Optimization of Ultrasound-assisted Alkali Extraction of Bound Polyphenol from Rosa sterilis and Its Antioxidant Activity
-
摘要: 为探究无籽刺梨在药食同源领域的应用及开发,以无籽刺梨为原料,在单因素实验的基础上,采用Box-Behnken法优化无籽刺梨结合多酚的提取工艺,以无籽刺梨游离多酚和VC为对照,研究结合多酚对DPPH·、ABTS+·、·OH的清除率和总还原力。结果表明:无籽刺梨结合多酚最优提取工艺为液料比24 mL/g、碱液浓度8 mol/L、超声时间47 min、超声功率250 W,该条件下结合多酚提取量为135.73 mg GAE/g,与回归模型预测值(135.77 mg GAE/g)接近,模型拟合极好。所得结合多酚对DPPH·、ABTS+·的IC50值分别为0.0599、0.0121 mg/mL,显著(P<0.05)优于游离多酚(0.168、0.0395 mg/mL)。在浓度0.02~0.1 mg/mL范围内,结合多酚·OH清除能力较强,与VC无显著差异(P>0.05)。结合多酚总还原力优于游离多酚,2种多酚的总还原力与溶液浓度呈线性依赖关系。提取的结合多酚可作为天然抗氧化剂使用,为无籽刺梨加工利用提供理论依据。Abstract: In order to investigate the application and development of Rosa sterilis in the field of drug-food homology, the Box-Behnken method was used to optimize the extraction process of bound polyphenols from Rosa sterilis based on the single factor experiment. The study also examined the clearance rate of DPPH·, ABTS+·, and ·OH and total reducing power of bound polyphenols and free polyphenols from Rosa sterilis, with VC as controls. The results showed that the optimal extraction conditions for the bound polyphenols of Rosa sterilis were a liquid-to-solid ratio of 24 mL/g, a lye concentration of 8 mol/L, an ultrasonic time of 47 min, and an ultrasonic power of 250 W. Under these conditions, the amount of extracted bound polyphenols was 135.73 mg GAE/g, which was close to the predicted value of the regression model (135.77 mg GAE/g). The model fit was excellent. The IC50 values of the bound polyphenols to DPPH· and ABTS+· were 0.0599 and 0.0121 mg/mL, respectively. These values were significantly (P<0.05) lower than those of the free polyphenols (0.168, 0.0395 mg/mL). In the concentration range of 0.02~0.1 mg/mL, the scavenging ability of bound polyphenols on ·OH was strong, which was not significantly different from that of VC (P>0.05). The total reducing power of the bound polyphenols was higher than that of the free polyphenols. Additionally, the total reducing power of both polyphenols was significantly influenced by the concentration of the solution. The extracted bound polyphenols can be used as natural antioxidants, providing a theoretical basis for the processing and utilization of Rosa sterilis.
-
无籽刺梨(Rosa sterilis S.D.Shi)属蔷薇科蔷薇属落叶灌木植物,因果实无籽得名无籽刺梨[1],又名无子刺梨、金刺梨和搭钩刺梨等,主要分布于我国贵州、云南、四川等地。刺梨含有丰富的多酚、黄酮、VC、膳食纤维等营养成分,具有抗氧化、降血脂、降血糖等作用[2−3]。多酚具有良好的抗氧化性,是常用的天然抗氧化剂,可分为游离多酚和结合多酚。游离多酚可以被人体直接消化吸收,结合多酚不能被口腔、胃、小肠直接吸收,而是直接到达结肠,由肠道微生物转化利用[4]。
结合多酚大多存在于植物不溶性膳食纤维中,通过酯键、糖苷键、醚苷键等与其他物质(如纤维素、木质素等)结合,存在于提取完游离多酚后的残渣中,无法直接用有机溶剂萃取,因此常被忽视。常见的结合多酚释放方式有酸法、碱法、酶法[5−7],通过处理使膳食纤维中共价键水解断裂,结合多酚可以以游离形式被有机溶剂萃取。张兴杰[8]采用酸法、碱法、酶法分别提取菠萝蜜膳食纤维中的结合多酚,结果表明碱法提取的结合多酚含量最高、种类最多。Wu等[9]采用超声波辅助酶解法提取西兰花多酚,结果表明超声辅助提取较单一水解提取有明显优势。钟烜钰等[10]采用超声波辅助碱解法提取火龙果皮中的多酚,结果表明,结合多酚对α-淀粉酶和α-葡萄糖苷酶的抑制活性均强于游离多酚。许涵婷等[11]研究荔枝果渣可溶性膳食纤维去除结合多酚前后的结构和功能性质的变化,结果表明去除结合多酚后,膳食纤维多种功能性质显著降低,结合多酚与荔枝果渣可溶性膳食纤维的生物活性息息相关。目前,对超声辅助碱法提取无籽刺梨结合多酚及其抗氧化活性的研究鲜有报道。
综上所述,本研究采用超声辅助碱法提取无籽刺梨结合多酚,通过超声辅助的手段,以提高多酚提取量。同时,在最佳提取工艺的基础上,对无籽刺梨结合多酚体外抗氧化活性进行研究,以期为无籽刺梨结合多酚进一步利用提供理论基础。
1. 材料与方法
1.1 材料与仪器
无籽刺梨 云南省宣威市;石油醚(60~90 ℃)、没食子酸 上海麦克林生化科技有限公司;碱性蛋白酶(200 U/mg)、耐高温α-淀粉酶(4000 U/g)、糖化酶(10W U/g) 南京都莱生物技术有限公司;福林酚、DPPH、ABTS 上海源叶生物科技有限公司;氢氧化钠、盐酸 云南杨林工业开发区汕滇药业有限公司;无水乙醇、过氧化氢、抗坏血酸、硫酸亚铁、水杨酸、冰醋酸 天津风船化学试剂科技有限公司;以上试剂均为分析纯。
DNF-4K热泵干燥机 北京东南风科技有限公司;SB-400DTY超声波清洗机 宁波新芝超声设备有限公司;TG16-WS离心机 湖南迈克尔实验仪器有限公司;SpectraMax-M5酶标仪 上海美谷分子仪器有限公司。
1.2 实验方法
1.2.1 无籽刺梨结合多酚提取
1.2.1.1 不溶性膳食纤维提取
参考李晗等[12]和Dong等[13]的方法稍作修改。将新鲜无籽刺梨剪枝、洗净后整果破碎,60 ℃烘干至含水量低于6%,粉碎,过60目筛。样品加入石油醚(液料比15 mL/g)浸泡24 h脱脂,50 ℃干燥1 h,4 ℃保存。脱脂刺梨粉分散于蒸馏水中,加入2 mol/L NaOH溶液调pH至9.0,加6%碱性蛋白酶(按脱脂刺梨粉质量计算),55 ℃水浴30 min。加3 mol/L冰醋酸调pH至4.8,加入8%耐高温α-淀粉酶,55 ℃水浴1 h。加入10%糖化酶,55 ℃水浴30 min。样品静置冷却至室温,5500 r/min离心10 min,弃去上清液,沉淀用无水乙醇洗至中性,50 ℃干燥3 h,得无籽刺梨不溶性膳食纤维。
1.2.1.2 结合多酚提取
参考Zheng等[14]的方法稍作修改。准确称取0.10 g无籽刺梨不溶性膳食纤维,按设定液料比加入所需浓度的NaOH溶液,设置超声参数进行超声提取。超声提取结束后加6 mol/L盐酸调pH至2.0,加入两倍体积无水乙醇,4000 r/min离心20 min,上清液即结合多酚粗提液。
1.2.1.3 结合多酚提取量测定
参考Zheng等[14]的方法稍作修改。取10 μL多酚粗提液于离心管中,加入90 μL无水乙醇稀释,加入200 μL福林酚显色剂,静置3 min,加入20%碳酸钠溶液500 μL,37 ℃避光水浴30 min,765 nm处测吸光度。绘制没食子酸标准曲线,得回归方程y=6.6033x+0.0071,R2=0.9993。式中,y为结合多酚浓度(对应的没食子酸浓度),x为所测吸光度。采用公式(1)计算结合多酚提取量。
结合多酚提取量 (mg GAE/g)=c×V×10m (1) 式中:c为结合多酚浓度,mg/mL;V为反应液体积,mL;10为稀释倍数;m为不溶性膳食纤维质量,g;mg GAE/g表示结合多酚提取量所对应的没食子酸当量。
1.2.1.4 单因素实验
固定条件为液料比20 mL/g,碱液浓度9 mol/L,超声时间40 min,超声功率250 W,超声温度40 ℃,研究液料比(10、15、20、25、30 mL/g)、碱液浓度(7、8、9、10、11 mol/L)、超声时间(20、30、40、50、60 min)、超声功率(150、200、250、300、350 W)对结合多酚提取量的影响。
1.2.1.5 响应面优化试验
根据单因素实验结果,以液料比、碱液浓度、超声时间和超声功率为响应面试验参数,无籽刺梨结合多酚提取量为响应值,设计响应面优化试验,试验因素水平见表1。
表 1 响应面试验因素水平Table 1. Factors and levels of Box-Behnken test水平 因素 A 液料比(mL/g) B 碱液浓度(mol/L) C 超声时间(min) D 超声功率(W) −1 20 7 40 200 0 25 8 50 250 1 30 9 60 300 1.2.2 无籽刺梨结合多酚抗氧化活性研究
将1.2.1中最优提取工艺下的结合多酚粗提液使用乙酸乙酯1:1萃取3次,合并乙酸乙酯相蒸发浓缩至干,复溶于甲醇[13]。以无籽刺梨游离多酚(提取方法参照宋亚星等[15])和VC为对照,以IC50值(自由基清除率50%时所对应的样品浓度,mg/mL)判断样品抗氧化性强弱,IC50值越高,抗氧化性越弱。
1.2.2.1 DPPH·清除率测定
参考Addo等[16]的方法稍作修改。准确称取DPPH,使用无水乙醇配制成0.1 mg/mL的DPPH溶液,置于4 ℃冰箱冷藏备用。将DPPH溶液200 μL分别与浓度0.02、0.04、0.06、0.08、0.1、0.2、0.4、0.6、0.8、1.0 mg/mL样品200 μL混匀,37 ℃避光水浴30 min,517 nm处测吸光度,同时设置对照组和空白组。按公式(2)计算DPPH·清除率:
DPPH⋅清除率(%)=(1−A−A0AM)×100 (2) 式中:A为实验组(样品溶液+DPPH溶液)吸光值;A0为对照组(样品溶液+等DPPH溶液体积的溶剂)吸光值;AM为空白组(等样品溶液体积的溶剂+DPPH溶液)吸光值。
1.2.2.2 ABTS+·清除率测定
参考Zongo等[17]的方法稍作修改。将7 mmol/L ABTS溶液与4.9 mmol/L K2S2O8溶液按照体积比1:1混合均匀,避光反应16 h,使用去离子水稀释至其于734 nm处吸光度为0.70±0.02,标为ABTS工作液。将ABTS工作液200 μL分别与浓度0.005、0.01、0.02、0.04、0.06、0.08、0.1、0.2、0.4 mg/mL样品200 μL混匀,室温反应6 min,734 nm处测吸光度,同时设置对照组和空白组。按公式(3)计算ABTS+·清除率:
ABTS+⋅清除率(%)=(1−A−A0AM)×100 (3) 式中:A为实验组(样品溶液+ABTS工作液)吸光值;A0为对照组(样品溶液+等ABTS溶液体积的溶剂)吸光值;AM为空白组(等样品溶液体积的溶剂+ABTS工作液)吸光值。
1.2.2.3 ·OH清除率测定
参考Wang等[18]的方法稍作修改。将样品配制成浓度0.02、0.04、0.06、0.08、0.1、0.3、0.7、1.0 mg/mL样品液,取样品液200 μL依次加入9 mmol/L硫酸亚铁200 μL、9 mmol/L水杨酸-乙醇溶液200 μL,混匀,再加入8.8 mmol/L过氧化氢200 μL,37 ℃避光反应30 min,510 nm处测吸光度,同时设置对照组和空白组。按公式(4)计算·OH清除率:
⋅OH清除率(%)=(1−A−A0AM)×100 (4) 式中:A为实验组(样品溶液+反应液)吸光值;A0为对照组(样品溶液+等反应液体积的溶剂)吸光值;AM为空白组(等样品溶液体积的溶剂+反应液)吸光值。
1.2.2.4 总还原力测定
参考蒋雨心等[19]的方法稍作修改。将样品配制成0.5、1.0、2.0、3.0、4.0、5.0、6.0 mg/mL样品液,取样品液100 μL,加入0.01 g/mL铁氰化钾溶液100 μL,混匀,50 ℃水浴20 min。加入100 μL三氯乙酸(10%)终止反应,加入0.001 g/mL氯化铁溶液100 μL,静置显色10 min,700 nm处测吸光度,吸光度越大,样品Fe3+还原能力越强。
1.3 数据处理
以上处理均做3次平行实验。利用软件Design-Expert 13设计响应面试验,IBM SPSS Statistics 26进行显著性分析(P<0.05),Origin 2021绘图。
2. 结果与分析
2.1 单因素实验
2.1.1 液料比对结合多酚提取的影响
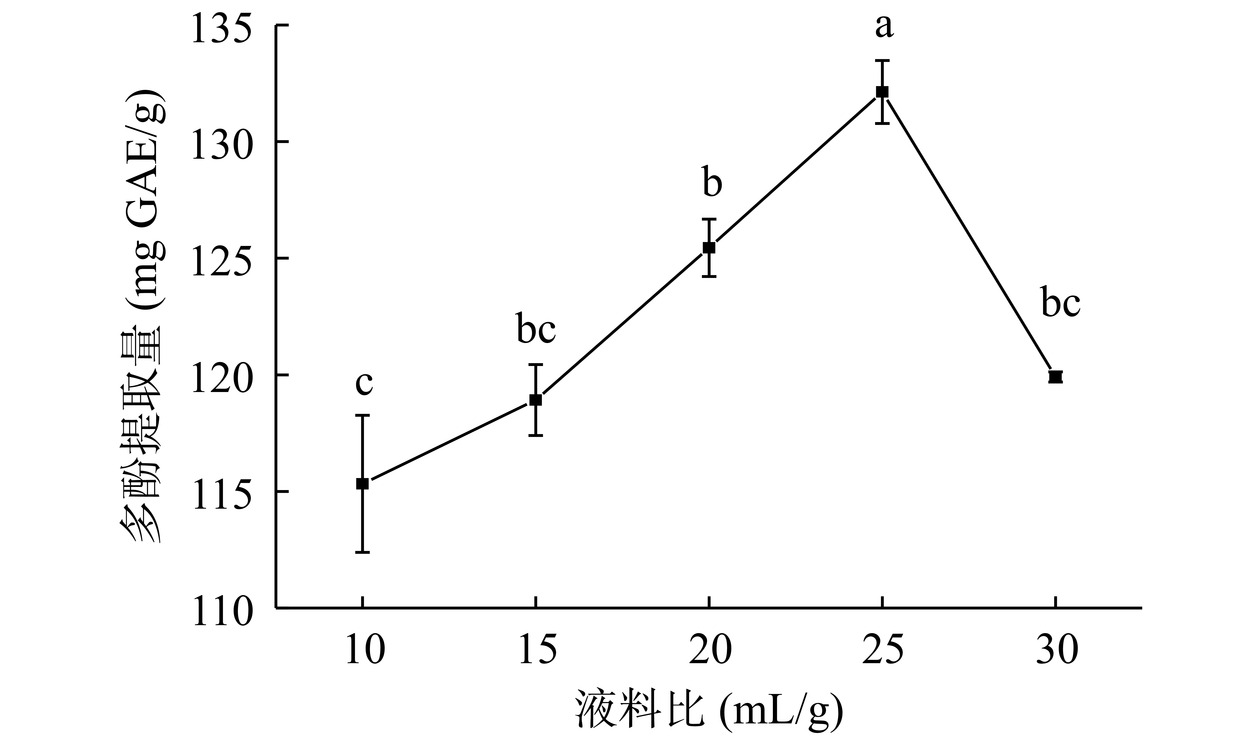
由图1可知,液料比10~25 mL/g范围内,结合多酚提取量显著增加(P<0.05),于25 mL/g达到最大(132.13 mg GAE/g),继续提高液料比,结合多酚提取量减少。原因为液料比过低,体系黏度大,原料在溶剂中流动性差,二者未能充分接触,反应不完全;增大液料比,在超声作用下,原料充分分散在溶剂中,结合多酚提取量显著增加(P<0.05);液料比过高,过多的溶剂会消耗超声波能量,原料吸收的能量少[20],结合多酚提取量减少。综上所述,选择20、25、30 mL/g三个水平进行响应面优化试验。
2.1.2 碱液浓度对结合多酚提取的影响
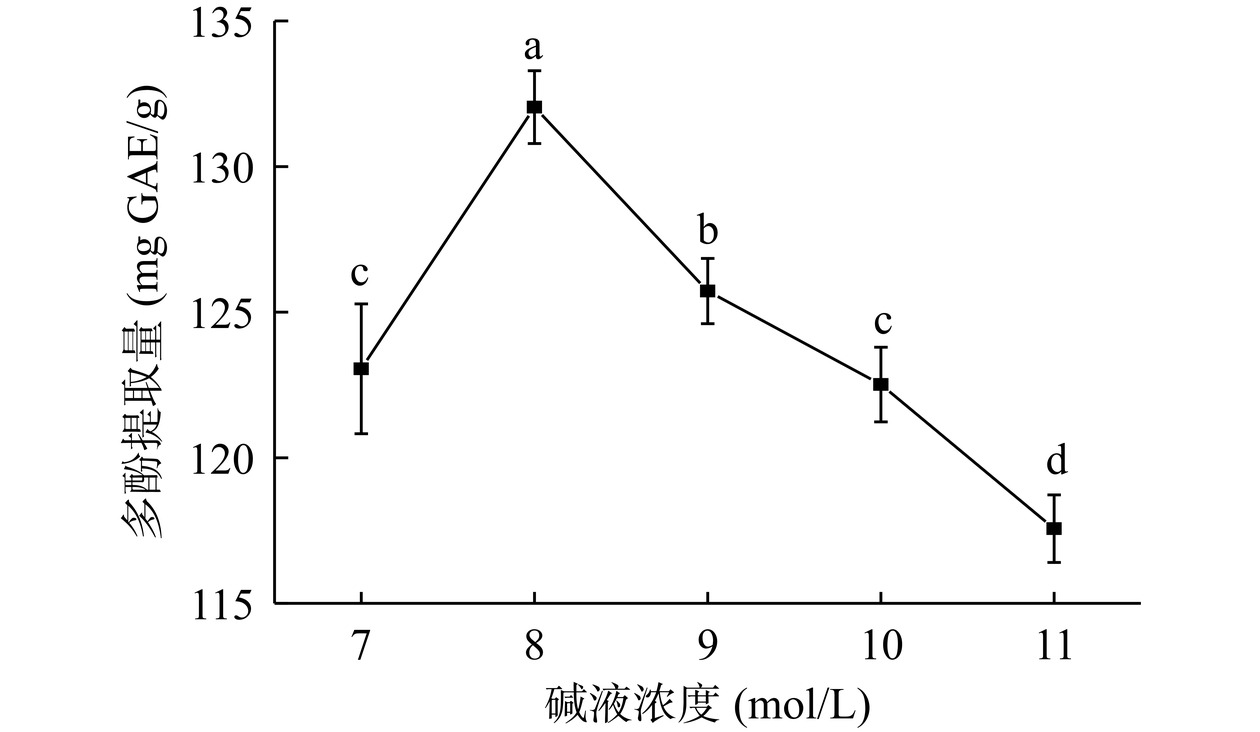
由图2可知,碱液浓度7~8 mol/L范围内,结合多酚提取量显著增加(P<0.05),于8 mol/L达到最大(132.04 mg GAE/g),继续增大碱液浓度,结合多酚提取量显著减少(P<0.05)。主要原因是碱液浓度低,膳食纤维中的酯键、酚苷未能完全水解;而碱液浓度过高会导致其他化学键水解断裂,非多酚类物质溶出,杂质成分增多,结合多酚提取量减少。此结果与张兴杰[8]碱法提取菠萝蜜结合多酚,Zheng等[14]碱法提取绿豆皮结合多酚结果类似。综上所述,选择7、8、9 mol/L三个水平进行响应面优化试验。
2.1.3 超声时间对结合多酚提取的影响
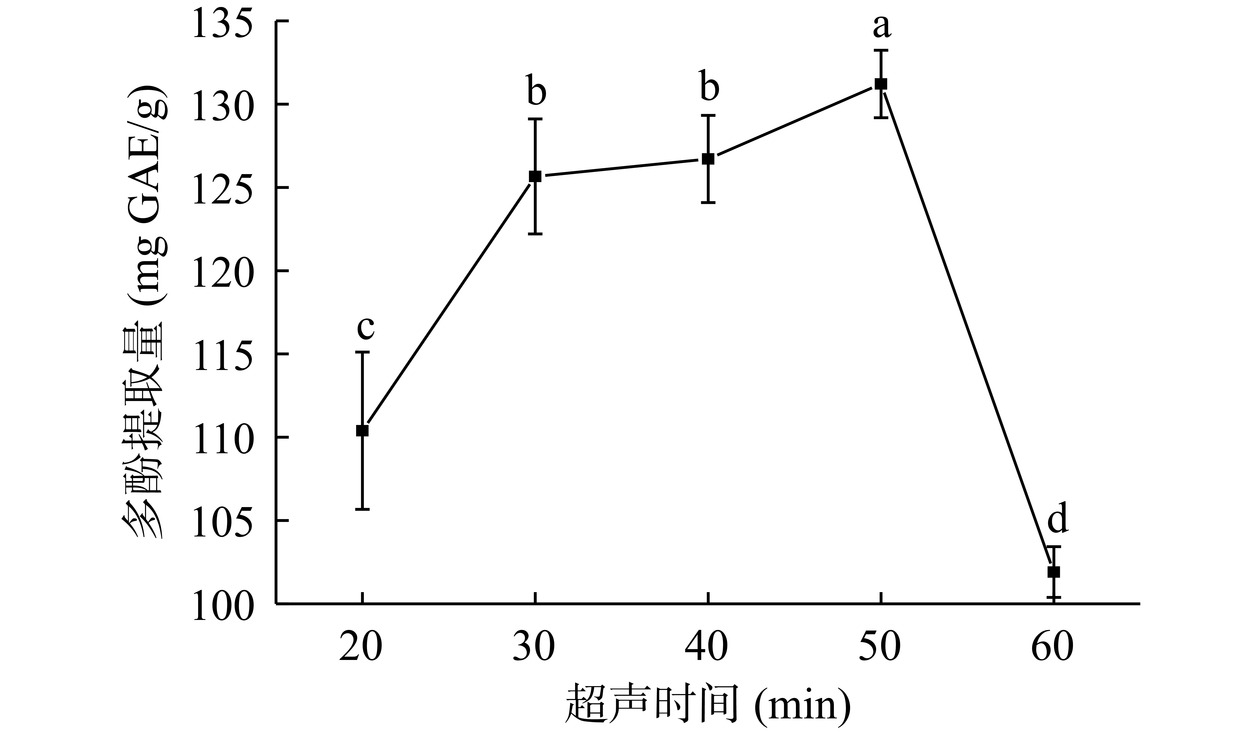
由图3可知,超声时间20~50 min范围内,结合多酚提取量增加,于50 min达到最大(131.21 mg GAE/g),继续延长超声时间(60 min),结合多酚提取量显著减少(P<0.05)。原因是超声时间过短,结合多酚未能有效溶出;超声时间延长,细胞壁破坏程度增大,结合多酚提取量增加;超声时间过长,液流动作和剪切作用使分子分散、膨胀,体系黏度增加,并形成较大的传质阻力[21],结合多酚提取量减少。综上所述,选择40、50、60 min三个水平进行响应面优化试验。
2.1.4 超声功率对结合多酚提取的影响
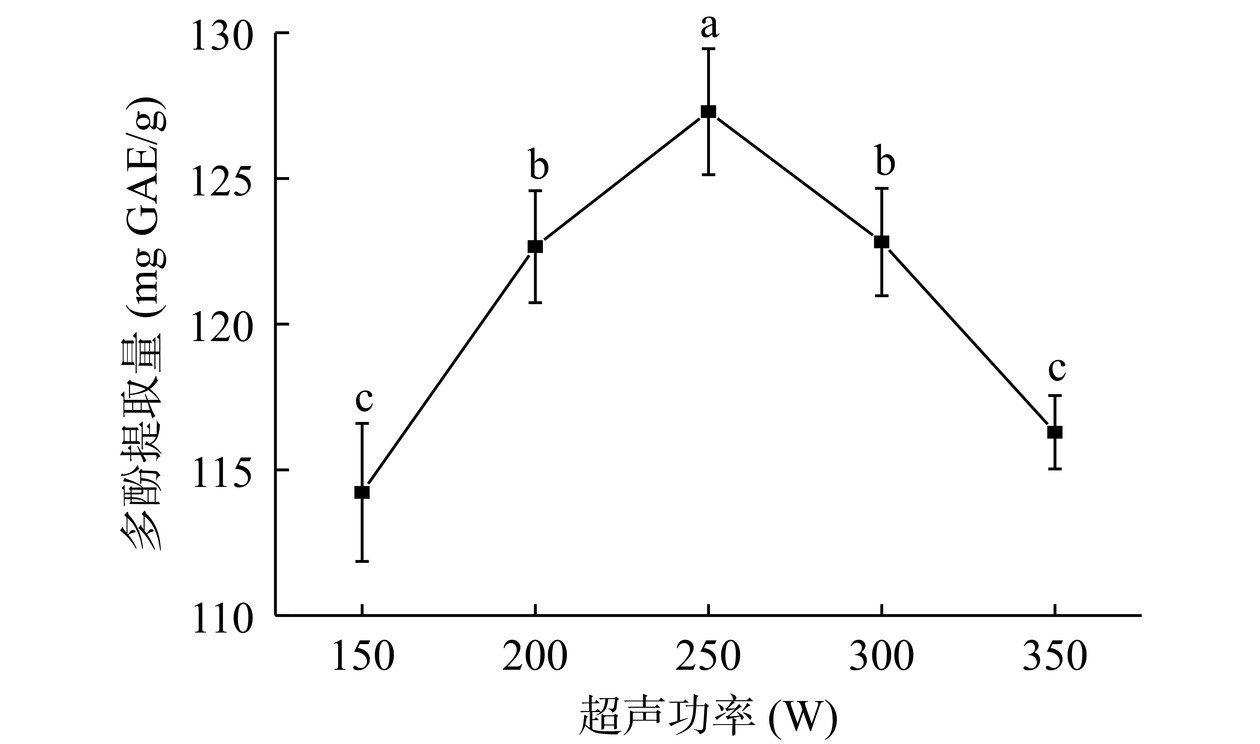
由图4可知,超声功率150~250 W范围内,结合多酚提取量显著增加(P<0.05),于250 W达到最大(127.29 mg GAE/g),继续增大超声功率,结合多酚提取量显著减少(P<0.05)。超声功率增大,机械效应增加,破坏细胞壁,结合多酚提取量增加,过强的超声功率产生的空化效应会使化学键断裂,同时产生大量热量,破坏体系中活性成分[22−23]。综上所述,选择200、250、300 W三个水平进行响应面优化试验。
2.2 响应面优化试验
2.2.1 响应面试验结果及方差分析
响应面试验设计及结果如表2所示。通过回归拟合得到的回归方程为:Y=133.6−3.4A+2.31B−9.58C−0.5536D+1.75AB+0.1834AC+2.62AD−0.31BC+6.86BD+0.8184CD−7.78A2−4.48B2−13.74C2−9.26D2。
表 2 响应面试验设计及结果Table 2. Design and results of response surface test试验号 A B C D 提取量(mg GAE/g) 1 1 0 −1 0 118.23±1.54 2 0 1 0 1 129.09±1.83 3 0 −1 0 1 116.16±0.91 4 −1 −1 0 0 120.73±0.68 5 −1 0 0 −1 126.79±1.72 6 1 0 0 −1 111.70±1.44 7 1 0 1 0 99.72±0.71 8 −1 0 0 1 114.97±0.46 9 0 1 1 0 106.76±0.62 10 0 1 −1 0 126.25±1.97 11 0 −1 1 0 103.90±1.24 12 0 0 1 1 101.00±2.13 13 0 0 1 −1 102.09±1.47 14 0 0 −1 −1 123.48±2.53 15 0 0 0 0 135.72±1.68 16 0 0 0 0 131.51±0.95 17 0 −1 0 −1 123.89±1.53 18 0 0 0 0 131.89±2.64 29 1 0 0 1 110.35±2.07 20 −1 0 1 0 105.12±1.83 21 0 1 0 −1 109.39±2.35 22 1 1 0 0 127.10±0.72 23 0 −1 −1 0 122.15±1.43 24 0 0 0 0 135.08±2.61 25 −1 1 0 0 128.38±1.52 26 1 −1 0 0 112.46±1.66 27 −1 0 −1 0 124.36±0.73 28 0 0 0 0 133.79±1.96 29 0 0 −1 1 119.12±2.04 表3为响应面二次模型方差分析,模型F=16.41,P<0.0001,表明模型极显著,失拟项P=0.0647>0.05,不显著,表明方程拟合良好。模型结果表明,超声时间(C)对无籽刺梨结合多酚提取量影响极显著(P<0.0001),料液比(A)影响较显著(P<0.01),碱液浓度(B)影响显著(P<0.05)。各因素对无籽刺梨结合多酚提取的影响大小不同:超声时间(C)>液料比(A)>碱液浓度(B)>超声功率(D)。碱液浓度和超声功率交互项(BD)影响较显著(P<0.01),二次项C2、D2影响极显著(P<0.0001),A2、B2影响较显著(P<0.01)。决定系数R2=0.9425,校正后R2adj=0.8851,表明模型适用于分析预测无籽刺梨结合多酚提取量。变异系数C.V.=3.13%,精密度Adeq=12.82>4,表明试验结果可靠。
表 3 响应面模型方差分析Table 3. Response surface model variance analysis方差来源 平方和 自由度 均方差 F值 P值 显著性 模型 3179.79 14 227.13 16.41 <0.0001 *** A 138.62 1 138.62 10.01 0.0069 ** B 63.86 1 63.86 4.61 0.0497 * C 1101.78 1 1101.78 79.58 <0.0001 *** D 3.68 1 3.68 0.2656 0.6143 AB 12.20 1 12.20 0.8812 0.3638 AC 0.1346 1 0.1346 0.0097 0.9229 AD 27.43 1 27.43 1.98 0.1811 BC 0.3844 1 0.3844 0.0278 0.8700 BD 188.24 1 188.24 13.60 0.0024 ** CD 2.68 1 2.68 0.1935 0.6667 A² 392.36 1 392.36 28.34 0.0001 ** B² 130.42 1 130.42 9.42 0.0083 ** C² 1224.72 1 1224.72 88.46 <0.0001 *** D² 556.35 1 556.35 40.19 <0.0001 *** 残差 193.82 14 13.84 失拟项 179.79 10 17.98 5.12 0.0647 不显著 纯误差 14.04 4 3.51 总和 3373.61 28 R²=0.9425,R2adj=0.8851,C.V.=3.13%,Adeq=12.82 注:“***”表示P<0.0001,影响极显著;“**”表示P<0.01,影响较显著;“*”表示P<0.05,影响显著。 2.2.2 各因素交互作用
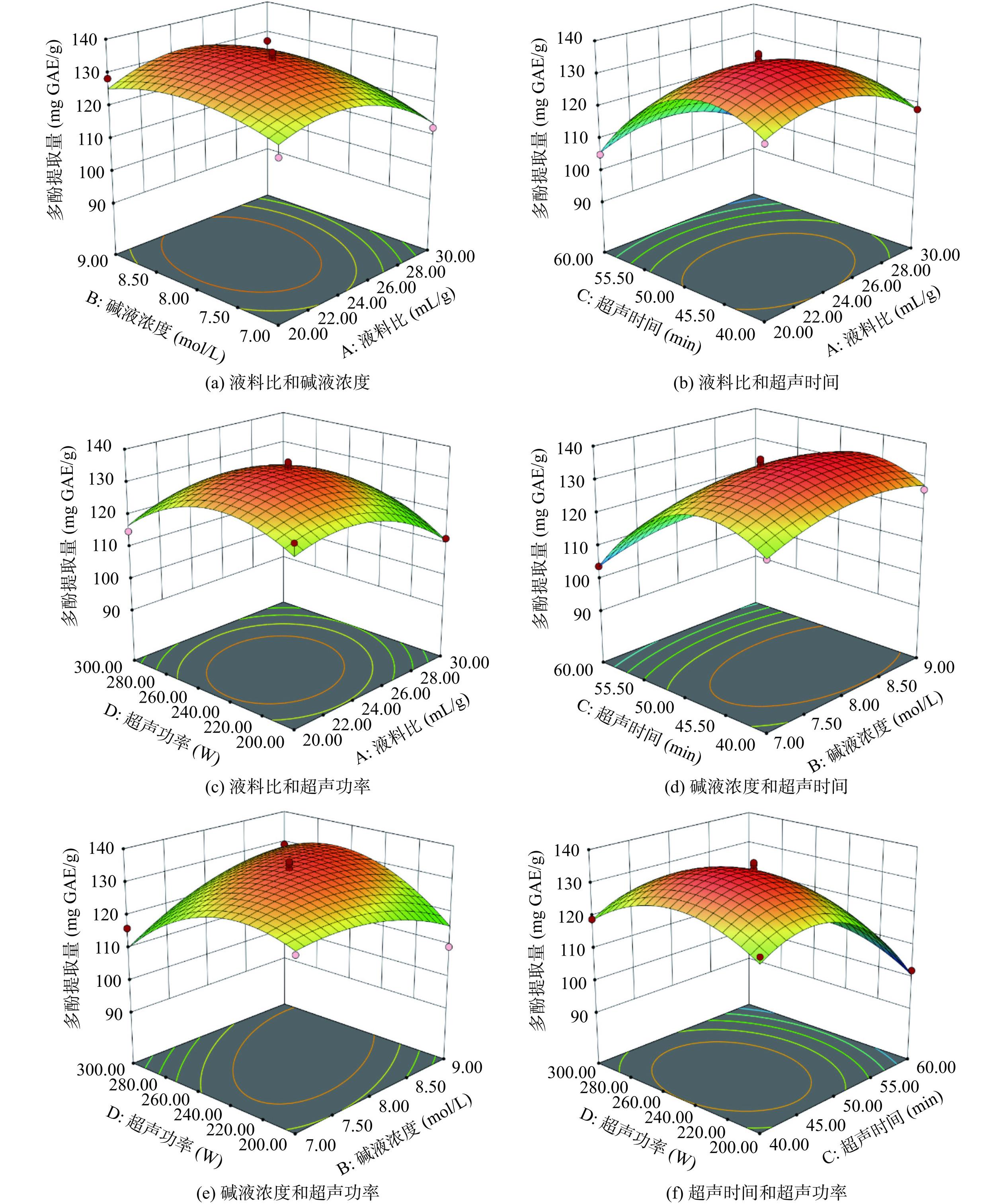
响应面3D曲面图和等高线图反映了各因素交互作用对无籽刺梨结合多酚提取量的影响。3D曲面越陡峭,表明该因素对结合多酚提取量影响越显著,等高线图越接近椭圆,表明因素交互作用越显著[24]。图5可知超声功率和碱液浓度(e)的等高线图更椭圆,表明该交互作用项对无籽刺梨结合多酚提取量的影响显著,与方差分析中回归模型系数显著性结果一致。各交互作用项对无籽刺梨结合多酚提取量影响程度排序为:BD>AD>AB>CD>BC>AC。
2.2.3 验证实验
根据模型拟合结果,超声辅助碱法提取无籽刺梨结合多酚的最佳提取工艺条件为:液料比24.12 mL/g、碱液浓度8.35 mol/L、超声时间46.74 min、超声功率254.74 W,此条件下结合多酚提取量预测值为135.77 mg GAE/g。根据实际情况对最佳试验条件进行简化处理,简化后工艺条件为:液料比24 mL/g、碱液浓度8 mol/L、超声时间47 min、超声功率250 W,在此条件下进行验证实验,重复3次,测得无籽刺梨结合多酚提取量为135.73±0.23 mg GAE/g,与预测值(135.77 mg GAE/g)相差0.04 mg GAE/g,试验结果具有可靠性。Zheng等[14]应用碱法在绿豆皮中提取结合多酚,最佳提取率为37.638 mg FAE/g,冉莎等[25]应用碱法在竹笋膳食纤维中提取结合多酚,最佳提取量为26.68 mg GAE/g,无籽刺梨中结合多酚提取量高于绿豆皮和竹笋,有进一步研究价值。
2.3 无籽刺梨结合多酚抗氧化活性测定
2.3.1 无籽刺梨结合多酚DPPH·清除能力
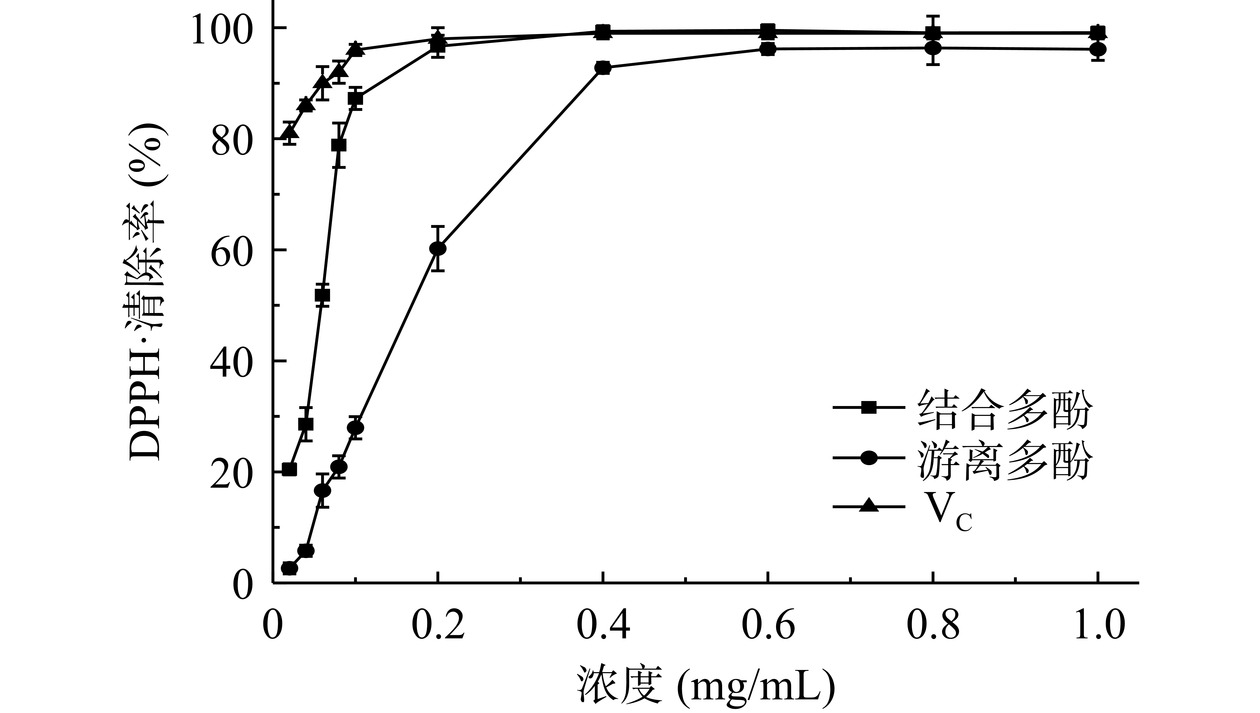
图6可知,浓度0.02~0.4 mg/mL范围内,结合多酚对DPPH·清除率由20.5%升高至96.5%,此后稳定在98.0%~99.0%。结合多酚对DPPH·清除率IC50值为0.0599 mg/mL。结合多酚浓度大于0.2 mg/mL时,和VC一样达到DPPH·最大清除率。浓度0.02~0.6 mg/mL范围内,游离多酚对DPPH·清除率由2.6%升高至92.5%,此后稳定在95.0%~96.0%。游离多酚对DPPH·清除率IC50值为0.168 mg/mL,该值大于结合多酚对DPPH·清除率IC50值,表明无籽刺梨结合多酚较游离多酚有较好的DPPH·清除能力。
2.3.2 无籽刺梨结合多酚ABTS+·清除能力
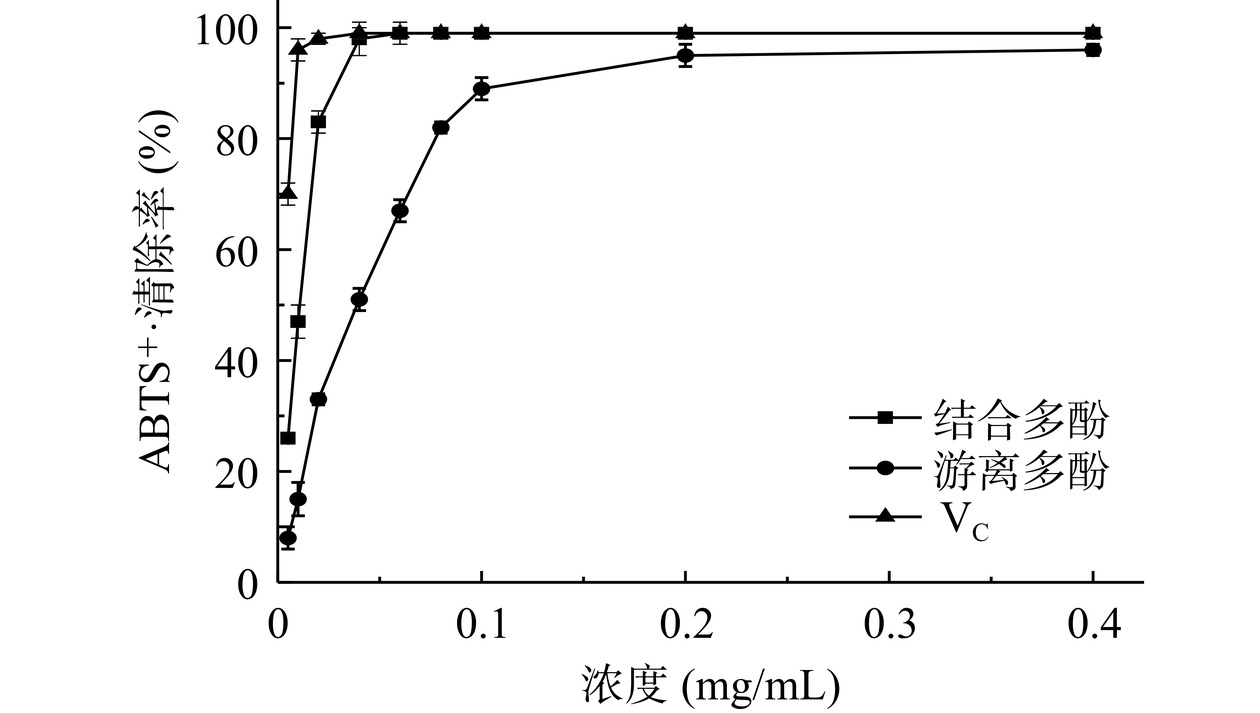
图7可知,浓度0.005~0.04 mg/mL范围内,结合多酚对ABTS+·清除率由26.0%升高至98.5%,此后稳定在98.0%~99.0%。结合多酚对ABTS+·清除率IC50值为0.0121 mg/mL。结合多酚浓度大于0.04 mg/mL时,和VC一样达到ABTS+·最大清除率。浓度0.005~0.2 mg/mL范围内,游离多酚对ABTS+·清除率由8.5%升高至95.0%,此后稳定在95.0%~96.0%。游离多酚对ABTS+·清除率IC50值为0.0395 mg/mL,该值大于结合多酚对ABTS+·清除率IC50值,表明无籽刺梨结合多酚较游离多酚有较好的ABTS+·清除能力。
2.3.3 无籽刺梨结合多酚·OH清除能力
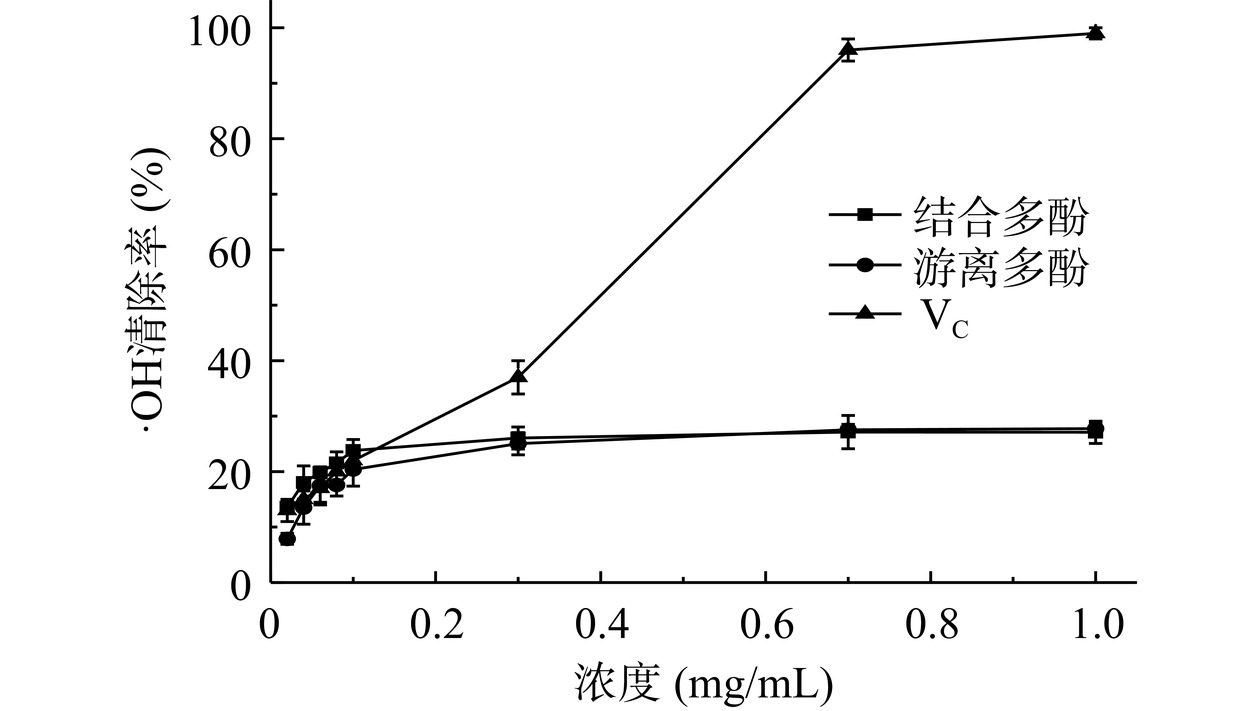
人体生化反应会产生大量活性氧,大部分活性氧代谢产生水,小部分未被代谢的活性氧会造成组织或细胞受损。·OH与DNA、脂质、蛋白质等物质结合会发生反应,使机体细胞或组织氧化,造成代谢紊乱,引发疾病[26−27]。图8可知,浓度0.02~0.3 mg/mL范围内,结合多酚对·OH清除率由13.5%升高至26.0%,此后稳定在26.0%~27.0%。浓度0.02~0.3 mg/mL范围内,游离多酚对·OH清除率由7.5%升高至25.0%,此后稳定在25.0%~27.0%。无籽刺梨2种多酚对·OH清除能力与浓度依赖关系弱,随浓度增加变化不明显;2种多酚对·OH清除能力较弱,两者清除能力无明显差别。
2.3.4 无籽刺梨结合多酚总还原力
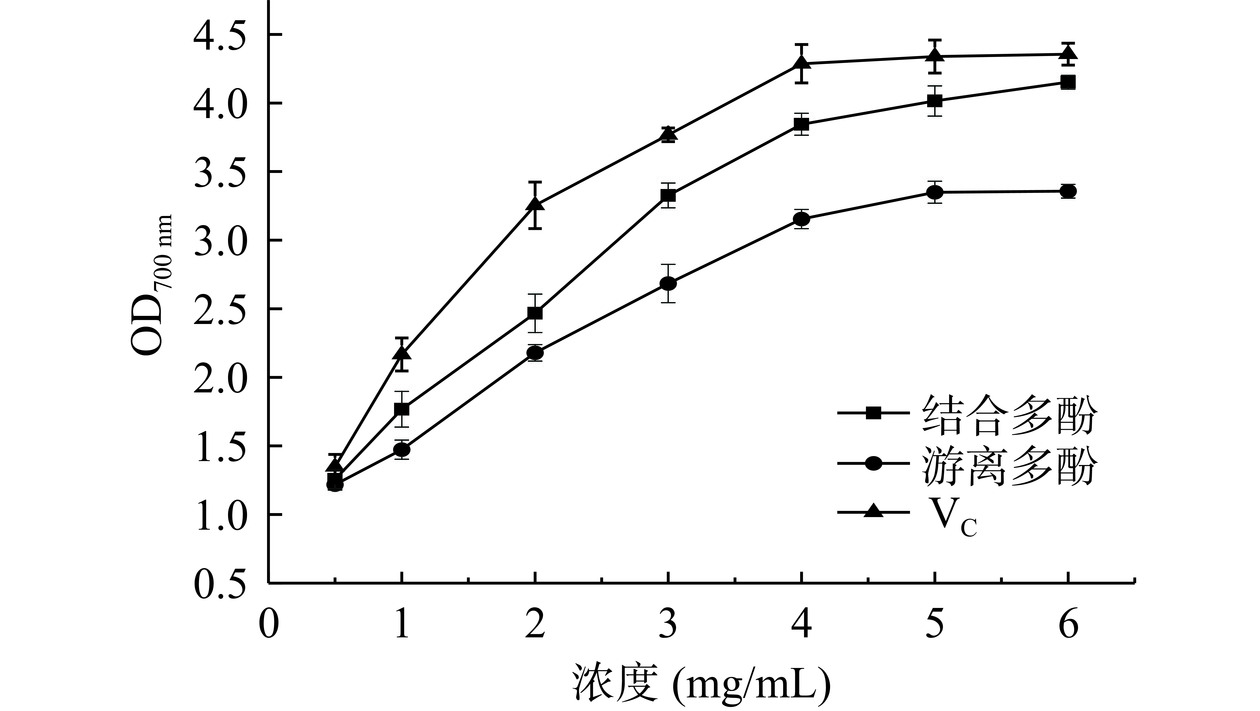
还原力是抗氧化活性的重要指标,还原力强表示样品为优良电子供体,抗氧化物可通过提供电子将自由基转化为稳定物质,中断自由基连锁反应。已有研究表明,植物中抗氧化成分活性与还原能力存在直接关联[28−29]。图9可知,浓度为0.5 mg/mL时,2种多酚还原力无显著差别(P>0.05)。在浓度1~4 mg/mL范围内,结合多酚700 nm处吸光度由1.77升高至3.85,此后稳定在4.0~4.1;游离多酚吸光度由1.47升高至3.15,此后稳定在3.3~3.4。Fe3+还原能力由大到小为VC>结合多酚>游离多酚,2种多酚的总还原力与溶液浓度呈线性依赖关系。
3. 讨论与结论
超声辅助碱法有利于无籽刺梨结合多酚溶出,原因是超声波处理使样品细胞壁破坏程度增大,相比单一碱处理效率更高,结合多酚释放更多。钟烜钰等[10]通过扫描电镜观察到超声辅助提取是破坏细胞壁和释放结合多酚的关键。无籽刺梨结合多酚抗氧化活性总体优于游离多酚,原因可能与两种多酚的物质组成有关。黄丹思[30]研究表明刺梨结合多酚抗氧化活性明显优于游离多酚,原因是结合多酚中总黄酮和没食子酸含量高于游离多酚,总黄酮和没食子酸含量显著影响抗氧化活性,结合多酚是刺梨不溶性膳食纤维中多酚的重要组成部分。彭春彦等[31]研究表明海带、坛紫菜和裙带菜结合多酚抗氧化能力均优于游离多酚。本文采用响应面法对超声辅助碱法提取无籽刺梨结合多酚进行工艺优化,得到最佳工艺参数为液料比24 mL/g、碱液浓度8 mol/L、超声时间47 min、超声功率250 W,该条件下结合多酚提取量为135.73 mg GAE/g。抗氧化实验表明,无籽刺梨结合多酚对DPPH·、ABTS+·、·OH均有一定的清除能力,其对DPPH·、ABTS+·清除的IC50值分别为0.0599、0.0121 mg/mL,优于无籽刺梨游离多酚。·OH清除试验表明,低浓度下(0.02~0.1 mg/mL)无籽刺梨结合多酚对·OH清除力与VC无显著差异(P>0.05),浓度0.1~1.0 mg/mL范围内,·OH清除能力不及VC。总还原力由大到小为VC>结合多酚>游离多酚,2种多酚的总还原力与溶液浓度呈线性依赖关系。无籽刺梨中结合多酚含量高,抗氧化性良好,可作为天然食品抗氧化剂应用,有待进一步研究利用。
-
表 1 响应面试验因素水平
Table 1 Factors and levels of Box-Behnken test
水平 因素 A 液料比(mL/g) B 碱液浓度(mol/L) C 超声时间(min) D 超声功率(W) −1 20 7 40 200 0 25 8 50 250 1 30 9 60 300 表 2 响应面试验设计及结果
Table 2 Design and results of response surface test
试验号 A B C D 提取量(mg GAE/g) 1 1 0 −1 0 118.23±1.54 2 0 1 0 1 129.09±1.83 3 0 −1 0 1 116.16±0.91 4 −1 −1 0 0 120.73±0.68 5 −1 0 0 −1 126.79±1.72 6 1 0 0 −1 111.70±1.44 7 1 0 1 0 99.72±0.71 8 −1 0 0 1 114.97±0.46 9 0 1 1 0 106.76±0.62 10 0 1 −1 0 126.25±1.97 11 0 −1 1 0 103.90±1.24 12 0 0 1 1 101.00±2.13 13 0 0 1 −1 102.09±1.47 14 0 0 −1 −1 123.48±2.53 15 0 0 0 0 135.72±1.68 16 0 0 0 0 131.51±0.95 17 0 −1 0 −1 123.89±1.53 18 0 0 0 0 131.89±2.64 29 1 0 0 1 110.35±2.07 20 −1 0 1 0 105.12±1.83 21 0 1 0 −1 109.39±2.35 22 1 1 0 0 127.10±0.72 23 0 −1 −1 0 122.15±1.43 24 0 0 0 0 135.08±2.61 25 −1 1 0 0 128.38±1.52 26 1 −1 0 0 112.46±1.66 27 −1 0 −1 0 124.36±0.73 28 0 0 0 0 133.79±1.96 29 0 0 −1 1 119.12±2.04 表 3 响应面模型方差分析
Table 3 Response surface model variance analysis
方差来源 平方和 自由度 均方差 F值 P值 显著性 模型 3179.79 14 227.13 16.41 <0.0001 *** A 138.62 1 138.62 10.01 0.0069 ** B 63.86 1 63.86 4.61 0.0497 * C 1101.78 1 1101.78 79.58 <0.0001 *** D 3.68 1 3.68 0.2656 0.6143 AB 12.20 1 12.20 0.8812 0.3638 AC 0.1346 1 0.1346 0.0097 0.9229 AD 27.43 1 27.43 1.98 0.1811 BC 0.3844 1 0.3844 0.0278 0.8700 BD 188.24 1 188.24 13.60 0.0024 ** CD 2.68 1 2.68 0.1935 0.6667 A² 392.36 1 392.36 28.34 0.0001 ** B² 130.42 1 130.42 9.42 0.0083 ** C² 1224.72 1 1224.72 88.46 <0.0001 *** D² 556.35 1 556.35 40.19 <0.0001 *** 残差 193.82 14 13.84 失拟项 179.79 10 17.98 5.12 0.0647 不显著 纯误差 14.04 4 3.51 总和 3373.61 28 R²=0.9425,R2adj=0.8851,C.V.=3.13%,Adeq=12.82 注:“***”表示P<0.0001,影响极显著;“**”表示P<0.01,影响较显著;“*”表示P<0.05,影响显著。 -
[1] 时圣德. 贵州蔷薇属新分类群[J]. 贵州科学,1985(1):8−9. [SHI S D. New taxa of Rosaceae from Guizhou[J]. Guizhou Science,1985(1):8−9.] SHI S D. New taxa of Rosaceae from Guizhou[J]. Guizhou Science, 1985(1): 8−9.
[2] HAO M, ZHANG F, LIU X, et al. Qualitative and quantitative analysis of catechin and quercetin in flavonoids extracted from Rosa roxburghii Tratt[J]. Tropical Journal of Pharmaceutical Research,2018,17(1):71−76. doi: 10.4314/tjpr.v17i1.11
[3] 付阳洋, 刘佳敏, 卢小鸾, 等. 刺梨主要活性成分及药理作用研究进展[J]. 食品工业科技,2020,41(13):328−335, 342. [FU Y Y, LIU J M, LU X L, et al. Research progress on main active components and pharmacological effects of Rosa roxburghii Tratt[J]. Science and Technology of Food Industry,2020,41(13):328−335, 342.] FU Y Y, LIU J M, LU X L, et al. Research progress on main active components and pharmacological effects of Rosa roxburghii Tratt[J]. Science and Technology of Food Industry, 2020, 41(13): 328−335, 342.
[4] FULGENCIO S C. Dietary fiber as a carrier of dietary antioxidants:An essential physiological function[J]. Journal of Agricultural & Food Chemistry,2011,59(1):43−49.
[5] ZHUANG G, TANG D, CHEN Y. Research progress of bound polyphenols in improving intestinal oxidative stress and intestinal barrier[J]. Science and Technology of Food Industry,2022,43(11):440−448.
[6] ZHANG H, TROISE A D, QI Y, et al. Insoluble dietary fibre scavenges reactive carbonyl species under simulated physiological conditions:The key role of fibre-bound polyphenols[J]. Food Chemistry, 2021:129018.
[7] DOMINGGUEZ R, MARINA M, PLAZA M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace[J]. Food Chemistry, 2020:128086.
[8] 张兴杰. 菠萝蜜膳食纤维中结合多酚的提取工艺优化及其消化酵解特性的研究[D]. 南昌:南昌大学, 2022. [ZHANG X J. Study on the optimization of the extraction process of the bound polyphenols in jackfruit dietary fiber, and its characteristics of digestion and fermentation[D]. Nanchang:Nanchang University, 2022.] ZHANG X J. Study on the optimization of the extraction process of the bound polyphenols in jackfruit dietary fiber, and its characteristics of digestion and fermentation[D]. Nanchang: Nanchang University, 2022.
[9] WU H, ZHU J, YANG L, et al. Ultrasonic-assisted enzymatic extraction of phenolics from broccoli (Brassica oleracea L. var. italica) inflorescences and evaluation of antioxidant activity in vitro[J]. Food Sci Technol Int,2015,21(4):306−319. doi: 10.1177/1082013214536174
[10] 钟烜钰, 王弘, 朱杰, 等. 超声-碱解联合提取法促进火龙果皮多酚的释放及其生物活性研究[C]. 中国食品科学技术学会第十九届年会论文摘要集, 2022:2. [ZHONG X Y, WANG H, ZHU J, et al. Ultrasound-alkaline combined extraction improves the release of polyphenols from pitahaya (Hylocereus undatus ‘Foo-Lon’) peel and its bioactivity study[C]. Abstracts of the 19th Annual Meeting of CIFST, 2022:2.] ZHONG X Y, WANG H, ZHU J, et al. Ultrasound-alkaline combined extraction improves the release of polyphenols from pitahaya (Hylocereus undatus ‘Foo-Lon’) peel and its bioactivity study[C]. Abstracts of the 19th Annual Meeting of CIFST, 2022: 2.
[11] 许涵婷, 唐语谦, 胡腾根, 等. 荔枝果渣可溶性膳食纤维去结合酚前后结构和功能性质的比较[J]. 现代食品科技,2023,39(8):206−212. [XU H T, TANG Y Q, HU T G, et al. Comparison of the structure and functional properties of soluble dietary fiber from lychee pomace before and after the removal of bound phenolics[J]. Modern Food Science and Technology,2023,39(8):206−212.] XU H T, TANG Y Q, HU T G, et al. Comparison of the structure and functional properties of soluble dietary fiber from lychee pomace before and after the removal of bound phenolics[J]. Modern Food Science and Technology, 2023, 39(8): 206−212.
[12] 李晗, 范方宇, 戚建华, 等. 超声辅助酶法提取无籽刺梨渣膳食纤维及理化性质评价[J]. 食品科技,2021,46(4):194−201. [LI H, FAN F Y, QI J H, et al. Ultrasonic assisted enzymatic extraction of dietary fiber from Rosa sterilis pomace and its physicochemical properties[J]. Food Science and Technology,2021,46(4):194−201.] LI H, FAN F Y, QI J H, et al. Ultrasonic assisted enzymatic extraction of dietary fiber from Rosa sterilis pomace and its physicochemical properties[J]. Food Science and Technology, 2021, 46(4): 194−201.
[13] DONG R, LIU S, ZHENG Y, et al. Release and metabolism of bound polyphenols from carrot dietary fiber and their potential activity in in vitro digestion and colonic fermentation[J]. Food & Function,2020,11(7):6652−6665.
[14] ZHENG Y, LIU S, XIE J, et al. Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber[J]. LWT-Food Science and Technology,2020,132:109943. doi: 10.1016/j.lwt.2020.109943
[15] 宋亚星, 郭占京, 黄宏妙, 等. Box-Behnken响应面优化超声辅助提取藿香蓟多酚及抗氧化活性研究[J]. 饲料研究,2023,46(11):75−80. [SONG Y X, GUO Z J, HUANG H M, et al. Optimization of ultrasonic-assisted extraction of polyphenols from Ageratum conyzoides L. by Box-Behnken response surface and evaluation of antioxidation activity[J]. Feed Research,2023,46(11):75−80.] SONG Y X, GUO Z J, HUANG H M, et al. Optimization of ultrasonic-assisted extraction of polyphenols from Ageratum conyzoides L. by Box-Behnken response surface and evaluation of antioxidation activity[J]. Feed Research, 2023, 46(11): 75−80.
[16] ADDO P, POUDINEH Z, SHEARER M, et al, Relationship between total antioxidant capacity, cannabinoids and terpenoids in hops and cannabis[J]. Plants, 2023, 12(6):1225.
[17] ZONGO E, BUSUIOC A, MEDA R, et al. Exploration of the antioxidant and anti-inflammatory potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach. ) Milne-Redh, traditionally used in the treatment of hepatitis in the Hauts-Bassins region of Burkina Faso[J]. Pharmaceuticals,2023,16(1):133. doi: 10.3390/ph16010133
[18] WANG Z, LIU Z, WU C, et al. Computational analysis on antioxidant activity of four characteristic structural units from persimmon tannin[J]. Materials,2023,16(1):320.
[19] 蒋雨心, 邓岚, 范方宇. 鱼腥草根总黄酮超声辅助酶法提取工艺优化及其抗氧化活性研究[J]. 食品工业科技,2023,44(6):226−234. [JIANG Y X, DENG L, FAN F Y. Ultrasonic assisted extraction process of flavonoids from Houttuynia cordata Thunb and its antioxidant activity[J]. Science and Technology of Food Industry,2023,44(6):226−234.] JIANG Y X, DENG L, FAN F Y. Ultrasonic assisted extraction process of flavonoids from Houttuynia cordata Thunb and its antioxidant activity[J]. Science and Technology of Food Industry, 2023, 44(6): 226−234.
[20] 王丹丹, 董文江, 赵建平, 等. 剪切乳化辅助酶法提取咖啡果皮可溶性膳食纤维[J]. 热带作物学报,2019,40(3):567−575. [WANG D D, DONG W J, ZHAO J P, et al. Extraction of SDF from coffee peel by shearing emulsification assisted enzymatic hydrolysis[J]. Chinese Journal of Tropical Crops,2019,40(3):567−575.] doi: 10.3969/j.issn.1000-2561.2019.03.022 WANG D D, DONG W J, ZHAO J P, et al. Extraction of SDF from coffee peel by shearing emulsification assisted enzymatic hydrolysis[J]. Chinese Journal of Tropical Crops, 2019, 40(3): 567−575. doi: 10.3969/j.issn.1000-2561.2019.03.022
[21] 杨宗玲, 李晗, 范方宇, 等. 超声辅助酶法提取无籽刺梨果渣中黄酮的工艺优化及其抗氧化活性[J]. 食品工业科技,2021,42(13):184−192. [YANG Z L, LI H, FAN F Y, et al. Ultrasound-assisted enzymatic extraction of flavonoids from Rosa sterilis pomace and its antioxidant activity[J]. Science and Technology of Food Industry,2021,42(13):184−192.] YANG Z L, LI H, FAN F Y, et al. Ultrasound-assisted enzymatic extraction of flavonoids from Rosa sterilis pomace and its antioxidant activity[J]. Science and Technology of Food Industry, 2021, 42(13): 184−192.
[22] 张孟凡, 岳丽, 敬思群, 等. 超声辅助-酶解协同作用提取红枣渣膳食纤维及其促消化作用[J]. 食品工业科技,2019,40(7):205−212. [ZHANG M F, YUE L, JING S Q, et al. Extraction of dietary fiber from red jujube residue by ultrasonic-enzymatic hydrolysis synergistic action and its promoting digestion function[J]. Science and Technology of Food Industry,2019,40(7):205−212.] ZHANG M F, YUE L, JING S Q, et al. Extraction of dietary fiber from red jujube residue by ultrasonic-enzymatic hydrolysis synergistic action and its promoting digestion function[J]. Science and Technology of Food Industry, 2019, 40(7): 205−212.
[23] 马艳, 姜亦超, 董格格, 等. 超声辅助提取玄米茶多酚工艺的优化及其抗氧化活性研究[J]. 食品工业,2023,44(8):22−27. [MA Y, JIANG Y C, DONG G G, et al. Optimization on process of polyphenols from genmaicha by ultrasonic-assisted extraction and its antioxidant activities study[J]. The Food Industry,2023,44(8):22−27.] MA Y, JIANG Y C, DONG G G, et al. Optimization on process of polyphenols from genmaicha by ultrasonic-assisted extraction and its antioxidant activities study[J]. The Food Industry, 2023, 44(8): 22−27.
[24] 付镓榕, 徐荣, 郭刚军, 等. 单因素结合响应面法优化魔芋多酚含量测定方法[J]. 食品工业,2023,44(6):299−304. [FU J R, XU R, GUO G J, et al. Optimization of content determination method of polyphenols from konjac by single factor and response surface methodology[J]. The Food Industry,2023,44(6):299−304.] FU J R, XU R, GUO G J, et al. Optimization of content determination method of polyphenols from konjac by single factor and response surface methodology[J]. The Food Industry, 2023, 44(6): 299−304.
[25] 冉莎, 张甫生, 李彬, 等. 竹笋膳食纤维中结合多酚的提取工艺优化及抗氧化活性分析[J]. 食品工业科技,2023,44(13):233−241. [RAN S, ZHANG F S, LI B, et al. Extraction optimization and antioxidant activity of bound polyphenols in bamboo shoot dietary fiber[J]. Science and Technology of Food Industry,2023,44(13):233−241.] RAN S, ZHANG F S, LI B, et al. Extraction optimization and antioxidant activity of bound polyphenols in bamboo shoot dietary fiber[J]. Science and Technology of Food Industry, 2023, 44(13): 233−241.
[26] 王萍, 刘慧, 付湘晋, 等. 13种植物多酚提取物抗氧化活性分析及其对冻藏未漂洗鱼糜品质的影响研究[J]. 食品安全质量检测学报,2023,14(16):50−56. [WANG P, LIU H, FU X J, et al. Study on the analysis of antioxidant activity of 13 kinds of plant polyphenol extracts and its effect on the quality of frozen unwashed surimi[J]. Journal of Food Safety and Quality,2023,14(16):50−56.] WANG P, LIU H, FU X J, et al. Study on the analysis of antioxidant activity of 13 kinds of plant polyphenol extracts and its effect on the quality of frozen unwashed surimi[J]. Journal of Food Safety and Quality, 2023, 14(16): 50−56.
[27] ELENA O, CRISTINA N, MARGHERITA L, et al. Antioxidant activity of coatings containing eugenol for flexible aluminium foils to preserve food shelf-life[J]. Food Packaging and Shelf Life, 2023, 39:101145.
[28] YILDIRIM A, MAVI A, KARA A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts[J]. Journal of Agricultural and Food Chemistry,2001,49(8):4083−4089. doi: 10.1021/jf0103572
[29] 黄忠有, 黄锁义, 喻巧容, 等. 薏苡根不同极性组分的抗氧化活性分析[J]. 中国野生植物资源,2019,38(1):17−20. [HUANG Z Y, HUANG S Y, YU Q R, et al. Research on different part antioxidant activity of Coix seed[J]. Chinese Wild Plant Resources,2019,38(1):17−20.] doi: 10.3969/j.issn.1006-9690.2019.01.005 HUANG Z Y, HUANG S Y, YU Q R, et al. Research on different part antioxidant activity of Coix seed[J]. Chinese Wild Plant Resources, 2019, 38(1): 17−20. doi: 10.3969/j.issn.1006-9690.2019.01.005
[30] 黄丹思. 刺梨果渣多酚的提取鉴定、抗氧化活性及其体外消化酵解规律的研究[D]. 广州:华南理工大学, 2022. [HUANG D S. Study on the extraction, identification and antioxidant activity of polyphenols from Rosa roxburghii Tratt fruit pomace and their in vitro digestion and colonic fermentation[D]. Guangzhou:South China University of Technology, 2022.] HUANG D S. Study on the extraction, identification and antioxidant activity of polyphenols from Rosa roxburghii Tratt fruit pomace and their in vitro digestion and colonic fermentation[D]. Guangzhou: South China University of Technology, 2022.
[31] 彭春彦, 谢星, 李一华, 等. 海带、坛紫菜和裙带菜游离和结合酚抗氧化和酶抑制活性比较[J]. 食品与发酵工业,2023,49(4):110−116. [PENG C Y, XIE X, LI Y H, et al. Antioxidant and enzyme inhibition activities of free and bound phenolics of Lanminaria japonica, Porphyra haitanensis, and Undaria pinnatifida[J]. Food and Fermentation Industries,2023,49(4):110−116.] PENG C Y, XIE X, LI Y H, et al. Antioxidant and enzyme inhibition activities of free and bound phenolics of Lanminaria japonica, Porphyra haitanensis, and Undaria pinnatifida[J]. Food and Fermentation Industries, 2023, 49(4): 110−116.
-
期刊类型引用(2)
1. 顾丹丹,董雪,张金秀,王晓茹,赵宗硕,王立安. 小麦羊肚菌菌粮制备工艺优化及营养成分、理化性质和抗氧化活性分析. 食品工业科技. 2025(04): 237-245 .  本站查看
本站查看
2. 马莉,季爱兵,曾胤,严亮. 蘑菇多糖生物活性及提取研究进展. 热带农业科学. 2024(06): 123-130 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:
 下载:
下载:
