Advances in Understanding the Role Mechanism of Dietary Fiber in Mitigating Colitis
-
摘要: 结肠炎是一种持续时间长、病因不明、反复发作的炎症性肠道疾病,通常伴随着肠道细胞损伤、肠道免疫及肠道菌群异常等症状。大量研究表明,膳食纤维作为一种益生元,其代谢产物可选择性地改善肠道菌群的组成,进而提高肠道短链脂肪酸的水平、降低炎症因子表达、增强肠道免疫屏障功能,从而改善机体的炎症反应。膳食纤维这种通过其代谢产物发挥抗炎的作用,为辅助治疗结肠炎提供了新的研究思路。本文概述了结肠炎的发病机制,膳食纤维及其代谢产物与肠道菌群、炎症因子、免疫细胞之间的作用机制,旨在为膳食纤维缓解结肠炎和开发功能性食品提供理论依据。Abstract: Colitis is an inflammatory intestinal disease that lasts for a long time, has an unknown cause, and occurs repeatedly. It is usually accompanied by symptoms such as intestinal cell damage, intestinal immunity, and abnormal intestinal flora. Numerous studies have shown that dietary fiber, as a prebiotic, its metabolites can selectively improve the composition of the intestinal flora, which in turn improves the level of intestinal short-chain fatty acids, reduces the expression of inflammatory factors, and enhances the function of the intestinal immune barrier, thereby improving the inflammatory response of the organism. This anti-inflammatory effect of dietary fiber through its metabolites provides new research ideas to assist in the treatment of colitis. This paper outlines the pathogenesis of colitis, and the mechanism of interaction between dietary fiber and its metabolites with intestinal flora, inflammatory factors and immune cells, aiming to provide theoretical basis for the alleviation of colitis by dietary fiber and the development of functional foods.
-
Keywords:
- dietary fiber /
- colitis /
- intestinal microbiota /
- immune barrier /
- mechanism of action
-
结肠炎是一种病因复杂的肠道慢性疾病,能造成腹痛、腹泻、发热等,严重者还会出现贫血、脱水等症状。近年来,结肠炎的发病率逐年上升,发病周期长,其发病因素和发病机制尚未明确。据流行病学调查[1]分析,饮食结构不合理、膳食纤维缺乏是引发结肠炎的重要因素。膳食纤维是一种不能被肠道消化酶分解的复合碳水混合物,但可以被肠道菌群分解成短链脂肪酸、脂多糖等物质,促进炎症因子的分泌,起到降低炎症、保护肠黏膜屏障等作用。因此,本文重点综述了膳食纤维的代谢产物以及如何作用于炎症及其相关机制等内容,旨在为膳食纤维的功能性研究提供理论指导。
1. 结肠炎及其发病机制
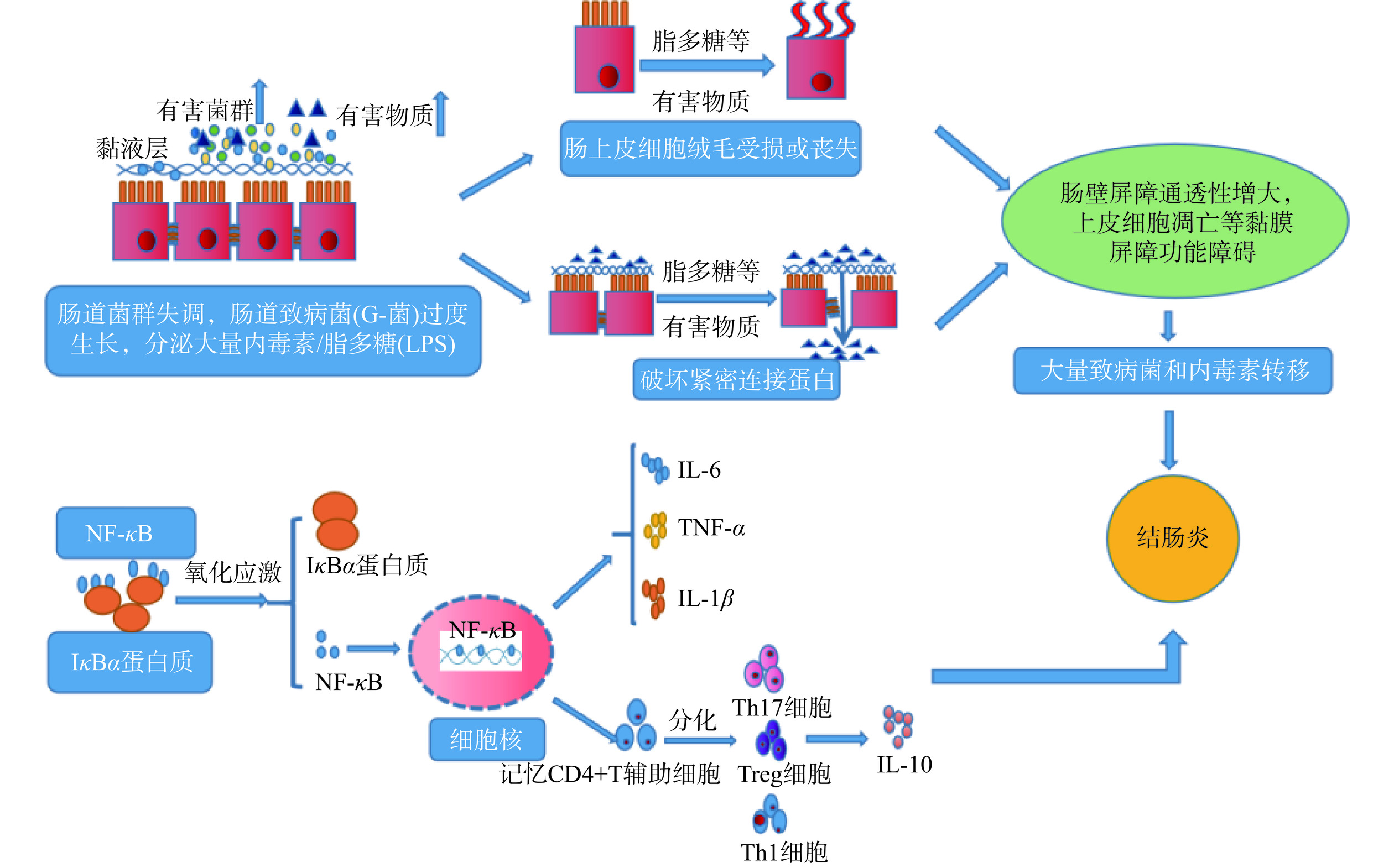
结肠炎是由肠道内炎症因子的释放、肠毒素及有害菌群的增加、短链脂肪酸的异常变化,促使肠道上皮细胞受损及细胞间的紧密连接蛋白遭到破坏,而引发的一种肠道炎症性疾病[2]。如图1所示,当肠道发生炎症时,肠道菌群紊乱,双歧杆菌、玫瑰杆菌等益生菌数量减少;同时,振荡杆菌、脱硫弧菌等有害菌数量增加,增大了有害菌群与肠道黏液层的接触面积,并刺激肠道分泌大量的脂多糖等有害物质[3]。脂多糖等能诱导多种炎症因子的释放,还能激活NO合成酶系统(iNOS),破坏肠道上皮细胞的绒毛膜蛋白,增大肠道黏膜的通透性,使有害物质透过肠道屏障,加重炎症反应[4]。当肠道发生炎症时,Toll等受体蛋白的活性增强,激活NF-κB和TLR信号通路,促进炎症因子的表达[5]。其中,NF-κB是重要的炎症转录因子,能与DNA或部分蛋白质结合,调控细胞多种编码因子基因的表达,促进促炎因子和趋化因子的释放。NF-κB还能刺激多种神经细胞和免疫细胞的增殖、分化与凋亡,产生更多的抑炎因子IL-10等[6]。在静息作用下,NF-κB还能与IκB家族的蛋白质结合,使NF-κB失去活性,其中起主要作用的蛋白质有IκBα、IκBβ和IκBe[7]。当IκB激酶存在时,能与IκBα蛋白上的结合位点结合,激活IκBα蛋白的活性,使蛋白结合位点上的NF-κB被释放,进入细胞核中与DNA相结合,调控编码基因的表达;一方面能调控促炎编码基因,促进TNF-α、IL-6等炎症因子的释放[8];另一方面还能促进机体T细胞、B细胞的分化,产生更多的IL-6、TNF-α、IL-10等炎症因子,破坏细胞的结构,降低免疫细胞对致病菌的吞噬作用,加重炎症反应。
目前,临床上常用来缓解炎症反应的抗菌药物有左氧氟沙星、头孢呋辛、美沙拉嗪等;还有一些免疫制剂,如硫唑嘌呤、甲氨蝶呤以及环孢素A等。然而,长期使用这些药物不仅能破坏肠道菌群平衡,还能引起肠胃不适、肝功能损伤等[9]。Arboleya等[10]在研究抗生素对肠道菌群影响时,证实孕妇长期使用抗菌药物,能影响新生儿的短链脂肪酸的含量和肠道菌群的数量。Yuliia等[11]在研究长期使用抗生素对短链脂肪酸和肠道屏障完整性的影响时,证实头孢曲松能促进肠道致病菌的增加,减少短链脂肪酸的含量,加速炎症反应。因此,寻找和开发具有缓解结肠炎作用的天然物质已成为国内外学者的研究热点,膳食纤维作为一种益生元,目前尚未发现在缓解结肠炎中的副作用,而且还具有多种对人体有益的生理功能。
2. 膳食纤维
2.1 膳食纤维分类及来源
膳食纤维是一种能被肠道菌群酵解成丁酸、乙酸、脂多糖等多种小分子物质的碳水化合物,主要包括抗性淀粉、木质素和非淀粉多糖等多糖类物质[12]。膳食纤维主要来源于水果、蔬菜、豆类、谷类以及化学合成。根据其溶水性,可分为可溶性膳食纤维(soluble dietary fiber,SDF)和不可溶性膳食纤维(insoluble dietary fiber,IDF)。SDF主要有低聚糖、果胶、菊粉、葡聚糖等物质,可被肠道微生物酵解成丁酸、丙酸等,能调控各种免疫细胞,激活各种免疫机制,促进炎症因子的表达,抑制炎症反应[13]。IDF主要包括木质素、纤维素和植物蜡等,能促进肠道消化、减少肠道有害物质的留存和预防便秘等[14]。
2.2 膳食纤维的抗炎作用
为了明确膳食纤维的摄入对结肠炎的影响,Valcheva等[15]发现摄入富含果聚糖的菊粉,能调控肠道菌群的丰度和短链脂肪酸的含量,影响肠道菌群的功能性,从而缓解结肠炎;廖勇等[16]通过动物实验研究魔芋润肠复合膳食纤维作用时,表明膳食纤维能通过调控炎症因子的释放和NF-κB等炎症通路的表达来缓解炎症反应。综上所述,摄入膳食纤维可有效的改善炎症症状;且膳食纤维主要是通过促进肠道益生菌的增殖、提高肠道短链脂肪酸的含量,促进抑炎因子的释放及其相关通路的表达,以缓解炎症反应。因此,增加膳食纤维的摄入,不仅能缓解结肠炎、便秘带来的炎症症状,还能维持肠道内环境的稳态,对结肠炎的治疗和预防具有重要的意义。
3. 膳食纤维的降解
3.1 膳食纤维的代谢途径
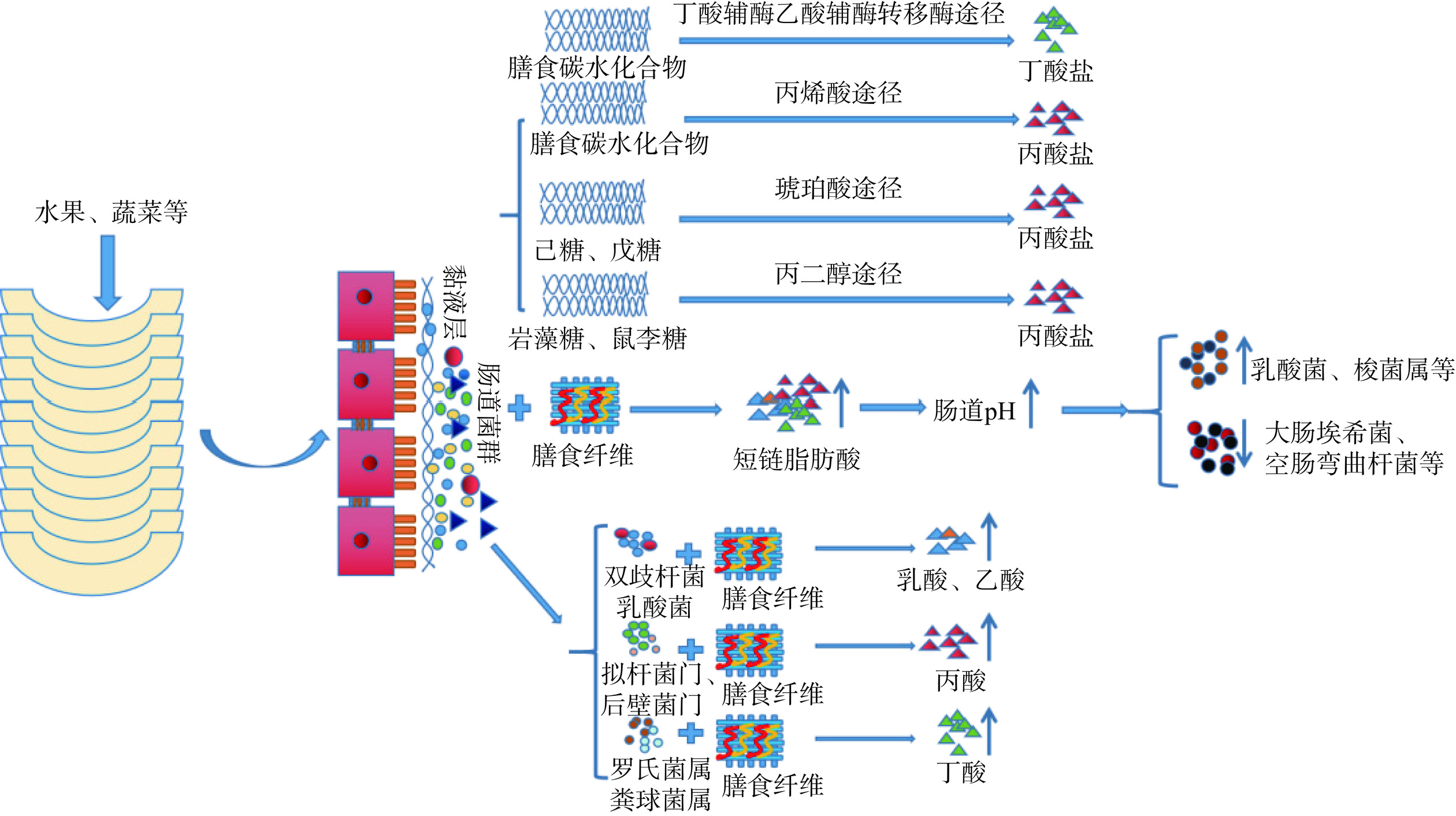
膳食纤维是一种多糖类物质,能被肠道菌群发酵产生多种盐类物质和能量,在不同的肠道环境下,肠道菌群的作用也不同(如图2)。不同类型膳食纤维能降解成多种类型的糖类化合物,若降解成戊糖、己糖等单糖,可通过琥珀酸途径产生丙酸盐;若降解成岩藻糖、鼠李糖等脱氧核糖,则通过丙烯酸酯途径和丙二醇途径产生丙酸盐。膳食纤维发酵产生的二羟基丙酮磷酸,可用于形成甲基乙二醇,促进1,2-丙二醇的生成。此外,在缺氧的条件下,肠道菌群可降解乳酸,产生1,2-丙二醇,再转化成丙醛和丙酰辅酶A,促进肠道丙酸盐的形成;在二氧化碳分压较低时,己糖、戊糖等多糖能通过琥珀酸途径转化生成甲基丙二酰辅酶A,促进生成丙酸盐[17]。肠道内丁酸盐的形成,需要两分子的乙酰辅酶a缩合,生成乙酰辅酶a,然后将其还原为β-羟基丁基辅酶a和巴豆酰辅酶a,最后还原为丁基辅酶a,生成丁酸盐。值得注意的是,在外源性衍生的乙酸酯的条件下,乙酸辅酶转移酶能将丁基辅酶a转化为丁酸酯和乙酰辅酶a,也能促进丁酸盐的形成[18]。膳食纤维降解产生的丁酸盐、丙酸盐等,能被肠道上皮细胞吸收,通过血液循环运送到不同的部分,发挥不同的作用;不仅能抑制T细胞分化成Th1和Th2等效应T细胞,还能抑制组蛋白去乙酰化酶的活性,影响不同功能的基因表达,从而影响肠道细胞的增殖与分化,加强肠道屏障功能[19]。综上所述,膳食纤维发酵生成的盐类物质,能够被肠道上皮细胞吸收,为其增殖提供能量,也能维持肠道免疫屏障的完整性,缓解炎症反应。
3.2 膳食纤维降解的影响因素
膳食纤维不能被消化系统分泌的消化酶分解,但能特异性地激活肠道菌群的多糖利用位点基因(PUL),产生相关特异性的糖苷酶,降解膳食纤维,生成多种单糖、短链脂肪酸和少量脂肪等[20]。多项研究表明,膳食纤维发酵生成的代谢产物与肠道菌群的种类有关[21],而且其代谢产物还能反作用于肠道菌群,通过改变肠道pH,抑制大肠埃希菌、空肠弯曲杆菌等有害菌的生长,促进乳酸菌、梭菌属类等益生菌的生长[22](如图2)。例如,山杏膳食纤维在肠道中分解时,发现人乳酸杆菌、双歧杆菌能分解野山杏果肉膳食纤维,不仅促进肠道乙酸的形成,还体现出金黄色葡萄球菌能产生更多的丁酸和异丁酸,证实不同肠道菌群的产酸能力不同[23]。黄苇等[24]在研究菊粉通过肠道菌群调控动物脂代谢时,表明拟杆菌门、厚壁菌门均能降解菊粉膳食纤维,产生丙酸;赵文婧等[25]在研究膳食纤维与肠道菌群的关系时,表明罗氏菌群、粪球菌群均能降解膳食纤维,产生丁酸。综上所述,目前多数研究集中在乳酸杆菌、双歧杆菌等少量细菌的作用,且数据均来自膳食纤维体外发酵实验,但是其他肠道菌群的作用及其如何分解膳食纤维的机制还尚待进一步研究。
4. 膳食纤维抗结肠炎作用机制
膳食纤维发酵产生的单糖、短链脂肪酸等代谢物质具有多种免疫调节功能,不仅能对免疫细胞产生直接作用,而且还能影响肠道菌群的丰度,对缓解炎症产生间接作用。肠道菌群的丰度主要能影响膳食纤维在肠道中的发酵,生成丁酸、乙酸等不同的代谢产物,从而产生不同的抗炎效果。
4.1 调控机体免疫作用
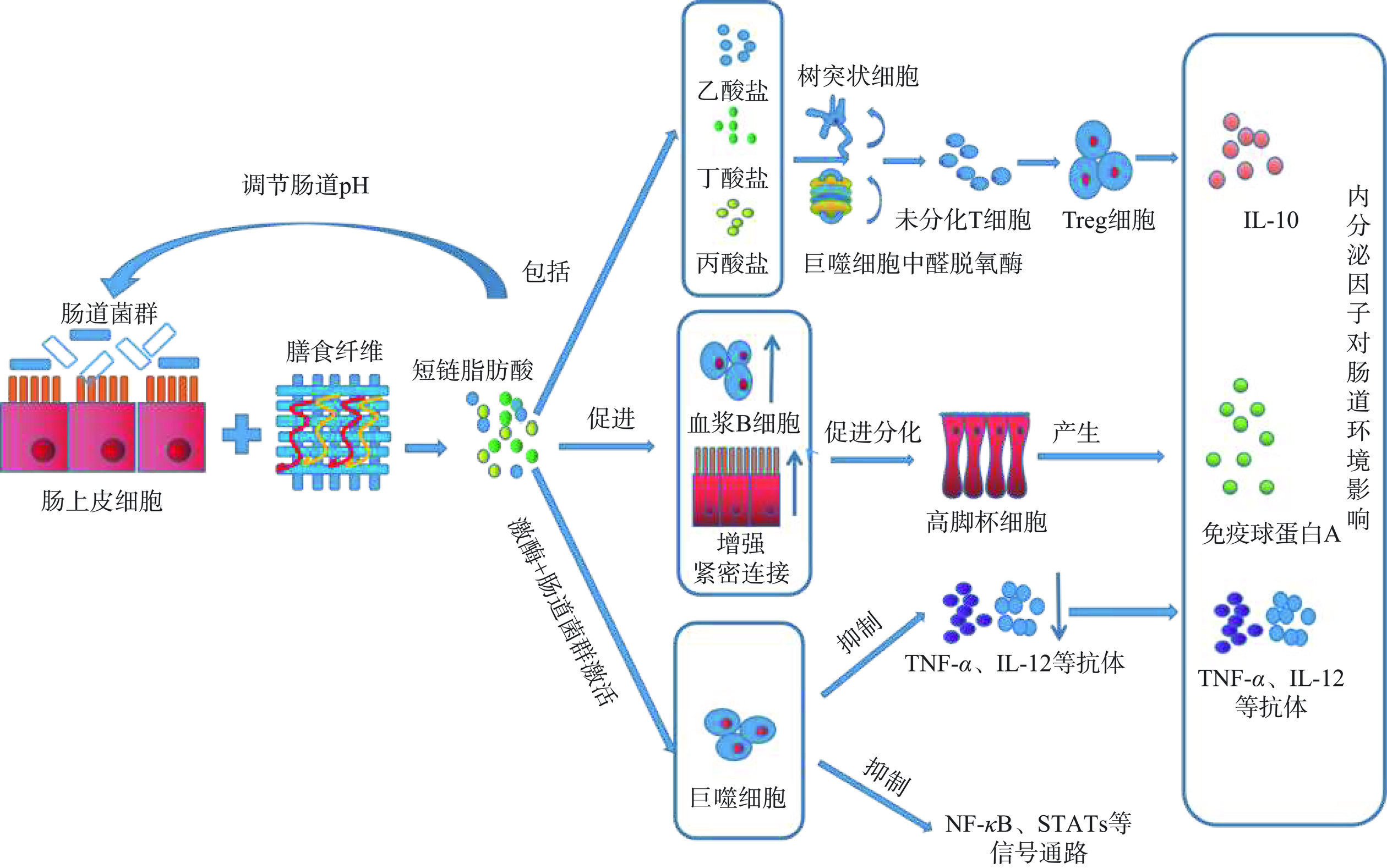
膳食纤维在肠道菌群的作用下,不仅能产生多种短链脂肪酸,还能促进记忆CD4+T辅助细胞的分化。其次,为了维持肠道内环境的稳定,短链脂肪酸还能刺激机体分泌IL-10、免疫球蛋白等物质,减少肠道致病菌与肠道上皮细胞间的直接作用,增强机体的免疫作用(如图3)。其具体作用机制主要涉及三个方面,一方面是短链脂肪酸能加强血浆B细胞的代谢,能通过调控细胞核编码蛋白基因来改变紧密连接蛋白的结构,加强肠道屏障功能,还促进高脚杯细胞的分化并产生免疫球蛋白A,增强肠道黏液层,抵御肠道致病菌的侵袭[26−28]。其次,膳食纤维降解产生的丁酸盐、丙酸盐、乙酸盐等物质,还能提高树突状细胞和巨噬细胞中醛脱氧酶的活性,促进未分化的T细胞分化成Treg细胞,促进IL-10炎症因子的产生,参与肠道炎症反应[29−30]。另一方面,膳食纤维降解产生丁酸盐,不仅促进吞噬细胞分化,还能抑制TNF-α、IL-12等炎症因子的产生,抑制NF-κB、STATs等信号通路,从而达到缓解结肠炎的作用[31−32]。黄超勇等[33]还探明了菊粉缓解肠道炎症反应,不仅能提高肠道丁酸、丙酸等短链脂肪酸的含量,降低肠道大肠杆菌的数量,还能促进肠道抗炎因子IL-10的分泌,降低促炎因子COX-2、IL-2和TNF-α的表达以及抑制NF-κB的表达通路。综上所述,大多数研究证实,膳食纤维发酵产生的短链脂肪酸,不仅能促进肠道免疫细胞的分化,还能刺激肠道免疫细胞分泌抗炎物质,抑制肠道炎症通路的表达,达到缓解结肠炎的作用。不同来源的膳食纤维缓解结肠炎的作用机制不同,这可能与膳食纤维结构不同有关,因此,可进一步研究不同结构的膳食纤维对结肠炎的作用,更有助于了解膳食纤维抗结肠炎的机制。
4.2 修复肠道屏障作用
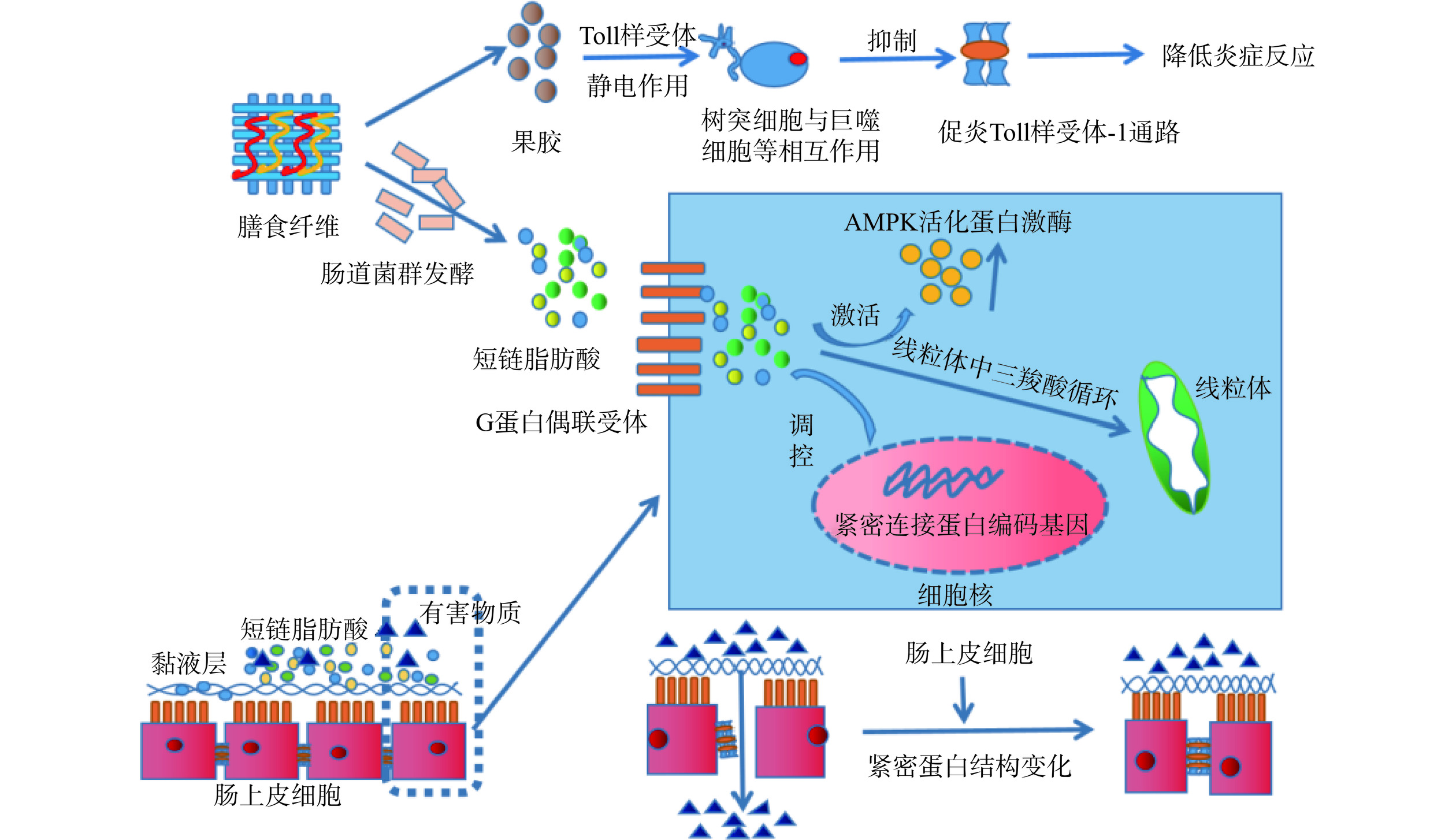
肠黏膜屏障是由肠黏膜上皮细胞、肠黏液层、肠黏膜淋巴组织和肠道菌群共同构成。它们之间相互作用,共同维持肠道通透性,阻碍致病菌、肠毒素等有害物质对肠道的侵害(如图4)。膳食纤维中的果胶类物质能与树突细胞和巨噬细胞中的Toll样受体结合,减少与炎症因子的结合,通过静电相互作用,抑制促炎Toll样受体-1的通路,影响炎症因子的释放[34]。膳食纤维发酵的产物,还可以与一些膜蛋白受体结合,激活Toll样受体-1等相关的免疫通路,抑制有害物质破坏黏膜屏障作用,抑制炎症反应。例如,短链脂肪酸能激活肠细胞膜表面的G蛋白偶联受体等,通过膜蛋白运输,进入细胞内,通过线粒体中的三羧酸循环,为细胞呼吸作用提供能量,促进肠道细胞的增殖与分化,增强肠道屏障功能[35]。短链脂肪酸还能调控细胞核中的紧密连接蛋白编码基因,使细胞间的紧密连接蛋白结构发生变化[36],激活细胞内AMPK活化蛋白激酶,促进黏蛋白的分泌,强化肠道黏液层[37]。此外,膳食纤维的代谢产物还能作用于细胞间的紧密连接蛋白,调控黏膜屏障的通透性,加强对肠道的保护作用。在研究大麦叶不溶性膳食纤维的功能时,不溶性膳食纤维增强闭合蛋白和Mucin2的活性,降低Claudin-1蛋白的活性,证明了膳食纤维能通过调控紧密连接蛋白的活性,抑制结肠炎[38]。Tong等[39]在研究短链脂肪酸缓解炎症的作用时,也得到类似的结论。膳食纤维发酵产生的代谢产物不仅能调节肠道的酸碱度,抑制有害菌的生长,还能减少致病菌分解黏液蛋白,加强肠道的保护作用。然而,大多数文献的研究对象都是可溶性膳食纤维,具有局限性。研究表明,可溶性膳食纤维发酵产生的短链脂肪酸能调控跨膜蛋白、ZO-1、闭合蛋白等部分膜蛋白,但并未完全阐明所有的膜蛋白,以及它们之间复杂的机制关系,还需做大量的实验来证明。此外,不可溶性膳食纤维也能调控紧密连接蛋白,增强肠道屏障功能,但报道的文献较少,其中复杂的机制还需要进一步的探索。
4.3 调控肠道菌群发挥抗炎作用
肠道菌群能降解膳食纤维产生多种短链脂肪酸、色氨酸、胆汁酸等代谢物质;这些代谢产物不仅能进一步分解,抑制炎症细胞分化和炎症因子的释放,还能影响肠道的酸碱度,调控肠道菌群的丰度和多样性,进一步加强膳食纤维的降解,从而增强膳食纤维的抗结肠炎作用[40]。由表1可知,膳食纤维的种类很多,即使同一类型结构不同的膳食纤维对肠道菌群的影响不同,产生的短链脂肪酸不同,对缓解结肠炎的作用也不同;但多数研究表明,膳食纤维能促进双歧杆菌、嗜黏蛋白阿克曼氏菌等益生菌的增殖,抑制大肠杆菌、球菌等致病菌的增殖;且经肠道菌群降解后,产生丁酸、乙酸、丙酸等短链脂肪酸,通过影响多种代谢和炎症通路,达到缓解结肠炎的作用。如菊粉型果糖能诱导肠道代谢产物的改变,增加丁酸含量,改变双歧杆菌和毛螺菌科的丰度,缓解结肠炎的症状反应[15];Akihito等[41]发现,水溶性膳食纤维能增加肠道脆弱拟杆菌的比例和免疫球蛋白A的产生,从而增强肠道免疫功能。另外,肠道菌群能进一步分解胆汁酸,产生次级胆汁酸脱氧胆酸和石胆酸能激活机体免疫反应,抑制T细胞分化,减少IL-17、IL-22促炎因子的产生[42];Paik等[43]研究发现,石胆酸能在3α-羟基固醇脱氧酶和3β-羟基固醇脱氧酶的作用下,分解产生异石胆酸和胆汁酸衍生物3-oxoLCA,代谢产物能透过肠道上皮细胞,进入肠道屏障的固有层,抑制T细胞的分化和IL-17、IL-22等炎症因子的释放;嗜黏蛋白阿克曼氏菌不仅能增加肠道黏液层的厚度,恢复肠道屏障功能,还能增加肠道内短链脂肪酸的产生,促进调节性T细胞的分化,缓解肠道炎症[44]。综上所述,不同分子量和结构的膳食纤维,发酵产生短链脂肪酸的能力不同,而且对肠道菌群多样性的影响也不同,这可能与其结构的复杂程度和支链的长短有关[45]。目前,大多数的研究都停留在膳食纤维类型对肠道菌群动态平衡的影响,下一步应加强对膳食纤维化学结构与菌群变化、结肠炎之间的关系研究,理清膳食纤维化学结构是如何影响肠道菌群动态变化及炎症反应的,进一步了解膳食纤维是如何通过影响肠道菌群的丰度来发挥抗结肠炎作用。
表 1 不同膳食纤维对肠道菌群的影响Table 1. Effects of different dietary fibers on intestinal microbial communities膳食纤维 肠道菌群 短链脂肪酸 参考文献 褐藻膳食纤维 梭状芽孢杆菌、双歧杆菌上升
肠杆菌科细菌、卵磷脂酶阴性梭状芽孢杆菌下降乙酸、丙酸含量增加 [46−48] 低聚糖和低聚半乳糖 双歧杆菌上升
肠杆菌、粪球菌、沙门氏菌、瘤胃球菌、脱盐球菌、协同菌、
脱盐菌荷兰菌下降丁酸、乙酸含量减少 [49−50] 果胶低聚糖 双歧杆菌、乳酸杆菌数量增加拟杆菌、梭菌数量减少 乙酸、乳酸、丙酸含量增加 [51−52] 菊粉 螺杆菌属、狄氏副拟杆菌属相对丰度增加乳球菌属细菌相对丰度降低狄氏
副拟杆菌、双歧杆菌数量增加琥珀酸含量增加 [53−54] 抗性淀粉 促进双歧杆菌、乳酸菌生长抑制梭状芽孢杆菌、拟杆菌生长 丁酸、丙酸含量增加 [45,55] 阿拉伯木聚糖 促进双歧杆菌、乳酸杆菌生长
抑制大肠杆菌、沙门氏菌、拟杆菌生长乙酸的含量增加 [56] 魔芋低聚糖 乳杆菌、双歧杆菌数量增加大肠杆菌、肠球菌数量减少 丙酸含量升高
乙酸、丙酸含量降低[57−58] 瓜尔豆胶 双歧杆菌属、瘤胃球菌属数量增加 丙酸、丁酸含量增加 [59] 海藻多糖 乳酸菌、双歧杆菌数量增加 乙酸、丙酸、丁酸含量增加 [60−61] 乳低聚糖 双歧杆菌、乳酸杆菌数量增加
大肠杆菌的数量减少乙酸盐含量提高 [62] 5. 结论
膳食纤维的来源广泛,蔬菜、水果、谷类食物中均有且含量丰富。膳食纤维能以直接或者间接的方式参与抗炎作用,能够抑制相关炎症因子的表达和相关炎症通路的激活。膳食纤维发挥抗炎的机制主要表现在以下三个方面。首先,膳食纤维代谢产生的短链脂肪酸能激活相关的免疫蛋白受体,激活Toll样受体-1等多条免疫通路,还能促进T细胞、淋巴细胞等的增殖与分化,产生IL-4、IL-10等抗炎因子,缓解炎症反应。第二,膳食纤维能影响肠道上皮细胞间的紧密连接蛋白,促进肠道免疫细胞分泌黏液,增强肠道的黏液层,加强肠道的屏障功能。第三,膳食纤维能影响肠道菌群的丰度,一方面能促进益生菌的增殖,另一方面能促进益生菌对膳食纤维的酵解,发挥抗炎作用。此外,不同结构的膳食纤维,其作用的靶点可能不同,产生的免疫作用可能不同。膳食纤维的抗结肠炎效果还与膳食纤维的结构、作用于肠道的时间以及代谢产物有关,这为开发膳食纤维饮料、膳食纤维餐粉等功能性产品提供了新的思路。
目前,在膳食纤维抗结肠炎机制还有一些尚未解决的问题,包括:a.膳食纤维的功效可能受其聚合度和糖苷键的影响,现阶段缺乏对不同聚合度和不同连接方式的膳食纤维特性的直接比较和详细的描述。b.现阶段膳食纤维功能性的研究大部分来源于动物实验和少量的人体实验,后期的实验应该加大临床试验,侧重于膳食对人类结肠炎作用的探索,制定膳食纤维使用的标准剂量。c.进一步研究不同肠道菌群与膳食纤维结构之间的关系,阐明膳食纤维与肠道菌群代谢作用的靶点及肠道菌群相关基因表达的影响,并探讨其中的作用机制。
-
表 1 不同膳食纤维对肠道菌群的影响
Table 1 Effects of different dietary fibers on intestinal microbial communities
膳食纤维 肠道菌群 短链脂肪酸 参考文献 褐藻膳食纤维 梭状芽孢杆菌、双歧杆菌上升
肠杆菌科细菌、卵磷脂酶阴性梭状芽孢杆菌下降乙酸、丙酸含量增加 [46−48] 低聚糖和低聚半乳糖 双歧杆菌上升
肠杆菌、粪球菌、沙门氏菌、瘤胃球菌、脱盐球菌、协同菌、
脱盐菌荷兰菌下降丁酸、乙酸含量减少 [49−50] 果胶低聚糖 双歧杆菌、乳酸杆菌数量增加拟杆菌、梭菌数量减少 乙酸、乳酸、丙酸含量增加 [51−52] 菊粉 螺杆菌属、狄氏副拟杆菌属相对丰度增加乳球菌属细菌相对丰度降低狄氏
副拟杆菌、双歧杆菌数量增加琥珀酸含量增加 [53−54] 抗性淀粉 促进双歧杆菌、乳酸菌生长抑制梭状芽孢杆菌、拟杆菌生长 丁酸、丙酸含量增加 [45,55] 阿拉伯木聚糖 促进双歧杆菌、乳酸杆菌生长
抑制大肠杆菌、沙门氏菌、拟杆菌生长乙酸的含量增加 [56] 魔芋低聚糖 乳杆菌、双歧杆菌数量增加大肠杆菌、肠球菌数量减少 丙酸含量升高
乙酸、丙酸含量降低[57−58] 瓜尔豆胶 双歧杆菌属、瘤胃球菌属数量增加 丙酸、丁酸含量增加 [59] 海藻多糖 乳酸菌、双歧杆菌数量增加 乙酸、丙酸、丁酸含量增加 [60−61] 乳低聚糖 双歧杆菌、乳酸杆菌数量增加
大肠杆菌的数量减少乙酸盐含量提高 [62] -
[1] SAMANTHA K G, MEGAN R, BALAZS B, et al. Dietary fibre in gastrointestinal health and disease[J]. Nature Reviews. Gastroenterology & Hepatology,2021,18(2):101−116.
[2] 叶雪珂, 单国顺, 付郁, 等. 溃疡性结肠炎发病机制及中西医治疗的研究进展[J]. 中华中医药学刊,2022,40(9):158−162. [YE X K, SHAN G S, FU Y, et al. Research progress on the pathogenesis and treatment of ulcerative colitis with traditional Chinese and western medicine[J]. Chinese Journal of Traditional Chinese Medicine,2022,40(9):158−162.] YE X K, SHAN G S, FU Y, et al. Research progress on the pathogenesis and treatment of ulcerative colitis with traditional Chinese and western medicine[J]. Chinese Journal of Traditional Chinese Medicine, 2022, 40(9): 158−162.
[3] SURIANO F, NYSTROM E E. L, SERGI D, et al. Diet, microbiota, and the mucus layer:The guardians of our health[J]. Frontiers in Immunology,2022,13:953196. doi: 10.3389/fimmu.2022.953196
[4] LEE G J, HAN S D, JO V S, et al. Characteristics and pathogenic role of adherent-invasive Escherichia coli in inflammatory bowel disease:Potential impact on clinical outcomes[J]. PLoS One,2019,14(4):216165.
[5] 贺雅静, 谢勇. Toll样受体信号通路与TAM受体在炎症性肠病中的作用[J]. 中国免疫学杂志,2021,37(10):1271−1273. [HE Y J, XIE Y. The role of toll like receptor signaling pathway and TAM receptor in inflammatory bowel disease[J]. Chinese Journal of Immunology,2021,37(10):1271−1273.] doi: 10.3969/j.issn.1000-484X.2021.10.024 HE Y J, XIE Y. The role of toll like receptor signaling pathway and TAM receptor in inflammatory bowel disease[J]. Chinese Journal of Immunology, 2021, 37(10): 1271−1273. doi: 10.3969/j.issn.1000-484X.2021.10.024
[6] ZHOU Y F, CUI C P, MA X Y, et al. Nuclear factor κB (NF-κB)-mediated inflammation in multiple sclerosis[J]. Frontiers in Immunology,2020,11:391−391. doi: 10.3389/fimmu.2020.00391
[7] SOCHA M W, MALINOWSKI B, PUK O, et al. The role of NF-κB in uterine spiral arteries remodeling, insight into the cornerstone of preeclampsia[J]. International Journal of Molecular Sciences,2021,22(2):704. doi: 10.3390/ijms22020704
[8] NICOLAS R, CLIFF V W, HERMAN W, et al. Pseudorabies virus infection of epithelial cells leads to persistent but aberrant activation of the NF-κB pathway, inhibiting hallmark NF-κB-induced pro-inflammatory gene expression[J]. Journal of Virology, 2020, 94(10): e00196.
[9] 郭玲, 罗雪梅. 硫唑嘌呤治疗炎症性肠病的疗效及骨髓抑制相关不良反应分析研究[J]. 中南药学,2021,19(1):137−142. [GUO L, LUO X M. Study on the efficacy of azathioprine in the treatment of inflammatory bowel disease and the analysis of adverse reactions related to bone marrow suppression[J]. Zhongnan Pharmaceutical,2021,19(1):137−142.] GUO L, LUO X M. Study on the efficacy of azathioprine in the treatment of inflammatory bowel disease and the analysis of adverse reactions related to bone marrow suppression[J]. Zhongnan Pharmaceutical, 2021, 19(1): 137−142.
[10] ARBOLEYA S, SANCHEZ B, SOLIS G, et al. Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota:A functional inference study[J]. Ijms,2016,17(5):649. doi: 10.3390/ijms17050649
[11] YULLIIA H, TAISA D, IZUMI K, et al. The long-term consequences of antibiotic therapy:Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity[J]. PLoS One,2019,14(8):220642−220642.
[12] 李珊, 孙万成, 罗毅皓. 非淀粉多糖对肠道菌群的调节作用及其对代谢疾病影响的研究概述[J]. 食品研究与开发,2021,42(19):219−224. [LI S, SUN W C, LUO Y H. Overview of the regulatory effects of non starch polysaccharides on gut microbiota and their effects on metabolic diseases[J]. Food Research and Development,2021,42(19):219−224.] doi: 10.12161/j.issn.1005-6521.2021.19.031 LI S, SUN W C, LUO Y H. Overview of the regulatory effects of non starch polysaccharides on gut microbiota and their effects on metabolic diseases[J]. Food Research and Development, 2021, 42(19): 219−224. doi: 10.12161/j.issn.1005-6521.2021.19.031
[13] 王静, 李超君, 陆学洲, 等. 膳食纤维生理功能、制备方法及其在食品加工中的应用[J]. 保鲜与加工,2023,23(4):74−80. [WANG J, LI C J, LU X Z, et al. Physiological functions, preparation methods, and applications of dietary fiber in food processing[J]. Preservation and Processing,2023,23(4):74−80.] doi: 10.3969/j.issn.1009-6221.2023.04.012 WANG J, LI C J, LU X Z, et al. Physiological functions, preparation methods, and applications of dietary fiber in food processing[J]. Preservation and Processing, 2023, 23(4): 74−80. doi: 10.3969/j.issn.1009-6221.2023.04.012
[14] 耿宁宁, 戴竹青, 牛丽影, 等. 膳食纤维调节肠道微生物对机体健康的影响研究进展[J]. 江苏农业科学,2021,49(7):51−56. [GENG N N, DAI Z Q, NIU L Y, et al. Research progress on the effects of dietary fiber regulation on gut microbiota and body health[J]. Jiangsu Agricultural Science,2021,49(7):51−56.] GENG N N, DAI Z Q, NIU L Y, et al. Research progress on the effects of dietary fiber regulation on gut microbiota and body health[J]. Jiangsu Agricultural Science, 2021, 49(7): 51−56.
[15] VALCHEVA R, KOLEVA P, MARTINEZ I, et al. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels[J]. Gut Microbes,2019,10(3):334−357. doi: 10.1080/19490976.2018.1526583
[16] 廖勇, 贺海波, 李洁, 等. 基于TLR4/MyD88/NF-κB信号通路和肠黏膜屏障保护因子探讨魔芋润肠复合膳食纤维治疗便秘大鼠作用机制[J]. 中药药理与临床,2022,38(6):75−83. [LIAO Y, HE H B, LI J, et al. Based on TLR4/MyD88/NF- κB exploration of the signaling pathway and intestinal mucosal barrier protective factors in the treatment of constipation in rats using konjac runchang composite dietary fiber[J]. Pharmacology and Clinical Application of Traditional Chinese Medicine,2022,38(6):75−83.] LIAO Y, HE H B, LI J, et al. Based on TLR4/MyD88/NF- κB exploration of the signaling pathway and intestinal mucosal barrier protective factors in the treatment of constipation in rats using konjac runchang composite dietary fiber[J]. Pharmacology and Clinical Application of Traditional Chinese Medicine, 2022, 38(6): 75−83.
[17] 吕玉红, 郭瑞瑞, 孙心悦, 等. 肠道菌群利用膳食纤维及其与人体健康关系研究进展[J]. 中国酿造,2021,40(3):6−10. [LÜ Y H, GUO R R, SUN X Y, et al. Research progress on the utilization of dietary fiber by gut microbiota and its relationship with human health[J]. Brewing in China,2021,40(3):6−10.] doi: 10.11882/j.issn.0254-5071.2021.03.002 LÜ Y H, GUO R R, SUN X Y, et al. Research progress on the utilization of dietary fiber by gut microbiota and its relationship with human health[J]. Brewing in China, 2021, 40(3): 6−10. doi: 10.11882/j.issn.0254-5071.2021.03.002
[18] NOGALl A, VALDES A M, MENNI C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health[J]. Gut Microbes,2021,13(1):21−24.
[19] 冯焱, 闫丽欢, 冯江浩, 等. 膳食纤维与短链脂肪酸对肠道微生物以及宿主健康的影响[J]. 粮食与饲料工业,2021(4):37−41. [FENG Y, YAN L H, FENG J H, et al. The effects of dietary fiber and short chain fatty acids on gut microbiota and host health[J]. The Grain and Feed Industry,2021(4):37−41.] FENG Y, YAN L H, FENG J H, et al. The effects of dietary fiber and short chain fatty acids on gut microbiota and host health[J]. The Grain and Feed Industry, 2021(4): 37−41.
[20] MAKKI K, DEEHAN E C, WALTER J, et al. The impact of dietary fiber on gut microbiota in host health and disease[J]. Cell Host Microbe,2018,23(6):705−715. doi: 10.1016/j.chom.2018.05.012
[21] 施宇萌, 梁富强, 郭锐林, 等. 米糠不溶性膳食纤维结合酚结构特性及其对肠道菌群的影响[J]. 食品工业科技,2023,44(10):1−10. [SHI Y M, LIANG F Q, GUO R L, et al. The structural characteristics of insoluble dietary fiber bound phenols in rice bran and its impact on gut microbiota[J]. Science and Technology of Food Industry,2023,44(10):1−10.] SHI Y M, LIANG F Q, GUO R L, et al. The structural characteristics of insoluble dietary fiber bound phenols in rice bran and its impact on gut microbiota[J]. Science and Technology of Food Industry, 2023, 44(10): 1−10.
[22] HAN X Y, YANG D, ZHANG S, et al. Characterization of insoluble dietary fiber from Pleurotus eryngii and evaluation of its effects on obesity-preventing or relieving effects via modulation of gut microbiota[J]. Journal of Future Foods,2023,3(1):55−56. doi: 10.1016/j.jfutfo.2022.09.009
[23] 尼格尔热依·亚迪卡尔, 彭禛菲, 岳明, 等. 野山杏果肉不溶性膳食纤维对高脂血症大鼠肠道菌群及短链脂肪酸的影响[J]. 食品科技,2022,47(1):190−195. [NIAGARAI A, PENG S F, YUE M, et al. The effect of insoluble dietary fiber from wild apricot pulp on gut microbiota and short chain fatty acids in hyperlipidemic rats[J]. Food Technology,2022,47(1):190−195.] doi: 10.3969/j.issn.1005-9989.2022.1.spkj202201029 NIAGARAI A, PENG S F, YUE M, et al. The effect of insoluble dietary fiber from wild apricot pulp on gut microbiota and short chain fatty acids in hyperlipidemic rats[J]. Food Technology, 2022, 47(1): 190−195. doi: 10.3969/j.issn.1005-9989.2022.1.spkj202201029
[24] 黄苇, 黎力之, 廖晓鹏, 等. 菊粉通过肠道菌群调控动物脂代谢及其在动物生产中的应用[J]. 中国畜牧杂志,2021,57(6):36−40. [HUANG W, LI L Z, LIAO X P, et al. Inulin regulates animal lipid metabolism through gut microbiota and its application in animal production[J]. Chinese Journal of Animal Husbandry,2021,57(6):36−40.] HUANG W, LI L Z, LIAO X P, et al. Inulin regulates animal lipid metabolism through gut microbiota and its application in animal production[J]. Chinese Journal of Animal Husbandry, 2021, 57(6): 36−40.
[25] 赵文婧, 陈立英, 刘霞, 等. 膳食纤维经肠道微生态途径抑制结肠癌的研究进展[J]. 太原师范学院学报(自然科学版),2021,20(4):76−83. [ZHAO W J, CHEN L Y, LIU X, et al. Research progress on the inhibitory effect of dietary fiber on colon cancer through the gut microbiota pathway[J]. Journal of Taiyuan Normal University (Natural Science Edition),2021,20(4):76−83.] ZHAO W J, CHEN L Y, LIU X, et al. Research progress on the inhibitory effect of dietary fiber on colon cancer through the gut microbiota pathway[J]. Journal of Taiyuan Normal University (Natural Science Edition), 2021, 20(4): 76−83.
[26] WU W, SUN M, CHEN F, et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43.[J]. Mucosal Immunology,2017,10(4):946−956. doi: 10.1038/mi.2016.114
[27] 陈文轩, 张哲, 孙亚星, 等. 短链脂肪酸在炎症性肠病中的作用研究进展[J]. 中国免疫学杂志,2023,39(1):185−188. [CHEN W X, ZHANG Z, SUN Y X, et al. Research progress on the role of short chain fatty acids in inflammatory bowel disease[J]. Chinese Journal of Immunology,2023,39(1):185−188.] CHEN W X, ZHANG Z, SUN Y X, et al. Research progress on the role of short chain fatty acids in inflammatory bowel disease[J]. Chinese Journal of Immunology, 2023, 39(1): 185−188.
[28] 阿依木古丽·艾尼, 靳瑾. 短链脂肪酸在炎症性肠病中的作用研究进展[J]. 现代医药卫生,2023,39(10):1727−1731. [AINI A, JIN J. Research progress on the role of short chain fatty acids in inflammatory bowel disease[J]. Modern Medicine and Health,2023,39(10):1727−1731.] AINI A, JIN J. Research progress on the role of short chain fatty acids in inflammatory bowel disease[J]. Modern Medicine and Health, 2023, 39(10): 1727−1731.
[29] RASTOGI S, MOHANTY S, SHARMA S, et al. Possible role of gut microbes and hosts immune response in gut–lung homeostasis[J]. Frontiers in Immunology,2022,13:954339. doi: 10.3389/fimmu.2022.954339
[30] SUN M M, WU M, CHENG L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis[J]. Nature Communications,2018,9(1):3555. doi: 10.1038/s41467-018-05901-2
[31] 康宇婷, 赵志军, 安童童, 等. 短链脂肪酸对NR8383肺泡巨噬细胞炎性反应的影响[J]. 中国病原生物学杂志,2019,14(6):691−696. [KANG Y T, ZHA Z J, AN T T, et al. The effect of short chain fatty acids on the inflammatory response of NR8383 alveolar macrophages[J]. Chinese Journal of Pathogenic Biology,2019,14(6):691−696.] KANG Y T, ZHA Z J, AN T T, et al. The effect of short chain fatty acids on the inflammatory response of NR8383 alveolar macrophages[J]. Chinese Journal of Pathogenic Biology, 2019, 14(6): 691−696.
[32] SHRIYA B, MAHESH G. Dietary fiber from fruit waste as a potential source of metabolites in maintenance of gut milieu during ulcerative colitis:A comprehensive review[J]. Food Research International,2023,164: 112329. doi: 10.1016/J.FOODRES.2022.112329
[33] 黄超勇, 罗子林, 黎力之, 等. 菊粉对单胃动物肠道免疫功能的调节及应用研究进展[J]. 中国畜牧杂志,2022,58(5):77−81. [HUANG C Y, LUO Z L, LI L Z, et al. Research progress on the regulation and application of inulin on intestinal immune function in monogastric animals[J]. Chinese Journal of Animal Husbandry,2022,58(5):77−81.] HUANG C Y, LUO Z L, LI L Z, et al. Research progress on the regulation and application of inulin on intestinal immune function in monogastric animals[J]. Chinese Journal of Animal Husbandry, 2022, 58(5): 77−81.
[34] SAHASRABUDHEN M, BEUKEMA M, TIAN L, et al. Dietary fiber pectin directly blocks toll-like receptor 2-1 and prevents doxorubicin-induced ileitis[J]. Front in Immunol, 2018, 9:383.
[35] 刘帅, 李红霞, 董秀山. 短链脂肪酸对肠道动力影响的研究进展[J]. 中国微生态学杂志,2021,33(12):1476−1482. [LIU S, LI H X, DONG X S. Research progress on the effects of short chain fatty acids on intestinal motility[J]. Chinese Journal of Microbiology,2021,33(12):1476−1482.] LIU S, LI H X, DONG X S. Research progress on the effects of short chain fatty acids on intestinal motility[J]. Chinese Journal of Microbiology, 2021, 33(12): 1476−1482.
[36] WANG R X, LEE J S, CAMPBELL E L, et al. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin[J]. Proceedings of the National Academy of Sciences of the United States of America,2020,117(21):11648−11657.
[37] GONZALEZ A, KRIEG R, MASSEY H D, et al. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression[J]. Nephrol Dial Transplant,2019,34(5):783−794. doi: 10.1093/ndt/gfy238
[38] TIAN M, LI D, MA C, et al. Barley leaf insoluble dietary fiber alleviated dextran sulfate sodium-induced mice colitis by modulating gut microbiota[J]. Nutrients,2021,13(3):846. doi: 10.3390/nu13030846
[39] TONG L C, WANG Y, WANG Z B, et al. Propionate ameliorates dextran sodium sulfate-Induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress[J]. Front Pharmacol,2016,7:253.
[40] CRISTOFORI F, DARGENIO V N, DARGENIO C, et al. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation:A door to the body[J]. Frontiers in Immunology,2021,12:578386. doi: 10.3389/fimmu.2021.578386
[41] AKIHITO N, SASAKI S, ITOH K, et al. A soluble fiber diet increases Bacteroides fragilis group abundance and immunoglobulin a production in the gut[J]. Applied and Environmental Microbiology,2020,86(13):e00405−20.
[42] 尚玮璇, 刘璐, 雷素珍, 等. 功能性碳水化合物通过调节肠道菌群和代谢物改善非酒精性脂肪肝的作用机制[J]. 食品与发酵工业,2022,48(14):311−318. [SHANG W X, LIU L, LEI S Z, et al. The mechanism by which functional carbohydrates improve non-alcoholic fatty liver by regulating gut microbiota and metabolites[J]. Food and Fermentation Industry,2022,48(14):311−318.] SHANG W X, LIU L, LEI S Z, et al. The mechanism by which functional carbohydrates improve non-alcoholic fatty liver by regulating gut microbiota and metabolites[J]. Food and Fermentation Industry, 2022, 48(14): 311−318.
[43] PAIK D, YAO L, ZHANG Y, et al. Human gut bacteria produce TH17-modulating bile acid metabolites[J]. Nature,2022,603(7903):907−912. doi: 10.1038/s41586-022-04480-z
[44] 陈菁青, 郑建华, 董巧燕, 等. 嗜黏蛋白阿克曼氏菌在肠道疾病和代谢紊乱中的关键作用[J]. 实验动物科学, 2023, 40(5):80−85. [CHEN Q Q, ZHENG J H, DONG Q Y, et al. The key role of Actinobacteria mucophila in intestinal diseases and metabolic disorders[J]. Experimental Animal Science, 2023, 40(5):80−85.] CHEN Q Q, ZHENG J H, DONG Q Y, et al. The key role of Actinobacteria mucophila in intestinal diseases and metabolic disorders[J]. Experimental Animal Science, 2023, 40(5): 80−85.
[45] 张金秀, 胡新中, 马蓁. 不同类型抗性淀粉的多尺度结构特征与肠道菌群调节功能研究进展[J]. 食品科学,2022,43(17):24−35. [ZHANG J X, HU X Z, MA Q. Research progress on multi-scale structural characteristics and gut microbiota regulation function of different types of resistant starch[J]. Food Science,2022,43(17):24−35.] doi: 10.7506/spkx1002-6630-20220401-007 ZHANG J X, HU X Z, MA Q. Research progress on multi-scale structural characteristics and gut microbiota regulation function of different types of resistant starch[J]. Food Science, 2022, 43(17): 24−35. doi: 10.7506/spkx1002-6630-20220401-007
[46] BAI S F, CHEN H H, ZHU L Y, et al. Comparative study on the in vitro effects of Pseudomonas aeruginosa and seaweed alginates on human gut microbiota[J]. PLoS One,2017,12(2):e0171576. doi: 10.1371/journal.pone.0171576
[47] QICHAO C, MIN L, PENGYU Z, et al. Fucoidan and galactooligosaccharides ameliorate high-fat diet induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism[J]. Nutrition,2019,65:50−59. doi: 10.1016/j.nut.2019.03.001
[48] MOEMI N T, TAKASHI K, MIYU T, et al. Detection and isolation of low molecular weight alginate and laminaran-susceptible gut indigenous bacteria from ICR mice[J]. Carbohydrate Polymers,2020,238:116−205.
[49] LIU F T, LI P, CHEN M X, et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population[J]. Scientific Reports,2017,7(1):11789. doi: 10.1038/s41598-017-10722-2
[50] 王海松, 任鹏飞. 不同单糖组成的低聚糖对人肠道菌群的调节作用[J]. 中国食品学报,2020,20(7):44−52. [WANG H S, REN P F. The regulatory effect of oligosaccharides composed of different monosaccharides on human intestinal microbiota[J]. Chinese Journal of Food Science,2020,20(7):44−52.] WANG H S, REN P F. The regulatory effect of oligosaccharides composed of different monosaccharides on human intestinal microbiota[J]. Chinese Journal of Food Science, 2020, 20(7): 44−52.
[51] SINGH R P, PRAKASH S, BHATIA R, et al. Generation of structurally diverse pectin oligosaccharides having prebiotic attributes[J]. Food Hydrocolloids,2020,108:105988. doi: 10.1016/j.foodhyd.2020.105988
[52] CHERBUTH C, MICHELC, LECANNU G. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats[J]. The Journal of Nutrition,2003,133(1):21−7. doi: 10.1093/jn/133.1.21
[53] 高珊, 杨镭镭, 施媛, 等. 饮食添加益生元菊粉改善烧伤大鼠肠道菌群紊乱和骨骼肌分解代谢[J]. 华中科技大学学报(医学版),2023,52(1):60−67. [GAO S, YANG L L, SHI Y, et al. Dietary supplementation of probiotic inulin improves intestinal microbiota disorder and skeletal muscle catabolism in burned rats[J]. Journal of Huazhong University of Science and Technology (Medical Edition),2023,52(1):60−67.] GAO S, YANG L L, SHI Y, et al. Dietary supplementation of probiotic inulin improves intestinal microbiota disorder and skeletal muscle catabolism in burned rats[J]. Journal of Huazhong University of Science and Technology (Medical Edition), 2023, 52(1): 60−67.
[54] 向岑, 赵玙璠, 荣耀, 等. 菊粉对小鼠抗疲劳作用及对肠道微生物的影响[J]. 食品研究与开发,2020,41(23):68−72. [XIANG C, ZHAO Y P, RONG Y, et al. The anti fatigue effect of inulin on mice and its effect on intestinal microbiota[J]. Food Research and Development,2020,41(23):68−72.] doi: 10.12161/j.issn.1005-6521.2020.23.012 XIANG C, ZHAO Y P, RONG Y, et al. The anti fatigue effect of inulin on mice and its effect on intestinal microbiota[J]. Food Research and Development, 2020, 41(23): 68−72. doi: 10.12161/j.issn.1005-6521.2020.23.012
[55] DALILE B, VAN O L, VERVLIET B, et al. The role of short-chain fatty acids in microbiota-gut-brain communication[J]. Nature Reviews. Gastroenterology & Hepatology, 2019, 16(8) :461−478.
[56] 赵萌菲, 王琳燚, 袁艳枝, 等. 小麦阿拉伯木聚糖的益生功能及对肠道微生态的调节[J]. 中国食物与营养,2019,25(4):12−16. [ZHAO M F, WANG L Y, YUAN Y Z, et al. The probiotic function of wheat arabinoxylan and its regulation on intestinal microbiota[J]. Chinese Food and Nutrition,2019,25(4):12−16.] ZHAO M F, WANG L Y, YUAN Y Z, et al. The probiotic function of wheat arabinoxylan and its regulation on intestinal microbiota[J]. Chinese Food and Nutrition, 2019, 25(4): 12−16.
[57] 穆晓燕, 郑艳, 朱新鹏. 魔芋低聚糖生理作用及应用的研究进展[J]. 现代商贸工业,2019,40(11):219−222. [MU X Y, ZHENG Y, ZHU X P. Research progress on the physiological effects and applications of konjac oligosaccharides[J]. Modern Commercial Industry,2019,40(11):219−222.] MU X Y, ZHENG Y, ZHU X P. Research progress on the physiological effects and applications of konjac oligosaccharides[J]. Modern Commercial Industry, 2019, 40(11): 219−222.
[58] ZENG Y, ZHANG J G, ZHANG Y, et al. Prebiotic, immunomodulating, and antifatigue effects of konjac oligosaccharide[J]. Journal of Food Science,2018,83(12):3110−3117. doi: 10.1111/1750-3841.14376
[59] 吴尘萱, 丰硕, 刘军, 等. 部分水解瓜尔豆胶对高脂高糖饮食诱导小鼠代谢紊乱的调节作用[J]. 食品科学技术学报,2021,39(5):63−73. [WU C X, FENG S, LIU J, et al. The regulatory effect of partially hydrolyzed guar gum on metabolic disorders induced by a high fat and high sugar diet in mice[J]. Journal of Food Science and Technology,2021,39(5):63−73.] WU C X, FENG S, LIU J, et al. The regulatory effect of partially hydrolyzed guar gum on metabolic disorders induced by a high fat and high sugar diet in mice[J]. Journal of Food Science and Technology, 2021, 39(5): 63−73.
[60] 林晓娟, 苏志琛, 陈继承. 海带多糖的结构特征、生物活性及其应用[J]. 现代食品,2021(24):49−52. [LIN X J, SU Z S, CHEN J C. The structural characteristics, biological activity, and application of kelp polysaccharides[J]. Modern Food,2021(24):49−52.] LIN X J, SU Z S, CHEN J C. The structural characteristics, biological activity, and application of kelp polysaccharides[J]. Modern Food, 2021(24): 49−52.
[61] HYUNBIN S, JAE-HAN B, JI S S, et al. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation[J]. Journal of Functional Foods,2019,57:408−416. doi: 10.1016/j.jff.2019.04.014
[62] FATIMA E, THOMAS J. Linkage-specific detection and metabolism of human milk oligosaccharides in Escherichia coli[J]. Cell Chemical Biology,2018,25(10):1292−1303. doi: 10.1016/j.chembiol.2018.06.002





 下载:
下载:
 下载:
下载:



