Inhibition of Proliferation of Esophageal Cancer Cells by Fucoidan Based on Nrf2/ROS Signaling Pathway
-
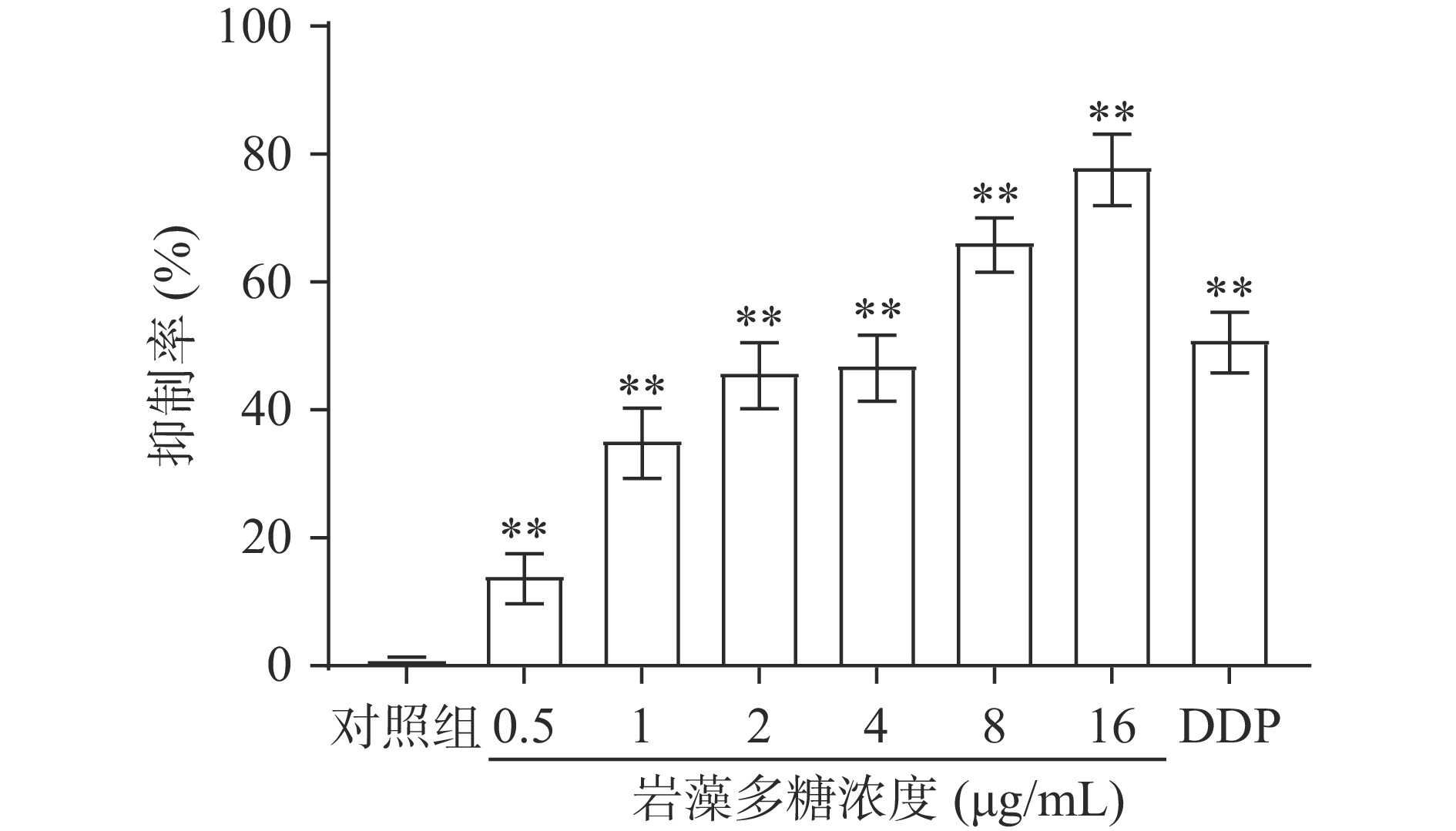
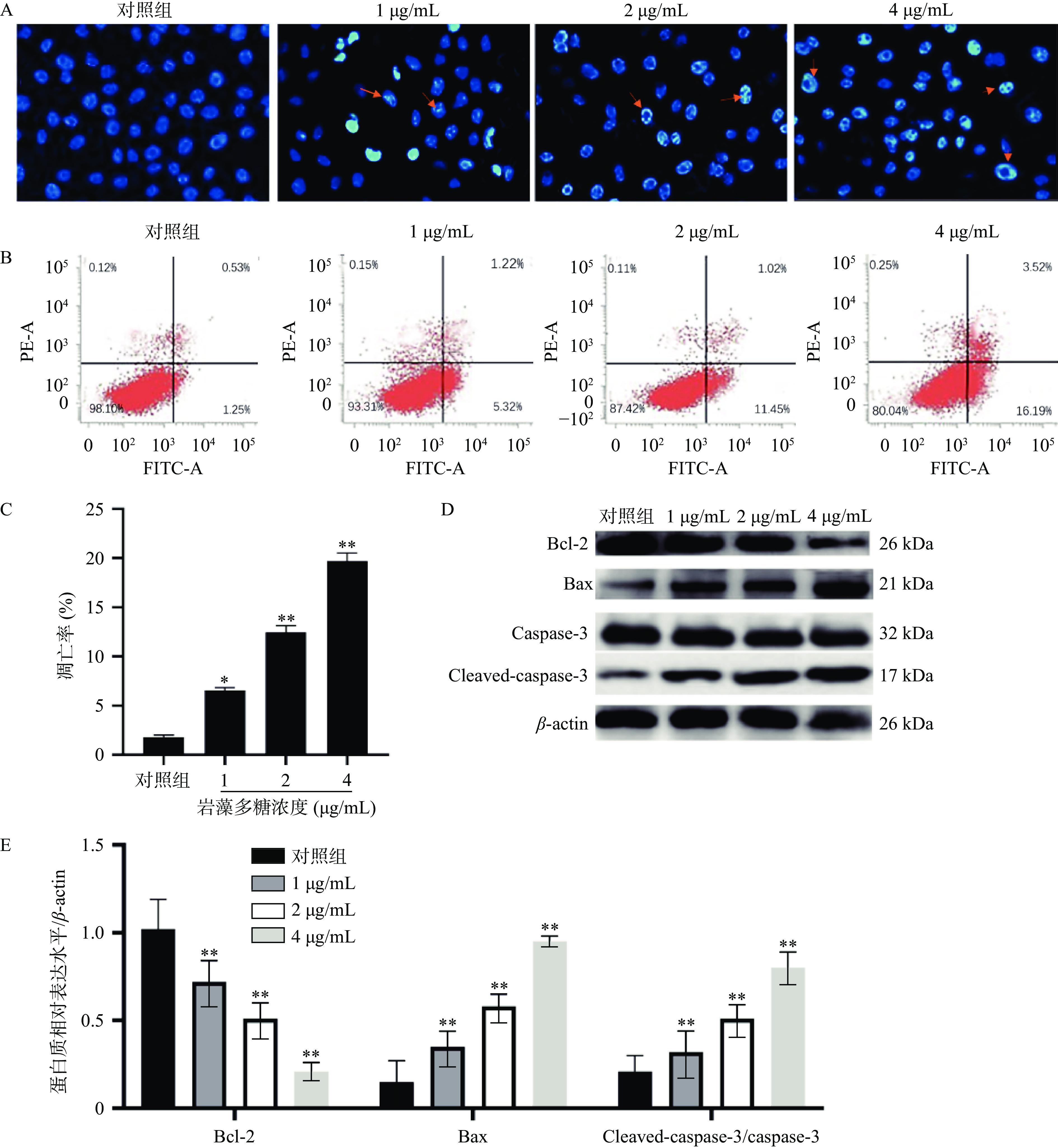
摘要: 目的:探讨岩藻多糖对食管癌增殖的影响,并分析其机制。方法:MTT法分析细胞增殖抑制率,Hoechst 33258染色和流式细胞术检测细胞凋亡,DCFH-DA探针检测ROS水平,Western blot法分析Nrf2、HO-1、NQO-1、Bcl-2、Bax、caspase-3水平,研究岩藻多糖对细胞增殖以及Nrf2/ROS信号通路的影响。裸鼠成瘤实验验证岩藻多糖对瘤体的瘤重、瘤体积及Nrf2、HO-1、NQO-1水平的影响。结果:1~16 µg/mL岩藻多糖极显著抑制ECA109细胞增殖,48 h IC50为3.26 µg/mL。与对照组(0.1% DMSO)相比,1、2、4 µg/mL岩藻多糖处理后的ECA109细胞出现核凝聚、染色质不规则收缩、凋亡小体等明显的凋亡特征,(极)显著促进ECA109细胞凋亡,极显著下调Bcl-2表达水平,极显著上调Bax、Cleaved-caspase-3表达水平,极显著增加ROS水平,极显著降低Nrf2、HO-1、NQO-1蛋白水平(P<0.05,P<0.01);Nrf2过表达能显著下调岩藻多糖抑制ECA109细胞增殖效果,显著下调ROS水平,显著上调Nrf2、HO-1、NQO-1蛋白水平(P<0.05)。体内实验显示,50、100 mg/kg岩藻多糖极显著抑制瘤体体积、瘤体质量,下调瘤体Nrf2、HO-1、NQO-1水平(P<0.05)。结论:岩藻多糖抑制ECA109细胞增殖,对体内移植瘤抑瘤效果显著,其机制与调控Nrf2/ROS信号通路有关。
-
关键词:
- 岩藻多糖 /
- Nrf2/ROS信号通路 /
- 食管癌 /
- 细胞增殖 /
- 移植瘤
Abstract: Objective: To explore the effect of fucoidan on esophageal cancer and analyze its mechanism. Methods: MTT assay was used to analyze the inhibition rate of cell proliferation, Hoechst33258 staining and flow cytometry were used to detect cell apoptosis, and DCFH-DA probe was used to detect ROS level, and Western blot was used to analyze levels of Nrf2, HO-1, NQO-1, Bcl-2, Bax and caspase-3, which were used to observe its effects on fucoidan-regulated cell proliferation and Nrf2/ROS signaling pathway. The tumor formation experiment in nude mice verified the effects of fucoidan on tumor weight, tumor volume and levels of Nrf2, HO-1 and NQO-1 in nude mice. Results: The proliferation of ECA109 cells was significantly inhibited by fucoidan from 1 to 16 µg/mL, and the IC50 was 3.26 µg/mL at 48 h. Compared with the control group (0.1%DMSO), ECA109 cells treated with 1, 2, and 4 µg/mL fucoidan showed obvious apoptotic characteristics, such as nuclear condensation, irregular chromatin contraction and apoptotic bodies, which (extremely) significantly promoted the apoptosis of ECA109 cells and significantly down-regulated the expression level of Bcl-2.The expression levels of Bax and Cleaved-caspase-3 were significantly increased, ROS levels were significantly increased, and Nrf2, HO-1, and NQO-1 protein levels were significantly decreased (P<0.05, P<0.01). Nrf2 overexpression could significantly down-regulate the inhibitory effect of fucoidan on ECA109 cell proliferation, significantly down-regulate ROS levels, and significantly up-regulate Nrf2, HO-1, NQO-1 protein levels (P<0.05). In vivo experiments showed that 50 mg/kg and 100 mg/kg of fucoidan significantly inhibited tumor volume and mass, and down-regulated the levels of Nrf2, HO-1 and NQO-1 in tumors (P<0.05). Conclusion: Fucoidan inhibits the proliferation of ECA109 cells and has significant anti-tumor effect on transplanted tumor in vivo, and its mechanism is related to the regulation of Nrf2/ROS signal pathway.-
Keywords:
- fucoidan /
- Nrf2/ROS signal pathway /
- esophageal cancer /
- cell proliferation /
- transplanted tumor
-
食道癌(Esophageal cancer,EC)是最常见、最致命的恶性肿瘤之一,虽然治疗技术有所改进,但EC患者的预后仍然不佳[1]。此外,由于频繁的局部侵袭和远处转移,食管癌的5年生存率很低,约为10%[2]。已有的研究显示,活性氧(ROS)在包括癌症在内的多种疾病中发挥重要作用[3],高水平的ROS可以增强氧化应激,增加肿瘤组织辐射、化疗敏感性[4]。而ROS与转录因子NF-E2相关因子2(Nrf2)之间存在一定的联系,可以通过调控下游血红素加氧酶-1(HO-1)、烟酰胺腺嘌呤二核苷酸磷酸(NADPH)和醌氧化还原酶-1(NQO-1)水平控制氧化应激[5−7]。因此,通过调控Nrf2/ROS信号通路对癌症的治疗具有一定的意义。
岩藻多糖是从海带等海洋海藻中提取的岩藻糖胶提取物,具有多维抗癌活性,并且已被发现对正常细胞无毒[8]。岩藻多糖主要成分为α-L-岩藻糖,含量为34%~44%,其他单糖组成包括半乳糖、木糖、甘露糖、糖醛酸等[9]。作为膳食补充剂和传统药物,能与多种癌症相关的细胞信号通路相互作用,包括PI3K/AKT,MAPK和caspase信号通路,抑制癌细胞增殖、迁移和侵袭,诱导细胞凋亡[10]。鉴于上述功能,岩藻多糖可能是对抗食管癌的潜在候选者[11]。尽管关于岩藻多糖抗癌活性的报道较多,但它们对诱导细胞凋亡的机制的影响,包括食管癌或任何肿瘤系统中的Nrf2/ROS干扰,研究甚少。本研究重点分析岩藻多糖对食管癌细胞增殖、凋亡的影响,并分析岩藻多糖和Nrf2/ROS信号通路在调节食管癌细胞增殖和凋亡中的作用,为岩藻多糖的开发提供依据。
1. 材料与方法
1.1 材料与仪器
人食管癌ECA109细胞系 齐氏生物科技有限公司;BALB/c裸鼠 90只SPF级5~6周龄,雄性,体重(16~20 g) 中国科学院上海动物实验中心,动物合格证号SCXK(沪)2017-0005。标准动物房饲养,温度(22±5) ℃,湿度57%±8%,光暗交替各12 h,自由获得标准的饲料和水。本研究动物实验均经漯河医学高等专科学校动物实验伦理委员会批准(伦理编号:20220009);岩藻多糖(纯度93%) 南京景竹生物科技有限公司;兔抗人Nrf2、HO-1、NQO-1、Bcl-2、Bax、caspase-3抗体、HRP标记的羊抗兔IgG二抗 美国Sigma公司;pcDNA3.1-Nrf、对照质粒pcDNA3.1 上海吉玛制药有限公司。
CB-S170 CO2培养箱 德国Binder公司;DMIL LED倒置荧光显微镜 德国徕卡公司;ELx800全自动酶标仪 美国BioTek公司;BIO-RAD伯乐电泳成像系统 美国BIO-RAD公司。
1.2 实验方法
1.2.1 细胞培养
细胞在补充有10% FBS和1%青霉素/链霉素的DMEM中于37 ℃和5% CO2条件下培养。
1.2.2 MTT法检测细胞抑制率
将ECA109细胞(4×103个细胞/孔)接种到96孔板中,贴壁后,37 ℃下用不同浓度(0、0.5、1、2、4、8、16 µg/mL)的岩藻多糖处理细胞。另设阳性对照组(2 mg/L,顺铂,DDP)、对照组(0.1% DMSO)和空白对照组(只加培养基),均孵育48 h,加入终浓度为5 mg/mL的MTT溶液在37 ℃孵育4 h,DMSO(150 µL/孔)溶解甲臜晶体。分光光度计在490 nm波长处检测吸光度[12],采用以下公式计算增殖抑制率。
1.2.3 细胞分组
将ECA109细胞(2×105细胞/孔)接种在6孔板中,细胞贴壁后,随机分组:对照组(0.1%DMSO)、岩藻多糖(0、1、2和4 µg/mL)组。
1.2.4 Hoechst 33258染色分析细胞凋亡
参照“1.2.3”分组,处理48 h后,PBS洗涤3次,多聚甲醛(4%)室温固定15 min,100 µL 0.5 µg/mL Hoechst 33258溶液染色15 min。采用荧光显微镜随机选择的视野中观察染色的细胞。细胞核浓缩和破碎即为凋亡细胞[13]。
1.2.5 流式细胞术检测细胞凋亡
参照“1.2.3”分组,处理48 h。PBS洗涤3次,5 μL Annexin V-FITC和5 μL PI避光孵育15 min,使用流式细胞仪检测细胞凋亡,计算细胞凋亡率。细胞凋亡率(%)=早期凋亡率(%)+晚期凋亡率(%)[14]。
1.2.6 Western blot检测Bcl-2、Bax、caspase-3、Nrf2、HO-1、NQO-1蛋白水平
参照“1.2.3”分组,处理48 h。采用含有蛋白酶抑制剂的裂解液裂解细胞,并提取总蛋白,BCA试剂盒定量。在10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离后,将蛋白质转移到聚偏二氟乙烯(PVDF)膜上,5% BSA封闭1 h,加入Bcl-2(1:2000)、Bax(1:10000)、caspase-3(1:1000)、Nrf2(1:2000)、HO-1(1:2000)、NQO-1(1:10000)抗体,4 ℃孵育过夜。TBST洗膜3次,加入相应的羊抗兔抗体(1:1000),室温下反应1 h,TBST冲洗膜3次。采用Image J分析软件进行分析,目的蛋白与GAPDH灰度值的比值表示目的蛋白的相对含量[15]。
1.2.7 DCFH-DA法检测ROS水平
参照“1.2.3”分组,处理48 h。将细胞与含有10 μmol/L DCFH-DA的无血清培养基在37 ℃下孵育20 min,然后用无血清培养基洗涤两次以去除细胞外DCFH-DA。倒置荧光显微镜下拍照分析,荧光酶标仪检测荧光强度[16]。
1.2.8 细胞转染及分组处理
将ECA109细胞(2×105细胞/孔)接种在6孔板中,细胞贴壁后,依据实验需要设置5组,包括:对照组、pcDNA3.1-Nrf2组、pcDNA3.1组、岩藻多糖组、岩藻多糖+pcDNA3.1-Nrf2组。依据分组采用Lipofectamine 2000将pcDNA3.1-Nrf2、pcDNA3.1分别转染至ECA109细胞[17],转染6 h后,对照组、pcDNA3.1-Nrf2组、pcDNA3.1组只加新鲜培养基,而岩藻多糖组、岩藻多糖+pcDNA3.1-Nrf2组加入终浓度为4 μg/mL的岩藻多糖,均继续处理48 h,Western blot实验检测Nrf2、HO-1、NQO-1蛋白水平,MTT法分析抑制率,DCFH-DA荧光探针分析ROS水平。
1.2.9 裸鼠急性毒性实验
预实验摸索岩藻多糖最小剂量(Dm)和最大剂量(Dn),采用等比数列(药物浓度比值r=0.70)设置5组(n=10),腹腔注射,隔日一次,持续14 d。SPSS 21.0软件计算LD50[18]。
1.2.10 移植瘤实验
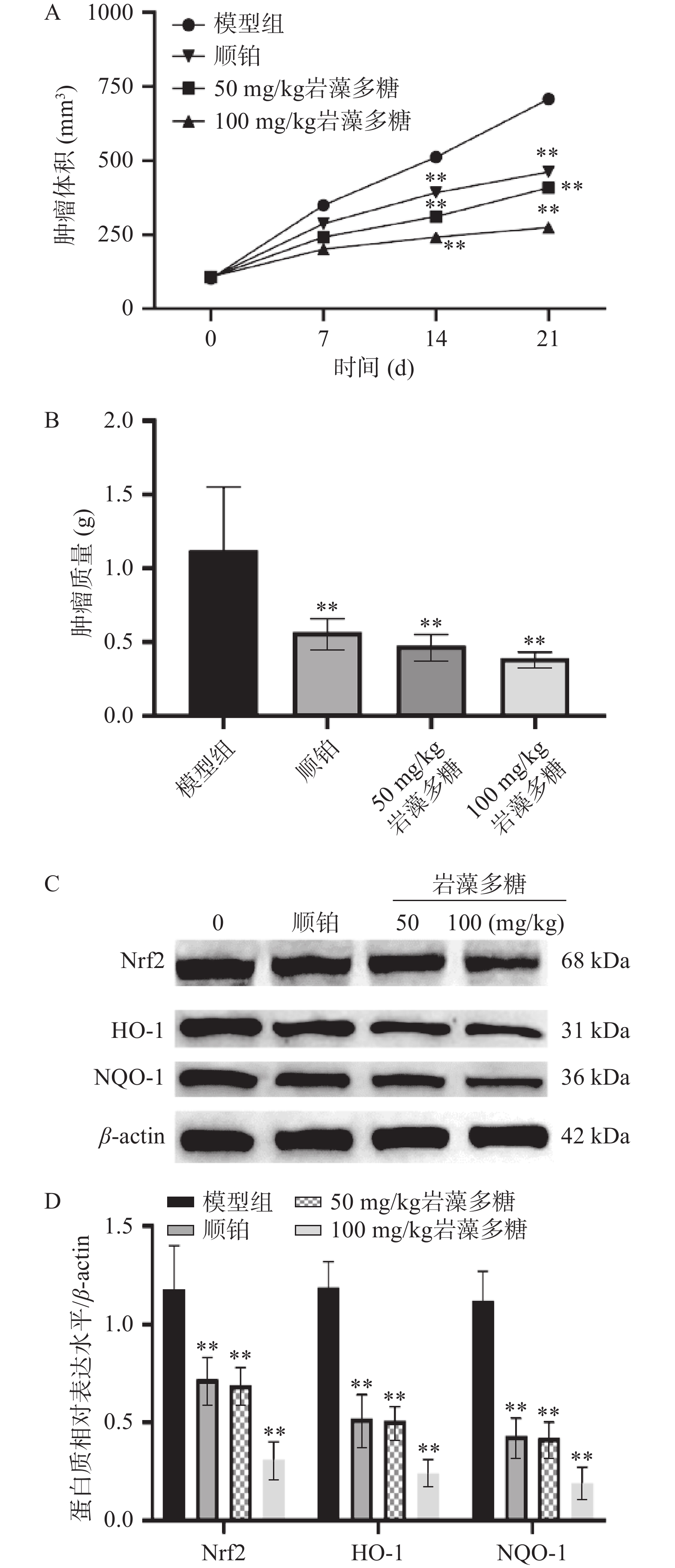
裸鼠随机分为模型组(生理盐水)、岩藻多糖组(50、100 mg/kg)、顺铂组(20 mg/kg,预实验获得)。将0.2 mL含3×107个ECA109细胞接种到BALB/c裸鼠的侧腹,每组6只。肿瘤达到100~200 mm3时,认为移植瘤造模成功。参照分组给药剂量进行腹腔给药(1次/2 d),游标卡尺评估肿瘤大小。肿瘤体积(mm3)=宽度(mm)2×长度(mm)/2。第21 d,裸鼠给予安乐死,并进行解剖、拍照和称重[19]。参照“1.2.6”提取总蛋白,并采用Western blot分析肿瘤中的Nrf2、NQO1与HO-1水平。
1.3 数据处理
SPSS 21.0软件进行统计学分析,采用单因素方差分析,组间比较采用LSD-t法,P<0.05为有统计学差异。
2. 结果与分析
2.1 岩藻多糖对ECA109细胞给药浓度的筛选
为了检测岩藻多糖对细胞增殖的影响,采用MTT法检测了吸光度,并计算增殖抑制率。结果表明,0.5、1、2、4、8、16 µg/mL岩藻多糖以剂量依赖的方式抑制ECA109细胞系的细胞增殖(图1)。处理48 h后,岩藻多糖对ECA109细胞系的IC50值为3.26 µg/mL。因此选择岩藻多糖浓度为1、2和4 µg/mL进行后续实验。
2.2 岩藻多糖对ECA109细胞凋亡的影响
本研究中,采用Hoechst 33258染色法检测岩藻多糖处理的ECA109细胞的形态学变化。与对照组相比,荧光显微镜下观察到岩藻多糖处理后的ECA109细胞出现核凝聚、染色质不规则收缩、凋亡小体等明显的凋亡特征[20](图2A)。流式细胞术分析进一步显示,岩藻多糖能显著提高细胞凋亡率,且呈剂量依赖性(图2B、2C)。在岩藻多糖刺激的ECA109细胞中,caspase-3切割(cleaved)活化之后生的成Cleaved-caspase-3显著增加(P<0.01),说明岩藻多糖促进了ECA109细胞凋亡,同时,Bcl-2表达水平降低,Bax表达水平升高,进一步说明岩藻多糖可以通过调节细胞凋亡相关蛋白水平诱导细胞凋亡(图2D、2E)。
2.3 岩藻多糖对ECA109细胞ROS水平及Nrf2/ROS信号通路的影响
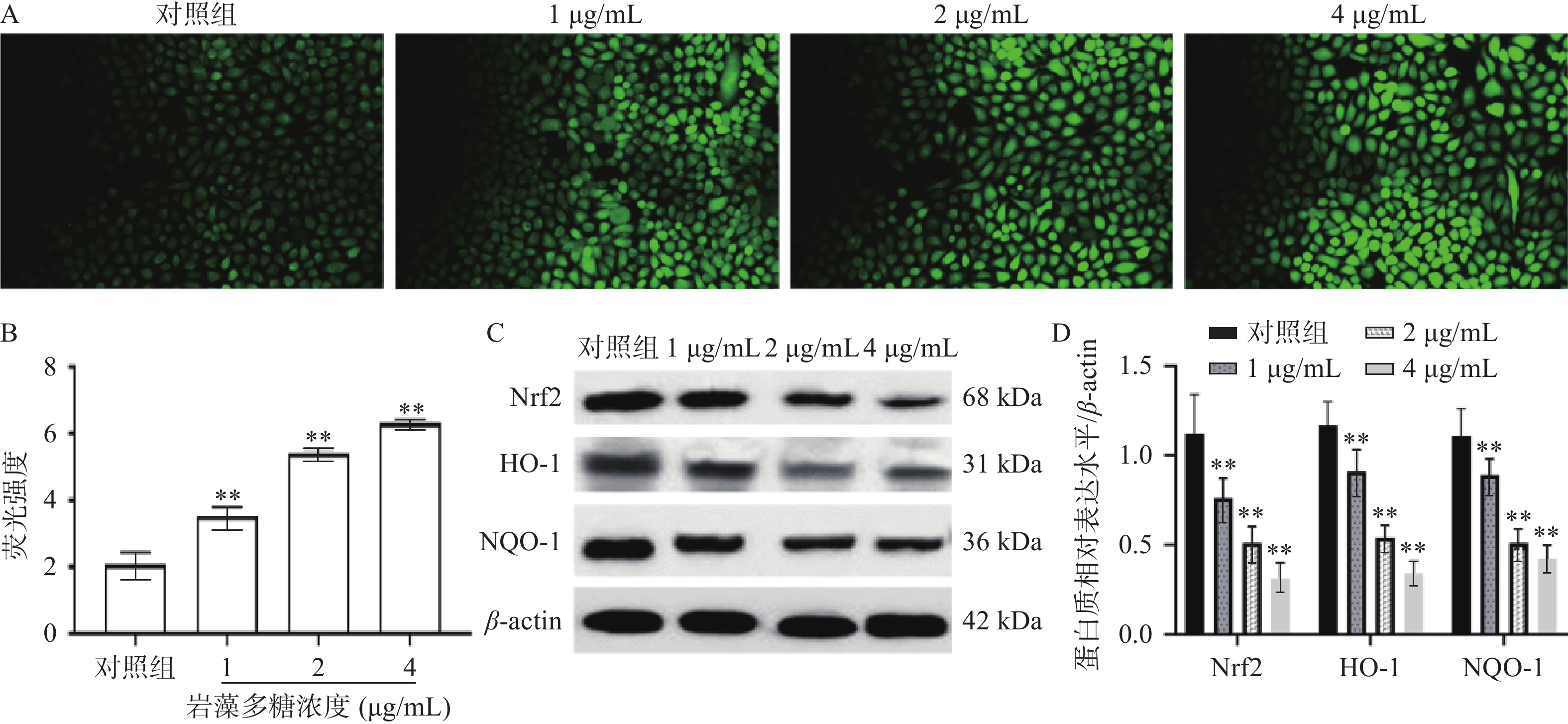
细胞内活性氧正常情况下保持着平衡[21],而在受到凋亡刺激下活性氧水平会增高,影响细胞正常活动而破坏细胞结构[22]。为观察岩藻多糖对ECA109细胞的氧化应激影响,本研究检测了ECA109细胞中ROS的水平。如图3A、3B所示,1、2、4 µg/mL岩藻多糖处理ECA109细胞48 h ROS的水平极显著增加(P<0.01),说明岩藻多糖会以浓度依赖的方式影响细胞内ROS的水平。
Nrf2是研究最多的细胞防御氧化应激的信号通路[23],当ROS水平升高时,Nrf2与其抑制剂Keap1解离,进入细胞核[24],通过HO-1启动下游蛋白的表达,抵抗氧化应激[25]。Western blot实验分析,ECA109细胞暴露于不同浓度的岩藻多糖48 h后,Nrf2、HO-1、NQO-1蛋白水平显著降低(图3C、3D),表明岩藻多糖能通过抑制ECA109细胞中的Nrf2/ROS 信号通路来激活氧化应激,导致细胞损伤,并诱发ECA109细胞凋亡。
2.4 Nrf2过表达对ECA109细胞增殖的影响
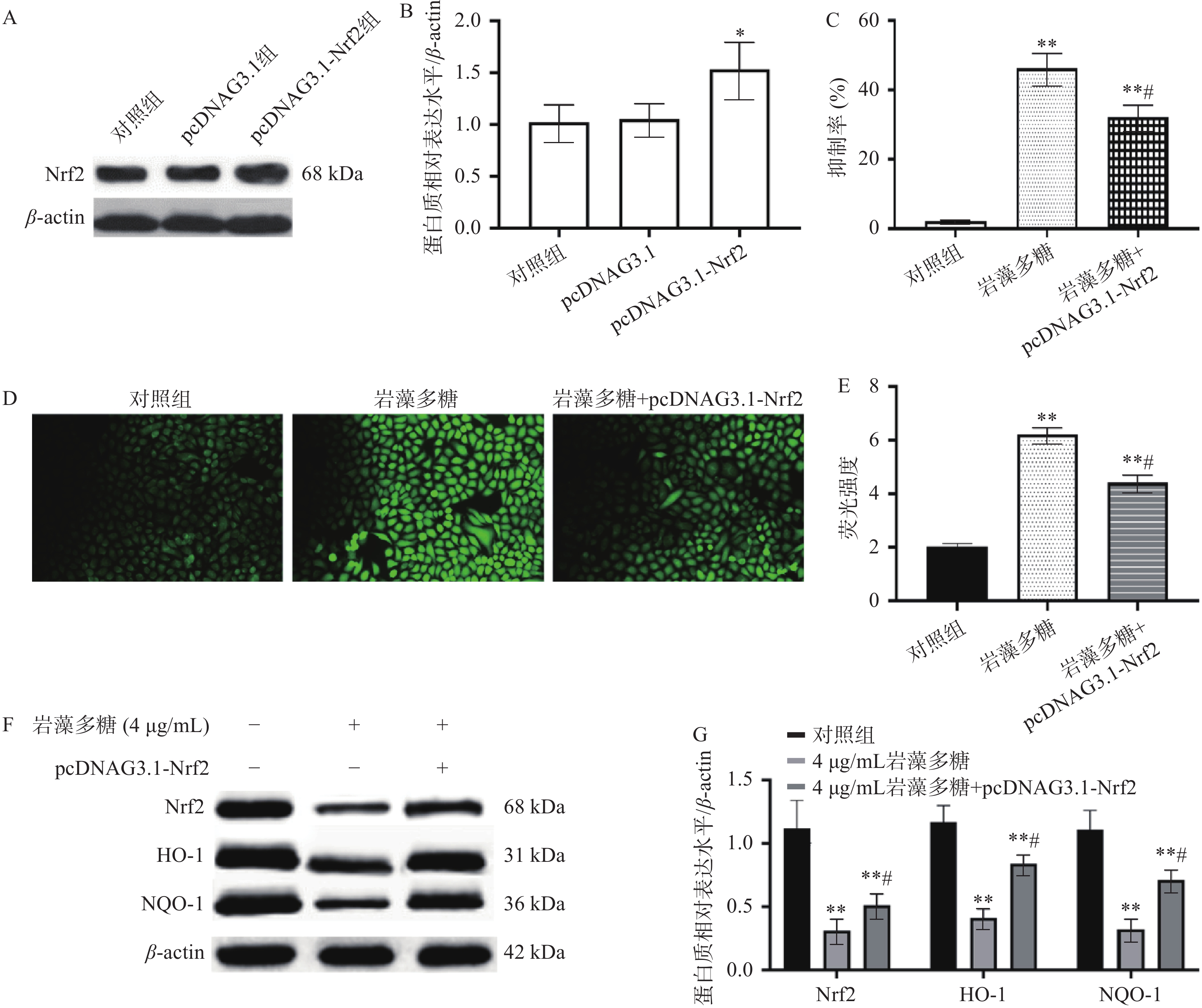
Nrf2是一种细胞保护性转录因子,对细胞或组织具有一定的保护作用[26],可以使正常细胞和癌细胞免受氧化损伤[27],这意味着它可以抑制恶性转化,同时也会对治疗产生耐药性[28]。本研究中,与对照组比较,pcDNAG3.1组Nrf2表达水平无统计学差异(P>0.05),pcDNAG3.1-Nrf2组Nrf2表达水平显著增加(图4A、4B,P<0.05),说明pcDNAG3.1-Nrf2转染是成功的;与对照组比较,岩藻多糖组(4 μg/mL)和岩藻多糖(4 μg/mL)+pcDNAG3.1-Nrf2组增殖抑制率显著增加(P<0.01)(图4C),与岩藻多糖组(4 μg/mL)比较,岩藻多糖(4 μg/mL)+pcDNAG3.1-Nrf2组抑制率显著降低(P<0.05),说明Nrf2过表达影响了岩藻多糖对ECA109细胞抑制效果。而且与岩藻多糖组(4 μg/mL)比较,Nrf2、NQO1与HO-1水平显著上调(P<0.05)(图4F、4G),ROS水平显著下调(图4D、4E),这也说明Nrf2表达水平参与了岩藻多糖对ECA109细胞的调控,并影响ROS水平。
2.5 裸鼠急性毒性实验
SPSS21.0软件分析显示,伴随岩藻多糖浓度的增加,裸鼠死亡率增加(表1),裸鼠体内实验LD50为973.96 mg/kg,95%可信限为783.86~1173.00 mg/kg。后续选择LD50的1/20、1/10,即50、100 mg/kg进行实验,而且其毒性会变小,能进行正常的实验。
表 1 急性毒性实验Table 1. Acute toxicity test剂量(mg/kg) 死亡数 死亡率(%) 435 0 0 622 2 20 888 5 50 1268 6 60 1812 10 100 2.6 岩藻多糖抑制食管癌肿瘤的增长
肿瘤细胞的增殖和过度生长被证明是由于Nrf2的过度激活而发生的[29],可以防止癌细胞经历细胞凋亡和死亡,从而使其对化学疗法或放射疗法产生拮抗[30]。本研究中,与模型组比较,第14、21 d,顺铂组、岩藻多糖组(50、100 mg/kg)肿瘤体积显著下调(图5A,P<0.01),肿瘤质量极显著降低(图5B),Nrf2、HO-1与NQO-1水平极显著下调(图5C、5D,P<0.01),说明岩藻多糖在体内能增强氧化应激,抑制肿瘤增殖。
3. 讨论与结论
本研究重点主要分析岩藻多糖对食管癌细胞及肿瘤组织的影响,并分析其机制。其中,1~16 µg/mL岩藻多糖能显著抑制ECA109细胞增殖,显著促进ECA109细胞凋亡,其原因可能是通过调节凋亡相关蛋白水平发挥作用,包括Bcl-2、Bax和Cleaved-caspase-3。体内实验也显示,50、100 mg/kg岩藻多糖抑制瘤体体积、瘤体质量,这些都能说明岩藻多糖对食管癌细胞和肿瘤组织具有较好的抑制作用。
有研究显示,Nrf2通过其靶向基因对转化的恶性细胞具有促癌作用,通过刺激SOD1、CAT、HO-1等基因的表达,参与保护癌细胞免受内在或外在氧化应激的影响[31]。在癌前病变或癌细胞中,Nrf2引起代谢改变,例如无氧糖酵解、改变戊糖磷酸途径和脂肪酸生物合成等,还可以通过增强抗凋亡基因Bcl-2以及增殖基因CDCA4的表达而导致癌细胞的增殖和细胞凋亡的抑制[32]。已有文献证明了Nrf2在各种恶性肿瘤、神经退行性疾病、心血管疾病、衰老、炎症或光氧化应激中的防御作用[33]。本研究中,1、2、4 µg/mL岩藻多糖处理后的ECA109细胞中ROS水平显著上调,Nrf2、HO-1、NQO-1蛋白水平显著降低,说明岩藻多糖可能是通过调控Nrf2/ROS信号通路发挥抗癌作用。而且通过Nrf2过表达实验能进一步证明上述的观点,其中Nrf2过表达能下调岩藻多糖抑制ECA109细胞增殖效果,下调ROS水平,上调Nrf2、HO-1、NQO-1蛋白水平。同样,体内实验也显示,岩藻多糖能下调瘤体Nrf2、HO-1、NQO-1水平,也说明了岩藻多糖拮抗食管癌的机制。
综上所述,岩藻多糖能通过抑制Nrf2/ROS信号通路上调ROS水平,并对食管癌ECA109细胞造成损伤,抑制细胞增殖,诱导细胞凋亡。同样体内实验中,岩藻多糖能增强氧化应激,抑制肿瘤组织增殖,这些都为岩藻多糖的开发提供依据,同时也为食管癌疾病的研究提供参考。
-
表 1 急性毒性实验
Table 1 Acute toxicity test
剂量(mg/kg) 死亡数 死亡率(%) 435 0 0 622 2 20 888 5 50 1268 6 60 1812 10 100 -
[1] ISHIHARA R, TANI Y, OKUBO Y, et al. Endoscopic landmarks corresponding to anatomical landmarks for esophageal subsite classification[J]. DEN Open,2023,4(1):e273.
[2] ZHANG X, YANG R, WANG H, et al. Inhibition of p62-Keap1-Nrf2 pathway activation by realgar promotes the inhibition of esophageal cancer cell proliferation, migration, and ferroptosis[J]. Current Drug Delivery,2024,21(2):236−248. doi: 10.2174/1567201820666221226105655
[3] TANG D, ZHENG Y, WANG G, et al. HPV18 E6 inhibits α-ketoglutarate-induced pyroptosis of esophageal squamous cell carcinoma cells via the P53/MDH1/ROS/GSDMC pathway[J]. FEBS Open Bio,2023,13(8):1522−1535. doi: 10.1002/2211-5463.13666
[4] KHALATBANI A, CASTLE J D, LI T, et al. Direct and indirect effects of alcohol and its toxic metabolite acetaldehyde on human esophageal myofibroblasts and epithelial cells[J]. Alcohol, Clinical & Experimental Research, 2023, 47(7):1297-1311.
[5] WANG X D, SU S Y, ZHU Y Q, et al. Metabolic Reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors[J]. Nature Communications,2023,14(1):5778. doi: 10.1038/s41467-023-41470-9
[6] WANG X T, WU H K, AN J F, et al. Cyclovirobuxine D alleviates aldosterone-induced myocardial hypertrophy by protecting mitochondrial function depending on the mutual regulation of Nrf2-SIRT3[J]. Biomed Pharmacother,2023,167:115618. doi: 10.1016/j.biopha.2023.115618
[7] RADWAN S M, ALQULALY M, ELSAEED M Y, et al. L-carnitine reverses methotrexate-induced nephrotoxicity in experimental rat model:Insight on SIRT1/PGC-1α/Nrf2/HO-1 axis[J]. Journal of Applied Toxicology:JAT,2023,43(11):1667−1675. doi: 10.1002/jat.4503
[8] MUNEERUNQSEE N, TANASAWET S, SUKKETSIRI W. Antimetastatic effect of fucoidan against non-small cell lung cancer by suppressing non-receptor tyrosine kinase and extracellular signal-related kinase pathway[J]. Nutrition Research and Practice,2023,17(5):844−854. doi: 10.4162/nrp.2023.17.5.844
[9] GEORGE A, SHRIVASTAV P S. Fucoidan, a brown seaweed polysaccharide in nanodrug deliveryy[J]. Drug Delivery and Translational Research,2023,3(10):2427−2446.
[10] USOLTSEVA R V, ZUEVA A O, MALYARENKO O S, et al. Structure and metabolically oriented efficacy of fucoidan from bown alga Sargassum muticum in the model of colony formation of melanoma and breast cancer cells[J]. Marine Drugs,2023,21(9):486−498. doi: 10.3390/md21090486
[11] SANNIYASI E, GOPAL R K, DAMODHARAN R, et al. In vitro anticancer potential of laminarin and fucoidan from brown seaweeds[J]. Scientific Reports,2023,13(1):14452−14467. doi: 10.1038/s41598-023-41327-7
[12] CUI G M, WU J R, LIN J Y, et al. Graphene-based nanomaterials for breast cancer treatment:Promising therapeutic strategies[J]. Journal of Nanobiotechnology,2021,19(1):211−232. doi: 10.1186/s12951-021-00902-8
[13] YAN W J, MA X C, ZHAO X Y, et al. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro[J]. Drug Design, Development and Therapy,2018,12:3961−3972. doi: 10.2147/DDDT.S181939
[14] HICKS H M, NASSAR V L, LUND J, et al. The effects of Aurora Kinase inhibition on thyroid cancer growth and sensitivity to MAPK-directed therapies[J]. Cancer Biology & Therapy,2024,25(1):2332000−2332013.
[15] MA J, LI J Y, CHEN X J, et al. Ojeok-san enhances platinum sensitivity in ovarian cancer by regulating adipocyte paracrine IGF1 secretion[J]. Adipocyte,2024,13(1):2282566−2282579. doi: 10.1080/21623945.2023.2282566
[16] RUAN S Y, LIU R, CHEN X, et al. Determination of H2O2 and its antioxidant activity by BCM@Au NPs ratiometric SERS sensor[J]. Talanta,2024,268(Pt1):125323.
[17] PETRONEK M S, TEFERI N, LEE C Y, et al. MRI detection and therapeutic enhancement of ferumoxytol internalization in Glioblastoma cells[J]. Nanomaterials (Basel),2024,14(2):189−199. doi: 10.3390/nano14020189
[18] GAO G P, LI J Y, CAO Y, et al. Design, synthesis, and biological evaluation of novel 4,4'-bipyridine derivatives acting as CDK9-Cyclin T1 protein-protein interaction inhibitors against triple-negative breast cancer[J]. European Journal of Medicinal Chemistry ,2023,261:115858. doi: 10.1016/j.ejmech.2023.115858
[19] KNUDTSON J F, MCLAUGHLIN J E, SULTANA M, et al. CD44 variant 6 is involved in the attachment and invasion of endometrial cells to peritoneum[J]. F & S Science,2020,1(2):188−194.
[20] RAJENDRAPRASAD G, RODRIGUEZ-CALADO S, BARISIC M. SiR-DNA/SiR-Hoechst-induced chromosome entanglement generates severe anaphase bridges and DNA damage[J]. Life Science Alliance,2023,6(12):e202302260. doi: 10.26508/lsa.202302260
[21] TU Y, ZHANG W, FAN G, et al. Paclitaxel-loaded ROS-responsive nanoparticles for head and neck cancer therapy[J]. Drug Delivery,2023,30(1):2189106. doi: 10.1080/10717544.2023.2189106
[22] LIU W, HE Y Y, GUO Z J, et al. Discovery of potent tubulin inhibitors targeting the colchicine binding site via structure-based lead optimization and antitumor evaluation[J]. Journal of Enzyme Inhibition and Medicinal Chemistry,2023,38(1):2155815−2155832. doi: 10.1080/14756366.2022.2155815
[23] CHENG L, ZHAI H Q, DU J, et al. Lobetyolin inhibits cell proliferation and induces cell apoptosis by downregulating ASCT2 in gastric cancer[J]. Cytotechnology,2023,75(5):435−448. doi: 10.1007/s10616-023-00588-w
[24] LIANG C, ZHANG H Y, WANG Y Q, et al. TMED2 induces cisplatin resistance in breast cancer via targeting the KEAP1-Nrf2 pathway[J]. Current Medical Science,2023,43(5):1023−1032. doi: 10.1007/s11596-023-2777-7
[25] JOMOVA K, RAPTOVA R, ALOMAR S Y, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants:Chronic diseases and aging[J]. Archives of Toxicology,2023,97(10):2499−2574. doi: 10.1007/s00204-023-03562-9
[26] HSIEH P L, TSAI K L, CHOU W C, et al. Cisplatin triggers oxidative stress, apoptosis and pro-inflammatory responses by inhibiting the SIRT1-mediated Nrf2 pathway in chondrocytes[J]. Environmental Toxicology,2023,38(10):2476−2486. doi: 10.1002/tox.23885
[27] NAIR B, ADITHYA J K, CHANDRABABU G, et al. Modulation of carcinogenesis with selected GRAS nutraceuticals via Keap1-Nrf2 signaling pathway[J]. Phytotherapy Research:PTR,2023,37(10):4398−4413. doi: 10.1002/ptr.7940
[28] LEE J, ROH J L. Targeting Nrf2 for ferroptosis-based therapy:Implications for overcoming ferroptosis evasion and therapy resistance in cance[J]. Biochimica Et Biophysica Acta-Molecular Basis of Disease,2023,1869(7):166788. doi: 10.1016/j.bbadis.2023.166788
[29] LU C, XUE L, LUO K, et al. Colon-accumulated gold nanoclusters alleviate intestinal inflammation and prevent secondary colorectal carcinogenesis via Nrf2-dependent macrophage reprogramming[J]. ACS Nano,2023,17(18):18421−18432. doi: 10.1021/acsnano.3c06025
[30] DENG C, XIONG L, CHEN Y, et al. Metformin induces ferroptosis through the Nrf2/HO-1 signaling in lung cancer[J]. BMC Pulmonary Medicine,2023,23(1):360−375. doi: 10.1186/s12890-023-02655-6
[31] SHEN X F, CHEN H Q, ZHANG H, et al. A natural sesquiterpene lactone isolinderalactone attenuates lipopolysaccharide-induced inflammatory response and acute lung injury through inhibition of NF-κB pathway and activation Nrf2 pathway in macrophages[J]. International Immunopharmacology, 2023, 124(Pt B):110965-110978.
[32] WANG Z Y, LI W J, WANG X, et al. Isoliquiritigenin induces HMOX1 and GPX4-mediated ferroptosis in gallbladder cancer cells[J]. Chinese Medical Journal,2023,136(18):2210−2220. doi: 10.1097/CM9.0000000000002675
[33] WANG L H, CHENG F Y, PAN R M, et al. FGF2 rescued cisplatin-injured granulosa cells through the NRF2-autophagy pathway[J]. International Journal of Molecular Sciences,2023,24(18):14215−14225. doi: 10.3390/ijms241814215
-
期刊类型引用(0)
其他类型引用(1)





 下载:
下载:
 下载:
下载:



