Research Progress on Anticancer Effects of Sulforaphane
-
摘要: 癌症是威胁人类健康的头号杀手,其致死率占所有疾病之首。目前,治疗癌症的方式不可避免地存在相关副作用,因此开发安全、有效的抗癌药物成为人们关注的焦点。萝卜硫素作为十字花科植物中生物活性高的天然化合物之一,具有抗癌、抗氧化、抗病毒等特性,其中,治疗癌症的作用机理被人们广泛研究。萝卜硫素可以通过调控细胞周期、抑制细胞的迁移和侵袭以及抑制凋亡相关蛋白的表达从而抑制癌细胞的生长,也可以通过抗炎和抗氧化,抑制癌细胞的发生和发展。与传统治疗癌症的方法相比,萝卜硫素具有安全、易获得的特点,在抗癌方面有较大的发展潜力。本文综述了近两年萝卜硫素抗癌作用机制,为萝卜硫素的抗癌作用的进一步研究提供了相应的理论依据。Abstract: Cancer is the leading cause of mortality in human health, with the highest mortality rate among all diseases. Unfortunately, most of the current cancer treatments inevitably come with certain side effects. Therefore, a significant amount of research is focused on the development of safe and effective anticancer drugs. Sulforaphane is one of the most bioactive natural compounds isolated from cruciferous plants. Numerous studies show its anti-cancer, antioxidant, and antiviral properties, as well as its anticancer mechanisms. Sulforaphane can inhibit the growth of cancer cells by regulating the cell cycle, inhibiting cell migration and invasion, and inhibiting the expression of apoptosis related proteins. It can also inhibit the occurrence and development of cancer cells through anti-inflammatory and antioxidant measures. Some reports also indicate that sulforaphane can impede the development of cancer cells through its anti-inflammatory and antioxidant effects. Compared to most traditional cancer treatment methods, sulforaphane is much safer and more reliable. It is also more readily available and has great anticancer potential. This paper review the anticancer mechanisms of sulforaphane over the past two years and provide the corresponding theoretical basis for further research on the anticancer effects of sulforaphane.
-
Keywords:
- sulforaphane(SFN) /
- anti-cancer properties /
- anti-inflammatory /
- antioxidant /
- mechanism of action
-
《2020年癌症统计》表明,2020年癌症的确诊病例达到1930万例,癌症的死亡病例将近1000万例,占所有疾病死亡率之首[1]。在我国,癌症也是导致死亡的主要原因,大数据表明,2022年我国癌症的确诊病例预估为480万例,癌症的死亡病例预估为321万例,采取有效的方式预防和治疗癌症尤为重要[2]。癌症治疗手段主要包括手术、化疗和放疗,通常采用两种方式相结合的方式来获得更好的预后效果[3],但是无法避免对机体的损伤,产生消化系统不良反应、骨髓抑制、内分泌失调等情况,使患者备受煎熬,治疗效果十分受限。开发天然化合物作为抗癌药物辅助治疗,减少癌细胞的耐药性及机体的损伤,对提高肿瘤患者的存活率和生活质量有重要意义[4]。
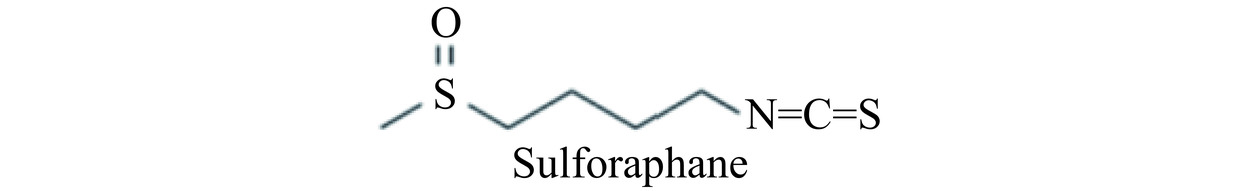
近年来随着对食物抗癌作用的深入研究,发现十字花科植物的摄入量与癌症呈负相关趋势[5]。在十字花科植物如花椰菜、卷心菜和芥蓝等植物中,萝卜硫素(Sulforaphane,SFN)是其中抗癌活性最好的化合物之一[6]。SFN是一种天然的异硫氰酸盐,分子式为C6H11NOS2,相对分子质量为117.3,化学结构式见图1[7]。SFN的前体是硫代葡萄糖苷,后者主要分布在十字花科植物的种子和幼苗中。植物受到损伤时分泌黑芥子酶,水解硫代葡萄糖苷生成异硫氰酸酯,主要为SFN[8]。
SFN具有安全、有效、低成本的特点,并且可以通过膳食补充获得,因而被广泛关注[9]。研究表明通过抑制Ⅰ相代谢酶、诱导Ⅱ相代谢酶的活性,减轻一些致癌物、毒素、活性氧和其他炎症诱导产生的机体损伤[10],且近些年来研究发现SFN具有多种药理作用,如抗癌[6]、抗氧化[11]、抗衰老[12]、抗病毒[13]、抗糖尿病[14]、抗血栓[15]等。因此,SFN能够有效的发挥抗癌作用,对于多发性骨髓瘤[16]、结肠癌[17]、胃癌[18]、乳腺癌[19]、卵巢癌[20]等癌细胞都具有明显的抑制作用。本文综述了SFN通过抑制癌细胞周期,抑制癌细胞迁移与侵袭,诱导癌细胞凋亡,调控多种炎症通路、抗氧化途径,以及与其他药物联合使用而抑制肿瘤的发展(如表1所示),期待为SFN及其衍生物作为抗癌药物研究提供参考。
表 1 SFN的抗癌作用及其机制Table 1. Anti-cancer effects and mechanisms of SFN作用机制 癌症类型 评价模型 作用途径 参考文献 抑制癌细胞周期 人星形细胞瘤 1321N1 上调P21蛋白表达,G2/M期阻滞 [28] 甲状腺癌 FTC133、8305C、BCPAP、K1 上调P21蛋白表达,抑制Cdk1、Cdk2、CyclinB1和Cdc25C表达 [27] 急性白血病 上调P21蛋白表达,抑制CDC2和CDC2/cyclin B表达 [29] 乳腺癌 MCF-7、MDA-MB-231 降低RB磷酸化 [31] 抑制癌细胞迁移与侵袭 甲状腺癌 FTC133、8305C、BCPAP、K1 抑制PI3K/Akt信号通路,促进E-cadherin的表达 [27] 肝母细胞瘤 U87MG、U373MG 激活ERK1/2信号通路,降低MMP-2表达 [33] 乳腺癌 4T1 下调HDA5C表达,抑制Vimentin、MMP-9的表达 [35] 乳腺癌 MCF-7 改变PML结构,抑制癌细胞转移 [37] 促进癌细胞凋亡 胃癌 BGC-823、MGC-803 激活P53蛋白,上调Bax表达 [18] 肝癌 HepG2 Bax/Bcl比值上升 [25] 结肠癌 SW-480 上调Bax合成 [44] 结肠癌 HT29 激活caspase 8介导的外源凋亡途径 [17] 抑制炎症发展 肝癌 HepG2 降低IL-6的表达 [56] 胃癌 SCG7901 抑制IL-6、IL-8、TNF-α [59] 调节抗氧化 结肠癌 HCT116 介导Nrf2信号通路中抗氧化酶表达以及抗凋亡蛋白表达 [62] 乳腺癌 骨髓间充质干细胞 激活Nrf2途径,促进HO-1,抑制COX-2 [64] 1. 萝卜硫素抑制癌细胞周期
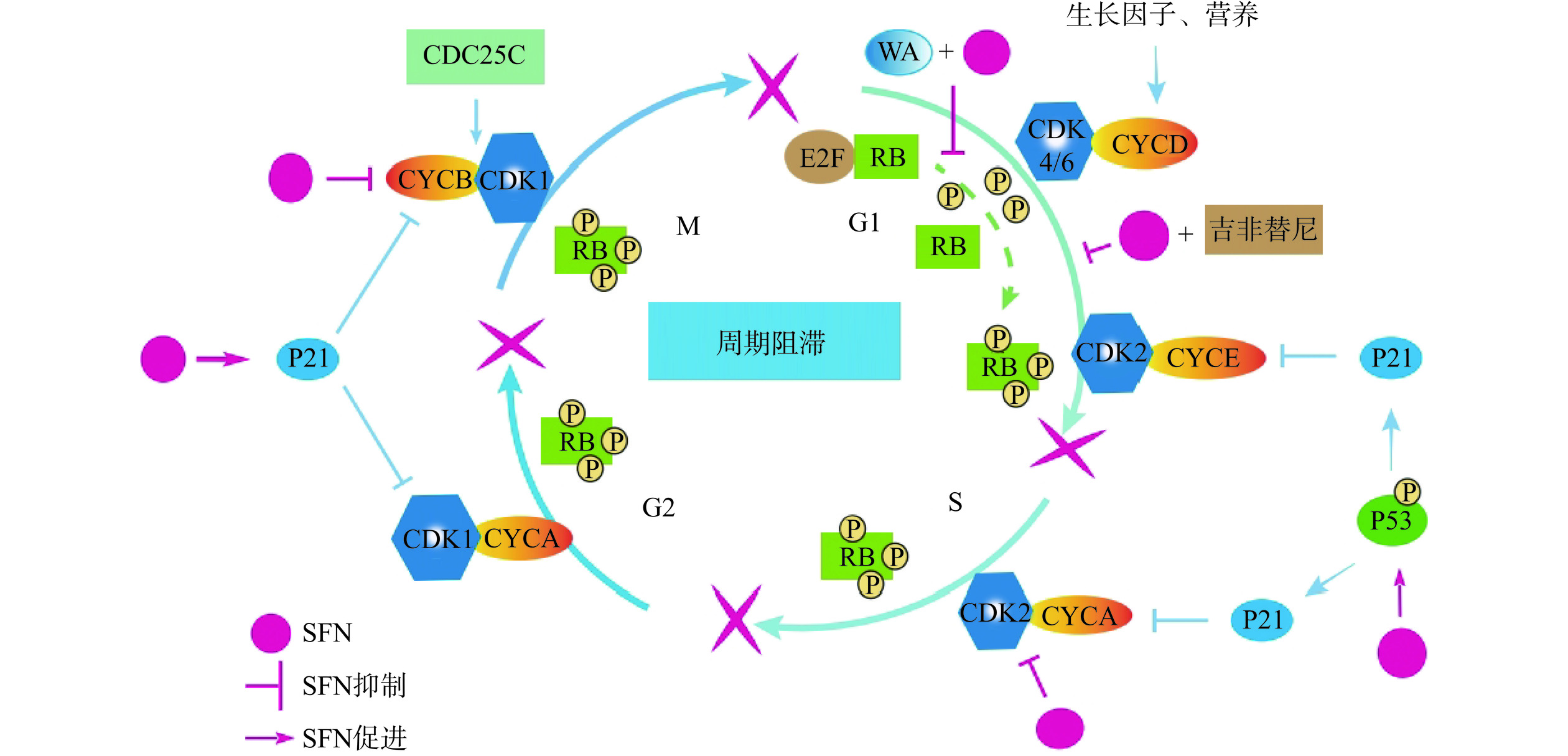
细胞增殖是细胞正常的生命活动,连续分裂的细胞具有细胞周期。而癌细胞无限增殖,可能与细胞周期失控有关。细胞周期的调节依赖于细胞周期蛋白(cyclin)对细胞周期蛋白依赖性激酶(CDK)的结合与激活,从而驱动细胞周期[21−22]。不同阶段,不同的cyclin结合不同CDK发挥作用,比如G2/M期,cyclinB与CDK1结合,进入M期进程[23],而这些细胞周期调控蛋白容易成为周期阻滞药物的作用靶点,抑制细胞增殖[24]。SFN抑制人肝癌HepG2细胞cyclin B1蛋白表达,将细胞周期阻滞在G2/M期[25]。SFN与帕拉替尼联用,使胃癌SCG-7901细胞的细胞周期停滞在G0/G1期,抑制胃癌细胞增殖[19]。吉非替尼和SFN联用,使吉非替尼耐药肺癌细胞PC9/AB11的周期阻滞在G1期[26](图2)。
“基因保卫者”P53(抑癌基因)与其下游靶蛋白P21(细胞周期蛋白依赖性激酶抑制剂)参与细胞周期的调控。当细胞受到损伤,P53被激活,P21迅速表达,抑制cyclin和CDK的结合,从而抑制细胞周期[23],因此P21可以作为分子靶点抑制癌细胞增殖。研究发现SFN能够促进P21蛋白表达,抑制癌细胞中CDK的表达,抑制癌细胞的细胞周期[27−28]。高浓度SFN与其水解酶提取物提高人星形细胞瘤1321N1细胞中非P53调控的P21蛋白表达,导致G2/M期阻滞,抑制肿瘤增殖[28]。在胃癌BGC-823和MGC-803细胞中,SFN上调P53蛋白和P21蛋白的表达,抑制CDK2蛋白表达,将胃癌细胞周期阻滞在S期[18]。SFN提高甲状腺癌FTC133、8305C、BCPAP和K1细胞中P21蛋白表达,抑制Cdk1、Cdk2、CyclinB1和Cdc25C基因的表达,使癌细胞周期阻滞在G2/M期[27]。而对于急性白血病而言,P53蛋白不发生突变,SFN激活P53信号通路,诱导下游P21蛋白表达,抑制CDC2和CDC2/cyclin B的表达,细胞停滞在G2/M期,停止增殖[29]。
在细胞周期G1早期,RB(视网膜母细胞瘤蛋白)与转录因子E2F结合,以非磷酸化形式存在;G1晚期,cyclinE-CDK2复合物使RB磷酸化,E2F发生转录,细胞进入S期[30]。RB过度磷酸化与细胞周期失去抑制相关,SFN和WA(一种类固醇内酯)联合使用降低RB磷酸化,使乳腺癌细胞MCF-7和MDA-MB-231细胞周期阻滞在G1期[31]。
以上研究表明,萝卜硫素针对不同肿瘤细胞,通过抑制相关周期调控蛋白表达,诱导肿瘤细胞凋亡,阻碍肿瘤细胞增殖,达到抗肿瘤效果。
2. 萝卜硫素抑制癌细胞的迁移与侵袭抑制癌症
癌细胞的迁移与侵袭是加速癌症恶化的重要因素,也是癌症治疗复发的重要原因。抑制癌细胞的迁移与侵袭,防止癌细胞扩散,会减轻癌细胞扩散产生的不良后果。癌细胞转移过程中会发生上皮间质转化(EMT)过程,该过程使细胞从相对稳定的状态转变为具有转移和侵袭能力的状态,涉及胞内胞外多种复杂的信号通路如PI3K/Akt、ERK1/2、Wnt/β-catenin等,以及EMT过程相关重要靶点的变化,如E-cadherin表达减少,Vimentin、Twist、MMP-2、MMP-9、Snail表达的增加等,促进EMT途径[32]。
研究发现SFN通过抑制PI3K/Akt信号通路抑制Vimentin和E-cadherin的转录抑制因子Slug、Twist的表达,促进下游E-cadherin的表达,抑制甲状腺癌FTC133、8305C、BCPAP和K1细胞的EMT途径,阻碍癌细胞的迁移与侵袭[27]。此外,SFN与吉非替尼联合使用抑制PI3K/AKT信号通路,抑制癌细胞的EMT途径,从而克服肺癌细胞PC9/AB11的耐药性[26]。基质金属蛋白酶(MMP)能够水解细胞外基质(ECM),破坏细胞表面的组织学屏障,对于肿瘤细胞的迁移与侵袭起到关键性作用,因此靶向MMP-2、MMP-9能抑制肿瘤迁移与侵袭。有研究表明SFN通过激活ERK1/2信号通路降低MMP-2的活性,并上调糖蛋白粘附分子CD44v6,抑制肝母细胞瘤U87MG和U373MG细胞伪足的形成,抑制肿瘤细胞的迁移和侵袭[33−34]。HDA5C在乳腺癌中异常高表达,研究发现SFN通过下调HDA5C的表达,可以抑制Vimentin、MMP-9的表达,上调E-cadherin的表达,从而抑制乳腺癌细胞4T1的迁移和侵袭[35]。
miRNA是一种非编码RNA,其异常表达参与癌症的发生和发展。miR-29a-3p被发现在不同的癌症中抑制癌细胞的增殖、转移和凋亡,具有抑制癌症发展的作用。研究发现SFN上调胃癌GC细胞中miR-29a-3p的表达使Wnt/β-catenin信号通路失活,其中,ECM能够激活Wnt/β-catenin信号通路,加速癌细胞的转移,而SFN促进miR-29a-3p的成熟,后者协助SFN破坏ECM的结构,抑制Wnt/β-catenin信号通路[36]。
人乳腺癌细胞MCF-7中含有早幼粒细胞白血病蛋白(PML),促使MCF-7细胞增殖、迁移以及生长,SFN改变PML蛋白中半胱氨酸残基,使其结构发生改变,失去功能,降低致癌特性[37]。蛋白精氨酸转移酶5(PRMT5)与甲基小体蛋白50(MEP50)表达量升高与间皮瘤细胞的迁移与侵袭有关,SFN通过降低PRMT5和MEP50的表达,抑制间皮瘤转移[38]。SFX-01(SFN中添加α-环糊精复合物配制而成的稳定试剂),能够抑制异种移植肿瘤的生长,并且抑制乳腺癌肿瘤起始干细胞的转移,对于患者可能具有更好的治疗效果[39]。创伤愈合实验和Transwell实验表明SFN能够抑制肝癌HepG2细胞的迁移与侵袭[25]。
上述研究结果显示,SFN通过抑制相关信号通路、靶向EMT途径标志物,抑制EMT途径,以及促进相关miRNA的表达,抑制癌细胞的迁移和侵袭。
3. 萝卜硫素促进癌细胞凋亡抑制癌症
对于正常细胞而言,细胞凋亡是受基因调控的为了维持内环境稳定的自主有序的死亡。而对于癌细胞而言,凋亡受到抑制,癌细胞的无限增殖是癌症发展的基础。因此,诱导癌细胞凋亡在抗肿瘤方面发挥至关重要的作用。
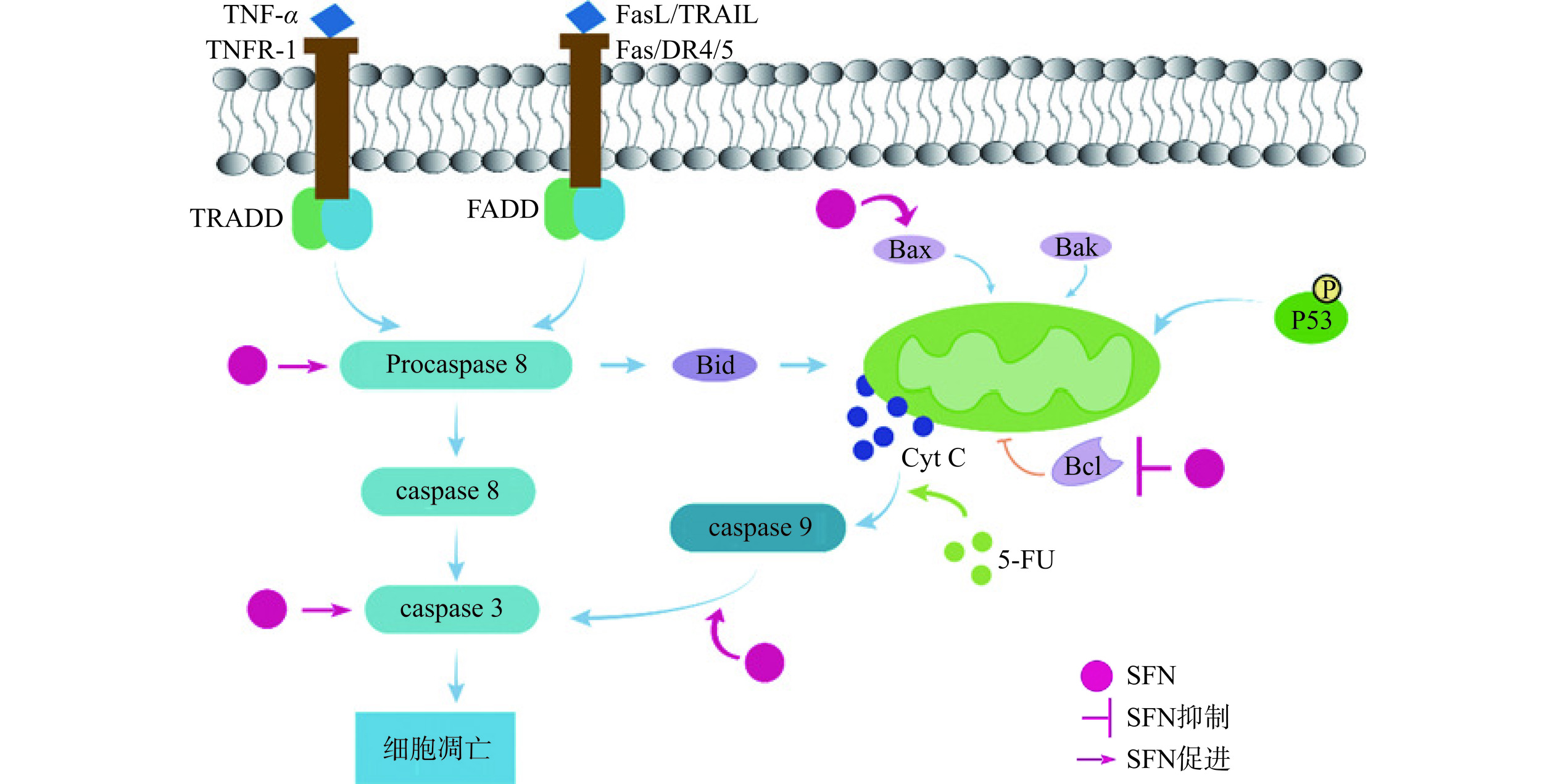
细胞凋亡主要可以分为外源凋亡途径(又称死亡受体通路)和内源凋亡途径。其中外源凋亡途径是由凋亡信号分子和细胞表面受体结合启动的,经过一系列途径,活化半胱氨酸依赖性天冬氨酸蛋白酶(caspase),最终诱导细胞凋亡。内源凋亡途径是由线粒体中的细胞色素C(CytC)介导的通路,进而激活caspase家族,导致细胞凋亡[40−41]。
SFN通过介导内源凋亡途径,使癌细胞凋亡。内源性凋亡途径中CytC的释放受Bcl-2蛋白家族影响[42],SFN通过激活P53的表达,诱导Bcl-2蛋白家族中促凋亡蛋白Bax的表达,促使胃癌BGC-823和MGC-803细胞发生内源性细胞凋亡[18]。研究发现SFN可以诱导肝癌HepG2细胞中Bax/Bcl比值的上升,促进肝癌HepG2细胞的内源凋亡途径[25]。此外,SFN可以加速食管鳞状细胞癌(ESCC)细胞中caspase 9的裂解促进癌细胞凋亡,说明SFN可以提高caspase依赖的内源凋亡途径[43]。5-FU是有效治疗结肠癌的化疗药物,但是癌细胞会产生耐药性,SFN通过上调Bax的合成,增强了5-FU对结肠癌SW-480细胞的诱导凋亡能力[44](图3)。
有些癌症的治疗可以通过细胞凋亡的内源和外源途径双重作用来抑制其发展。SFN类似物与5-FU联合使用诱导结肠癌HT29细胞的凋亡,其中,SFN类似物可以激活caspase 8介导的外源凋亡途径,5-FU可以激活caspase 9介导的线粒体内源凋亡途径,因此联合治疗效果更好[17]。PARP(与DNA修复相关的酶)能够被caspase 3水解,促进DNA降解,SFN可以促进肝母细胞瘤(HB)中caspase 3的表达升高,PARP蛋白的裂解,加速肝母细胞瘤的外源凋亡途径;SFN还能促进Bax的表达,抑制抗凋亡蛋白Bcl-2的表达,促进HB的内源凋亡途径[34]。
由此可见,SFN既能上调caspase介导的外源凋亡途径,也能促进线粒体介导的内源凋亡途径,还能与其他药物联合,共同发挥诱导肿瘤细胞的凋亡的作用。
4. 萝卜硫素抑制炎症的发展抑制癌症
持续的炎症可能会导致正常细胞向恶性细胞转化,增加肿瘤发生的可能性[45];而一些肿瘤的发展也会过度表达IL-1β、iNOS、TNF-α等促炎症因子,激活相关炎症通路如NF-κB信号通路等,进而加剧恶性肿瘤的发展[46]。因此,抑制炎症的发生也格外重要。
4.1 萝卜硫素调控NF-κB炎症通路抑制癌症及其并发症
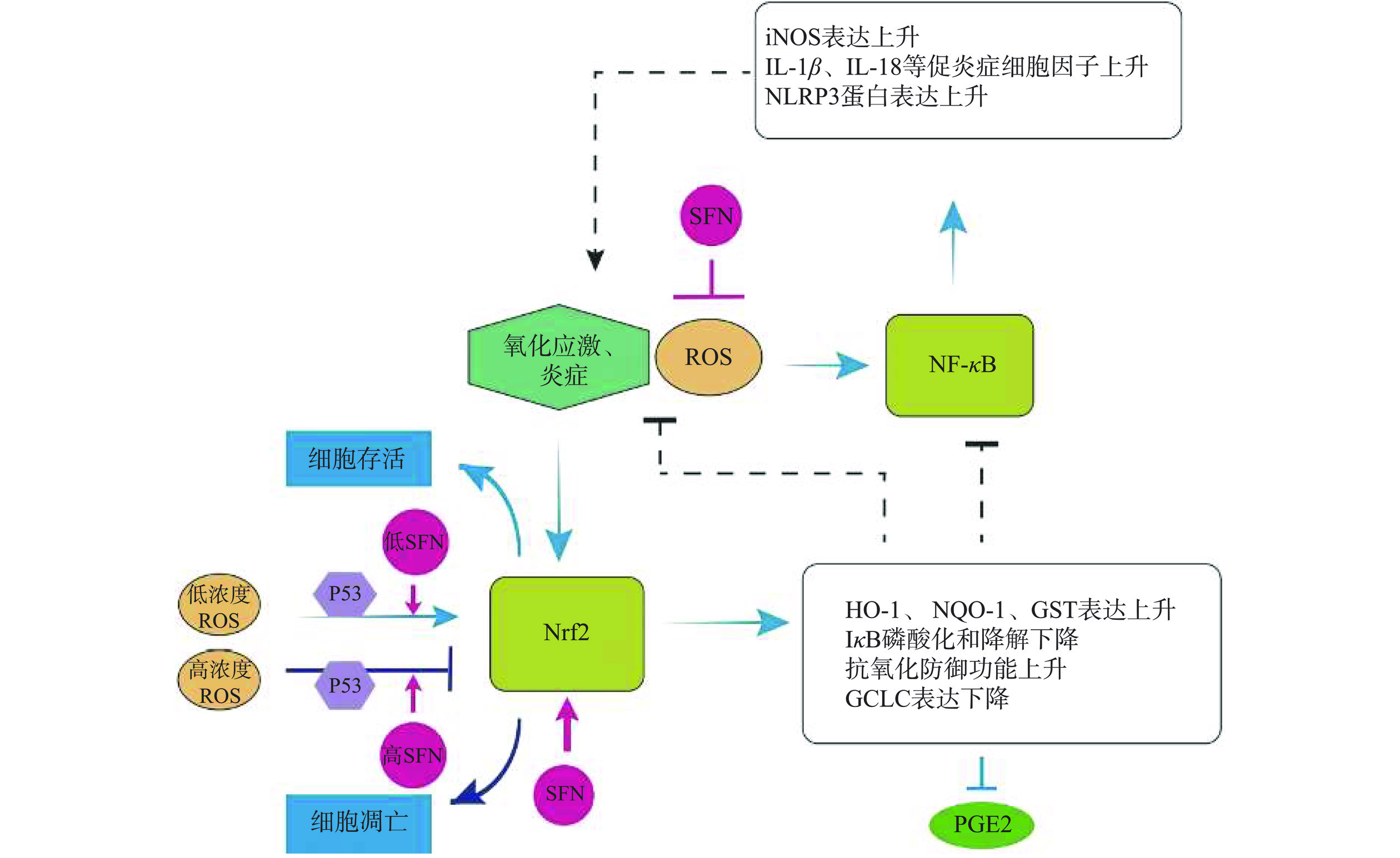
NF-κB是经典的炎症信号通路,当机体受到如促炎症介质因子(TNF-α)、脂多糖(LPS)、活性氧(ROS)或其他物理化学因素等诱导后,与细胞膜表面TNF-R受体相结合,将信号向下游传递,导致NF-κB蛋白三聚体复合物中的IκB蛋白磷酸化并从中解离,NF-κB暴露出核定位信号序列,从细胞质转移到细胞核内,启动下游炎症相关基因的表达,引发许多慢性炎症的发展,这可能会导致癌症的发生[45]。SFN可以抑制镉诱导的人正常肺上皮BEAS-2R细胞产生ROS,而抑制NF-κB信号通路的发生,抑制炎性微环境的发生,最终抑制BEAS-2R细胞向恶性细胞的转化,抑制肺癌的发生[47]。NLRP3炎症小体是NF-κB信号通路的靶蛋白,可以激活促炎症细胞因子IL-1β、IL-18等的表达,从而诱导炎症反应[48]。SFN抑制NF-κB的核转位,抑制其下游靶基因的表达,如IL-1β、iNOS、TNF-α等促炎因子,对于抑制癌症的发生与发展有着非常重要的作用(图4)。
4.2 萝卜硫素调控炎症细胞因子抑制癌症
近些年,抑制细胞因子(TNF、IL-1β和IL-6)来抑制炎症和癌症的发展也被广泛研究,炎症细胞因子过表达与肿瘤生长、转移、血管生成相关[49],因此,药物靶向炎症细胞因子能够缓解肿瘤的发展[50]。肠道炎症与巨噬细胞的表型有关,M1型巨噬细胞可以使IL-1β、iNOS表达,释放促炎症因子,加剧炎症反应,M2型可有效的消除炎症[51],而SFN可以促进IL-10的表达,进而使STAT3磷酸化,激活STAT3信号通路,使巨噬细胞从M1表型转换为M2表型,对肠道炎症起到保护作用,防止其发生恶性病变[52]。癌性骨痛(CIBP)会使免疫细胞产生IL-6、TNF-α等促炎症因子,加剧疼痛和肿瘤的发展[53]。SFN不仅可以通过上调Nrf2信号通路,抑制促炎症因子的表达,减缓CIBP外,SFN还能上调MOR的表达,增强吗啡对CIBP的缓解作用[54]。癌症导致的代谢紊乱与炎症密不可分,癌症患者的IL-6高表达会加速癌症的进程[55]。SFN会降低肝癌HepG2细胞中IL-6的表达量,同样抑制癌症的发展[56]。IL-8高表达会促进癌细胞的转移[57],TNF-α会增加癌细胞的增殖、抑制凋亡、促进血管的生成以及促进迁移和侵袭等特性[58]。SFN可以抑制胃癌细胞SCG7901中的IL-6、IL-8、TNF-α的合成,并且抑制相关的炎症信号通路,抑制胃癌的发展[59]。
5. 萝卜硫素调节抗氧化途径抑制癌症
Keap1-Nrf2信号通路是比较经典的抗氧化通路。正常状态下,Keap1与Nrf2相互作用,使得Nrf2被泛素化降解;在炎症或者氧化应激的状态下,Keap1与Nrf2解偶联,导致Nrf2转移至细胞核,启动下游抗氧化酶基因的表达,使细胞免受伤害。有研究表明,增强Nrf2能够预防癌症和其他疾病,因此,调控Keap1-Nrf2信号通路抑制抗氧化过程,最终达到抗肿瘤的目的[60]。p53对于Nrf2信号通路的调控是双相的,当存在较低浓度的ROS时,p53促进对Nrf2信号通路的调控,上调抗氧化基因的表达,清除ROS;而当细胞持续处于氧化应激时,高浓度p53抑制Nrf2抗氧化促细胞存活途径,促进细胞凋亡[61]。因而,对于含有p53的结肠癌HCT116细胞,SFN起到了双相生长的作用[62],即低浓度(≤5 μmol/L)促进细胞增殖,高浓度(≥10 μmol/L)抑制细胞增殖,这是由于低浓度的SFN可以介导Nrf2信号通路中抗氧化酶的表达以及抗凋亡蛋白的表达,而高浓度的SFN会促进凋亡相关基因的表达促使细胞凋亡,并且在含有p53基因的HCT116细胞中效果显著。SFN可以使BEAS-2BR的恶性转化细胞恢复自噬,抑制组成型Nrf2的活性,下调抗凋亡蛋白的表达,抑制凋亡抗性,抑制肿瘤的发生[47]。研究发现SFN与阿霉素联合使用,SFN会降低阿霉素治疗骨髓间充质干细胞(MDSCs)产生的耐药性[63],对抑制乳腺癌有更强的作用效果。SFN抑制MDSCs的作用机制是通过激活Nrf2途径,促进下游血红素氧合酶(HO-1)、谷氨酰半胱氨酸连接酶(GCLC)等酶的表达,进而抑制COX-2的表达,抑制其下游产物PGE2的表达,从而抑制MDSCs的积累[64]。细胞内发生氧化应激会积累大量的ROS,影响线粒体的电子传递,继而引发细胞凋亡、坏死[65]。Nrf2可以调节ROS的数量而调节细胞内的氧化应激反应[66],最终能够抑制癌症的发展。研究显示,日常摄食蔬菜获得的SFN含量一般≤2 μmol/L,且<2 μmol/L的SFN可以通过降低胃黏膜MNP01细胞中的ROS而消除细胞的氧化应激反应,其机理同样是激活Nrf2途径提高抗氧化酶的表达,发挥细胞保护活性预防癌症的发生[67]。SFN能够激活Keap1-Nrf2信号通路,激活下游靶基因包括HO-1、NQO-1、GSTs等抗氧化基因,降低癌细胞中ROS的含量以及降低氧化应激和炎症反应,抑制相关癌症的进程。
6. 其他机制
MicroRNA是一组约22个核苷酸构成的短链非编码RNA,其作用是通过与目标mRNA结合并使其降解,达到基因沉默的目的[68]。有研究发现,miRNA作为肿瘤的抑制基因或者癌基因,其调控异常导致肿瘤的发生,因此,靶向miRNA可以作为肿瘤治疗的新策略[69]。在胃癌SGC7901细胞中,SFN上调miR-4521的表达,后者靶向PIK3R3抑制胃癌细胞自噬,从而抑制胃癌的发展[70]。Georgikou等[71]制备的SFN衍生物,能够调控NF-κB信号通路,并上调miR27b-5p和miR29b-1-5p表达,对多种癌细胞的生长具有明显的抑制作用,且几乎无副作用。有研究发现,将厚朴酚和SFN制成杂化物CT1-3,其抗癌特性比二者单独给药的效果要好,且几乎没有毒副作用,CT1-3既能抑制抗凋亡蛋白Bcl-2的表达、促进促凋亡蛋白Bax的表达,促进线粒体介导的细胞凋亡,又能抑制癌细胞的迁移与侵袭[72]。Gasparello 等[73]发现靶向miR-15b-5p的肽-核酸能够抑制结肠癌HT29细胞侵袭而促进细胞凋亡,SFN联合肽-核酸使用促进凋亡的效果更强。
他莫昔芬和氟维司群是治疗晚期雌激素受体阳性(ER+)乳腺癌的药物,但是副作用也较为明显,会使STAT3发生磷酸化,磷酸化的STAT3会使ALDH+乳腺癌肿瘤起始干细胞的数目和致癌性均增加,而SFX-01可以靶向STAT3,抑制其磷酸化,SFX-01与抗雌激素药物联合使用对晚期ER+细胞有较好的治疗效果[39]。SFN和帕拉替尼联合使用可以通过介导AMPK通路诱导SCG-7901胃癌细胞凋亡[19]。肺癌PC9/AB11细胞对吉非替尼具有耐药性,有研究表明,SFN和二氢咖啡酸(1:1)联合使用对结肠癌HT29细胞具有较强的毒性作用,其机制为SFN和二氢咖啡酸能够促进细胞内ROS的产生,进而激活细胞内一系列信号通路,上调P21的表达,下调cyclin D1,阻断细胞周期,且ROS大量产生会导致细胞色素C的释放,引发内源凋亡途径[74]。研究表明,将SFN和顺铂包裹在纳米粒子中,建立SFN-CDPP-NPs体系能有效的抑制乳腺癌的进程,其作用机理是能降低乳腺癌细胞中GSH的含量,诱导DNA损伤,且能够高效靶向肿瘤部位,提高抗癌特性[75]。
高浓度的SFN可以导致急性髓系白血病细胞U-937发生铁下垂,包括谷胱甘肽(GSH)的含量下降,谷胱甘肽过氧化物酶4(GPX4)表达量降低以及脂质过氧化等特征,诱导AML细胞发生非caspase依赖性死亡[76],SFN能够抑制急性髓系白血病细胞U937的生长,与视黄酸联合使用能够增强U937细胞产生ROS,诱导细胞凋亡[77]。TAp63α是P53家族的转录因子,TAp63α能够促进结直肠癌干细胞的生成,其下游靶基因是Lgr5,Lgr5可以促进β-catinen的积累,SFN可以靶向这一系列途径,抑制结直肠癌干细胞的合成[6]。研究发现,SFN还能够抑制三阴性乳腺癌(TNBC)的发展,其原因可能是SFN靶向抑制乳腺肿瘤起始干细胞的形成来抑制乳腺癌的进程[77]。对于表皮鳞状细胞癌,2型谷氨转氨酶(TG2)的封闭构象是维持癌细胞具有侵袭特性的重要酶,SFN的直接作用靶点TG2,SFN可以共价结合TG2,使其保持开放构象而抑制TG2的活性[78]。HIF-1α被认为在低氧条件下可以激活糖酵解途径,SFN可以降低HIF1-α的含量并阻断其靶向作用而抑制低氧条件下膀胱癌细胞系的糖酵解途径,抑制癌细胞的增殖[79]。
7. 结论与展望
由此可见,SFN能够抑制细胞周期,抑制癌细胞的迁移和侵袭,通过内源凋亡途径和外源凋亡途径诱导癌细胞的凋亡,通过调控NF-κB信号通路和Nrf2信号通路抑制炎症来抑制癌症的发生。此外,研究表明,SFN及其制剂与某些药物联合使用能够有效抑制癌细胞的数量和致癌性。因此,开发SFN作为抗癌药物具有非常广阔的前景。
目前正在开发和研究提高花椰菜中SFN的含量以及制备良好的SFN运输载体,提高SFN的应用效率。Amer等[80]发现用一定浓度的茉莉酮酸甲酯(MeJA)和水杨酸(SA)作为外源诱导因子处理花椰菜后,产生的SFN含量更高,并用SFN提取物处理MDA-MB-231乳腺癌细胞系发现,SFN可以更好地提升促凋亡基因表达,诱导MDA-MB-231细胞凋亡,这对于未来使用纯化的SFN或SFN制剂进行体外实验提供了更好的思路。Gu等[81]制备一种纳米载体,将疏水的SFN包封在其中,并且利用透明质酸对乳腺肿瘤干细胞表面标记物CD44+的特异性,对乳腺癌干细胞具有靶向效应。对于SFN靶向癌细胞的体内体外实验及未来SFN临床应用的高效性提供了更具实用意义的研究方向。
虽然SFN在抗癌方面表现出了极大的优势,但是就目前的研究而言,仍然存在许多不足。比如SFN在体内的稳定性可能会影响其治疗效果,目前关于SFN在体内的药代动力学鲜有人探究。并且高浓度的SFN对正常细胞有所损伤,如何避免SFN破坏正常细胞,高效针对癌细胞靶部位也是亟待解决的问题。SFN与多种抗癌药物联合治疗能够展现出协同效应,且比单独给药效果更好,而且SFN能够克服某些癌细胞的耐药性的特点,然而有些联合治疗的机制还需要进一步研究。下一步关于SFN的给药体系,以最佳的给药剂量、最小的毒副作用进行临床治疗是亟待解决的问题。
-
表 1 SFN的抗癌作用及其机制
Table 1 Anti-cancer effects and mechanisms of SFN
作用机制 癌症类型 评价模型 作用途径 参考文献 抑制癌细胞周期 人星形细胞瘤 1321N1 上调P21蛋白表达,G2/M期阻滞 [28] 甲状腺癌 FTC133、8305C、BCPAP、K1 上调P21蛋白表达,抑制Cdk1、Cdk2、CyclinB1和Cdc25C表达 [27] 急性白血病 上调P21蛋白表达,抑制CDC2和CDC2/cyclin B表达 [29] 乳腺癌 MCF-7、MDA-MB-231 降低RB磷酸化 [31] 抑制癌细胞迁移与侵袭 甲状腺癌 FTC133、8305C、BCPAP、K1 抑制PI3K/Akt信号通路,促进E-cadherin的表达 [27] 肝母细胞瘤 U87MG、U373MG 激活ERK1/2信号通路,降低MMP-2表达 [33] 乳腺癌 4T1 下调HDA5C表达,抑制Vimentin、MMP-9的表达 [35] 乳腺癌 MCF-7 改变PML结构,抑制癌细胞转移 [37] 促进癌细胞凋亡 胃癌 BGC-823、MGC-803 激活P53蛋白,上调Bax表达 [18] 肝癌 HepG2 Bax/Bcl比值上升 [25] 结肠癌 SW-480 上调Bax合成 [44] 结肠癌 HT29 激活caspase 8介导的外源凋亡途径 [17] 抑制炎症发展 肝癌 HepG2 降低IL-6的表达 [56] 胃癌 SCG7901 抑制IL-6、IL-8、TNF-α [59] 调节抗氧化 结肠癌 HCT116 介导Nrf2信号通路中抗氧化酶表达以及抗凋亡蛋白表达 [62] 乳腺癌 骨髓间充质干细胞 激活Nrf2途径,促进HO-1,抑制COX-2 [64] -
[1] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020:globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA-A: A Cancer Journal for Clinicians,2021,71(3):209−249.
[2] XIA C F, DONG X S, LI H, et al. Cancer statistics in China and United States, 2022:profiles, trends, and determinants[J]. Chinese Medical Journal,2022,135(5):584−590.
[3] ZHANG Y, TAN L X, LI C, et al. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by dss[J]. AMB Express,2020,10(1):119−127.
[4] IAHTISHAM-UL-HAQ, KHAN S, AWAN K A, et al. Sulforaphane as a potential remedy against cancer:Comprehensive mechanistic review[J]. Journal of Food Biochemistry,2021,46(3):e13886.
[5] AUNE D, GIOVANNUCCI E, BOFFETTA P, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies[J]. International Journal of Epidemiology,2017,46(3):1029−1056.
[6] CHENG L, WAN K, LIANG H, et al. Sulforaphane and sulforaphane[A]. Glucosinolates:Properties, Recovery, and Applications, 2020, 281−232.
[7] SINHA S, SHARMA S, SHARMA A, et al. Sulforaphane-cisplatin combination inhibits the stemness and metastatic potential of tnbcs via down regulation of sirtuins-mediated emt signaling axis[J]. Phytomedicine,2021,84:153492.
[8] VANDUCHOVA A, ANZENBACHER P, ANZENBACHEROVA E. Isothiocyanate from broccoli, sulforaphane, and its properties[J]. Journal of Medicinal Food,2018,22(2):121−126.
[9] DINKOVA-KOSTOVA A T, FAHEY J W, KOSTOV R V, et al. KEAP1 and Done? targeting the NRF2 pathway with sulforaphane[J]. Trends in Food Science & Technology, 2017, 69(Pt B):257−269.
[10] JAMES D, DEVARAJ S, BELLUR P, et al. Novel concepts of broccoli sulforaphanes and disease:induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli[J]. Nutrition Reviews,2012,70(11):654−665.
[11] MAHN A, CASTILLO A. Potential of sulforaphane as a natural immune system enhancer:A review[J]. Molecules,2021,26(3):752−783.
[12] SE-RAN J, CHEEMA A, BOSE C, et al. Multi-omic analysis reveals the anti-aging impact of sulforaphane on the microbiome and metabolome[J]. ResearchGate, 2020.
[13] LI Z S, LIU Y M, FANG Z Y, et al. Natural sulforaphane from broccoli seeds against influenza a virus replication in mdck cells[J]. Natural Product Communications, 2019, 14(6):1934578X1985822.
[14] AXELSSON A S, TUBBS E, MECHAM B, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes[J]. Science Translational Medicine,2017,9(394):eaah4477.
[15] JAYAKUMAR T, CHEN W F, LU W J, et al. A novel antithrombotic effect of sulforaphane via activation of platelet adenylate cyclase:ex vivo and in vivo studies[J]. The Journal of Nutritional Biochemistry,2013,24(6):1086−1095.
[16] JAKUBIKOVA J, CERVI D, OOI M, et al. Anti-tumor activity and signaling events triggered by the isothiocyanates, sulforaphane and phenethyl isothiocyanate, in multiple myeloma[J]. Haematologica,2011,96(8):1170−1179.
[17] MILCZAREK M, POGORZELSKA A, WIKTORSKA K. Synergistic interaction between 5-fu and an analog of sulforaphane-2-oxohexyl isothiocyanate-in an in vitro colon cancer model[J]. Molecules,2021,26(10):3019−3032. doi: 10.3390/molecules26103019
[18] WANG Y, WU H Z, DONG N N, et al. Sulforaphane induces s-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells[J]. Scientific Reports,2021,11(1):2504. doi: 10.1038/s41598-021-81815-2
[19] YI H X, LI Z M, LIU X X, et al. Therapeutic mechanism of lapatinib combined with sulforaphane on gastric cancer[J]. Evidence-Based Complementary and Alternative Medicine,2021,eCAM:9933274.
[20] KAN S F, WANG J, SUN G X. Sulforaphane regulates apoptosis-and proliferation related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer[J]. International Journal of Molecular Medicine,2018,42(5):2447−2458.
[21] JAMASBI E, HAMELIAN M, HOSSAIN M A, et al. The cell cycle, cancer development and therapy[J]. Mol Biol Rep. 2022, 49(11):10875−10883.
[22] BLOOM J, CROSS F R. Multiple levels of cyclin specificity in cell-cycle control[J]. Molecular Cell Biology,2007,8(2):149−160.
[23] MATTHEWS H K, BERTOLI C, DE BRUIN RAM. Cell cycle control in cancer[J]. Nature Reviews Molecular Cell Biology,2021,23(1):74−88.
[24] LAPENNA S, GIORDANO A. Cell cycle kinases as therapeutic targets for cancer[J]. Nature Reviews Drug Discovery,2009,8(7):547−566.
[25] HUANG B, LEI S X, WANG D, et al. Sulforaphane exerts anticancer effects on human liver cancer cells via induction of apoptosis and inhibition of migration and invasion by targeting MAPK7 signalling pathway[J]. Journal of BUON,2020,25(2):959−964.
[26] MENG W, MENG J, ZHANG F, et al. Sulforaphane overcomes t790m-mediated gefitinib resistance in vitro through epithelial-mesenchymal transition[J]. Journal of Physiology and Pharmacology,2021,72(5):741−749.
[27] WANG L P, TIAN Z F, YANG Q, et al. Sulforaphane inhibits thyroid cancer cell growth and invasiveness through the reactive oxygen species-dependent pathway[J]. Oncotarget,2015,6(28):25917−25931. doi: 10.18632/oncotarget.4542
[28] TOMASELLO B, DOMENICA M, DI M, et al. Rapha myr, a blend of sulforaphane and myrosinase, exerts antitumor and anoikis- sensitizing effects on human astrocytoma cells modulating sirtuins and dna methylation[J]. International Journal of Molecular Sciences,2020,21(15):5328−5353. doi: 10.3390/ijms21155328
[29] 王凡平, 乔彩娟, 孙彦威, 等. 莱菔硫烷诱导急性髓系白血病KG1a和KG1细胞G2/M期阻滞的作用和相关机制[J]. 中国实验血液学杂志,2021,29(4):1050−1055. [WANG F P, QIAO C J, SUN Y W, et al. Effect and mechanism of sulforaphane on G2/ M phase arrest of acute Myeloid Leukemia KG1a and KG1 Cells[J]. Journal of Experimental Hematology,2021,29(4):1050−1055.] WANG F P, QIAO C J, SUN Y W, et al. Effect and mechanism of sulforaphane on G2/ M phase arrest of acute Myeloid Leukemia KG1a and KG1 Cells[J]. Journal of Experimental Hematology, 2021, 29(4): 1050−1055.
[30] SUSKI J M, BRAUN M, STRMISKA V, et al. Targeting cell-cycle machinery in cancer[J]. Cancer Cell,2021,39(6):759−778. doi: 10.1016/j.ccell.2021.03.010
[31] ROYSTON K J, PAUL B, NOZELL S, et al. Withaferin a and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms[J]. Experimental Cell Research,2018,368(1):67−74. doi: 10.1016/j.yexcr.2018.04.015
[32] DAVIS F M, STEWART T A, THOMPSON E W, et al. Targeting emt in cancer:opportunities for pharmacological intervention[J]. Trends in Pharmacological Sciences,2014,35(9):479−488. doi: 10.1016/j.tips.2014.06.006
[33] LI C L, YAN Z, PENG X H, et al. Sulforaphane inhibits invasion via activating erk1/2 signaling in human glioblastoma u87mg and u373mg cells[J]. PLoS One,2014,9(2):e90520. doi: 10.1371/journal.pone.0090520
[34] LIN J S, XU Y L, ZHAO X, et al. Anticancer activity of sulforaphane against human hepatoblastoma cells involves apoptosis, autophagy and inhibition ofβ-catenin signaling pathway[J]. Archives of Medical Science,2020(1):1−9.
[35] 谢金芳, 曹春雨, 任雪, 等. SFN对小鼠乳腺癌4T1细胞上皮-间质转化, 增殖和迁移的影响研究[J]. 中国癌症杂志,2021,31(7):605−615. [XIE J F, CAO C Y, REN X, et al. Effects of sulforaphane on epithelial-mesenchymal transition, proliferation and migration of mouse breast cancer 4T1 cells[J]. China Oncology,2021,31(7):605−615.] XIE J F, CAO C Y, REN X, et al. Effects of sulforaphane on epithelial-mesenchymal transition, proliferation and migration of mouse breast cancer 4T1 cells[J]. China Oncology, 2021, 31(7): 605−615.
[36] HAN S C, WANG Z, LIU J N, et al. Mir-29a-3p-dependent col3a1 and col5a1 expression reduction assists sulforaphane to inhibit gastric cancer progression[J]. Biochemical Pharmacology,2021,188:114539. doi: 10.1016/j.bcp.2021.114539
[37] ALHAZMI N, PAI C P, ALBAQAMI A, et al. The promyelocytic leukemia protein isoform pml1 is an oncoprotein and a direct target of the antioxidant sulforaphane (sfn)[J]. Biochimica ET Biophysica Acta-Molecular Cell Research,2020,1867(8):118707. doi: 10.1016/j.bbamcr.2020.118707
[38] EZEKA G, ADHIKARY G, KANDASAMY S, et al. Sulforaphane inhibits prmt5 and mep50 function to suppress the mesothelioma cancer cell phenotype[J]. Molecular Carcinogenesis,2021,60(7):429−439. doi: 10.1002/mc.23301
[39] SIMÕES B M, SANTIAGO-GÓMEZ A, CHIODO C, et al. Targeting stat3 signaling using stabilised sulforaphane (sfx-01) inhibits endocrine resistant stem-like cells in er-positive breast cancer[J]. Oncogene,2020,39(25):4896−4908. doi: 10.1038/s41388-020-1335-z
[40] VERMEULEN K, BOCKSTAELE D R, BERNEMAN Z N. Apoptosis:mechanisms and relevance in cancer[J]. Annals of Hematology,2005,84(10):627−639. doi: 10.1007/s00277-005-1065-x
[41] PENG F, LIAO M R, et al. Regulated cell death (rcd) in cancer:key pathways and targeted therapies[J]. Signal Transduction and Targeted Therapy,2022,7(1):286−352. doi: 10.1038/s41392-022-01110-y
[42] GROSS A, MCDONNELL J M, KORSMEYER S J. Bcl-2 family members and the mitochondria in apoptosis[J]. Genes & Development,1999,13(15):1899−1911.
[43] LU Z M, REN Y D, YANG L, et al. Inhibiting autophagy enhances sulforaphane-induced apoptosis via targeting Nrf2 in esophageal squamous cell carcinoma[J]. Acta Pharmaceutica Sinica,2020,11(5):1246−1260.
[44] 顾文燕, 李丽, 吴敏. SFN通过调节Bax表达降低结肠癌细胞株5-氟尿嘧啶的耐药[J]. 中国现代中药,2019,21(4):458−463. [GU W Y, LI L, WU M. Effect of sulforaphane on decrease of drug resistance of colon cancer cells to 5-fu via regulating bax expression[J]. Modern Chinese Medicine,2019,21(4):458−463.] GU W Y, LI L, WU M. Effect of sulforaphane on decrease of drug resistance of colon cancer cells to 5-fu via regulating bax expression[J]. Modern Chinese Medicine, 2019, 21(4): 458−463.
[45] MOSS S F, BLASER M J. Mechanisms of disease:Inflammation and the origins of cancer[J]. Nature Clinical Practice Oncology,2005,2(2):90−97,113. doi: 10.1038/ncponc0081
[46] CANDIDO J, HAGEMANN T. Cancer-related inflammation[J]. Journal of Clinical Immunology,2013,33(1):79−84.
[47] SIM H, LEE W, CHOO S, et al. Sulforaphane alleviates particulate matter-induced oxidative stress in human retinal pigment epithelial cells[J]. Frontiers in Medicine,2021,8:685032. doi: 10.3389/fmed.2021.685032
[48] BRYAN N B, DORFLEUTNER A, ROJANASAKUL Y, et al. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain[J]. Journal of Immunology,2009,182(5):3173−3182. doi: 10.4049/jimmunol.0802367
[49] GALDIERO M R, MARONE G, MANTOVANI A. Cancer inflammation and cytokines[J]. Cold Spring Harbor Perspectives in Biology,2018,10(8):a028662. doi: 10.1101/cshperspect.a028662
[50] PROPPER D J, BALKWILL F R. Harnessing cytokines and chemokines for cancer therapy[J]. Nature Reviews Clinical Oncology,2022,19(4):237−253. doi: 10.1038/s41571-021-00588-9
[51] ZHU W, YU J B, NIE Y, et al. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases[J]. Immunological Investigations,2014,43(7):638−652. doi: 10.3109/08820139.2014.909456
[52] SUN Y Y, TANG J Q, LI C, et al. Sulforaphane attenuates dextran sodium sulphate induced intestinal inflammation via IL-10/stat3 signaling mediated macrophage phenotype switching[J]. Food Science and Human Wellness,2022,11(1):129−142. doi: 10.1016/j.fshw.2021.07.014
[53] LOZANO-ONDOUA A N, SYMONS-LIGUORI A M, VANDERAH T W. Cancer-induced bone pain:mechanisms and models[J]. Neuroscience Letters, 2013, 557(pt A):52−59.
[54] FU J, XU M, XU L S, et al. Sulforaphane alleviates hyperalgesia and enhances analgesic potency of morphine in rats with cancer-induced bone pain[J]. European Journal of Pharmacology,2021,909:174412. doi: 10.1016/j.ejphar.2021.174412
[55] WHITE J P. IL-6, cancer and cachexia:Metabolic dysfunction creates the perfect storm[J]. Translation Cancer Research,2017,6(S2):S280−S285. doi: 10.21037/tcr.2017.03.52
[56] AL-BAKHEIT A, ABU-QATOUSEH L. Sulforaphane from broccoli attenuates inflammatory hepcidin by reducing IL-6 secretion in human HepG2 cells[J]. Journal of Functional Foods,2020,75(2020):1−7.
[57] FREUND A, CHAUVEAU C, BROUILLET J P, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells[J]. Oncogene,2003,22(2):256−265. doi: 10.1038/sj.onc.1206113
[58] BUCK I, MORCEAU F, GRIGORAKAKI C, et al. Linking anemia to inflammation and cancer:The crucial role of TNF-α[J]. Biochemical Pharmacology,2008,77(10):1572−1579.
[59] 杨艳华, 梁丽琴. SFN对胃癌细胞生物学特征的影响及其机制研究[J]. 中国药师,2019,22(5):840−845. [YANG Y H, LIANG L Q. Effect of sulforaphane on biological activity of gastric cancer cells and underlying mechanisms[J]. Chinese Pharmacist,2019,22(5):840−845.] YANG Y H, LIANG L Q. Effect of sulforaphane on biological activity of gastric cancer cells and underlying mechanisms[J]. Chinese Pharmacist, 2019, 22(5): 840−845.
[60] SPORN M B, LIBY K T. Nrf2 and cancer:the good, the bad and the importance of context[J]. Nature Reviews Cancer,2012,12(8):564−571. doi: 10.1038/nrc3278
[61] CHEN W M, JIANG T, WANG H H, et al. Does Nrf2 contribute to p53-mediated control of cell survival and death?[J]. Antioxidants & Redox Signaling,2012,17(12):1670−1675.
[62] GWON Y, OH J, KIM J S. Sulforaphane induces colorectal cancer cell proliferation through nrf2 activation in a p53-dependent manner[J]. Applied Biological Chemistry,2020,63(1):86−96. doi: 10.1186/s13765-020-00578-y
[63] RONG Y, YUAN C H, QU Z, et al. Doxorubicin resistant cancer cells activate myeloid-derived suppressor cells by releasing pge2[J]. Scientific Reports,2016,6(1):23824. doi: 10.1038/srep23824
[64] RONG Y, HUANG L X, YI K Z, et al. Co-administration of sulforaphane and doxorubicin attenuates breast cancer growth by preventing the accumulation of myeloid-derived suppressor cells-sciencedirect[J]. Cancer Letter,2020,493:189−196. doi: 10.1016/j.canlet.2020.08.041
[65] AHMED S M, LUO L, NAMANI A, et al. Nrf2 signaling pathway:pivotal roles in inflammation[J]. Biochimica et Biophysica Acta-Molecular Basis of Disease,2017,1863(2):585−597. doi: 10.1016/j.bbadis.2016.11.005
[66] MPAB C, ECLB C, IA B, et al. Dietary supplementation with sulforaphane ameliorates skin aging through activation of the Keap1-Nrf2 pathway[J]. The Journal of Nutritional Biochemistry,2021,98:108817. doi: 10.1016/j.jnutbio.2021.108817
[67] SANTOS P, MACHADO A, GRANDIS R D, et al. Effects of sulforaphane on the oxidative response, apoptosis, and the transcriptional profile of human stomach mucosa cells in vitro[J]. Genetic Toxicology and Environmental Mutagenesis,2020,854:503201.
[68] LIN S B, GREGORY R I. MicroRNA biogenesis pathways in cancer[J]. Nature reviews Cancer,2015,15(6):321−333. doi: 10.1038/nrc3932
[69] RUPAIMOOLE R, SLACK F J. MicroRNA therapeutics:towards a new era for the management of cancer and other diseases[J]. Nature Reviews Drug Discovery,2017,16(3):203−222. doi: 10.1038/nrd.2016.246
[70] PENG Z T, GU P. Sulforaphane suppresses autophagy during the malignant progression of gastric carcinoma via activating miR-4521/PIK3R3 pathway[J]. Human & Experimental Toxicology,2021,40(12):S711−S720.
[71] GEORGIKOU C, BUGLIONI L, BREMERICH M, et al. Novel broccoli sulforaphane-based analogues inhibit the progression of pancreatic cancer without side effects[J]. Biomolecules,2020,10(5):769−785. doi: 10.3390/biom10050769
[72] CHENG T, CHEN J, HUANG X F, et al. CT1-3, a novel magnolol-sulforaphane hybrid suppresses tumorigenesis through inducing mitochondria-mediated apoptosis and inhibiting epithelial mesenchymal transition[J]. European Journal of Medicinal Chemistry,2020,199:112441. doi: 10.1016/j.ejmech.2020.112441
[73] GASPARELLO J, GAMBARI L, PAPI C, et al. High levels of apoptosis are induced in the human colon cancer ht-29 cell line by co-administration of sulforaphane and a peptide nucleic acid targeting mir-15b-5p[J]. Nucleic Acid Therapeutics,2020,30(3):164−174. doi: 10.1089/nat.2019.0825
[74] SANTANA-GÁLVEZ J, VILLELA-CASTREJÓN J, SERNA-SALDÍVAR S O, et al. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells[J]. International Journal of Molecular Sciences,2020,21(9):3108−3128. doi: 10.3390/ijms21093108
[75] XU Y, HAN X Y, LI Y Y, et al. Sulforaphane mediates glutathione depletion via polymeric nanoparticles to restore cisplatin chemosensitivity[J]. ACS nano,2019,13(11):13445−13455. doi: 10.1021/acsnano.9b07032
[76] GRECO G, SCHNEKENBURGER M, CATANZARO E, et al. Discovery of Sulforaphane as an inducer of ferroptosis in U-937 leukemia cells:expanding its anticancer potential[J]. Cancers (Basel),2021,14(1):76−92. doi: 10.3390/cancers14010076
[77] AKIYOSHI S, KIKUCHI H, KURIBAYASHI F, et al. Sulforaphane displays the growth inhibition, cytotoxicity and enhancement of retinoic acid-induced superoxide-generating activity in human monoblastic U937 cells[J]. Fundamental Toxicological Sciences,2019,6(8):319−325. doi: 10.2131/fts.6.319
[78] RORKE E A, ADHIKARY G, SZMACINSKI H, et al. Sulforaphane covalently interacts with the transglutaminase 2 cancer maintenance protein to alter its structure and suppress its activity[J]. Molecular Carcinogenesis,2021,61(1):19−32.
[79] XIA Y, KANG T W, JUNG D Y, et al. Sulforaphane inhibits nonmuscle invasive bladder cancer cells proliferation through suppression of hif-1α-mediated glycolysis in hypoxia[J]. Journal of Agricultural and Food Chemistry,2019,67(28):7844−7854. doi: 10.1021/acs.jafc.9b03027
[80] AMER M A, MOHAMED T R, RAHMAN R A A, et al. Studies on exogenous elicitors promotion of sulforaphane content in broccoli sprouts and its effect on the MDA-MB-231 breast cancer cell line[J]. Annals of Agricultural Sciences,2021,66(1):46−52. doi: 10.1016/j.aoas.2021.02.001
[81] GU H F, REN F Z, MAO X Y, et al. Mineralized and GSH-responsive hyaluronic acid based nano-carriers for potentiating repressive effects of sulforaphane on breast cancer stem cells-like properties[J]. Carbohydrate Polymers,2021,269:118294−118305. doi: 10.1016/j.carbpol.2021.118294
-
期刊类型引用(0)
其他类型引用(2)





 下载:
下载:
 下载:
下载:



