Effects of Carbon Nitride Combined with Chitosan on Lipid Metabolism of Salmonella Typhimurium
-
摘要: 目的:以鼠伤寒沙门氏菌为研究对象,从细菌脂质代谢层面探究氮化碳-壳聚糖复合溶胶作用下的杀菌机理。方法:基于透射电子显微镜和蛋白泄露分析氮化碳和壳聚糖联用对细菌细胞壁膜的破坏,并采用脂质代谢组学,通过差异代谢物功能及其调控网络从细菌脂质代谢水平探究杀菌机理。结果:与单独使用氮化碳或壳聚糖相比,复合溶胶对鼠伤寒沙门氏菌细胞壁膜的损伤程度更大。脂质代谢中明显上调和下调的差异代谢物分别为203个和95个,主要集中在与细胞膜构成骨架相关的脂肪酸类、甘油磷脂类和糖脂类中。代谢物功能和调控网络分析表明,复合溶胶处理后,细菌脂肪酸生物合成、甘油磷脂代谢、亚油酸代谢、四烯酸代谢等代谢通路被破坏,细胞的能量代谢、物质运输和信号传导等生长代谢过程产生紊乱,进而导致细菌死亡。结论:本研究从脂质代谢角度揭示,氮化碳与壳聚糖联合破坏沙门氏菌的代谢通路,特别是与脂质代谢相关的通路,导致细菌代谢紊乱并死亡。这为光催化材料的杀菌机制提供了理论依据。Abstract: Objective: To investigate the bactericidal mechanism of a composite sol made of carbon nitride and chitosan on Salmonella typhimurium by studying its effects on lipid metabolism. Methods: Transmission electron microscopy and protein leakage concentration were utilized to evaluate the magnitude of cell membrane damage induced by carbon nitride-chitosan composite sol at a cellular level. In addition, lipid metabolomics was employed to investigate the bactericidal process by examining several metabolite functions and regulatory networks associated with bacterial lipid metabolism. Results: The composite sol of carbon nitride-chitosan caused more extensive damage to the cell membrane of Salmonella typhimurium. In lipid metabolism, the significantly up-regulated in 203 metabolites and down-regulated in 95 metabolites. These metabolites were particularly involved in the metabolism of fatty acids, glycerophospholipids, and glycolipids. Metabolite function analysis showed that the composite sol treatment disrupted various metabolic pathways in bacteria. These pathways included fatty acid biosynthesis, glycerol phospholipid metabolism, linoleic acid metabolism, tetraenoic acid metabolism, as well as pathways related to cell energy metabolism, material transport, and signal conduction. These disturbances finally resulted in the demise of the bacteria. Conclusion: This study revealed from the perspective of lipid metabolism that the combination of carbon nitride and chitosan disrupts the metabolic pathways of Salmonella, especially those related to lipid metabolism, leading to bacterial metabolic disruption and death. It provides a theoretical basis for comprehending the mechanisms by which photocatalytic materials exert their antibacterial properties.
-
Keywords:
- Salmonella /
- targeted lipid metabolomics /
- graphene carbon nitride /
- chitosan /
- photocatalysis
-
沙门氏菌(Salmonella)是革兰氏阴菌,无芽孢、无荚膜、兼性厌氧,是引起细菌性食物中毒的常见致病菌[1]。在全球细菌性食物中毒事件中,沙门氏菌位居榜首[2]。在中国,约70%~80%的细菌性食物中毒事件由沙门氏菌引起,每年中毒超过300万人次[3]。其中,90%以上是由于食用了被污染的禽肉制品、猪肉、牛肉制品、乳制品、蛋制品等[1]。因此,加强食品加工过程的沙门氏菌污染防控对提升食品安全、保障人民健康意义重大。目前食品工业中常用的杀菌技术主要包括热杀菌(巴氏杀菌、高温杀菌等)、非热杀菌(辐照杀菌、超声杀菌、高压脉冲电场杀菌、低温等离子体杀菌等)和化学杀菌(含氯杀菌剂、醇类杀菌剂、天然提取物等)[4]。物理杀菌通常能够较好地控制微生物污染,但会影响食品品质和口感,化学杀菌剂易造成设备腐蚀,且易产生细菌抗性[5]。
光催化杀菌是近年来最潜力的“绿色”杀菌技术,具有高效广谱性、无毒无害、不易产生耐药性等优势[6]。光催化杀菌是通过光照射在具有光响应的纳米材料上,激发产生光生载流子,经氧化还原反应产生活性氧物种(Reactive oxygen species,ROS),包括羟基自由基(·OH)、过氧化氢(H2O2)、超氧阴离子自由基(O2−·)等,这些活性氧物种通过破坏细胞膜和胞内物质,致使细菌死亡[7]。常见的光催材料包括二氧化钛、氧化锌、纳米银、氧化亚铜等,它们具有载流子浓度高、载流子迁移率高等优点,但均含有金属成分,可能导致环境中重金属残留[8]。石墨烯氮化碳(Graphene carbon nitride,g-C3N4)作为一种非金属半导体纳米光催化剂,具有化学性质稳定、比表面积大、表面可修饰、无毒无害等特点,近年来在食品抗菌领域备受关注。然而,石墨烯氮化碳材料可见光响应范围窄、光生载流子快速复合等缺点限制了其光催化性能[9]。一些研究发现,g-C3N4与天然大分子物质如壳聚糖[10]、魔芋葡聚糖[11]、玉米醇溶蛋白[12]等联用可以提高杀菌性能[13]。
壳聚糖具有可降解、无毒、抗菌和可成膜等优点,但壳聚糖溶液杀菌能力较弱、壳聚糖薄膜机械强度较低、阻隔性能较差,在食品加工领域的使用受到限制[14]。前人研究发现,氮化碳和壳聚糖联用可显著提高杀菌效果,可能与破坏细胞膜的完整性和通透性有关[15],但缺乏明确的数据支撑。本研究利用靶向脂质代谢组学对细菌脂质代谢物进行研究,尤其是对与细胞膜相关的脂质差异代谢物进行分析,以期为氮化碳和壳聚糖复合溶胶的杀菌机制提供理论参考。
1. 材料与方法
1.1 材料与仪器
鼠伤寒沙门氏菌(Salmonella typhimurium,S. typhimurium保藏号ATCC 14028) 浙江省农业科学院实验室保藏菌种;脑心浸液肉汤(BHI)培养基 北京普纳德科技有限公司;磷酸盐缓冲液(PBS) 广州索璞生物科技有限公司;壳聚糖 脱乙酰≥95%,上海源叶生物科技有限公司;冰醋酸 色谱纯,上海凌峰化学试剂有限公司;三聚氰胺 上海泰坦科技股份有限公司;所有分离用有机溶剂均为国产分析纯。
TS-200B型冷冻恒温振荡器 金坛市国旺试验仪器厂;SPX-250B-Z型生化培养箱 上海博远实业有限公司医疗设备厂;DW-L2000型全自动微生物平皿螺旋接种仪、DW-V9型全自动菌落计数仪 杭州大微生物技术有限公司;CEL-HXF300-T3型氙灯光源系统 北京中教金源科技有限公司;230型VTALBOYS旋涡混合器 美国Troemner公司;BIOFUGE PRIMOR ST8R型高速离心机 美国Thermo Fisher Scientific 公司;H-7650型透射电子显微镜 日立(中国)有限公司;RO-D4型磁力搅拌器 拓赫机电科技(上海)有限公司。
1.2 实验方法
1.2.1 菌种活化
将贮存于−80 ℃的沙门氏菌常温解冻,取100 µL菌液于5 mL BHI中,置于37 ℃振荡培养箱中过夜培养22 h。
1.2.2 菌悬液制备
将过夜培养后的菌液进行8000 r/min、4 ℃离心5 min,弃上清,加入PBS对菌沉淀洗涤2次并重悬,最终获取浓度为109 CFU/mL的菌悬液,置于4 ℃备用。
1.2.3 g-C3N4纳米片制备
参考Bian等[16]的方法,将三聚氰胺置于坩埚中,在马弗炉中加热至520 ℃,煅烧4 h。待冷却至室温后,研磨得到淡黄色的g-C3N4纳米片(CN)。
1.2.4 壳聚糖氮化碳复合溶胶制备
参考Qu等[17]的方法,将1.6 g壳聚糖充分溶解于100 mL 1%(v/v)醋酸中,以500 r/min的速率磁力搅拌3 h,得到1.6%(w/v)壳聚糖(CS)溶液。在CS中加入50 mg的g-C3N4纳米片,以300 r/min的速率搅拌5 h,得到壳聚糖氮化碳(CN-CS)复合溶胶。
1.2.5 杀菌处理
参照Cai等[18]的方法稍作修改,将2 mL浓度为109 CFU/mL的菌悬液加入18 mL CN-CS复合溶胶中,避光反应30 min,建立吸附解吸平衡。随后以氙灯为光源进行光催化杀菌。以PBS缓冲液为对照组,CS、CN、对照组的杀菌处理方法参照上述步骤。探究各实验组在避光反应结束后、光照20 min、光照40 min时的细菌死亡率,每个时间点取平行样本3个进行细菌计数,实验重复3次。
1.2.6 细菌死亡率测定
各时间点反应结束后,取1 mL样品加入9 mL PBS缓冲液中,梯度稀释至适当浓度,在XLT4琼脂培养基上螺旋接种。将接种后的平板置于生化培养箱中,37 ℃恒温培养12 h,采用全自动菌落计数仪计数。根据计数结果计算细菌死亡率,计算公式为:
Y(%)=N0−NtN0×100 式中:Y表示细菌死亡率(%);N0表示初始细菌数(CFU/mL);Nt表示处理后的细菌数(CFU/mL)。
1.2.7 细胞水平的分析
1.2.7.1 细菌细胞结构观察
参考刘骁等[19]的方法对细菌进行前处理,并用透射电子显微镜对处理前后的细菌切片进行观察。
1.2.7.2 蛋白泄露测定
参考Wang等[20]的方法并稍作修改,将处理后的菌液在4 ℃条件下进行离心(8000 r/min、5 min),取上清。蛋白浓度检测方法参照BCA蛋白浓度测定试剂盒上的说明书测定。
1.2.8 脂质代谢组分析
1.2.8.1 样品制备
将1.2.5处理后的沙门氏菌8000 r/min、4 ℃离心5 min,弃上清,用PBS缓冲液洗涤菌泥2遍,在液氮中快速冷冻。样品送至上海凌恩科技有限公司进行脂质代谢组学测序。
1.2.8.2 色谱条件
色谱柱选用 Thermo Accucore C30,进样体积5 µL,流速为0.35 mL/min;柱温设定为40 ℃,流动相A为(乙腈:水=60:40)+0.1%甲酸+10 mmol/L乙酸铵,流动相B为(异丙醇:乙腈=90:10)+0.1%甲酸+10 mmol/L乙酸铵,分析采用梯度洗脱。具体洗脱程序如表1所示。
表 1 色谱梯度洗脱程序Table 1. Chromatographic gradient elution procedure时间(min) A(%) B(%) 初始 70 30 2 70 30 5 57 43 5.1 45 55 11 30 70 16 1 99 18 1 99 18.1 70 30 20 70 30 1.2.8.3 质谱条件
鞘气为0 psi,辅助气速率在正离子模式下为10 L/min,负离子模式下为7 L/min,电喷雾电压为3.5 kV,毛细管温度为320 ℃,加热器温度为350 ℃,扫描范围为114~1700 m/z,自动增益控制目标离子数为3e+6个,归一化碰撞能为22、24、28 eV,最大等待注入时间为100 ms,隔离窗口为1 m/z,自动增益控制目标离子数(MS2)为2e+5个,动态排除为6 s。
1.2.8.4 定性与定量分析
下机原始数据使用CD3.1数据处理软件进行数据预处理。首先通过保留时间、质荷比等参数简单筛选,对于不同样本根据保留时间偏差和质量偏差(Part per million,ppm)进行峰对齐,使鉴定更准确;随后根据设置的ppm、信噪比(Signal-to-noise ratio,S/N)、加合离子等信息进行峰提取,同时对峰面积进行定量。然后比对Lipidmaps和Lipiblast谱图数据库进行数据库检索(搜库),得到脂类物质的定性定量结果。然后保留QC样本中变异系数(Coefficient of Variance,CV)小于30%的脂质化合物作为最终的鉴定结果,进行后续分析。
1.3 数据处理
所有实验样本均设置3个平行样本,实验结果以“平均值±标准差”表示。实验数据均通过GraphPad Prism 8.0软件进行绘图。
2. 结果与分析
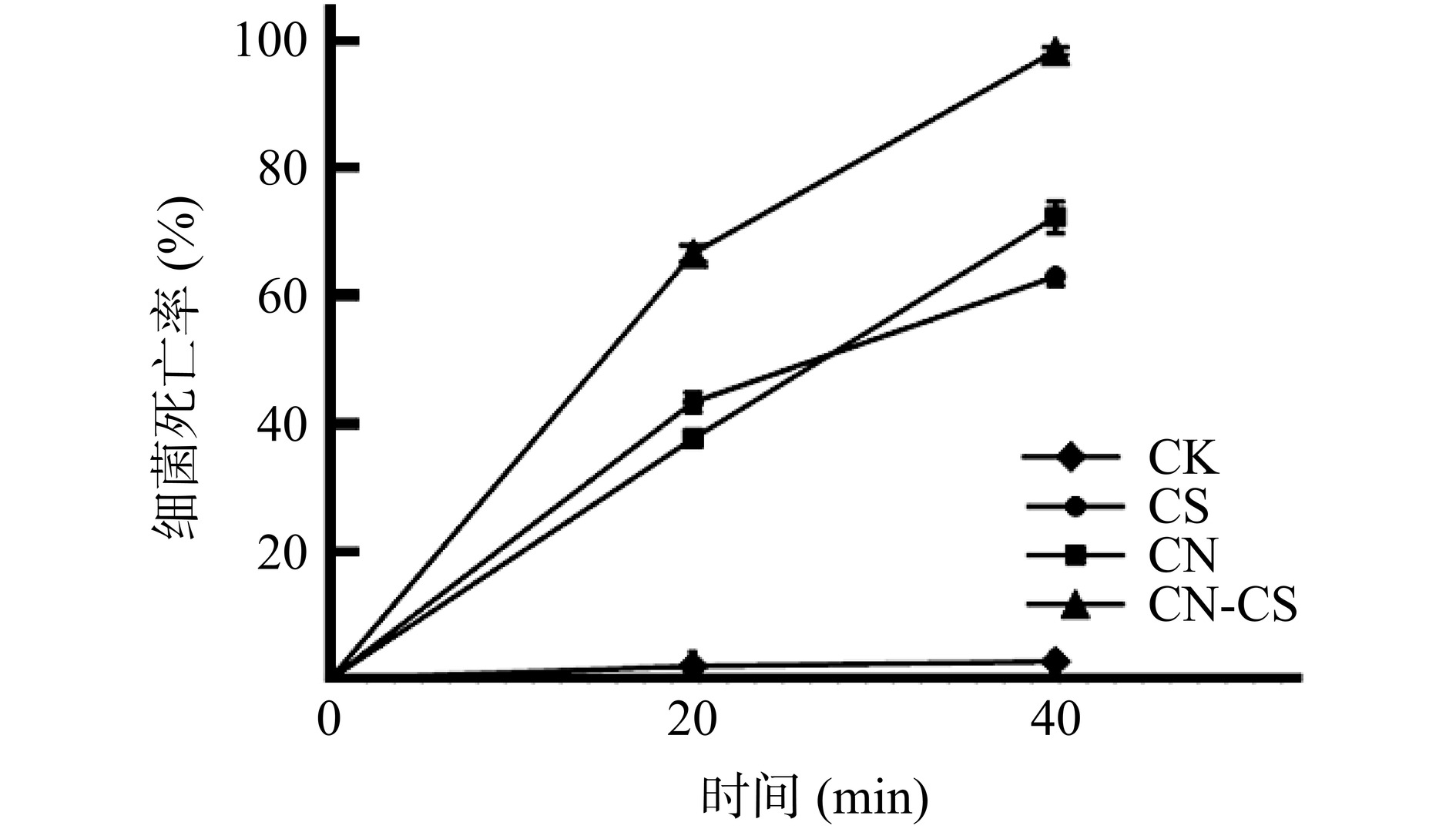
2.1 CN-CS复合溶胶对沙门氏菌的杀菌效果
如图1所示,CN-CS复合溶胶的杀菌率在40 min时达到98.45%,而单独使用氮化碳(CN)和壳聚糖(CS)的杀菌率分别仅为72.36%和63.06%,复合溶胶的杀菌效果有所提高。推测壳聚糖带正电荷,可与细菌细胞膜表面的负电荷发生静电吸附作用,影响细胞膜的稳定性和通透性,加速活性氧物质进入细菌体内,从而提高了细菌死亡率[21]。多项研究也证实壳聚糖与氮化碳联用可增强杀菌效果,如赵鹏程[22]制备的氮化碳气凝胶对大肠杆菌的去除率为47.1%,壳聚糖氮化碳复合气凝胶的细菌去除率提高至66.4%;Praseetja等[23]研究发现添加壳聚糖后,氮化碳对大肠杆菌的抑菌圈由5±2.28 mm扩大至24±1.67 mm。
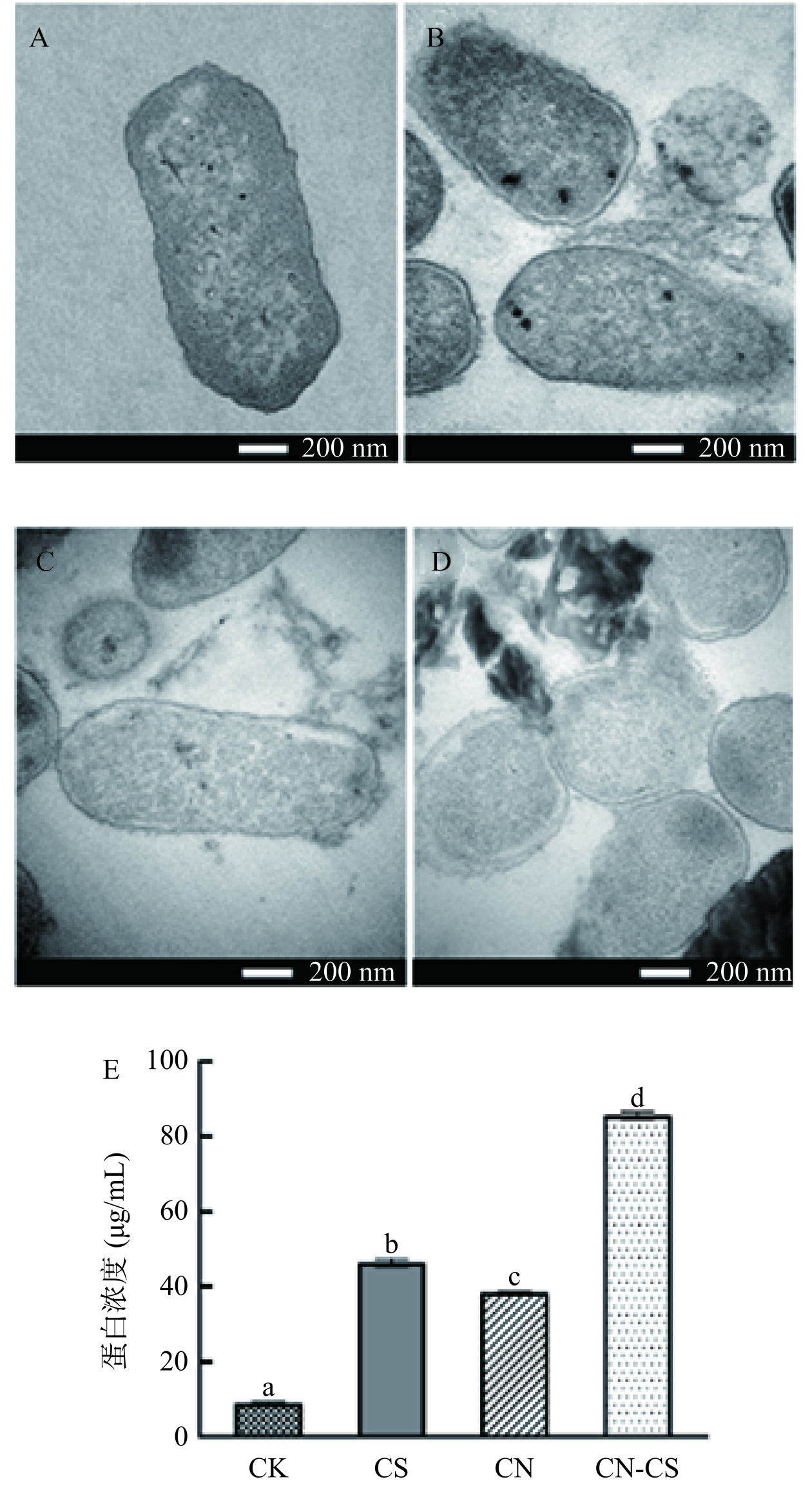
2.2 氮化碳与壳聚糖联用对沙门氏菌细胞壁膜的影响
如图2A所示,对照组中沙门氏菌细胞结构完整,细胞壁膜轮廓清晰,细胞质电子密度分布均匀,呈规则的棒状。经CS溶液和CN分别处理的沙门氏菌(图2B和图2C),细胞壁膜界限模糊,细胞质和细胞膜之间间隙变宽,出现类似“质壁分离”的现象,并伴随着少量的胞内物质泄漏。经CN-CS复合溶胶处理的沙门氏菌细胞壁膜出现明显破裂,细胞内容物泄露(图2D)。蛋白质泄露是评价细菌细胞膜完整性的重要指标。如图2E所示,CN-CS复合溶胶处理组蛋白浓度显著高于对照组和单一处理组(P<0.05),说明复合溶胶对沙门氏菌细胞膜的破坏程度更大,证实壳聚糖和g-C3N4具有协同杀菌作用[24]。
2.3 氮化碳与壳聚糖联用对沙门氏菌脂质代谢的影响
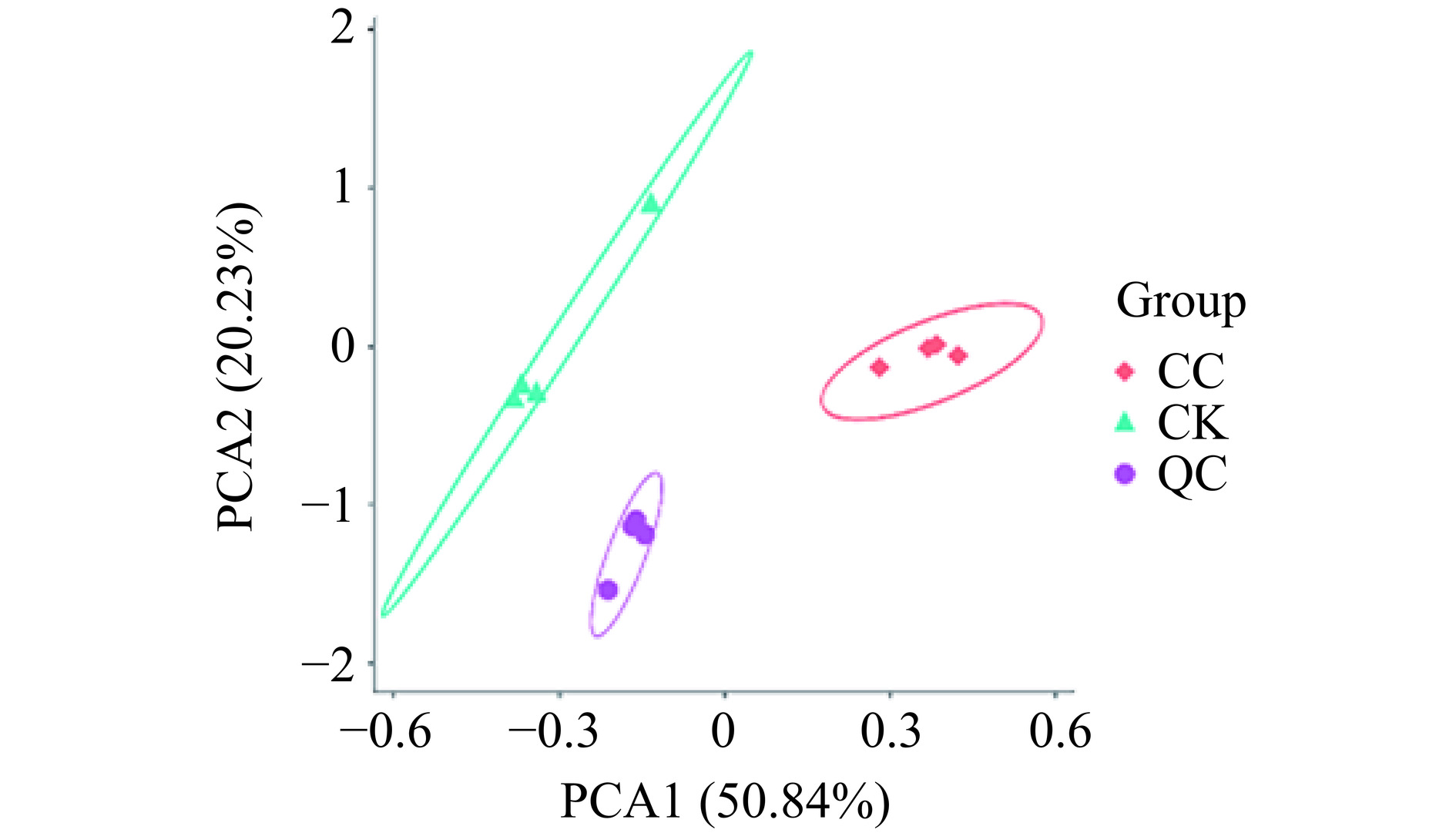
2.3.1 主成分分析
根据PCA分析的结果(图3),第一主成分(PC1)的贡献率为50.84%,第二主成分(PC2)的贡献率为20.23%。质控样本(QC)之间基本重合,表明检测的稳定性和可靠性良好,同时,对照组(CK)和处理组(CC)两组样品均在置信椭圆内,且椭圆之间无交叉,说明处理前后的样品代谢物之间有明显的差异[25]。
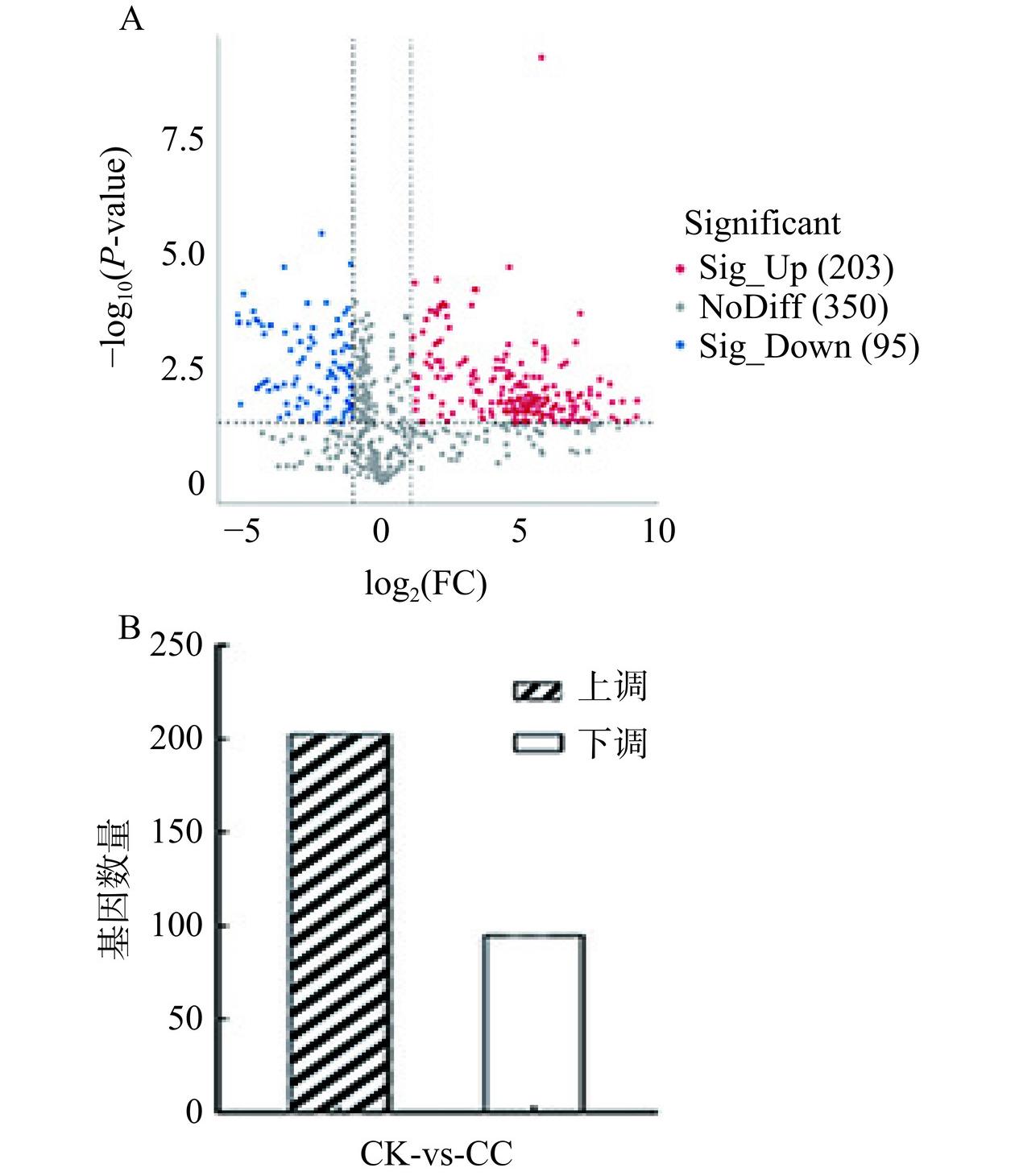
2.3.2 差异代谢物分析
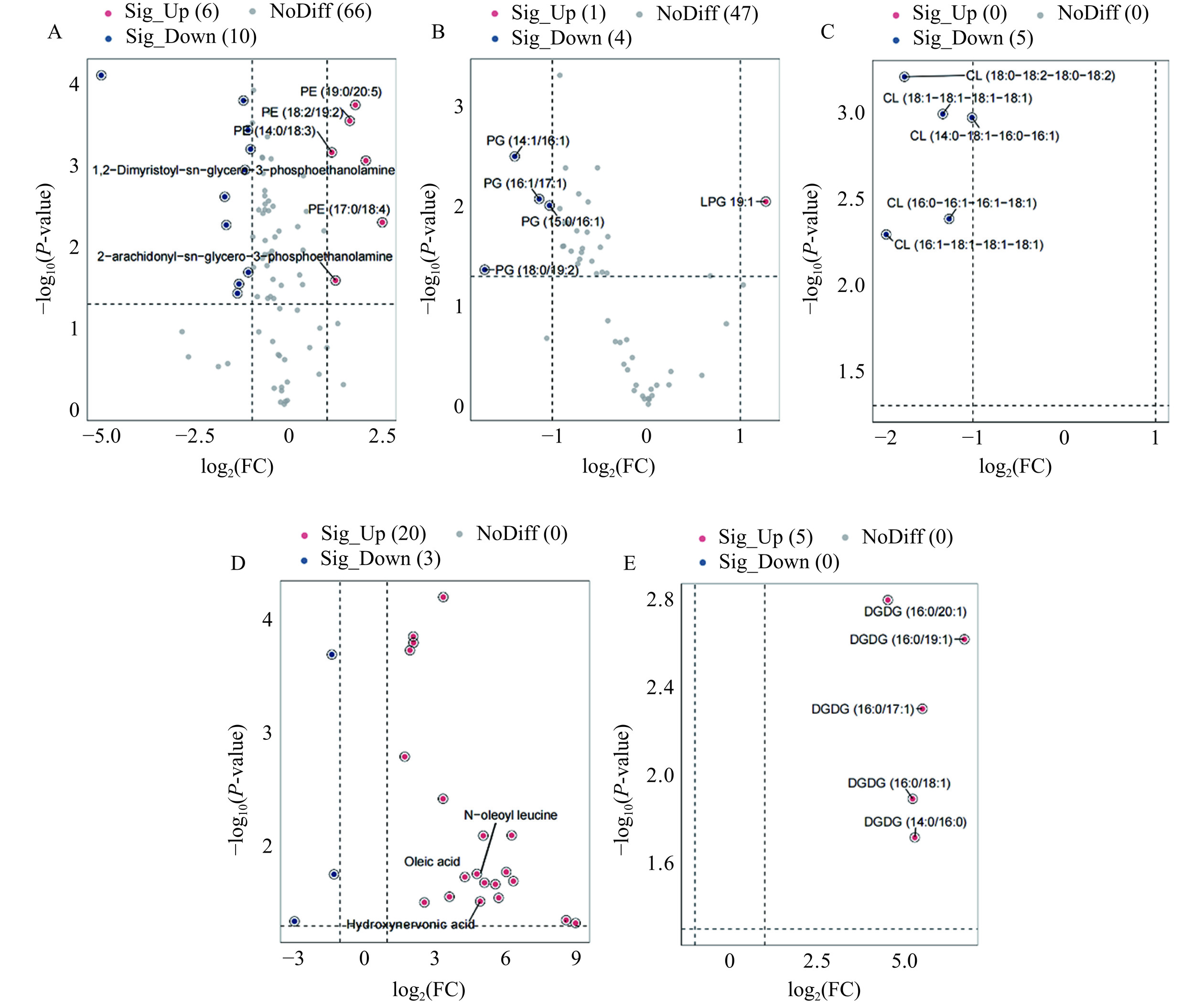
共检测出650种差异代谢物,基于OPLS-DA模型的VIP、单变量分析的FC和相应的筛选标准,在筛选条件为|log2FC|≥1 & VIP≥1 & P-value≤0.05的情况下,得到298个差异代谢物,其中203个上调,95个下调(图4)。
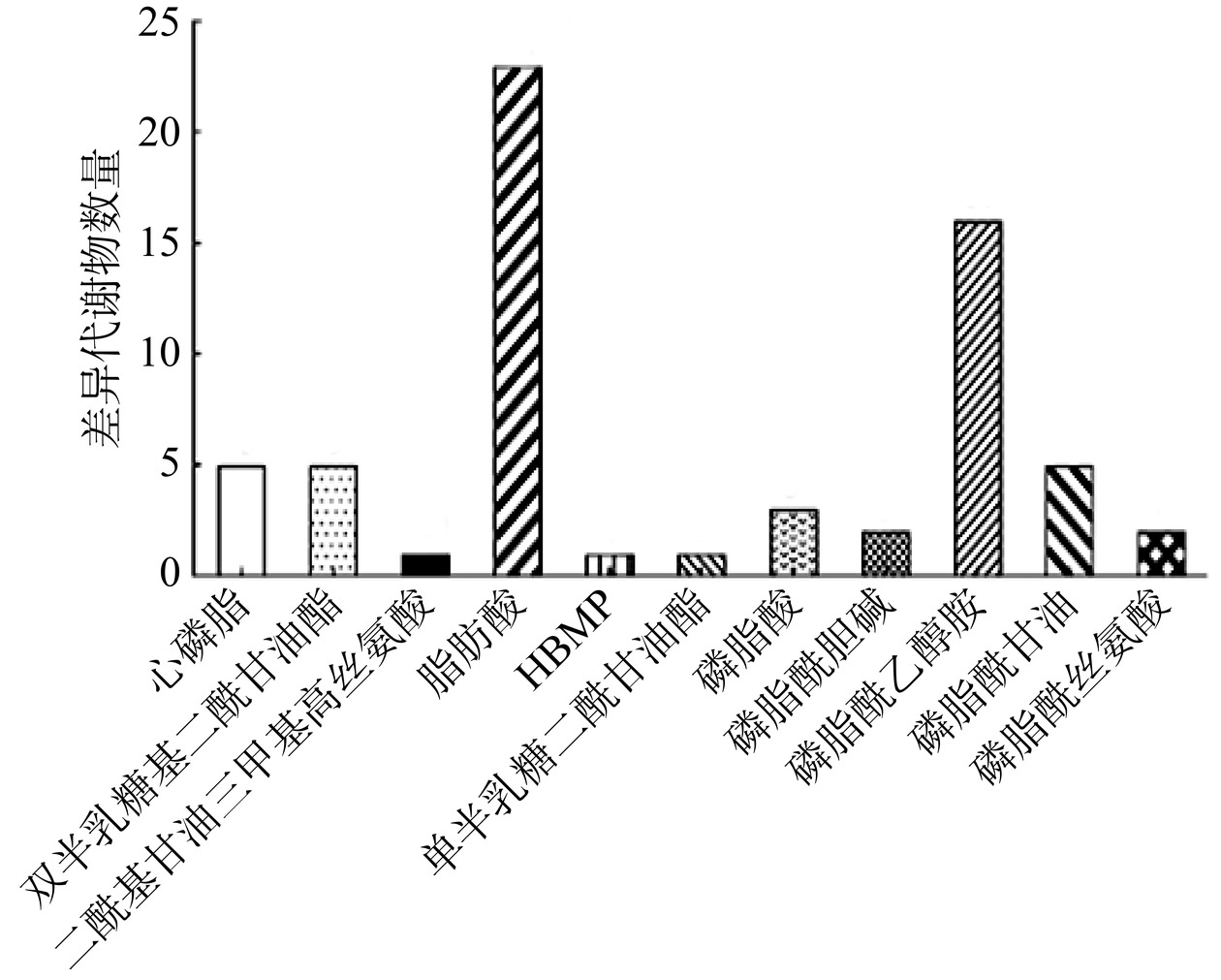
将不同处理组中筛选出的差异代谢物与LIPID MAPS数据库脂质代谢物的代谢功能进行注释,结果如图5所示。差异代谢物数量最多的脂质种类为脂肪酸(Fatty Acids,FA)和磷脂酰乙醇胺(Phosphatidylethanolamine,PE),分别为23种和16种,其次为心磷脂(Cardiolipin,CL),磷脂酰甘油(Phosphatidylglycerol,PG)和双半乳糖基二酰甘油酯(Digalactosyldiacylglycerol,DGDG),分别注释到5种差异代谢物。FA作为细胞膜的主要构成成分,对细胞膜的合成、能量储存和信号分子的产生有重要作用[26]。PE作为甘油磷脂中的一类化合物,是细胞膜中的主要磷脂,可以抑制脂肪酸的氧化代谢[25]。
细菌细胞膜的脂质主要由磷脂(PL)、脂肪酸(FA)类和糖脂(SL)组成。在PL中,差异代谢物占比最高为PE(57.14%),然后依次是PG(17.86%)、CL(17.86%)和磷脂酰胆碱(Phosphatidylcholine,PC)(7.14%)。处理组中PE差异代谢物中有62.5%(10/16)显著下调,PG中有80%(4/5)显著下调,CL中100%显著下调(图6A~图6C)。推测可能是由于光催化产生的活性氧物种首先攻击细胞膜,破坏与细胞膜骨架结构相关的PE、PG、CL分子的分布排列,造成相关代谢物的合成和分解过程被打破[27]。细菌为修复受损的细胞膜会增加PE分子合成,因此差异代谢物PE(14:0/18:3)、2-水杨酰-sn-甘油-3-磷酰乙醇胺(2-arachidonyl-sn-glycero-3-phosphoethanolamine)、PE(18:2/19:2)、PE(19:0/20:5)、1,2-二肉豆蔻酰-sn-丙三基-3-磷脂酰乙醇胺 (1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine)和PE (17:0/18:4)上调(表2)。Jaureguiberry等[28]和Bleijerveld等[29]也发现了细菌通过上调PE分子合成应对细胞膜损伤。在FA中,差异代谢物中有86.96%发生了上调(图6D)。细菌为适应外界环境压力和提高细胞膜的稳定性会上调FA代谢物含量(表2)[25],如N-油酰基亮氨酸(N-oleoyl leucine)、油酸(Oleic acid)、羟基神经酸(Hydroxynervonic acid)等。Gao等[30]、Hann等[31]研究也证明细胞通过上调FA来提高细胞活力、抑制细胞的凋亡。在SL中,差异代谢物均发生了上调(图6E),这可能是由于为应对细胞膜发生的变化,细菌可通过上调DGDG的合成和代谢以维持细胞膜的稳定性和流动性[32],特别是DGDG16:0系列差异代谢物,具体变化情况如表2所示。Jouhet等[33]研究表明细胞在受到损伤时会增加DGDG的含量来取代脂质PE、PC、PG在细胞中的作用。综上,氮化碳与壳聚糖联用会对干扰沙门氏菌脂质代谢,尤其影响细胞膜的脂质合成,破坏细胞膜的稳定性。
表 2 差异代谢物的变化情况Table 2. Changes in differential metabolites类别 差异代谢物 代谢物丰度(CK) 代谢物丰度(CC) log2FC P-value VIP 磷脂酰乙醇胺(PE) 1-myristoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine 3087908.46 1432344.06 −1.1↓ 0.020 1.01 PE (14:0/17:1) 2885324683.00 1332356332.66 −1.11↓ 0.0003 1.14 PE (16:0/16:0) 2517183793.00 1072114357.76 −1.23↓ 0.0004 1.13 PE (18:1/18:1) 117169606.56 56798413.41 −1.04↓ 0.001 1.13 2-arachidonyl-sn-glycero-3-phosphoethanolamine 1215612.65 2854045.99 1.23↑ 0.026 1.00 PE (8:0/17:1) 16986639.91 6661624.78 −1.35↓ 0.028 1.06 PE (19:0/20:5) 42248613.82 143513299.46 1.76↑ 0.0002 1.11 1-(1Z-octadecenyl)-2-hexadecanoyl-sn-glycero-3-phosphoethanolamine 34873973.64 1062719.78 −5.03↓ 0.0002 1.06 PE (18:2/19:2) 775575709.26 2370849090.00 1.61↑ 0.0001 1.11 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine 224078.85 927729.27 2.04↑ 0.001 1.13 磷脂酰乙醇胺(PE) PE (16:1/17:1) 201851919.86 76886490.46 −1.39↓ 0.037 1.01 PE (19:2/19:2) 18491023.19 8074389.05 −1.19↓ 0.001 1.08 PE (17:0/18:4) 390138502.36 2176832972.33 2.48↑ 0.005 1.10 PE (14:0/18:3) 534098811.03 1170628764.33 1.13↑ 0.0004 1.09 PE (16:0/19:2) 13905032.28 4294103.88 −1.69↓ 0.005 1.07 PE (15:0/18:0) 10843408.41 3262759.20 −1.73↓ 0.002 1.10 磷脂酰甘油(PG) PG (14:1/16:1) 1788260.26 676148.58 −1.4↓ 0.003 1.05 PG (16:1/17:1) 38161050.56 17229962 −1.14↓ 0.008 1.09 PG (15:0/16:1) 29848174.48 14544686.93 −1.03↓ 0.010 1.06 LPG 19:1 9156632.41 22109917.67 1.27↑ 0.009 1.09 PG (18:0/19:2) 10523087.88 3183318.08 −1.72↓ 0.043 1.11 心磷脂(CL) CL (18:1-18:1-18:1-18:1) 55278628.76 21954608.13 −1.33↓ 0.001 1.13 CL (16:0-16:1-16:1-18:1) 47438290.51 19806600.51 −1.26↓ 0.004 1.08 CL (14:0-18:1-16:0-16:1) 450132091.5 222021608.7 −1.01↓ 0.001 1.12 CL (16:1-18:1-18:1-18:1) 13622773.8 3510470.44 −1.95↓ 0.005 1.11 CL (18:0-18:2-18:0-18:2) 10404845.37 3083243.79 −1.75↓ 0.001 1.13 磷脂酰胆碱(PC) PC (17:0/18:1) 1503911.11 25462730.84 4.08↑ 0.005 1.143 PC(o-18:1(9Z)/20:1(11Z)) 2699159.92 115663.72 −4.54↓ 0.009 1.14 脂肪酸(FA) Lesquerolic acid 116010.3 8910461.25 2.10↑ 0.0003 1.14 trans-2-hexacosenoyl-CoA 999032.26 3912696.15 5.57↑ 0.021 1.14 cis-Jasmone 3461457.19 14854527.95 5.71↑ 0.029 1.14 margaric acid 131100.07 6229211.37 4.79↑ 0.017 1.14 ximenic acid 233758.5 12310554.53 3.63↑ 0.028 1.14 behenic acid 200839.08 5570467.07 6.33↑ 0.020 1.14 2-hydroxy-22-methyltetracosanoic acid 315097.76 3904991.98 3.37↑ 0.0002 1.14 tricosanedioic acid 100448.33 8103206.32 8.56↑ 0.044 1.14 azelaic acid 766489.2 7977751.66 −1.25↓ 0.018 1.07 hydroxynervonic acid 271216.02 102377842.7 6.03↑ 0.017 1.14 pimelic acid 1501382.33 630086.84 5.11↑ 0.021 1.14 N-oleoyl leucine 98225.4 6428706.94 4.93↑ 0.030 1.14 oleic acid 2244503 77654566.5 −1.34↓ 0.0002 1.12 docosanedioic acid 58039.14 1776556.08 1.74↑ 0.002 1.10 phenolic phthiocerol 54157540.28 21380555.21 2.11↑ 0.0001 1.13 2-hydroxy-3-methylhexadecanoyl-CoA 700423.22 2341788.02 5.06↑ 0.010 1.15 dodecanedioic acid 502918.81 2171774.43 2.57↑ 0.031 1.12 Sterculic acid 57384.02 1922927.59 −2.92↓ 0.046 1.12 juniperic acid 5459746.83 32546886.16 3.36↑ 0.004 1.14 N-(3-carboxypropanoyl)-N-hydroxycadaverine 5522095.26 726042.98 8.96↑ 0.047 1.14 4-one 5535226.15 56835725.72 4.28↑ 0.019 1.14 nervonic acid 85646.73 42880055.06 2.10↑ 0.0002 1.14 ricinelaidic acid 1098354.91 21474771.64 5.57↑ 0.021 1.14 双半乳糖基二酰甘油酯(DGDG) DGDG (16:0/17:1) 202384.85 9264710.16 5.51↑ 0.005 1.15 DGDG (16:0/18:1) 1690561.83 63609468.54 5.23↑ 0.013 1.15 DGDG (16:0/19:1) 611374.84 64373339.7 6.71↑ 0.002 1.15 DGDG (16:0/20:1) 912986.79 20963232.46 4.52↑ 0.002 1.15 DGDG (14:0/16:0) 381739.93 14980271.89 5.29↑ 0.020 1.13 注:向上箭头代表差异代谢物CC组较CK组上调,向下箭头代表差异代谢物CC组较CK组下调。 2.3.3 差异代谢物的调控网络分析
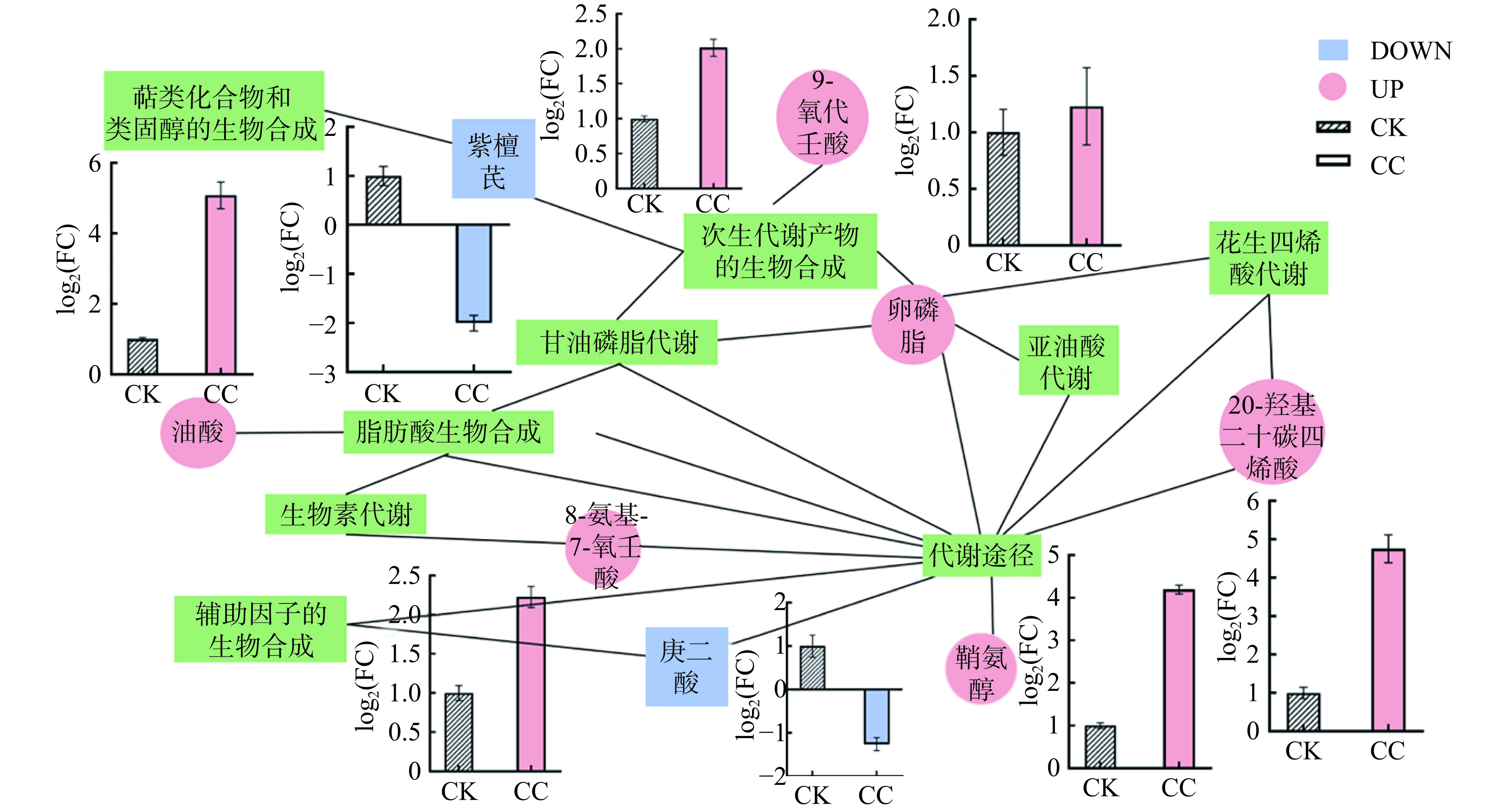
基于差异代谢物和代谢通路构建代谢物调控分子网络图,如图7所示,CN-CS复合溶胶对细菌脂质代谢干扰的作用机制主要体现在细胞膜、氧化应激、信号传导等方面。首先,光催化作用产生的活性氧物种首先攻击细菌细胞膜,主要通过影响脂肪酸生物合成、甘油磷脂代谢、亚油酸代谢三条通路来影响细胞膜的组成和功能。细胞膜受到损伤时会提高卵磷脂合成,以修补破坏的细胞膜[34]。脂肪酸的生物合成通路和甘油磷脂代谢的变化也会破坏细菌的能量代谢和细胞膜的物质转换[25]。同时,细菌通过调整次生代谢物的生物合成通路来适应外部环境的变化,如细菌通过上调差异代谢物9-氧代壬酸(9-Oxononanoic acid)应对活性氧物质对细胞的氧化还原作用[35]。此外,差异代谢物普遍对细菌信号传导和氧化应激起调控作用,如与花生四烯酸代谢通路相关的20-羟基二十碳四烯酸(20-HETE)在处理后上调,这是由于细菌受到ROS攻击后产生氧化应激,细菌通过上调20-HETE抑制细菌体内的氧化应激水平,从而提高细胞膜的稳定性[36]。鞘氨醇代谢增高影响脂质代谢,对细胞的生长存活、信号传导有重要影响[37]。
3. 结论
光催化杀菌是食品工业领域具有应用潜力的新型杀菌技术,利用天然大分子物质提高光催化效率是提升杀菌性能的有效手段,然而,相关杀菌机制研究还较少。本研究利用了壳聚糖的天然杀菌性能,制备了具有光催化杀菌特性的复合溶胶,并通过靶向脂质代谢组探究了复合溶胶对沙门氏菌脂质脂代谢的作用机制。研究结果表明,CN-CS复合溶胶对沙门氏菌灭活率在40 min时达98%以上,分别是CN和CS溶液的1.36倍和1.56倍;细菌生理特征分析表明,复合溶胶通过破坏沙门氏菌的细胞膜结构,导致胞内蛋白质外泄,进而引发细菌死亡;脂质代谢结果表明,经CN-CS复合溶胶处理后的沙门氏菌产生了298个差异代谢物,其中203个上调,95个下调,这些脂质差异代谢物主要是与细胞膜相关的脂肪酸类、甘油磷脂类和糖脂类;对差异代谢物深入分析发现,其主要集中在能量代谢、物质转运和氧化应激方面,能够通过破坏甘油磷脂代谢、脂肪酸生物合成、亚油酸代谢通路来破坏细胞膜的完整性,通过干扰细胞膜信号传递、物质转运和能量代谢等,造成细菌死亡。本研究为光催化材料和壳聚糖的抗菌机制提供了有力的数据支撑。然而,本研究尚未探索最佳复合溶胶的灭菌组分和条件,仍需进一步结合细胞膜脂质代谢关键点,探究最佳杀菌方案,以期在食品工业中的应用。
-
表 1 色谱梯度洗脱程序
Table 1 Chromatographic gradient elution procedure
时间(min) A(%) B(%) 初始 70 30 2 70 30 5 57 43 5.1 45 55 11 30 70 16 1 99 18 1 99 18.1 70 30 20 70 30 表 2 差异代谢物的变化情况
Table 2 Changes in differential metabolites
类别 差异代谢物 代谢物丰度(CK) 代谢物丰度(CC) log2FC P-value VIP 磷脂酰乙醇胺(PE) 1-myristoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine 3087908.46 1432344.06 −1.1↓ 0.020 1.01 PE (14:0/17:1) 2885324683.00 1332356332.66 −1.11↓ 0.0003 1.14 PE (16:0/16:0) 2517183793.00 1072114357.76 −1.23↓ 0.0004 1.13 PE (18:1/18:1) 117169606.56 56798413.41 −1.04↓ 0.001 1.13 2-arachidonyl-sn-glycero-3-phosphoethanolamine 1215612.65 2854045.99 1.23↑ 0.026 1.00 PE (8:0/17:1) 16986639.91 6661624.78 −1.35↓ 0.028 1.06 PE (19:0/20:5) 42248613.82 143513299.46 1.76↑ 0.0002 1.11 1-(1Z-octadecenyl)-2-hexadecanoyl-sn-glycero-3-phosphoethanolamine 34873973.64 1062719.78 −5.03↓ 0.0002 1.06 PE (18:2/19:2) 775575709.26 2370849090.00 1.61↑ 0.0001 1.11 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine 224078.85 927729.27 2.04↑ 0.001 1.13 磷脂酰乙醇胺(PE) PE (16:1/17:1) 201851919.86 76886490.46 −1.39↓ 0.037 1.01 PE (19:2/19:2) 18491023.19 8074389.05 −1.19↓ 0.001 1.08 PE (17:0/18:4) 390138502.36 2176832972.33 2.48↑ 0.005 1.10 PE (14:0/18:3) 534098811.03 1170628764.33 1.13↑ 0.0004 1.09 PE (16:0/19:2) 13905032.28 4294103.88 −1.69↓ 0.005 1.07 PE (15:0/18:0) 10843408.41 3262759.20 −1.73↓ 0.002 1.10 磷脂酰甘油(PG) PG (14:1/16:1) 1788260.26 676148.58 −1.4↓ 0.003 1.05 PG (16:1/17:1) 38161050.56 17229962 −1.14↓ 0.008 1.09 PG (15:0/16:1) 29848174.48 14544686.93 −1.03↓ 0.010 1.06 LPG 19:1 9156632.41 22109917.67 1.27↑ 0.009 1.09 PG (18:0/19:2) 10523087.88 3183318.08 −1.72↓ 0.043 1.11 心磷脂(CL) CL (18:1-18:1-18:1-18:1) 55278628.76 21954608.13 −1.33↓ 0.001 1.13 CL (16:0-16:1-16:1-18:1) 47438290.51 19806600.51 −1.26↓ 0.004 1.08 CL (14:0-18:1-16:0-16:1) 450132091.5 222021608.7 −1.01↓ 0.001 1.12 CL (16:1-18:1-18:1-18:1) 13622773.8 3510470.44 −1.95↓ 0.005 1.11 CL (18:0-18:2-18:0-18:2) 10404845.37 3083243.79 −1.75↓ 0.001 1.13 磷脂酰胆碱(PC) PC (17:0/18:1) 1503911.11 25462730.84 4.08↑ 0.005 1.143 PC(o-18:1(9Z)/20:1(11Z)) 2699159.92 115663.72 −4.54↓ 0.009 1.14 脂肪酸(FA) Lesquerolic acid 116010.3 8910461.25 2.10↑ 0.0003 1.14 trans-2-hexacosenoyl-CoA 999032.26 3912696.15 5.57↑ 0.021 1.14 cis-Jasmone 3461457.19 14854527.95 5.71↑ 0.029 1.14 margaric acid 131100.07 6229211.37 4.79↑ 0.017 1.14 ximenic acid 233758.5 12310554.53 3.63↑ 0.028 1.14 behenic acid 200839.08 5570467.07 6.33↑ 0.020 1.14 2-hydroxy-22-methyltetracosanoic acid 315097.76 3904991.98 3.37↑ 0.0002 1.14 tricosanedioic acid 100448.33 8103206.32 8.56↑ 0.044 1.14 azelaic acid 766489.2 7977751.66 −1.25↓ 0.018 1.07 hydroxynervonic acid 271216.02 102377842.7 6.03↑ 0.017 1.14 pimelic acid 1501382.33 630086.84 5.11↑ 0.021 1.14 N-oleoyl leucine 98225.4 6428706.94 4.93↑ 0.030 1.14 oleic acid 2244503 77654566.5 −1.34↓ 0.0002 1.12 docosanedioic acid 58039.14 1776556.08 1.74↑ 0.002 1.10 phenolic phthiocerol 54157540.28 21380555.21 2.11↑ 0.0001 1.13 2-hydroxy-3-methylhexadecanoyl-CoA 700423.22 2341788.02 5.06↑ 0.010 1.15 dodecanedioic acid 502918.81 2171774.43 2.57↑ 0.031 1.12 Sterculic acid 57384.02 1922927.59 −2.92↓ 0.046 1.12 juniperic acid 5459746.83 32546886.16 3.36↑ 0.004 1.14 N-(3-carboxypropanoyl)-N-hydroxycadaverine 5522095.26 726042.98 8.96↑ 0.047 1.14 4-one 5535226.15 56835725.72 4.28↑ 0.019 1.14 nervonic acid 85646.73 42880055.06 2.10↑ 0.0002 1.14 ricinelaidic acid 1098354.91 21474771.64 5.57↑ 0.021 1.14 双半乳糖基二酰甘油酯(DGDG) DGDG (16:0/17:1) 202384.85 9264710.16 5.51↑ 0.005 1.15 DGDG (16:0/18:1) 1690561.83 63609468.54 5.23↑ 0.013 1.15 DGDG (16:0/19:1) 611374.84 64373339.7 6.71↑ 0.002 1.15 DGDG (16:0/20:1) 912986.79 20963232.46 4.52↑ 0.002 1.15 DGDG (14:0/16:0) 381739.93 14980271.89 5.29↑ 0.020 1.13 注:向上箭头代表差异代谢物CC组较CK组上调,向下箭头代表差异代谢物CC组较CK组下调。 -
[1] 尹文琴, 李瑞锐. 一起鼠伤寒沙门菌感染引起的食物中毒流行病学调查报告[J]. 食品安全导刊,2023(27):25−27,37. [YIN W Q, LI R R. Report of an epidemiologic investigation of food poisoning caused by Salmonella typhimurium infection[J]. Food Safety Guide,2023(27):25−27,37.] YIN W Q, LI R R. Report of an epidemiologic investigation of food poisoning caused by Salmonella typhimurium infection[J]. Food Safety Guide, 2023(27): 25−27,37.
[2] 吴宪. 我国食品沙门氏菌污染率与引起的发病率统计分析[D]. 大连:大连理工大学, 2021. [WU X. Statistical analysis of the contamination rate and morbidity caused by Salmonella in food in China[D]. Dalian:Dalian University of Technology, 2021.] WU X. Statistical analysis of the contamination rate and morbidity caused by Salmonella in food in China[D]. Dalian: Dalian University of Technology, 2021.
[3] 陈崟珺. 食品微生物污染源与传播途径分析及风险评估[J]. 中国食品工业, 2023(21):82−85. [CHEN Y J. Analysis and risk assessment of sources and transmission pathways of food microbial contamination[J] China Food Industry, 2023(21):82−85.] CHEN Y J. Analysis and risk assessment of sources and transmission pathways of food microbial contamination[J] China Food Industry, 2023(21): 82−85.
[4] 金锋. 新型食品加工技术对食品营养的影响[J]. 中国食品工业,2023(15):106−107,110. [JIN F. The impact of new food processing technologies on food nutrition[J]. China Food Industry,2023(15):106−107,110.] JIN F. The impact of new food processing technologies on food nutrition[J]. China Food Industry, 2023(15): 106−107,110.
[5] 王潇栋, 孔阳芷, 张艳玲, 等. 杀菌技术的作用机制及在食品领域中的应用[J]. 中国酿造,2022,41(2):1−8. [WANG X D, KONG Y Z, ZHANG Y L, et al. Mechanism of action of sterilization technology and its application in food field[J]. China Brewing,2022,41(2):1−8.] doi: 10.11882/j.issn.0254-5071.2022.02.001 WANG X D, KONG Y Z, ZHANG Y L, et al. Mechanism of action of sterilization technology and its application in food field[J]. China Brewing, 2022, 41(2): 1−8. doi: 10.11882/j.issn.0254-5071.2022.02.001
[6] ZHENG Q M, AIELLO A, CHOI Y S, et al. 3D printed photoreactor with immobilized graphitic carbon nitride:A sustainable platform for solar water purification[J]. Journal of Hazardous Materials,2020,399:123097. doi: 10.1016/j.jhazmat.2020.123097
[7] DENG Y, LI Z, TANG R, et al. What will happen when microorganisms ''meet'' photocatalysts and photocatalysis?[J]. Environmental Science:Nano,2020,7(3):702−723.
[8] GUO Q, ZHOU C Y, MA Z B, et al. Fundamentals of TiO2 photocatalysis:Concepts, mechanisms, and challenges[J]. Adv Mater,2019,31(50):e1901997. doi: 10.1002/adma.201901997
[9] DONG J Q, ZHANG Y, HUSSAIN M I, et al. G-C3N4:Properties, pore modifications, and photocatalytic applications[J]. Nanomaterials (Basel),2021,12(1):121. doi: 10.3390/nano12010121
[10] YUE L, ZHENG M H, KHAN I M, et al. Chlorin e6 conjugated chitosan as an efficient photoantimicrobial agent[J]. International Journal of Biological Macromolecules,2021,183:1309−1316. doi: 10.1016/j.ijbiomac.2021.05.085
[11] 倪永升. 纳米石墨相氮化碳基食品包装膜的制备及杀菌机制研究[D]. 咸阳:西北农林科技大学, 2023. [NI Y S. Preparation and bactericidal mechanism of nano graphite phase carbon nitride based food packaging film[D]. Xianyang:Northwest A&F University, 2023.] NI Y S. Preparation and bactericidal mechanism of nano graphite phase carbon nitride based food packaging film[D]. Xianyang: Northwest A&F University, 2023.
[12] 杨欧, 张晓湘, 徐小涵, 等. 抗氧化型壳聚糖/大豆蛋白复合食用膜的制备与应用[J]. 食品工业科技, 2024, 45(6):210−218. [YANG O, ZHANG X X, XU X H, et al. Preparation and application of antioxidant chitosan/soy protein composite edible film [J] Science and Technology of Food Industry, 2024, 45(6):210−218.] YANG O, ZHANG X X, XU X H, et al. Preparation and application of antioxidant chitosan/soy protein composite edible film [J] Science and Technology of Food Industry, 2024, 45(6): 210−218.
[13] LI Z H, BAI H, WEI J L, et al. One-step synthesis of melamine-sponge functionalized carbon nitride for excellent water sterilization via photogenerated holes and photothermal conversion[J]. Journal of Colloid and Interface Science,2022,610:893−904. doi: 10.1016/j.jcis.2021.11.126
[14] ABDElHACK M E, ELSSAADONY M T, SHAFI M E, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications:A review[J]. International Journal of Biological Macromolecules,2020,164:2726−2744. doi: 10.1016/j.ijbiomac.2020.08.153
[15] MONTASER A S, WASSEL A R, ALSHAYEA O N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane [J]. International Journal of Biological Macromolecules,2018,124:802−809.
[16] BIAN C H, WANG Y Y, YI Y Y, et al. Enhanced photocatalytic activity of S-doped graphitic carbon nitride hollow microspheres:Synergistic effect, high-concentration antibiotic elimination and antibacterial behavior[J]. Journal of Colloid and Interface Science,2023,643:256−266. doi: 10.1016/j.jcis.2023.04.034
[17] QU B, LUO Y C. Chitosan-based hydrogel beads:Preparations, modifications and applications in food and agriculture sectors - A review[J]. Int J Biol Macromol,2020,152:437−448. doi: 10.1016/j.ijbiomac.2020.02.240
[18] CAI L, WEI X, FEMG H, et al. Antimicrobial mechanisms of g-C3N4 nanosheets against the oomycetes Phytophthora capsici:Disrupting metabolism and membrane structures and inhibiting vegetative and reproductive growth[J]. Journal of Hazardous Materials,2021,417:126121. doi: 10.1016/j.jhazmat.2021.126121
[19] 刘骁, 孟茜, 张明莉, 等. 等离子体活化水对腐败希瓦氏菌杀菌效果及机理[J]. 食品科学,2023,44(9):25−31. [LIU X, MENG X, ZHANG M L, et al. The bactericidal effect and mechanism of plasma activated water on spoilage Shigella[J]. Food Science,2023,44(9):25−31.] doi: 10.7506/spkx1002-6630-20220520-264 LIU X, MENG X, ZHANG M L, et al. The bactericidal effect and mechanism of plasma activated water on spoilage Shigella[J]. Food Science, 2023, 44(9): 25−31. doi: 10.7506/spkx1002-6630-20220520-264
[20] WANG Y X, MALKKMES M J, JIANG C, et al. Antibacterial mechanism and transcriptome analysis of ultra-small gold nanoclusters as an alternative of harmful antibiotics against Gram-negative bacteria[J]. Journal of Hazardous Materials,2021,416:126236. doi: 10.1016/j.jhazmat.2021.126236
[21] PRIYADARSHI R, RHIM J-W. Chitosan-based biodegradable functional films for food packaging applications[J]. Innovative Food Science & Emerging Technologies, 2020, 62: 102346.
[22] 赵鹏程. 壳聚糖衍生物氮化碳可见光催化灭活抗性菌及去除抗性基因的研究[D]. 合肥:合肥工业大学, 2022. [ZHAO P C. Research on visible light catalyzed inactivation of resistant bacteria and removal of resistance genes by chitosan derivative nitrogen doped carbon [D]. Hefei:Hefei University of Technology, 2022.] ZHAO P C. Research on visible light catalyzed inactivation of resistant bacteria and removal of resistance genes by chitosan derivative nitrogen doped carbon [D]. Hefei: Hefei University of Technology, 2022.
[23] PRASEETJA P K, GODWIN M A, ALSALHI M S, et al. Porous chitosan-infused graphitic carbon nitride nanosheets for potential microbicidal and photo-catalytic efficacies[J]. International Journal of Biological Macromolecules,2023,238:124120. doi: 10.1016/j.ijbiomac.2023.124120
[24] ZHAO C, YAN Q, WANG S, et al. Regenerable g-C(3)N(4)-chitosan beads with enhanced photocatalytic activity and stability[J]. RSC Adv,2018,8(48):27516−27524. doi: 10.1039/C8RA04293D
[25] CESARI A B, PAULUCCI N S, BIASUTTI M A, et al. Changes in the lipid composition of Bradyrhizobium cell envelope reveal a rapid response to water deficit involving lysophosphatidylethanolamine synthesis from phosphatidylethanolamine in outer membrane[J]. Research in Microbiology,2018,169(6):303−312. doi: 10.1016/j.resmic.2018.05.008
[26] YUAN L, FAN L Y, DAI H C, et al. Multi-omics reveals the increased biofilm formation of Salmonella typhimurium M3 by the induction of tetracycline at sub-inhibitory concentrations[J]. Science of The Total Environment,2023,899:165695. doi: 10.1016/j.scitotenv.2023.165695
[27] LUO W, WANG J Q, SUN L, et al. Metabolome analysis shows that ultrasound enhances the lethality of chlorine dioxide against Salmonella Typhimurium by disrupting its material and energy metabolism[J]. Food Research International,2022,162:112135. doi: 10.1016/j.foodres.2022.112135
[28] JAUREGUIBERRY M S, TRICERRI M A, SANCHEZ S A, et al. Role of plasma membrane lipid composition on cellular homeostasis:Learning from cell line models expressing fatty acid desaturases[J]. Acta Biochimica et Biophysica Sinica,2014,46(4):273−282. doi: 10.1093/abbs/gmt155
[29] BLEIJERVELD O B, BROUWERS J F H M, VAANDRAGER A B, et al. The CDP-ethanolamine pathway and phosphatidylserine decarboxylation generate different phosphatidylethanolamine Molecular Species[J]. Journal of Biological Chemistry,2007,282(39):28362−28372. doi: 10.1074/jbc.M703786200
[30] GAO Y, SHABALINA I G, BRAZ G R F, et al. Establishing the potency of N-acyl amino acids versus conventional fatty acids as thermogenic uncouplers in cells and mitochondria from different tissues[J]. Biochimica et Biophysica Acta (BBA)- Bioenergetics,2022,1863(4):148542. doi: 10.1016/j.bbabio.2022.148542
[31] HANN R M, EVANS J E, MCCLUER R H, et al. Gangliosides in membranes from torpedo electric organ[J]. Lipids,1996,31(6):627−33. doi: 10.1007/BF02523833
[32] WANG Y, GAO X F, YANG H S. Integrated metabolomics of “big six” Escherichia coli on pea sprouts to organic acid treatments[J]. Food Research International,2022,157:111354. doi: 10.1016/j.foodres.2022.111354
[33] JOUHET J, MARECHAL E, BALDAN B, et al. Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria[J]. J Cell Biol,2004,167(5):863−874. doi: 10.1083/jcb.200407022
[34] CHEN X, WENG M F, LAN M J, et al. Superior antibacterial activity of sulfur-doped g-C3N4 nanosheets dispersed by Tetrastigma hemsleyanum Diels & Gilg's polysaccharides-3 solution[J]. International Journal of Biological Macromolecules,2021,168:453−463. doi: 10.1016/j.ijbiomac.2020.11.155
[35] XU J S, DANG D K, TRAN V T, et al. Liquid-phase exfoliation of graphene in organic solvents with addition of naphthalene[J]. Journal of Colloid and Interface Science,2014,418:37. doi: 10.1016/j.jcis.2013.12.009
[36] PENG S Y, LI Y J, HUANG M J, et al. Metabolomics reveals that CAF-derived lipids promote colorectal cancer peritoneal metastasis by enhancing membrane fluidity[J]. International Journal of Biological Sciences,2022,18(5):1912−1932. doi: 10.7150/ijbs.68484
[37] CAI F Y, JIN S X, CHEN G J. The effect of lipid metabolism on CD4+ T Cells[J]. Mediators Inflamm,2021,2021:6634532.





 下载:
下载:
 下载:
下载:



