Progress on Bioavailability of Calcium and Calcium-Peptide Complexes
-
摘要: 钙是人体内十分重要的营养元素,对维持人体健康起到了至关重要的作用。小肠是钙吸收的主要部位,食物中的钙经胃酸溶解为离子形式后在微碱性的肠道环境内可能会形成沉淀,导致钙的吸收下降和生物利用度降低。而长期钙摄入不足则会导致佝偻病、骨质疏松症等一系列骨代谢疾病。因此,选择能够提供高可溶性和生物利用度的钙的食物或补充剂十分重要。部分有机钙盐如葡萄糖酸钙、柠檬酸钙等可以在肠道中形成过饱和溶液从而增强钙的吸收。此外,多肽螯合钙也被证实有非常好的钙吸收率和生物利用度,可以作为新型的钙补充剂。本文综述了食物基质成分对钙吸收和生物利用度的影响,并对部分有机钙盐和多肽螯合钙的促钙吸收机理进行了总结,旨在为钙的吸收与钙补充剂的研发提供新的思路。Abstract: Calcium is an essential nutrient for human health and plays a crucial role in various physiological processes such as bone formation, muscle contraction, nerve transmission, and blood clotting. Calcium absorption mainly occurs in the small intestine where the calcium enters the enterocytes through active transport or passive diffusion. However, the free calcium ions dissolved in gastric acid may form insoluble precipitates with other dietary components (such as phytate, oxalate, fiber) in the slightly alkaline intestinal environment, leading to decreased calcium absorption and bioavailability. Decreased calcium absorption can result in a range of bone metabolic diseases, such as rickets and osteoporosis. Therefore, it is important to choose foods or supplements that can provide highly soluble and bioavailable forms of calcium. Some organic calcium salts such as calcium gluconate and calcium citrate have been shown to form supersaturated solutions in the intestinal tract, which can enhance calcium absorption by increasing the concentration gradient across the intestinal epithelium. Furthermore, calcium-peptide complexes are proven to be very effective for improving calcium absorption without causing any side effects. Calcium-peptide complexes can serve as a novel calcium supplement. In this paper, the effects of food components on calcium absorption and bioavailability are reviewd, and the mechanisms underlying the enhancement of calcium absorption by some organic salts (such as calcium gluconate and calcium citrate) and peptides are summarized. And the potential applications of calcium-peptide complexes in human nutrition are also discussed, aiming to provide new insights that can aid in the development of safe and effective calcium supplements
-
Keywords:
- calcium /
- calcium salts /
- calcium-peptide complexes /
- bioavailability
-
人体中正常的生理过程会涉及22种无机元素,其中钙是含量最丰富的无机元素之一,占人体总重的1.5%~2.2%[1]。钙与人体内许多重要的生物学功能息息相关,如肌肉收缩、神经传导、腺体分泌和骨结构支撑等[2−3]。人体中大约99%的钙储存在骨骼和牙齿中,剩余1%的钙则存在于细胞内液和细胞外液中[4]。当身体的钙摄入量不足时,钙就会从骨骼中释放出来,患骨质疏松症等疾病的风险就会增加[5]。充足的钙摄入可以降低慢性疾病的风险,且因为在预防骨质疏松症、高血压、结肠癌和其他疾病风险方面的作用使得钙赢得了“超级营养物质”的称号[6−7]。食物是人体钙摄入的主要来源,且人体中绝大部分的钙(90%左右)都是在小肠当中被吸收。然而并不是所有食物中的钙都能被完全吸收,食物中的钙组分经胃酸消化溶解成离子形式后转运至微碱性的十二指肠[8],随着小肠肠段pH的不断升高,离子钙发生沉淀,造成钙的生物利用度降低。生物利用度是指食物中的某种营养成分被实际吸收和利用的部分所占的比例[9]。人体中最常见的钙的可利用形式是离子钙[10],钙的生物利用度可以被很多内源性因素如年龄、疾病、激素、生理条件(怀孕、哺乳、绝经等)和肠道微生物等影响,也可以被膳食中其他营养成分(如脂肪、植酸盐)等外源性因素所影响。
部分有机钙盐(如葡萄糖酸钙、柠檬酸钙)是比较良好的钙补充剂,其可以在动力学层面(如沉淀速率、钙盐复合物形成和解离速率等)抑制钙离子在碱性肠道环境中产生的沉淀[11]。此外,多肽和一些氨基酸(如天冬氨酸和谷氨酸)[12]被视作很好的钙结合配体,而且无论是氨基酸螯合钙还是多肽螯合钙,二者都能在小肠中pH不断升高的环境下保持可溶的状态。然而螯合钙的吸收率主要取决于其所结合的配体。与氨基酸相比,肽配合物有着许多优势,比如能量消耗少、运输速度加快、载体不容易饱和等[13]。从牛奶当中提取出来的酪蛋白磷酸肽(CPPs)已经被证实可以和钙离子结合形成可溶性的稳定复合物从而增强钙的吸收[14−15]。因此,多肽螯合钙可能是改善人体胃肠道钙吸收的合适钙补充剂候选者。
本文综述了食物基质成分对钙的生物利用度的影响,并就葡萄糖酸钙、柠檬酸钙等有机钙盐和多肽螯合钙的促钙吸收机制进行了总结,旨在为钙离子的吸收和新一代钙补充剂的研发提供思路。
1. 钙的生理功能
钙离子是人体内分布最广泛的矿物质,也是研究最多的、与人体健康息息相关的矿物质元素之一[1]。人类刚出生时体内的钙含量为25~30 g,成年后体内的钙含量可以达到1000~1500 g[16]。钙在人体内无法自发合成,生长发育所需要的钙以及日常钙损失的补偿都需要从外源性食物中获得[17]。99%的钙都以羟基磷灰石的形式存在于骨骼和牙齿中,并通过对骨骼系统提供刚性支持来发挥重要的结构功能[18]。剩余1%的钙主要存在于软组织和体液当中(如血液和细胞外液)。在软组织当中,钙主要存在于细胞质中的各个细胞器[19];在血液中,钙以三种不同的形式存在:50%以游离阳离子形式存在,40%与血浆蛋白结合,10%溶解后与其他离子(如柠檬酸盐和乳酸)结合形成复合物[20]。健康受试者的血清钙浓度通常维持在1.0~1.2 mmol/L左右,并经由甲状旁腺激素、维生素D和降钙素的作用控制,分别调节肠道钙吸收、肾脏钙排泄或重吸收、以及骨骼钙流失与钙沉积等[21]。除结构功能外,钙还参与很多生理功能,如受精、血液凝固、肌肉收缩、神经冲动传导、激素分泌、细胞死亡、免疫反应、细胞分化和酶活力调节等[22−24]。获得和保留体内所需量的钙对于儿童骨骼的发育、强度和密度以及预防老年人骨质流失和骨质疏松性骨折至关重要。摄入足够的钙也有助于降低不同慢性疾病的风险,如高血压、高胆固醇血症、结肠癌、肾结石和腹部肥胖等[25−27]。当然,仅摄入足量的钙并不一定能满足实现这些生理功能,因为人体摄入的钙需要被吸收之后才能发挥作用。
2. 钙的生物利用度
评估一种营养物质的生物利用度首先要评估其生物可及性。生物可及性通常适用于体外矿物质生物利用度研究,指食物基质经胃肠道消化后所能释放出来的矿物质营养组分[28],但是并不包括矿物质在肠道内的吸收[29]。基于此,Cian等[30]和Wang等[31]通过体外模拟消化实验中的可溶性组分来确定营养物质的生物可及性。部分钙盐和钙肽复合物可以显著提高钙离子进入肠腔内的生物可及性[32],但这并不意味着其一定会提高钙的生物利用度,这是因为一种营养物质的生物利用度除了包含其生物可及性,还包括其正常生理代谢过程中经肠道细胞吸收和利用的部分[17],甚至是穿越肠道屏障到达目标组织的部分[29]。钙的生物利用度受内源性因素和外源性因素两方面的影响。内源性因素主要包括如年龄、疾病[33−34]、激素[35]、肠道微生物[36]、生理条件(怀孕、哺乳、绝经等)[37]等方面,外源性因素(表1)主要是指日常膳食营养。正常生理状态下钙的生物利用度主要受外源性因素的影响。常量营养素和微量营养素会在一定程度上改善或紊乱钙的吸收。
表 1 影响钙生物利用度的外源性因素Table 1. Exogenous factors affecting calcium bioavailability2.1 脂肪
维生素D是钙吸收过程中必不可少的激素之一[38−40],而脂肪对脂溶性维生素D的生物利用度有一定程度的影响。一般来说,脂类可以促进食物亲脂性成分的吸收,是脂溶性微量营养素传递的关键物质。首先,脂质作为疏水相可以溶解脂溶性营养物质,促使其从食物基质中扩散;然后通过刺激胆汁分泌,产生消化酶催化水解脂质并释放脂肪酸、单甘油酯和磷脂,进一步溶解亲脂性营养物质并产生大量胶束;最后,这些脂质介导亲脂性营养物质,避免了肠上皮细胞中维生素D的积累,从而增加胃肠道对维生素D的吸收。整个过程取决于脂肪(甘油三酯)的数量和脂肪酸的类型等[41]。在体外研究中检测了两种高脂肪饮食—富含饱和或单不饱和脂肪酸的饮食,以及低脂肪饮食对钙吸收的影响。结果表明富含饱和脂肪酸饮食对钙吸收作用的促进效果最好[42]。然而,尽管如此,饱和脂肪酸对骨骼健康的影响小于同等比例的单不饱和脂肪酸或脂肪含量正常的饮食。有研究发现添加单不饱和脂肪酸的小鼠Calbindin-D9k(钙结合蛋白)肠道基因表达水平和小梁状软骨体积厚度显著高于未修饰饮食的小鼠。因此,富含单不饱和脂肪酸的饮食对骨骼健康最有益,并能适度增加肠道钙的吸收[43]。
2.2 乳糖
乳制品是乳糖和钙的主要膳食来源[44]。低乳制品饮食是钙缺乏的一个重要因素,其会对骨代谢产生不利影响[45]。生理浓度下乳糖对牛奶中钙的吸收没有显著影响[46−47],而高剂量的乳糖(50 g/d)在一定程度上会影响钙吸收[48]。
2.3 膳食纤维
膳食纤维本身(纤维素、半纤维素、木质素和非纤维素多糖)似乎对钙的生物利用度没有直接影响。一些难以消化的膳食纤维已被证明可以通过增强细菌发酵来降低肠道中的pH,增加钙离子的溶解度[49]。还可以通过酸化肠道环境,限制有关磷酸盐的形成,从而增加钙吸收,因为钙可以和部分磷酸盐产生沉淀[50]。膳食纤维摄入对骨骼结构和代谢的影响与性别有关,其原因可能是膳食纤维降低了肠道中β-葡萄糖醛酸酶的活性,导致雌激素重吸收的减少,从而降低骨密度[51]。
2.4 磷
饮食中钙与磷的比例应该保持在1.3:1到2:1之间。低于或高于这一比例会造成人体一系列的疾病,如生长发育迟缓、食欲减退、皮肤屏障或肌肉力量受损,严重情况下甚至可以导致死亡[52]。低磷饮食即使钙充足,也会导致表观消化率和肾脏磷排泄的下降,以及钙排泄的显著增加[52]。钙磷比2:1是维持骨骼生长发育和骨保护素含量平衡的最佳比例[53]。
2.5 植酸和草酸
食物中的植酸盐又被称作肌醇六磷酸盐(IP6),是植物中磷和肌醇的一种储存形式[54]。植酸主要存在于谷物、豆类、坚果和种子中,在胃中酸性pH的条件下可以与钙形成可溶性配合物,在微碱性的肠道中发生沉淀[55]。与植酸类似,草酸作为一种存在于蔬菜中的强有机酸,其可以与钙形成水不溶性盐,造成钙生物利用度的降低[56]。
2.6 酪蛋白
酪蛋白在消化过程中会产生酪蛋白磷酸肽(CPPs),CPPs除了具有抗菌、防龋齿、细胞调节和免疫调节特性外,还可以转运钙离子并增加其生物利用度[57]。Sun等[58]和Luo等[59]的研究已经证实,CPPs可以阻止肠道内钙离子的沉淀,且从CPPs混合物中分离出来的单体肽“Ser(p)-Ser(p)-Ser(p)-Glu-Glu”具有最大的钙结合能力,它可以同时结合6个钙离子。通过核壳细胞外微粒递送的CPPs-壳聚糖低聚糖-三聚磷酸是钙补充剂中一种很有前途的成分,其可以逐步释放钙离子,增加钙的吸收时间,通过调节钙的可控释放增强其在肠道中的生物利用度[60]。
3. 钙补充剂
通常情况下,人主要通过膳食的方式来摄入和补充体内所需要的钙。如果膳食钙的摄入量不足,钙补充剂可以帮助预防或治疗钙缺乏症[61],特别是对于老年人或绝经后的妇女,服用适当的钙补充剂可以预防骨质疏松症[51,62]。钙补充剂可以以不同形式的钙盐存在,此外,已经有大量的动物和细胞实验证明食物蛋白中的多肽与钙的螯合产物作为钙补充剂的应用潜力[1,63]。
3.1 钙盐
比较常见的钙盐有碳酸钙、葡萄糖酸钙、柠檬酸钙和乳酸钙。与其他钙盐相比,碳酸钙的钙元素含量最高(40%),同时也是市场上较为便宜的钙源[64−65],但是其溶解度较差,生物利用度较低,且长期服用副作用明显,会给人体带来比较严重的负担[66]。相比于碳酸钙,羟基羧酸钙盐(葡萄糖酸钙、柠檬酸钙等)具有更高的生物利用度。
钙主要在小肠当中被吸收。食物当中的钙经胃酸消化溶解成离子形式后转运至碱性环境的小肠当中,容易产生离子钙的沉淀(如钙与食物中的植酸、草酸和磷酸等结合),导致生物利用率降低[67]。一些天然配体,如部分水解的蛋白质、多肽以及羟基羧酸盐等可以与小肠中已经沉淀的钙复合物发生竞争性结合,增强钙的吸收效果,尽管部分配体可能与钙发生强络合从而降低游离钙的浓度[11]。Pak等[68]发现,相较于柠檬酸钙(二柠檬酸三钙,柠檬酸和钙摩尔比为1:1.5)化合物,由氢氧化钙和柠檬酸制备的“柠檬酸钙”混合物(柠檬酸和钙的摩尔比为1:1.25)具有更高的溶解度以及钙生物利用度,这充分证明了钙在肠道中的“存在形式”对其生物利用度的重要性。在模拟肠道环境的条件下,葡萄糖酸钠在饱和乳酸钙溶液中等温溶解会自发产生十分稳定的葡萄糖酸钙过饱和溶液,钙离子活度不变,浓度增加,这主要是由于羟基羧酸盐配体可以和沉淀物的固体表面发生反应[69−70]。肠道中钙盐的过饱和溶液的形成对增强钙离子吸收的重要性还没有得到足够多的研究。过饱和钙盐溶液中高浓度的钙离子会在肠道中形成较高的渗透压,从而增加钙在小肠中扩散的驱动力[71]。此外,尽管和氯化钙一样,柠檬酸钙、葡萄糖酸钙会在胃酸中完全解离,但是后者钙的吸收效率却远高于氯化钙[72]。这可能与羟基羧酸钙盐的解离速率有关,其在胃中发生解离后在肠道中重组形成羟基羧酸钙复合物的速率比钙离子在碱性肠道环境下的沉淀速率更高,因此具有更高的生物利用度。
3.2 多肽螯合钙
氨基酸和多肽可以帮助保持钙的离子形式,增加钙离子在碱性肠道环境下的溶解度,对钙的吸收有着积极的影响[73]。Asp和Glu是钙结合能力最强的氨基酸[74],但是与食物中的活性蛋白质多肽相比,其钙的结合能力依然较弱。Vavrusova等[75]的研究表明,Asp和Glu可以互相结合成四种不同的二肽(Asp-Asp/Asp-Glu/Glu-Glu/Glu-Asp),四种二肽与钙的结合常数Ka表明,在两种离子强度下,与单个氨基酸相比,它们对钙具有更高的亲和力,对Ser和二肽Ser-Glu钙亲和力的研究也发现了类似的结果。迄今为止,研究人员已经从多种不同的食物来源中分离得到了具有活性的多肽螯合钙,如太平洋鳕鱼皮蛋白[76],南极磷虾蛋白[77],罗非鱼蛋白[78],大豆蛋白[79]和蛋清蛋白[80]等。钙肽螯合物是十分理想的钙补充剂,容易被胃肠道吸收,且具有十分良好的稳定性[32]。酪蛋白磷酸肽(CPPs)因为其良好的补钙效果现在已经实现商业化生产[15]。
4. 多肽螯合钙的结合机理和构效关系
一般来说,多肽和钙之间的结合反应是在特定的温度和时间下将多肽溶液与氯化钙搅拌混合来完成的。反应过程中需要调节pH来使相应的钙结合基团去质子化,这是因为去质子化残基更有利于结合钙离子[81]。在胃肠道消化过程中,由于胃中的强酸性环境,导致多肽的钙离子结合位点发生质子化,不利于多肽和钙的结合;而在小肠的碱性环境中,由于pH大于多肽部分氨基酸残基的pKa,使得多肽中钙离子的结合位点发生去质子化,更有利于和钙的结合[82]。这就意味着尽管小肠当中的碱性环境会降低游离钙的溶解度甚至产生沉淀,但却有利于多肽和钙的结合,这也是多肽螯合钙优于其他钙补充剂的原因之一。
4.1 多肽和钙的结合原则
多肽与钙结合的先决条件是作为电子供体的多肽和电子受体的钙之间,存在一个或多个可能的结合位点,因此肽可以被看作是路易斯酸,钙可以看做是路易斯碱,肽与钙之间发生的反应是因为路易斯酸碱之间的相互作用。在结构上,钙离子属于钙肽螯合物的一部分[5]。钙与肽结合形成复合物后,钙的物理化学性质会发生一定程度的变化,配体多肽和钙之间互相共享部分各自的属性,同时保留部分自己独有的物理化学性质[83]。
钙肽复合物的形成主要受以下因素的影响:多肽链中能形成共价键的官能团、空间位阻、以及形成复合物所需要的能量限制[84],其特异性与氨基酸的空间分布与排列有关[85],即钙肽复合物中可以形成共价配位键的官能团的位置。因此,通过改变多肽序列中的氨基酸残基,可以增加或减少钙与多肽之间的相互作用[86]。此外,多肽表面的电荷还会影响其与钙离子结合之后的稳定性[87]。原子结构、共价键或离子键、钙或多肽之间连接的配位层都会影响多肽螯合钙的生物利用度[83]。
4.2 多肽和钙的结合位点
钙与肽的结合主要依赖于多肽链上的活性位点,分子间作用力主要包含共价键、氢键、静电引力和疏水相互作用[1,88-90]。目前的研究表明,多肽链上结合钙离子的主要活性位点为磷酸基、羧基和氨基[91−92]。
4.2.1 磷酸基
磷酸化修饰有助于钙离子与多肽的结合,而磷酸化丝氨酸是钙离子的主要结合位点[93]。其中最具代表性的磷酸基-钙结合模式是酪蛋白磷酸肽(CPPs)和卵黄高磷蛋白磷酸肽(PPPs)[94−95]。
CPPs作为钙鳌合肽的作用机制已经被广泛研究。CPPs是酪蛋白衍生的磷酸化肽,包含一段高极性序列(SpSpSpEE),由三个磷酸化丝氨酸残基和两个谷氨酸残基组成,是CPPs中钙的结合活性位点,能够促进钙的吸收[96]。PPPs比CPPs含有更丰富的磷酸化丝氨酸残基,已经有研究证明了PPPs的钙结合能力以及同磷酸钙盐沉淀竞争性结合以增加钙离子溶解度的关键因素是PPPs中磷酸基团的含量,且最佳含量为35%左右[97]。
磷酸肽与钙结合性质之间的关系已经得到了广泛的研究。Jiang等[98]的研究表明磷酸肽的分子大小对钙离子的结合至关重要。小于1 kDa的磷酸肽片段和没有磷酸化的丝氨酸与钙离子的结合能力很弱,而1~3 kDa的磷酸肽片段甚至比CPPs具有更强的钙结合能力。Luo等[99]的研究也得到了类似的结果,具有较强钙结合能力的P-CP(磷酸化鱼骨胶原肽)其分子量大小主要分布在0.5~3 kDa范围内。Zong等[100]以0~3个连续或不连续的磷酸化丝氨酸为样品,合成6种不同的磷酸肽,实验结果表明含有3个磷酸化丝氨酸残基的磷酸肽的钙结合能力最强,且连续的磷酸化丝氨酸残基比不连续的磷酸化丝氨酸残基具备更强的钙结合能力。
4.2.2 羧基和氨基
根据钙与肽的结合常数,一些非磷酸化的肽可能比CPPs具有更强的钙结合能力,表明相对于活性肽中的磷酸基团而言,氨基酸序列对钙结合力的影响可能占据了主导地位[79−80]。迄今为止,已经发现众多不含磷酸基团的、具有较强钙结合能力的生物活性肽。Bao等[101]研究了大豆蛋白水解物中钙结合能力与羧基含量之间的关系。结果表明,结合钙的含量随着羧基含量的增加呈线性增加,且最可能的结合位点位于Asp和Glu的羧基。Zhang等[102]从太平洋鳕鱼骨凝胶水解分离出一种新的钙结合KGDPGLSPGK,通过圆二色谱、傅立叶红外光谱(FTIR)等技术对肽和钙的结合位点进行了表征,发现Asp羧基的氧原子和Lys侧链氨基的氮原子参与了螯合作用。Liu等[103]从小麦胚芽蛋白中分离出多肽FVDVT,与钙结合后,肽链中N-H键的伸缩振动向高波数方向移动,表明由于诱导效应或偶极场效应,N-H键附近的电子云密度增加;FTIR中C=O和COO—的伸缩振动变化表明FVDVT中的酰胺键和羧基氧参与了和钙的结合。Cui等[104]对NDEELNK肽进行了研究,发现当其结合钙离子后酰胺Ⅰ带(C=O的伸缩振动)从1668.12 cm−1转移到了1667.90 cm−1,酰胺Ⅱ带(N-H弯曲和C-N伸缩振动)从1544.31 cm−1转移到了1557.67 cm−1,表明多肽NDEELNK中的羧基氧和氨基氮和钙离子之间发生了结合反应。对乳清蛋白水解产物二肽GY的核磁共振研究结果显示,GY与钙结合后两个氨基酸的α-碳处的质子信号发生了0.025 ppm的化学位移,表明GY中的两个氨基酸残基都与钙离子产生了相互作用,且作用位点为N端氨基和C端羧基[105]。
4.3 多肽和钙的结合方式
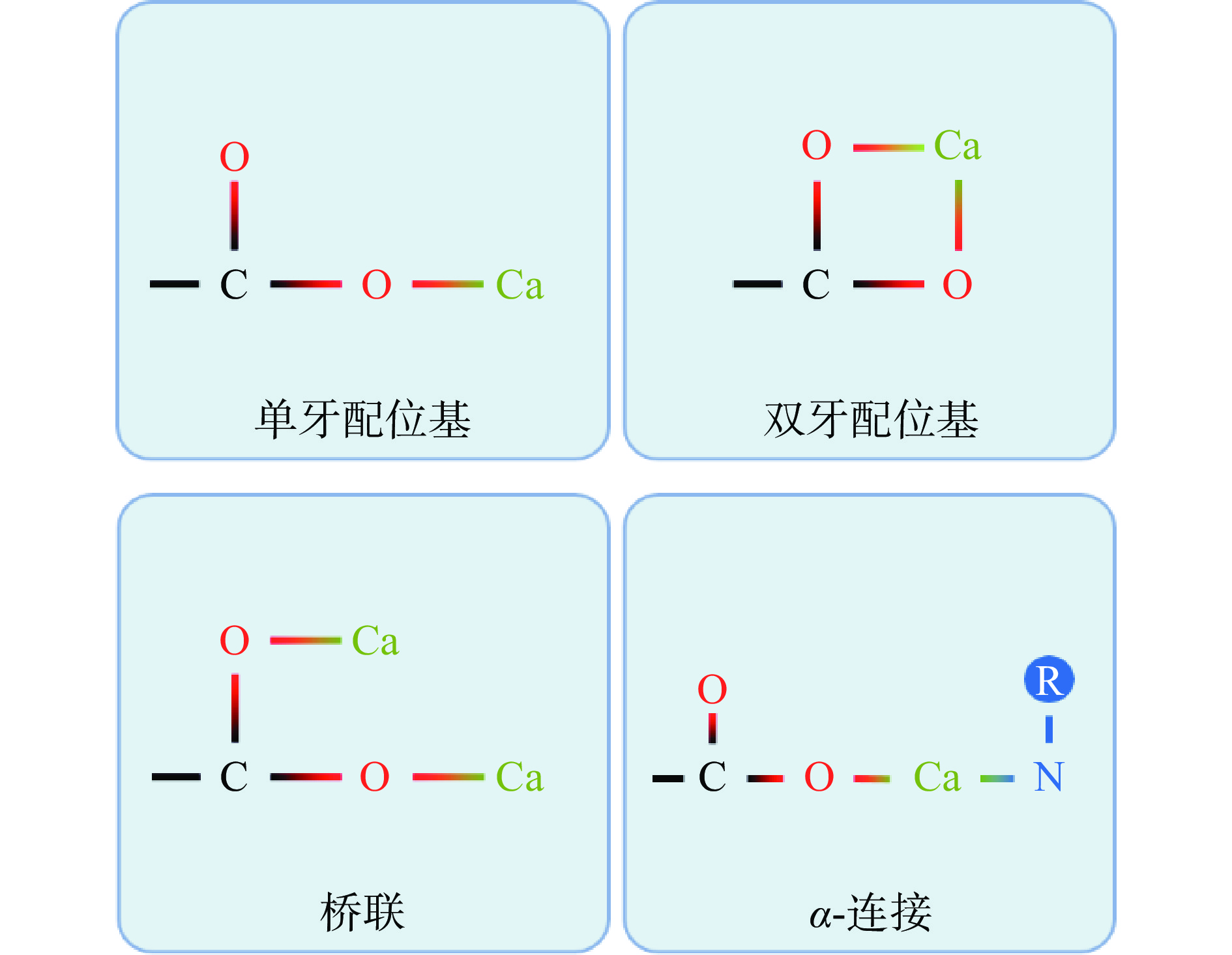
钙离子与多肽上的羧基基团(-COO−-)连接的主要方式有单牙配位基、双牙配位基、桥联和α-连接(图1)。单牙配位基指钙离子与羧基基团的一个氧原子发生相互作用,双牙配位基指钙离子与羧基基团上的两个氧原子发生相互作用。如果羧基基团上的两个氧原子分别与两个钙离子发生相互作用,则为桥联连接。如果钙离子与羧基基团中的一个氧原子和另一个配体中的其他原子(O、N、S等)发生结合,则称为α-连接[106]。来自太平洋鳕鱼骨的十肽与钙离子有三种结合模式,分别为单牙配位基、双牙配位基和α-连接[102]。
5. 多肽螯合钙的吸收途径
小肠是钙离子吸收的主要部位,正常生理条件下通过小肠吸收的钙约占钙吸收总量的90%。多肽螯合钙在肠道中的吸收机制尚不完全明确。一种可能的情况是钙肽复合物在肠道中释放钙离子,另一种情况是钙肽复合物以整体的形式通过肠道中肽的吸收方式被吸收[81]。
5.1 钙离子的吸收途径
跨细胞途径(Transcellular pathway)和细胞旁路途径(Paracellular pathway)是小肠钙离子吸收的两种主要细胞机制(图2)。过去的研究认为两种吸收途径是两个相互独立的体系,最近的研究表明,跨细胞途径和细胞旁路途径之间可能存在协同作用来维持机体内的钙稳态。跨细胞途径主要负责钙摄入量较低或对钙的需求量较高(如妊娠期和哺乳期)时钙的吸收,当小肠肠腔内的钙离子浓度远高于血浆钙离子浓度时,将通过细胞旁路途经来吸收钙离子[107]。
5.1.1 跨细胞途径
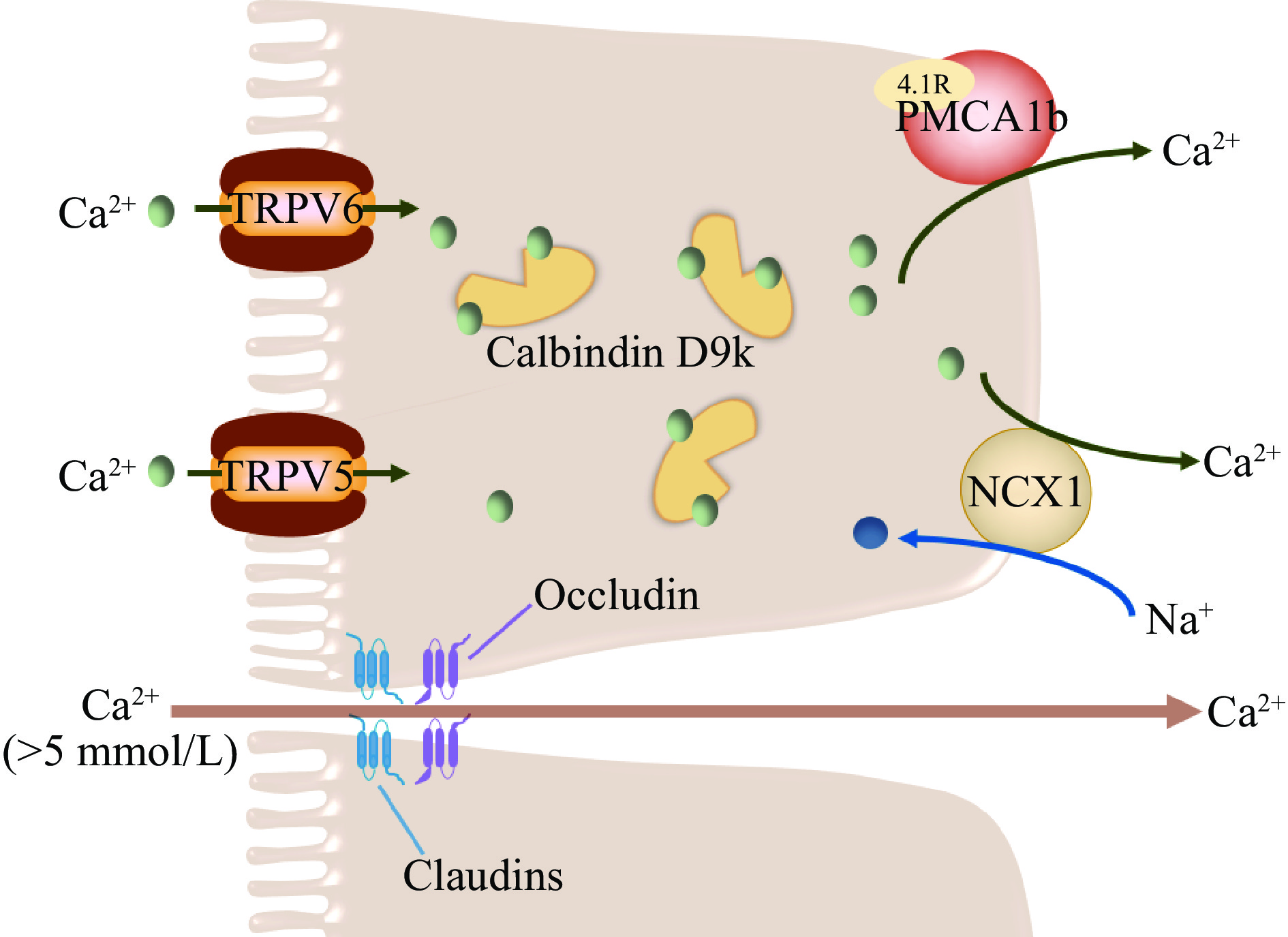
跨细胞吸收途径是一种主动的、转运量饱和的、顺浓度梯度的吸收方式,其主要发生在十二指肠和空肠,受维生素D调控,且钙离子需要跨越粘膜-浆膜两侧细胞质膜肠道屏障[108]。跨上皮细胞途径主要涉及三个主要钙离子运输步骤[109]:a. 钙离子穿过小肠上皮细胞顶端刷状缘进入细胞;b. 从刷状缘转运至细胞基底膜;c. 穿过细胞基底膜排出细胞。
5.1.1.1 从顶端刷状缘进入细胞
钙离子穿过刷状缘进入细胞的过程主要受位于小肠上皮顶端的钙离子通道蛋白TRPV6调控,TRPV6缺陷小鼠会产生钙离子吸收障碍,导致体重下降、骨密度降低等一系列问题的发生[110]。TRPV6为瞬时受体电位TRP(transient receptor potential)的超家族成员,属于香草酸型瞬时受体电位亚家族(TRPV)。上皮钙离子通道结构上包含3个结构域:N-端由327个亲水性氨基酸组成,有3个锚定蛋白结合位点和数个潜在的蛋白激酶C磷酸化位点;中间为6次跨膜域,N-端含有2个潜在的糖基化位点和一段额外的疏水性肽链,可以形成离子孔道;C-端由151个亲水性氨基酸组成,含有潜在的蛋白激酶A和蛋白激酶C磷酸化位点[111−112]。
5.1.1.2 从刷状缘转运至细胞基底膜
细胞内钙离子浓度受到十分精细的调控,其浓度必须保持在10−7 mol/L以下,才能维持细胞内正常的信号转导,同时防止细胞凋亡。一旦大量的钙离子进入小肠上皮细胞,细胞需要有缓冲多余钙的机制,并能够将这些钙离子从粘膜侧转运至浆膜侧[113]。细胞内钙离子的转运主要由钙结合蛋白Calbindin D9k(CB-D9k)介导,其含有两个EF-hand钙离子结构域,因此每摩尔CB-D9k可以结合2摩尔钙离子,对钙离子有着极高的亲和力[114]。CB-D9k主要在十二指肠绒毛尖端处聚集表达[115]。
5.1.1.3 穿过细胞基底膜排出细胞
钙离子穿过细胞基底膜排出细胞是一个能量依赖的过程。P型初级活性转运体ATP2B1(即PMCA1b)和二级活性转运体SLC8A1(即NCX1)主要负责将细胞质中的钙从小肠细胞基底膜转运至细胞外[116]。PMCA1b是细胞基底膜主要的钙离子转运体,负责80%的胞浆钙离子转运[108,117]。PMCA1b可以被胞浆钙离子、钙调素和CB-D9k有效激活,被钒酸盐抑制[118−119]。最近的研究表明4.1R蛋白可能是PMCA1b活性作用的关键蛋白。Liu等[120]的研究发现,4.1R蛋白敲除小鼠的PMCA1b表达下调,小肠钙吸收受损,导致小鼠出现低钙血症和骨质减少。NCX1转运体负责大约20%的胞浆钙离子转运。PMCA1b和NCX1并不是相互独立的两个基底膜钙离子转运体,二者之间存在协同作用,当用KB-R7943等小分子对NCX1的表达进行抑制,发现PMCA1b的活性也受到了抑制,反之亦然[121]。
5.1.2 细胞旁路途径
与跨细胞途径不同,细胞旁路途经是一种不饱和的、被动的扩散吸收途径,主要发生在小肠上皮细胞的紧密连接和细胞间隙中,受小肠管腔内的电化学梯度和紧密连接的完整性驱动[122]。大鼠十二指肠细胞和Caco-2单层细胞体外实验显示,当小肠肠腔内的钙离子浓度超过5 mmol/L时,细胞旁路途径的钙通量明显增加[123−124]。Fujita等[125]认为,细胞旁路途经是一种可调节的、跨紧密连接的转运方式,在生理条件下可以与跨细胞途径发生协调互补作用。紧密连接是位于肠上皮细胞顶端和基底膜之间的特殊膜域,通过其对电荷和分子大小的选择性,对离子、蛋白质和其他大分子在肠道内的运动形成屏障。Claudins是一个由24个成员组成的蛋白家族,分子量从20~27 kDa不等,已经被鉴定为紧密连接中形成跨膜屏障和孔道的主要跨膜组分[126]。最近的一项研究表明,Claudin-2蛋白敲除动物的细胞旁路途径钙吸收减少,证明了Claudin-2蛋白在细胞旁路吸收途径中的重要性[127]。含有带电氨基酸的细胞外环可以插入胞外空间,形成具有不同选择性的离子通道孔径[128]。Claudin-2和Claudin-12蛋白在其细胞外环处含有带负电荷的氨基酸,可以吸引钙离子[129−130]。
5.2 钙离子吸收的节段异质性
钙的吸收发生在整个胃肠道,但其在每个节段的吸收和转运效率存在异质性(表2)。当钙摄入量充足或较高时,钙的吸收效率取决于膳食钙在胃肠道中所处的节段位置、在不同节段位置的停留时间以及膳食钙的溶解度[131]。
表 2 钙离子吸收的节段异质性Table 2. Segmental heterogeneity of calcium absorption5.2.1 胃
胃中通常不存在钙的吸收,但是胃可以为膳食钙的溶解提供酸性环境[132]。胃壁细胞产生的胃酸增加了膳食钙的溶解度,并允许钙离子与其他食物成分形成复合物。参与表达胃酸产生的基因(Cckbr、Tcirg1、Snx10等)如果被破坏,会对正常的胃酸分泌产生影响,同时会导致骨密度降低、低钙血症等[133−134]。临床数据显示胃切除的术后病人也会产生相同的症状[38,135]。这些发现表明正常的胃酸分泌对维持人体的钙稳态起到了不可替代的作用。
5.2.2 十二指肠
食物经胃消化后产生的酸性食糜会进入十二指肠,并由十二指肠腺分泌的HCO3−和碱性粘液中和。由于此时食糜中的游离钙含量较高,十二指肠中pH的即时变化并不会对游离钙的浓度产生十分大的影响,且与小肠中其他远端肠段相比,十二指肠中钙的溶解度最高[138−139]。然而由于食物在十二指肠中仅停留2~3 min,十二指肠中的钙吸收量仅占整个肠道钙吸收总量的8%[140]。
十二指肠中既存在钙离子的跨细胞吸收途径,也存在细胞旁路吸收途径。当机体钙摄入不足时,1,25(OH)2D3上调钙选择性转运受体TRPV6和钙结合蛋白Calbindin D9k,促进钙离子的跨细胞途径吸收[141−142],但因为跨细胞吸收途径可以饱和,因此该途径下钙的吸收随着钙摄入量的增加而减少[143]。当肠腔内的游离钙浓度大于5 mmol/L时,十二指肠中钙离子的吸收主要为细胞旁路途径[123]。
5.2.3 空肠
进入空肠的钙离子在接近空肠远端的过程中逐渐暴露于较高的pH(6.6~8),从而导致空肠肠腔内的不溶性钙增加[138]。高钙饮食的大鼠其膳食钙在整个空肠停留的时间为40~45 min,约为十二指肠停留时间的20倍,因此空肠中膳食钙的吸收量要高于十二指肠(17% vs 7%)[144]。与十二指肠相同,空肠中同时存在钙离子的跨细胞吸收途径和细胞旁路吸收途径,其中细胞旁路途径负责了空肠中80%的钙吸收,跨细胞吸收途径(15%)主要发生在空肠近端[144]。最近的研究显示,与新生小鼠相比,成年小鼠空肠中TRPV6的表达要低得多,这是因为当新生小鼠的十二指肠跨细胞吸收途径没有发育完全时,由空肠的跨细胞吸收途径来维持新生小鼠体内的钙稳态[145−146]。
5.2.4 回肠
回肠是绝大部分钙离子被吸收的地方,虽然回肠中pH大于8,导致钙的溶解度和吸收率降低,但钙的吸收总量并没有减少,这是因为食物在回肠中的停留时间最长[138]。高钙饮食饲喂的成年大鼠中,钙在其十二指肠、空肠和回肠中的停留时间分别为3、43和141 min[66]。当回肠腔内少量的未沉淀的游离钙被吸收后,不溶性的钙沉淀会进一步解离为游离钙,从而进一步被吸收[17]。因此尽管回肠中钙的转运速度要比十二指肠和空肠中慢得多,但是回肠当中膳食钙的长时间停留保证了缓慢而稳定的细胞旁路吸收途径,使得回肠成为了大量钙吸收(65%~88%)的肠道节段[147]。
回肠中钙离子的吸收主要为细胞旁路途经,现有的研究表明回肠中跨细胞钙吸收十分有限[143]。与其他节段相比,回肠中参与细胞旁路运输的紧密连接蛋白Claudin-2和Claudin-12的表达量要高出很多[129],而跨细胞途径中的TRPV6和Calbindin D9k的表达量几乎缺失,PMCA1b的表达量极低[148]。
5.3 肽的吸收途径
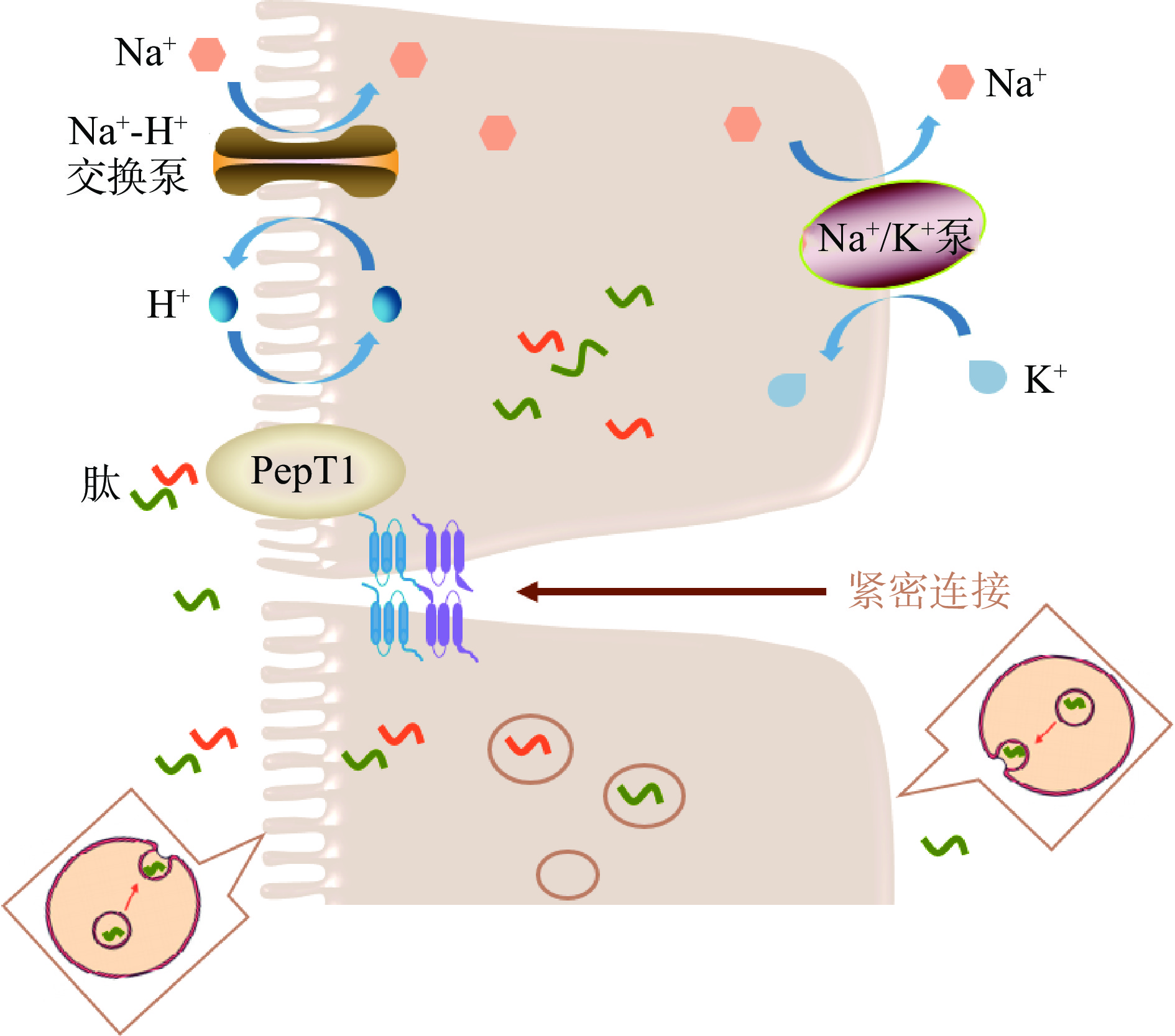
多肽螯合钙中活性肽的序列大小一般为2~20个氨基酸残基[149],其需要抵抗胃酸的消化和胃蛋白酶的活性,并最终进入肠道被吸收[150]。因此,了解肠道中肽的细胞转运途径有助于了解多肽螯合钙的促钙吸收机制(图3)。肠道中肽的吸收途径主要有三种[113]:a. PepT1介导的二肽和三肽的转运吸收;b. 细胞内囊泡介导的寡肽的胞吞作用;c. 寡肽和二肽、三肽的细胞旁路吸收。
5.3.1 PepT1介导的二肽和三肽的转运吸收
PepT1是一种广泛存在的特异性肽转运蛋白,具有由710个氨基酸残基组成的12个跨膜结构域,其中肽结合腔大小约为13×12×11Å,因此其只能结合二肽和三肽,对于四肽及以上的多肽的结合存在空间限制[151]。PepT1介导的二肽和三肽的转运受小肠上皮细胞顶端刷状缘膜上的质子梯度驱动。PepT1蛋白从肠腔内摄取二肽/三肽和H+后,细胞内的H+通过刷状缘膜上的Na+-H+交换泵离开细胞,Na+进入细胞。随后Na+通过基底膜外侧上的Na+-K+泵排出。三个Na+被运输出细胞的同时两个有K+进入细胞,以恢复细胞的电化学梯度[152]。有研究表明芳香族或碱性氨基酸残基可能在PepT1识别中起关键作用[153]。
5.3.2 细胞内囊泡介导的寡肽的胞吞作用
一种细胞内囊泡介导的运输系统,即胞吞作用,也存在于小肠上皮细胞中。一些寡肽,特别是含有高比例疏水性或碱性氨基酸残基的寡肽,可能与小肠上皮细胞膜表面发生相互作用,并通过胞吞作用进行运输,但是这一吸收途径在小肠肽吸收当中所占的比例很小,因为在胞吞作用后肽可能会被细胞质内的肽酶水解[149]。
5.3.3 寡肽和二肽、三肽的细胞旁路吸收
小肠上皮细胞的闭合蛋白Occludin、紧密连接蛋白Claudins和其他辅助蛋白相互结合,构成了小肠中的紧密连接[149]。紧密连接中存在一定大小的孔径,可以允许二肽、三肽以及寡肽(3~5个氨基酸残基)的运输[154]。
6. 结论与展望
本文综述了食物基质成分对钙的生物利用度的影响,阐述了部分钙盐以及钙肽螯合物的促钙吸收作用,并对多肽螯合钙中钙肽结合的构效关系、多肽螯合物的吸收机制进行了总结。食物基质中的很多成分均对钙的生物利用度有影响,其中植酸、草酸和磷酸盐等物质可以和钙形成沉淀,从而抑制钙的吸收。钙离子对人体的重要性已经在很多研究中得到了证实,且人体内钙稳态的失衡可以诱发一系列的疾病。钙补充剂可以以不同形式的钙盐存在,但葡萄糖酸钙、柠檬酸钙等有机钙盐对钙生物利用度的影响尚未得到比较明确的阐释。除部分有机钙盐外,多肽螯合钙有着十分显著的促钙吸收潜能,已经有越来越多的研究对钙肽结合的机理与构效关系、以及多肽鳌合钙中钙离子的吸收机制进行了研究。因此,在现有研究的基础上,未来对钙离子吸收的研究可以聚焦在以下几个方面:a. 不同钙盐中的钙在肠道中的“存在形式”以及肠道中过饱和钙盐的形成对钙离子生物利用度的影响;b. 多肽螯合钙中钙的吸收形式,钙离子是否可以在肠道中从复合物释放,亦或是以钙肽复合物整体的形式被吸收。c. 由于部分有机钙盐、氨基酸和多肽均具有促钙吸收作用,因此可以探究钙盐-氨基酸或钙盐-多肽复合物是否具有促钙吸收潜质。
-
表 1 影响钙生物利用度的外源性因素
Table 1 Exogenous factors affecting calcium bioavailability
表 2 钙离子吸收的节段异质性
Table 2 Segmental heterogeneity of calcium absorption
-
[1] ZHANG H, ZHAO L, SHEN Q, et al. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability[J]. LWT,2021,144:111264. doi: 10.1016/j.lwt.2021.111264
[2] VANNUCCI L, FOSSI C, QUATTRINI S, et al. Calcium intake in bone health:A focus on calcium-rich mineral waters[J]. Nutrients,2018,10(12):1930. doi: 10.3390/nu10121930
[3] LIAO W, CHEN H, JIN W, et al. Three newly isolated calcium-chelating peptides from tilapia bone collagen hydrolysate enhance calcium absorption activity in intestinal Caco-2 cells[J]. Journal of Agricultural and Food Chemistry,2020,68(7):2091−2098. doi: 10.1021/acs.jafc.9b07602
[4] MINE Y, SHAHIDI F. Nutraceutical proteins and peptides in health and disease[M]. Boca Raton:CRC Press, 2005.
[5] ZHANG M, LIU K. Calcium supplements and structure-activity relationship of peptide-calcium chelates:A review[J]. Food Science and Biotechnology,2022,31(9):1111−1122. doi: 10.1007/s10068-022-01128-6
[6] CHANG G R L, TU M Y, CHEN Y H, et al. KFP-1, a novel calcium-binding peptide isolated from kefir, promotes calcium influx through TRPV6 channels[J]. Molecular Nutrition & Food Research,2021,65(22):e2100182.
[7] MILLER G D, JARVIS J K, MCBEAN L D. The importance of meeting calcium needs with foods[J]. Journal of the American College of Nutrition, 2001, 20(2 Suppl):168S-185S.
[8] CHAROENPHUN N, CHEIRSILP B, SIRINUPONG N, et al. Calcium-binding peptides derived from tilapia ( Oreochromis niloticus) protein hydrolysate[J]. European Food Research and Technology,2013,236(1):57−63. doi: 10.1007/s00217-012-1860-2
[9] SHI J, LE MAGUER M. Lycopene in tomatoes:Chemical and physical properties affected by food processing[J]. Critical Reviews in Biotechnology,2000,20(4):293−334. doi: 10.1080/07388550091144212
[10] STRAUB D A. Calcium supplementation in clinical practice:A review of forms, doses, and indications[J]. Nutrition in Clinical Practice:Official Publication of the American Society for Parenteral and Enteral Nutrition,2007,22(3):286−296. doi: 10.1177/0115426507022003286
[11] VAVRUSOVA M, SKIBSTED L H. Calcium nutrition. Bioavailability and fortification[J]. LWT-Food Science and Technology, 2014, 59(2, Part 2):1198-1204.
[12] VOGEL H J. Calcium-binding protein protocols[M]. Humana Press, 2002.
[13] RÉRAT A, NUNES C S, MENDY F, et al. Amino acid absorption and production of pancreatic hormones in non-anaesthetized pigs after duodenal infusions of a milk enzymic hydrolysate or of free amino acids[J]. British Journal of Nutrition,1988,60:121−136. doi: 10.1079/BJN19880082
[14] MCDONAGH D, FITZGERALD R J. Production of caseinophosphopeptides (CPPs) from sodium caseinate using a range of commercial protease preparations[J]. International Dairy Journal,1998,8(1):39−45. doi: 10.1016/S0958-6946(98)00019-3
[15] PEREGO S, DEL FAVERO E, DE LUCA P, et al. Calcium bioaccessibility and uptake by human intestinal like cells following in vitro digestion of casein phosphopeptide-calcium aggregates[J]. Food and Function,2015,6(6):1796−1807. doi: 10.1039/C4FO00672K
[16] HEANEY R P. Calcium intake and disease prevention[J]. Arquivos Brasileiros de Endocrinologia e Metabologia,2006,50(4):685−693. doi: 10.1590/S0004-27302006000400014
[17] SHKEMBI B, HUPPERTZ T. Calcium absorption from food products:food matrix effects[J]. Nutrients,2021,14(1):180. doi: 10.3390/nu14010180
[18] ILICH J Z, KERSTETTER J E. Nutrition in bone health revisited:A story beyond calcium[J]. Journal of the American College of Nutrition,2000,19:715−737. doi: 10.1080/07315724.2000.10718070
[19] RUTTER G A, FASOLATO C, RIZZUTO R. Calcium and organelles:A two-sided story[J]. Biochemical and Biophysical Research Communications,1998,253(3):549−557.
[20] WILLIAMS G R, DOWNS J W, WOLF C E, et al. Evaluation of strontium interference in calcium measurement procedures and content in supplements as measured by ICP-MS[J]. The Journal of Applied Laboratory Medicine,2023,8(2):307−318. doi: 10.1093/jalm/jfac092
[21] WANG L, MANSON J E, SESSO H D. Calcium intake and risk of cardiovascular disease[J]. American Journal of Cardiovascular Drugs,2012,12(2):105−116. doi: 10.2165/11595400-000000000-00000
[22] PARK Y W. Overview of bioactive components in milk and dairy products[M]. John Wiley & Sons, Inc, 2009.
[23] ZHU K, PRINCE R L. Calcium and bone[J]. Clinical Biochemistry,2012,45(12):936−942. doi: 10.1016/j.clinbiochem.2012.05.006
[24] RIZZOLI R. Dairy products, yogurts, and bone health[J]. The American Journal of Clinical Nutrition, 2014, 99(5 Suppl):1256S−1262S.
[25] TORRES M R S G, FRANCISCHETTI E A, GENELHU V, et al. Effect of a high-calcium energy-reduced diet on abdominal obesity and cardiometabolic risk factors in obese Brazilian subjects[J]. International Journal of Clinical Practice,2010,64(8):1076−1083. doi: 10.1111/j.1742-1241.2009.02312.x
[26] TORRES M R S G, SANJULIANI A F. Does calcium intake affect cardiovascular risk factors and/or events?[J]. Clinics (Sao Paulo, Brazil),2012,67(7):839−844. doi: 10.6061/clinics/2012(07)22
[27] VINAROVA L, VINAROV Z, TCHOLAKOVA S, et al. The mechanism of lowering cholesterol absorption by calcium studied by using an in vitro digestion model.[J]. Food & Function,2016,7(1):151−163.
[28] FERNÁNDEZ-JALAO I, SÁNCHEZ-MORENO C, ANCOS B D. Influence of food matrix and high-pressure processing on onion flavonols and antioxidant activity during gastrointestinal digestion[J]. Journal of Food Engineering,2017,213:60−68.
[29] CARBONELL-CAPELLA J M, BUNIOWSKA M, BARBA F J, et al. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables:A review[J]. Comprehensive Reviews in Food Science and Food Safety,2014,13(2):155−171. doi: 10.1111/1541-4337.12049
[30] CIAN R E, GARZÓN A G, ANCONA D B, et al. Chelating properties of peptides from red seaweed pyropia columbina and its effect on iron bio-accessibility[J]. Plant Foods for Human Nutrition (Dordrecht, Netherlands),2016,71(1):96−101. doi: 10.1007/s11130-016-0533-x
[31] WANG X, ZHOU J, TONG P S, et al. Zinc-binding capacity of yak casein hydrolysate and the zinc-releasing characteristics of casein hydrolysate-zinc complexes[J]. Journal of Dairy Science,2011,94(6):2731−2740. doi: 10.3168/jds.2010-3900
[32] BUDSEEKOAD S, YUPANQUI C T, SIRINUPONG N, et al. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate[J]. Journal of Functional Foods,2018,49:333−341. doi: 10.1016/j.jff.2018.07.041
[33] NIE X, JIN H, WEN G, et al. Estrogen regulates duodenal calcium absorption through differential role of estrogen receptor on calcium transport proteins[J]. Digestive Diseases and Sciences,2020,65(12):3502−3513. doi: 10.1007/s10620-020-06076-x
[34] WEBER D R, O’BRIEN K O, SCHWARTZ G J. Evidence of disordered calcium metabolism in adolescent girls with type 1 diabetes:An observational study using a dual-stable calcium isotope technique[J]. Bone,2017,105:184−190. doi: 10.1016/j.bone.2017.09.001
[35] WONGDEE K, RODRAT M, TEERAPORNPUNTAKIT J, et al. Factors inhibiting intestinal calcium absorption:Hormones and luminal factors that prevent excessive calcium uptake[J]. The Journal of Physiological Sciences:JPS,2019,69(5):683−696.
[36] D’AMELIO P, SASSI F. Gut microbiota, immune system, and bone[J]. Calcified Tissue International,2018,102(4):415−425. doi: 10.1007/s00223-017-0331-y
[37] MOUSA A, NAQASH A, LIM S. Macronutrient and micronutrient intake during pregnancy:An overview of recent evidence[J]. Nutrients,2019,11(2):443. doi: 10.3390/nu11020443
[38] KATRIEN C, LIEVE V, MATTHIAS L, et al. Thin bones:Vitamin D and calcium handling after bariatric surgery[J]. Bone Reports,2018,8:57−63. doi: 10.1016/j.bonr.2018.02.002
[39] CHRISTAKOS S, VELDURTHY V, PATEL N, et al. Intestinal regulation of calcium:Vitamin D and bone physiology[J]. Advances in Experimental Medicine and Biology,2017,1033:3−12.
[40] SIRICHAKWAL P P, KAMCHANSUPPASIN A, AKOH C C, et al. Vitamin D status is positively associated with calcium absorption among postmenopausal thai women with low calcium intakes[J]. The Journal of Nutrition,2015,145(5):990−995. doi: 10.3945/jn.114.207290
[41] MAURYA V K, AGGARWAL M. Factors influencing the absorption of vitamin D in GIT:An overview[J]. Journal of Food Science and Technology,2017,54(12):3753−3765. doi: 10.1007/s13197-017-2840-0
[42] BANDALI E, WANG Y, LAN Y, et al. The influence of dietary fat and intestinal pH on calcium bioaccessibility:An in vitro study[J]. Food & Function,2018,9(3):1809−1815.
[43] WANG Y, DELLATORE P, DOUARD V, et al. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice[J]. Nutrition Research,2016,36(7):742−750. doi: 10.1016/j.nutres.2016.03.002
[44] SZILAGYI A, ISHAYEK N. Lactose intolerance, dairy avoidance, and treatment options[J]. Nutrients,2018,10(12):1994. doi: 10.3390/nu10121994
[45] HODGES J K, CAO S, CLADIS D P, et al. Lactose intolerance and bone health:The challenge of ensuring adequate calcium intake[J]. Nutrients,2019,11(4):E718. doi: 10.3390/nu11040718
[46] GRIESSEN M, COCHET B, INFANTE F, et al. Calcium absorption from milk in lactase-deficient subjects[J]. The American Journal of Clinical Nutrition,1989,49(2):377−384. doi: 10.1093/ajcn/49.2.377
[47] TREMAINE W J, NEWCOMER A D, LAWRENCE RIGGS B, et al. Calcium absorption from milk in lactase-deficient and lactase-sufficient adults[J]. Digestive Diseases and Sciences,1986,31(4):376−378. doi: 10.1007/BF01311672
[48] COCHET B, JUNG A, GRIESSEN M, et al. Effects of lactose on intestinal calcium absorption in normal and lactase-deficient subjects[J]. Gastroenterology,1983,84(5Pt1):935−940.
[49] COUDRAY C, BELLANGER J, CASTIGLIA-DELAVAUD C, et al. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men[J]. European Journal of Clinical Nutrition,1997,51(6):375−380. doi: 10.1038/sj.ejcn.1600417
[50] ALBARRACÍN M, WEISSTAUB A R, ZULETA A, et al. Extruded whole grain diets based on brown, soaked and germinated rice. Effects on the lipid profile and antioxidant status of growing Wistar rats. Part II[J]. Food & Function,2016,7(6):2729−2735.
[51] DAI Z, ZHANG Y, LU N, et al. Association between dietary fiber intake and bone loss in the framingham offspring study[J]. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research,2018,33(2):241−249. doi: 10.1002/jbmr.3308
[52] KIEFER-HECKER B, KIENZLE E, DOBENECKER B. Effects of low phosphorus supply on the availability of calcium and phosphorus, and musculoskeletal development of growing dogs of two different breeds[J]. Journal of Animal Physiology and Animal Nutrition,2018,102(3):789−798. doi: 10.1111/jpn.12868
[53] ZHAO L, LI M, SUN H. Effects of dietary calcium to available phosphorus ratios on bone metabolism and osteoclast activity of the OPG /RANK/RANKL signalling pathway in piglets[J]. Journal of Animal Physiology and Animal Nutrition,2019,103(4):1224−1232. doi: 10.1111/jpn.13115
[54] SILVA E O, BRACARENSE A P F R L. Phytic acid:From antinutritional to multiple protection factor of organic systems[J]. Journal of Food Science,2016,81(6):R1357−1362.
[55] LESJAK M, K S SRAI S. Role of dietary flavonoids in iron homeostasis[J]. Pharmaceuticals,2019,12(3):119. doi: 10.3390/ph12030119
[56] LO D, WANG H I, WU J, et al. Anti-nutrient components and their concentrations in edible parts in vegetable families[J]. CABI Reviews,2018,2018:1−30.
[57] NAQVI M A, IRANI K A, KATANISHOOSHTARI M, et al. Disorder in milk proteins:Formation, structure, function, isolation and applications of casein phosphopeptides[J]. Current Protein & Peptide Science,2016,17(4):368−379.
[58] SUN S, LIU F, LIU G, et al. Effects of casein phosphopeptides on calcium absorption and metabolism bioactivity in vitro and in vivo[J]. Food & Function,2018,9(10):5220−5229.
[59] LUO M, XIAO J, SUN S, et al. Deciphering calcium-binding behaviors of casein phosphopeptides by experimental approaches and molecular simulation[J]. Food & Function,2020,11(6):5284−5292.
[60] ZHU B, HOU T, HE H. Calcium-binding casein phosphopeptides-loaded chitosan oligosaccharides core-shell microparticles for controlled calcium delivery:Fabrication, characterization, and in vivo release studies[J]. International Journal of Biological Macromolecules,2020,154:1347−1355. doi: 10.1016/j.ijbiomac.2019.11.014
[61] REID I R, BRISTOW S M, BOLLAND M J. Calcium supplements:Benefits and risks[J]. Journal of Internal Medicine,2015,278(4):354−368.
[62] GENANT H K, COOPER C, POOR G, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis[J]. Osteoporosis International,1999,10(4):259−264. doi: 10.1007/s001980050224
[63] EFSA Panel on Food Additives and Nutrient Sources Added to Food (EFSA ANS Panel), MAGED Y, PETER A, et al. Evaluation of di-calcium malate, used as a novel food ingredient and as a source of calcium in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes[J]. EFSA Journal,2018,16(6):e05291.
[64] BAILEY R L, DODD K W, GOLDMAN J A, et al. Estimation of total usual calcium and vitamin D intakes in the United States[J]. The Journal of Nutrition,2010,140(4):817−822. doi: 10.3945/jn.109.118539
[65] KELLER J L, LANOU A, BARNARD N D. The consumer cost of calcium from food and supplements[J]. Journal of the American Dietetic Association,2002,102(11):1669−1671. doi: 10.1016/S0002-8223(02)90355-X
[66] BRONNER F, PANSU D. Nutritional aspects of calcium absorption[J]. The Journal of Nutrition,1999,129(1):9−12.
[67] VAVRUSOVA M, RAITIO R, ORLIEN V, et al. Calcium hydroxy palmitate:Possible precursor phase in calcium precipitation by palmitate[J]. Food Chemistry,2013,138(4):2415−2420. doi: 10.1016/j.foodchem.2012.12.012
[68] PAK C Y, HARVEY J A, HSU M C. Enhanced calcium bioavailability from a solubilized form of calcium citrate[J]. The Journal of Clinical Endocrinology and Metabolism,1987,65(4):801−805. doi: 10.1210/jcem-65-4-801
[69] VAVRUSOVA M, SKIBSTED L H. Spontaneous supersaturation of calcium D-gluconate during isothermal dissolution of calcium L-lactate in aqueous sodium D-gluconate[J]. Food & Function,2014,5(1):85−91.
[70] VAVRUSOVA M, LIANG R, SKIBSTED L H. Thermodynamics of dissolution of calcium hydroxycarboxylates in water[J]. Journal of Agricultural and Food Chemistry,2014,62(24):5675−5681. doi: 10.1021/jf501453c
[71] BRONNER F. Current concepts of calcium absorption:An overview[J]. The Journal of Nutrition,1992,122(Suppl_3):641−643.
[72] GOSS S L, LEMONS K A, KERSTETTER J E, et al. Determination of calcium salt solubility with changes in pH and P(CO(2)), simulating varying gastrointestinal environments[J]. The Journal of Pharmacy and Pharmacology,2007,59(11):1485−1492.
[73] TANG N, SKIBSTED L H. Calcium binding to amino acids and small glycine peptides in aqueous solution:Toward peptide design for better calcium bioavailability[J]. Journal of Agricultural and Food Chemistry,2016,64(21):4376−4389. doi: 10.1021/acs.jafc.6b01534
[74] LUMB R F, MARTELL A E. Metal chelating tendencies of glutamic and aspartic acids[J]. Journal of Physical Chemistry, 1953.
[75] VAVRUSOVA M, SKIBSTED L H. Calcium binding to dipeptides of aspartate and glutamate in comparison with orthophosphoserine[J]. Journal of Agricultural and Food Chemistry,2013,61(22):5380−5384. doi: 10.1021/jf400741e
[76] WU W, LI B, HOU H, et al. Isolation and identification of calcium-chelating peptides from Pacific cod skin gelatin and their binding properties with calcium[J]. Food & Function,2017,8(12):4441−4448.
[77] HOU H, WANG S, ZHU X, et al. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex[J]. Food Chemistry,2018,243:389−395. doi: 10.1016/j.foodchem.2017.09.152
[78] HE J, GUO H, ZHANG M, et al. Purification and characterization of a novel calcium-binding heptapeptide from the hydrolysate of tilapia bone with its osteogenic activity[J]. Foods (Basel, Switzerland),2022,11(3):468.
[79] LÜ Y, LIU H, REN J, et al. The positive effect of soybean protein hydrolysates-calcium complexes on bone mass of rapidly growing rats[J]. Food & Function,2013,4(8):1245−1251.
[80] BAO Z, ZHANG P, SUN N, et al. Elucidating the calcium-binding site, absorption activities, and thermal stability of egg white peptide-calcium chelate[J]. Foods,2021,10(11):2565. doi: 10.3390/foods10112565
[81] CAETANO-SILVA M E, NETTO F M, BERTOLDO-PACHECO M T, et al. Peptide-metal complexes:Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals[J]. Critical Reviews in Food Science and Nutrition,2021,61(9):1470−1489. doi: 10.1080/10408398.2020.1761770
[82] PORATH J. Amino acid side chain interaction with chelate-liganded crosslinked dextran, agarose and TSK gel. A mini review of recent work[J]. Journal of Molecular Recognition,1990,3(3):123−127. doi: 10.1002/jmr.300030306
[83] BELL C, ATKINS P. Principles and applications of metal chelation[M]. Clarendon Press, 1977.
[84] ASHMEAD S D. The chemistry of ferrous bis-glycinate chelate[J]. Archivos Latinoamericanos De Nutricion, 2001, 51(1 Suppl 1):7−12.
[85] LIN Y, CAI X, WU X, et al. Fabrication of snapper fish scales protein hydrolysate-calcium complex and the promotion in calcium cellular uptake[J]. Journal of Functional Foods,2020,65:103717. doi: 10.1016/j.jff.2019.103717
[86] CARLTON D D, SCHUG K A. A review on the interrogation of peptide-metal interactions using electrospray ionization-mass spectrometry[J]. Analytica Chimica Acta,2011,686(1-2):19−39. doi: 10.1016/j.aca.2010.11.050
[87] UDECHUKWU M C, DOWNEY B, UDENIGWE C C. Influence of structural and surface properties of whey-derived peptides on zinc-chelating capacity, and in vitro gastric stability and bioaccessibility of the zinc-peptide complexes[J]. Food Chemistry,2018,240:1227−1232. doi: 10.1016/j.foodchem.2017.08.063
[88] SI K, GONG T, DING S, et al. Binding mechanism and bioavailability of a novel phosvitin phosphopeptide (Glu-Asp-Asp-pSer-pSer) calcium complex[J]. Food Chemistry,2023,404:134567. doi: 10.1016/j.foodchem.2022.134567
[89] ZHANG Y Y, STOCKMANN R, NG K, et al. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability:A review[J]. Comprehensive Reviews in Food Science and Food Safety,2021,20(1):652−685. doi: 10.1111/1541-4337.12669
[90] KHARE E, HOLTEN-ANDERSEN N, BUEHLER M J. Transition-metal coordinate bonds for bioinspired macromolecules with tunable mechanical properties[J]. Nature Reviews Materials,2021,6(5):421−436.
[91] CHEN M, JI H, ZHANG Z, et al. A novel calcium-chelating peptide purified from Auxis thazard protien hydrolysate and its binding properties with calcium[J]. Journal of Functional Foods,2019,60:103447. doi: 10.1016/j.jff.2019.103447
[92] ZHANG X, JIA Q, LI M, et al. Isolation of a novel calcium-binding peptide from phosvitin hydrolysates and the study of its calcium chelation mechanism[J]. Food Research International,2021,141:110169. doi: 10.1016/j.foodres.2021.110169
[93] FERRARETTO A, GRAVAGHI C, FIORILLI A, et al. Casein-derived bioactive phosphopeptides:Role of phosphorylation and primary structure in promoting calcium uptake by HT-29 tumor cells[J]. FEBS letters,2003,551(1-3):92−98. doi: 10.1016/S0014-5793(03)00741-5
[94] SUN N, WANG Y, BAO Z, et al. Calcium binding to herring egg phosphopeptides:Binding characteristics, conformational structure and intermolecular forces[J]. Food Chemistry,2020,310:125867.
[95] ZHAO M, LI S, AHN D U, et al. Phosvitin phosphopeptides produced by pressurized hea-trypsin hydrolysis promote the differentiation and mineralization of MC3T3-E1 cells via the OPG/RANKL signaling pathways[J]. Poultry Science,2021,100(2):527−536. doi: 10.1016/j.psj.2020.10.053
[96] PEREGO S, ZABEO A, MARASCO E, et al. Casein phosphopeptides modulate calcium uptake and apoptosis in Caco2 cells through their interaction with the TRPV6 calcium channel[J]. Journal of Functional Foods,2013,5(2):847−857. doi: 10.1016/j.jff.2013.01.032
[97] JIANG B, MINE Y. Preparation of novel functional oligophosphopeptides from hen egg yolk phosvitin[J]. Journal of Agricultural and Food Chemistry,2000,48(4):990−994. doi: 10.1021/jf990600l
[98] JIANG B, MINE Y. Phosphopeptides derived from hen egg yolk phosvitin:effect of molecular size on the calcium-binding properties[J]. Bioscience, Biotechnology, and Biochemistry,2001,65(5):1187−1190. doi: 10.1271/bbb.65.1187
[99] LUO J, YAO X, SOLADOYE O P, et al. Phosphorylation modification of collagen peptides from fish bone enhances their calcium-chelating and antioxidant activity[J]. LWT,2022,155:112978. doi: 10.1016/j.lwt.2021.112978
[100] ZONG H, PENG L, ZHANG S, et al. Effects of molecular structure on the calcium-binding properties of phosphopeptides[J]. European Food Research and Technology,2012,235(5):811−816.
[101] BAO X L, LV Y, YANG B C, et al. A study of the soluble complexes formed during calcium binding by soybean protein hydrolysates[J]. Journal of Food Science,2008,73(3):C117−121. doi: 10.1111/j.1750-3841.2008.00673.x
[102] ZHANG K, LI J, HOU H, et al. Purification and characterization of a novel calcium-biding decapeptide from pacific cod ( Gadus macrocephalus) bone:Molecular properties and calcium chelating modes[J]. Journal of Functional Foods,2019,52:670−679. doi: 10.1016/j.jff.2018.11.042
[103] LIU F R, WANG L, WANG R, et al. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide-calcium complex[J]. Journal of Agricultural and Food Chemistry,2013,61(31):7537−7544. doi: 10.1021/jf401868z
[104] CUI P, LIN S, JIN Z, et al. In vitro digestion profile and calcium absorption studies of a sea cucumber ovum derived heptapeptide-calcium complex[J]. Food & Function,2018,9(9):4582−4592.
[105] ZHAO L, HUANG Q, HUANG S, et al. Novel peptide with a specific calcium-binding capacity from whey protein hydrolysate and the possible chelating mode[J]. Journal of Agricultural and Food Chemistry,2014,62(42):10274−10282. doi: 10.1021/jf502412f
[106] AN J, ZHANG Y, YING Z, et al. The formation, structural characteristics, absorption pathways and bioavailability of calcium-peptide chelates[J]. Foods (Basel, Switzerland),2022,11(18):2762.
[107] HOENDEROP J G J, NILIUS B, BINDELS R J M. Calcium absorption across epithelia[J]. Physiological Reviews,2005,85(1):373−422. doi: 10.1152/physrev.00003.2004
[108] DIAZ DE BARBOZA G, GUIZZARDI S, TOLOSA D T N. Molecular aspects of intestinal calcium absorption[J]. World Journal of Gastroenterology,2015,21(23):7142−7154. doi: 10.3748/wjg.v21.i23.7142
[109] PÉREZ A V, PICOTTO G, CARPENTIERI A R, et al. Minireview on regulation of intestinal calcium absorption[J]. Digestion,2008,77(1):22−34. doi: 10.1159/000116623
[110] BIANCO S D, PENG J B, TAKANAGA H, et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the trpv6 calcium channel gene[J]. Journal of Bone and Mineral Research,2006,22(2):274−285.
[111] DEN DEKKER E, HOENDEROP J G J, NILIUS B, et al. The epithelial calcium channels, TRPV5 & TRPV6:From identification towards regulation[J]. Cell Calcium,2003,33(5-6):497−507. doi: 10.1016/S0143-4160(03)00065-4
[112] MCGOLDRICK L L, SINGH A K, SAOTOME K, et al. Opening of the human epithelial calcium channel TRPV6[J]. Nature,2018,553(7687):233−237. doi: 10.1038/nature25182
[113] WONGDEE K, CHANPAISAENG K, TEERAPORNPUNTAKIT J, et al. Intestinal calcium absorption[J]. Comprehensive Physiology,2021,11(3):2047−2073.
[114] SCHWALLER B. Cytosolic Ca2+ buffers are inherently Ca2+ signal modulators[J]. Cold Spring Harbor Perspectives in Biology,2020,12(1):a035543. doi: 10.1101/cshperspect.a035543
[115] HONG E J, JEUNG E B. Biological significance of calbindin-D9k within duodenal epithelium[J]. International Journal of Molecular Sciences,2013,14(12):23330−23340. doi: 10.3390/ijms141223330
[116] FLEET J C. Regulation of intestinal calcium and phosphate absorption[M]. ACADEMIC Press, 2018:329-342.
[117] GHIJSEN W E J M, DE J M D, VAN O C H. Kinetic properties of Na+/Ca2+ exchange in basolateral plasma membranes of rat small intestine[J]. BBA-Biomembranes, 1983.
[118] GUÉGUEN L, POINTILLART A. The bioavailability of dietary calcium[J]. Journal of the American College of Nutrition, 2000:119S-136S.
[119] SHARMA R V, BUTTERS C A, MCELDOON J P, et al. Characterization of Ca2+ uptake in plasma membrane vesicles isolated from guinea pig ileum smooth muscle[J]. Cell Calcium,1987,8(1):65−77. doi: 10.1016/0143-4160(87)90037-6
[120] LIU C, WENG H, CHEN L, et al. Impaired intestinal calcium absorption in protein 4.1R-deficient mice due to altered expression of plasma membrane calcium atpase 1b (PMCA1b)[J]. Journal of Biological Chemistry,2013,288(16):11407−11415. doi: 10.1074/jbc.M112.436659
[121] DORKKAM N, WONGDEE K, SUNTORNSARATOON P, et al. Prolactin stimulates the L-type calcium channel-mediated transepithelial calcium transport in the duodenum of male rats[J]. Biochemical and Biophysical Research Communications,2013,430(2):711−716.
[122] TSUCHITA H, SUZUKI T, KUWATA T. The effect of casein phosphopeptides on calcium absorption from calcium-fortified milk in growing rats[J]. The British Journal of Nutrition,2001,85(1):5−10. doi: 10.1079/BJN2000206
[123] CHAROENPHANDHU N, LIMLOMWONGSE L, KRISHNAMRA N. Prolactin directly enhanced Na+/K+- and Ca2+-ATPase activities in the duodenum of female rats[J]. Canadian Journal of Physiology and Pharmacology,2006,84(5):555−563. doi: 10.1139/y05-161
[124] JANTARAJIT W, THONGON N, PANDARANANDAKA J, et al. Prolactin-stimulated transepithelial calcium transport in duodenum and Caco-2 monolayer are mediated by the phosphoinositide 3-kinase pathway[J]. American Journal of Physiology. Endocrinology and Metabolism,2007,293(1):E372−384.
[125] FUJITA H, SUGIMOTO K, INATOMI S, et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes[J]. Molecular Biology of the Cell,2008,19(5):1912−1921. doi: 10.1091/mbc.e07-09-0973
[126] TSUKITA S, FURUSE M, ITOH M. Multifunctional strands in tight junctions[J]. Nature Reviews. Molecular Cell Biology,2001,2(4):285−293. doi: 10.1038/35067088
[127] CURRY J N, SAURETTE M, ASKARI M, et al. Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease[J]. The Journal of Clinical Investigation,2020,130(4):1948−1960. doi: 10.1172/JCI127750
[128] COLEGIO O R, ITALLIE C V, RAHNER C, et al. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture[J]. American Journal of Physiology-Cell Physiology,2003,284(6):C1346−C1354.
[129] HIROKI F, HIDEKI C, HIROSHI Y, et al. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine[J]. Journal of Histochemistry & Cytochemistry, 2007:933-944.
[130] VAN ITALLIE C M, FANNING A S, ANDERSON J M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins[J]. American Journal of Physiology. Renal Physiology,2003,285(6):F1078−1084. doi: 10.1152/ajprenal.00116.2003
[131] ALEXANDER R T, RIEVAJ J, DIMKE H. Paracellular calcium transport across renal and intestinal epithelia[J]. Biochemistry and Cell Biology = Biochimie et Biologie Cellulaire,2014,92(6):467−480. doi: 10.1139/bcb-2014-0061
[132] KOPIC S, GEIBEL J P. Gastric acid, calcium absorption, and their impact on bone health[J]. Physiological Reviews,2013,93(1):189−268. doi: 10.1152/physrev.00015.2012
[133] YE L, MORSE L R, ZHANG L, et al. Osteopetrorickets due to Snx10 deficiency in mice results from both failed osteoclast activity and loss of gastric acid-dependent calcium absorption[J]. PLoS Genetics,2015,11(3):e1005057. doi: 10.1371/journal.pgen.1005057
[134] SCHINKE T, SCHILLING A F, BARANOWSKY A, et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass[J]. Nature Medicine,2009,15(6):674−681. doi: 10.1038/nm.1963
[135] CARRASCO F, BASFI-FER K, ROJAS P, et al. Calcium absorption may be affected after either sleeve gastrectomy or Roux-en-Y gastric bypass in premenopausal women:A 2-y prospective study[J]. The American Journal of Clinical Nutrition,2018,108(1):24−32. doi: 10.1093/ajcn/nqy071
[136] SUGITA M, MATSUMOTO M, TSUKANO Y, et al. Gastric emptying time after breakfast in healthy adult volunteers using ultrasonography[J]. Journal of Anesthesia,2019,33(6):697−700. doi: 10.1007/s00540-019-02694-6
[137] LU Z, DING L, LU Q, et al. Claudins in intestines:Distribution and functional significance in health and diseases[J]. Tissue Barriers,2013,1(3):e24978. doi: 10.4161/tisb.24978
[138] DUFLOS C, BELLATON C, PANSU D, et al. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats[J]. The Journal of Nutrition,1995,125(9):2348−2355. doi: 10.1093/jn/125.9.2348
[139] FLEET J C, SCHOCH R D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors[J]. Critical Reviews in Clinical Laboratory Sciences,2010,47(4):181−195. doi: 10.3109/10408363.2010.536429
[140] MORGAN E L, MACE O J, HELLIWELL P A, et al. A role for Cav1.3 in rat intestinal calcium absorption[J]. Biochemical and Biophysical Research Communications,2003,312(2):487−493. doi: 10.1016/j.bbrc.2003.10.138
[141] FLEET J C, EKSIR F, HANCE K W, et al. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,2002,283(3):G618−625. doi: 10.1152/ajpgi.00269.2001
[142] KARBACH U. Paracellular calcium transport across the small intestine[J]. The Journal of Nutrition, 1992, 122(3 Suppl):672−677.
[143] PANSU D, BELLATON C, ROCHE C, et al. Duodenal and ileal calcium absorption in the rat and effects of vitamin D[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,1983,244(6):G695−G700. doi: 10.1152/ajpgi.1983.244.6.G695
[144] CRAMER C F, COPP D H. Progress and rate of absorption of radiostrontium through intestinal tracts of rats[J]. Experimental Biology and Medicine,1959,102(2):514−517. doi: 10.3181/00379727-102-25301
[145] BEGGS M R, LEE J J, BUSCH K, et al. TRPV6 and Cav1.3 mediate distal small intestine calcium absorption before weaning[J]. Cellular and Molecular Gastroenterology and Hepatology,2019,8(4):625−642. doi: 10.1016/j.jcmgh.2019.07.005
[146] RIEVAJ J, PAN W, CORDAT E, et al. The Na+/H+ exchanger isoform 3 is required for active paracellular and transcellular Ca2+ transport across murine cecum[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,2013,305(4):G303−G313. doi: 10.1152/ajpgi.00490.2012
[147] WASSERMAN R H. Vitamin D and the dual processes of intestinal calcium absorption[J]. The Journal of Nutrition,2004,134(11):3137−3139. doi: 10.1093/jn/134.11.3137
[148] HOENDEROP J G, VENNEKENS R, MÜLLER D, et al. Function and expression of the epithelial Ca(2+) channel family:Comparison of mammalian ECaC1 and 2[J]. The Journal of Physiology, 2001, 537(Pt 3):747-761.
[149] KORHONEN H. Milk-derived bioactive peptides:From science to applications[J]. Journal of Functional Foods,2009,1(2):177−187. doi: 10.1016/j.jff.2009.01.007
[150] WANG X, ZHANG Z, XU H, et al. Preparation of sheep bone collagen peptide-calcium chelate using enzymolysis-fermentation methodology and its structural characterization and stability analysis[J]. RSC Advances,2020,10(20):11624−11633. doi: 10.1039/D0RA00425A
[151] SHEN W, MATSUI T. Intestinal absorption of small peptides:A review[J]. International Journal of Food Science & Technology,2019,54(6):1942−1948.
[152] FERNÁNDEZ-MUSOLES R, ISIDRA R, JUAN B S, et al. Bioavailability of antihypertensive lactoferricin B-derived peptides:Transepithelial transport and resistance to intestinal and plasma peptidase[J]. International Dairy Journal,2013,32(2):169−174.
[153] MEREDITH D, PRICE R A. Molecular modeling of PepT1-towards a structure[J]. The Journal of Membrane Biology,2006,213(2):79−88. doi: 10.1007/s00232-006-0876-6
[154] KATIMBA H A, WANG R, CHENG C. Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans[J]. Critical Reviews in Food Science and Nutrition, 2021:1−21.
-
期刊类型引用(1)
1. 蔡梦婷,刘荔,黄若楠,孙学宁,曾名湧. 亲水胶体调控全牡蛎-大豆分离蛋白热诱导凝胶的形成机制. 食品工业科技. 2024(23): 129-139 .  本站查看
本站查看
其他类型引用(1)





 下载:
下载:
 下载:
下载:



