Effect of Fomes officinalis Ames Polysaccharides on Intestinal Flora and Immune Function in Exercise-induced Immunosuppression Rats
-
摘要: 目的:探讨不同剂量阿里红多糖(Fomes officinalis Ames polysaccharides,FOP)对运动性免疫抑制(Exercise-induced immunosuppression,EIS)大鼠肠道菌群及免疫功能的保护作用。方法:50只7~8周龄SPF级雄性SD大鼠随机分为对照组(NC组)、运动训练组(Ex组)、运动训练+低剂量FOP组(LFOP组,40 mg/(kg·d))、运动训练+中剂量FOP组(MFOP组,60 mg/(kg·d))和运动训练+高剂量FOP组(HFOP组,80 mg/(kg·d))。Ex组和各剂量FOP组采用6周跑台训练构建EIS模型,LFOP、MFOP、HFOP组每次训练后灌胃FOP。干预结束后,酶联免疫吸附试验检测血清免疫球蛋白G(Immunoglobulin G,IgG)、免疫球蛋白M(Immunoglobulin M,IgM)、白介素6(Interleukin-6,IL-6)、白介素10(Interleukin-10,IL-10)、干扰素γ(Interferon-γ,INF-γ)、肿瘤坏死因子α(Tumor necrosis factor-α,TNF-α)、脂多糖(Lipopolysaccharide,LPS)水平及肠道短链脂肪酸含量;细胞分析仪检测血清CD4+、CD8+的数量;16S rDNA检测大鼠肠道菌群结构变化;Western blot检测结肠组织紧密连接蛋白(Zonula occludens-1,ZO-1)、封闭蛋白(Occludin)、紧密连接蛋白4(Claudin4)蛋白表达。结果:与Ex组比较,LFOP、MFOP、HFOP组大鼠血清IgG、IgM、INF-γ、TNF-α、CD4+和CD8+极显著上升(P<0.01),血清IL-6、IL-10和LPS水平极显著降低(P<0.01);肠道短链脂肪酸含量和拟杆菌门、疣微菌门及乳杆菌属、拟杆菌属、毛螺菌属(未分类)、艾克曼菌属相对丰度极显著上升(P<0.01);结肠组织ZO-1、Occludin、Claudin4蛋白表达极显著上调(P<0.01)。此外,MFOP、HFOP组大鼠脾脏指数、胸腺指数、肠道菌群α多样性较Ex组极显著上升(P<0.01)。结论:FOP可能通过减轻炎症改善肠道菌群结构及活性,提高机体免疫功能,进而抑制EIS的发生发展。Abstract: Objective: To investigate the protective effects of different doses of Fomes officinalis Ames polysaccharides (FOP) on intestinal flora and immune function in exercise-induced immunosuppression (EIS) rats. Methods: Fifty SPF male SD rats aged 7 to 8 weeks were randomly divided into control group (NC group), exercise training group (Ex group), exercise training+low-dose FOP group (LFOP group, 40 mg/(kg·d)), exercise training+medium-dose FOP group (MFOP group, 60 mg/(kg·d)) and exercise training+high-dose FOP group (HFOP group, 80 mg/(kg·d)). Ex group and FOP group were trained on the treadmill for 6 weeks to construct EIS model. LFOP, MFOP and HFOP groups were given FOP after each training. After the intervention, serum IgG, IgM, IL-6, IL-10, INF-γ, TNF-α, LPS levels and intestinal short-chain fatty acid content were detected by enzyme-linked immunosorbent assay. The number of serum CD4+ and CD8+ was detected by cell analyzer. 16S rDNA was used to detect the structural changes of intestinal flora. Western blot was used to detect the protein expression of ZO-1, Occludin, Claudin4 in the colon tissue. Results: Compared with Ex group, serum IgG, IgM, INF-γ, TNF-α, CD4+ and CD8+ of rats in LFOP, MFOP and HFOP groups were significantly increased (P<0.01), while serum levels of IL-6, IL-10 and LPS were significantly decreased (P<0.01). Intestinal short-chain fatty acid content and the relative abundance of Bacteroidetes, Verrucobacteria and Lactobacillus, Bacteroidetes, Spirillum (not classified) and Ekmanella were significantly increased (P<0.01). The expression of ZO-1, Occludin, Claudin4 proteins in colon tissue was significantly up-regulated (P<0.01). In addition, spleen index, thymus index, intestinal flora alpha diversity in MFOP and HFOP groups were significantly increased compared with Ex group (P<0.01). Conclusion: FOP may improved the structure and activity of intestinal flora by reducing inflammation, enhanced the body's immune function, and then inhibited the development of EIS.
-
研究证实,长期从事高强度运动(70%~80% HRmax,>60 min)可能通过炎症和氧化应激反应、免疫功能障碍以及宿主病原体防御障碍诱导运动性免疫抑制(Exercise-induced immunosuppression,EIS)[1]。动物实验证实[2],长期高强度运动后的免疫细胞如淋巴细胞、自然杀伤细胞、单核巨噬细胞的数量、密度及活性出现明显下降,中性粒细胞功能减退,免疫器官出现萎缩和结构改变[3]。EIS的主要征象包括淋巴细胞计数紊乱、自然杀伤细胞活性降低、淋巴细胞转化减低及分泌型免疫球蛋白A减少等。现有研究表明,肠道菌群结构及稳定性可能是影响机体免疫功能的重要因素[4],但EIS的发生发展与肠道微生物群的关系尚未明了。此外,运动可调控宿主代谢和免疫功能,是改变肠道微生物群的潜在外部因素[5]。一项对照研究显示[6],运动是橄榄球运动员肠道微生物群多样性的驱动因素,肠道微生物群多样性与运动员基础免疫能力密切相关。
天然产物多糖提取物可通过免疫系统修饰对个体免疫功能产生有益作用[7]。此外,有研究证实,天然产物多糖提取物可能同时影响宿主肠道菌群结构与免疫系统功能,如,大蒜多糖可以通过调控小鼠菌群-免疫轴促进免疫器官发育,并改善免疫功能与肠道环境[8]。阿里红(Fomes officinalis Ames)系多孔菌科层孔菌属植物,是我国西北地区的常见药食同源天然产物,常用于治疗上呼吸道感染、牙周疾病、胃痛、急慢性肾炎、尿道结石、类风湿关节炎等。阿里红富含多糖成分,阿里红多糖(Fomes officinals Ames polysaccharides,FOP)的免疫调节[9]、抗肿瘤[10]、抗衰老[11]、抗炎[12]等功能已得到证实。课题组前期研究发现,FOP可有效促进机体疲劳恢复,加快自由基清除,并显著改善机体的抗氧化能力及免疫状态[13]。研究证实,EIS与肠道菌群结构密切相关,EIS可以引起肠道通透性增加、肠道黏膜炎症反应和微生物群落失调等,进而诱导肠道菌群失衡,可见肠道菌群在免疫调节中也扮演着重要角色[14]。然而,现阶段鲜见FOP对EIS肠道菌群及免疫功能的干预研究。
本实验采用长期力竭训练构建EIS大鼠模型,首先通过检测大鼠血清免疫指标、体重、免疫器官脏器指数及血清炎症反应指标验证FOP的抗免疫抑制、抗炎效果,随后检测肠道菌群多态性、肠道菌群结构、血清脂多糖水平及肠道屏障功能。以期系统地验证FOP对EIS大鼠肠道菌群及免疫功能的干预效果,为天然产物在运动实践中的应用提供实验依据。
1. 材料与方法
1.1 材料与仪器
SPF级雄性SD大鼠 50只,7~8周龄,平均体重(224.71±19.85)g,北京华阜康生物科技股份有限公司[SCXK(京) 2019-0008];阿里红多糖 实验室自制,经硫酸-苯酚法测定多糖含量为75.81%;免疫球蛋白G(Immunoglobulin G,IgG)试剂盒、免疫球蛋白M(Immunoglobulin M,IgM)试剂盒、白介素6(Interleukin-6,IL-6)试剂盒、白介素10(Interleukin-10,IL-10)试剂盒、干扰素γ(Interferon-γ,INF-γ)试剂盒、肿瘤坏死因子α(Tumor necrosis factor-α,TNF-α)试剂盒 南京建成生物工程研究所;大鼠短链脂肪酸(Short chain fatty acid,SCFA)试剂盒 上海瑞番生物科技有限公司;脂多糖(LPS)酶联免疫试剂盒 上海江莱生物科技有限公司;小鼠抗大鼠CD4+ R-PE抗体、小鼠抗大鼠CD8+ R-PE抗体 美国Caltag公司;RIPA裂解液、电泳缓冲液、6.6×8.5 cm PVDF膜 上海碧云天生物技术有限公司;QIAampDNA粪便DNA试剂盒 德国Qiagen公司;BCA蛋白浓度测定试剂盒、紧密连接蛋白(Zonula occludens-1,ZO-1)抗体、闭合蛋白(Occludin)抗体、紧密连接蛋白4(Claudin4)抗体、甘油醛-3-磷酸脱氢酶(GAPDH)内参抗体、HRP标记山羊抗兔IgG 美国CST公司。
ZH-PT/5S型大鼠跑台 安徽正华生物仪器设备有限公司;imark Bio-Rad酶标仪 美国Bio-Rad公司;BK-3100VET智能细胞分析仪 山东博科科学仪器有限公司;5424R高速冷冻离心机 德国艾本德股份公司;BioSpectrometer fluorescence荧光分光光度计 德国艾本德股份公司;AlphaImager EP凝胶成像系统 美国ProteinSimple公司。
1.2 实验方法
1.2.1 干预药物制备
参考米仁沙·牙库甫等[9]研究中对FOP的制备方法,即采用水提醇沉法,经DEAE-52、Sepharose CL-6B和Sephadex G-100柱层析对粗多糖进行纯化、精制。将精制阿里红多糖产物加水溶解,以硫酸-苯酚法测定得到多糖含量为78.92%。
1.2.2 动物分组及给药
动物于实验室条件(室内恒温23±0.5 ℃,空气湿度50%~55%,光照/黑暗交替循环各12 h)适应性喂养3 d后,随机分为5组,每组10只,分别为对照组(NC组)、运动训练组(Ex组)、运动训练+低剂量阿里红多糖补充组(LFOP组)、运动训练+中剂量阿里红多糖补充组(MFOP组)和运动训练+高剂量阿里红多糖补充组(HFOP组)。运动方案参照覃飞等[3]建立运动性免疫抑制模型大鼠方案:运动干预前Ex组及LFOP、MFOP、HFOP组进行为期3 d跑台适应性训练,正式跑台训练坡度0°、递增跑速(第1周为10 m/min,第2周为20 m/min,自第3周开始,跑台速度每周递增5 m/min,直至第6周达到40 m/min),运动至力竭,1次/d,6次/周,周日休息,共持续6周。运动力竭判断标准为大鼠不能以既定强度坚持运动、四肢无力、头部低垂,声光电刺激均无反应。NC组不接受运动训练干预,正常饲养。LFOP、MFOP、HFOP组每次训练后1 h,称体重,分别灌胃40、60、80 mg/(kg·d)FOP水溶液2 mL;Ex组灌胃等量生理盐水。本研究经喀什地区医共体伦理委员会审批核准(审批编号:(2022)第(55)号)。
1.2.3 动物处置、取样
末次跑台训练后2 h,按摩大鼠下腹部刺激排便,无菌镊子取新鲜粪便于无菌冻存管中,液氮急速低温后转移至−80 ℃超低温冰箱保存备用。随后处死大鼠并取材:10%水合氯醛麻醉,腹主动脉取血5 mL,加EDTA抗凝,−80 ℃保存待测;5 mL 4000 r/min离心20 min,取血清,−80 ℃保存待测;迅速取大鼠脾脏、胸腺、结肠组织,称重、计算脾脏指数及胸腺指数(脏器指数(%)=脏器重量/体重×100),剔除筋膜及外覆脂肪组织,生理盐水反复漂洗,吸水纸擦干水分,液氮急速低温后−80 ℃保存待测。
1.2.4 组织匀浆制备
分别取部分脾脏、胸腺及结肠组织,剪碎,加入PBS匀浆介质,2000 r/min匀浆2 min,匀浆液转移至离心管,4 ℃、6000 r/min离心10 min,取上清−80 ℃保存。
1.2.5 酶联免疫吸附试验
取预先制备血清及结肠组织匀浆液,按照酶联免疫吸附法试剂盒说明书检测血清IgG、IgM、IL-6、IL-10、INF-γ、TNF-α、LPS水平及结肠组织SCFA水平。
1.2.6 血清CD4+、CD8+检测
取待测血清100 μL,加20 μL CD4+-FITC/CD8+-PE单抗,摇匀、避光、25 ℃孵育1 h,加入红细胞裂解液,PBS洗涤,智能细胞分析仪检测CD4+、CD8+的数量,计算CD4+/CD8+比值。
1.2.7 肠道微生物DNA提取及16S rDNA测序
取大鼠粪便,采用Qiagen粪便基因组DNA试剂盒提取大鼠粪便样本DNA,1%琼脂糖凝胶电泳验证粪便样本DNA完整性。使用通用引物341F、806R从提取的DNA中扩增细菌核糖体RNA基因的V3-V4区。F端序列(5′-3′):CCTACGGGRSGCAGCAG;R端序列(5′-3′):GGACTACHVGGGTWTCTAAT。PCR反应体系:25 μL(2.5 μL 10×PCR缓冲液II+0.5单位DNA Polymerase High Fidelity+引物0.4 μmol/L+10 ng模板DNA)。热循环条件:95 ℃预变性2 min;95 ℃变性、55 ℃退火、72 ℃延伸共30 s,27个循环;72 ℃稳定延伸5 min;4 ℃保存。PCR反应产物经Illumina测序平台上机测序,获得的原始数据经高质量质控及嵌合体去除后得到有效数据。根据不同的相似度水平,对有效数据中所有的序列进行归类获得可操作分类单元(OTU)。根据已有数据对每个OTU的代表性序列进行物种注释,得到相应的物种信息和物种丰度分布。
1.2.8 蛋白印迹检测
取大鼠脾脏、胸腺匀浆液,RIPA法提取组织总蛋白,4 ℃,12000 r/min离心20 min;取上清液采用BCA法测定蛋白浓度。配制十二烷基硫酸钠聚丙烯酰胺凝胶(Sodium dodecyl sulfate polyacrylamide gel electrophoresis,SDS-PAGE),浓缩胶恒压80 V电泳30 min,分离胶恒压110 V电泳至溴酚蓝到达分离胶底部,20 μg上样,用电转法将蛋白质转移到PVDF膜上。5%脱脂牛奶室温封闭1 h,加入一抗,4 ℃摇床孵育过夜;TBST洗膜10 min×3次,加二抗,37 ℃孵育40 min;TBST洗膜10 min×5次。ECL法显影,凝胶成像系统成像后使用Quantity One 3.0软件分析,以甘油醛-3-磷酸脱氢酶(Glyceraldehyde-3-phosphate dehydrogenase,GAPDH)作为内参,计算ZO-1、Occludin及Claudin4蛋白相对表达量。
1.3 数据处理
采用SPSS 25.0统计学软件进行统计分析,数据以平均数±标准差(X±SD)表示,数据处理均采用one-way ANOVA进行方差分析,P<0.05为差异显著,P<0.01为差异极显著。
2. 结果与分析
2.1 阿里红多糖对运动免疫抑制大鼠免疫功能、体重及脏器指数的影响
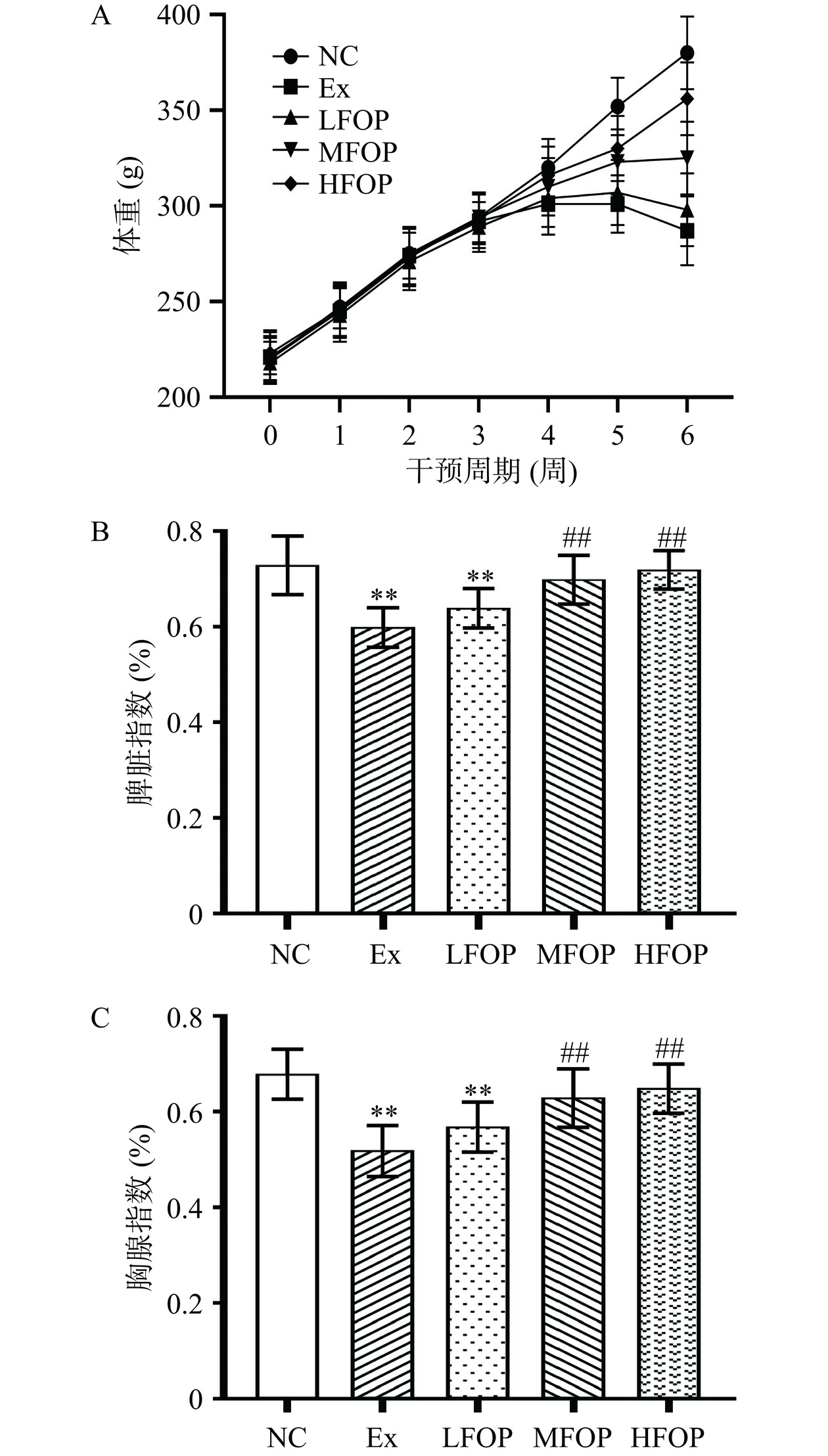
脾脏指数、胸腺指数在一定程度上可反映机体在特定条件下的免疫状况,一般情况下,外周免疫器官指数下降说明机体免疫性能减弱。长期力竭训练或运动疲劳可导致动物脾脏指数和(或)胸腺指数降低,此外,免疫抑制还可能造成机体生长障碍和体重下降[15]。如图1所示,与NC组比较,Ex组大鼠脾脏指数及胸腺指数极显著降低(P<0.01),自第5周体重出现下降趋势,且第5周、第6周体重极显著降低(P<0.01);补充6周FOP后,相比较Ex组,MFOP、HFOP组第5周、第6周体重和免疫器官指数极显著上升(P<0.01)。如斯塔木·托合尼牙孜等[16]研究表明,FOP可提高免疫抑制小鼠体重及免疫器官指数。以上研究表明,FOP可改善EIS大鼠生长障碍及免疫器官功能。
IgG和IgM是血清免疫球蛋白的重要亚型结构,是反映机体免疫水平的敏感标志物。此外,IgG和IgM也是反映机体运动训练期间免疫功能的敏感指标[17]。如表1所示,与NC组比较,Ex组大鼠血清IgG、IgM水平极显著降低(P<0.01);补充6周FOP后,相比较Ex组,LFOP、MFOP、HFOP组大鼠血清IgG和IgM水平均极显著上升(P<0.01)。前期研究显示,40~80 mg/(kg·d) FOP可有效提高运动疲劳小鼠血清免疫球蛋白水平[15]。米仁沙·牙库甫等[9]也发现,FOP的FOPS-a、FOPS-b组分均可促进小鼠脾脏淋巴细胞分泌IgG。表明FOP可改善EIS大鼠的免疫功能。
表 1 各组大鼠血清IgG、IgM及T淋巴细胞亚群标志物($\overline {\rm X} $±SD,n=10)Table 1. Serum IgG, IgM, and T lymphocyte subpopulation markers of rats in each group ($\overline {\rm X} $±SD, n=10)组别 IgG(μg/mL) IgM(μg/mL) CD4+(%) CD8+(%) CD4+/CD8+ NC组 12.51±2.39 136.85±16.11 48.17±4.22 27.63±3.45 1.73±0.20 Ex组 7.02±1.47** 67.88±8.05** 26.74±3.95** 16.86±3.72** 1.58±0.17* LFOP组 9.54±1.42**## 87.41±12.07**## 37.09±4.14**## 22.03±4.51**## 1.68±0.19# MFOP组 11.39±1.66## 120.59±10.50*## 44.51±5.06*## 25.95±3.83## 1.71±0.12# HFOP组 11.05±1.59## 109.64±14.7**## 43.24±4.67*## 23.67±5.02*## 1.83±0.14# 注:与NC组相比,**代表差异极显著(P<0.01),*代表差异显著(P<0.05);与Ex组相比,##代表差异极显著(P<0.01),#代表差异显著(P<0.05);表2同。 CD4+和CD8+是T淋巴细胞的两类重要亚群,CD4+/CD8+比例失调可诱导机体免疫平衡失调。研究证实,长期力竭运动可导致CD4+/CD8+比值降低,进而限制机体T淋巴细胞免疫平衡[18]。如表1所示,与NC组比较,Ex组大鼠血清CD4+、CD8+和CD4+/CD8+比值显著或极显著降低(P<0.05,P<0.01);补充6周FOP后,相比较Ex组,LFOP、MFOP、HFOP组大鼠血清CD4+、CD8+和CD4+/CD8+比值均显著或极显著上升(P<0.05,P<0.01)。沙依拜·沙比提等[19]研究表明,200 mg/kg FOP可提高荷瘤小鼠外周血CD4+ T淋巴细胞和CD4+/CD8+比值。表明FOP可改善EIS大鼠T淋巴细胞免疫平衡。
2.2 阿里红多糖对运动免疫抑制大鼠炎症反应及脂多糖水平的影响
IL-6、IL-10、INF-γ及TNF-α等细胞因子可作为炎症信号蛋白介导免疫细胞增殖、活化、渗透等生物过程,是炎症反应的重要生物标志物[20]。长期力竭训练和(或)运动疲劳可严重抑制细胞免疫功能并激活广泛的炎症反应[3]。如表2所示,6周高强度运动训练后,与NC组比较,Ex组大鼠血清IL-6、IL-10水平极显著上升(P<0.01),血清INF-γ及TNF-α水平极显著降低(P<0.01);补充6周FOP后,相比较Ex组,LFOP、MFOP、HFOP组大鼠血清IL-6、IL-10水平极显著降低(P<0.01),血清INF-γ、TNF-α水平极显著上升(P<0.01)。木妮然·吐尔逊江等[12]研究发现,粗提FOP及FOP-a多糖组分均可有效抑制Aβ25-35诱导的BV-2细胞炎症反应;前期研究也显示,40~80 mg/(kg·d) FOP可显著降低EIS小鼠血清IL-6和TNF-α水平[16]。以上结果表明,FOP可改善EIS大鼠炎症反应,进而缓解机体免疫抑制现象。
表 2 各组大鼠血清炎症反应标志物及脂多糖水平($\overline {\rm X} $±SD,n=10)Table 2. Serum inflammatory response markers and lipopolysaccharide levels in rats of each group ($\overline {\rm X} $±SD, n=10)组别 IL-6(pg/mL) IL-10(pg/mL) INF-γ(pg/mL) TNF-α(pg/mL) LPS(ng/mL) NC组 124.57±10.71 73.45±8.70 590.23±78.15 35.41±5.07 30.55±4.72 Ex组 242.59±17.36** 148.62±15.63** 402.76±62.35** 21.57±4.93** 64.38±6.08** LFOP组 204.35±19.06**## 119.91±13.48**## 498.54±50.98**## 27.83±4.90**## 48.84±6.71**## MFOP组 160.31±16.58**## 91.53±11.34**## 533.09±68.47**## 32.16±4.44*## 37.46±5.53**## HFOP组 193.82±22.09**## 106.57±14.09**## 516.17±49.45**## 33.52±3.81*## 39.78±5.64**## 研究发现,LPS通过影响核因子κB和激活蛋白-1等转录因子激活炎症细胞因子TNF-α、IL-1β、IL-6和C反应蛋白的生成[21]。在炎症环境中,LPS等病原体可诱导一系列急性免疫失衡,并通过介导T细胞和巨噬细胞功能调控机体免疫功能[22]。如表2所示,6周高强度运动训练后,与NC组比较,Ex组大鼠血清LPS水平极显著上升(P<0.01);补充6周FOP后,相比较Ex组,LFOP、MFOP、HFOP组大鼠血清LPS水平极显著降低(P<0.01)。目前尚无直接证据证明FOP对血清LPS的调控作用。但部分侧面证据显示,天然产物多糖提取物可显著降低机体血清LPS水平,有报道显示,多糖提取物可抑制LPS诱导的多器官炎症反应[23]。也有研究发现,LPS可通过与巨噬细胞膜表面的CD14受体偶联,进而将LPS转运至Toll样受体4及髓样分化蛋白2形成蛋白复合体并诱导炎症反应[24]。Yatoo等[25]研究表明,植物多糖可能通过抑制血清及肠道LPS表达减轻炎症反应。由此推测,FOP可能通过降低LPS减轻EIS大鼠炎症反应。
2.3 阿里红多糖对运动免疫抑制大鼠肠道菌群多态性的影响
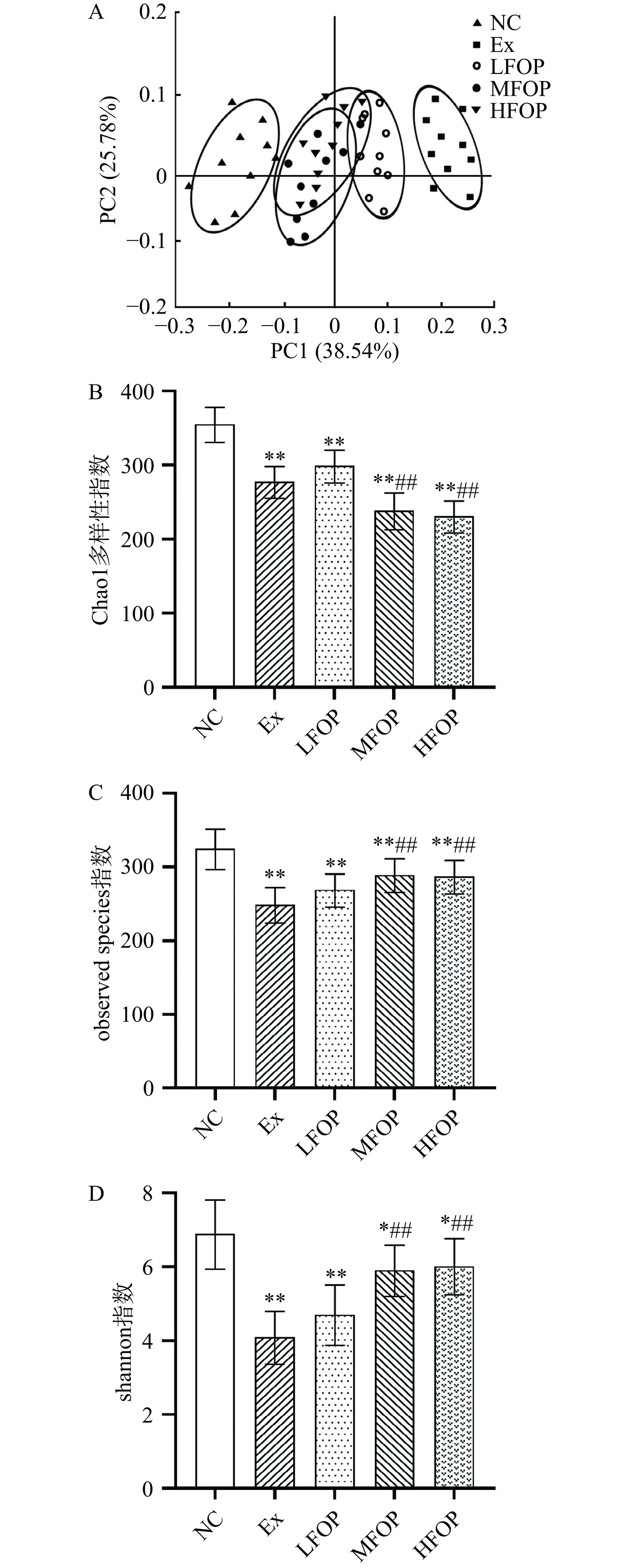
肠道菌群多样性可反映菌群微生态的稳定性和对外界致病菌的抵御能力,α多样性及β多样性是评估肠道菌群多样性的金标准。长期高强度运动可能诱导训练期运动员的胃肠道应激反应,并引发肠道微生物群结构性及代谢性紊乱[26]。EIS可能造成机体肠道菌群α、β多样性降低,且肠道菌群α、β多样性与运动训练强度和周期密切相关[27]。大鼠肠道菌群结构α多样性分析发现(图2B~图2D),与NC组比较,Ex组大鼠肠道Chao1、observed species、shannon指数均极显著降低(P<0.01),表明6周高强度运动训练造成大鼠肠道菌群α多样性降低;补充6周FOP后,相比较Ex组,MFOP和HFOP组大鼠肠道observed species、shannon指数均极显著上升(P<0.01),Chao1指数极显著降低(P<0.01)。主坐标分析(PCoA)结果表明(图2A),NC组样本集中于横坐标的负半轴,Ex组样本集中于横坐标的正半轴;补充6周FOP后,FOP各剂量组样本集中于NC组和Ex组间,表明各组样本肠道菌群β多样性存在显著差异。天然植物性多糖(黄芪多糖[28]、灵芝多糖[29]等)对EIS肠道菌群结构的调节效应已得到证实。上述机制仍需进一步研究,FOP对EIS大鼠肠道菌群多样性的影响可能与血清细胞因子功能、巨噬细胞吞噬能力和脾淋巴细胞指数的改善有关。这提示FOP有利于改善EIS大鼠肠道菌群的相对多样性。
2.4 阿里红多糖对运动免疫抑制大鼠肠道菌群结构的影响
2.4.1 门水平相对丰度
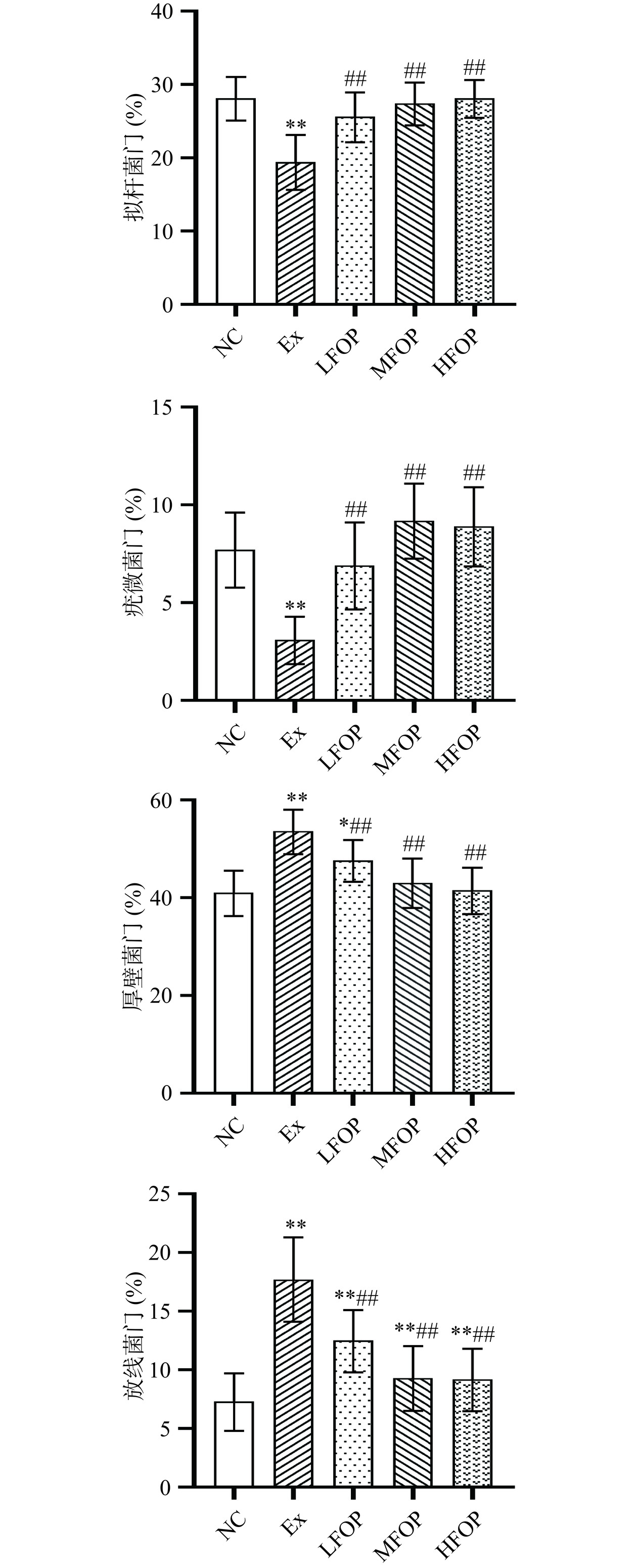
研究显示,长期高强度运动训练或极端饮食条件可影响机体肠道微生物多样性及结构,促进有利于炎症的微生态失调现象,并对机体代谢平衡产生负面影响[30]。Mao等[31]研究表明,3周高强度运动训练可显著降低小鼠肠道拟杆菌门、疣微菌门丰度。大鼠肠道菌群门水平分析发现(图3),长期高强度运动训练大鼠拟杆菌门和疣微菌门相对丰度极显著降低(P<0.01),厚壁菌门和放线菌门相对丰度极显著上升(P<0.01),该结果与既往研究基本一致。厚壁菌门和放线菌门具有促炎特性,参与调控蛋白质分解时的毒性物质释放,与肠道健康和免疫功能密切相关。拟杆菌门和疣微菌门分别主导胃肠道复杂碳水化合物[32]、多糖类物质(如粘多糖、纤维素)[33]的降解,其相对丰度下降可能直接扰乱机体的能量代谢平衡。由此,长期力竭运动诱导的EIS可能与拟杆菌门和疣微菌门相对丰度下降、厚壁菌门和放线菌门相对丰度上升有关。补充6周FOP后,LFOP、MFOP、HFOP组大鼠肠道拟杆菌门和疣微菌门相对丰度较Ex组极显著上升(P<0.01),厚壁菌门和放线菌门相对丰度较Ex组极显著降低(P<0.01)。天然产物提纯物尤其是多糖类物质可调理免疫抑制大鼠肠道菌群结构,该现象可能与天然产物多糖的生物学活性有关[34]。此外,膳食中多糖成分摄入可促进短链脂肪酸(Short chain fatty acids,SCFAs)合成,其机制可能与拟杆菌门等产SCFAs菌群增殖有关,但FOP对拟杆菌属的作用效应仍有待进一步验证。提示FOP可诱导EIS大鼠肠道益生菌增殖并抑制有害菌在肠道中的定殖,从而稳定EIS大鼠肠道菌群结构。
2.4.2 属水平相对丰度
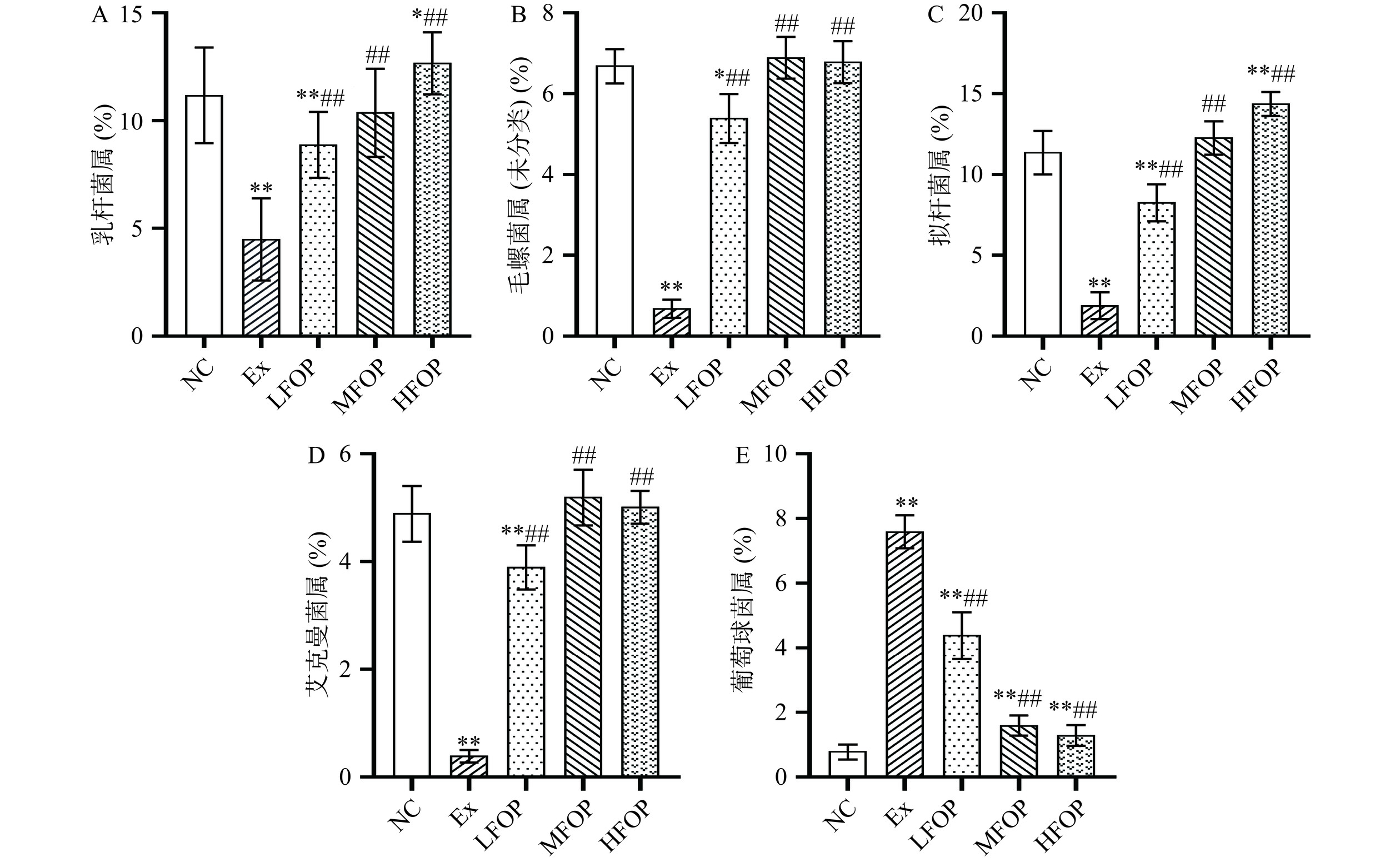
力竭运动可能导致肠道中抗炎菌群(如拟杆菌属、栖粪杆菌属和罗斯氏菌属)丰度减少,有害菌群丰度增加,且有害菌丰度与肠道通透性密切相关[35]。进一步对大鼠肠道菌群属水平上分析发现(图4),Ex组葡萄球菌属相对丰度极显著上升(P<0.01),乳杆菌属、毛螺菌属(未分类)、拟杆菌属及艾克曼菌属相对丰度极显著降低(P<0.01)。乳杆菌和艾克曼氏菌为机体肠道内有益菌,可通过激活巨噬细胞活化作用,增强巨噬细胞毒性效果,进而参与机体免疫调节过程[36]。毛螺菌属(未分类)为潜在有益菌,广泛参与多种碳水化合物的代谢调控过程,同时可能与宿主自身免疫稳态密切相关。葡萄球菌是抗菌物质中毒和病原微生物入侵条件下成为次级的微生物区系的主要细菌,可通过多种途径发起炎症反应并诱导免疫抑制[37]。可见,长期力竭运动可能通过抑制乳杆菌属、毛螺菌属(未分类)、拟杆菌属及艾克曼菌属等有益菌丰度诱导EIS反应。补充6周FOP后,LFOP、MFOP、HFOP组大鼠肠道乳杆菌属、拟杆菌属、毛螺菌属(未分类)及艾克曼菌属相对丰度较Ex组极显著上升(P<0.01),葡萄球菌属相对丰度较Ex组极显著降低(P<0.01)。表明FOP可促进EIS大鼠肠道有益菌生长,有效恢复长期力竭运动造成的肠道菌群变化,促进肠道菌群属水平结构平衡。
2.5 阿里红多糖对运动免疫抑制大鼠肠屏障功能的影响
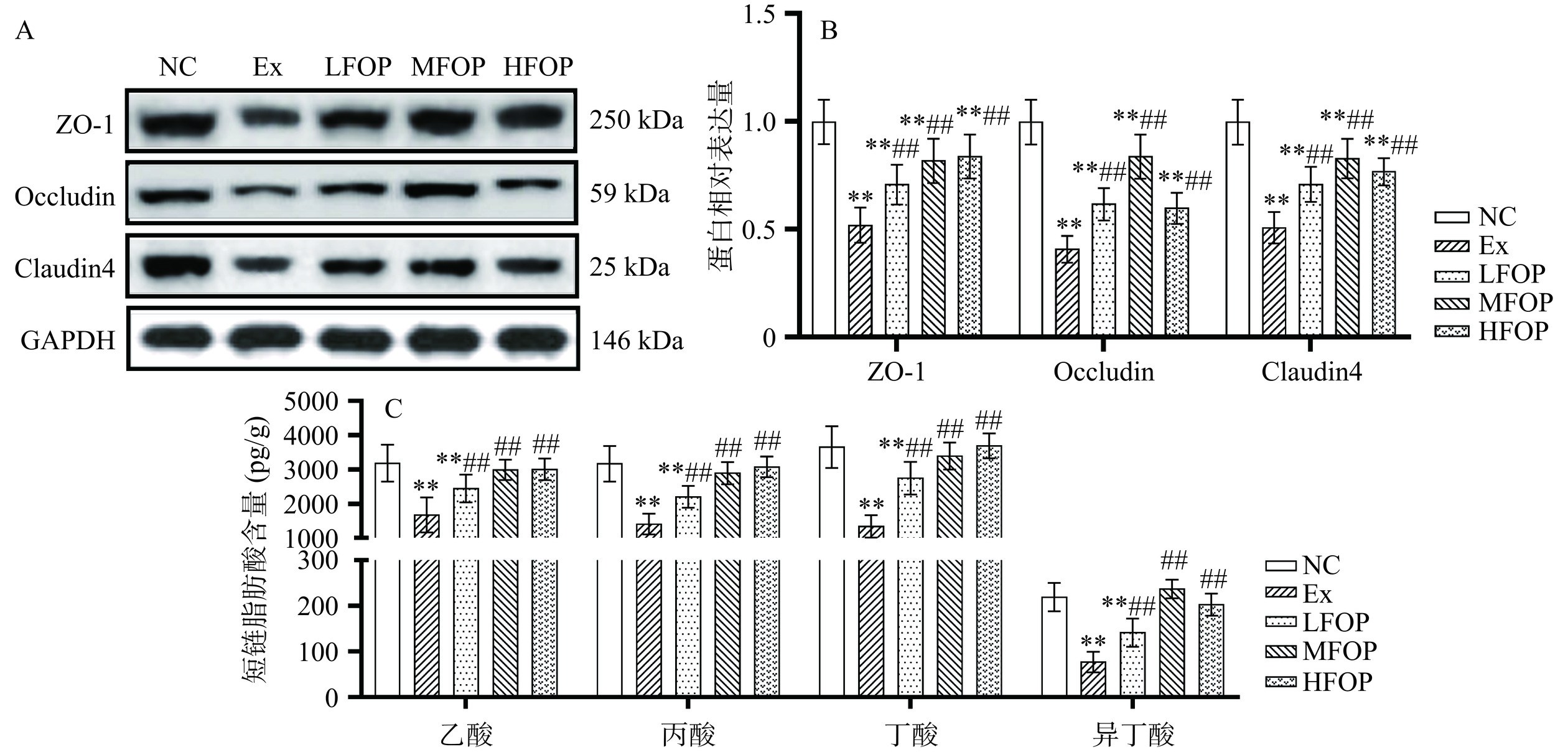
高强度运动刺激可通过发起肠道屏障损伤诱导致病性物质内毒素移位,进而引发广泛的肠易激反应[38]。紧密连接蛋白主要由ZO、Occludin及Claudins构成,其活性与机体肠道组织屏障功能密切相关[39]。Davison等[40]研究表明,14 d高强度运动训练可通过降低青年男性肠道上皮细胞Occludin、Claudin4蛋白表达及磷酸化水平增加肠道通透性。本研究发现(图5),与NC组比较,Ex组大鼠结肠组织ZO-1、Occludin、Claudin4蛋白相对表达量极显著下调(P<0.01),表明6周高强度运动训练提高大鼠肠道通透性并诱导肠屏障功能障碍。本结果与既往研究基本一致。补充6周FOP后,与Ex组比较,LFOP、MFOP、HFOP组大鼠结肠组织ZO-1、Occludin、Claudin4蛋白相对表达量极显著上调(P<0.01)。阿里红黄酮提取物可显著上调小鼠肠组织ZO、Occludin和Claudins的表达水平[41],但FOP对EIS肠道组织紧密连接蛋白的影响尚未明确。提示FOP可降低EIS大鼠肠道通透性并改善肠屏障功能。
长期高强度训练可抑制SCFAs合成并影响肠道细胞能量代谢和增殖能力调控机体肠道屏障功能,同时参与介导肠道菌群结构功能与机体免疫耐受性的调节过程[42]。Ruiz等[43]研究表明,高强度急性运动降低Wistar大鼠盲肠丙酸、丁酸及戊酸水平。如图5C所示,6周高强度运动训练后,与NC组比较,Ex组大鼠肠道组织乙酸、丙酸、丁酸及异丁酸水平均极显著降低(P<0.01)。上述结果表明,高强度运动训练可能与SCFAs水平呈负相关关系,其机制可能与剧烈运动后肠道通透性增加及肠道“渗漏”现象有关。补充6周FOP后,与Ex组比较,LFOP、MFOP、HFOP组大鼠肠道组织乙酸、丙酸、丁酸及异丁酸水平均极显著上调(P<0.01)。植物性多糖可能通过聚糖降解旁通路上调SCFAs生成并促进能源代谢物整合,从而改善机体整体免疫能力。陈亮[44]研究亦发现,植物性多糖提升免疫抑制小鼠肠道中乙酸、丙酸、丁酸和戊酸等SCFAs的含量。综上,FOP可能有效调节EIS大鼠肠道短链脂肪酸合成能力。
3. 结论
综上所述,6周力竭运动造成大鼠免疫功能抑制和炎症反应激活现象,同时诱导肠道菌群多态性、肠道菌群结构及肠道屏障功能紊乱。本研究中,FOP干预可有效改善EIS大鼠免疫功能、抑制炎症反应,提高肠道菌群相对多样性并促进肠道有益菌生长、抑制有害菌定殖,激活肠屏障功能及短链脂肪酸合成能力。因此,FOP可有效调节EIS大鼠肠道菌群及免疫功能。但其具体机制仍有待进一步研究,以期探析FOP通过调控肠道菌群结构,为防治EIS提供理论及实验依据。
-
表 1 各组大鼠血清IgG、IgM及T淋巴细胞亚群标志物(¯X±SD,n=10)
Table 1 Serum IgG, IgM, and T lymphocyte subpopulation markers of rats in each group (¯X±SD, n=10)
组别 IgG(μg/mL) IgM(μg/mL) CD4+(%) CD8+(%) CD4+/CD8+ NC组 12.51±2.39 136.85±16.11 48.17±4.22 27.63±3.45 1.73±0.20 Ex组 7.02±1.47** 67.88±8.05** 26.74±3.95** 16.86±3.72** 1.58±0.17* LFOP组 9.54±1.42**## 87.41±12.07**## 37.09±4.14**## 22.03±4.51**## 1.68±0.19# MFOP组 11.39±1.66## 120.59±10.50*## 44.51±5.06*## 25.95±3.83## 1.71±0.12# HFOP组 11.05±1.59## 109.64±14.7**## 43.24±4.67*## 23.67±5.02*## 1.83±0.14# 注:与NC组相比,**代表差异极显著(P<0.01),*代表差异显著(P<0.05);与Ex组相比,##代表差异极显著(P<0.01),#代表差异显著(P<0.05);表2同。 表 2 各组大鼠血清炎症反应标志物及脂多糖水平(¯X±SD,n=10)
Table 2 Serum inflammatory response markers and lipopolysaccharide levels in rats of each group (¯X±SD, n=10)
组别 IL-6(pg/mL) IL-10(pg/mL) INF-γ(pg/mL) TNF-α(pg/mL) LPS(ng/mL) NC组 124.57±10.71 73.45±8.70 590.23±78.15 35.41±5.07 30.55±4.72 Ex组 242.59±17.36** 148.62±15.63** 402.76±62.35** 21.57±4.93** 64.38±6.08** LFOP组 204.35±19.06**## 119.91±13.48**## 498.54±50.98**## 27.83±4.90**## 48.84±6.71**## MFOP组 160.31±16.58**## 91.53±11.34**## 533.09±68.47**## 32.16±4.44*## 37.46±5.53**## HFOP组 193.82±22.09**## 106.57±14.09**## 516.17±49.45**## 33.52±3.81*## 39.78±5.64**## -
[1] MOIR H, HUGHES G, POTTER S, et al. Exercise-induced immunosuppression:roles of reactive oxygen species and 5'-AMP-activated protein kinase dephosphorylation within immune cells[J]. Journal of Applied Physiology,2010,108(5):1284−1292. doi: 10.1152/japplphysiol.00737.2009
[2] XIAO W, CHEN P, LIU X, et al. The impaired function of macrophages induced by strenuous exercise could not be ameliorated by BCAA supplementation[J]. Nutrients,2015,7(10):8645−8656. doi: 10.3390/nu7105425
[3] 覃飞, 赵杰修, 王松涛, 等. 低强度激光对运动性免疫抑制大鼠的干预效果研究[J]. 中国体育科技,2019,55(7):71−80. [QIN F, ZHAO J X, WANG S T, et al. The effect of low power laser on exercise-induced immunosuppression in rats[J]. China Sport Science and Technology,2019,55(7):71−80.] QIN F, ZHAO J X, WANG S T, et al. The effect of low power laser on exercise-induced immunosuppression in rats[J]. China Sport Science and Technology, 2019, 55(7): 71−80.
[4] WANG J, ZHU N, SU X, et al. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis[J]. Cells,2023,12(5):793. doi: 10.3390/cells12050793
[5] HUGHES L, HOLSCHER D. Fueling gut microbes:A review of the interaction between diet, exercise, and the gut microbiota in athletes[J]. Advances in Nutrition,2021,12(6):2190−2215. doi: 10.1093/advances/nmab077
[6] BERMON S, PETRIZ B, KAJÉNIENÉ A, et al. The microbiota:an exercise immunology perspective[J]. Exercise Immunology Review,2015,21(5):70−79.
[7] 孟庆龙, 金莎, 刘雅婧, 等. 植物多糖药理功效研究进展[J]. 食品工业科技,2020,41(11):335−341. [MENG Qinglong, JIN Sha, LIU Yajing, et al. Research progress in pharmacological efficacy of plant polysaccharides[J]. Science and Technology of Food Industry,2020,41(11):335−341.] MENG Qinglong, JIN Sha, LIU Yajing, et al. Research progress in pharmacological efficacy of plant polysaccharides[J]. Science and Technology of Food Industry, 2020, 41(11): 335−341.
[8] 赵人杰, 白芯嫣, 项璐, 等. 大蒜多糖对健康小鼠肠道菌群及免疫功能的影响[J]. 中国食品学报,2023,23(8):132−141. [ZHAO Renjie, BAI Xinyan, XIANG Lu, et al. Effects of garlic polysaccharide on intestinal flora and immune function of healthy mice[J]. Journal of Chinese Institute of Food Science and Technology,2023,23(8):132−141.] ZHAO Renjie, BAI Xinyan, XIANG Lu, et al. Effects of garlic polysaccharide on intestinal flora and immune function of healthy mice[J]. Journal of Chinese Institute of Food Science and Technology, 2023, 23(8): 132−141.
[9] 米仁沙·牙库甫, 祖丽胡玛尔·阿卜杜合力力, 木尼萨·迪力夏提, 等. 阿里红多糖组分对小鼠脾淋巴细胞免疫功能的影响[J]. 食品安全质量检测学报,2018,9(16):4369−4374. [ISHAREN Yakufu, ZULIHUMAER Abuduhelili, MUNISA Dilixiati, et al. Effects of polysaccharide from Fomes officinals Ames on immune function of murine splenic lymphocytes[J]. Journal of Food Safety & Quality,2018,9(16):4369−4374.] ISHAREN Yakufu, ZULIHUMAER Abuduhelili, MUNISA Dilixiati, et al. Effects of polysaccharide from Fomes officinals Ames on immune function of murine splenic lymphocytes[J]. Journal of Food Safety & Quality, 2018, 9(16): 4369−4374.
[10] 沙依拜·沙比提, 丛媛媛, 古丽尼歌尔·阿布都米吉提, 等. 阿里红多糖体内抗肿瘤作用及其机制研究[J]. 中国实验动物学报,2021,29(2):197−203. [SHAYIBAI Shabiti, CONG Yuanyuan, GULINIGEER Abudumijiti, et al. In vivo anti-tumor activities of Fomes officinalis polysaccharide and the underlying mechanisms[J]. Acta Laboratorium Animalis Scientia Sinica,2021,29(2):197−203.] SHAYIBAI Shabiti, CONG Yuanyuan, GULINIGEER Abudumijiti, et al. In vivo anti-tumor activities of Fomes officinalis polysaccharide and the underlying mechanisms[J]. Acta Laboratorium Animalis Scientia Sinica, 2021, 29(2): 197−203.
[11] 帕丽达·阿不力孜, 如斯塔木·托合尼牙孜, 丛媛媛, 等. 阿里红多糖抗衰老作用研究[J]. 中华中医药杂志,2013,28(2):340−342. [PALIDA Abulizi, ROUSITAMU Tuoheniyazi, CONG Yuanyuan, et al. Study on the anti-aging effect of Fomes officinalis Ames polysaccharides[J]. China Journal of Traditional Chinese Medicine and Pharmacy,2013,28(2):340−342.] PALIDA Abulizi, ROUSITAMU Tuoheniyazi, CONG Yuanyuan, et al. Study on the anti-aging effect of Fomes officinalis Ames polysaccharides[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2013, 28(2): 340−342.
[12] 木妮然·吐尔逊江, 依明·尕哈甫, 丛媛媛, 等. 阿里红多糖对Aβ_(25-35)诱导的小胶质细胞炎症反应的影响[J]. 食品安全质量检测学报,2018,9(22):5943−5948. [MUNIRAN Tuerxunjiang, YIMING Gahafu, CONG Yuanyuan, et al. Effects of Fomes officinais Ames polysaccharides on Aβ_(25-35) induced inflammatory response of microglia[J]. Journal of Food Safety & Quality,2018,9(22):5943−5948.] MUNIRAN Tuerxunjiang, YIMING Gahafu, CONG Yuanyuan, et al. Effects of Fomes officinais Ames polysaccharides on Aβ_(25-35) induced inflammatory response of microglia[J]. Journal of Food Safety & Quality, 2018, 9(22): 5943−5948.
[13] 王凤华, 孔海军, 黄玲. 维药阿里红多糖对运动性免疫抑制改善的作用机制研究[J]. 世界科学技术-中医药现代化,2018,9(22):5943−5948. [WANG Fenghua, KONG Haijun, HUANG Ling, et al. Effect of inhibition of Uygur Fomes officinalis polysaccharides on antioxidant capacity and exercise immunity[J]. Journal of Food Safety & Quality,2018,9(22):5943−5948.] WANG Fenghua, KONG Haijun, HUANG Ling, et al. Effect of inhibition of Uygur Fomes officinalis polysaccharides on antioxidant capacity and exercise immunity[J]. Journal of Food Safety & Quality, 2018, 9(22): 5943−5948.
[14] NIEMAN C, SCHERR J, LUO B, et al. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists:A randomized, crossover trial[J]. PLoS One,2014,9(11):e113725. doi: 10.1371/journal.pone.0113725
[15] 孔海军, 王凤华, 孔令生, 等. 维药阿里红多糖对运动疲劳小鼠Th1/Th2平衡及Foxp3 Scurfin蛋白表达调控研究[J]. 食品与药品,2017,19(5):305−309. [KONG Haijun, WANG Fenghua, KONG Lingsheng, et al. Effects of polysaccharide from Fomes officinalis Ames on Th1/Th2 balance and Foxp3 scurfin expression in exercise-induced physical fatigue mice[J]. Food and Drug,2017,19(5):305−309.] KONG Haijun, WANG Fenghua, KONG Lingsheng, et al. Effects of polysaccharide from Fomes officinalis Ames on Th1/Th2 balance and Foxp3 scurfin expression in exercise-induced physical fatigue mice[J]. Food and Drug, 2017, 19(5): 305−309.
[16] 如斯塔木·托合尼牙孜, 赛力曼·哈德尔, 刘纪杉, 等. 不同产地阿里红多糖的含量比较研究[J]. 中华中医药杂志,2012,27(10):2565−2567. [RUSITAMU Tuoheniyazi, SAILIMAN Hadeer, LIU Jibin, et al. Comparative study on the polysaccharide contents of Fomes officinalis Ames from different regions[J]. China Journal of Traditional Chinese Medicine and Pharmacy,2012,27(10):2565−2567.] RUSITAMU Tuoheniyazi, SAILIMAN Hadeer, LIU Jibin, et al. Comparative study on the polysaccharide contents of Fomes officinalis Ames from different regions[J]. China Journal of Traditional Chinese Medicine and Pharmacy, 2012, 27(10): 2565−2567.
[17] 金其贯, 胡要娟, 金爱娜, 等. 不同模式的低氧运动训练对大鼠肠道体液免疫功能的影响[J]. 中国运动医学杂志,2015,34(8):764−769. [JIN Qiguan, HU Yaojuan, JIN Aina, et al. Effect of different modes of hypoxic training on the humoral immune function of intestine in rats[J]. Chinese Journal of Sports Medicine,2015,34(8):764−769.] JIN Qiguan, HU Yaojuan, JIN Aina, et al. Effect of different modes of hypoxic training on the humoral immune function of intestine in rats[J]. Chinese Journal of Sports Medicine, 2015, 34(8): 764−769.
[18] DIRKSEN C, HANSEN BR, KOLTE L, et al. T-lymphocyte subset dynamics in well-treated HIV-infected men during a bout of exhausting exercise[J]. Infect Dis (Lond),2015,47(12):919−923. doi: 10.3109/23744235.2015.1069392
[19] 沙依拜·沙比提, 米仁沙·牙库甫, 古丽尼歌尔·阿布都米吉提, 等. 阿里红多糖对S_(180)荷瘤小鼠肿瘤生长的影响[J]. 中药材,2021,44(7):1744−1748. [SHAYIBAI Shabiti, MIRENSHA Yakufu, GULINIGEER Abudumijiti, et al. The effect of Fomes officinalis Ames polysaccharides on tumor growth in S180 tumor bearing mice[J]. Journal of Chinese Medicinal Materials,2021,44(7):1744−1748.] SHAYIBAI Shabiti, MIRENSHA Yakufu, GULINIGEER Abudumijiti, et al. The effect of Fomes officinalis Ames polysaccharides on tumor growth in S180 tumor bearing mice[J]. Journal of Chinese Medicinal Materials, 2021, 44(7): 1744−1748.
[20] 姜一弘, 张丹, 张天择, 等. 核因子κB(NF-κB)信号通路在炎症与肿瘤中作用的研究进展[J]. 细胞与分子免疫学杂志,2018,34(12):1130−1135. [JIANG Yihong, ZHANG Dan, ZHANG Tianze, et al. Nuclear factor-κB (NF-κB) research progress on the role of signaling pathways in inflammation and tumors[J]. Chinese Journal of Cellular and Molecular Immunology,2018,34(12):1130−1135.] JIANG Yihong, ZHANG Dan, ZHANG Tianze, et al. Nuclear factor-κB (NF-κB) research progress on the role of signaling pathways in inflammation and tumors[J]. Chinese Journal of Cellular and Molecular Immunology, 2018, 34(12): 1130−1135.
[21] CANTINI N, SCHEPETKIN I A, DANILENKO N V, et al. Pyridazinones and structurally related derivatives with anti-inflammatory activity[J]. Molecules,2022,27(12):3749. doi: 10.3390/molecules27123749
[22] VAN N D, NGUYEN T L L, JIN Y, et al. 6'-Sialylactose abolished lipopolysaccharide-induced inflammation and hyper-permeability in endothelial cells[J]. Arch Pharm Res,2022,45(11):836−848. doi: 10.1007/s12272-022-01415-0
[23] DONG N, LI X, XUE C, et al. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-kappaB/MAPK signaling pathway[J]. J Cell Physiol,2020,235(7-8):5525−5540. doi: 10.1002/jcp.29452
[24] CIESIELSKA A, MATYJEK M, KWIATKOWSKA K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling[J]. Cell Mol Life Sci,2021,78(4):1233−1261. doi: 10.1007/s00018-020-03656-y
[25] YATOO M I, GOPALAKRISHNAN A, SAXENA A, et al. Anti-Inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-A review[J]. Recent Pat Inflamm Allergy Drug Discov,2018,12(1):39−58. doi: 10.2174/1872213X12666180115153635
[26] CLARK A, MACH N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet:A systematic review for athletes[J]. Journal of the International Society of Sports Nutrition,2016,13(11):43.
[27] CAI Y, LIU Y, WU Z, et al. Effects of diet and exercise on circadian rhythm:Role of gut microbiota in immune and metabolic systems[J]. Nutrients,2023,15(12):2743. doi: 10.3390/nu15122743
[28] 胡晓燕, 郝选明. 补充黄芪多糖对递增负荷训练大鼠免疫指标的影响[J]. 武汉体育学院学报,2012,46(2):59−64. [HU Xiaoyan, HAO Xuanming. Effects of Astragalus polysaccharide intaking on immune index of rats during long-term incremental exercise[J]. Journal of Wuhan Institute of Physical Education,2012,46(2):59−64.] HU Xiaoyan, HAO Xuanming. Effects of Astragalus polysaccharide intaking on immune index of rats during long-term incremental exercise[J]. Journal of Wuhan Institute of Physical Education, 2012, 46(2): 59−64.
[29] 李晓勇. 灵芝多糖对运动疲劳及运动性免疫抑制影响[J]. 中国食用菌,2020,39(2):45−48. [LI Xiaoyong. Effect of Ganoderma lucidum polysaccharide on exercise fatigue and exercise-induced immunosuppression[J]. Edible Fungi of China,2020,39(2):45−48.] LI Xiaoyong. Effect of Ganoderma lucidum polysaccharide on exercise fatigue and exercise-induced immunosuppression[J]. Edible Fungi of China, 2020, 39(2): 45−48.
[30] MOTIANI K, COLLADO C, ESKELINEN J, et al. Exercise training modulates gut microbiota profile and improves endotoxemia[J]. Medicine & Science in Sports & Exercise,2020,52(1):94−104.
[31] MAO Y H, WANG M, YUAN Y, et al. Konjac glucomannan counteracted the side effects of excessive exercise on gut microbiome, endurance, and strength in an overtraining mice model[J]. Nutrients,2023,15(19):4206. doi: 10.3390/nu15194206
[32] 郭慧玲, 邵玉宇, 孟和毕力格, 等. 肠道菌群与疾病关系的研究进展[J]. 微生物学通报,2015,42(2):400−410. [GUO Huiling, SHAO Yuyu, MENGHEBILIGE, et al. Research on the relation between gastrointestinal microbiota and disease[J]. Microbiology,2015,42(2):400−410.] GUO Huiling, SHAO Yuyu, MENGHEBILIGE, et al. Research on the relation between gastrointestinal microbiota and disease[J]. Microbiology, 2015, 42(2): 400−410.
[33] ZHAI Q, FENG S, ARJAN N, et al. A next generation probiotic, Akkermansia muciniphila[J]. Critical Reviews in Food Science and Nutrition,2019,59(19):3227−3236. doi: 10.1080/10408398.2018.1517725
[34] TANG C, DING R, SUN J, et al. The impacts of natural polysaccharides on intestinal microbiota and immune responses-a review[J]. Food & Function,2019,10(5):2290−2312.
[35] BONOMINI R, PLAZA J, JORQUERA C, et al. Effect of intensity and duration of exercise on gut microbiota in humans:A systematic review[J]. Int J Environ Res Public Health,2022,19(15):9518. doi: 10.3390/ijerph19159518
[36] 林璋, 祖先鹏, 谢海胜, 等. 肠道菌群与人体疾病发病机制的研究进展[J]. 药学学报,2016,51(6):843−852. [LIN Zhang, ZU Xianpeng, XIE Haisheng, et al. Research progress in mechanism of intestinal microorganisms in human diseases[J]. Acta Pharmaceutica Sinica,2016,51(6):843−852.] LIN Zhang, ZU Xianpeng, XIE Haisheng, et al. Research progress in mechanism of intestinal microorganisms in human diseases[J]. Acta Pharmaceutica Sinica, 2016, 51(6): 843−852.
[37] KELLY A M, LEECH J M, DOYLE S L, et al. Staphylococcus aureus-induced immunosuppression mediated by IL-10 and IL-27 facilitates nasal colonisation[J]. PLoS Pathog,2022,18(7):e1010647. doi: 10.1371/journal.ppat.1010647
[38] MCKENNA J, GORINI F, GILLUM L, et al. High-altitude exposures and intestinal barrier dysfunction[J]. American Journal of Physiology-regulatory Integrative and Comparative Physiology,2022,322(3):192−203. doi: 10.1152/ajpregu.00270.2021
[39] 袁榴翼, 李小锦, 尹清晟, 等. 中药干预肠道菌群改善肠黏膜屏障功能的研究进展[J]. 中草药,2018,49(8):1932−1938. [YUAN Liuyi, LI Xiaojin, YIN Qingsheng, et al. Research progress on Chinese materia medica intervening intestinal flora to improve intestinal mucosal barrier function[J]. Acta Pharmaceutica Sinica,2018,49(8):1932−1938.] YUAN Liuyi, LI Xiaojin, YIN Qingsheng, et al. Research progress on Chinese materia medica intervening intestinal flora to improve intestinal mucosal barrier function[J]. Acta Pharmaceutica Sinica, 2018, 49(8): 1932−1938.
[40] DAVISON G, MARCHBANK T, MARCH D S, et al. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers[J]. Am J Clin Nutr,2016,104(2):526−536. doi: 10.3945/ajcn.116.134403
[41] 沙爱龙. 阿里红黄酮对衰老模型小鼠的抗衰老作用[J]. 中国应用生理学杂志,2016,32(2):121−123,127. [SHA Ailong. Effects of the Fomes officinalis flavonoids on anti-senile action in the aging model mice[J]. Chinese Journal of Applied Physiology,2016,32(2):121−123,127.] SHA Ailong. Effects of the Fomes officinalis flavonoids on anti-senile action in the aging model mice[J]. Chinese Journal of Applied Physiology, 2016, 32(2): 121−123,127.
[42] MARTIN C, MARINELLI L, BLOTTIÈRE M, et al. SCFA:Mechanisms and functional importance in the gut[J]. Proceedings of the Nutrition Society,2021,80(1):37−49. doi: 10.1017/S0029665120006916
[43] RUIZ P, MASSOT M, RODRÍGUEZ M J, et al. A cocoa diet can partially attenuate the alterations in microbiota and mucosal immunity induced by a single session of intensive exercise in rats[J]. Front Nutr,2022,34(9):861533.
[44] 陈亮. 鼠李糖乳杆菌胞外多糖的分离纯化及其免疫调节作用研究[D]. 杭州:浙江工商大学, 2023:35−37. [CHEN Liang. Isolation and purification of exopolysaccharide from Lactobacillus rhamnosus and its immunomodulatory effects[D]. Hangzhou:Zhejiang Gongshang University, 2023:35−37.] CHEN Liang. Isolation and purification of exopolysaccharide from Lactobacillus rhamnosus and its immunomodulatory effects[D]. Hangzhou: Zhejiang Gongshang University, 2023: 35−37.
-
期刊类型引用(1)
1. 刘馨泽,冯琳,孙恺婧,孙莹,杨雪,李光哲,吴巍,陈长宝,李玉,金鑫,万茜淋. 维药苦白蹄的本草考证及现代药理学分析. 生物技术进展. 2024(06): 920-928 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:
 下载:
下载:



