Polysaccharide of Atractylodes macrocephala Koidz Alleviate Kidney Injury Induced by Cyclophosphamide in Mice through Arachidonic Acid Metabolic Pathway
-
摘要: 本研究旨在探讨白术多糖(PAMK)对环磷酰胺(CTX)诱导的小鼠肾脏损伤的影响及潜在的作用机制。选用100只42~43日龄雄性C57BL/6小鼠随机分为四组,白术多糖(PAMK)组和白术多糖+环磷酰胺(PAMK+CTX)组灌胃200 mg/kg PAMK,1次/d;对照(Control)组和环磷酰胺(CTX)组给予等量的生理盐水。实验第25~27 d,CTX组和PAMK+CTX组腹腔注射100 mg/kg CTX,1次/d;Control组和PAMK组注射等量生理盐水。实验第35 d采集肾脏进行组织学观察,氧化应激检测和转录组测序。结果显示,与CTX组相比,PAMK+CTX组肾脏损伤有所缓解;肾脏中丙二醛量显著下降(P<0.05)、谷胱甘肽过氧化物酶、超氧化物歧化酶、过氧化氢酶活性和总抗氧化能力显著升高(P<0.05)。为了进一步探究PAMK缓解CTX诱导肾损伤的调控机制,本实验进行了肾脏转录组测序。结果显示,在Control组vs CTX组和CTX组vs PAMK+CTX组分别鉴定到493个和333个差异表达基因(DEGs)。两组DEGs功能富集分析结果发现DEGs富集在花生四烯酸代谢等信号通路。花生四烯酸通路相关基因检测结果显示,与Control组相比,CTX组Cyp2b9 、PTGS1、NF-κB和Mfsd2a mRNA表达量显著升高(P<0.05);与CTX组相比,PAMK+CTX组Cyp2c65 、BCL6 mRNA表达量显著升高(P<0.05),Cyp2b9 、PTGS1 和Mfsd2a mRNA表达量显著降低(P<0.05)。综上所述,PAMK可能通过花生四烯酸代谢通路缓解小鼠肾脏氧化应激,从而降低CTX诱导的小鼠肾脏损伤。Abstract: This study aimed to investigate the effects of the polysaccharide of Atractylodes macrocephala Koidz (PAMK) on cyclophosphamide (CTX)-induced renal injury in mice and its potential underlying mechanisms. One hundred male C57BL/6 mice, aged 42~43 days, were randomly divided into four groups, with five repetitions in each group and five mice in each repetition. The PAMK group and PAMK+CTX group were orally administered 200 mg/kg PAMK once daily, while the control group and CTX group were given an equivalent amount of saline. From days 25~27 of the experiment, the CTX group and PAMK+CTX group were intraperitoneally injected with 100 mg/kg CTX once daily, while the control group and PAMK group were injected with an equivalent amount of saline. On day 35 of the experiment, the kidneys were collected for histological observation, oxidative stress detection, and transcriptome sequencing. The results showed that compared with the CTX group, the renal injury in the PAMK+CTX group was alleviated. The content of malondialdehyde (MDA) in the kidneys was significantly decreased (P<0.05), while the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (T-AOC) were significantly increased (P<0.05). To further explore the regulatory mechanisms of PAMK in alleviating CTX-induced renal injury, transcriptome sequencing of the kidneys was performed. The results showed that compared with the control group, 493 differentially expressed genes (DEGs) were identified in the CTX group vs control group comparison, and 333 DEGs were identified in the CTX group vs PAMK+CTX group comparison. Functional enrichment analysis of the DEGs in both groups revealed significant enrichment in signaling pathways related to arachidonic acid metabolism. The expression levels of arachidonic acid pathway-related genes were examined, and it was found that compared with the control group, the mRNA expression levels of Cyp2b9, PTGS1, NF-κB and Mfsd2a were significantly increased in the CTX group (P<0.05). On the other hand, in the PAMK+CTX group, the expression levels of Cyp2c65 and BCL6 were significantly increased (P<0.05), while the expression levels of Cyp2b9, PTGS1and Mfsd2a were significantly decreased (P<0.05). In conclusion, PAMK may alleviate oxidative stress in the kidneys of mice and reduce CTX-induced renal injury through the arachidonic acid metabolism pathway.
-
白术多糖(Polysaccharide of Atractylodes macrocephala Koidz,PAMK)是中药白术最主要的有效生物活性成分之一。研究表明,白术多糖具有抗炎、抗氧化、降血糖与免疫调节等多种生物活性[1−3]。在免疫方面,PAMK通过改善肝脂代谢和增加抗氧化酶活性,有效地保护肝脏免受高能量低蛋白饮食引起的损伤,进一步减轻肝脏负担,从而显著提升免疫系统的功能[4],并通过介导信号通路减轻肝脏炎症损伤和氧化应激[5]。此外,PAMK还可以通过提高白细胞数量、修复骨髓、脾脏及胸腺的组织结构、提高白细胞功能等途径减轻环磷酰胺对小鼠白细胞的抑制和破坏作用,并对正常小鼠的淋巴细胞功能具有明显的提升作用[6]。
肾脏是哺乳动物重要的排泄器官,在维持体内平衡中起着至关重要的作用。环磷酰胺(Cyclophosphamide,CTX)是一种常用的抗癌药物,但它也会产生许多副作用,包括尿毒性或肾毒性[7]。CTX的代谢转化导致两种代谢物的形成:磷酰胺芥末和丙烯醛,磷酰胺芥被认为具有抗肿瘤活性,而丙烯醛作为一种生物半衰期短的高活性代谢物,可能是CTX诱导肾损伤的原因[8−9]。丙烯醛可以产生高活性氧(ROS),干扰组织抗氧化防御系统,对哺乳动物细胞具有致突变性[10−12],为了避免这些氧化毒性副作用,当前常用抗氧化剂对丙烯醛进行解毒,比如芍药苷已被用于缓解CTX引起的肾损害[13]。Gunes等[14]发现香芹酚能够作为一种抗氧化剂,保护生物分子,如膜脂,免受自由基诱导的损伤,并且能够减少CTX注射后大鼠的氧化应激的反应。然而,PAMK对CTX诱导的小鼠肾损伤的影响尚未见报道。
本研究拟通过注射CTX建立小鼠肾损伤模型,以探究PAMK对CTX诱导的小鼠肾损伤的影响,并通过转录组测序掌握PAMK缓解CTX诱导的小鼠肾损伤的作用机制,为后续研究PAMK缓解CTX诱导的机体损伤提供参考。
1. 材料与方法
1.1 材料与仪器
PAMK 纯度95%,中国西安杨凌慈源生物技术公司;注射用环磷酰胺国药准字H14023686 山西普德药业股份有限公司;SPF级C57BL/6雄性小鼠 42~43日龄,100只,购自广东省医学实验动物中心;超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)、总抗氧化能力(T-AOC)、丙二醛(MDA)、过氧化氢酶(CAT)试剂盒 南京建成生物工程研究所;预混型定量用反转录试剂盒 日本TAKARA公司;Trizol试剂 美国Thermo Fisher公司;SYBR Green Master Mix PCR 中国康润生物技术公司。
Epoch超微量微孔板分光光度计 美国伯腾仪器有限公司;7500 REAL TIME PCR SYSTEM实时荧光定量PCR仪、VERITI梯度PCR仪 美国ABI公司;Micro 21R高速冷冻离心机 美国Thermo Fisher公司;Bioanalyzer 2100 2100生物分析系统 美国Agilent公司;NovaSeq 6000基因DNA测序仪 美国Illumina公司。
1.2 实验方法
1.2.1 试验设计与样品采集
100只42~43日龄雄性C57BL/6小鼠被随机分为四组,分别为对照组(Control)、环磷酰胺组(CTX)、白术多糖组(PAMK)、白术多糖+环磷酰胺组(PAMK+CTX),25只/组,每组5个重复,每个重复5只。实验期间,PAMK组和PAMK+CTX组灌胃200 mg/kg PAMK 1次/d;Control组和CTX组给予等量的生理盐水。实验第25~27 d,CTX组和PAMK+CTX组腹腔注射100 mg/kg CTX 1次/d;Control组和PAMK组注射等量生理盐水。实验第35 d,每组随机选取5只小鼠采集肾脏组织,取部分肾脏组织立即冻入液氮,−80 ℃保存备用;剩余组织样品于10%中性缓冲福尔马林中固定保存。本试验所有小鼠均接受人道处理,试验经仲恺农业工程学院实验动物伦理委员会批准,批准协议号为:2023030501。
1.2.2 肾脏组织学及超微形态学观察
肾脏组织在10%中性缓冲福尔马林溶液中固定48 h,包埋石蜡,切成5~6 μm厚的切片。常规组织学检查,石蜡切片苏木精-伊红染色法(HE染色),HE染色切片在光学显微镜下观察并用Case Viewer软件进行图像采集。
1.2.3 肾脏抗氧化指标检测
取0.2 g肾脏组织加入1.8 mL磷酸盐缓冲液(PBS),经组织破碎仪破碎制成10%组织匀浆,3000 r/min离心10 min,取上清待测(n=5)。按照试剂盒的说明,检测组织中超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)、总抗氧化能力(T-AOC)、丙二醛(MDA)、过氧化氢酶(CAT)含量。
1.2.4 RNA提取及文库构建
用Trizol试剂提取肾脏组织总RNA,利用NanoDrop ND-1000分析总RNA质量和纯度(浓度>50 ng/μL、RIN值>7.0、OD260/280>1.8),并通过Bioanalyzer 2100仪器检测RNA完整性。每个样本分别取5 μg RNA用于转录组文库构建,运用Epicenter Ribo-Zero Gold试剂盒去除样本中的核糖体RNA(rRNA),剩余RNA纯化后进行反转录以构建cDNA文库。通过联川生物技术有限公司的Illumina Novaseq™ 6000平台对构建的小鼠肾组织cDNA文库进行双端测序,测序模式为PE150。测序完成后,使用Cutadapt软件删除包含连接物污染、低质量碱基和未定义碱基读数的原始数据(raw reads)。利用FastQC软件(http://www.bioinformatics babraham.ac.uk/projects/fastqc/)获得高质量的clean reads,并且将该数据与小鼠(Mus musculus)基因组序列进行序列比对。利用Tophat2检测测序数据的饱和度,同时对比对上的reads进行统计分析,以评估clean reads在内含子(intron)、外显子(exon)和基因间隔区(intergenic)的分布情况。
1.2.5 差异表达基因GO功能及KEGG通路富集分析
采用DESeq2软件对两组间基因差异表达进行分析,以P<0.05且|log2(Fold change)|≥1为阈值,筛选差异表达基因。利用Goseq R软件包和Kobas在线工具(http://kobas.cbi.pku.edu.cn/genelist/)分别对差异表达基因进行GO功能和KEGG功能富集通路分析。GO功能富集包括生物过程、细胞成分和分子功能。以P<0.05作为筛选显著富集的GO条目或KEGG信号通路的标准。利用在线网站Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html)对两组KEGG富集的通路进行交集分析。
1.2.6 转录组测序基因实时荧光定量PCR检测
根据RNA-Seq结果挑选花生四烯酸通路相关基因CYB2B9、CYP2C65、NF-κB、PTGS1、ALOX5、ALOX12、BCL6、MFSD2A进行实时荧光定量PCR。使用Trizol试剂,严格按照说明书提取相同批次的RNA,按照TaKaRa逆转录试剂盒说明书将RNA逆转录为cDNA。根据GenBank中各基因序列,利用Oligo 7软件设计引物,引物信息见表1。引物均由上海生工生物工程有限公司合成。在实时定量PCR采用SYBR Green嵌合荧光法,反应体系包括SYBR Green Master Mix 10 μL,上游引物0.5 μL,下游引物0.5 μL,cDNA 1 μL,RNase free ddH2O 8 μL。反应程序为:95 ℃预变性2 min;95 °C变性15 s,60 ℃退火30 s,72 ℃延伸30 s,40个循环。采用2−ΔΔCt法计算相对表达量。
表 1 引物信息Table 1. Information of the primers基因 引物序列(5’→3’) BCL6 F:CCTTCTTCGGAGGATGAGATTG R:GGGACCTGTTCACGAGATTATT Mfsd2a F:CCCTCATGGAGCGTAATCTAATC R:CTTATTCTGTCGCCGCTTCT NF-κB F:GCTGCGGATTCTACACCAGA R:CCACTCATCCAAGCGCCTAT ACTB F:TCATCCATGGCGAACTGGTG R:TATAAAACCCGGCGGCGCA ALOX5 F:CAGGATTCCCAACAGCGTAG R:CAGCCTTCAGTTTCAACCAGAC ALOX12 F:TGATGAGCACAGGTGGAGGA R:GCAGGGCATCTTTAGCATAG PTGS1 F:CAGTGCGGTCCAACCTTATC R:CAGAGGGCAGAATGCGAGTA Cyp2b9 F:CTGGATCCCACCTTTCTCTTC R:GGAACTGGTCATCTGTGTAGTC Cyp2c65 F:CCCTGTGTTCACTCTGTACTTG R:AACTCCTCTTCCAGCAAACTC 1.3 数据处理
利用GraphPad Prism 8.0软件进行统计、作图。根据one-way ANOVA比较平均值,并通过Tukey检验进行组间多重比较,数值以“平均值±标准误”表示。P<0.05表示差异显著。
2. 结果与分析
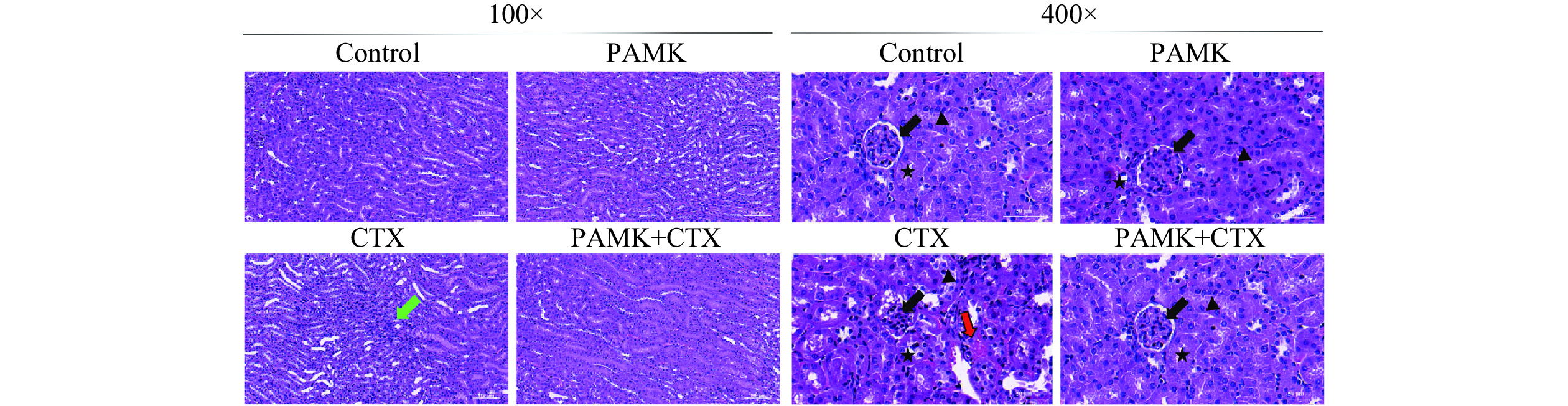
2.1 PAMK对CTX诱导的小鼠肾脏组织结构的影响
各组小鼠的肾组织切片如图1所示,Control组和PAMK组小鼠肾脏结构更为完整,肾小管包括近曲小管及远曲小管各部位上皮细胞无变性,排列有序,肾小囊明显,肾小体、肾小球等结构均正常,管腔形态大小正常,未见异状。CTX组出现了明显的病理现象,如肾小球萎缩、肾小管上皮细胞脱落、周围有少许出血点和炎性细胞浸润;与CTX组相比,PAMK+CTX组肾组织病变明显好转。
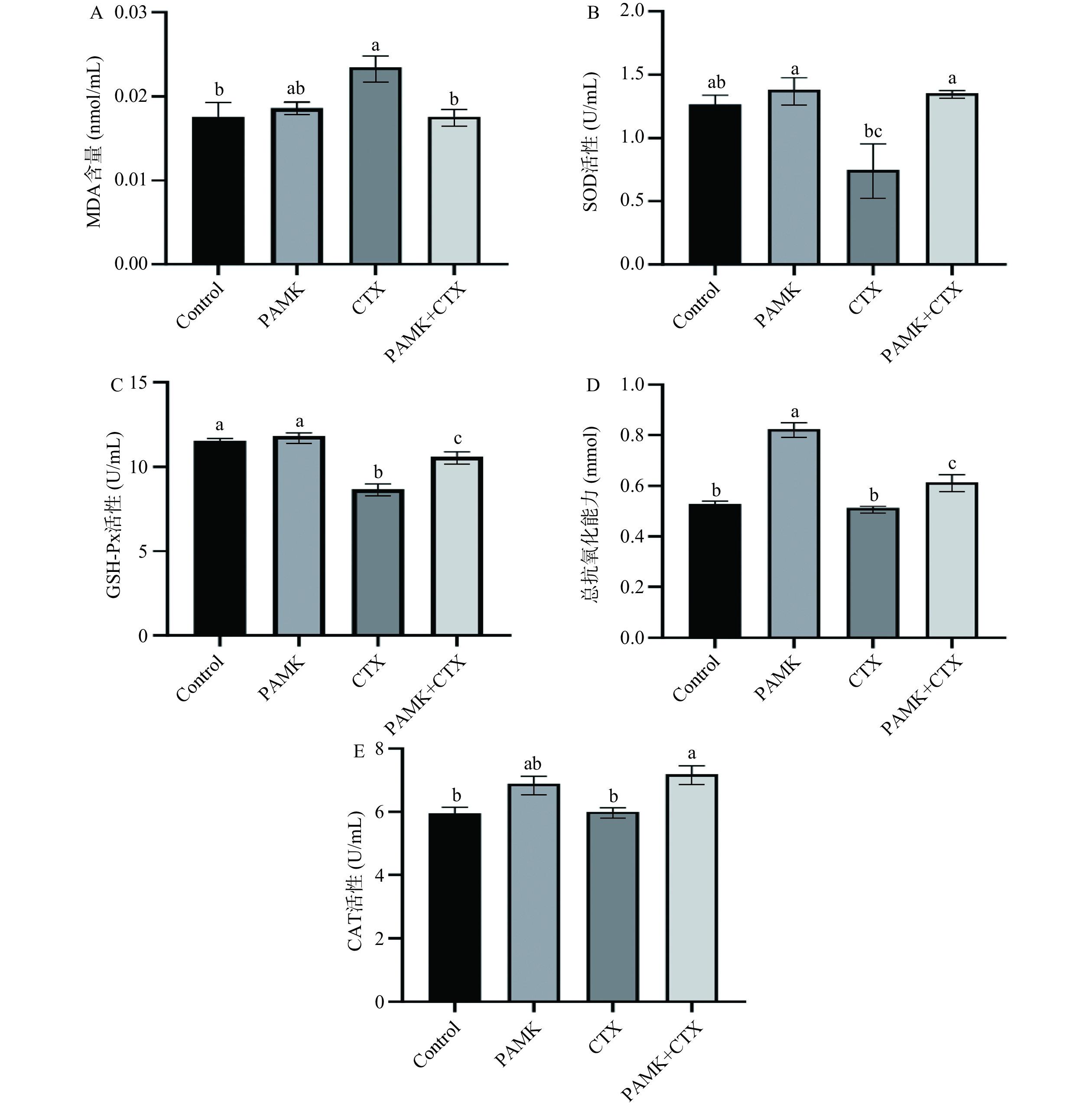
2.2 PAMK对CTX诱导的小鼠肾脏抗氧化指标的影响
肾脏组织中MDA含量、GSH-Px、SOD、CAT活性和总抗氧化能力检测结果如图2所示,与Control组相比,注射CTX后的小鼠肾组织的SOD、GSH-Px活性显著降低(P<0.05);MDA含量显著升高(P<0.05);相较于CTX组,CTX+PAMK组小鼠肾组织的GSH-Px、SOD、CAT活性和总抗氧化能力显著升高(P<0.05);MDA含量显著下降(P<0.05)。与Control组相比,PAMK组小鼠肾组织中的总抗氧化能力显著升高(P<0.05),GSH-Px、SOD、CAT活性无显著差异。
2.3 转录组测序数据分析
本研究构建的9个cDNA文库中获得了37125316~43483558条清洁读数(clean reads),有效比对率在94.36%以上,所有样本的Q20%值均>99.90%,Q30%值均>97.81%,GC含量(RNA序列中,含有鸟嘌呤(Guanine,简称G)和胞嘧啶(Cytosine,简称C)这两种碱基的比例在47.50%~50.00%,说明测序质量的错误率低,数据可靠性高,可用于后续分析。通过对各样本的mRNA区域分布进行统计分析,结果显示,发现各样本中外显子(exon)、内含子(intron)和基因间隔区域(intergenic)所占比例相似,其中由外显子转录而来的mRNAs片段比例最高,占95%以上(图3)。
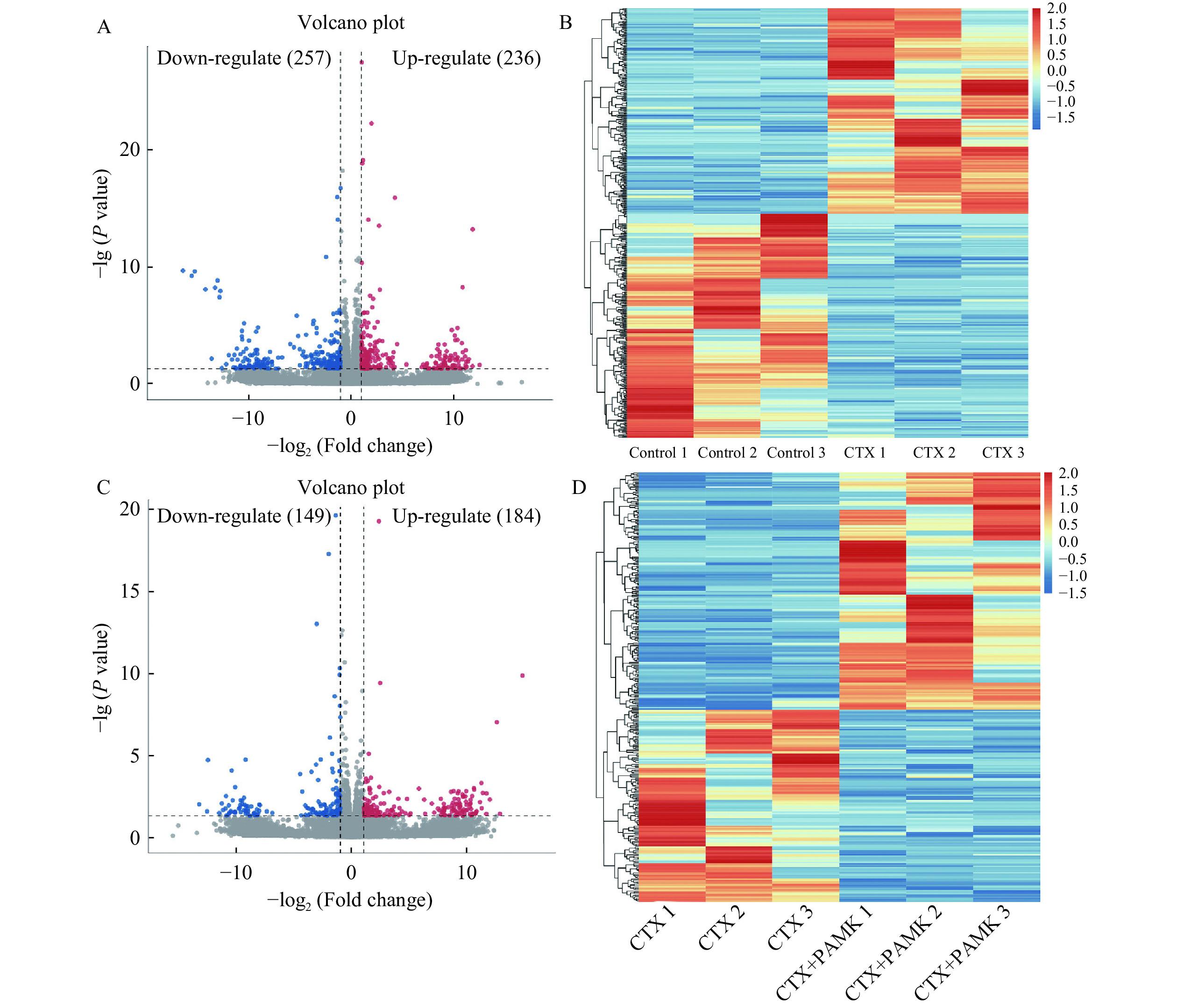
2.4 差异表达基因分析
通过DESeq2软件对转录组测序数据进行分析,结果发现,Control组vs CTX组共获得493个差异表达基因,其中236个基因上调,257个基因下调(图4A~图4B);CTX组vs PAMK+CTX组共获得333个差异表达基因,其中184个基因上调,149个基因下调(图4C~图4D)。
2.5 差异表达基因GO功能与KEGG通路富集分析
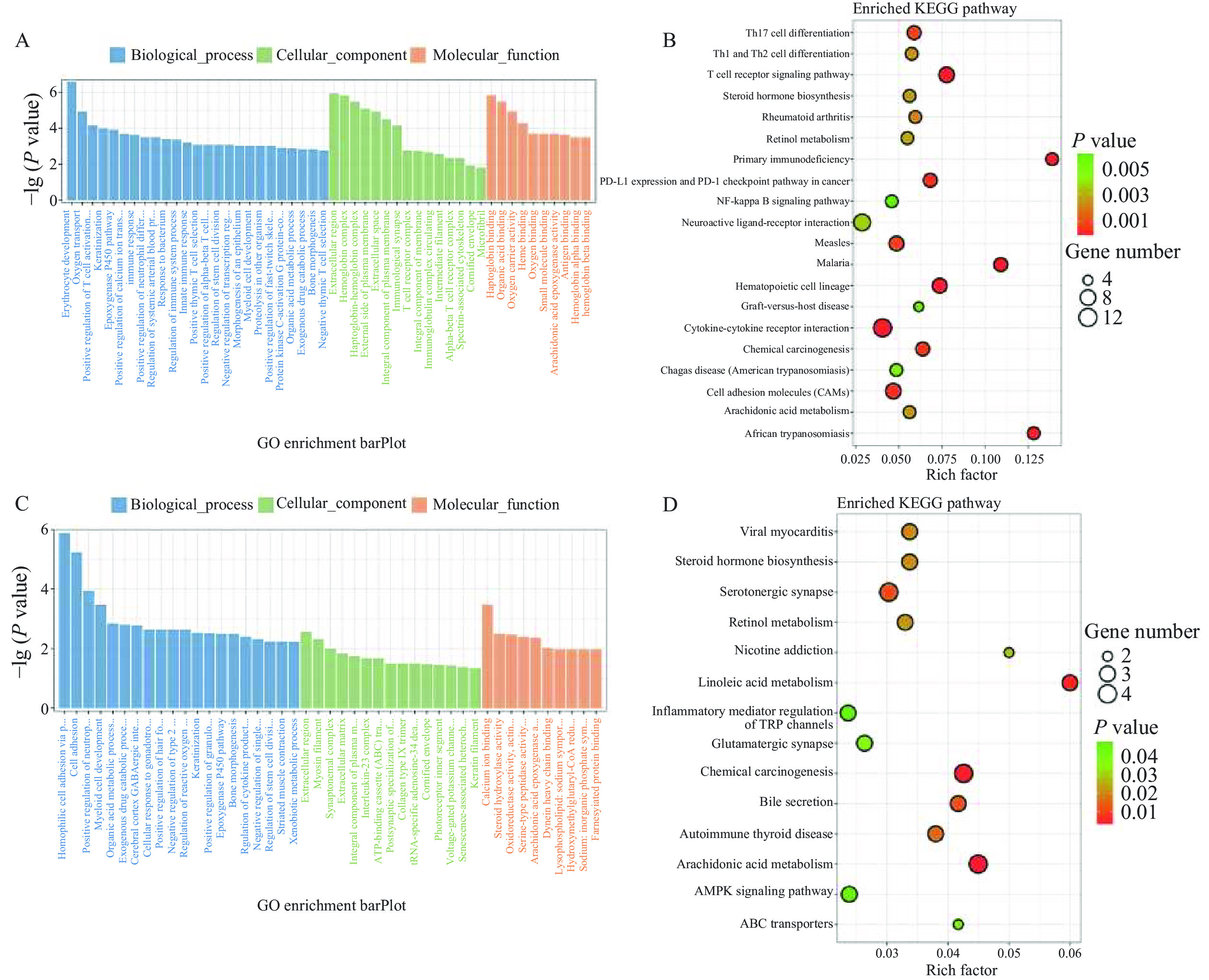
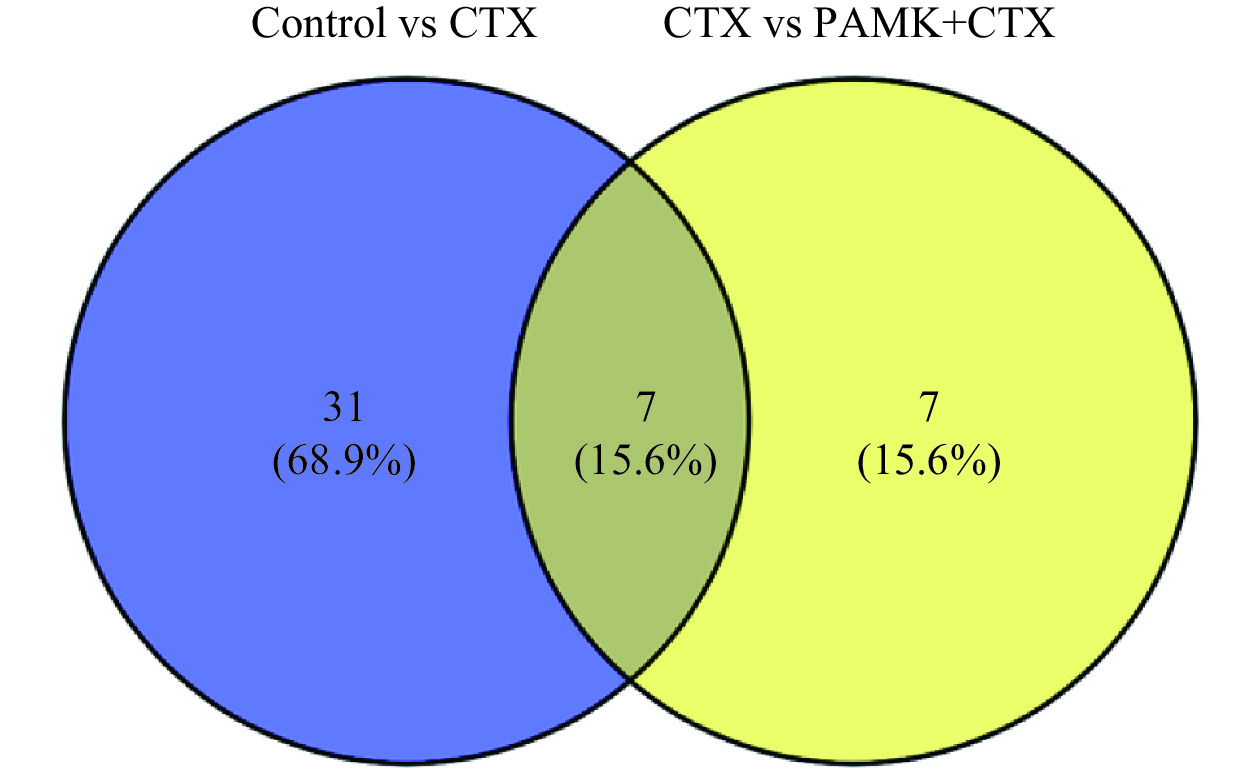
GO功能富集分析发现,差异表达基因多富集在与炎症和免疫反应相关的GO条目,其中Control组vs CTX组主要富集在环氧合酶P450途径、花生四烯酸环氧合酶活性、转录调控区DNA结合的负调控等途径(图5A);CTX组vs PAMK+CTX组主要富集在花生四烯酸环氧合酶活性、细胞色素P450途径、丝氨酸型肽酶活性等途径(图5C)。KEGG通路富集分析结果显示,Control组vs CTX组差异表达基因主要富集在花生四烯酸代谢(T细胞受体、NF-κB等信号通路(图5B);CTX组vs PAMK+CTX组主要富集在花生四烯酸代谢、亚油酸代谢、TRP 通道的炎症介质调节等信号通路(图5D)。表2和图6结果显示,Control组vs CTX组、CTX vs PAMK+CTX组的KEGG富集通路中有交集的通路有7个。其中花生四烯酸代谢通路在Control组vs CTX组和CTX组 vs PAMK+CTX组中的富集最显著。
表 2 Control vs CTX和CTX vs PAMK+CTX KEGG交集通路Table 2. Intersection pathways of Control vs CTX, CTX vs PAMK+CTX KEGGID 信号通路 mmu00590 Arachidonic acid metabolism(花生四烯酸代谢) mmu05204 Chemical carcinogenesis(化学致癌) mmu00591 Linoleic acid metabolism(亚油酸代谢) mmu04726 Serotonergic synapse(血清素能突触) mmu05320 Autoimmune thyroid disease(自身免疫性甲状腺疾病) mmu00140 Steroid hormone biosynthesis(类固醇激素生物合成) mmu00830 Retinol metabolism(视黄醇代谢) 2.6 实时荧光定量PCR检测
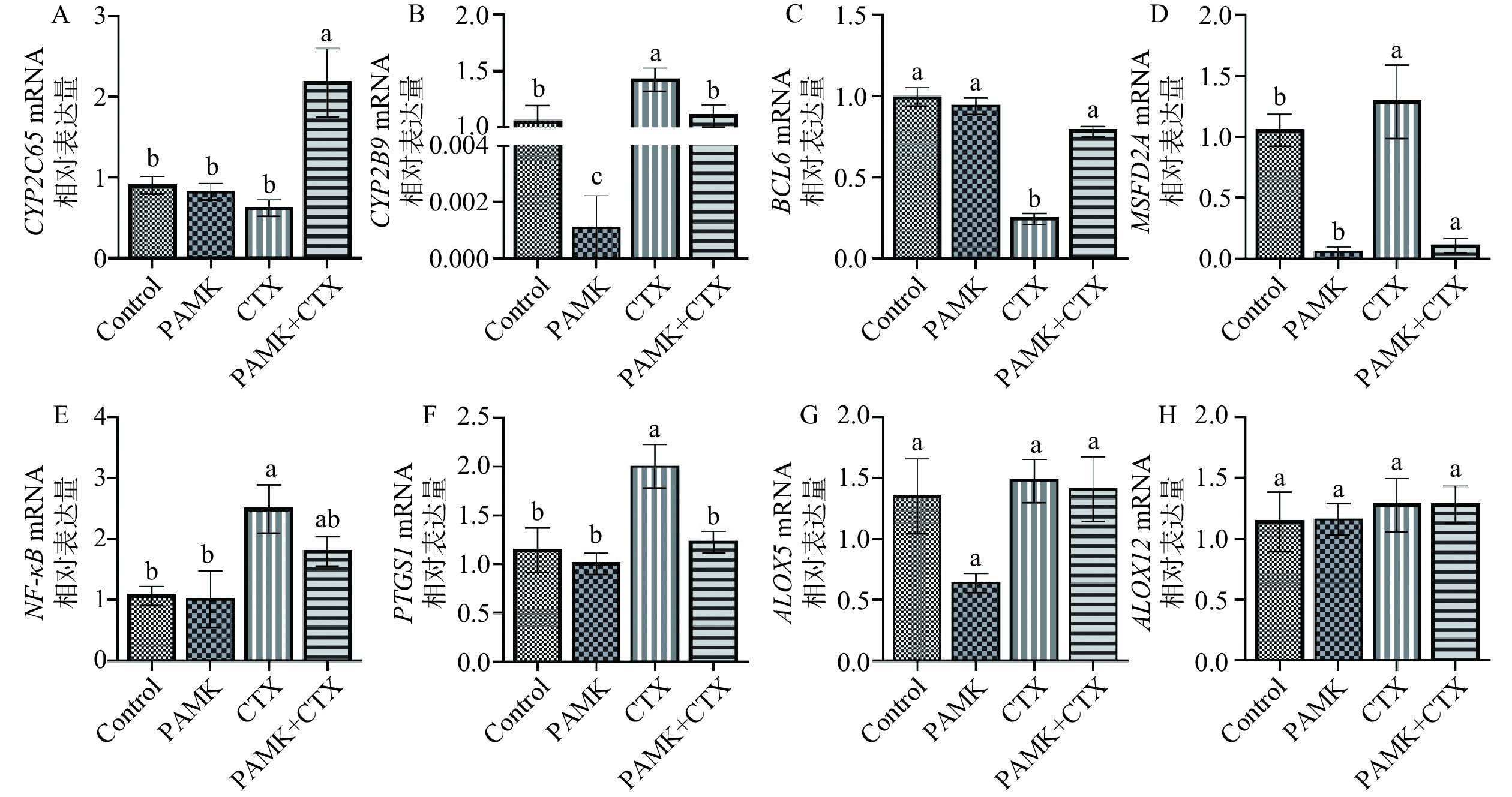
用实时荧光定量检测Control组vs CTX组和CTX组vs PAMK+CTX组关键基因CYP2C65、CYP2B9、PTGS1、ALOX5、ALOX12、NF-κB和抗氧化基因BCL6、MFSD2A。由图7可知,与Control组相比,CTX组Cyp2b9、PTGS1、NF-κB和Mfsd2a mRNA表达量显著升高(P<0.05),BCL6 mRNA表达量显著降低(P<0.05),Cyp2c65、ALOX5和ALOX12 mRNA表达量无显著差异(P>0.05);与CTX组相比,PAMK+CTX组Cyp2c65、BCL6 mRNA表达量显著升高(P<0.05),Cyp2b9、PTGS1和Mfsd2a mRNA表达量显著降低(P<0.05);ALOX5、ALOX12和NF-κB mRNA表达量无明显差异。
3. 讨论与结论
肾脏能清除有毒物质,如果肾脏功能不正常,会影响机体有害物质的排泄,使毒素的积累不能立即清除,从而可能导致各种疾病。目前肾脏疾病的防治已成为众多学者关注的焦点[15]。通过观察小鼠肾组织病理切片,HE染色结果显示CTX组肾小球萎缩,肾小管腔变形,周围有少许出血点并有炎性细胞浸润,导致肾组织出现明显的病理损伤。与CTX组相比,经白术多糖治疗后,肾脏损伤程度明显改善,主要表现为肾小管结构清晰,周围炎性细胞明显减少,表明PAMK治疗逆转了CTX致肾脏组织炎症损伤。
本试验发现CTX处理后,MDA含量增加、SOD、GSH-Px、CAT活性降低。经过PAMK治疗后,MDA的积累减少,小鼠机体内SOD、GSH-Px、CAT活性和总抗氧化能力显著提高。CTX的使用与机体发生氧化应激密切相关,有研究表明CTX会引起动物体内MDA升高,抑制GSH-Px、CAT和SOD的活性,削弱机体的抗氧化能力,破坏氧化系统和抗氧化系统的平衡,导致机体发生氧化应激现象[16]。氧化应激反应是一种高响应性的防御机制,如果长期处于氧化应激状态,可能会导致多种疾病的发生[17]。SAKR等[18]发现了注射环磷酰胺可引起大鼠肾血管的扩张和充血,肾小管上皮细胞的空泡化以及肾小球的萎缩,并推测引起这些病理变化的原因可能是环磷酰胺诱导的氧化应激造成的。说明了CTX处理的小鼠产生了氧化应激反应。近些年来,中药白术中的主要活性物质白术多糖被证明有抗氧化、抗炎及免疫调节等作用,如陈浩祥等[19]的研究发现,对岭南黄鸡饲喂白术多糖可显著提高血清中SOD与GSH-Px的活性,降低机体MDA含量,从而缓解CTX引起的机体氧化应激与肝细胞凋亡情况。上述结果表明了PAMK能增强CTX处理下的抗氧化能力,减少脂质过氧化程度,缓解由CTX引起的氧化应激。
为了探究PMAK对CTX诱导的肾脏损伤的基因调控机制,本研究进行了转录组测序分析。研究结果显示,存在大量与抗氧化相关的DEGs,其中BCL6、Mfsd2a在本实验的转录组测序数据中有显著性差异。BCL6的表达水平相对于CTX组显著上调,这种上调的程度与CTX诱导的小鼠氧化应激的减轻呈正相关,同时PAMK具有抗氧化的功能,BCL6的上调可能与PAMK抗氧化应激反应的激活相关,从而减轻了CTX诱导的氧化应激损伤。并且,有研究发现BCL6通过负调控NLRP3转录来减轻血管紧张素 II或脂多糖诱导的人肾小管上皮细胞炎症,BCL6在自发性高血压大鼠中的过表达降低了SHR的血压、NLRP3的表达和肾皮质炎症[20]。另外,有研究表明,主要Mfsd2a的下调可能通过抑制Caveola介导的细胞内转运,维持血脑屏障的完整性和抗氧化功能,从而保护中枢神经系统的正常功从而维持了血脑屏障的完整性和抗氧化功能[21]。本实验的转录组测序结果与该研究一致,进一步支持了Mfsd2a下调有助于PAMK缓解CTX诱导的小鼠氧化应激反应。综上,表明PAMK通过调节抗氧化途径来发挥其保护作用,从而减轻小鼠肾脏氧化损伤。
为了进一步探究PAMK缓解CTX诱导的肾损伤的机制,本研究进一步将两组DEGs进行富集分析发现,两组交集通路中最显著的是花生四烯酸代谢通路,其中花生四烯酸代谢通路已经有研究发现与炎症发生与缓解密切相关。花生四烯酸(AA)作为细胞膜脂质的主要成分,是细胞膜脂含量的主要组成部分,主要由三种酶代谢:环氧合酶、脂氧合酶和细胞色素P450(CYP450)酶[22]。基于这三种代谢途径,AA可以转化为各种代谢产物,引发不同的炎症反应。在肾脏中,前列腺素、血栓素、白三烯和羟基二碳四烯酸(HETEs)是AA产生的主要代谢物。前列腺素和白三烯水平升高导致肾脏炎症损伤[23−25]。花生四烯酸可以通过花生四烯酸5-氧化酶的催化转化为5-羟基花生四烯酸,然后5-羟基花生四烯酸可以进一步转化为白三烯,白三烯是一类强炎症介质,能够引起血管扩张、增加血管通透性和促进炎症细胞的吸附和迁移[26]。PTGS1(也称为COX-1)是一种环氧合酶酶类,它参与花生四烯酸的代谢,催化花生四烯酸转化为前列腺素H2的反应,PTGS1可能导致前列腺素和血栓素的过量产生,引发炎症反应和相关病理变化[27]。实时荧光定量结果显示,PTGSI、ALOX5和ALOX12 CTX组中mRNA的表达量均呈现增高的趋势。表明了CTX可能通过氧合酶和脂氧合酶途径诱导小鼠肾脏炎症损伤。此外,NF-κB是调控机体炎症反应的关键性转录因子,有研究发现缺血再灌注所致急性肾损伤大鼠的肾组织NF-κB信号通路相关基因NF-κB 表达量显著上升[28]。提示CTX可能激活肾脏内的NF-κB通路导致小鼠肾脏损伤,上述结果证实,在PAMK+CTX组中PTGS1、AOLX5、ALOX12、NF-κB的mRNA表达量均有下降的趋势,PAMK在CTX诱导的肾损伤中发挥了关键的肾脏保护作用。
此外,AA还可以通过细胞色素P450单加氧酶产生的19-HETE和20-HETE调节肾离子运输;由CYP450酶产生的环氧二十碳三烯酸(EETs)也在炎症过程中肾脏损伤中起着重要作用,CYP450途径产生的EETs能够促进肾小管细胞的增殖和分化,促进肾损伤后的修复。研究发现,EETs还具有抗炎和抗氧化作用,可以减轻肾损伤引起的炎症和氧化应激反应,有助于保护肾脏免受进一步的损伤用[29−31]。并且,显著富集通路中的DEGs,大多参与CYP450通路,通过对肾脏的转录组分析,共同调控基因可能是PAMK的调控靶点,两组相关的关键基因Cyp2c65、Cyp2b9mRNA表达量呈相反的趋势与测序结果一致。Cyp2b9是细胞色素P450家族的一员,众所周知,它通过产生超氧化物自由基而产生氧化应激[32]。Lu等[33]研究结果表明,肝脏在HFD(高脂饮食)的作用与Cyp2b家族相关。此外,研究发现LPS诱导肝脏和十二指肠中Cyp2c、Cyp2j总含量的强烈抑制;同样处理条件下,在十二指肠,Cyp2c65和Cyp2j6的表达显著减少[34]。这与上述实验结果一致,CTX组Cyp2c65表达量显著减少,PAMK+CTX组表达量显著上升,说明了PAMK可以通过Cyp2c65、Cyp2b9调控花生四烯酸代谢通路影响代谢性产物,缓解CTX诱导的小鼠肾损伤。P450酶具有许多生理相关功能,包括调节心脏血管张力[35−36],肾脏离子转运介导[37],血管抗炎特性[38],以及控制胰腺肽激素的分泌[39]。CYP2J成员已被证明在AA和亚油酸以及各种外源性药物的代谢中具有活性[40−41]。有研究发现CYP2J酶可能参与脂肪酸代谢,并可能影响许多心血管和肾脏代谢过程[42−43]。同时PAMK具有调节脂代谢酶的活性,提高抗氧化能力,PAMK对CTX所致的炎症和氧化应激有正向的调节作用[44]。另外,细胞色素P450途径产生的EETs加快对肾脏损伤细胞的增殖分化,促进血管新生,恢复肾脏基本功能,及时将代谢废物和有害物质排出体外[45]。以上结果表明,在CTX诱导的小鼠肾损伤过程中,PAMK可能通过影响花生四烯酸环氧合酶的活性缓解小鼠肾脏损伤。
综上所述,本研究结果表明PAMK可能通过调节花生四烯酸代谢通路,缓解小鼠肾脏的氧化应激反应,从而降低CTX引起的肾脏损伤。这一发现揭示了PAMK在维持肾脏形态和结构完整性方面的重要作用,这些结果为进一步探索PAMK的调控机制以及开发相关治疗方法提供参考依据。
-
表 1 引物信息
Table 1 Information of the primers
基因 引物序列(5’→3’) BCL6 F:CCTTCTTCGGAGGATGAGATTG R:GGGACCTGTTCACGAGATTATT Mfsd2a F:CCCTCATGGAGCGTAATCTAATC R:CTTATTCTGTCGCCGCTTCT NF-κB F:GCTGCGGATTCTACACCAGA R:CCACTCATCCAAGCGCCTAT ACTB F:TCATCCATGGCGAACTGGTG R:TATAAAACCCGGCGGCGCA ALOX5 F:CAGGATTCCCAACAGCGTAG R:CAGCCTTCAGTTTCAACCAGAC ALOX12 F:TGATGAGCACAGGTGGAGGA R:GCAGGGCATCTTTAGCATAG PTGS1 F:CAGTGCGGTCCAACCTTATC R:CAGAGGGCAGAATGCGAGTA Cyp2b9 F:CTGGATCCCACCTTTCTCTTC R:GGAACTGGTCATCTGTGTAGTC Cyp2c65 F:CCCTGTGTTCACTCTGTACTTG R:AACTCCTCTTCCAGCAAACTC 表 2 Control vs CTX和CTX vs PAMK+CTX KEGG交集通路
Table 2 Intersection pathways of Control vs CTX, CTX vs PAMK+CTX KEGG
ID 信号通路 mmu00590 Arachidonic acid metabolism(花生四烯酸代谢) mmu05204 Chemical carcinogenesis(化学致癌) mmu00591 Linoleic acid metabolism(亚油酸代谢) mmu04726 Serotonergic synapse(血清素能突触) mmu05320 Autoimmune thyroid disease(自身免疫性甲状腺疾病) mmu00140 Steroid hormone biosynthesis(类固醇激素生物合成) mmu00830 Retinol metabolism(视黄醇代谢) -
[1] XU D N, TIAN Y B. Selenium and polysaccharides of Atractylodes macrocephala Koidz play different roles in improving the immune response induced by heat stress in chickens[J]. Biological Trace Element Research,2015,168(1):235−241. doi: 10.1007/s12011-015-0351-2
[2] XU D N, LI W Y, HUANG Y M, et al. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz. (PAMK) on immune response in chicken spleen under heat stress[J]. Biological Trace Element Research,2014,160(2):232−237. doi: 10.1007/s12011-014-0056-y
[3] XU D N, LI B X, CAO N, et al. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function[J]. Oncotarget,2017,8(41):70394−70405. doi: 10.18632/oncotarget.19709
[4] MIAO Y F, GAO X N, XU D N, et al. Protective effect of the new prepared Atractylodes macrocephala Koidz polysaccharide on fatty liver hemorrhagic syndrome in laying hens[J]. Poultry Science,2020,100(2):938−948.
[5] GUO S X, LI W Y, CHEN F Y, et al. Polysaccharide of Atractylodes macrocephala Koidz regulates LPS-mediated mouse hepatitis through the TLR4-MyD88-NFκB signaling pathway[J]. International Immunopharmacology,2021,98:107692. doi: 10.1016/j.intimp.2021.107692
[6] 相雪莲, 许丹宁, 曹楠, 等. 白术多糖对环磷酰胺诱导的免疫抑制小鼠白细胞数量及功能的修复作用[J]. 中国兽医杂志, 2020, 56(7):36−41, 2. [XIANG X L, XU D N, CAO N, et al. Restorative effects of atractylodes polysaccharide on leukocyte count and function in cyclophosphamide-induced immunocompromised mice[J]. Chinese Journal of Veterinary Medicine, 2015, 56 (7):36−41, 2.] XIANG X L, XU D N, CAO N, et al. Restorative effects of atractylodes polysaccharide on leukocyte count and function in cyclophosphamide-induced immunocompromised mice[J]. Chinese Journal of Veterinary Medicine, 2015, 56 (7): 36−41, 2.
[7] SAKTHIVEL K M, GURUVAYOORAPPAN C. Acacia ferruginea inhibits cyclophosphamide-induced immunosuppression and urotoxicity by modulating cytokines in mice[J]. Journal of Immunotoxicology,2015,12(2):154−163. doi: 10.3109/1547691X.2014.914988
[8] OLAYINKA E T, ORE A, OLA O S, et al. Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats[J]. Medical Sciences,2015,3(3):78−92. doi: 10.3390/medsci3030078
[9] OHNO Y, ORMSTAD K. Formation, toxicity and inactivation of acrolein during biotransformation of cyclophosphamide as studied in freshly isolated cells from rat liver and kidney[J]. Archives of Toxicology,1985,57(2):99−103. doi: 10.1007/BF00343118
[10] HALDAR S, DRU C, BHOWMICK N A. Mechanisms of hemorrhagic cystitis[J]. American Journal of Clinical and Experimental Urology,2014,2(3):199−208.
[11] KAWANISHI M, MATSUDA T, NAKAYAMA A, et al. Molecular analysis of mutations induced by acrolein in human fibroblast cells using supF shuttle vector plasmids[J]. Mutation Research,1998,417(2-3):65−73. doi: 10.1016/S1383-5718(98)00093-X
[12] MYTHILI Y, SUDHARSAN P T, SELVAKUMAR E, et al. Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury[J]. Chemico-Biological Interactions,2004,151(1):13−19. doi: 10.1016/j.cbi.2004.10.004
[13] LIU Q, LIN X M, LI H, et al. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis[J]. Biomedicine & Pharmacotherapy,2016,84:1899−1905.
[14] GUNES S, AYHANCI A, SAHINTURK V, et al. Carvacrol attenuates cyclophosphamide-induced oxidative stress in rat kidney[J]. Canadian Journal of Physiology and Pharmacology,2017,95(7):844−849. doi: 10.1139/cjpp-2016-0450
[15] GORIN Y. The kidney:An organ in the front line of oxidative stress-associated pathologies[J]. Antioxidants & Redox Signaling,2016,25(12):639−641.
[16] 潘航, 俞佳, 史悦, 等. 口服人参茎叶皂苷减缓环磷酰胺诱导的氧化应激作用研究[J]. 中兽医医药杂志,2015,34(1):45−47. [PAN H, YU J, SHI Y, et al. Study on the protective effect of oral ginsenoside on cyclophosphamide-induced oxidative stress[J]. Chinese Journal of Veterinary Medicine,2015,34(1):45−47.] PAN H, YU J, SHI Y, et al. Study on the protective effect of oral ginsenoside on cyclophosphamide-induced oxidative stress[J]. Chinese Journal of Veterinary Medicine, 2015, 34(1): 45−47.
[17] YU Y, WU B, JIANG L M, et al. Comparative analysis of toxicity reduction of wastewater in twelve industrial park wastewater treatment plants based on battery of toxicity assays[J]. Scientific Reports,2019,9(1):3751. doi: 10.1038/s41598-019-40154-z
[18] SAKR S A, El-MESSADY F A. Cyclophosphamide induced histological and immunohistochemical alterations in kidney of albino rats:The ameliorative effect of fennel oil[J]. International Journal of Sciences,2017,3(8):78−87. doi: 10.18483/ijSci.1383
[19] 陈浩祥, 杨舒展, 陆智儿, 等. 白术多糖缓解环磷酰胺诱导的岭南黄鸡氧化应激和肝脏细胞凋亡[J]. 动物营养学报,2023,35(3):1976−1984. [CHEN H X, YANG S Z, LU Z E, et al. Protective effects of Atractylodes polysaccharide on oxidative stress and hepatocyte apoptosis induced by cyclophosphamide in lingnan yellow chickens[J]. Journal of Animal Nutrition,2023,35(3):1976−1984.] CHEN H X, YANG S Z, LU Z E, et al. Protective effects of Atractylodes polysaccharide on oxidative stress and hepatocyte apoptosis induced by cyclophosphamide in lingnan yellow chickens[J]. Journal of Animal Nutrition, 2023, 35(3): 1976−1984.
[20] CHEN D, XIONG X Q, ZANG Y H, et al. BCL6 attenuates renal inflammation via negative regulation of NLRP3 transcription[J]. Cell Death & Disease,2017,8(10):e3156.
[21] BEN-ZVI A, LACOSTE B, KUR E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier[J]. Nature,2014,509(7501):507−511. doi: 10.1038/nature13324
[22] WANG T Q, FU X J, CHEN Q F, et al. Arachidonic acid metabolism and kidney inflammation[J]. International Journal of Molecular Sciences,2019,20(15):3683. doi: 10.3390/ijms20153683
[23] VAN DORP D A. Essential fatty acid metabolism[J]. The Proceedings of the Nutrition Society,1975,34(3):279−286. doi: 10.1079/PNS19750050
[24] SPERLING R I, BENINCASO A I, KNOELL C T, et al. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils[J]. The Journal of Clinical Investigation,1993,91(2):651−660. doi: 10.1172/JCI116245
[25] DE JONGE H W, DEKKERS D H, LAMERS J M. Polyunsaturated fatty acids and signalling via phospholipase C-beta and A2 in myocardium[J]. Molecular and Cellular Biochemistry,1996,157(1−2):199−210. doi: 10.1007/BF00227899
[26] RADMARK O, WERZ O, STEINHILBER D, et al. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease[J]. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids,2015,1851(4):331−339. doi: 10.1016/j.bbalip.2014.08.012
[27] PERRONE M G, SCILIMATI A, SIMONE L, et al. Selective COX-1 inhibition:A therapeutic target to be reconsidered[J]. Current Medicinal Chemistry,2010,17(32):3769−3805. doi: 10.2174/092986710793205408
[28] 袁思宇. 丹红注射液对缺血再灌注所致急性肾损伤大鼠的保护及机制研究[D]. 广州:广东药科大学, 2020. [YUAN S Y. Protective effects and mechanism of danhong injection on acute renal injury in rats induced by ischemia-reperfusion[D]. Guangzhou:Guangdong Pharmaceutical University. 2020.] YUAN S Y. Protective effects and mechanism of danhong injection on acute renal injury in rats induced by ischemia-reperfusion[D]. Guangzhou: Guangdong Pharmaceutical University. 2020.
[29] CALDER P C. Marine omega-3 fatty acids and inflammatory processes:Effects, mechanisms and clinical relevance[J]. Biochimica et Biophysica Acta,2015,1851(4):469−484. doi: 10.1016/j.bbalip.2014.08.010
[30] YATES C M, CALDER P C, ED R G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease[J]. Pharmacology & Therapeutics,2014,141(3):272−282.
[31] RAND A A, BARNYCH B, MORISSEAU C, et al. Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids[J]. Proceedings of the National Academy of Sciences of the United States of America,2017,114(17):4370−4375.
[32] IIDA M, ANNA C H, HARTIS J, et al. Changes in global gene and protein expression during early mouse liver carcinogenesis induced by non-genotoxic model carcinogens oxazepam and Wyeth-14, 643[J]. Carcinogenesis,2003,24(4):757−770. doi: 10.1093/carcin/bgg011
[33] LU Y F, SHAO M M, XIANG H J, et al. Integrative transcriptomics and metabolomics explore the mechanism of kaempferol on improving nonalcoholic steatohepatitis[J]. Food & Function,2020,11(11):10058−10069.
[34] GRAVES J P, BRADBURY J A, GRUZDEV A, et al. Expression of Cyp2c/Cyp2j subfamily members and oxylipin levels during LPS-induced inflammation and resolution in mice[J]. Federation of American Societies for Experimental Biology,2019,33(12):14784−14797. doi: 10.1096/fj.201901872R
[35] CAMPBELL W B, HARDER D R. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone[J]. Circulation Research,1999,84(4):484−488. doi: 10.1161/01.RES.84.4.484
[36] FISSLTHALER B, POPP R, KISS L, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries[J]. Nature,1999,401(6752):493−497. doi: 10.1038/46816
[37] ZOU A P, DRUMMOND H A, ROMAN R J. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats[J]. Hypertension, 1996, 27 (3Pt2):631-635.
[38] NODE K, RUAN X L, DAI J, et al. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids[J]. The Journal of Biological Chemistry,2001,276(19):15983−15989. doi: 10.1074/jbc.M100439200
[39] FALCK J R, MANNA S, MOLTZ J, et al. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets[J]. Biochemical and Biophysical Research Communications,1983,114(2):743−749. doi: 10.1016/0006-291X(83)90843-4
[40] MA J, QU W, SCARBOROUGH P E, et al. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney[J]. The Journal of Biological Chemistry,1999,274(25):17777−17788. doi: 10.1074/jbc.274.25.17777
[41] QU W, BRADBURY J A, TSAO C C, et al. Cytochrome P450 CYP2J9, a new mouse arachidonic acid omega-1 hydroxylase predominantly expressed in brain[J]. The Journal of Biological Chemistry,2001,276(27):25467−25479. doi: 10.1074/jbc.M100545200
[42] LUO G, ZELDIN D C, BLAISDELL J A, et al. Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid[J]. Archives of Biochemistry and Biophysics,1998,357(1):45−57. doi: 10.1006/abbi.1998.0806
[43] WANG H, ZHAO Y, BRADBURY J A, et al. Cloning, expression, and characterization of three new mouse cytochrome p450 enzymes and partial characterization of their fatty acid oxidation activities[J]. Molecular Pharmacology,2004,65(5):1148−1158. doi: 10.1124/mol.65.5.1148
[44] 钱隆, 刘洋, 李冰心, 等. 白术多糖可能通过Toll样受体4信号通路缓解环磷酰胺诱导的雏鹅肝脏损伤[J]. 动物营养学报,2019,31(2):764−774. [QIAN L, LIU Y, LI B X, et al. Atractylodes polysaccharide may alleviate cyclophosphamide-induced liver injury in goslings via toll-like receptor 4 signaling pathway[J]. Journal of Animal Nutrition,2019,31(2):764−774.] QIAN L, LIU Y, LI B X, et al. Atractylodes polysaccharide may alleviate cyclophosphamide-induced liver injury in goslings via toll-like receptor 4 signaling pathway[J]. Journal of Animal Nutrition, 2019, 31(2): 764−774.
[45] VAN OPDENBOSCH N, LAMKANFI M. Caspases in cell death, inflammation, and disease[J]. Immunity,2019,50(6):1352−1364. doi: 10.1016/j.immuni.2019.05.020
-
期刊类型引用(5)
1. 沃佳美雪,徐晓敏,贾素霞,胡文凯,卢芳,刘树民. 整合16S rRNA测序技术和代谢组学探究白鲜皮对斑马鱼幼鱼的肝毒性机制. 中草药. 2025(01): 177-190 .  百度学术
百度学术
2. 王启民,赵威,黄婉佳. 真武汤加减治疗糖尿病肾病用药规律研究. 河南中医. 2024(07): 1006-1011 .  百度学术
百度学术
3. 沈嘉淼,蔡军涛,李杰明,吕帅宜,胡玉龙,董春红. 中药多糖防治肾损伤作用机制的研究进展. 中国药科大学学报. 2024(04): 454-462 .  百度学术
百度学术
4. 熊志伟,王云,曹华斌,彭成诚,杨帆,代雪艳,幸程鸿,刘灵莉,李静妮,胡爱明. 钼、镉联合暴露介导氧化应激和铁死亡致绵羊肾损伤. 畜牧兽医学报. 2024(12): 5802-5812 .  百度学术
百度学术
5. 杨佳佳,杨玉兰. 基于数据挖掘探析中医药治疗脾肾阳虚型慢性肾功能衰竭用药规律. 中医临床研究. 2024(35): 99-104 .  百度学术
百度学术
其他类型引用(3)





 下载:
下载:
 下载:
下载:



