Determination of Ofloxacin Residues in Honey by Indirect Competition ELISA
-
摘要: 为了建立间接竞争ELISA法检测氧氟沙星(Ofloxacin,OFLX)在蜂蜜中的残留,本研究采用活性酯法将OFLX与载体蛋白偶联,得到抗OFLX完全抗原(OFLX-BSA)和检测抗原(OFLX-OVA)。采用OFLX-BSA免疫BALB/c小鼠,之后用杂交瘤等技术制备抗OFLX单克隆抗体。通过优化反应条件,建立间接竞争ELISA方法,并对该法的准确度、精密度和特异性进行判定。结果显示OFLX与载体蛋白偶联成功;得到一株抗OFLX的杂交瘤细胞株(3D7),单克隆抗体的IC50值为1.17 ng/mL;该法在蜂蜜中的OFLX平均添加回收率为84.1%,其批间变异系数均大于批内的变异系数,与马波沙星的交叉反应率为52.9%,与其他竞争反应物没有交叉反应。本研究建立的间接竞争ELISA方法能够满足OFLX在蜂蜜中残留的测定需求。
-
关键词:
- 氧氟沙星 /
- 免疫原 /
- 单克隆抗体 /
- 酶联免疫吸附试验(ELISA) /
- 蜂蜜
Abstract: In order to establish an indirect competitive ELISA for the detection of ofloxacin (OFLX) residues in honey, in this study, OFLX was coupled to carrier proteins using the active ester method to obtain anti-OFLX complete antigen (OFLX-BSA) and detection antigen (OFLX-OVA). BALB/c mice were immunized with OFLX-BSA, after which anti-OFLX monoclonal antibodies were prepared by hybridoma and other techniques. An indirect competitive ELISA method was developed by optimizing the reaction conditions and the accuracy, precision and specificity of the method were determined. The results showed that OFLX was successfully coupled to the carrier protein, a hybridoma cell line (3D7) against OFLX was obtained. And the IC50 value of the monoclonal antibody was 1.17 ng/mL. The average recovery rate of OFLX spiked in honey by this method was 84.1%, and its intra-batch coefficients of variation were greater than inter-batch ones in all batches. The cross-reaction rate with marbofloxacin was 52.9%, and no cross-reaction with the other competing reactants. The indirect competitive ELISA method developed in this study is able to fulfill the requirements for determination of OFLX residues in honey.-
Keywords:
- ofloxacin /
- immunogen /
- monoclonal antibody /
- enzyme linked immunosorbent assay (ELISA) /
- honey
-
氧氟沙星(Ofloxacin,OFLX)属于氟喹诺酮类(Fluoroquinolones,FQs)合成抗菌药物,具有抗菌谱广、抗菌活性强、组织渗透性好等优势,对多种致病菌均有良好的抗菌效果,在兽医行业被广泛用于治疗和预防各种传染病,还能促进动物生长,减少成本,为此被作为兽医药物和饲料添加剂在动物养殖中过度使用[1−4]。随着我国蜂蜜的出口量逐年增加,其质量问题受到越来越多的关注,在蜂蜜的实际生产流程中,OFLX可以防治蜜蜂的腐臭病和麻痹症等,并能提升蜂蜜的产能[5−6]。而随着OFLX在动物生产中的滥用,经食物链和环境进入人体,对人类健康构成各种潜在危险,如菌群失调、直接的毒性反应(恶心、呕吐、眩晕、心悸和心血管系统问题)、过敏反应(皮肤损伤和光降解产物的毒性)、某些酶活性抑制等[7−10],世界卫生组织(World health organization,WHO)称抗菌药耐药性(Antimicrobial resistance,AMR)已成为一个全球健康问题,到2050年,多重AMR导致的死亡人数预计将超过1000万[11]。因此,应对OFLX进行严格的监测和控制,2023年我国农业农村部发布了“食品中41种兽药最大残留限量(Maximum residue limits,MRLs)”(GB 31650.1-2022),其中规定所有食品动物(包含鱼)靶组织(肌肉、肝、肾、脂肪、蛋和奶)中OFLX的MRLs均为2 ng/mL,蜂蜜为5 ng/mL。因此,发展检测OFLX残留的分析方法尤为重要。
目前,传统的检测OFLX残留方法包括高效液相色谱法(High performance liquid chromatography,HPLC)[12−15]、超高液相色谱串联质谱法(Ultra HPLC-MS/MS,UHPLC-MS/MS)[16−19]、化学发光法(ChemiLuminescence,CL)[20]、毛细管电泳法(Capillary electrophoresis)[21]、微生物检测法(Microbiological assay)[22]等。虽然这些方法都是经过验证并被广泛接受的方法,但这些方法通常费时、费力,且需要繁杂的样品预处理、大量的设备投资等,并不适用OFLX大规模的现场筛查[23−24]。酶联免疫吸附试验(Enzyme linked immunosorbent assay,ELISA)在动物源性食品检测中具有灵敏、特异、简便的特点,适用于大量样品的现场分析检测。鉴于今年我国关于OFLX在动物源性食品中的MRLs更加严格的规定,目前国内尚无关于OFLX免疫分析方法检测蜂蜜中残留的报道,本研究建立了一种灵敏、特异的蜂蜜中检测OFLX的间接竞争ELISA法,将为此提供更有力的技术支撑。
1. 材料与方法
1.1 材料与仪器
OFLX、水溶性碳二亚胺盐酸盐(EDC)、N-羟基琥珀酰亚胺(N-Hydroxy succinimide,NHS)、弗氏佐剂、牛血清白蛋白(Bovine albumin,BSA) Sigma公司;鸡卵白蛋白(Ovalbumin,OVA) 上海源叶生物科技有限公司;羊抗鼠酶标二抗 洛阳华美生物工程有限公司;马波沙星(Marbofloxacin,MAR)、环丙沙星(Ciprofloxacin,CIP)、恩诺沙星(Enrofloxacin,ENR)、洛美沙星(Lomefloxacin,LOM)、沙拉沙星(Sarafloxacin,SAR) 上海阿拉丁生化科技股份有限公司;替马沙星(Temafloxacin,TEM) 上海麦克林生化科技股份有限公司;蜂蜜 周口市川汇区当地某大型超市;SPF级BALB/c小鼠(许可证号:SCXK(豫)2021-0009,动物伦理审批号:ZKNU2023041) 郑州大学医学院实验动物中心;SP2/0细胞 河南省动物免疫学重点实验室提供。
Thermo Scientific NanoDrop 2000c紫外分光光度计、Multiskan酶标仪 美国Thermo Electron公司;Tanon-1600凝胶成像仪、VE-180A电泳仪 上海Tanon科技有限公司;N-1000核酸蛋白分析仪 德国BECKMAN公司。
1.2 实验方法
1.2.1 免疫原的制备与鉴定
1.2.1.1 OFLX免疫原的合成
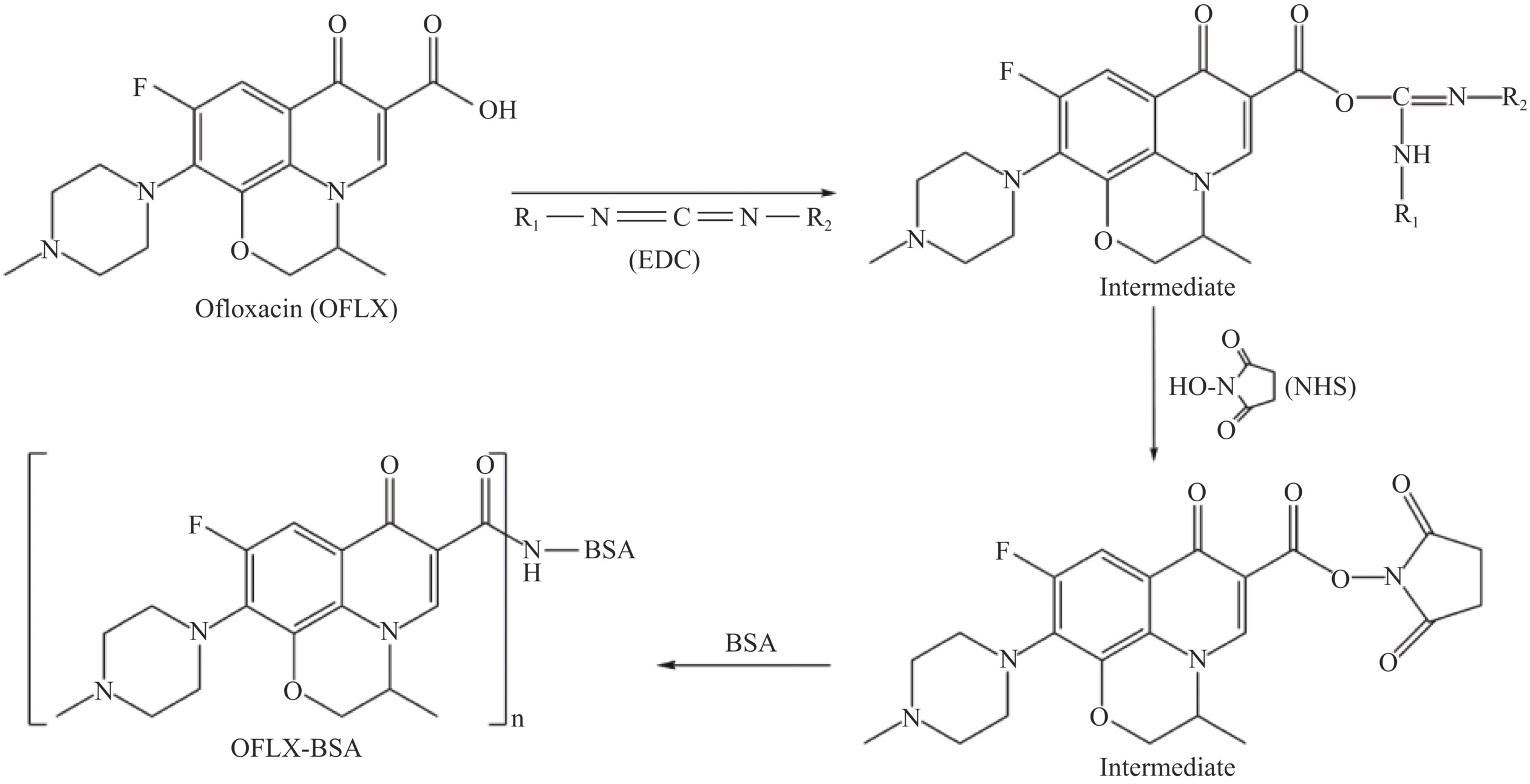
利用活性酯法合成OFLX-BSA[25]。36.5 mg OFLX溶解在3 mL N,N-二甲基甲酰胺(N,N-Dimethylformamide,DMF)中,再加入15.6 mg EDC与11.6 mg NHS,待完全溶解后,添加双蒸水2 mL,在暗环境中,混合液作用18 h,得到OFLX中间反应产物(A液)。55.0 mg BSA充分溶解在1.8 mL PBS溶液中(B液),在冰浴条件下,将A液逐滴添加到B液中,继续反应12 h。将上述混合液放入透析袋中,用PBS缓冲液(pH7.4)透析72 h,每天更换透析液2次,收获免疫原(OFLX-BSA),以此方法制备检测原(OFLX-OVA),3900 r/min离心5 min,−20 ℃保存备用。具体的反应路线见图1。
1.2.1.2 OFLX免疫原的鉴定
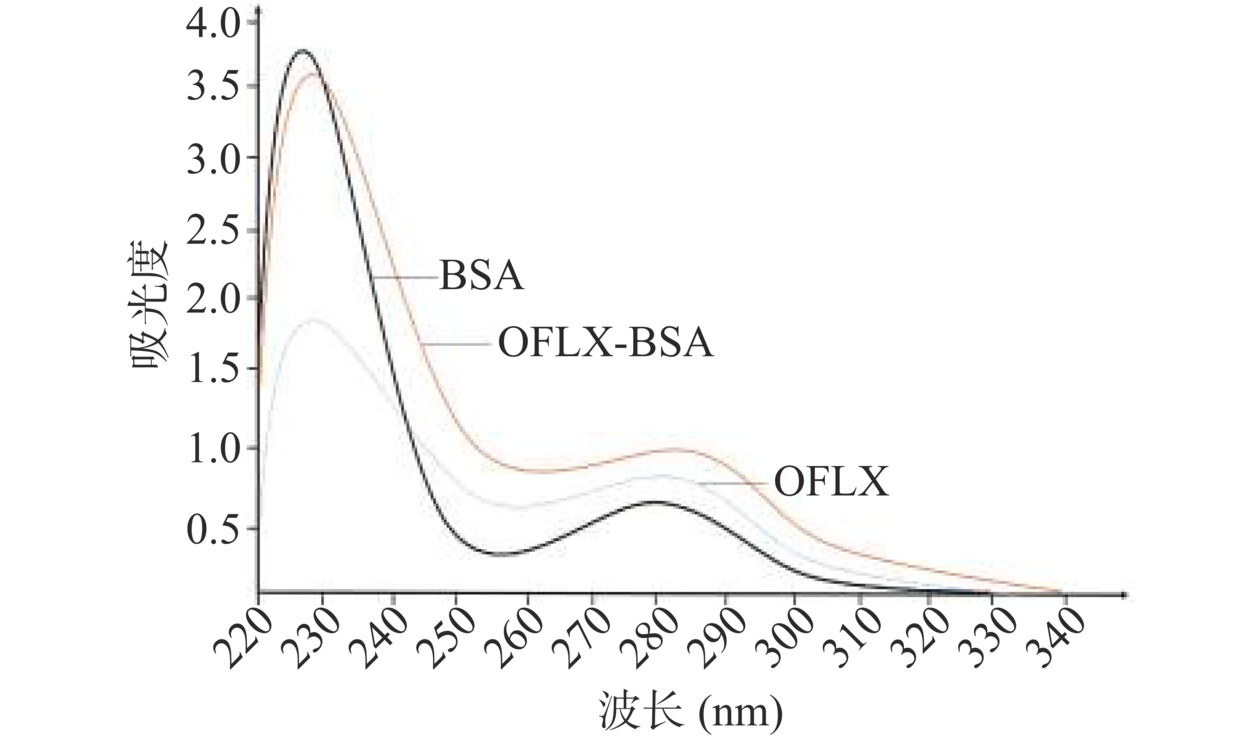
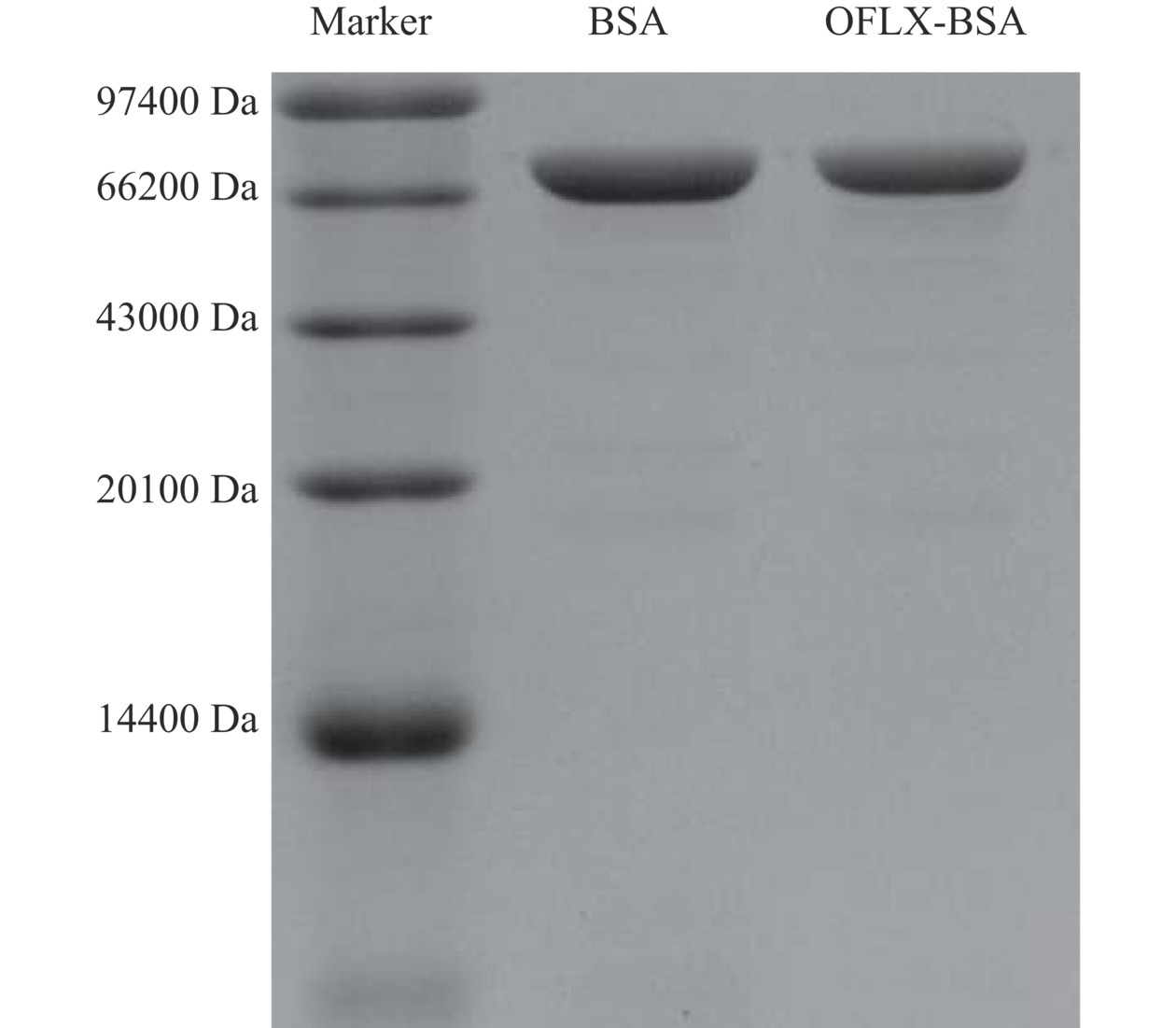
紫外扫描鉴定:将OFLX、BSA、OFLX-BSA统一调整到0.8 mg/mL,在波长220~350 nm范围内,用核酸蛋白分析仪分别对这3种物质进行测定,比较BSA和抗OFLX-BSA免疫原的特征峰,判断OFLX与载体蛋白(BSA)是否偶联成功[26]。SDS-PAGE鉴定:制备5%浓缩胶和12%分离胶,在浓缩胶的样孔中依次加入Marker、BSA和OFLX-BSA,浓缩胶和分离胶的电压分别为70、82 V,电泳完毕后,进一步染色及脱色。通过对比BSA与OFLX-BSA的泳动速率判定OFLX与BSA是否偶联成功。
1.2.1.3 OFLX多克隆抗体的鉴定
间接ELISA测定OFLX多克隆抗体的效价:将检测抗原(OFLX-OVA)用5 μg/mL碳酸盐缓冲液(Carbonate buffer solution,CBS)稀释浓度至2.0 μg/mL,包被量50 μL/孔,37 ℃孵育2 h,PBST(含0.5 mL/L吐温-20的PBS溶液)洗板4次,5%猪血清以220 μL/孔加入,4 ℃反应12 h,洗板4次,自然晾干,待测;每孔均加入一系列倍比稀释(200、400、800、1600、3200、6400、12800)的OFLX多克隆抗体50 μL,并设阴性对照和空白对照,37 ℃分别孵育15 min,PBST洗板4次;1:1000稀释的羊抗鼠酶标二抗以50 μL/孔加入,37 ℃孵育30 min后,PBST洗板6次;加入显色液,6 min后,加入终止液,立即把ELISA板放入酶标仪进行测定。间接竞争ELISA测定OFLX多克隆抗体的敏感性:用OFLX多克隆抗体对OFLX的半数抑制浓度(50% Concentration of inhibition,IC50)判定其敏感性。操作步骤同间接ELISA,区别在于对ELISA板包被完成后,分别加入OD450 nm值为1.0的OFLX的多克隆抗体和浓度分别为2、4、8、16、32、64、128 ng/mL的OFLX标准品。
1.2.2 OFLX单克隆抗体(monoclonal antibody,mAb)制备
1.2.2.1 免疫动物
使用300 μL PBS(含240 μg OFLX-BSA)和300 μL的弗氏完全佐剂(Complete Freund’s Adjuvant,FCA)充分乳化后,免疫3只BALB/c小鼠,注射途径为背部皮下多点注射(注射剂量为150 μL/只)。间隔21 d之后,将OFLX-BSA用相同的剂量与弗氏不完全佐剂(Incomplete Freund’s Adjuvant,FIA)乳化后,用同样的免疫途径进行免疫,每次间隔时间为18 d,4次免疫程序完成后,对小鼠行使尾静脉采血,用PBS溶液稀释离心后,获得OFLX多抗血清(Polyclonal antibody serum,pAbs),用间接ELISA、间接竞争ELISA分别检测OFLX pAbs的效价及敏感性[27],选择效价和抑制率最好的小鼠腹腔注射100 μg OFLX-BSA,3 d后处死小鼠。
1.2.2.2 OFLX mAb的制备
无菌条件下制备免疫小鼠的脾细胞,用PEG 2000对SP2/0细胞和脾脏细胞进行融合,产生杂交瘤细胞。通过有限稀释筛选和克隆阳性杂交瘤,筛选出具有抗OFLX的单克隆杂交瘤株,给预先用液体石蜡处理的BALB/c小鼠进行腹腔注射,注射剂量为0.55 mL/只,10 d后获得腹水。用饱和(NH4)2SO4进一步沉淀纯化,−20 ℃保存待检[27]。
1.2.3 间接竞争ELISA检测方法的建立
间接竞争ELISA的反应步骤与方法1.2.1.3的间接ELISA操作过程相同,主要区别在于ELISA板包被完毕后,每孔均加入一系列倍比稀释的OFLX标准品(0、100、200、400、800、1600、3200、6400 pg/mL)和OD450 nm值为1.0左右的mAbs 50 μL[28]。
1.2.4 间接竞争ELISA检测OFLX实验条件的优化
1.2.4.1 方阵滴定试验确定OFLX-OVA和OFLX mAb最佳工作浓度
将OFLX-OVA、OFLX mAb进行一系列稀释,分别于横向、纵向加入到96孔酶标板中,测定各个反应孔的OD450 nm值,选取数值为1.0左右,且与相邻数值变化较大的孔所对应的OFLX-OVA浓度和OFLX mAb稀释倍数为最佳的OFLX-OVA包被浓度和OFLX mAb工作浓度。
1.2.4.2 其他实验条件的优化
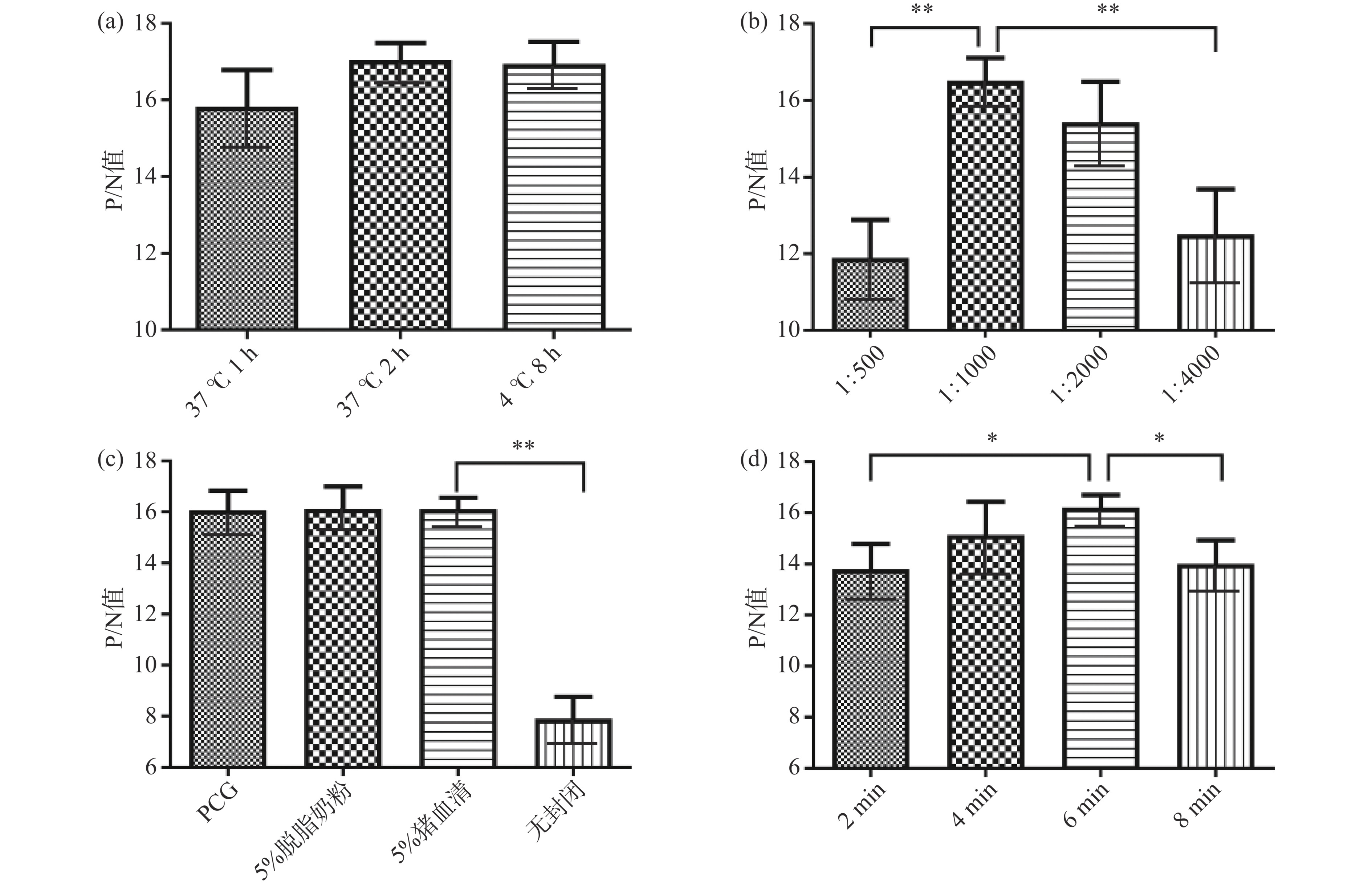
按照1.2.4.1步骤确定的最优包被浓度将OFLX-OVA包被在酶标板,设定OFLX-OVA的包被时间(37 ℃ 1 h、37 ℃ 2 h、4 ℃ 8 h)、酶标二抗的稀释度(1:500、1:1000、1:2000、1:4000)、封闭条件[聚乙二醇(Polyethylene glycol,PCG)、5%脱脂奶粉、5%猪血清、无封闭]及3,3',5,5'-四甲基联苯胺(3,3',5,5'-Tetramethylbenzidine,TMB)显色时间(2、4、6、8 min)等实验条件,间接ELISA测定OD450 nm值,根据确定的两者反应浓度,计算P/N值(P为阳性孔OD450 nm值,N为阴性孔OD450 nm值),选择P/N值最高的工作条件为最佳[29]。
1.2.5 方法的性能测定
1.2.5.1 准确度测定
用添加回收试验来判定方法的准确度。首先对蜂蜜样品进行预处理:蜂蜜样品5 g,用5 mL碱性双蒸水(pH11.0)进行溶解,加入10 mL乙酸乙酯后振荡12 min,5500 r/min离心8 min。抽取上清液4 mL,在45 ℃条件下旋转蒸干,加入2 mL正己烷进行溶解蒸干存留物,再加入2 mL PBS溶液,去除上层的正己烷,保留下清液待测。分别将2、5、15 ng/mL OFLX加入蜂蜜样品中,检测时用0.5%乙酸乙腈提取OFLX。准确度根据回收率及变异系数(Coefficient of variation,CV)进行判定[30]。
1.2.5.2 精密度测定
测定批内、批间误差来判定方法的精密度。OFLX标准品的浓度分别设为4、8、24 ng/mL,在同一批次作6次重复,重复操作6个批次。
1.2.5.3 OFLX mAb特异性测定
应用交叉反应(Cross reaction,CR)来表示,用间接竞争ELISA分别测定其类似物(马波杀星、环丙沙星、恩诺沙星、洛美沙星、沙拉沙星和替马沙星)的IC50值,其计算公式如下:
CR(%)=OFLXIC50值其他化合物IC50值×100 1.3 数据处理
本实验中的所有未标明数据均为3次重复的平均值±标准差,并采用Microsoft Excel 软件进行数据统计,运用GraphPad Prism软件进行作图。
2. 结果与分析
2.1 OFLX-BSA的鉴定结果
2.1.1 OFLX-BSA的紫外扫描结果
BSA、OFLX的吸收峰分别在278、287 nm的位置,与BSA、OFLX的紫外吸收曲线相比,OFLX-BSA的吸收峰同时具备BSA和OFLX的紫外吸收特征,并向长波长段生了偏移(图2),表明OFLX与BSA成功偶联。
2.1.2 SDS-PAGE鉴定结果
图3显示,BSA的向下泳动速率略大于OFLX-BSA。分析物分子质量的大小与电泳条带泳动速率呈反比,据此进一步证实BSA与OFLX偶联成功。
2.2 OFLX多克隆抗体的制备与鉴定
2.2.1 OFLX pAbs的效价鉴定结果
OFLX-BSA免疫BALB/c小鼠的免疫程序完成后,使用间接ELISA检测OFLX pAbs的效价,表1显示,1号和3号小鼠的pAbs效价都在6.4×103以上,2号小鼠达到1.28×104,表明偶联物(OFLX-BSA)的免疫原性较好。
表 1 免疫小鼠的pAbs效价检测Table 1. pAbs potency assay in immunized mice稀释倍数 pAbs效价 No.1 No.2 No.3 2.0×102 2.261 3.011 2.063 4.0×102 1.872 2.324 1.621 8.0×102 1.435 1.718 1.128 1.6×103 1.031 1.332 0.746 3.2×103 0.637 0.880 0.335 6.4×103 0.235 0.439 0.189 1.28×104 0.122 0.198 0.092 阴性 0.082 0.084 0.081 空白 0.71 0.069 0.066 IC50(ng/mL) 17.52 12.42 19.74 2.2.2 OFLX 多抗血清的敏感性鉴定结果
表1显示2号被免小鼠的抑制最优,根据横坐标:OFLX的稀释浓度的对数值,纵坐标:B/B0值(B、B0分别为OFLX各浓度、0 ng/mL浓度对应的OD450 nm值),得到线性回归方程:y=−0.393x+0.930,R2=0.990,当y值等于0.5时,所对应的x数值即为IC50,推算出抗OFLX pAbs的IC50为12.42 ng/mL。
2.3 OFLX单克隆抗体的制备
将免疫OFLX-BSA小鼠的脾细胞和骨髓瘤细胞融合,在HAT培养基培养12 d时,分别取各个孔细胞上清,用间接ELISA筛选出强阳性孔(OD450 nm值>2.0),用间接竞争ELISA对强阳性孔进一步筛选,使用有限稀释法对筛选出抑制好的强阳性孔进行亚克隆,最终收获了杂交瘤细胞株3D7,经诱生腹水法获得了OFLX mAb,其效价为2.4×105。
2.4 间接竞争ELISA检测OFLX方法反应条件的优化及建立
选取OD450 nm数值在1.0左右,且与相邻的OD450 nm值变化较大的孔所对应包被抗原浓度和单克隆抗体稀释度。表2显示,用方阵滴定法测定的数值1.013符合上述要求,其所对应的OFLX-OVA包被浓度和OFLX mAb工作浓度为最优反应条件,最终确定ELISA包被OFLX-OVA的最佳包被浓度为2 μg/mL、OFLX mAb的稀释度为1.6×104倍。
表 2 方阵滴定法确定OFLX-OVA包被浓度和OFLX mAb稀释倍数Table 2. Square-array titration to determine coating concentration of OFLX-OVA and dilution factor of OFLX mAbOFLX mAb稀释度 包被原浓度(μg/mL) 8 6 4 2 1 0.5 1.0×103 3.313 2.853 2.587 2.208 1.791 1.112 2.0×103 2.885 2.474 2.393 2.137 1.532 0.826 4.0×103 2.619 2.319 2.226 1.859 1.163 0.684 8.0×103 2.526 2.188 1.821 1.511 0.944 0.378 1.6×104 2.196 1.786 1.440 1.013 0.723 0.245 3.2×104 1.838 1.275 1.128 0.740 0.505 0.189 6.4×104 1.682 1.036 0.782 0.539 0.286 0.150 空白对照(BC) 0.079 0.073 0.077 0.059 0.067 0.070 阴性对照(NC) 0.064 0.069 0.066 0.068 0.074 0.065 结合步骤1.2.3筛选确定的反应条件,选择P/N值最高的包被条件为最优OFLX-OVA包被条件。图4a显示,37 ℃孵育1 h、37 ℃孵育2 h、4 ℃孵育8 h的P/N值差异不显著(P>0.05),37 ℃孵育2 h的P/N值最大,确定作为最优OFLX-OVA包被条件;选择不同稀释度的羊抗鼠酶标二抗做对比实验,稀释浓度为1:1000时的P/N值最大,与稀释浓度为1:2000的P/N值差异不明显,但与1:500、1:4000对应的P/N值差异极显著(P<0.01),确定羊抗鼠酶标二抗浓度稀释1:1000时作为最优条件(图4b);图4c显示,3种封闭液的P/N值间差异不显著(P>0.05),但5%猪血清与无封闭条件存在极显著差异(P<0.01)且P/N值最大,因此将5%猪血清作为最佳封闭液;室温条件下,加入显色液后,随着显色时间的延长,P和N值均在不断的增加,当作用时间为6 min时,P/N值最大(图4d),与作用时间为4 min的P/N值无显著差异(P>0.05),但与作用时间为2、8 min的P/N值间有显著差异(P<0.05),最终确定显色液作用时间为6 min。
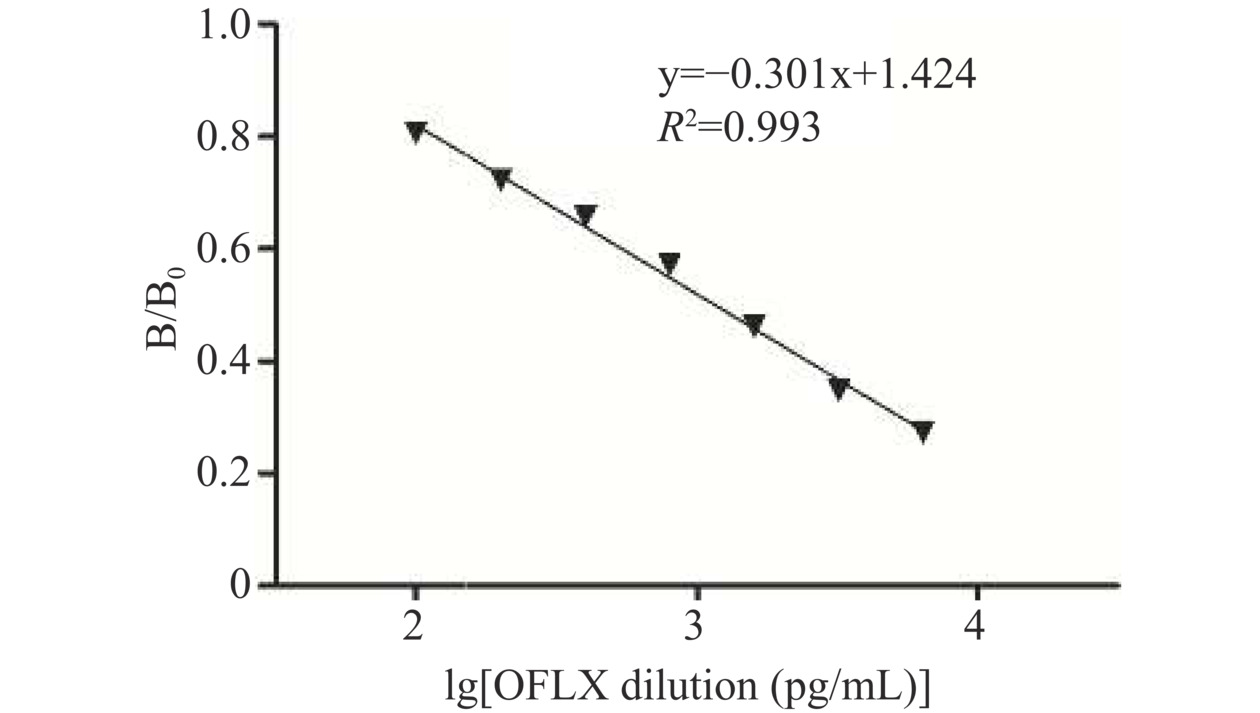
依据实验数据绘制OFLX的标准曲线,得到标准曲线的回归方程:y=−0.301x+1.424,R2=0.993(图5),IC50为1.17 ng/mL,线性范围为0.12~11.65 ng/mL。
2.5 间接竞争ELISA方法的鉴定结果
2.5.1 方法准确度鉴定结果
阴性蜂蜜样的回收率在78.0%~89.9%之间,平均为84.1%,CV为5.3%~9.7%,平均CV为7.3%(表3)。
表 3 OFLX不同添加浓度在蜂蜜中的回收率Table 3. Recovery of OFLX different spiked concentrations in honeyOFLX添加量(ng/mL) 测定值(ng/mL) 回收率(%) 变异系数(%) 2 1.56±0.15 78.0±7.5 9.7 5 4.22±0.29 84.4±5.8 6.9 15 13.49±0.71 89.9±4.7 5.3 2.5.2 方法精密度鉴定结果
阴性蜂蜜样的批内CV在4.1%~7.6%之间,平均批内CV为5.9%;批间CV在4.6%~8.8%之间,平均批间CV为6.8%,且批间CV均大于批内CV,且均不超过10%(表4),表明该法的精密度较好。
表 4 间接竞争ELISA检测蜂蜜样品的精密度Table 4. Precision of the indirect competition ELISA for the detection of honey samplesOFLX添加量(ng/mL) 批次 测定值(ng/mL) 批内变异系数(%) 测定值(ng/mL) 批间变异系数(%) 4 6 3.29 7.6 3.22±0.28 8.8 8 6 6.82 6.0 6.69±0.47 7.1 24 6 22.52 4.1 21.99±1.01 4.6 2.5.3 方法特异性鉴定结果
该法与MAR的CR为52.9%,与CIP、ENR、LOM、SAR和TEM等竞争物没有CR(表5),进一步表明该法的特异性较好。
表 5 OFLX 单克隆抗体与OFLX类似物的交叉反应率Table 5. Cross-reactivity rate of OFLX mAb and its analogue竞争物 IC50(ng/mL) CR(%) 氧氟沙星(Ofloxacin,OFLX) 1.17 100.0 马波沙星(Marbofloxacin,MAR) 2.21 52.9 环丙沙星(Ciprofloxacin,CIP) >1.5×103 <0.01 恩诺沙星(Enrofloxacin,ENR) >1.5×103 <0.01 洛美沙星(Lomefloxacin,LOM) >1.5×103 <0.01 沙拉沙星(Sarafloxacin,SAR) >1.5×103 <0.01 替马沙星(Temafloxacin,TEM) >1.5×103 <0.01 3. 讨论与结论
OFLX作为半抗原(Hapten)没有免疫原性,需与大分子载体结合才能刺激动物机体产生针对OFLX的多抗血清,而多抗血清的敏感性是判定该研究成败的核心[31−32]。PINACHO等[33]用NHS法将OFLX与BSA进行偶联,获得的完全抗原免疫新西兰白兔,得到最优抗血清的IC50值为1.84 ng/mL;李彬彬等[34]、潘孝成[35]均用碳二亚胺法使OFLX与载体蛋白发生偶联,分别免疫小鼠、长耳白兔后,制备所得的抗OFLX血清IC50值为19.97、180.32 ng/mL。而本研究得到的多抗血清、OFLX mAb的IC50分别为12.42、1.17 ng/mL,与他们相比,本研究多抗血清鉴定核心的敏感性相对较好。栗慧等[24]先后用碳二亚胺法合成OFLX-BSA,免疫原免疫小鼠,细胞融合技术等得到OFLX mAb,其IC50为14.58 ng/mL;张运尚等[36]基于OFLX单链抗体的间接竞争ELISA检测方法中IC50达到1.66 ng/mL,低于本研究所得单克隆抗体的敏感性。此外,为提高检测OFLX的灵敏度,一些免疫传感器的纳米材料被引入到其残留检测中,如UCNPs[37]、APTMS[38]、MWCNTs[39]等。这些免疫传感器的引入均相对于传统ELISA方法提高了灵敏度,但也增加了额外的免疫探针制备过程和检测步骤。
本研究对检测OFLX间接竞争ELISA方法的6个单因素反应条件进行优化,建立了最佳OFLX间接竞争ELISA方法,优化后的反应条件分别为:OFLX mAb的稀释倍数1:1.6×104,OFLX-OVA的包被浓度2 μg/mL、包被条件37 ℃作用2 h,酶标二抗的稀释倍数1:1000,封闭液用5%猪血清,TMB显色时间6 min。在此反应条件优化的基础上,建立的ELISA方法IC50为1.17 ng/mL,检测范围为0.12~11.65 ng/mL,检测反应时间大约需要50 min。有鉴于此,本研究所建立的间接竞争ELISA方法具有较高的准确度、精密度和灵敏度,且性能很稳定,可用于蜂蜜中OFLX残留检测,能够满足国内最新规定的相关测定要求。
-
表 1 免疫小鼠的pAbs效价检测
Table 1 pAbs potency assay in immunized mice
稀释倍数 pAbs效价 No.1 No.2 No.3 2.0×102 2.261 3.011 2.063 4.0×102 1.872 2.324 1.621 8.0×102 1.435 1.718 1.128 1.6×103 1.031 1.332 0.746 3.2×103 0.637 0.880 0.335 6.4×103 0.235 0.439 0.189 1.28×104 0.122 0.198 0.092 阴性 0.082 0.084 0.081 空白 0.71 0.069 0.066 IC50(ng/mL) 17.52 12.42 19.74 表 2 方阵滴定法确定OFLX-OVA包被浓度和OFLX mAb稀释倍数
Table 2 Square-array titration to determine coating concentration of OFLX-OVA and dilution factor of OFLX mAb
OFLX mAb稀释度 包被原浓度(μg/mL) 8 6 4 2 1 0.5 1.0×103 3.313 2.853 2.587 2.208 1.791 1.112 2.0×103 2.885 2.474 2.393 2.137 1.532 0.826 4.0×103 2.619 2.319 2.226 1.859 1.163 0.684 8.0×103 2.526 2.188 1.821 1.511 0.944 0.378 1.6×104 2.196 1.786 1.440 1.013 0.723 0.245 3.2×104 1.838 1.275 1.128 0.740 0.505 0.189 6.4×104 1.682 1.036 0.782 0.539 0.286 0.150 空白对照(BC) 0.079 0.073 0.077 0.059 0.067 0.070 阴性对照(NC) 0.064 0.069 0.066 0.068 0.074 0.065 表 3 OFLX不同添加浓度在蜂蜜中的回收率
Table 3 Recovery of OFLX different spiked concentrations in honey
OFLX添加量(ng/mL) 测定值(ng/mL) 回收率(%) 变异系数(%) 2 1.56±0.15 78.0±7.5 9.7 5 4.22±0.29 84.4±5.8 6.9 15 13.49±0.71 89.9±4.7 5.3 表 4 间接竞争ELISA检测蜂蜜样品的精密度
Table 4 Precision of the indirect competition ELISA for the detection of honey samples
OFLX添加量(ng/mL) 批次 测定值(ng/mL) 批内变异系数(%) 测定值(ng/mL) 批间变异系数(%) 4 6 3.29 7.6 3.22±0.28 8.8 8 6 6.82 6.0 6.69±0.47 7.1 24 6 22.52 4.1 21.99±1.01 4.6 表 5 OFLX 单克隆抗体与OFLX类似物的交叉反应率
Table 5 Cross-reactivity rate of OFLX mAb and its analogue
竞争物 IC50(ng/mL) CR(%) 氧氟沙星(Ofloxacin,OFLX) 1.17 100.0 马波沙星(Marbofloxacin,MAR) 2.21 52.9 环丙沙星(Ciprofloxacin,CIP) >1.5×103 <0.01 恩诺沙星(Enrofloxacin,ENR) >1.5×103 <0.01 洛美沙星(Lomefloxacin,LOM) >1.5×103 <0.01 沙拉沙星(Sarafloxacin,SAR) >1.5×103 <0.01 替马沙星(Temafloxacin,TEM) >1.5×103 <0.01 -
[1] AHADI H, SHOKRZADEH M, HOSSEINI-KHAH Z, et al. Conversion of antibacterial quinolone drug levofloxacin to potent cytotoxic agents[J]. Journal of Biochemical and Molecular Toxicology,2023,37(6):e23334. doi: 10.1002/jbt.23334
[2] LI Y F, ZHOU J H, GU J X, et al. Enhanced antibacterial activity of levofloxacin/hydroxypropyl-β-cyclodextrin inclusion complex:In vitro and in vivo evaluation[J]. Colloids and Surfaces B-Biointerfaces,2022,215:112514. doi: 10.1016/j.colsurfb.2022.112514
[3] EZELARAB H A A, ABBAS S H, HASSAN H A, et al. Recent updates of fluoroquinolones as antibacterial agents[J]. Arch Pharm (Weinheim),2018,351(9):e1800141. doi: 10.1002/ardp.201800141
[4] ZHANG Z H, LIU X N, LI N, et al. Effect of ofloxacin levels on growth, photosynthesis and chlorophyll fluorescence kinetics in tomato[J]. Plant Physiology and Biochemistry,2023,194:374−382. doi: 10.1016/j.plaphy.2022.11.029
[5] 马永强, 张丝瑶, 遇世友, 等. 蜂蜜中抗生素残留的检测方法研究进展[J]. 食品工业科技,2022,43(20):454−463. [MA Y Q, ZHANG S Y, YU S Y, et al. Advances in the detection methods of antibiotic residues in honey[J]. Science and Technology of Food Industry,2022,43(20):454−463.] MA Y Q, ZHANG S Y, YU S Y, et al. Advances in the detection methods of antibiotic residues in honey[J]. Science and Technology of Food Industry, 2022, 43(20): 454−463.
[6] 贾英杰. 液质联用技术在蜂蜜兽药残留检测中的应用[J]. 食品工业,2019,40(7):284−286. [JIA Y J. Application of liquid chromatography-mass spectrometry detection technology in veterinary drug residues in honey[J]. The Food Industry,2019,40(7):284−286.] JIA Y J. Application of liquid chromatography-mass spectrometry detection technology in veterinary drug residues in honey[J]. The Food Industry, 2019, 40(7): 284−286.
[7] BHATT S, CHATTERJEE S. Fluoroquinolone antibiotics:Occurrence, mode of action, resistance, environmental detection, and remediation-A comprehensive review[J]. Environmental Pollution,2022,315:120440. doi: 10.1016/j.envpol.2022.120440
[8] MAJALEKAR P P, SHIROTE P J. Fluoroquinolones:Blessings or curses[J]. Current Drug Targets,2020,21(13):1354−1370. doi: 10.2174/1389450121666200621193355
[9] BERTINO J J R, FISH D. The safety profile of the fluoroquinolones[J]. Clinical Therapeutics,2000,22(7):798−817. doi: 10.1016/S0149-2918(00)80053-3
[10] DE SARRO A, DE SARRO G. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects[J]. Current Medicinal Chemistry,2001,8(4):371−384. doi: 10.2174/0929867013373435
[11] ZHANG D D, CUI Y W, ZHANG X P. Estimating factors related to fluoroquinolone resistance based on one health perspective:Static and dynamic panel data analyses from Europe[J]. Frontiers in Pharmacology,2019,10:1145. doi: 10.3389/fphar.2019.01145
[12] TIMOFEEVA I, TIMOFEEV S, MOSKVIN L, et al. A dispersive liquid-liquid microextraction using a switchable polarity dispersive solvent. automated HPLC-FLD determination of ofloxacin in chicken meat[J]. Analytica Chimica Acta,2017,949:35−42. doi: 10.1016/j.aca.2016.11.018
[13] LIU Y F, CAI D, LI X, et al. Occurrence, fate, and risk assessment of antibiotics in typical pharmaceutical manufactories and receiving water bodies from different regions[J]. PLoS One,2023,18(1):e0270945. doi: 10.1371/journal.pone.0270945
[14] QIN Y Q, REN X Y, JU H Y, et al. Occurrence and distribution of antibiotics in a tropical mariculture area of Hainan, China:Implications for risk assessment and management[J]. Toxics,2023,11(5):421. doi: 10.3390/toxics11050421
[15] 范广宇, 唐秀, 张琳, 等. 高效液相色谱-串联质谱法测定鲆鲽类水产品中喹诺酮的不确定度评定[J]. 食品工业科技,2021,42(20):248−254. [FANG G Y, TANG X, ZHANG L, et al. Uncertainty evaluation of determination of quinolones in flatfish by high performance liquid chromatography-tandem[J]. Science and Technology of Food Industry,2021,42(20):248−254.] FANG G Y, TANG X, ZHANG L, et al. Uncertainty evaluation of determination of quinolones in flatfish by high performance liquid chromatography-tandem[J]. Science and Technology of Food Industry, 2021, 42(20): 248−254.
[16] HE L X, HE L Y, GAO F Z, et al. Antibiotics, antibiotic resistance genes and microbial community in grouper mariculture[J]. Science of the Total Environment,2022,808:152042. doi: 10.1016/j.scitotenv.2021.152042
[17] WANG X L, JIAO Y N, WANG G L, et al. Occurrence of quinolones in cultured fish from Shandong Province, China and their health risk assessment[J]. Marine Pollution Bulletin,2022,180:113777. doi: 10.1016/j.marpolbul.2022.113777
[18] WANG R, ZHANG C X, LI Z Y, et al. Detection of fluoroquinolone and sulfonamide residues in poultry eggs in Kunming city, southwest China[J]. Poultry Science,2022,101(6):101892. doi: 10.1016/j.psj.2022.101892
[19] FANG L F, YE P P, FANG B, et al. Pollution characteristics and ecological risk assessment of antibiotics in vegetable field in Kaizhou, Chongqing[J]. Huan Jing Ke Xue,2022,43(11):5244−5252.
[20] KACZMAREK M, STANINSKI K, STODOLNY M. New chemiluminescent method of levofloxacin and ofloxacin determination based on terbium (III)-sensitized fluoroquinolone-KBrO3 reaction[J]. Luminescence,2021,36(8):1945−1952. doi: 10.1002/bio.4128
[21] URBANIAK B, MILANOWSKI B, LULEK J, et al. Study of ofloxacin enantiomers dissolution from selected solid dosage forms selected solid dosage forms using high performance capillary electrophoresis method[J]. Acta Poloniae Pharmaceutica,2017,74(3):955−968.
[22] EV LDA S, SCHAPOVA L E E. Microbiological assay for determination of ofloxacin injection[J]. Journal of Pharmaceutical and Biomedical Analysis,2002,27(1−2):91−96. doi: 10.1016/S0731-7085(01)00513-1
[23] LIU Y Z, ZHAO G X, WANG P, et al. Production of the broad specific monoclonal antibody against sarafloxacin for rapid immunoscreening of 12 fluoroquinolones in meat[J]. Journal of Environmental Science and Health Part B,2013,48(2):139−146. doi: 10.1080/03601234.2013.727668
[24] 栗慧, 薛瑛辉, 冯亚宁, 等. 氧氟沙星单克隆抗体的制备及生物条形码检测技术的研究[J]. 食品安全质量检测学报,2023,14(9):169−176. [LI H, XUE Y H, FENG Y N, et al. Preparation of ofloxacin monoclonal antibody and study on bio-bar codes technology[J]. Journal of Food Safety and Quality,2023,14(9):169−176.] LI H, XUE Y H, FENG Y N, et al. Preparation of ofloxacin monoclonal antibody and study on bio-bar codes technology[J]. Journal of Food Safety and Quality, 2023, 14(9): 169−176.
[25] BYZOVA N A, SMIRNOVA N I, ZHERDEV A V, et al. Rapid immunochromatographic assay for ofloxacin in animal original food stuffs using native antisera labeled by colloidal gold[J]. Talanta,2014,119:125−32. doi: 10.1016/j.talanta.2013.10.054
[26] 谢倩, 李庆, 李佳楠. 日落黄酶联免疫试剂盒的制备及应用[J]. 现代食品科技,2021,37(6):326−332. [XIE Q, LI Q, LI J N. Preparation and application of ELISA kit for detection of sunset yellow[J]. Modern Food Science and Technology,2021,37(6):326−332.] XIE Q, LI Q, LI J N. Preparation and application of ELISA kit for detection of sunset yellow[J]. Modern Food Science and Technology, 2021, 37(6): 326−332.
[27] 刘河冰, 秦誉, 邢维维, 等. 蘑菇中鹅膏毒肽间接竞争ELISA检测方法的建立[J]. 食品工业科技,2022,43(5):294−301. [LIU H B, QIN Y, XING W W, et al. Establishment of indirect competitive ELISA method for detecting amanitin in mushroom[J]. Science and Technology of Food Industry,2022,43(5):294−301.] LIU H B, QIN Y, XING W W, et al. Establishment of indirect competitive ELISA method for detecting amanitin in mushroom[J]. Science and Technology of Food Industry, 2022, 43(5): 294−301.
[28] 司艳芳, 李鹏, 郭东光, 等. 西马特罗多克隆抗体的制备及其免疫学检测方法的建立[J]. 河南农业科学,2020,49(6):157−164. [SI Y F, LI P, GUO D G, et al. Development of cimaterol polyclonal antibody and establishment of immunological detection method[J]. Journal of Henan Agricultural Sciences,2020,49(6):157−164.] SI Y F, LI P, GUO D G, et al. Development of cimaterol polyclonal antibody and establishment of immunological detection method[J]. Journal of Henan Agricultural Sciences, 2020, 49(6): 157−164.
[29] YANG X D, WANG Y B, SONG C M, et al. Hapten synthesis and the development of an ultrasensitive indirect competitive ELISA for the determination of diethylstilbestrol in food samples[J]. Scientific Reports,2020,10(1):3270. doi: 10.1038/s41598-020-59112-1
[30] 孙亚宁, 李青梅, 杨苏珍, 等. 脱氧雪腐镰刀菌烯醇单克隆抗体的制备及间接竞争ELISA方法建立[J]. 中国兽医学报,2021,41(4):689−696. [SUN Y N, LI Q M, YANG S Z, et al. Preparation of monoclonal antibody against deoxynivalenol and establishment of indirect competitive ELISA[J]. Chinese Journal of Veterinary Science,2021,41(4):689−696.] SUN Y N, LI Q M, YANG S Z, et al. Preparation of monoclonal antibody against deoxynivalenol and establishment of indirect competitive ELISA[J]. Chinese Journal of Veterinary Science, 2021, 41(4): 689−696.
[31] 蔡陈芳, 周常义, 曾磊, 等. 杀草丹半抗原及其人工抗原的制备与鉴定[J]. 食品工业科技,2019,40(14):284−291. [CAI C F, ZHOU C Y, ZENG L, et al. Synthesis and identification of thiobencarb hapten and artificial antigen[J]. Science and Technology of Food Industry,2019,40(14):284−291.] CAI C F, ZHOU C Y, ZENG L, et al. Synthesis and identification of thiobencarb hapten and artificial antigen[J]. Science and Technology of Food Industry, 2019, 40(14): 284−291.
[32] 郭东光, 陈明艳, 冯春花, 等. 齐帕特罗人工抗原合成及其多克隆抗体制备[J]. 动物医学进展,2022,43(5):6−12. [GUO D G, CHEN M Y, FENG C H, et al. Synthesis of zilpaterol complete antigen and preparation of polyclonal antibody[J]. Progress in Veterinary Medicine,2022,43(5):6−12.] doi: 10.3969/j.issn.1007-5038.2022.05.002 GUO D G, CHEN M Y, FENG C H, et al. Synthesis of zilpaterol complete antigen and preparation of polyclonal antibody[J]. Progress in Veterinary Medicine, 2022, 43(5): 6−12. doi: 10.3969/j.issn.1007-5038.2022.05.002
[33] PINACHO D G, SANCHEZ-BAEZA F, MARCO M P. Molecular modeling assisted hapten design to produce broad selectivity antibodies for fluoroquinolone antibiotics[J]. Analytical Chemistry,2012,84(10):4527−4534. doi: 10.1021/ac300263m
[34] 李彬彬, 侯玉泽, 邓瑞广, 等. 氧氟沙星人工抗原的合成与多克隆抗体的制备[J]. 食品科技,2009,34(9):6−10. [LI B B, HOU Y Z, DENG R G, et al. Research on Synthesis and identification of Ofloxacin artificial antigen[J]. Food Science and Technology,2009,34(9):6−10.] LI B B, HOU Y Z, DENG R G, et al. Research on Synthesis and identification of Ofloxacin artificial antigen[J]. Food Science and Technology, 2009, 34(9): 6−10.
[35] 潘孝成. 氧氟沙星与二氟沙星的抗体制备及ELISA方法建立[D]. 合肥:安徽农业大学, 2007. [PAN X C. Preparation of antibodies and establishment of ELISA methods for ofloxacin and difloxacin[D]. Hefei:Anhui Agricultural University, 2007.] PAN X C. Preparation of antibodies and establishment of ELISA methods for ofloxacin and difloxacin[D]. Hefei: Anhui Agricultural University, 2007.
[36] 张运尚, 王方雨, 胡曼, 等. 氧氟沙星单链抗体的制备及间接竞争ELISA的建立[J]. 黑龙江畜牧兽医,2021,616(4):116−123. [ZHANG Y S, WANG F Y, HU M, et al. Preparation of anti-ofloxacin single chain antibody fragment and establishment of indirect competitive ELISA method[J]. Heilongjiang Animal Science and Veterinary Medicine,2021,616(4):116−123.] ZHANG Y S, WANG F Y, HU M, et al. Preparation of anti-ofloxacin single chain antibody fragment and establishment of indirect competitive ELISA method[J]. Heilongjiang Animal Science and Veterinary Medicine, 2021, 616(4): 116−123.
[37] NAMPI P P, VAKUROV A, SAHA S, et al. Surface modified hexagonal up conversion nano-particles for the development of competitive assay for biodetection[J]. Biomaterials Advances,2022,136:212763. doi: 10.1016/j.bioadv.2022.212763
[38] SULLIVAN M V, HENDERSON A, HAND R A, et al. A molecularly imprinted polymer nano-particle-based surface plasmon resonance sensor platform for antibiotic detection in river water and milk[J]. Analytical and Bioanalytical Chemistry,2022,414(12):3687−3696. doi: 10.1007/s00216-022-04012-8
[39] SHINKO E I, FARAFONOVA O V, SHANIN I A, et al. Determination of the fluoroquinolones levofloxacin and ciprofloxacin by a piezoelectric immunosensor modified with multiwalled carbon nanotubes (MWCNTs)[J]. Analytical Letters,2022,55(7):1164−1177. doi: 10.1080/00032719.2021.1991364
-
期刊类型引用(1)
1. 刘顺. 畜牧养殖产品中喹诺酮类药物残留检测技术研究进展. 畜禽业. 2024(11): 24-28+33 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:
 下载:
下载:



