Protective Effect and Mechanism of Paeonia suffruticosa Seed Ethanol Extract on Photoaging of HaCaT Cells Induced by UVB
-
摘要: 目的:研究牡丹籽乙醇提取物对UVB诱导人永生化角质形成细胞(HaCaT)光老化的保护作用及机制。方法:通过超高效液相色谱-四级杆飞行时间质谱(UPLC-Q-TOF-MS)法分析牡丹籽乙醇提取物存在的抗光老化活性成分,采用UVB刺激HaCaT细胞建立光老化细胞模型,噻唑蓝比色(MTT)法测定细胞活力,细胞划痕法检测牡丹籽乙醇提取物对细胞迁移能力的影响,通过酶联免疫吸附试验(ELISA)检测影响衰老的白细胞介素-1(Interleukin-1,IL-1)、白细胞介素-6(Interleukin-6,IL-6)、白细胞介素-22(Interleukin-22,IL-22)、干扰素-γ(Interferon-γ,IFN-γ)和转化生长因子-β(Tansforming growth factor-β,TGF-β)等细胞因子,以活性氧(Reactive oxygen species,ROS)和核因子-κB(Nuclear factor-κB,NF-κB)的水平为指标,研究不同浓度的牡丹籽乙醇提取物对HaCaT细胞的抗光老化活性及其机制。结果:含量最多的前五种成分为单硬脂酸甘油酯(10.73%)、芥酸酰胺(3.17%)、5-[(2R,3S)-6-羟基-2-(4-羟基苯基)-4-[(E)-2-(4-羟基苯基)乙烯基]-2,3-二氢1-苯并呋喃-3-基]苯-1,3-二醇(2.20%)、α,α-海藻糖(1.90%)和芍药内酯苷(1.45%)。经不同浓度牡丹籽乙醇提取物处理后的HaCaT细胞活力均在80%以上,其中浓度为6.25 μg/mL的牡丹籽乙醇提取物对HaCaT细胞无显著毒性,并且浓度为12.5 μg/mL(H组)和6.25 μg/mL(L组)的牡丹籽乙醇提取物均能抑制HaCaT细胞的迁移能力,H组能显著降低细胞IL-1、IL-6、IL-22、IFN-γ和TGF-β水平(P<0.05)从而抵抗皮肤衰老,L组能减少ROS产生,降低NF-κB蛋白因子的含量(P<0.05),从而有效地抑制HaCaT细胞的光老化反应。结论:牡丹籽乙醇提取物具有抗光老化作用,可以改善UVB诱导的氧化应激和炎症反应。Abstract: Objective: To study the protective effect and mechanism of ethanol extract from Paeonia suffruticosa seed on photoaging of HaCaT cells induced by UVB. Method: The anti-photoaging active ingredients in the ethanol extract of Paeonia suffruticosa seed were analyzed using ultra-performance liquid chromatography/quadrupole time-of-flight-tandem mass spectrometry (UPLC-Q-TOF-MS), and the photoaging cell model was established by stimulating HaCaT cells with UVB. Cell viability was determined by the multiple table tournament (MTT) method. The effect of ethanol extract from Paeonia suffruticosa seed on cell migration was detected by cell scratch assay. The related cytokines affecting senescence such as interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-22 (IL-22), interferon-γ (IFN-γ), and tansforming growth factor-β (TGF-β) were detected by enzyme-linked immunosorbent (ELISA) experiment, and the levels of reactive oxygen species (ROS) and nuclear factor-κ-gene binding (NF-κB) were used as indicators to study the anti-photoaging activity and mechanism of Paeonia suffruticosa seed ethanol extract at different concentrations on HaCaT cells. Results: The top five components with the highest content were 1-stearoylglycerol (10.73%), erucamide (3.17%), 5-[(2R,3S)-6-hydroxy-2-(4-hydroxyphenyl)-4-[(E)-2-(4-hydroxyphenyl)ethenyl]-2,3-dihydro-1-benzofuran-3-yl]benzene-1,3-diol (2.20%), α,α-trehalose (1.90%) and albiflorin (1.45%). After treatment with different concentrations of Paeonia suffruticosa seed ethanol extract, the viability of HaCaT cells was above 80%. The concentration of 6.25 μg/mL of Paeonia suffruticosa seed ethanol extract showed no significant cytotoxicity to HaCaT cells. Both the concentrations of 12.5 μg/mL (H group) and 6.25 μg/mL (L group) of Paeonia suffruticosa seed ethanol extract could inhibit the migration ability of HaCaT cells. The H group significantly reduced the levels of IL-1, IL-6, IL-22, IFN-γ, and TGF-β in cells (P<0.05), thereby resisting skin aging. The L group reduced ROS production and decreased the content of NF-κB protein factor (P<0.05), effectively inhibiting the photoaging response of HaCaT cells. Conclusion: Ethanol extract from Paeonia suffruticosa seed can improve oxidative stress and inflammation induced by UVB, and has the effect of anti-photoaging.
-
Keywords:
- ethanol extract /
- Paeonia suffruticosa seed /
- photoaging /
- HaCaT cell
-
牡丹(Paeonia suffruticosa)为芍药属牡丹组植物,在我国具有悠久的栽培与药用历史[1],据民族药记载,除牡丹组植物的根和丹皮外,花、叶和种子对皮肤护理具有良好的疗效[2−3]。黄酮类化合物是牡丹重要的活性成分[4],其抗氧化效果较为理想,具有很好的推广应用价值[5−6]。目前,国内对于牡丹籽的研究并没有深入,仅对其脂肪酸组成、油脂提取工艺等方面进行了初步的研究。牡丹籽中生物活性成分组成、提取、应用及作用机制等方面都有待于进一步研究。牡丹籽油不仅具有极高的营养价值,还具有高抗氧化能力以及抗衰老能力,可抑制细胞衰老。研究发现牡丹籽油对DPPH自由基具有良好的清除能力,可见牡丹籽油中含有抗氧化成分,证实了其具有抗氧化活性[7−8]。梁栋等[9]通过体外实验研究发现牡丹籽油在0.1%浓度下对UVA诱导的HDFn细胞基质金属蛋白酶-1(Matrix Metalloproteinase-1,MMP-1)合成具有抑制作用,证明了牡丹籽油在UVA诱导下具有抗光老化功效。然而,此研究中并未对牡丹籽油的抗光老化机制进行分析,同时欠缺牡丹籽油对UVB诱导的抗光老化的相关机制研究。
了解皮肤老化的成因是研究抗光老化的基础。HaCaT细胞作为人类永生化表皮细胞,常作为研究光老化的体外模型[10]。UVB照射常作为评估皮肤光老化的方法。UVB能量较强,穿透能力较浅,主要影响皮肤表皮细胞[11]。UVB照射导致皮肤中细胞外相关基质成分发生改变,基质成分结构改变引起功能丧失,使皮肤失去弹性、形成皱纹,引发过早老化[12]。同时UVB引起皮肤炎症,诱导皮肤角质层细胞生成白细胞介素(Interleukin,ILs)和肿瘤坏死因子-β(Tumor necrosis factor-β,TNF-β),这些炎性细胞因子促使生成过多的ROS加剧皮肤细胞的氧化应激,进一步介导炎性因子分泌,加剧皮肤光老化程度。此外,炎症因子还能加快MMPs的活化,加快皮肤胶原蛋白的降解,从而加速皮肤老化[13]。
为了更全面深入研究牡丹籽乙醇提取物的抗老化功效及机制,本研究通过UPLC-Q-TOF-MS分析牡丹籽乙醇提取物的成分,并利用UVB诱导HaCaT细胞体外构建抗光老化模型,探究牡丹籽乙醇提取物对HaCaT细胞迁移能力影响,以IL-1、IL-6、IL-22、IFN-γ和TGF-β这五个细胞因子、活性氧和NF-κB的浓度水平为指标,观察不同浓度的牡丹籽乙醇提取物对HaCaT细胞的抗光老化活性,阐明牡丹籽乙醇提取物对HaCaT细胞的抗光老化活性及其调控机制,为牡丹籽抗光老化功能性食品的开发与利用提供科学依据。
1. 材料与方法
1.1 材料与仪器
牡丹籽 山东省菏泽市某产业园区;人角质形成细胞系HaCaT细胞 上海复祥生物科技有限公司;4′,6-二脒基-2-苯基吲哚(DAPI)、牛血清白蛋白(BSA) Sigma公司;高糖Dulbecco改良Eagle培养基(DMEM)、PBS、胎牛血清(FBS)、链霉素溶液 Gibco公司;噻唑四氮唑(MTT) 上海阿拉丁生化科技有限公司;总氮测定试剂盒、DCFH-DA荧光探针、中性红染色液 上海碧云天生物有限公司;IFN-γ、IL-1、IL-6、IL-22、TGF-β的ELISA试剂盒 江苏酶标生物技术有限公司;Cy3连接的山羊抗兔IgG(H+L) 武汉赛维尔生物技术有限公司;抗NF-κB/p65抗体 Affinity公司。
HH-2数显恒温水浴锅 常州国华电器有限公司;DHG-9023A电热恒温鼓风干燥箱 苏州江东精密仪器有限公司;RE-52AA旋转蒸发仪 上海亚荣生化仪器厂;ACQUITY UPLC H-CLASS高效液相色谱仪 Waters公司;IX71倒置荧光显微镜 Olympus公司;CellXpert C170 CO2培养箱 Eppendorf公司;多功能酶标仪 Tecan公司;SS-04B型UVB仪 上海希格玛高技术有限公司。
1.2 实验方法
1.2.1 牡丹籽乙醇提取物制备
称取200 g牡丹籽粉碎,加入1 L无水乙醇,浸取24 h,待药材与乙醇充分浸润后,在水浴锅中以60 ℃恒温加热回流3 h,然后用纱布过滤,取上清液。将提取液经45 ℃旋转蒸发仪浓缩至膏状,转移至70 ℃烘箱烘干,放入4 ℃冰箱中备用[14]。
1.2.2 超高效液相色谱-四级杆飞行时间质谱
在UPLC-Q-TOF-MS(Thermo,Ultimate 3000 LC,Q Exactive HF),C18柱(Zorbax Eclipse C18,1.8 μm×2.1 mm×100 mm)上进行。流动相为A相(0.1%甲酸水溶液)和B相(乙腈),恒定流速为0.8 mL/min,进样体积为10 μL,梯度洗脱条件为95%~85% A,0~3.5 min;85%~70% A,3.5~6 min;70% A,6~6.5 min;70%~30% A,6.5~12 min;30% A,12~12.5 min;30%~0% A,12.5~18 min;0% A,18~25 min;0%~95% A,25~26 min;95% A,26~30 min。将柱温度设置为30 ℃。质谱分析条件:采用电喷雾离子源(ESI);正负离子扫描模式;扫描范围:100~1000 m/z;正离子模式下,毛细管电压:5500 V;去簇电压:80 V;去溶剂气温度:350 ℃;溶剂气流速:600 L/h;碎裂电压:(30±15)eV。负离子模式下,毛细管电压:4500 V;去簇电压:80 V;去溶剂气温度:350 ℃;溶剂气流速:600 L/h;碎裂电压:(−30±15)eV。通过Thermo Xcalibur 4.1软件对数据进行初步处理,将准确的分子量测试值与理论值进行匹配,并在Chem Spider和mz Cloud数据库中进行搜索,然后通过二级质谱的碎片离子分析和鉴定化合物的结构式,结合数据库和文献报道相关化合物及其对照品的相同或类似成分的碎片离子信息进行对比分析、确定成分[15]。
1.2.3 细胞培养
细胞在含有10% FBS和1%链霉素溶液的DMEM培养基中进行常规传代和培养,并在含有5%的二氧化碳的37 ℃培养箱中生长[16]。
1.2.4 细胞存活率测试
MTT试验用于检测不同浓度牡丹籽乙醇提取物对HaCaT细胞的毒性[17]。将HaCaT细胞在96孔板(每孔100 μL)中以5×104个细胞/mL的浓度培养24 h。之后,用100 μL含有不同浓度牡丹籽乙醇提取物(100、50、25、12.5、6.25 μg/mL)基础培养基替换每孔的原完全培养基液体,并在培养箱中培养24 h。药物作用结束后,除去含有不同药物浓度的原培养基,向每孔加入100 μL MTT(用无血清培养基稀释到0.5 mg/mL),并在培养箱中培养3 h。最后,除去96孔板的上清液,向每孔加入100 μL DMSO。用酶标仪在570 nm下检测每孔的吸光度A值。
1.2.5 细胞迁移划痕试验
HaCaT细胞被播种在24孔板中(1×106个细胞/孔,1 mL培养基/孔)。在培养箱中培养24 h后,弃去上清液,向每孔加入1 mL含有不同浓度牡丹籽乙醇提取物的培养基。培养2 h后,UVB照射300 mJ/cm2,持续15 min,并设置空白组和模型组,空白组不做照射处理,模型组给予照射。然后,用吸头排查孔底,清除刮出的细胞,并在倒置显微镜下拍照。将24孔板在37 ℃培养箱中培养24 h,在同一位置用倒置显微镜再次拍照。使用GraphPad prism 8.0.2计算划痕愈合率[18]。划痕愈合率(%)=(0 h划痕宽度−24 h划痕宽度)/0 h划痕宽度×100。划痕愈合率越高,表示细胞迁移能力越强。
1.2.6 IFN-γ、IL-1、IL-6、IL-22和TGF-β的ELISA检测
HaCaT细胞被播种在6孔板中(6×105个细胞/孔,1 mL培养基/孔)。在培养箱中培养24 h后,弃去上清液,向每孔加入1 mL含有不同浓度牡丹籽乙醇提取物的培养基。培养2 h后,UVB照射300 mJ/cm2,持续15 min,并设置空白组和模型组,空白组不做照射处理,模型组给予照射。在培养箱中培养24 h后,用ELISA试剂盒检测不同组的上清液中IFN-γ、IL-1、IL-6、IL-22和TGF-β的表达水平[19−21]。
1.2.7 细胞中ROS的检测
HaCaT细胞被播种在6孔板中(6×105个细胞/孔,1 mL培养基/孔)。在培养箱中培养24 h后,将6孔板中的旧培养基换成含有不同浓度牡丹籽乙醇提取物的1 mL培养基。培养2 h后,UVB照射300 mJ/cm2,持续15 min,并设置空白组和模型组,空白组不做照射处理,模型组给予照射。培养24 h后,弃去上清液,加入1 mL的DCFH-DA(溶于无血清培养基,终浓度为0.01 mol/L)溶液。30 min后,用PBS清洗6孔板三次,以减少荧光背景。最后,在倒置的荧光显微镜下对6孔板进行拍照(FITC刺激荧光)[22]。
1.2.8 免疫荧光染色
HaCaT细胞被播种在12孔板中(1×105个细胞/孔,1 mL培养基/孔)。在培养箱中培养24 h后,将12孔板中的旧培养基换成含有不同浓度牡丹籽乙醇提取物的1 mL培养基。培养2 h后,UVB照射300 mJ/cm2,持续15 min,并设置空白组和模型组,空白组不做照射处理,模型组给予照射。24 h后,用4%多聚甲醛溶液固定12孔板中的细胞,然后用Triton X-100处理细胞的通透性,再用1%牛血清白蛋白(BSA)在室温下封锁1 h。最后,在4 ℃下用抗NF-κB/p65抗体(1% BSA,1:200)孵育细胞过夜。过夜孵化后,用Cy3共轭山羊抗兔IgG(H+L)在黑暗中孵化2 h(1% BSA,1:800)。最后,使用DAPI进行双重染色并在倒置荧光显微镜下拍照[23−24]。
1.3 数据处理
数据以平均值±标准差(SD)表示。每组样品样本数为3。统计学意义分析采用单因素方差分析,与空白组相比P<0.05(*)显著,P<0.01(**)极显著。与模型组相比P<0.05(#)显著,P<0.01(##)极显著。采用GraphPad Prism 8.0.2软件对数据进行分析及绘图处理。
2. 结果与分析
2.1 牡丹籽乙醇提取物主要成分
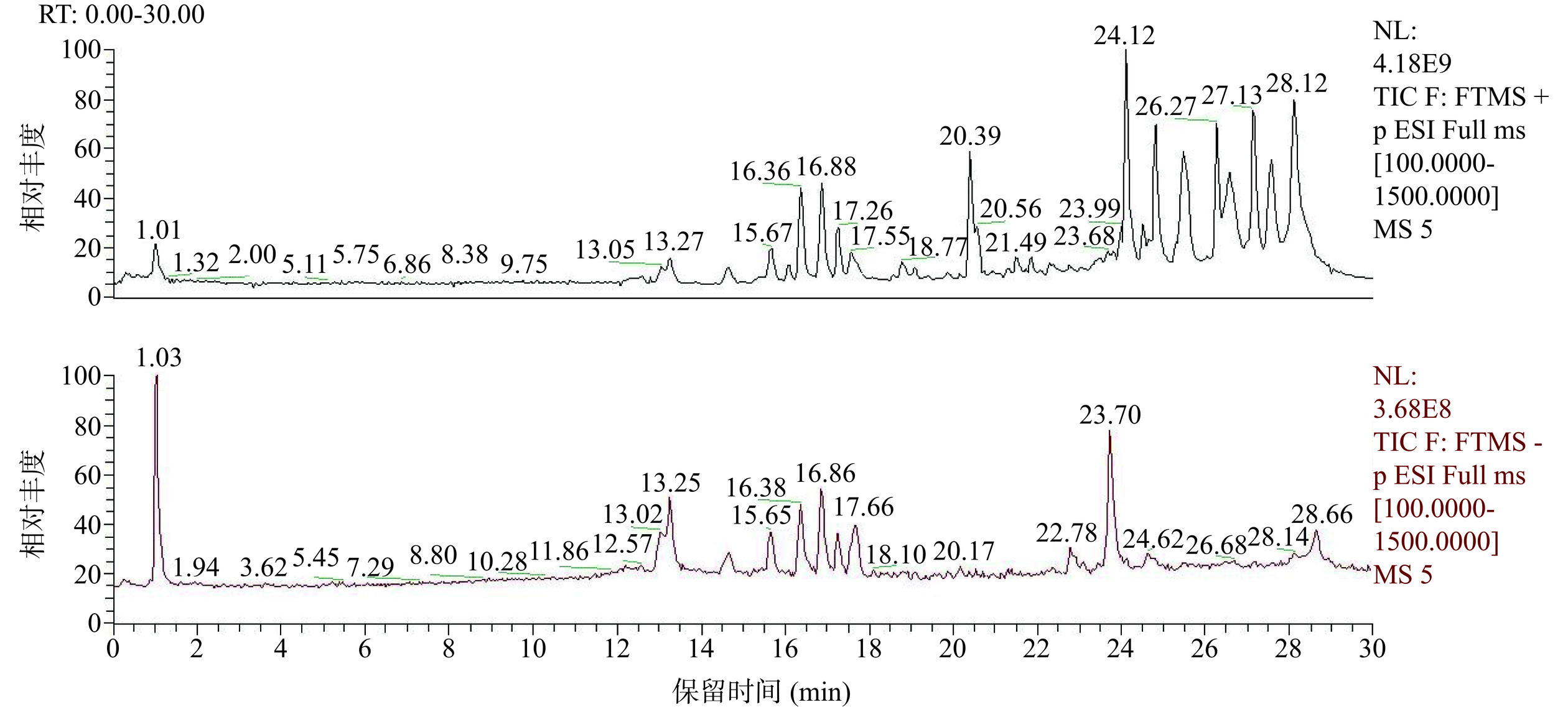
用高效液相色谱-质谱法对乙醇提取的牡丹籽提取物进行化学成分分析。图1分别为用乙醇提取的牡丹籽提取物正、负总离子色谱图。表1为采用Xcalibur4.1软件(Theromo Fisher Inc.)进行低分辨率质谱分析得到的牡丹籽乙醇提取物的化学成分。
表 1 牡丹籽乙醇提取物的化学成分Table 1. Chemical constituents of Paeonia suffruticosa seed ethanol extract序号 保留时间
(min)化合物名称 分子式 相对分子质量
(m/z)正离子(m/z)
[M+H]+负离子(m/z)
[M-H]−误差(ppm) 1 1.043 α,α-海藻糖 C12H22O11 342.11575 341.10449 −1.34 2 1.051 棉籽糖 C18H32O16 504.16915 503.16190 0.23 3 12.19 芍药内酯苷 C23H28O11 480.16218 481.16931 −2.05 4 12.989 虎杖苷 C20H22O8 390.13065 391.13791 −2.09 5 13.286 芍药苷 C23H28O11 480.16336 481.16561 0.42 6 15.34 白藜芦醇 C14H12O3 228.0781 229.08562 −2.38 7 15.995 木犀草苷 C21H20O11 286.0477 287.05423 −1.88 8 16.711 霉酚酸 C17H20O6 320.12509 321.13251 −2.82 9 16.899 5-[(2R,3S)-6-羟基-2-(4-羟基苯基)-4-[(E)-2-(4-
羟基苯基)乙烯基]-2,3-二氢1-苯并呋喃-3-基]苯-1,3-二醇C28H22O6 454.14066 453.13422 −2.16 10 17.28 木犀草素 C15H10O6 286.04716 287.05405 −2 11 19.041 N,N-二甲基癸烷基-N-氧化胺 C12H27NO 201.20885 202.21610 −2.05 12 20.577 双(4-乙基亚苯基)山梨醇 C24H30O6 414.203 415.19223 −2.98 13 22.49 9-O-10(E),12(E)-十八碳二烯酸 C18H30O3 294.21858 295.22574 −3.1 14 23.424 棕榈油酸 C16H30O2 254.22379 255.23106 −3.12 15 23.765 2,2'-亚甲基双-(4-甲基-6-叔丁基苯酚) C23H32O2 340.24013 339.23270 −0.3 16 24.155 油酰胺 C18H35NO 281.27095 282.27835 −3.26 17 24.439 羽扇豆醇 C30H50O 426.38509 427.39233 −2.52 18 24.837 油乙酰胺 C20H39NO 309.30222 310.30951 −3.06 19 24.855 单硬脂酸甘油酯 C21H42O4 358.30727 359.29651 −2.9 20 25.632 芥酸酰胺 C22H43NO 337.33336 338.34073 −3.28 经分析得到20种化合物,其中通过筛选与软件匹配契合度90%以上,并且比较峰面积得到相对含量占比前五种主要的化合物为单硬脂酸甘油酯(10.73%)、芥酸酰胺(3.17%)、5-[(2R,3S)-6-羟基-2-(4-羟基苯基)-4-[(E)-2-(4-羟基苯基)乙烯基]-2,3-二氢1-苯并呋喃-3-基]苯-1,3-二醇(2.20%)、α,α-海藻糖(1.90%)和芍药内酯苷(1.45%)。其中单硬脂酸甘油酯具有抗氧化和抗衰老的功效[25−29]。
2.2 牡丹籽乙醇提取物对HaCaT细胞增殖的影响
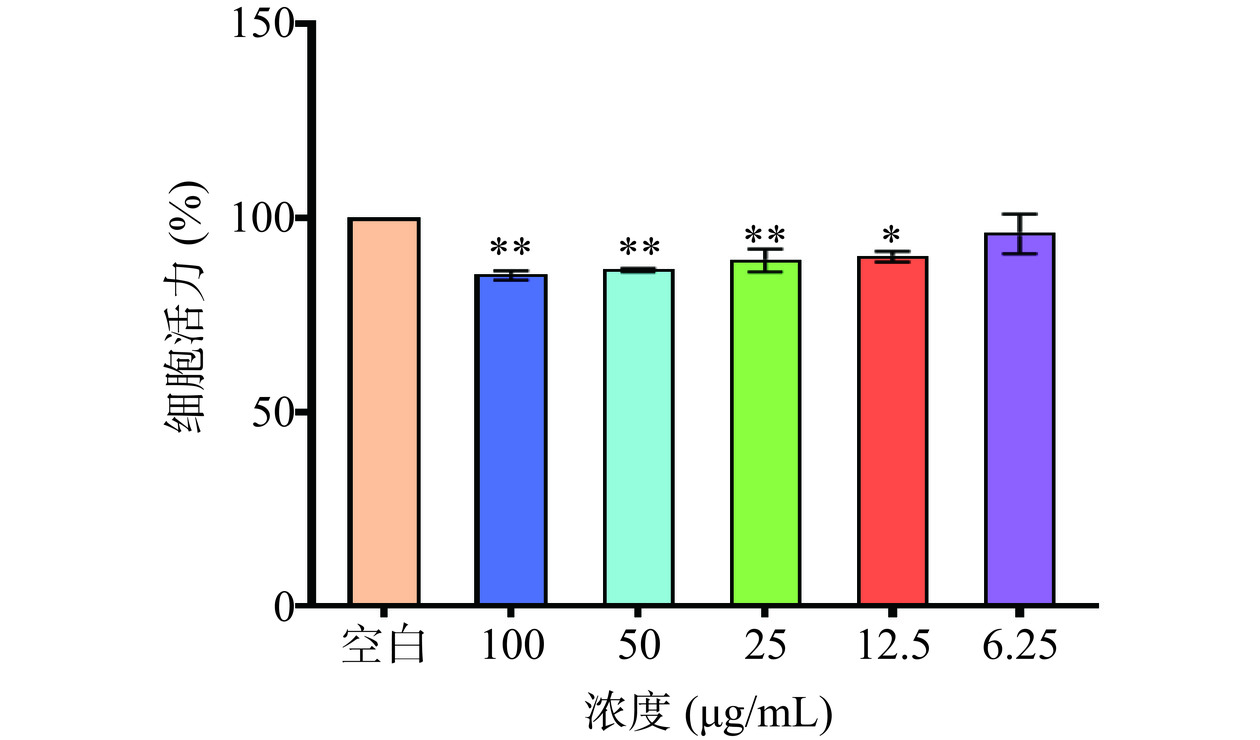
不同的牡丹籽乙醇提取物浓度对UVB诱导的HaCaT细胞的保护效果不同,为了寻找牡丹籽乙醇提取物对HaCaT细胞的最佳处理浓度,用MTT法检测不同浓度的牡丹籽乙醇提取物对HaCaT细胞活性的影响。结果显示,经过不同浓度的牡丹籽乙醇提取物处理后的HaCaT细胞的活性均在80%以上,最低为85.1%,并且与空白组相比,12.5 μg/mL(H组)对HaCaT细胞的活力有显著的细胞毒性(P<0.05),6.25 μg/mL(L组)对HaCaT细胞的活力没有显著的细胞毒性作用(P>0.05),而其他组的牡丹籽乙醇提取物浓度对HaCaT细胞有极显著的细胞毒性(P<0.01),故选择12.5 μg/mL(H组)和6.25 μg/mL(L组)作为后续实验的浓度。其中,6.25 μg/mL的牡丹籽乙醇提取物对细胞的毒性作用最小,而100 μg/mL的牡丹籽乙醇提取物对细胞的毒性作用最大(图2)。这是由于牡丹籽油中含有丰富的不饱和脂肪酸、少量的不皂化物以及微量元素等,具有一定的抗炎、抗氧化、抑菌等功效[30]。
2.3 牡丹籽乙醇提取物对HaCaT细胞抗光老化作用的影响
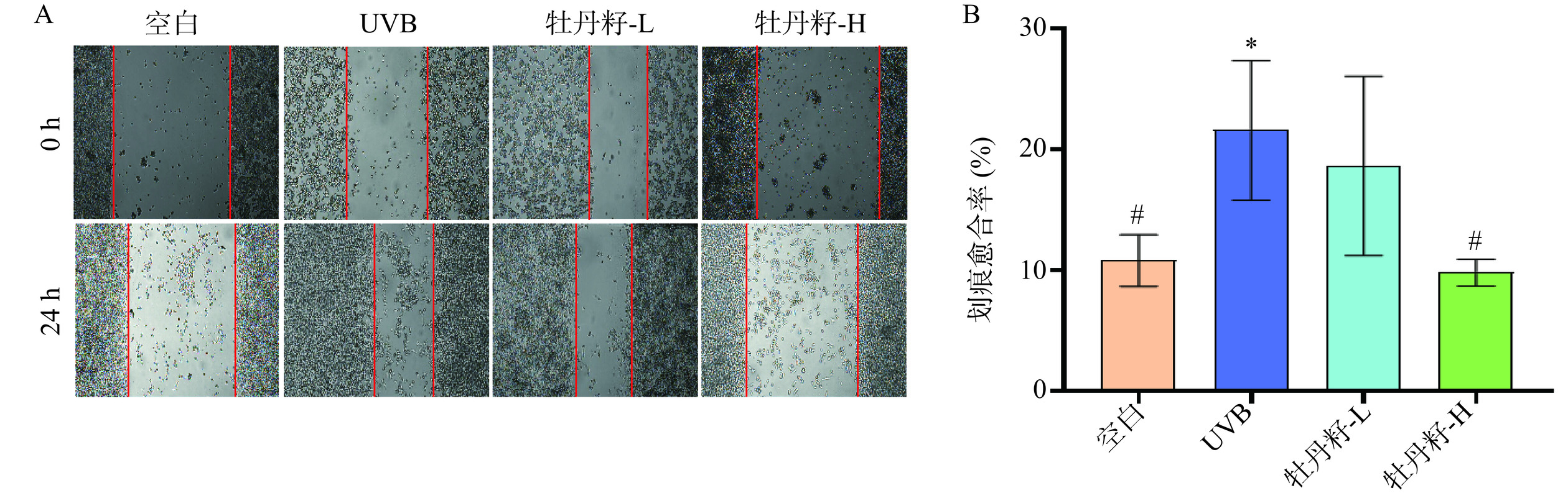
研究表明,当受到外界刺激时,皮肤损伤会导致一系列的生理生化反应,包括炎症等应激反应会被激活,并对损伤部位做出反应,从而促进创伤愈合[31]。通过细胞划痕法检测不同浓度牡丹籽乙醇提取物对HaCaT细胞迁移的影响。在细胞划痕实验中,图3A可以看出,与空白组相比,UVB造模的HaCaT细胞有明显的迁移趋势;与UVB组相比,高浓度的牡丹籽乙醇提取物可以降低UVB刺激的HaCaT细胞的迁移率,两种浓度的牡丹籽乙醇提取物均可以抑制UVB诱导的HaCaT细胞产生的炎症,从而抑制HaCaT细胞的迁移,其中高浓度牡丹籽乙醇提取物的划痕愈合率差异显著(P<0.05),低浓度牡丹籽乙醇提取物的划痕愈合率差异无统计学意义(P>0.05)(图3B)。这些结果证实了牡丹籽乙醇提取物可以抑制HaCaT细胞的迁移能力。
2.4 牡丹籽乙醇提取物对UVB诱导HaCaT细胞中IL-1、IL-6、IL-22、IFN-γ和TGF-β的影响
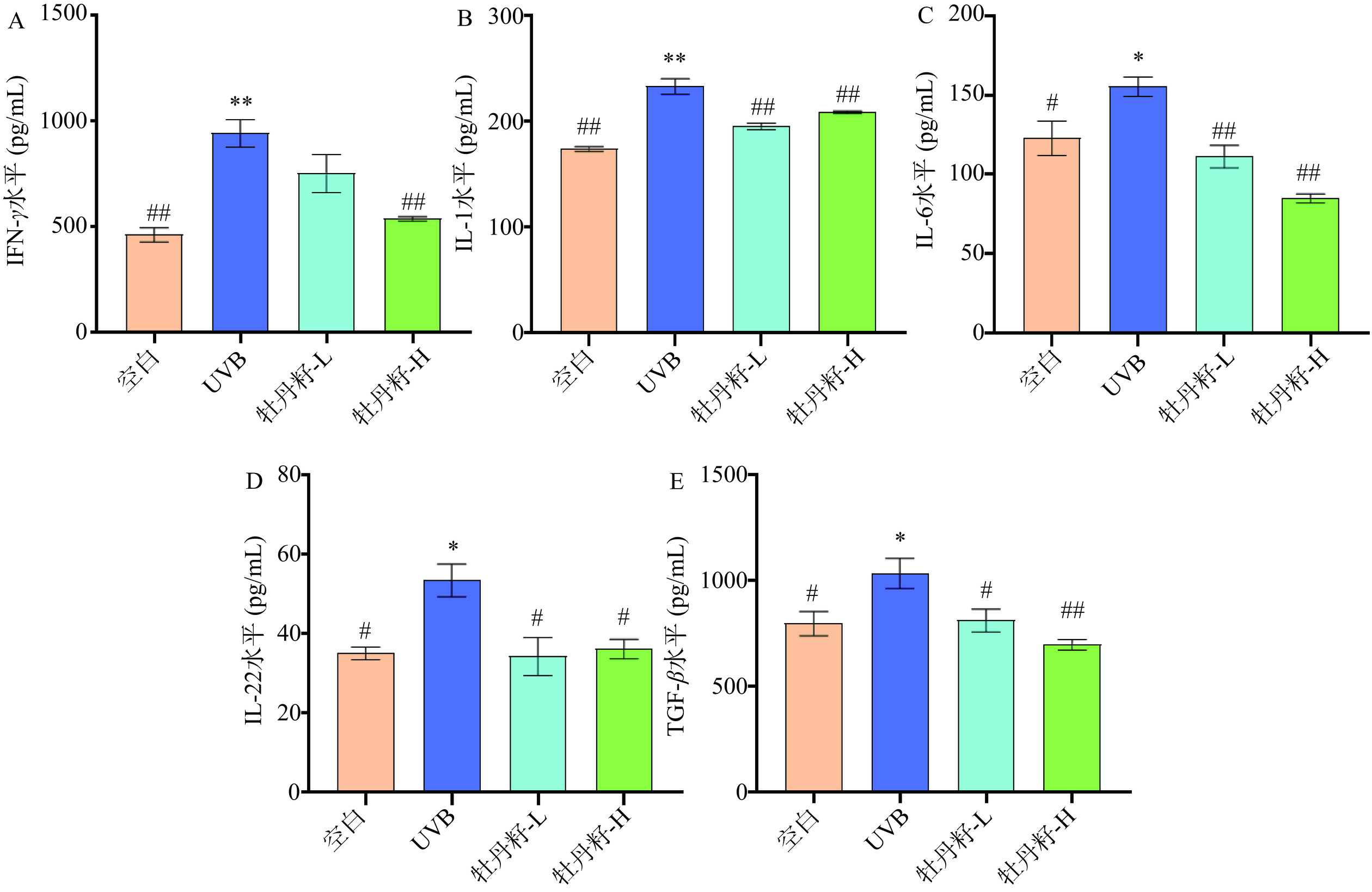
UVB诱导HaCaT细胞,可激活NF-κB转录因子,促进IL-1、IL-6等多种炎症因子的表达[32−33]。IL-22可促进HaCaT细胞过度增殖和炎性反应[34]。IFN-γ具有促炎作用,IFN-γ的表达量下降有利于机体抵抗炎症[35]。TGF-β是炎症发展的关键介质,介导炎症反应[36]。UVB辐射HaCaT细胞后,细胞内ROS水平上升,诱导细胞释放大量促炎因子,增加真皮层MMPs的表达,并持续激活NF-κB通路,导致皮肤衰老[37]。IL-1、IL-6、IL-22、IFN-γ和TGF-β的表达量下降有利于机体抵抗因炎症诱导的皮肤衰老。如图4A~4E所示,与空白组相比,在经过UVB辐射后HaCaT细胞中IFN-γ、IL-1、IL-6、IL-22和TGF-β的水平都显著升高(P<0.05),与UVB组相比,牡丹籽乙醇提取物抑制了UVB处理的HaCaT细胞中的IFN-γ、IL-1、IL-6、IL-22和TGF-β的表达,其中高浓度牡丹籽乙醇提取物处理后的HaCaT细胞的IFN-γ、IL-1、IL-6和TGF-β的水平都呈现极显著性降低(P<0.01),IL-22水平呈现显著性降低(P<0.05),而低浓度组HaCaT细胞中的IL-1、IL-6的水平呈现极显著性降低(P<0.01),IL-22、TGF-β水平呈现显著性降低(P<0.05)。该结果证明了牡丹籽乙醇提取物能抑制HaCaT细胞中炎症因子的表达,避免NF-κB通路的持续激活。这与叶希韵[38]的研究一致,牡丹籽乙醇提取物可能通过调节NF-κB信号通路,抑制炎症因子如IFN-γ、IL-1、IL-6、IL-22和TGF-β的表达发挥其抗炎作用从而延缓皮肤衰老。
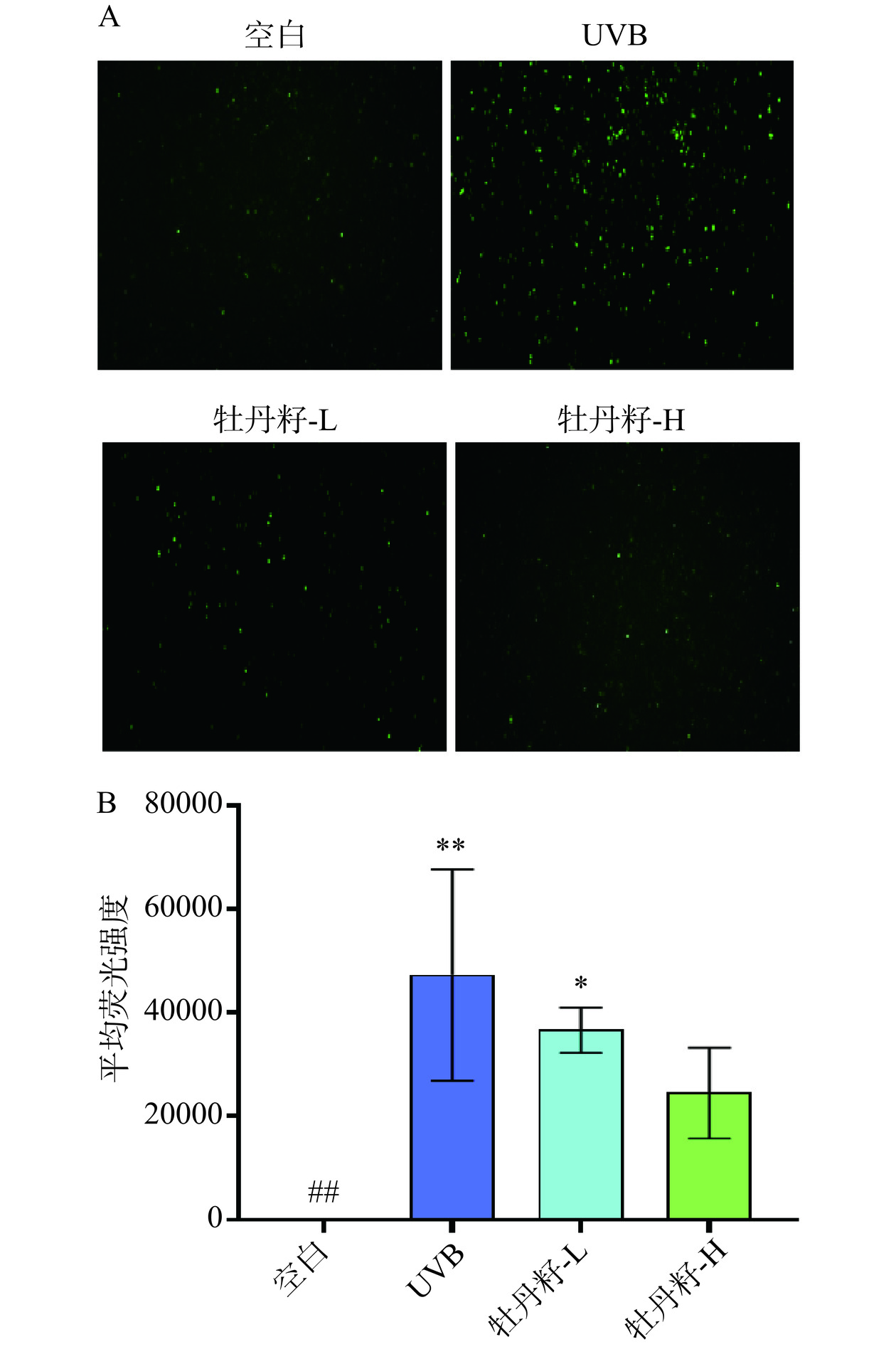
2.5 牡丹籽乙醇提取物对UVB诱导HaCaT细胞ROS水平的影响
氧的正常代谢的天然副产物是ROS,其在细胞信号传导和体内平衡中发挥重要作用。研究发现在UVB的刺激下细胞内ROS水平显著提升,该现象会引起氧化应激造成细胞结构严重损害,诱发炎症反应,导致细胞老化和衰退从而诱发衰老[39]。图5A中UVB照射后的HaCaT细胞的荧光强度有所提高,证明其ROS水平上升,并且给予不同浓度的牡丹籽乙醇提取物治疗后信号减弱,ROS水平下降,并且如图5B所示,模型组的HaCaT细胞内,ROS释放量与空白组相比呈现极显著提高(P<0.01),在经过给予不同浓度的牡丹籽乙醇提取物共同培养细胞后,低浓度组和高浓度组的HaCaT细胞的ROS释放量与UVB组相比均下降。说明牡丹籽乙醇提取物能抑制UVB诱导的细胞氧化应激,降低细胞内ROS释放量,缓解UVB导致的细胞老化和衰退。这与Kong等[40]的研究结果一致,含有芍药苷的牡丹籽乙醇提取物能够显著抑制UVB诱导的HaCaT细胞中ROS的产生。且ROS的降低可能是通过抑制HaCaT细胞中的p38和p53通路的激活,这是因为ROS-p38-p53通路参与紫外线诱导的皮肤细胞损伤与凋亡[41]。
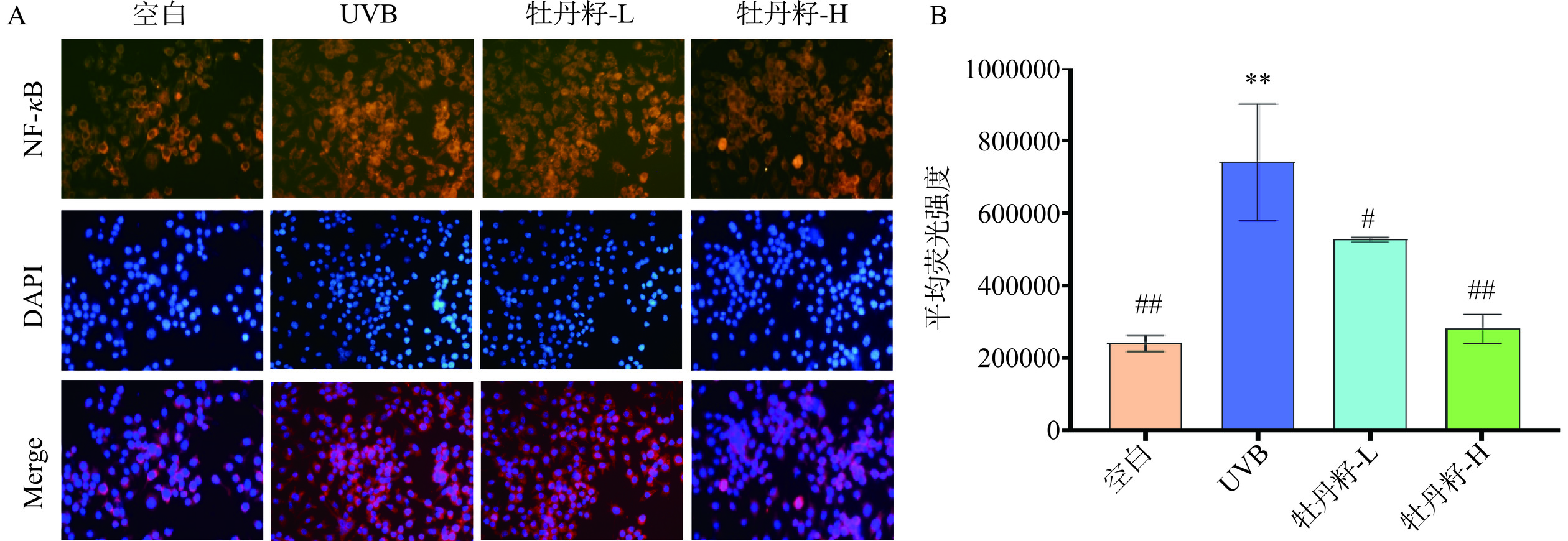
2.6 牡丹籽乙醇提取物对HaCaT细胞中NF-κB蛋白因子的影响
NF-κB蛋白因子参与细胞各种免疫和炎症途径,以及细胞增殖、分化和凋亡等多种生物学过程,NF-κB信号通路能促进炎症因子TNF-α、IL-6等的表达释放,因此抑制NF-κB的表达能减少细胞内炎症反应[42]。免疫荧光测定结果见图6,如图6A所示,经过UVB辐射后的HaCaT细胞中NF-κB蛋白因子较空白组相比,荧光强度有所提高,证明UVB诱导后的HaCaT细胞中的NF-κB水平上升,在经过不同浓度的牡丹籽乙醇提取物治疗后,荧光强度降低,并且图6B中细胞在接受UVB刺激时,细胞内分泌的NF-κB蛋白因子与空白组相比极显著增加(P<0.01),在经过不同浓度牡丹籽乙醇提取物培养后,不同浓度的牡丹籽乙醇提取物均能有效降低细胞NF-κB蛋白因子的表达,其中效果极显著的是牡丹籽高浓度组(P<0.01),低浓度组与UVB模型组相比降低效果显著(P<0.05)。郭婷[43]的研究也证实了牡丹籽油可能通过调控MAPK信号通路,抑制NF-κB的活性,下调炎症因子的表达,发挥抗炎作用。
3. 结论
本研究通过UPLC-Q-TOF-MS对牡丹籽进行化学成分分析,从牡丹籽乙醇提取物中分析得到主要成分为单硬脂酸甘油酯、芥酸酰胺等化合物。采用MTT实验评估牡丹籽乙醇提取物对HaCaT细胞的增殖作用,结果表明,牡丹籽乙醇提取物对HaCaT细胞增殖的影响较小,无明显毒性。利用UVB照射HaCaT细胞模型,划痕试验证明了牡丹籽乙醇提取物能抑制UVB诱导的HaCaT细胞迁移。另外,通过ELISA试验证实了牡丹籽乙醇提取物可以抑制促进细胞衰老的 IL-1、IL-6、IL-22、IFN-γ 和 TGF-β 等细胞因子的分泌。对UVB照射后的HaCaT细胞中ROS的测定,证实了牡丹籽乙醇提取物能有效去除细胞中的ROS,缓解UVB诱导的细胞受损,延缓细胞衰老。此外,免疫荧光实验也证明牡丹籽乙醇提取物能抑制NF-κB的活性,从而下调TNF-α、IL-6等炎症因子的表达,发挥抗炎作用。综上所述,牡丹籽乙醇提取物具有抵御UVB刺激、保护皮肤的作用,本研究为牡丹籽在食品、化妆品领域中的应用提供了一定的理论基础,为后续研发提供了思路。
-
表 1 牡丹籽乙醇提取物的化学成分
Table 1 Chemical constituents of Paeonia suffruticosa seed ethanol extract
序号 保留时间
(min)化合物名称 分子式 相对分子质量
(m/z)正离子(m/z)
[M+H]+负离子(m/z)
[M-H]−误差(ppm) 1 1.043 α,α-海藻糖 C12H22O11 342.11575 341.10449 −1.34 2 1.051 棉籽糖 C18H32O16 504.16915 503.16190 0.23 3 12.19 芍药内酯苷 C23H28O11 480.16218 481.16931 −2.05 4 12.989 虎杖苷 C20H22O8 390.13065 391.13791 −2.09 5 13.286 芍药苷 C23H28O11 480.16336 481.16561 0.42 6 15.34 白藜芦醇 C14H12O3 228.0781 229.08562 −2.38 7 15.995 木犀草苷 C21H20O11 286.0477 287.05423 −1.88 8 16.711 霉酚酸 C17H20O6 320.12509 321.13251 −2.82 9 16.899 5-[(2R,3S)-6-羟基-2-(4-羟基苯基)-4-[(E)-2-(4-
羟基苯基)乙烯基]-2,3-二氢1-苯并呋喃-3-基]苯-1,3-二醇C28H22O6 454.14066 453.13422 −2.16 10 17.28 木犀草素 C15H10O6 286.04716 287.05405 −2 11 19.041 N,N-二甲基癸烷基-N-氧化胺 C12H27NO 201.20885 202.21610 −2.05 12 20.577 双(4-乙基亚苯基)山梨醇 C24H30O6 414.203 415.19223 −2.98 13 22.49 9-O-10(E),12(E)-十八碳二烯酸 C18H30O3 294.21858 295.22574 −3.1 14 23.424 棕榈油酸 C16H30O2 254.22379 255.23106 −3.12 15 23.765 2,2'-亚甲基双-(4-甲基-6-叔丁基苯酚) C23H32O2 340.24013 339.23270 −0.3 16 24.155 油酰胺 C18H35NO 281.27095 282.27835 −3.26 17 24.439 羽扇豆醇 C30H50O 426.38509 427.39233 −2.52 18 24.837 油乙酰胺 C20H39NO 309.30222 310.30951 −3.06 19 24.855 单硬脂酸甘油酯 C21H42O4 358.30727 359.29651 −2.9 20 25.632 芥酸酰胺 C22H43NO 337.33336 338.34073 −3.28 -
[1] 胡云飞. 基于药物分析组合技术研究凤丹药材的道地性[D]. 合肥:安徽中医药大学, 2015. [HU Y F. Research on the authenticity of Fengdan medicinal materials based on drug analysis combination technology[D]. Hefei:Anhui University of Chinese Medicine, 2015.] HU Y F. Research on the authenticity of Fengdan medicinal materials based on drug analysis combination technology[D]. Hefei: Anhui University of Chinese Medicine, 2015.
[2] 田给林, 赵贵红. 牡丹鲜花在食品中的应用现状及展望[J]. 食品研究与开发,2010,31(4):4. [TIAN G L, ZHAO G H. Food of peony flowers's exploitation and development prospects[J]. Food Research and Development,2010,31(4):4.] TIAN G L, ZHAO G H. Food of peony flowers's exploitation and development prospects[J]. Food Research and Development, 2010, 31(4): 4.
[3] 龙正莉, 杨立新, 杨蓉, 等. 牡丹组植物的药用民族植物学研究与考证[J]. 广西植物,2021,41(2):10. [LONG Z L, YANG L X, YANG R, et al. Medicinal ethnobotany research on Paeonia sect. Moutan through textual evidence[J]. Guihaia,2021,41(2):10.] LONG Z L, YANG L X, YANG R, et al. Medicinal ethnobotany research on Paeonia sect. Moutan through textual evidence[J]. Guihaia, 2021, 41(2): 10.
[4] 王晓, 时新刚, 郑成超, 等. 牡丹花提取物清除活性氧及对·OH引发的DNA损伤的保护作用[J]. 食品与发酵工业,2004,30(7):55−58. [WANG X, SHI X G, ZHENG C C, et al. Effects of extract from peony flowers on removal of reactive oxygen species and preventing dna damage caused by hydroxyl radical[J]. Food & Fermentation Industries,2004,30(7):55−58.] WANG X, SHI X G, ZHENG C C, et al. Effects of extract from peony flowers on removal of reactive oxygen species and preventing dna damage caused by hydroxyl radical[J]. Food & Fermentation Industries, 2004, 30(7): 55−58.
[5] 刘建华, 董福英. 牡丹花营养成分分析及其评价[J]. 山东科学,1999,12(4):3. [LIU J H, DONG F Y. The analysis and appreciation for the nutritions of peony flower[J]. Shandong Science,1999,12(4):3.] LIU J H, DONG F Y. The analysis and appreciation for the nutritions of peony flower[J]. Shandong Science, 1999, 12(4): 3.
[6] 周畅. 牡丹籽油作为化妆品基础油的开发研究[D]. 上海:上海交通大学, 2015. [ZHOU C. Research on the development of peony seed oil as a base oil for cosmetics[D]. Shanghai:Shanghai Jiao Tong University, 2015.] ZHOU C. Research on the development of peony seed oil as a base oil for cosmetics[D]. Shanghai: Shanghai Jiao Tong University, 2015.
[7] 向芳, 张国豪. 皮肤衰老的研究进展[J]. 贵州医药,2011,35(12):1138−1140. [XIANG F, ZHANG G H. Advances in the study of skin aging[J]. Guizhou Medical Journal,2011,35(12):1138−1140.] XIANG F, ZHANG G H. Advances in the study of skin aging[J]. Guizhou Medical Journal, 2011, 35(12): 1138−1140.
[8] ZHANG X, WANG H, XU Y, et al. Advances on the anti-inflammatory activity of oleanolic acid and derivatives[J]. Mini-Reviews in Medicinal Chemistry,2021,21(15):2020−2038. doi: 10.2174/1389557521666210126142051
[9] 梁栋, 房盟盟, 马康, 等. ‘凤丹’牡丹籽油抗光老化功效[J]. 林业科技通讯,2023(2):76−78. [LIANG D, FANG M M, MA K, et al. Anti photoaging effect of 'Fengdan' peony seed oil[J]. Forest Science and Technology,2023(2):76−78.] LIANG D, FANG M M, MA K, et al. Anti photoaging effect of 'Fengdan' peony seed oil[J]. Forest Science and Technology, 2023(2): 76−78.
[10] 周源, 李文宇, 范润哥, 等. 光老化人角质形成细胞模型的构建[J]. 广西医科大学学报,2022,39(2):5. [ZHOU Y, LI W Y, FAN R G, et al. Construction of photoaging human keratinocyte model[J]. Journal of Guangxi Medical University,2022,39(2):5.] ZHOU Y, LI W Y, FAN R G, et al. Construction of photoaging human keratinocyte model[J]. Journal of Guangxi Medical University, 2022, 39(2): 5.
[11] BENNETT M F, ROBINSON M K, BARON E D, et al. Skin Immune systems and inflammation:Protector of the skin or promoter of aging?[J]. Journal of Investigative Dermatology Symposium Proceedings,2008,13(1):15−19. doi: 10.1038/jidsymp.2008.3
[12] XIAO Z, YANG S, CHEN J, et al. Trehalose against UVB-induced skin photoaging by suppressing MMP expression and enhancing procollagen I synthesis in HaCaT cells[J]. Journal of Functional Foods,2020,74:104198. doi: 10.1016/j.jff.2020.104198
[13] 王学红, 尹星星, 陆杰, 等. 树莓果油抑制UVB诱导HaCaT细胞光老化的作用研究[J]. 西南农业学报,2023,36(2):9. [WANG X H, YIN X X, LU J, et al. Inhibition effects of raspberry pulp oil on photoaging of HaCaT cells induced by UVB[J]. Southwest China Journal of Agricultural Sciences,2023,36(2):9.] WANG X H, YIN X X, LU J, et al. Inhibition effects of raspberry pulp oil on photoaging of HaCaT cells induced by UVB[J]. Southwest China Journal of Agricultural Sciences, 2023, 36(2): 9.
[14] 张红玉, 王成章, 原姣姣, 等. 牡丹籽壳提取物及不同极性部位的抗氧化活性研究[J]. 中国油脂,2016,41(7):4. [ZHANG H Y, WANG C Z, YUAN J J, et al. Antioxidant activities of peony seed shell extract and different polarity fractions[J]. China Oils and Fats,2016,41(7):4.] ZHANG H Y, WANG C Z, YUAN J J, et al. Antioxidant activities of peony seed shell extract and different polarity fractions[J]. China Oils and Fats, 2016, 41(7): 4.
[15] 符史良, 陈洪, 谢建英, 等. 桐花树叶醇提物的气相色谱-质谱分析[J]. 湛江海洋大学学报,2006,26(4):2. [FU S L, CHEN H, XIE J Y, et al. Gas chromatography-mass spectrometry (GC-MS) analysis of the alcoholic extracts of the leaves of Tongkat Ali[J]. Journal of Guangdong Ocean University,2006,26(4):2.] FU S L, CHEN H, XIE J Y, et al. Gas chromatography-mass spectrometry (GC-MS) analysis of the alcoholic extracts of the leaves of Tongkat Ali[J]. Journal of Guangdong Ocean University, 2006, 26(4): 2.
[16] 苏华振, 魏明. 寻常型银屑病皮损中Notch1表达及对体外培养HaCaT细胞的影响[J]. 中国医药生物技术,2019,14(5):412−420. [SU H Z, WEI M. Expression of Notch1 in skin lesions of patients with psoriasis vulgaris and its effects on the growth of HaCaT cells in vitro[J]. Chinese Medicinal Biotechnology,2019,14(5):412−420.] SU H Z, WEI M. Expression of Notch1 in skin lesions of patients with psoriasis vulgaris and its effects on the growth of HaCaT cells in vitro[J]. Chinese Medicinal Biotechnology, 2019, 14(5): 412−420.
[17] ZHANG H, SHAN Y, WU Y, et al. Berberine suppresses LPS-induced inflammation through modulating Sirt1/NF-κB signaling pathway in RAW264. 7 cells[J]. International Immunopharmacologyl,2017,52:93−100.
[18] 李敏, 丁毅, 郭琼, 等. Hsa-let-7b-5p靶向ΔNp63抑制HaCaT细胞的增殖, 迁移和侵袭[J]. 中国组织化学与细胞化学杂志,2020,29(4):6. [LI M, DING Y, GUO Q, et al. Hsa-let-7b-5p inhibits proliferation, migration and invasion of HaCaT cells by targeting ΔNp63[J]. Chinese Journal of Histochemistry and Cytochemistry,2020,29(4):6.] LI M, DING Y, GUO Q, et al. Hsa-let-7b-5p inhibits proliferation, migration and invasion of HaCaT cells by targeting ΔNp63[J]. Chinese Journal of Histochemistry and Cytochemistry, 2020, 29(4): 6.
[19] 孙璇, 李萍, 孙静, 等. 槐提取物和维生素C组合物对紫外照射诱导的HaCaT细胞光损伤的保护作用[J]. 食品工业科技,2024,45(1):303−309. [SUN X, LI P, SUN J, et al. Protective effect of Sophora japonica L. extract and vitamin C composition on photodamage of HaCat cells induced by ultraviolet irradiation[J]. Science and Technology of Food Industry,2024,45(1):303−309.] SUN X, LI P, SUN J, et al. Protective effect of Sophora japonica L. extract and vitamin C composition on photodamage of HaCat cells induced by ultraviolet irradiation[J]. Science and Technology of Food Industry, 2024, 45(1): 303−309.
[20] 曾颖, 付桂莉. 黄芩提取物抑制IL-22诱导的人角质形成细胞系HaCaT过度增殖[J]. 基础医学与临床,2023,43(1):123−129. [ZENG Y, FU G L. Scutellaria extract inhibits IL-22-induced hyperproliferation of human keratinocyte cell line HaCat[J]. Basic and Clinical Medicine,2023,43(1):123−129.] ZENG Y, FU G L. Scutellaria extract inhibits IL-22-induced hyperproliferation of human keratinocyte cell line HaCat[J]. Basic and Clinical Medicine, 2023, 43(1): 123−129.
[21] 郑柳怡, 谢凯枫, 杨九妹, 等. 玉屏风颗粒对HaCaT特应性皮炎细胞模型的保护作用研究[J]. 广东药科大学学报,2021,37(2):66−71. [ZHENG L Y, XIE K F, YANG J M, et al. Protective effect of Yupingfeng granule on HaCaT cell model of atopic dermatitis[J]. Journal of Guangdong Pharmaceutical,2021,37(2):66−71.] ZHENG L Y, XIE K F, YANG J M, et al. Protective effect of Yupingfeng granule on HaCaT cell model of atopic dermatitis[J]. Journal of Guangdong Pharmaceutical, 2021, 37(2): 66−71.
[22] 张俊焱. 藏药翁布化合物及精油对紫外线UVB所致HaCat细胞损伤的保护作用研究[D]. 西宁:青海师范大学, 2022. [ZHANG J Y. Study on the protective effect of Tibetan medicine Wengbu compounds and essential oils on UVB-induced HaCat cell damage[D]. Xining:Qinghai Normal University, 2022.] ZHANG J Y. Study on the protective effect of Tibetan medicine Wengbu compounds and essential oils on UVB-induced HaCat cell damage[D]. Xining: Qinghai Normal University, 2022.
[23] LI M, DONG L, DU H, et al. Potential mechanisms underlying the protective effects of Tricholoma matsutake Singer peptides against LPS-induced inflammation in RAW264.7 macrophages[J]. Food Chemistry,2021,353:129452. doi: 10.1016/j.foodchem.2021.129452
[24] 苗淼, 刘佳, 谈静, 等. 芍药苷抑制NF-κB通路减少银屑病HaCaT细胞炎症的作用研究[J]. 中医药信息,2023,40(3):47−51. [MIAO N, LIU J, TAN J, et al. Paeoniflorin lnhibiting NF-κB pathway and reducing lnflammation of HaCaT cells in treatment of psoriasis[J]. Information on Traditional Chinese Medicine,2023,40(3):47−51.] MIAO N, LIU J, TAN J, et al. Paeoniflorin lnhibiting NF-κB pathway and reducing lnflammation of HaCaT cells in treatment of psoriasis[J]. Information on Traditional Chinese Medicine, 2023, 40(3): 47−51.
[25] 张彤. 九种野生牡丹种子和籽油化学成分及其生物活性研究[D]. 洛阳:河南科技大学2022. [ZHANG T. Study on the chemical composition and biological activity of seeds and seed oil of nine wild peony species[D]. Luoyang:Henan University of Science and Technology, 2022.] ZHANG T. Study on the chemical composition and biological activity of seeds and seed oil of nine wild peony species[D]. Luoyang: Henan University of Science and Technology, 2022.
[26] JATTUJAN P, SRISIRIRUNG S, WATCHARAPORN W, et al. 2-Butoxytetrahydrofuran and palmitic acid from Holothuria scabra enhance C. elegans lifespan and healthspan via DAF-16/FOXO and SKN-1/NRF2 signaling pathways[J]. Pharmaceuticals (Basel),2022,15(11):1374. doi: 10.3390/ph15111374
[27] APAZA TICONA L, PEÑA-ROJAS G, ANDÍA-AYME V, et al. Anti-glycative and anti-inflammatory effects of macamides isolated from Tropaeolum tuberosum in skin cells[J]. Natural Product Research,2022,36(22):5803−5807. doi: 10.1080/14786419.2021.2016751
[28] LIU W, YIN D X, ZHANG T, et al. Major fatty acid compositions and antioxidant activity of cultivated Paeonia ostii under different nitrogen fertilizer application[J]. Chemistry & Biodiversity,2020,17(12):e2000617.
[29] 周景瑞, 王洪琳, 张琴, 等. 贵州地区牡丹籽油脂肪酸组成分析[J]. 安徽农业科学,2021,49(19):177−179. [ZHOU J R, WANG H L, ZHANG Q, et al. Analysis of fatty acid composition of peony seed oil in Guizhou regions[J]. Journal of Anhui Agricultural Sciences,2021,49(19):177−179.] doi: 10.3969/j.issn.0517-6611.2021.19.046 ZHOU J R, WANG H L, ZHANG Q, et al. Analysis of fatty acid composition of peony seed oil in Guizhou regions[J]. Journal of Anhui Agricultural Sciences, 2021, 49(19): 177−179. doi: 10.3969/j.issn.0517-6611.2021.19.046
[30] 侯天兰, 王顺利, 米生权, 等. 牡丹籽油营养成分和功能作用研究进展[J]. 中国油脂,2021,46(8):51−55,71. [HOU T L, WANG S L, MI S Q, et al. Progress on nutritional components and functional activities of peony seed oil[J]. China Oils and Fats,2021,46(8):51−55,71.] HOU T L, WANG S L, MI S Q, et al. Progress on nutritional components and functional activities of peony seed oil[J]. China Oils and Fats, 2021, 46(8): 51−55,71.
[31] GURTNER G C, WERNER S, BARRANDON Y, et al. Wound repair and regeneration[J]. Nature,2008,453(7193):314−321. doi: 10.1038/nature07039
[32] ZHANG J, DOU W, ZHANG E, et al. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,2014,306(1):G27−G36. doi: 10.1152/ajpgi.00465.2012
[33] HIRANO T. IL-6 in inflammation, autoimmunity and cancer[J]. International Immunology,2021,33(3):127−148. doi: 10.1093/intimm/dxaa078
[34] 孙奇. IL-22—炎症性疾病关键因子[J]. 免疫学杂志,2011,27(9):821−825. [SUN Q. IL-22:The key cytokine in inflammatory diseases[J]. Immunological Journal,2011,27(9):821−825.] SUN Q. IL-22: The key cytokine in inflammatory diseases[J]. Immunological Journal, 2011, 27(9): 821−825.
[35] 章昊旻, 王子, 郭元睿, 等. 清疕饮调控S1P/S1PR5信号通路对HaCaT炎症模型的影响[J]. 环球中医药,2023,16(12):2434−2443. [ZHANG H M, WANG Z, GUO Y R, et al. Qingbi decoction regulate and control the S1P/S1PR5 signaling pathway to influence HaCaT inflammation model[J]. Global Traditional Chinese Medicine,2023,16(12):2434−2443.] doi: 10.3969/j.issn.1674-1749.2023.12.006 ZHANG H M, WANG Z, GUO Y R, et al. Qingbi decoction regulate and control the S1P/S1PR5 signaling pathway to influence HaCaT inflammation model[J]. Global Traditional Chinese Medicine, 2023, 16(12): 2434−2443. doi: 10.3969/j.issn.1674-1749.2023.12.006
[36] STEWART A G, THOMAS B, KOFF J. TGF-β:Master regulator of inflammation and fibrosis[J]. Respirology,2018,23(12):1096−1097. doi: 10.1111/resp.13415
[37] 甘嘉荷, 廖勇. 皮肤炎症性衰老与治疗策略[J]. 实用皮肤病学杂志,2022,15(1):35−40. [GAN J H, LIAO Y. Skin inflammaging and treatment strategies[J]. Journal of Practical Dermatology,2022,15(1):35−40.] GAN J H, LIAO Y. Skin inflammaging and treatment strategies[J]. Journal of Practical Dermatology, 2022, 15(1): 35−40.
[38] 叶希韵. 紫外线致皮肤光老化研究进展[J]. 生物学教学,2015,40(11):2−5. [YE X Y. Research progress of skin photoaging caused by ultraviolet rays[J]. Biology Teaching,2015,40(11):2−5.] YE X Y. Research progress of skin photoaging caused by ultraviolet rays[J]. Biology Teaching, 2015, 40(11): 2−5.
[39] 刘少英, 孟祥璟, 张祥奎, 等. 皮肤光老化机制及抗光老化药物[J]. 生理科学进展,2018,49(4):265−269. [LIU S Y, MENG X J, ZHANG X K, et al. The mechanism of skin photoaging and anti photoaging drugs[J]. Progress in Physiological Sciences,2018,49(4):265−269.] LIU S Y, MENG X J, ZHANG X K, et al. The mechanism of skin photoaging and anti photoaging drugs[J]. Progress in Physiological Sciences, 2018, 49(4): 265−269.
[40] KONG L, WANG S, WU X, et al. Paeoniflorin attenuates ultraviolet B-induced apoptosis in human keratinocytes by inhibiting the ROS-p38-p53 pathway[J]. Molecular Medicine Reports,2016,13(4):3553−3558. doi: 10.3892/mmr.2016.4953
[41] 刘爱华. p53和p38对UV诱导的细胞凋亡的抑制效应及其机制研究[D]. 广州:第一军医大学, 2008. [LIU A H. Study on the inhibitory effects and mechanisms of p53 and p38 on UV-induced cell apoptosis[D]. Guangzhou:First Military Medical University, 2008.] LIU A H. Study on the inhibitory effects and mechanisms of p53 and p38 on UV-induced cell apoptosis[D]. Guangzhou: First Military Medical University, 2008.
[42] 杨玲, 胡中华. 中波紫外线对HaCaT细胞NF-κB及miRNA表达的影响及调控机制研究[J]. 皮肤病与性病,2023,45(1):1−6. [YANG L, HU Z H. Expression levels of NF-κB and microRNA and involved mechanisms in HaCaT cells induced by ultraviolet B[J]. Dermatology and Venereology,2023,45(1):1−6.] YANG L, HU Z H. Expression levels of NF-κB and microRNA and involved mechanisms in HaCaT cells induced by ultraviolet B[J]. Dermatology and Venereology, 2023, 45(1): 1−6.
[43] 郭婷. 牡丹籽油抗炎作用的分子机理研究[D]. 长沙:中南林业科技大学, 2020. [GUO T. Study on the molecular mechanism of anti-inflammatory effect of peony seed oil[D]. Changsha:Central South University of Forestry and Technology, 2020.] GUO T. Study on the molecular mechanism of anti-inflammatory effect of peony seed oil[D]. Changsha: Central South University of Forestry and Technology, 2020.





 下载:
下载:
 下载:
下载:



