Synthesis and Application of Lipase Immobilized on Mussel-inspired Polymer Microspheres
-
摘要: 为构建新型固定化酶催化体系,以聚甲基丙烯酸缩水甘油酯为载体,利用贻贝仿生技术——多巴胺/聚乙烯亚胺共沉积进行修饰,采用扫描电镜(SEM)、能谱(EDS)、Zeta电位及红外光谱(FT-IR)表征所得材料,并研究其固定化近平滑假丝酵母CICC 33470所产脂肪酶的表征及酶学性质。最佳酶固定化条件为:固定化温度为30 ℃,固定化pH为7.0,固定化时间为5 h,初始酶活为337.76 U/mL,载体添加量为0.2 g。固定化酶最佳反应温度为50 ℃,最佳反应pH为8.0,最佳反应时间为10 min,最优条件下固定化酶酶活为484.42±5.97 U/g-载体。固定化酶的稳定性明显提高,重复使用8次后,固定化酶仍有39.22%的初始酶活。进一步将固定化酶用于催化乙酰丙酸与十二醇的酯化反应,转化率可达75.94%,充分证明聚甲基丙烯酸缩水甘油酯经修饰后是固定化脂肪酶的优良载体,为未来扩大脂肪酶的应用范围提供了基础数据。
-
关键词:
- 甲基丙烯酸缩水甘油酯 /
- 固定化脂肪酶 /
- 多巴胺/聚乙烯亚胺共沉积 /
- 近平滑假丝酵母 /
- 酯化反应
Abstract: In order to construct a new type of immobilized enzyme catalytic system, poly (glycidyl methacrylate) was used as a support, and was modified with dopamine/polyethyleneimine by the mussel-inspired co-deposition method. The resulted material was characterized by scanning electron microscope (SEM), energy spectroscopy (EDS), Zeta potential and Fourier transform infrared spectrometer (FT-IR), respectively. Then, the resulted material was applied for the immobilization of lipase, which was fermented via Candida parapsilosis CICC 33470. The characterization and enzymatic properties of immobilized enzyme were also investigated. The optimal immobilized conditions were the immobilization temperature of 30 ℃, the immobilization pH of 7.0, the immobilization time of 5 h and the immobilization initial enzyme acticity of 337.76 U/mL, together with the carrier addition of 0.2 g. It was indicated that the maximum enzyme activity reached 484.42±5.97 U/g-support, as the optimal reaction temperature, reaction pH and reaction time were 50 ℃, 8.0 and 10 min, respectively. In addition, the immobilized lipase exhibited excellent stability, and it kept 39.22% of original enzyme activity after eight reuse cycles. Furthermore, when the immobilized lipase was used to catalyze the esterification reaction of levulinic acid and lauryl alcohol, the conversion rate could reach 75.94%. It was illuminated that the modified poly (glycidyl methacrylate) could serve as a good support for lipase immobilization, and the results provided basic data for expanding the application areas of lipase in the future. -
脂肪酶是一种能够催化酯水解、酯化和酯交换反应的工业酶,这使其成为食品、制药和生物能源行业中炙手可热的生物催化剂[1−3]。然而,游离的脂肪酶不能满足工业生物催化剂的所有要求,存在有机溶剂耐受性低、热稳定性差,以及难以回收利用等问题[4],这都极大地制约了脂肪酶的进一步应用。为获得适用范围更广、性能更加优越的脂肪酶,人们主要从两方面入手开展相关研究工作。一方面,可以通过分子改造提高脂肪酶本身的环境耐受性,但是存在改造成本高、研究过程繁琐等问题[5−6]。另一方面,采用固定化酶技术,将酶固定在载体上,使酶能够重复使用并增加其稳定性,从而简化回收过程,在实际应用中具有更大优势[7−8]。使用化学方法(如交联法)固定化酶是研究热点之一,然而常用的戊二醛、京尼平、环氧氯丙烷等交联剂会影响酶分子的构象,进而造成酶活的损失,在一定程度上限制了固定化酶的进一步应用。因此,减少使用或不使用交联剂,而是利用材料表面的粘附性及氢键等作用力进行固定化酶,是解决上述问题的主要方法之一。
以多巴胺(dopamine,DA)沉积为代表的贻贝仿生技术,因其绿色、环保的优点受到越来越多的关注,并已初步应用于固定化酶的研究中[9]。该技术的主要反应机制是,在有氧和弱碱性条件下,多巴胺可自发在材料表面氧化自聚形成聚多巴胺(polydopamine,PDA)涂层。聚多巴胺凭借优良的粘附特性以及丰富的反应基团(如邻苯二酚基团),通过迈克尔加成或席夫碱反应再次键合功能分子,从而实现进一步的功能化修饰[10]。例如,Liu等[11]使用多巴胺与聚乙烯亚胺(polyethyleneimine,PEI)共沉积作为柔性链修饰氧化石墨烯,用于固定化腺苷酸环化酶,沉积时间需16 h。Zhai等[12]以聚多巴胺/聚乙烯亚胺共沉积修饰二氧化硅微球,用于固定化碳酸酐酶,固定化酶在10次循环之后有初始酶活的95.10%,沉积时间需12 h。因此寻找合适的载体并尽量缩短沉积时间,对制备和应用固定化酶具有积极意义。
甲基丙烯酸缩水甘油酯的合成方法简单,具有良好的生物相容性,已被用于β-葡萄糖苷酶[13]、纤维素酶[14]及葡萄糖氧化酶/过氧化氢酶共固定化[15]的研究中,而将其与贻贝仿生技术相结合并应用于脂肪酶的固定化具有较强的创新性和重要的研究意义。因此,本研究采用聚甲基丙烯酸缩水甘油酯(poly (glycidyl methacrylate),PGMA)微球作为固定化酶的载体,在其表面通过多巴胺(DA)和聚乙烯亚胺(PEI)共沉积的方法进行贻贝仿生改性,实现在不使用任何交联剂的前提下,利用载体表面的特异性官能团直接与脂肪酶结合,进行酶的固定化工艺研究。进一步评估固定化酶的酶学性能,包括催化活性、稳定性和重复利用性等。最后,研究固定化酶催化酯化反应的效果。该固定化酶策略具有反应条件较温和及催化效率较高等优点,在大规模工业催化领域具有广阔的实际应用前景。
1. 材料与方法
1.1 材料与仪器
近平滑假丝酵母(Candida parapsilosis)CICC 33470 购买自中国工业微生物菌种保藏中心;盐酸多巴胺(98%)、聚乙烯亚胺(99%)、甲基丙烯酸缩水甘油酯(97%) 上海阿拉丁试剂有限公司;聚乙烯吡咯烷酮、棕榈酸对硝基苯酯 均为分析纯,上海麦克林生化科技股份有限公司;偶氮二异丁腈、磷酸氢二钠、磷酸二氢钠 均为分析纯,天津市科密欧化学试剂有限公司;三羟甲基氨基甲烷(99%) 国药集团化学试剂有限公司。
SpectraMax Plus384 酶标仪 上海仪电科学仪器股份有限公司;Spectrum 10 傅里叶变换红外光谱仪 铂金埃尔默仪器有限公司;JSM 7800F型场发射扫描电子显微镜 日本电子株式会社;Zeta sizer 3000HSA激光粒度仪 英国马尔文仪器公司。
1.2 实验方法
1.2.1 近平滑假丝酵母脂肪酶的制备
根据石莹[16]的方法活化冻干菌粉。用接种环蘸取活化后的近平滑假丝酵母菌液,在麦芽汁琼脂培养基上划线,于28 ℃的培养箱中培养48 h,待有单菌落出现并生长良好后,用接种环挑取一个单菌落,接种到装液量为20%的摇瓶中,28 ℃,200 r/min培养14 h,作为种子液。将种子液以10%接种量(v/v)接种至摇瓶中,200 r/min培养14 h,将得到的发酵液在4 ℃,8000 r/min离心10 min,上清液即为脂肪酶酶液。
1.2.2 贻贝仿生功能化PGMA-OH的制备及表征
1.2.2.1 聚甲基丙烯酸缩水甘油酯(PGMA)的制备
向三口烧瓶中加入81 g无水乙醇、9 g水和3 g聚乙烯吡咯烷酮(PVP K-30),待固体超声溶解后,加入10 g甲基丙烯酸缩水甘油酯单体和0.2 g偶氮二异丁腈。在通氮气的条件下将体系加热至70 ℃,磁力搅拌24 h,反应结束后,用去离子水和乙醇交替洗涤微球,冷冻真空干燥得到的微球粉末记为PGMA。
1.2.2.2 PGMA-OH的制备
取3 g PGMA微球加入100 mL硫酸水溶液(0.1 mol/L)中,70 ℃下磁力搅拌反应48 h。用去离子水和乙醇交替洗涤PGMA反应后得到的微球,冷冻真空干燥得到的微球粉末记为PGMA-OH。
1.2.2.3 贻贝仿生功能化PGMA-OH的制备
在20 mL的Tris-HCl缓冲液(0.05 mol/L,pH为8.5)中加入0.5 g PGMA-OH。之后,迅速加入40 mg聚乙烯亚胺(PEI)及多巴胺(DA)。25 ℃条件下,磁力搅拌6 h。反应结束后,用去离子水和乙醇交替洗涤至上清液无色,以PGMA-OH@PDA/PEI表示冷冻真空干燥得到的微球粉末。
1.2.2.4 扫描电子显微镜与能谱分析
干燥后的PGMA、PGMA-OH及PGMA-OH@PDA/PEI样品置于载物台上,真空条件下喷金处理,将处理好的样品放入观察室,拍摄样品形貌照片,分析样品形貌,对元素分布进行分析[17]。
1.2.2.5 红外光谱分析
采用KBr压片法,将干燥后的PGMA、PGMA-OH及PGMA-OH@PDA/PEI与KBr按照1%的比例充分混合、研磨,压片后置于红外光谱仪上测试[18]。
1.2.2.6 Zeta电位分析
将PGMA-OH及PGMA-OH@PDA/PEI分散于蒸馏水中,稀释后至1 mg/L后,80 W超声10 min进行测试[19]。
1.2.3 固定化酶的制备及表征
1.2.3.1 固定化酶的制备及工艺条件优化
将0.2 g PGMA-OH@PDA/PEI溶解于5 mL PBS缓冲液(0.02 mol/L,pH为7.0)中,然后与5 mL脂肪酶酶液混合,放入摇床中,200 r/min固定化5 h,在4 ℃,6000 r/min的条件下离心5 min,用PBS缓冲液洗涤三次,冷冻真空干燥得到固定化酶粉末。分别在不同固定化温度(20、25、30、35、40 ℃)、不同固定化pH(5.0、6.0、7.0、8.0、9.0)、不同固定化时间(3、4、5、6、7 h)、不同初始酶活(112.59、225.18、337.76、450.35、562.93 U/mL)及不同载体添加量(0.05、0.10、0.15、0.20、0.25 g)条件下进行实验,测定固定化酶酶活的变化规律。
1.2.3.2 固定化酶的红外光谱分析
采用KBr压片法,将冷冻真空干燥所得最佳酶固定化条件下制备出的固定化酶样品与KBr按照1%的比例充分混合、研磨,压片后置于红外光谱仪上测试[18]。
1.2.4 游离酶及固定化酶的酶学性质研究
1.2.4.1 游离酶及固定化酶的最佳反应温度及温度稳定性
当pH为7.0时,将游离酶及固定化酶分别置于不同温度(30、40、50、60、70 ℃)下反应,确定最佳反应温度后,以处理前的酶活力定义为100%,测定8 h内,游离酶与固定化酶的相对酶活。
1.2.4.2 游离酶及固定化酶的最佳反应pH及pH稳定性
在最佳反应温度下,将游离酶及固定化酶分别置于不同pH(6.0、7.0、8.0、9.0、10.0、11.0)反应,确定最佳反应pH后,以处理前测定的酶活力定义为100%,测定2 h内、4 ℃下,游离酶与固定化酶的相对酶活。
1.2.4.3 游离酶及固定化酶的最佳反应时间
在最佳反应温度及最佳反应pH条件下,将游离酶及固定化酶分别反应不同时间(5、10、15、20、25 min),确定最佳反应时间。
1.2.4.4 固定化酶的重复利用性
在最佳反应条件下,依据固定化酶酶活测定方法,将制备的固定化酶进行8次循环实验。向3 mL PBS缓冲液(0.02 mol/L)中加入0.022 g固定化酶及200 μL棕榈酸对硝基苯酯,在50 ℃下反应10 min,每次反应结束后用PBS缓冲液洗涤三次,之后投入下一次循环。以第一次循环测定的酶活力定义为100%,测定固定化酶的相对酶活。
1.2.4.5 游离及固定化酶的储藏稳定性
将游离酶和固定化酶分别置于4 ℃环境中储藏1个月,每5 d测定酶活力,以储藏当天(第0 d)的酶活力定义为100%,计算出各储藏天数的相对酶活。
1.2.5 固定化酶在催化乙酰丙酸与十二醇酯化反应中的应用
按照王立晖等[20]的方法,向无溶剂体系中,加入摩尔比为1:10的乙酰丙酸及十二醇,测定4 h内,乙酰丙酸在不同反应温度(35、40、45、50、55 ℃)下的转化率。在最佳反应温度下,改变乙酰丙酸和十二醇的摩尔比(1:2.5、1:5、1:10、1:15、1:20、1:25),测定4 h内乙酰丙酸的转化率。在上述最优条件下,测定乙酰丙酸在不同反应时间(3、6、9、12、15、18、21、24 h)下的转化率。
1.2.6 酶水解活性的测定
利用改进的棕榈酸对硝基苯酯(p-NPP,5.0 mg/mL)的方法对脂肪酶的水解活性进行测定[21]。在3 mL的PBS缓冲液(0.02 mol/L,pH7.0)中,加入200 μL的游离酶(或0.022 g固定化酶)和200 μL的p-NPP。混匀后在50 ℃下反应10 min,过滤取上清液,稀释适当的倍数后,使用酶标仪测定上清液在410 nm处的吸光值。根据上清液中对硝基苯酚(p-NP)的含量,确定游离酶(或固定化酶)的水解酶活。酶活力(U)定义为:单位时间内(min),释放单位产物(1 nmol)所需的酶量,定义为1个酶活单位。固定化酶酶活(U/g-载体)定义为:单位时间内(min),单位载体(g)上,释放单位产物(1 nmol)所需的酶量。
1.2.7 乙酰丙酸转化率的测定
利用热乙醇法,分别测定反应前后乙酰丙酸含量,按照公式(1)计算乙酰丙酸的转化率[20]。
(1) 式中,A0为反应前乙酰丙酸含量,A1为反应后乙酰丙酸含量。
1.3 数据处理
每个样品设置三组平行,利用Origin 8.0软件进行数据分析,测定结果以平均值±标准差表示,使用SPSS 22.0进行差异显著性分析。
2. 结果与分析
2.1 载体的表征
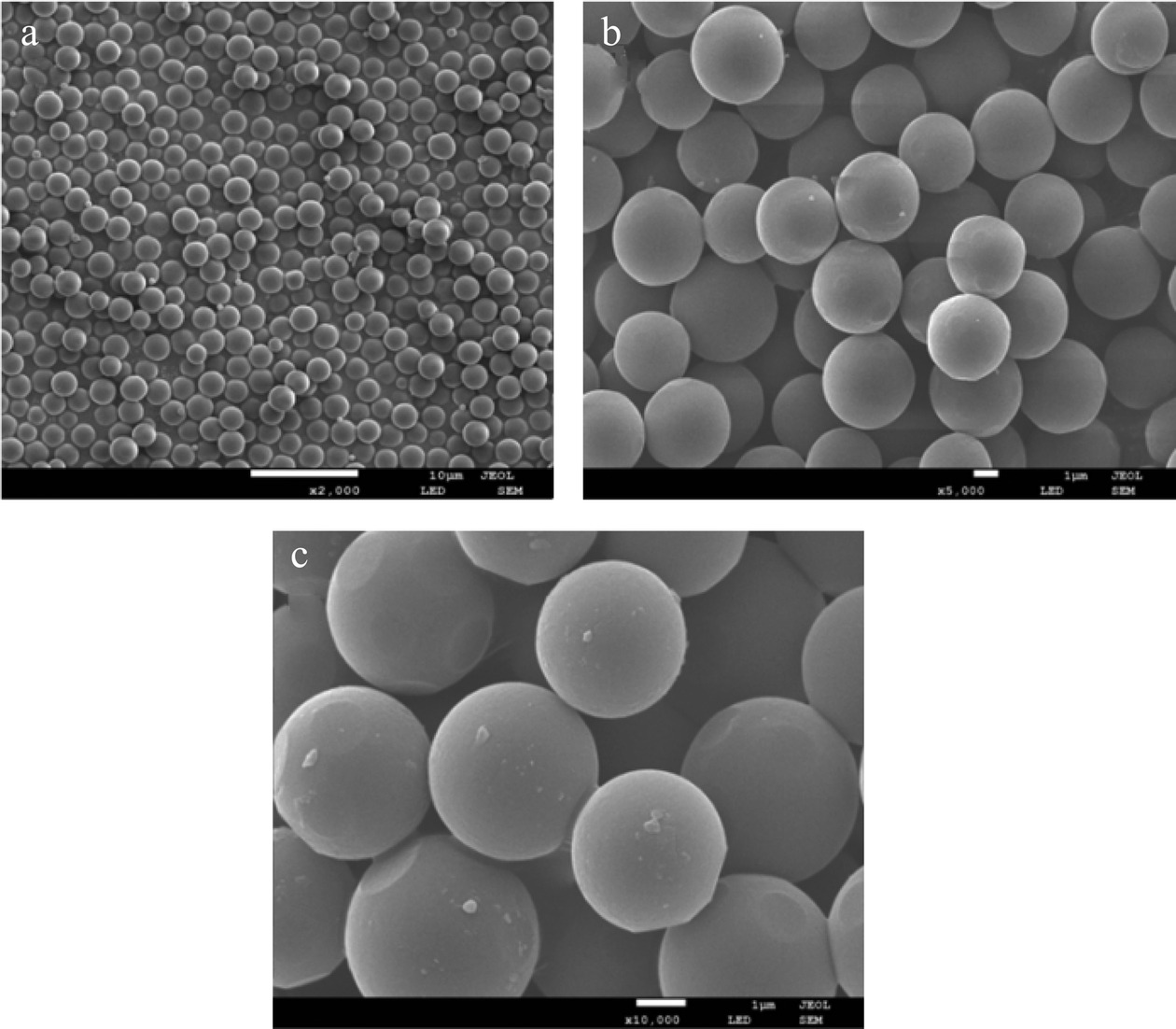
2.1.1 载体的扫描电镜图与能谱分析
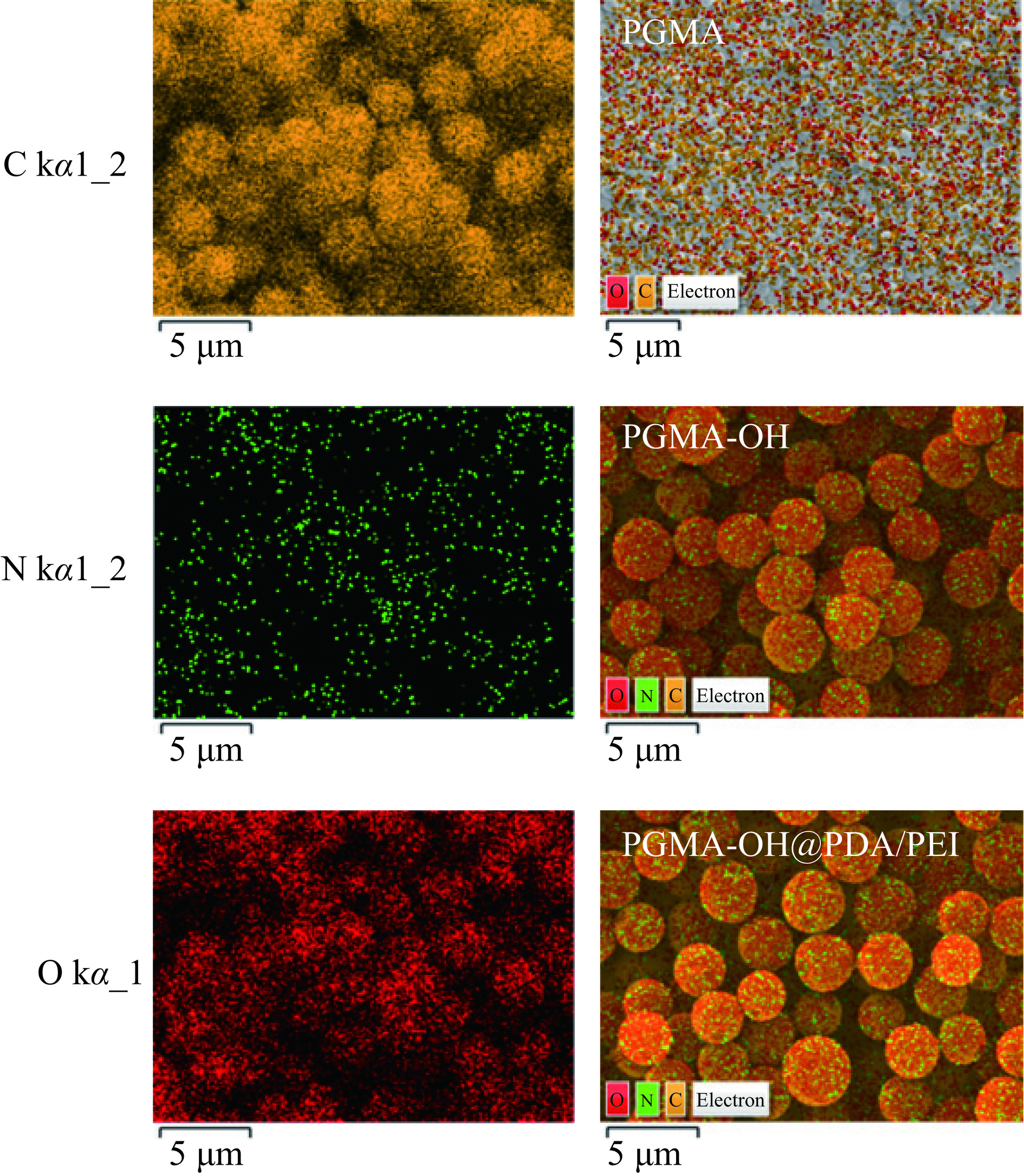
通过图1的扫描电镜图可以看出,与PGMA相比,PGMA-OH表面形貌没有发生明显的变化,是粒径大小为1 μm的光滑的微球。在微球表面进行聚多巴胺/聚乙烯亚胺涂覆之后,微球表面产生了细小的颗粒,使其表面变得粗糙。由能谱图(图2)可知,与PGMA相比,PGMA-OH的C元素含量由69.99%提高到77.50%,N元素含量为0.44%,O元素含量由30.01%下降到22.06%,这可能是因为,PGMA中环氧基在酸性条件下开环之后在其表面产生丰富的羟基[22],导致C、O、N元素含量发生变化。聚多巴胺/聚乙烯亚胺修饰PGMA-OH能在其表面引入更加丰富的氨基[23],因此与PGMA-OH相比,PGMA-OH@PDA/PEI的C元素含量由77.50%上升到84.67%,N元素含量由0.44%上升到0.83%,而O元素含量由22.06%下降到14.50%,证明了聚多巴胺/聚乙烯亚胺的成功涂覆。
2.1.2 载体的红外光谱图
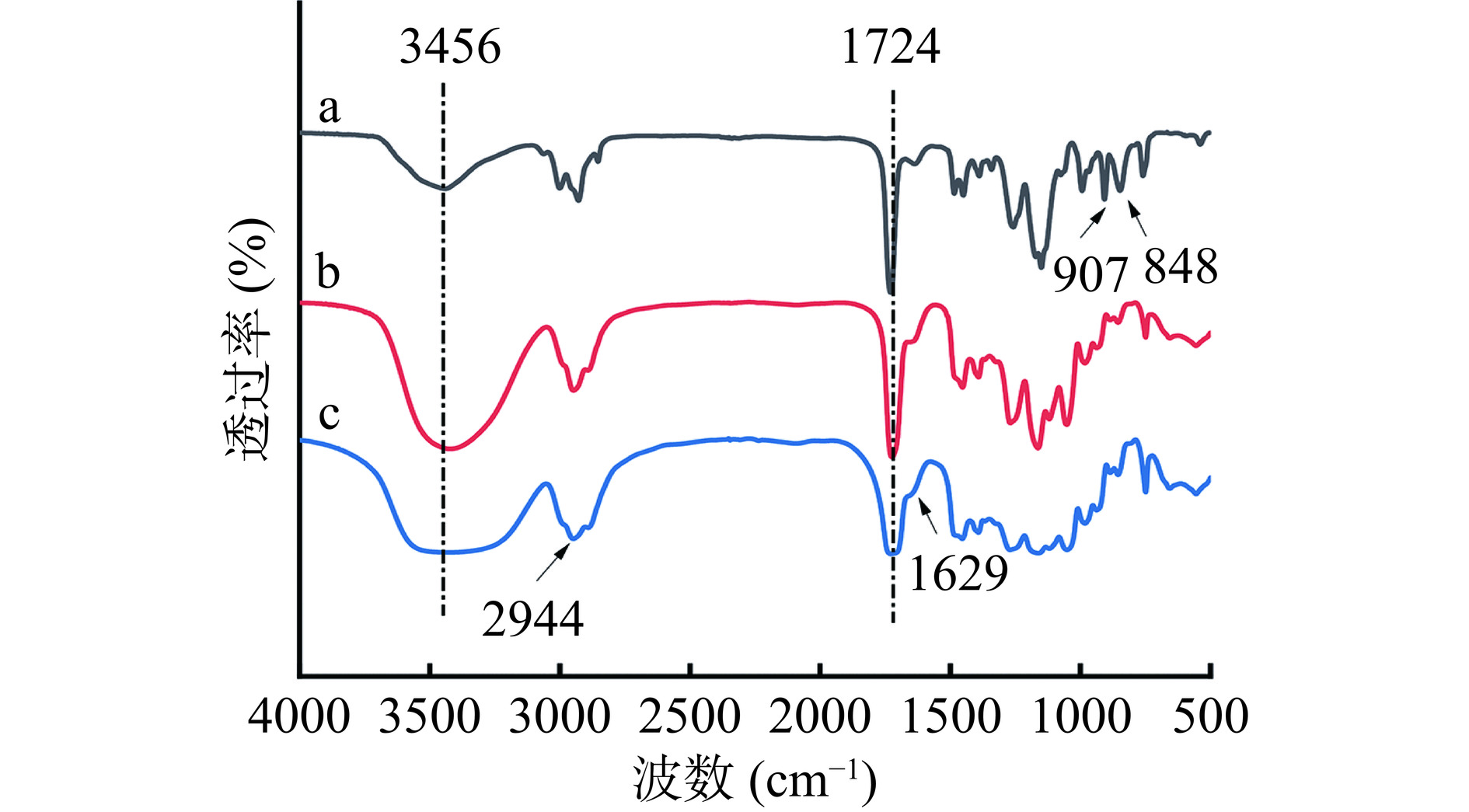
通过图3a的红外光谱图可以看出,1724 cm−1是PGMA中-C=O的特征吸收峰,848和907 cm−1是环氧基的特征吸收峰[24]。图3b中,3456 cm−1是-OH的弯曲振动峰,与图3a相比,该峰强度显著增强,说明反应形成了更多的-OH,且848和907 cm−1处环氧基特征峰的消失,表明环氧基成功开环,这些均证明载体的表面水解成功[24]。通过图3c可以看出,2944 cm−1为-CH2-键的特征峰,苯环骨架的特征吸收峰出现在1629 cm−1处[23],这些结果表明,聚多巴胺/聚乙烯亚胺成功地涂覆在PGMA-OH表面。
2.1.3 载体的 Zeta 电位分析
当 pH 为 7.0 时,与PGMA-OH相比,PGMA-OH@PDA/PEI电势值由−10.30±1.83 mV变为−13.67±0.55 mV,当pH为8.0时,与PGMA-OH相比,PGMA-OH@PDA/PEI电势值由−11.56±0.80 mV变为−16.97±1.72 mV。电势值的变化可能与贻贝仿生功能化修饰有关,聚乙烯亚胺带有正电荷,而多巴胺带有更多负电荷[10],共沉积时二者共同作用导致PGMA-OH表面带有较多负电荷。Zeta电位分析结果同样证明聚多巴胺/聚乙烯亚胺成功地涂覆在PGMA-OH表面。
2.2 固定化酶制备工艺优化
2.2.1 固定化温度
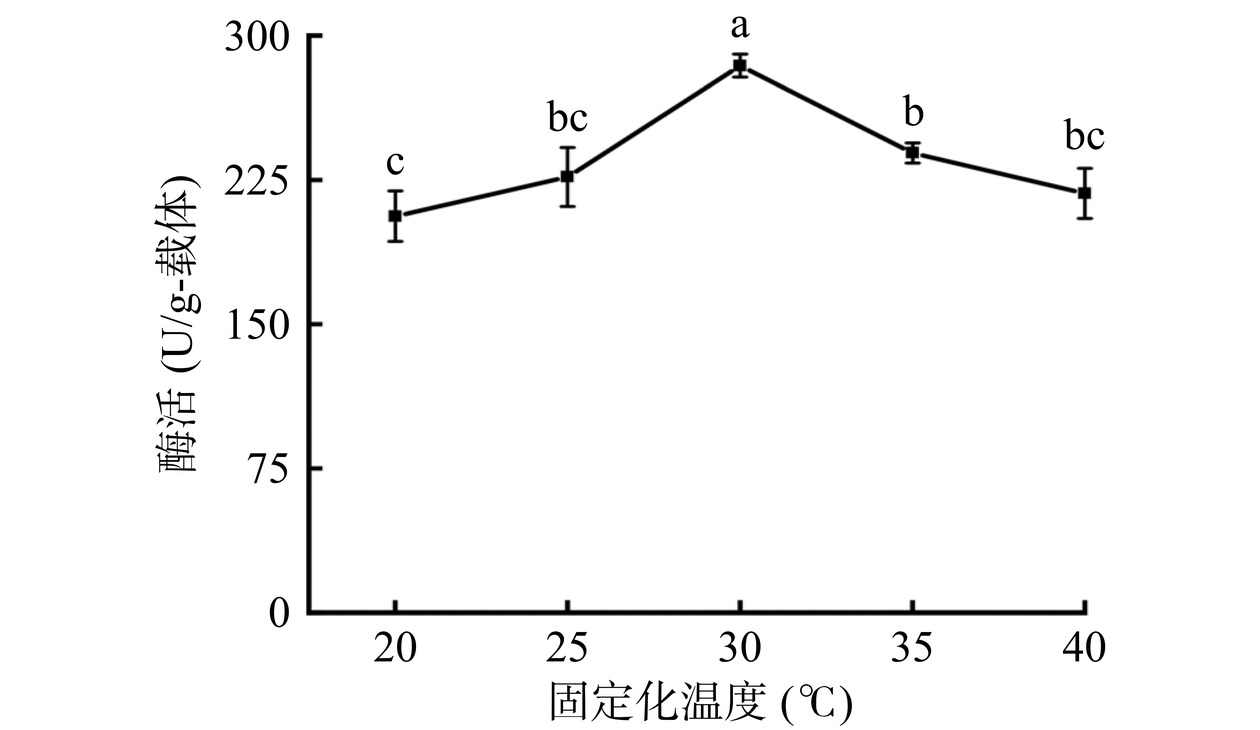
从图4可以看出,当固定化温度为20 ℃时,固定化酶的酶活较低,这可能是因为低温时席夫碱反应的反应速率较慢[25]。与25 ℃相比,当固定化温度为30 ℃时,固定化酶酶活显著升高(P<0.05),达到284.40±5.91 U/g-载体。继续升高温度至40 ℃,可能由于温度对脂肪酶的酶分子构象产生了影响[26],导致固定化酶酶活降低。因此,选择固定化温度为30 ℃进行后续实验。
2.2.2 固定化pH
从图5可以看出,固定化pH处于5.0至7.0范围内,固定化酶酶活随pH的增加而升高,在pH为7.0时,达到最高值,且显著高于pH为5.0和6.0的酶活数据(P<0.05)。进一步提高pH至9.0,固定化酶酶活明显降低,这可能是因为pH的变化导致部分酶失活,这与田家英等[27]的研究结果类似。因此,选择固定化pH7.0进行后续实验。
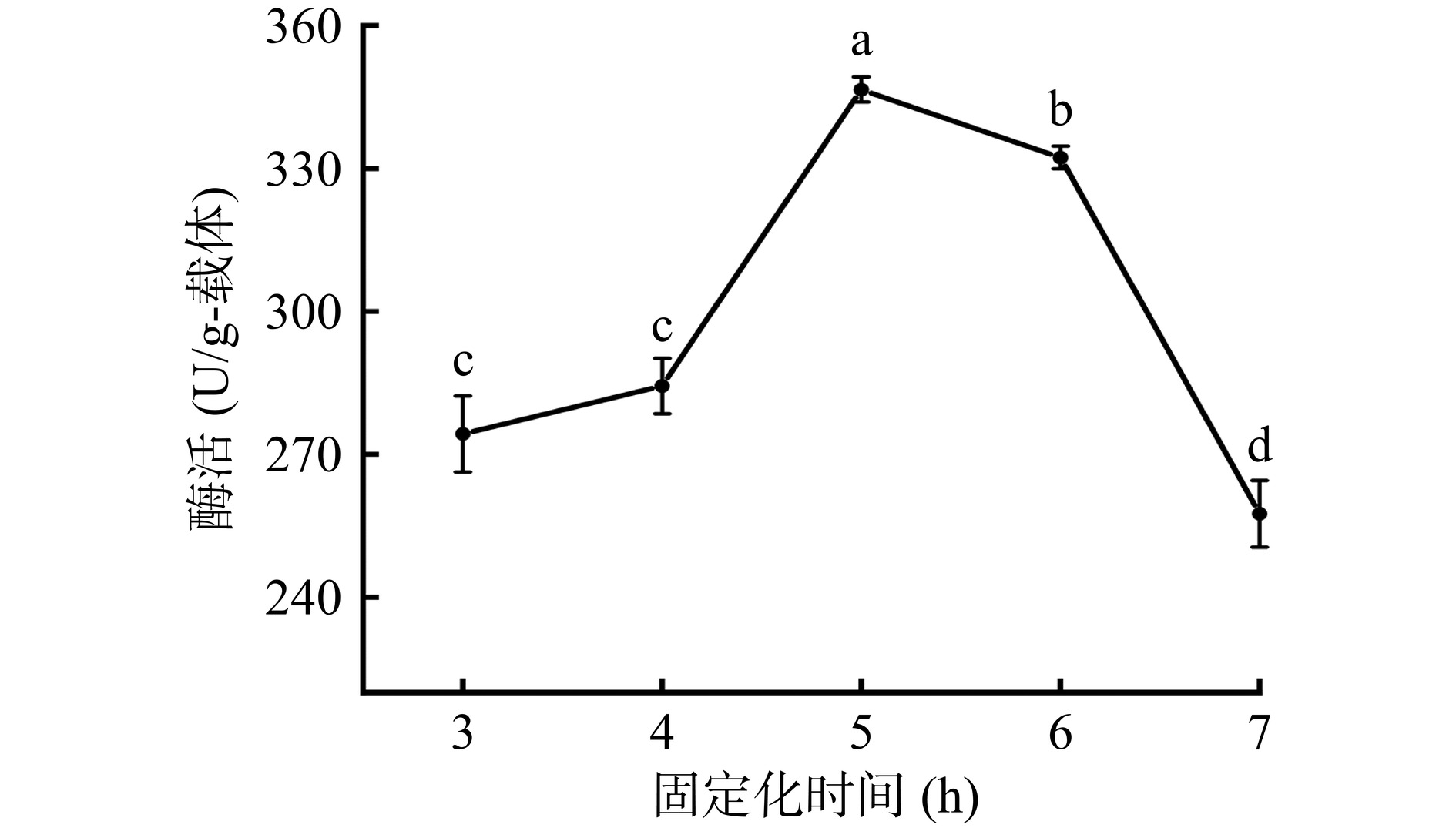
2.2.3 固定化时间
从图6可以看出,随着固定化时间的增加,固定化酶酶活呈现先上升后下降的变化规律。当固定化时间为5 h时,酶活达到346.55±2.62 U/g-载体,显著高于固定化时间为3 h或4 h的酶活数据(P<0.05)。当固定化时间为7 h时,固定化酶的酶活显著下降(P<0.05)。这种现象出现的原因可能是由于时间的增加,酶分子与PDA/PEI涂层发生共价结合的比例提高,因此酶活呈现出上升趋势;当酶与载体的连接位点达到饱和后,即使继续增加固定化时间,酶分子暴露出的活性位点也非常有限甚至减少,故而酶活表现出下降趋势[28]。因此,选择固定化时间为5 h进行后续实验。
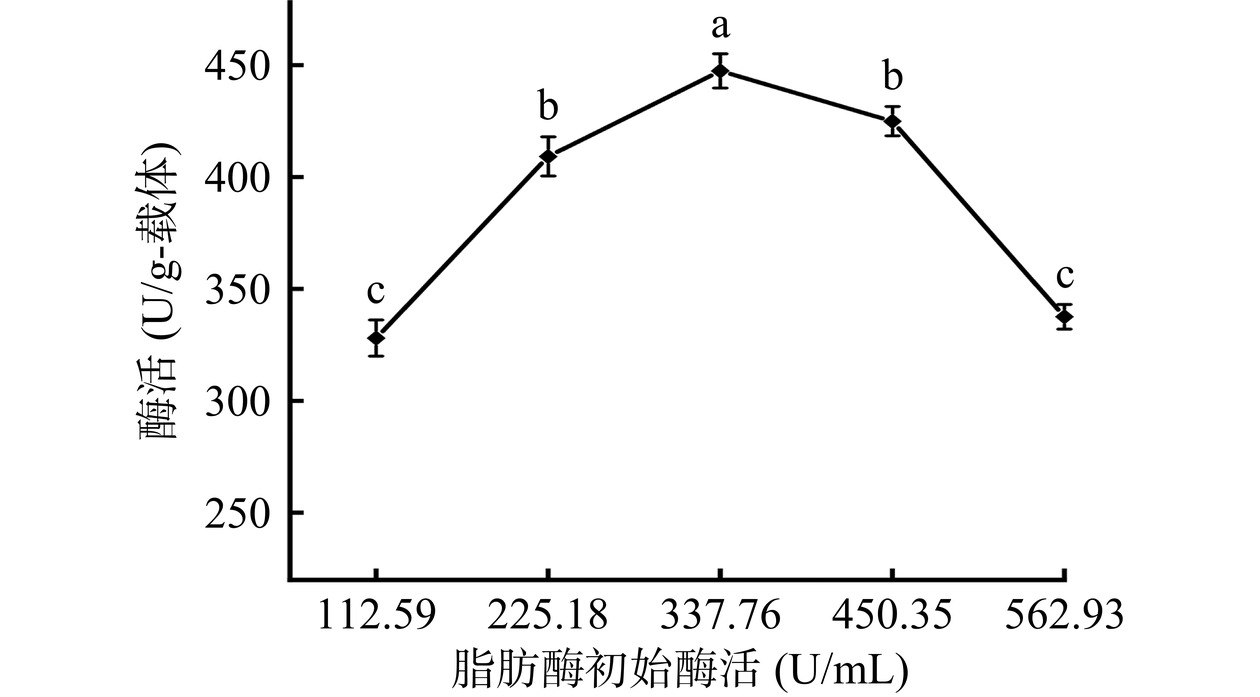
2.2.4 初始酶活
由图7可以看出,当初始酶活为337.76 U/mL时,固定化酶活达到444.05±0.87 U/g-载体。进一步增加初始酶活至562.93 U/mL,固定化酶酶活显著下降(P<0.05),这是因为初始酶活为337.76 U/mL时,载体的反应位点可能被酶分子完全占据,继续增加初始酶活,造成酶与载体的空间位阻增加,发生酶促反应时酶和底物接触不完全,因而导致酶活降低[23]。因此,选择初始酶活为337.76 U/mL进行后续实验。
2.2.5 载体添加量
如图8所示,当载体添加量从0.05 g增加至0.20 g时,由于酶与载体的连接位点增加[13],固定化酶酶活显著提高(P<0.05),达到444.19±2.26 U/g-载体。继续增加载体添加量至0.25 g,酶活显著降低(P<0.05)。这可能因为,当固定化体系中载体含量较高时,载体与酶分子间的共价作用减弱,从而导致酶活下降[28]。因此,选择载体添加量为0.20 g来进行后续实验。
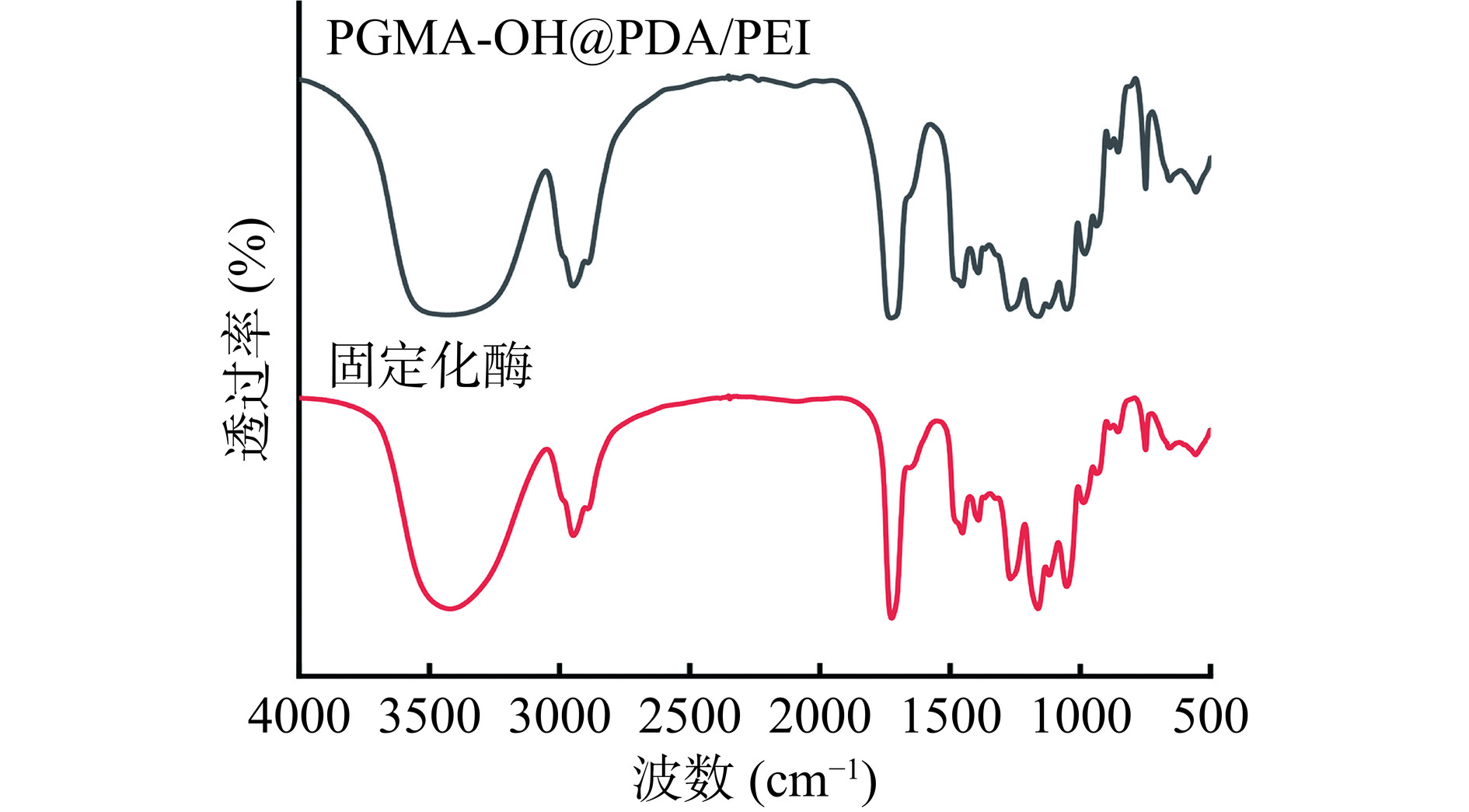
2.3 固定化酶的表征
通过图9可以看出,2944 cm−1处-CH2-键的特征峰减弱,并且固定化脂肪酶之后,1276、1167及1042 cm−1衍射峰强度增加,表明脂肪酶与PGMA-OH@PDA/PEI存在着相互作用力,结合图3,实验结果证明PGMA-OH@PDA/PEI成功固定化脂肪酶。
2.4 游离酶和固定化酶的酶学性质研究
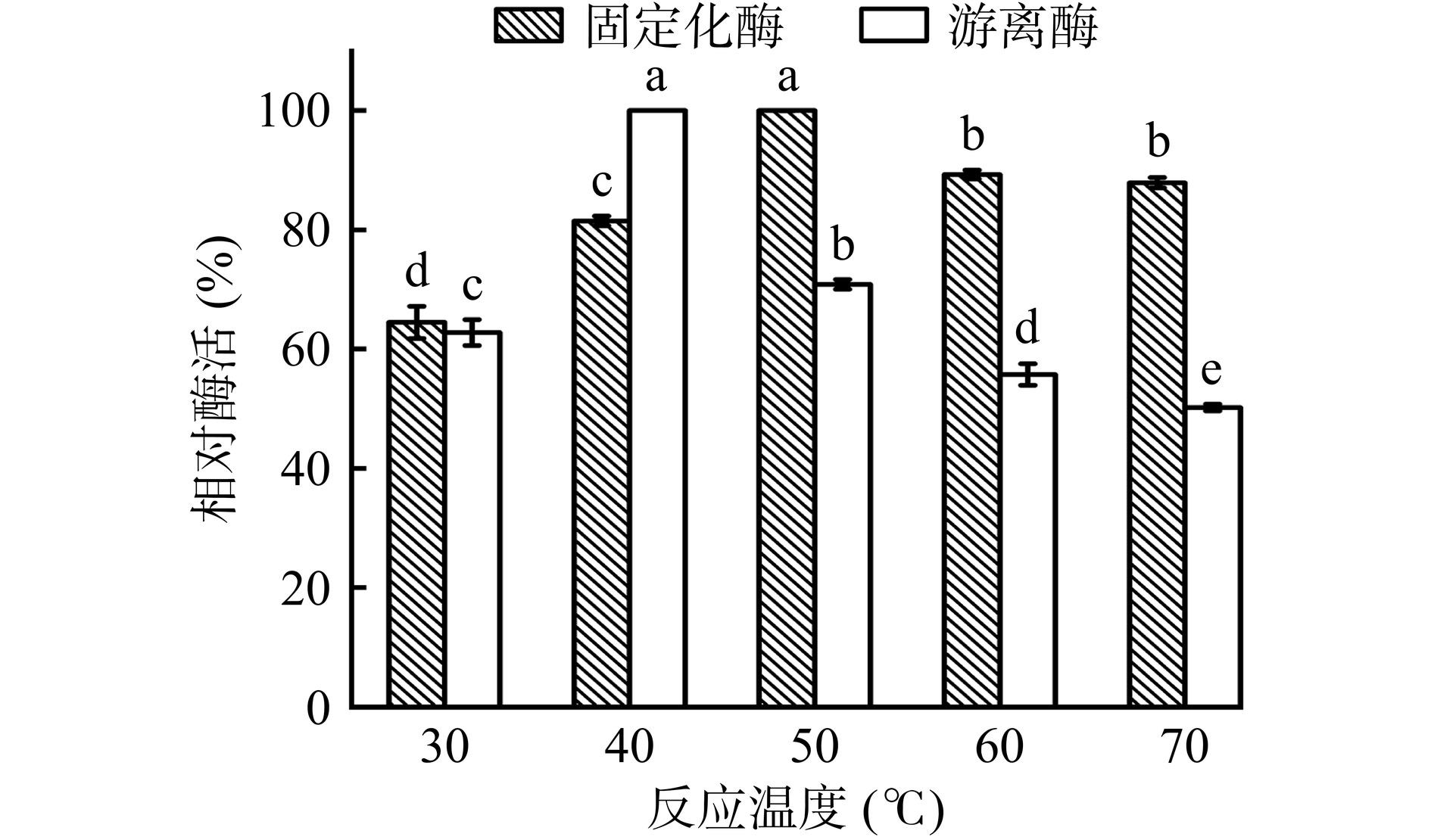
2.4.1 游离及固定化酶的最佳反应温度及温度稳定性
由图10可知,游离酶的最佳反应温度为40 ℃,固定化酶的最佳反应温度为50 ℃。对固定化酶而言,与50 ℃相比,60 ℃其酶活显著下降至89.28%(P<0.05),与60 ℃相比,70 ℃固定化酶酶活差异不显著(P>0.05)。对游离酶而言,与40 ℃相比,反应温度为50、60及70 ℃酶活显著下降(P<0.05)。这是因为,将酶固定在载体上时,PDA/PEI涂层能稳定酶的结构,因此固定化酶对高温表现出较高的抵抗力,实验结果与林海蛟等[29]的研究结果相似。同时,最佳反应温度的提高也有利于固定化酶的实际工业化生产[30]。
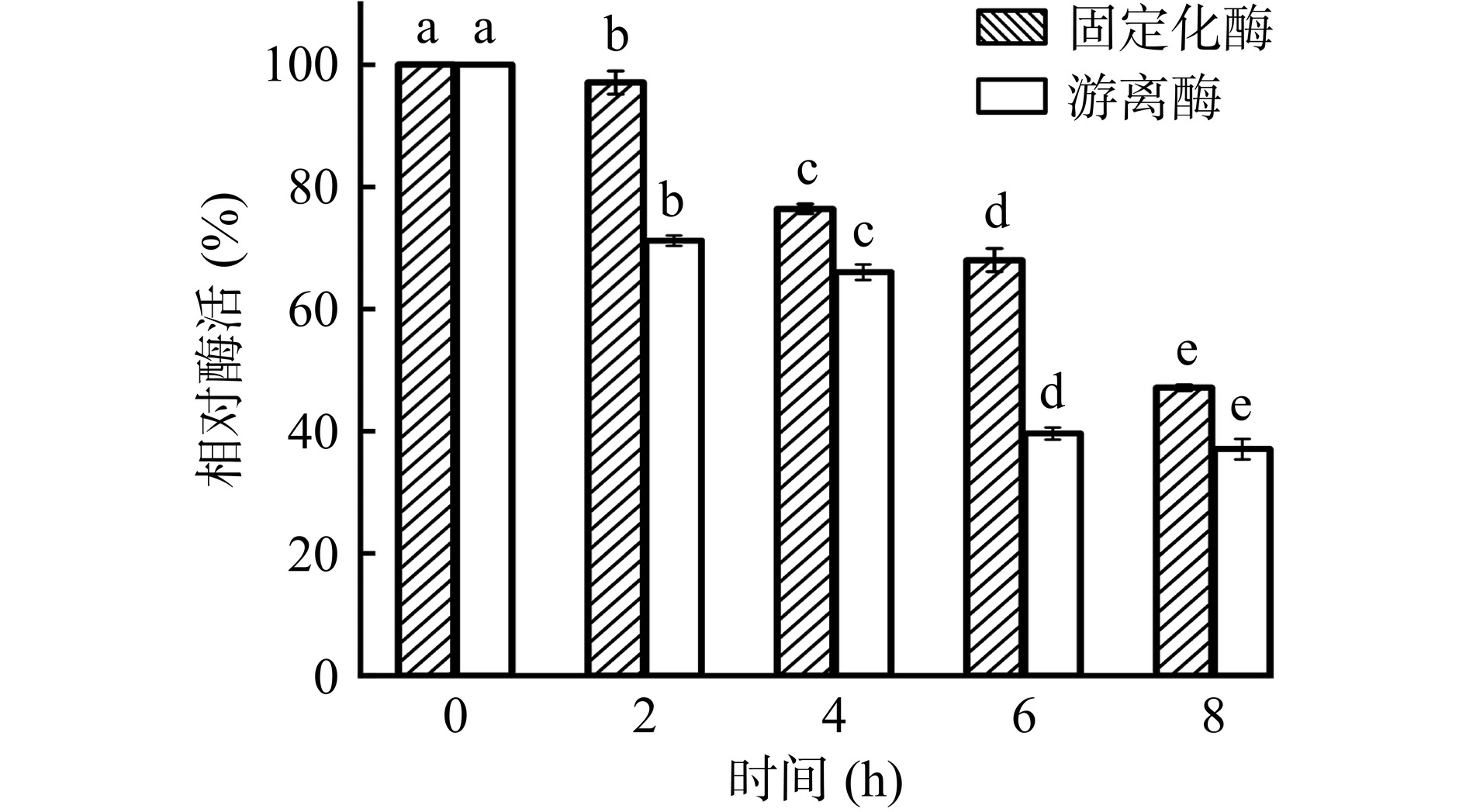
由图11可知,在50 ℃下,固定化酶在前6 h内能保留初始酶活的60%以上,而游离酶在40 ℃放置同样的时间,仅为初始酶活的40%。与0 h相比,在50 ℃放置8 h后,固定化酶的酶活显著下降(P<0.05),此时固定化酶有47.61%的相对酶活,而游离酶在40 ℃放置8 h后酶活显著下降(P<0.05)为初始酶活的36.58%。从结构上看,当酶的活性中心长时间暴露在较高温度下时,酶分子从有序结构到无序结构转变是引起酶失活的主要原因[31]。与游离酶相比,固定化酶中的酶分子被限制在载体表面,载体表面对酶分子空间结构的限制效应,可以使固定化酶保持良好的活性构象,避免因温度变化而导致失活[32]。
2.4.2 游离及固定化酶的最佳反应pH及pH稳定性
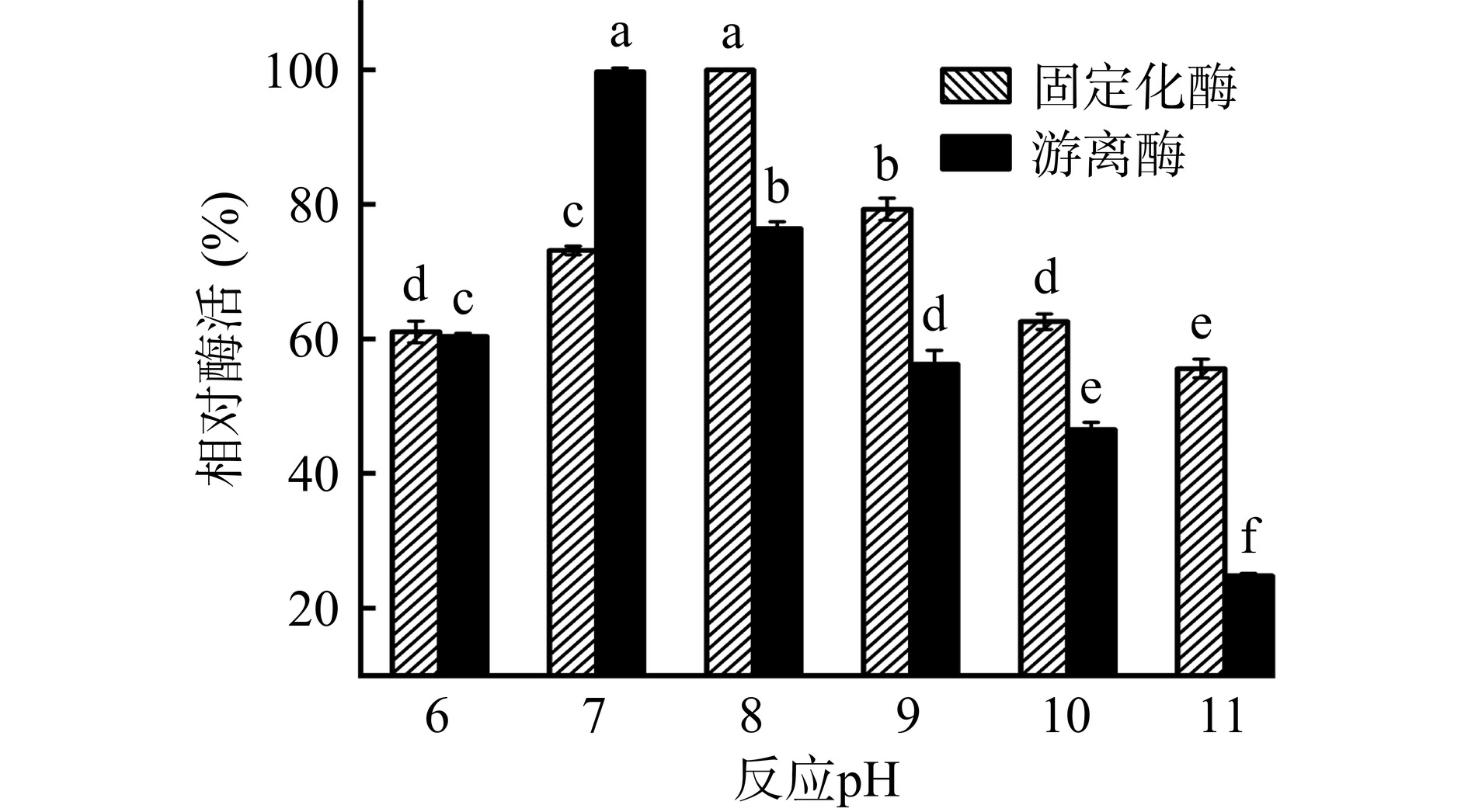
由图12可知,游离酶最佳反应pH为7.0,而固定化酶为8.0。对游离酶而言,与pH为7.0相比,pH增加至8.0、9.0、10.0及11.0时酶活显著下降(P<0.05),对固定化酶而言,与pH为8.0相比,pH增加至9.0、10.0及11.0时酶活显著下降(P<0.05)。通过Zeta电位分析,在固定化条件下(pH为7.0),载体表面存在很多负电荷,电势值为−13.67±0.55 mV,载体表面电荷分布不均会引起pH的变化[33]。由于载体表面带负电,会吸引反应体系中的阳离子,造成固定化酶周围H+浓度升高,呈酸性,为维持反应体系中微环境的动态平衡,整个反应体系要向碱性方向移动,因此固定化酶的最佳反应pH提高至8.0。同时,Suo等[32]使用磁性ZIF-90固定化脂肪酶,所得固定化酶最佳反应pH提高至7.5,而游离酶为7.0。除此之外,Zheng等[23]在固定化柚苷酶的过程中发现,强酸和强碱会破坏酶与底物结合位点的结构,使二者不能有效结合,造成酶活的下降。
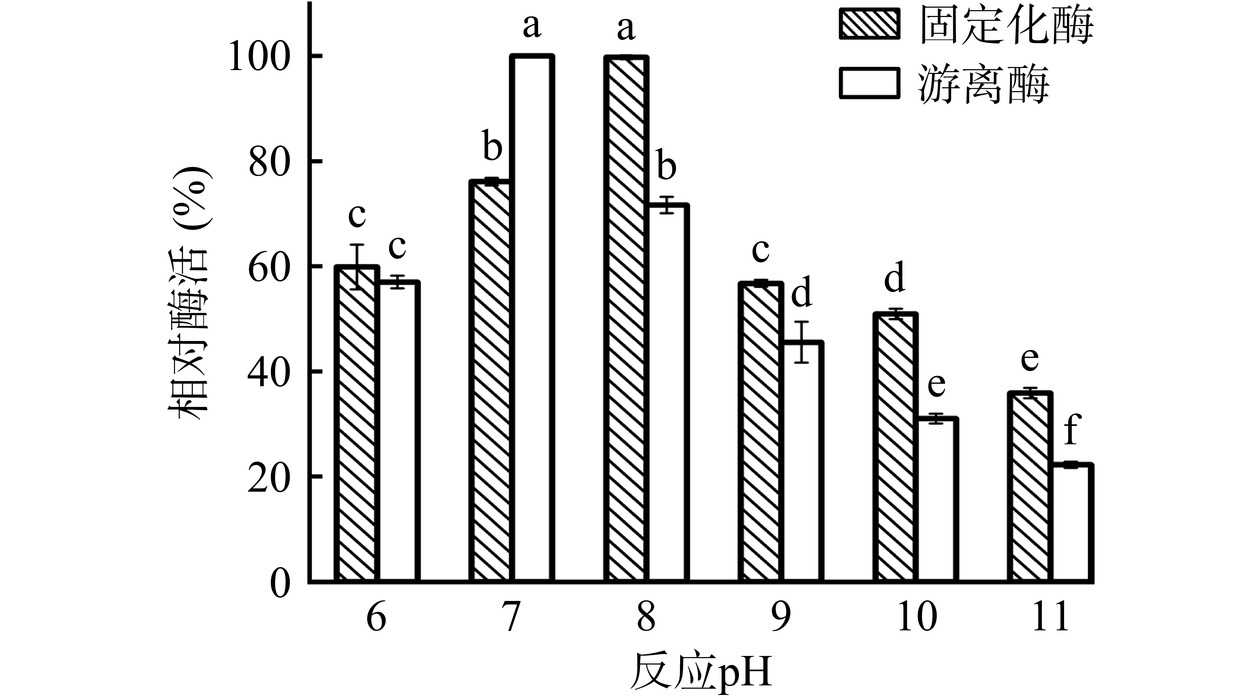
由图13 可知,与 pH 为 7.0 相比,在 pH 为 6.0的缓冲液中放置2 h后,游离酶与固定化酶的酶活显著下降(P<0.05),均保持在各自初始酶活的60%左右。而在 pH 为分别为 9.0、10.0 及 11.0 的缓冲液中放置相同的时间,游离酶与固定化酶酶活均呈现明显的下降趋势,但固定化酶的pH稳定性强于游离酶,这可能是由于载体为酶分子提供了相对稳定的微环境,可以更好地维持酶分子的空间构象而有效保持酶活[34]。
2.4.3 游离及固定化酶的最佳反应时间
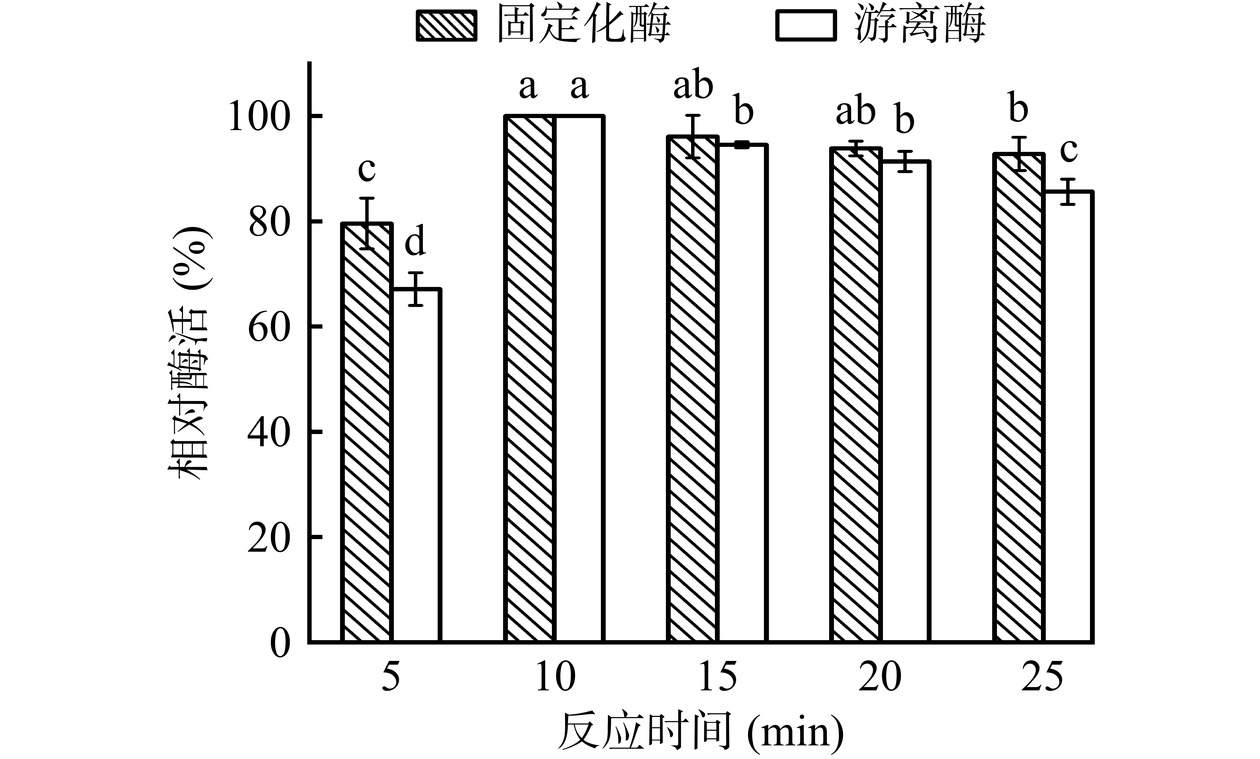
如图14所示,与反应时间5 min相比,当反应时间为10 min时,游离酶及固定化酶的酶活显著上升(P<0.05)。与反应时间为10 min相比,当反应时间增加至15 min,游离酶酶活显著下降(P<0.05)。进一步延长反应时间至25 min,游离酶及固定化酶的酶活均呈下降趋势。这可能是由于PDA/PEI涂层与酶的共价结合能够保护酶活性中心[35],因此固定化酶酶活的下降趋势较游离酶缓慢,最优条件下固定化酶酶活为484.42±5.97 U/g-载体。
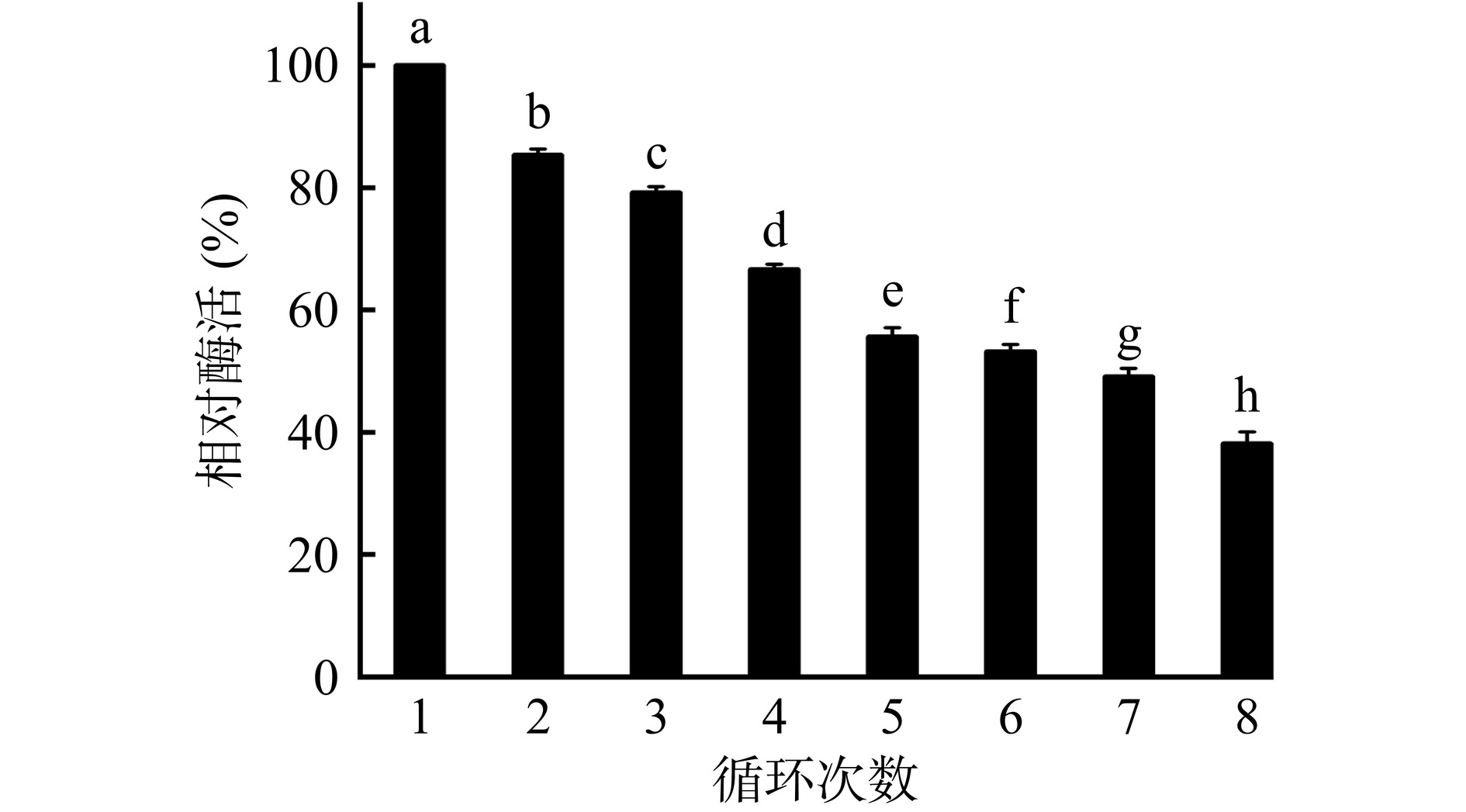
2.4.4 固定化酶的重复利用性
如图15所示,与第1次循环相比,经过5次循环使用后,固定化酶酶活显著下降(P<0.05),但仍保留有50%以上的初始酶活。Maharani等[36]以二氧化硅固定化脂肪酶,在5次循环后,固定化酶酶活仅保留最初酶活的30.28%。刘振山等[37]制备出的脂肪酶杂化纳米花在5次循环之后仅能保持40%左右的酶活力。这些实验结果表明,可能是由于多次搅拌、洗涤,使得酶分子从载体上脱落,造成固定化酶酶活的损失[38]。而本研究中的固定化酶在循环使用8次后,仍保留有初始酶活的39.22%,说明贻贝仿生修饰策略能有效提高固定化酶的重复利用性。
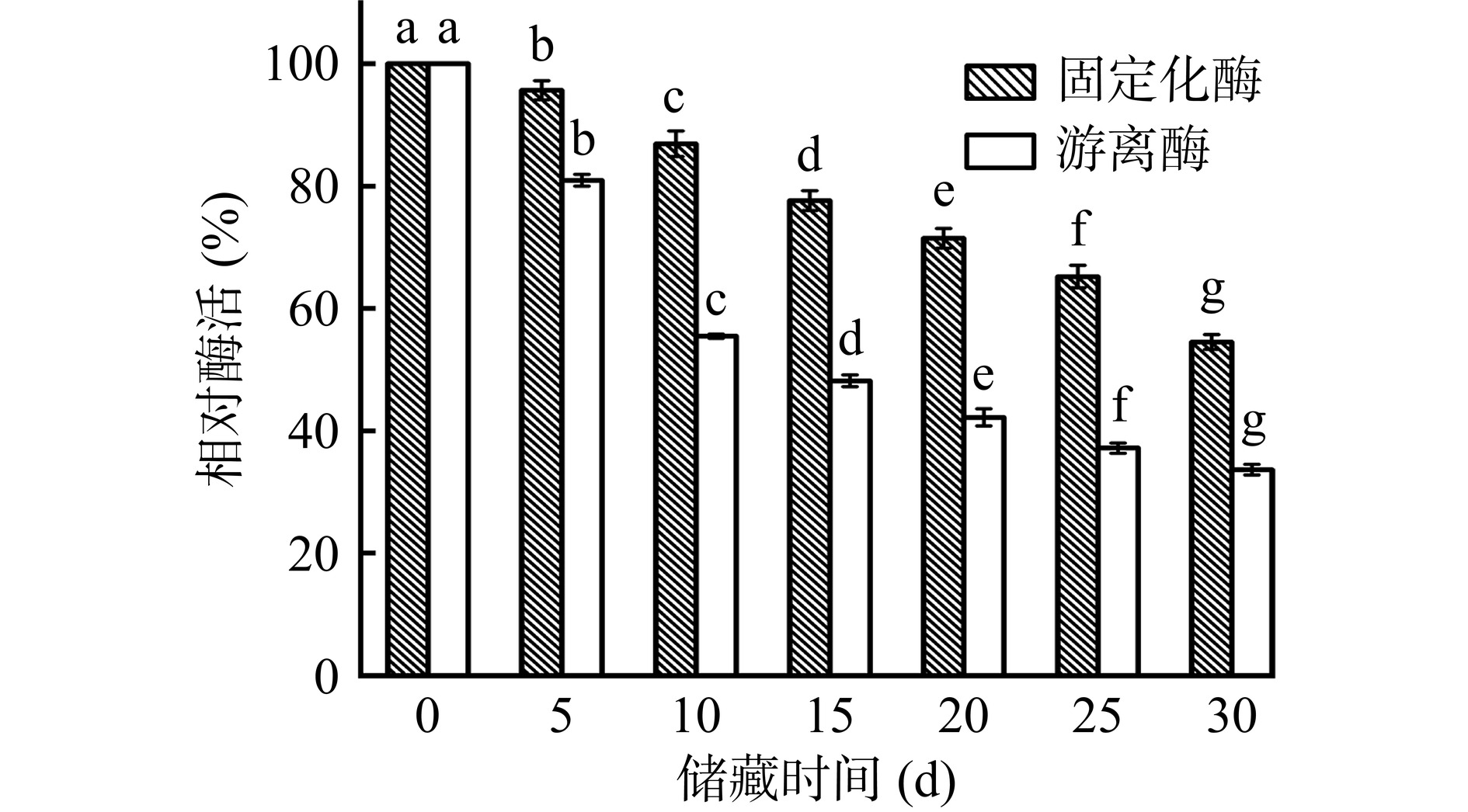
2.4.5 游离及固定化酶的储藏稳定性
如图16所示,与储藏0 d相比,固定化酶在4 ℃条件下储存30 d后,其酶活显著下降(P<0.05),但仍有初始酶活的54.52%,而储存后的游离酶酶活仅为初始酶活的33.63%。储藏过程中,与游离酶相比,固定化酶酶活的变化趋势较缓慢,这可能是因为载体表面贻贝仿生涂层对脂肪酶存在束缚作用,在长期储存过程中保持了其活性构象,可以有效减少储藏过程中酶分子的失活[38]。
2.5 固定化酶在催化乙酰丙酸与十二醇酯化反应中的应用
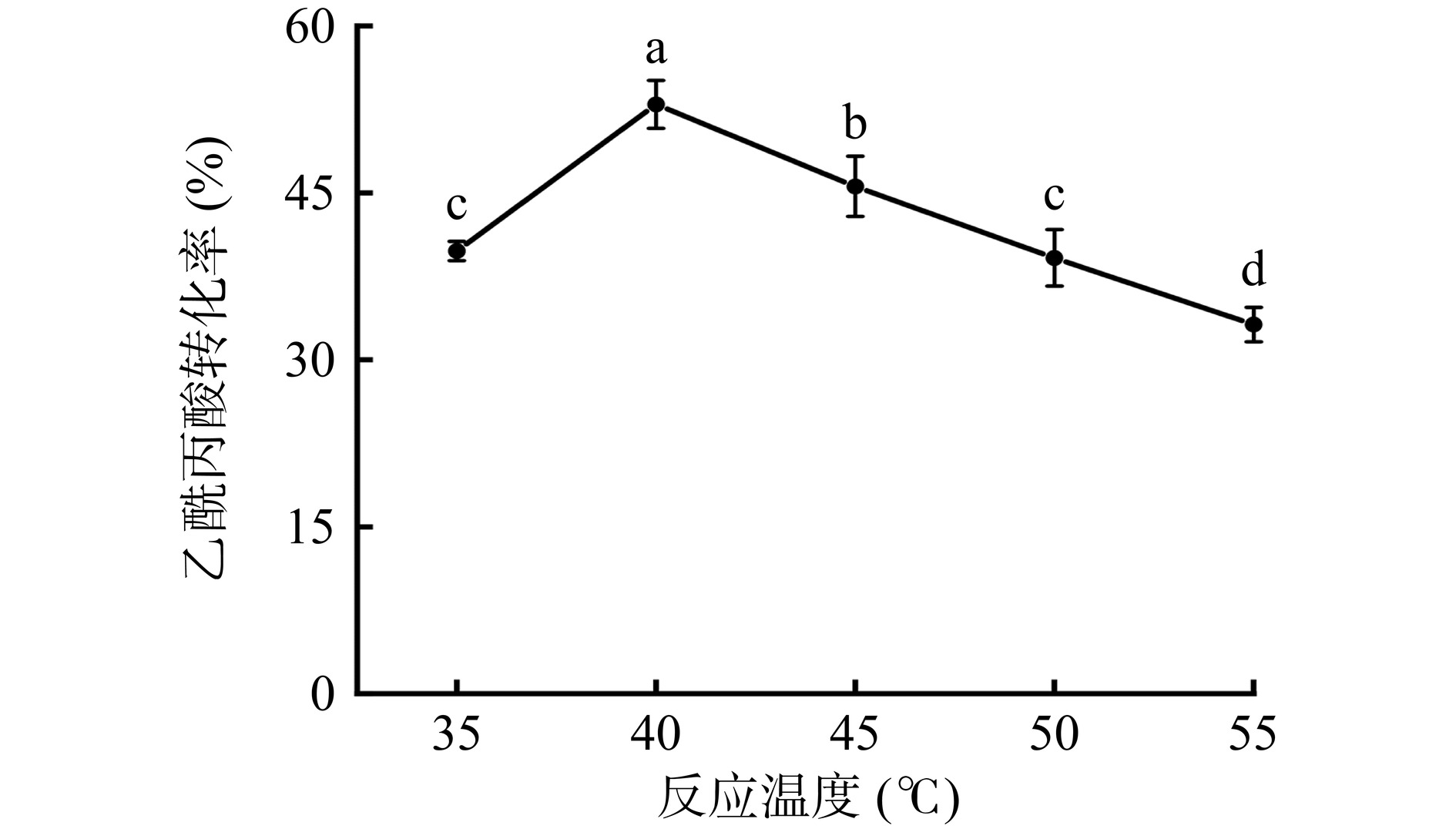
2.5.1 反应温度对乙酰丙酸转化率的影响
从图17可以看出,乙酰丙酸的转化率随温度的升高呈现先上升后降低的趋势。与35 ℃相比,当反应温度升高到40 ℃时,乙酰丙酸转化率显著上升至52.96%(P<0.05),这可能是因为温度的升高促进了产物合成[39]。而当反应温度继续增加到45、50、55 ℃,乙酰丙酸转化率下降,与40 ℃相比具有显著性差异(P<0.05)。温度的升高可能造成固定化酶出现了一定程度的变性甚至失活,最终导致乙酰丙酸转化率降低。
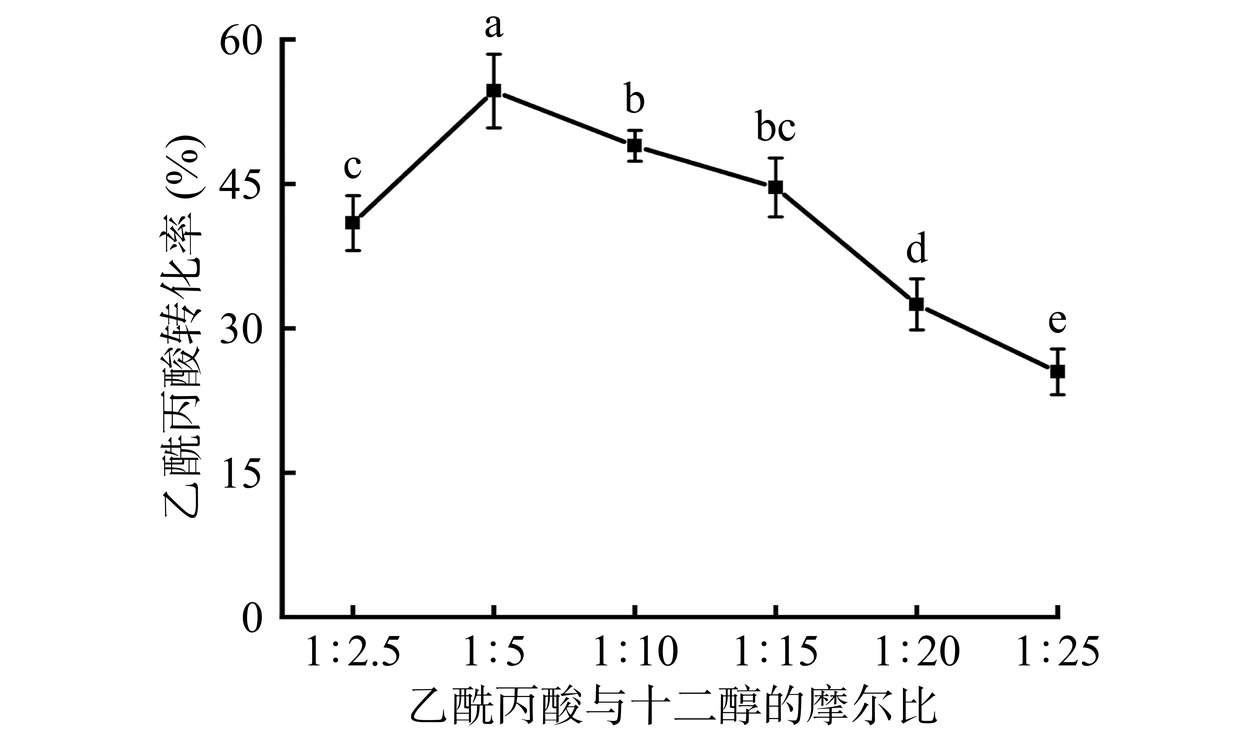
2.5.2 乙酰丙酸与十二醇的摩尔比对乙酰丙酸转化率的影响
从图18可以看出,与摩尔比为1:2.5相比,摩尔为1:5时,乙酰丙酸的转化率由40.93%显著提高至54.64%(P<0.05),这主要是因为酯化反应朝着正反应的方向进行。而继续增加十二醇的含量,反应体系中的固定化酶分子构象发生了变化[20],导致乙酰丙酸转化率降低。
2.5.3 反应时间对乙酰丙酸转化率的影响
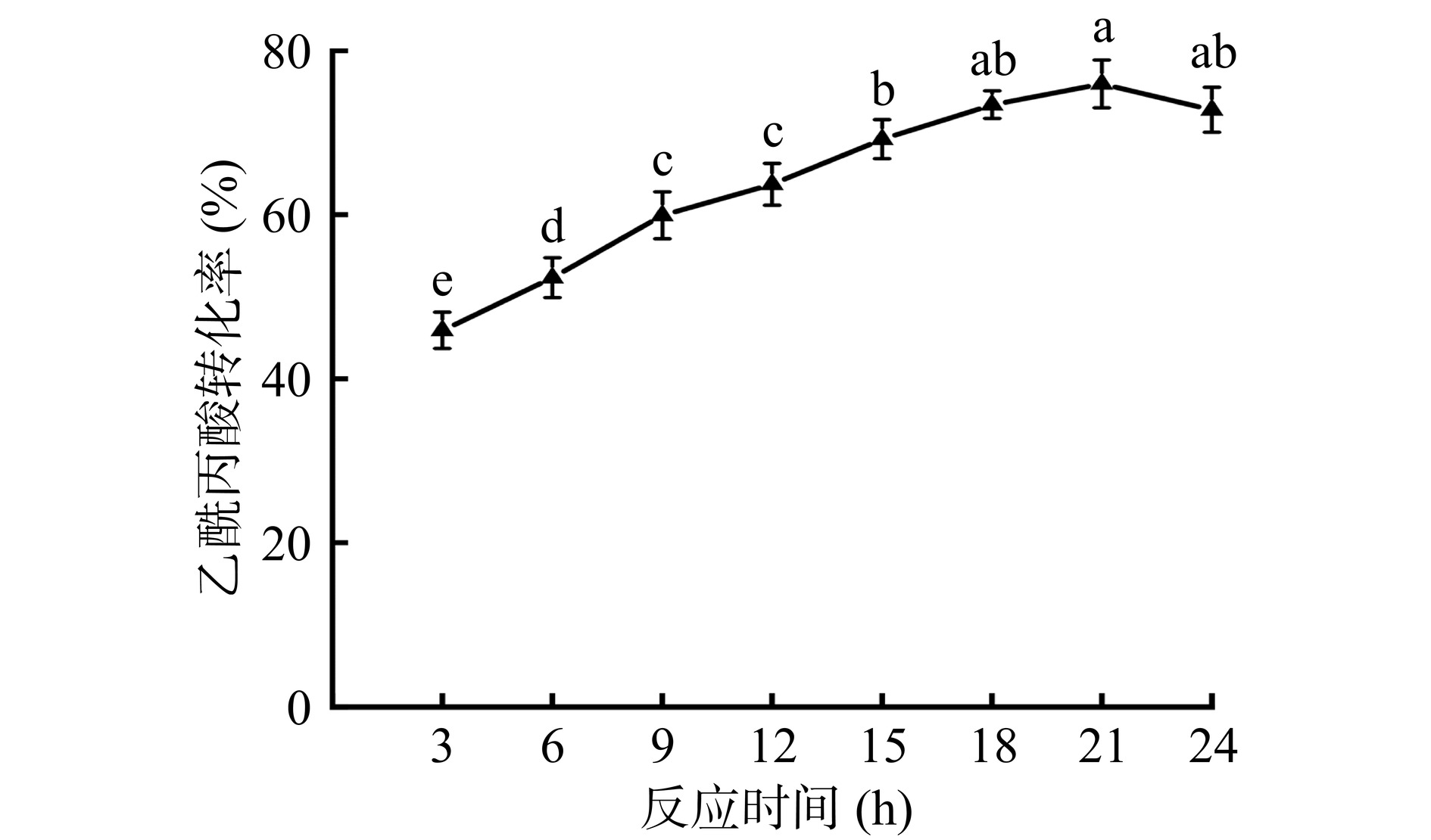
由图19可知,随着反应时间延长到21 h,乙酰丙酸的转化率逐渐增加,此时最大的乙酰丙酸转化率为75.94%。继续延长反应时间至24 h,与21 h的实验数据相比,此时乙酰丙酸转化率差异不显著(P>0.05)。因此,本实验所得固定化酶催化条件为反应温度40 ℃、乙酰丙酸/十二醇的摩尔比1:5、反应时间21 h,与王立晖等[20]的研究结果(构建核壳结构磁性树枝状纤维形有机硅材料,用于南极假丝酵母脂肪酶B的固定化研究,所得固定化酶在50 ℃、乙酰丙酸/十二醇的摩尔比1:10、反应时间22 h条件下,催化乙酰丙酸的转化率为85.05%)相比,本研究制备得到固定化酶在较低的催化温度和较少的十二醇用量条件下,用较短的反应时间,达到了较高的转化率,不仅反应条件温和,且在一定程度上减低了反应成本,具有良好的应用前景。
3. 结论
本研究通过探索载体与酶分子之间的构效关系,实现了固定化酶的高效制备并应用于催化酯化反应。使用扫描电镜观察聚多巴胺/聚乙烯亚胺共沉积前后PGMA-OH表面的变化,并用能谱检测聚多巴胺/聚乙烯亚胺共沉积前后PGMA-OH微球C、N、O元素含量的变化,采用红外光谱分析共沉积前后及固定化前后PGMA-OH表面官能团的变化,以证明PGMA-OH@PDA/PEI的成功制备并成功固定化脂肪酶,Zeta电位结果表明PGMA-OH@PDA/PEI在pH为7.0时电势值为−13.67±0.55 mV。最佳酶固定化条件为:固定化温度为30 ℃,固定化pH为7.0,固定化时间为5 h,初始酶活为337.76 U/mL,载体添加量为0.2 g。使用该固定化策略,固定化酶的最佳反应温度为50 ℃,最佳反应pH为8.0,最佳反应时间为10 min,最优条件下固定化酶酶活为484.42±5.97 U/g-载体。与游离酶相比,固定化酶的稳定性有了明显提高,在重复使用8次之后,固定化酶仍有39.22%的初始酶活。进一步将固定化酶用于催化乙酰丙酸与十二醇的酯化反应,转化率可达75.94%,通过固定化载体的设计构建,为脂肪酶的工业化应用提供了新的技术路线和数据支持。
-
-
[1] CAVALCANTE F, NETO F S, FALCO I, et al. Opportunities for improving biodiesel production via lipase catalysis[J]. Fuel,2020,288:119577.
[2] 路雪纯, 辛嘉英, 张帅, 等. 脂肪酶固定化及其在食品领域中应用的研究进展[J]. 食品工业科技,2021,42(17):423−431. [LU X C, XIN J Y, ZHANG S, et al. Research progress in immobilization of lipase and its application in food industry[J]. Science and Technology of Food Industry,2021,42(17):423−431.] doi: 10.13386/j.issn1002-0306.2020080096 LU X C, XIN J Y, ZHANG S, et al. Research progress in immobilization of lipase and its application in food industry[J]. Science and Technology of Food Industry, 2021, 42(17): 423−431. doi: 10.13386/j.issn1002-0306.2020080096
[3] PAITAID P, H KITTIKUN A. Enhancing immobilization of Aspergillus oryzae ST11 lipase on polyacrylonitrile nanofibrous membrane by bovine serum albumin and its application for biodiesel production[J]. Preparative Biochemistry & Biotechnology,2020,51(6):1−14.
[4] GUO H, LEI B S, YU J W, et al. Immobilization of lipase by dialdehyde cellulose crosslinked magnetic nanoparticles[J]. International Journal of Biological Macromolecules,2021,185:287−296. doi: 10.1016/j.ijbiomac.2021.06.073
[5] LAN D M, ZHAO G, HOLZMANN N, et al. Structure-guided rational design of a mono- and diacylglycerol lipase from Aspergillus oryzae:A single residue mutant increases the hydrolysis ability[J]. Journal of Agricultural and Food Chemistry,2021,69(18):5344−5352. doi: 10.1021/acs.jafc.1c00913
[6] WANG R, WANG S, XU Y, et al. Engineering of a thermo-alkali-stable lipase from Rhizopus chinensis by rational design of a buried disulfide bond and combinatorial mutagenesis[J]. Journal of Industrial Microbiology & Biotechnology,2020,47(12):1019−1030.
[7] CHEN G, KHAN M I, HE W S, et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods:Current status and future prospects[J]. Comprehensive Reviews in Food Science and Food Safety,2022,21(3):2688−2714. doi: 10.1111/1541-4337.12965
[8] REMONATTO D, MIOTTI R H, MONTI R, et al. Applications of immobilized lipases in enzymatic reactors:A review[J]. Process Biochemistry,2022,114(5):1−20.
[9] 王彩红, 孙婧, 季书馨, 等. 聚乙烯亚胺/多巴胺改性氧化硅固定碳酸酐酶[J]. 化工学报,2019,70(5):1887−1893. [WANG C H, SUN J, JI S X, et al. Immobilization of carbonic anhydrase on polyethylenimine/dopamine co-deposited SiO2[J]. CIESC Journal,2019,70(5):1887−1893.] doi: 10.11949/j.issn.0438-1157.20181423 WANG C H, SUN J, JI S X, et al. Immobilization of carbonic anhydrase on polyethylenimine/dopamine co-deposited SiO2[J]. CIESC Journal, 2019, 70(5): 1887−1893. doi: 10.11949/j.issn.0438-1157.20181423
[10] 李梦坤. 两性荷电复合纳滤膜的制备及性能研究[D]. 南京:南京理工大学, 2018. [LI M K. Research on fabrication and performance of the amphoteric charged composite nanofiltration membranes[D]. Nanjing:Nanjing University of Science & Technology, 2018.] LI M K. Research on fabrication and performance of the amphoteric charged composite nanofiltration membranes[D]. Nanjing: Nanjing University of Science & Technology, 2018.
[11] LIU T L, RAO Y, ZHOU W F, et al. Improved adenylate cyclase activity via affinity immobilization onto co-modified GO with bio-inspired adhesive and PEI[J]. Colloids and Surfaces B: Biointerfaces,2021,205:111888. doi: 10.1016/j.colsurfb.2021.111888
[12] ZHAI T T, WANG C H, GU F J, et al. Dopamine/polyethylenimine-modified silica for enzyme immobilization and strengthening of enzymatic CO2 conversion[J]. ACS Sustainable Chemistry & Engineering,2020,8(40):15250−15257.
[13] DENG X, HE T, LI J, et al. Enhanced biochemical characteristics of β-glucosidase via adsorption and cross-linked enzyme aggregate for rapid cellobiose hydrolysis[J]. Bioprocess and Biosystems Engineering,2020,43:2209−2217. doi: 10.1007/s00449-020-02406-5
[14] 吴非, 吴泽楠, 孙秋月, 等. 纳米磁酶水酶法在磁流化床中提取大豆油脂的数值模拟及应用[J]. 农业工程学报,2022,38(6):302−311. [WU F, WU Z N, SUN Q Y, et al. Numerical simulation and application of nano-magnetic enzyme hydroenzymatic method for soybean oil and grease extraction in a magneto-fluidized bed[J]. Transactions of the Chinese Society of Agricultural Engineering,2022,38(6):302−311.] doi: 10.11975/j.issn.1002-6819.2022.06.034 WU F, WU Z N, SUN Q Y, et al. Numerical simulation and application of nano-magnetic enzyme hydroenzymatic method for soybean oil and grease extraction in a magneto-fluidized bed[J]. Transactions of the Chinese Society of Agricultural Engineering, 2022, 38(6): 302−311. doi: 10.11975/j.issn.1002-6819.2022.06.034
[15] 廖灵通. 葡萄糖氧化酶/过氧化氢酶共固定化材料技术的研究[D]. 济南:齐鲁工业大学, 2020. [LIAO L T. Studies of the immobilization technology for co-immobilized glucose oxidase and catalase[D]. Ji'nan:Qilu University of Technology, 2020.] LIAO L T. Studies of the immobilization technology for co-immobilized glucose oxidase and catalase[D]. Ji'nan: Qilu University of Technology, 2020.
[16] 石莹. 长链脂肪酸转运蛋白对热带假丝酵母产二元酸的影响研究[D]. 济南:齐鲁工业大学, 2017. [SHI Y. The effect of Long chain fatty acid transport protein on the production of dicarboxylic acid in Candida tropicalis[D]. Ji'nan:Qilu University of Technology, 2017.] SHI Y. The effect of Long chain fatty acid transport protein on the production of dicarboxylic acid in Candida tropicalis[D]. Ji'nan: Qilu University of Technology, 2017.
[17] 徐国财. 纳米科技导论[M]. 北京:高等教育出版社, 2005. [XU G C. Introduction to nanotechnology[M]. Beijing:Higher Education Press, 2005.] XU G C. Introduction to nanotechnology[M]. Beijing: Higher Education Press, 2005.
[18] 王琳, 刘容旭, 刘丹怡, 等. 壳聚糖涂覆磁性纳米颗粒对纤维素酶的固定化[J]. 食品工业科技,2022,43(16):74−80. [WANG L, LIU R X, LIU D Y, et al. Immobilization of cellulase by chitosan-coated magnetic nanoparticles[J]. Science and Technology of Food Industry,2022,43(16):74−80.] doi: 10.13386/j.issn1002-0306.2021110183 WANG L, LIU R X, LIU D Y, et al. Immobilization of cellulase by chitosan-coated magnetic nanoparticles[J]. Science and Technology of Food Industry, 2022, 43(16): 74−80. doi: 10.13386/j.issn1002-0306.2021110183
[19] 翟静雅. 阳离子染料与海泡石复合颜料的制备及作用机理的研究[D]. 北京:中国地质大学, 2021. [ZHAI J Y. Preparation of cationic dyes/sepiolite composite pigments and study of the mechanism[D]. Beijing:China University of Geosciences, 2021.] ZHAI J Y. Preparation of cationic dyes/sepiolite composite pigments and study of the mechanism[D]. Beijing: China University of Geosciences, 2021.
[20] 王立晖, 刘焕, 李赫宇, 等. 核壳结构磁性树枝状纤维形有机硅固定化脂肪酶制备及其应用[J]. 化工学报,2021,72(9):4861−4871. [WANG L H, LIU H, LI H Y, et al. Preparation and application of core-shell hydrophobic magnetic dendritic fibrous organosilica immobilized lipase[J]. CIESC Journal,2021,72(9):4861−4871.] doi: 10.11949/0438-1157.20210283 WANG L H, LIU H, LI H Y, et al. Preparation and application of core-shell hydrophobic magnetic dendritic fibrous organosilica immobilized lipase[J]. CIESC Journal, 2021, 72(9): 4861−4871. doi: 10.11949/0438-1157.20210283
[21] 罗旭聪. 基于共价有机框架材料构建生物催化剂及性能研究[D]. 天津:河北工业大学, 2020. [LUO X C. Construction of biocatalyst based on covalent organic frame materials and the catalytic performance[D]. Tianjin:Hebei University of Technology, 2020.] LUO X C. Construction of biocatalyst based on covalent organic frame materials and the catalytic performance[D]. Tianjin: Hebei University of Technology, 2020.
[22] 孙西同. 磁性高分子复合材料的制备及对Cr(Ⅵ)的吸附性能研究[D]. 北京:中国科学院大学, 2015. [SUN X T. Preparation of magnetic polymer composite and its adsorption properties for Cr(VI)[D]. Beijing:University of Chinese Academy of Sciences, 2015.] SUN X T. Preparation of magnetic polymer composite and its adsorption properties for Cr(VI)[D]. Beijing: University of Chinese Academy of Sciences, 2015.
[23] ZHENG X Y, LI Q, TIAN J, et al. Novel strategy of mussel-inspired immobilization of naringinase with high activity using a polyethylenimine/dopamine co-deposition method[J]. ACS Omega,2021,6(4):3267−3277. doi: 10.1021/acsomega.0c05756
[24] 朱俐燕. 多肽配基定向化固定抗体的研究[D]. 天津:天津大学, 2013. [ZHU L Y. Research on the oriented immobilization using peptide ligand[D]. Tianjin:Tianjing University, 2013.] ZHU L Y. Research on the oriented immobilization using peptide ligand[D]. Tianjin: Tianjing University, 2013.
[25] WANG Z, ZHANG W J, WEN S, et al. Rapid co-deposition of dopamine and polyethyleneimine triggered by CuSO4/H2O2 oxidation to fabricate nanofiltration membranes with high selectivity and antifouling ability[J]. Separation and Purification Technology,2023,305:122409. doi: 10.1016/j.seppur.2022.122409
[26] 徐珊, 李任强, 张继福, 等. 使用国产环氧树脂LXEP-120固定化脂肪酶研究[J]. 广西师范大学学报(自然科学版),2018,36(4):108−118. [XU S, LI R Q, ZHANG J F, et al. Immobilization of lipase using domestic epoxy resin LXEP-120[J]. Journal of Guangxi Normal University (Natural Science Edition),2018,36(4):108−118.] XU S, LI R Q, ZHANG J F, et al. Immobilization of lipase using domestic epoxy resin LXEP-120[J]. Journal of Guangxi Normal University (Natural Science Edition), 2018, 36(4): 108−118.
[27] 田家英, 张江华, 崔励, 等. 固定化脂肪酶制备条件优化及催化应用[J]. 大连工业大学学报,2022,41(6):408−413. [TIAN J Y, ZHANG J H, CUI L, et al. Optimization of conditions for preparing immobilized lipase and its catalytic performance[J]. Journal of Dalian Polytechnic University,2022,41(6):408−413.] doi: 10.19670/j.cnki.dlgydxxb.2022.6015 TIAN J Y, ZHANG J H, CUI L, et al. Optimization of conditions for preparing immobilized lipase and its catalytic performance[J]. Journal of Dalian Polytechnic University, 2022, 41(6): 408−413. doi: 10.19670/j.cnki.dlgydxxb.2022.6015
[28] YANG S J, ZOU L Y, LIU C, et al. Codeposition of levodopa and polyethyleneimine:reaction mechanism and coating construction[J]. ACS Applied Materials & Interfaces,2020,12(48):54094−54103.
[29] 林海蛟, 张继福, 张云, 等. 基于大孔吸附树脂先交联后吸附法固定化脂肪酶[J]. 广西师范大学学报(自然科学版),2020,38(4):101−108. [LIN H J, ZHANG J F, ZHANG Y, et al. Immobilization of lipase by crosslinking and then adsorption method using macroporous adsorbent resin[J]. Journal of Guangxi Normal University (Natural Science Edition),2020,38(4):101−108.] LIN H J, ZHANG J F, ZHANG Y, et al. Immobilization of lipase by crosslinking and then adsorption method using macroporous adsorbent resin[J]. Journal of Guangxi Normal University (Natural Science Edition), 2020, 38(4): 101−108.
[30] YU C, LI Q, TIAN J, et al. A facile preparation of immobilized naringinase on polyethyleneimine-modified Fe3O4 magnetic nanomaterials with high activity[J]. RSC Advances,2021,11(24):14568−14577. doi: 10.1039/D1RA01449H
[31] 刘慧强. 水解酶催化两种C-N键反应及奥美拉唑的合成[D]. 南昌:东华理工大学, 2013. [LIU H Q. Study on hydrolases catayltic two kinds of C-N formation reactions and synthesis of omeprazole[D]. Nanchang:East China Institute of Technology, 2013.] LIU H Q. Study on hydrolases catayltic two kinds of C-N formation reactions and synthesis of omeprazole[D]. Nanchang: East China Institute of Technology, 2013.
[32] SUO H B, GENG X Y, SUN Y H, et al. Surface modification of magnetic ZIF-90 nanoparticles improves the microenvironment of immobilized lipase and its application in esterification[J]. Langmuir,2022,38(49):15384−15393. doi: 10.1021/acs.langmuir.2c02672
[33] 龙思诗. 米曲霉FS0179脂肪酶固定化及应用研究[D]. 福州:福建师范大学, 2017. [LONG S S. Immobilization and application of lipase from Aspergillus oryzae FS0179[D]. Fuzhou:Fujian Normal University, 2017.] LONG S S. Immobilization and application of lipase from Aspergillus oryzae FS0179[D]. Fuzhou: Fujian Normal University, 2017.
[34] ZHAO J X, MA M M, YAN X H, et al. Green synthesis of polydopamine functionalized magnetic mesoporous biochar for lipase immobilization and its application in interesterification for novel structured lipids production[J]. Food Chemistry,2022,379:132148.
[35] BAYRAMOGLU G, CELIKBICAK O, KILIC M, et al. Immobilization of Candida rugosa lipase on magnetic chitosan beads and application in flavor esters synthesis[J]. Food Chemistry,2022,366:130699. doi: 10.1016/j.foodchem.2021.130699
[36] MAHARANI C A, SUHARTI S, WONORAHARDJO S. Optimizing the immobilization of lipase enzyme (Aspergillus oryzae) in the silica and silica-cellulose matrix by adsorption method[J]. Journal of Physics Conference Series,2020,1595:12011.
[37] 刘振山, 时祎, 陈剑雄, 等. 嗜热脂肪酶-无机杂化纳米花的制备及其性能研究[J]. 食品与发酵工业,2022,48(15):1−8. [LIU Z S, SHI Y, CHEN J X, et al. Research about the preparation and characterisation of inorganic thermophilic lipase hybrid nanoflowers[J]. Food and Fermentation Industries,2022,48(15):1−8.] doi: 10.13995/j.cnki.11-1802/ts.030010 LIU Z S, SHI Y, CHEN J X, et al. Research about the preparation and characterisation of inorganic thermophilic lipase hybrid nanoflowers[J]. Food and Fermentation Industries, 2022, 48(15): 1−8. doi: 10.13995/j.cnki.11-1802/ts.030010
[38] JIANG Y J, LIU H, WANG L H, et al. Virus-like organosilica nanoparticles for lipase immobilization:Characterization and biocatalytic applications[J]. Biochemical Engineering Journal,2019,144:125−134. doi: 10.1016/j.bej.2019.01.022
[39] JIANG Y J, ZHAI J Q, ZHOU L Y, et al. Enzyme@silica hybrid nanoflowers shielding in polydopamine layer for the improvement of enzyme stability[J]. Biochemical Engineering Journal,2018,132:196−205. doi: 10.1016/j.bej.2018.01.028
-
期刊类型引用(12)
1. 付晨青,郭潇潇,刘徐冬雨,李国辉,韩蕊,王秀萍. 贮藏温度对鲜食紫苏采后品质的影响及货架期预测模型的构建. 北方园艺. 2024(02): 86-93 .  百度学术
百度学术
2. 郭慧静,王飞,李自芹,赵志永,宋方圆. 壳聚糖/H_2O_2雾化处理对伽师瓜贮藏品质的影响. 中国食品添加剂. 2024(05): 243-249 .  百度学术
百度学术
3. 刘志旭,朱璇,赵亚婷,张昱,石慧敏,栾若林,刘嘉宝. 高浓度CO_2短时处理对西梅采后贮藏品质及抗氧化代谢的影响. 食品工业科技. 2024(12): 311-318 .  本站查看
本站查看
4. 周旭,杨倩倩,张进,李博岩. 基于便携式近红外光谱仪的黄桃腐败时间快速预测. 食品与机械. 2024(05): 101-106+187 .  百度学术
百度学术
5. 李自芹,李文绮,陈雅,张正红,党富民,赵志永,雷用东. 延迟预冷结合过氧化氢熏蒸对蟠桃贮藏品质及生理特性的影响. 新疆农业科学. 2024(04): 908-915 .  百度学术
百度学术
6. 黄嘉瑜. 食品贮藏条件对植物源性食品中抗氧化剂含量的影响. 中外食品工业. 2024(01): 86-88 .  百度学术
百度学术
7. 侯文焕,唐兴富,廖小芳,赵艳红. 贮藏温度对玫瑰茄果实品质及生理特性的影响. 食品研究与开发. 2024(17): 23-28 .  百度学术
百度学术
8. 张怡,黄雯,张映曈,凌军,李鹏霞,程顺昌,杨青松,周宏胜. 6-苄氨基嘌呤处理对‘苏翠1号’梨货架期品质的影响. 食品研究与开发. 2024(23): 9-17 .  百度学术
百度学术
9. 林景兰,陈日檬,高平,黄国方. 加工过程对紫薯火龙果复合饮料抗氧化性能的影响. 广东化工. 2023(18): 48-50 .  百度学术
百度学术
10. 陈廷慧,曾加庆,罗炜华,王娅玲,龚霄,魏长宾. 不同贮藏温度对黄秋葵采后品质及木质化的影响. 保鲜与加工. 2023(10): 7-14 .  百度学术
百度学术
11. 武海霞,徐玉婷,张永兰,赵彩平. 不同品种桃果实采后常温贮藏性评价. 陕西农业科学. 2023(10): 49-53 .  百度学术
百度学术
12. 何辉,乔勇进,柳洪入,刘晨霞,王春芳,钟耀广,李佳荷,胡留申. 气调贮藏增强桃果活性氧清除能力减轻冷害并改善芳香品质. 食品科学. 2023(23): 165-176 .  百度学术
百度学术
其他类型引用(6)





 下载:
下载:


 下载:
下载:



