Comparative on the Hypoglycemic and Lipid-lowering Activities in Vitro of Crude Polysaccharides from Fermented Bean Flour by Two Types of Bacteria
-
摘要: 枯草芽孢杆菌和干酪乳杆菌常用于发酵豆制品的生产。为研究这两种细菌发酵对豆粉粗多糖降血糖、降血脂生物活性的影响。本研究制备了8种不同的两种细菌发酵豆粉的粗多糖样品,比较分析了发酵前后豆粉粗多糖的组成、水溶能力和体外降血糖降血脂活性变化差异。结果表明:两种细菌发酵豆粉的粗多糖中糖醛酸含量均增加,且枯草芽孢杆菌发酵豆粉的粗多糖糖醛酸含量(4.05%~22.46%)显著(P<0.05)高于干酪乳杆菌发酵豆粉的粗多糖(2.63%~10.72%)。两种细菌发酵可以改善豆粉粗多糖的水溶性。两种细菌发酵均能增加豆粉粗多糖体外降血糖和降血脂的生物活性。干酪乳杆菌发酵大豆、豇豆和蚕豆豆粉的粗多糖对α-淀粉酶抑制能力显著(P<0.05)高于枯草芽孢杆菌发酵。枯草芽孢杆菌发酵豇豆、红豆、绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖结合甘氨脱氧胆酸钠能力高于干酪乳杆菌发酵。两种细菌发酵绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖对牛磺脱氧胆酸钠的结合能力均显著提高(P<0.05)。本研究为开发具有降血糖降血脂功能的富含活性多糖的发酵豆类食品提供了理论基础。Abstract: Bacillus subtilis and Lactobacillus casei were commonly used in the production of fermented bean products. The effects of fermentation by two types of bacteria on the hypoglycemic and lipid-lowering activities of crude polysaccharides from bean flour were analyzed. In this study, 8 kinds of crude polysaccharide samples from fermented edible beans by the two types of bacteria were prepared. And the composition, water solubility, in vitro hypoglycemic and lipid-lowering activity of crude polysaccharides from before and after bean flour fermentation by the two types of bacteria were compared and analyzed. The results showed that the content of uronic acid in the crude polysaccharide from fermented bean flour increased. The content of uronic acid in the crude polysaccharides from fermented bean flour by Bacillus subtilis (4.05%~22.46%) was significantly higher than that of the crude polysaccharides from fermented bean flour by Lactobacillus casei (2.63%~10.72%) (P<0.05). The water solubility of crude polysaccharides from fermented bean flour by the two types of bacteria was improved. The hypoglycemic and lipid-lowering activities in vitro of crude polysaccharides were increased through the fermentation process by Bacillus subtilis and Lactobacillus casei. The α-amylase inhibition rates of crude polysaccharides from soybean, cowpea and broad bean by Lactobacillus casei fermentation were significantly higher than that of crude polysaccharides by Bacillus subtilis fermentation (P<0.05). The binding capacities of sodium glycodeoxycholate with the crude polysaccharides from cowpea, red bean, mung bean, broad bean, kidney bean, lentil and pea by Bacillus subtilis fermentation were higher than that of crude polysaccharides by Lactobacillus casei fermentation. The binding capacities of sodium taurocholate with the crude polysaccharides from mung bean, broad bean, kidney bean, lentil and pea by the two types of bacteria fermentation were significantly improved (P<0.05). This study provided a theoretical basis for the development of fermented bean foods riched in active polysaccharides with the functions of hypoglycemic and lipid-lowering activities.
-
Keywords:
- bean flour /
- fermentation /
- polysaccharides /
- hypoglycemic activity /
- lipid-lowering activity
-
传统饮食讲究“五谷宜为养,失豆则不良”[1]。食用豆类中除含有丰富的蛋白质、脂肪和维生素等营养物质外,还含有功能多糖、黄酮和皂苷等生物活性成分[2],且豆类多糖在抗氧化、抑菌、降血糖和降血脂等方面表现出良好的作用[3]。糖尿病是一种以高血糖为病症的慢性代谢性疾病,此病有较强的药物依赖性,目前所用到的药物如阿卡波糖等都在不同程度上有副作用[4]。高效低毒的具有调节血糖和血脂作用的天然活性成分已成为科学研究关注的焦点。Bai等[5]研究了6种豆科植物粗多糖的化学组成,结果表明较高含量的粗多糖和具有特定结构的粗多糖对降血糖具有一定的作用。Wu等[6]研究发现红豆多糖通过激活磷酸肌醇3-激酶(Phosphatidylin-ositol-3-kinase, PI3K)/(蛋白激酶B)AKT信号通路调节葡萄糖代谢和氧化应激。Wu等[2]从绿豆、红豆、豌豆和豇豆中提取了四种豆类多糖,以400 mg/kg b.w./d的剂量给链脲佐菌素(Streptozocin, STZ)诱发的糖尿病小鼠口服,比较其降血糖活性,研究结果表明,红豆和豇豆具有显著的降血糖活性。付王威等[1]研究建立Ⅱ型糖尿病模型大鼠,发现白扁豆非淀粉多糖(NS-DLP)对Ⅱ型糖尿病大鼠具有降血糖、降血脂作用。
微生物发酵技术可以有效提高多种生物活性物质释放,如酚类和多糖等[7]。微生物在生长代谢过程中分泌的各种生物酶类能破坏植物细胞的结构,使活性成分能够较大程度地溶出,提高其生物利用度[8]。枯草芽孢杆菌和乳酸菌在发酵豆制品中应用广泛。枯草芽孢杆菌可产纤维素酶和蛋白酶等酶类,能够破坏植物细胞壁,使活性物质大量溶出[9],同时能够改善产品风味和色泽等感官品质,增强其营养价值和保健价值。乳酸菌是一类多功能的益生菌,其具有抗氧化和增强免疫力等功效[10]。Tian等[11]采用新型乳酸益生菌DU-106发酵铁皮石斛多糖,DU-106改变了铁皮石斛多糖的单糖组成和分子量,改进了铁皮石斛多糖的免疫刺激活性。杨壮等[10]选取7种乳杆菌进行固态发酵,利用副干酪乳杆菌ATCC 334固态发酵黄精多糖,显著提高了黄精多糖的得率和抗氧化活性。黄文利等[12]利用粗壮脉纹孢菌发酵豆渣,研究表明发酵后豆渣粗多糖含量提高,多糖的微观结构得到了改善,具有作为优质膳食纤维的潜力。微生物发酵作为一种生物活性成分的有效修饰方法,越来越广泛地应用于天然产物的结构修饰[13]。目前,有关微生物发酵豆类的多糖在降血糖、降血脂等方面的活性研究较少,限制了发酵豆类功能食品的开发利用。
本研究选取大豆、豇豆、红豆、绿豆、扁豆、豌豆、蚕豆和菜豆8种常见的食用豆子作为研究对象,用枯草芽孢杆菌(ATCC 6051)和干酪乳杆菌(NRRL B-441)固态发酵豆粉,比较研究了8种豆粉发酵前后粗多糖的化学组成、水溶性质、体外降血糖和降血脂的生物活性。本研究为开发具有降血糖降血脂功能的富含活性多糖的发酵豆类食品提供了新思路,也为传统发酵豆制品的营养学研究提供了理论基础,对食用豆类加工产业经济的发展具有重要的意义。
1. 材料与方法
1.1 材料与仪器
蚕豆(CD-035)、豌豆(汉豌1号)、大豆(MD-065)、豇豆(鄂豇豆7号)、红豆(红珍珠)、绿豆(绿宝石)、扁豆(德扁3号)、菜豆(SJ-0052) 均由湖北省食用豆类植物自然科技资源中心提供;菌种枯草芽孢杆菌(Bacillus subtilis subsp. subtilis)ATCC 6051、菌种干酪乳杆菌(Lactobacillus casei)NRRL B-441 保藏于湖北省豆类(蔬菜)植物工程技术研究中心;LB培养基 实验室自制(蛋白胨1 g,酵母粉0.5 g,NaCl 1 g,蒸馏水100 mL,琼脂2 g);MRS培养基(5.12 g MRS培养基,100 mL蒸馏水,琼脂2 g) 实验室自制;标准葡萄糖、浓硫酸、蒽酮乙酸乙酯、半乳糖醛酸、间羟基联苯、四硼酸钠/硫酸、无水乙醇、α-淀粉酶、淀粉、磷酸缓冲液(Phosphate Buffer,PB)、3,5-二硝基水杨酸(3,5-Dinitrosalicylic acid,DNS)、甘氨脱氧胆酸钠(sodium glycocholate)、牛磺脱氧胆酸钠(Sodium taurodeoxycholate hydrate)、三氯乙酸(Trichloroacetic acid,TCA)、牛血清白蛋白(Bovine albumin,BSA)、考马斯亮蓝G-250 国药集团化学试剂有限公司。
FA124C分析天平 上海力辰科技;TGL-16A离心机 长沙平凡仪器;SPX-150B-Z型生化培养箱 上海博讯实业有限公司医疗设备厂;SMF01磨粉机 苏泊尔电器公司;FreeZone 6 Plus冷冻干燥机 美国Labconco公司;1510全波长酶标仪 Thermo Fisher公司;JP-020超声波清洗机 深圳市洁盟清洗设备有限公司。
1.2 实验方法
1.2.1 种子液制备
枯草芽孢杆菌ATCC 6051接种于LB培养基,于37 ℃下培养2 d得到枯草芽孢杆菌种子液[14]。干酪乳杆菌NRRL B-441接种于MRS培养基,选取干酪乳杆菌单菌落于MRS液体培养基,37 ℃静置培养24 h,得到干酪乳杆菌种子液[15]。
1.2.2 固态发酵
将8种豆子用磨粉机磨碎过筛(直径小于1 mm),分别称取24 g置于250 mL锥形瓶内,121 ℃灭菌,烘干至恒重,冷却[14]。以5%接种量分别将枯草芽孢杆菌和干酪乳杆菌种子液接种于豆粉,搅拌均匀,置于37 ℃培养箱发酵培养7 d。发酵后的豆粉样品经过真空冷冻干燥(真空度0.040 mbar,温度−85 ℃,时间12 h)后置于−20 ℃冰箱储存备用。
1.2.3 粗多糖的提取
1.2.3.1 样品脱脂
将未发酵和发酵2种豆粉,分别取24 g于锥形瓶,按料液比1:10(g/mL)加入95%的乙醇,室温振荡6 h,6000 r/min离心10 min,收集沉淀置于60 ℃烘箱烘干[16],备用。
1.2.3.2 样品水提
采用水提醇沉法进行多糖提取[17]。取脱脂后的样品,按料液比1:20(g/mL)添加蒸馏水,95 ℃水浴提取6 h,冷却后6000 r/min离心10 min,收集上清液。
1.2.3.3 蛋白质去除
收集的上清液中加入等体积3% TCA溶液于4 ℃静置过夜,8000 r/min离心10 min,沉淀蛋白质,收集上清液[18]。
1.2.3.4 粗多糖沉淀
收集的上清液中加入等体积无水乙醇,4 ℃静置过夜,8000 r/min离心10 min,收集沉淀,真空冷冻干燥得到粗多糖。
1.2.3.5 粗多糖得率的计算
称量冷冻干燥后的粗多糖,按以下公式计算豆粉发酵前和发酵后粗多糖得率。
粗多糖得率(%)=冻干后粗多糖的质量原料质量×100 (1) 1.2.4 粗多糖组成测定
1.2.4.1 总糖含量测定
参考王勋等[19]的方法,采用蒽酮比色法测定豆粉粗多糖中总糖含量。配制0.1 mg/mL标准葡萄糖溶液,分别取标准葡萄糖溶液0、0.2、0.4、0.6、0.8、1.0 mL,分别加蒸馏至2 mL,加入蒽酮乙酸乙酯0.5 mL,再加入5 mL浓硫酸,沸水浴5 min后冷却,在620 nm波长下测定吸光度,以总糖浓度为横坐标,吸光值为纵坐标,制作标准曲线。分别将未发酵和两种菌发酵后的样品配制成0.5 mg/mL的样品溶液,取0.5 mL样品溶液,加1.5 mL蒸馏水,加0.5 mL蒽酮乙酸乙酯和5 mL浓硫酸,620 nm下测定样品吸光度,记录吸光度值,根据标准曲线计算粗多糖中总糖含量(%)。葡萄糖标准曲线为y=4.28x+0.061,R2=0.996。
总糖含量(%)=CNVm×100 (2) 式中,C为样品质量浓度,mg/mL;N为稀释倍数;V为样品定容体积,mL;m 为样品质量,mg。
1.2.4.2 糖醛酸含量测定
最大吸收波长测定:采用间羟基联苯比色法测定粗多糖样品中糖醛酸的含量[20]。配制1 mg/mL标准半乳糖醛酸溶液,采用间羟基联苯比色法测定各粗多糖样品中糖醛酸含量。取半乳糖醛酸标准溶液0.5 mL,加蒸馏水定容至10 mL,取1 mL该定容溶液,冰水浴,缓慢加入四硼酸钠/硫酸溶液6 mL,涡旋混匀,沸水浴5 min后冷却,加0.1 mL间羟基联苯溶液,充分涡旋混匀5 min,超声除气泡,在200~800 nm波长内扫描,确定最大吸收波长。
标准曲线:分别取标准半乳糖醛酸溶液0.1、0.2、0.3、0.4、0.5、0.6 mL,定容至10 mL,各取1 mL,后续同确定最大波长操作,在最大波长下测定吸光度值,以糖醛酸浓度为横坐标,吸光值为纵坐标,作标准曲线。半乳糖醛酸标准曲线为y=2.7543x+0.0486,R2=0.9956。
样品测定:取粗多糖样品20 mg,用蒸馏水配制成浓度为0.5 mg/mL的样品溶液。取样品溶液1 mL,同确定最大波长操作方法,计算粗多糖中糖醛酸含量(%)。
糖醛酸含量(%)=CVm×100 (3) 式中,C为样品质量浓度,mg/mL;V为样品定容体积,mL;m为样品质量,mg。
1.2.4.3 蛋白质含量测定
配制0.1 mg/mL BSA作为标准品,采用考马斯亮蓝法测定各豆粉粗多糖样品中的蛋白质含量[21]。分别取BSA溶液0、0.2、0.4、0.6、0.8、1.0 mL,分别加蒸馏水至1 mL,加入5 mL考马斯亮蓝,室温反应5 min,记录各反应液在595 nm波长下的吸光值,以蛋白质浓度为横坐标,吸光值为纵坐标,作标准曲线。取样品20 mg溶于40 mL水中配成0.5 mg/mL的样品溶液,后续操作同标准曲线步骤,根据标准曲线计算样品中蛋白质含量(%)。蛋白质标准曲线为y=0.8057x+0.1207,R2=0.99。
蛋白质含量(%)=CNVm×100 (4) 1.2.5 粗多糖水溶性测定(WSC)
参照张子木等[22]的方法。称取粗多糖样品50 mg,加1 mL蒸馏水,混合涡旋振荡18 h,9300 r/min离心。取上清液加3倍无水乙醇于4 ℃过夜,回收沉淀,真空冷冻干燥后称重。
溶水能力(%)=上清液种碳水化合物的质量样品质量×100 (5) 1.2.6 粗多糖体外降血糖、降血脂活性测定
1.2.6.1 抑制α-淀粉酶活性测定
参照Deng等[23]的方法,α-淀粉酶活性抑制体系见表1。将粗多糖样品用0.1 mol/mL PB缓冲液(pH6.8)配制成0.5、1.0、2.0、4.0 mg/mL的样品溶液,取0.4 mL粗多糖样品溶液,分别加0.2 mL 1 %淀粉溶液(0.1 mol/mL PB配制,已预温),40 ℃水浴5 min,再加入0.2 mL 0.5 mg/mL α-淀粉酶溶液(3700 U/mg)(0.1 mol/mL PB配制),40 ℃水浴10 min,加入0.4 mL DNS,95 ℃沸水浴4 min,立即冰浴,于波长540 nm处测定其吸光度值A2。用阿卡波糖做阳性对照组,结果按公式(6)计算样品粗多糖对α-淀粉酶的抑制率(%)。
表 1 α-淀粉酶活性抑制体系Table 1. Activity inhibition system of α-amylase组别 0.4 mL样品溶液 0.4 mL PB溶液 0.2 mL淀粉溶液 0.2 mL α-淀粉酶溶液 0.2 mL PB溶液 0.4 mL DNS A0 − √ √ √ − √ A1 √ − √ − √ √ A2 √ − √ √ − √ 注:“−”表示不添加;“√”表示添加该试剂。 α-淀粉酶抑制率(%)=(1−A2−A1A0)×100 (6) 1.2.6.2 胆酸盐结合能力测定
胆酸盐标准曲线绘制:参考于美汇等[24]的实验方法。分别取不同浓度的标准胆酸盐溶液各1 mL(甘胺脱氧胆酸钠0.03、0.06、0.12、0.18、0.24、0.3 mmol/L;牛磺脱氧胆酸钠0.05、0.1、0.15、0.2、0.25、0.3 mmol/mL),加入3 mL 60 %的H2SO4溶液于70 ℃水浴40 min,冰浴5 min,在387 nm下测定吸光度。以胆酸盐浓度为横坐标,吸光度值为纵坐标,绘制胆酸盐标准曲线。甘氨脱氧胆酸钠标准曲线为y=0.1616x+0.0516,R2=0.9962,牛磺脱氧胆酸钠标准曲线为y=0.14x+0.053,R2=1。
胆酸盐结合能力:参照Long等[25]测定多糖胆酸盐结合能力的实验方法。将粗多糖样品用0.1 mol/mL PB(pH6.3)溶液配制成0.5、1.0、2.0、4.0 mg/mL的样品溶液,分别取200 μL发酵前后粗多糖样品溶液,加入200 μL 10 mg/mL胃蛋白酶(0.1 mol/mL PB配制pH6.3),加入600 μL 0.01 mol/L HCL溶液,37 ℃恒温振荡消化1 h,模拟胃环境;用0.1 mol/mL NaOH溶液调节pH至6.3,加入800 μL 10 mg/mL胰蛋白酶(0.1 mol/mL PB配制pH6.3),37 ℃恒温振荡消化1 h,模拟肠道环境;结束分别加入800 μL 0.4 mmol/mL甘氨脱氧胆酸钠(0.1 mol/mL PB配制pH6.3),800 μL 0.5 mmol/mL牛磺脱氧胆酸钠(0.1 mol/mL PB配制pH6.3),37 ℃恒温振荡1 h。结束8000 r/min离心10 min,取1 mL离心后上清液,加3 mL 60% H2SO4,在387 nm处测定样品吸光度。甘氨脱氧胆酸钠和牛磺脱氧胆酸钠结合率按式(7)、式(8)计算。
甘氨胆酸钠结合率(%)=C1−C2C1×100 (7) 式中:C1为甘氨脱氧胆酸钠加入量,μmol;C2为甘氨脱氧胆酸钠剩余量,μmol。
牛磺胆酸钠结合率(%)=C3−C4C3×100 (8) 式中:C3为牛磺脱氧胆酸钠加入量,μmol;C4为牛磺脱氧胆酸钠剩余量,μmol。
1.3 数据处理
实验样品均重复3次。运用Microsoft Excel 2010整理数据,结果采用平均值和标准差表示(¯x±s)。采用SPSS 25软件,通过单因素方差ANOVA分析比较多组间的值以确定统计学上有无显著性差异。P<0.05表示具有统计学意义。Origin Pro 2020绘制相关图示。GrapPad软件计算IC50值。
2. 结果与分析
2.1 两种细菌发酵豆粉粗多糖组分
发酵前后8种豆粉粗多糖组分见表2。未发酵豇豆、红豆、绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖得率显著高于枯草芽孢杆菌发酵豆粉(P<0.05),干酪乳杆菌发酵豆粉粗多糖得率显著高于枯草芽孢杆菌发酵豆粉(P<0.05)。未发酵豆粉中蛋白质含量较高,蛋白质含量以及蛋白质与多糖的结合方式可能会影响粗多糖样品的得率和多糖纯度[26]。未发酵豆粉的粗多糖中总糖含量为2.77%~20.62%,枯草芽孢杆菌发酵后豆粉的粗多糖中总糖含量为14.39%~57.95%,干酪乳杆菌发酵后豆粉的粗多糖中总糖含量为8.45%~45.89%。发酵过程提高了豆粉粗多糖样品中的多糖纯度。大豆、红豆、绿豆、蚕豆、菜豆和扁豆豆粉经过两种细菌发酵后,粗多糖样品中总糖含量显著增加(P<0.05)。枯草芽孢杆菌发酵后的豇豆、红豆、绿豆、蚕豆、菜豆和扁豆豆粉的粗多糖样品中总糖含量显著高于干酪乳杆菌发酵豆粉(P<0.05)。两种细菌发酵豆粉的粗多糖中总糖含量的提高可能是由于微生物在发酵过程中产生胞外酶(纤维素酶和果胶酶等)破坏细胞壁,促进胞内多糖物质溶出[27]。8种未发酵豆粉粗多糖中含有少量糖醛酸(1.27%~5.58%),两种细菌发酵豆粉的粗多糖中糖醛酸含量都增加,其中发酵大豆、绿豆、蚕豆、菜豆和豌豆豆粉的粗多糖中糖醛酸含量增加显著(P<0.05)。枯草芽孢杆菌发酵豆粉的粗多糖糖醛酸含量(4.05%~22.46%)显著(P<0.05)高于干酪乳杆菌发酵豆粉的粗多糖(2.63%~10.72%)。豆粉发酵过程中,微生物能代谢豆粉中的部分蛋白质,提取的豆粉粗多糖采用TCA方法去除粗多糖中蛋白质。蚕豆、菜豆、豌豆和大豆豆粉发酵后蛋白质含量降低。大多数游离蛋白质能被TCA去除,但可能存在微量的糖蛋白,发酵过程可能会改变糖蛋白间肽键,发酵豆粉的粗多糖中蛋白质含量显著减少[28]。本研究结果表明通过枯草芽孢杆菌和干酪乳杆菌发酵能显著提高豆粉粗多糖中总糖和糖醛酸含量。
表 2 两种细菌发酵前后豆粉粗多糖组成Table 2. Composition of crude polysaccharides from before and after bean flour fermentation by two types of bacteria样品 得率(%) 总糖(%) 糖醛酸(%) 蛋白质(%) 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 蚕豆 3.78±0.02b 1.62±0.01c 5.03±0.22a 8.51±2.63c 52.83±0.43a 35.26±2.90b 1.27±0.09c 7.63±0.17a 4.83±0.13b 1.31±0.68a − 0.15±0.97b 菜豆 5.34±0.16b 4.10±0.23c 6.25±0.13a 20.62±0.69c 41.93±1.87a 29.47±1.42b 5.58±0.12c 17.58±1.06a 8.52±0.12b 0.62±1.07a − 0.01±0.01a 扁豆 2.38±0.10b 0.93±0.21c 4.76±0.73a 20.36±0.48c 55.20±5.51a 39.50±0.18b 3.19±0.15b 5.50±0.33a 5.23±0.31a − − − 豌豆 1.50±0.02b 0.24±0.02c 1.96±0.31a 13.41±0.79b 14.39±0.65b 45.89±5.55a 3.02±0.06c 22.46±0.45a 5.28±0.23b 6.82±0.50a 0.60±0.57c 1.81±0.56b 大豆 0.74±0.01b 0.61±0.01b 1.25±0.05a 10.70±0.23c 15.57±1.03b 32.52±0.95a 4.24±0.06c 12.52±0.12a 10.72±0.17b 16.10±1.97a 7.60±1.72b 8.81±3.53b 豇豆 4.15±0.08b 0.93±0.05c 9.62±0.54a 14.18±1.18b 57.95±8.73a 20.09±8.20b 2.04±0.14b 7.41±0.85a 3.00±0.11b − − − 红豆 4.34±0.25b 2.89±0.41c 7.80±0.56a 4.71±1.18c 43.11±0.54a 20.03±0.99b 1.89±0.06b 8.53±3.03a 2.90±0.04b − − − 绿豆 4.06±0.73b 1.48±0.03c 6.95±0.38a 2.77±1.29c 36.64±0.19a 8.45±2.64b 1.46±0.09c 4.05±0.05a 2.63±0.04b − − − 注:“−”表示未检出;同行不同字母表示同一指标具有显著性差异(P<0.05)。 2.2 两种细菌发酵豆粉粗多糖水溶性(WSC)
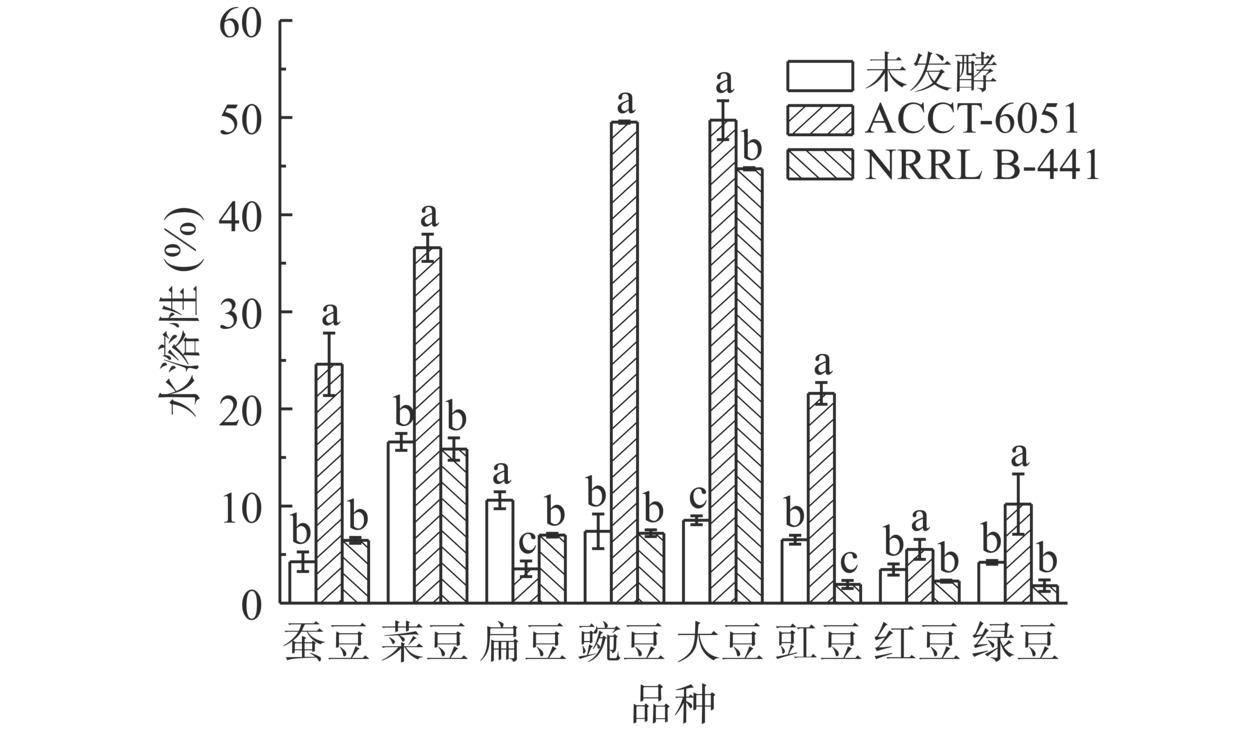
发酵前后8种豆粉粗多糖水溶性见图1。未发酵豆粉粗多糖的水溶性为3.47%~16.60%,枯草芽孢杆菌发酵豆粉的粗多糖水溶性为3.53%~49.73%,干酪乳杆菌发酵豆粉的粗多糖水溶性为1.80%~44.73%。枯草芽孢杆菌发酵大豆、豇豆、红豆、绿豆、蚕豆、菜豆和豌豆豆粉的粗多糖水溶性显著(P<0.05)高于未发酵豆粉粗多糖,也显著(P<0.05)高于干酪乳杆菌发酵后豆粉的粗多糖水溶性。微生物发酵豆粉的粗多糖中糖醛酸的增加可能有利于提高粗多糖的水溶性。多糖分子量、单糖组成以及单糖的连接方式都会影响其水溶性[29]。扁豆多糖的水溶性降低可能与其多糖的分子量、单糖组成以及单糖的连接方式有关。Jia等[30]研究利用绿色木霉菌发酵脱脂米糠来制备可溶性膳食纤维,微生物发酵处理显著提高了可溶性膳食纤维的溶解性。多糖溶解度越高,在食品工业中的应用前景越广泛[31]。本研究结果表明枯草芽孢杆菌发酵能有效提高豆粉粗多糖的水溶性。
2.3 两种细菌发酵豆粉粗多糖体外降血糖降血脂活性
2.3.1 抑制α-淀粉酶活性
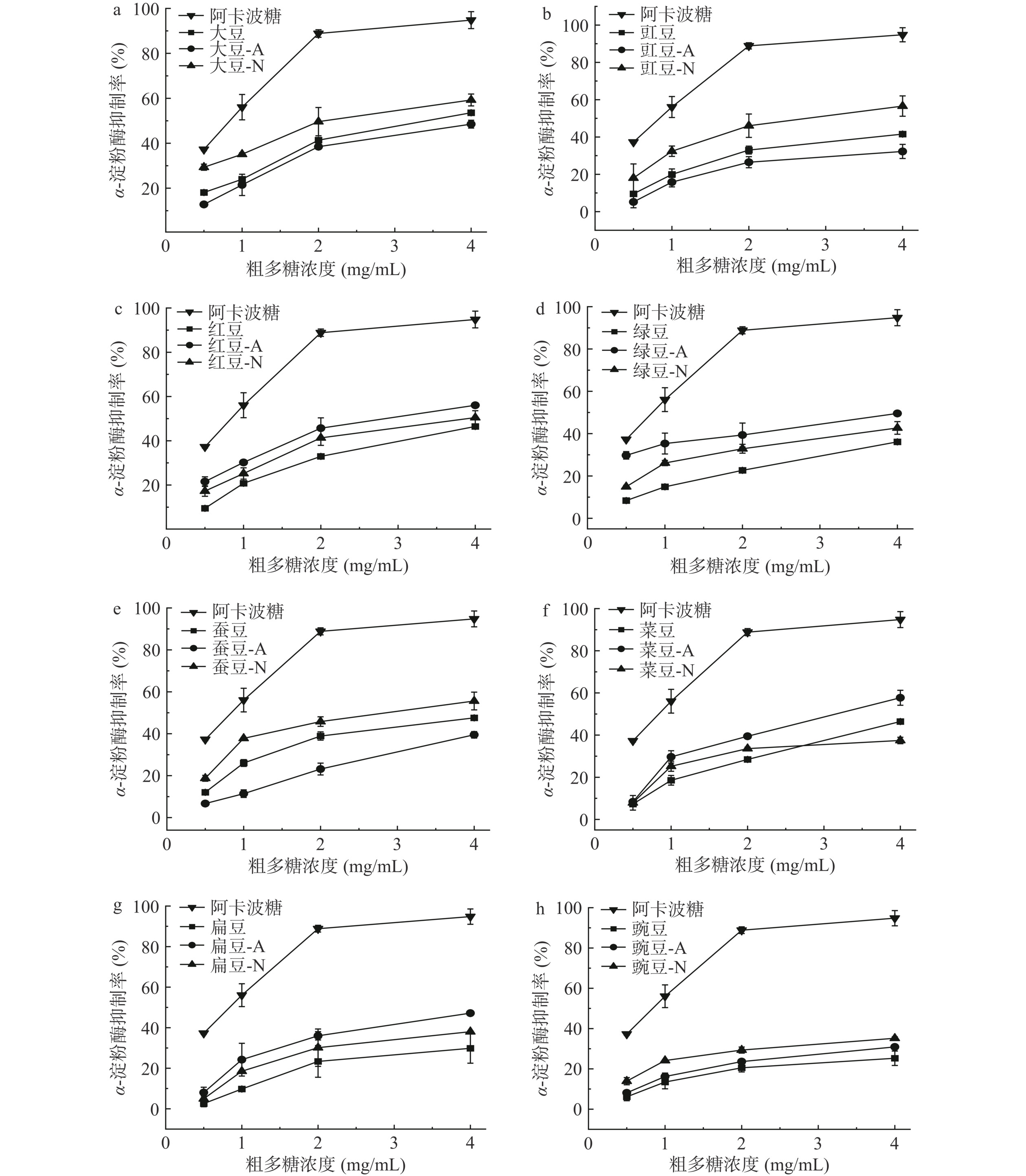
α-淀粉酶将碳水化合物降解为单糖,使得胰岛素和血糖升高。通过抑制α-淀粉酶的活性,可以减缓碳水化合物的水解,以此来降低餐后血糖的上升,此方式是预防和治疗Ⅱ型糖尿病比较有效的手段[32]。两种细菌发酵豆粉的粗多糖对α-淀粉酶活性抑制率如图2所示。研究结果表明,发酵前后豆粉的粗多糖对α-淀粉酶的抑制作用呈剂量依赖性,随着豆粉粗多糖质量浓度的增加,抑制α-淀粉酶能力增加,均低于阳性对照(阿卡波糖)的抑制能力。由粗多糖对α-淀粉酶的抑制曲线经回归处理可得出粗多糖对α-淀粉酶抑制率的IC50值(Half aximal inhibitory concentration,IC50),IC50值见表3。发酵与未发酵豆粉的粗多糖对α-淀粉酶抑制率的IC50值之间差异显著。枯草芽孢杆菌发酵红豆、绿豆、菜豆和扁豆豆粉的粗多糖对α-淀粉酶抑制能力显著高于其未发酵豆粉的粗多糖(P<0.05);干酪乳杆菌发酵大豆、绿豆和蚕豆豆粉的粗多糖对α-淀粉酶抑制能力显著高于其未发酵豆粉的粗多糖(P<0.05);干酪乳杆菌发酵大豆、豇豆和蚕豆豆粉的粗多糖对α-淀粉酶抑制能力显著高于枯草芽孢杆菌(P<0.05)。不同豆粉粗多糖对α-淀粉酶抑制率之间存在差异。陈鹏等[33]研究表明多糖的糖苷键种类、连接方式以及空间结构都会影响多糖的降血糖和降血脂生物活性。枯草芽孢杆菌发酵大豆、红豆和菜豆豆粉的粗多糖(质量浓度4 mg/mL)对α-淀粉酶抑制率均超过50%,其中发酵菜豆豆粉的粗多糖对α-淀粉酶抑制率最高(57.71%)。干酪乳杆菌发酵大豆、豇豆、红豆和蚕豆豆粉的粗多糖(质量浓度4 mg/mL)对α-淀粉酶抑制率均超过50%,其中发酵大豆豆粉的粗多糖对α-淀粉酶抑制率最高(59.27%)。本研究结果表明微生物发酵能提高豆粉粗多糖对α-淀粉酶的抑制率。Ji等[34]研究指出具有较高糖醛酸含量的多糖能呈现相对分散的状态,进而有利于发挥多糖的降血糖活性。因此,发酵后大豆和菜豆豆粉的粗多糖均含有较高糖醛酸,这可能有利于增强豆粉粗多糖的降血糖活性。目前市场上常用的阿卡波糖等降糖药物,过度的抑制活性有一定的副作用,如肠胃胀气和腹泻等[35]。常食用枯草芽孢杆菌和干酪乳杆菌发酵后的豆制品可能对稳定血糖具有积极的作用。
表 3 两种细菌发酵前后豆粉粗多糖降血糖降血脂活性显著性分析Table 3. Significance analysis of hypoglycemic and lipid-lowering activities of crude polysaccharides from before and after bean flour fermentation by two kinds of bacteria样品 α-淀粉酶抑制率IC50(mg/mL) 甘氨胆酸钠结合率IC50(mg/mL) 牛磺胆酸钠结合率IC50(mg/mL) 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 大豆 3.31±0.12a 3.97±0.25a 2.28±0.52b 0.20±0.03b 1.06±0.04a 0.06±0.01c 388.40±0.60b 576.00±13.00a 192.40±5.06c 豇豆 5.52±0.28ab 9.74±3.68a 2.71±0.77b 71.36±0.76a 0.44±0.04c 16.56±0.11b 488.60±8.62b 577.30±1.10a 281.90±2.00c 红豆 4.49±0.08a 2.74±0.28b 3.77±0.76a 20.04±0.27a 9.58±0.64c 14.82±1.05b 259.30±1.03b 366.80±6.30a 362.20±5.40a 绿豆 7.84±0.55a 4.77±1.16b 6.18±0.68b 4.22±0.12a 1.72±0.16c 3.19±0.11b 17.17±0.98a 4.14±0.42c 12.30±0.82b 蚕豆 4.05±0.14b 5.92±0.69a 2.66±0.32c 32.93±1.16a 4.92±0.15c 14.29±0.05b 65.19±2.26a 6.18±0.04b 7.17±0.96b 菜豆 4.59±0.02b 2.91±0.27c 7.00±0.70a 39.60±0.52a 0.47±0.03c 9.89±0.23b 6.28±0.27a 1.77±0.15c 4.18±0.04b 扁豆 9.82±3.76a 4.12±0.08b 6.17±0.72ab 64.77±0.98a 1.66±0.08c 6.54±0.21b 45.04±1.73a 1.71±0.08b 2.06±0.04b 豌豆 18.69±5.61a 12.22±1.81a 11.87±2.07a 51.81±1.92a 6.20±0.02c 11.26±0.59b 503.30±2.85a 367.40±11.6b 149.70±1.60c 注:同行不同字母表示同一指标具有显著性差异(P<0.05)。 2.3.2 甘氨脱氧胆酸钠结合率
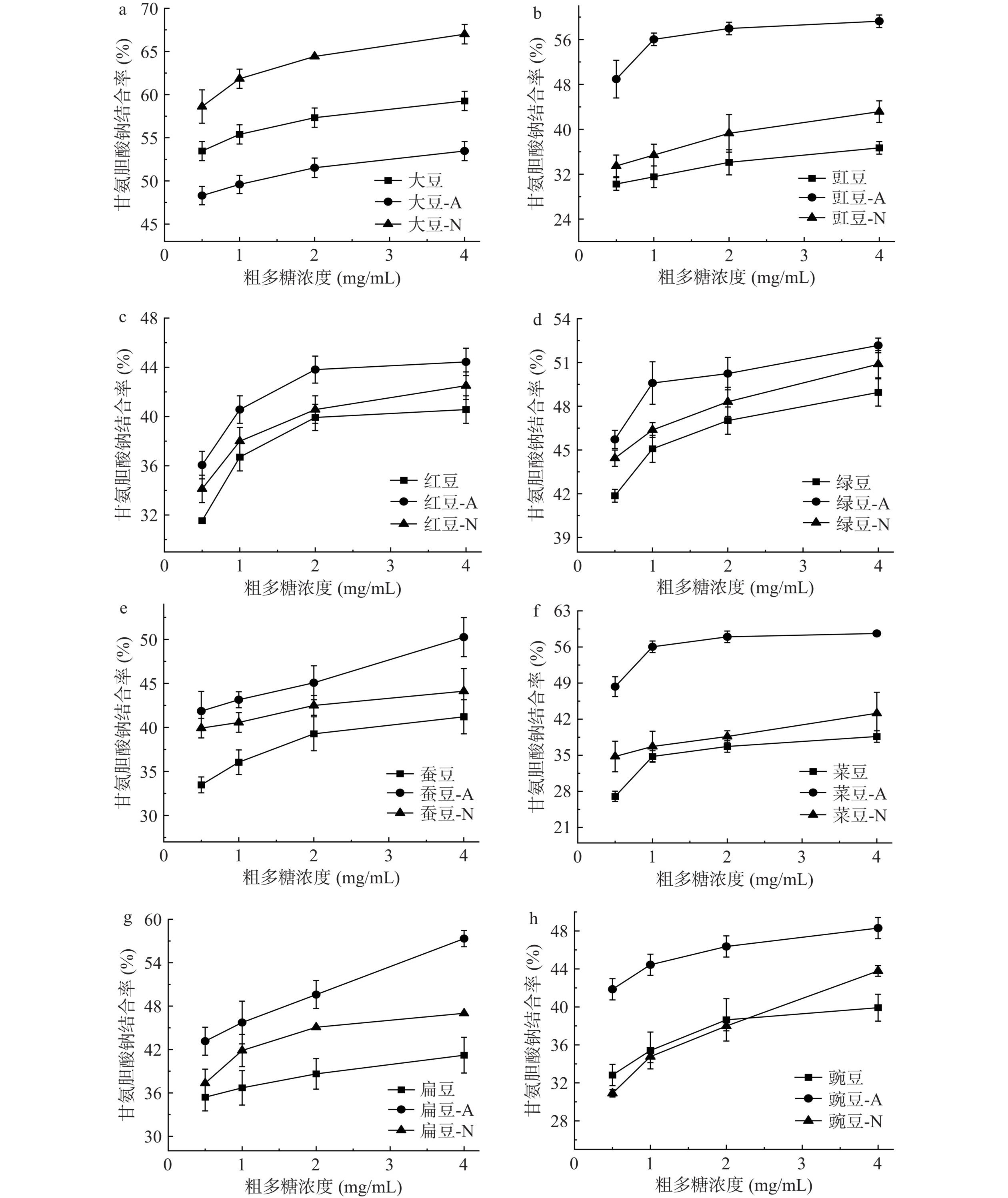
胆汁酸一般都以盐及结合型的形式存在人体内,并且以胆汁酸的形式排出体外,粗多糖通过其形成的黏性网络吸附胆酸盐,促进了胆汁酸的排泄,使得胆固醇向胆汁酸的转化过程加快,从而使机体内的胆固醇含量减少,以达到降脂的效果[36]。两种细菌发酵豆粉的粗多糖结合甘氨胆酸钠的能力比较如图3所示。随着豆粉粗多糖质量浓度的增加,两种细菌发酵前后豆粉的粗多糖对甘氨脱氧胆酸钠的结合率呈现先增加后稳定的趋势。质量浓度为0~2 mg/mL时,豆粉粗多糖对甘氨脱氧胆酸钠的结合率快速增加。质量浓度在2~4 mg/mL时,豆类粗多糖对甘氨脱氧胆酸钠的结合率增加趋于稳定。计算粗多糖对甘氨脱氧胆酸钠结合率IC50值见表3。未发酵豆粉的粗多糖和两种细菌发酵豆粉的粗多糖对甘氨脱氧胆酸钠结合率之间差异显著(P<0.05)。枯草芽孢杆菌发酵豆粉的粗多糖结合甘氨脱氧胆酸钠的能力显著提高(P<0.05)。通过比较豆粉粗多糖对甘氨脱氧胆酸钠结合率的IC50值。比较豇豆、红豆、绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖结合甘氨脱氧胆酸钠能力,枯草芽孢杆菌发酵>干酪乳杆菌发酵>未发酵豆粉。多糖分子结构特征如分子量、表观粘度以及单糖组成等都会影响多糖结合胆汁酸的能力[37]。Hu等[38]研究表明高分子量多糖由于其粘度大,对胆汁酸结合具有流体动力学限制作用。不同微生物发酵对多糖的修饰机制不同,也表现出不同的胆汁酸结合能力。当粗多糖质量浓度为1 mg/mL时,未发酵大豆豆粉的粗多糖结合甘氨脱氧胆酸钠的能力超过50%;枯草芽孢杆菌发酵豇豆和菜豆豆粉的粗多糖结合甘胺脱氧胆酸钠的能力均超过50%,分别达56.03%和56.04%;干酪乳杆菌发酵大豆豆粉的粗多糖结合率超过50%,达61.84%。刘荣等[39]研究发现对水溶性黑木耳多糖样品进行除蛋白处理后,多糖分子对胆酸盐的结合能力增强了,可能是由于多糖中的蛋白质阻碍了多糖分子和胆酸盐的结合。本研究对粗多糖样品进行了TCA除蛋白质处理,两种细菌发酵后豆粉的粗多糖样品中的蛋白质含量显著降低。本研究结果表明通过枯草芽孢杆菌和干酪乳杆菌发酵处理,豆粉粗多糖结合甘氨脱氧胆酸钠的能力显著提高。
2.3.3 牛磺脱氧胆酸钠结合率
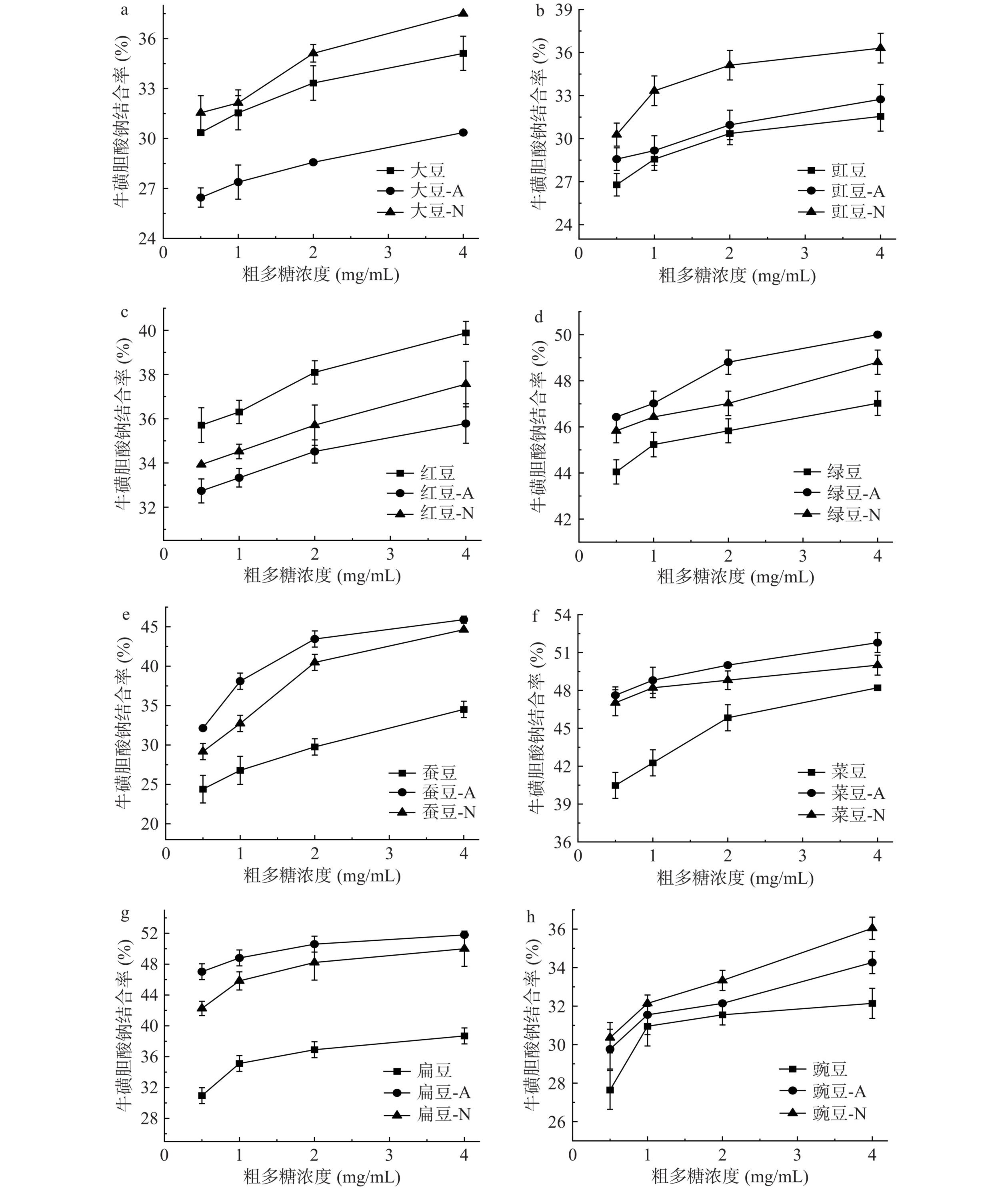
两种细菌发酵前后豆粉的粗多糖结合牛磺脱氧胆酸钠的能力如图4所示。8种豆粉粗多糖均可以结合牛磺脱氧胆酸钠,且多糖质量浓度与牛磺脱氧胆酸钠结合率之间呈现明显的剂量效应关系。计算粗多糖对牛磺脱氧胆酸钠结合率IC50值见表3。比较发酵前后豆粉的粗多糖对牛磺脱氧胆酸钠结合能力IC50值,枯草芽孢杆菌和干酪乳杆菌发酵绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖结合牛磺脱氧胆酸钠的能力显著提高(P<0.05)。枯草芽孢杆菌发酵菜豆和绿豆豆粉的粗多糖结合牛磺脱氧胆酸钠能力显著高于干酪乳杆菌发酵豆粉的粗多糖(P<0.05)。郑娟霞等[40]研究表明多糖的分子基团能与胆酸盐络合,起到降血脂作用。不同豆粉粗多糖的分子基团差异会影响其与胆酸盐的结合能力。当质量浓度为4 mg/mL时,未发酵豆粉的粗多糖结合牛磺脱氧胆酸钠的能力均未超过50%;枯草芽孢杆菌发酵菜豆和扁豆豆粉的粗多糖结合牛磺脱氧胆酸钠的能力均超过50%,分别达51.78%和51.79%;干酪乳杆菌发酵扁豆豆粉的粗多糖结合率超过50%。Pei等[28]比较了发酵前后蓝金银花多糖和胆酸盐的结合能力,研究表明发酵后蓝金银花的多糖与胆酸盐的结合能力更好,可能是由于益生菌发酵修饰多糖分子结构增强其功能活性。Qin等[41]研究表明,发酵茶多糖相比未发酵茶多糖表现出更高的胆汁酸结合能力。本研究结果表明枯草芽孢杆菌和干酪乳杆菌发酵有利于提高豆粉粗多糖结合牛磺脱氧胆酸钠的能力。
3. 结论
本研究用枯草芽孢杆菌和干酪乳杆菌发酵大豆、豇豆、红豆、绿豆、扁豆、豌豆、 蚕豆和菜豆8种不同豆粉制备了粗多糖样品,比较分析了两种细菌发酵前后豆粉的粗多糖样品的组成、水溶性和降血糖降血脂活性变化。发酵大豆、绿豆、蚕豆、菜豆和豌豆豆粉的粗多糖糖醛酸含量显著增加(P<0.05),且枯草芽孢杆菌发酵豆粉粗多糖的糖醛酸含量和水溶性均高于干酪乳杆菌发酵。发酵可以改善豆粉粗多糖的水溶性,且枯草芽孢杆菌发酵高于干酪乳杆菌发酵。发酵豆粉的粗多糖表现出不同的体外降血糖和降血脂活性。比较豆类粗多糖对α-淀粉酶抑制率IC50值,如干酪乳杆菌发酵大豆、豇豆和蚕豆豆粉的粗多糖对α-淀粉酶抑制能力显著高于枯草芽孢杆菌发酵(P<0.05)。当豆粉粗多糖质量浓度为4 mg/mL时,枯草芽孢杆菌发酵菜豆豆粉的粗多糖对α-淀粉酶抑制率最高,达57.71%;而干酪乳杆菌发酵大豆豆粉的粗多糖对α-淀粉酶抑制率最高,达59.26%。发酵豇豆、红豆、绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖结合甘氨脱氧胆酸钠能力,枯草芽孢杆菌发酵后>干酪乳杆菌发酵后>未发酵豆粉。发酵绿豆、蚕豆、菜豆、扁豆和豌豆豆粉的粗多糖对牛磺脱氧胆酸钠的结合能力相比于未发酵豆粉粗多糖均显著提高(P<0.05)。枯草芽孢杆菌和干酪乳杆菌发酵能增加豆类粗多糖体外降血糖和降血脂的生物活性。
-
表 1 α-淀粉酶活性抑制体系
Table 1 Activity inhibition system of α-amylase
组别 0.4 mL样品溶液 0.4 mL PB溶液 0.2 mL淀粉溶液 0.2 mL α-淀粉酶溶液 0.2 mL PB溶液 0.4 mL DNS A0 − √ √ √ − √ A1 √ − √ − √ √ A2 √ − √ √ − √ 注:“−”表示不添加;“√”表示添加该试剂。 表 2 两种细菌发酵前后豆粉粗多糖组成
Table 2 Composition of crude polysaccharides from before and after bean flour fermentation by two types of bacteria
样品 得率(%) 总糖(%) 糖醛酸(%) 蛋白质(%) 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 蚕豆 3.78±0.02b 1.62±0.01c 5.03±0.22a 8.51±2.63c 52.83±0.43a 35.26±2.90b 1.27±0.09c 7.63±0.17a 4.83±0.13b 1.31±0.68a − 0.15±0.97b 菜豆 5.34±0.16b 4.10±0.23c 6.25±0.13a 20.62±0.69c 41.93±1.87a 29.47±1.42b 5.58±0.12c 17.58±1.06a 8.52±0.12b 0.62±1.07a − 0.01±0.01a 扁豆 2.38±0.10b 0.93±0.21c 4.76±0.73a 20.36±0.48c 55.20±5.51a 39.50±0.18b 3.19±0.15b 5.50±0.33a 5.23±0.31a − − − 豌豆 1.50±0.02b 0.24±0.02c 1.96±0.31a 13.41±0.79b 14.39±0.65b 45.89±5.55a 3.02±0.06c 22.46±0.45a 5.28±0.23b 6.82±0.50a 0.60±0.57c 1.81±0.56b 大豆 0.74±0.01b 0.61±0.01b 1.25±0.05a 10.70±0.23c 15.57±1.03b 32.52±0.95a 4.24±0.06c 12.52±0.12a 10.72±0.17b 16.10±1.97a 7.60±1.72b 8.81±3.53b 豇豆 4.15±0.08b 0.93±0.05c 9.62±0.54a 14.18±1.18b 57.95±8.73a 20.09±8.20b 2.04±0.14b 7.41±0.85a 3.00±0.11b − − − 红豆 4.34±0.25b 2.89±0.41c 7.80±0.56a 4.71±1.18c 43.11±0.54a 20.03±0.99b 1.89±0.06b 8.53±3.03a 2.90±0.04b − − − 绿豆 4.06±0.73b 1.48±0.03c 6.95±0.38a 2.77±1.29c 36.64±0.19a 8.45±2.64b 1.46±0.09c 4.05±0.05a 2.63±0.04b − − − 注:“−”表示未检出;同行不同字母表示同一指标具有显著性差异(P<0.05)。 表 3 两种细菌发酵前后豆粉粗多糖降血糖降血脂活性显著性分析
Table 3 Significance analysis of hypoglycemic and lipid-lowering activities of crude polysaccharides from before and after bean flour fermentation by two kinds of bacteria
样品 α-淀粉酶抑制率IC50(mg/mL) 甘氨胆酸钠结合率IC50(mg/mL) 牛磺胆酸钠结合率IC50(mg/mL) 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 未发酵 枯草发酵 干酪发酵 大豆 3.31±0.12a 3.97±0.25a 2.28±0.52b 0.20±0.03b 1.06±0.04a 0.06±0.01c 388.40±0.60b 576.00±13.00a 192.40±5.06c 豇豆 5.52±0.28ab 9.74±3.68a 2.71±0.77b 71.36±0.76a 0.44±0.04c 16.56±0.11b 488.60±8.62b 577.30±1.10a 281.90±2.00c 红豆 4.49±0.08a 2.74±0.28b 3.77±0.76a 20.04±0.27a 9.58±0.64c 14.82±1.05b 259.30±1.03b 366.80±6.30a 362.20±5.40a 绿豆 7.84±0.55a 4.77±1.16b 6.18±0.68b 4.22±0.12a 1.72±0.16c 3.19±0.11b 17.17±0.98a 4.14±0.42c 12.30±0.82b 蚕豆 4.05±0.14b 5.92±0.69a 2.66±0.32c 32.93±1.16a 4.92±0.15c 14.29±0.05b 65.19±2.26a 6.18±0.04b 7.17±0.96b 菜豆 4.59±0.02b 2.91±0.27c 7.00±0.70a 39.60±0.52a 0.47±0.03c 9.89±0.23b 6.28±0.27a 1.77±0.15c 4.18±0.04b 扁豆 9.82±3.76a 4.12±0.08b 6.17±0.72ab 64.77±0.98a 1.66±0.08c 6.54±0.21b 45.04±1.73a 1.71±0.08b 2.06±0.04b 豌豆 18.69±5.61a 12.22±1.81a 11.87±2.07a 51.81±1.92a 6.20±0.02c 11.26±0.59b 503.30±2.85a 367.40±11.6b 149.70±1.60c 注:同行不同字母表示同一指标具有显著性差异(P<0.05)。 -
[1] 付王威, 吴睿婷, 万敏, 等. 白扁豆非淀粉多糖对Ⅱ型糖尿病大鼠的降血糖降血脂作用[J]. 现代食品科技,2021,37(8):1−7,35. [FU W W, WU R T, WAN M, et al. Hypoglycemic and hypolipidemic effects of non-starch polysaccharide from dolichos lablab L in type II diabetic rats[J]. Modern Food Science and Technology,2021,37(8):1−7,35.] FU W W, WU R T, WAN M, et al. Hypoglycemic and hypolipidemic effects of non-starch polysaccharide from dolichos lablab L in type II diabetic rats[J]. Modern Food Science and Technology, 2021, 37(8): 1−7,35.
[2] WU G J, LIU D, WAN Y J, et al. Comparison of hypoglycemic effects of polysaccharides from four legume species[J]. Food Hydrocolloids,2019,90:299−304. doi: 10.1016/j.foodhyd.2018.12.035
[3] HOU D, YOUSAF L, XUE Y, et al. Mung bean (Vigna radiata L.):bioactive polyphenols, polysaccharides, peptides, and health benefits[J]. Nutrients,2019,11(6):1238. doi: 10.3390/nu11061238
[4] 崔珏, 李超, 尤健, 等. 白茅根多糖改善糖尿病小鼠糖脂代谢作用的研究[J]. 食品科学,2012,33(19):302−305. [CUI J, LI C, YOU J, et al. Effects of imperata cylindrica polysaccharides on glucose and lipid metabolism in diabetic mice[J]. Food Science,2012,33(19):302−305.] CUI J, LI C, YOU J, et al. Effects of imperata cylindrica polysaccharides on glucose and lipid metabolism in diabetic mice[J]. Food Science, 2012, 33(19): 302−305.
[5] BAI Z Y, MENG J X, HUANG X J, et al. Comparative study on antidiabetic function of six legume crude polysaccharides[J]. International Journal of Biological Macromolecules,2020,154:25−30. doi: 10.1016/j.ijbiomac.2020.03.072
[6] WU G J, BAI Z Y, WAN Y J, et al. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway[J]. Food Hydrocolloids,2020,101:105456. doi: 10.1016/j.foodhyd.2019.105456
[7] 刘袆帆, 林诺怡, 成坚, 等. 微生物发酵法制备活性多糖的研究概述[J]. 食品与发酵工业,2021,47(3):281−287. [LIU H F, LIN N Y, CHENG J, et al. Isolation, advances in preparation of polysaccharides by microbial fermentation[J]. Food and Fermentation Industries,2021,47(3):281−287.] LIU H F, LIN N Y, CHENG J, et al. Isolation, advances in preparation of polysaccharides by microbial fermentation[J]. Food and Fermentation Industries, 2021, 47(3): 281−287.
[8] LIU X, HOU R L, XU K Q, et al. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus[J]. International Journal of Biological Macromolecules,2019,123:468−476. doi: 10.1016/j.ijbiomac.2018.11.069
[9] LIU F R, CHEN Z X, SHAO J J, et al. Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ[J]. Food Bioscience,2017,20:141−148. doi: 10.1016/j.fbio.2017.10.002
[10] 杨壮, 刘怡琳, 李隆熙, 等. 固态发酵制备黄精多糖的工艺优化、理化特性及抗氧化活性[J]. 食品与发酵工业, 1−9[2024-04-10] DOI:10.13995/j.cnki.11-1802/ts.035769. [YANG Z, LIU Y L, LI L X, et al. Process optimization, physicochemical properties and antioxidant activity of Polygonatum sibiricum polysaccharide prepared by solid-state fermentation[J]. Food and Fermentation Industries, 1−9[2024-04-10] DOI:10.13995/j.cnki.11-1802/ts.035769.] YANG Z, LIU Y L, LI L X, et al. Process optimization, physicochemical properties and antioxidant activity of Polygonatum sibiricum polysaccharide prepared by solid-state fermentation[J]. Food and Fermentation Industries, 1−9[2024-04-10] DOI: 10.13995/j.cnki.11-1802/ts.035769.
[11] TIAN W N, DAI L W, LU S M, et al. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide:Structure and immunoregulatory activities[J]. International Journal of Biological Macromolecules,2019,135:1034−1042. doi: 10.1016/j.ijbiomac.2019.05.203
[12] 黄文利, 欧阳耀铭, 卢玲, 等. 粗壮脉纹孢菌发酵前后豆渣多糖理化性质和结构特性[J]. 食品工业科技,2022,43(3):26−32. [HUANG W L, OUYANG Y M, LU L, et al. Functional components and structural characteristics of soybean dreg polysaccharides fermented by neurospora crassa[J]. Science and Technology of Food Industry,2022,43(3):26−32.] doi: 10.13386/j.issn1002-0306.2021040038 HUANG W L, OUYANG Y M, LU L, et al. Functional components and structural characteristics of soybean dreg polysaccharides fermented by neurospora crassa[J]. Science and Technology of Food Industry, 2022, 43(3): 26−32. doi: 10.13386/j.issn1002-0306.2021040038
[13] YANG J J, ZHANG X, DAI J F, et al. Effect of fermentation modification on the physicochemical characteristics and anti-aging related activities of Polygonatum kingianum polysaccharides[J]. International Journal of Biological Macromolecules,2023,235:123661. doi: 10.1016/j.ijbiomac.2023.123661
[14] 屈雅宁, 许梦粤, 唐双庆, 等. 枯草芽孢杆菌发酵对豆类粗多糖结构与抗氧化活性的影响[J]. 食品工业科技:2023, 44(17):129−138. [QU Y N, XU M Y, TANG S Q, et al. Effects of the structure and antioxidant activity of legume crude polysaccharides after Bacillus subtilis fermentation[J]. Science and Technology of Food Industry:2023, 44(17):129−138.] QU Y N, XU M Y, TANG S Q, et al. Effects of the structure and antioxidant activity of legume crude polysaccharides after Bacillus subtilis fermentation[J]. Science and Technology of Food Industry: 2023, 44(17): 129−138.
[15] LI S L, JIN Z Y, HU D J, et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour[J]. LWT,2020,125:109264−109264. doi: 10.1016/j.lwt.2020.109264
[16] 马高兴, 王晗, 杨文建, 等. 不同提取工艺对杏鲍菇多糖结构特征及免疫活性的影响[J]. 食品科学,2022,43(17):42−49. [MA G X, WANG H, YANG W J, et al. Effects of different extraction processes on structural characteristic and immunomodulatory activity of Pleurotus eryngii polysaccharide[J]. Food Science,2022,43(17):42−49.] MA G X, WANG H, YANG W J, et al. Effects of different extraction processes on structural characteristic and immunomodulatory activity of Pleurotus eryngii polysaccharide[J]. Food Science, 2022, 43(17): 42−49.
[17] 李思涵, 王君巧, 聂少平. 沙苑子多糖的提取纯化与结构解析[J]. 食品科学,2023,44(10):310−316. [LI S H, WANG J Q, NIE S P. Isolation, purification and structural characterization of polysaccharides from Semen astragali complanati[J]. Food Science,2023,44(10):310−316.] LI S H, WANG J Q, NIE S P. Isolation, purification and structural characterization of polysaccharides from Semen astragali complanati[J]. Food Science, 2023, 44(10): 310−316.
[18] 陈越, 宋振康, 张海悦. 三氯乙酸法脱除龙葵果多糖中蛋白质的工艺优化[J]. 食品与发酵工业,2020,46(24):198−203. [CHEN Y, SONG Z K, ZHANG H Y, et al. Study on optimization of response surface for the removement of protein from polysaccharide of Solanum nigrum fruit by chloroacetic acid method[J]. Food and Fermentation Industries,2020,46(24):198−203.] CHEN Y, SONG Z K, ZHANG H Y, et al. Study on optimization of response surface for the removement of protein from polysaccharide of Solanum nigrum fruit by chloroacetic acid method[J]. Food and Fermentation Industries, 2020, 46(24): 198−203.
[19] 王勋, 顾玉梅, 米生喜, 等. 胶磨破碎和冷冻破碎对芒果浆品质的影响[J/OL]. 现代食品科技, 1−11[2024-04-11]. https://doi.org/10.13982/j.mfst.1673-9078.2024.5.0588. [WANG X, GU Y M, MI S X, et al. Effects of colloid milling and freezing milling on the quality of mango pulp[J/OL]. Modern Food Science and Technology, 1−11[2024-04-11]. https://doi.org/10.13982/j.mfst.1673-9078.2024.5.0588.] WANG X, GU Y M, MI S X, et al. Effects of colloid milling and freezing milling on the quality of mango pulp[J/OL]. Modern Food Science and Technology, 1−11[2024-04-11]. https://doi.org/10.13982/j.mfst.1673-9078.2024.5.0588.
[20] 王轶帆, 邓媛元, 张雁, 等. 龙眼多糖与燕麦多糖的结构特征及其益生活性比较[J]. 中国食品学报,2020,20(12):62−71. [WANG Y F, DEN Y Y, ZHANG Y, et al. Comparison of the structural characteristics of longan polysaccharides and oat polysaccharides and their beneficial life properties[J]. Journal of Chinese Institute of Food Science and Technology,2020,20(12):62−71.] WANG Y F, DEN Y Y, ZHANG Y, et al. Comparison of the structural characteristics of longan polysaccharides and oat polysaccharides and their beneficial life properties[J]. Journal of Chinese Institute of Food Science and Technology, 2020, 20(12): 62−71.
[21] CHEN S H, CHEN H X, TIAN J G, et al. Chemical modification, antioxidant and α-amylase inhibitory activities of corn silk polysaccharides[J]. Carbohydrate Polymers,2013,98(1):428−437. doi: 10.1016/j.carbpol.2013.06.011
[22] 张子木, 黄秀芳, 张琴, 等. 壶瓶碎米荠多糖硫酸化结构修饰及抗氧化活性研究[J]. 中国粮油学报,2021,36(12):28−33. [ZHANG Z M, HUANG X F, ZHANG Q, et al. Study on sulfated structure modification and antioxidant activity of cardamine hupingshanensis polysaccharide[J]. Journal of the Chinese Cereals and Oils Association,2021,36(12):28−33.] ZHANG Z M, HUANG X F, ZHANG Q, et al. Study on sulfated structure modification and antioxidant activity of cardamine hupingshanensis polysaccharide[J]. Journal of the Chinese Cereals and Oils Association, 2021, 36(12): 28−33.
[23] DENG Y J, HUANG L X, ZHANG C H, et al. Novel polysaccharide from chaenomeles speciosa seeds:Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation[J]. International Journal of Biological Macromolecules,2020,153:755−766. doi: 10.1016/j.ijbiomac.2020.03.057
[24] 于美汇, 赵鑫, 尹红力, 等. 碱提醇沉黑木耳多糖体外和体内降血脂功能[J]. 食品科学,2017,38(1):232−237. [YU M H, ZHAO X, YIN H L, et al. In vitro and in vivo hypolipidemic effect of Auricularia auricular polysaccharides[J]. Food Science,2017,38(1):232−237.] YU M H, ZHAO X, YIN H L, et al. In vitro and in vivo hypolipidemic effect of Auricularia auricular polysaccharides[J]. Food Science, 2017, 38(1): 232−237.
[25] LONG H R, GU X Y, ZHOU N, et al. Physicochemical characterization and bile acid-binding capacity of water-extract polysaccharides fractionated by stepwise ethanol precipitation from Caulerpa lentillifera[J]. International Journal of Biological Macromolecules,2020,150:654−661. doi: 10.1016/j.ijbiomac.2020.02.121
[26] CHEN R Z, LUO S J, WANG C X, et al. Effects of ultra-high pressure enzyme extraction on characteristics and functional properties of red pitaya (Hylocereus polyrhizus) peel pectic polysaccharides[J]. Food Hydrocolloids,2021,121:107016. doi: 10.1016/j.foodhyd.2021.107016
[27] FILANNINO P, CAVOSKI I, THLIEN N, et al. Lactic acid fermentation of cactus cladodes (Opuntia ficusindica L.) generates flavonoid derivatives with antioxidant and anti-inflammatory properties[J]. PLoS One,2016,11(3):e0152575. doi: 10.1371/journal.pone.0152575
[28] PEI F Y, CAO X B, WANG X M, et al. Structural characteristics and bioactivities of polysaccharides from blue honeysuckle after probiotic fermentation[J]. LWT,2022,165:113764. doi: 10.1016/j.lwt.2022.113764
[29] HUANG F, HONG R Y, ZHANG R F, et al. Physicochemical and biological properties of longan pulp polysaccharides modified by Lactobacillus fermentum fermentation[J]. International Journal of Biological Macromolecules,2019,125:232−237. doi: 10.1016/j.ijbiomac.2018.12.061
[30] JIA M Y, CHEN J J, LIU X Z, et al. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation[J]. Food Hydrocolloids,2019,94:468−474. doi: 10.1016/j.foodhyd.2019.03.047
[31] GAN L Z, LI X G, WANG H B, et al. Structural characterization and functional evaluation of a novel exopolysaccharide from the moderate halophile Gracilibacillus sp. SCU50[J]. International Journal of Biological Macromolecules,2020,154:1140−1148. doi: 10.1016/j.ijbiomac.2019.11.143
[32] YANG Y, ZHANG J L, SHEN L H, et al. Inhibition mechanism of diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) against α-amylase and α-glucosidase[J]. Food Chemistry,2021,359:129934. doi: 10.1016/j.foodchem.2021.129934
[33] 陈鹏, 颜军, 梁立, 等. 银耳多糖的降解、磷酸酯化修饰及其抗氧化活性[J]. 食品工业科技,2019,40(9):34−37,43. [GHEN P, YAN J, LIANG L, et al. Degradation, phosphate modification of Tremella fuciformis polysaccharide and its antioxidant activity[J]. Science and Technology of Food Industry,2019,40(9):34−37,43.] GHEN P, YAN J, LIANG L, et al. Degradation, phosphate modification of Tremella fuciformis polysaccharide and its antioxidant activity[J]. Science and Technology of Food Industry, 2019, 40(9): 34−37,43.
[34] JI X L, GUO J H, CAO T Z, et al. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources[J]. Food Science and Human Wellness,2023,12(6):1969−1980. doi: 10.1016/j.fshw.2023.03.017
[35] ZHONG Q W, ZHOU T S, QIU W H, et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida[J]. Food Chemistry,2021,341:128148. doi: 10.1016/j.foodchem.2020.128148
[36] 纪慧杰, 朱彩平. 石榴皮多糖的提取及组成、体外降脂活性研究[J]. 食品与发酵工业,2023,49(4):161−168. [JI H J, ZHU C P. Study on the extraction and composition of pomegranate peel polysaccharide and hypolipidemic activity in vitro[J]. Food and Fermentation Industries,2023,49(4):161−168.] JI H J, ZHU C P. Study on the extraction and composition of pomegranate peel polysaccharide and hypolipidemic activity in vitro[J]. Food and Fermentation Industries, 2023, 49(4): 161−168.
[37] LI Q Y, DOU Z M, DUAN Q F, et al. A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods:Antioxidant and bile acid-binding capacity[J]. Food Science and Human Wellness,2024,13(1):494−505. doi: 10.26599/FSHW.2022.9250043
[38] HU J L, NIE S P, LI C, et al. In vitro effects of a novel polysaccharide from the seeds of Plantago asiatica L. on intestinal function[J]. International Journal of Biological Macromolecules,2013,54:264−269. doi: 10.1016/j.ijbiomac.2012.12.011
[39] 刘荣, 王蕾, 栾淑莹, 等. 水溶性黑木耳多糖体外结合胆酸盐能力的分析[J]. 食品工业科技,2015,36(17):358−361. [LIU R, WANG L, LUAN S Y, et al. In vitro binding of bile salts by water-soluble AAP[J]. Science and Technology of Food Industry,2015,36(17):358−361.] LIU R, WANG L, LUAN S Y, et al. In vitro binding of bile salts by water-soluble AAP[J]. Science and Technology of Food Industry, 2015, 36(17): 358−361.
[40] 郑娟霞, 陈文宁, 月金玲, 等. 海带多糖降血脂活性研究进展[J]. 食品与机械,2020,36(6):220−225. [ZHENG J X, CHEN W N, YUE J L, et al. Progress in the research on the lipid-lowering activity of polysacchairdes from kelp[J]. Food & Machinery,2020,36(6):220−225.] ZHENG J X, CHEN W N, YUE J L, et al. Progress in the research on the lipid-lowering activity of polysacchairdes from kelp[J]. Food & Machinery, 2020, 36(6): 220−225.
[41] QIN H N, HUANG L, TENG J W, et al. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation[J]. Food Chemistry,2021,353:129419. doi: 10.1016/j.foodchem.2021.129419
-
期刊类型引用(0)
其他类型引用(1)





 下载:
下载:
 下载:
下载:



