体包封率。
1.2.2.5 低速离心法对包封率测定影响
分别制备0.3 mg/mL AP-D和AP-L。取AP-D适量,分别于4000 r/min的速度下离心10、15、20、30 min,取离心后上清液于340 nm处测定紫外吸收,并计算上清液中芹菜素含量,考察离心分离游离芹菜素的效果。另取超声好的脂质体适量,分别于4000 r/min的速度下离心20、30 min,取离心后上清液(获得含芹菜素的脂质体)按“1.2.2.4”项所述方法分别测定两次离心后芹菜素脂质体的包封率。
1.2.3 薄膜分散-超声法单因素实验
1.2.3.1 水化温度对包封率的影响
固定芹菜素质量6 mg,芹菜素磷脂质量比1:20,磷脂胆固醇质量比6:1,无水乙醇6 mL,PBS 20 mL,超声时间20 min,考察水化温度在20、37、50、60 ℃时对AP-L包封率的影响。
1.2.3.2 超声时间对包封率和粒径分布的影响
固定芹菜素质量6 mg,芹菜素磷脂质量比1:20,磷脂胆固醇质量比6:1,无水乙醇6 mL,PBS 20 mL,水化温度50 ℃,考察超声时间在0、10、20、30、40 min时对AP-L包封率和粒径分布的影响。
1.2.3.3 芹菜素磷脂质量比对包封率的影响
固定芹菜素质量6 mg,磷脂胆固醇质量比6:1,无水乙醇6 mL,PBS 20 mL,水化温度50 ℃,超声时间20 min,考察芹菜素磷脂质量比1:8、1:10、1:15、1:20、1:25、1:30对AP-L包封率的影响。
1.2.3.4 磷脂胆固醇质量比对包封率的影响
固定芹菜素质量6 mg,芹菜素磷脂质量比1:20,无水乙醇6 mL,PBS 20 mL,水化温度50 ℃,超声时间20 min,考察磷脂胆固醇质量比在2:1、4:1、6:1、8:1、10:1时对AP-L包封率的影响。
1.2.3.5 乙醇用量对包封率的影响
固定芹菜素质量为6 mg,芹菜素磷脂质量比1:20,磷脂胆固醇质量比6:1,PBS 20 mL,水化温度50 ℃,超声时间20 min,考察乙醇用量在4、6、8、10 mL时对AP-L包封率的影响。
1.2.3.6 PBS用量对包封率的影响
固定芹菜素质量6 mg,芹菜素磷脂质量比1:20,磷脂胆固醇质量比6:1,无水乙醇6 mL,水化温度50 ℃,超声时间20 min,考察PBS用量在20、30、40、50、60 mL时对AP-L包封率的影响。
1.2.4 薄膜分散-超声法Box-Behnken响应面试验
根据单因素实验结果,芹菜素磷脂质量比(A)、磷脂胆固醇质量比(B)和乙醇用量(C)三个因素需要进一步确定最佳用量,因此以这三个因素作为自变量,每个因素设置三个水平,以包封率为响应值,利用Design-Expert 13软件选择Box-Behnken响应面模型进行试验设计,具体见表2。根据响应面试验结果,预测最佳用量,并按照预测的最佳用量,制备3组AP-L,测定包封率与预测值比较。
表 2 Box-Behnken响应面模型的因素和水平Table 2. Factors and levels of Box-Behnken response surface model水平 因素 A芹菜素磷脂质量比
(W/W)B磷脂胆固醇质量比
(W/W)C乙醇用量
(mL)−1 1:20 4:1 4 0 1:25 6:1 6 1 1:30 8:1 8 1.2.5 壳聚糖修饰芹菜素脂质体
1.2.5.1 壳聚糖修饰脂质体的制备及包封率的测定
精密称取壳聚糖1 g,溶解在100 mL体积分数为1%乙酸溶液中,磁力搅拌24 h后用0.1 mol/L的氢氧化钠调节pH至5.5[21]。取适量制备好的AP-L,磁力搅拌下(25 r/min)逐滴滴入等体积上述壳聚糖溶液中,搅拌2 h后使高分子化合物与AP-L结合完全,得到壳聚糖修饰的芹菜素脂质体(Chitosan apigenin liposomes,CS-AP-L),并按“1.2.2.4”项所述方法测定脂质体包封率。壳聚糖修饰的空白脂质体(Blank chitosan liposomes,BL-CS-L)按上述相同方法制备,并按“1.2.2.4”项所述方法配制离心前后溶液作为CS-AP-L包封率测定时的空白对照。
1.2.5.2 修饰前后脂质体外观及显微观察
将空白脂质体(Blank liposomes,BL-L)、AP-L、BL-CS-L、CS-AP-L分别装于小西林瓶中进行外观观察;并取适量AP-L、CS-AP-L滴至载玻片上,自然晾干后盖上盖玻片,于光学显微镜下观察脂质体微观形态[22],观察壳聚糖修饰前后脂质体微观形态变化。
1.2.5.3 修饰前后脂质体粒径分布及Zeta电位的测定
取适量BL-L、AP-L、BL-CS-L和CS-AP-L用蒸馏水稀释40倍,混匀后25 ℃下使用布鲁克海文粒径仪测定脂质体粒径、多分散系数和Zeta电位。
1.2.6 贮藏稳定性
根据杨红艳等[22]报道的方法并做适当调整,将AP-L、CS-AP-L放置于常温条件下,分别于0、5、10、15、20、25、30 d时按“1.2.2.4”项所述方法测定包封率,进而计算脂质体的渗漏率,分析脂质体的贮藏稳定性。渗漏率计算公式如下:
渗漏率(%)=(1−EtE0)×100 (2) 式中:Et表示在t时脂质体的包封率,%;E0表示为初始时脂质体的包封率,%。
1.2.7 DPPH自由基清除率
根据李亮等[23]报道的方法并做适当调整,向1 mL DPPH乙醇溶液(0.15 mmol/L)中分别加入含不同浓度芹菜素(0.1、0.2、0.4、0.6、0.8 mg/mL)的AP-D、AP-L和CS-AP-L,测定其DPPH自由基清除率。BL-L和BL-CS-L加入量与AP-L和CS-AP-L保持一致,用于测定不含芹菜素的空白脂质体的DPPH自由基清除能力。VC作阳性对照。具体操作如下:将混合后的溶液涡旋振荡混匀1 min,于25 ℃下避光静置30 min,乙醇稀释至4 mL后在517 nm处测得混合溶液的吸光值,测得A样。空白组为乙醇代替DPPH溶液,测得A空。对照组为PBS代替样品,测得A对照。DPPH自由基清除率计算如下:
DPPH自由基清除率(%)=(1−A样−A空A对照)×100 (3) 式中:A样表示为样品和DPPH混合溶液的吸光度;A空表示为样品和乙醇混合溶液的吸光度;A对照表示为PBS和DPPH混合溶液的吸光度。
1.2.8 ABTS+自由基清除率
按照试剂盒说明书所述方法,分别测定含不同浓度芹菜素(0.40、1.30、2.20、3.00、4.62 mg/mL)的AP-D、AP-L、CS-AP-L的ABTS+自由基清除率。BL-L和BL-CS-L加入量与AP-L和CS-AP-L保持一致,用于测定不含芹菜素的空白脂质体的ABTS+自由基清除能力。VC作阳性对照。ABTS+自由基清除率计算如下:
ABTS+自由基清除率(%)=(1−A测−A对照A空)×100 (4) 式中:A测表示为样品和ABTS工作液反应后的吸光度;A对照表示为样品的吸光度;A空表示总ABTS工作液的吸光度。
1.3 数据处理
所有实验平行测定三次,数据以平均值±标准偏差表示,采用Design Expert 13软件设计响应面试验,使用Excel 2016软件进行绘图并作数据统计分析。
2. 结果与分析
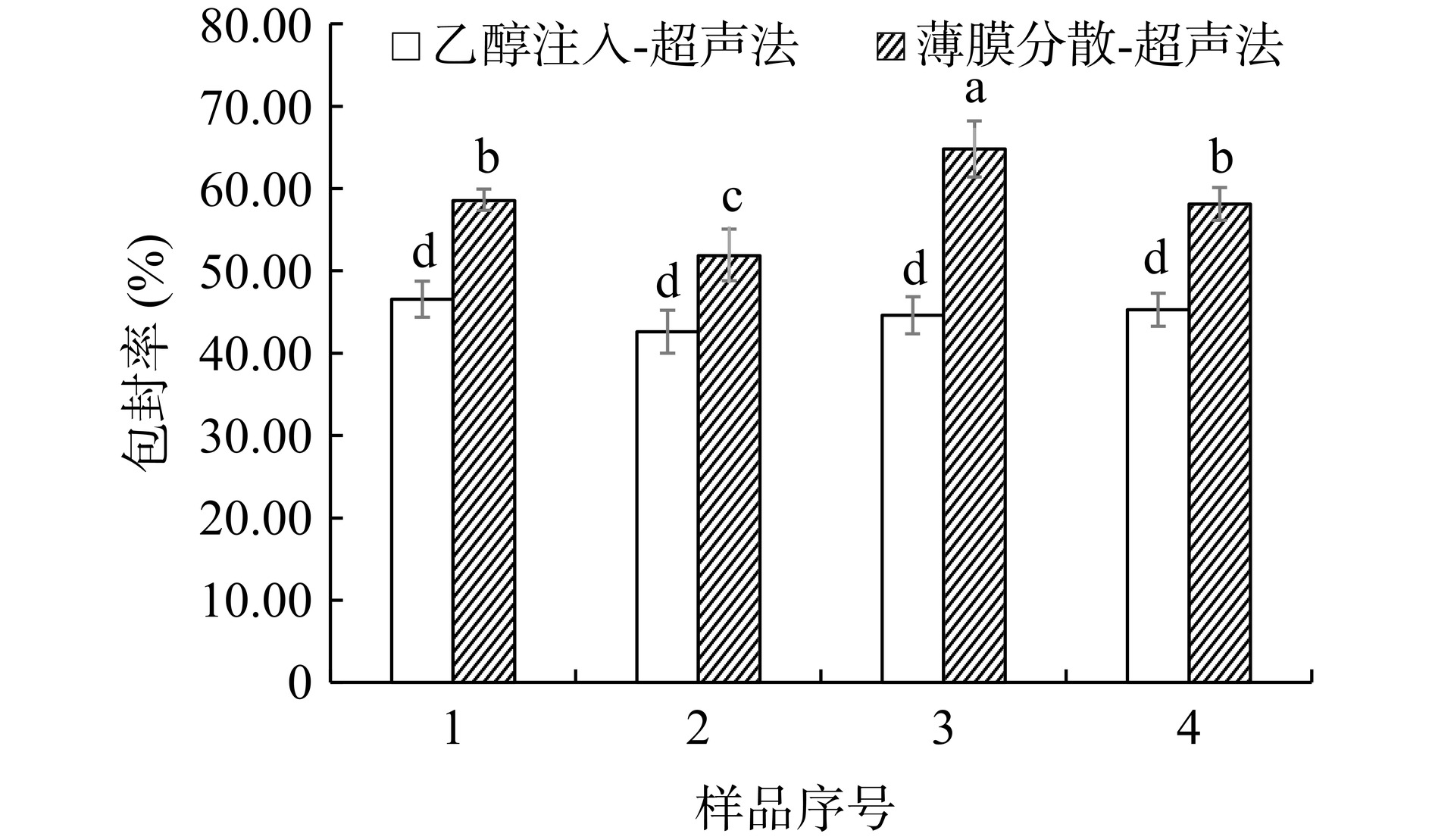
2.1 AP-L不同制备方法比较
如图1所示,在所有样品中,薄膜分散-超声法制备的脂质体的包封率均显著高于乙醇注入-超声法(P<0.05)。说明芹菜素更适合用薄膜分散-超声法制备脂质体。这可能是因为芹菜素具有一定的脂溶性,在成膜的过程中,芹菜素可以嵌入脂质分子发生有序且均匀的排列,从而在水化过程中更易形成包封率较高、分布均匀的脂质体[24]。因此,本文选用薄膜分散-超声法制备AP-L。
2.2 AP-L包封率测定方法比较
2.2.1 芹菜素标准曲线
芹菜素乙醇溶液的标准曲线方程为:A=71.651c+0.0013(R2=0.9999)。该方程在芹菜素浓度为4.8~12 µg/mL范围内呈现良好的线性关系。
2.2.2 过膜法
AP-D和AP-L过膜后,滤液中芹菜素的含量及芹菜素脂质体的包封率如表3所示。由表中数据可以看出AP-D经0.22 μm滤膜过滤后,滤液中芹菜素含量为0.26 μg/mL,表明过膜法几乎可以完全除去不溶芹菜素。但AP-L经过两次过膜后,包封率由47.42%±2.72%降为34.14%±2.03%,由此判断过膜可能对脂质体有吸附作用,导致包封率测定值偏低。
2.2.3 低速离心法
AP-D和AP-L离心后上清液中芹菜素的含量及脂质体的包封率如表4所示。可以看出AP-D经4000 r/min速度离心20 min后,上清液中芹菜素含量仅为0.38 μg/mL,表明低速离心基本可以完全去除不溶芹菜素。AP-L经4000 r/min速度分别离心20、30 min后,包封率分别为56.45%±1.58%和57.09%±2.43%,包封率无显著变化,表明离心法测定包封率可行。因此,本文选择低速离心法测定脂质体包封率。
表 4 低速离心法对AP-L包封率测定的影响Table 4. Effect of the low-speed centrifugation on the determination of the encapsulation efficiency of AP-L离心时间(min) AP-D上清液中芹菜素浓度(μg/mL) AP-L包封率(%) 10 0.64±0.01 − 15 0.41±0.02 − 20 0.38±0.01 56.45±1.58 30 0.36±0.23 57.09±2.43 2.3 薄膜分散-超声法单因素实验
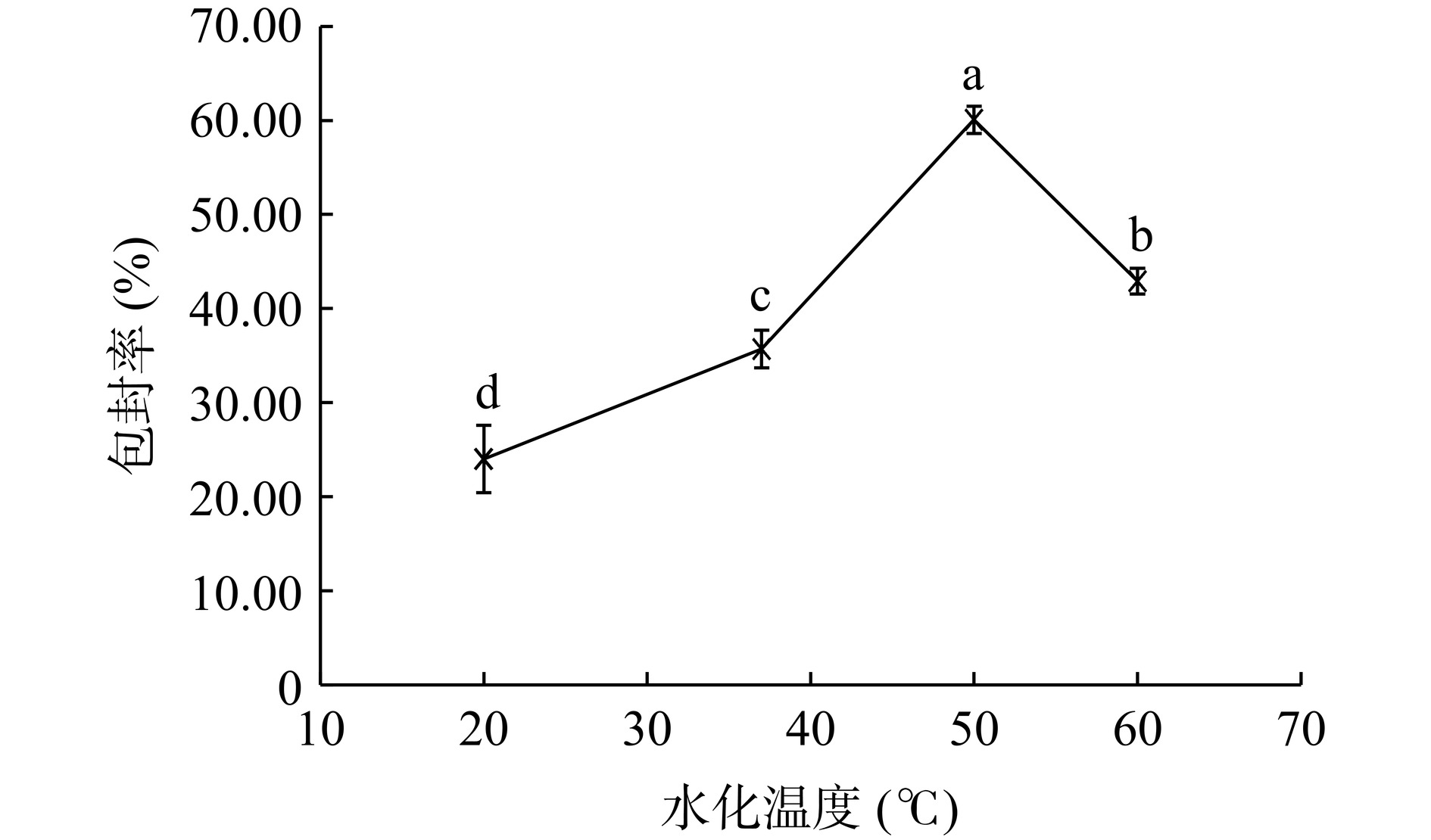
2.3.1 水化温度对包封率的影响
如图2所示,随着水化温度的升高,脂质体的包封率先升高再降低,水化温度为50 ℃时,包封率最高,达到50%以上,与其他温度下的包封率具有显著性差异(P<0.05)。表明脂质体的制备过程中,水化温度不宜太低或过高。温度太低,达不到磷脂的相变温度,磷脂不容易由凝胶态向液晶态转变,流动性低,不利于形成脂质囊泡包裹芹菜素[25]。而过高的温度可能会使磷脂氧化和分解,导致脂质囊泡破裂以及芹菜素泄露[26]。因此,水化温度定为50 ℃。
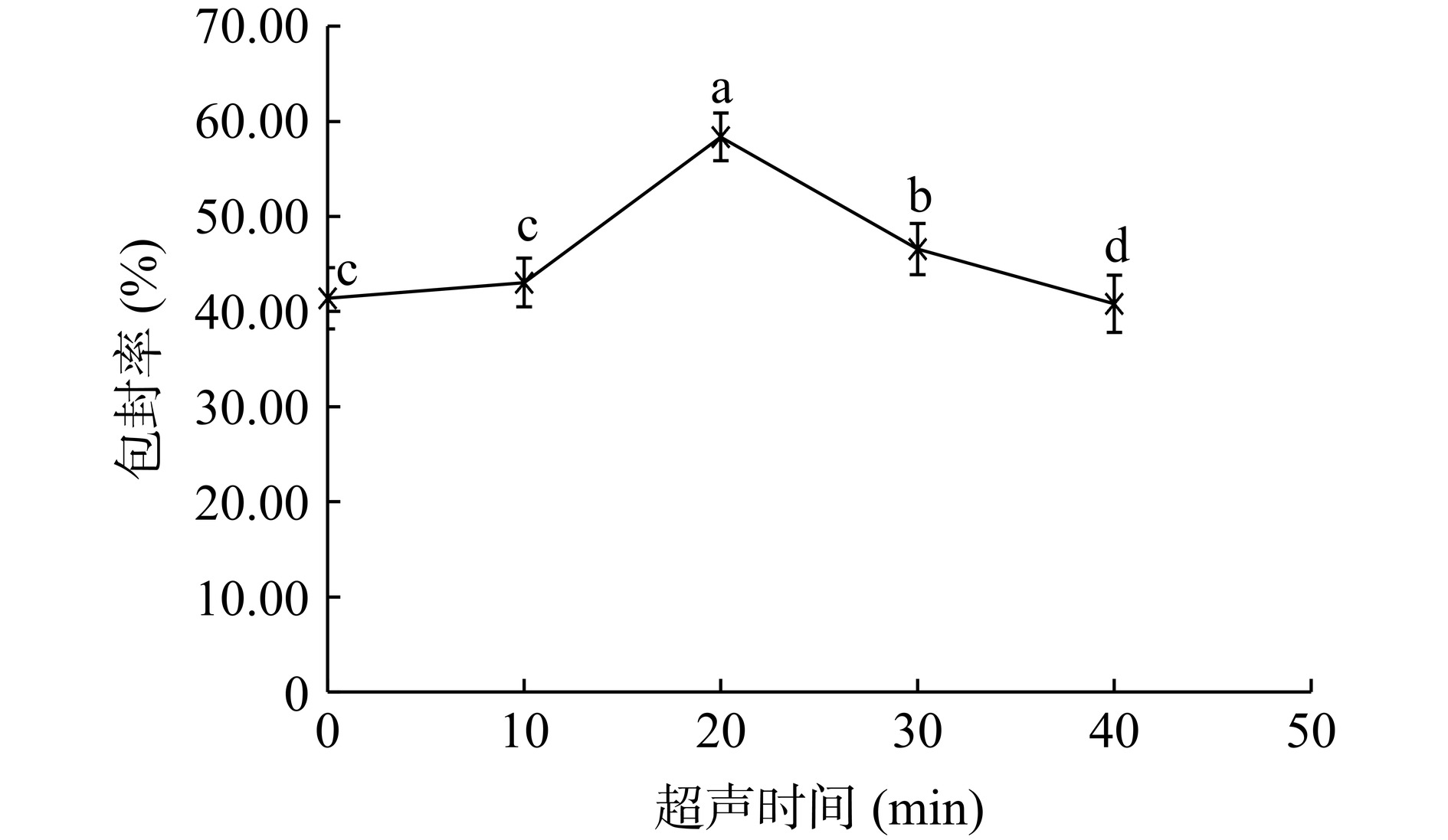
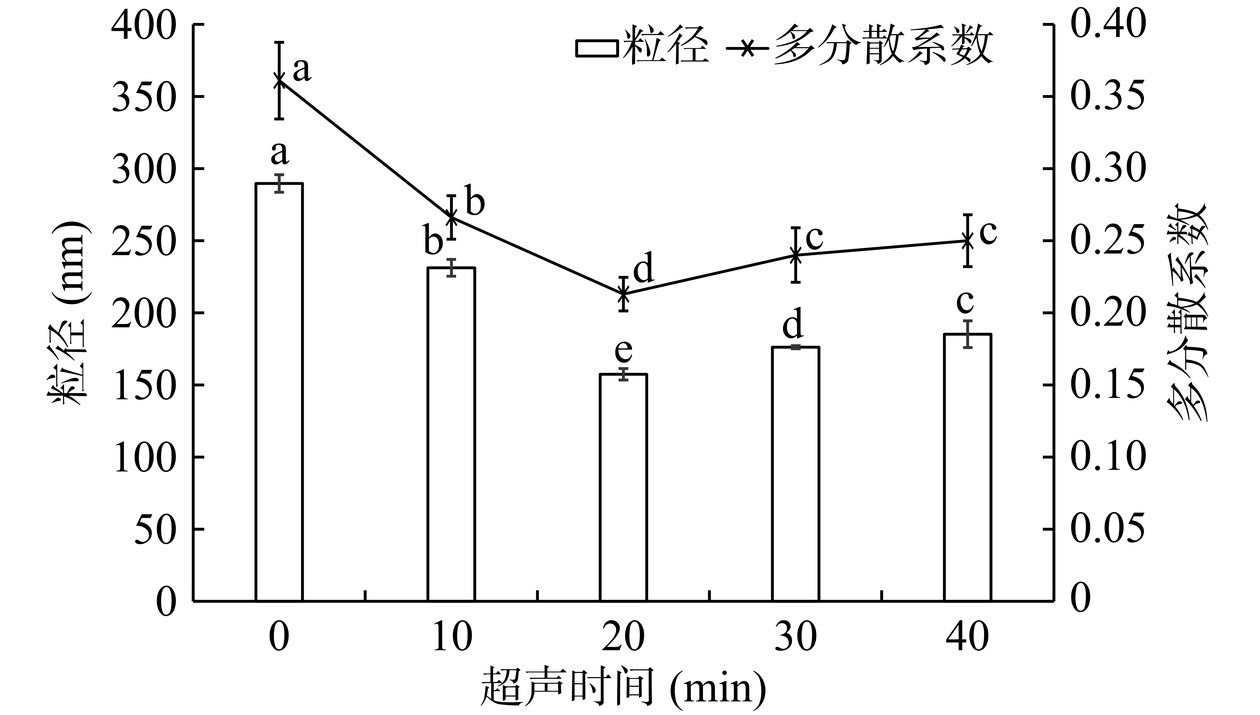
2.3.2 超声时间对包封率和粒径分布的影响
如图3所示,超声时间为20 min时,脂质体的包封率最高,达到50%以上,与其他超声时间下的包封率具有显著性差异(P<0.05)。此外,本文测定了不同超声时间下脂质体的粒径分布,结果如图4所示。从图中可以看出,不超声或超声时间过短,脂质体粒径较大,且分布不均匀,表明此时形成的脂质体囊泡较为松散,对芹菜素包封的能力有限。超声时间达到20 min时,超声波的振荡作用促使脂质分子进一步聚集,脂质体的粒径进一步缩小,此时,形成的脂质体较为均一稳定,芹菜素包封率达到最高。超声时间达到40 min时,脂质体的粒径再次变大,均匀度也再次变差,表明过度的超声可能导致脂质囊泡的破裂和芹菜素的泄露,因而包封率再次降低。因此,超声时间定为20 min。
2.3.3 芹菜素磷脂质量比对包封率的影响
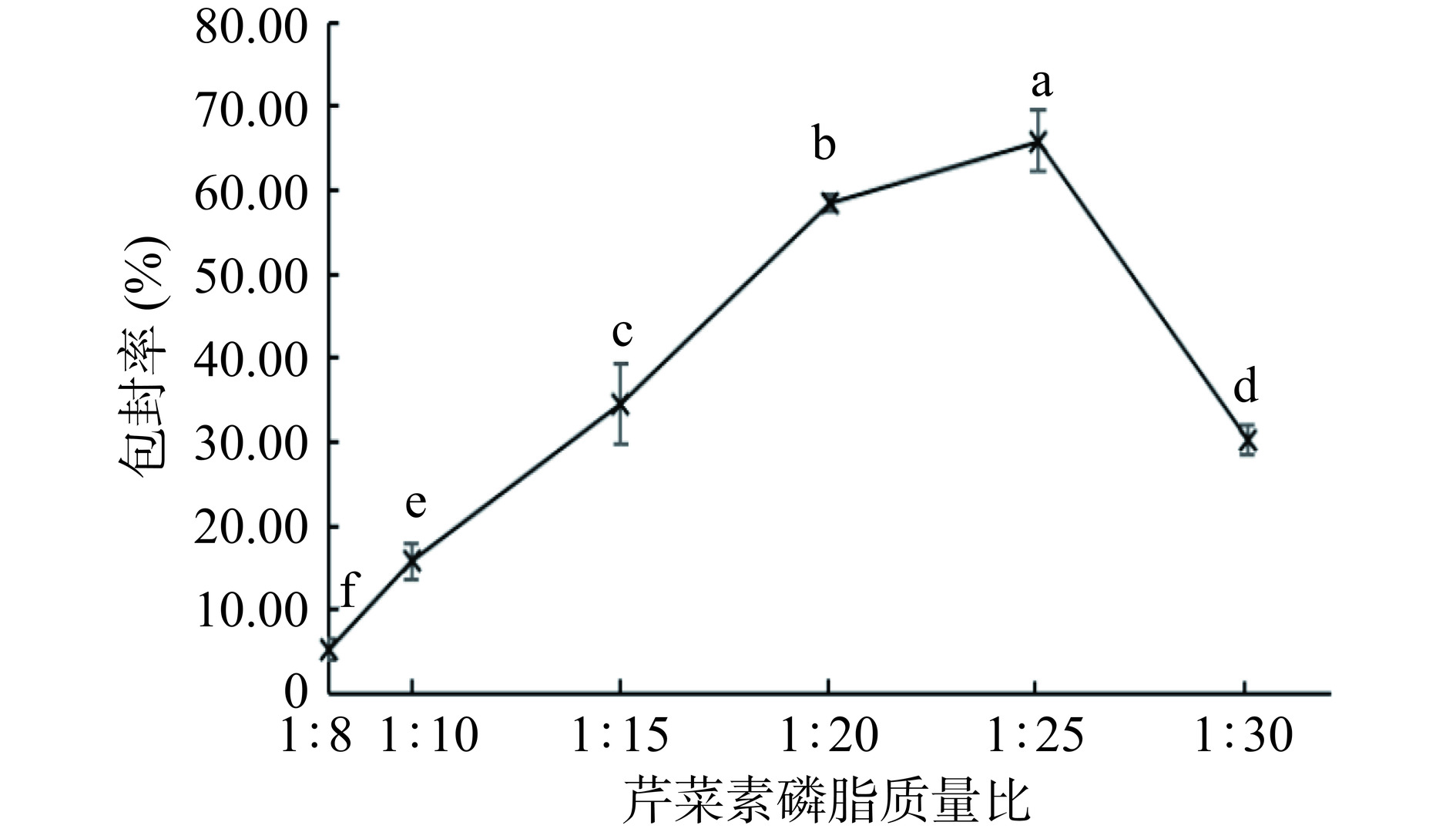
如图5所示,随着芹菜素磷脂质量比增大,芹菜素包封率先增加再减小,当芹菜素磷脂质量比为1:25时,包封率最高。当芹菜素磷脂质量比较低时,磷脂含量较少,在溶液中的浓度较低,可能会导致形成的脂质囊泡少且粒径小,难以提供足够的空间来包裹芹菜素。当芹菜素磷脂质量比过高时,磷脂含量过多,过多的磷脂可能会在制备过程中分散困难,导致包封率下降[27]。芹菜素磷脂质量比在1:20~1:30之间,包封率存在最大值,可作为后续优化试验中芹菜素磷脂质量比的选取范围。
2.3.4 磷脂胆固醇质量比对包封率的影响
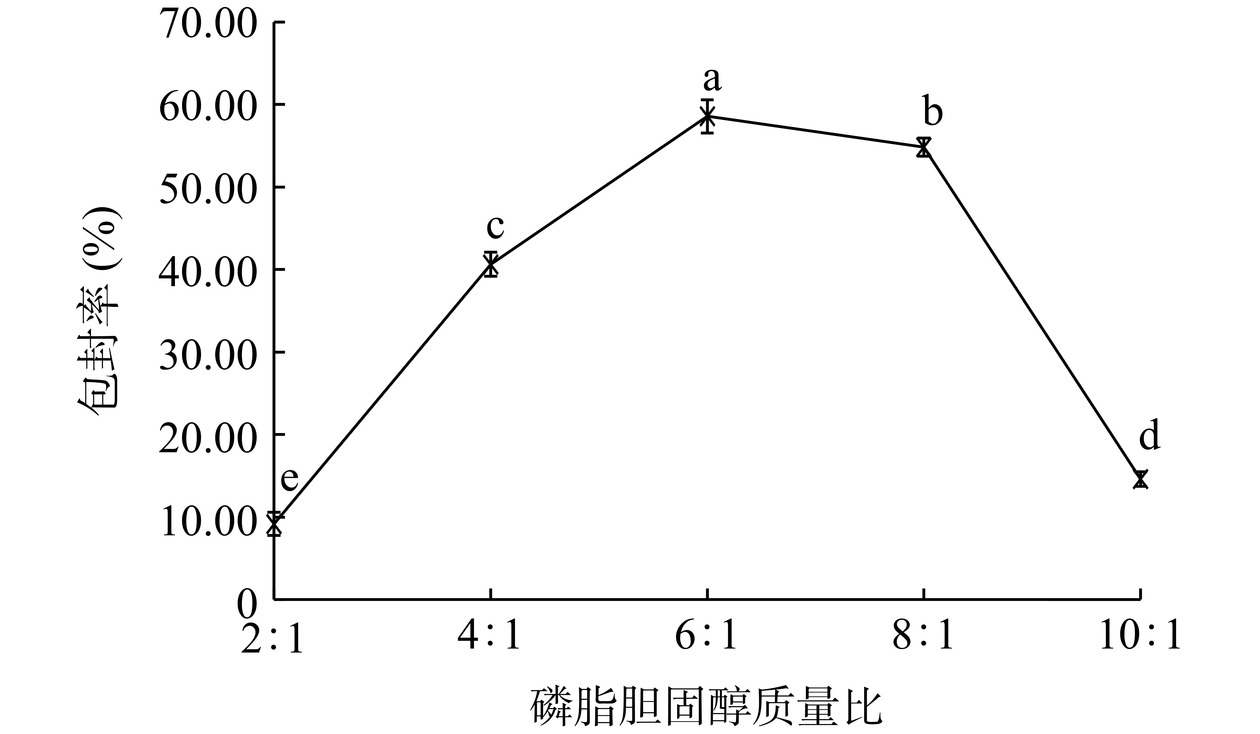
如图6所示,随着磷脂胆固醇质量比的增加,包封率呈现先增高再减小的趋势,比例在6:1时,包封率最高。胆固醇可调节脂质体膜的流动性,对于维持脂质体膜的稳定性有着重要的作用。当磷脂胆固醇质量比较低时,胆固醇含量过高,不仅会增加磷脂的刚性,使其不易弯曲形成脂质囊泡,而且会和芹菜素抢占脂质囊泡的疏水空间,导致芹菜素包封率不高[28]。而当磷脂胆固醇质量比较高时,胆固醇含量过低,脂质体膜的流动性较大,稳定性较差,导致芹菜素容易泄露进而使包封率较低[29]。磷脂胆固醇比在4:1~8:1之间,包封率可达最大值,可作为后续优化试验中磷脂胆固醇质量比的选取范围。
2.3.5 乙醇用量对包封率的影响
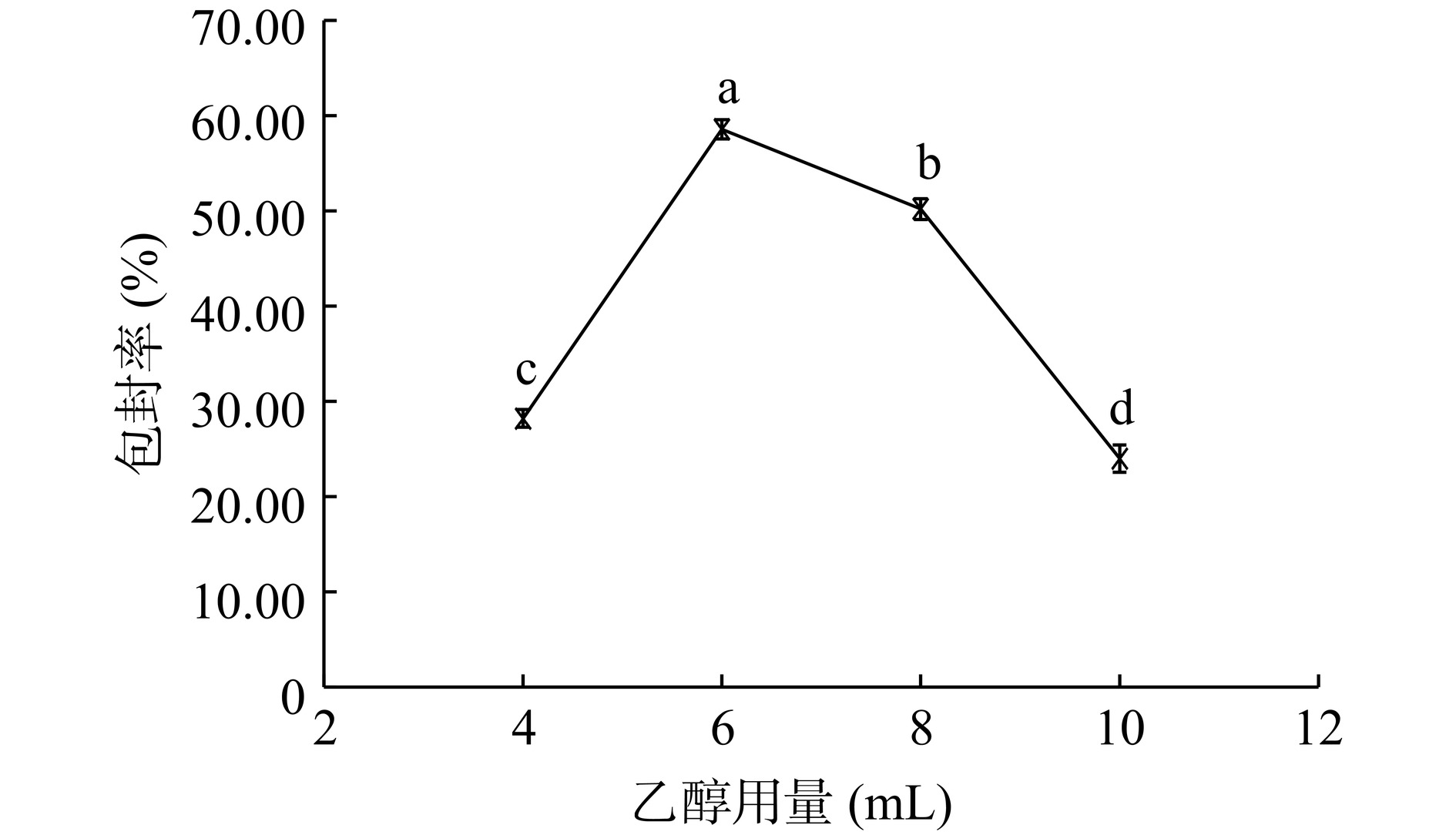
如图7所示,随着乙醇用量的增加,脂质体的包封率先增加再减小,乙醇用量在6 mL时,包封率最高。乙醇用量对脂质膜的形成十分关键,乙醇用量少可能导致形成的磷脂膜较厚,在随后的水化过程中膜不易分散形成脂质囊泡。而乙醇用量多则可能导致形成的磷脂膜较稀薄,在随后的水化过程中脂质分子间不易聚集或形成的脂质囊泡较小,不易包封芹菜素。乙醇用量在4~8 mL之间,包封率可达最大值,可作为后续优化试验中乙醇用量的选取范围。
2.3.6 PBS用量对包封率的影响
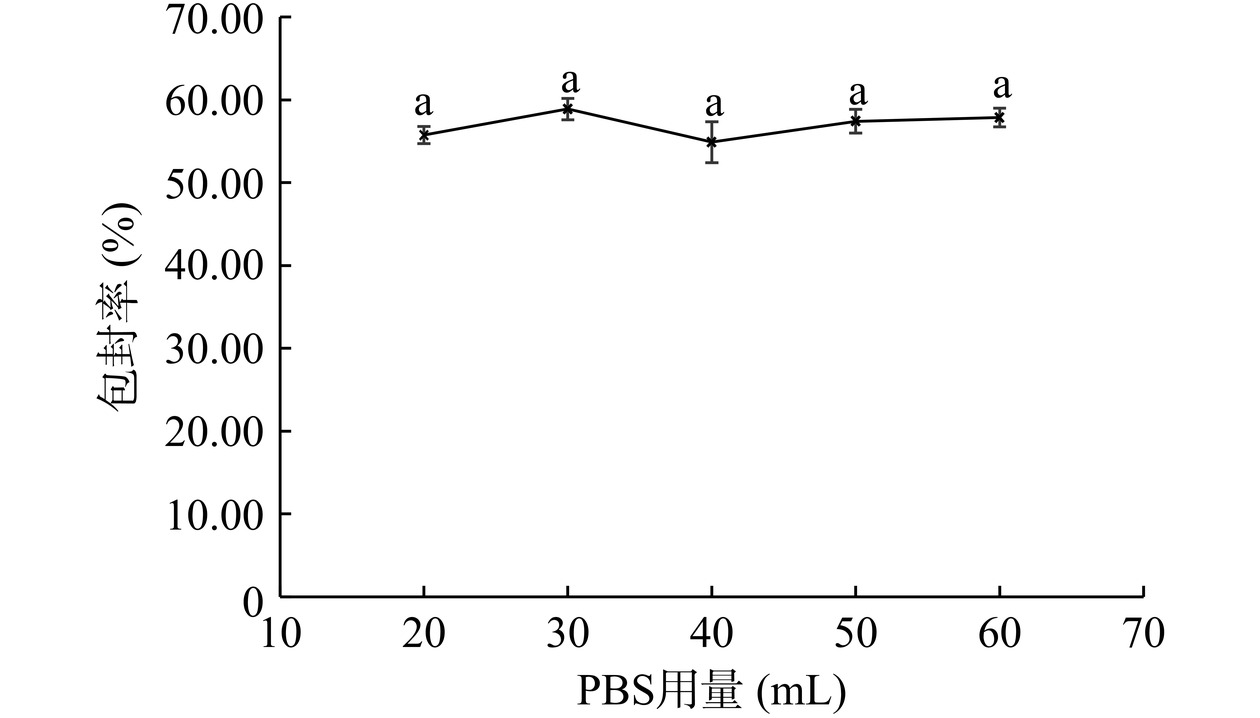
如图8所示,随着PBS用量的改变,脂质体包封率变化无显著性差异(P>0.05),均在50%以上。表明PBS用量不是脂质体制备的主要影响因素,因此PBS用量可以在20~60 mL之间。为尽可能提高芹菜素在溶液中的浓度,本文选择PBS用量为20 mL。
2.4 Box-Behnken响应面试验结果分析
2.4.1 Box-Behnken响应面模型建立及分析
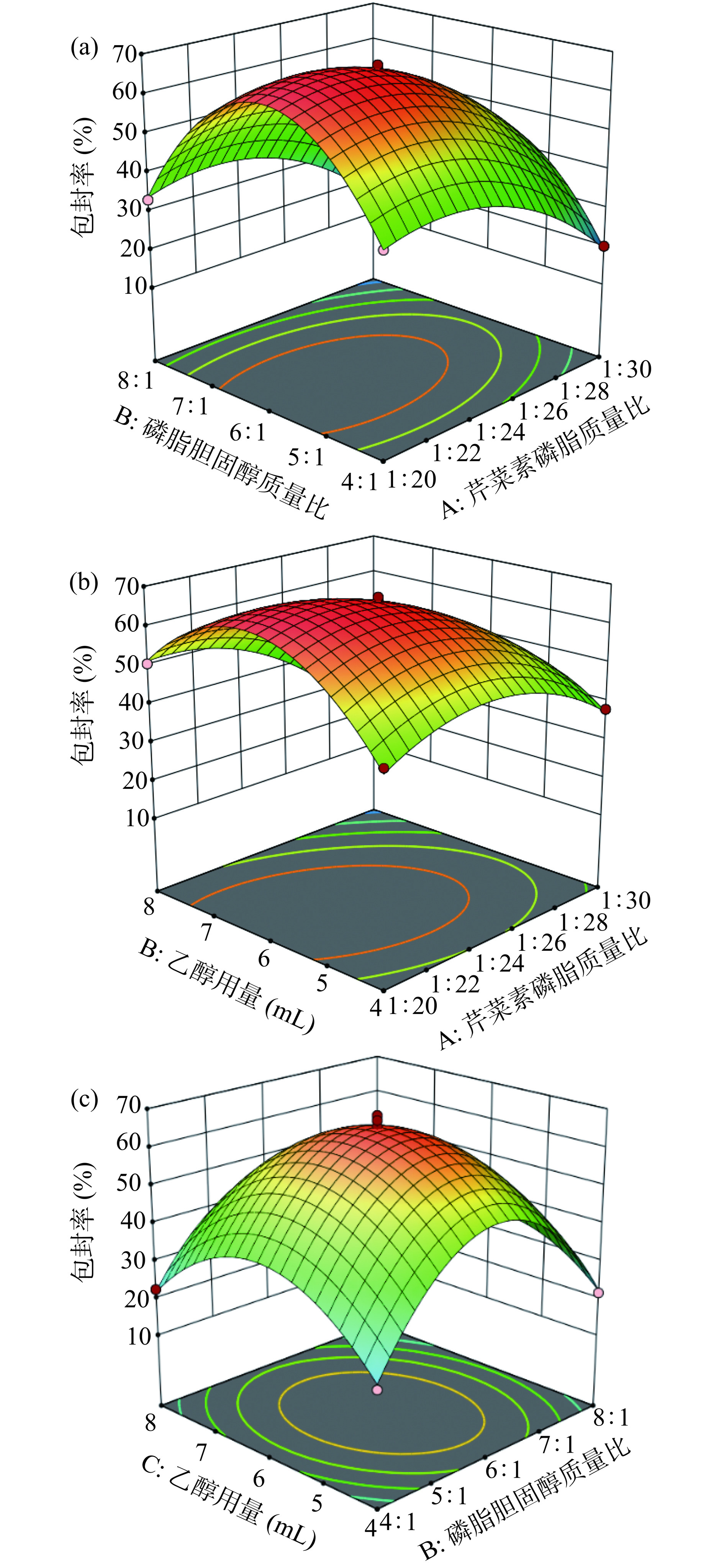
Box-Behnken响应面试验结果见表5。采用Design Expert 13软件对试验数据进行拟合,结果显示二元多次方程拟合最佳,拟合的包封率Y与影响因素A、B、C之间关系的方程为Y=66.19−9.83A−2.67B−2.91C−0.6375AB−7.53AC−3.12BC−10.51A2−28.40B2−18.88C2。模型的方差分析见表6,从表6可以看出该模型具有高度的显著性(P<0.0001),失拟项不显著(P=0.0771),表明该模型拟合精度好,可以进行后续的优化设计。多元决定系数R2=0.9982,校正决定系数R2Adj=0.9959,与预测决定系数R2Pred=0.9767的差值小于0.2,C.V.为3.45%<10%,有效信号与噪声比值(Adeq Precision)=54.2623>4,表明该模型可以充分说明工艺过程,试验的可信度和精确度高,适应性较好。
表 5 Box-Behnken响应面设计试验结果Table 5. Experimental results of Box-Behnken response surface design试验号 因素 包封率(%) A芹菜素磷脂
质量比(W/W)B磷脂胆固醇
质量比(W/W)C乙醇用量
(mL)1 1:20 6:1 8 50.57 2 1:20 8:1 6 53.14 3 1:25 6:1 6 66.48 4 1:30 4:1 6 20.15 5 1:25 4:1 8 22.49 6 1:25 4:1 4 20.04 7 1:25 6:1 6 65.42 8 1:30 6:1 4 38.09 9 1:25 6:1 6 65.23 10 1:30 8:1 6 15.45 11 1:25 6:1 6 67.04 12 1:25 4:1 6 40.39 13 1:25 8:1 8 11.53 14 1:25 8:1 4 21.57 15 1:25 6:1 6 66.78 16 1:20 6:1 4 43.38 17 1:30 6:1 8 15.18 表 6 响应面模型方差分析Table 6. ANOVA for response surface model方差来源 平方和 自由度 均方 F值 P值 显著性 模型 7044.41 9 782.71 432.42 <0.0001 *** A芹菜素磷脂质量比 772.44 1 772.44 426.75 <0.0001 *** B磷脂胆固醇质量比 57.14 1 57.14 31.57 0.0008 *** C乙醇用量 67.92 1 67.92 37.52 0.0005 *** AB 1.63 1 1.63 0.8981 0.3748 AC 226.50 1 226.50 125.14 <0.0001 *** BC 39.00 1 39.00 21.55 0.0024 ** A2 464.65 1 464.65 256.71 <0.0001 *** B2 3396.64 1 3396.64 1876.53 <0.0001 *** C2 1500.86 1 1500.86 829.18 <0.0001 *** 残差 12.67 7 1.81 失拟项 10.00 3 3.33 5.00 0.0771 不显著 纯误差 2.67 4 0.6673 总差 7057.08 16 R2=0.9982,R2Adj=0.9959,R2Pred=0.9767 注:***表示差异极显著(P<0.001),**表示差异非常显著(P<0.01),*表示差异显著(P<0.05)。 2.4.2 Box-Behnken响应面设计结果分析
根据表6分析可以看出芹菜素磷脂质量比、磷脂胆固醇质量比、乙醇用量三个因素对包封率的影响极为显著(P<0.001)。在两两之间交互作用中,芹菜素磷脂质量比和乙醇用量的交互作用对包封率的影响最为显著(P<0.001),其次为磷脂胆固醇质量比和乙醇用量的交互作用(P<0.01),芹菜素磷脂质量比和磷脂胆固醇质量比的交互作用不显著(P>0.05)。如图9所示,与图9a相比,图9b和图9c响应面曲面陡峭度更大,表明芹菜素磷脂质量比和乙醇用量的交互作用以及磷脂胆固醇质量比和乙醇用量的交互作用对包封率的影响更大。且从图中可以看出,随着各交互因素水平的提升,包封率均呈现先增大后减小的趋势。
2.4.3 Box-Behnken响应面最佳条件验证
根据Box-Behnken响应面模型预测,最优的AP-L制备条件为芹菜素磷脂质量比为1:23.998,磷脂胆固醇质量比为6.067:1,乙醇用量为6.294 mL,芹菜素脂质体包封率预测值为66.981%。结合实际操作情况,将最终实验条件修正为芹菜素磷脂质量比1:24,磷脂胆固醇质量比6:1,乙醇用量为6.3 mL。在此条件下进行3组平行验证实验,得到AP-L包封率为66.50%±1.00%。
2.5 壳聚糖修饰芹菜素脂质体
2.5.1 修饰前后脂质体包封率的变化
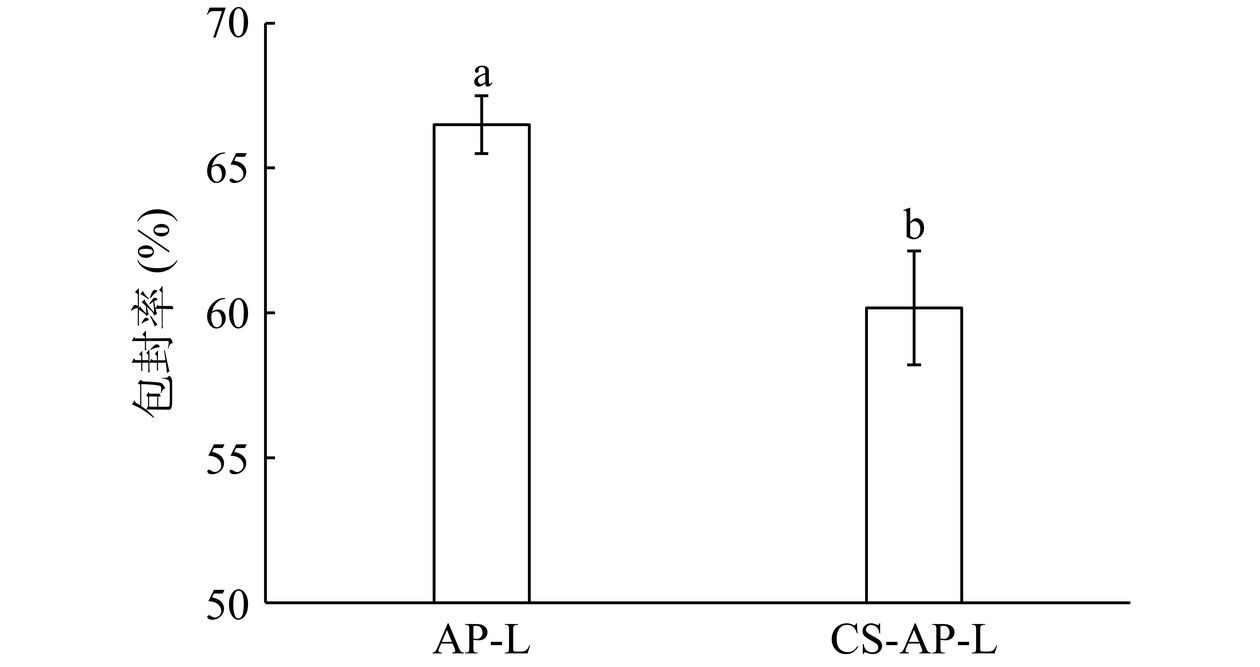
从图10可以看出,AP-L和CS-AP-L的包封率分别为66.50%±1.00%和60.17%±1.97%,CS-AP-L的包封率较AP-L的包封率显著降低(P<0.05),但仍然可以达到60%。包封率的降低可能是结合过程中部分脂质体结构被破坏造成的。
2.5.2 修饰前后脂质体外观及显微观察
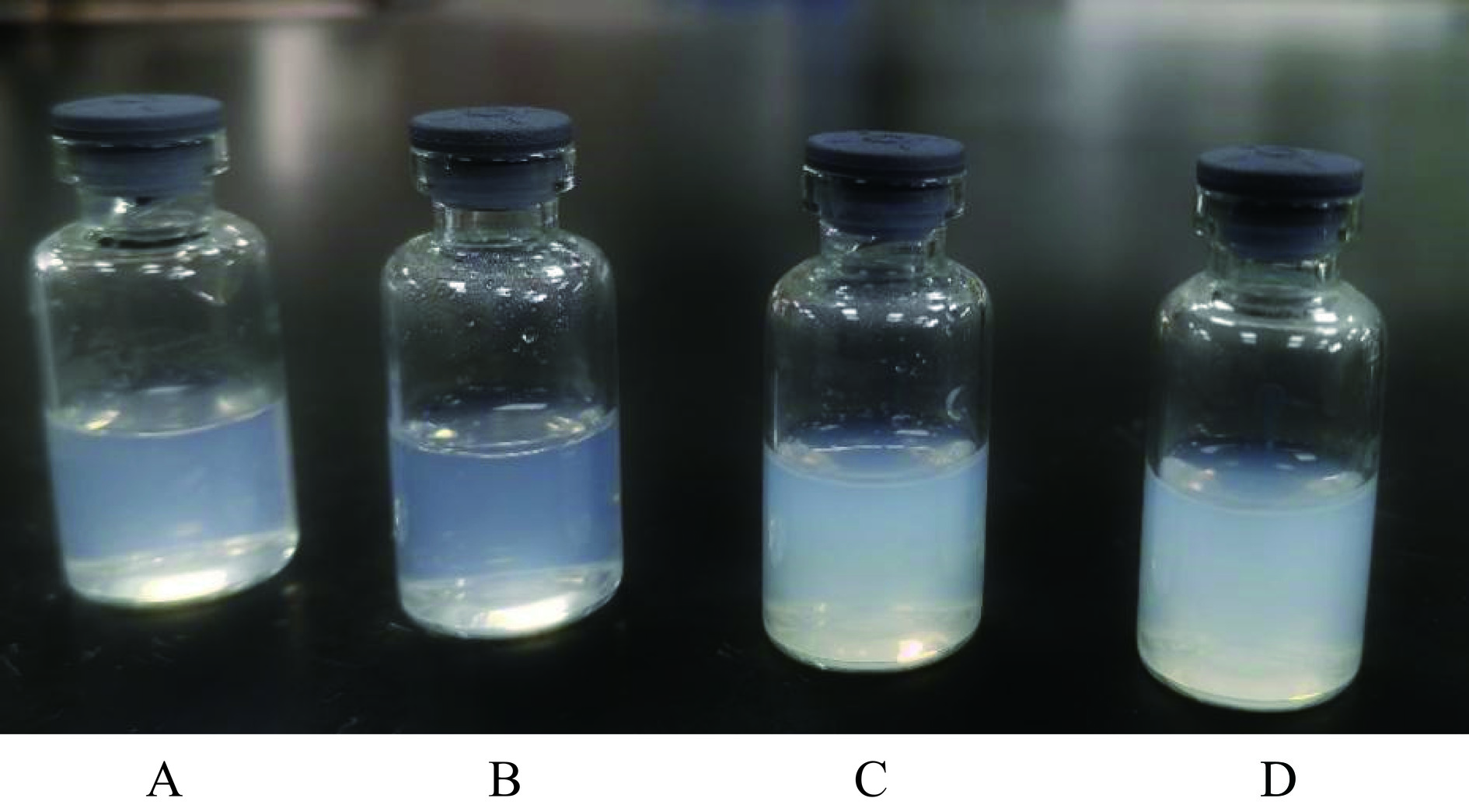
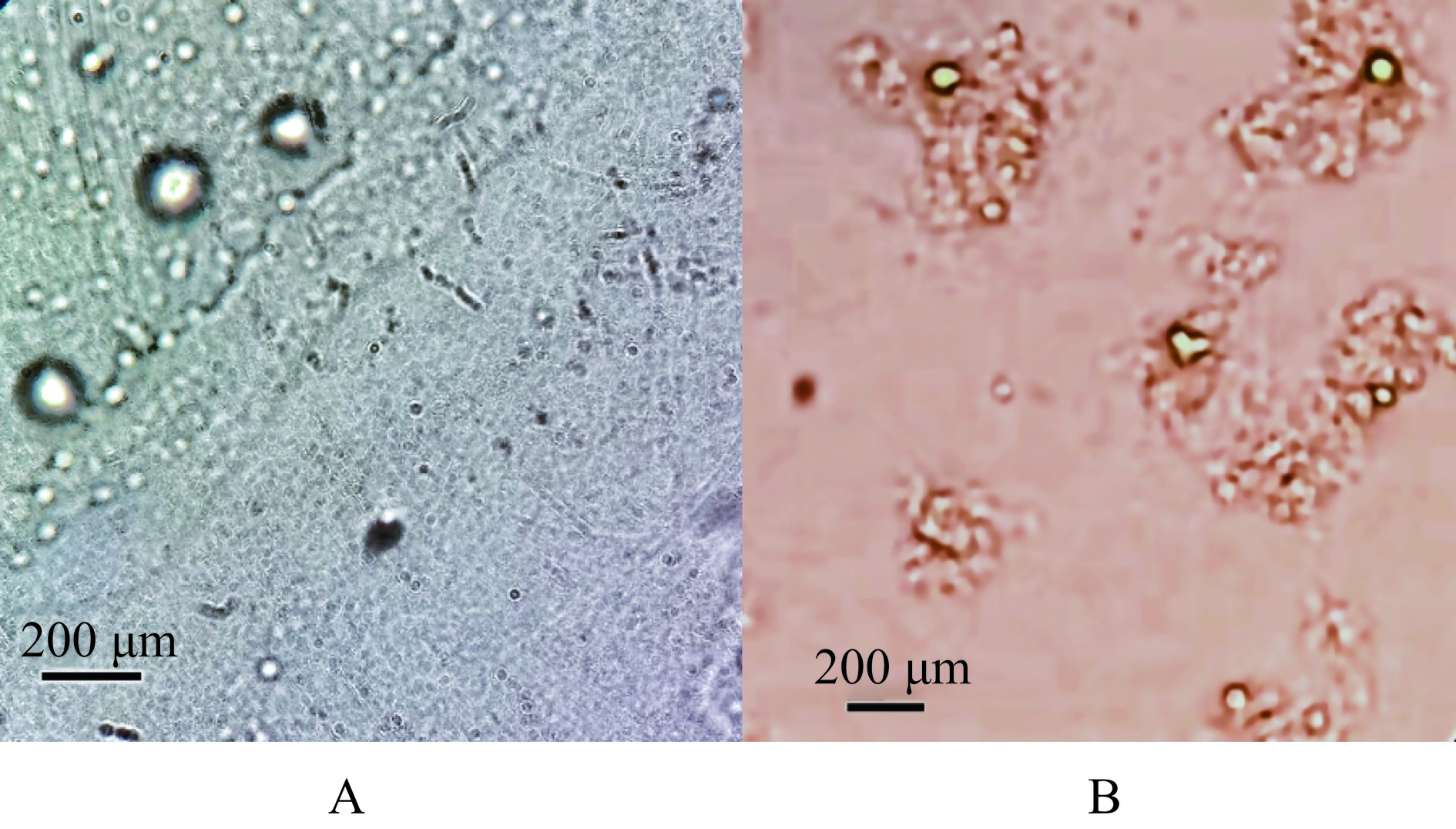
由图11可以看出,BL-L、AP-L、BL-CS-L、CS-AP-L均为泛着淡蓝乳光的均一溶液。图12为AP-L和CS-AP-L的显微镜图片,可以看出AP-L为球形或类球形空心囊泡,囊泡外侧为一圈排列紧密且有序的磷脂分子。而CS-AP-L的图片则显示壳聚糖分子吸附聚集在脂质体膜外侧,将脂质囊泡包裹在其内部。
2.5.3 修饰前后脂质体粒径分布及Zeta电位的测定
从表7可以看出,与BL-L相比,包裹了芹菜素的AP-L粒径显著增大(P<0.05),而多分散系数和电位几乎无变化,表明包裹芹菜素对脂质体颗粒分布的均匀度和脂质分子的排列秩序影响不大。壳聚糖修饰后,带正电荷的壳聚糖以静电吸附的方式吸附在带负电荷的脂质体膜表面,粒径较未修饰脂质体显著增加(P<0.05),脂质体表面的电荷也由负值变为正值,而多分散系数较未修饰脂质体无明显变化,表明修饰对脂质体粒径分布的均匀度影响不大。与未修饰脂质体一样,CS-AP-L的粒径显著大于BL-CS-L的粒径(P<0.05),电位无显著性差异(P>0.05)。
表 7 BL-L、AP-L、BL-CS-L、CS-AP-L的粒径、多分散系数和电位Table 7. Particle size, PDI and zeta potential of BL-L, AP-L, BL-CS-L and CS-AP-L样品 粒径(nm) 多分散系数 电位 BL-L 139.94±4.34d 0.235±0.018a −38.17±0.23b AP-L 157.34±1.87c 0.240±0.025a −38.38±0.13b BL-CS-L 499.40±57.73b 0.313±0.026a 39.23±1.09a CS-AP-L 564.22±39.7a 0.292±0.022a 39.57±1.61a 2.6 贮藏稳定性
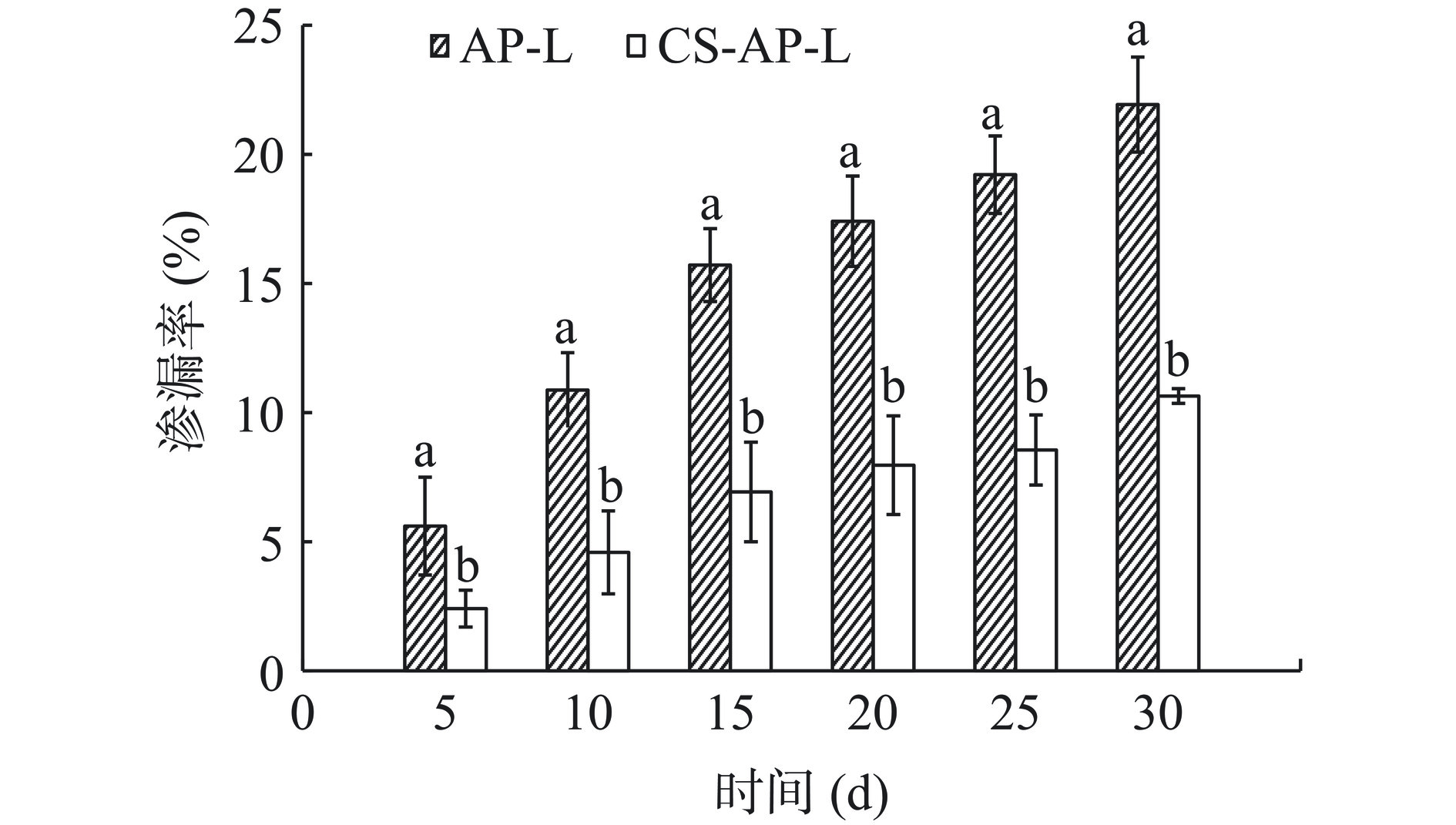
AP-L、CS-AP-L在不同时间点的渗漏率如图13所示。结果显示,随着贮藏时间的延长,AP-L和CS-AP-L均有不同程度的芹菜素泄露。每个时间点下,CS-AP-L的渗漏率均显著低于AP-L的渗漏率(P<0.05)。贮藏30 d后,AP-L芹菜素渗漏率为21.92%±4.84%,CS-AP-L的芹菜素渗漏率为10.64%±0.28%。表明壳聚糖修饰可以显著提高脂质体的稳定性。
2.7 自由基清除率
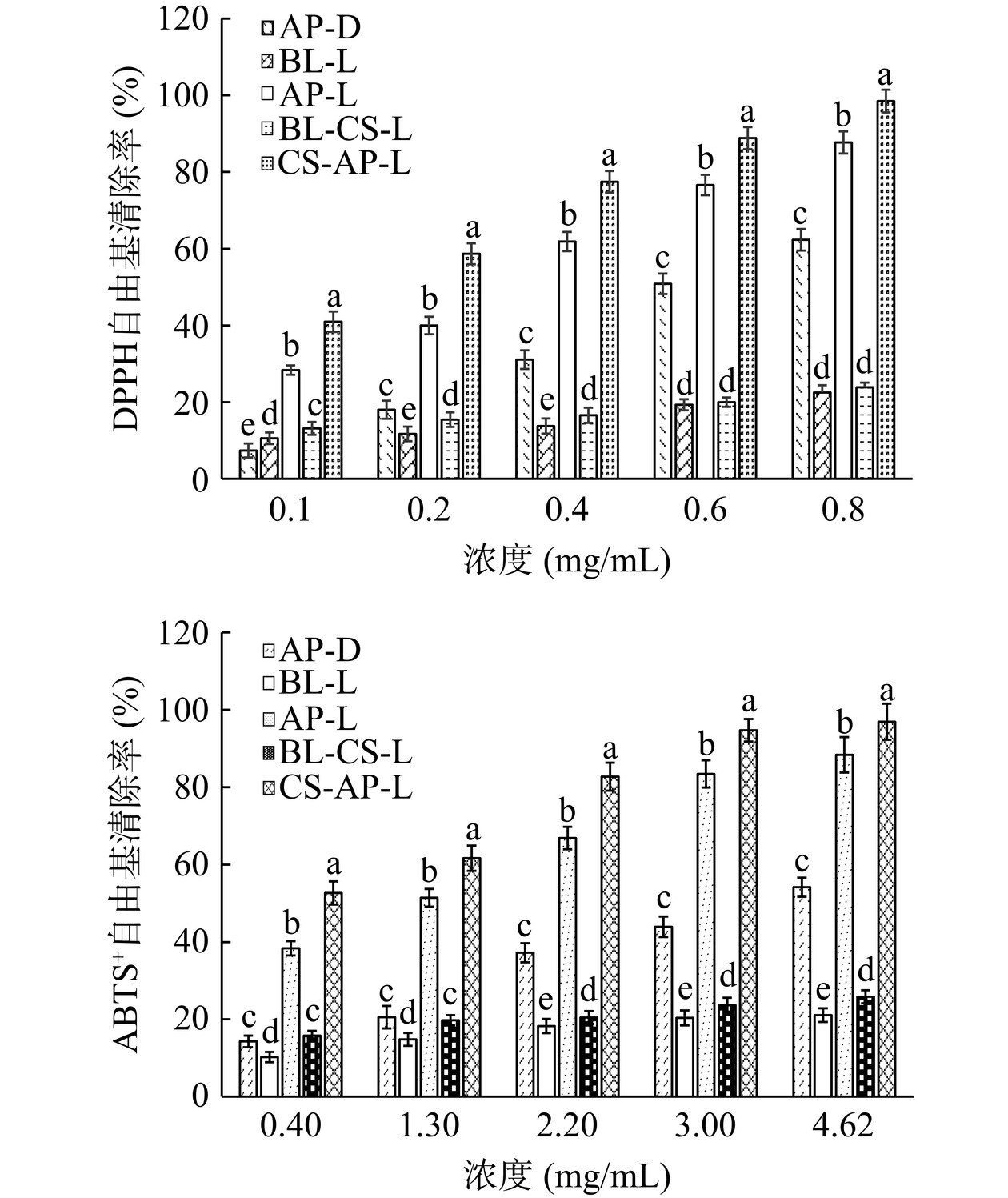
表8列出了VC、AP-D、AP-L、CS-AP-L的DPPH和ABTS+自由基半数清除浓度(IC50)。根据表中所列数据,虽然AP-D、AP-L和CS-AP-L的DPPH自由基和ABTS+自由基IC50均显著高于VC的(P<0.05),但AP-L和CS-AP-L的DPPH自由基和ABTS+自由基IC50较AP-D的显著降低(P<0.05)。芹菜素通过酚羟基提供氢离子来减少氧自由基的产生,具有优异的抗氧化能力[30−31]。AP-D的IC50高,可能是因为芹菜素在水中的溶解性差,导致其提供氢离子的能力减弱,从而限制了抗氧化能力的发挥[32]。而AP-L可以提高芹菜素在水中的溶解度,从而使芹菜素分子可以提供更多的氢离子充分与DPPH或者ABTS+自由基接触,进而加强了芹菜素的DPPH和ABTS+自由基清除能力[33],因此IC50显著降低。在CS-AP-L中,壳聚糖修饰增强了脂质体的稳定性,可以更好保护包裹其中的芹菜素,减少芹菜素自身降解,从而获得更高的自由基清除能力,IC50进一步降低。
表 8 VC、AP-D、AP-L、CS-AP-L的DPPH和ABTS+自由基半数清除浓度Table 8. IC50 of DPPH and ABTS+ free radical of VC, AP-D,AP-L, CS-AP-L样品 DPPH IC50(mg/mL) ABTS+ IC50(mg/mL) VC 0.017±0.001d 0.11±0.01d AP-D 0.59±0.14a 4.62±0.86a AP-L 0.25±0.06b 1.30±0.21b CS-AP-L 0.17±0.02c 0.38±0.02c 从图14可以看出,AP-D、AP-L、CS-AP-L的DPPH和ABTS+自由基清除率均随着浓度的提高而提高。在同一浓度下,AP-L、CS-AP-L的DPPH和ABTS+自由基清除率均显著高于AP-D(P<0.05),其中CS-AP-L的清除率最高。不含芹菜素的BL-L和CS-BL-L均有一定的DPPH和ABTS+自由基清除能力。在实验浓度范围内,BL-L的DPPH自由基清除率从10.58%±1.22%提高到21.52%±2.88%,ABTS+自由基清除率从10.23%±1.42%提高到21.13%±1.69%,CS-BL-L的DPPH自由基清除率从12.18%±1.71%提高到25.73%±1.37%,ABTS+自由基清除率从15.80%±1.34%提高到25.89%±1.74%。总体上二者的DPPH和ABTS+自由基清除率不高且随用量增加变化不大,表明脂质分子和壳聚糖分子本身虽然具有一定的抗氧化能力[23-34],可以与芹菜素发挥协同清除自由基的作用,但不是主要作用,AP-L和CS-AP-L自由基清除能力的提高主要是因为促进了芹菜素自身DPPH和ABTS+自由基清除能力。
3. 结论
游离芹菜素在水中的溶解度极低,实验测得约0.38 μg/mL。本文制备的AP-L包封率可以达到60%,即芹菜素在水中的溶解度可以达到180 μg/mL,表明脂质体的包裹可以有效提高芹菜素的溶解度。AP-L更适用于薄膜分散-超声法制备,超声有助于脂质体形成更均一的小单室脂质体。脂质体包封率用低速离心法测定更准确。所得AP-L粒径大小合适,分布均匀。AP-L制备的最佳用量为:芹菜素6 mg,芹菜素磷脂质量比1:24,磷脂胆固醇质量比6:1,乙醇用量6.3 mL,PBS用量20 mL。壳聚糖修饰使AP-L的粒径变大,但不影响粒径分布的均匀度,粒子表面电位由负变正。除此之外,壳聚糖修饰可以改善AP-L易发生聚集、融合、裂解而导致芹菜素泄露的缺陷,提高脂质体的稳定性。AP-L和CS-AP-L均可以提高芹菜素的自由基清除能力,CS-AP-L的自由基清除能力最高,表明制备脂质体并用壳聚糖修饰具有提高芹菜素抗氧化能力的潜力。
-
表 1 AP-L不同成分具体用量
Table 1 Accurate amount of different ingredients in AP-L
样品序号 芹菜素质量
(mg)芹菜素磷脂
质量比磷脂胆固醇
质量比无水乙醇
(mL)PBS(mL) 1 6 1:20 6:1 6 20 2 6 1:20 8:1 6 20 3 6 1:25 6:1 6 20 4 6 1:25 8:1 6 20 表 2 Box-Behnken响应面模型的因素和水平
Table 2 Factors and levels of Box-Behnken response surface model
水平 因素 A芹菜素磷脂质量比
(W/W)B磷脂胆固醇质量比
(W/W)C乙醇用量
(mL)−1 1:20 4:1 4 0 1:25 6:1 6 1 1:30 8:1 8 表 3 过膜法对AP-L包封率测定的影响
Table 3 Effect of the membrane filtration on the determination of the encapsulation efficiency of AP-L
表 4 低速离心法对AP-L包封率测定的影响
Table 4 Effect of the low-speed centrifugation on the determination of the encapsulation efficiency of AP-L
离心时间(min) AP-D上清液中芹菜素浓度(μg/mL) AP-L包封率(%) 10 0.64±0.01 − 15 0.41±0.02 − 20 0.38±0.01 56.45±1.58 30 0.36±0.23 57.09±2.43 表 5 Box-Behnken响应面设计试验结果
Table 5 Experimental results of Box-Behnken response surface design
试验号 因素 包封率(%) A芹菜素磷脂
质量比(W/W)B磷脂胆固醇
质量比(W/W)C乙醇用量
(mL)1 1:20 6:1 8 50.57 2 1:20 8:1 6 53.14 3 1:25 6:1 6 66.48 4 1:30 4:1 6 20.15 5 1:25 4:1 8 22.49 6 1:25 4:1 4 20.04 7 1:25 6:1 6 65.42 8 1:30 6:1 4 38.09 9 1:25 6:1 6 65.23 10 1:30 8:1 6 15.45 11 1:25 6:1 6 67.04 12 1:25 4:1 6 40.39 13 1:25 8:1 8 11.53 14 1:25 8:1 4 21.57 15 1:25 6:1 6 66.78 16 1:20 6:1 4 43.38 17 1:30 6:1 8 15.18 表 6 响应面模型方差分析
Table 6 ANOVA for response surface model
方差来源 平方和 自由度 均方 F值 P值 显著性 模型 7044.41 9 782.71 432.42 <0.0001 *** A芹菜素磷脂质量比 772.44 1 772.44 426.75 <0.0001 *** B磷脂胆固醇质量比 57.14 1 57.14 31.57 0.0008 *** C乙醇用量 67.92 1 67.92 37.52 0.0005 *** AB 1.63 1 1.63 0.8981 0.3748 AC 226.50 1 226.50 125.14 <0.0001 *** BC 39.00 1 39.00 21.55 0.0024 ** A2 464.65 1 464.65 256.71 <0.0001 *** B2 3396.64 1 3396.64 1876.53 <0.0001 *** C2 1500.86 1 1500.86 829.18 <0.0001 *** 残差 12.67 7 1.81 失拟项 10.00 3 3.33 5.00 0.0771 不显著 纯误差 2.67 4 0.6673 总差 7057.08 16 R2=0.9982,R2Adj=0.9959,R2Pred=0.9767 注:***表示差异极显著(P<0.001),**表示差异非常显著(P<0.01),*表示差异显著(P<0.05)。 表 7 BL-L、AP-L、BL-CS-L、CS-AP-L的粒径、多分散系数和电位
Table 7 Particle size, PDI and zeta potential of BL-L, AP-L, BL-CS-L and CS-AP-L
样品 粒径(nm) 多分散系数 电位 BL-L 139.94±4.34d 0.235±0.018a −38.17±0.23b AP-L 157.34±1.87c 0.240±0.025a −38.38±0.13b BL-CS-L 499.40±57.73b 0.313±0.026a 39.23±1.09a CS-AP-L 564.22±39.7a 0.292±0.022a 39.57±1.61a 表 8 VC、AP-D、AP-L、CS-AP-L的DPPH和ABTS+自由基半数清除浓度
Table 8 IC50 of DPPH and ABTS+ free radical of VC, AP-D,AP-L, CS-AP-L
样品 DPPH IC50(mg/mL) ABTS+ IC50(mg/mL) VC 0.017±0.001d 0.11±0.01d AP-D 0.59±0.14a 4.62±0.86a AP-L 0.25±0.06b 1.30±0.21b CS-AP-L 0.17±0.02c 0.38±0.02c -
[1] 杨一帆, 梁艺瑶, 刘保保, 等. 转铁蛋白修饰雷公藤甲素脂质体的制备与体外评价[J]. 中草药,2022,53(3):687−695. [YANG Yifan, LIANG Yiyao, LIU Baobao, et al. Preparation of transferrin modified triptolide liposome and in vitro evaluation[J]. Chinese Traditional and Herbal Drugs,2022,53(3):687−695.] YANG Yifan, LIANG Yiyao, LIU Baobao, et al. Preparation of transferrin modified triptolide liposome and in vitro evaluation[J]. Chinese Traditional and Herbal Drugs, 2022, 53(3): 687−695.
[2] WANG W, LI Y, WANG H M, et al. The preparation of apigenin nanoparticles and the study of their anti-inflammatory and anti-tumor activities in vitro[J]. Separations,2023,10(1):16.
[3] 江靖雯, 闫佩瑶, 匡佳妮, 等. 芹菜素抗肿瘤药理作用机制的研究进展[J]. 中国药学杂志,2023,58(6):469−474. [JIANG Jingwen, YAN Peiyao, KUANG Jiani, et al. Research advances in antitumor mechanism of apigenin[J]. Chinese Pharmaceutical Journal,2023,58(6):469−474.] JIANG Jingwen, YAN Peiyao, KUANG Jiani, et al. Research advances in antitumor mechanism of apigenin[J]. Chinese Pharmaceutical Journal, 2023, 58(6): 469−474.
[4] 高国际, 龙玲, 张海霞. 芹菜素对机体免疫功能调节作用的研究进展[J]. 农产品加工,2022(12):83−87. [GAO Guoji, LONG Ling, ZHANG Haixia. Research progress of apigenin on the regulation of immune function[J]. Aem Roducts Rocecing,2022(12):83−87.] GAO Guoji, LONG Ling, ZHANG Haixia. Research progress of apigenin on the regulation of immune function[J]. Aem Roducts Rocecing, 2022(12): 83−87.
[5] ZHANG J J, LIU D P, HUANG Y T, et al. Biopharmaceutics classification and intestinal absorption study of apigenin[J]. International Journal of Pharmaceutics,2012,436(1):311−317.
[6] 李娇娇, 赵梦楠, 糜丹丹, 等. 藤黄酸纳米制剂的制备及其抗肿瘤活性[J]. 现代肿瘤医学,2023,31(24):4503−4507. [LI Jiaojiao, ZHAO Mengnan, MI Dandan, et al. Preparation and anti tumor activity of gambogic acid nanopreparations[J]. Morden Oncology,2023,31(24):4503−4507.] LI Jiaojiao, ZHAO Mengnan, MI Dandan, et al. Preparation and anti tumor activity of gambogic acid nanopreparations[J]. Morden Oncology, 2023, 31(24): 4503−4507.
[7] 韩露, 吴港城, 马子宾, 等. 氨基酸锌脂质体及前体脂质体的工艺优化[J]. 中国食品添加剂,2023,34(12):68−76. [HAN Lu, WU Gangcheng, MA Zibin, et al. Process optimization of zinc amino acid liposomes and precursor liposomes[J]. China Food Additives,2023,34(12):68−76.] HAN Lu, WU Gangcheng, MA Zibin, et al. Process optimization of zinc amino acid liposomes and precursor liposomes[J]. China Food Additives, 2023, 34(12): 68−76.
[8] ZHANG H Y, WEI S R, ZHANG Y, et al. Improving cellular uptake and bioavailability of periplocymarin-linoleic acid prodrug by combining PEGylated liposome[J]. Drug Delivery,2022,29(1):2491−2497. doi: 10.1080/10717544.2022.2104406
[9] VEMURI S, RHODES C T. Preparation and characterization of liposomes as therapeutic delivery systems:A review[J]. Pharmaceutica Acta Helvetiae,1995,70(2):95−111. doi: 10.1016/0031-6865(95)00010-7
[10] VUILLEMARD J C. Recent advances in the large-scale production of lipid vesicles for use in food products:Microfluidization[J]. [J]. Jounal of Microencapsulation,1991,8(4):547. doi: 10.3109/02652049109021878
[11] 许海丹, 吕秀阳, 李英华. 卵磷脂稳定性的研究进展[J]. 科技通报,2006,22(2):267−270. [XU Haidan, LÜ Xiuyang, LI Yinghua. Recent advances on lecithin stability[J]. Bulettin of Science and Technology,2006,22(2):267−270.] XU Haidan, LÜ Xiuyang, LI Yinghua. Recent advances on lecithin stability[J]. Bulettin of Science and Technology, 2006, 22(2): 267−270.
[12] 房佳慧, 杨喆, 纪新元, 等. 凹凸棒石/壳聚糖载药微球的制备及性能[J]. 非金属矿,2023,46(3):23−27. [FANG Jiahui, YANG Zhe, JI Xinyuan, et al. Preparation and performance of attapulgite/chitosan drug-loaded microspheres[J]. Non-Metallic Mines,2023,46(3):23−27.] FANG Jiahui, YANG Zhe, JI Xinyuan, et al. Preparation and performance of attapulgite/chitosan drug-loaded microspheres[J]. Non-Metallic Mines, 2023, 46(3): 23−27.
[13] TAI K, RAPPOLT M, MAO L, et al. The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan[J]. Food Hydrocolloids,2020(99):105355.
[14] SEBAALY C, HAYDAR S, GREIGE-GERGES H. Eugenol encapsulation into conventional liposomes and chitosan-coated liposomes:A comparative study[J]. Jounal of Drug Delivery Science Technology,2022,67:102942. doi: 10.1016/j.jddst.2021.102942
[15] MOHAMMAD R I S, NAYMUL K, XIE J H, et al. Colonic delivery of pelargonidin-3-O-glucoside using pectin-chitosannanoliposome:Transport mechanism and bioactivity retention[J]. International Journal of Biological Macromolecules,2020,159:341−355. doi: 10.1016/j.ijbiomac.2020.05.076
[16] WANG X W, CHENG F Y, WANG X J, et al. Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes[J]. International Journal of Biological Macromolecules,2021,168:59−66. doi: 10.1016/j.ijbiomac.2020.12.003
[17] WU Y J, MOU B L, SONG S H, et al. Curcumin-loaded liposomes prepared from bovine milk and krill phospholipids:Effects of chemical composition on storage stability, in-vitro digestibility and anti-hyperglycemic properties[J]. Food Reserch International,2020,136:109301. doi: 10.1016/j.foodres.2020.109301
[18] LIANG R, CHEN L, YOKOYAMA W, et al. Niosomes consisting of Tween-60 and cholesterol improve the chemical stability and antioxidant activity of (−)-epigallocatechin gallate under intestinal tract conditions[J]. Journal of Agriculture Food Chemistry,2016,64(48):9180−9188. doi: 10.1021/acs.jafc.6b04147
[19] 赵茜茜, 王英豪, 肖志勇, 等. 姜黄素-胡椒碱共载脂质体的制备及其体外抗肿瘤活性评价[J]. 中国医药工业杂志,2023,54(2):230−236. [ZHAO Qianqian, WANG Yinghao, XIAO Zhiyong, et al. Preparation and evaluation of in vitro antitumor activity of curcumin and piperine co-loaded liposomes[J]. Chinese Journal of Pharmaceuticals,2023,54(2):230−236.] ZHAO Qianqian, WANG Yinghao, XIAO Zhiyong, et al. Preparation and evaluation of in vitro antitumor activity of curcumin and piperine co-loaded liposomes[J]. Chinese Journal of Pharmaceuticals, 2023, 54(2): 230−236.
[20] 郭丽珊, 李晰月, 姚成丽, 等. 维生素K1混合胶束包封率测定方法的适用性研究[J]. 中国医药工业杂志,2023,54(9):1367−1373. [GUO Lishan, LI Xiyue, YAO Chengli, et al. Applicability study of determination methods for encapsulation efficiency of vitamin K1-loaded mixed micelles[J]. Chinese Journal of Pharmaceuticals,2023,54(9):1367−1373.] GUO Lishan, LI Xiyue, YAO Chengli, et al. Applicability study of determination methods for encapsulation efficiency of vitamin K1-loaded mixed micelles[J]. Chinese Journal of Pharmaceuticals, 2023, 54(9): 1367−1373.
[21] 魏征, 杨森, 罗正康, 等. 牛血清白蛋白/壳聚糖双层修饰载肉桂醛脂质体的制备[J]. 中国药房,2022,33(7):848−852. [WEI Zheng, YANG Sen, LUO Zhengkang, et al. Preparation of cinnamaldehyde loaded liposomes bilayer-modified by bovine serum albumin/chitosan[J]. China Pharmacy,2022,33(7):848−852.] WEI Zheng, YANG Sen, LUO Zhengkang, et al. Preparation of cinnamaldehyde loaded liposomes bilayer-modified by bovine serum albumin/chitosan[J]. China Pharmacy, 2022, 33(7): 848−852.
[22] 杨红艳, 陈子明, 黄丽平, 等. 盐酸莫西沙星脂质体的制备及包封率测定方法研究[J]. 中国医院药学杂志,2018,38(10):1051−1055. [YANG Hongyan, CHEN Ziming, HUANG Liping, et al. Preparation of moxifloxacin hydrochloride liposomes and entrapment efficiency determination[J]. Chinese Journal of Hospital Pharmacy,2018,38(10):1051−1055.] YANG Hongyan, CHEN Ziming, HUANG Liping, et al. Preparation of moxifloxacin hydrochloride liposomes and entrapment efficiency determination[J]. Chinese Journal of Hospital Pharmacy, 2018, 38(10): 1051−1055.
[23] 李亮, 袁传勋, 王敏, 等. PEG修饰EGCG/Cur复合脂质体的制备与抗氧化研究[J]. 食品工业科技,2023,44(9):88−95. [LI Liang, YUAN Chuanxun, WANG Min, et al. Preparation and antioxidant study of PEG-modified EGCG/Cur composite liposomes[J]. Science and Technology of Food Industry,2023,44(9):88−95.] LI Liang, YUAN Chuanxun, WANG Min, et al. Preparation and antioxidant study of PEG-modified EGCG/Cur composite liposomes[J]. Science and Technology of Food Industry, 2023, 44(9): 88−95.
[24] 侯丽芬, 谷克仁, 吴永辉. 不同制剂脂质体制备方法的研究进展[J]. 河南工业大学学报(自然科学版),2016,37(5):118−124. [HOU Lifen, GU Keren, WU Yonghui. Research progress of the preparation methods of liposome about different formulations[J]. Journal of Henan University of Technology(Natural Science Edition),2016,37(5):118−124.] HOU Lifen, GU Keren, WU Yonghui. Research progress of the preparation methods of liposome about different formulations[J]. Journal of Henan University of Technology(Natural Science Edition), 2016, 37(5): 118−124.
[25] 邢宇, 李雄, 于颖. 银杏内酯B脂质体的处方与制备工艺研究[J]. 中国现代应用药学,2021,38(6):697−703. [XING Yu, LI Xiong, YU Ying. Study on formulation and preparation process of ginkgolide B liposome[J]. Chinese Journal of Modern Applied Pharmacy,2021,38(6):697−703.] XING Yu, LI Xiong, YU Ying. Study on formulation and preparation process of ginkgolide B liposome[J]. Chinese Journal of Modern Applied Pharmacy, 2021, 38(6): 697−703.
[26] 刘淼, 高悦, 李康帆, 等. 聚乙二醇修饰木犀草素脂质体的制备及其理化性质[J]. 现代食品科技,2021,37(10):118−125,316. [LIU Miao, GAO Yue, LI Kangfan, et al. Preparation and physicochemical properties of polyethylene glycol-modified luteolin liposome[J]. Modern Food Science & Technology,2021,37(10):118−125,316.] LIU Miao, GAO Yue, LI Kangfan, et al. Preparation and physicochemical properties of polyethylene glycol-modified luteolin liposome[J]. Modern Food Science & Technology, 2021, 37(10): 118−125,316.
[27] 万子腾, 张跃, 姜建鑫, 等. 壳聚糖修饰绿原酸脂质体的制备及其体外透皮性能考察[J]. 中国医药工业杂志,2023,54(5):760−765. [WAN Ziteng, ZHANG Yue, JIANG Jianxin, et al. Preparation of chitosan-modified chlorogenic acid liposomes and investigation on their transdermal properties in vitro[J]. Chinese Journal of Pharmaceuticals,2023,54(5):760−765.] WAN Ziteng, ZHANG Yue, JIANG Jianxin, et al. Preparation of chitosan-modified chlorogenic acid liposomes and investigation on their transdermal properties in vitro[J]. Chinese Journal of Pharmaceuticals, 2023, 54(5): 760−765.
[28] 曹振大, 梁蓉. 表没食子儿茶素没食子酸酯脂质体的制备及其透皮性能研究[J]. 日用化学工业,2020,50(9):609−614. [CAO Zhenda, LIANG Rong. Study on the preparation and transdermal performance of epigallocatechin gallate liposome[J]. China Surfactant Detergent & Cosmetics,2020,50(9):609−614.] CAO Zhenda, LIANG Rong. Study on the preparation and transdermal performance of epigallocatechin gallate liposome[J]. China Surfactant Detergent & Cosmetics, 2020, 50(9): 609−614.
[29] 叶晓莉, 吴梅君, 王丛瑶, 等. 冰片修饰的葛根素脂质体的制备及脑组织分布研究[J]. 中国现代应用药学,2023,40(5):632−637. [YE Xiaoli, WU Meijun, WANG Congyao, et al. Preparation and brain distribution of borneol modified puerarin liposomes[J]. Chinese Journal of Modern Applied Pharmacy,2023,40(5):632−637.] YE Xiaoli, WU Meijun, WANG Congyao, et al. Preparation and brain distribution of borneol modified puerarin liposomes[J]. Chinese Journal of Modern Applied Pharmacy, 2023, 40(5): 632−637.
[30] DARSHAN R T, ARUN T P, ANIL M P, et al. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential[J]. European Journal of Pharmaceutics Science,2016,108:36−49.
[31] GUO J Y, GAN C F, CHENG B, et al. Exploration of binding mechanism of apigenin to pepsin:Spectroscopic analysis, molecular docking, enzyme activity and antioxidant assays[J]. Spectrochimica Acta A,2023,290:122281. doi: 10.1016/j.saa.2022.122281
[32] MOHAMMED F A, MOHAMMED M A, FARHAT F, et al. Development and characterization of calcium-alginate beads of apigenin:In vitro antitumor, antibacterial, and antioxidant activities[J]. Marine Drugs,2021,19(467):1−16.
[33] 刘鑫岳, 陈晓平, 焦丽蓉, 等. 脂质包衣的姜黄素/玉米醇溶蛋白纳米粒的制备及性能表征[J]. 食品与发酵工业,2022,48(5):130−135. [LIU Xinyue, CHEN Xiaoping, JIAO Lirong, et al. Fabrication and characterization of lipid coated curcumin/zein nanoparticles[J]. Food and Fermentation Industries,2022,48(5):130−135.] LIU Xinyue, CHEN Xiaoping, JIAO Lirong, et al. Fabrication and characterization of lipid coated curcumin/zein nanoparticles[J]. Food and Fermentation Industries, 2022, 48(5): 130−135.
[34] ANG S S, THOO Y Y, SIOW L F. Encapsulation of hydrophobic apigenin into small unilamellar liposomes[J]. Food and Bioprocess Technology,2023,13:1−16.





 下载:
下载:
 下载:
下载:



