Simultaneously Detecting of 13 Illegally Added Drugs in Health Foods for Improving Sexual and Prostate Function by High Performance Liquid Chromatography
-
摘要: 为了同时检测13种非法添加化学药物,建立适用于改善性功能和前列腺功能类保健食品的高效液相色谱分析方法。将样品经涡旋振荡1 min再甲醇超声提取10 min后,以甲醇-磷酸三乙胺缓冲液为流动相,经Agilent 5 HC-C18(2)色谱柱(250 mm×4.6 mm,5 µm)梯度分离,检测波长为218 nm和245 nm,柱温30 ℃,进样量10 μL。结果表明,该方法在线性范围内的相关系数(r)均大于0.999,检测限为0.76~4.61 mg/kg,平均回收率为92.4%~100.7%,相对标准偏差为0.44%~1.06%。运用本方法对65批次改善性功能或前列腺功能类保健食品进行筛查,结果在2批样品中检测出盐酸达泊西汀(分别为15.26 mg/片、6.34 mg/粒),1批样品中检测出盐酸育亨宾(10.12 mg/粒)。综上,该方法具有操作简单,灵敏度和准确度高,实用性强的特点,适用于改善性功能和前列腺功能的保健品中非法添加药物的日常监管检验。Abstract: To simultaneously detect 13 illegally added chemical drugs, a high performance liquid chromatography (HPLC) analytical method applicable to health food products for improving sexual function and prostate function was established. The samples were processed by vortex shaking for 1 min, followed by methanol extraction for 10 min under the ultrasonic condition. Then, the sample was separated on an Agilent 5 HC-C18 (2) column (250 mm×4.6 mm, 5 µm) with a gradient separation using methanol-phosphate-triethylamine buffer as the mobile phase. The detection wavelengths were of 218 nm and 245 nm, the column temperature was 30 ℃, and the sample size was 10 µL. Results showed that the correlation coefficients (r) in the linear range were all greater than 0.999, the detection limits were 0.76~4.61 mg/kg, the average recoveries were 92.4%~100.7%, and the relative standard deviations were 0.44%~1.06%. The method was applied to the rapid screening of 65 batches of health foods for improving sexual and prostate function, and results indicated that paroxetine hydrochloride was detected in 2 batches of samples (15.26 mg/tablet, 6.34 mg/pellet, respectively) and yohimbine hydrochloride was detected in 1 batch of samples (10.12 mg/pellet). In summary, the method is easy to conduct and characterized with high sensitivity and reliability, which is suitable for daily supervision and inspection of illegal added drugs in health foods for improving sexual and prostate function.
-
随着社会经济水平的提高以及人们保健意识的增强,我国已成为全球第二大保健食品消费市场,行业规模达4000亿元[1−2]。面对巨大的市场需求和利益诱惑,一些不良商家为了增强产品功效,利用某些化学药物功能性强、见效快的特点,将其非法添加至保健品中以提高销量,谋取非法暴利[3−4]。其中非法添加化学药物的现象在改善性功能和前列腺功能类保健品中尤为严重[5−6]。消费者在不知情的情况下长期或过量服用此类保健品,可能引发诸多不良反应,或对身体造成不可逆的伤害,甚至可能危及生命[7−9]。
近年来,国家食品药品监督管理局连续颁布了多个补充检验方法和补充项目批准件,涉及非法添加改善性功能或前列腺功能化学药物的5个国家标准[10−14]中收载了90种化学药物,均为5型磷酸二酯酶(PDE5)抑制剂及其衍生物。然而,临床上用于改善性功能和前列腺功能的药物种类并不限于PDE5。此外还有5-羟色胺再摄取抑制剂、α-肾上腺素受体阻滞剂和5α-还原酶抑制剂等[15−17]。同时,不法分子通过添加现行检测标准涵盖范围以外的具有相同功效的药物以躲避监管的现象国内外均有报道[18−20]。因此,鉴于现有检测标准中筛查的药物种类尚不全面,有必要根据药物的功效和市场监测的反馈情况,扩充上述三类常用于改善性功能或前列腺功能的化学物,开发出同时检测多种化学物的高通量检测方法,实现最大范围的应检尽检。
目前,高效液相色谱法[20−21]和高效液相色谱-串联质谱法等[22]常用于食品中违法添加物的检测。液相色谱-串联质谱法虽灵敏度高、分辨率好,但较强的基质效应通常需要单独的样品净化过程[23],且操作复杂,仪器价格昂贵,检测和维护成本大,难实现基层推广[24]。高效液相色谱-二极管阵列检测法(HPLC-PDA)通过峰保留时间和紫外特征光谱图,可同时对多组分进行定性、定量分析,具有分离效能好、定量准确、普适性强、操作简便、维护成本低等特点和基层检验机构推广的潜力[25−27]。宁霄等[28]建立了液相色谱-高分辨质谱法同时测定300种非法添加药物的检测方法,但针对5-羟色胺再摄取抑制剂、α-肾上腺素受体阻滞剂和5α-还原酶抑制剂这3类药物仅涉及7种,并且该研究中所采用的方法需通过样品萃取处理结合基质匹配校准才能减小基质效应对方法性能的影响,流程繁琐,操作复杂。徐硕等[6]建立了检测补肾壮阳和改善前列腺功能保健品中17种非法添加药物的高效液相色谱法-二极管阵列检测法,该方法分为2个HPLC系统对3种α-肾上腺素受体阻滞剂和3种5α-还原酶抑制剂进行分离,但存在操作繁琐的问题,且仍未涉及5-羟色胺再摄取抑制剂。由此,尚未有研究同时涵盖5-羟色胺再摄取抑制剂、α-肾上腺素受体阻滞剂和5α-还原酶抑制剂这3类化学药物。
为了扩充现行检测标准针对改善性功能和前列腺功能的化学药物的筛查范围,并且创建一个简便准确的HPLC方法,因此本研究以5-羟色胺再摄取抑制剂(盐酸达泊西汀、盐酸舍曲林、盐酸氟西汀、盐酸帕罗西汀)、α-肾上腺素受体阻滞剂(盐酸妥拉唑林、乌拉地尔、盐酸育亨宾、盐酸特拉唑嗪、盐酸阿夫唑嗪、盐酸哌唑嗪、甲磺酸酚妥拉明、甲磺酸多沙唑嗪)和5α-还原酶抑制剂(非那雄胺)3类共计13种化学药物为研究对象,建立了HPLC-PDA的定性定量分析方法。方法已用于实际工作,可为基层政府监管及检测机构提供技术支撑。
1. 材料与方法
1.1 材料与仪器
盐酸妥拉唑林(纯度:100%)、乌拉地尔(纯度:99.8%)、盐酸育亨宾(纯度:99.3%)、盐酸特拉唑嗪(纯度:91.9%)、盐酸哌唑嗪(纯度:99.9%)、甲磺酸酚妥拉明(纯度:99.7%)、甲磺酸多沙唑嗪(纯度:97.8%)、盐酸帕罗西汀(纯度:97.3%)、盐酸氟西汀(纯度:99.8%)、盐酸舍曲林(纯度:99.9%)、非那雄胺(纯度:99.5%) 购自中国食品药品检定研究院;盐酸阿呋唑嗪(纯度:94.38%)、盐酸达泊西汀(纯度:99.02%) 购自Hong Kong Institute of standard substance;乙腈、甲醇 色谱纯,Sigma公司;磷酸 色谱纯,诺尔施科技有限公司;三乙胺 色谱纯,科密欧化学试剂有限公司;实验室用水为超纯水;65批改善性功能或改善前列腺功能的保健品样品 包括市售样品20批、稽查样品25批、网络销售样品20批,样品类型涉及胶囊剂、颗粒剂、片剂、口服液、保健酒。
Shimadzu LC-2050C 3D高效液相色谱仪(配有Prominence SPD-M20A PDA 检测器) 日本岛津公司;Mettler Toledo XSE205电子天平、SevenMulti型pH测量仪 瑞士梅特勒-托利多公司;JP-060S型超声仪 深圳市洁盟清洗设备有限公司;Eppendorf 5430R高速冷冻离心机 德国艾本德公司;ULPHW-IV型超纯水仪 四川优普超纯水科技有限公司。
1.2 实验方法
1.2.1 标准溶液配制
称取13种化学药物的标准品适量,配制成混合标准溶液,其中盐酸妥拉唑林、乌拉地尔、盐酸育亨宾、盐酸特拉唑嗪、盐酸阿呋唑嗪、盐酸哌唑嗪、甲磺酸酚妥拉明、甲磺酸多沙唑嗪、盐酸达泊西汀、盐酸氟西汀、盐酸舍曲林、非那雄胺的浓度均为500 μg/mL,盐酸帕罗西汀的浓度为800 μg/mL。分别取混合标准溶液0.1、0.2、0.5、1、2、4、10 mL用甲醇稀释至10 mL,配制成系列混合标准溶液s1~s7,用于测定13种化合物的线性关系。
1.2.2 样品前处理
液体样品:根据参考文献[6,29−30]的方法并稍作修改。取待测样品适量,充分混匀。精密量取混匀试样2 mL置10 mL具塞玻璃比色管中,加入甲醇6 mL涡旋振荡1 min再超声提取10 min后,冷却至室温,用甲醇定容至10 mL。摇匀后转移提取液至离心管中,在转速7000 r/min,温度20 ℃的条件下离心5 min,上清液通过0.22 µm微孔有机滤膜过滤,取滤液作为供试品溶液。
固体样品:取胶囊或片剂等固体样品适量,将其研碎并混匀。精密称取1.0000 g试样,置10 mL具塞玻璃比色管中,后续操作步骤同液体样品。
1.2.3 色谱条件
色谱柱:Agilent 5 HC-C18(2)柱(250 mm×4.6 mm,5 µm);柱温:30 ℃;进样体积:10 µL;流速:1.0 mL/min;检测波长:245 nm(盐酸特拉唑嗪、盐酸阿呋唑嗪、盐酸哌唑嗪、甲磺酸多沙唑嗪)和218 nm(盐酸妥拉唑林、乌拉地尔、盐酸育亨宾、甲磺酸酚妥拉明、盐酸帕罗西汀、盐酸达泊西汀、盐酸氟西汀、盐酸舍曲林和非那雄胺);流动相:A为甲醇,B为磷酸三乙胺溶液(含0.1%三乙胺,磷酸调pH至3.0),梯度洗脱程序见表1。
表 1 梯度洗脱程序Table 1. Mobile phase gradient elution procedures时间(min) 流动相A(%) 流动相B(%) 0 15 85 12 35 65 24 42 58 26 58 42 45 75 25 1.2.4 定性实验
在初步判定是阳性的样品中加入相应的对照品溶液,若样品中疑似峰与对照品色谱峰能完全重合,并且疑似峰与对照品色谱峰紫外吸收特征谱图相似度大于99.0%,则判定该样品是阳性样品。
拖尾因子T的计算公式如下:
T=W0.05h2d1 式中,W0.05h为5%峰高处的峰宽;d1为峰顶在5%峰高处横坐标平行线的投影点至峰前沿与此平行线交点的距离。
1.2.5 定量实验
配制检出成分的对照品溶液,使对照品色谱峰面积与阳性样品中检出成分峰面积接近,以外标法计算阳性样品中检出成分的含量。
1.3 数据处理
采用Microsoft Excel 2016进行数据处理,计算平均值和相对标准偏差(RSD);采用Origin 2021绘制百分比堆积条形图、热图、色谱图。
2. 结果与分析
2.1 前处理方法的优化
2.1.1 提取溶剂的优化
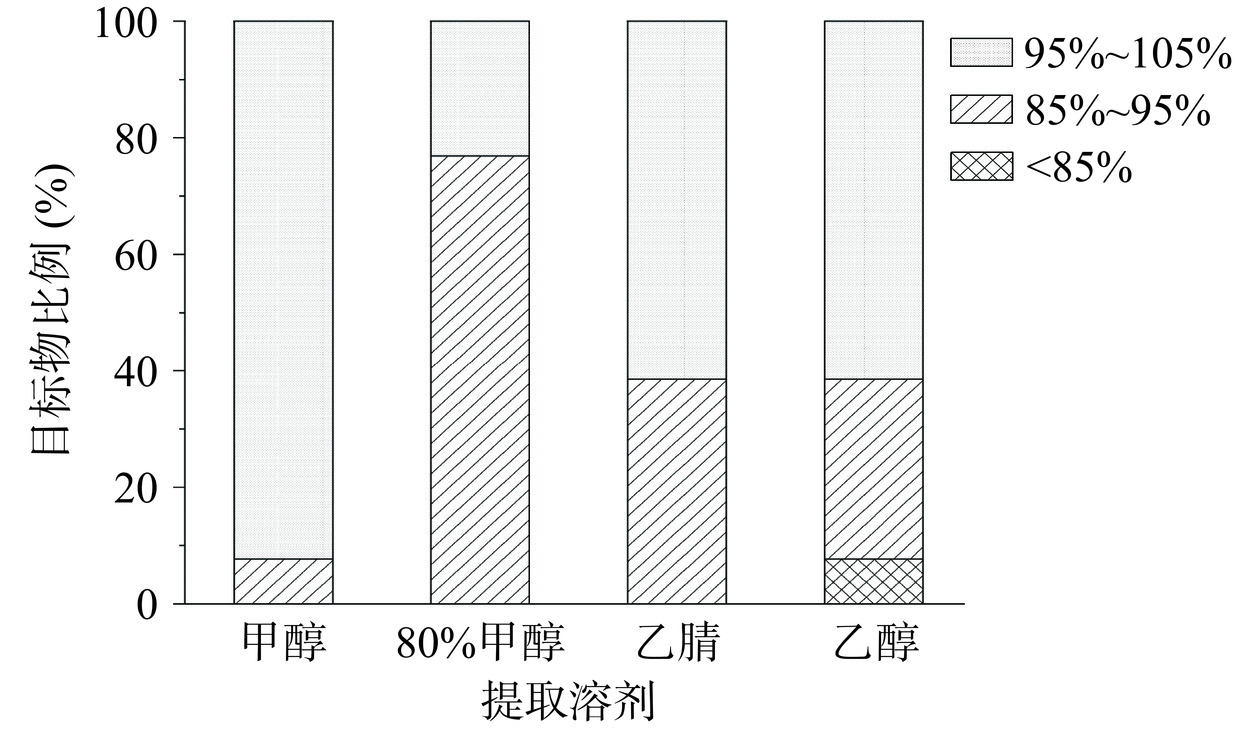
13种化合物的理化性质不同,因此选择提取溶剂需综合各化合物的极性及溶解性[31−32]。以市售改善性功能的保健品为测试样品,通过空白样品加标方式,以回收率为指标,在100 mg/kg加标水平下,考察了甲醇、80%甲醇、乙醇、乙腈四种提取溶剂对13种目标化合物的提取效果。结果如图1显示,以甲醇作为提取溶剂时只有盐酸帕罗西汀的回收率为93.5%,其余12个化合物的回收率都在95%~105%之间。当将甲醇浓度稀释到80%时,目标化合物的提取效果普遍变差。而乙腈和乙醇作为提取溶剂时,提取效果均不佳,尤其是乙醇对盐酸妥拉唑林的提取效果极差,回收率低至43.3%。实验结果与徐硕等[6]的报道相似,其也采用高效液相色谱-二极管阵列法检测了补肾壮阳和改善前列腺功能保健品中非法添加的17种化学药物,发现大多数化合物在甲醇中具有良好的溶解性,采用甲醇作为提取溶剂时全部药物的回收率较高。综上,本研究以甲醇作为提取溶剂。
2.1.2 提取方式和提取时间的优化
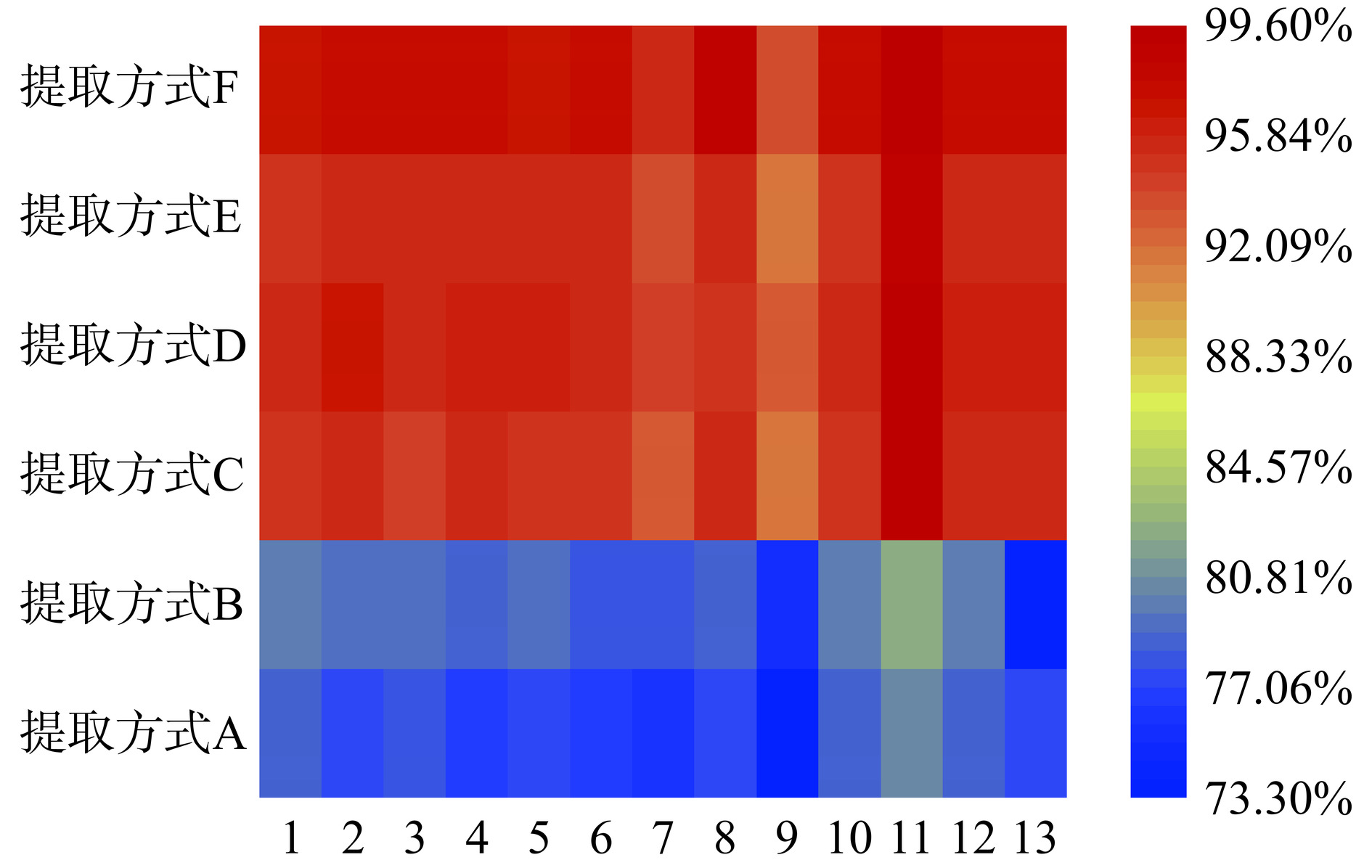
实验以空白样品加标的方式,在100 mg/kg加标水平下,以回收率为指标,对提取方式和提取时间进行考察。如图2所示,仅采用超声提取时,超声10 min和15 min大多数化合物的回收率较低,在70%~80%之间。当超声时间延长至20 min时,各化合物的回收率明显提高,13种化合物的回收率均达到90%以上;采用涡旋振荡和超声结合进行提取时,涡旋振荡1 min再超声10 min,各化合物的回收率均可达到95%左右。这一结果也验证了袁利杰等[33]的研究结论,其在采用HPLC检测非法添加物时也发现,相比单独使用超声提取,涡旋振荡结合超声的提取方式可明显提升样品的提取效率。这一现象的原因可能在于,涡旋振荡可以迅速使样品和提取液混合均匀从而增大其接触面积,提高提取效率。然而,继续增加超声时间,回收率不再发生明显变化。这与邵瑞婷等[19]在采用超高效液相色谱-串联质谱法测定调节三高类保健食品中59种非法添加药物时的试验结果一致。因此,综合样品的提取效率和回收率,以涡旋振荡1 min再超声10 min作为最佳提取方法。
![]() 图 2 不同提取方式和时间对13种非法添加化学药物的提取效果注:1.盐酸妥拉唑林;2.乌拉地尔;3.盐酸育亨宾;4.盐酸特拉唑嗪;5.盐酸阿夫唑嗪;6.盐酸哌唑嗪;7.甲磺酸酚妥拉明;8.甲磺酸多沙唑嗪;9.盐酸帕罗西汀;10.盐酸达泊西汀;11.盐酸氟西汀;12.盐酸舍曲林;13.非那雄胺;提取方式A:超声10 min;提取方式B:超声15 min;提取方式C:超声20 min;提取方式D:涡旋振荡1 min超声10 min;提取方式E:涡旋振荡1 min超声15 min;提取方式F:涡旋振荡1 min超声20 min;图3~图5同。Figure 2. Effects of different extraction methods and time on the 13 illegally added chemical drugs
图 2 不同提取方式和时间对13种非法添加化学药物的提取效果注:1.盐酸妥拉唑林;2.乌拉地尔;3.盐酸育亨宾;4.盐酸特拉唑嗪;5.盐酸阿夫唑嗪;6.盐酸哌唑嗪;7.甲磺酸酚妥拉明;8.甲磺酸多沙唑嗪;9.盐酸帕罗西汀;10.盐酸达泊西汀;11.盐酸氟西汀;12.盐酸舍曲林;13.非那雄胺;提取方式A:超声10 min;提取方式B:超声15 min;提取方式C:超声20 min;提取方式D:涡旋振荡1 min超声10 min;提取方式E:涡旋振荡1 min超声15 min;提取方式F:涡旋振荡1 min超声20 min;图3~图5同。Figure 2. Effects of different extraction methods and time on the 13 illegally added chemical drugs2.2 色谱条件的优化
2.2.1 色谱柱的选择
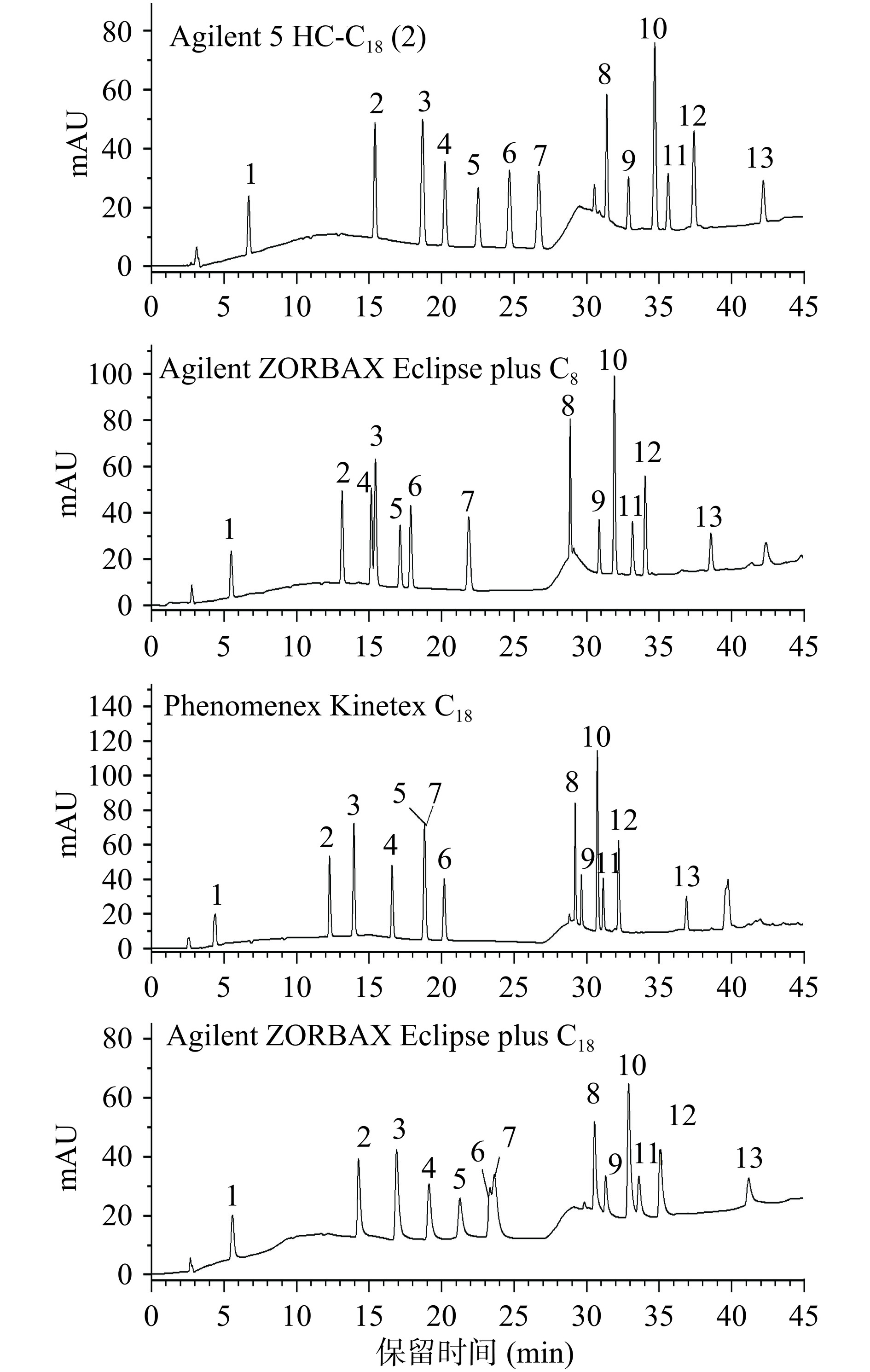
为了使13种极性不同的目标化合物在同一色谱条件下实现良好的分离,实验首先考察了色谱柱对13种化合物的分离效果的影响。由图3可知,虽然C8和C18色谱柱都属于弱极性色谱柱,但C8的极性相对更强,对13种化合物的分离效果不佳,盐酸特拉唑嗪和盐酸育亨宾无法有效分离。而其他3种C18色谱柱因硅胶键合类型和碳载量有所不同,分离效果也存在较大差异,使用Phenomenex Kinetex C18时盐酸阿夫唑嗪和甲磺酸酚妥拉明完全重叠,使用Agilent ZORBAX Eclipse plus C18时盐酸哌唑嗪和甲磺酸酚妥拉明无法有效分离,而使用Agilent 5 HC-C18(2)柱时,13种化合物均能实现基线分离,并且具有较好的峰形。因此,本实验确定选用Agilent 5 HC-C18(2)色谱柱进行后续检测。
2.2.2 流动相的选择
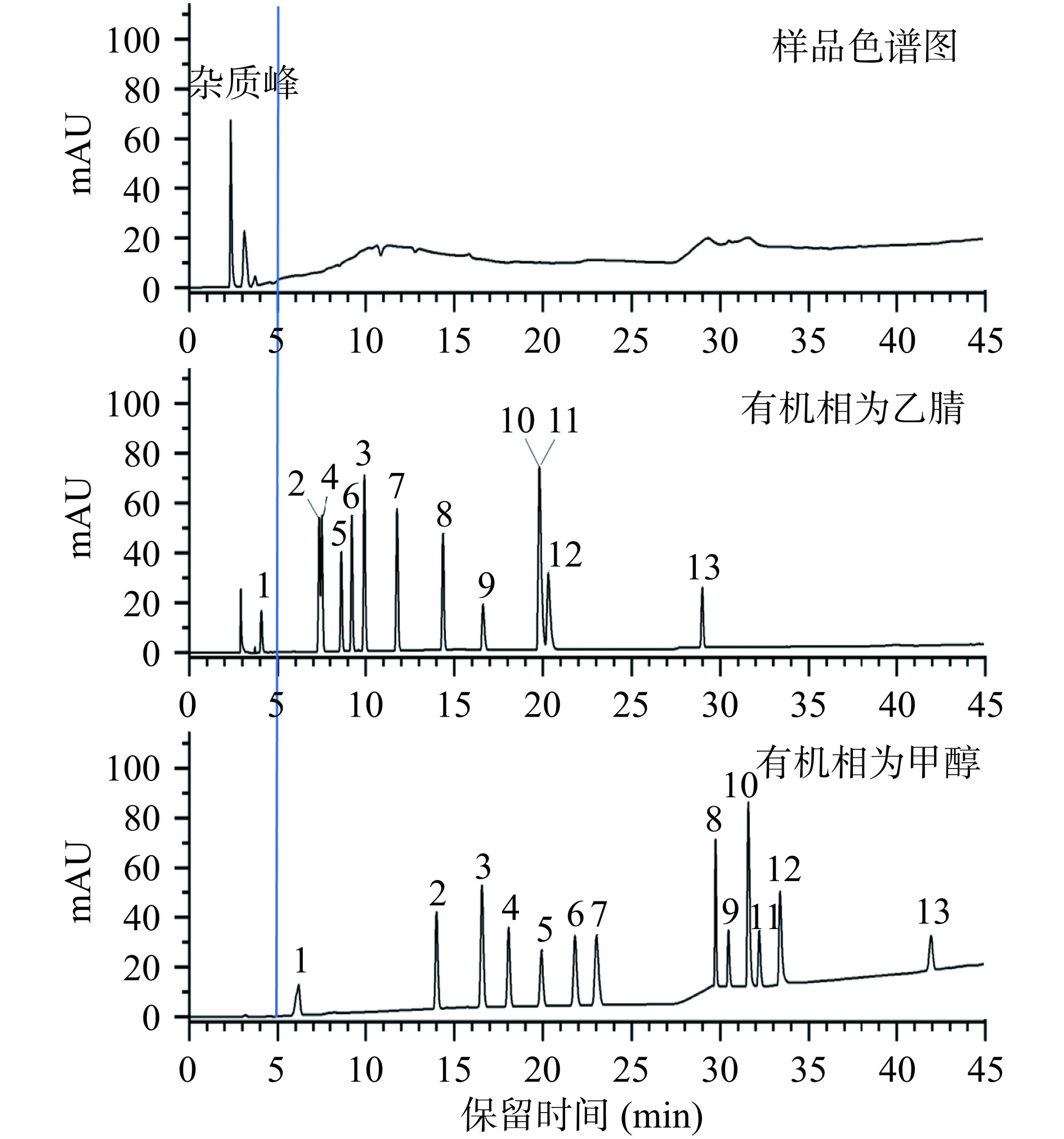
流动相对目标化合物的分离度、灵敏度和峰型影响较大。本研究对甲醇和乙腈2种有机相进行了考察。由于甲醇和乙腈的极性不同,对13种化合物的洗脱能力也不同。由图4显示,乙腈的洗脱能力相比更强,所以本研究中13种化合物出峰时间整体更早,其中盐酸妥拉唑林在2.9 min就已出峰,但大多数样品的主要杂质峰也集中在5 min之前。过早出峰易造成目标化合物色谱峰和样品杂质峰无法有效分离,从而影响分析的准确性。此外,使用乙腈为有机相时,乌拉地尔与盐酸特拉唑嗪分离度低,盐酸达泊西汀和盐酸氟西汀完全重叠。然而,当甲醇作为有机相时,出峰最早的盐酸妥拉唑林出峰时间延迟到6.7 min,相对远离了样品中的主要杂质峰,使之不易受干扰,并且13种化合物均能完全分离。
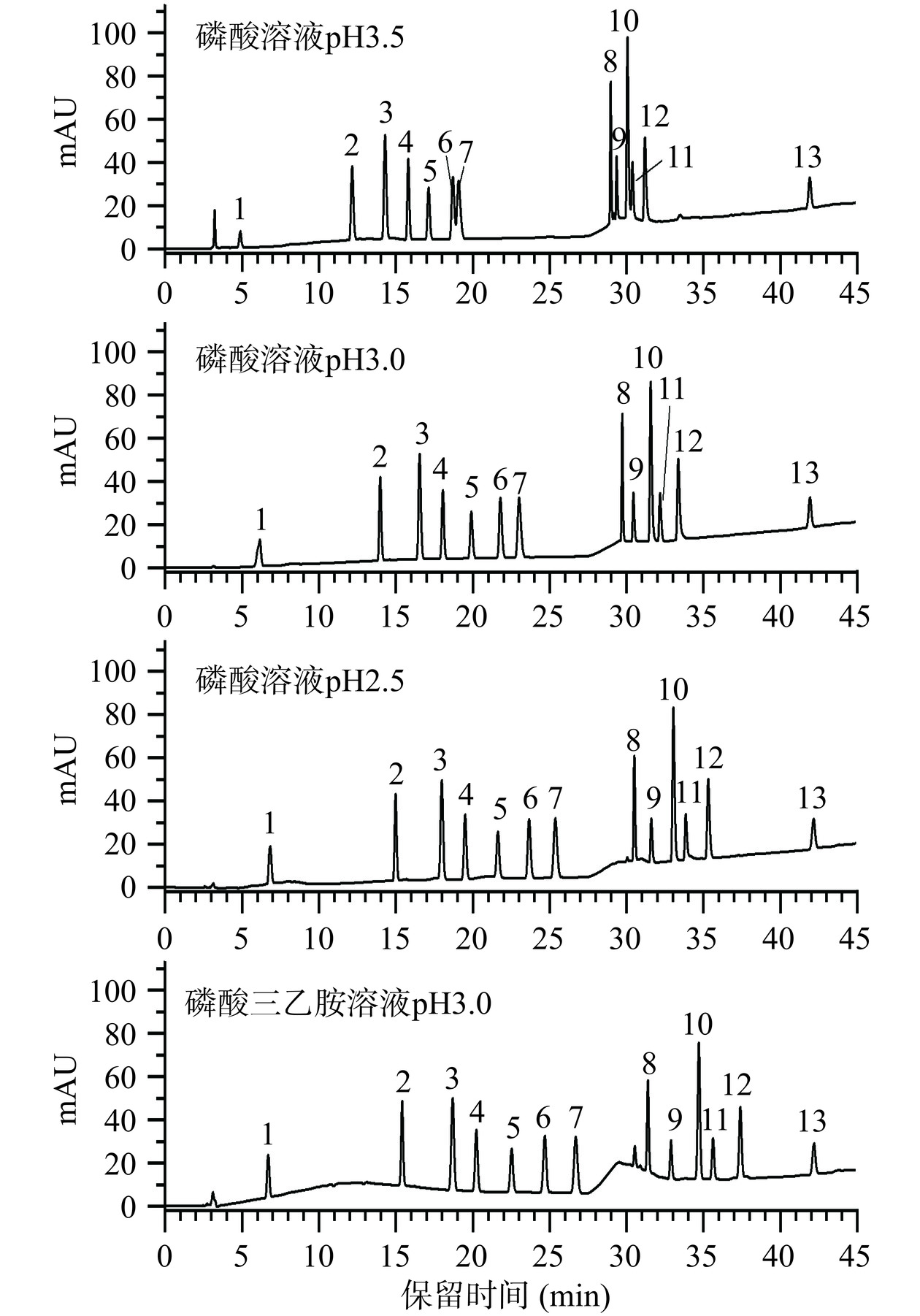
由于13种化合物大多数属于弱碱性物质,因此本研究尝试在酸性条件下进行分离。为进一步确定其最佳pH,考察了磷酸溶液pH为2.5、3.0、3.5时对各化合物的分离效果的影响,见图5。结果显示,当pH为3.5时盐酸哌唑嗪和甲磺酸酚妥拉明无法达到基线分离,并且盐酸妥拉唑林的响应值明显较低,当pH≤3.0时各化合物均能完全分离,但大多数化合呈现不同程度的峰后拖尾。拖尾因子(T)是一个重要的色谱参数,用于评价色谱峰的对称性和分离效果。由表2可知,当pH为3.0时12号峰拖尾因子(T)为1.67存在严重的后拖尾现象,1号峰的拖尾因子小于0.8有明显的峰前拖尾,当pH降低至2.5时各化合物的拖尾现象有一定改善,但仍有色谱峰9、10、11、12存在明显的后拖尾现象。而三乙胺能与C18键合相中游离的硅羟基结合,增强目标化合物的保留,减少拖尾现象,故向流动相中加入三乙胺。如图5和表2所示,在甲醇-磷酸三乙胺的流动相中,各化合物均能有效分离,并具有尖锐且对称的峰形。文献[34−36]中也发现,三乙胺可作为“扫尾剂”减少色谱峰的拖尾现象,起到改善峰形的作用。因此,本实验推荐以甲醇为有机相,以磷酸-三乙胺溶液(磷酸调节pH为3.0)为水相,作为分离13种目标化合物的流动相体系。
表 2 13种非法添加化学药物在不同流动相体系下拖尾因子(T)Table 2. Tailing factors (T) of 13 illegally added chemical drugs in different mobile phase systems流动相组成 1 2 3 4 5 6 7 8 9 10 11 12 13 甲醇-磷酸溶液pH3.0 0.78 1.16 1.14 1.13 1.14 1.15 1.23 1.18 1.31 1.46 1.29 1.67 1.01 甲醇-磷酸溶液pH2.5 1.09 1.09 1.08 1.08 1.08 1.09 1.11 1.16 1.24 1.30 1.37 1.30 1.01 甲醇-磷酸三乙胺溶液pH3.0 1.06 1.05 1.03 1.04 1.04 1.04 1.03 1.07 1.07 1.04 1.05 1.04 1.02 注:1.盐酸妥拉唑林;2.乌拉地尔;3.盐酸育亨宾;4.盐酸特拉唑嗪;5.盐酸阿夫唑嗪;6.盐酸哌唑嗪;7.甲磺酸酚妥拉明;8.甲磺酸多沙唑嗪;9.盐酸帕罗西汀;10.盐酸达泊西汀;11.盐酸氟西汀;12.盐酸舍曲林;13.非那雄胺;以峰面积定量法拖尾因子可接受范围为0.8~1.2。 2.2.3 检测波长的选择
本实验使用二极管阵列检测器(PDA)对13种目标化合物进行了紫外全波长扫描。盐酸特拉唑嗪、盐酸阿呋唑嗪、盐酸哌唑嗪、甲磺酸多沙唑嗪的最大吸收波长均在240~250 nm之间,并且都在245 nm波长下有较大吸收。因此,选取245 nm为上述4种化合物的最佳检测波长。
盐酸帕罗西汀、盐酸达泊西汀、盐酸氟西汀、盐酸舍曲林和非那雄胺的最大吸收波长都在195 nm的低波段,但在200 nm左右的低波段流动相有较大的吸收值,基线波动大,背景噪音干扰强。但是,盐酸妥拉唑林、乌拉地尔、盐酸育亨宾、甲磺酸酚妥拉明、盐酸帕罗西汀、盐酸达泊西汀、盐酸氟西汀、盐酸舍曲林和非那雄胺9种化合物均在218 nm有较大吸收,并且背景噪音相对较小。因此,选取218 nm为上述9种化合物的最佳检测波长。
2.3 方法学验证
2.3.1 线性关系考察、检测限和定量限
在以甲醇-磷酸三乙胺缓冲液为流动相,经Agilent 5 HC-C18(2)色谱柱(250 mm×4.6 mm,5 µm)梯度分离,检测波长为218 nm和245 nm,柱温30 ℃,进样量10 µL的优化条件下,对13种化学药物的系列混合对照品溶液进行分析,并以质量浓度(µg/mL)为横坐标(x),峰面积为纵坐标(y),绘制标准曲线,并建立线性回归方程。结果显示,盐酸达泊西汀在8~800 µg/mL质量浓度范围内,甲磺酸多沙唑嗪在5~200 µg/mL质量浓度范围内,其余11种化学药物在5~500 µg/mL质量浓度范围内呈良好的线性,相关系数r≧0.999。其线性回归方程和相关系数(r)见表3。
表 3 标准曲线、线性范围、检测限和定量限Table 3. Regression equations, linear ranges, LODs and LOQs化合物 线性范围(µg/mL) 回归方程 r LOD
(mg/kg)LOQ
(mg/kg)盐酸妥拉唑林 5~500 y=19736.0x+92000.2 0.9999 3.12 10.41 乌拉地尔 5~500 y=33270.5x+217146 0.9996 1.06 3.52 盐酸育亨宾 5~500 y=48139.4x+349669 0.9993 0.76 2.54 盐酸特拉唑嗪 5~500 y =71926.2x+127235 1.0000 0.90 2.95 盐酸阿夫唑嗪 5~500 y=62938.3x+255922 0.9998 1.15 3.85 盐酸哌唑嗪 5~500 y=78163.3x+165238 1.0000 0.95 3.25 甲磺酸酚妥拉明 5~500 y=41031.3x+175272 0.9998 1.10 3.60 甲磺酸多沙唑嗪 5~200 y=58752.0x+97853.1 0.9998 1.14 3.78 盐酸帕罗西汀 8~800 y=15668.3x+105422 0.9996 4.61 15.36 盐酸达泊西汀 5~500 y=64769.7x+344273 0.9997 1.40 4.70 盐酸氟西汀 5~500 y=19882.4x+84166.8 0.9998 1.01 3.35 盐酸舍曲林 5~500 y=37235.1x+228286 0.9996 1.12 3.72 非那雄胺 5~500 y=17615.4x+98621.3 0.9996 1.50 4.98 取未检出13种目标化合物的阴性样品,以加标浓度为25 mg/kg的加标样品测定信噪比,按照以信噪比为3计算检出限(LOD),以信噪比为10计算定量限(LOQ),各种化合物的LOD和LOQ见表3。
2.3.2 回收率和精密度试验
选取不含13种化学药物的阴性样品,在低(s1浓度水平)、中(s2浓度水平)、高(s4浓度水平)三个添加水平下进行加标回收实验,每个添加水平分别重复制备6份平行测试样,在最优色谱条件下测定。以回收率评价方法的准确度,以各加标水平下6个平行实验结果的相对标准偏差(RSD)值评估方法的精密度。由表4可知,13种化学药物的回收率在92.4%~100.7%之间,RSD在0.44%~1.06%之间(n=6),均符合GB 5009.295-2023《食品安全国家标准 化学分析方法验证通则》的评价标准,说明该试验方法准确度和精密度良好,可完全满足实际样品的分析要求。
表 4 13种非法添加化学物的加标回收率和精密度(n=6)Table 4. Recovery and precision of 13 illegally added chemical drugs (n=6)化合物 加标浓度
(µg/mL)平均回收率
(%)精密度
(%)盐酸妥拉唑林 5 98.9 0.81 10 97.5 0.49 50 97.2 0.85 乌拉地尔 5 99.2 0.53 10 98.3 0.50 50 97.8 0.98 盐酸育亨宾 5 98.2 0.74 10 97.5 0.54 50 97.1 1.02 盐酸特拉唑嗪 5 99.0 0.68 10 98.1 0.50 50 97.8 0.97 盐酸阿呋唑嗪 5 99.6 0.62 10 98.6 0.52 50 97.9 0.92 盐酸哌唑嗪 5 99.0 0.67 10 98.0 0.58 50 97.6 0.98 甲磺酸酚妥拉明 5 96.2 0.90 10 95.5 0.44 50 97.0 0.94 甲磺酸多沙唑嗪 5 97.4 0.95 10 97.0 0.67 50 97.6 1.02 盐酸帕罗西汀 8 92.9 0.92 16 92.4 0.65 80 94.6 0.58 盐酸达泊西汀 5 96.3 0.85 10 96.8 0.47 50 97.6 1.06 盐酸氟西汀 5 100.2 0.83 10 100.7 0.69 50 98.2 0.83 盐酸舍曲林 5 97.7 0.52 10 97.8 0.84 50 98.1 1.03 非那雄胺 5 99.1 0.76 10 98.6 0.56 50 98.0 1.02 2.3.3 方法稳定性试验
选取回收率试验中中等加标水平的样品分别在0、2、4、8、12和24 h进行检测,记录13种化学药物的峰面积并计算RSD。13种化学药物的RSD均小于2%,表明此方法的稳定性较好,可满足定量分析的要求。
2.4 实际样品检测
采用本方法对市售20批样品、网络销售20批样品和稽查样品25批样品,共计65批样品进行检测分析。每个样品分别制备3个平行测试样,测试结果取平均值。实验结果显示,在2批样品(片剂、胶囊剂)中检测出盐酸达泊西汀疑似峰,1批片剂样品中检测出盐酸育亨宾疑似峰。通过加标实验进一步确证,各疑似峰与相对应的对照品色谱峰完全重叠,对比紫外特征光谱图,盐酸达泊西汀色谱峰相似度分别为99.3%、99.6%,盐酸育亨宾色谱峰相似度为99.2%。故判定为阳性样品。盐酸达泊西汀阳性样品(片剂、胶囊)中盐酸达泊西汀含量分别为15.26 mg/片、6.34 mg/粒,盐酸育亨宾阳性样品中盐酸育亨宾含量为10.12 mg/粒。65批改善性功能或前列腺功能的保健品中检测出2种国家检测标准以外的非法添加药物,表明扩充其非法添加药物的筛查范围具有十分重要的现实意义,可进一步加强对保健食品质量安全的监管。此外,3批阳性样品中的2批来自网络销售,表明网络销售的改善性功能或前列腺功能的保健品存在较大的健康风险,需扩大样品采集范围,全面开展网络销售产品隐患排查,加强对网络销售产品的监查力度。
3. 结 论
本研究建立了一种采用HPLC-PDA测定改善性功能或改善前列腺功能类保健品中13种非法添加化学药物的高通量分析方法,涵盖了国家标准筛查范围以外的5-羟色胺再摄取抑制、α-肾上腺素受体阻滞剂和5α-还原酶抑制剂3类非法添加化学药物。通过优化样品前处理条件和色谱条件,获得了良好的样品提取效果和色谱分离效果。优化后的方法,经验证表明其准确度、精密度、检测限、定量限等参数均能满足实际样品的检测要求。该方法应用于不同渠道获得的65批样品的分析,在其中检测出盐酸达泊西汀和盐酸育亨宾2种国家标准以外的非法添加药物,阳性样品检出率约为5%。综上,该方法具有操作简单、准确性高和实用性强等优点,可为打击保健食品中非法添加化学药行为的监督检验工作和相关食品安全标准制修订提供有力的技术支持。
-
图 2 不同提取方式和时间对13种非法添加化学药物的提取效果
注:1.盐酸妥拉唑林;2.乌拉地尔;3.盐酸育亨宾;4.盐酸特拉唑嗪;5.盐酸阿夫唑嗪;6.盐酸哌唑嗪;7.甲磺酸酚妥拉明;8.甲磺酸多沙唑嗪;9.盐酸帕罗西汀;10.盐酸达泊西汀;11.盐酸氟西汀;12.盐酸舍曲林;13.非那雄胺;提取方式A:超声10 min;提取方式B:超声15 min;提取方式C:超声20 min;提取方式D:涡旋振荡1 min超声10 min;提取方式E:涡旋振荡1 min超声15 min;提取方式F:涡旋振荡1 min超声20 min;图3~图5同。
Figure 2. Effects of different extraction methods and time on the 13 illegally added chemical drugs
表 1 梯度洗脱程序
Table 1 Mobile phase gradient elution procedures
时间(min) 流动相A(%) 流动相B(%) 0 15 85 12 35 65 24 42 58 26 58 42 45 75 25 表 2 13种非法添加化学药物在不同流动相体系下拖尾因子(T)
Table 2 Tailing factors (T) of 13 illegally added chemical drugs in different mobile phase systems
流动相组成 1 2 3 4 5 6 7 8 9 10 11 12 13 甲醇-磷酸溶液pH3.0 0.78 1.16 1.14 1.13 1.14 1.15 1.23 1.18 1.31 1.46 1.29 1.67 1.01 甲醇-磷酸溶液pH2.5 1.09 1.09 1.08 1.08 1.08 1.09 1.11 1.16 1.24 1.30 1.37 1.30 1.01 甲醇-磷酸三乙胺溶液pH3.0 1.06 1.05 1.03 1.04 1.04 1.04 1.03 1.07 1.07 1.04 1.05 1.04 1.02 注:1.盐酸妥拉唑林;2.乌拉地尔;3.盐酸育亨宾;4.盐酸特拉唑嗪;5.盐酸阿夫唑嗪;6.盐酸哌唑嗪;7.甲磺酸酚妥拉明;8.甲磺酸多沙唑嗪;9.盐酸帕罗西汀;10.盐酸达泊西汀;11.盐酸氟西汀;12.盐酸舍曲林;13.非那雄胺;以峰面积定量法拖尾因子可接受范围为0.8~1.2。 表 3 标准曲线、线性范围、检测限和定量限
Table 3 Regression equations, linear ranges, LODs and LOQs
化合物 线性范围(µg/mL) 回归方程 r LOD
(mg/kg)LOQ
(mg/kg)盐酸妥拉唑林 5~500 y=19736.0x+92000.2 0.9999 3.12 10.41 乌拉地尔 5~500 y=33270.5x+217146 0.9996 1.06 3.52 盐酸育亨宾 5~500 y=48139.4x+349669 0.9993 0.76 2.54 盐酸特拉唑嗪 5~500 y =71926.2x+127235 1.0000 0.90 2.95 盐酸阿夫唑嗪 5~500 y=62938.3x+255922 0.9998 1.15 3.85 盐酸哌唑嗪 5~500 y=78163.3x+165238 1.0000 0.95 3.25 甲磺酸酚妥拉明 5~500 y=41031.3x+175272 0.9998 1.10 3.60 甲磺酸多沙唑嗪 5~200 y=58752.0x+97853.1 0.9998 1.14 3.78 盐酸帕罗西汀 8~800 y=15668.3x+105422 0.9996 4.61 15.36 盐酸达泊西汀 5~500 y=64769.7x+344273 0.9997 1.40 4.70 盐酸氟西汀 5~500 y=19882.4x+84166.8 0.9998 1.01 3.35 盐酸舍曲林 5~500 y=37235.1x+228286 0.9996 1.12 3.72 非那雄胺 5~500 y=17615.4x+98621.3 0.9996 1.50 4.98 表 4 13种非法添加化学物的加标回收率和精密度(n=6)
Table 4 Recovery and precision of 13 illegally added chemical drugs (n=6)
化合物 加标浓度
(µg/mL)平均回收率
(%)精密度
(%)盐酸妥拉唑林 5 98.9 0.81 10 97.5 0.49 50 97.2 0.85 乌拉地尔 5 99.2 0.53 10 98.3 0.50 50 97.8 0.98 盐酸育亨宾 5 98.2 0.74 10 97.5 0.54 50 97.1 1.02 盐酸特拉唑嗪 5 99.0 0.68 10 98.1 0.50 50 97.8 0.97 盐酸阿呋唑嗪 5 99.6 0.62 10 98.6 0.52 50 97.9 0.92 盐酸哌唑嗪 5 99.0 0.67 10 98.0 0.58 50 97.6 0.98 甲磺酸酚妥拉明 5 96.2 0.90 10 95.5 0.44 50 97.0 0.94 甲磺酸多沙唑嗪 5 97.4 0.95 10 97.0 0.67 50 97.6 1.02 盐酸帕罗西汀 8 92.9 0.92 16 92.4 0.65 80 94.6 0.58 盐酸达泊西汀 5 96.3 0.85 10 96.8 0.47 50 97.6 1.06 盐酸氟西汀 5 100.2 0.83 10 100.7 0.69 50 98.2 0.83 盐酸舍曲林 5 97.7 0.52 10 97.8 0.84 50 98.1 1.03 非那雄胺 5 99.1 0.76 10 98.6 0.56 50 98.0 1.02 -
[1] MA M H, ZHANG J N, MA X L, et al. Using UHPLC-HRMS-based comprehensive strategy to efficiently and accurately screen and identify illegal additives in health-care foods[J]. Food Research International,2023,170:110315.
[2] BRACCI E L, DAVIS C R, MURPHY K J. Developing a mediterranean healthy food basket and an updated australian healthy food basket modelled on the australian guide to healthy eating[J]. Nutrients,2023,15(7):1692. doi: 10.3390/nu15071692
[3] 余晓琴, 刘美, 李澍才, 等. 新型食品中非法添加挖掘及应对策略研究[J]. 中国食品卫生杂志,2023,35(9):1357−1363. [YU X Q, LIU M, LI S C, et al. Research on mining hidden rules of new unconventional illegal food addition andcoping strategies[J]. Chinese Journal of Food Hygiene,2023,35(9):1357−1363.] YU X Q, LIU M, LI S C, et al. Research on mining hidden rules of new unconventional illegal food addition andcoping strategies[J]. Chinese Journal of Food Hygiene, 2023, 35(9): 1357−1363.
[4] 陈东洋, 张昊, 冯家力, 等. 保健食品中违禁药物检验技术研究进展[J]. 色谱,2020,38(8):880−890. [CHEN D Y, ZHANG H, FENG J L, et al. Advances in technologies for determination of illegal drugs in health food[J]. Chinese Journal of Chromatography,2020,38(8):880−890.] CHEN D Y, ZHANG H, FENG J L, et al. Advances in technologies for determination of illegal drugs in health food[J]. Chinese Journal of Chromatography, 2020, 38(8): 880−890.
[5] 胡青, 孙健, 季申. 中药和食品中非法添加化学药品新趋势及监管对策[J]. 中国食品药品监管,2022(3):88−95. [HU Q, SUN J, JI S. Illegally added chemicals in traditional Chinese medicines and foods:New trends and regulatory countermeasures[J]. China Food & Drug Administration Magazine,2022(3):88−95.] HU Q, SUN J, JI S. Illegally added chemicals in traditional Chinese medicines and foods: New trends and regulatory countermeasures[J]. China Food & Drug Administration Magazine, 2022(3): 88−95.
[6] 徐硕, 金鹏飞, 徐文峰, 等. HPLC-DAD法检测补肾壮阳和改善前列腺功能保健品中非法添加的17种化学药物[J]. 中国药师,2022,25(6):1108−1114. [XU S, JIN P F, XU W F, et al. Determination of seventeen chemical drugs illegally adulterated in health foods for invigorating kidney-yang and improving prostatic function by HPLC-DAD[J]. China Pharmacist,2022,25(6):1108−1114.] XU S, JIN P F, XU W F, et al. Determination of seventeen chemical drugs illegally adulterated in health foods for invigorating kidney-yang and improving prostatic function by HPLC-DAD[J]. China Pharmacist, 2022, 25(6): 1108−1114.
[7] ZHANG C F, LI C L, GENG Q, et al. Reasons and treatment strategy for discontinuation of dapoxetine treatment in premature ejaculation patients in China:A retrospective observational study[J]. Andrologia,2022,54(7):14425.
[8] LELIEFELD H H J, DEBRUYNE F M J, REISMAN Y. The post-finasteride syndrome:Possible etiological mechanisms and symptoms[J]. International Journal of Impotence Research, 2023: 37697052.
[9] VARITSARA M, CHINTHURAN T, FATEMEH A, et al. 26355 Alopecia areata:A rare side effect of prazosin[J]. Journal of the American Academy of Dermatology,2021,85(3S):98.
[10] 国家食品药品监督管理局. BJS 2008016补肾壮阳类中成药中西地那非及其类似物的检测[S]. 北京:中国标准出版社, 2008. [National Food and Medical Products Administration. BJS 2008016 Detection of sildenafil and analogues in Chinese patent medicines for invigorating kidney-yang[S]. Beijing:Standards Press of China, 2008.] National Food and Medical Products Administration. BJS 2008016 Detection of sildenafil and analogues in Chinese patent medicines for invigorating kidney-yang[S]. Beijing: Standards Press of China, 2008.
[11] 国家食品药品监督管理局. BJS 2009030补肾壮阳类中成药中PDE5型抑制剂的快速检测方法[S]. 北京:中国标准出版社, 2009. [National Food and Medical Products Administration. BJS 2009030 Rapid detection method of PDE5 inhibitors in Chinese patent medicines for invigorating kidney-yang[S]. Beijing:Standards Press of China, 2009.] National Food and Medical Products Administration. BJS 2009030 Rapid detection method of PDE5 inhibitors in Chinese patent medicines for invigorating kidney-yang[S]. Beijing: Standards Press of China, 2009.
[12] 国家食品药品监督管理局. BJS 201710保健食品中75种非法添加化学药物的检测[S]. 北京:中国标准出版社, 2017. [National Food and Medical Products Administration. BJS 201710 Detection of 75 illegally added chemical drugs in health food [S]. Beijing:Standards Press of China, 2017.] National Food and Medical Products Administration. BJS 201710 Detection of 75 illegally added chemical drugs in health food [S]. Beijing: Standards Press of China, 2017.
[13] 国家食品药品监督管理局. BJS 201805食品中那非物质的测定[S]. 北京:中国标准出版社, 2018. [National Food and Medical Products Administration. BJS 201805 Determination of nafils substances in foods[S]. Beijing:Standards Press of China, 2018.] National Food and Medical Products Administration. BJS 201805 Determination of nafils substances in foods[S]. Beijing: Standards Press of China, 2018.
[14] 中华人民共和国海关总署. SN/T 5357-2021 出口保健食品中多种非法添加物的测定液相色谱-质谱/质谱法[S]. 北京:中国标准出版社, 2021. [General Administration of Customs of the People's Republic of China. SN/T 5357-2021 Determination of multi-groups of illegal additives in health foods for export-LC-MS/MS method[S]. Beijing:Standards Press of China, 2018.] General Administration of Customs of the People's Republic of China. SN/T 5357-2021 Determination of multi-groups of illegal additives in health foods for export-LC-MS/MS method[S]. Beijing: Standards Press of China, 2018.
[15] VIEIRALVES R R, FAVORITO L A. Dapoxetine and premature ejaculation[J]. International Brazilian Journal of Urology,2023,49(4):511−514. doi: 10.1590/s1677-5538.ibju.2023.9908
[16] 刘竹芳, 黄文柱, 刘敏, 等. 5α还原酶抑制剂联用α受体阻断剂对老年良性前列腺增生下尿路症状的效果及护理要点[J]. 中国药业,2017,26(12):38−40. [LIU Z F, HUANG W Z, LIU M, et al. Clinical effect of 5α reductase inhibitors combined with α receptor blockers in treating lower urinary tract symptoms in elderly patients with benign prostatic hyperplasia and its main nursing points[J]. China Pharmaceuticals,2017,26(12):38−40.] doi: 10.3969/j.issn.1006-4931.2017.12.010 LIU Z F, HUANG W Z, LIU M, et al. Clinical effect of 5α reductase inhibitors combined with α receptor blockers in treating lower urinary tract symptoms in elderly patients with benign prostatic hyperplasia and its main nursing points[J]. China Pharmaceuticals, 2017, 26(12): 38−40. doi: 10.3969/j.issn.1006-4931.2017.12.010
[17] MANDOUR A A, ELKAEED E B, HAGRAS M, et al. Virtual screening approach for the discovery of selective 5α-reductase type II inhibitors for benign prostatic hyperplasia treatment[J]. Future Medicinal Chemistry, 2023, 15(23): 2149−2163.
[18] WANG X, ZHAO J J, ZHANG Q, et al. A chemometric strategy for accurately identifying illegal additive compounds in health foods by using ultra-high-performance liquid chromatography coupled to high resolution mass spectrometry[J]. Analytical Methods:Advancing Methods and Applications,2021,13(14):1731−1739.
[19] 邵瑞婷, 丁学妍, 姜洁. 超高效液相色谱-串联质谱法测定调节三高类保健食品中59种非法添加药物[J]. 食品科学,2024,45(9):232−242. [SHAO R T, DING X Y, JIANG J. Determination of 59 illegal addition of drugs in three kinds of regulated health food by ultrahigh performance liquid chromatography-tandem mass spectrometry[J]. Food Science,2024,45(9):232−242.] doi: 10.7506/spkx1002-6630-20230628-221 SHAO R T, DING X Y, JIANG J. Determination of 59 illegal addition of drugs in three kinds of regulated health food by ultrahigh performance liquid chromatography-tandem mass spectrometry[J]. Food Science, 2024, 45(9): 232−242. doi: 10.7506/spkx1002-6630-20230628-221
[20] XIE X J, ZHANG Y, YUE Z F, et al. Multi-fingerprint profiling analysis for screening and quantification of illegal adulterated antidiabetics in a functional food using HPLC coupled to diode array detection/fluorescence detection[J]. Microchemical Journal,2019,149:103995. doi: 10.1016/j.microc.2019.103995
[21] 胡燕. 高效液相色谱法检测茶叶中5种非法添加合成着色剂[J]. 食品与发酵科技,2023,59(4):137−142. [HU Y. Determination of five synthetic colorants illegally added in teasamples by high performance liquid chromatography[J]. Food and Fermentation Science & Technology,2023,59(4):137−142.] HU Y. Determination of five synthetic colorants illegally added in teasamples by high performance liquid chromatography[J]. Food and Fermentation Science & Technology, 2023, 59(4): 137−142.
[22] XU D M, LAI G Y, CHEN Y, et al. Simultaneous determination of 21 illegally added compounds in health foods by ultra high performance liquid chromatography-tandem mass spectrometry with solid phase extraction[J]. Chinese Journal of Chromatography,2019,37(7):778−785. doi: 10.3724/SP.J.1123.2019.01016
[23] WU Z Q, JIANG X Q, YANG Y Q, et al. Amphiphilic polymers facilitated solid phase extraction coupled with ultra-performance liquid chromatography-tandem mass spectrometry for direct extraction and analysis of zearalenone and zearalanone in corn juice samples[J]. Journal of Separation Science,2023,46(14):e2300112. doi: 10.1002/jssc.202300112
[24] PENG S, CHAO W, LEI X, et al. Simultaneous determination of eight phenolic acids in rapeseed by accelerated solvent extraction-solid phase extraction ultra-high-performance liquid chromatography-tandem mass spectrometry[J]. Food Analytical Methods,2022,15(10):2625−2632. doi: 10.1007/s12161-022-02310-6
[25] HASSAN Y A A, AYAD M F, HUSSEIN L A, et al. Hydrophilic interaction liquid chromatography (HILIC) with DAD detection for the determination of relatively non polar fungicides in orange samples[J]. Microchemical Journal,2023,193:109196. doi: 10.1016/j.microc.2023.109196
[26] SOUMIA B E, DOMENICA M, KATIA A, et al. Determination of astaxanthin and astaxanthin esters in three samples of shrimp waste (Parapenaeus longirostris) by high performance liquid chromatography coupled photo-diode array and mass spectrometry detection[J]. Natural Product Research,2023,193:1−8.
[27] SUMIA A, BUSHRA S, RAFIQUE M A, et al. Salting-out assisted liquid-liquid microextraction and reverse-phase chromatographic quantification of two neonicotinoid insecticides from fruits and vegetables[J]. Journal of Chromatographic Science,2023,61(9):875−884. doi: 10.1093/chromsci/bmad055
[28] 宁霄, 金绍明, 李志远, 等. 功能性老年乳粉中300种非法添加药物及其类似物的液相色谱-高分辨质谱分析[J]. 色谱,2023,41(11):960−975. [NING X, JIN S M, LI Z Y, et al. Liquid chromatography-high resolution mass spectrometry analysis of 300 illegally added drugs and their analogues in functional milk powder for the elderly[J]. Chinese Journal of Chromatography,2023,41(11):960−975.] doi: 10.3724/SP.J.1123.2023.05013 NING X, JIN S M, LI Z Y, et al. Liquid chromatography-high resolution mass spectrometry analysis of 300 illegally added drugs and their analogues in functional milk powder for the elderly[J]. Chinese Journal of Chromatography, 2023, 41(11): 960−975. doi: 10.3724/SP.J.1123.2023.05013
[29] 周建峰, 王晓园, 隋玉杰, 等. QuEChERS-超高效液相色谱-串联质谱法快速筛查食疗膳食中40种非法添加药物[J]. 分析科学学报,2023,39(4):461−468. [ZHOU J F, WANG X Y, SUI Y J, et al. Determination of 40 kinds of illegally additives in medicine dietary by QuEChERS-ultra-performance liquid chromatography-tandem mass spectrometry[J]. Journal of Analytical Science,2023,39(4):461−468.] ZHOU J F, WANG X Y, SUI Y J, et al. Determination of 40 kinds of illegally additives in medicine dietary by QuEChERS-ultra-performance liquid chromatography-tandem mass spectrometry[J]. Journal of Analytical Science, 2023, 39(4): 461−468.
[30] XU H B, ZHANG S P, DU R Y, et al. Ultra-high performance liquid chromatography-orbitrap high-resolution mass spectrometry for rapid screening and identification of 32 illegally added drugs in slimming and anti-impotence health foods[J]. Chinese Journal of Chromatography,2022,40(6):531−540. doi: 10.3724/SP.J.1123.2021.12009
[31] 李莲微, 杨晓聪, 姚闽娜, 等. 超高效液相色谱-串联质谱法同时测定动物源性食品中9种多肽类抗生素残留[J]. 食品工业科技,2022,43(17):330−337. [LI L W, YANG X C, YAO M N, et al. Determination of nine polypeptide antibiotics residues in animal tissue food by ultra-high performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry,2022,43(17):330−337.] LI L W, YANG X C, YAO M N, et al. Determination of nine polypeptide antibiotics residues in animal tissue food by ultra-high performance liquid chromatography-tandem mass spectrometry[J]. Science and Technology of Food Industry, 2022, 43(17): 330−337.
[32] VARSHA R, JASHBIR S, JAYAPRAKASHA G K, et al. Op-timization of extraction solvent and fast blue BB assay for comparative analysis of antioxidant phenolics from Cucumis melo L.[J]. Plants,2021,10(7):1379. doi: 10.3390/plants10071379
[33] 袁利杰, 赵光升, 张培毅, 等. UPLC-MS/MS测定小麦粉及其制品与小麦粉添加剂中新型非法添加物乙酰氧肟酸[J]. 现代食品科技,2024,40(1):281−287. [YUAN L J, ZHAO G S, ZHANG P Y, et al. Determination of new illegal additive acetohydroxamic acid in wheat flour and its products and wheat flour additive by UPLC-MS/MS[J]. Modern Food Science and Technology,2024,40(1):281−287.] YUAN L J, ZHAO G S, ZHANG P Y, et al. Determination of new illegal additive acetohydroxamic acid in wheat flour and its products and wheat flour additive by UPLC-MS/MS[J]. Modern Food Science and Technology, 2024, 40(1): 281−287.
[34] 寻坚兰. 琥珀酸噁泼西汀高效液相色谱分析方法开发[D]. 上海:上海交通大学, 2020:29-30. [XUN J L. Developing a method for the determination of esreboxetine succinate by high performance liquid chromatography[D]. Shanghai:Shanghai Jiao Tong University, 2020:29-30.] XUN J L. Developing a method for the determination of esreboxetine succinate by high performance liquid chromatography[D]. Shanghai: Shanghai Jiao Tong University, 2020: 29-30.
[35] HINAL H, KASHYAP T, SANJAY C, et al. HPTLC method development and validation for the simultaneous estimation of nortriptyline HCl and pregabalin in their combined dosage form[J]. Journal of Chromatographic Science, 2023.
[36] FU X X, ZHONG L L, WANG R, et al. Exploration of a high-performance liquid chromatography method for antibiotic drug monitoring in melioidosis patients and establishment of its sampling procedure[J]. Results in Chemistry,2023,6:101187. doi: 10.1016/j.rechem.2023.101187
-
期刊类型引用(0)
其他类型引用(1)





 下载:
下载:
 下载:
下载:



