Research Progress on Bioactivity and Mechanism of Tea Polyphenols
-
摘要: 茶多酚是茶树体内一类具有酚羟基结构的多元酚类混合物的总称,是茶叶主要功能成分,在绿茶中含量相对较高。茶多酚生物活性研究广受关注,在功能食品、药物研发、保鲜防腐等领域具备广阔应用前景。茶多酚具有抗氧化、抗癌、降血脂、调节血糖、抑菌、抗辐射等多种生物活性,其作用机制主要涉及调控蛋白激酶B(Protein kinase B,AKT)、核因子κB(Nuclear factor-kappa B,NF-κB)、表皮生长因子受体(Epithelial growth factor receptor,EGFR)、腺苷酸活化蛋白激酶(Adenylate activated protein kinase,AMPK)等信号通路及相关蛋白。通过分析近年来相关研究文献,对茶多酚物质特性、生物活性及作用机制、应用领域等进行综述,以期为含茶多酚功能食品、天然药物等产品开发提供借鉴。Abstract: Tea polyphenols are a class of polyphenolic mixtures with phenolic hydroxyl structure in tea plants, which are the main functional component of tea, and the content is relatively high in green tea. Tea polyphenol bioactivity research gains popularity, in the functional food, drug development, preservation and preservation of preservatives and other areas with broad application prospects. Tea polyphenols have a variety of biological activities such as antioxidant, anticancer, hypolipidemic, blood sugar regulation, antibacterial, anti-radiation and so on. Their mechanism of action mainly includes regulating protein kinase B (AKT), nuclear factor-kappa B (NF-κB), epithelial growth factor receptor (EGFR), adenylate activated protein kinase (AMPK) and other signalling pathways and related proteins. By analyzing the relevant research literature in recent years, we review the material properties, biological activities, mechanisms and applications of tea polyphenols, with a view to providing reference for the development of tea polyphenol-containing functional foods and natural medicines.
-
作为茶树原产地,我国每年茶叶产量与出口量居世界各国之首,2022年茶叶年出口量为38.94万吨,其中绿茶出口量为31.39万吨,为六大茶类之最。近年来统计数据显示,绿茶年出口量及出口额远超其它五大茶类之和[1−2]。绿茶富含多种有益人体健康的功能成分,如:茶多酚、氨基酸、咖啡碱、茶多糖等。其中,茶多酚是绿茶中含量最多的一类活性物质,不仅参与绿茶品质形成,还具备多种生物活性,如抗氧化[3]、抗炎症[4]、抗肿瘤[5]、降血脂、降血糖[6]以及抗病毒[7]等,是茶叶功能成分研究领域的热点。
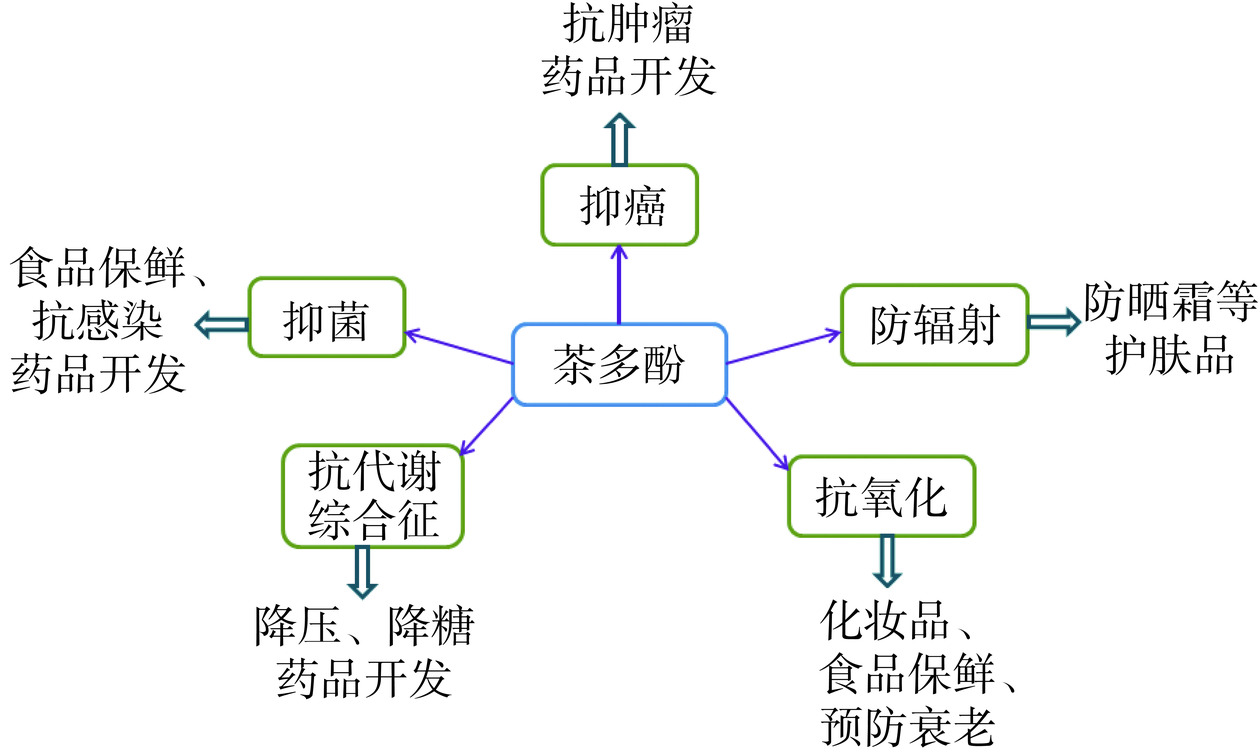
如今全世界已有超过360多种的含茶多酚类保健食品获得相关批准文号,它们多以胶囊、片剂、茶等形式呈现[8]。我国茶多酚、茶氨酸、茶黄素等功能成分通常作为原料,用于功能食品、日化产品等加工。目前,我国的茶多酚终端产品仍存在品类单一、产品附加值不高等问题[9],尤其是在茶多酚功能化利用研发方面,与欧美日发达国家相比仍存在一定差距。因此,本文综述了茶多酚生物活性研究进展,旨在为我国茶多酚类产品研发及功能化应用提供借鉴。茶多酚类主要产品研发如图1所示。
1. 茶多酚的组成与理化性质
1.1 茶多酚化学成分组成
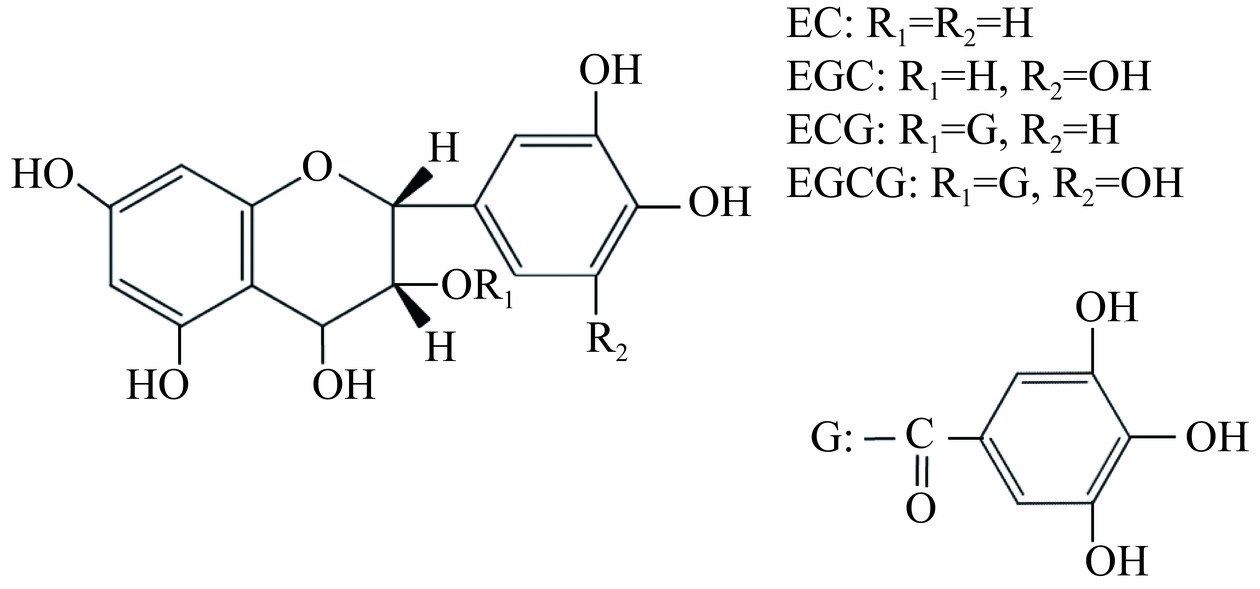
茶多酚占茶叶干物质总量的18%~36%,是构成茶汤滋味的主要组分,目前已从茶多酚类物质中分离出多种化学成分,主要包括:儿茶素类化合物;黄酮类化合物;花青素、花白素类化合物;酚酸和缩酚酸类化合物等四大类[10]。其中儿茶素类物质占茶多酚总量的70%以上,最常见的为:表没食子儿茶素没食子酸酯(Epi-gallocatechin-3-gallate,L-EGCG)、表没食子儿茶素(Epi-gallocatechin,L-EGC)、表儿茶素没食子酸酯(Epi-catechin-3-gallate,L-ECG)、表儿茶素(Epi-catechin,L-EC)等4种[10],其化学结构式如图2所示。茶叶中儿茶素种类除了常见的表型儿茶素外,还有许多含量很少的反式儿茶素类化合物。此外,茶多酚及其衍生物还包括众多由4-羰基-3羟基碳环为特征的苷类物质[11]。茶多酚主要化学成分组成如表1所示。
表 1 茶多酚主要化学成分组成Table 1. Main chemical composition of tea polyphenols类别 化合物 分子式 参考文献 类别 化合物 分子式 参考文献 儿茶素类 表没食子儿茶素-3-没食子酸酯 C22H18O11 [12−13] 异牡荆素-2-O-鼠李糖苷 C27H30O14 没食子儿茶素没食子酸酯 C22H18O11 山奈酚-3-O-葡萄糖苷 C21H20O11 表儿茶素-3-没食子酸酯 C22H18O10 山奈酚-3-O-半乳糖苷 C21H20O11 儿茶素 C15H14O6 山奈酚-3-O-阿拉伯糖苷 C20H18O10 表儿茶素 C15H14O6 芹菜素-7-O-新橙皮苷 C27H30O14 儿茶素没食子酸酯 C22H18O10 杨梅素-3-O-芸香糖苷 C27H30O17 表没食子儿茶素 C15H14O7 异鼠李素-7-O-葡萄糖苷 C22H22O12 没食子儿茶素 C15H14O7 异鼠李素-3-O-葡萄糖苷 C22H22O12 黄酮、黄酮苷类 山奈素 C15H10O6 [12−15] 花青素、花白素类 飞燕草素-3-O-葡萄糖苷 C21H21ClO12 [10] 槲皮素 C15H10O7 飞燕草花青素 C15H11O7 杨梅素 C15H10O8 芙蓉花青素 C15H11O6 山奈酚 C15H10O6 翘摇紫苷元 C15H11O7 芦丁 C27H30O16 芙蓉花白素 C15H14O7 原花青素 B2 C30H26O12 飞燕草花白素 C15H14O8 槲皮素-3-O-葡萄糖苷 C21H20O12 飞燕草素-3-(6-对香豆酰基半乳糖苷) C30H27O14 槲皮素-3-O-半乳糖苷 C21H20O12 酚酸、缩酚酸类 没食子酸 C7H6O5 [12] 槲皮素-3-O-鼠李糖苷 C21H20O11 绿原酸 C16H18O9 槲皮素-3-O-葡萄糖苷-7-O-鼠李糖苷 C27H30O16 对香豆酸 C9H8O3 槲皮素-5-O-β-D-葡萄糖苷 C21H20O12 咖啡酸 C9H8O4 木犀草素-6-C-葡萄糖苷 C21H20O11 对香豆-3-鸡纳酸 C15H18O8 木犀草素-8-C-阿拉伯糖苷 C21H20O11 异绿原酸 C25H24O12 杨梅素-3-O-葡萄糖苷 C21H20O13 间双没食子酸 C14H10O9 杨梅素-3-O-半乳糖苷 C21H20O13 异阿魏酸 C10H10O4 柚皮素-7-O-芸香糖苷 C27H32O14 对香豆酰奎宁酸 C16H18O8 1.2 茶多酚的理化性质
儿茶素类物质为白色固体,易溶于热水,黄酮类物质为亮黄色结晶,易溶于有机溶剂,但这两类物质均难溶于苯、氯仿等有机溶剂,儿茶素易与金属阳离子发生络合反应,黄酮苷类物质易发生水解反应形成黄酮或黄酮醇[10]。相比儿茶素和黄酮类物质,花青素类物质在紫光下呈暗棕色,花白素类物质则为无色,酚酸、缩酚酸类物质多为白色结晶,这几类物质水溶解性均较强[10]。茶多酚含有多个羟基,氧化性较强,在多酚氧化酶(Polyphenol oxidase,PPO)、过氧化物酶(Peroxidase,POD)等作用下,茶多酚易发生氧化、聚合反应,最终形成茶黄素、茶红素等物质[16]。
2. 茶多酚的生物活性
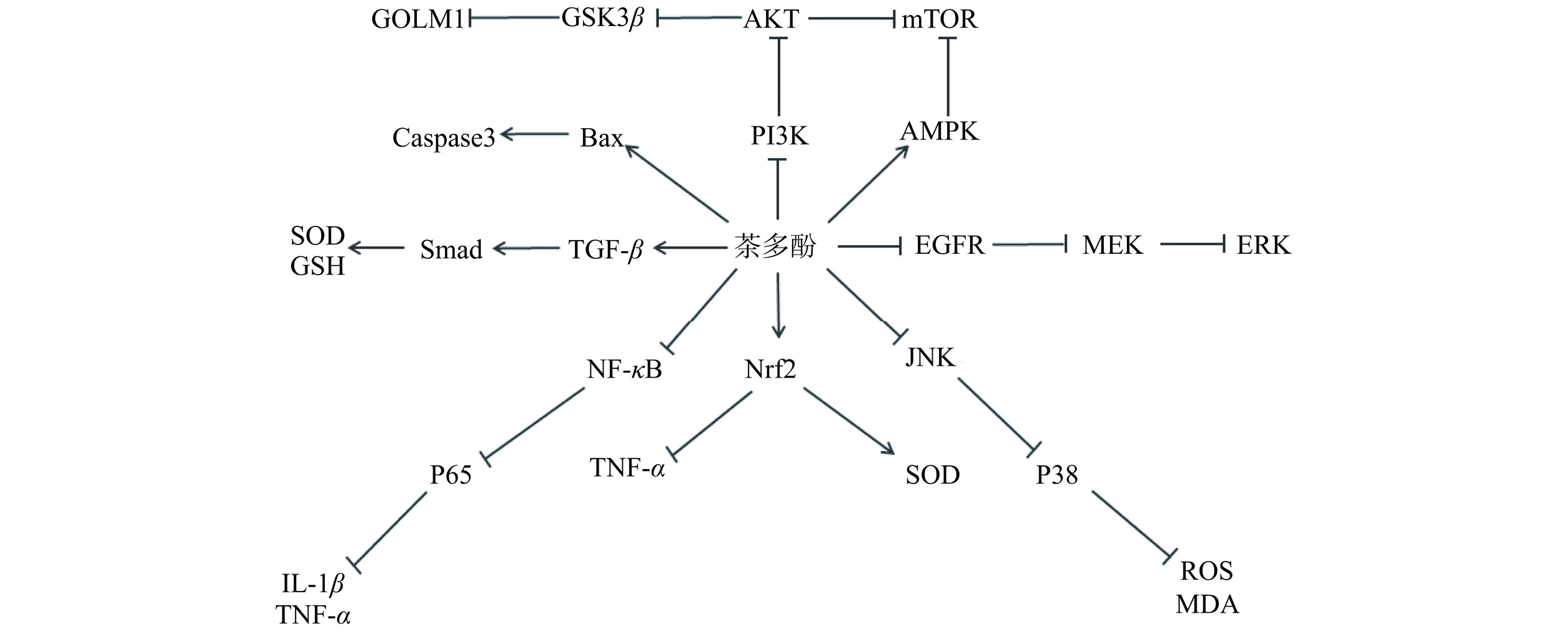
茶多酚生物活性包括:抗氧化、抗癌、降血脂、调节血糖、抑菌、抗辐射等,茶多酚主要生物活性作用机制相关信号通路如图3所示。
2.1 茶多酚抗氧化活性及作用机制
作为茶叶中提取的一类天然抗氧化活性物质,生物学研究表明,茶多酚可以通过调节多种酶蛋白及信号通路中靶蛋白的表达,提高细胞的抗氧化能力,并且在预防氧化损伤、皮肤衰老与皱纹产生方面作用显著[17−18]。
超氧化物歧化酶(Superoxide dismutase,SOD)、谷胱甘肽过氧化物酶(Glutathione peroxidase,GSH-Px)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)是调控细胞抗氧化机制起主要作用的酶蛋白。Yin等[19]通过茶多酚体内抗氧化实验表明,茶多酚可通过上调SOD、GSH-Px、核转录因子2(Nuclear factor erythroid 2-related factor2,Nrf2)等蛋白表达,同时下调肿瘤坏死因子α(Tumor necrosis factor-α,TNF-α)、肌酸激酶同工酶等酶蛋白表达,进而增强鸡心肌细胞的抗氧化能力,以缓解氧化应激造成的损伤。Yu等[20]通过体内外实验证明ECG可通过促进Nrf2核转移、血红素加氧酶1(Heme oxygenase-1,HO-1)蛋白表达,抑制NF-κB通路中P65磷酸化,从而增强SOD活性。在奶牛乳腺细胞中,茶多酚可通过降低丙二醛(Malondialdehyde,MDA)、活性氧(Reactive oxygen species,ROS)积累来增加抗氧化能力,这可能与茶多酚下调P38、JNK等相关蛋白磷酸化水平有关[21]。
Nrf2、信号传导及转录激活因子(Signal transducers and activators of transcription,STAT)、EGFR 等信号通路及其下游靶蛋白参与细胞内抗氧化机制的调控。Huang等[22]通过对冠心病模型小鼠研究表明,EGCG可激活冠心病小鼠体内Nrf2/HO-1抗氧化信号通路,显著提高心肌组织中SOD活性,降低ROS活性,减轻冠心病小鼠动脉粥样硬化氧化应激造成的损伤。研究表明,EGFR信号通路对衰老皮肤的改善作用可能是通过其抗氧化途径进行的[23]。Chen等[24]研究发现在氧化衰老模型小鼠皮肤组织中EGFR信号通路中非受体型酪氨酸蛋白激酶(Janus Kinase,JAK)、STAT靶蛋白表达下调,通过20 mg/kg EGCG处理8周后,发现小鼠皮肤组织中EGFR/JAK/STAT信号通路被显著激活,且衰老皮肤状况得到显著改善。
2.2 茶多酚抗癌活性及作用机制
癌症是继心血管疾病之后的全球第二大致死病因。随着植物活性成分不断开发,茶多酚对癌细胞增殖、迁移的抑制作用越来越受到研究人员的重视。茶多酚主要从调控细胞周期[25]、细胞信号转导[26]、血管生成[27]等方面抑制肿瘤细胞生长并诱导其凋亡。近年来研究表明,茶多酚可有效降低乳腺癌、肝癌、皮肤癌、口腔癌等的发病风险[28]。
细胞周期调控异常与细胞正常凋亡受到抑制是导致恶性肿瘤发生的常见现象。Li等[25]通过5 mg/mL茶多酚饲喂乳腺肿瘤模型小鼠27周后,发现小鼠乳腺肿瘤细胞周期处于G1期状态,且乳腺肿瘤较对照组变小,这可能与茶多酚通过下调小鼠体内细胞周期蛋白、细胞周期蛋白依赖性激酶等蛋白表达有关。流行病学调查显示,每天茶多酚的摄入量与女性乳腺癌发生的风险呈负相关[29],每天喝不少于5杯绿茶的乳腺癌患者乳腺癌复发率为16.7%,而每天喝低于4杯绿茶乳腺癌患者其复发率为24.3%,表明茶多酚摄入量越多则乳腺癌复发率则越低[30]。细胞凋亡蛋白酶(Caspase)作为细胞凋亡直接效应蛋白,通过引起细胞内结构蛋白和功能蛋白的降解诱导细胞凋亡,该信号通路可由促凋亡基因Bax(Bcl-2 Assaciated X protein,Bax)调控[31]。大量研究结果表明,茶多酚可使Bax、Caspase9、Caspase3等蛋白表达水平上调,并让B淋巴细胞瘤-2基因(B-cell lymphoma-2,Bcl2)等蛋白表达水平受到抑制,进而促进肿瘤细胞凋亡[32−34]。Bae等[35]通过用5 μmol/L EGCG处理多发性骨髓瘤细胞96 h,Caspase3信号通路被激活促使多发性骨髓瘤细胞凋亡。茶多酚的另一种化合物山奈酚对肿瘤细胞的抑制同样通过激活Caspase3信号通路进行,研究表明山奈酚可通过上调Caspase3蛋白表达,降低肿瘤细胞核转录因子p53、Bcl2等基因表达进而抑制肿瘤生长[36]。
肿瘤的生长与新血管的形成密不可分,在缺乏新血管形成的环境中,肿瘤细胞便会凋亡或坏死[27]。血管内皮生长因子(Vascular endothelial growth factor,VEGF)在肿瘤细胞中过表达且特异性促进肿瘤细胞的增殖与迁移,诱导肿瘤血管生成。研究表明EGCG抑制VEGF及其受体在细胞中的表达,进而抑制肿瘤生长和血管形成,这一方面可能与趋化因子配体中巨噬细胞的迁移和分化受到EGCG的限制,另一方面可能与磷脂酰肌醇3激酶(Phosphoinositide-3-Kinase,PI3K)/AKT信号通路受到抑制,使VEGF蛋白表达降低有关[37−38]。EGFR是调控肿瘤发生的重要细胞因子之一,肿瘤细胞的侵袭、转移、侵润、增殖等均与EGFR及其磷酸化蛋白的过表达有关[39]。近年来研究表明,EGFR信号通路中:雷帕霉素靶蛋白(Mammalian target of rapamycin,mTOR)、STAT3、丝裂原活化蛋白激酶激酶(Mitogen-activated protein kinase kinase,MEK)、细胞外调节蛋白激酶(Extracellular regulated protein,ERK)等靶蛋白磷酸化水平均可被EGCG显著下调,进而抑制肿瘤细胞的增殖与侵袭[40−41]。
高尔基膜蛋白1(Golgi membrane protein 1,GOLM1)是控制肿瘤发生的主要靶点之一,在肿瘤细胞中过表达,增强肿瘤细胞的增殖和侵袭能力。Xie等[42]通过研究证明,EGCG能增强对人乳腺癌细胞迁移的抑制能力,这可能与GOLM1上游AKT/糖原合成-3-激酶(Glycogen synthase kinase-3,GSK3β)信号通路被EGCG抑制进而下调GOLM1在肿瘤细胞内表达有关。细胞外基质重塑是肿瘤细胞增殖与侵袭的必要步骤,基质金属蛋白酶(Matrix metalloproteinase,MMPs)在该过程中参与细胞外基质重塑过程[43]。研究表明,当用50 μmol/L EGCG每日腹腔注射,连续3周,可显著抑制肿瘤小鼠体内MMP9蛋白及其mRNA表达水平[44],Bretaudeau等[45]通过体外实验表明人舌鳞状细胞癌细胞内MMP1、MMP2等蛋白表达水平受EGCG显著抑制。
2.3 茶多酚调节代谢综合症活性及作用机制
代谢综合症是由体内代谢紊乱引起的,可导致多种不良健康后果,主要表现为:高血压、血脂异常、胰岛素抵抗、肥胖等[46]。大量研究表明,多酚类物质可通过降低血压、改善糖脂代谢、降低肥胖,从而预防和改善代谢综合症[47−50]。通过对茶叶提取物没食子酸、黄烷醇类等进行实验,表明以多酚类为主的茶叶提取物能有效预防高糖高脂诱导的代谢紊乱大鼠高血压发生,这可能与多酚上调小鼠动脉组织中抗氧化酶表达,进而使体内促炎、促氧化标记物表达降低有关[51]。
胰岛素抵抗作为公认的代谢综合症致病因素,其致病机理源于胰岛素抵抗会导致糖脂代谢紊乱以及进一步造成的氧化应激损伤和炎症反应[52]。在经过茶多酚饲喂的胰岛素抵抗大鼠模型体内α-葡萄糖苷酶活性显著被抑制,血糖含量显著下降,脂质积累量降低,葡萄糖转运蛋白-4表达及易位增加,有效增加葡萄糖摄取能力[53−54]。夏燕平等[47]通过用800 mg/kg茶多酚对代谢综合症模型大鼠灌胃16周后发现,大鼠体内游离脂肪酸、胆固醇、甘油三酯水平下降,且胰岛素抵抗现象得以改善。王晓芹等[55]通过对胰岛素抵抗大鼠饲喂茶多酚8周发现,大鼠肝组织形态恢复至正常水平,血液中抗氧化指标 SOD、谷胱甘肽(Glutathione,GSH)含量升高,血脂指标因子含量明显下降,炎症因子:白细胞介素6(Interleukin-6,IL-6)、白细胞介素1β(Interleukin-1β,IL-1β)、TNF-α含量下降,血脂水平降低,炎症反应减轻,胰岛素抵抗现象明显改善,该机制可能与茶多酚对肝脏细胞转化生长因子β(Transforming growth factor-beta,TGF-β)/Smad信号通路激活有关。同时,用100 mg/kg EGCG饲喂胰岛素代谢紊乱的大鼠模型10周后,在修复受损胰岛β细胞、促进胰岛素分泌、降低血糖等方面可达到与二甲双胍相似效果[56]。
另外,Wen等[57]通过基因组学、代谢组学对茶多酚在肝功能保护方面研究发现,茶多酚对高脂血症所引起代谢功能紊乱的改善作用同样显著,可显著降低血清胆固醇、甘油三酯和甘油磷酸酯在肝脏的积累。Li等[58]通过实验也证明茶多酚可显著降低小鼠体重、脂肪组织质量、血清甘油三酯和胆固醇水平,并且通过降低炎症因子表达水平改善脂肪堆积、肝损伤、葡萄糖耐受不良和内毒素血症等症状,进而保护高脂诱导的肥胖小鼠肝功能免受代谢紊乱造成的损伤。研究表明,通过100 mg/kg EGCG处理,可显著减轻瘦素受体敲除的肥胖大鼠体重,这可能是EGCG通过激活AMPK信号通路,降低血清中甘油三酯、甘油二酯和胆固醇水平,并显著改善葡萄糖耐受不良等途径完成的[59]。
2.4 茶多酚抑菌活性及作用机制
茶多酚对自然界中多种革兰氏阴性和阳性菌均具有良好的抑制能力[60−62],在食品防腐保鲜、医疗卫生领域应用广泛。茶多酚的抑菌活性主要体现在:破坏微生物细胞膜通透性、破坏微生物酶活性、抑制微生物遗传物质合成、抑制微生物ATP合成等四方面[63]。近年来,多项研究表明茶多酚对金黄色葡萄球菌、枯草芽孢杆菌、铜绿假单胞菌、白色念珠菌等微生物抑制作用显著[60,62]。
在茶多酚的主要抑菌机制中,破坏细菌细胞膜通透性,促使膜内物质外流,是茶多酚最主要的抑菌途径。Wang等[64]通过茶多酚微乳液对铜绿假单胞杆菌和白色念珠菌抑菌活性研究表明,茶多酚可增加细菌细胞膜通透性,导致细胞内电解质、蛋白质和核酸外流,加速细胞死亡。同样,在大肠杆菌体内,超过50 mg/L的茶多酚处理会破坏其细胞膜完整性,导致细胞内蛋白质泄漏,促使大肠杆菌裂解死亡[65]。
同时,茶多酚可通过抑制细菌体内关键酶蛋白的合成,起到抑制细菌增殖与生长的作用。非核糖体肽合成酶是控制赭曲霉素合成的关键基因,茶多酚可通过抑制非核糖体肽合成酶的表达及赭曲霉素菌株的生长,进而使黑茶贮藏风险系数降低[66]。Gao等[67]通过代谢组学和蛋白组学途径对EGCG抗猪链球菌研究表明,病菌经EGCG处理后许多参与DNA复制、细胞壁和细胞膜合成以及毒力的差异表达相关蛋白下调。此外,通过抑制细菌体内能量合成也是茶多酚主要抑菌机制之一。研究表明:在经过2 g/L的茶多酚溶液处理之后,金黄色葡萄球菌和铜绿假单胞菌磷代谢水平受到严重抑制,进而通过抑制ATP的合成,使细菌失去生长所需的能量来源,加速细菌细胞凋亡[68]。近期研究表明,低浓度的茶多酚对益生菌的生长增殖具有促进作用,这对茶多酚在益生菌产品及食品安全领域研究具有积极意义[69]。
2.5 茶多酚抗辐射活性及作用机制
作为人体免疫的第一道防线,皮肤直接与外部环境接触。外部应激如:生物类、物理类、化学类因素等均会在不同程度上造成皮肤损伤。在诸多的外部因素中,辐射损伤更为常见,主要包括紫外辐射与电离辐射。茶多酚的抗辐射活性主要体现在:可减少辐射诱导的DNA损伤和凋亡;清除体内ROS并增强内源性细胞抗氧化系统。
紫外辐射可造成皮肤光老化,不仅会导致角质层细胞氧化应激,严重的还会造成皮肤细胞损伤及凋亡。紫外辐射造成的皮肤光老化主要表现为,细胞周期停滞不前,大量细胞集中在G1期,Jia等[70]使用25 μg/mL EGCG对受紫外辐射的人皮肤成纤维细胞细胞周期研究表明,EGCG可显著缓解G1期细胞增加,进而延缓因紫外辐射诱导的皮肤成纤维细胞衰老。一项研究表明,连续一周向辐射模型小鼠注射60 mg/kg 花青素后,小鼠衰老细胞内G0/G1期细胞比例下降,G2/M期、S期细胞比例显著增加[71]。李彤等[72]通过5% EGCG混合基质处理紫外诱导损伤大鼠模型,发现EGCG可通过抑制皮肤脂质过氧化并下调皮肤组织中凋亡蛋白含量抑制皮肤细胞凋亡,进而保护皮肤组织免受紫外辐射损伤。另外一项研究也表明,50 μmol/L原花青素处理紫外辐射皮肤细胞后,细胞内SOD、GSH-Px活性增强,有效抑制脂质过氧化,对紫外辐射诱导的皮肤细胞损伤具有良好的保护作用[73]。
电离辐射作用在人体不仅会导致皮肤衰老,还会带来许多疾病,对人体健康存在潜在威胁。研究表明在电离辐射小鼠模型中,小鼠的细胞活力显著下降,经茶多酚处理后细胞活力、肝细胞增殖能力呈剂量依赖性上升,相关凋亡蛋白表达受到抑制,进而保护机体免受电离辐射损伤[74]。细胞信号转导可通过调控ROS及相关抗氧化酶蛋白活性,从而对受电离辐射损伤的细胞组织起到保护作用。Xie等[75]通过使用2 μmol/L EGCG对电离辐射诱导的小鼠肠道损伤模型研究表明,EGCG可通过激活Nrf2信号通路及其下游抗氧化蛋白,清除小鼠体内ROS进而减少辐射诱导的 DNA 损伤和细胞凋亡,起到保护肠道作用。另外,Han等[76]研究结果表明,茶多酚类化合物的另一类单质茶黄素,同样通过激活Nrf2及其下游靶蛋白,降低ROS水平,进而改善电离辐射诱导的损伤。
2.6 茶多酚的其他生物活性
除以上生物活性功能之外,茶多酚还具备多种其他功效。在抗病毒方面,茶多酚一方面可破坏病毒包膜的完整性,同时抑制病毒神经氨酸酶(Neuraminidase,NA)活性,进而阻断病毒对宿主细胞的吸附,导致病毒细胞穿透能力丧失[77];另一方面,可通过与宿主细胞神经氨酸酶结合,进一步抑制病毒在宿主细胞的释放,从而抑制病毒的复制与扩散[78]。
此外,在细胞组织损伤保护方面茶多酚也发挥重要作用,连续用25 mg/kg EGCG注射HBV诱导的肝损伤模型小鼠6周后,小鼠体内肝细胞和巨噬细胞自噬功能被激活,进而改善肝损伤和纤维化[79]。而在糖尿病诱导的心肌组织损伤模型中,EGCG通过调节AMPK/mTOR信号通路激活细胞自噬,减轻心肌细胞纤维化,使心肌功能障碍、心肌肥厚和损伤等症状得以改善[80−81]。研究表明,10 μmol/L EGCG处理氧化损伤模型人血管内皮细胞24 h后,细胞内PI3K/AKT信号通路下调,mTOR磷酸化水平受到抑制,进而介导细胞自噬,增强抵抗氧化损伤能力[82]。
另外,EGCG在行为保护方面作用同样显著。用50 mg/kg EGCG对产后抑郁症小鼠模型腹腔注射10 d后,小鼠体内海马信号蛋白表达下调,GSK3β磷酸化水平上调,小鼠焦虑和抑郁行为得到缓解[83],表明茶多酚具有一定的神经保护功能。一项对阿尔茨海默症(Alzheimers disease,AD)大鼠模型研究也表明,通过250 mg/kg EGCG对AD大鼠进行灌胃7周后,大鼠海马Tua蛋白(Microtubule associated protein tau)磷酸化被抑制,β淀粉样蛋白表达下调,乙酰胆碱酯含量提高,进而改善AD大鼠抗氧化系统和学习记忆功能[84]。
3. 总结与展望
茶多酚作为茶树体内次级代谢产物,包含多种单体化合物且具备多羟基结构,因此具有多种生物活性。第一,茶多酚通过增强体内抗氧化酶活性与阴离子自由基含量,起到抗氧化功能。第二,茶多酚通过调控细胞周期蛋白、细胞信号转导、肿瘤血管生成及MMPS、Caspase蛋白酶表达等途径抑制癌细胞的侵袭并促使其凋亡,起到抗肿瘤功能。第三,茶多酚通过改善胰岛素抵抗改善体内糖脂代谢紊乱,并通过AMPK信号通路显著降低胆固醇、甘油三酯、磷酸甘油酯等含量,减少脂肪在体内的积累。第四,茶多酚通过破坏微生物膜结构、微生物酶活性,抑制微生物DNA、RNA复制,并调控能量代谢,起到抑菌作用。第五,茶多酚通过调控细胞周期、凋亡蛋白表达以及Nrf2信号通路,清除体内ROS并减少DNA损伤与细胞凋亡,起到抗辐射功能。而茶多酚的生物活性机制主要涉及到的信号通路包括:EGFR、PI3K/AKT/mTOR、AMPK、NF-κB、Nrf2等。
当前对茶多酚的研究主要集中在儿茶素类化合物方面,对黄酮类、酚酸类等其他茶多酚类物质的研究较为薄弱,为了更加全面了解茶多酚的生物活性,应加强茶多酚类物质中其他单体物质的功效研究。此外,茶多酚类物质多个羟基具有不稳定性,在进入细胞体内前易被氧化,如何使茶多酚被氧化前进入靶器官、靶细胞内发挥其最大功效也将成为今后的研究热点之一。另外,在研究茶多酚的某一目标信号通路作用机制的同时,要做到系统性、全面性,以防止茶多酚破坏靶细胞、靶器官以外的细胞与器官组织,从而使茶多酚生物活性达到最大化利用。本文通过综述近年来茶多酚类物质生物活性研究进展,总结了茶多酚主要功能及作用机制,以期为茶多酚类功能食品、日化产品、新型保鲜材料等产品开发提供一些借鉴。
-
表 1 茶多酚主要化学成分组成
Table 1 Main chemical composition of tea polyphenols
类别 化合物 分子式 参考文献 类别 化合物 分子式 参考文献 儿茶素类 表没食子儿茶素-3-没食子酸酯 C22H18O11 [12−13] 异牡荆素-2-O-鼠李糖苷 C27H30O14 没食子儿茶素没食子酸酯 C22H18O11 山奈酚-3-O-葡萄糖苷 C21H20O11 表儿茶素-3-没食子酸酯 C22H18O10 山奈酚-3-O-半乳糖苷 C21H20O11 儿茶素 C15H14O6 山奈酚-3-O-阿拉伯糖苷 C20H18O10 表儿茶素 C15H14O6 芹菜素-7-O-新橙皮苷 C27H30O14 儿茶素没食子酸酯 C22H18O10 杨梅素-3-O-芸香糖苷 C27H30O17 表没食子儿茶素 C15H14O7 异鼠李素-7-O-葡萄糖苷 C22H22O12 没食子儿茶素 C15H14O7 异鼠李素-3-O-葡萄糖苷 C22H22O12 黄酮、黄酮苷类 山奈素 C15H10O6 [12−15] 花青素、花白素类 飞燕草素-3-O-葡萄糖苷 C21H21ClO12 [10] 槲皮素 C15H10O7 飞燕草花青素 C15H11O7 杨梅素 C15H10O8 芙蓉花青素 C15H11O6 山奈酚 C15H10O6 翘摇紫苷元 C15H11O7 芦丁 C27H30O16 芙蓉花白素 C15H14O7 原花青素 B2 C30H26O12 飞燕草花白素 C15H14O8 槲皮素-3-O-葡萄糖苷 C21H20O12 飞燕草素-3-(6-对香豆酰基半乳糖苷) C30H27O14 槲皮素-3-O-半乳糖苷 C21H20O12 酚酸、缩酚酸类 没食子酸 C7H6O5 [12] 槲皮素-3-O-鼠李糖苷 C21H20O11 绿原酸 C16H18O9 槲皮素-3-O-葡萄糖苷-7-O-鼠李糖苷 C27H30O16 对香豆酸 C9H8O3 槲皮素-5-O-β-D-葡萄糖苷 C21H20O12 咖啡酸 C9H8O4 木犀草素-6-C-葡萄糖苷 C21H20O11 对香豆-3-鸡纳酸 C15H18O8 木犀草素-8-C-阿拉伯糖苷 C21H20O11 异绿原酸 C25H24O12 杨梅素-3-O-葡萄糖苷 C21H20O13 间双没食子酸 C14H10O9 杨梅素-3-O-半乳糖苷 C21H20O13 异阿魏酸 C10H10O4 柚皮素-7-O-芸香糖苷 C27H32O14 对香豆酰奎宁酸 C16H18O8 -
[1] 潘蓉, 赵学尽, 杜建斌, 等. 2021年中国茶叶进出口贸易情况简析[J]. 中国茶叶,2022,44(3):25−30. [PAN Rong, ZHAO Xuejin, DU Jianbin, et al. A brief analysis on tea import and export trade in China during 2021[J]. China Tea,2022,44(3):25−30.] PAN Rong, ZHAO Xuejin, DU Jianbin, et al. A brief analysis on tea import and export trade in China during 2021[J]. China Tea, 2022, 44(3): 25−30.
[2] 潘蓉, 余玉庚, 刘兰, 等. 2022年中国茶叶进出口贸易结构简析[J]. 中国茶叶,2023,45(4):31−35. [PAN Rong, YU Yugeng, LIU Lan, et al. A brief analysis on tea Import and export trade in China during 2022[J]. China Tea,2023,45(4):31−35.] PAN Rong, YU Yugeng, LIU Lan, et al. A brief analysis on tea Import and export trade in China during 2022[J]. China Tea, 2023, 45(4): 31−35.
[3] YAN Z M, ZHONG Y Z, DUAN Y H, et al. Antioxidant mechanism of tea polyphenols and its impact on health benefits[J]. Animal Nutrition,2020,2(6):115−123.
[4] HELIEH O S. Chronic inflammatory diseases and green tea polyphenols[J]. Nutrients,2017,6(9):561−574.
[5] LUZ J R D, LÓPEZ J A, FERREIRA M P, et al. In vitro antithrombotic, antitumor and antiangiogenic activities of green tea polyphenols and its main constituent epigallocatechin-3-gallate[J]. Processes,2022,11(1):76−81. doi: 10.3390/pr11010076
[6] WAN C P, OU Y J, LI M X, et al. Effects of green tea polyphenol extract and epigallocatechin-3-O-gallate on diabetes mellitus and diabetic complications:Recent advances[J]. Critical Reviews in Food Science and Nutrition,2022,19:21−29.
[7] 高婷, 袁芳艳, 刘泽文, 等. 茶多酚的抗菌抗病毒作用[J]. 动物医学进展,2022,43(4):107−111. [GAO Ting, YUAN Fangyan, LIU Zewen, et al. Antibacterial and antiviral effects of tea polyphenols[J]. Progress in Veterinary Medicine,2022,43(4):107−111.] doi: 10.3969/j.issn.1007-5038.2022.04.020 GAO Ting, YUAN Fangyan, LIU Zewen, et al. Antibacterial and antiviral effects of tea polyphenols[J]. Progress in Veterinary Medicine, 2022, 43(4): 107−111. doi: 10.3969/j.issn.1007-5038.2022.04.020
[8] 张文娟, 刘雪娜, 李丽维, 等. 茶多酚生理机制及其保健食品研发进展[J]. 食品研究与开发,2023,44(5):217−224. [ZHANG Wenjuan, LIU Xuena, LI Liwei, et al. Physiological mechanism of tea polyphenols and development of their health food[J]. Food Research and Development,2023,44(5):217−224.] ZHANG Wenjuan, LIU Xuena, LI Liwei, et al. Physiological mechanism of tea polyphenols and development of their health food[J]. Food Research and Development, 2023, 44(5): 217−224.
[9] 郑艳超, 於天, 郑志刚, 等. 茶黄素生物活性与开发应用的研究进展[J]. 中草药,2020,51(23):6095−6101. [ZHENG Yanchao, YU Tian, ZHENG Zhigang, et al. Research progress on biological activity and application development of theaflavins[J]. Chinese Traditional and Herbal Drugs,2020,51(23):6095−6101.] ZHENG Yanchao, YU Tian, ZHENG Zhigang, et al. Research progress on biological activity and application development of theaflavins[J]. Chinese Traditional and Herbal Drugs, 2020, 51(23): 6095−6101.
[10] 宛晓春. 茶叶生物化学[M]. 第三版. 北京:中国农业出版社, 2003:8−20. [WAN Xiaochun. Tea biochemistry[M]. The third edition. Beijing:China Agriculture Press, 2003:8−20.] WAN Xiaochun. Tea biochemistry[M]. The third edition. Beijing: China Agriculture Press, 2003: 8−20.
[11] XING L J, ZHANG H, QI R L, et al. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols[J]. Journal of Agricultural and Food Chemistry,2019,67(4):1029−1043. doi: 10.1021/acs.jafc.8b06146
[12] 游小妹, 韩奥迪, 李鑫磊, 等. 黄化茶树新品种‘茗冠’多茶类品质差异分析[J]. 食品工业科技,2023,44(23):287−297. [YOU Xiaomei, HAN Aodi, LI Xinlei, et al. Analysis of metabolites difference of the albino tea tree variety 'Mingguan'[J]. Science and Technology of Food Industry,2023,44(23):287−297.] YOU Xiaomei, HAN Aodi, LI Xinlei, et al. Analysis of metabolites difference of the albino tea tree variety 'Mingguan'[J]. Science and Technology of Food Industry, 2023, 44(23): 287−297.
[13] 朱婉, 吴颖, 黎晓湘, 等. 基于广泛靶向代谢组学结合高效液相色谱法分析‘紫娟’和‘迎霜’茶树花代谢产物差异[J]. 浙江大学学报(农业与生命科学版),2023,49(6):825−839. [ZHU Wan, WU Ying, LI Xiaoxiang, et al. Analysis of differential metabolites between 'Zijuan' and 'Yingshuang' tea flowers based on widely targeted metabolomics combined with high performance liquid chromatography[J]. Journal of Zhejiang University (Agriculture and Life Sciences),2023,49(6):825−839.] ZHU Wan, WU Ying, LI Xiaoxiang, et al. Analysis of differential metabolites between 'Zijuan' and 'Yingshuang' tea flowers based on widely targeted metabolomics combined with high performance liquid chromatography[J]. Journal of Zhejiang University (Agriculture and Life Sciences), 2023, 49(6): 825−839.
[14] 龚雪蛟, 秦琳, 黄颖博, 等. 茶园施肥模式对茶叶黄酮类及糖苷类代谢物含量的影响[J]. 植物营养与肥料学报,2022,28(10):1867−1883. [GONG Xuejiao, QIN Lin, HUANG Yingbo, et al. Effects of fertilization patterns on flavonoids and glycoside metabolites in tea[J]. Journal of Plant Nutrition and Fertilizers,2022,28(10):1867−1883.] GONG Xuejiao, QIN Lin, HUANG Yingbo, et al. Effects of fertilization patterns on flavonoids and glycoside metabolites in tea[J]. Journal of Plant Nutrition and Fertilizers, 2022, 28(10): 1867−1883.
[15] 赵熙, 赵洋, 杨培迪, 等. 茶树种质资源汝城白毛茶的代谢物差异研究[J]. 热带作物学报,2023,44(1):83−91. [ZHAO Xi, ZHAO Yang, YANG Peidi, et al. Metabonomic analysis of metabolic differences in rucheng baimaocha tea germplasm[J]. Chinese Journal of Tropical Crops,2023,44(1):83−91.] ZHAO Xi, ZHAO Yang, YANG Peidi, et al. Metabonomic analysis of metabolic differences in rucheng baimaocha tea germplasm[J]. Chinese Journal of Tropical Crops, 2023, 44(1): 83−91.
[16] ITO A, YANASE E. Study into the chemical changes of tea leaf polyphenols during Japanese black tea processing[J]. Food Research International (Ottawa, Ont.),2022,160:1−35.
[17] WANG J P, JIA R, CELI P, et al. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs[J]. Food & Function,2020,11(1):534−543.
[18] JAGDEO J, KURTTI A, HERNANDEZ S, et al. Novel vitamin C and E and green tea polyphenols combination serum improves photoaged facial skin[J]. Journal of Drugs in Dermatology:JDD,2021,20(9):996−1003. doi: 10.36849/JDD.5818
[19] YIN B, LIAN R, LI Z, et al. Tea polyphenols enhanced the antioxidant capacity and induced hsps to relieve heat stress injury[J]. Oxidative Medicine and Cellular Longevity,2021,2021:1−13.
[20] YU J, LI W, XIAO X, et al. (−)-Epicatechin gallate blocks the development of atherosclerosis by regulating oxidative stress in vivo and in vitro[J]. Food & Function,2021,12(18):8715−8727.
[21] XU R, ZHU M R, CAO J W, et al. Tea polyphenols protect the mammary gland of dairy cows by enhancing antioxidant capacity and regulating the TGF-β1/p38/JNK pathway[J]. Metabolites,2022,12(11):1−13.
[22] HUANG X Y, CHU Y, REN H, et al. Antioxidation function of EGCG by activating Nrf2/HO-1 pathway in mice with coronary heart disease[J]. Contrast Media & Molecular Imaging,2022,2022:1−8.
[23] 李佳, 安苗青, 吕晨豪, 等. 重组人源胶原蛋白对D-半乳糖致衰老小鼠的抗衰老作用及机制研究[J]. 食品工业科技,2023,44(10):343−352. [LI Jia, AN Miaoqing, LÜ Chenhao, et al. Anti-aging effects and mechanisms of recombinant human-derived collagen on aging mouse induced by d-galactose[J]. Science and Technology of Food Industry,2023,44(10):343−352.] LI Jia, AN Miaoqing, LÜ Chenhao, et al. Anti-aging effects and mechanisms of recombinant human-derived collagen on aging mouse induced by d-galactose[J]. Science and Technology of Food Industry, 2023, 44(10): 343−352.
[24] CHEN J M, LI Y F, ZHU Q Q, et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by D-galactose[J]. Mechanisms of Ageing and Development,2017,164:1−7. doi: 10.1016/j.mad.2017.03.007
[25] LI S Z, WU H X, TOLLEFSBOL T O. Combined broccoli sprouts and green tea polyphenols contribute to the prevention of estrogen receptor-negative mammary cancer via cell cycle arrest and inducing apoptosis in HER2/neu mice[J]. The Journal of Nutrition,2021,151(1):73−84. doi: 10.1093/jn/nxaa315
[26] ZHAO X, SHI X, LIU Q Q, et al. Tea polyphenols alleviates acetochlor-induced apoptosis and necroptosis via ROS/MAPK/NF-κB signaling in Ctenopharyngodon idellus kidney cells[J]. Aquatic Toxicology,2022,246:1−13.
[27] PARANGI S, O'REILLY M, CHRISTOFORI G, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth[J]. Proceedings of the National Academy of Sciences-PNAS,1996,93(5):2002−2007. doi: 10.1073/pnas.93.5.2002
[28] TRISHA A T, SHAKIL M H, TALUKDAR S, et al. Tea polyphenols and their preventive measures against cancer:Current trends and directions[J]. Foods,2022,11(21):3349−3368. doi: 10.3390/foods11213349
[29] INOUE M, ROBIEN K, WANG R, et al. Green tea intake, MTHFR/TYMS genotype and breast cancer risk:The Singapore Chinese health study[J]. Carcinogenesis,2008,29(10):1967−1972. doi: 10.1093/carcin/bgn177
[30] FRITZ H, SEELY D, KENNEDY D A, et al. Green tea and lung cancer:A systematic review[J]. Integrative Cancer Therapies,2013,12(1):7−24. doi: 10.1177/1534735412442378
[31] WOLF P, SCHOENIGER A, EDLICH F. Edlich, pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochimica et biophysica acta[J]. Molecular Cell Research,2022,21(11):1−20.
[32] YIN Z F, LI J, KANG L, et al. Epigallocatechin-3-gallate induces autophagy-related apoptosis associated with LC3B II and Beclin expression of bladder cancer cells[J]. Journal of Food Biochemistry,2021,45(6):e13758−e13765.
[33] KANG Q, ZHANG X, CAO N, et al. EGCG enhances cancer cells sensitivity under Coγ radiation based on miR-34a/Sirt1/p53[J]. Food and Chemical Toxicology,2019,133:1−9.
[34] LA X, ZHANG L, LI Z, et al. (-)-Epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway[J]. Journal of Agricultural and Food Chemistry,2019,67(9):2510−2518. doi: 10.1021/acs.jafc.8b06665
[35] BAE J, KUMAZOE M, SU J, et al. The anti-cancer effect of epigallocatechin-3-O-gallate against multiple myeloma cells is potentiated by 5,7-dimethoxyflavone[J]. FEBS Open Bio,2023,13(11):2147−2156. doi: 10.1002/2211-5463.13708
[36] NANDI S K, PRADHAN A, DAS B, et al. Kaempferol attenuates viability of ex-vivo cultured post-NACT breast tumor explants through downregulation of p53 induced stemness, inflammation and apoptosis evasion pathways[J]. Pathology-Research and Practice,2022,237:154029. doi: 10.1016/j.prp.2022.154029
[37] CHEN L, GUO X, HU Y, et al. Epigallocatechin-3-gallate sensitises multidrug-resistant oral carcinoma xenografts to vincristine sulfate[J]. FEBS Open Bio,2020,10(7):1403−1413. doi: 10.1002/2211-5463.12905
[38] WANG J, MAN G C W, CHAN T H, et al. A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer[J]. Cancer Letters,2018,412:10−20. doi: 10.1016/j.canlet.2017.09.054
[39] KJÆR I M, OLSEN D A, BRANDSLUND I, et al. Dysregulated EGFR pathway in serum in early-stage breast cancer patients:A case control stud[J]. Scientific Reports,2020,10(1):6714−6122. doi: 10.1038/s41598-020-63375-z
[40] MINNELLI C, CIANFRUGLIA L, LAUDADIO E, et al. Effect of epigallocatechin-3-gallate on EGFR signaling and migration in non-small cell lung cancer[J]. International Journal of Molecular Sciences,2021,22(21):1−14.
[41] WENG L X, WANG G H, YAO H, et al. Epigallocatechin gallate inhibits the growth of salivary adenoid cystic carcinoma cells via the EGFR/Erk signal transduction pathway and the mitochondria apoptosis pathway[J]. Neoplasma,2017,64(4):563−570. doi: 10.4149/neo_2017_410
[42] XIE L, YI J, SONG Y J, et al. Suppression of GOLM1 by EGCG through HGF/HGFR/AKT/GSK-3β/β-catenin/c-Myc signaling pathway inhibits cell migration of MDA-MB-23[J]. Food and Chemical Toxicology,2021,157:1−10.
[43] KAI F B, DRAIN A P, WEAVER V M. The extracellular matrix modulates the metastatic journey[J]. Developmental Cell,2019,49(3):332−345. doi: 10.1016/j.devcel.2019.03.026
[44] LUO K W, WEI C, LUNG W Y, et al. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9[J]. The Journal of Nutritional Biochemistry,2017,41:56−64. doi: 10.1016/j.jnutbio.2016.12.004
[45] BRETAUDEAU C, BAUD S, DUPONT-DESHORGUE A, et al. AG-9, an Elastin-derived peptide, increases in vitro oral tongue carcinoma cell invasion, through an increase in MMP-2 secretion and MT1-MMP expression, in a RPSA-dependent manner[J]. Biomolecules,2020,11(1):1−14. doi: 10.3390/biom11010001
[46] 吴铁良. 代谢综合症诊治进展[J]. 现代预防医学,2010,37(16):3200−3201. [WU Tieliang. Progress in diagnosis and treatment of metabolic syndrome[J]. Modern Preventive Medicine,2010,37(16):3200−3201.] WU Tieliang. Progress in diagnosis and treatment of metabolic syndrome[J]. Modern Preventive Medicine, 2010, 37(16): 3200−3201.
[47] 夏燕萍, 俞茂华, 陈蔚, 等. 茶多酚改善代谢综合症大鼠糖脂代谢的作用机制研究[J]. 中国现代医学杂志,2016,26(17):1−6. [XIA Yanping, YU Maohua, CHEN Wei, et al. Effect of tea polyphenols on improving insulin resistance of rats with metabolic syndrome[J]. China Journal of Modern Medicine,2016,26(17):1−6.] doi: 10.3969/j.issn.1005-8982.2016.17.001 XIA Yanping, YU Maohua, CHEN Wei, et al. Effect of tea polyphenols on improving insulin resistance of rats with metabolic syndrome[J]. China Journal of Modern Medicine, 2016, 26(17): 1−6. doi: 10.3969/j.issn.1005-8982.2016.17.001
[48] XU L L, LI W W, CHEN Z Q, et al. Inhibitory effect of epigallocatechin-3-O-gallate on α-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells[J]. International Journal of Biological Macromolecules,2019,125:605−611. doi: 10.1016/j.ijbiomac.2018.12.064
[49] 杨宽, 钱卫东, 秦蓓. 茶多酚对高脂血症大鼠血脂代谢和肝组织MDA\T-SOD含量的影响[J]. 中国油脂,2019,44(1):70−73,96. [YANG Kuan, QIAN Weidong, QIN Bei. Effects of tea polyphenols on blood lipid metabolism and contents of MDA and T-SOD in liver tissue of hyperlipidemia rat[J]. China Oils and Fats,2019,44(1):70−73,96.] doi: 10.3969/j.issn.1003-7969.2019.01.016 YANG Kuan, QIAN Weidong, QIN Bei. Effects of tea polyphenols on blood lipid metabolism and contents of MDA and T-SOD in liver tissue of hyperlipidemia rat[J]. China Oils and Fats, 2019, 44(1): 70−73,96. doi: 10.3969/j.issn.1003-7969.2019.01.016
[50] CHEN R H, LAI X F, XIANG L M, et al. Aged green tea reduces high-fat diet-induced fat accumulation and inflammation via activating the AMP-activated protein kinase signaling pathway[J]. Food & Nutrition Research,2022,66:1−12.
[51] MARIO D L F M, MARÍA D L F F, MARTA R C, et al. Supplementation with two new standardized tea extracts prevents the development of hypertension in mice with metabolic syndrome[J]. Antioxidants,2022,11(8):1573−1590. doi: 10.3390/antiox11081573
[52] YANG C S, ZHANG J, ZHANG L, et al. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea[J]. Molecular Nutrition & Food Research,2016,60(1):160−174.
[53] CHENG J, TAN Y, ZHOU J, et al. Green tea polyphenols ameliorate metabolic abnormalities and insulin resistance by enhancing insulin signalling in skeletal muscle of Zucker fatty rats[J]. Clinical Science (London, England:1979),2020,134(10):1167−1180. doi: 10.1042/CS20200107
[54] KAN L, CAPUANO E, FOGLIANO V, et al. Inhibition of α-glucosidases by tea polyphenols in rat intestinal extract and Caco-2 cells grown on Transwell[J]. Food chemistry,2021,361:1−8.
[55] 王晓芹, 邓小燕, 于晓斌, 等. 茶多酚通过降脂/抗炎/抗氧化以及调控TGF-β/Smad信号通路缓解2型糖尿病[J]. 中药药理与临床,2018,34(3):46−50. [WANG Xiaoqin, DENG Xiaoyan, YU Xiaobin, et al. Green tea polyphenols ameliorates type 2 diabetes mellitus through lipid-lowering, anti-oxidation, anti-inflammation and regulating TGF-β/Smad signal pathway[J]. Pharmacology and Clinics of Chinese Materia,2018,34(3):46−50.] WANG Xiaoqin, DENG Xiaoyan, YU Xiaobin, et al. Green tea polyphenols ameliorates type 2 diabetes mellitus through lipid-lowering, anti-oxidation, anti-inflammation and regulating TGF-β/Smad signal pathway[J]. Pharmacology and Clinics of Chinese Materia, 2018, 34(3): 46−50.
[56] ZHU T T, LI M H, ZHU M L, et al. Epigallocatechin-3-gallate alleviates type 2 diabetes mellitus via β-cell function improvement and insulin resistance reduction[J]. Iranian Journal of Basic Medical Sciences,2022,25(4):483−488.
[57] WEN J J, LI M Z, CHEN C H, et al. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota[J]. Food Chemistry,2023,404:1−13.
[58] LI A, WANG J, KOU R X, et al. Polyphenol-rich oolong tea alleviates obesity and modulates gut microbiota in high-fat diet-fed mice[J]. Frontiers in Nutrition,2022,9:1−14.
[59] WU G H, CHENG H J, GUO H M, et al. Tea polyphenol EGCG ameliorates obesity-related complications by regulating lipidomic pathway in leptin receptor knockout rats[J]. The Journal of Nutritional Biochemistry,2023,118:1−18.
[60] 段宙位, 李鹏, 何艾, 等. 不同方法提取的鹧鸪茶多酚抗氧化及抑菌性比较[J]. 热带作物学报,2021,42(3):847−853. [DUAN Zhouwei, LI Peng, HE Ai, et al. Antioxidant and bacteriostasis activity of flavanoid from Mallotus oblongifolius by different extraction methods[J]. Chinese Journal of Tropical Crops,2021,42(3):847−853.] doi: 10.3969/j.issn.1000-2561.2021.03.033 DUAN Zhouwei, LI Peng, HE Ai, et al. Antioxidant and bacteriostasis activity of flavanoid from Mallotus oblongifolius by different extraction methods[J]. Chinese Journal of Tropical Crops, 2021, 42(3): 847−853. doi: 10.3969/j.issn.1000-2561.2021.03.033
[61] 李峰, 邓江丽, 陈雯雯, 等. 儿茶素对野油菜黄单胞菌的抑菌作用[J]. 云南农业大学学报(自然科学),2021,36(2):215−222. [LI Feng, DENG Jiangli, CHEN Wenwen, et al. Inhibitory effect of catechin against Xanthomonas campestris[J]. Journal of Yunnan Agricultural University (Natural Science),2021,36(2):215−222.] LI Feng, DENG Jiangli, CHEN Wenwen, et al. Inhibitory effect of catechin against Xanthomonas campestris[J]. Journal of Yunnan Agricultural University (Natural Science), 2021, 36(2): 215−222.
[62] 陈琛, 徐尤美, 蔺蓓蓓, 等. 秦岭绿茶茶多酚抑菌活性及其机理研究[J]. 四川农业大学学报,2019,37(6):821−827. [CHEN Chen, XU Youmei, LIN Beibei, et al. Antibacterial activity and mechanism of green tea polyphenols from Qinling Mountains[J]. Journal of Sichuan Agricultural University,2019,37(6):821−827.] CHEN Chen, XU Youmei, LIN Beibei, et al. Antibacterial activity and mechanism of green tea polyphenols from Qinling Mountains[J]. Journal of Sichuan Agricultural University, 2019, 37(6): 821−827.
[63] 毕可, 刘月, 杨杰, 等. 茶多酚结合热处理对枯草杆菌芽孢细胞结构与能量代谢的影响[J]. 中国食品学报,2023,23(3):138−146. [BI Ke, LIU Yue, YANG Jie, et al. Effect of tea polyphenols combined with heat treatment on the cellular structure and energy metabolism of Bacillus subtilis[J]. Journal of Chinese Institute of Food Science and Technology,2023,23(3):138−146.] BI Ke, LIU Yue, YANG Jie, et al. Effect of tea polyphenols combined with heat treatment on the cellular structure and energy metabolism of Bacillus subtilis[J]. Journal of Chinese Institute of Food Science and Technology, 2023, 23(3): 138−146.
[64] WANG W, CHEN Y F, WEI Z F, et al. Microemulsion of ginnamon essential oil formulated with tea polyphenols, gallic acid, and tween 80:Antimicrobial properties, stability and mechanism of action[J]. Microorganisms,2022,11(1):2−17. doi: 10.3390/microorganisms11010002
[65] 冉强三, 金纪玥, 冯萃敏, 等. EGCG-Cu对水中大肠杆菌的杀灭性能研究[J]. 应用化工,2021,50(5):1227−1230. [RAN Qiangsan, JIN Jiyue, FENG Cuimin, et al. Effect of tea polyphenols combined with heat treatment on the cellular structure and energy metabolism of Bacillus subtilis[J]. Applied Chemical Industry,2021,50(5):1227−1230.] doi: 10.3969/j.issn.1671-3206.2021.05.015 RAN Qiangsan, JIN Jiyue, FENG Cuimin, et al. Effect of tea polyphenols combined with heat treatment on the cellular structure and energy metabolism of Bacillus subtilis[J]. Applied Chemical Industry, 2021, 50(5): 1227−1230. doi: 10.3969/j.issn.1671-3206.2021.05.015
[66] ZHAO Y Q, JIA W B, LIAO S Y, et al. Dietary assessment of ochratoxin A in Chinese dark tea and inhibitory effects of tea polyphenols on ochratoxigenic Aspergillus niger[J]. Frontiers in Microbiology,2022,13:1−11.
[67] GAO T, YE F, TAN Y Q, et al. Metabolomics and proteomics analyses revealed mechanistic insights on the antimicrobial activity of epigallocatechin gallate against Streptococcus suis[J]. Frontiers in Cellular and Infection Microbiology,2022,12:1358−1372.
[68] 钱丽红. 几种天然保鲜剂的抑菌机理[D]. 上海:上海海洋大学, 2010. [QIAN Lihong. Antimicrobial mechanisms of several natural preservatives[D]. Shanghai:Shanghai Ocean University, 2010.] QIAN Lihong. Antimicrobial mechanisms of several natural preservatives[D]. Shanghai: Shanghai Ocean University, 2010.
[69] 江福林, 卢云浩, 何强. 茶多酚对植物乳杆菌\金黄色葡萄球菌和大肠杆菌生长的双向调节作用[J]. 食品工业科技,2023,44(22):152−159. [JIANG Fulin, LU Yunhao, HE Qiang. Dual-directional regulation of tea polyphenols on the growth of Lactobacillus plantarum, Staphylococcus aureus, and Escherichia coli[J]. Science and Technology of Food Industry,2023,44(22):152−159.] JIANG Fulin, LU Yunhao, HE Qiang. Dual-directional regulation of tea polyphenols on the growth of Lactobacillus plantarum, Staphylococcus aureus, and Escherichia coli[J]. Science and Technology of Food Industry, 2023, 44(22): 152−159.
[70] JIA Y Y, MAO Q Y, YANG J Y, et al. (-)-Epigallocatechin-3-gallate protects human skin fibroblasts from ultraviolet a induced photoaging[J]. Clin Cosmet Investig Dermatol,2023,16:149−159. doi: 10.2147/CCID.S398547
[71] 陈彩云, 纪雨含, 李宁, 等. 紫薯花青素调节p53-p21Waf1/Cip1信号通路对辐射致造血干/祖细胞衰老的保护[J/OL]. 食品科学:1−10 [2023-08-25]. http://kns.cnki.net/kcms/detail/11.2206.ts.20221208.0801.001.html. [CHEN Caiyun, JI Yuhan, LI Ning, et al. Protective effect of solanum tuberdsm anthocyanin against radiation-induced hematopoietic stem/progenitor cell senescence via p53-p21Waf1/Cip1 signaling pathway[J/OL]. Food Science: 1−10 [2023-08-25]. http://kns.cnki.net/kcms/detail/11.2206.ts.20221208.0801.001.html.] CHEN Caiyun, JI Yuhan, LI Ning, et al. Protective effect of solanum tuberdsm anthocyanin against radiation-induced hematopoietic stem/progenitor cell senescence via p53-p21Waf1/Cip1 signaling pathway[J/OL]. Food Science: 1−10 [2023-08-25]. http://kns.cnki.net/kcms/detail/11.2206.ts.20221208.0801.001.html.
[72] 李彤, 卢浩, 陈际名, 等. 表没食子儿茶素没食子酸酯对紫外线损伤小鼠皮肤的保护作用[J]. 中国皮肤性病学杂志,2016,30(11):1107−1111. [LI Tong, LU Hao, CHEN Jiming, et al. Effect of EGCG on protection of mice skin injury induced by ultraviolet A and ultraviolet B[J]. The Chinese Journal of Dermatovenereology,2016,30(11):1107−1111.] LI Tong, LU Hao, CHEN Jiming, et al. Effect of EGCG on protection of mice skin injury induced by ultraviolet A and ultraviolet B[J]. The Chinese Journal of Dermatovenereology, 2016, 30(11): 1107−1111.
[73] 董丽红, 罗牡康, 张名位, 等. 荔枝果壳原花青素对中波紫外线诱导HaCaT细胞氧化损伤的保护作用[J]. 食品科学,2022,43(21):233−240. [DONG Lihong, LUO Mukang, ZHANG Mingwei, et al. Protective effect of procyanidins from litchi pericarp on ultraviolet B-induced oxidative damage in HaCaT cells[J]. Food Science,2022,43(21):233−240.] DONG Lihong, LUO Mukang, ZHANG Mingwei, et al. Protective effect of procyanidins from litchi pericarp on ultraviolet B-induced oxidative damage in HaCaT cells[J]. Food Science, 2022, 43(21): 233−240.
[74] 陈晨. 电离辐射诱发miRNA表达谱改变及EGCG辐射防护分子机制的初步研究[D]. 郑州:郑州大学, 2017. [CHEN Chen. Ionizing radiation induced changes of miRNA expression profile and the preliminary study on antiradiation effect of epigallocatechin gallate[D]. Zhengzhou:Zhengzhou University, 2017.] CHEN Chen. Ionizing radiation induced changes of miRNA expression profile and the preliminary study on antiradiation effect of epigallocatechin gallate[D]. Zhengzhou: Zhengzhou University, 2017.
[75] XIE L W, CAI S, ZHAO T S, et al. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo[J]. Free Radical Biology and Medicine,2020,161:175−186. doi: 10.1016/j.freeradbiomed.2020.10.012
[76] HAN X D, ZHANG J L, XUE X L, et al. Theaflavin ameliorates ionizing radiation-induced hematopoietic injury via the NRF2 pathway[J]. Free Radical Biology and Medicine,2017,113:59−70. doi: 10.1016/j.freeradbiomed.2017.09.014
[77] KIM M, KIM S Y, LEE H W, et al. Inhibition of influenza virus internalization by (−)-epigallocatechin-3-gallate[J]. Antiviral Research,2013,100(2):460−472. doi: 10.1016/j.antiviral.2013.08.002
[78] MULLER P, DOWNARD K M. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry[J]. Journal of Pharmaceutical and Biomedical Analysis,2015,111:222−230. doi: 10.1016/j.jpba.2015.03.014
[79] HE M J, CHU T H, WANG Z T, et al. Inhibition of macrophages inflammasome activation via autophagic degradation of HMGB1 by EGCG ameliorates HBV-induced liver injury and fibrosis[J]. Frontiers in Immunology,2023,14:1−16.
[80] JIA Q, YANG R, MEHMOOD S, et al. Epigallocatechin-3-gallate attenuates myocardial fibrosis in diabetic rats by activating autophagy[J]. Experimental Biology and Medicine (Maywood, N.J.),2022,247(17):1591−1600. doi: 10.1177/15353702221110646
[81] GUI L M, WANG F X, HU X K, et al. Epigallocatechin gallate protects diabetes mellitus rats complicated with cardiomyopathy through TGF-β1/JNK signaling pathway[J]. Current Pharmaceutical Design,2022,28(33):2758−2770. doi: 10.2174/1381612828666220902115437
[82] MENG J, CHEN Y, WANG J, et al. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway[J]. Annals of Translational Medicine,2020,8(5):200−211. doi: 10.21037/atm.2020.01.92
[83] XU F, WU H, XIE L H, et al. Epigallocatechin-3-gallate alleviates gestational stress-induced postpartum anxiety and depression-like behaviors in mice by downregulating semaphorin3A and promoting GSK3β phosphorylation in the hippocampus[J]. Frontiers in Molecular Neuroscience,2023,15:1−12.
[84] NAN S J, WANG P, ZHANG Y Z, et al. Epigallocatechin-3-gallate provides protection against Alzheimers disease induced learning and memory impairments in rats[J]. Molecular Nutrition & Food Research,2021,15:2013−2024.
-
期刊类型引用(13)
1. 高树财,闫格,胡桂芳,高火亮. 茶饮料中茶多酚含量测定方法的研究. 标准科学. 2025(01): 99-103 .  百度学术
百度学术
2. 邓婷婷,皮锦蝉,彭小平,姚于飞,李义全,李文娟. 茶多酚基于活性氧-线粒体途径发挥心血管保护作用的研究进展. 食品工业科技. 2025(04): 434-442 .  本站查看
本站查看
3. 蔡妙莹,钟雪莲,胡新涛,袁炎茹,梁花. 改性纤维中总多酚含量的测定. 中国纤检. 2025(03): 66-69 .  百度学术
百度学术
4. 苟祎,夏丽飞,马玉青,杨恺清,吴致远,普金霞,叶红,申时全. 不同花期紫娟茶树花的品质分析及加工工艺研究. 食品科技. 2025(02): 96-104 .  百度学术
百度学术
5. 黄珊由美,林东艺,马成英,荣杰峰,孙威江,黄艳. 茶饮料泡沫生成机理及控制技术研究进展. 茶叶科学. 2025(02): 181-190 .  百度学术
百度学术
6. 田宝明,叶芃,孔俊涛,杨开,孙培龙,陈红平,王舰,张相春. 茶多酚自组装纳米泡腾片的研制. 中国茶叶加工. 2024(02): 33-40 .  百度学术
百度学术
7. 陈俊婕,劳颖仪,陈晓维,余元善,温靖,吴继军,徐玉娟,肖更生,杨继国,唐延天,胡腾根. 茶多酚的功能活性及稳态化研究进展. 中国果菜. 2024(08): 25-31 .  百度学术
百度学术
8. 葛天睿,黄雪君,张娜,陈小强,沙如意,毛建卫. 茶多酚在医药和食品领域的应用研究进展. 食品安全质量检测学报. 2024(17): 176-184 .  百度学术
百度学术
9. 朱旋,田芸,孙海燕,王瑞,金文刚,陈琛. 茶多酚结合超高压处理对鲜切天麻贮藏品质的影响. 北方园艺. 2024(20): 83-90 .  百度学术
百度学术
10. 韩舒婷,华梓延,房耀维,杨光,侯晓月,周文梅,唐明,刘姝. 嗜热链球菌FUA329发酵绿茶浸提液及贮藏特性评价. 食品工业科技. 2024(21): 322-328 .  本站查看
本站查看
11. 辛皎瑜,钟雅静,沈启东,敖娜,梁玉文,付晶,刘春朋. 鹧鸪源大肠杆菌分离鉴定及茶多酚抑菌效果研究. 东北农业大学学报. 2024(07): 51-59 .  百度学术
百度学术
12. 陈晓真,韦怡含,蓝平,封余贤,黎笑笑,关欣,冯杰,何日梅. 茶多酚的提取纯化技术研究进展. 中国酿造. 2024(11): 20-25 .  百度学术
百度学术
13. 吴彤,刘丽莉,程伟伟,丁玥,徐宝成. NaCl和茶多酚对碱诱导鸡蛋清凝胶流变学特性及结构的影响. 食品与发酵工业. 2024(23): 194-201 .  百度学术
百度学术
其他类型引用(8)





 下载:
下载:
 下载:
下载:



