Regulation of Yeast Extract-Curcumin on Intestinal Flora in Mice with Acute Alcoholic Liver Injury
-
摘要: 目的:研究酵母抽提物、姜黄素+酵母抽提物复合物对小鼠急性酒精性肝损伤的预防保护作用及其对肠道菌群的调节作用,并探讨其保护作用机制。方法:建立急性酒精性肝损伤小鼠模型,分别给予酵母抽提物和姜黄素+酵母抽提物复合物,检测小鼠血清中谷丙转氨酶(Alanine aminotrans-ferase,ALT)、谷草转氨酶(Aspartate transaminase,AST)和肝组织中总谷胱甘肽(Toal glutathione,T-GSH)、谷胱甘肽过氧化物酶(Glutathione peroxidase,GSH-Px)、丙二醛(Malonicdialdehyde,MDA)、总胆固醇(Total cholesterol,TC)、甘油三酯(Triglyceride,TG)的变化,观察肝组织病理切片损伤情况,分析肠道菌群等指标。结果:与模型组相比,酵母抽提物、姜黄素+酵母抽提物复合物能够降低小鼠ALT、AST、MDA、TC和TG的水平,提高GSH-Px、T-GSH的水平;肝组织切片表明,受试物明显改善酒精引起的肝细胞脂肪浸润和炎症浸润;肠道菌群分析表明,酒精性肝损伤小鼠肠道菌群结构发生变化,姜黄素+酵母抽提物复合物能有效恢复因酒精改变的不动杆菌属、普雷沃菌属、瘤胃球菌属的相对丰度,效果优于酵母抽提物。结论:酵母抽提物、姜黄素+酵母抽提物复合物对小鼠急性酒精性肝损伤有明显的保护作用,其机制可能与调节氧化应激、脂质代谢、肠道菌群有关。Abstract: Objective: This study aimed to investigate the protective effect and mechanism of yeast extract and curcumin-yeast extract on acute alcoholic liver injury in mice. Methods: The mouse model of acute alcoholic liver injury was established, and the mice were administered with yeast extract and curcumin-yeast extract. Changes in ALT, AST, T-GSH, GSH-Px, and MDA levels were observed in the serum and liver tissue, respectively. Pathological sections of liver tissue were examined to observe the damage. The intestinal flora of mice was analyzed. Results: Compared with the model group, both yeast extract and curcumin-yeast extract reduced the levels of ALT, AST, MDA, TC, and TG, increased the activities of GSH-Px and T-GSH, and alleviated fatty infiltration and inflammatory infiltration of hepatocytes. Curcumin-yeast extract effectively restored the abundance of Acinetobacter, Prevotella, and Ruminococcus, which had been altered by alcohol. The restorative effect was superior to that of the yeast extract group. Conclusion: Yeast extract and curcumin-yeast extract showed significant protective effects against acute alcoholic liver injury in mice. This mechanism may be associated with the regulation of oxidative stress, lipid metabolism, and intestinal flora.
-
Keywords:
- yeast extract /
- curcumin /
- acute alcoholic liver injury /
- intestinal flora
-
酒精性肝病(Alcoholic liver disease,ALD)是由于长期大量饮酒而导致的肝脏疾病[1],肝脏作为人体重要的代谢和解毒器官,在受到损害后若不及时治疗,会由最初的酒精性肝损伤逐渐发展为酒精性脂肪肝等,严重威胁着人类的生命健康[2]。酒精性肝病的发病率在逐年增长,占总肝病发病率的14.8%,2015年较2000年我国酒精性肝病流行率上升3.9倍[3]。目前ALD治疗途径主要是戒酒、营养疗法和药物疗法,严重的需进行肝脏移植,轻度酒精肝患者应多摄入蛋白质、维生素、矿物质等营养素,以增强免疫力的食物为主[4]。
ALD发病机制涉及氧化应激、脂质代谢、肠道菌群等各方面[5−6]。其中,氧化应激是诱发ALD的主要机制[7]。在肝脏组织中,酒精在铁离子的参与下进行氧化反应,会产生大量活性氧(Reactive oxygen species,ROS),损害线粒体内过氧化氢酶(Catalase,CAT)和超氧化物歧化酶(Superoxide dismutase,SOD)活性,引起线粒体损伤,细胞膜发生氧化,脂质过氧化产物累积,促发细胞凋亡与炎症,最终导致肝损伤[8]。与此同时,大量饮酒还会改变肠道菌群的结构及其在体内的分布,引起肠道粘膜损伤,减少有益细菌并增加有害细菌的丰度,有害菌产生的毒素随门静脉进入肝脏,加重肝细胞损伤[9−10]。因此,通过减少氧化应激、调节脂代谢和肠道菌群,可缓解急性酒精性肝损伤[11]。
酵母抽提物(Yeast extract)是酵母在酶或附加食品级酶的共同作用下,通过酶水解和自溶得到的产物,富含多种氨基酸、多肽、核苷酸和B族维生素等小分子营养物质[12]。酵母抽提物含有丰富的谷胱甘肽,具有清除自由基和维持细胞生物功能的作用,因其具有保健、调味和营养三大功能在功能性食品、饲料营养添加剂和生物培养基营养添加剂等领域应用广泛[13]。有研究表明,布拉迪酵母等可以改变肠道菌群组成,增加双歧杆菌和乳酸杆菌的相对丰度,酵母中的β-葡聚糖也被报道可以调节肠道菌群从而改善宿主代谢健康[14−15]。姜黄为药食两用植物,其主要活性成分是姜黄素,具有抗炎、抗氧化、护肝等多种功能,可通过激活Nrf2信号通路减轻氧化应激,增强肝脏抗氧化能力,同时调节PPAR-γ、FAS、c-Ski信号通路改善脂肪的沉积[16−17]。有研究表明[18−19],姜黄素能减轻肝脂肪变性,还能改善肠道屏障功能和减少内毒素产生,通过上调有益细菌的数量并下调机会性病原体的数量来调控肠道失衡。以上研究显示,姜黄素与酵母抽提物可能通过调节肠道菌群来实现护肝作用,但二者联合的作用机制研究鲜有报道。
因此,本文研究酵母抽提物、姜黄素+酵母抽提物复合物对急性酒精性肝损伤小鼠的保护作用及其对肠道菌群的调节作用,为其在护肝及调节肠道菌群的功能性食品领域的产品开发提供理论依据。
1. 材料与方法
1.1 材料与仪器
无特殊病原体(Specific pathogen free,SPF)级昆明小鼠 雄性,体重(20±2) g,购自三峡大学实验动物中心,许可证号为[SYXK(鄂)2017-0061],动物实验伦理批准号(2022003A),于温度(23±2) ℃、湿度55%~65%环境中,分笼饲养,普通饲料喂养,自由进食和饮水;酵母抽提物 安琪酵母股份有限公司;姜黄素+酵母抽提物复合物 以35%姜黄素加65%酵母抽提物混合均匀,安琪纽特股份有限公司;谷丙转氨酶(ALT)、谷草转氨酶(AST)、谷胱甘肽过氧化物酶(GSH-Px)、总谷胱甘肽(T-GSH)、丙二醛(MDA)、总胆固醇(TC)、甘油三酯(TG)测试试剂盒 南京建成生物工程研究所;无水乙醇 分析纯,国药集团化学试剂有限公司;4%多聚甲醛 上海碧云天生物技术有限公司。
HH-S4数显恒温水浴锅 江苏金仪科技有限公司;ME-203电子天平 托利多仪器(上海)有限公司;H1750R高速冷冻离心机 湖南湘仪实验室仪器开发有限公司;SuPerMax3100多功能酶标仪 上海闪谱生物;CX43型显微镜 奥林巴斯有限公司。
1.2 实验方法
1.2.1 造模与样品采集
24只SPF级雄性成年昆明小鼠,适应性喂养7 d后随机分成4组,分别为正常对照组(KB组)、急性酒精性肝损伤模型组(MX组)、酵母抽提物组(FH组,给予酵母抽提物0.45 g/kg BW)、姜黄素+酵母抽提物复合物组(GH组,给予复合物0.45 g/kg BW)。正常对照组和模型组灌胃0.3%羧甲基纤维素钠溶液,其余两组连续灌胃相应受试物,灌胃体积按0.01 mL/g确定。连续灌胃8 d,第7 d开始早晚给予50%酒精,连续灌胃2 d。随后,禁食15 h,无菌条件下采集各组小鼠新鲜粪便,−80 ℃保存备用。内眦静脉丛取血,全血室温静置2 h,4000 r/min离心10 min,分离血清,−80 ℃保存备用。另取部分肝组织,4%多聚甲醛固定,用于病理学检测,其余肝组织用预冷生理盐水清洗后,冻存于−80 ℃冰箱,备用[20−21]。本实验所涉及所有实验动物饲养均符合国家实验动物福利指南及实验动物伦理规范。
1.2.2 肝功能指标的检测
取小鼠血清适量,按谷丙转氨酶、谷草转氨酶试剂盒操作方法检测ALT、AST的含量。
1.2.3 氧化应激指标的检测
根据谷胱甘肽过氧化物酶、总谷胱甘肽、丙二醛检测试剂盒操作方法,检测肝脏中GSH-Px、T-GSH、MDA的含量。
1.2.4 脂质代谢指标的检测
取小鼠血清适量,按照总胆固醇、甘油三酯试剂盒操作方法检测TC、TG的含量。
1.2.5 肝组织病理学检测
将4%多聚甲醛固定的肝组织采用石蜡包埋,切片后,苏木素-伊红(Hematoxylin-eosin,HE)染色,光学显微镜下观察小鼠肝组织细胞的病理学变化。
1.2.6 肠道菌群检测
测序部分,提取小鼠粪便样品的DNA,以16S rDNA基因高变区V3-V4 341F(5′-CCTAYGGGRBGCASCAG-3′)和806R(5′-GGACTACNNGGGTATCTAAT-3′)为引物进行PCR扩增,使用NEBNext®UltraTM IIDNA Library Prep Kit建库试剂盒进行文库构建,将构建好的文库进行Qubit和q-PCR定量,待文库合格后,使用NovaSeq 6000进行上机测序;信息分析部分,对测序数据进行拼接、质控,使用DADA2模块进行降噪,从而获得最终的扩增子序列变异(Amplicon Sequence Variants,ASVs)。
1.3 数据处理
数据均以平均值±标准偏差表示,n=6,生理指标数据统计分析采用Excel和Minitab,多组间比较采用单因素方差分析(one-way ANOVA),组内两两比较采用LSD法,图形绘制采用Origin 2019。
2. 结果与分析
2.1 受试物对急性酒精性肝损伤小鼠血清ALT、AST的影响
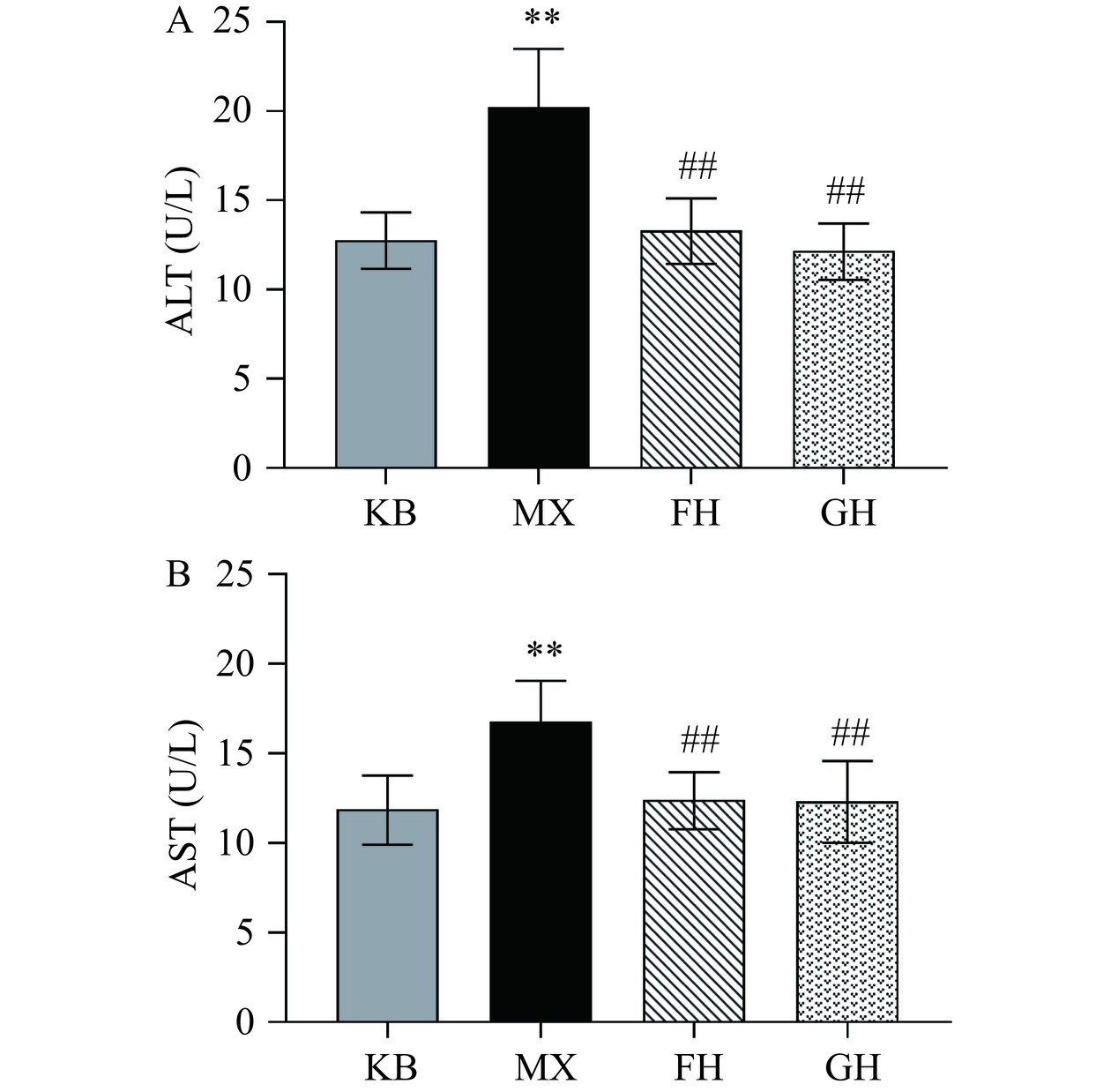
本研究首先分析了各组小鼠血清中ALT、AST水平,结果如图1所示。与KB组相比,MX组小鼠血清中ALT、AST水平均升高约1.5倍;与MX组相比,FH组和GH组小鼠血清中的ALT、AST均极显著降低(P<0.01),其中FH组的ALT浓度降低了34.16%,AST浓度降低了26.19%,GH组的ALT浓度降低了39.60%,AST浓度降低了26.79%。血清转氨酶ALT、AST是评价肝功能最常用的生化指标,ALT和AST主要存在于包括肝脏在内的多种组织中,当肝脏受损时,转氨酶便会进入血液,导致血清中ALT和AST水平升高[22]。
上述结果表明,50%酒精灌胃2 d导致小鼠肝脏转氨酶升高,肝脏受损。酵母抽提物、姜黄素+酵母抽提物复合物两组均恢复了ALT和AST的水平,对酒精导致的肝损伤具有保护作用。
2.2 受试物对急性酒精性肝损伤小鼠肝组织氧化应激的影响
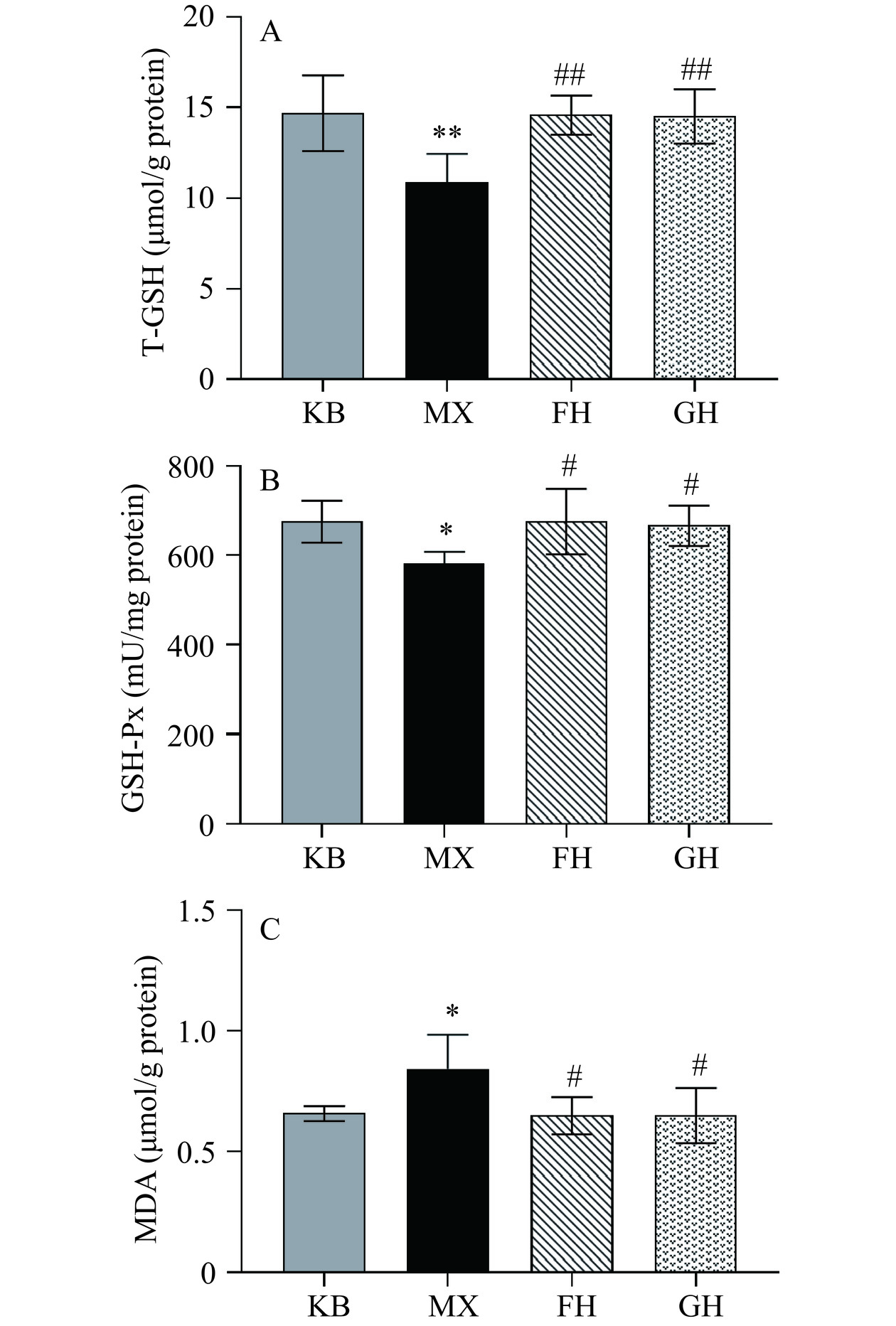
本研究中各组小鼠肝脏中氧化应激指标T-GSH、GSH-Px和MDA水平检测结果如图2所示。与KB组相比,MX组的T-GSH浓度极显著降低(P<0.01),GSH-Px的浓度显著降低(P<0.05),MDA的显著浓度升高(P<0.05);与MX组相比,FH组和GH组小鼠的T-GSH和GSH-Px的浓度显著升高(P<0.05),MDA的浓度显著降低(P<0.05)。其中,FH组的T-GSH浓度升高了35.19%,GSH-Px的浓度升高了16.45%,MDA的浓度降低了22.62%;GH组的T-GSH和GSH-Px的浓度分别升高了34.25%、15.02%,MDA的浓度则降低了22.62%。
上述结果表明,50%酒精灌胃2 d会导致小鼠肝脏中T-GSH和GSH-Px的浓度降低,MDA的浓度升高,表现出氧化应激损伤。酵母抽提物、姜黄素+酵母抽提物复合物可提高小鼠肝脏中总谷胱甘肽、谷胱甘肽过氧化物酶的浓度,降低丙二醛的浓度,有效预防了酒精导致的小鼠肝脏的氧化应激损伤。
2.3 受试物对急性酒精性肝损伤小鼠血脂的影响
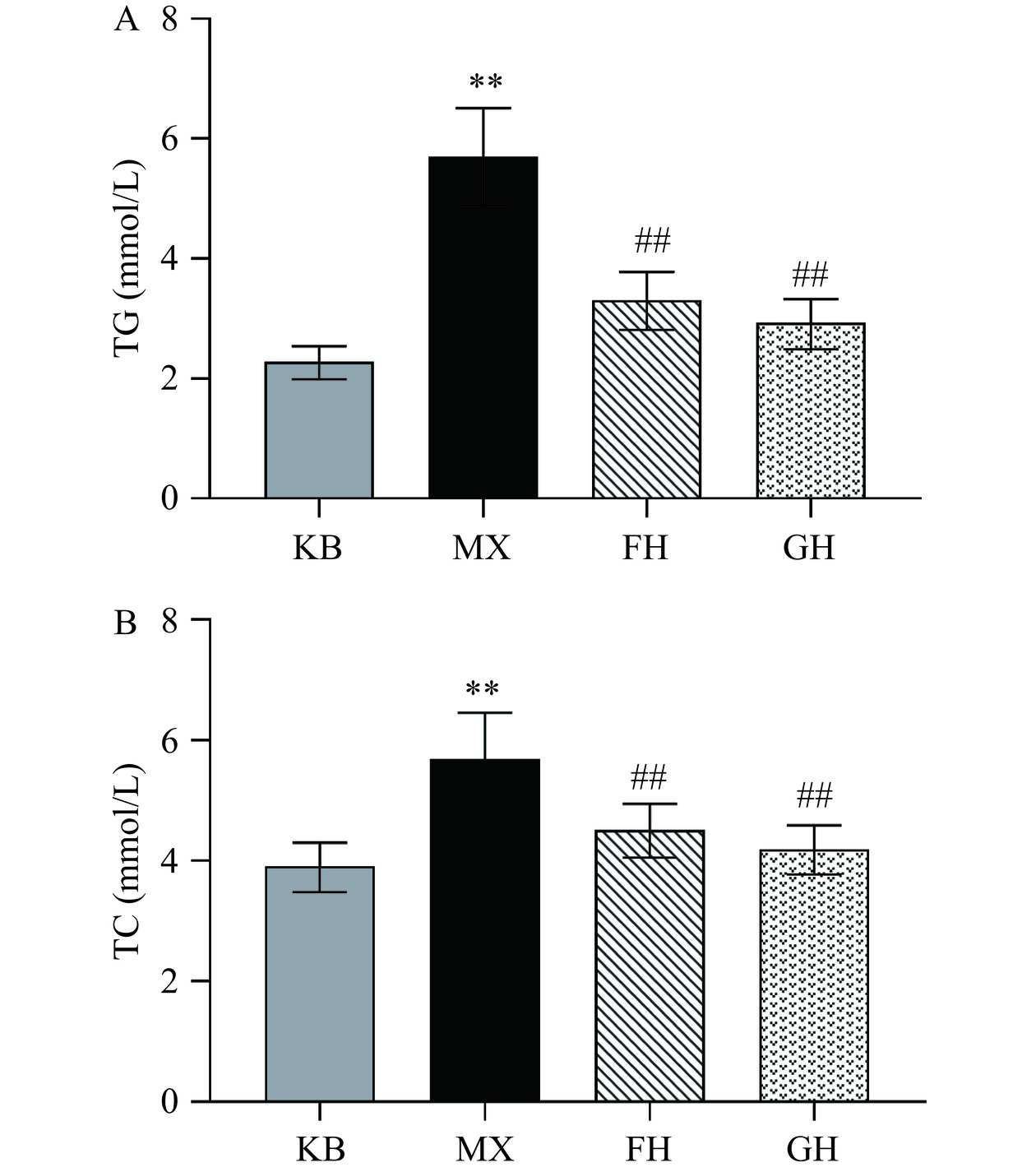
对小鼠血清中TG和TC的含量进行测定,结果如图3所示。与KB组相比,MX组小鼠血清中TG组和TC组水平极显著升高(P<0.01);与MX组相比,FH组和GH组的TG、TC水平极显著降低(P<0.01)。其中FH组的TG浓度降低了41.87%,TC浓度降低了20.61%,GH组的TG和TC浓度分别降低了48.56%、26.25%。酒精对肝脏的损害也反映在脂肪的积累上,可以通过确定相应脂肪的含量,反映酒精对肝脏的损害程度。
上述结果表明,50%酒精灌胃2 d会导致小鼠体内脂肪堆积。酵母抽提物、姜黄素+酵母抽提物复合物有效预防了酒精导致的小鼠血脂水平升高,对酒精导致的肝损伤具有保护作用。
2.4 受试物对急性酒精性肝损伤小鼠肝组织的影响
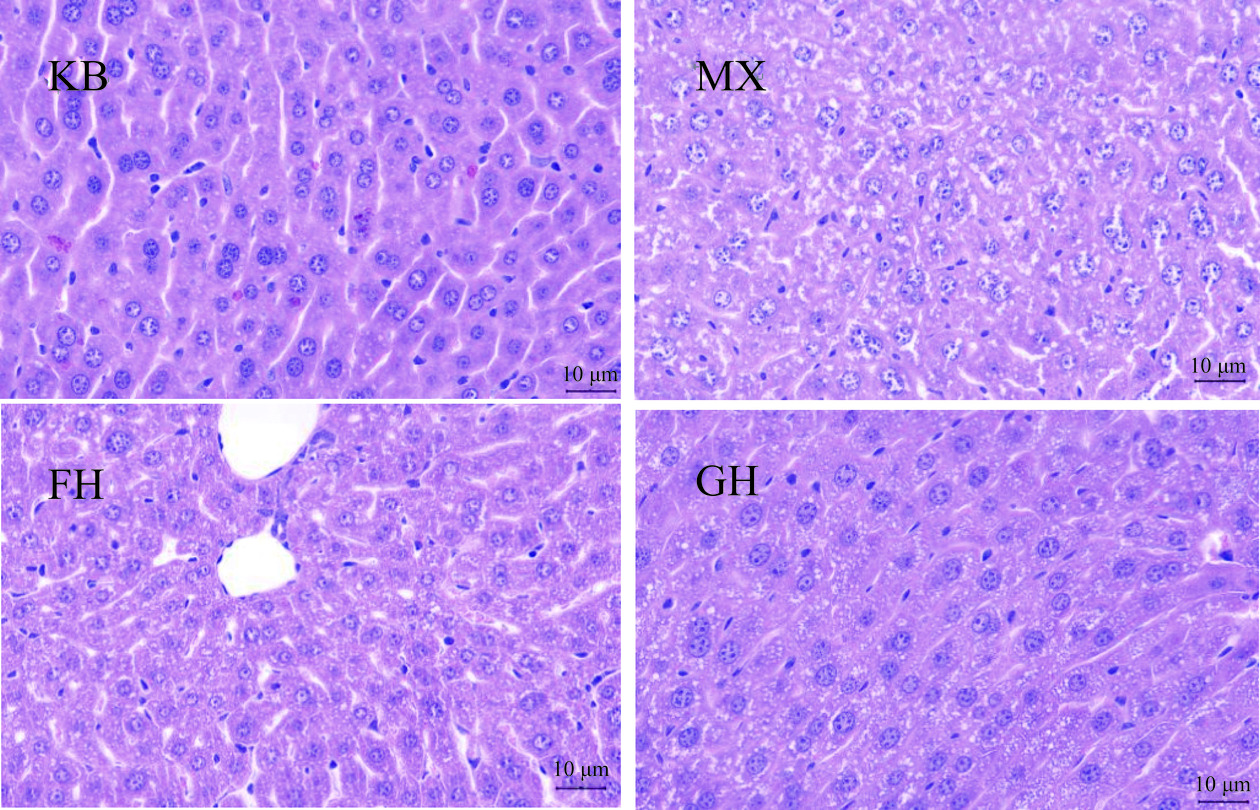
如图4所示,各组小鼠肝组织病理学检测,光镜下观察可见,KB组小鼠肝小叶结构清晰,肝细胞索呈放射状排列,肝细胞无水肿、变性及坏死,汇管区无炎症细胞浸润。而MX组肝细胞有炎症浸润并且伴有脂肪浸润,细胞内出现脂滴的异常病理改变。与MX组比较,GH组和FH组小鼠肝细胞病变程度明显减轻,GH组肝细胞索的排列情况明显优于FH组。
上述结果表明,50%酒精灌胃2 d会导致小鼠肝细胞索被破坏、肝细胞肿胀和脂滴等损伤,结合生理指标的变化,说明酒精性肝损伤模型造模成功。酵母抽提物、姜黄素+酵母抽提物复合物明显改善了酒精引起的肝细胞水肿和脂肪变性,且姜黄素+酵母抽提物复合物的效果优于酵母抽提物。
2.5 受试物对急性酒精性肝损伤小鼠肠道菌群的影响
2.5.1 ASV分析
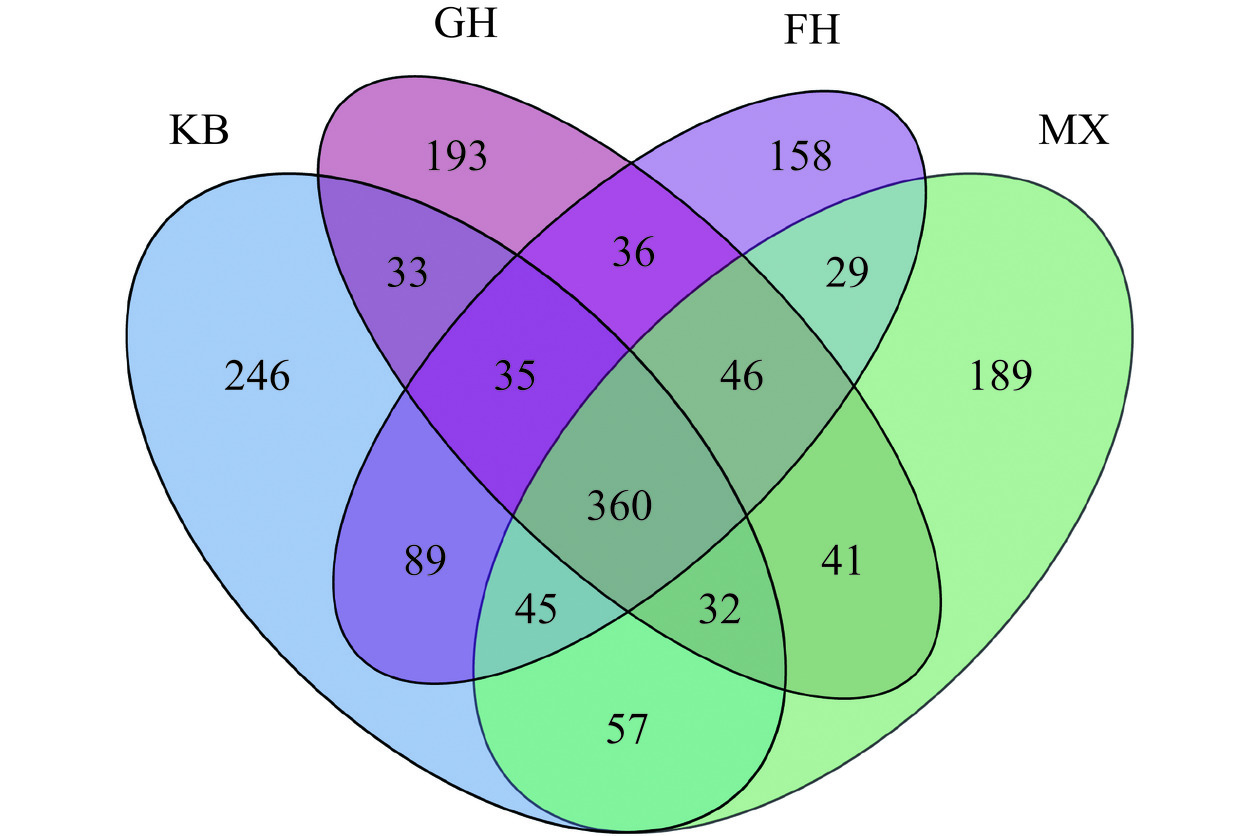
使用DADA2降噪后产生的每个去重的序列称为扩增序列变异(Amplicon sequence variants,ASVs),相当于以100%相似度聚类,ASVs数量越大说明微生物群落的多样性越大。绘制ASVs的Venn图,如图5所示,KB、MX、GH、FH组的ASVs数量分别为897、772、776、798个,各组独有的ASVs数量分别为246、189、193、158,各组共有的ASVs数量为360个。酒精会降低小鼠肠道菌群多样性,各组ASVs的数量之间的差异说明各组小鼠肠道菌群的相似度存在差异。
2.5.2 Alpha多样性分析
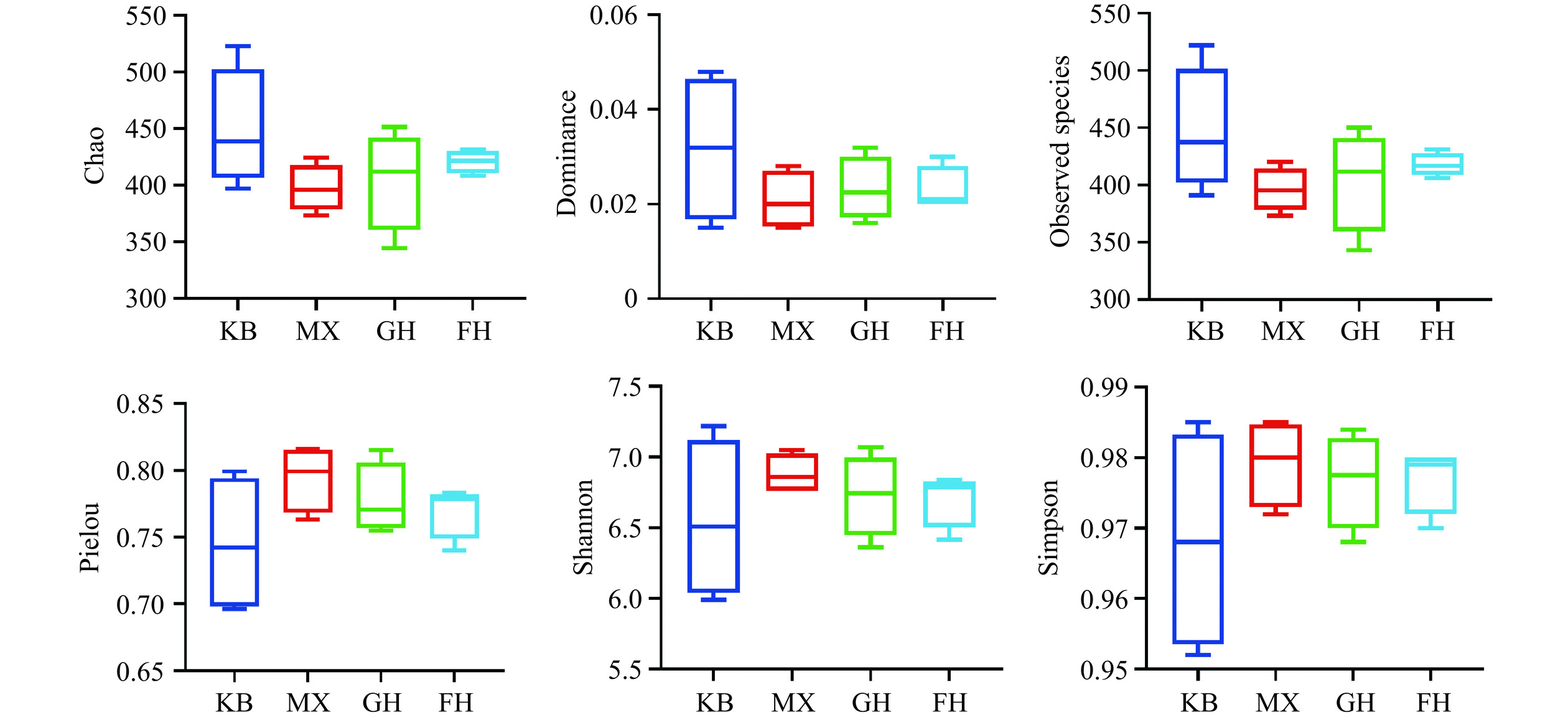
Alpha多样性是反映物种丰富度和均匀度的综合指标,选择Chao指数、Dominance指数、Observed species指数、Pielou指数、Shannon指数、Simpson指数评价其Alpha多样性。Chao反映了群落丰富度;Dominance和Pielou反映了群落的物种均匀度;Observed species直观观测到的物种数目,指数越大观测到的物种越多;Shannon和Simpson则反映了群落多样性[23]。如图6所示,Chao、Dominance、Observed species指数由高到低分别为KB组、FH组和GH组、MX组,其它指数无明显趋势,说明酒精对小鼠肠道菌群有一定影响,酵母抽提物、姜黄素+酵母抽提物复合物使肝损伤小鼠肠道菌群的丰富度和均匀度有所升高,但未见统计学差异。
2.5.3 Beta多样性分析
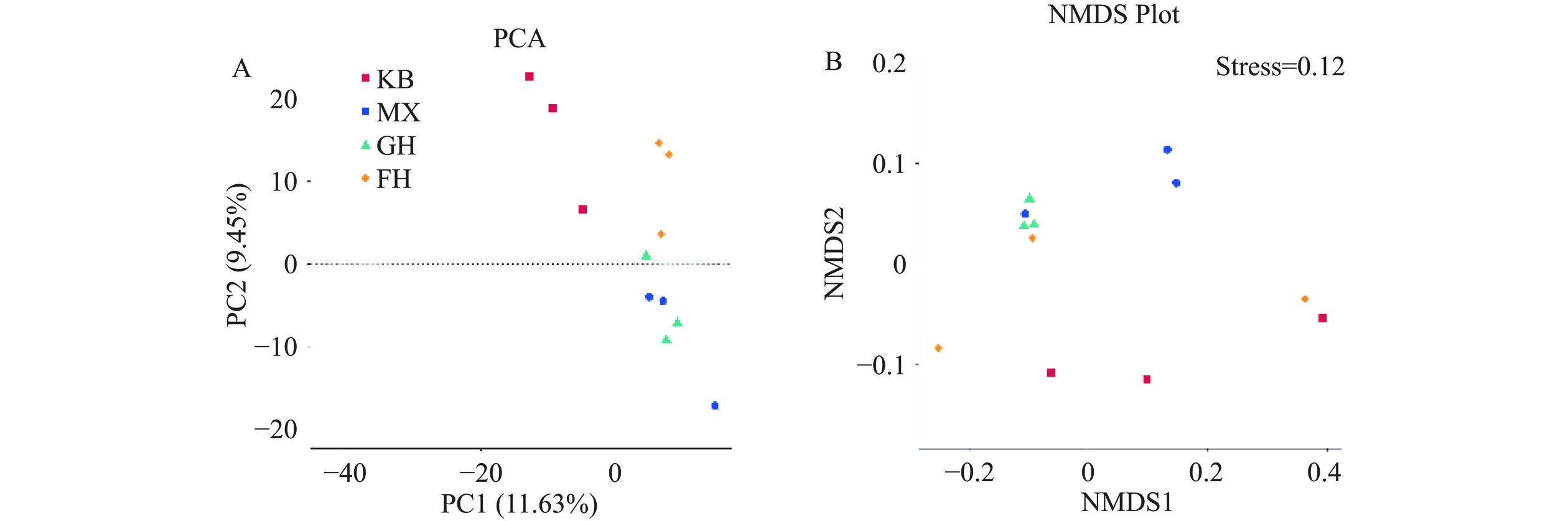
Beta多样性是对不同微生物群落间的物种多样性进行组间比较来反映样本间微生物群落结构的差异,可通过主成分分析(Principal component analysis,PCA)、非度量多维尺度分析(Non-metric multidimensional scaling,NMDS)来直观地反映样本间的差异,且NMDS的Stress值小于0.2[24]。如图7所示,肠道菌群群落组成差异较小的样本在图中分布得更近,MX组与KB组区分明显,说明酒精会改变小鼠肠道菌群的结构,FH组的样本分布与MX组分离,趋向于KB组,GH组样本分离不明显,说明酵母抽提物干预了酒精损伤小鼠的肠道菌群结构,对酒精损伤小鼠肠道菌群紊乱具有预防保护作用。
2.5.4 小鼠肠道菌群群落组成分析
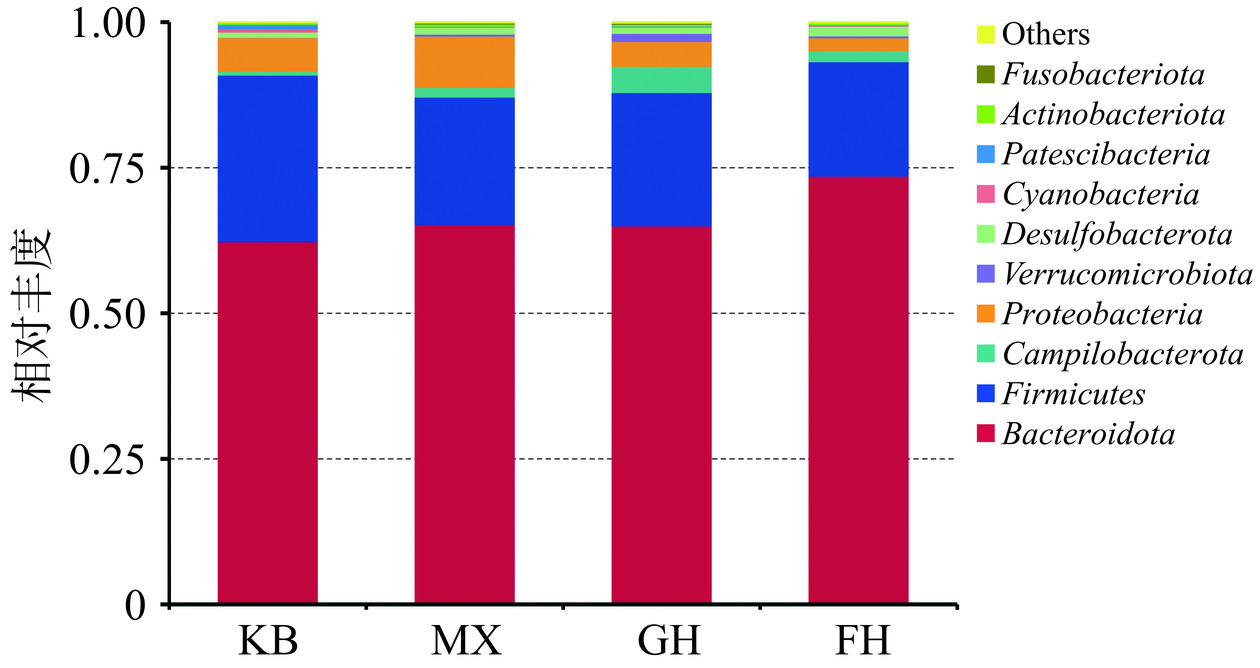
根据分类学结果,可获得各组在分类学水平上的组成情况,在门、属水平绘制物种相对丰度柱形累加图进行可视化分析,更加直观的分析各组在某一分类学水平上的群落物种组成、各组菌群组成的相似性和差异性。如图8所示,在门水平上,与KB组相比,MX组厚壁菌门(Firmicutes)相对丰度极显著降低(P<0.01),拟杆菌门(Bacteroidota)、变形菌门(Proteobacteria)、放线菌门(Actinobacteriota)的相对丰度显著升高(P<0.05);与MX组相比,GH组厚壁菌门(Firmicutes)的相对丰度得到恢复(P<0.05),变形菌门、放线菌门的相对丰度显著降低(P<0.05),拟杆菌门的相对丰度降低,但未见统计学差异;FH组变形菌门和放线菌门的相对丰度显著降低(P<0.05),而在厚壁菌门的调节效果上则GH组效果更优。
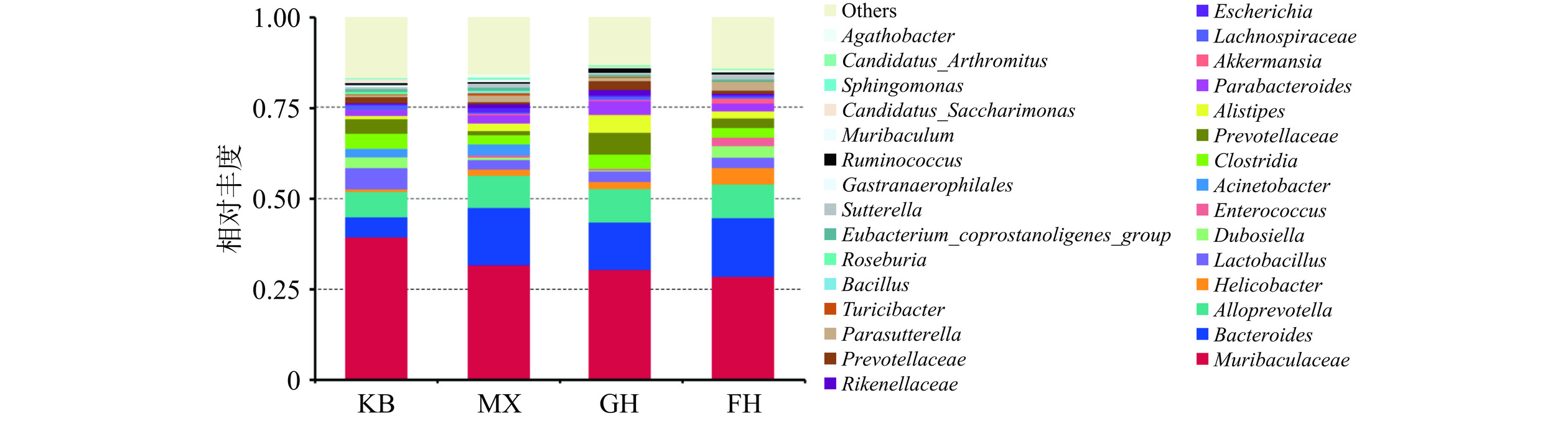
如图9所示,在属水平上,与KB组相比,MX组不动杆菌属(Acinetobacter)等显著增加(P<0.05),普雷沃菌属(Prevotella)、乳杆菌属(Lactobacillus)、瘤胃球菌属(Ruminococcus)等显著减少(P<0.05);与MX组相比,GH组不动杆菌属极显著减少(P<0.01),普雷沃菌属、瘤胃球菌属极显著增加(P<0.01);FH组不动杆菌属显著减少(P<0.05),乳杆菌属、普雷沃菌属、瘤胃球菌属等相对丰度升高,但未见统计学差异。相比于FH组来说,添加姜黄素的GH组在普雷沃菌属、瘤胃球菌属的调节作用上效果更优。小鼠肠道菌群的优势菌主要是拟杆菌门、厚壁菌门以及变形菌门,在肠道中参与碳水化合物和脂质的代谢,对宿主的健康有非常重要的影响[25−26]。相关研究表明过敏、多发性硬化症等的发生均与普雷沃氏菌丰度减少有关,普雷沃氏菌可参与治疗肥胖、代谢综合征、炎性肠病等,但作用及机制等还需进一步探究验证[27];瘤胃球菌属是肠道中分解碳水化合物最有效的细菌属之一,有着稳定肠道屏障、逆转腹泻、降低结直肠癌风险、调节免疫系统等作用[28−29]。
3. 讨论与结论
本研究通过建立小鼠急性酒精性肝损伤模型,探讨了酵母抽提物、姜黄素+酵母抽提物复合物对酒精诱导肝损伤的预防保护作用及对小鼠肠道菌群的调节作用。理想的急性酒精性肝损伤动物模型应包括其过程的所有方面,包括显著的血清ALT、AST浓度升高、脂肪变性、肝中性粒细胞浸润和肝损伤[30]。本研究采用50%酒精灌胃2 d的方式制备急性酒精性肝损伤模型,结果发现,与正常对照组相比,模型组的AST和ALT活力显著升高约1.5倍,且模型组的肝细胞有炎症浸润并且伴有脂肪浸润,细胞内出现脂滴的异常病理改变,这些结果表明,在酒精诱导下成功建立了小鼠急性酒精性肝损伤模型。
已有研究表明酵母抽提物具有调节免疫力、抗氧化、调节肠道稳态等益生作用,富硒酵母联合维生素E能够提高小鼠体内抗氧化水平蛋白CAT、GPX4、核Nrf2、SOD1的表达,下调TNF-α和NF-κB p65的表达,从而改善肝脏损伤程度[31−32];酵母源β-葡聚糖可以通过激活Nrf2信号通路和抑制NF-κB信号通路从而起到抗氧化和抗炎的作用,诱导肠道菌群的组成和结构提升与抗氧化相关基因(Gsta、Nqol、Prdx5和Trxl)的表达,进而提高SOD酶活力,抑制NF-κB的活化,降低IFN-γ、NLRP3和HMGBl的表达,进而发挥护肝作用[33];姜黄素可通过抑制ROS的产生,上调Trx-1和下调TXNIP,抑制NLRP3炎症小体活化,从而预防小鼠急性肝损伤[34],证明了酵母抽提物和姜黄素有助于开发预防急性酒精性肝损伤的保健品。
ALD的发病机制涉及氧化应激、脂质代谢、肠道菌群等各方面,氧化应激是酒精造成肝脏损伤的重要原因之一[35]。MDA是氧化应激导致脂质过氧化产物的一种,其含量可以反映机体内脂质过氧化的程度以及自由基攻击导致肝细胞损伤的程度[36]。GSH-Px是一种过氧化物酶,其主要作用是催化谷胱甘肽还原二氧化氢,从而清除过氧化物,通过检测GSH-Px的活力和MDA的水平来反映机体或者细胞的脂质氧化损伤程度,间接判断肝细胞的氧化应激水平[37−38]。急性酒精性肝损伤引起脂质代谢紊乱[39],主要表现在TG和TC的堆积,过量酒精摄入会抑制TG和TC的代谢,TG在肝脏堆积,导致肝脏发生脂肪变性,同时酒精还会影响血清中脂质的转运[40]。本研究中酵母抽提物组和姜黄素+酵母抽提物复合物组T-GSH、GSH-Px、TG、TC的含量明显升高,MDA的含量明显降低,表明酵母抽提物、姜黄素+酵母抽提物复合物有助于机体清除酒精代谢过程中产生的自由基,缓解了机体由急性酒精引起的氧化应激和脂质代谢紊乱,从而预防肝损伤。
许多研究证明急性酒精性肝损伤与肠道菌群失调紧密相关,肝脏作为第一个接触微生物产物进入门静脉循环的器官,可能受到肠道微生物群及其变化的多种影响[41−42]。酒精导致细菌群落结构发生变化,引起肠道益生菌减少,致病菌过度生长,进而导致肠道内容物中内毒素增加,且肠黏膜屏障受损,肠道内细菌、毒素等有害物质随门静脉系统进入肝脏,进而导致肝损伤[43−44]。因此,通过调节肠道菌群来预防急性酒精性肝损伤是一个可行的策略,梁璇[45]通过补充肠道益生菌可恢复酒精导致的肠道菌群失调,增强肠道屏障功能,降低NF-κB蛋白的表达,降低血清内的炎症因子,达到预防酒精性肝损伤的效果;Li等[46]通过口服姜黄素能减轻大鼠的肝脏异位脂肪沉积,缓解炎症因子,改善肠道屏障完整性,降低厚壁菌门/拟杆菌门的比例,恢复肠道微生物群的组成。本文研究结果显示,在门水平上,酒精处理显著降低了小鼠肠道菌群的丰度和均匀度,降低厚壁菌门的丰度,增加了拟杆菌门、变形菌门和放线菌门的丰度;在属水平上,酒精使普雷沃菌属、瘤胃球菌属相对丰度降低,但姜黄素+酵母抽提物复合物组能有效预防,且效果优于酵母抽提物组。
酒精性肝损伤的早期预防尤为重要,故需要找到能有效预防急性酒精性肝损伤的途径。本文研究了酵母抽提物、姜黄素+酵母抽提物复合物对急性酒精性肝损伤小鼠的保护作用以及肠道菌群的调节作用。结果发现:酵母抽提物、姜黄素+酵母抽提物复合物可显著降低ALT、AST水平,恢复肝组织中T-GSH和GSH-Px的含量,降低MDA以及TG、TC的水平,表明酵母抽提物、姜黄素+酵母抽提物复合物可提高机体的抗氧化能力,缓解机体由急性酒精引起的氧化应激和脂质代谢紊乱;并有效恢复了由酒精引起的小鼠肠道菌群变化,提高了普雷沃菌属、瘤胃球菌属等有益菌的丰度。
本研究为其在保健品应用方面提供实验依据,在预防急性酒精性肝损伤保健食品的开发方面具有重要的意义和广阔的前景,后期应对急性酒精性肝损伤代谢通路有影响的关键菌或其关键代谢产物进一步研究。
-
-
[1] ZHU L, LI H D, XU J J, et al. Advancements in the alcohol-associated liver disease model[J]. Biomolecules,2022,12(8):1−26.
[2] SONG X, LIU Z, ZHANG J, et al. Antioxidative and hepatoprotective effects of enzymatic and acidic-hydrolysis of Pleurotus geesteranus mycelium polysaccharides on alcoholic liver diseases[J]. Carbohydrate Polymers,2018,201:75−86. doi: 10.1016/j.carbpol.2018.08.058
[3] 庄辉. 中国肝病的疾病负担[R]. 厦门:中华医学会, 2023. [ZHUANG H. Disease burden of liver disease in China[R]. Xiamen:Chinese Medical Association, 2023.] ZHUANG H. Disease burden of liver disease in China[R]. Xiamen: Chinese Medical Association, 2023.
[4] LIU J, SONG H, LIU Y, et al. Discovery of kokumi peptide from yeast extract by LC-Q-TOF-MS/MS and sensomics approach[J]. Journal of the Science of Food and Agricuture,2015,95(15):3183−3194. doi: 10.1002/jsfa.7058
[5] LI H, SHI J, ZHAO L, et al. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 mixture prevents chronic alcoholic liver injury in mice by protecting the intestinal barrier and regulating gut microbiota and liver-related pathways[J]. Journal of Agricultural and Food Chemistry,2021,69(1):183−197. doi: 10.1021/acs.jafc.0c06346
[6] HOU R, LIU X, YAN J, et al. Characterization of natural melanin from Auricularia auricula and its hepatoprotective effect on acute alcohol liver injury in mice[J]. Food and Function,2019,10(2):1017−1027. doi: 10.1039/C8FO01624K
[7] AVILA M A, DUFOUR J F, GERBES A L, et al. Recent advances in alcohol-related liver disease (ALD):Summary of a gut round table meeting[J]. Gut,2020,69(4):764−780. doi: 10.1136/gutjnl-2019-319720
[8] 曲航, 高鑫, 伊娟娟, 等. 食源性天然产物对酒精性肝损伤的防护作用研究进展[J]. 食品科学,2020,41(17):283−290. [QU H, GAO X, YI J J. Research progress on the protective effect of foodborne natural products on alcoholic liver injury[J]. Food Science,2020,41(17):283−290.] doi: 10.7506/spkx1002-6630-20190920-262 QU H, GAO X, YI J J. Research progress on the protective effect of foodborne natural products on alcoholic liver injury[J]. Food Science, 2020, 41(17): 283−290. doi: 10.7506/spkx1002-6630-20190920-262
[9] PARK J, KIM S, LEE N Y, et al. Role of microbiota-derived metabolites in alcoholic and non-alcoholic fatty liver diseases[J]. International Journal of Molecular Sciences,2021,23(1):426. doi: 10.3390/ijms23010426
[10] SHARMA S P, SUK K T, KIM D J. Significance of gut microbiota in alcoholic and non-alcoholic fatty liver diseases[J]. World Journal of Gastroenterology,2021,27(37):6161−6179. doi: 10.3748/wjg.v27.i37.6161
[11] HARTMANN P, SEEBAUER C T, SCHNABL B. Alcoholic liver disease:The gut microbiome and liver cross talk[J]. Alcoholism:Clinical and Experimental Research,2015,39(5):763−775. doi: 10.1111/acer.12704
[12] 石拓, 刘晓倩, 徐庆阳. 酵母抽提物生产工艺的研究进展[J]. 发酵科技通讯,2019,48(1):5−8. [SHI T, LIU X Q, XU Q Y. Research progress of yeast extract production technology[J]. Bulletin of Fermentation Science and Technology,2019,48(1):5−8.] SHI T, LIU X Q, XU Q Y. Research progress of yeast extract production technology[J]. Bulletin of Fermentation Science and Technology, 2019, 48(1): 5−8.
[13] 努热孜姑丽·托合提卡地尔, 杨雅, 徐磊, 等. 小茴香提取物对酒精性肝损伤小鼠的保护作用[J]. 中国食品添加剂,2022,33(10):175−180. [NUREZIGULI T, YANG Y, XU L, et al. Protective effect of fennel extract on alcohol-induced liver injury in mice[J]. Chinese Food Additives,2022,33(10):175−180.] NUREZIGULI T, YANG Y, XU L, et al. Protective effect of fennel extract on alcohol-induced liver injury in mice[J]. Chinese Food Additives, 2022, 33(10): 175−180.
[14] 阿荣, 张洁. 肠道菌群失调与糖尿病相关性的研究进展[J]. 中国当代医药,2023,30(13):43−49. [A R, ZHANG J. Research progress on the correlation between intestinal flora disorder and diabetes mellitus[J]. China Modern Medicine,2023,30(13):43−49.] doi: 10.3969/j.issn.1674-4721.2023.13.011 A R, ZHANG J. Research progress on the correlation between intestinal flora disorder and diabetes mellitus[J]. China Modern Medicine, 2023, 30(13): 43−49. doi: 10.3969/j.issn.1674-4721.2023.13.011
[15] 李祥, 陈贵杰, 康贻军, 等. 酵母甘露糖蛋白体外发酵及其代谢产物的抗炎活性[J]. 食品科学,2023,44(2):212−221. [LI X, CHEN G J, KANG Y J, et al. Anti-inflammatory activity of yeast mannoglycoprotein and its metabolites in vitro fermentation[J]. Food Science,2023,44(2):212−221.] doi: 10.7506/spkx1002-6630-20220209-037 LI X, CHEN G J, KANG Y J, et al. Anti-inflammatory activity of yeast mannoglycoprotein and its metabolites in vitro fermentation[J]. Food Science, 2023, 44(2): 212−221. doi: 10.7506/spkx1002-6630-20220209-037
[16] 孟瑞媛, 卯明彩, 宋晓, 等. 姜黄素对黄曲霉毒素B1诱导的斑马鱼肝损伤的修复作用[J]. 农产品质量与安全,2023,1(2):33−39. [MENG R Y, MAO M C, SONG X, et al. Effects of curcumin on liver injury induced by aflatoxins B1 in zebrafish[J]. Quality and Safety of Agro-Products,2023,1(2):33−39.] doi: 10.3969/j.issn.1674-8255.2023.02.006 MENG R Y, MAO M C, SONG X, et al. Effects of curcumin on liver injury induced by aflatoxins B1 in zebrafish[J]. Quality and Safety of Agro-Products, 2023, 1(2): 33−39. doi: 10.3969/j.issn.1674-8255.2023.02.006
[17] 刘啸昂, 唐辉, 刘盼盼, 等. 姜黄素对HepG2细胞脂质沉积的改善作用及机制研究[J]. 上海中医药大学学报,2021,35(1):50−54. [LIU X A, TANG H, LIU P P, et al. Effect and mechanism of curcumin on lipid deposition in HepG2 cells[J]. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai,2021,35(1):50−54.] LIU X A, TANG H, LIU P P, et al. Effect and mechanism of curcumin on lipid deposition in HepG2 cells[J]. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai, 2021, 35(1): 50−54.
[18] 苟梦星, 卢安琪, 翟江洋. 姜黄素的功能特性及其在食品领域的应用现状[J]. 食品科技,2021,46(11):264−268. [GOU M X, LU A Q, ZHAI J Y. Functional properties of curcumin and its application in food field[J]. Food Science and Technology,2021,46(11):264−268.] doi: 10.3969/j.issn.1005-9989.2021.11.spkj202111040 GOU M X, LU A Q, ZHAI J Y. Functional properties of curcumin and its application in food field[J]. Food Science and Technology, 2021, 46(11): 264−268. doi: 10.3969/j.issn.1005-9989.2021.11.spkj202111040
[19] FENG D, ZOU J, SU D F, et al. Curcumin prevents high-fat diet-induced hepatic steatosis in Apo E-/-mice by improving intestinal barrier function and reducing endotoxin and liver TLR4/NF-κB inflammation[J]. Nutrition Metabolism,2019,16(1):79. doi: 10.1186/s12986-019-0410-3
[20] LOUVET A, MATHURIN P. Alcoholic liver disease:mechanisms of injury and targeted treatment[J]. Nature Reviews Gastroenterology and Hepatology,2015,12(4):231−242. doi: 10.1038/nrgastro.2015.35
[21] 王康乐. 云芝胞内糖肽不同组分抗酒精性肝损伤的活性评价[D]. 无锡:江南大学, 2018. [WANG K L. Evaluation of the activity of different components of intracellular glycopeptides in antialcoholic liver injury[D]. Wuxi:Jiangnan University, 2018.] WANG K L. Evaluation of the activity of different components of intracellular glycopeptides in antialcoholic liver injury[D]. Wuxi: Jiangnan University, 2018.
[22] DJAKPO D K, WANG Z Q, SHRESTHA M. The significance of transaminase ratio (AST/ALT) in acute myocardial infarction[J]. Archives of Medical Sciences:Atherosclerotic Diseases,2020,5:279−283. doi: 10.5114/amsad.2020.103028
[23] 宋新玲. 秀珍菇菌丝体多糖结构解析及其改善小鼠酒精性肝损伤的机制研究[D]. 泰安:山东农业大学, 2022. [SONG X L. Structure analysis of polysaccharides from mycelium of Pleurotus geesteranus and its mechanism of improving alcoholic liver injury in mice[D]. Taian:Shandong Agricultural University, 2022.] SONG X L. Structure analysis of polysaccharides from mycelium of Pleurotus geesteranus and its mechanism of improving alcoholic liver injury in mice[D]. Taian: Shandong Agricultural University, 2022.
[24] 祁程, 姜李欢, 阿卜杜萨拉木·努尔麦麦提, 等. 基于SMRT测序技术的塔里木马鹿冬季肠道菌群多样性分析[J]. 野生动物学报,2024,45(1):58−66. [QI C, JIANG L H, NUERMAIMAITI A, et al. Analysis of intestinal flora diversity of Tarim red deer in winter based on SMRT sequencing technology[J]. Chinese Journal of Wildlife,2024,45(1):58−66.] doi: 10.12375/ysdwxb.20240108 QI C, JIANG L H, NUERMAIMAITI A, et al. Analysis of intestinal flora diversity of Tarim red deer in winter based on SMRT sequencing technology[J]. Chinese Journal of Wildlife, 2024, 45(1): 58−66. doi: 10.12375/ysdwxb.20240108
[25] MUNOZ R, TEELING H, AMANN R, et al. Ancestry and adaptive radiation of Bacteroidetes as assessed by comparative genomics[J]. Systematic and Applied Microbiology,2020,43:126065. doi: 10.1016/j.syapm.2020.126065
[26] MOHAMED Q R, ASSAFI M S. The association between body mass index and the oral Firmicutes and Bacteroidetes profiles of healthy individuals[J]. Malaysian Family Physician,2021,16(3):36−43. doi: 10.51866/oa1129
[27] TETT A, PASOLLI E, MASETTI G, et al. Prevotella diversity, niches and interactions with the human host[J]. Nature Reviews Microbiology,2021,19(9):585−599. doi: 10.1038/s41579-021-00559-y
[28] HENKE M T, KENNY D J, CASSILLY C D, et al. Ruminococcus Gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide[J]. Proceedings of the National Academy of Sciences of the United States of America,2019,116(26):12672−12677.
[29] CHU H H, CHOU H C, TUNG Y L, et al. Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants[J]. Gastroenterology,2018,154(1):154−167. doi: 10.1053/j.gastro.2017.09.006
[30] PAZ A L, HAO F, NELSON L J, et al. Alcoholic liver disease:utility of animal models[J]. World Journal of Gastroenterology,2018,24(45):5063−5075. doi: 10.3748/wjg.v24.i45.5063
[31] 李子燕. S. C-AF工程菌减轻AFB1诱导小鼠肝损伤的作用及机制研究[D]. 南昌:南昌大学, 2023. [LI Z Y. Effect and mechanism of S. C-AF engineered bacteria on liver injury induced by AFB1 in mice [D]. Nanchang:Nanchang University, 2023.] LI Z Y. Effect and mechanism of S. C-AF engineered bacteria on liver injury induced by AFB1 in mice [D]. Nanchang: Nanchang University, 2023.
[32] 王跃. 富硒酵母联合维生素E减轻镉诱导小鼠肝损伤的研究[D]. 宜春:宜春学院, 2022. [WANG Y. Effects of selenium enriched yeast combined with vitamin E on cadmium-induced liver injury in mice [D]. Yichun:Yichun University, 2022.] WANG Y. Effects of selenium enriched yeast combined with vitamin E on cadmium-induced liver injury in mice [D]. Yichun: Yichun University, 2022.
[33] 李盼盼. 微生物源β-葡聚糖改善肝损伤的机制研究[D]. 无锡:江南大学, 2023. [LI P P. Study on the mechanism of microbial-derived β-glucan to improve liver injury [D]. Wuxi:Jiangnan University, 2023.] LI P P. Study on the mechanism of microbial-derived β-glucan to improve liver injury [D]. Wuxi: Jiangnan University, 2023.
[34] 张维, 张运超, 胡丞铭, 等. 姜黄素对四氯化碳诱导小鼠急性肝损伤的预防机制[J]. 武汉轻工大学学报,2023,42(6):37−46. [ZHANG W, ZHANG Y C, HU C M, et al. Preventive mechanism of curcumin on acute liver injury induced by carbon tetrachloride in mice[J]. Journal of Wuhan Polytechnic University,2023,42(6):37−46.] ZHANG W, ZHANG Y C, HU C M, et al. Preventive mechanism of curcumin on acute liver injury induced by carbon tetrachloride in mice[J]. Journal of Wuhan Polytechnic University, 2023, 42(6): 37−46.
[35] KIM H G, HUANG M, XIN Y, et al. The epigenetic regulator SIRT6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice[J]. Journal of Hepatology,2019,71(5):960−969. doi: 10.1016/j.jhep.2019.06.019
[36] MORITA M, ISHIDA N, UCHIYAMA K, et al. Fatty liver induced by free radicals and lipid peroxidation[J]. Free Radical Research,2012,46(6):758−765. doi: 10.3109/10715762.2012.677840
[37] SONG Y, ZHAO G Z, ZHAO B X, et al. Effect of electroacupuncture intervention at different time-points on levels of HSP70, MDA, SOD and GSH-Px of liver in rats with simulated weightlessness[J]. Acupuncture Research,2015,40(5):383−387.
[38] 彭月梅, 叶状, 汪飞燕, 等. 毒害艾美耳球虫谷胱甘肽过氧化物酶EnGPX的原核表达与分析[J]. 畜牧兽医学报,2024,55(2):846−853. [PENG Y M, YE Z, WANG F Y, et al. Prokaryotic expression and analysis of glutathione peroxidase EnGPX in toxic Eimeria[J]. Acta Veterinaria et Zootechnica Sinica,2024,55(2):846−853.] PENG Y M, YE Z, WANG F Y, et al. Prokaryotic expression and analysis of glutathione peroxidase EnGPX in toxic Eimeria[J]. Acta Veterinaria et Zootechnica Sinica, 2024, 55(2): 846−853.
[39] YOU M, ARTEEL G E. Effect of ethanol on lipid metabolism[J]. Journal of Hepatology,2019,70(2):237−248. doi: 10.1016/j.jhep.2018.10.037
[40] JEON S, CARR R. Alcohol effects on hepatic lipid metabolism[J]. Journal of Lipid Research,2020,61(4):470−479. doi: 10.1194/jlr.R119000547
[41] 程晓阳, 廖明, 何全光, 等. 三叶青超微粉对酒精性肝损伤大鼠肠道菌群的调节作用[J]. 食品工业科技,2023,44(18):415−424. [CHENG X Y, LIAO M, HE Q G, et al. Regulation effect of ultramicro powder of trifoliate on intestinal flora in rats with alcoholic liver injury[J]. Science and Technology of Food Industry,2023,44(18):415−424.] CHENG X Y, LIAO M, HE Q G, et al. Regulation effect of ultramicro powder of trifoliate on intestinal flora in rats with alcoholic liver injury[J]. Science and Technology of Food Industry, 2023, 44(18): 415−424.
[42] JONES R M, NEISH A S. Gut microbiota in intestinal and liver disease[J]. Annual Review of Pathology,2021,16:251−275. doi: 10.1146/annurev-pathol-030320-095722
[43] CHENG Y, WANG H H, HU Y Y, et al. Structural shifts of gut flora in rat acute alcoholic liver injury and jianpi huoxue decoction's effect displayed by ERIC-PCR fingerprint[J]. Chinese Journal of Integrative Medicine,2011,17(5):361−368. doi: 10.1007/s11655-011-0603-8
[44] XIAO J, ZHANG R, ZHOU Q, et al. Lychee (Litchi chinensis sonn.) pulp phenolic extract provides protection against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, intestinal barrier dysfunction, and liver inflammation[J]. Journal of Agricultural and Food Chemistry,2017,65(44):9675−9684. doi: 10.1021/acs.jafc.7b03791
[45] 梁璇, 张正, 单妍, 等. 肠道益生菌补充对酒精高糖高脂致肝损伤大鼠肝功能和肠道菌群的影响[J]. 中国临床保健杂志,2021,24(1):106−111. [LIANG X, ZHANG Z, SHAN Y, et al. Effects of intestinal probiotics supplementation on liver function and intestinal flora in rats with liver injury induced by alcohol high sugar and high fat[J]. Chinese Journal of Clinical Healthcare,2021,24(1):106−111.] doi: 10.3969/J.issn.1672-6790.2021.01.028 LIANG X, ZHANG Z, SHAN Y, et al. Effects of intestinal probiotics supplementation on liver function and intestinal flora in rats with liver injury induced by alcohol high sugar and high fat[J]. Chinese Journal of Clinical Healthcare, 2021, 24(1): 106−111. doi: 10.3969/J.issn.1672-6790.2021.01.028
[46] LI R F, YAO Y R, GAO P F, et al. The therapeutic efficacy of curcumin vs. metformin in modulating the gut microbiota in NAFLD rats:A comparative study[J]. Frontiers in Microbiology,2021(11):555293.
-
期刊类型引用(2)
1. 李嘉灏,曾瑶英,熊玉帛,佘杰海,余焯均,郭航宇,熊雯,龚林,周文化. 基于HS-SPME-GC-MS及电子舌对预制梅菜扣肉关键性风味与过熟味评价分析. 食品工业科技. 2024(21): 234-245 .  本站查看
本站查看
2. 郑训培,覃业优,巢瑾,蒋立文,李跑,刘洋. 常温低温发酵对二次发酵辣椒酱品质的影响比较. 食品安全质量检测学报. 2023(22): 210-219 .  百度学术
百度学术
其他类型引用(0)





 下载:
下载:
 下载:
下载:



