Screening of α-Glucosidase Inhibitory Peptides from Tea Leaves using Ultrafiltration Affinity Combined with Liquid Chromatography-Mass Spectrometry and Molecular Docking Technology
-
摘要: 目的:筛选茶叶中具有抑制α-葡萄糖苷酶活性的肽段。方法:采用响应面法优化茶叶酶解产物制备工艺,超滤亲和法分离与α-葡萄糖苷酶结合的茶多肽,液相色谱-质谱联用对分离肽段进行序列测定,生物信息学方法进行虚拟筛选。结果:茶叶酶解产物最佳制备工艺为碱性蛋白酶酶解温度50 ℃,酶解时间3 h,液料比10:1 (mL/g),对α-葡萄糖苷酶抑制率为57.29%,从中鉴定出624条肽段,筛选出一条四肽LIGF具有α-葡萄糖苷酶抑制活性,在 5 mg/mL的浓度下对α-葡萄糖苷酶的最大抑制率为88.13%,IC50值为1.22 mg/mL。分子对接显示,LIGF与α-葡萄糖苷酶能形成5个氢键,结合能为−3.51 kJ,具有高的亲和力、稳定性以及与α-葡萄糖苷酶结合的能力。结论:LIGF具有成为II型糖尿病治疗药物的潜在价值。
-
关键词:
- 超滤亲和 /
- 液质联用 /
- 分子对接 /
- 茶叶 /
- α-葡萄糖苷酶抑制肽
Abstract: Objective: To screen for tea peptides with inhibitory activity against α-glucosidase. Methods: The response surface method was used to optimize the preparation process of tea peptides. Affinity ultrafiltration was used to isolate tea peptides that bind with α-glucosidase, and liquid chromatography-mass spectrometry was used to determine the sequence of the isolated peptides. Virtual screening was performed using bioinformatics methods. Results: The optimal preparation process of tea leaf enzymatic hydrolysis products was alkaline protease hydrolysis temperature of 50 ℃, enzymatic hydrolysis time of 3 h, and a liquid-to-solid ratio of 10:1 (mL/g). The α-glucosidase inhibitory rate was 57.29%. From this, 624 peptide segments were identified, and LIGF was selected for its α-glucosidase inhibitory activity. At a concentration of 5 mg/mL, LIGF exhibited a maximum inhibition rate of 88.13% against α-glucosidase and an IC50 value of 1.22 mg/mL. Molecular docking showed that LIGF could form 5 hydrogen bonds with α-glucosidase, and the binding energy was −3.51 kJ, indicating a high affinity, stability and ability to bind to α-glucosidase. Conclusion: LIGF had potential value as a therapeutic drug for type II diabetes. -
糖尿病(Diabetes Mellitus)是一种复杂的代谢紊乱疾病,其特征是由胰岛素分泌不足或胰岛素作用受损而导致血糖水平较高[1]。截至2021年,全球成人糖尿病患者人数已达到5.37亿,其中90%以上属于Ⅱ型糖尿病(T2DM)[2]。在健康人体内,胰岛素帮助细胞从血液中吸收葡萄糖,以维持正常的血糖水平,而T2DM患者的胰岛素功能障碍阻止细胞对葡萄糖的吸收,导致高血糖症状的发生。
α-葡萄糖苷酶抑制剂和肠上皮细胞钠葡萄糖共转运蛋白(SGLT1)抑制剂均可用于治疗T2DM[1]。葡萄糖苷酶抑制剂通过减缓小肠中碳水化合物的消化而延缓餐后血糖水平上升,对餐后血糖水平难以控制的患者更有效[3]。然而,患者对这类药物有严重的不良反应,如胃肠道气胀、腹泻、肠壁囊肿和低血糖等[4]。因此,开发更有效的生物活性剂作为合成药物的替代品,预防和治疗T2DM至关重要。
Zhang 等[5]通过通过液相色谱电喷雾串联质谱分析、虚拟筛选和鉴定合成,从山茶籽饼蛋白中制备具有α-葡萄糖苷酶抑制作用的活性肽(LLVLYYEY和LLLLPSYSEF),IC50值分别为 0.33 和 1.11 mmol/L。绿茶,白茶和葡萄茶籽,凤凰丹丛茶以及红茶中提取的肽均可对T2DM型糖尿病α-葡萄糖苷酶具有抑制潜力[6-8]。经过酶解处理后产生的肽段,如绿茶和乌龙茶在碱性酶、胃蛋白酶和胰蛋白酶的作用下,可以产生具有抗氧化和抗菌活性的肽段[9-10]。茶渣蛋白中也获得了抗菌肽、抗氧化肽、降血脂肽和具有ACE抑制活性的茶多肽[11-14]。目前生物信息学技术已广泛应用于抗菌肽、抗氧化肽、肽激素、肽药物等领域。然而,关于茶叶蛋白α-葡萄糖苷酶抑制肽的分子对接和活性预测方面的信息仍然有限。α-葡萄糖苷酶抑制肽的信息较少[15-17]。本研究采用响应面法优化茶叶酶解产物制备工艺,结合亲和超滤-液相色谱-质谱技术和生物信息学技术,筛选出具有抑制α-葡萄糖苷酶活性的茶多肽,旨在为治疗糖尿病的肽段开发提供参考。
1. 材料与方法
1.1 材料与仪器
茶树鲜叶(平阳特早) 采自陕西省西乡县东裕茶园;α-葡萄糖苷酶(10万U/mg) 美国Sigma有限公司;乙腈(色谱纯) 天津科密欧化工有限公司;磷酸氢二钾(分析纯) 天津晶科化工有限公司;磷酸二氢钾(分析纯)、三氟乙酸(分析纯) 天津大茂化工有限公司;碱性蛋白酶(20 万U/g) 河南美罗实业有限公司;对-硝基苯基-α-D-吡喃葡萄糖苷、阿卡波糖 分析纯,南京都莱生物技术有限公司;乙腈(质谱级) 美国费雪化学有限公司;甲酸、碳酸氢铵、二硫苏糖醇、碘乙酰胺 质谱级,美国西格玛奥德里奇有限公司。
FA2104分析天平 上海恒平科技有限公司;Multifuge-X3R高速冷冻离心机 美国热电科技有限公司;DK-98-ⅡA恒温水浴锅 天津泰斯特有限公司;1260高效液相色谱仪 美国安捷伦有限公司;Ultimate 3000毛细管高效液相色谱仪、Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer电喷雾-组合型离子阱Orbitrap质谱仪 美国赛默飞世尔科技有限公司。
1.2 实验方法
1.2.1 粗茶多肽的制备工艺
称取茶叶粉末,按照40:1(mL/g)的液料比加入蒸馏水,90 ℃提取40 min,过滤,弃去提取液。取茶叶渣烘干后,按照一定液料比加入蒸馏水,用0.1 mol/L NaOH调节pH为8.5,加入2%碱性蛋白酶,调节温度酶解一定时间,结束后于沸水浴中灭酶,冷却至室温,用0.1 mol/L HCl将提取液pH调整为7,8000 r/min离心15 min,取上清液冷冻干燥,备用。
1.2.2 单因素实验
固定液料比为30:1(mL/g),分别在45、50、55、60、65 ℃提取3 h,考察提取温度对α-葡萄糖苷酶抑制率的影响;固定液料比为30:1(mL/g),提取温度55 ℃,提取时间分别为1.5、2.0、2.5、3、3.5 h的条件下,考察时间对α-葡萄糖苷酶抑制率的影响;固定提取温度55 ℃,提取时间3 h,考察液料比分别为5:1、10:1、20:1、30:1、40:1(g/mL)的条件下,对α-葡萄糖苷酶抑制率的影响。
1.2.3 响应面试验
在单因素实验基础上,选取提取温度(A)、提取时间(B)和液料比(C)三个因素为自变量,以茶叶酶解产物对α-葡萄糖苷酶的抑制率(α-glucosidase inhibition rate,GIR)为响应值,使用Design-Expert 8.0软件中Box-Behnken进行三因素三水平响应面设计,因素水平编码表见表1。
表 1 响应面试验因素水平表Table 1. Factors and levels in response surface experiments水平 A:提取温度(℃) B:提取时间(h) C:液料比(mL/g) −1 50 2.5 10:1 0 55 3 20:1 1 60 3.5 30:1 1.2.4 粗茶多肽GIR测定
GIR的测定参照Wu等[18]的方法,并做适当修改。将响应面法得到的粗茶多肽制备成5 mg/mL的样品溶液,与α-葡萄糖苷酶(10 U/mL)和10 mmol/L PBS(pH6.8)缓冲液按照1:1:4(v:v:v)混匀,37 ℃水浴5 min;加入与样品溶液等体积的pNPG(1 mmol/L)37 ℃水浴5 min,加入1 mL 0.2 mmol/L Na2CO3溶液中止反应,冷却至室温后,405 nm测定吸光值。阿卡波糖溶液作为阳性对照,计算公式如下:
$$\rm GIR(\text{%})=1-(A-A_{0})/A\times 100 $$ 其中,A为样品组的吸光值;A0为空白组的吸光值。
1.2.5 超滤亲和法分离α-葡萄糖苷酶抑制肽组分
参考Chen等[19]的研究,采用超滤亲和法分离α-葡萄糖苷酶抑制肽。取100 μL 2 mg/mL粗茶多肽溶液与200 μL 10 U/mL α-葡萄糖苷酶,37 ℃搅拌30 min使其混合,30 kDa超滤离心,10000 r/min离心10 min,弃去上清液,沉淀加入200 μL 50%乙腈,将结合后的多肽溶解,10000 r/min离心10 min,取上清,冷冻干燥,得到α-葡萄糖苷酶的抑制肽组分,使用HPLC-MS/MS系统分析。
1.2.6 HPLC-MS/MS分析条件
采用HPLC-MS/MS对分离的α-葡萄糖苷酶抑制肽进行序列测定。HPLC条件为:色谱柱Acclaim Pe C18(150 μm×150 mm,1.9 μm);柱温25 ℃;A相(0.1%甲酸),B相(80%乙腈),流速为0.6 mL/min,进样量10 μL。电喷雾质谱条件:正离子电离模式,扫描范围m/z 100~1500 Da,毛细管电压2 kV,ESI+离子源温度320 ℃,碰撞气为氮气。一级质谱参数设置:分辨率70000(FWHM);自动增益控制(AGC)目标值3×106;最大注射时间100 ms;扫描范围为100~1500 m/z。二级质谱参数设置:分辨率75000(FWHM);AGC目标值1×105;最大注射时间50 ms;选择前20个离子进行碎裂;碰撞能量值28。质谱原始文件使用Byonic蛋白质组学分析软件,检索蛋白质数据库,得到氨基酸序列。
1.2.7 分离后肽段GIR测定
将分离后不同分子量区间的多肽样品冷冻干燥后,用PBS配制成5 mg/mL的样品溶液,同“1.2.4”项下方法测定GIR。将阿卡波糖、LIGF和EFGAF用PBS分别配制成1、2、3、5 mg/mL的样品溶液,同“1.2.4”项下方法测定GIR。
1.2.8 生物活性及理化性质预测
使用多种生物信息学工具对肽段进行性质预测和评估。首先采用Peptide Ranker对肽段的一级结构进行分析,预测其稳定性[20],其次使用ToxinPred(http://crdd.osdd.net/raghava/toxinpred/)、Innovagen(https://www.innovagen.com/service/peptide-property-calculator)和Protparam(https://web.expasy.org/protparam/)等在线工具,预测肽段的潜在毒性、水溶性、等电点和稳定性[19]。最后,使用ADMETLab 2.0(http://admet.scbdd.com)服务平台,对肽段在人体内的吸收、分布、代谢、排泄和毒性等性质进行模拟评价[21]。
1.2.9 分子对接
在PDB蛋白数据库中选取α-葡萄糖苷酶晶体蛋白(PDB ID: 3AJ7)[2]。使用Autodock软件对蛋白结构进行优化,并定义活性位点。进行多肽与葡萄糖苷酶的分子对接,并根据几何和能量匹配做出评价分析[22]。采用Consensus score对多肽-酶的结合模型进行打分排序,以筛选出最佳结合模型。最后使用PyMOL软件对最佳结合模型进行作图,以便更好地观察和分析结合情况。
1.3 数据处理
每组实验设置3次平行测定,实验结果表示为平均值±标准差。使用IBM SPSS Statistics进行数显著性分析,采用GraphPad Prism 9.4进行图表绘制。
2. 结果与分析
2.1 单因素实验
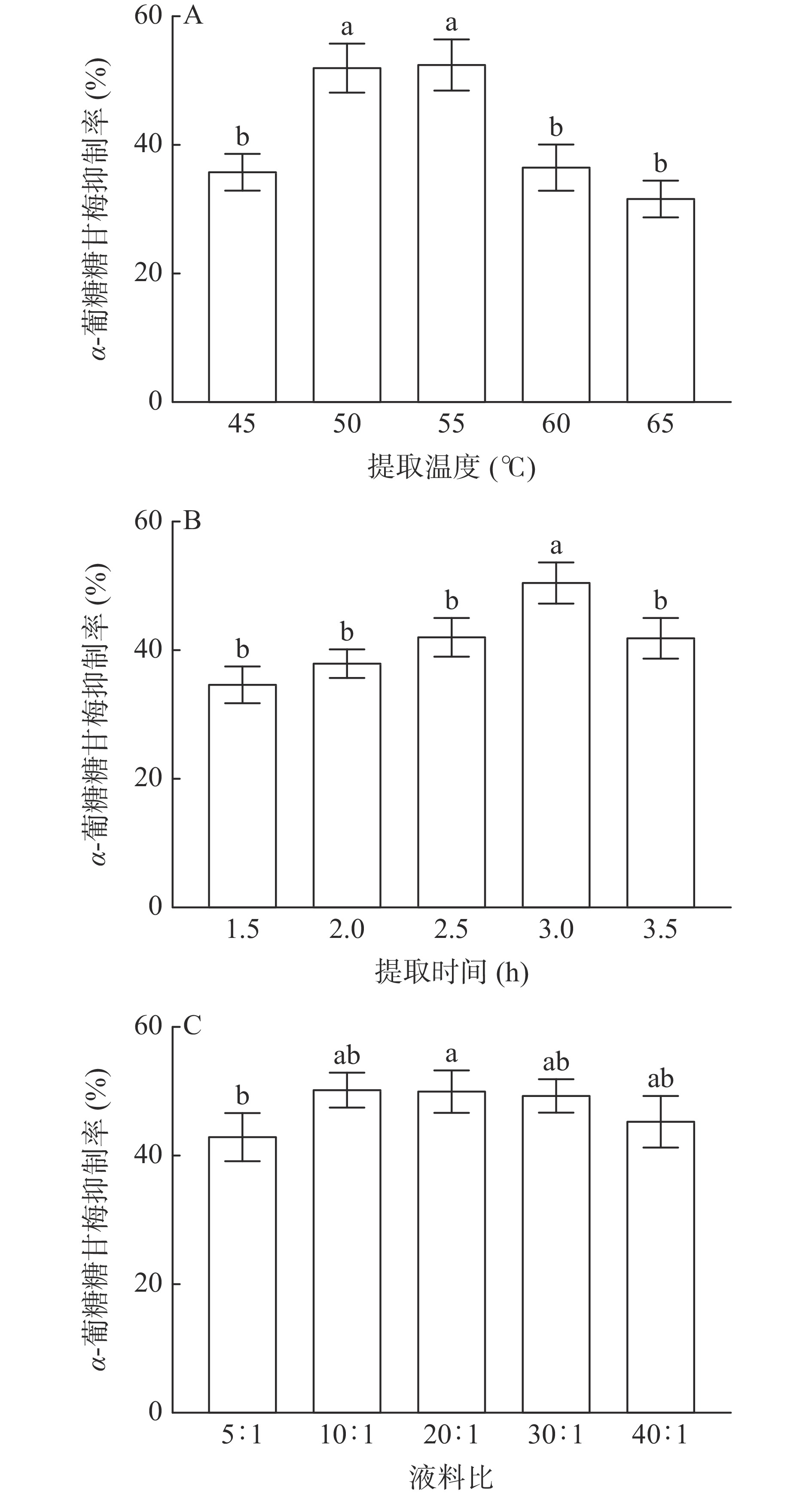
考察了茶叶酶解产物制备工艺中提取温度、提取时间和料液比对α-葡萄糖苷酶抑制率的影响,结果如图1所示。
图1A可知,酶解温度在50和55 ℃时,产物的α-葡萄糖苷酶抑制率分别为52.13%和52.54%,温度降低或升高会显著降低产物活性,这可能是由于温度过低使反应不完全,温度过高导致蛋白酶变性和失活[23]。因此,酶解温度选择为50、55、60 ℃。
在图1B中可见,随着时间的延长,酶解产物对α-葡萄糖苷酶的抑制率逐渐升高,在3 h时达到最高50.51%,超过3 h后,产物活性开始下降,说明提取时间过长,茶渣底物的浓度会降低,这可能会影响酶促反应,导致反应速率下降,降低产品活性[24],所以时间选择2.5、3和3.5 h。如图1C所示,当液料比为20:1,产物对α-葡萄糖苷酶的抑制率最高为50.06%,但在20:1~40:1(g/mL)之间,产物对α-葡萄糖苷酶的抑制率变化并不显著。但当液料比为40:1(g/mL)时,可能因为酶的相对浓度降低导致产物酶解效率和活性较低[24],因此选择10:1,20:1和30:1(g/mL)进行响应面试验。
2.2 响应面结果分析
通过响应面法对茶叶酶解产物制备工艺进行优化,共进行了17组实验,结果见2。
从表2可知,以α-葡萄糖苷酶的抑制率为响应值,回归分析得到二次回归方程:Y=50.83−10.25A−1.48B−0.48C−0.17AB+0.57AC+1.48BC−5.64A2−10.57B2+0.60C2。对方程进行方差分析,结果见表3。
表 2 茶叶酶解产物制备工艺的响应面试验结果Table 2. Response surface experimental results for tea peptide preparation process实验号 A B C Y:GIR(%) 1 0 0 0 51.53±2.86 2 1 −1 0 28.95±1.96 3 −1 0 1 54.32±3.03 4 −1 1 0 55.72±2.13 5 1 1 0 36.25±1.07 6 0 0 0 53.48±1.98 7 0 1 1 48.68±2.88 8 −1 0 -1 57.61±3.35 9 −1 −1 0 55.34±2.5 10 0 0 0 52.4±2.65 11 0 1 -1 49.72±3.37 12 0 −1 -1 45.89±3.62 13 0 −1 1 43.48±2.75 14 0 0 0 50.87±1.97 15 1 0 1 36.55±2.7 16 1 0 -1 37.09±1.69 17 0 0 0 50.64±1.76 表 3 回归模型方差分析Table 3. Analysis of variance (ANOVA) for regression models方差来源 自由度 平方和 均方差 F值 P值 显著性 模型 1086.06 9 120.67 65.62 <0.0001 ** A温度 885.15 1 885.15 481.30 <0.0001 ** B时间 34.90 1 34.90 18.98 0.0033 ** C液料比 6.62 1 6.62 3.60 0.0995 AB 11.97 1 11.97 6.51 0.0380 * AC 1.89 1 1.89 1.03 0.3444 BC 0.4692 1 0.4692 0.2551 0.6290 A2 71.98 1 71.98 39.14 0.0004 ** B2 54.10 1 54.10 29.42 0.0010 ** C2 6.65 1 6.65 3.62 0.0989 残差 12.87 7 1.84 失拟项 7.41 3 2.47 1.81 0.2854 纯误差 5.46 4 1.37 总误差 1098.93 16 注:*代表P<0.05;**代表P<0.01。 由表3可知,模型P<0.0001,回归模型极其显著;失拟项P=0.9585,不显著,决定系数R2=0.9949,预测值R2=0.9883与调整后的R2差值小于0.2,说明模型拟合程度良好,能够较准确地预测和分析实际情况。模型的一次项A、B,二次项A2、B2对响应值的影响极其显著(P<0.01);交互项AB对响应值的影响显著(P<0.05);影响茶多肽提取的因素的作用顺序为A>B>C,即酶解温度>提取时间>液料比。
响应面法预测粗茶叶酶解产物的最佳制备工艺为:酶解温度50 ℃,酶解时间3 h,液料比15:1(mL/g),对α-葡萄糖苷酶抑制率为58.2%。实际实验组中最佳工艺为酶解温度50 ℃,酶解时间3 h,液料比10:1(mL/g),酶解产物对α-葡萄糖苷酶抑制率为57.41%。为检验该模型的可靠性,根据优化所得到最佳条件,开展3组验证实验,得到对α-葡萄糖苷酶抑制率为56.82%±2.27%,与预测值无显著差异。
2.3 肽段分子量的筛选
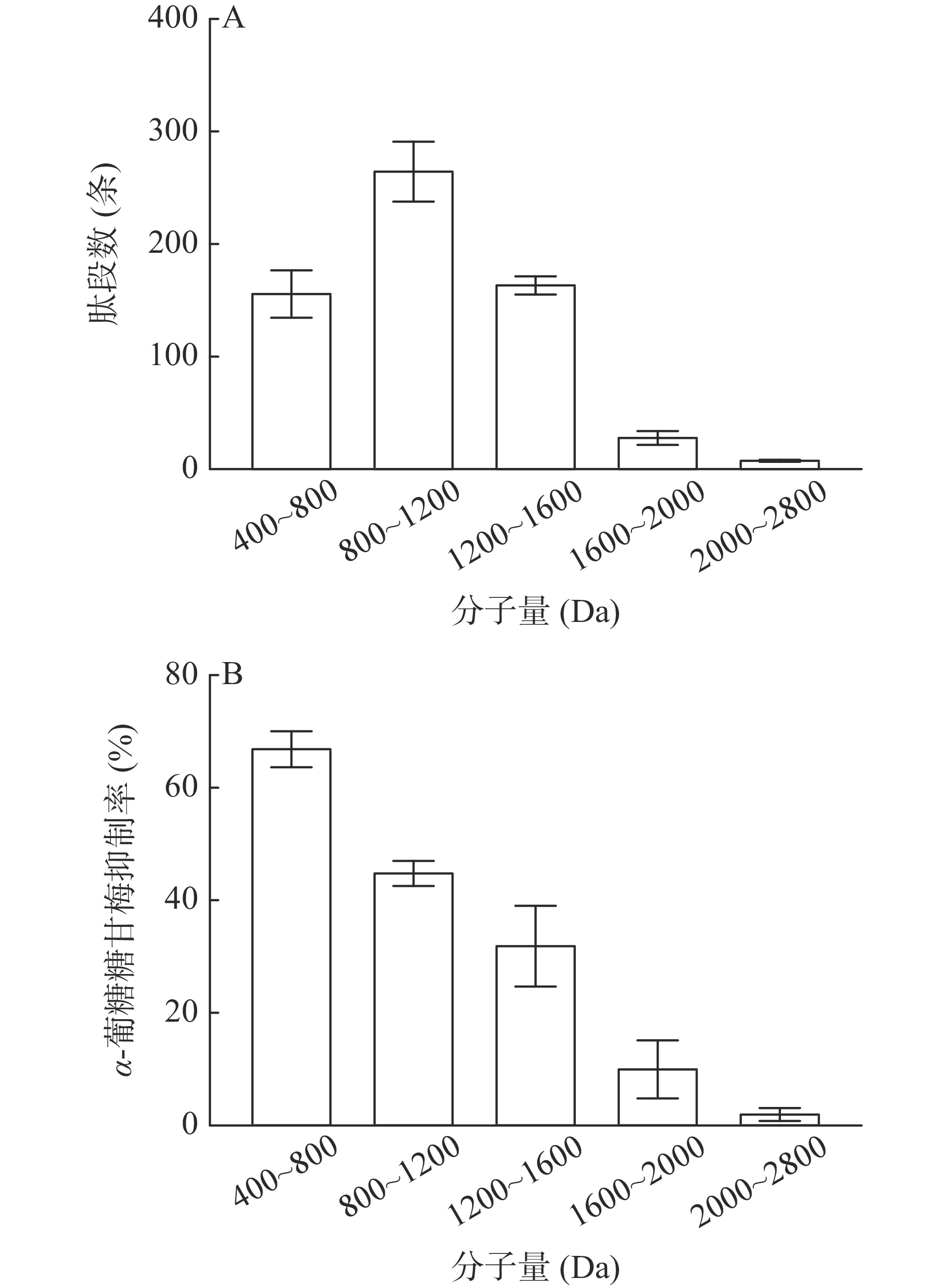
经超滤亲和分离-液相色谱-质谱技术分离并鉴定,得到624条分子量在400~2800 Da的肽段。图2展示了茶叶酶解产物中肽段分子量和对α-葡萄糖苷酶的抑制活性。这些肽段主要为低聚肽,其中266条肽段的分子量为800~1200 Da,平均分子量为988 Da,占总肽段的42.63%,其对α-葡萄糖苷酶的抑制活性为67.04%。有157条400~800 Da的肽段,平均分子量为670 Da,抑制率显著降低至45.86%(P<0.05)。1600~2000和2000~2800 Da肽段,对α-葡萄糖苷酶的抑制活性分别为9.84%和3.04%。因此,400~800 Da的肽段具有较强的α-葡萄糖苷酶抑制活性。
2.4 生物活性及理化性质评估
结合分子量小于800 Da和氨基酸残基小于7个的生物活性肽筛选标准[25],选择具有较强α-葡萄糖苷酶抑制活性的低聚肽(400~800 Da)进行生物信息学虚拟筛选。使用Peptide Ranker预测,结果显示可能具有生物活性的肽段为35条。采用Toxinpred和Innovagen在线工具判断35条肽段的毒性、溶解性、等电点和稳定性,结果显示所有肽段均无毒,但仅有9条肽段水溶性较好。根据Expasy预测,筛选出6条肽段稳定性指数数小于40且等电点在0.7~4.9之间的肽段,该性质肽段有助于多肽酶抑制剂的活性和稳定性[26-27]。
采用ADMET服务平台评估6条肽段的人体胃肠道吸收(HIA)和血脑屏障穿透(BBB)等特性[28]。结果表明,6条肽段的BBB特性、肠道稳定性都较好,均不是CYP4503A4酶的抑制剂。这表明这些多肽具有穿过血脑屏障的能力,在通过肠道吸收后,不会被代谢酶CYP4503A4降解,能够保持稳定性,也不会干扰其他药物的代谢。结合HIA和30%口服利用度分析,选择表现优秀的两个茶肽LIGF和EPDAF进行分子对接。
2.5 质谱分析
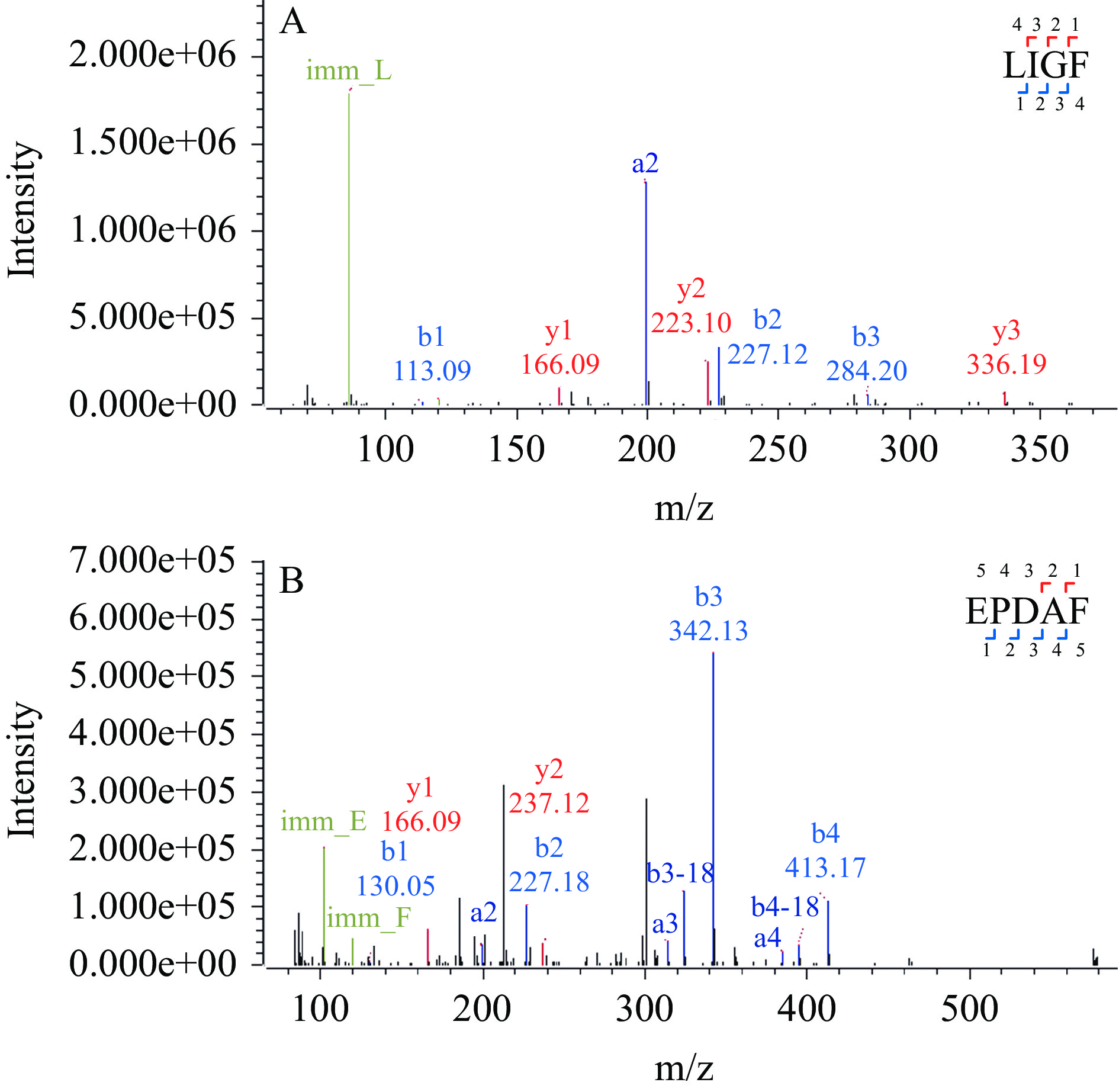
一级质谱确定肽段LIGF和EFGAF的相对分子质量分别为448.28和569.26。通过UniProt数据库查询,两个肽段分别来源于山茶(Camellia sinensis)Spatacsin_C (UniProtKB:A0A7J7I5Q8)和Peptidase_S8(UniProtKB:A0A7J7HMN5)的结构域蛋白,分别由基因HYC85_001559 和HYC85_011168编码。
二级质谱图提供肽段的碎片离子质荷比(m/z),完整肽段在质谱中从肽键位置被打碎为碎片离子,靠近肽段N端的为b离子,靠近C端的为y离子。图3A通过b离子(m/z 113.09,227.18,284.20)和y离子(m/z 166.09,223.10,336.19),确定肽段序列为LIGF(Leu-IIe-Gly-Phe)。图3B通过b离子(m/z 130.05,227.12,342.13,413.17)和y离子(m/z 166.09,237.12),确定肽段序列为EFGAF(Glu-Phe-Gly-Ala-Phe)。
2.6 配体与受体的分子对接
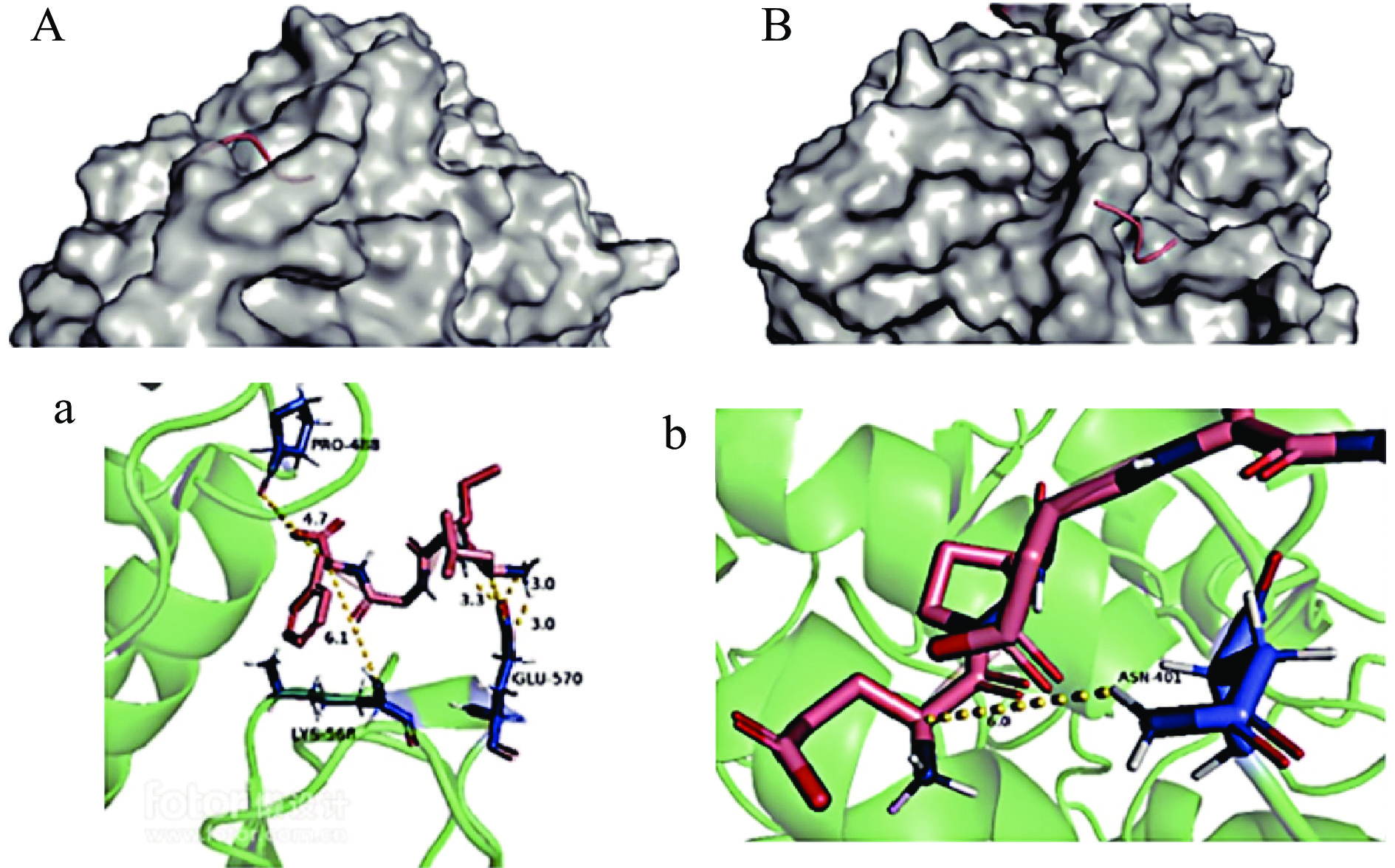
采用分子对接技术构建两种茶肽配体与α-葡萄糖苷酶蛋白受体的结合模型,结果如图4所示。
图4A展示了配体LIGF与α-葡萄糖苷酶受体对接后的复合物整体外部构象。在图4a中,结合多肽N端的苯丙氨酸(F)与α-葡萄糖苷酶上的Pro-488和Lys-568残基形成2个氢键,亮氨酸(L)与Glu-570残基形成3个氢键。该对接结果的Dockingscore为-3.51 kJ。图4B展示了配体EPDAF与α-葡萄糖苷酶受体对接后的复合物整体外部构象。在图4b中,复合物多肽N端谷氨酸(E)与α-葡萄糖苷酶的Asn-401残基形成1个氢键。该对接结果的Dockingscore为-1.31 kJ。通过对比对接结果可知,LIGF序列形成的氢键较多,与α-葡萄糖苷酶结合的能力更强,能够形成更加稳定的复合体[25,29]。
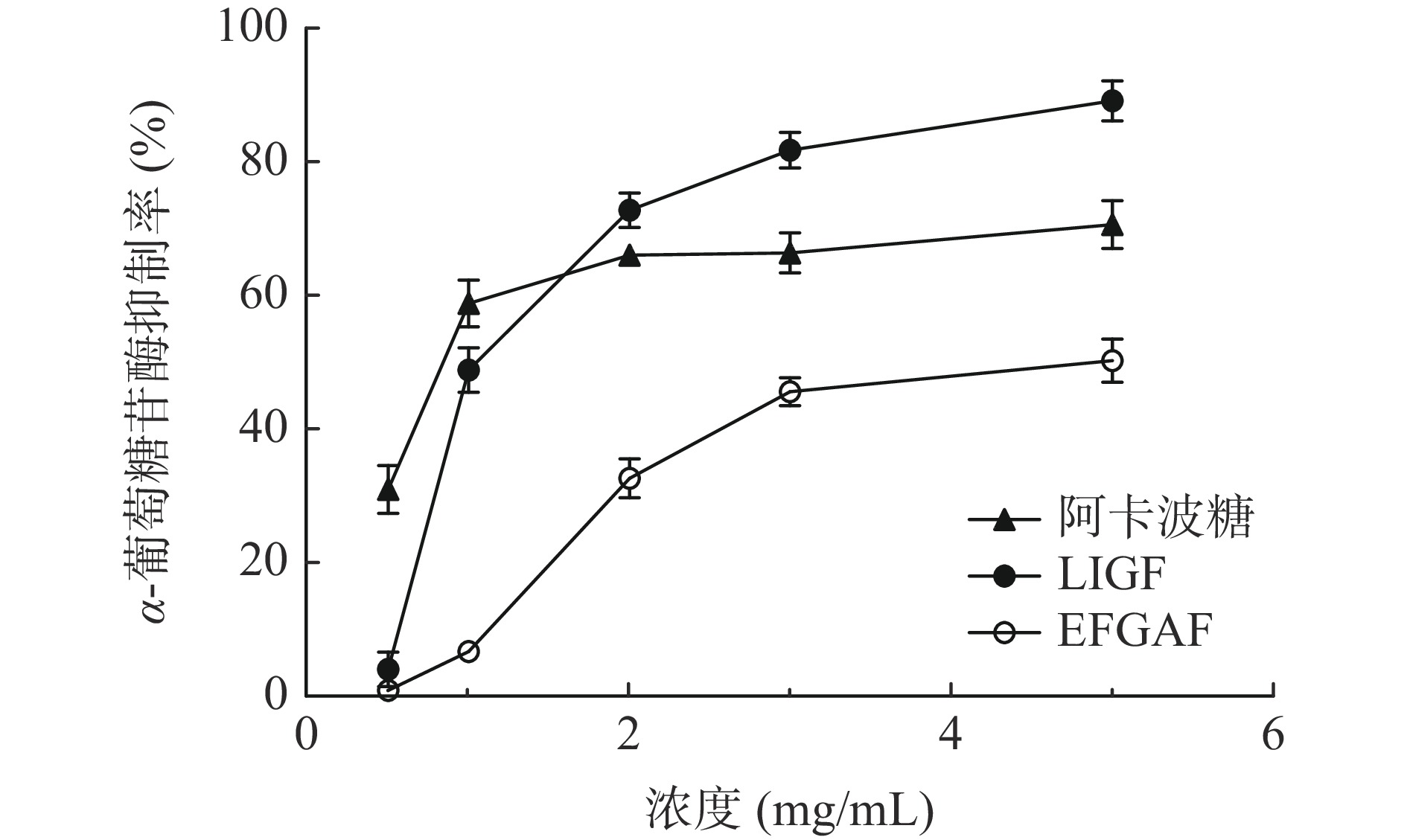
2.7 茶肽对α-葡萄糖苷酶的抑制作用
图5展示了茶肽和阿卡波糖在不同浓度下对α-葡萄糖苷酶的抑制效果。结果显示LIGF对α-葡萄糖苷酶的最大抑制率为88.13%,比阿卡波糖的最大抑制率高19.59%。阿卡波糖、LIGF和EFGAF的IC50值分别为0.98、1.22和4.10 mg/mL。当浓度高于2 mg/mL时,LIGF对α-葡萄糖苷酶的抑制率高于阿卡波糖,这表明在一定浓度范围内,LIGF具有比阿卡波糖更强的α-葡萄糖苷酶抑制作用。
3. 结论
本研究采用响应面法确定了茶叶酶解产物的最佳制备工艺。结果表明,碱性蛋白酶解温度50 ℃,酶解时间3 h,液料比10:1(g/mL)条件下,酶解液对α-葡萄糖苷酶抑制率为57.61%。经超滤亲和分离-液相色谱-质谱技术分离并鉴定,得到624条分子量在400~2800 Da的肽段。选择具有较强α-葡萄糖苷酶抑制活性的低聚肽(400~800 Da)进行生物信息学虚拟筛选,得到茶肽LIGF和EPDAF。5 mg/mL LIGF对α-葡萄糖苷酶的最大抑制率为88.13%,比阿卡波糖的最大抑制率高19.59%,IC50值为1.22 mg/mL。分子对接分析显示,LIGF(亮氨酸-异亮氨酸-甘氨酸-苯丙氨酸)与α-葡萄糖苷酶能形成5个氢键,结合能为-3.51 kJ,具有高的亲和力、稳定性、以及与α-葡萄糖苷酶结合的能力。LIGF具有成为Ⅱ型糖尿病治疗药物的潜在价值。
-
表 1 响应面试验因素水平表
Table 1 Factors and levels in response surface experiments
水平 A:提取温度(℃) B:提取时间(h) C:液料比(mL/g) −1 50 2.5 10:1 0 55 3 20:1 1 60 3.5 30:1 表 2 茶叶酶解产物制备工艺的响应面试验结果
Table 2 Response surface experimental results for tea peptide preparation process
实验号 A B C Y:GIR(%) 1 0 0 0 51.53±2.86 2 1 −1 0 28.95±1.96 3 −1 0 1 54.32±3.03 4 −1 1 0 55.72±2.13 5 1 1 0 36.25±1.07 6 0 0 0 53.48±1.98 7 0 1 1 48.68±2.88 8 −1 0 -1 57.61±3.35 9 −1 −1 0 55.34±2.5 10 0 0 0 52.4±2.65 11 0 1 -1 49.72±3.37 12 0 −1 -1 45.89±3.62 13 0 −1 1 43.48±2.75 14 0 0 0 50.87±1.97 15 1 0 1 36.55±2.7 16 1 0 -1 37.09±1.69 17 0 0 0 50.64±1.76 表 3 回归模型方差分析
Table 3 Analysis of variance (ANOVA) for regression models
方差来源 自由度 平方和 均方差 F值 P值 显著性 模型 1086.06 9 120.67 65.62 <0.0001 ** A温度 885.15 1 885.15 481.30 <0.0001 ** B时间 34.90 1 34.90 18.98 0.0033 ** C液料比 6.62 1 6.62 3.60 0.0995 AB 11.97 1 11.97 6.51 0.0380 * AC 1.89 1 1.89 1.03 0.3444 BC 0.4692 1 0.4692 0.2551 0.6290 A2 71.98 1 71.98 39.14 0.0004 ** B2 54.10 1 54.10 29.42 0.0010 ** C2 6.65 1 6.65 3.62 0.0989 残差 12.87 7 1.84 失拟项 7.41 3 2.47 1.81 0.2854 纯误差 5.46 4 1.37 总误差 1098.93 16 注:*代表P<0.05;**代表P<0.01。 -
[1] LEHMANN A, HORNBY P J. Intestinal SGLT1 in metabolic health and disease[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,2016,310(11):887−898.
[2] THAO T T P, BUI T Q, HAI N T T, et al. Newly synthesised oxime and lactone derivatives from Dipterocarpus alatus dipterocarpol as anti-diabetic inhibitors: Experimental bioassay-based evidence and theoretical computation-based prediction[J]. RSC Advances,2021,11(57):35765−35782.
[3] 崔艳荣. 大柴胡汤对2型糖尿病模型大鼠氧化应激致胰岛β细胞损伤的影响[D]. 兰州: 甘肃中医药大学, 2019 CUI Y R. Effect of Dachaihu decoction on oxidative stress induced beta-cell injury of isletin rats with type 2 diabetes[D]. Lanzhou: Gansu University of Chinese Medicine, 2019.
[4] 田文国, 刘毅, 盖晓红, 等. 地黄治疗2型糖尿病作用机制的研究进展[J]. 中草药,2022,53(23):7575−7584. [TIAN W G, LIU Y, GAI X H, et al. Research progress on mechanism of rehmanniae radix in treatment of type 2 diabetes mellitus[J]. Chinese Traditional and Herbal Drugs,2022,53(23):7575−7584. TIAN W G, LIU Y, GAI X H, et al. Research progress on mechanism of rehmanniae radix in treatment of type 2 diabetes mellitus[J]. Chinese Traditional and Herbal Drugs, 2022, 53(23): 7575-7584.
[5] ZHANG Y, WU F, HE Z, et al. Optimization and molecular mechanism of novel α-glucosidase inhibitory peptides derived from camellia seed cake through enzymatic hydrolysis[J]. Foods,2023,12(2):393.
[6] 祝子坪, 欧燕清, 刘家全, 等. 超声辅助酶解茶渣蛋白制备抗氧化活性肽[J]. 分子植物育种,2020,18(2):573−578. [ZHU Z P, OU Y Q, LIU J Q, et al. Preparation of antioxidant peptides from proteins in tea residues through ultrasonic-assisted enzymolysis[J]. Molecular Plant Breeding,2020,18(2):573−578. ZHU Z P, OU Y Q, LIU J Q, et al. Preparation of antioxidant peptides from pr-oteins in tea residues through ultrasonic-assisted enzymolysis[J]. Molecular Plant Breeding, 2020, 18(2): 573-578.
[7] PATIL S P, GOSWAMI A, KALIA K, et al. Plant-derived bioactive peptides: A treatment to cure diabetes[J]. International Journal of Peptide Research and Therapeutics,2020,26(2):955−968. doi: 10.1007/s10989-019-09899-z
[8] WANG R, ZHAO H, PAN X, et al. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein[J]. Food Science & Nutrition,2019,7(5):1848−1856.
[9] LIN Y C, HSU P K. Evidence based study of hypoglycemic potential of bitter melon peptide[J]. American Journal of Biomedical Science & Research,2020,9(1):60−63.
[10] AYIM I, MA H, ALI Z S et al. Preparation of antioxidant peptides from tea (Camellia sinensis L.) residue[J]. Journal of food Measurement and Characterization,2018,12(3):2128−2137. doi: 10.1007/s11694-018-9828-y
[11] ZHANG H, CHEN Y, GUO Y, et al. Label-free quantification proteomics reveals the active peptides from protein degradation during anaerobic fermentation of tea[J]. LWT-Food Science and Technology,2021,150(39):111950.
[12] LAI X, PAN S, ZHANG W, et al. Properties of ACE inhibitory peptide prepared from protein in green tea residue and evaluation of its anti-hypertensive activity[J]. Process Biochemistry,2020,92(5):277−287.
[13] 代成, 谭梓铭, 张阳, 等. 超声波预处理酶解制备茶渣蛋白ACE抑制肽及其活性分析[J]. 食品工业科技,2022,43(16):192−200. [DAI C, TAN Z M, ZHANG Y, et al. Ultrasonic pre-treatment for the preparation of ACE-inhibitory peptides from the tea residue protein through enzymatic hydrolysis and its stability[J]. Science and Technology of Food Industry,2022,43(16):192−200. DAI C, TAN Z M, ZHANG Y, et al. Ultrasonic pre-treatment for the preparation of ACE-inhibitory peptides from the tea residue protein through enzymatic hydrolysis and its stability[J]. Science and Technology of Food Industry, 2022, 43(16): 192-200.
[14] 杜梦珂. 富硒碱性茶蛋白ACE抑制肽的制备、分离纯化及结构鉴定[D]. 上海: 上海师范大学, 2018 DU M K. Study on the preparation, purification and identification of ACE inhibitory activity peptides from Se-enriched tea protein[D]. Shanghai: Shanghai Normal University, 2018.
[15] WEN Y. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review[J]. Trends in Food Science & Technology,2020,105(1):308−322.
[16] 叶灏铎, 苗建银, 李龙星, 等. 勐库大叶茶蛋白降血脂肽的酶解制备及活性分析[J]. 食品工业科技,2022,43(9):212−221. [YE H D, MIAO J Y, LI L X, et al. Preparation and activity of hypolipidemic peptides from Mengkudayecha protein by enzymatic hydrolysis[J]. Science and Technology of Food Industry,2022,43(9):212−221. YE H D, MIAO J Y, LI L X, et al. Preparation and activity of hypolipidemic peptides from Mengkudayecha protein by enzymatic hydrolysis[J]. Science and Technology of Food Industry, 2022, 43(9): 212-221.
[17] 頡宇, 胡锦灵, 赵宏飞, 等. 基于生物信息学定向制备柠条籽蛋白抗氧化肽的工艺优化[J]. 食品科学,2020,41(20):278−284. [JIE Y, HU J L, ZHAO H F, et al. Optimization of bioinformatics-based directional preparation of antioxidant peptide from Caragana seed protein[J]. Food Science,2020,41(20):278−284. JIE Y, HU J L, ZHAO H F, et al. Optimization of bioinformatics-based directional preparation of antioxidant peptide from Caragana seed protein[J]. Food Science, 2020, 41(20): 278-284.
[18] WU J L, WEI Z J, CHEN G, et al. Rapid screening for α-glucosidase inhibitors from gymnema sylvestre by affinity ultrafiltration-HPLC-MS[J]. Frontiers in Pharmacology,2019,8:228.
[19] CHEN G, GUO M. Rapid screening for α-glucosidase inhibitors from gymnema sylvestre by affinity ultrafiltration-HPLC-MS[J]. Front Pharmacol,2017,8:228. doi: 10.3389/fphar.2017.00228
[20] HASSAN N M, ALHOSSARY A, MU Y, et al. Protein-ligand blind docking using quickvina-W with inter-process Spatio-Temporal integration[J]. Scientific Reports,2017,7(1):15451.
[21] CATHERINE M, HASLA N J, GIANLUCA P, et al. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity[J]. PLoS One,2012,7(10):e45012. doi: 10.1371/journal.pone.0045012
[22] JI D, XU M, AGYEI D, et al. Physicochemical characterisation, molecular docking, and drug-likeness evaluation of hypotensive peptides encrypted in flaxseed proteome[J]. Current Research in Food Science,2020,3:41−50. doi: 10.1016/j.crfs.2020.03.001
[23] 陈海君, 秦惠玉, 龙飞, 等. 超滤亲和结合液相色谱-质谱联用和分子对接技术筛选毛菊苣种子中高亲和性α-葡萄糖苷酶抑制剂[J]. 分析化学,2017,45(6):889−897. [CHEN H J, QIN H Y, LONG F, et al. Screening of high-affinity α-glucosidase inhibitors from cichorium glandulosum Boiss. et hout seed based on ultrafiltration liquid chromatography-mass spectrometry and molecular docking[J]. Chinese Journal of Analytical Chemistry,2017,45(6):889−897. CHEN H J, QIN H Y, LONG F, et al. Screening of high-affinity α-glucosidase inhibitors from cichorium glandulosum Boiss. et hout seed based on ultrafiltration liquid chromatography-mass spectrometry and molecular docking[J]. Chinese Journal of Analytical Chemistry, 2017, 45(6): 889-897.
[24] REZAUL K, SHAHEEN S, AMARCHAND C M. Anti-amylolytic activity of fresh and cooked okra (Hibiscus esculentus L.) pod extract[J]. Biocatalysis and Agricultural Biotechnology,2014,3(4):373−377. doi: 10.1016/j.bcab.2014.07.006
[25] SPIGNO G, TRAMELLI L, DMD F. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics[J]. Journal of Food Engineering,2007,81(1):200−208. doi: 10.1016/j.jfoodeng.2006.10.021
[26] CUI Q, XING N I, ZENG L, et al. Optimization of protein extraction and decoloration conditions for tea residues[J]. Horticultural Plant Journal,2017,3(4):172−176. doi: 10.1016/j.hpj.2017.06.003
[27] 李志明, 张舒, 孟维洪, 等. 食源性α-葡萄糖苷酶抑制肽: 构效关系、安全性及生物利用度[J/OL]. 食品科学: 1−18 [2023-07-06]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221101.1612.020.html ZHANG S, MENG W H, et al. Food-derived α-glucosidase inhibitory peptides: Structure-activity relationship, safety and bioavailability[J/OL]. Food Science: 1−18 [2023-07-06]. http://kns.cnki.net/kcms/detail/11.2206.TS.20221101.1612.020.html
[28] 李琳琳, 曹新志, 刘芳, 等. 蚕丝蛋白水解液中多肽相对分子质量分布的测定[J]. 食品工业科技,2014,35(7):95−99. [LI L L, CAO X Z, LIU F, et al. Determination of molecular weight distribution of peptides in silk protein hydrolysate[J]. Science and Technology of Food Industry,2014,35(7):95−99. LI L L, CAO X Z, LIU F, et al. Determination of molecular weight distribution of peptides in silk protein hydrolysate[J]. Science and Technology of Food Industry, 2014, 35(7): 95-99.
[29] MELISSA R P, STEPHANIE M C, et al. Isoelectric point separations of peptides and proteins[J]. Proteomes,2017,5(1):4.
-
期刊类型引用(2)
1. 张保生,查磊,赵妍,张梦珂,余盼玲,徐保婷,陈明杰. 十二种食用菌蛋白及其酶解产物降血糖活性. 菌物学报. 2024(06): 151-161 .  百度学术
百度学术
2. 刘虹,刘鸿靖. 液质联用技术在药品质量分析中的应用. 实验室检测. 2024(10): 147-149 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:
 下载:
下载:



