Effects of Matrices on the Structure and Properties of Composite Films of Soy Protein Isolates/Xanthan Gum
-
摘要: 本文研究大豆分离蛋白(SPI)和黄原胶(XG)与不同基底添加物成膜的结构和性能。以SPI和XG为基材,加入海藻酸钠(SA)、羧甲基纤维素(CMC)、明胶(GEL)、果胶(PEC)和琼脂(Agar)制备复合膜,对复合膜厚度、机械性能、透氧性和不透明度进行测定,并对其结构进行表征。结果表明,添加1.5 g不同添加物的复合膜厚度显著增高(P<0.05),拉伸强度最大为SPI/XG-Agar膜5.90±0.32 MPa(P<0.05),透氧性显著降低(P<0.05),不透明度最低为SPI/XG-Agar膜0.45±0.09 Abs600/mm(P<0.05)。SEM显示,SPI/XG-Agar复合膜表面平坦光滑且无颗粒,而其他复合膜表面有颗粒和褶皱。红外光谱显示,基材之间均有良好的相容性。SPI/XG-Agar复合膜的拉伸强度、不透明度及SEM结果优于其他复合膜。Abstract: The structures and properties of the composite films formed by soy protein isolate (SPI) and xanthan gum (XG) with different other matrices were investigated. The composite films were prepared with SPI and XG by adding sodium alginate (SA), carboxymethyl cellulose (CMC), gelatin (GEL), pectin (PEC) and agar (Agar), respectively. The thickness, mechanical properties, oxygen permeability and opacity of the composite films were measured, and their structures were characterized. The results showed that the thicknesses of composite films with 1.5 g of different other matrices were significantly higher (P<0.05) than the film of SPI/XG, and the maximum tensile strength was 5.90±0.32 MPa for SPI/XG-Agar film (P<0.05), the oxygen permeabilities of them were significantly reduced (P<0.05), and the lowest opacity was 0.45±0.09 Abs600/mm for SPI/XG-Agar film (P<0.05). The photograph of SEM showed that the surface of SPI/XG-Agar composite film was flat, smooth and free of particles, while the other composite films were with particles and folds on the surface. The infrared spectroscopy analysis showed that there was good compatibility between all the matrices. The results of tensile strength, opacity and SEM for the SPI/XG-Agar film were superior to other films.
-
Keywords:
- soy protein isolate /
- xanthan gum /
- composite film /
- mechanical property /
- matrices
-
大豆分离蛋白(Soy protein isolate,SPI)、黄原胶(Xanthan gum,XG)、海藻酸钠(Sodium alginate,SA)、羧甲基纤维素(Carboxymethylcellulose,CMC)、明胶(Gelatin,GEL)、果胶(Pectin,PEC)和琼脂(Agar)均是良好的成膜材料。SPI具有质优价廉、来源丰富、成膜良好等特点,其分子结构为紧密卷曲的球状,在水中相对稳定,但SPI膜具有机械强度低、阻隔性能较差、易溶于水、容易吸湿[1],且易滋生细菌的特点,导致其应用十分有限[2−4]。XG又称汉生胶,是一种由革兰氏阴性菌弯曲黄单胞菌NRRL B-1459产生的胞外多糖,可作为稳定剂、增稠剂和乳化剂等[5],然而关于SPI和XG复合膜的研究较少。SA是从褐藻或马尾藻中提取的一种天然多糖物质,具有良好的生物降解性、凝胶性、成膜性和相容性[6]。可与蛋白、多糖和脂类等大分子物质复合制成具有良好性能的复合膜[7]。Zhu等[8]在SPI/SA复合膜的基础上研究不同添加量的硬脂酸对复合膜的影响,发现硬脂酸能改善膜的水蒸气渗透性和接触角,但断裂伸长率、水蒸气透过率显著下降。CMC具有良好水溶性[9],它由一个疏水多糖主链和亲水羧基组成,使其具有两亲性[10]。Han等[11]在SPI的基础上添加CMC,使复合膜具有更强的拉伸强度,并降低了水蒸气渗透性,但透明度显著下降。GEL经胶原蛋白水解而成,三股螺旋结构经水解后变为单链分子。在其结构中可以观察到大量的氨基(-NH2)、羧基(-COOH)和羟基(-OH)[12],使GEL膜具有很强的机械和阻隔性能,其结构中存在的不同氨基酸容易吸收紫外辐射并保护包装食品免受氧化损伤[13]。Bai等[14]研究不同添加比的GEL和SPI对复合膜的影响,发现在SPI基质中加入10%~30%的明胶,薄膜具有良好的视觉性能、高表面疏水性、力学性能和显著的隔水性,但复合膜仍然具有高度的水敏感性。PEC是一种存在于自然界中的天然可溶性的复杂的阴离子多糖,广泛存在于植物的初生细胞层和细胞壁[15],常用作胶凝剂、稳定剂及饮品中的增稠剂[16],且来源广泛。Amado等[17]研究SPI与不同高氧基PEC及pH对复合膜的影响,表明随着高甲氧基PEC的添加比例增高,改善了复合膜的机械性能,但复合膜的水溶性增加。Agar是红藻类(红藻科)的胶状产物,是加工相同主链结构的相关多糖的异质复杂混合物,具有可再生性和生物降解性[18−19],以及巨大的胶凝能力,是一种安全无毒,且成膜性好的可食性多糖。Tian等[20]将Agar加入到SPI中制膜,发现Agar与SPI之间存在氢键相互作用,具有优越的力学性能,但随着Agar的添加比例增加,复合膜表面出现的褶皱越多。SPI与XG以及SA、CMC、GEL、PEC、Agar都具有良好的相容性,但单一基质或两者结合制成的复合膜均无法满足食品包装要求。将三种成膜基材结合起来,利用其相互作用可提高复合膜的相关性能。
目前,物理、化学、酶法、纳米改性方法存在操作复杂、安全性低和成本高等问题,二元复合膜仍有结构和性能的不足,而三元复合制备复合膜的研究鲜有报道。本研究以SPI和XG为基材,分别与SA、CMC、GEL、PEC、Agar制备三元共混改性复合膜,并对其进行结构和性能测定,以揭示基材之间的相互作用以及对复合膜的性能影响,以期为SPI膜应用发展提供理论基础。
1. 材料与方法
1.1 材料与仪器
SPI(蛋白质含量≥90%) 购自哈尔滨高科技大豆食品有限公司;黄原胶 购自国药集团化学试剂有限公司;焦磷酸钠、甘油 购自上海迈瑞尔技术有限公司;海藻酸钠 购自成都市科龙化工试剂厂;羧甲基纤维素 购自天津市元力化工有限公司;明胶 购自上海明胶厂;果胶 购自北京索莱宝科技有限公司;琼脂 购自中国北京兰杰柯科技有限公司。
STA449F3同步热分析仪 德国NETZSCH公司;Nexus 870红外光谱仪 美国Nicolet仪器公司;ESCALAB 250Xi X射线光电子能谱仪 中国赛默飞世尔科技公司;JSM-5600LV扫描电子显微镜 日本电子光学公司;AD300L-H高速分散均质机 中国上海昂尼仪器仪表有限公司;102-2A电热鼓风干燥箱 中国北京科伟永兴仪器有限公司。
1.2 实验方法
1.2.1 不同基底材料复合膜的制备
按一定比例分别称取1.5 g SPI、0.018 g的黄原胶、2 g甘油和0.009 g的焦磷酸钠,加入70 mL去离子水,再分别加入1.5 g的SA、CMC、GEL、PEC和Agar,用高速分散均质机8000 r/min搅拌5 min,得到均匀的混合溶液。将混合溶液置于100 ℃烘箱烘烤1 h,取出放至室温,使用超声细胞破碎仪超声处理,功率360 W,超声30 min,得到均匀透明的膜液,在培养皿中倒入20 mL膜液均匀流延在培养皿上成膜,膜厚度约0.1~0.5 mm,放置在50 ℃烘箱干燥48 h成膜。揭下的复合膜放入真空袋中密封,室温下保存,备用。
1.2.2 复合膜物理性能测定
1.2.2.1 复合膜厚度测定
参考Liu等[21]的方法测定膜厚度。使用千分尺随机测量膜样品上10个位置的厚度,精确到0.001 mm,计算平均值。
1.2.2.2 复合膜机械性能测定
参考Rachtanapun等[22]的方法稍作修改,测量薄膜的拉伸强度(Tensile stress,TS)和断裂伸长率(Elongation at break,EAB),将膜切成3.2×9.5 cm的条带,初始夹具距离为40 mm,以30 mm/min的速度进行拉伸,至复合膜破裂。TS和EAB的计算公式如下:
TS(MPa)=FD×L (1) 式中:TS为拉伸强度,MPa;F为最大力,N;D为膜的厚度,mm;L为样品膜宽度,mm。
EAB(%)=L−L0L0×100 (2) 式中:EAB为断裂伸长率,%;Lo为薄膜的最初长度,mm;L为薄膜拉伸断裂前的最终长度,mm。
1.2.2.3 复合膜含水率及水溶性测定
参考Chen等[23]的方法测定复合膜的含水率(Moisture content,MC)及水溶性(Water solubility,WS)。计算公式如下:
MC(%)=M1−M2M1×100 (3) 式中:M1为复合膜初始重量;M2为烘干后复合膜重量。
WS(%)=W1−W2W1×100 (4) 式中:W1为烘干后复合膜重量;W2为浸泡烘干后复合膜重量。
1.2.2.4 复合膜水蒸气透过率测定
参考Li等[24]的方法稍作修改,称取5 g无水CaCl2装入40 mm×25 mm称量瓶中,用橡皮筋将复合膜固定于瓶口处,测定膜的水蒸气透过率(Water vapor permeability,WVP)。计算公式如下:
WVP(g⋅mm⋅m−2⋅s−1⋅Pa−1)=Δm×dA×Δp×t (5) 式中:WVP为水蒸气透过率;∆m为水分透过质量,g;d为膜厚,mm;A为水蒸气透过的面积,m2;t为间隔时间,s;∆p为膜两侧的压强差,∆p=3179 Pa。
1.2.2.5 复合膜透氧性测定
参考Zhou等[25]的方法测定复合膜的透氧性(Oxygen permeability,OP)。计算公式如下:
OP(g⋅s−1⋅m−2)=mt−m0t×A (6) 式中:OP为复合膜的透氧性;mt为薄膜的最终质量,g;m0为初始质量,g;t为时间间隔,s;A为称量瓶瓶口面积,m2。
1.2.2.6 复合膜透光率测定
用紫外-可见分光光度计检测样品的透光率,扫描范围为200~800 nm。
1.2.2.7 复合膜颜色
参考Yong等[26]的方法测定复合膜的色差。计算公式如下:
ΔE=√ΔL∗2+Δa∗2+Δb∗2 (7) 式中:ΔL*=L*−L0*、Δa*=a*−a0*、Δb*=b*−b0*;L*、a*、b*为不同基底复合膜的值;L0*、a0*、b0*为标准白板的值。
1.2.2.8 复合膜不透明度测定
参照Liu等[27]的方法测定复合膜的不透明度(Opacity,O)。计算公式如下:
O(Abs600/mm)=Abs600d (8) 式中,O为不透明度;Abs600为复合膜在600 nm处的吸光度;d为膜的厚度,mm。
1.2.2.9 复合膜热重分析
参考Riahi等[28]的方法,精确称量6 mg样品置于陶瓷坩埚中,以10 ℃/min的加热速率从30 ℃升温至600 ℃,以氮气作为保护气体。
1.2.3 复合膜结构的表征
1.2.3.1 复合膜扫描电镜分析
参考Salarbashi等[29]的方法对复合膜进行扫描电镜分析。测试条件:抽真空30 s,喷金20 s,加速电压为20 kV,放大倍数2000×。
1.2.3.2 复合膜傅里叶变换红外光谱分析
参考Vicentini等[30]的方法使用傅里叶变换红外光谱仪对薄膜进行光谱表征,扫描范围设置400~4000 cm−1,每个样品重复扫描32次,分辨率为0.1 cm−1。
1.2.3.3 复合膜X-射线衍射分析
参考Huang等[31]的方法对复合膜进行X-射线衍射分析。测试条件:模式为2θ/θ,步长为0.02°/min,扫描范围为5°~100°。
1.3 数据处理
实验数据表示为平均值±标准偏差(Mean±SD),数据处理和分析使用IBM SPSS 23.0和Excel进行。采用单因素方差分析(ANOVA)来检验实验结果。P<0.05,为差异有统计学意义。使用Origin 2023 b绘图,每组实验重复3次。
2. 结果与分析
2.1 复合膜物理性能
2.1.1 薄膜的厚度及机械性能
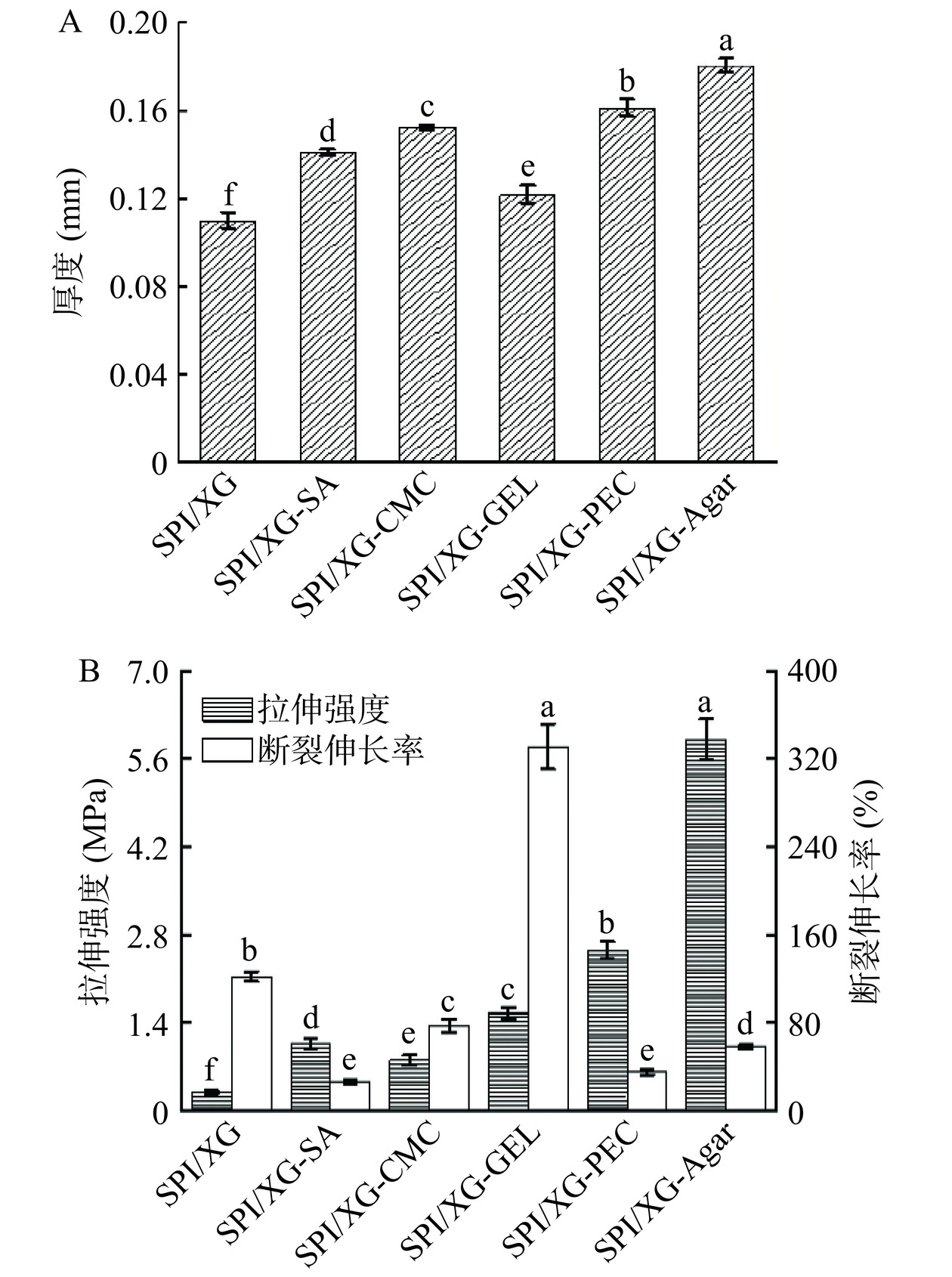
厚度是影响食品包装膜的机械性能和水蒸气透过率的重要因素[26]。如图1A可知,SPI/XG膜的厚度最小,SPI/XG-Agar膜的厚度最大,加入不同基底材料后,复合膜的厚度都显著增加(P<0.05),厚度大小顺序为SPI/XG-Agar膜>SPI/XG-PEC膜>SPI/XG-CMC膜>SPI/XG-SA膜>SPI/XG-GEL膜>SPI/XG膜,这表明膜的厚度主要与成膜基质有关,造成不同复合膜厚度之间的差异可能是不同基材与SPI/XG的胶体性质以及组分之间的相互作用[32]。
食品包装薄膜需要优良的性质来保证食品的运输及安全性,TS和EAB是衡量机械性能的重要参数,可用于评价包装膜的实用性[33]。由图1B可知,不同基底材料的复合膜的TS和EAB具有显著差异(P<0.05)。不同基底复合膜的TS大小依次为SPI/XG-Agar膜>SPI/XG-PEC膜>SPI/XG-GEL膜>SPI/XG-SA膜>SPI/XG-CMC膜>SPI/XG膜。膜的TS大小与膜分子结晶结构以及氢键有着密切的关系[34]。SPI/XG-Agar膜表现出最高的抗拉强度,其抗拉强度的提高可能是由于SPI与Agar之间分子间氢键的形成,以及复合膜紧凑均匀的三维结构,而其EAB相较于其他基底材料低的原因可能是Agar占据了更多的空隙位点,减少了水分子与基质的交联[35]。而SPI/XG膜的TS最低可能是因为没有添加其他基材相互作用,导致拉伸强度低。从EAB来看,SPI/XG-GEL膜的EAB最大,而SPI/XG-SA膜的EAB最低。不同基底复合膜的EAB大小顺序为SPI/XG-GEL膜>SPI/XG膜>SPI/XG-CMC膜>SPI/XG-Agar膜>SPI/XG-PEC膜>SPI/XG-SA膜,其中SPI/XG-GEL膜的EAB最大,可能是GEL大分子松弛,SPI/XG与GEL相互作用的结果[36−37]。SPI/XG-SA膜EAB最低可能与MC的降低和蛋白质链间空间位阻相互作用的增加有关,原因是SPI和海藻酸钠能够形成良好的网络结构,增强了蛋白质链之间的空间位阻,这导致EAB降低[38]。
2.1.2 复合膜的含水率及水溶性
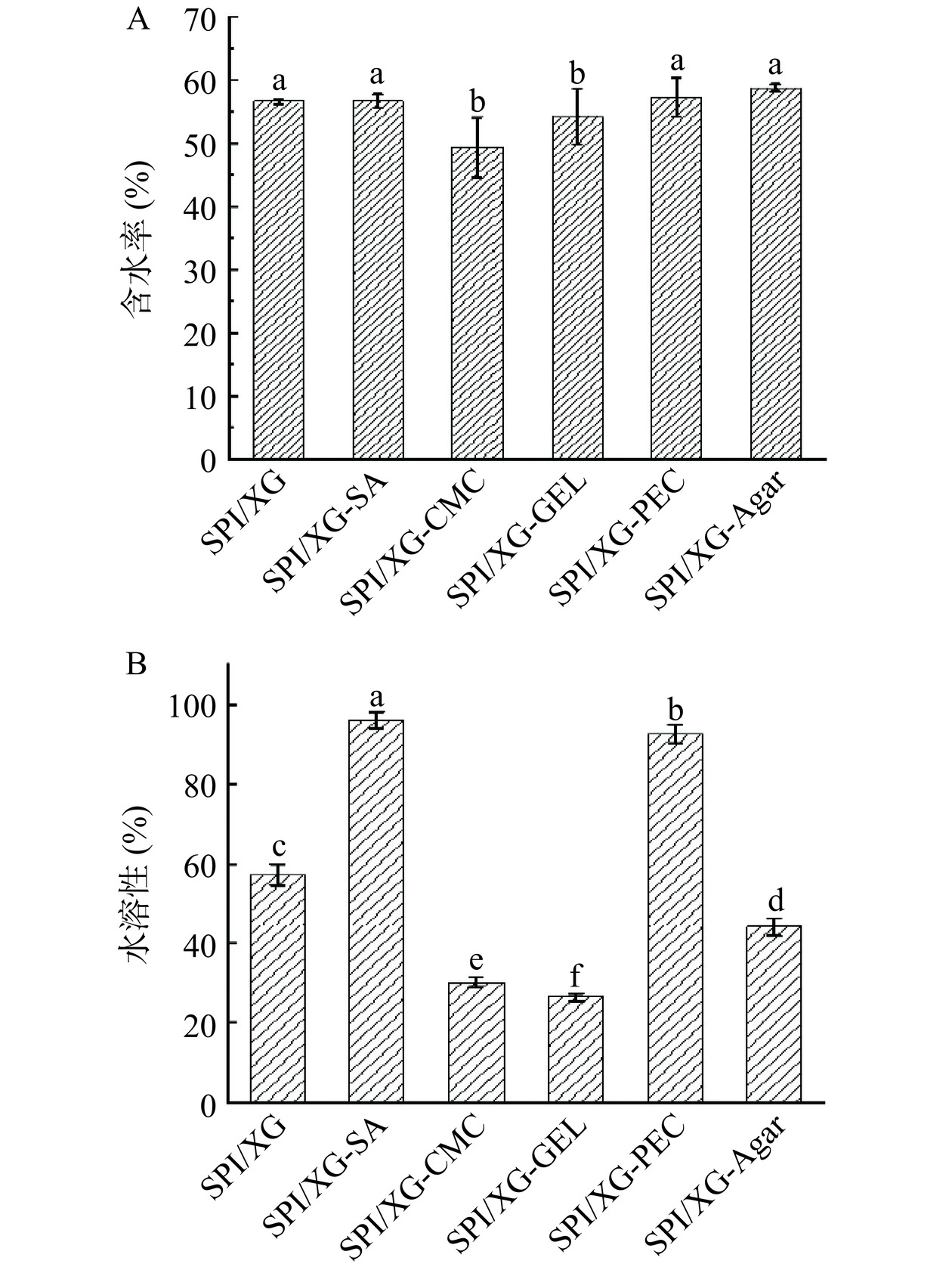
包装薄膜的水分含量与控制微生物繁殖生长和包装内食品的质量密切相关[39]。由图2A可知,MC最大的为SPI/XG-Agar膜达到了58.73%,而SPI/XG-CMC膜的MC最低为49.35%。SPI/XG-CMC膜、SPI/XG-GEL膜的MC相较于SPI/XG膜、SPI/XG-SA膜、SPI/XG-PEC膜和SPI/XG-Agar膜低,可能是因为复合膜成膜基质之间形成更多的氢键,使得复合膜内部结构更稳定,抑制了与水分子之间的相互作用,使MC显著降低[40]。
WS可用以评价复合膜的耐水性和物理阻隔性,对于水活度高的产品需要提高薄膜的水不溶性以保证薄膜的完整性及食品的最佳状态。由图2B可知,WS较低的有SPI/XG-CMC膜、SPI/XG-GEL膜和SPI/XG-Agar膜,而最高的为SPI/XG-SA膜,其WS高的原因可能是SA具有很大的亲水性、内部结构松散且SA与SPI/XG间的相互作用较弱,从而增加薄膜的WS[41−42]。
2.1.3 复合膜的水蒸气透过率及透氧性
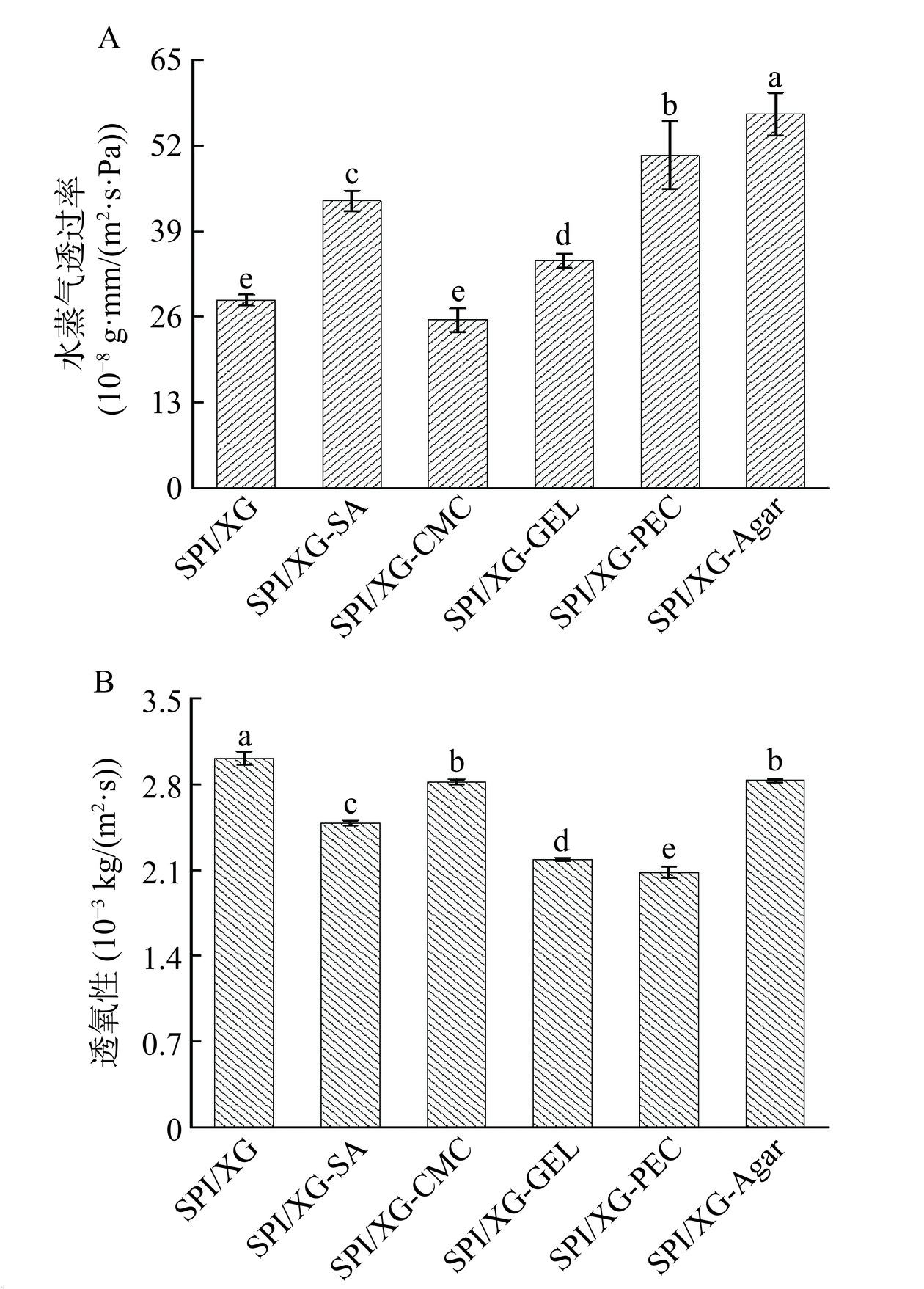
WVP是包装膜的一个关键特性。因为食品在储存过程中需要减少与外界的水汽交换[43],而水蒸气的传递行为包括水的吸附和水的扩散。由图3A可知,WVP最大的是SPI/XG-Agar膜,而WVP最小的是SPI/XG-CMC膜,可能是SPI/XG与CMC发生了交联反应,从而使水蒸气通过膜的曲径比其他膜更长以及疏水性更强[44],而其他基材与SPI/XG交联具有亲水性,导致WVP高,这也与Galus等[32]的研究结果相似。
OP对于维持食品质量极为重要,因为食品在流通过程中容易被氧化。由图3B可知,SPI/XG膜的OP值最高,这可能是由于SPI的主要组成为球蛋白,导致形成的蛋白网络结构松散,从而OP值高。而其他膜的OP值比SPI/XG膜低的原因可能是在SPI和XG的基础上加入了其他的基底材料,使SPI和XG与相应的基底材料进行结合,形成了大量的氢键,使膜具有亲水性,这使得它们对氧和其他非极性化合物具有阻隔性,从而使其他膜相较于SPI/XG膜的OP值低。
2.1.4 复合膜的透光率
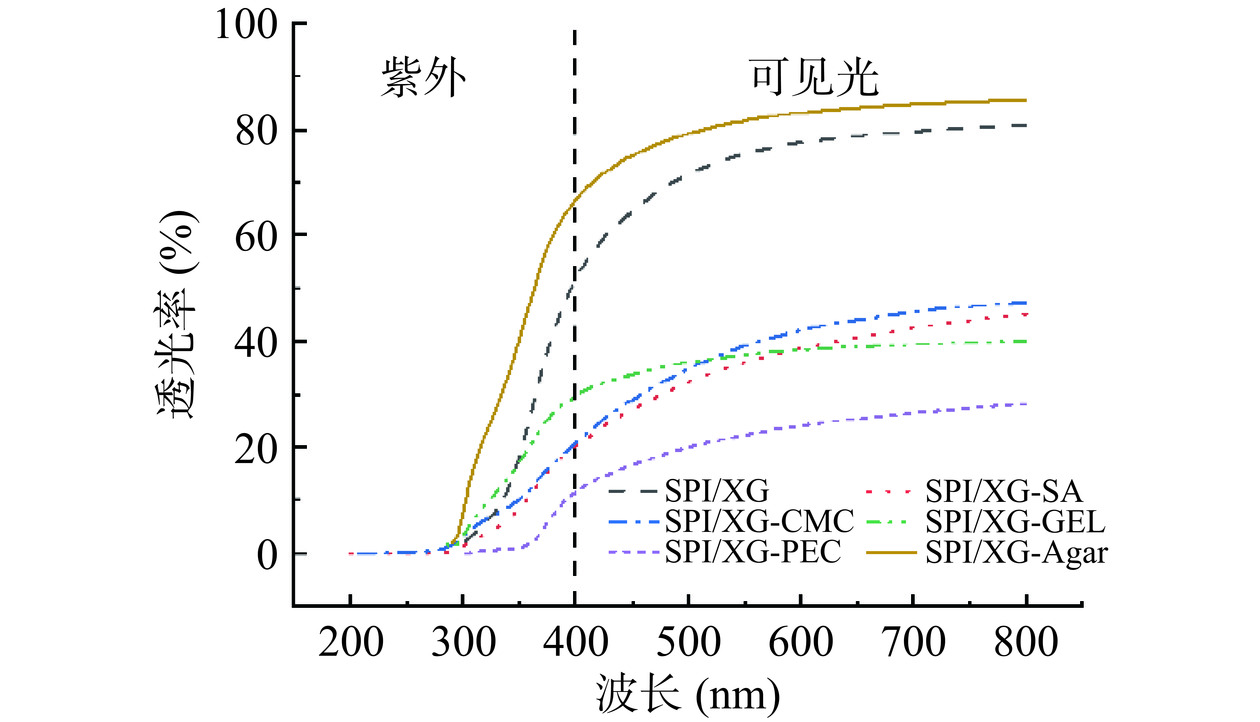
食品的外观是影响消费者购买的最重要评价指标。良好的透明度可以确保食品的真实性质得到反映。紫外线会引起许多光化学反应,对食物有害,特别是光敏食品[45]。而如果包装膜具有紫外-可见光阻隔性能,则能有效保护包装食品免受光辐射的影响[46]。不同基底材料膜的透光率如图4所示。在波长为350 nm时,SPI/XG-Agar膜的透光率最高达到40.03%,而SPI/XG-PEC膜的透光率最低为0.99%。其大小顺序为SPI/XG-Agar膜>SPI/XG膜>SPI/XG-GEL膜>SPI/XG-CMC膜>SPI/XG-SA膜>SPI/XG-PEC膜。在波长为300 nm时,6种膜的透光率均接近于0,这表明6种材料都具有一定的防紫外线性能。而在波长为600 nm时,SPI/XG-Agar膜的透光率最高为83.17%,SPI/XG-PEC膜的透光率最低为24.03%。其大小顺序为SPI/XG-Agar膜>SPI/XG膜>SPI/XG-CMC膜>SPI/XG-SA膜>SPI/XG-GEL膜>SPI/XG-PEC膜。综上所述,SPI/XG-Agar膜相对于其他基材的抗紫外能力弱,但在可见光范围的透光率远远高于其他基材。造成这样的原因可能是Agar和SPI/XG相容性较好,形成均匀的网络,并且光线更容易通过[36]。而其他膜的透光率较低可能是基材存在多种结构,从而吸收紫外线/可见光[46]。虽然SPI/XG-PEC膜的透光率最低,但仍可以清晰地显示其背后的文本。
2.1.5 复合膜的颜色及不透明度
颜色是食品包装膜的重要表现特性,会直接影响消费者对于包装食品的接受度。表1列出了不同膜的颜色参数及不透明度。从表中可以看出SPI/XG-SA膜、SPI/XG-GEL膜和SPI/XG-PEC膜的L*没有显著差异(P>0.05),但a*、b*和ΔE具有显著差异(P<0.05)。相比SPI/XG-GEL膜,SPI/XG-SA膜和SPI/XG-PEC膜的b*值较大,偏黄色,这主要是因为它们的基质有轻微的黄色。ΔE值大小表示感觉色差程度,当ΔE值大于3时,人的视觉器官对于色差就会感觉明显,而表中6种膜的ΔE均大于5,表明膜的色差都很明显。同时,从表中可以看出,SPI/XG-Agar膜的透明度最高,而SPI/XG-PEC膜的透明度最低,透明度的差异主要与膜内部结构相关[47]。
表 1 复合膜的颜色参数及不透明度Table 1. Color parameters and opacities of the composite films膜 L* a* b* ΔE 不透明度(Abs600/mm) SPI/XG 88.108±2.226ab 0.834±0.465c 11.886±4.941ab 11.742±5.076b 1.163±0.075d SPI/XG-SA 85.206±2.351c 1.860±0.549a 13.616±3.386a 14.426±3.990a 2.953±0.115c SPI/XG-CMC 87.200±1.545b 1.429±0.382b 10.464±0.952b 10.716±1.181b 3.120±0.285bc SPI/XG-GEL 85.293±1.791c 0.003±0.190d 3.517±0.708d 6.038±1.156c 3.330±0.262b SPI/XG-PEC 84.931±0.773c 0.779±0.121c 7.662±0.710c 9.760±0.917b 3.873±0.057a SPI/XG-Agar 88.757±0.510a 0.567±0.084c 4.077±0.818d 5.473±0.793c 0.447±0.093e 注:表中的数值均表示为平均值±标准偏差;同列不同小写字母表示有显著性差异(P<0.05)。 2.1.6 复合膜的热重分析
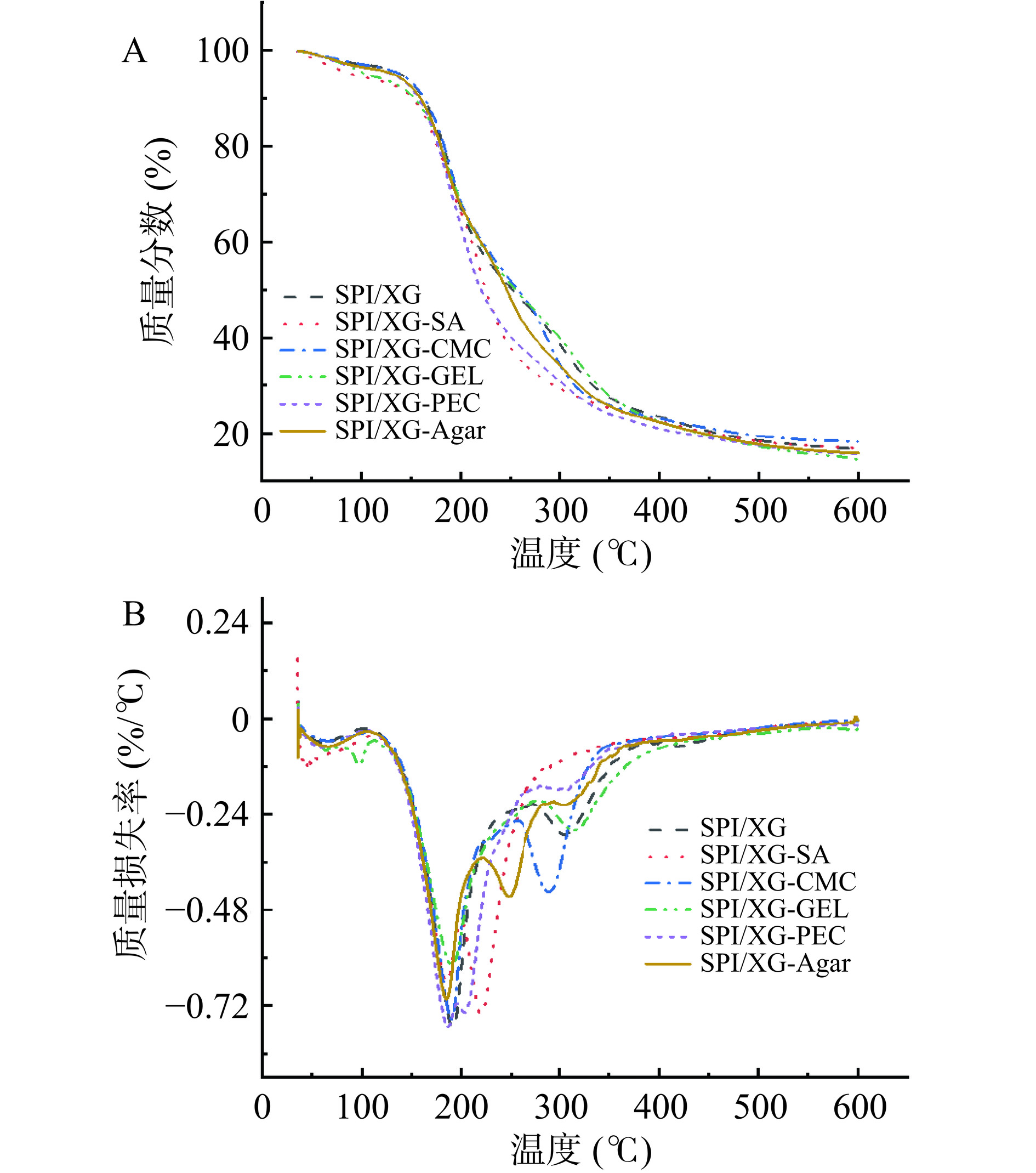
热重分析(Thermogravimetric analysis,TGA)是指在一定的升温速率条件下,对样品进行连续检测,得到样品的质量变化和温度之间的函数关系,可反映材料的热稳定性。各复合膜的TGA,如图5A所示,可划分为三个阶段,第一主要的降解阶段(35~120 ℃)是由于复合膜中吸收的游离水和结合水的损失以及氢键的断裂[48],样品质量损失率约为10%。第二降解阶段(120~196 ℃)中,可归因于甘油的降解、焦磷酸钠的热分解[49],样品质量损失率约为20%;第三阶段的降解表现为两种情况,第一种情况膜在(196~256 ℃)时出现第三个波谷(图5B ),分别是SPI/XG-CMC膜、SPI/XG-SA膜、SPI/XG-PEC膜和SPI/XG-Agar膜,样品质量损失率约为20%,其最大分解速率时的温度分别为225.78、220.57、203.58和248.32 ℃,造成它们提前产生波谷的原因可能是添加的基底材料开始热分解;第二种情况是SPI/XG膜、SPI/XG-GEL膜(196~390 ℃),它们的最大分解速率时的温度分别为307.31 ℃和315.9 ℃,这两种膜的质量减轻主要是膜内主要材质开始分解且质量快速损失,主要是蛋白质主链的分解以及相应材质的分解,样品质量损失率约为40%。其中第一种情况的膜进行了第四次降解阶段(256~414 ℃),它们的最大分解速率时的温度与第二种情况的第三次降解的最高分解速率时的温度近似,这表明第一种情况膜中添加的基质比SPI更早热分解。
2.2 复合膜结构的表征
2.2.1 复合膜的扫描电镜分析
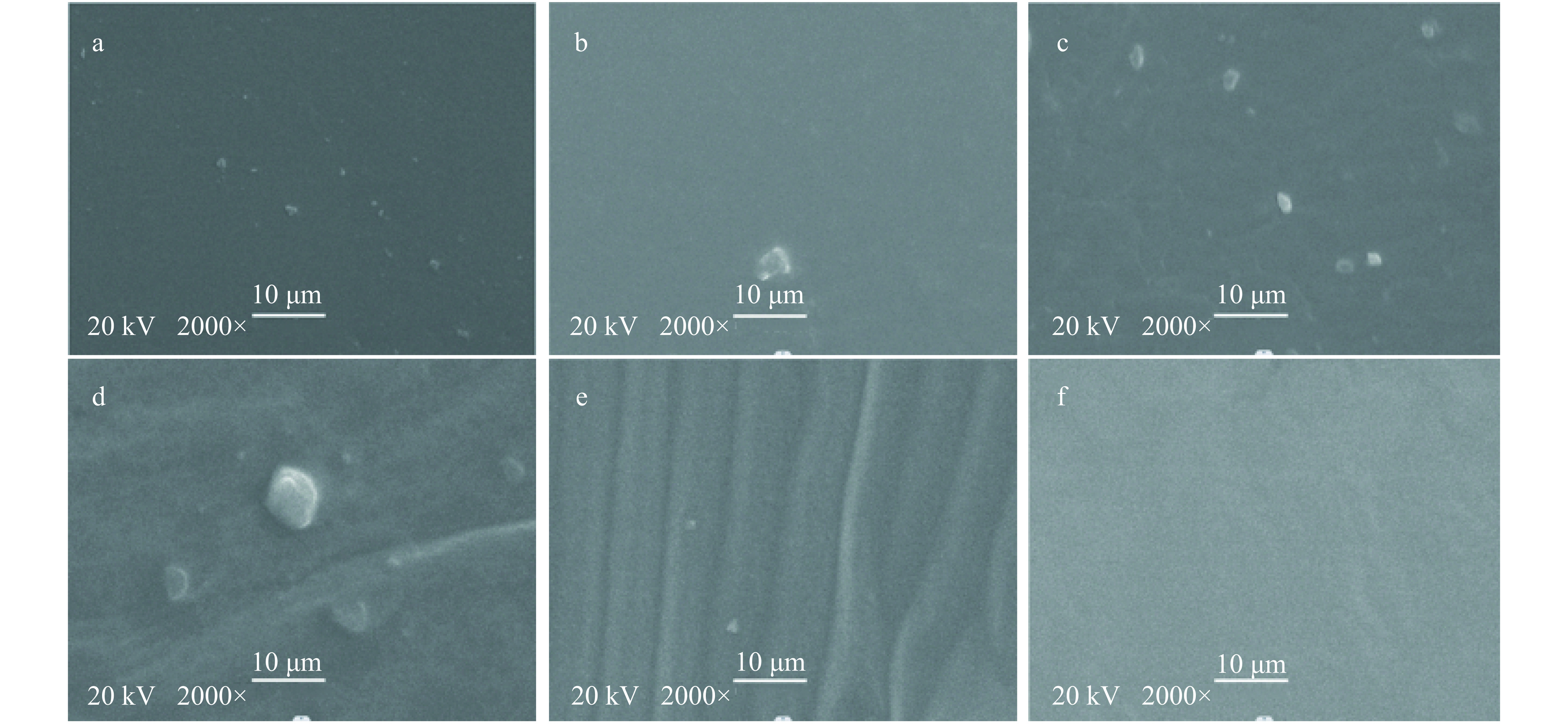
扫描电镜(Scanning electron microscopy,SEM)可以清晰地观察出复合膜结构、形态、尺寸、分布等信息,从而根据共混高聚物中两相断裂性质的差异来判断材料间的相容性[50]。生物相容性较好时复合膜表面应呈现平整光滑的图像,若相容性不好,可能会观察到明显的分界或表面颗粒析出[51]。图6(A~E)有明显的颗粒物,说明复合膜基质间相容性较差,同时,图6(D和E)出现褶皱,也代表GEL和PEC与SPI的相容性较差。图6(F)中没有明显颗粒且无褶皱,同时薄膜表面较为平坦光滑,表明Agar与SPI的相容性较好,形成较为致密的结构,这也从机械性能有所体现。
2.2.2 复合膜的红外光谱分析
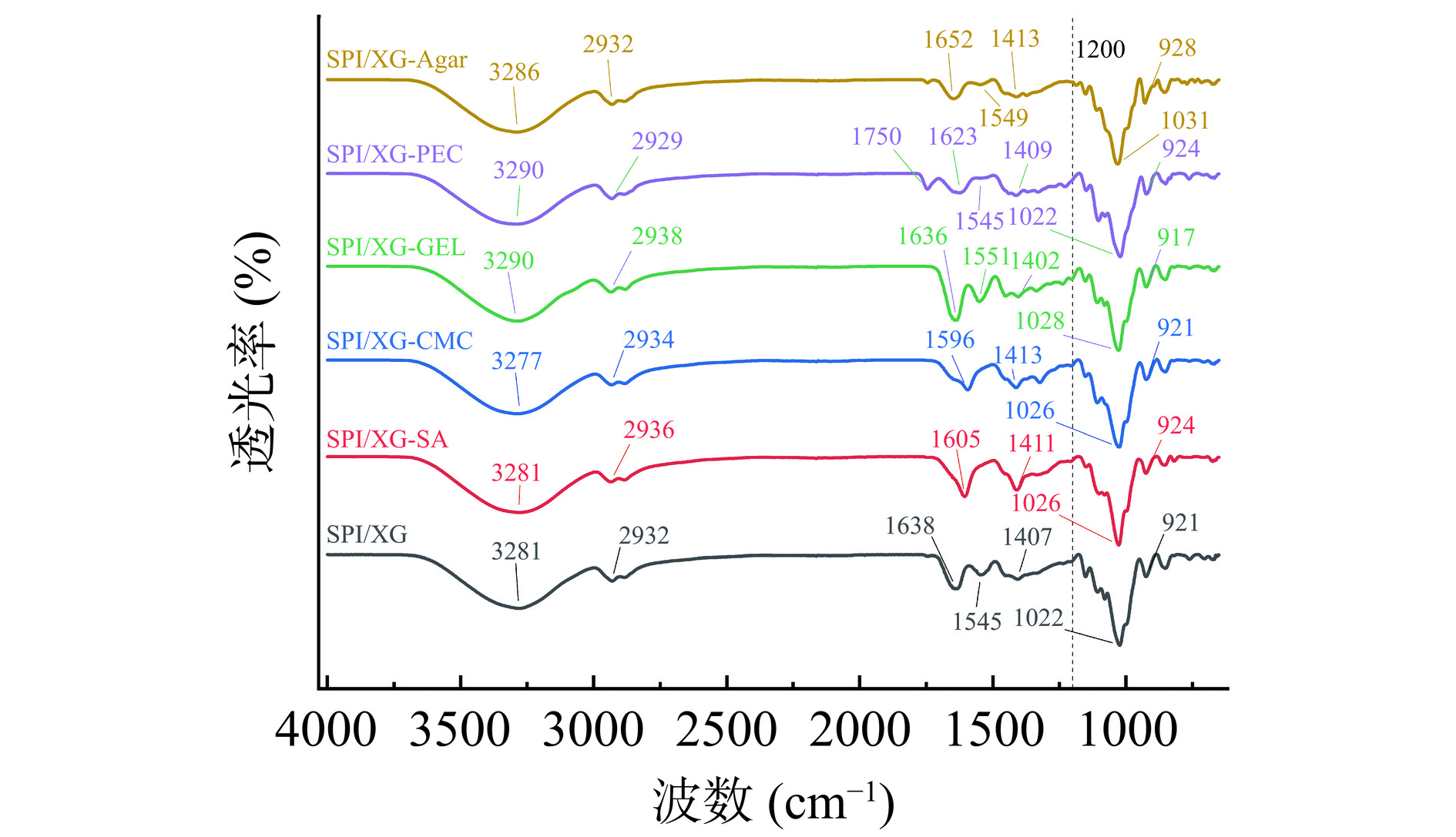
傅里叶红外变换光谱分析是一种微区分技术,根据光谱中出现的峰的位置反映测试样品的化学结构[52]。同时,红外光谱可以反映复合薄膜基质之间的相互作用情况,不同成膜基质混合会使复合膜的峰型发生移动和变化[53]。如图7所示,所有膜都在3280 cm−1左右出现强宽峰,这是由于-OH键的伸缩振动[54]。在2900 cm−1附近出现波峰是因为C-H键的伸缩振动,以及在1600 cm−1左右出现波峰是由于C=O键的伸缩振动。加入其他基底材料后,吸收峰(3281 cm−1和2932 cm−1)向高波数偏移,这可能是SPI/XG与不同基材之间存在氢键相互作用,SPI和XG分子链上的活性位点可能是羰基中的氧原子[20]。SPI/XG膜、SPI/XG-GEL膜、SPI/XG-PEC膜和SPI/XG-Agar膜有明显的三个酰胺特征峰:1652 cm−1处出现C=O伸缩振动峰(酰胺I带)、1549 cm−1处出现N-H弯曲峰(酰胺II带)、1413 cm−1处出现N-H弯曲振动峰和1200 cm−1处出现C-N伸缩振动峰(酰胺III带)[38],说明复合膜基质已成功地与增塑剂甘油结合。SPI/XG-PEC膜在1750 cm−1处的峰与酯的C-O和C=O键有关。所有膜在波数1032 cm−1左右出现波峰是由于SPI与甘油发生氢键作用。结果表明,各曲线具有极高的相似性,基质之间相容性良好。
2.2.3 复合膜的X-射线衍射分析
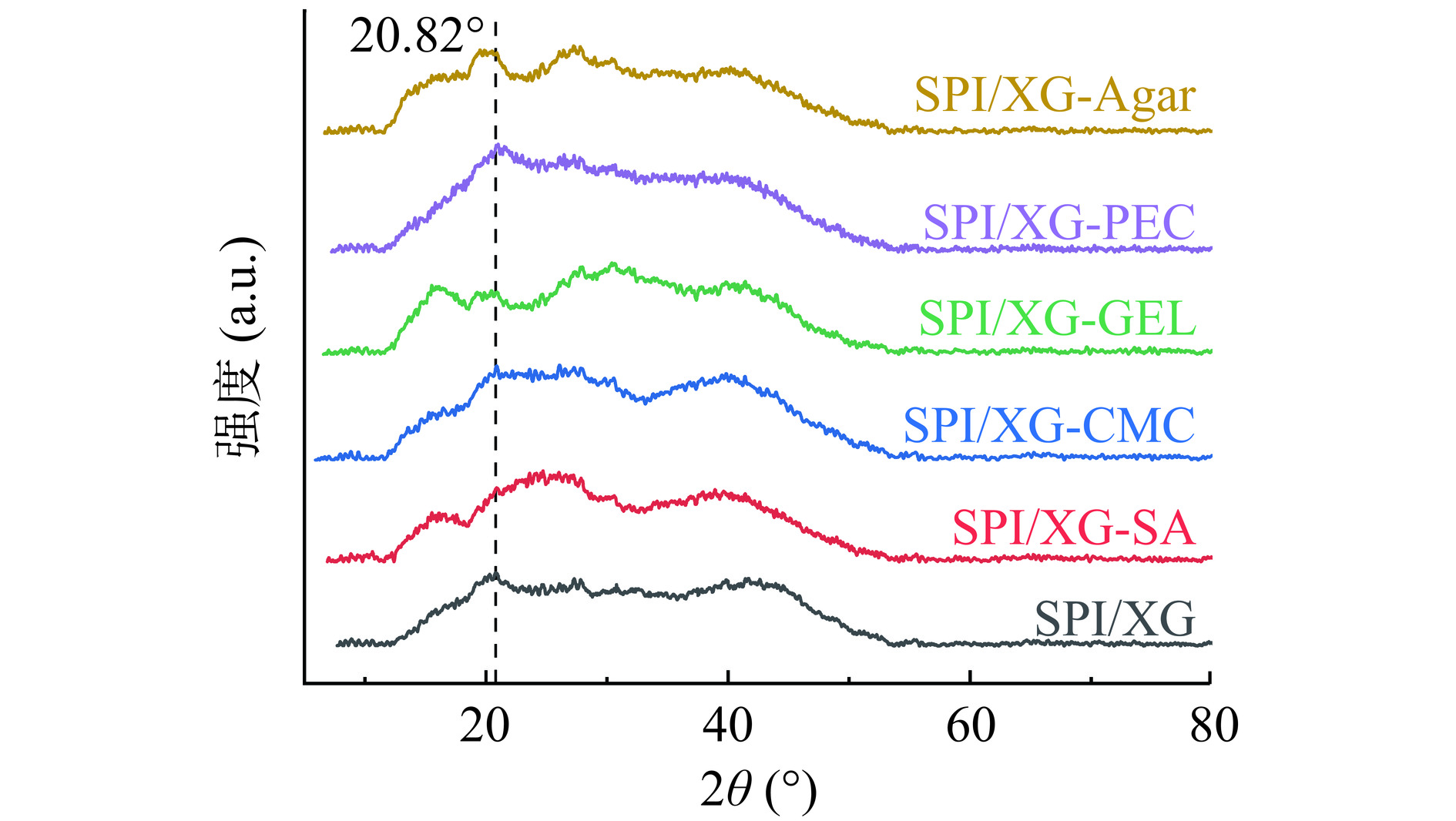
通过X射线衍射研究样品的结晶度。结晶度较高的聚合物表现出更尖、更高的峰,而更低、更宽的峰则代表聚合物存在非晶态区域,即无定形区域[55]。如图8所示,在2θ为20°左右时,SPI膜有强的衍射峰,代表了SPI二级结构的-β-折叠[56]。而SPI/XG-SA膜、SPI/XG-GEL膜和SPI/XG-Agar膜的强衍射峰在15°左右出现,膜的强衍射峰位置不同,表明成膜物质之间发生了交联,SA、GEL和Agar的加入影响了SPI/XG的结晶行为,从而改变了其非晶结构。而CMC和PEC的加入并没有改变SPI/XG的晶体结构。
3. 结论
本文以SPI/XG为成膜基质,添加SA、CMC、GEL、PEC和Agar等不同类型的基底材料制膜,对不同类型的复合膜的物理性能、阻隔性能等进行了测试。结果表明,基于不同成膜基质制备的复合膜的水溶性、不透明度、颜色外观、拉伸强度等性能表现具有较大的差异。通过对膜进行红外光谱分析发现,5种基底材料在成膜基质中分散较好,且基底材料中大量的羟基与成膜基质之间形成了氢键作用。此外,Agar对复合膜的微观结构有一定的影响,能够改善膜材料的相容性,从而改善复合膜的机械性能。其中,通过上述测试发现,SPI/XG-Agar膜不透明度为最低,颜色透明度高,外观和机械性能较为优异,因此SPI/XG-Agar膜,在食品包装领域具有一定的应用潜力。
-
表 1 复合膜的颜色参数及不透明度
Table 1 Color parameters and opacities of the composite films
膜 L* a* b* ΔE 不透明度(Abs600/mm) SPI/XG 88.108±2.226ab 0.834±0.465c 11.886±4.941ab 11.742±5.076b 1.163±0.075d SPI/XG-SA 85.206±2.351c 1.860±0.549a 13.616±3.386a 14.426±3.990a 2.953±0.115c SPI/XG-CMC 87.200±1.545b 1.429±0.382b 10.464±0.952b 10.716±1.181b 3.120±0.285bc SPI/XG-GEL 85.293±1.791c 0.003±0.190d 3.517±0.708d 6.038±1.156c 3.330±0.262b SPI/XG-PEC 84.931±0.773c 0.779±0.121c 7.662±0.710c 9.760±0.917b 3.873±0.057a SPI/XG-Agar 88.757±0.510a 0.567±0.084c 4.077±0.818d 5.473±0.793c 0.447±0.093e 注:表中的数值均表示为平均值±标准偏差;同列不同小写字母表示有显著性差异(P<0.05)。 -
[1] OU S Y, KWOK K C, KANG Y J. Changes in in vitro digestibility and available lysine of soy protein isolate after formation of film[J]. Journal of Food Engineering,2004,64(3):301−305. doi: 10.1016/j.jfoodeng.2003.10.013
[2] 王晨, 秦烨, 王志平. 大豆分离蛋白膜改性研究进展[J]. 食品工业,2019,40(3):244−248. [WANG C, QIN Y, WANG Z P. Research advances in modification of soy protein isolate film[J]. Food Industry,2019,40(3):244−248.] WANG C, QIN Y, WANG Z P. Research advances in modification of soy protein isolate film[J]. Food Industry, 2019, 40(3): 244−248.
[3] 毕会敏, 徐柠檬, 范方宇. 花青素/大豆分离蛋白智能包装膜特性及鱼肉新鲜度监测[J]. 食品与发酵工业,2023,49(7):232−240. [BI H M, XU N M, FAN F Y. Characteristics of smart packaging films of anthocyanin/soy protein isolate and monitoring of fish meat freshness[J]. Food and Fermentation Industry,2023,49(7):232−240.] BI H M, XU N M, FAN F Y. Characteristics of smart packaging films of anthocyanin/soy protein isolate and monitoring of fish meat freshness[J]. Food and Fermentation Industry, 2023, 49(7): 232−240.
[4] 尹兴, 朱俊南, 陈林, 等. 可食性大豆分离蛋白膜的制备及其性能研究[J]. 包装工程,2020,41(23):83−89. [YIN X, ZHU J N, CHEN L, et al. Preparation and properties of edible soy protein isolate film[J]. Packaging Engineering,2020,41(23):83−89.] YIN X, ZHU J N, CHEN L, et al. Preparation and properties of edible soy protein isolate film[J]. Packaging Engineering, 2020, 41(23): 83−89.
[5] ELELLA M H, GODA E S, GAB A M A, et al. Xanthan gum-derived materials for applications in environment and eco-friendly materials:A review[J]. Journal of Environmental Chemical Engineering,2021,9(1):104702. doi: 10.1016/j.jece.2020.104702
[6] 李瑞, 刘华, 高梦祥, 等. 不同添加剂对海藻酸钠复合膜性能的影响[J]. 塑料工业,2023,51(8):122−130,146. [LI R, LIU H, GAO M X, et al. Effects of different additives on properties of sodium alginate composite film[J]. China Plastics Industry,2023,51(8):122−130,146.] doi: 10.3969/j.issn.1005-5770.2023.08.021 LI R, LIU H, GAO M X, et al. Effects of different additives on properties of sodium alginate composite film[J]. China Plastics Industry, 2023, 51(8): 122−130,146. doi: 10.3969/j.issn.1005-5770.2023.08.021
[7] 任邦来, 秦娜娜. 不同浓度海藻酸钠处理对油桃保鲜效果的影响[J]. 中国食物与营养,2016,22(9):38−41. [REN B L, QIN N N. Effects of coating with different concentrations of sodium alginate on nectarine fresh-keeping[J]. China Food and Nutrition,2016,22(9):38−41.] doi: 10.3969/j.issn.1006-9577.2016.09.009 REN B L, QIN N N. Effects of coating with different concentrations of sodium alginate on nectarine fresh-keeping[J]. China Food and Nutrition, 2016, 22(9): 38−41. doi: 10.3969/j.issn.1006-9577.2016.09.009
[8] ZHU X, CHEN H, HE M, et al. Effect of stearic acid on properties of soy protein isolate/sodium alginate films[J]. Transactions of the Chinese Society for Agricultural Machinery,2022,53(5):406−412.
[9] 姚曜, 孙振炳, 李晓宝, 等. 羧甲基纤维素复合膜的研究现状[J]. 包装工程,2022,43(1):10−16. [YAO Y, SUN Z B, LI X B, et al. Research status of carboxymethyl cellulose composite film[J]. Packaging Engineering,2022,43(1):10−16.] YAO Y, SUN Z B, LI X B, et al. Research status of carboxymethyl cellulose composite film[J]. Packaging Engineering, 2022, 43(1): 10−16.
[10] TOǦRUL H, ARSLAN N. Carboxymethyl cellulose from sugar beet pulp cellulose as a hydrophilic polymer in coating of mandarin[J]. Journal of Food Engineering,2004,62(3):271−279. doi: 10.1016/S0260-8774(03)00240-1
[11] HAN J, SHIN S H, PARK K M, et al. Characterization of physical, mechanical, and antioxidant properties of soy protein-based bioplastic films containing carboxymethylcellulose and catechin[J]. Food Science and Biotechnology,2015,24(3):939−945. doi: 10.1007/s10068-015-0121-0
[12] 杨帅帅. 功能性明胶复合膜的制备及表征[D]. 西宁:青海民族大学, 2018. [YANG S S. Preparation and characterization of functional gelatin composite films[D]. Xining:Qinghai Minzu University, 2018.] YANG S S. Preparation and characterization of functional gelatin composite films[D]. Xining: Qinghai Minzu University, 2018.
[13] 梁宇雯. 纳米银/明胶抗菌膜制备及其性能研究[D]. 北京:北京化工大学, 2022. [LIANG Y W. Preparation of nano-silver/gelatin antibacterial film and properties[D]. Beijing:Beijing University of Chemical Technology, 2022.] LIANG Y W. Preparation of nano-silver/gelatin antibacterial film and properties[D]. Beijing: Beijing University of Chemical Technology, 2022.
[14] BAI H, XU J, LIAO P, et al. Mechanical and water barrier properties of soy protein isolate film incorporated with gelatin[J]. Journal of Plastic Film & Sheeting,2012,29(2):174−188.
[15] 任雯琪. 高强度果胶基食品包装薄膜的制备及性能研究[D]. 西安:陕西科技大学, 2023. [REN W Q. Preparation and performance of high-strength pectin-based food packaging film[D]. Xi'an:Shaanxi University of Science and Technology, 2023.] REN W Q. Preparation and performance of high-strength pectin-based food packaging film[D]. Xi'an: Shaanxi University of Science and Technology, 2023.
[16] 黄晟. 果胶修饰鱼明胶制备高性能亲水胶体及其应用研究[D]. 南昌:南昌大学, 2022. [HUANG S. Pectin-modified fish gelatin to prepare high-performance hydrophilic colloids and its application[D]. Nanchang:Nanchang University, 2022.] HUANG S. Pectin-modified fish gelatin to prepare high-performance hydrophilic colloids and its application[D]. Nanchang: Nanchang University, 2022.
[17] AMADO L R, SILVA K D S, MAURO M A. Effects of interactions between soy protein isolate and pectin on properties of soy protein-based films[J]. Journal of Applied Polymer Science,2019,137(21):48732.
[18] MOSTAFAVI F S, ZAEIM D. Agar-based edible films for food packaging applications-A review[J]. International Journal of Biological Macromolecule,2020,159:1165−1176. doi: 10.1016/j.ijbiomac.2020.05.123
[19] 王文娇. 淀粉/琼脂-花青素pH响应型包装膜的制备及其性能研究[D]. 泰安:山东农业大学, 2023. [WANG W J. Preparation and properties of starch/agar-anthocyanin pH responsive packaging films[D]. Taian:Shandong Agricultural University, 2023.] WANG W J. Preparation and properties of starch/agar-anthocyanin pH responsive packaging films[D]. Taian: Shandong Agricultural University, 2023.
[20] TIAN H, XU G, YANG B, et al. Microstructure and mechanical properties of soy protein/agar blend films:Effect of composition and processing methods[J]. Journal of Food Engineering,2011,107(1):21−26. doi: 10.1016/j.jfoodeng.2011.06.008
[21] LIU Y, QIN Y, BAI R, et al. Preparation of pH-sensitive and antioxidant packaging films based on kappa-carrageenan and mulberry polyphenolic extract[J]. International Journal of Biological Macromolecule,2019,134:993−1001. doi: 10.1016/j.ijbiomac.2019.05.175
[22] RACHTANAPUN P, KLUNKLIN W, JANTRAWUT P, et al. Characterization of chitosan film incorporated with curcumin extract[J]. Polymers,2021,13(6):963. doi: 10.3390/polym13060963
[23] CHEN H Z, ZHANG M, BHANDARI B, et al. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness[J]. Food Hydrocolloids,2020,100:105438. doi: 10.1016/j.foodhyd.2019.105438
[24] LI N, YANG X, LIN D. Development of bacterial cellulose nanofibers/konjac glucomannan-based intelligent films loaded with curcumin for the fresh-keeping and freshness monitoring of fresh beef[J]. Food Packaging and Shelf Life,2022,34:100989. doi: 10.1016/j.fpsl.2022.100989
[25] ZHOU S, LI N, PENG H, et al. The development of highly pH-sensitive bacterial cellulose nanofibers/gelatin-based intelligent films loaded with anthocyanin/curcumin for the fresh-keeping and freshness detection of fresh pork[J]. Foods,2023,12(20):3719. doi: 10.3390/foods12203719
[26] YONG H, WANG X, BAI R, et al. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix[J]. Food Hydrocolloids,2019,90:216−224. doi: 10.1016/j.foodhyd.2018.12.015
[27] LIU Z, LIN D, LOPEZ S P, et al. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan[J]. International Journal of Biological Macromolecule,2020,145:634−645. doi: 10.1016/j.ijbiomac.2019.12.109
[28] RIAHI Z, PRIYADARSHI R, RHIM J W, et al. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications[J]. Food Hydrocolloids,2021,112:106314. doi: 10.1016/j.foodhyd.2020.106314
[29] SALARBASHI D, TAFAGHODI M, BAZZAZ B S F, et al. pH-sensitive soluble soybean polysaccharide/SiO2 incorporated with curcumin for intelligent packaging applications[J]. Food Science & Nutrition,2021,9(4):2169−2179.
[30] VICENTINI N M, DUPUY N D, LEITZELMAN M, et al. Prediction of cassava starch edible film properties by chemometric analysis of infrared spectra[J]. Spectroscopy Letters,2005,38(6):749−767. doi: 10.1080/00387010500316080
[31] HUANG J, LIU J, CHEN M, et al. Immobilization of roselle anthocyanins into polyvinyl alcohol/hydroxypropyl methylcellulose film matrix:Study on the interaction behavior and mechanism for better shrimp freshness monitoring[J]. International Journal of Biological Macromolecule,2021,184:666−677. doi: 10.1016/j.ijbiomac.2021.06.074
[32] GALUS S, LENART A. Development and characterization of composite edible films based on sodium alginate and pectin[J]. Journal of Food Engineering,2013,115(4):459−465. doi: 10.1016/j.jfoodeng.2012.03.006
[33] RAMBABU K, BHARATH G, BANAT F, et al. Mango leaf extract incorporated chitosan antioxidant film for active food packaging[J]. International Journal of Biological Macromolecules,2019,126:1234−1243. doi: 10.1016/j.ijbiomac.2018.12.196
[34] 彭勇, 李云飞, 项凯翔. 绿茶多酚提高壳聚糖包装膜的抗氧化性能[J]. 农业工程学报,2013,29(14):269−276. [PENG Y, LI Y F, XIANG K X. Adding green tea polyphenols enhances antioxidant of chitosan film[J]. Journal of Agricultural Engineering,2013,29(14):269−276.] doi: 10.3969/j.issn.1002-6819.2013.14.034 PENG Y, LI Y F, XIANG K X. Adding green tea polyphenols enhances antioxidant of chitosan film[J]. Journal of Agricultural Engineering, 2013, 29(14): 269−276. doi: 10.3969/j.issn.1002-6819.2013.14.034
[35] YOSHIDA C M P, MACIEL V B V, MENDONÇA M E D, et al. Chitosan biobased and intelligent films:Monitoring pH variations[J]. LWT,2014,55(1):83−89. doi: 10.1016/j.lwt.2013.09.015
[36] 冯永莉. TG酶交联大豆蛋白—明胶可食性膜的性质及机理研究[D]. 天津:天津科技大学, 2022. [FENG Y L. Properties and mechanism of transglutaminase-crosslinked soy protein isolate-gelatin edible film[D]. Tianjin:Tianjin University of Science and Technology, 2022.] FENG Y L. Properties and mechanism of transglutaminase-crosslinked soy protein isolate-gelatin edible film[D]. Tianjin: Tianjin University of Science and Technology, 2022.
[37] CAO N, FU Y, HE J. Preparation and physical properties of soy protein isolate and gelatin composite films[J]. Food Hydrocolloids,2007,21(7):1153−1162. doi: 10.1016/j.foodhyd.2006.09.001
[38] YE Q, HAN Y, ZHANG J, et al. Bio-based films with improved water resistance derived from soy protein isolate and stearic acid via bioconjugation[J]. Journal of Cleaner Production,2019,214:125−131. doi: 10.1016/j.jclepro.2018.12.277
[39] LI K K, YIN S W, YANG X Q, et al. Fabrication and characterization of novel antimicrobial films derived from thymol-loaded zein-sodium caseinate (SC) nanoparticles[J]. Journal of Agricultural and Food Chemistry,2012,60(46):11592−11600. doi: 10.1021/jf302752v
[40] ZHAI X, SHI J, ZOU X, et al. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring[J]. Food Hydrocolloids,2017,69:308−317. doi: 10.1016/j.foodhyd.2017.02.014
[41] WANG X J, ULLAH N, SUN X C, et al. Development and characterization of bacterial cellulose reinforced biocomposite films based on protein from buckwheat distiller's dried grains[J]. International Journal of Biological Macromolecules,2017,96:353−360. doi: 10.1016/j.ijbiomac.2016.11.106
[42] 周佳豪, 熊熊, 刘成国, 等. 纳米纤维素添加量及干燥温度对海藻酸钠可食用膜性能的影响[J]. 食品研究与开发,2020,41(20):45−50. [ZHOU J H, XIONG X, LIU C G, et al. Effect of the amount of nanocelluose and drying temperature on the properties of sodium alginate edible films[J]. Food Research and Development,2020,41(20):45−50.] doi: 10.12161/j.issn.1005-6521.2020.20.008 ZHOU J H, XIONG X, LIU C G, et al. Effect of the amount of nanocelluose and drying temperature on the properties of sodium alginate edible films[J]. Food Research and Development, 2020, 41(20): 45−50. doi: 10.12161/j.issn.1005-6521.2020.20.008
[43] ZHENG T, YU X, PILLA S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films[J]. Carbohydrate Polymers,2017,157:1333−1340. doi: 10.1016/j.carbpol.2016.11.011
[44] SU J F, HUANG Z, YUAN X Y, et al. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions[J]. Carbohydrate Polymers,2010,79(1):145−153. doi: 10.1016/j.carbpol.2009.07.035
[45] GOUDARZI V, SHAHABI G I, BABAEI G A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite:Characterization[J]. International Journal of Biological Macromolecule,2017,95:306−313. doi: 10.1016/j.ijbiomac.2016.11.065
[46] PERALTA J, BITENCOURT C C M, MACIEL V B V, et al. Aqueous hibiscus extract as a potential natural pH indicator incorporated in natural polymeric films[J]. Food Packaging and Shelf Life,2019,19:47−55. doi: 10.1016/j.fpsl.2018.11.017
[47] VILLALOBOS R, CHANONA J, HERNÁNDEZ P, et al. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure[J]. Food Hydrocolloids,2005,19(1):53−61. doi: 10.1016/j.foodhyd.2004.04.014
[48] OUN A A, RHIM J W. Characterization of carboxymethyl cellulose-based nanocomposite films reinforced with oxidized nanocellulose isolated using ammonium persulfate method[J]. Carbohydrate Polymers,2017,174:484−492. doi: 10.1016/j.carbpol.2017.06.121
[49] 阳晖. 仙草胶对可食性蛋白膜功能特性的影响及作用机理[D]. 广州:华南农业大学, 2016. [YANG H. Effects of hsian-tsao gum upon functional properties of edible protein-based films and their mechanism[D]. Guangzhou:South China Agricultural University, 2016.] YANG H. Effects of hsian-tsao gum upon functional properties of edible protein-based films and their mechanism[D]. Guangzhou: South China Agricultural University, 2016.
[50] ALIZADEH S M, TAVASSOLI M, MOHAMMADIAN E, et al. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness[J]. International Journal of Biological Macromolecule,2021,166:741−750. doi: 10.1016/j.ijbiomac.2020.10.231
[51] 向飞, 夏玉婷, 肖满, 等. 纳米粒子对多糖基复合膜微观结构的影响研究进展[J]. 食品工业科技,2021,42:358−363. [XIANG F, XIA Y T, XIAO M, et al. Review of the influence of nanoparticles on the microstructure of polysaccharide based blend films[J]. Food Industry Science and Technology,2021,42:358−363.] XIANG F, XIA Y T, XIAO M, et al. Review of the influence of nanoparticles on the microstructure of polysaccharide based blend films[J]. Food Industry Science and Technology, 2021, 42: 358−363.
[52] 李庆钊, 林柏泉, 赵长遂, 等. 基于傅里叶红外光谱的高温煤焦表面化学结构特性分析[J]. 中国电机工程学报,2011,31:46−52. [LI Q Z, LIN B Q, ZHAO C S, et al. Chemical structure analysis of coal char surface based on Fourier-transform infrared spectrometer[J]. Chinese Journal of Electrical Engineering,2011,31:46−52.] LI Q Z, LIN B Q, ZHAO C S, et al. Chemical structure analysis of coal char surface based on Fourier-transform infrared spectrometer[J]. Chinese Journal of Electrical Engineering, 2011, 31: 46−52.
[53] 刘全娇. 鱼皮明胶—海藻酸钠复合膜的制备、性质和应用研究[D]. 合肥:合肥工业大学, 2018. [LIU Q J. Research on the preparation, properties and application of fish skin gelatin-sodium alginate composite films[D]. Hefei:Hefei University of Technology, 2018.] LIU Q J. Research on the preparation, properties and application of fish skin gelatin-sodium alginate composite films[D]. Hefei: Hefei University of Technology, 2018.
[54] SUN X, WU Q, PICHA D H, et al. Comparative performance of bio-based coatings formulated with cellulose, chitin, and chitosan nanomaterials suitable for fruit preservation[J]. Carbohydrate Polymers,2021,259:117764. doi: 10.1016/j.carbpol.2021.117764
[55] KHAMRAI M, BANERJEE S L, KUNDU P P. Modified bacterial cellulose based self-healable polyeloctrolyte film for wound dressing application[J]. Carbohydrate Polymers,2017,174:580−590. doi: 10.1016/j.carbpol.2017.06.094
[56] CHEN J, CHEN X, ZHU Q, et al. Determination of the domain structure of the 7S and 11S globulins from soy proteins by XRD and FTIR[J]. Journal of the Science of Food and Agriculture,2013,93(7):1687−1691. doi: 10.1002/jsfa.5950





 下载:
下载:
 下载:
下载:



