Molecular Mechanisms of Cucurbitacin B-induced Ferroptosis in Human Colorectal Cancer Cells
-
摘要: 为了探究葫芦素B(Cucurbitacin B,CuB)的抗结肠癌活性,并明确铁死亡在CuB发挥抗癌作用中的关键作用及其分子机制。本文将人结肠癌细胞HCT-116作为研究对象,评价了CuB的抗结肠癌活性,检测了铁死亡相关指标如细胞内铁离子浓度、谷胱甘肽(Glutathione,GSH)含量和乳酸脱氢酶(Lactatedehydrogenase,LDH)水平,并采用网络药理学分析、代谢组学分析、分子对接及分子动力学模拟,探究了CuB抑制结肠癌的作用机制。结果表明,CuB能显著(P<0.05)抑制人结肠癌细胞HCT-116增殖,半数抑制浓度(IC50)为64.48 nmol/L。此外,CuB可以降低细胞内GSH含量、促进总铁离子的积累和LDH的释放,并且铁死亡抑制剂Fer-1能够逆转CuB诱导的LDH释放。网络药理学分析和代谢组学分析结果表明,CuB抑制结肠癌的作用机制与铁死亡密切相关的谷胱甘肽代谢途径有关。随后,分子对接结果提示CuB能与谷胱甘肽代谢通路中的关键蛋白SLC7A11和GPX4分别以−4.819和−3.833的得分结合,分子动力学模拟结果表明CuB与SLC7A11的结合具有较好的结构稳定性、波动性以及能量稳定性。由此,本研究发现CuB可以通过诱导人结肠癌细胞HCT-116中铁死亡的发生来发挥抗癌作用。Abstract: In order to investigate the anti-colon cancer activity of cucurbitacin B (CuB) and clarify the key role and molecular mechanism of ferroptosis in the anticancer effect of CuB. This study used human colon cancer cells HCT-116 as the model to evaluate the anti-colon cancer activity of CuB, detected ferroptosis-related indicators, and investigated the mechanism of CuB in inhibiting colon cancer through network pharmacology analysis, metabolomics analysis, molecular docking, and molecular dynamics simulation. The results showed that CuB significantly (P<0.05) inhibited the proliferation of HCT-116 cells with an IC50 of 64.48 nmol/L. Additionally, CuB decreased the intracellular content of glutathione (GSH), promoted the accumulation of total iron ions, and enhanced the release of lactate dehydrogenase (LDH), all of which could be reversed by the ferroptosis inhibitor Fer-1. Results from network pharmacology and metabolomics analysed suggest that the mechanism of CuB's inhibition of colon cancer was linked to the GSH metabolic pathway closely related to ferroptosis. Furthermore, molecular docking revealed that CuB could bind to key proteins in the GSH metabolic pathway, SLC7A11 and GPX4, with scores of −4.819 and −3.833, respectively. Molecular dynamics simulations demonstrated that CuB had good structural stability, fluctuation, and energy stability when bound to SLC7A11. Consequently, this study uncovered that CuB exerted its anti-cancer effect by inducing ferroptosis in HCT-116 cells.
-
Keywords:
- cucurbitacin B /
- colorectal cancer /
- ferroptosis /
- molecular mechanisms
-
结肠癌是一种常见的恶性肿瘤,尽管近年来结肠癌的诊断和治疗取得了一定的进展,但该疾病的死亡率仍然很高。这主要是因为缺乏早期筛查和预后监测的生物标志物,导致大多数患者在就诊时已处于晚期,使得治疗效果大打折扣[1]。近年来,从动物、植物、微生物等生物来源中提取的具有广泛的生物活性和药理活性的天然产物分子,被认为在新型药物的开发中存在巨大潜力。葫芦素B(Cucurbitacin B,CUB)是一种来自葫芦科植物的四环三萜类化合物,在现有发现的八种不同结构的葫芦素中,它的生物活性最为活跃。研究报道,CuB可以通过调控信号通路发挥出强效的抗病毒、抗炎、抗癌等药理作用[2]。现有大量研究表明,CuB主要通过抑制细胞增殖、阻断细胞周期,诱导细胞凋亡和自噬发挥抗癌作用[3]。
铁死亡是Dixon于2012年提出的一种不同于自噬和细胞凋亡的新型细胞死亡方式[4−6],主要表现为细胞内铁的积累和脂质过氧化[7]。铁死亡过程由小分子诱导剂启动,如erastin[8]和RSL3[9],最终导致细胞内铁的积累、胱氨酸/谷氨酸反向转运蛋白的表达、活性氧的积累和脂质过氧化。此外,针对传统疗法存在的局限性(例如癌细胞的耐药性),铁死亡诱导剂(如erastin和RSL3)被认为能够增强癌细胞对传统癌症治疗药物的敏感性[10]。由于其独特的作用机制及克服当前癌症治疗难点存在的巨大潜力,铁死亡被视为癌症治疗的有效新途径。Huang等[11]的研究表明CuB通过诱导鼻咽癌细胞内铁离子的积累、谷胱甘肽的消耗和广泛的脂质过氧化,下调GPX4的表达等多条途径引发了铁死亡,进而发挥抗癌作用。目前,大量研究证实了CuB具有抑制结直肠癌细胞的生物活性,主要集中在JAK2/STAT3信号通路[12]、Notch信号通路[13]和细胞凋亡等[14],但CuB诱导人结直肠癌细胞铁死亡从而发挥抗癌作用的机制尚缺乏研究。
本研究选择人结肠癌细胞系HCT-116,用于探究CuB诱导人结肠癌细胞铁死亡的分子机制。通过测定不同浓度的CuB对HCT-116细胞毒作用及其对相关铁死亡指标(细胞内总铁浓度、细胞内谷胱甘肽含量和乳酸脱氢酶LDH释放量)的影响,并结合网络药理学、代谢组学分析、分子对接及分子动力学模拟来分析其作用机制。本研究丰富了CuB用于人结直肠癌的治疗的科学依据,同时,也初步阐明了葫芦素B诱导人结直肠癌细胞铁死亡的分子机制。
1. 材料与方法
1.1 材料与仪器
葫芦素B(98%) 中国麦克林科技有限公司;铁死亡抑制剂Fer-1(10 mmol/L) 美国Med Chemexpressshen生物科技有限公司;ATP试剂盒 赛默飞世尔科技(中国)有限公司;人结肠癌细胞株HCT-116 武汉普诺赛生命科技有限公司;DMEM高糖细胞培养基、FBS胎牛血清、青霉素-链霉素、胰蛋白酶-EDTA(0.05%) 美国Gibco公司;谷胱甘肽(GSH)含量检测试剂盒、乳酸脱氢酶LDH细胞毒性检测试剂盒 北京索莱宝生物科技有限公司;总铁离子测试法比色盒 武汉伊莱瑞特生物科技股份有限公司。
Thermo 371型CO2细胞培养箱 赛默飞世尔科技(中国)有限公司;SW-CJ-2型超净工作台 苏州净化设备有限公司;SpectraMax i3x型多功能酶标仪 美谷分子仪器(上海)有限公司;DGL-50B型高压蒸汽灭菌锅、LC-LXHR165A型高速冷冻离心机、HH-4型恒温水浴锅 上海力辰仪器科技有限公司;AB420型分析天平(0.0001 g) 三丰精密量仪(上海)有限公司。
1.2 实验方法
1.2.1 CuB储备液制备
将CuB溶解在DMSO中作为原液(10 mmol/L),并在-20 ℃保存。在实验前,CuB原液需要稀释到所需浓度(CuB在实验中的最终DMSO浓度应低于0.1%,以避免影响细胞生长)。
1.2.2 细胞培养
细胞在37 ℃、5% CO2环境下的培养箱中培养。在含10%(v/v)胎牛血清和1%(v/v)青霉素-链霉素的DMEM中培养人结肠癌细胞株HCT-116,培养基每2 d更换一次。所有实验都使用了10到20代的细胞。当无菌培养皿中装满细胞时,继续进行进一步的实验。
1.2.3 CuB对细胞增殖能力的影响
HCT-116细胞活力通过ATP试剂盒测定。将HCT-116细胞(5000个/孔)接种在96孔板中,并用不同浓度(0、50、100、150 nmol/L)的CuB处理24 h。处理后,在室温下向每孔加入0.1 mL的ATP释放因子,反应10 min,然后从第一孔的每孔中吸取0.1 mL细胞溶解液到第二孔板的对应孔中。第二孔板放置在温度低于25 °C的多孔自动荧光检测器中。向每孔加入20 μL ATP反应溶液,立即检测10 s的荧光强度。通过使用GraphPad Pro Prism 5.0软件计算IC50值来评估细胞增殖能力的影响。IC50的计算公式如下:
Y=100(1+IC50XHillSlope) 式中:X表示剂量或浓度,未转换为对数,nmol/L;Y表示归一化响应,从100%下降到0,随着X的增加而减少;IC50表示与X相同的浓度单位,nmol/L;HillSlope表示坡度系数。
1.2.4 谷胱甘肽的定量分析
将HCT-116细胞(1.5×106 个细胞/皿)接种在6孔板中,分别用不同浓度(0、50、100和150 nmol/L)的CuB处理24 h,然后洗涤并裂解细胞。按照GSH试剂盒的说明,对细胞内GSH含量进行测定,测量450 nm处的吸光度,生成GSH浓度和样品数据的标准曲线以计算GSH水平。
1.2.5 细胞内总铁浓度的测定
将HCT-116细胞(5×106个细胞/皿)接种在10 cm培养皿中,分别用不同浓度(0、50、100和150 nmol/L)的CuB处理24 h,处理过程中,观察细胞生长情况。处理完成后,用无血清DMEM培养基洗涤细胞,再用含有Triton X-100的缓冲液裂解细胞。根据制造商的说明,使用铁测定试剂盒测定细胞裂解液中的铁浓度。
1.2.6 LDH释放量的测定
将HCT-116细胞(5000个细胞/孔)接种在96孔板中,分别用不同的处理方式对HCT-116细胞进行处理,包括不同浓度CuB(0、50、100和150 nmol/L)作用以及不同浓度CuB和铁死亡抑制剂(0 nmol/L+Fer-1、50 nmol/L+Fer-1、100 nmol/L+Fer-1和150 nmol/L+Fer-1)共同作用,其中铁死亡抑制剂的作用终浓度都为10 nmol/L。处理24 h后,收集来自处理过的细胞的上清液,并根据制造商的说明使用LDH细胞毒性检测试剂盒在490 nm波长下测定LDH释放量。
1.2.7 网络药理学分析
通过检索DrugBank[15]、OMIM[16]、GeneCards[17]、PharmGkb[18]和TTD[19]数据库获得与结肠癌相关的靶点,并将获得的数据整合去除重复以获得更全面的癌症相关靶点。从PubChem网站获得葫芦素B的分子结构,并保存为.sdf格式[20]。同时,将葫芦素的.sdf格式文件导入SWISS Tartget Prediction[21]、BATMAN-TCM[22]、TCMSP[23]和Pharm Mapper[24]数据库进行CuB靶点的预测和采集,剔重后获得CuB的潜在靶点。随后,将CuB靶点与结肠癌相关靶点数据导入R语言CuB抗结肠癌关键靶点韦恩图;随后将获得的共同靶点导入STRING数据库,获取蛋白质相互作用关系,使用软件Cytoscape 3.7.2绘制蛋白质相互作用网络图;将物种设置为Homo Sapiens,以P<0.05为标准,选取分子功能(MF)、生物过程(BP)、细胞成分(CC)前10个结果,绘制GO富集直方图;以P<0.05为标准,选取10个有代表性的KEGG通路绘制KEGG气泡图[25]。
1.2.8 代谢组学分析
采用标准操作程序对人结肠癌细胞HCT-116进行代谢组学分析[26]。设置两组实验,包括对照组(0 nmol/L)和处理组(150 nmol/L),每组3个生物学重复,24 h后收集细胞,立即用液氮淬灭,用于后续实验。
将-80 ℃冰箱中保存的样品在冰上解冻。在细胞样品中加入500 μL甲醇水溶液(甲醇:水=4:1,V/V),涡旋3 min。再将样品置于液氮中快速冷冻5 min,后又取出置于冰上5 min,并于冰上解冻涡旋2 min,如此冻融循环重复3次。样品以12000 r/min离心10 min(4 ℃)后,取300 μL上清液,−20 ℃静置30 min,12000 r/min离心3 min(4℃)。准确吸取200 μL上清液进行LC-MS分析,所有样品均由LC-MS系统按照机器指令采集。
高效液相色谱-质谱分析用于分析和鉴定化合物的种类和含量。Aglient 1290 Infinity LC 超高效液相色谱系统使用Waters ACQUITY UPLC HSS T3 C18(1.8 μm,2.1 mm×100 mm)色谱柱,流速为0.4 mL/min、柱温40 ℃,进样量2 μL。流动相为0.1%甲酸水(流动相A)和含0.1%甲酸的乙腈(B),梯度洗脱程序为:0~11 min,5%~90% B;11~12 min,90% B;12~12.1 min,90%~5% B;12.1~14 min,5% B。Aglient QTOF/MS-6550质谱检测器,电喷雾离子源(ESI),分别采用正负离子模式采集数据。正离子喷雾电压为2.50 kV,负离子喷雾电压为−1.50 kV,辅气流速为8 L/min,鞘气流速为11 L/min,碎裂电压为135 V,雾化气电压为40 V,离子源温度为325 ℃。原始数据经格式转换、峰提取、对齐、保留校正时间和峰面积校正后进行过滤,再进行代谢物鉴定。
利用R语言中的MetaboAnalyst包(v1.0.1)建立正交偏最小二乘判别分析(Orthogonal偏最小二乘Discriminant Analysis,OPLS-DA)模型,从中获得预测变量重要性(VIP)值,P值由Student's t检验生成。VIP值>1,P<0.05的代谢物被认为具有统计学意义,标记为差异累积代谢物(differences cumulative Metabolites,DAMs),选择P<0.05的通路进行分析。
1.2.9 分子对接
本次对接研究使用Maestro 11.9程序预测SLC7A11、GPX4与CuB可能的结合行为[27]。GPX4和SLC7A11的三维结构从PDB数据库(https://www.rcsb.org/)获取并保存为.sdf格式,CuB的结构从PubChem(https://pubchem.ncbi.nlm.nih.gov/compound/5281316)获得。除去所有的水分子,在对接研究实验开始时加入极性氢。对接完成后,采用对接得分最高的模型进行进一步分析,一般认为结合能越低越稳定,得分也越高。
1.2.10 分子动力学模拟
用GROMACS软件包进行分子动力学模拟实验,选择CHARMM36立场进行后续模拟[28]。然后,将分子对接结果中的最佳结构导入GROMACS,通过GROMACS自带的预处理工具pdb2gmx为蛋白质分配原子类型和电荷,并生成拓扑文件。接下来,使用GROMACS中的editconf和genbox工具调整模拟盒子的大小,将蛋白质-配体复合物置于模拟盒子的中心,并添加适当数量的溶剂分子(TIP3P水模型)。为了模拟生物体内的条件,在模拟盒子中添加适当浓度的盐离子(Na+和Cl−),使用GROMACS中的genion工具替换部分溶剂分子以中和系统的总电荷。在系统准备完成后,进行能量最小化操作以消除系统中的不良接触和高能构象。能量最小化完成后,对模拟系统进行短时间的NVT(恒容恒温)和NPT(恒压恒温)平衡模拟,以使系统达到恒温和恒压条件。在NVT平衡模拟中,使用Berendsen温度耦合方法将系统加热至目标温度(310 K),并将其保持在该温度。接下来,在NPT平衡模拟中,使用Berendsen压力耦合方法将系统的压力调整至目标压力(1 bar)。在完成NVT和NPT平衡模拟之后,对模拟系统进行100 ns的生产模拟,以收集足够的轨迹数据。在生产模拟过程中,使用Nosé-Hoover温度耦合方法和Parrinello-Rahman压力耦合方法来分别维持系统的温度和压力。此外,定期保存系统的原子坐标信息(每10 ps保存一次),以便于后续分析。
1.3 数据处理
本研究实验数据均表示为“均值±标准差”,每项实验至少进行过三次独立生物学实验,以确保结果的稳定性。实验数据使用GraphPad Prism软件进行处理和分析,Student's t检验和方差分析(ANOVA)被用于统计比较,以评估实验组与对照组之间的差异,P<0.05被认为具有统计学意义。
2. 结果与分析
2.1 CuB对HCT-116细胞系增殖的影响
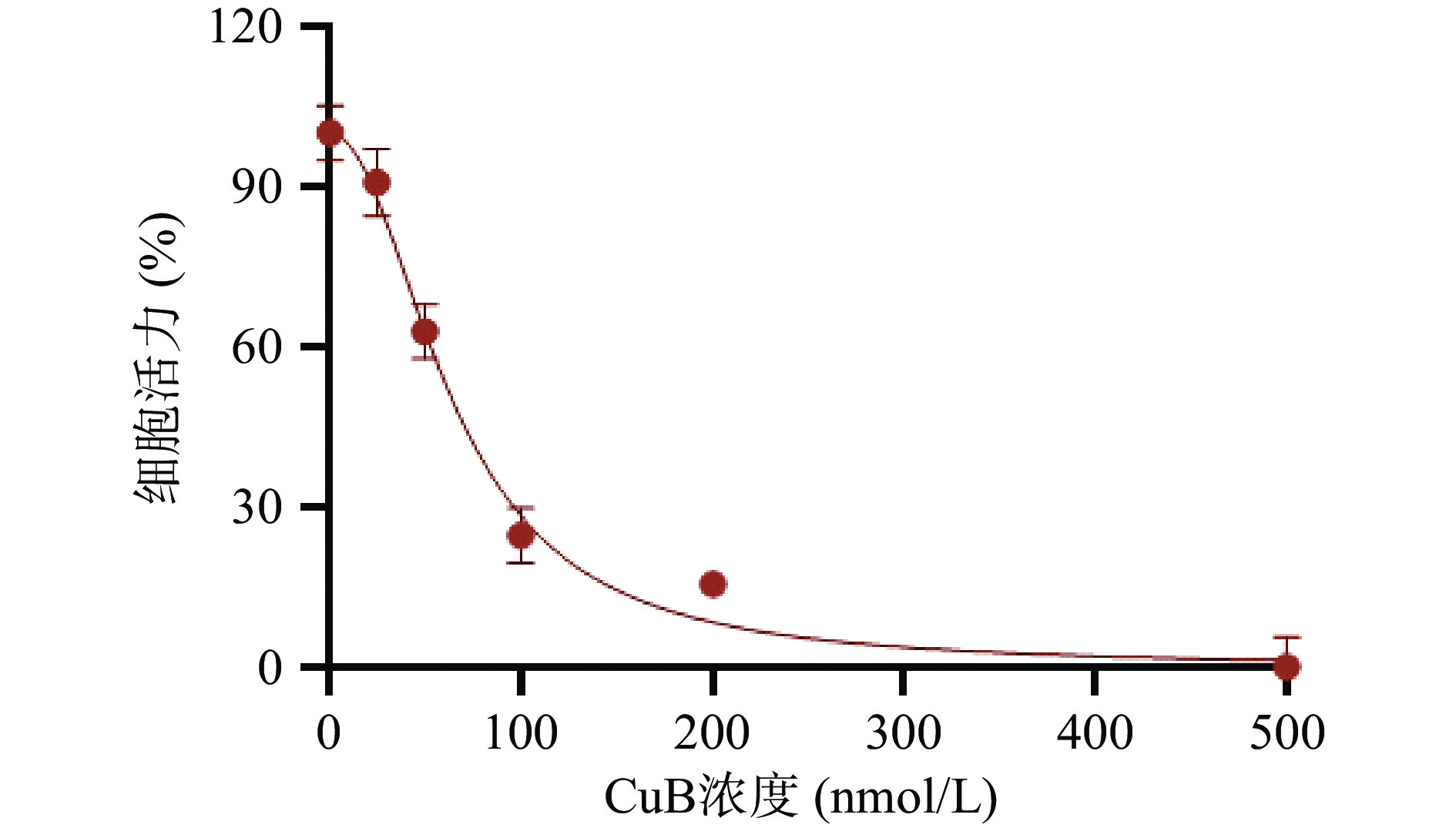
为了研究CuB是否有抗结肠癌作用,通过ATP法检测经CuB处理24 h后人结肠癌细胞株HCT-116的细胞活性。如图1所示,CuB可以抑制HCT-116的增殖,其半抑制浓度(IC50)为64.48 nmol/L,结果表明CuB具有较强的抗结肠癌作用。
2.2 CuB诱导HCT-116细胞铁死亡
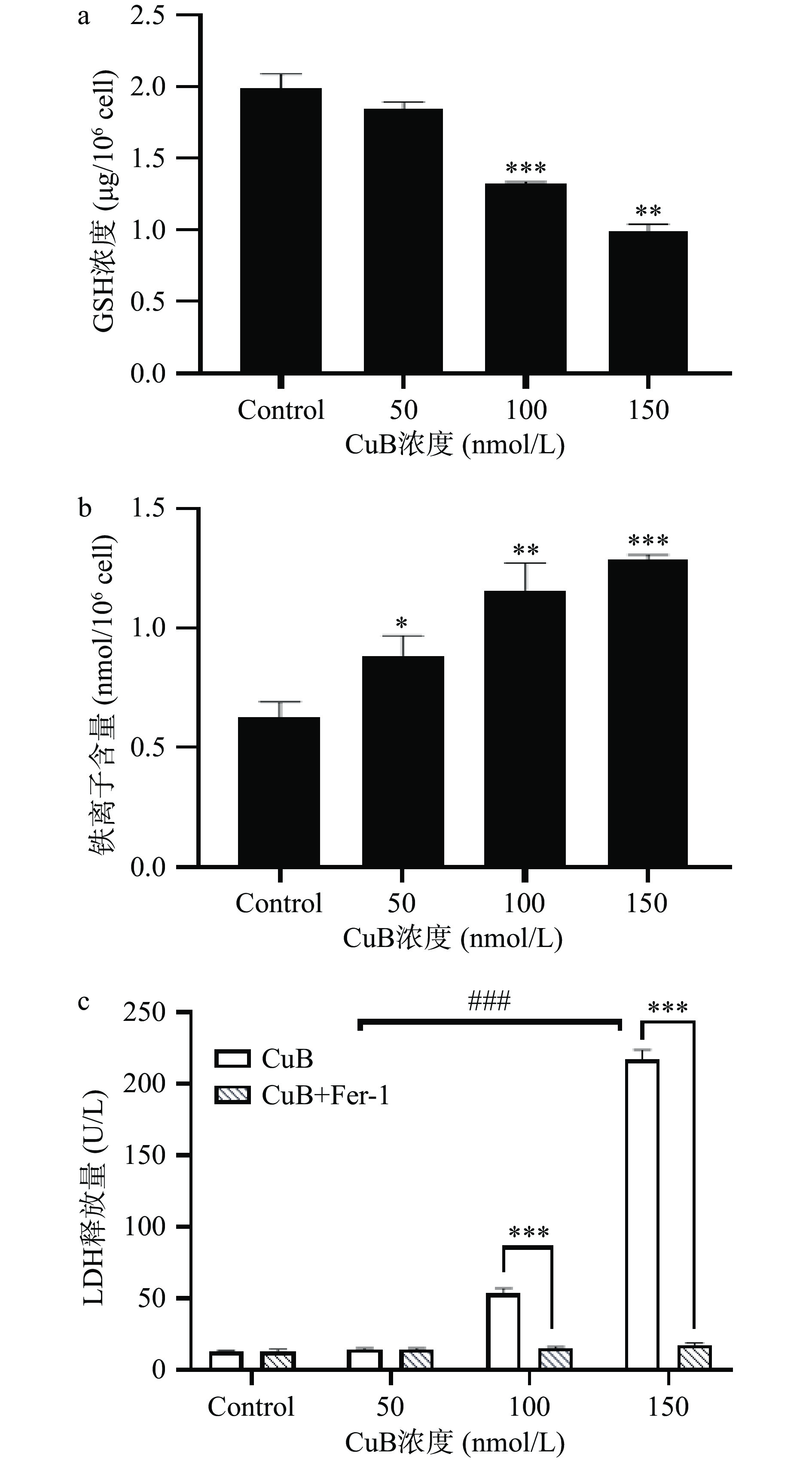
细胞内GSH水平降低会导致氧化应激,这也是铁死亡介导的细胞死亡的关键因素。细胞GSH依赖性抗氧化剂的失活会促进脂质过氧化物产物的积累,并进一步引发铁死亡[29]。如图2a所示,随着CuB作用浓度增加,细胞内GSH含量呈现出剂量依赖性下降,在CuB的浓度为100 mmol/L时,HCT-116细胞的细胞活性显著下降(P<0.01)。这表明CuB处理可以减少HCT-116细胞内的GSH含量,进而可能削弱细胞的抗氧化能力,导致细胞内脂质过氧化物的积累。
![]() 图 2 CuB诱导HCT-116细胞铁死亡的结果注:a. CuB对HCT-116细胞内GSH含量的影响(**P<0.01,***P<0.001);b. CuB对HCT-116细胞内总铁离子含量的影响(*P<0.05,**P<0.01,***P<0.001);c. CuB及铁死亡抑制剂Fer-1对HCT-116细胞LDH释放的影响(*表示有铁死亡抑制剂跟没有铁死亡抑制剂之间的显著性变化,***P<0.001,#表示不同浓度之间的给药组之间的显著性的变化,###P<0.001)。Figure 2. Results of CuB induced ferroptosis in HCT-116 cells
图 2 CuB诱导HCT-116细胞铁死亡的结果注:a. CuB对HCT-116细胞内GSH含量的影响(**P<0.01,***P<0.001);b. CuB对HCT-116细胞内总铁离子含量的影响(*P<0.05,**P<0.01,***P<0.001);c. CuB及铁死亡抑制剂Fer-1对HCT-116细胞LDH释放的影响(*表示有铁死亡抑制剂跟没有铁死亡抑制剂之间的显著性变化,***P<0.001,#表示不同浓度之间的给药组之间的显著性的变化,###P<0.001)。Figure 2. Results of CuB induced ferroptosis in HCT-116 cells铁在线粒体呼吸链中发挥着重要作用,并在铁死亡过程中扮演关键角色[30]。为了深入探讨CuB诱导铁死亡的机制,对CuB处理后的细胞内铁浓度进行检测。如图2b所示,随着CuB作用浓度的增加,细胞内铁浓度明显升高。这一发现意味着铁离子浓度的提高可能增加细胞对铁死亡的敏感性。铁离子水平的上升可能会影响线粒体呼吸链的正常功能,进一步导致细胞内氧化应激增加,最终促使铁死亡的发生[31]。
此外,本研究还检测了乳酸脱氢酶(LDH)的释放情况。LDH是一种细胞内酶,当细胞膜受到损伤时,LDH会从细胞内释放出来,因此LDH释放通常被用作评估细胞膜完整性和细胞死亡的指标[32]。如图2c所示,观察到LDH释放随CuB浓度的增加而显著(P<0.05)升高。这表明CuB处理可能导致细胞膜受损和细胞死亡。为了进一步证实CuB诱导的HCT-116细胞死亡是否与铁死亡有关,采用了铁死亡抑制剂Fer-1进行验证实验。比较了无Fer-1作用和有Fer-1作用的两组细胞在不同浓度CuB下的LDH释放情况,与无Fer-1作用组相比,Fer-1作用组在CuB作用浓度为100 nmol/L时能显著(P<0.05)降低细胞LDH的释放。这一结果表明,铁死亡抑制剂Fer-1能够有效抑制CuB诱导引起的细胞膜受损和细胞死亡。
2.3 网络药理学结果分析
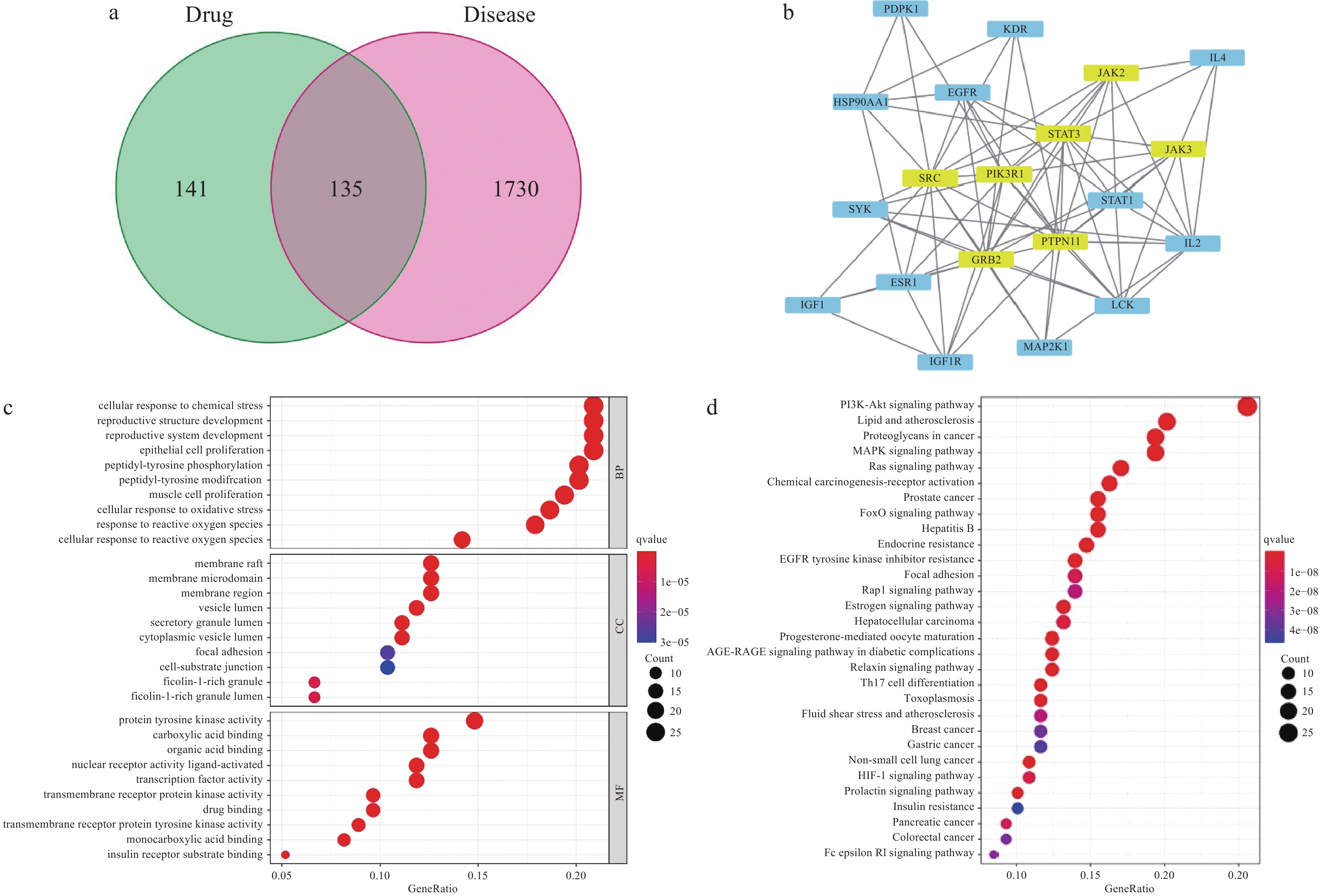
为了挖掘CuB的潜在作用靶点和信号通路,本研究进行了网络药理学分析。通过整合公共数据库中的信息,发现CuB有276个潜在靶标和1865个与结肠癌相关的基因。这些靶标和基因的交集通过韦恩图(图3a)展示。利用STRING数据库构建CuB与结肠癌靶点的交集蛋白质-蛋白质相互作用(PPI)网络,并使用Cytoscape软件筛选核心靶点。如图3b所示,在PPI网络中,发现了许多重要节点,如STAT3、EGFR、PIK3R1等。这些靶点在肿瘤发生、发展及治疗中扮演着重要角色。
R语言用于GO通路和KEGG通路的富集与筛选。图3c气泡图显示了P值低于0.05的前10条BP、CC和MF通路。这些通路主要涉及细胞氧化还原平衡、氨基酸代谢和脂质代谢等过程。此外,通过KEGG富集分析,发现130个差异代谢物映射到了159个KEGG通路中,前30条KEGGE通路如图3d所示,值得注意的是,一些KEGG通路与铁死亡高度相关,如PI3K-Akt信号通路[33]、脂质和动脉粥样硬化[34]以及MAPK信号通路[35]。
2.4 代谢组学结果分析
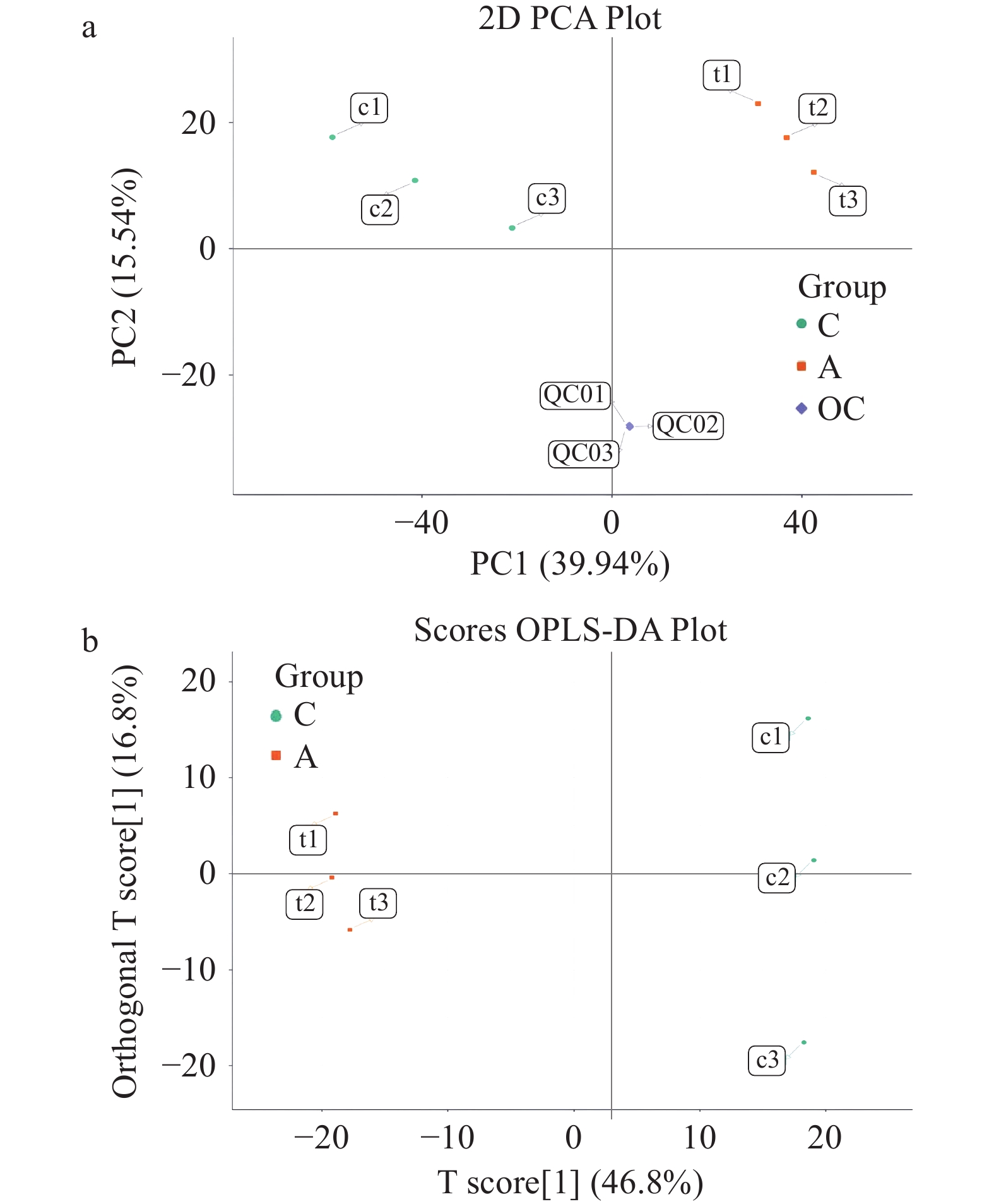
为了进一步探究CuB诱导人结直肠癌细胞铁死亡的分子机制,本文采用代谢组学分析作深入研究。通过主成分分析(Principal Component Analysis,PCA)和正交投影偏最小二乘法判别分析(OPLS-DA)评分图验证实验结果的可靠性[36−37]。PCA是一种无监督的多维统计分析方法,对鉴定到的代谢物进行PCA分析,可以从总体上反映样本组间和组内的变异度。空白处理组与CuB处理组在正负离子模式下的PCA得分图如图4a所示。图中PC1表示第一主成分,PC2表示第二主成分,图中的每个点表示一个样本,同一个组的样本使用同一种颜色表示,Group为分组。从而直观地观察到不同处理组间样本的差异以及同一组内样本的分布趋势,同一组间的样本聚集度高,说明样品的重复性好。由图4a可知,空白处理组和CuB处理组两组之间的样本有明显的区分,说明CuB处理明显引起HCT-116细胞中代谢物的变化,且这一变化在3个重复组中稳定存在。
进一步采用有监督且修正的判别分析统计方法正交偏最小二乘判别分析(Orthogonal Partial Least-squares Discrimination Analysis,OPLS-DA)进行组间、组内样本主成分分析,得分图如图4b所示。图4b中体现的信息与PCA得分图中相似,图中空白处理组和CuB处理组样本明显分离,即CuB处理引起HCT-116细胞中代谢物发生显著变化;纵坐标上则反映了组内的变异,可以观察到空白处理组与CuB处理组间样本相对聚集,生物学重复较好。
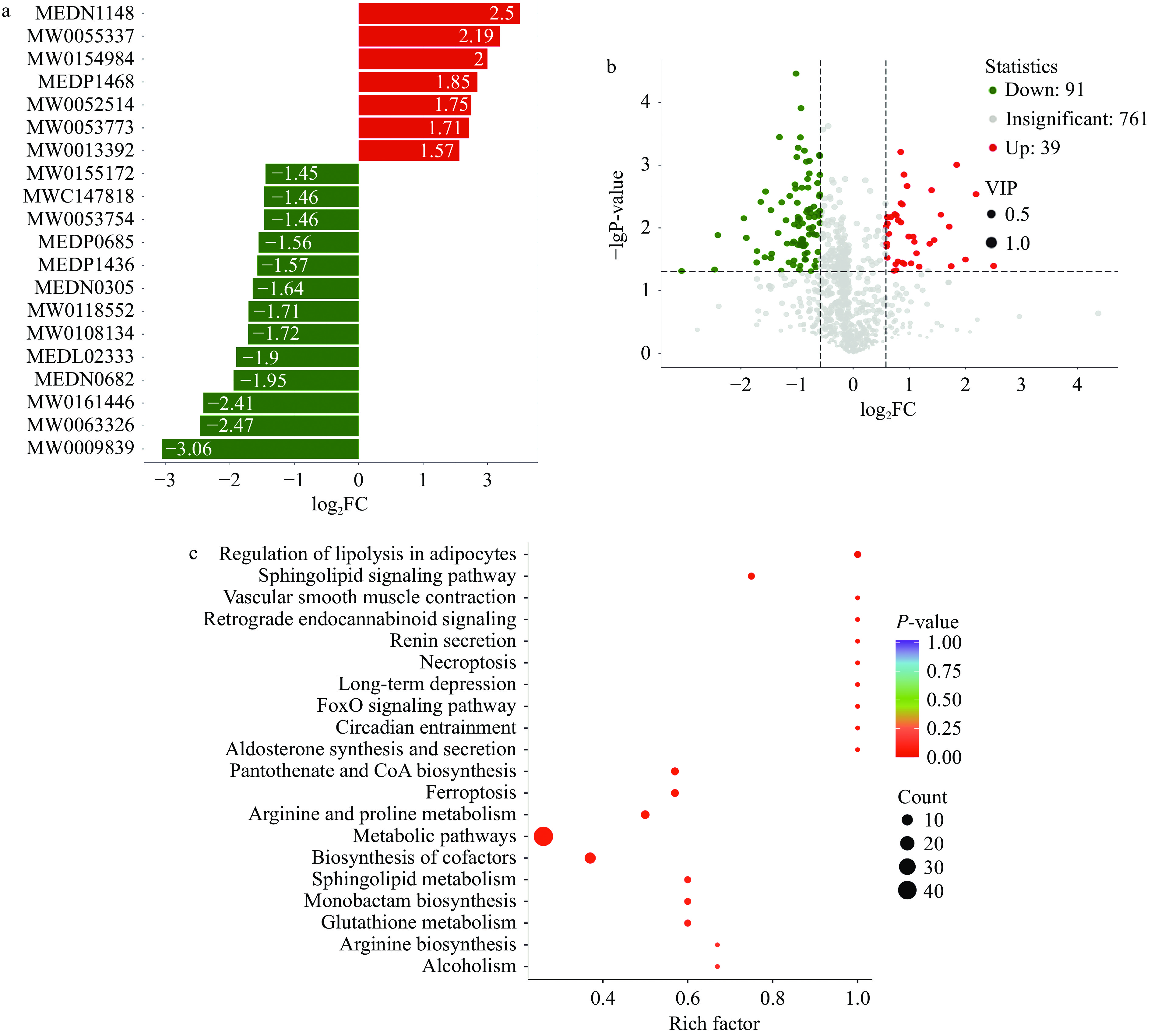
OPLS-DA模型中得到的变量权重值(Variable Importance for the Projection,VIP)能够用于挖掘具有生物学意义的差异代谢物,通常认为VIP>1的差异代谢物在模型中具有显著贡献。本实验以VIP>1和P<0.05为筛选显著差异代谢物的标准,共筛选到130种显著差异代谢物,其中上调的显著差异代谢物有39种,下调的显著差异代谢物有91种。正负离部分差异代谢物如表1所示。图5a为各分组比较中差异倍数前20代谢物表达的倍数变化结果展示,横坐标为差异代谢物的log2FC,即差异代谢物的差异倍数以2为底取对数的值,纵坐标为差异代谢物。红色代表代谢物含量上调,绿色代表代谢物含量下调。图5a可以观察到CuB处理后显著下调的差异代谢物明显多于上调的差异代谢物,推测CuB可能下调HCT-116细胞的基础代谢。
表 1 差异代谢物筛选结果Table 1. Screening results of differential metabolites指标 化合物 类型 MW0130472 (E)-2',6'-Dihydroxy 4',4-dimethoxychalcone up MW0063246 Pyrrolidinium,1-[(7R)-7-(acetyloxy)-4-
hydroxy-4-oxido-3,5,9-trioxa-4-
phosphapentacos-1-yl]-1-methyl-,inner saltdown MW0009839 Taprostene down MW0142142 2-{5-[3-(6-Benzoyl-1-propylnaphthalen-2-yloxy)
propoxy]indol-1-YL}ethanoic aciddown MW0000158 Cabergoline down MEDP1468 Dexpanthenol up MEDN1148 2-Hydroxyhexadecanoic acid up MW0012931 1-O-Hexadecyl-2-O-acetyl-sn-glyceryl-3-
phosphorylcholinedown MW0009945 Tolyfluanide down MW0140300 (2S)-2-(Benzenesulfonamido)-3-[[2-(4-piperidin-4-
ylpiperidin-1-yl)-4-propan-2-yl-1,3-thiazole-5-
carbonyl]amino]propanoic aciddown MW0111715 1,4-D-Xylobiose up MW0170018 Xanthohumol up MEDL02423 Creatine phosphate down MEDN0391 DHA up FDATN01264 Sodium 4-Aminosalicylate up 图5b展示了对照组和实验组差异代谢物的上调和下调分布。图5c以气泡图形式展现KEGG富集分析的结果,选择最显著富集的前20条通路进行绘图,结果如图5c所示通路主要富集在脂肪细胞中脂肪分解的调节、铁死亡、谷胱甘肽代谢等。这些结果表明,CuB对结肠癌代谢产生了显著影响,可能涉及到铁死亡通路的调节。
2.5 分子对接结果分析
分子对接技术作为一种计算化学方法,可以预测分子间的相互作用,它可以为深入了解药物与靶蛋白之间的结合模式提供依据。为了进一步探讨CuB抑制HCT-116结肠癌细胞的作用机制,特别是与铁死亡相关的信号通路,本研究选择了铁死亡信号通路中的两个关键蛋白GPX4和SLC7A11作为分子对接分析的对象。
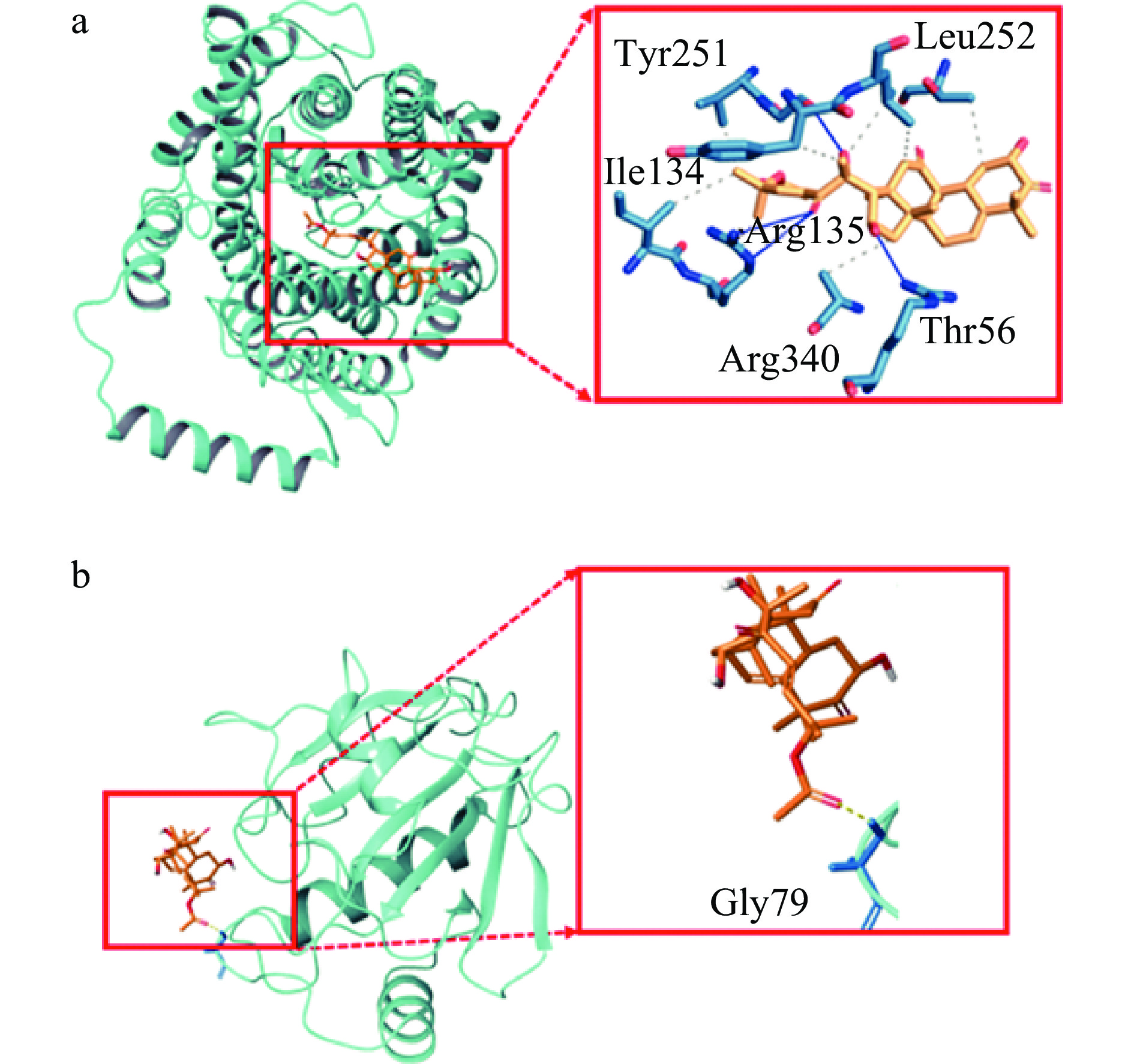
如图6a所示,CuB分别可以与SLC7A11蛋白中Tyr251,Leu252,Ile134,Arg135,Arg340,Thr56的6个氨基酸形成了6个氢键,与Tyr251,Arg135,Thr56这3个氨基酸形成4个疏水相互作用。如图6b所示,CuB与GPX4蛋白中的氨基酸Gly79形成1个氢键。分子对接结果如表2显示,CuB可能通过与铁死亡信号通路中的关键蛋白GPX4和SLC7A11相互作用来抑制HCT-116细胞的增殖,其中CuB与蛋白SLC7A11和GPX4的对接得分分别为-4.819和-3.833,结合能分别为-43.34 kcal/mol和-47.43 kcal/mol,表明CuB可与SLC7A11和GPX4蛋白形成稳定的结合。
表 2 CuB与蛋白质分子对接评分Table 2. Scores of the docking between CuB and protein化合物 靶点蛋白 氨基酸残基 对接得分 结合自由能
(kcal/mol)CuB SLC7A11 Tyr251,Leu252,Ile134,
Arg135,Arg340,Thr56−4.819 −43.34 CuB GPX4 Gly79 −3.833 −47.43 通过对分子对接结果的分析,发现CuB与SLC7A11结合的氢键主要涉及到SLC7A11蛋白的若干关键氨基酸残基。这些氨基酸在SLC7A11蛋白的功能和稳定性中起着重要作用,CuB与其结合可能会影响SLC7A11的正常功能,从而导致铁死亡信号通路的改变。与此同时,CuB与GPX4结合的模式也显示了相互作用的存在,但结合强度较弱,表明CuB对GPX4的作用可能较为有限。
2.6 分子动力学模拟结果分析
在分子对接过程中发现SLC7A11与CuB的得分更高。因此本研究选择对SLC7A11进行分子动力学模拟。
将SLC7A11与CuB进行100 ns的分子动力学模拟。模拟结果显示,体系在50 ns内达到了平衡。为了进一步评估模拟过程中蛋白质-配体复合物的稳定性,对模拟过程中的RMSD图、RMSF图、蛋白回转半径(Rg)变化趋势图和自由能形貌图等指标进行分析。
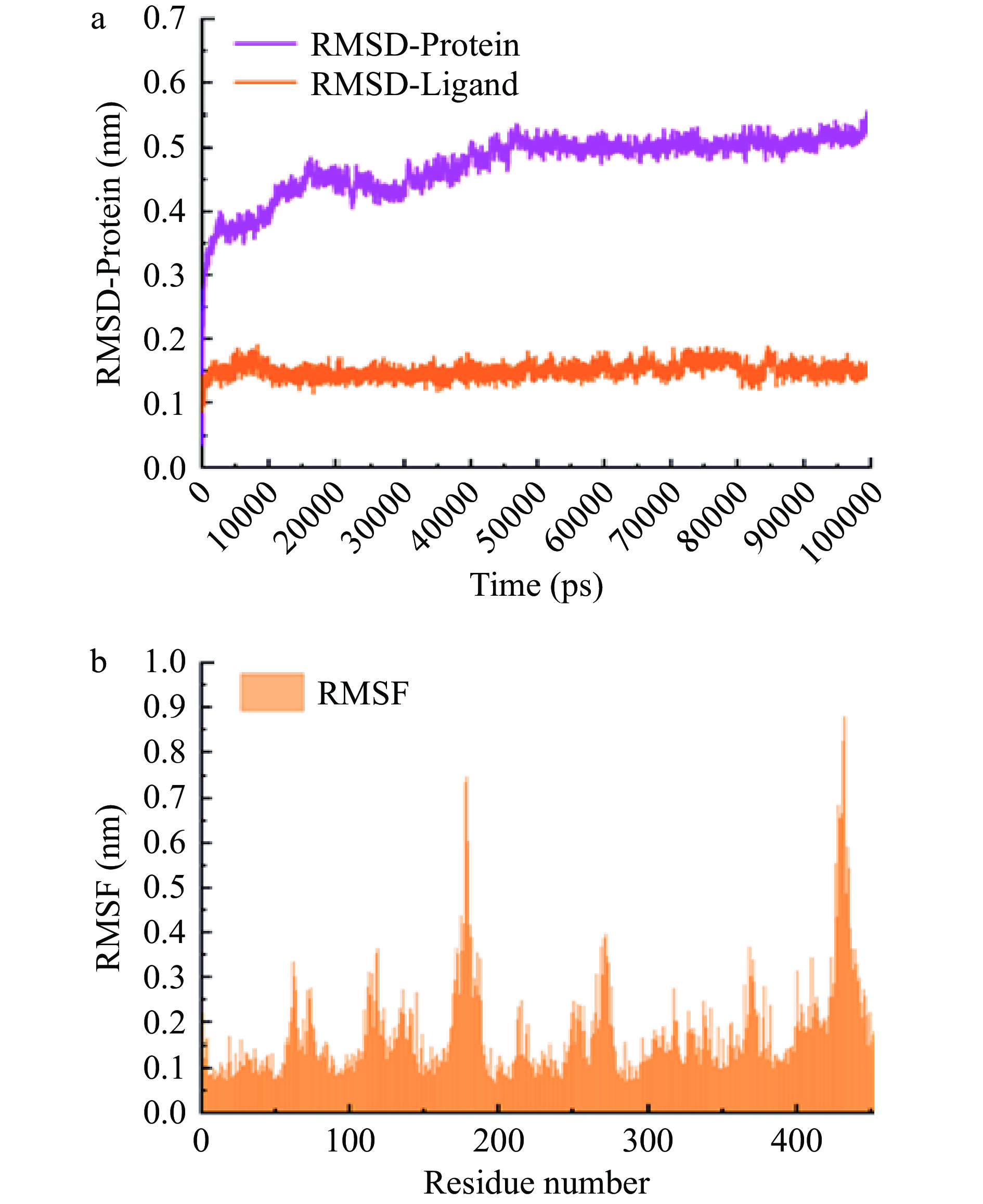
首先,计算模拟过程中蛋白质-配体复合物的RMSD值,以评估其结构稳定性。RMSD(Root Mean Square Deviation)是一种描述蛋白质结构在模拟过程中稳定性的指标。如图7a所示,在50 ns时,体系达到平衡,稳定在0.5 nm。这表明CuB与SLC7A11的结合在模拟过程中具有较好的稳定性。
随后,计算模拟过程中蛋白质各残基的RMSF值,以评估其在模拟过程中的波动情况。RMSF(Root Mean Square Fluctuation)是一种描述蛋白质各个残基在模拟过程中波动情况的指标。如图7b所示,在残基数量为200和450左右出现较大波动,表明这些区域的结构可能较为灵活,暗示这些区域可能是蛋白质结构和功能的关键部位。
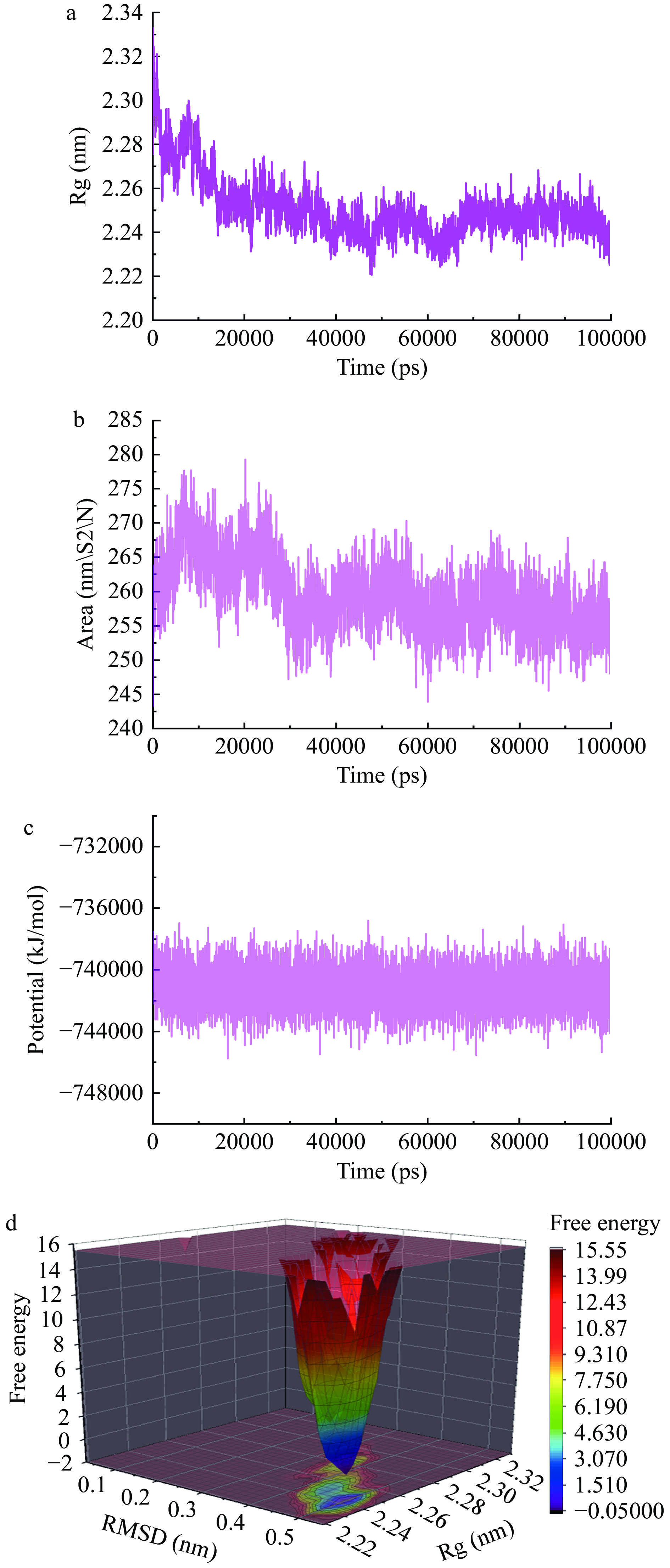
此外,分析模拟过程中蛋白质的回转半径(Rg)变化趋势,以评估蛋白质结构的紧密程度。结果如图8a所示,在50 ns时,Rg值达到2.24 nm并保持稳定,说明CuB与SLC7A11的结合在模拟过程中结构紧凑。蛋白质的回转半径(Rg)是一个描述蛋白质结构紧密程度的指标,通过观察Rg值的变化趋势可以更好地了解蛋白质-配体复合物的稳定性。
为了对模拟过程中的体系进行更全面的评估,对溶剂的可及表面积(SASA)进行分析。结果如图8b所示,SASA图可以用于描述蛋白质-配体复合物在模拟过程中的溶剂暴露程度。在50 ns时,SASA基本达到平衡,表明CuB与SLC7A11的结合在模拟过程中与溶剂的相互作用较为稳定。
接下来,计算模拟过程中体系的总势能变化曲线。总势能变化曲线可以用于评估体系在模拟过程中的能量稳定性。结果如图8c所示,体系能量一直在−740000 kJ/mol附近,表明CuB与SLC7A11结合在模拟过程中具有较好的能量稳定性。
最后,计算了模拟过程中体系的自由能形貌,以评估各个状态的稳定性。自由能形貌可描述体系中各个状态稳定性。如图8d所示,在Rg为2.24 nm,RMSD为0.5 nm时,自由能最低,与之前的RMSD和Rg达到稳定的结果保持一致。这进一步证实了CuB与SLC7A11结合在模拟过程中具有较好的稳定性。
综上所述,通过对SLC7A11与CuB进行分子动力学模拟及其相关指标的分析,发现CuB与SLC7A11的结合具有较好的结构稳定性、波动性以及能量稳定性。
3. 讨论与结论
CuB已经被证明可以经由多种信号通路发挥抑制多种类型癌症的作用,如非小细胞肺癌[38]、结直肠癌[39]、前列腺癌[40]、乳腺癌[41]等。目前,已证实葫芦素B具有抑制结直肠癌的作用,而大多数研究都集中在细胞凋亡。相比之下,对于葫芦素B在铁死亡诱导效应方面的研究报道较少,其中CuB诱发铁死亡发挥抗结直肠癌的研究尚无报道。因此,本文通过测定不同浓度的CuB对HCT-116细胞的毒作用及相关铁死亡指标初步明确了CuB介导铁死亡发挥的结直肠癌抑制作用,并通过网络药理学分析、代谢组学分析、分子对接技术和分子动力学模拟技术,进一步研究了CuB对人结肠癌细胞作用背后的铁死亡作用机制。
在本研究中,首先使用ATP法测定检测了CuB对HCT-116细胞的影响,结果显示CuB能够抑制HCT-116细胞的生长,其IC50值为64.48 nmol/L。随后,通过总铁离子浓度、GSH含量、加或不加铁死亡抑制剂Fer-1后的LDH释放实验,发现CuB能够显著影响HCT-116细胞内铁离子浓度、GSH含量和LDH水平。CuB处理后,细胞内铁离子浓度以剂量依赖性方式增加,GSH含量显著(P<0.05)降低。当加入铁死亡抑制剂Fer-1后,LDH释放水平明显下降。这些实验结果支持了CuB通过诱导铁死亡来抑制HCT-116细胞增殖的假设,这些结果表明CuB具有诱导HCT-116铁死亡的潜力。
在此基础上,进行了网络药理学分析,通过数据库检索获得CuB的276个关键靶点,并且基于CuB的靶点和结肠癌靶点相互作用网络图筛选,发现STAT3、EGFR、PIK3R1等关键靶点。基于GO和KEGG富集分析结果,推测铁死亡可能参与了CuB诱导的细胞死亡过程,CuB可能通过靶向作用这些关键靶点的方式,引发结肠癌细胞内铁的释放和蓄积,导致细胞内铁离子超载,从而引起铁死亡。另外,CuB可能通过抑制EGFR、JAK2、JAK3等靶点的信号通路,降低细胞内GSH含量,进而诱导氧化应激和细胞死亡。此外,CuB还可能通过激活STAT3、PIK3R1等靶点,诱导细胞凋亡和铁离子释放,从而增加细胞内铁含量,最终导致铁死亡。代谢组学分析则揭示了CuB对结肠癌细胞的代谢影响。KEGG通路的富集结果进一步聚焦于谷胱甘肽代谢与铁死亡,与本文中CuB显著(P<0.05)降低HCT-116细胞内谷胱甘肽的含量、铁死亡抑制剂Fer-1逆转由CuB诱发LDH释放分子而导致的细胞死亡相一致。胱氨酸/谷氨酸抗转运蛋白SLC7A11可以导入胱氨酸进行谷胱甘肽生物合成和抗氧化防御,并在多种人类癌症中过度表达[42]。网络药理学与代谢组学的结果都将CuB抑制HCT-116结肠癌的作用指向与铁死亡关系紧密的谷胱甘肽代谢途径,由此本研究通过分子对接探究了CuB与谷胱甘肽代谢途径中关键蛋白SLC7A11和GPX4的结合情况,分析发现SLC7A11蛋白能与CuB结合,并且有较低的结合能。进而继续采用分子动力学模拟分析进一步证实了CuB能与SLC7A11稳定结合。
总之,通过网络药理学、代谢组学、分子对接和分子动力学模拟等多种手段,对CuB诱导铁死亡抑制结肠癌细胞HCT-116增殖的机制进行了深入研究。这些实验结果揭示了CuB在结肠癌治疗中的潜在作用机制,为结肠癌的治疗提供了新的思路。同时也为后续研究提供了理论依据和方向,以期为结肠癌的治疗提供更多的选择。
-
图 2 CuB诱导HCT-116细胞铁死亡的结果
注:a. CuB对HCT-116细胞内GSH含量的影响(**P<0.01,***P<0.001);b. CuB对HCT-116细胞内总铁离子含量的影响(*P<0.05,**P<0.01,***P<0.001);c. CuB及铁死亡抑制剂Fer-1对HCT-116细胞LDH释放的影响(*表示有铁死亡抑制剂跟没有铁死亡抑制剂之间的显著性变化,***P<0.001,#表示不同浓度之间的给药组之间的显著性的变化,###P<0.001)。
Figure 2. Results of CuB induced ferroptosis in HCT-116 cells
表 1 差异代谢物筛选结果
Table 1 Screening results of differential metabolites
指标 化合物 类型 MW0130472 (E)-2',6'-Dihydroxy 4',4-dimethoxychalcone up MW0063246 Pyrrolidinium,1-[(7R)-7-(acetyloxy)-4-
hydroxy-4-oxido-3,5,9-trioxa-4-
phosphapentacos-1-yl]-1-methyl-,inner saltdown MW0009839 Taprostene down MW0142142 2-{5-[3-(6-Benzoyl-1-propylnaphthalen-2-yloxy)
propoxy]indol-1-YL}ethanoic aciddown MW0000158 Cabergoline down MEDP1468 Dexpanthenol up MEDN1148 2-Hydroxyhexadecanoic acid up MW0012931 1-O-Hexadecyl-2-O-acetyl-sn-glyceryl-3-
phosphorylcholinedown MW0009945 Tolyfluanide down MW0140300 (2S)-2-(Benzenesulfonamido)-3-[[2-(4-piperidin-4-
ylpiperidin-1-yl)-4-propan-2-yl-1,3-thiazole-5-
carbonyl]amino]propanoic aciddown MW0111715 1,4-D-Xylobiose up MW0170018 Xanthohumol up MEDL02423 Creatine phosphate down MEDN0391 DHA up FDATN01264 Sodium 4-Aminosalicylate up 表 2 CuB与蛋白质分子对接评分
Table 2 Scores of the docking between CuB and protein
化合物 靶点蛋白 氨基酸残基 对接得分 结合自由能
(kcal/mol)CuB SLC7A11 Tyr251,Leu252,Ile134,
Arg135,Arg340,Thr56−4.819 −43.34 CuB GPX4 Gly79 −3.833 −47.43 -
[1] LABIANCA R, BERETTA G D, KILDANI B, et al. Colon cancer[J]. Critical Reviews in Oncology/Hematology,2010,74(2):106−133. doi: 10.1016/j.critrevonc.2010.01.010
[2] 宫辰, 杨冰, 周钰通, 等. 葫芦素抗肿瘤作用及其机制研究进展[J]. 药物评价研究,2023,46(2):452−461. [GONG Chen, YANG Bing, ZHOU Yutong, et al. Research progress on the pharmacological effects and mechanisms of cucurbitacin against tumors[J]. Drug Evaluation Research,2023,46(2):452−461.] GONG Chen, YANG Bing, ZHOU Yutong, et al . Research progress on the pharmacological effects and mechanisms of cucurbitacin against tumors[J]. Drug Evaluation Research,2023 ,46 (2 ):452 −461 .[3] GARG S, KAUL S C, WADHWA R. Cucurbitacin B and cancer intervention:Chemistry, biology and mechanisms (Review)[J]. International Journal of Oncology,2018,52(1):19−37.
[4] DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis:An iron-dependent form of nonapoptotic cell death[J]. Cell,2012,149(5):1060−1072. doi: 10.1016/j.cell.2012.03.042
[5] MOU Y H, WANG J, WU J C, et al. Ferroptosis, a new form of cell death:Opportunities and challenges in cancer[J]. Journal of Hematology & Oncology,2019,12(1):34.
[6] LI J, CAO F, YIN H L, et al. Ferroptosis:Past, present and future[J]. Cell Death & Disease,2020,11(2):88.
[7] GASCHLER M M, ANDIA A A, LIU H, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation[J]. Nature Chemical Biology,2018,14(5):507−515. doi: 10.1038/s41589-018-0031-6
[8] LIANG C, ZHANG X L, YANG M S, et al. Recent progress in ferroptosis inducers for cancer therapy[J]. Advanced Materials,2019,31(51):e1904197. doi: 10.1002/adma.201904197
[9] PARK T J, PARK J H, LEE G S, et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes[J]. Cell Death & Disease,2019,10(11):835.
[10] STOCKWELL B R, JIANG X, GU W. Emerging mechanisms and disease relevance of ferroptosis[J]. Trends in Cell Biology,2020,30:478−490. doi: 10.1016/j.tcb.2020.02.009
[11] HUANG S, CAO B H, ZHANG J L, et al. Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B:Molecular mechanism and therapeutic potential[J]. Cell Death & Disease,2021,12(3):237.
[12] MAO D, LIU A H, WANG Z P, et al. Cucurbitacin B inhibits cell proliferation and induces cell apoptosis in colorectal cancer by modulating methylation status of BTG3[J]. Neoplasma,2019,66(4):593−602. doi: 10.4149/neo_2018_180929N729
[13] ZHANG H Y, ZHAO B, WEI H Z, et al. Cucurbitacin b controls M2 macrophage polarization to suppresses metastasis via targeting Jak-2/Stat3 signalling pathway in colorectal cancer[J]. Journal of Ethnopharmacology,2022,287:114915. doi: 10.1016/j.jep.2021.114915
[14] DANDAWATE P, SUBRAMANIAM D, PANOVICH P, et al. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway[J]. Scientific Reports,2020,10(1):1290. doi: 10.1038/s41598-020-57940-9
[15] WISHART D S, FEUNANG Y D, GUO A C, et al. Drug Bank 5.0:A major update to the Drug Bank database for 2018[J]. Nucleic Acids Res,2018,46(D1):1074−1082. doi: 10.1093/nar/gkx1037
[16] AMBERGER J S, BOCCHINI C A, SCHIETTECATTE F, et al. OMIM. org:Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders[J]. Nucleic Acids Research, 2015, 43(Database issue):789-798.
[17] STELZER G, ROSEN N, PLASCHKES I, et al. The gene cards suite:from gene data mining to disease genome sequence analyses[J]. Curr Protoc Bioinformatics,2016,54:1−33.
[18] BARBARINO J M, WHIRL-CARRILLO M, ALTMAN R B, et al. Pharm GKB:A worldwide resource for pharmacogenomic information[J]. Wiley Interdisciplinary Reviews systems Biology And Medicine,2014,10(4):289−306.
[19] CHEN X, JI Z L, CHEN Y Z. TTD:Therapeutic target database[J]. Nucleic Acids Research,2002,30(1):412−415. doi: 10.1093/nar/30.1.412
[20] KIM S, CHEN J, CHENG T J, et al. PubChem in 2021:New data content and improved web interfaces[J]. Nucleic Acids Research,2021,49:1388−1395. doi: 10.1093/nar/gkaa971
[21] BIASINI M, BIENERT S, WATERHOUSE A, et al. SWISS-MODEL:Modelling protein tertiary and quaternary structure using evolutionary information[J]. Nucleic Acids Research,2014,42:252−258. doi: 10.1093/nar/gku340
[22] LIU Z Y, GUO F F, WANG Y, et al. BATMAN-TCM:A bioinformatics analysis tool for molecular mechanism of traditional Chinese medicine[J]. Scientific Reports,2016,6:21146. doi: 10.1038/srep21146
[23] RU J L, LI P, WANG J N, et al. TCMSP:A database of systems pharmacology for drug discovery from herbal medicines[J]. Journal of Cheminformatics,2014,6:13. doi: 10.1186/1758-2946-6-13
[24] ZHANG J T, FAN F Y, LIU A F, et al. Icariin:A potential molecule for treatment of knee osteoarthritis[J]. Frontiers in Pharmacology,2022,13:811808. doi: 10.3389/fphar.2022.811808
[25] YANG X, LI Y H, LÜ R L, et al. Study on the multitarget mechanism and key active ingredients of herba siegesbeckiae and volatile oil against rheumatoid arthritis based on network pharmacology[J]. Evidence-based Complementary and Alternative Medicine,2019,2019:8957245.
[26] GUO H H, GUO H X, ZHANG L, et al. Metabolome and transcriptome association analysis reveals dynamic regulation of purine metabolism and flavonoid synthesis in transdifferentiation during somatic embryogenesis in cotton[J]. International Journal of Molecular Sciences,2019,20(9):2070. doi: 10.3390/ijms20092070
[27] ALONSO H, BLIZNYUK A A, GREADY J E. Combining docking and molecular dynamic simulations in drug design[J]. Medicinal Research Reviews,2006,26(5):531−568. doi: 10.1002/med.20067
[28] IMAN M, KABOUTARAKI H B, JAFARI R, et al. Molecular dynamics simulation and docking studies of selenocyanate derivatives as anti-leishmanial agents[J]. Combinatorial Chemistry & High Throughput Screening,2016,19(10):847−854.
[29] 张博文, 董君丽, 曹兵, 等. 铁死亡的表观遗传调控机制[J]. 生命的化学,2023,43(2):227−233. [ZHANG Bowen, DONG Junli, CAO Bing, et al. The epigenetic regulatory mechanisms of ferroptosis[J]. Chemistry of Life,2023,43(2):227−233.] ZHANG Bowen, DONG Junli, CAO Bing, et al . The epigenetic regulatory mechanisms of ferroptosis[J]. Chemistry of Life,2023 ,43 (2 ):227 −233 .[30] WANG Y Q, CHANG S Y, WU Q, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis[J]. Frontiers in Aging Neuroscience,2016,8:308.
[31] 李雅琪, 徐弘庭, 赵维坚, 等. 肝细胞癌中的铁死亡与氧化应激[J]. 生物医学,2023,13(3):293−302. [LI Yaqi, XU Hongting, ZHAO Weijian, et al. Ferroptosis and oxidative stress in hepatic cellular carcinoma[J]. Hans Journal of Biomedicine,2023,13(3):293−302.] doi: 10.12677/HJBM.2023.133034 LI Yaqi, XU Hongting, ZHAO Weijian, et al . Ferroptosis and oxidative stress in hepatic cellular carcinoma[J]. Hans Journal of Biomedicine,2023 ,13 (3 ):293 −302 . doi: 10.12677/HJBM.2023.133034[32] 高绪聪, 柴振海, 张宗鹏. 药物性肝损伤的生物标志物及其评价的研究进展[J]. 中国药理学与毒理学杂志, 2012, 5(26):692−696. [GAO Xucong, CHAI Zhenhai, ZHANG Zongpeng. Progress in biomarkers and evaluation of drug-induced liver injury[J]. Chinese Journal of Pharmacology and Toxicology, 2012, 5(26):692−696.] GAO Xucong, CHAI Zhenhai, ZHANG Zongpeng. Progress in biomarkers and evaluation of drug-induced liver injury[J]. Chinese Journal of Pharmacology and Toxicology, 2012, 5(26): 692−696.
[33] ERSAHIN T, TUNCBAG N, CETIN-ATALAY R. The PI3K/AKT/mTOR interactive pathway[J]. Molecular Biosystems,2015,11(7):1946−1954. doi: 10.1039/C5MB00101C
[34] ZHU Y H, XIAN X M, WANG Z Z, et al. Research progress on the relationship between atherosclerosis and inflammation[J]. Biomolecules,2018,8(3):80. doi: 10.3390/biom8030080
[35] FANG J Y, RICHARDSON B C. The MAPK signalling pathways and colorectal cancer[J]. The Lancet Oncology,2005,6(5):322−327. doi: 10.1016/S1470-2045(05)70168-6
[36] BOULESTEIX A L, STRIMMER K. Partial least squares:A versatile tool for the analysis of high-dimensional genomic data[J]. Briefings in Bioinformatics,2007,8(1):32−44.
[37] WANG B, LIU G L, FEI Q, et al. Orthogonal projection to latent structures combined with artificial neural networks in non-destructive analysis of Ampicillin powder[J]. Spectrochimica Acta, Part A, Molecular and Biomolecular Spectroscopy,2009,71(5):1695−1700. doi: 10.1016/j.saa.2008.06.021
[38] YUAN R, ZHAO W, WANG Q Q, et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis[J]. Pharmacological Research,2021,170:105748. doi: 10.1016/j.phrs.2021.105748
[39] CHAI Y T, XIANG K, WU Y Z, et al. Retracted:Cucurbitacin B inhibits the Hippo-YAP signaling pathway and exerts anticancer activity in colorectal cancer cells[J]. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research,2022,28:e938690.
[40] ALAFNAN A, KHALIFA N E, HUSSAIN T, et al. Cucurbitacin-B instigates intrinsic apoptosis and modulates notch signaling in androgen-dependent prostate cancer LNCaP cells[J]. Frontiers in Pharmacology,2023,14:1206981. doi: 10.3389/fphar.2023.1206981
[41] SINHA S, KHAN S, SHUKLA S, et al. Cucurbitacin B inhibits breast cancer metastasis and angiogenesis through VEGF-mediated suppression of FAK/MMP-9 signaling axis[J]. The International Journal of Biochemistry & Cell Biology,2016,77:41−56.
[42] KOPPULA P, ZHUANG L, GAN B. Cystine transporter SLC7A11/xCT in cancer:Ferroptosis, nutrient dependency, and cancer therapy[J]. Protein & Cell,2021,12(8):599−620.
-
期刊类型引用(1)
1. 黄素艳,曹荣,刘楠,孙永,周德庆,王珊珊. 提取方式对微拟球藻蛋白理化性质和功能特性的影响. 食品工业科技. 2025(01): 87-96 .  本站查看
本站查看
其他类型引用(0)





 下载:
下载:
 下载:
下载:



