Research Progress on Starch Digestibility Regulated by Multi-scale Structure and Physical Modification
-
摘要: 淀粉为人体生命活动提供必须的热量,但它通常有较高的血糖生成指数,消化后易导致血糖水平陡增,引发胰岛素抵抗,危害健康。淀粉的消化特性与其多尺度结构有关,通过改性改变淀粉的多尺度结构可以实现对淀粉消化特性的调控,而物理改性因安全简便、环境友好的特点受到越来越多的关注。基于此,本文概述了淀粉的消化特性,分析了淀粉多尺度结构与消化特性的关系,综述了水热处理、微波、挤压、高压均质、超声波、冷等离子和物理包封等物理改性对淀粉消化特性的调控,展望了淀粉消化特性的未来研究方向。Abstract: Starch provides essential calories for the body's vital activities, but it usually has a high glycemic index. The digestion of starch tends to lead to a steep increase in blood sugar levels, insulin resistance and health risk. The digestibility of starch is related to its multi-scale structure. The regulations of starch digestibility can be achieved by altering its multi-scale structure through the modifications. However, the physical modification has received more and more attention due to its safety, simplicity and environmental friendliness. Based on this, the digestibility of starch is outlined. The relationship between the multi-scale structure and digestive properties of starch is analyzed. The digestive properties of starch regulated by physical modifications, such as hydrothermal treatment, microwave, extrusion, high-pressure homogenization, ultrasonic, cold plasma and physical encapsulation are reviewed. The future research directions on starch digestibility are also provided.
-
Keywords:
- physical modification /
- starch /
- multi-scale structure /
- digestibility
-
淀粉是大米、高粱、小麦等粮食作物的主要成分,也是饮食中重要的碳水化合物来源,它经人体消化后水解为葡萄糖,转换为维持生命活动所必需的热量[1−2]。然而天然淀粉经人体消化吸收后易导致机体血糖水平陡增,增加Ⅱ型糖尿病的患病风险,严重危害人体健康。因此,淀粉消化特性的调控成为淀粉研究领域的热点,近年来受到众多国内外研究学者的关注[3]。研究显示,淀粉的消化特性主要与其多尺度结构有关,淀粉的多尺度结构是影响淀粉与消化酶结合的主要因素。因此,通过淀粉改性来有序化淀粉多尺度结构是实现对其消化特性调控的有效手段[4]。目前,常用的淀粉改性有物理改性、化学改性和酶改性。化学改性是目前广泛应用的淀粉改性方法,有定向高效的优点,但其改性副产物对环境存在威胁;酶法改性对环境友好,但它的成本较高。因此,安全简便、环境友好的物理改性受到越来越多的关注[5−6]。物理改性有水热处理(Hydrothermal treatment,HT)、微波处理(Microwave,MW)、挤压(Extrusion,ET)、高压均质(High pressure homogenization,HPH)和超声波处理(Ultrasonic technique,UT)等,除传统的物理改性方法外,冷等离子处理(Cold plasma,CP)和物理包封(Physical encapsulation,PE)等新兴方法也开始在淀粉消化特性调控领域开展研究应用[7]。基于此,本文首先介绍了淀粉的消化特性,并分析了淀粉多尺度结构对消化特性的影响,综述了物理改性对淀粉消化特性的调控,最后为淀粉消化特性的未来研究方向提出展望,以期为低消化性淀粉的食品开发提供参考。
1. 淀粉的消化过程及评价指标
1.1 淀粉的消化过程
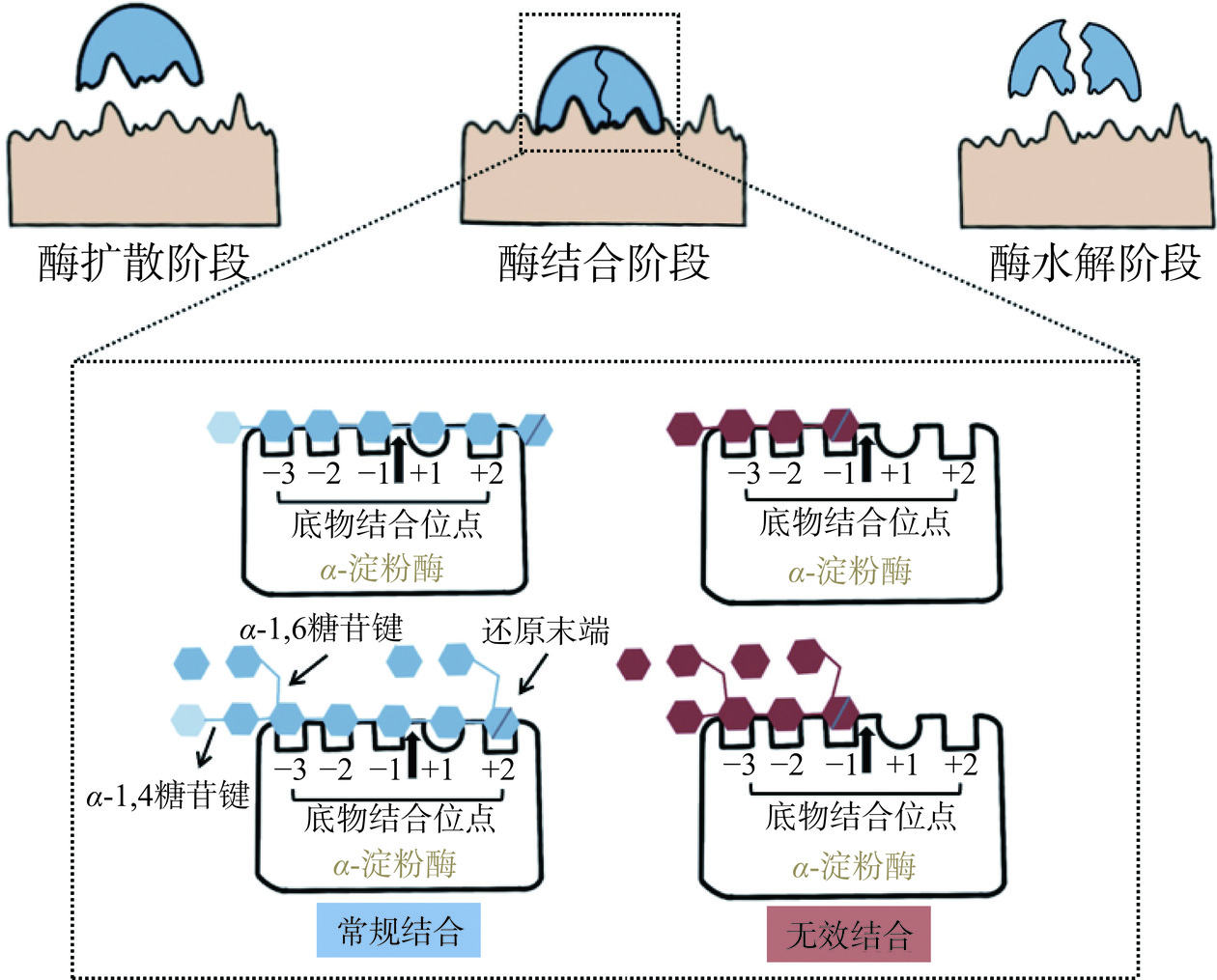
淀粉在消化酶的催化下消化水解,它的作用过程如图1所示,分为三个阶段:酶扩散阶段、酶结合阶段和酶水解阶段。其中酶结合阶段是影响淀粉消化特性的主要阶段[8]。α-淀粉酶是主要的淀粉消化酶,它有5个与淀粉分子结合的亚位点,作用于α-1,4糖苷键进行特异性降解[9]。在人体内,淀粉首先受口腔和小肠中唾液和胰腺α-淀粉酶的作用转化为麦芽糖和极限糊精等双糖,随后在麦芽糖酶-葡萄糖淀粉酶(Maltase-glucoamylase,MGAM)和蔗糖酶-异麦芽糖酶(Sucrase-isomaltase,SIM)的双重作用下水解为葡萄糖,进入血液循环后转换为维持生命活动所必需的热量[10]。根据淀粉在人体内的消化特点可将其分为快消化淀粉(Rapid digestible starch,RDS)、慢消化淀粉(Slowly digestible starch,SDS)和抗性淀粉(Resistant starch,RS)[11]。RDS指在小肠中20 min内快速水解的淀粉,它易引起人体餐后高血糖应答和胰岛素抵抗,增加糖尿病的患病风险;SDS指在小肠中20~120 min内缓慢水解的淀粉,它能够维持餐后血糖稳态,提高机体胰岛素敏感性,预防糖代谢综合症;RS指在小肠中120 min后也无法消化水解的淀粉,它能够促进肠道的蠕动和粪便、毒素的排出,稳定肠道菌群,避免肠应激综合症及结肠癌等疾病的发生[3,12]。
1.2 淀粉消化特性评价指标
淀粉的消化特性可以通过体外模拟消化实验或体内消化实验评价。在体外消化实验中,通常用过量的淀粉消化酶(α-淀粉酶和葡萄糖淀粉酶)在37 ℃恒温环境下消化淀粉,根据环境中葡萄糖随时间的变化量绘制消化曲线,体外环境中每小时由淀粉消化酶水解产生的葡萄糖量与淀粉样品总量的比值为平均消化速率(Rate of starch digestion,RSD),取样时间点已水解淀粉量占总淀粉量的百分比为水解率(Hydrolysis rate,HR),一定时间内淀粉样品的消化曲线下面积和参比样品的消化曲线下面积的比值为水解指数(Hydrolysis index,HI),RSD、HR和HI值是淀粉体外消化特性的评价指标[13−14]。在体内环境中,血液中吸收的葡萄糖量可以反映淀粉的消化程度。人体摄入某食物一定时间内体内血糖水平与食用当量的葡萄糖或白面包相比引起的血糖升高效应为血糖生成指数(Glycemic index,GI),淀粉的GI值与淀粉中RDS、SDS和RS的含量密切相关,因此,体内实验中通常以GI值、RDS、SDS和RS含量为指标评价淀粉的消化特性[15−16]。
2. 淀粉多尺度结构对消化特性的调控
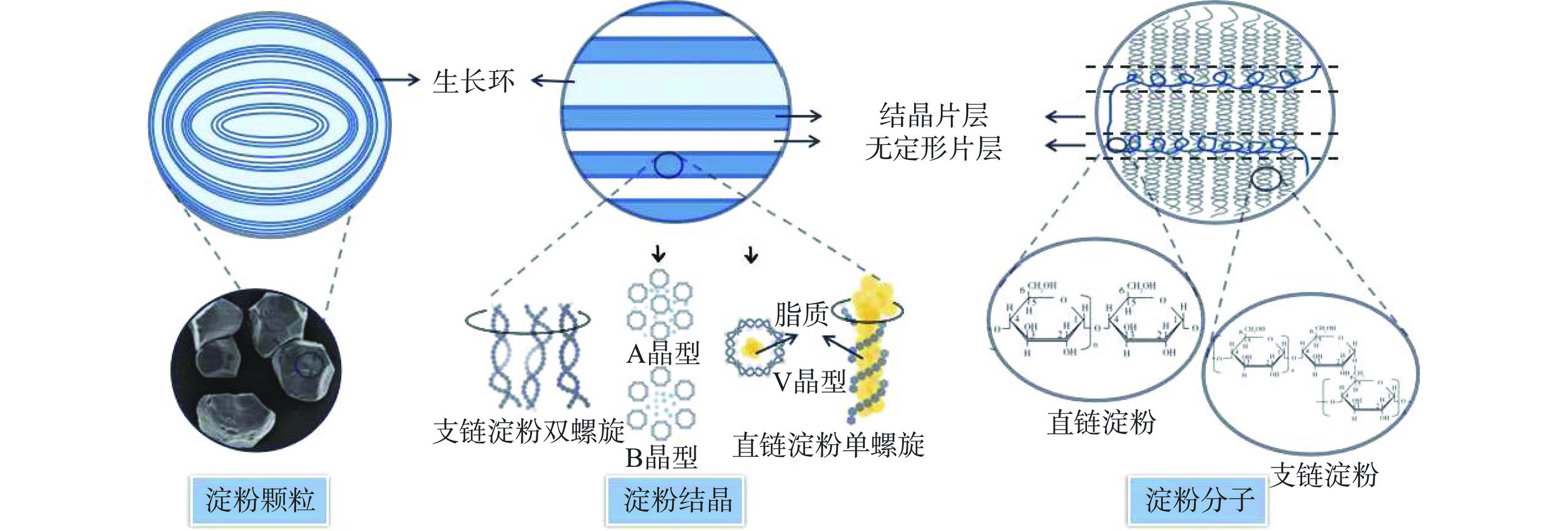
淀粉的结构呈现出多尺度的特点(图2),各尺度结构相互联系共同影响淀粉的消化特性[17]。对于生淀粉来说,其消化特性与颗粒、结晶和分子结构都显著相关,而熟淀粉在糊化过程中颗粒和结晶结构被破坏。因此,其消化特性主要由分子结构所决定[18]。同时,植物来源不同的淀粉由于多尺度结构的差异导致其与消化酶的结合存在差别,进而表现出不同的消化特性,表1总结了淀粉的消化特性与多尺度结构的关系。
表 1 淀粉的消化特性和多尺度结构Table 1. Digestive properties and multi-scale structure of different starches植物来源 淀粉种类 消化特性 多尺度结构 参考文献 谷类 大米淀粉 GI值55~85;
RS含量0.5%~2.5%颗粒呈多边形,多孔隙,3~8 μm,易与消化酶结合 [19] 玉米淀粉 GI值46~80;
RS含量3.3%~7.2%颗粒呈球形,5~20 μm [20−21] 小麦淀粉 GI值52~75;
RS含量0.5%~2.5%盘状颗粒,2~36 μm,A晶型,对消化酶敏感 [22−23] 豆类 绿豆淀粉 HI值44~51;
RS含量5.9%~10.9%直链淀粉含量高,支链淀粉以中长侧链为主,不易与消化酶结合 [24] 豌豆淀粉 SDS含量39.2%;
RS含量7.07%直链淀粉含量高,颗粒表面光滑 [25] 薯类 木薯淀粉 GI值70~85;
RS含量1.1%~2.8%颗粒呈椭圆形,10~40 μm [26−27] 甘薯淀粉 GI值44~78;
RS含量1.5%~3.2%颗粒呈圆形或多边形,9.5~18.5 μm [28] 马铃薯淀粉 GI值55~111;
RS含量0.8%~2.5%颗粒呈圆形,表面光滑,15~75 μm,B晶型,不易与消化酶结合 [27,29] 果实类 香蕉淀粉 RS含量高于马铃薯淀粉和玉米淀粉 支链淀粉中长侧链含量高 [30−31] 2.1 颗粒结构
淀粉的颗粒结构是淀粉消化过程中的第一道屏障,淀粉消化酶需要先吸附在淀粉颗粒表面随后扩散至内部作用于淀粉分子上的糖苷键催化其水解。因此,淀粉颗粒的粒径大小和表面形态显著影响淀粉的消化特性[32]。由于淀粉植物来源的不同,淀粉颗粒的粒径从小于10 μm到大于100 μm不等,颗粒也呈现出圆形、球形、多边形或不规则形等形态[33]。大米、玉米等谷物类淀粉的颗粒粒径较小,表面粗糙,有气孔与内部孔隙相连形成消化酶进入淀粉颗粒内部的通道,从而引起“由内而外”的消化模式,淀粉颗粒与消化酶的有效接触面积较大,消化速率较高[19−21]。而薯类淀粉的颗粒呈椭圆形且表面光滑,淀粉呈现出“由外而内”的消化模式,消化速率较低[27]。一些淀粉颗粒表面还存在少量蛋白质,这些颗粒表面蛋白相当于物理屏障,阻碍消化酶进入淀粉颗粒内部,能够延缓淀粉消化[34−35]。
2.2 结晶结构
淀粉具有半结晶结构,由结晶层和无定形层交替排列而成,其中结晶层由支链淀粉侧链构成,在氢键的作用下呈现出有序的双螺旋结构,抗消化性较强;而无定形层由直链淀粉和支链淀粉分支点构成,其结构无序松散,在消化过程中首先被水解[36]。X射线衍射图谱(Wide-angle X-ray diffraction,XRD)显示,天然淀粉的结晶类型分为A型、B型、C型和V型。A型是大米、玉米等谷物淀粉的结晶类型,其在XRD图谱的特征衍射峰是15°、17°、18°和23°(2θ),特点是结晶层的双螺旋结构较短,对消化酶更加敏感;B型是薯类、香蕉等高直链淀粉的结晶类型,XRD图谱的特征衍射峰是5.6°、17°、18°和23°(2θ),其支链淀粉侧链较长,且在无定形区有更多的分支点,抗消化性更强;C型是A型和B型的混合结晶类型,抗消化性介于A型和B型之间;V型是淀粉与脂质、碘、多酚等形成的复合物,脂质等物质占据了消化酶的结合位点,从而限制了淀粉分子与酶的结合,提高了淀粉的酶抗性[37−38]。研究发现,大米淀粉(A型)的RDS含量是马铃薯淀粉(B型)的三倍,且当相同基因型的大米淀粉由A型结晶转变至B型后,其消化率下降[39−40]。除结晶类型外,淀粉的消化特性也与其结晶度有关。结晶度高的淀粉具有更加紧凑有序的结晶结构,能够有效延缓淀粉酶在淀粉内部的扩散速度,使其具有较强的抗消化性[41]。
2.3 分子结构
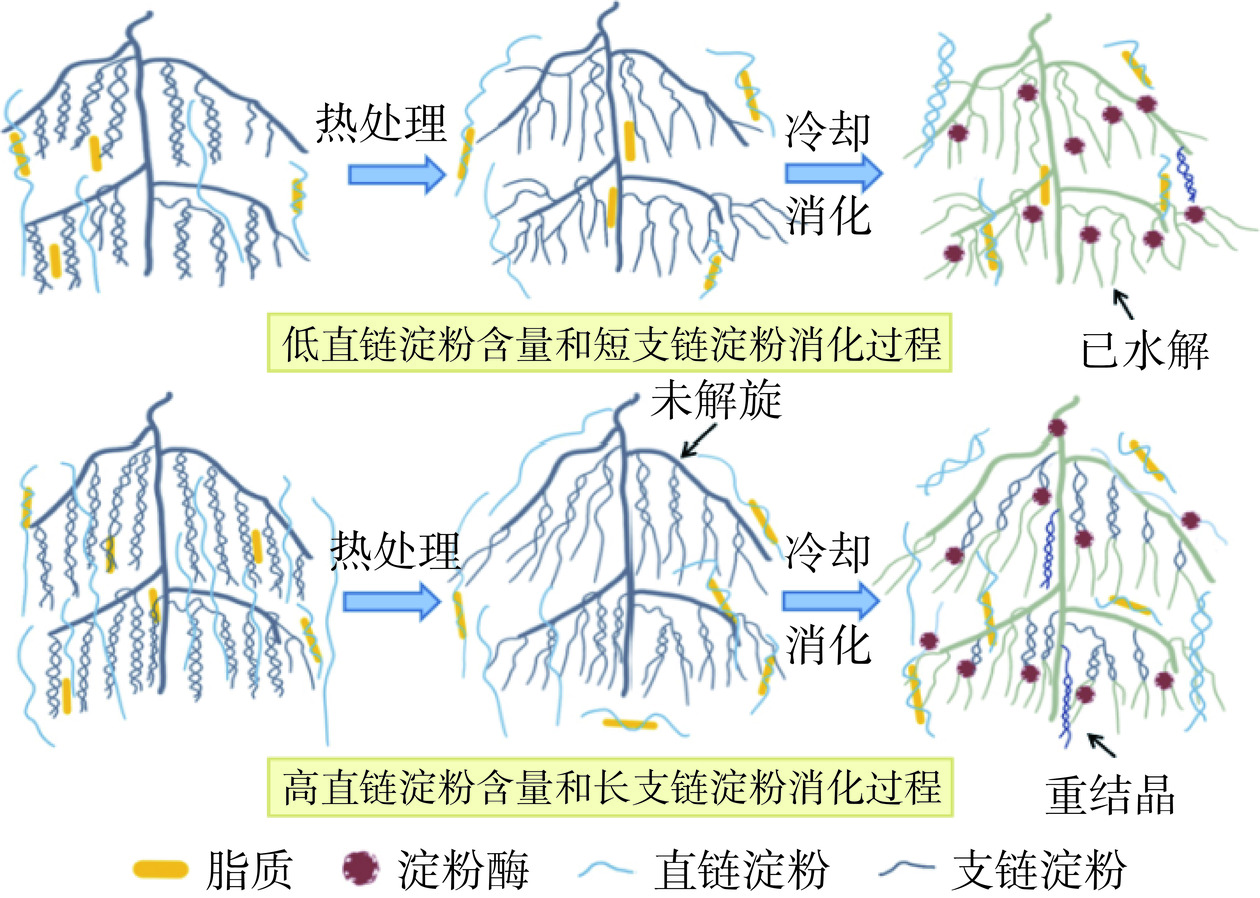
关于淀粉分子结构与消化特性的关系,目前的研究结论并不统一。大量研究结果表明,直链淀粉含量较高的淀粉具有更高的RS和SDS水平,消化速率更低[42−44]。这可能是由于直链淀粉相互缠绕成紧密结构,保护糖苷键免受淀粉消化酶的攻击[45]。然而也有研究发现,直链淀粉含量与SDS水平和消化速率无显著相关性[46]。为进一步确定淀粉分子结构与消化特性的关系,Yu等[47]分析了淀粉分子的链长分布后发现,短直链淀粉含量和直链淀粉分子尺寸与淀粉消化速率呈负相关。当直链淀粉含量不变而短直链分子数量增加时,淀粉的消化速率同样降低。这是由于短-中链直链分子相互交联形成了单独的凝胶网络,显著抑制了淀粉的消化[48]。因此,淀粉的消化特性受淀粉链长分布的影响。DP 6~12的淀粉分子易被酶消化水解,部分DP<12的短链淀粉分子受空间位阻的影响表现出延迟消化的特点,淀粉的消化速率先降低后上升,SDS含量先增加后减少,而DP>12的淀粉分子抗消化性更强。这是由于长链分子结构易形成双螺旋从而延缓消化[49−52]。淀粉的热加工过程中,颗粒和结晶结构被破坏,其消化特性主要受分子结构影响[18]。图3展示了淀粉热加工时的分子结构变化和酶解消化过程。热处理前淀粉的支链淀粉排列成双螺旋结构,直链淀粉穿插在其中,受热后支链淀粉双螺旋解开,直链淀粉流动性增强,冷却后溢出的直链淀粉与脂质形成复合物,直链淀粉、长支链淀粉重新缠绕为螺旋结构抵御淀粉消化酶的水解,而短支链淀粉则在淀粉消化酶的作用下水解为葡萄糖。
3. 物理改性对淀粉消化特性的调控
目前,对淀粉消化特性的调控主要有两种方式:构建延缓或阻碍淀粉消化酶进入淀粉颗粒的物理屏障和形成更加有序多尺度结构以抑制与淀粉消化酶的结合[53]。传统的物理改性通过热场、力场、磁场、电场等外力作用首先破坏淀粉的颗粒和结晶结构,增加淀粉分子的流动性,随后使淀粉分子重排,进而形成更有序的双螺旋结构或重结晶,增加淀粉的结晶度,从而抑制淀粉与消化酶的结合实现对消化特性的调控。而新兴的物理改性则可以通过构建物理屏障等方式调控淀粉的消化特性。不同物理改性对大米淀粉消化特性的调控在处理条件、调控机制及技术应用特点上存在差异,见表2。
表 2 物理改性调控淀粉消化特性对比Table 2. Comparison of digestive characteristics of starch regulated by physical modifications改性方法 处理条件 主要调控机制 特点 水热处理 ANN:高水分含量(40%~90%)低温(40~60 ℃)
HMT:低水分含量(10%~35%)高温(55~120 ℃)淀粉结晶熔融为橡胶态后重结晶,淀粉分子无序化,随后直链淀粉和支链淀粉相互交联 样品前处理简单;但改性时间较长,过程不易控制 微波处理 300 GHz~300 MHz电磁波 淀粉结晶先熔融后重结晶形成有序的双螺旋结构,
消化速率降低操作简单,改性时间短;但不适用于含水量高的淀粉样品 挤压 高温和压力下通过预设模具 淀粉在高温和压力的条件下糊化,结晶结构破坏,
淀粉分子重排并形成抗性淀粉,延缓消化能够制备出所需特定形状的RS,易于工业生产;但操作速度慢,模具损耗大 高压均质 50~150 MPa 剪切力使淀粉糖苷键断裂,直链淀粉含量增加,
促进V型结晶的形成改性时间短,效率高,无需添加溶剂;但能耗和成本较高 超声波处理 >5 W/cm2;40 kHz 低频率超声破坏淀粉颗粒和结晶结构,促进淀粉消化;高频率超声导致淀粉颗粒聚集,淀粉分子重排 改性时间短,操作简单,环境友好;但处理样品规模较小,不利于工业生产 冷等离子处理 低温低压、极高电场强度电
离气体由等离子体电离形成的自由基和高能电子诱导淀粉链解聚和交联 改性时间短,环境友好;但研究较少,应用前景有待开发 物理包封 将淀粉包封在海藻酸盐等多糖壳中 作为物理屏障保护淀粉颗粒不与淀粉酶接触;
限制淀粉颗粒膨胀糊化,延缓消化改性效果稳定,能有效提高RS含量;但壁材毒理安全性有待进一步研究 3.1 水热处理
水热处理包括韧化(Annealing,ANN)和湿热处理(Heat moisture treatment,HMT),两种改性的处理条件不同,对淀粉消化特性的调控作用也存在差异,详见表3。Zavareze等[54]研究发现HMT淀粉具有更高的体外消化速率,而Van等[2]的研究则观察到HMT淀粉的RDS含量减少,SDS和RS含量均增加,体外消化速率降低。造成这种差异的原因可能是由于处理过程中的高压灭菌导致淀粉糊化,淀粉的结晶结构被完全破坏,而未经高压处理的淀粉,结晶结构首先在加热过程中转变为流动的橡胶状,淀粉分子变得无序,随后直链淀粉和支链淀粉相互交联,形成了更为稳固的重结晶,从而抵抗淀粉消化酶的作用[55]。水分含量和处理时间影响水热处理对淀粉消化特性的调控作用。对10%、20%和30%水分含量的淀粉水热处理后发现,淀粉的体外消化速率随水分含量的增加而降低。这是由于当体系中水分含量增加,淀粉分子的流动性增强,从而促进了直链淀粉和支链淀粉间的交联和重结晶的形成[41]。因此,水热处理过程中淀粉分子相互交联形成的重结晶是延缓淀粉消化的主要因素。
表 3 水热处理对淀粉消化特性的调控Table 3. Regulation of starch digestion characteristics by hydrothermal treatment改性 实验条件 对淀粉消化特性的调控作用 调控机制 参考文献 湿热处理 水分含量15%~25%;
110 ℃高压灭菌1 h消化率均高于原淀粉,且25%水分含量的HMT淀粉消化率最高 高压高温导致淀粉糊化,结晶结构被完全破坏,结晶度下降 [54] 水分含量30%;110 ℃处理8 h RDS含量下降,RS含量上升 淀粉结晶结构先熔融后重结晶,淀粉分子相互交联,形成更稳固的结构 [2] 水分含量10%~30%;
110 ℃处理4 hRDS含量下降,RS和SDS含量上升 直链淀粉含量上升,促进直链淀粉-脂质复合 [41] 水分含量13%~18%;120 ℃高压
灭菌30/60 minRDS含量先上升后下降,SDS含量
上升淀粉结晶结构先熔融后重结晶,RS和RDS转化为SDS [56] 韧化 水分含量90%;45~55 ℃处理16 h 随温度升高消化率增加 淀粉颗粒表面气孔增多,结晶度降低 [57] 湿热韧化
联合处理韧化:水分含量80%;50 ℃处理24 h
湿热:水分含量25%;110 ℃处理8 h韧化-湿热:RDS和SDS含量上升,RS含量下降
湿热-韧化:RDS含量上升,SDS和RS含量下降韧化-湿热处理中HMT破坏了ANN淀粉的重结晶;湿热-韧化处理淀粉的结晶度与糊化焓降低,ANN无法使熔融的结晶结构重结晶 [58] 3.2 微波处理
微波处理对淀粉消化特性的调控与水热处理相似,但由于微波的高穿透性,它能够更加快速均匀地加热淀粉,操作时间更短,近年来逐渐受到研究者们的广泛关注[59]。为了确定微波处理对淀粉消化特性的影响,Sun等[60]对大米淀粉微波处理0、30和90 s后观察其RS含量变化。结果表明,微波处理30和90 s后淀粉中RS含量均显著增加,分别达到了46.87%和48.87%,消化特性得到改善。这是由于微波的作用下淀粉被迅速加热糊化,冷却后淀粉老化,淀粉分子显示出高度有序的双螺旋结构,形成了RS3抵抗淀粉消化酶的作用[61]。此外,研究发现淀粉样品的水分含量显著影响微波处理过程中对消化特性的作用,低水分含量淀粉微波后RS含量增加,RDS含量降低;而高水分含量淀粉RS含量变化则不显著[62−63]。这可能是由于高水分含量的淀粉更易发生糊化,淀粉颗粒表面产生裂纹和气孔,促进淀粉消化酶进入其内部参与消化[64]。由此可见,微波处理能够促进淀粉分子形成有序的双螺旋结构,从而形成抗性淀粉,然而当微波处理的淀粉含水量较高时,则不利于抗性淀粉的形成。
3.3 挤压
挤压是淀粉经过混合、蒸煮、捏合和剪切后从预设模具挤出的改性,它通过热处理和机械力作用使淀粉结构和性质改变[65]。研究发现,挤压处理能够降低淀粉的体外消化速率。对全谷物大米、碎米和挤压大米的淀粉消化率进行测定。结果显示挤压大米淀粉的消化速率(15.30%)低于全谷物大米淀粉(35.50%)和碎米淀粉(36.90%);同时挤压大米淀粉的HR(76.10%)和HI(2.91×10−2 min)也低于碎米淀粉(80.90%和6.42×10−2 min)[66]。由于挤压时淀粉首先在高温和压力的条件下糊化,结晶结构被破坏,淀粉分子流动性增强,随后淀粉分子重排并形成RS3,当有脂质存在时则形成RS5,从而对淀粉消化酶的水解产生抵抗力[67−68]。目前挤压已在抗性淀粉领域广泛应用,淀粉常被挤压加工成低GI重组米、预煮米糊、混合谷物棒等低GI产品。
3.4 高压均质
高压均质技术具有重现性高、瞬时高效的优点,近年来被广泛用于淀粉消化特性的研究。它通过高压在均质腔中形成压力差,从而对淀粉产生剪切、撞击、高频振荡、涡旋和气穴等机械力作用,改变淀粉的结构进而调控消化特性[69]。
高压均质对淀粉消化特性的调控作用与处理条件有关。在一定压力条件下,高压均质产生的机械力首先使淀粉分子链断裂,酶解速率加快;但随着压力的增加,剪切作用逐渐增强,支链淀粉脱分支使直链淀粉含量增加,淀粉酶的接触位点减少,导致RS含量上升,淀粉的抗消化性增强[70]。在实际应用中,高压均质技术常被用于RS5型抗性淀粉(淀粉和脂质/多酚复合物)的制备。高压均质下的淀粉-脂质/多酚复合物的形成分为两个阶段:第一个阶段淀粉分子链在高压的作用下断裂,分子流动性增强;第二阶段在高剪切力的作用下,脂质或多酚在淀粉悬浊液中分散,与淀粉分子充分作用,形成淀粉-脂质/多酚复合物,结构中直链淀粉分子呈紧密排列的单螺旋状,α-淀粉酶的扩散受到限制,从而延缓淀粉的消化[71]。
3.5 超声波处理
超声波处理是一种非热加工技术,已被广泛应用于食品原料加工、生物活性物质提取、食品发酵和降解等领域。由于其操作简便、环境友好的特点,近年来在调控淀粉消化特性领域也有着丰富的应用[72]。在超声波的空化作用下,淀粉的颗粒形貌、结晶结构和分子链结构发生变化,受此影响,淀粉消化酶与淀粉分子的结合状态改变,淀粉的消化特性被调控。
有研究显示,经超声波处理后的淀粉在体外消化实验表现出比对照组更高的GI值,这与超声波处理后淀粉的结晶度下降有关[73−74]。然而也有研究发现超声淀粉的GI值低于天然淀粉,且SDS和RS的含量均增加[75]。因此,推测超声波处理的频率和时间对淀粉的消化特性有重要影响。当淀粉在较低频率(0~300 W)超声波处理时,淀粉颗粒的表面破损,颗粒粒径减小,超声波作用于淀粉分子的糖苷键使其裂解,导致直链淀粉含量增加,淀粉结晶度降低,淀粉消化速率的增加;而随着超声频率和时间的增加,破碎的淀粉颗粒重新聚集形成粒径更大的颗粒,直链淀粉也重新排列形成高度有序的分子聚集结构或与脂肪酸形成V型结晶,限制淀粉分子与消化酶的接触,淀粉的RS含量增加,消化速率降低[76−77]。因此,超声波处理条件可能会使淀粉的结晶度和消化特性产生差异,而超声波具体如何促进淀粉分子链的重排以及V型结晶的形成还有待未来进一步的研究探索。
3.6 冷等离子处理
冷等离子(Cold plasma,CP)指非平衡状态的等离子,是气体(空气或任何气体混合物)在低温低压和极高电场强度环境中电离后的产物,包括自由电子、阳离子、阴离子、自由基、激发态的分子和原子,具有能耗少的优点,适用于淀粉等热敏感物质的改性[78]。CP是一种新兴的绿色改性,它不需要添加化学剂及催化剂,由等离子体电离形成的自由基和高能电子诱导淀粉分子链结构改变,如含氧基团的交联或接枝、淀粉链的解聚以及新官能团的形成。
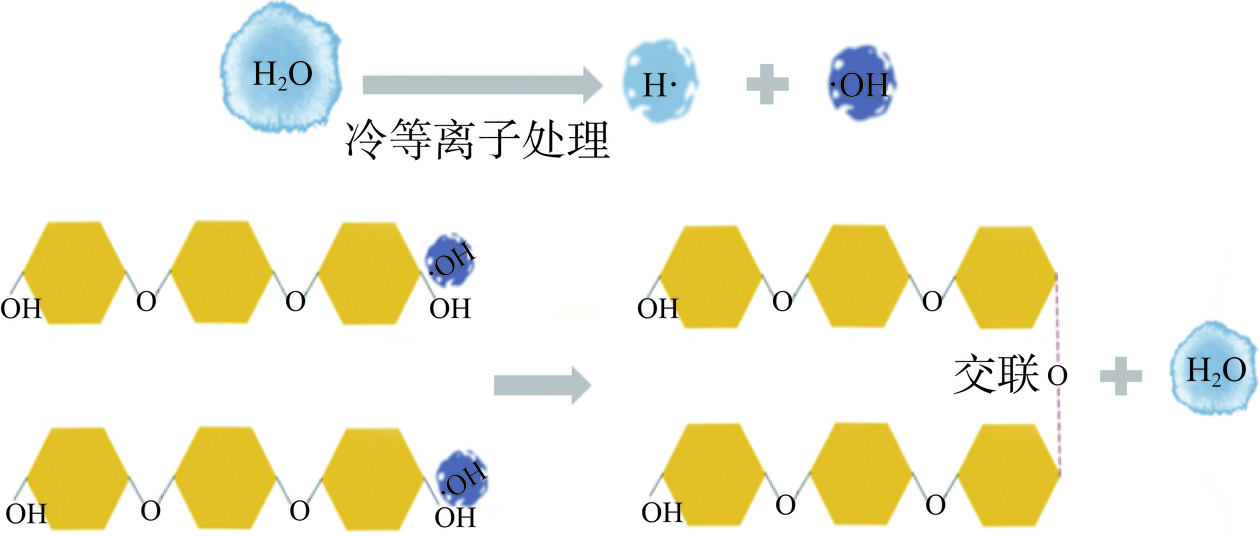
目前关于CP对淀粉消化特性影响的研究报道较少。Okyere等[79]对糯米淀粉进行CO2-Ar射频冷等离子处理后观察其消化特性的变化,结果显示,糯米淀粉的SDS含量由4.31%增加至5.51%。这可能是由于等离子体电离形成的羟基(-OH)会裂解糖苷键,在两条聚合链(C-OH)的还原端之间发生裂解并在这两条链之间形成新的C-O-C键,诱导淀粉分子交联,形成网络结构延缓淀粉酶的水解(图4)[80]。而Sun等[60]的研究则显示,CP处理后的淀粉有更高的消化率,可能与CP对支链淀粉的解聚和结晶度的降低有关。综上,CP对淀粉消化特性影响的研究仍在起步阶段,其作用机理也有待进一步研究。
3.7 物理包封
包裹在植物细胞中的天然淀粉其抗酶解性较强,不易被消化[53]。这是由于植物细胞外围的细胞壁能够作为物理屏障保护淀粉颗粒不与淀粉酶接触,表面结合位点提前与淀粉酶吸附结合,限制淀粉颗粒的膨胀糊化,从而使淀粉的抗消化性提高[81−82]。受此启发,将淀粉包封在多糖壳中可以模拟植物细胞壁的作用,阻止淀粉酶与淀粉分子接触从而降低淀粉的消化速率。Cui等[83]将淀粉包封在海藻酸盐微珠中,结果显示淀粉的RS含量(18.3%)高于对照淀粉(3.4%)。而进一步的研究表明海藻酸盐壳层强度随盐浓度的升高而增加,当海藻酸盐的浓度为0.50%时,壳层的强度最高,对淀粉颗粒的保护作用最好,RS的含量最高(28.82%)[84]。海藻酸盐壳限制了淀粉酶与淀粉的相互作用,降低了淀粉的消化速率。因此,将淀粉包裹在多糖聚合物壳中可以降低其消化率,是生产低GI淀粉食品的新兴方式。
4. 结论与展望
淀粉的消化特性与其多尺度结构有关。通过水热处理、挤压、高压均质、超声波、冷等离子等传统或新兴的物理改性可以改变淀粉的多尺度结构,进而实现对其消化特性的调控。传统物理改性调控淀粉消化特性虽然在工业中有较多应用,但其操作繁琐、效率低下。超声波、冷等离子等改性调控淀粉消化特性仅停留在研究层面,尚未投入工业生产,需不断完善,以促进其在抗性淀粉食品中应用。此外,有关淀粉消化特性的研究多数都是基于体外消化实验,未考虑胃肠道形状、蠕动频率和消化液释放等因素对淀粉消化特性的影响。因此,需建立更加完善的淀粉消化模型,为低GI淀粉食品的生产提供理论基础。
-
表 1 淀粉的消化特性和多尺度结构
Table 1 Digestive properties and multi-scale structure of different starches
植物来源 淀粉种类 消化特性 多尺度结构 参考文献 谷类 大米淀粉 GI值55~85;
RS含量0.5%~2.5%颗粒呈多边形,多孔隙,3~8 μm,易与消化酶结合 [19] 玉米淀粉 GI值46~80;
RS含量3.3%~7.2%颗粒呈球形,5~20 μm [20−21] 小麦淀粉 GI值52~75;
RS含量0.5%~2.5%盘状颗粒,2~36 μm,A晶型,对消化酶敏感 [22−23] 豆类 绿豆淀粉 HI值44~51;
RS含量5.9%~10.9%直链淀粉含量高,支链淀粉以中长侧链为主,不易与消化酶结合 [24] 豌豆淀粉 SDS含量39.2%;
RS含量7.07%直链淀粉含量高,颗粒表面光滑 [25] 薯类 木薯淀粉 GI值70~85;
RS含量1.1%~2.8%颗粒呈椭圆形,10~40 μm [26−27] 甘薯淀粉 GI值44~78;
RS含量1.5%~3.2%颗粒呈圆形或多边形,9.5~18.5 μm [28] 马铃薯淀粉 GI值55~111;
RS含量0.8%~2.5%颗粒呈圆形,表面光滑,15~75 μm,B晶型,不易与消化酶结合 [27,29] 果实类 香蕉淀粉 RS含量高于马铃薯淀粉和玉米淀粉 支链淀粉中长侧链含量高 [30−31] 表 2 物理改性调控淀粉消化特性对比
Table 2 Comparison of digestive characteristics of starch regulated by physical modifications
改性方法 处理条件 主要调控机制 特点 水热处理 ANN:高水分含量(40%~90%)低温(40~60 ℃)
HMT:低水分含量(10%~35%)高温(55~120 ℃)淀粉结晶熔融为橡胶态后重结晶,淀粉分子无序化,随后直链淀粉和支链淀粉相互交联 样品前处理简单;但改性时间较长,过程不易控制 微波处理 300 GHz~300 MHz电磁波 淀粉结晶先熔融后重结晶形成有序的双螺旋结构,
消化速率降低操作简单,改性时间短;但不适用于含水量高的淀粉样品 挤压 高温和压力下通过预设模具 淀粉在高温和压力的条件下糊化,结晶结构破坏,
淀粉分子重排并形成抗性淀粉,延缓消化能够制备出所需特定形状的RS,易于工业生产;但操作速度慢,模具损耗大 高压均质 50~150 MPa 剪切力使淀粉糖苷键断裂,直链淀粉含量增加,
促进V型结晶的形成改性时间短,效率高,无需添加溶剂;但能耗和成本较高 超声波处理 >5 W/cm2;40 kHz 低频率超声破坏淀粉颗粒和结晶结构,促进淀粉消化;高频率超声导致淀粉颗粒聚集,淀粉分子重排 改性时间短,操作简单,环境友好;但处理样品规模较小,不利于工业生产 冷等离子处理 低温低压、极高电场强度电
离气体由等离子体电离形成的自由基和高能电子诱导淀粉链解聚和交联 改性时间短,环境友好;但研究较少,应用前景有待开发 物理包封 将淀粉包封在海藻酸盐等多糖壳中 作为物理屏障保护淀粉颗粒不与淀粉酶接触;
限制淀粉颗粒膨胀糊化,延缓消化改性效果稳定,能有效提高RS含量;但壁材毒理安全性有待进一步研究 表 3 水热处理对淀粉消化特性的调控
Table 3 Regulation of starch digestion characteristics by hydrothermal treatment
改性 实验条件 对淀粉消化特性的调控作用 调控机制 参考文献 湿热处理 水分含量15%~25%;
110 ℃高压灭菌1 h消化率均高于原淀粉,且25%水分含量的HMT淀粉消化率最高 高压高温导致淀粉糊化,结晶结构被完全破坏,结晶度下降 [54] 水分含量30%;110 ℃处理8 h RDS含量下降,RS含量上升 淀粉结晶结构先熔融后重结晶,淀粉分子相互交联,形成更稳固的结构 [2] 水分含量10%~30%;
110 ℃处理4 hRDS含量下降,RS和SDS含量上升 直链淀粉含量上升,促进直链淀粉-脂质复合 [41] 水分含量13%~18%;120 ℃高压
灭菌30/60 minRDS含量先上升后下降,SDS含量
上升淀粉结晶结构先熔融后重结晶,RS和RDS转化为SDS [56] 韧化 水分含量90%;45~55 ℃处理16 h 随温度升高消化率增加 淀粉颗粒表面气孔增多,结晶度降低 [57] 湿热韧化
联合处理韧化:水分含量80%;50 ℃处理24 h
湿热:水分含量25%;110 ℃处理8 h韧化-湿热:RDS和SDS含量上升,RS含量下降
湿热-韧化:RDS含量上升,SDS和RS含量下降韧化-湿热处理中HMT破坏了ANN淀粉的重结晶;湿热-韧化处理淀粉的结晶度与糊化焓降低,ANN无法使熔融的结晶结构重结晶 [58] -
[1] SAIKRISHNA A, DUTTA S, SUBRAMANIAN V, et al. Ageing of rice:A review[J]. Journal of Cereal Science,2018,81:161−170. doi: 10.1016/j.jcs.2018.04.009
[2] VAN HUNG P, CHAU H T, PHI N T L. In-vitro digestibility and in-vivo glucose response of native and physically modified rice starches varying amylose contents[J]. Food Chemistry,2016,191:74−80. doi: 10.1016/j.foodchem.2015.02.118
[3] KUMAR A, SAHOO U, BAISAKHA B, et al. Resistant starch could be decisive in determining the glycemic index of rice cultivars[J]. Journal of Cereal Science,2018,79:348−353. doi: 10.1016/j.jcs.2017.11.013
[4] WEE M S M, HENRY C J. Reducing the glycemic impact of carbohydrates on foods and meals:Strategies for the food industry and consumers with special focus on Asia[J]. Comprehensive Reviews in Food Science & Food Safety,2020,19:670−702.
[5] AHMED J, THOMAS L, TAHER A, et al. Impact of high pressure treatment on functional, rheological, pasting and structural properties of lentil starch dispersions[J]. Carbohydrate Polymers,2016,152:639−647. doi: 10.1016/j.carbpol.2016.07.008
[6] NEELAM K, VIJAY S, LALIT S. Various techniques for the modification of starch and the applications of its derivatives[J]. International Research Journal of Pharmacy,2012,3(5):25−31.
[7] QADIR N, WANI I A. In-vitro digestibility of rice starch and factors regulating its digestion process:A review[J]. Carbohydrate Polymers,2022,291:119600. doi: 10.1016/j.carbpol.2022.119600
[8] LEHMANN U, ROBIN F. Slowly digestible starch-its structure and health implications:A review[J]. Trends in Food Science & Technology,2007,18:346−355.
[9] BELLO-PEREZ L A, FLORES-SILVA P C, AGAMA-ACEVEDOA E, et al. Starch digestibility:Past, present, and future[J]. Journal of the Science of Food Agriculture,2020,100:5009−5016. doi: 10.1002/jsfa.8955
[10] NICHOLS B L, AVERY S, SEN P, et al. The maltase-glucoamylase gene:Common ancestry to sucrase-isomaltase with complementary starch digestion activities[J]. Proceedings of the National Academy of Sciences United States of America,2003,100:1432−1437. doi: 10.1073/pnas.0237170100
[11] ENGLYST H N, KINGMAN S M, CUMMINGS J H. Classification and measurement of nutritionally important starch fractions[J]. European Journal of Clinical Nutrition,1992,46:S33−S50.
[12] HIRSCH S, BARRERA G, LEIVA L, et al. Variability of glycemic and insulin response to a standard meal, within and between healthy subjects[J]. Nutricion Hospitalaria,2013,28:541−544.
[13] LI C, YU W, GILBERT R G. The effects of starch molecular fine structure on thermal and digestion properties of rice starch[J]. Foods,2022,11:4012. doi: 10.3390/foods11244012
[14] 黄峻榕, 任瑞珍, 蒲华寅, 等. 慢消化淀粉的消化特性, 测定及制备[J]. 中国粮油学报,2015,30(3):134−139. [HUANG J, REN R Z, PU H Y, et al. Digestibility, determination and preparation of slowly digestible starch[J]. Journal of the Chinese Cereals and Oils Association,2015,30(3):134−139.] HUANG J, REN R Z, PU H Y, et al. Digestibility, determination and preparation of slowly digestible starch[J]. Journal of the Chinese Cereals and Oils Association, 2015, 30(3): 134−139.
[15] GIRI S, BANERJI A, LELE S S, et al. Starch digestibility and glycaemic index of selected Indian traditional foods:Effects of added ingredients[J]. International Journal of Food Properties,2017,20:S290−S305. doi: 10.1080/10942912.2017.1295387
[16] MEERA K, SMITA M, HARIPRIYA S, et al. Varietal influence on antioxidant properties and glycemic index of pigmented and non-pigmented rice[J]. Journal of Cereal Science,2019,87:202−208. doi: 10.1016/j.jcs.2019.03.005
[17] ZHONG Y, QU J, LI Z, et al. Rice starch multi-level structure and functional relationships[J]. Carbohydrate Polymers,2022,275:118777. doi: 10.1016/j.carbpol.2021.118777
[18] LIU Z D, WANG J, LI L, et al. Mechanistic insights into the role of starch multi-level structures in functional properties of high-amylose rice cultivars[J]. Food Hydrocolloids,2021,113(80):106441.
[19] KUMAR A, SAHOO S, SAHU S, et al. Rice with pulses or cooking oils can be used to elicit lower glycemic response[J]. Journal of Food Composition and Analysis,2018,71:1−7. doi: 10.1016/j.jfca.2018.05.003
[20] APE D I, NWOGU N A, UWAKWE E I, et al. Comparative proximate analysis of maize and sorghum bought from ogbete main market of Enugu State, Nigeria[J]. Greener Journal of Agricultural Sciences,2016,6(9):272−275. doi: 10.15580/GJAS.2016.9.101516167
[21] CAI C, ZHAO L, HUANG J, et al. Morphology, structure and gelatinization properties of heterogeneous starch granules from high-amylose maize[J]. Carbohydrate Polymers,2014,102(1):606−614.
[22] BIRT D F, BOYLSTON T, HENDRICH S, et al. Resistant starch:Promise for improving human health[J]. Advances in Nutrition,2013,4(6):587−601. doi: 10.3945/an.113.004325
[23] SLADE A J, MCGUIRE C, LOEFFLER D, et al. Development of high amylose wheat through tilling[J]. BMC Plant Biology,2012,12(1):69. doi: 10.1186/1471-2229-12-69
[24] 周淑蓝, 叶发银, 赵国华. 绿豆淀粉的性质, 改性及其应用[J]. 中国食品学报,2022,22(4):450−461. [ZHOU S L, YE F Y, ZHAO G H. The properties, modification and application of mung bean (Vigna radiata L. Wilczek) starch[J]. Journal of Chinese Institute of Food Science and Technology,2022,22(4):450−461.] ZHOU S L, YE F Y, ZHAO G H. The properties, modification and application of mung bean (Vigna radiata L. Wilczek) starch[J]. Journal of Chinese Institute of Food Science and Technology, 2022, 22(4): 450−461.
[25] 王志倩, 李言, 钱海峰, 等. 豌豆成分对血糖的影响研究进展[J]. 中国食品学报,2023,23(9):430−438. [[WANG Z Q, LI Y, QIAN H F, et al. Effects of pea constituents on blood glucose:A review[J]. Journal of Chinese Institute of Food Science and Technology,2023,23(9):430−438.] [WANG Z Q, LI Y, QIAN H F, et al. Effects of pea constituents on blood glucose: A review[J]. Journal of Chinese Institute of Food Science and Technology, 2023, 23(9): 430−438.
[26] AKYEREKO Y G, WIREKO-MANU F D, ODURO I. Influence of processing methods on food components and glycaemic index of cassava-based traditional foods[J]. Journal of Food and Nutrition Sciences,2020,8(1):6−14. doi: 10.11648/j.jfns.20200801.12
[27] ZHANG L, ZHAO Y, HU W, et al. Multiscale structures of cassava and potato starch fractions varying in granule size[J]. Carbohydrate Polymers,2018,200(15):400−407.
[28] NIU S, LI X Q, TANG R, et al. Starch granule sizes and degradation in sweet potatoes during storage[J]. Postharvest Biology and Technology,2019,150:137−147. doi: 10.1016/j.postharvbio.2019.01.004
[29] SINGH A, RAIGOND P, LAL M K, et al. Effect of cooking methods on glycemic index and in-vitro bioaccessibility of potato(Solanum tuberosum L.) carbohydrates[J]. LWT-Food Science and Technology,2020,127:109363. doi: 10.1016/j.lwt.2020.109363
[30] 徐亚元, 沈素晴, 李大婧, 等. 青香蕉微波干燥中淀粉糊化行为及消化特性的研究[J]. 食品工业科技,2022,43(3):88−96. [XU Y Y, SHEN S Q, LI D J, et al. Study on starch gelatinization behaviors and digestibility of green bananas during microwave drying[J]. Science and Technology of Food Industry,2022,43(3):88−96.] XU Y Y, SHEN S Q, LI D J, et al. Study on starch gelatinization behaviors and digestibility of green bananas during microwave drying[J]. Science and Technology of Food Industry, 2022, 43(3): 88−96.
[31] AGAMA-ACEVEDO E, NUÑEZ-SANTIAGO M C, ALVAREZ-RAMIREZ J, et al. Physicochemical, digestibility and structural characteristics of starch isolated from banana cultivars[J]. Carbohydrate Polymers,2015,124:17. doi: 10.1016/j.carbpol.2015.02.003
[32] KONG B, KIM J, KIM M, et al. Porcine pancreatic α-amylase hydrolysis of native starch granules as a function of granule surface area[J]. Biotechnology Progress,2003,19:1162−1166.
[33] ALLER E, ABETE I, ASTRUP A, et al. Starches, sugars and obesity[J]. Nutrients,2011,3:341−369. doi: 10.3390/nu3030341
[34] TANG M, WANG L, CHENG X, et al. Non-starch constituents influence the in-vitro digestibility of naked oat (Avena nuda L.) starch[J]. Food Chemistry,2019,297:124953. doi: 10.1016/j.foodchem.2019.124953
[35] CHI C, LI X, ZHANG Y, et al. Understanding the mechanism of starch digestion mitigation by rice protein and its enzymatic hydrolysates[J]. Food Hydrocolloids,2018,84:473−480. doi: 10.1016/j.foodhyd.2018.06.040
[36] COPELAND L, BLAZEK J, SALMAN H, et al. Form and functionality of starch[J]. Food Hydrocolloids,2009,23(6):1527−1534. doi: 10.1016/j.foodhyd.2008.09.016
[37] GUTIÉRREZ T J, TOVAR J. Update of the concept of type 5 resistant starch (RS5):Self-assembled starch V-type complexes[J]. Trends in Food Science & Technology,2021,109:711−724.
[38] HE H, ZHENG B, WANG H, et al. Insights into the multi-scale structure and in-vitro digestibility changes of rice starch-oleic acid/linoleic acid complex induced by heat-moisture treatment[J]. Food Research International,2020,137:109612. doi: 10.1016/j.foodres.2020.109612
[39] BUTARDO V M, FITZGERALD M A, BIRD A R, et al. Impact of down-regulation of starch branching enzyme IIb in rice by artificial microRNA- and hairpin RNA-mediated RNA silencing[J]. Journal of Experimental Botany,2011,62:4927−4941. doi: 10.1093/jxb/err188
[40] ZHANG G, AO Z, HAMAKER B. Slow digestion property of native cereal starches[J]. Biomacromolecules,2006,7:3252−3258. doi: 10.1021/bm060342i
[41] WANG H W, LIU Y F, CHEN L, et al. Insights into the multi-scale structure and digestibility of heat-moisture treated rice starch[J]. Food Chemistry,2018,242:323−329. doi: 10.1016/j.foodchem.2017.09.014
[42] ZHU L J, LIU Q Q, WILSON J D, et al. Digestibility and physicochemical properties of rice (Oryza sativa L.) flours and starches differing in amylose content[J]. Carbohydrate Polymers,2011,86:1751−1759. doi: 10.1016/j.carbpol.2011.07.017
[43] CHUNG H J, LIU Q A, LEE L, et al. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents[J]. Food Hydrocolloids,2011,25:968−975. doi: 10.1016/j.foodhyd.2010.09.011
[44] CAI J W, MAN J M, HUANG J, et al. Relationship between structure and functional properties of normal rice starches with different amylose contents[J]. Carbohydrate Polymers,2015,125:35−44. doi: 10.1016/j.carbpol.2015.02.067
[45] ZHANG G, HAMAKER B R. Review:Cereal carbohydrates and colon health[J]. Cereal Chemistry,2010,87:331−341. doi: 10.1094/CCHEM-87-4-0331
[46] ZHOU X, YING Y N, HU B L, et al. Physicochemical properties and digestibility of endosperm starches in four indica rice mutants[J]. Carbohydrate Polymers,2018,195:1−8. doi: 10.1016/j.carbpol.2018.04.070
[47] YU W, TAO K, GILBERT R G. Improved methodology for analyzing relations between starch digestion kinetics and molecular structure[J]. Food Chemistry,2018,264:284−292. doi: 10.1016/j.foodchem.2018.05.049
[48] GONG B, CHENG L, GILBERT R, et al. Distribution of short to medium amylose chains are major controllers of in-vitro digestion of retrograded rice starch[J]. Food Hydrocolloids,2019,96:634−643. doi: 10.1016/j.foodhyd.2019.06.003
[49] ZHANG B, ZHOU W, QIAO D, et al. Changes in nanoscale chain assembly in sweet potato starch lamellae by downregulation of biosynthesis enzymes[J]. Journal of Agricultural and Food Chemistry,2019,67:6302−6312. doi: 10.1021/acs.jafc.8b06523
[50] ZHU F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch[J]. Trends in Food Science & Technology,2018,78:234−242.
[51] ALHAMBRA C M, de GUZMAN M K, DHITAL S, et al. Long glucan chains reduce in vitro starch digestibility of freshly cooked and retrograded milled rice[J]. Journal of Cereal Science,2019,86:108−116. doi: 10.1016/j.jcs.2019.02.001
[52] MIAO M, XIONG S, JIANG B, et al. Improved the slow digestion property of maize starch using partially beta-amylolysis[J]. Food Chemistry,2014,152:128−132. doi: 10.1016/j.foodchem.2013.11.148
[53] DHITAL S, WARREN F J, BUTTERWORTH P J, et al. Mechanisms of starch digestion by α-amylase-structural basis for kinetic properties[J]. Critical Reviews in Food Science and Nutrition,2015,57:875−892.
[54] ZAVAREZE E D, STORCK C R, CASTRO L A S, et al. Effect of heat-moisture treatment on rice starch of varying amylose content[J]. Food Chemistry,2010,121:358−365. doi: 10.1016/j.foodchem.2009.12.036
[55] CAROLINA A A S, ALMEIDA M M A. Physicochemical properties, modifications and applications of starches from different botanical sources[J]. Food Science and Technology,2015,35(2):215−236. doi: 10.1590/1678-457X.6749
[56] SILVA W M F, BIDUSKI B, LIMA K O, et al. Starch digestibility and molecular weight distribution of proteins in rice grains subjected to heat-moisture treatment[J]. Food Chemistry,2017,219:260−267. doi: 10.1016/j.foodchem.2016.09.134
[57] DIAS A R G, ZAVAREZE E D, SPIER F, et al. Effects of annealing on the physicochemical properties and enzymatic susceptibility of rice starches with different amylose contents[J]. Food Chemistry,2010,123:711−719. doi: 10.1016/j.foodchem.2010.05.040
[58] ZENG F, MA F, KONG F S, et al. Physicochemical properties and digestibility of hydrothermally treated waxy rice starch[J]. Food Chemistry,2015,172:92−98. doi: 10.1016/j.foodchem.2014.09.020
[59] DEKA D, SIT N. Dual modification of taro starch by microwave and other heat moisture treatments[J]. International Journal of Biological Macromolecules,2016,92:416−422. doi: 10.1016/j.ijbiomac.2016.07.040
[60] SUN X, SALEH A S M, SUN Z, et al. Modification of multi-scale structure, physicochemical properties, and digestibility of rice starch through microwave and cold plasma treatments[J]. LWT-Food Science & Technology,2022,153:112483.
[61] FAN D, WANG L, CHEN W, et al. Effect of microwave on lamellar parameters of rice starch through small-angle X-ray scattering[J]. Food Hydrocolloids,2014,35:620−626. doi: 10.1016/j.foodhyd.2013.08.003
[62] LIU T, ZHANG B, WANG L, et al. Microwave reheating increases the resistant starch content in cooked rice with high water contents[J]. International Journal of Biological Macromolecules,2021,184:804−811. doi: 10.1016/j.ijbiomac.2021.06.136
[63] LI Y, HU A, WANG X, et al. Physicochemical and in-vitro digestion of millet starch:Effect of moisture content in microwave[J]. International Journal of Biological Macromolecules,2019,134:308−315. doi: 10.1016/j.ijbiomac.2019.05.046
[64] ZHAO K, LI B, XU M, et al. Microwave pretreated esterification improved the substitution degree, structural and physicochemical properties of potato starch esters[J]. LWT-Food Science & Technology,2018,90:116−123.
[65] ALAM M S, KAUR J, KHAIRA H, et al. Extrusion and extruded products:Changes in quality attributes as affected by extrusion process parameters:A review[J]. Critical Reviews in Food Science & Nutrition,2016,56:445−473.
[66] YANG W, ZHENG Y, SUN W, et al. Effect of extrusion processing on the microstructure and in-vitro digestibility of broken rice[J]. LWT-Food Science & Technology,2020,119:108835.
[67] ZHANG B, DHITAL S, GIDLEY M J. Densely packed matrices as rate determining features in starch hydrolysis[J]. Trends in Food Science & Technology,2015,43:18−31.
[68] CASTELLANOS-GALLO L, GALICIA-GARCIA T, ESTRADA-MORENO I, et al. Development of an expanded snack of rice starch enriched with amaranth by extrusion process[J]. Molecules,2019,24:1−22.
[69] MENG S, MA Y, SUN D W, et al. Properties of starch palmitic acid complexes prepared by high pressure homogenization[J]. Journal of Cereal Science,2014,59(1):25−32. doi: 10.1016/j.jcs.2013.10.012
[70] OYEYINKA S A, SINGH S, MA Y, et al. Effect of high-pressure homogenization on structural, thermal and rheological properties of Bambara starch complexed with different fatty acids[J]. RSC Advances,2016,6:80174−80180. doi: 10.1039/C6RA16452H
[71] LIU Y, CHEN L, XU H, et al. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization[J]. International Journal of Biological Macromolecules,2019,134:856−863. doi: 10.1016/j.ijbiomac.2019.05.083
[72] BONTO A P, TIOZON J R N, SREENIVASULU N, et al. Impact of ultrasonic treatment on rice starch and grain functional properties:A review[J]. Ultrasonics Sonochemistry,2021,71:105383. doi: 10.1016/j.ultsonch.2020.105383
[73] KUNYANEE K, LUANGSAKUL N. The effects of ultrasound-assisted recrystallization followed by chilling to produce the lower glycemic index of rice with different amylose content[J]. Food Chemistry,2020,323:126843. doi: 10.1016/j.foodchem.2020.126843
[74] KUNYANEE K, LUANGSAKUL N. The utilization of ultrasound and chilling treatment to reduce GI in Thai glutinous rice(RD6)[J]. International Journal of Agricultural Technology,2018,14:1365−1378.
[75] DANG L, THERDTHAI N, RATPHITAGSANTI W. Effects of ultrasonic and enzymatic treatment on physical and chemical properties of brown rice[J]. Journal of Food Process Engineering,2019,42:e13016. doi: 10.1111/jfpe.13016
[76] DING Y, LIANG Y, LUO F, et al. Understanding the mechanism of ultrasonication regulated the digestibility properties of retrograded starch following vacuum freeze drying[J]. Carbohydrate Polymers, 2020, 228:115350.
[77] KAUR H, GILL B S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals[J]. International Journal of Biological Macromolecules,2019,126:367−375. doi: 10.1016/j.ijbiomac.2018.12.149
[78] MOUTIQ R, MISRA N N, MENDONÇA A, et al. In-package decontamination of chicken breast using cold plasma technology:Microbial, quality and storage studies[J]. Meat Science,2020,159:107942. doi: 10.1016/j.meatsci.2019.107942
[79] OKYERE A Y, BOAKYE P G, BERTOFT E, et al. Structural characterization and enzymatic hydrolysis of radio frequency cold plasma treated starches[J]. Journal of Food Science,2022,87(2):686−698. doi: 10.1111/1750-3841.16037
[80] ZOU J J, LIU C J, ELIASSON B. Modification of starch by glow discharge plasma[J]. Carbohydrate Polymers,2004,55(1):23−26. doi: 10.1016/j.carbpol.2003.06.001
[81] ROVALINO-CÓRDOVA A M, FOGLIANO V, CAPUANO E. A closer look to cell structural barriers affecting starch digestibility in beans[J]. Carbohydrate Polymers,2018,181:994−1002. doi: 10.1016/j.carbpol.2017.11.050
[82] BHATTARAI R R, DHITAL S, MENSE A, et al. Intact cellular structure in cereal endosperm limits starch digestion in-vitro[J]. Food Hydrocolloids,2018,81:139−148. doi: 10.1016/j.foodhyd.2018.02.027
[83] CUI C, JIANG H, GUAN M, et al. Characterization and in-vitro digestibility of potato starch encapsulated in calcium alginate beads[J]. Food Hydrocolloids,2022,126:107458. doi: 10.1016/j.foodhyd.2021.107458
[84] CUI C, LI M, JI N, et al. Calcium alginate/curdlan/corn starch@calcium alginate macrocapsules for slowly digestible and resistant starch[J]. Carbohydrate Polymers,2022,285:119259. doi: 10.1016/j.carbpol.2022.119259
-
期刊类型引用(1)
1. 何芮迪,翟立公,潘苗苗,何礼喜,尹雪斌,杨丽萍. 低血糖生成指数食物研究进展及其潜在利用价值. 中国科学:生命科学. 2025(03): 518-528 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:
 下载:
下载:



