Extraction, Bacteriostatic Effect and Synergistic Mechanism of Bacteriostatic Effect of Taxifolin in Larch in Combination with Leucocyanidin
-
摘要: 本研究以落叶松为原料,采用超声辅助乙醇热浸提法提取花旗松素。以大肠杆菌和金黄色葡萄球菌分别作为典型供试菌,通过观察细菌形态结构,测定细菌生长量、细胞膜泄漏、抗氧化酶系活性等变化情况,分析花旗松素抑菌效果。最终获得纯度达90%、提取率0.35%的花旗松素,且花旗松素对两种菌株的最小抑菌浓度均为1.2 mg/mL,抑菌率分别为81.12%、83.95%。通过电镜扫描后发现菌体表面被破坏伴有内容物泄漏。在Escherichia coli中,培养至24 h的1/2 MIC、1 MIC和2 MIC实验组OD260测试值是同期对照组的1.18、1.52、1.88倍,检测到胞外β-半乳糖苷酶的相对活性分别为79.15%和70.1%。Bliss独立模型计算生长速率为0.0142,表明花旗松素和白屈菜红碱药物联用后对金黄色葡萄球菌表现为协同抑制作用。药物联用条件下,低浓度对细菌细胞膜破坏明显增强,抑菌效果更加显著,培养上清液中β-半乳糖苷酶活达87.01%,较2 MIC单独作用S. aureus酶活性高16.91%,24 h CAT、SOD和POD酶活较单独使用1 MIC浓度花旗松素对S. aureus作用要更高,分别是其1.28、1.25、1.11倍。研究将为花旗松素作为食品保鲜、防腐剂等产品的开发应用奠定理论基础。Abstract: Using larch as raw material, taxifolin was extracted by ultrasonic-assisted ethanol hot leaching. Escherichia coli and Staphylococcus aureus were used as typical test bacteria respectively, and the bacterial inhibitory effects of taxifolin was analyzed by observing the morphological structure of bacteria and measuring the changes in bacterial growth, cell membrane leakage and antioxidant enzyme system activities. The final product was obtained with 90% purity and 0.35% extraction rate of taxifolin. The minimum inhibitory concentration of taxifolin was 1.2 mg/mL for both strains, and the inhibition rate was 81.12% and 83.95%, respectively. After the electron microscopy analysis, the surface of the bacteria cells was disrupted and the contents inside the cells were leaked. In E. coli, the OD260 test values of 1/2 MIC, 1 MIC and 2 MIC were 1.18, 1.52 and 1.88 times higher than those of the control group after 24 h incubation. The relative activities of extracellular β-galactosidase were 79.15% and 70.1%, respectively, and the growth rate calculated by the the Bliss independent model was 0.0142. S. aureus showed a synergistic inhibition effect. Under the conditions of drug combination, the bacterial cell membrane disruption extent was significantly enhanced by the low concentration and the inhibition effect was more significant. The β-galactosidase activity in the culture supernatant was 87.01% higher than that of 2 MIC group alone for S. aureus by 16.91%, and the 24 h CAT, SOD and POD enzyme activities were higher than that of 1 MIC group alone for S. aureus, which were 1.28, 1.25 and 1.11 times higher, respectively. The study could lay the theoretical foundation for the development and application of taxifolin as a food preservation and preservative.
-
Keywords:
- taxifolin /
- antibacterial activity /
- drug combination /
- mechanism of inhibition
-
花旗松素(Taxifolin,TFL)又名二氢槲皮素,是一种具有多种天然活性物质的二氢黄酮类化合物。自然界中,广泛存在于落叶松、水飞蓟、红蓼、洋葱和黄芪等多种松科与蔷薇科的植物中,其中以落叶松中含量最高[1],根茎部尤甚。常用的提取方法包括:热水浸提法、乙醇回流法、酶诱导法、超声法和有机溶剂萃取法等[2−3]。未来也可能通过构建基因工程菌株大规模发酵生产花旗松素。因花旗松素结构中含有较多的酚羟基,可表现出多种生物学活性与较强的抗氧化作用,现已研究发现其含有抗菌、抗病毒、抗肿瘤、抗辐射及抗心血管系统疾病等功能,具备开发为防腐剂、保健品和食品的潜力[4−6]。此外,2021年4月,花旗松素已被我国卫健委认定为“新食品原料”,可见其市场前景十分广阔。
白屈菜红碱(Chelerythrine,CHE)是一种苯并菲啶型生物碱,具有抗炎抑菌、抗肿瘤、防治虫害等生物活性[7]。目前研究发现白屈菜红碱对多种典型病虫害都具有显著抑制作用,具备开发生物防治农药的潜力,用于减轻作物病害[8]。但因单独使用该药物抑菌所需浓度较高,因此若能找到其他抗菌剂与之联合使用,将降低药量并增强抑菌活性,提升产品性能。已有研究表明,生物碱和黄酮类化合物都具有高效抑菌能力。因此,本实验将白屈菜红碱与花旗松素联合使用观察其抑菌能力是否增强。
基于此,本研究取材落叶松根茎部,采用超声辅助乙醇热浸提法提取花旗松素,经醚类萃取、重结晶技术进行纯化,测定其纯度与提取率,研究其对革兰氏阳/阴性菌的抑菌能力和机理,并与白屈菜红碱联用进行了药物联合抑菌实验,探究其抑菌效能,以期为未来食品、日化及医药领域的产品开发与行业发展提供理论基础。
1. 材料与方法
1.1 材料与仪器
大肠杆菌(E. coli)、金黄色葡萄球菌(S. aeruse) 东北林业大学微生物学科菌种保藏中心;过氧化氢酶试剂盒、过氧化物酶试剂盒、超氧化物歧化酶试剂盒 苏州格锐思生物科技有限公司;花旗松素标准品 上海源叶生物科技有限公司;胰蛋白胨、酵母提取物 Oxoid公司;甲醇(色谱级) Fisher公司;无水乙醇、甲基叔丁基醚、二甲基亚砜、2-Nitrophenyl-β-D-galactopyranoside(邻硝基苯-β-D-吡喃半乳糖苷,ONPG)等化学试剂 分析纯,国药集团;Luria-Bertani(LB)培养基;落叶松 来自大兴安岭;白屈菜红碱(CHE)(纯度98%) 东北林业大学微生物实验室。
Agilent 1260高效液相色谱仪 美国安捷伦科技有限公司;5430R低温高速离心机 德国艾本德公司;JEM-2100透射电子显微镜 日本电子株式会社;TU-1901紫外分光光度计 北京普析通用仪器责任有限公司。
1.2 实验方法
1.2.1 花旗松素提取与纯度计算
采用超声辅助乙醇热浸提法提取花旗松素[9]。取200 g落叶松粉末加入2 L 90%乙醇,80 ℃水浴2 h后超声(功率300 W)震荡1 h过滤得滤液,利用旋转蒸发仪进行减压浓缩。取第一次提取液加入甲基叔丁基醚1.2 L,匀速搅拌20 min,静止30 min,分离得到甲基叔丁基醚萃取液,此步骤重复3~4次,将其减压浓缩,冻干得粗提物。再经三次重结晶[10],冻干得终产物。
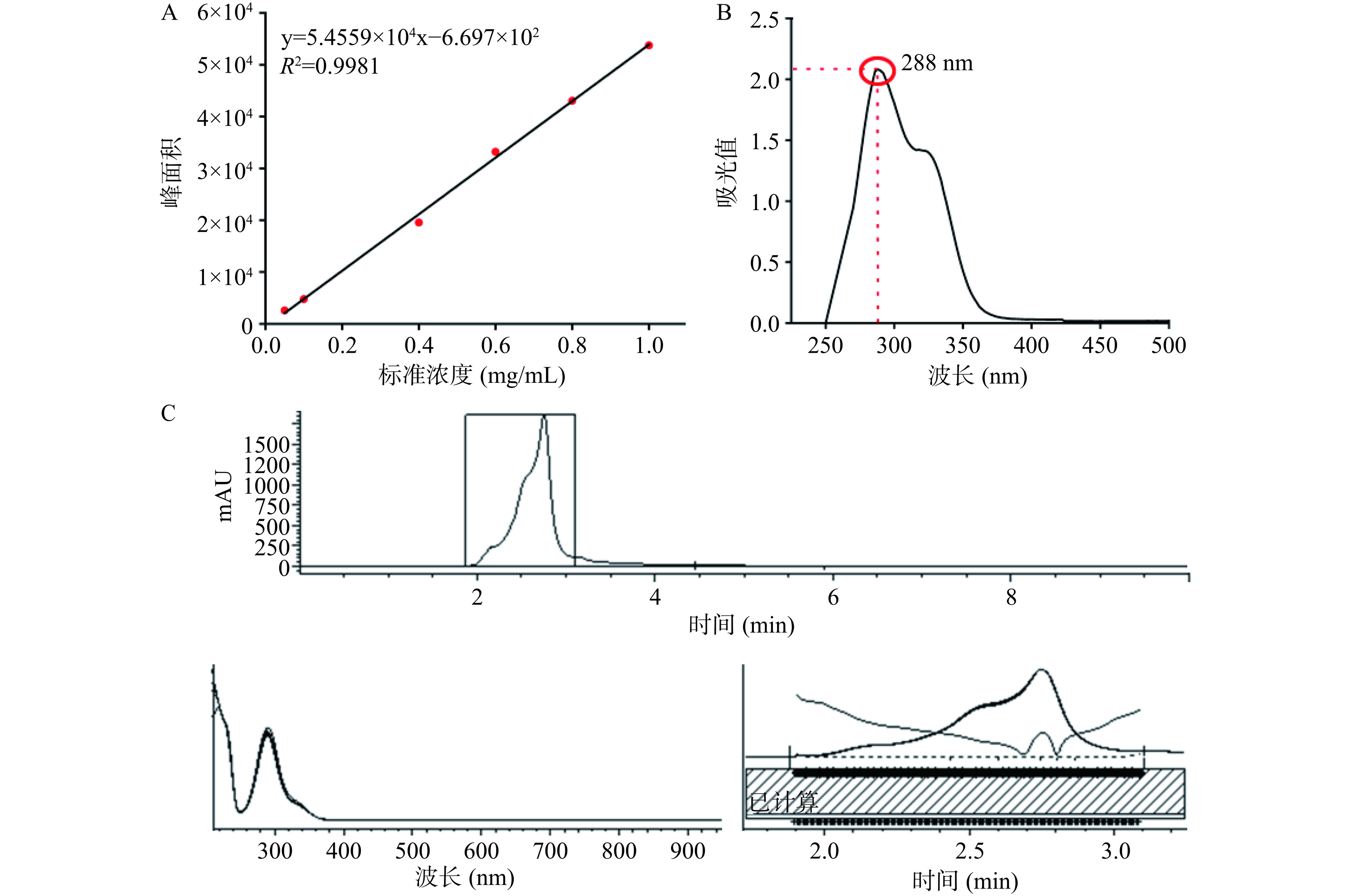
准确称取花旗松素标准品(HPLC≥98%)10 mg溶于乙醇中,定容至10 mL,制备成1 mg/mL的对照液,配制浓度分别为0.05、0.1、0.4、0.6、0.8、1 mg/mL的标准待测液,用于绘制花旗松素浓度标准曲线。花旗松素标准液及提取样品均采用高效液相色谱测试,条件如下:
柱温30 ℃、流动相40%水:60%甲醇、运行时间10 min、检测波长288 nm、流速1.0 mL/min。此条件下,花旗松素出峰时间约为(2.672±0.1)min[11−12]。
根据对应出峰时间的出峰面积,将其带入花旗松素浓度标准曲线得到样品纯度。另取适量花旗松素产物溶于去离子水,采用紫外分光光度计对其进行全光谱扫描,验证提取物为花旗松素。
1.2.2 花旗松素对供试菌MIC浓度测定及抑菌活性
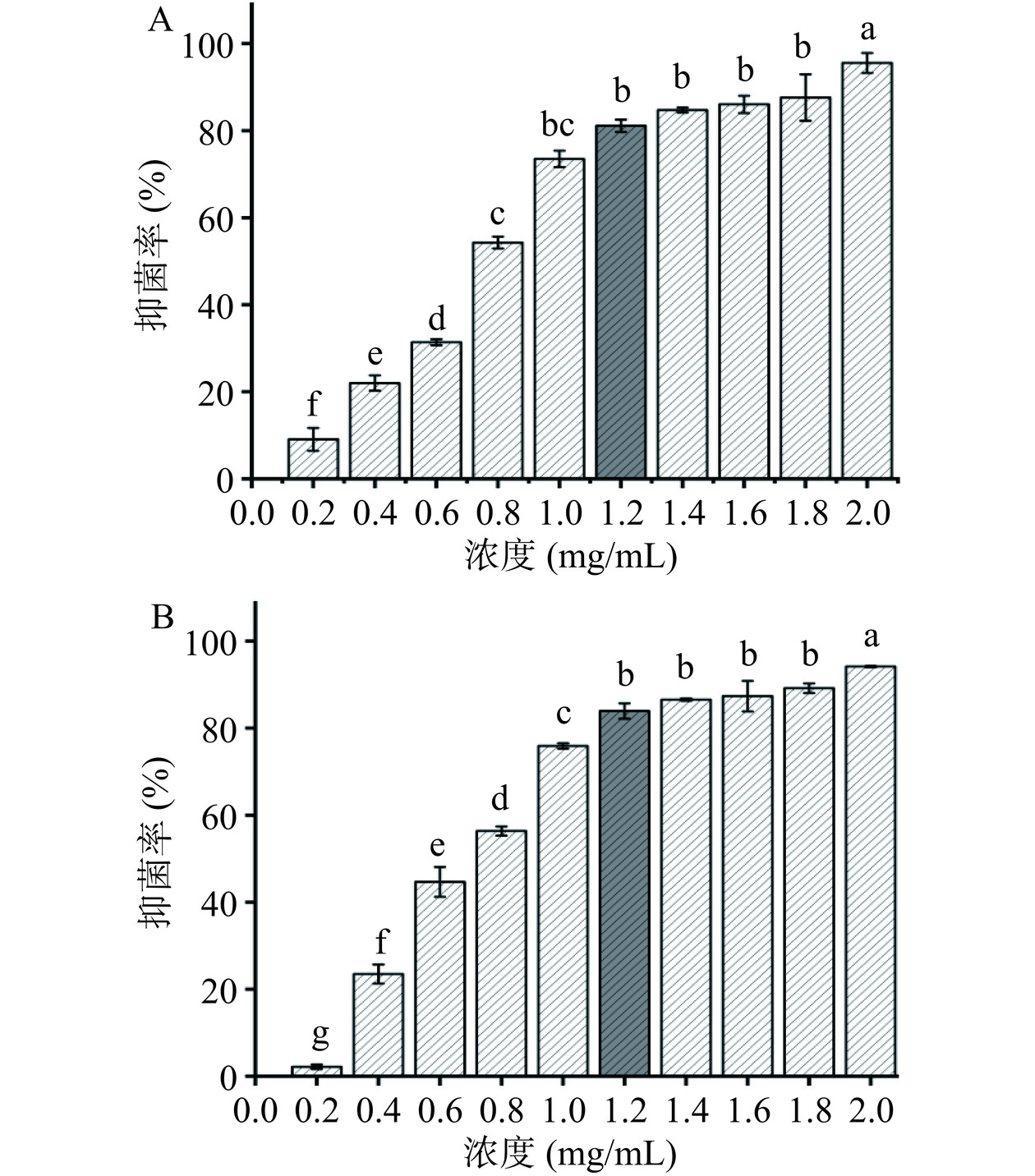
配制LB液体培养基:蛋白胨2 g,酵母提取物1 g,NaCl 2 g,蒸馏水200 mL,pH7.2~7.4,高压灭菌待用。用0.01 mol/L PBS溶解花旗松素,加入适量LB液体培养基,配制终浓度为0、0.2、0.4、0.6、0.8、1.0、1.2、1.4、1.6、1.8、2.0 mg/mL的花旗松素溶液,分别加入培养至对数生长期的E. coli、S. aureus菌液80 μL,终体积8 mL。各管充分混匀后置恒温培养箱37 ℃ 130 r/min振荡培养。24 h后取各组菌液测定OD600值。
供试菌抑菌率(%)=生长对照组OD600−实验组OD600生长对照组OD600−空白组OD600×100 抑制率>80%所含最低药物浓度为最小抑菌浓度(Minimum inhibitory concentration,MIC)[13]。
1.2.3 细菌形态学观察
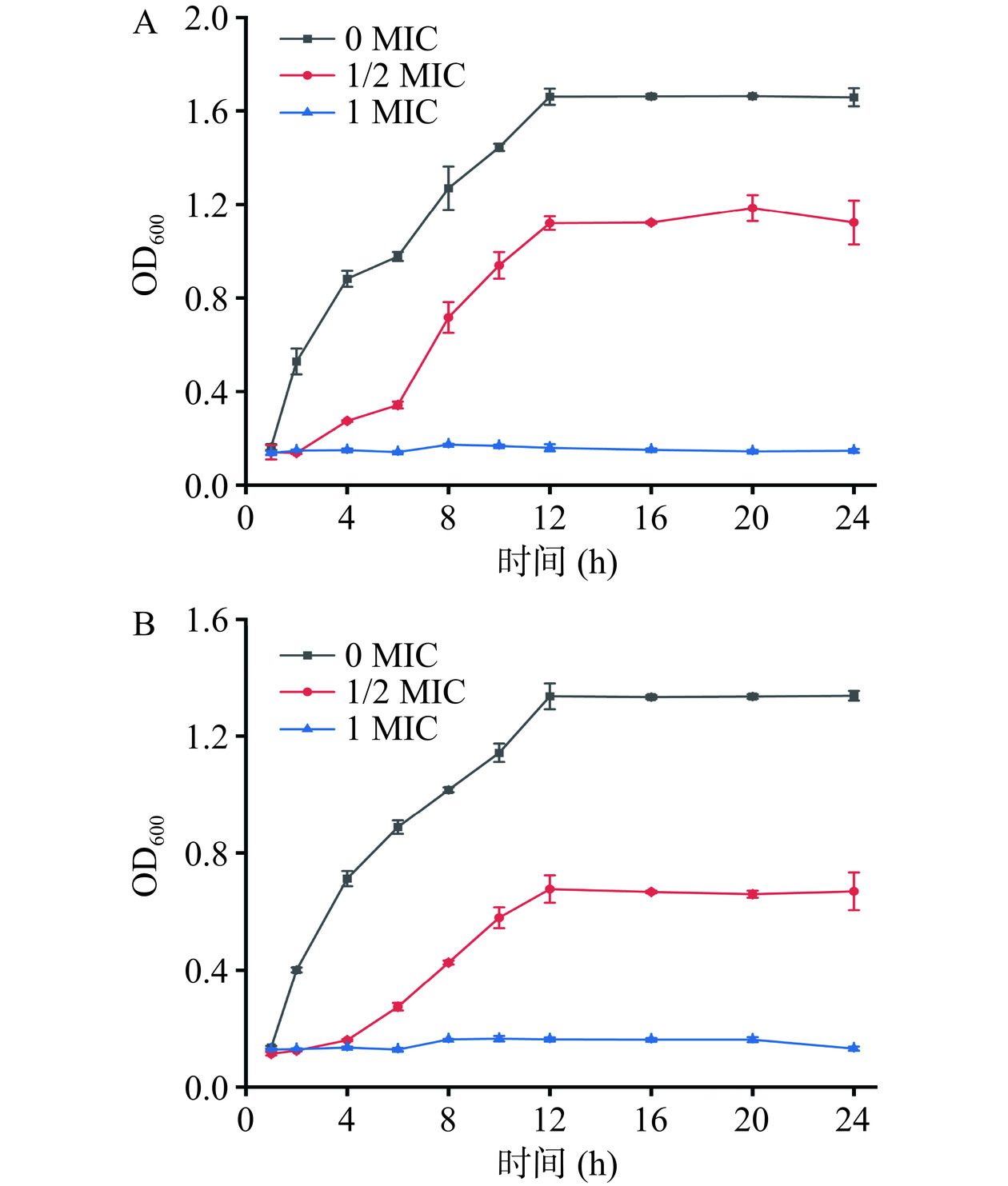
分别在培养至对数生长期的E. coli、S. aureus菌液中加入终浓度为0、0.5、1 MIC的花旗松素溶液,每组设置三个重复,混匀后置于37 ℃培养箱,130 r/min震荡培养,于1、2、4、6、8、10、12和24 h分别取样测定600 nm处吸光值[14],绘制生长曲线。另取适量菌液通过扫描电子显微镜和透射电子显微镜观察菌体形态变化,样品处理方法参照胡梅[15]。培养大肠杆菌、金黄色葡萄球菌与添加终浓度为1 MIC的二氢槲皮素培养至12、24 h。放入离心管中(设置三组管)离心后倒去上清液并收集大肠杆菌、金黄色葡萄球菌菌泥与经二氢槲皮素处理后的大肠杆菌、金黄色葡萄球菌菌泥。先将盖玻片用纯水和无水乙醇分别超声清洗15 min,将离心后菌泥接触到盖玻片上并均匀分散,等自然晾干,之后加入适量的3%戊二醛固定液将样品全部覆盖后放置−4 ℃冰箱固定12 h。使用现配制的乙醇(浓度为30%、40%、50%、60%、70%、80%、90%、95%、100%,每次浓度重复3次,更有效脱水作用)进行乙醇浓度梯度脱水,每次震荡脱水10~15 min即可。之后保存样品于−4 ℃冰箱样品自然晾干待测。
1.2.4 细菌胞内大分子物质泄露情况的测定
采用分光光度法测定花旗松素处理后菌体内大分子物质含量[16],以评价菌体被破坏的程度。将培养至对数期的菌体8000 r/min离心10 min,用0.01 mol/L PBS清洗3次并重悬,分别加入花旗松素使其终浓度为1/2、1、2 MIC,以无花旗松素的细菌培养液为对照,放置于37 ℃摇床130 r/min振荡培养,于1、4、8、24 h取混悬样液1 mL,8000 r/min离心10 min,取上清适当稀释后,立即于260、280 nm测定吸光值,每组重复三次。
1.2.5 细菌细胞质膜渗透性的测定
参照吴海霞[17]方法。制备待测菌液,分别加入不同浓度梯度的花旗松素(1/2、1、2 MIC)设置三组平行,再加入1 mg/mL的ONPG(邻硝基苯-β-D-吡喃半乳糖苷)混匀,37 ℃水浴,每隔1 h测于405 nm的吸光值OD2(共测4个小时时间段)。阴性对照为加入缓冲液,吸光度值为OD1。细胞质膜渗透性增加导致的吸光度值为OD=OD2−OD1。
重悬待测菌液,分别加入不同浓度花旗松素,使其终浓度为2 MIC,每组设置三组平行充分混匀,37 ℃水浴孵育1 h后离心,取上清液再加入1 mg/mL的ONPG(邻硝基苯-β-D-吡喃半乳糖苷),37 ℃条件下再水浴孵育4 h,测定在405 nm下的吸光度值OD2。细菌经超声波破碎(冰浴,功率200 W,超声5 s,间隔5 s)离心后取上清液,测吸光度值OD1作为阳性对照。上清液中β-半乳糖苷酶相对活性(%)=OD2/OD1×100,通过分光光度计测定β-半乳糖苷酶水解乳糖类似物ONPG产物的特征吸收评价花旗松素对细胞质膜渗透性的影响。
1.2.6 细菌胞内抗氧化酶系活性的测定
采用1.1中材料对花旗松素作用于细菌后的CAT酶、POD酶和SOD酶活性变化进行测定[18]。
取适量培养至对数生长期的E. coli、S. aureus,配制终浓度为1 MIC的TFL溶液,以0 MIC为对照,分别装于2 mL离心管内,每组设置3组平行重复,培养至1、4、8、24 h。将样品离心后加入1 mL提取液冰浴超声破胞,之后在4 ℃条件下12000 r/min离心10 min,取上清根据试剂盒说明书进行实验。
1.2.7 花旗松素与白屈菜红碱联合抑菌
使用Bliss独立模型计算药物联用作用指数[19],使用以下其公式计算协同指数:
S=(FAF0)(FBF0)−FABF0 式中,FAB:指在联用药物时细菌的生长速率(24 h测得OD值),A为花旗松素,B为白屈菜红碱。FA,FB:分别指有A、B时细菌的生长速率;F0:指没用药处理时细菌的生长速率;S:为药物联用作用指数,该参数若为正值,则代表药物联用对菌体具协同作用;负值则呈拮抗作用[20]。
1.2.8 花旗松素与白屈菜红碱的联合抑菌机制
同1.2.4、1.2.5和1.2.6操作,分别测定菌体的胞内大分子物质泄漏、细菌膜渗透性和抗氧化酶系的变化。实验中,将花旗松素样品变更为0.25 MIC的花旗松素和0.25 MIC的白屈菜红碱混合液。
1.3 数据处理
采用SPSS 26.0软件进行数据处理,采用t检验,以P<0.05为差异有统计学意义。Origin 2021软件进行绘图。
2. 结果与分析
2.1 花旗松素提取及纯度计算
根据高效液相色谱测定不同浓度花旗松素标准品所得峰面积,绘制标准曲线(图1A)。据此计算在落叶松中提取所得花旗松素样品(图1C)纯度为90%。经紫外分光光度计全光谱扫描(图1B),发现样品最大吸收峰值位于288 nm,这与刘文丛等[21]研究结果一致,可确定提取物为纯度较高的花旗松素。
2.2 花旗松素的MIC测定及抑菌活性
MIC表示受试物抑菌能力的指标[22],能更准确反映其抑菌活性。结果如图2所示,花旗松素对E. coli和S. aureus均具有较好的抑菌效果。当花旗松素的质量浓度达到1.2 mg/mL时,对两株细菌的抑菌活性大于80%,分别为81.12%和83.95%。
细菌的生长曲线反映了当前培养条件下的微生物群体生长规律。如图3所示,在细菌培养液中加入花旗松素后将严重影响大肠杆菌和金黄色葡萄球菌的生长,各时段菌液的OD600值均明显降低。相较未添加花旗松素的对照组菌液,加入1/2 MIC花旗松素后1 h即发生明显变化,迟缓期延长,12 h时近似达到细菌生长峰值,进入稳定期。花旗松素浓度为1 MIC时,细菌的生长即刻被抑制,生长速率极其缓慢,未见生长对数期。培养至8 h时的大肠杆菌组OD600值仅为0.173,较对照组下降了86.4%。由此可见,花旗松素对大肠杆菌和金黄色葡萄球菌的抑菌活性明显,细菌生长在1 MIC浓度下几乎被完全抑制。
2.3 花旗松素对菌体形态影响
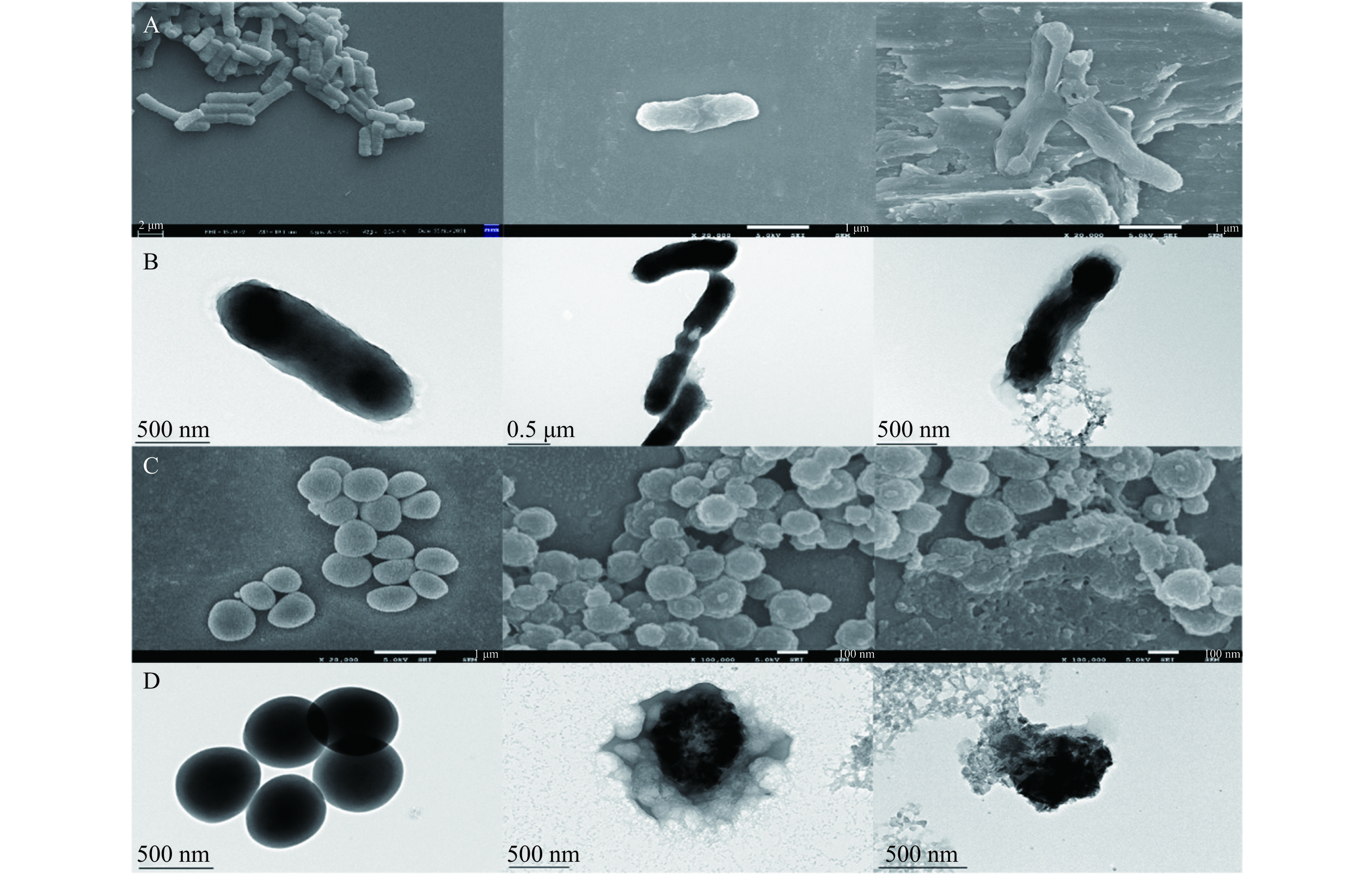
为观测花旗松素对供试菌作用后对其外观和微观结构的影响,研究加入2 MIC浓度的花旗松素,通过扫描电子显微镜和透射电子显微镜进行观察。如图4所示,正常培养的大肠杆菌和金黄色葡萄球菌外部光滑,细胞形态饱满,结构紧密,菌体之间界限清晰。但当培养液中添加花旗松素后,菌体表面逐渐粗糙,边缘模糊,伴有明显褶皱和凹陷,变形明显[23]。随处理时间延长,菌体形态更加不整,外壁不全,可见大量细胞碎片,出现消融迹象[24]。由此可见,花旗松素的作用可能是使细菌形态改变,细胞壁溶解,细胞膜破裂,并伴随大量内容物泄露,最终导致细菌消解死亡。
2.4 花旗松素对菌体核苷酸泄露影响
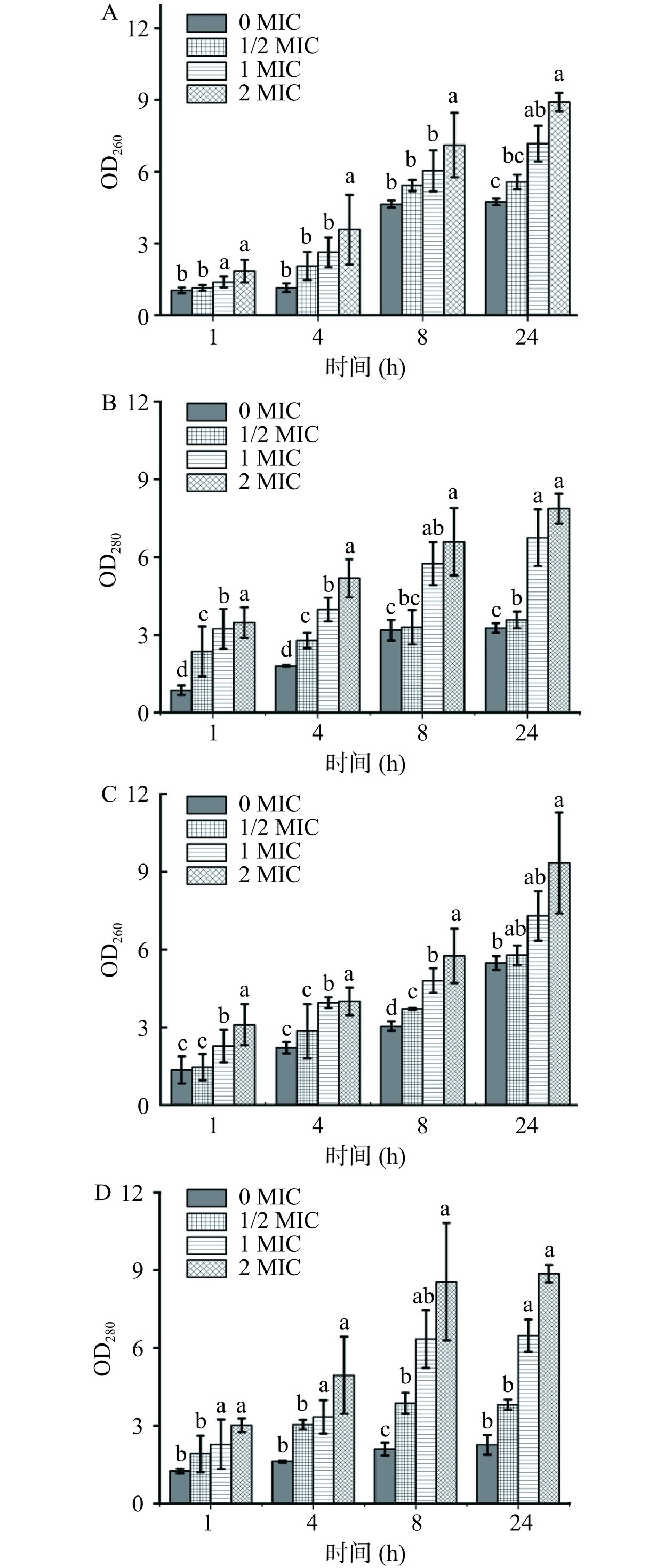
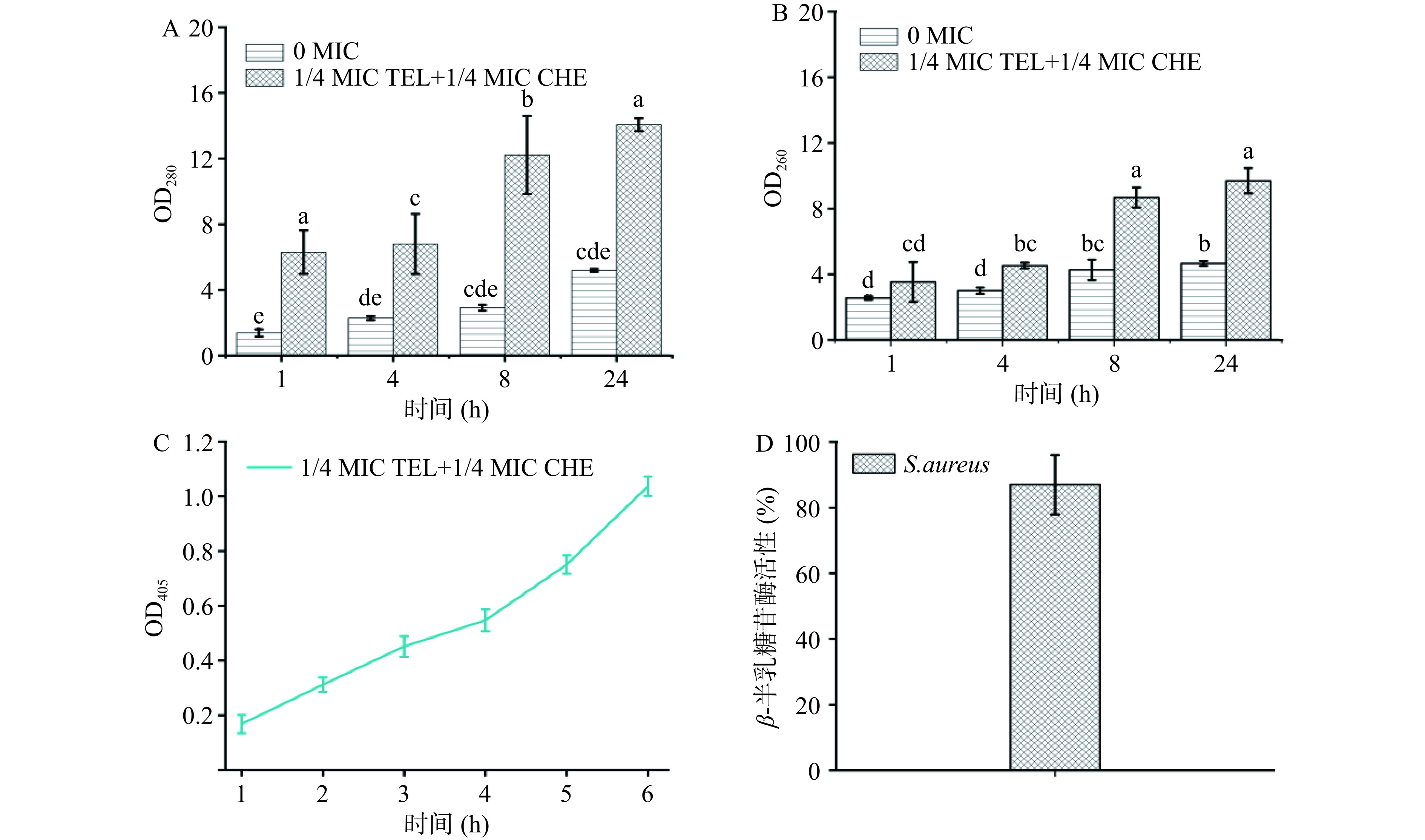
当菌体细胞膜受到损伤后,胞内大分子物质会向胞外渗出[23],因此根据核酸和蛋白质的含量变化可作为评价细胞膜完整性的指标。实验通过核酸、蛋白质等大分子物质在260、280 nm处的吸光值判断菌体胞内大分子泄漏情况[24−25]。结果如图5所示,在花旗松素作用下,两株菌的OD260和OD280值均有显著增加(P<0.05),且随浓度升高而增大。这说明随花旗松素浓度增加,细菌细胞膜的通透性更高,使正常情况下不能透过细胞膜的大分子物质大量泄漏,进而影响细胞正常生长,抑制菌体繁殖。
随培养时间推移,两株细菌对照组的OD260和OD280值也有所增加,这可能是由于细胞受到了轻微的破损或者机械损伤,但含有花旗松素的实验组明显较对照更高。以E. coli为例,培养至24 h的1/2、1、2 MIC实验组OD260测试值是同期对照组的1.18、1.52和1.88倍,表明菌体有大量内容物泄露,与花旗松素浓度呈正相关,且泄漏量随处理时间延长而增大。对比两株供试菌受到影响的程度,E. coli组OD260变化较S. aureus组更大,这可能是由于花旗松素对革兰氏阴性菌的细胞壁膜破坏更明显所致。
2.5 花旗松素对菌体细胞质膜渗透性影响
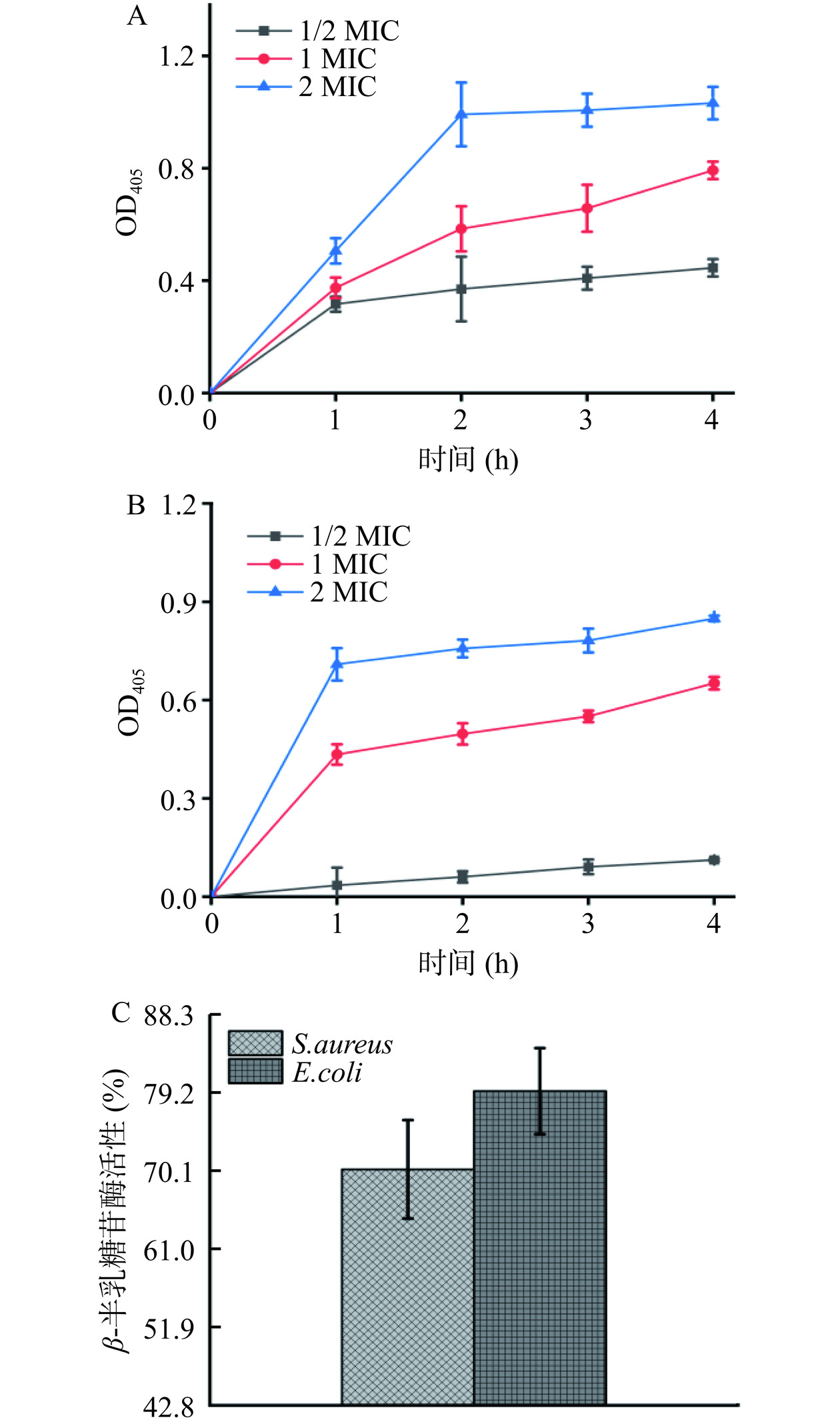
β-半乳糖苷酶是细菌胞质内的水解酶,可分解乳糖。研究发现,ONPG作为乳糖类似物可进入菌体细胞被β-半乳糖苷酶水解,产生半乳糖和在405 nm有特征吸收的邻-硝基苯酚。若细胞膜遭受破坏,膜通透性增强,ONPG可迅速进入菌体细胞[26]。因此可以通过测定OD405判断邻-硝基苯酚的产生量,进而评价花旗松素对细胞膜渗透性的作用。结果如图6所示,可见添加花旗松素后,E. coli和S. aureus的细胞质膜渗透性均有显著增加(P<0.05)。在培养作用初期,花旗松素对E. coli膜渗透性作用明显,随花旗松素浓度增大而增强。随处理时间延长,花旗松素对S. aureus膜渗透性影响逐渐增强,4 h时细菌膜渗透性较E. coli更高。
将花旗松素处理后的菌液离心测试β-半乳糖苷酶相对活性,观察胞外β-半乳糖苷酶泄漏情况。结果表明,2 MIC花旗松素处理E. coli和S. aureus后,检测到胞外β-半乳糖苷酶的相对活性分别为79.15%和70.1%,说明花旗松素的引入使菌体细胞膜受到破坏,导致上清液中有大量β-半乳糖苷酶泄漏,这与胞内大分子物质泄漏的测试结果一致。
2.6 花旗松素对菌体胞内抗氧化酶活性影响
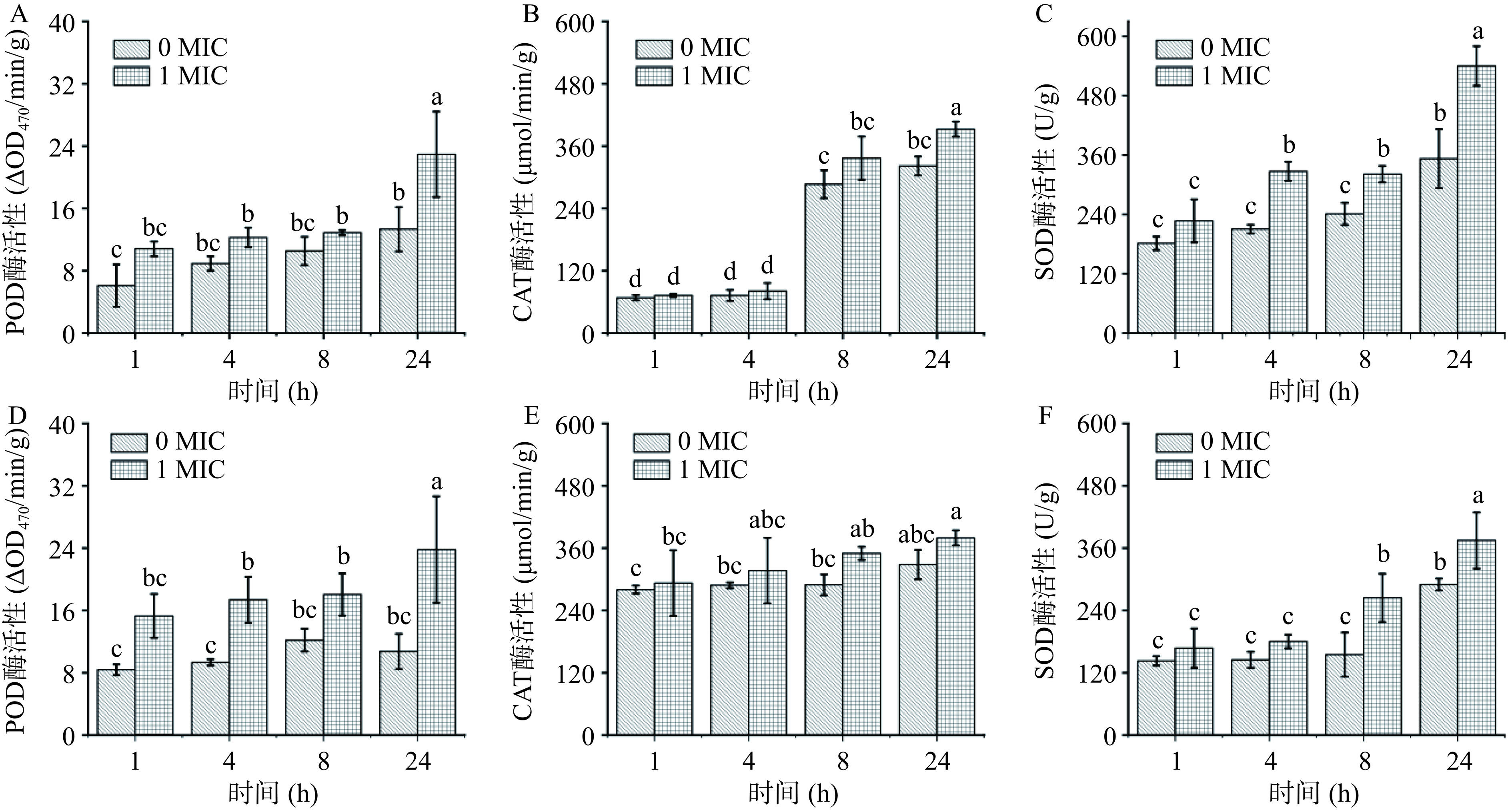
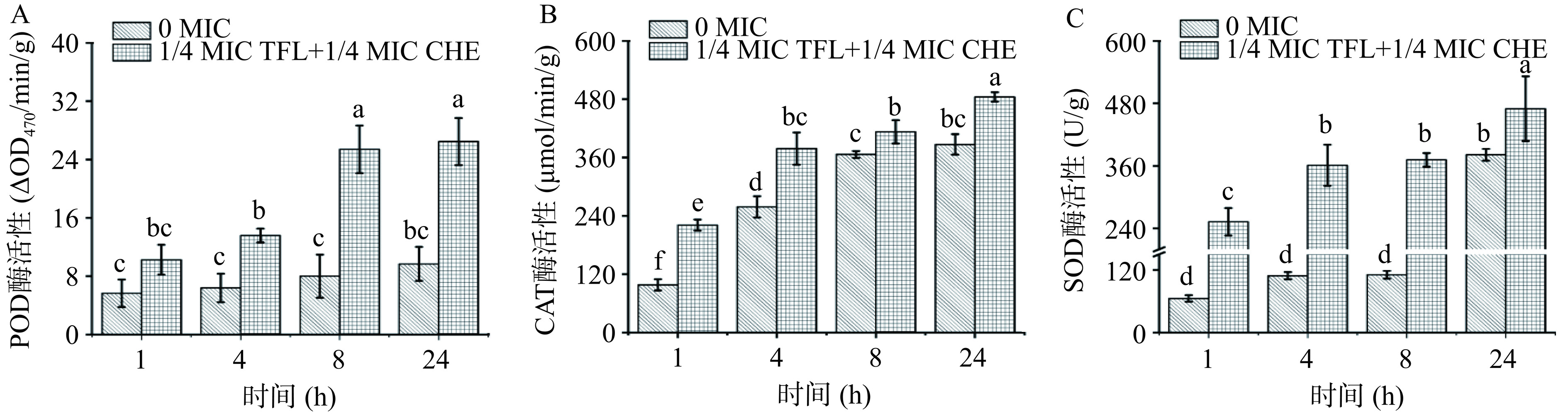
在遭受环境胁迫时,微生物胞内电子氧化呼吸链会受到影响,自由基代谢平衡被打破,诱导氧化应激[19]。过氧化物酶(POD)、过氧化氢酶(CAT)、超氧化物歧化酶(SOD)是胞内清除超氧自由基的抗氧化酶系,保护机体免受应激损伤[27]。如图7所示,在花旗松素作用下,E. coli和S. aureus胞内POD、CAT、SOD酶活较对照组明显升高,且随作用时间延长而增大。当花旗松素作用于E. coli到24 h时,各实验组差异最大,其POD、CAT、SOD酶活分别是对照组的1.72、1.22、1.53倍。在花旗松素对S. aureus作用中,CAT和SOD在8 h时最显著,分别是对照组的1.21、1.71倍,24 h时POD差异最大,是对照组的2.54倍。这说明花旗松素作用后使细菌均产生了不同水平的氧化压力,生成了更多的ROS造成菌体损伤,而细菌自身为应对这类刺激产生了更高水平的抗氧化酶。
2.7 花旗松素与白屈菜红碱联合抑菌效果
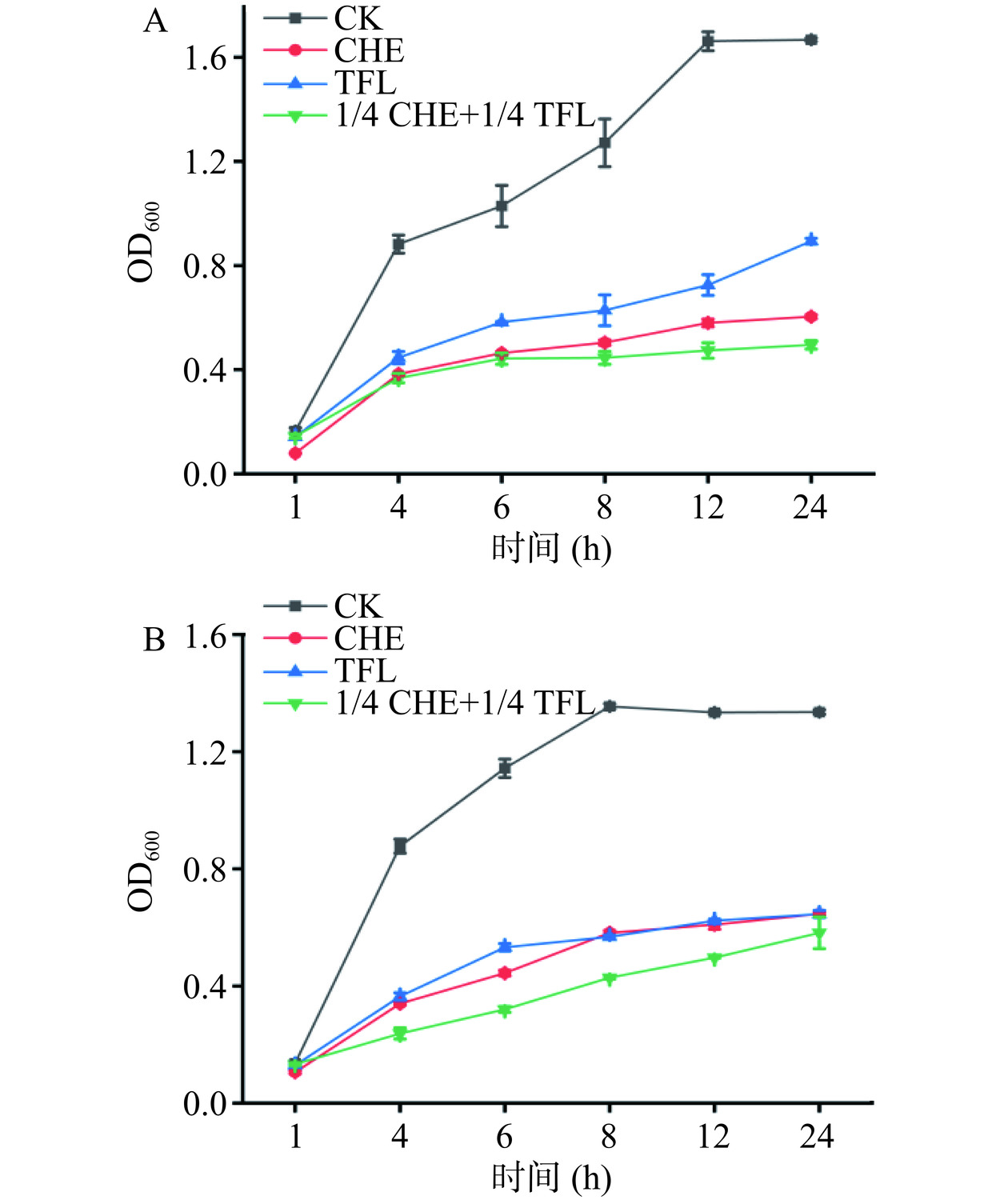
如图8,当将白屈菜红碱和花旗松素以1/4 CHE+1/4 TFL浓度混合用于联合抑菌时发现,对于E. coli而言,药物联用效果在前4 h中不明显,在6~24 h过程中较1/4 MIC白屈菜红碱和1/4 MIC花旗松素单独作用效果更显著。对S. aureus来说,两种药物联用后对细菌生长的抑制作用持续到24 h,效果明显。通过Bliss独立模型[28]计算药物联合作用对E. coli和S. aureus生长速率结果分别为−0.0279和0.0142,表明花旗松素和白屈菜红碱药物联用后对E. coli表现为拮抗作用,而对S. aureus表现为协同作用。后续实验将对药物联用于S. aureus的协同作用机制进行主要分析。
2.8 花旗松素与白屈菜红碱联合抑菌的探究
如图9,相较于前文中对花旗松素作用于S. aureus的胞内大分子物质泄漏情况,1/4白屈菜红碱+1/4花旗松素浓度的白屈菜红碱和花旗松素联用后,测得实验组OD260和OD280值明显较花旗松素单独作用更高,甚至分别是24 h 2 MIC浓度花旗松素的1.04倍和1.6倍。此外,通过在405 nm处测试邻-硝基苯酚的吸光度值发现,在药物联用作用下培养液吸光度值随菌体生长和培养时间延长而逐步上升,说明有大量的ONPG被β-半乳糖苷酶水解,表明细胞膜渗透性增强。而且,培养上清液中β-半乳糖苷酶活达87.01%,较2 MIC花旗松素单独作用S. aureus酶活性高16.91%,这也说明药物联用后对菌体细胞膜破坏更严重,导致β-半乳糖苷酶泄露量增多。
联合抑菌作用后胞内POD、CAT和SOD酶活变化如图10所示。相较对照组,各抗氧化酶活均有所上升。经比较分析,24 h CAT、SOD和POD酶活较单独使用1 MIC浓度花旗松素对S. aureus作用要更高,分别是其1.28、1.25、1.11倍。表明1/4 MIC浓度花旗松素和1/4 MIC浓度白屈菜红碱对菌体杀伤作用更大,氧化压力更强。
3. 结论
本实验通过在落叶松中利用超声辅助乙醇热浸提法得到纯度较高的花旗松素纯度为90%,将其用于抑菌研究。发现其对革兰氏阴性和阳性菌具有较好的抑菌效果,花旗松素对大肠杆菌与金黄色葡萄球菌MIC均为1.2 mg/mL,抑菌效果达到80%以上。并通过观察抑菌过程中的形态变化,发现明显褶皱、凹陷干瘪、菌体表面有不规则突起,均匀且出现空腔,开始出现消融现象,胞内大分子物质泄漏24 h OD260均与对照组相比增长了3.21、3.01倍,说明花旗松素对菌体细胞膜产生破坏作用,导致大量核苷酸泄露。2 MIC花旗松素处理E. coli和S. aureus后,检测到胞外β-半乳糖苷酶的相对活性分别为79.15%和70.1%,说明花旗松素的引入使菌体细胞膜受到破坏,导致上清液中有大量β-半乳糖苷酶泄漏,这与胞内大分子物质泄漏的测试结果一致。抗氧化酶活性测定中发现酶活性增强,与花旗松素破坏菌体产生了更多的ROS引起菌体应激性增强有关。与白屈菜红碱进行药物联合抑菌实验,发现二者对革兰氏阴性菌表现为拮抗作用,而革兰氏阳性菌具有协同作用,这可能是由于细菌细胞壁膜组成成分差异所致。在联合抑菌实验中,添加较低浓度的花旗松素和白屈菜红碱即可达到更好的抑菌效果,细胞膜渗透性大幅增加比单独作用S. aureus酶活性高16.91%,这也说明药物联用后对菌体细胞膜破坏更严重,导致β-半乳糖苷酶泄露量增多,1/4 MIC药物联用对菌体杀伤力更大氧化压力也随之增强,伴有大量的胞内大分子物质泄漏。这说明二者联用的协同抑菌效果主要是通过增强对细胞膜的破坏实现的。基于此,我们在下一步研究计划中将主要针对典型食源性致病菌,挖掘花旗松素及其与生物碱联用的广谱抑菌性能和机理,以扩展花旗松素产品的应用领域,制造可观的生态经济效益,使其成为特色林业产业发展的新亮点。
-
-
[1] 张星艳, 李新, 李虎玲, 等. 花旗松素植物来源, 提取方法和药理作用的研究进展[J]. 中草药,2022,2(3):23−35. [ZHANG X Y, LI X, LI H L, et al. Research progress on origin, extraction method and pharmacological activities of taxifolin[J]. Chinese Traditional and Herbal Drugs,2022,2(3):23−35.] ZHANG X Y, LI X, LI H L, et al. Research progress on origin, extraction method and pharmacological activities of taxifolin[J]. Chinese Traditional and Herbal Drugs, 2022, 2(3): 23−35.
[2] 逄弓一郎. 落叶松木中二氢槲皮素提取纯化工艺研究[D]. 哈尔滨:黑龙江中医药大学, 2009. [PANG G Y L. Study on technology of extraction and purification dihyroquercetin from larch wood[D]. Harbin:Heilongjiang University of Chinese Medicine, 2009.] PANG G Y L. Study on technology of extraction and purification dihyroquercetin from larch wood[D]. Harbin: Heilongjiang University of Chinese Medicine, 2009.
[3] LIU Z, GU H, YANG L. A novel approach for the simultaneous extraction of dihydroquercetin and arabinogalactan from Larix gmelinii by homogenate-ultrasound-synergistic technique using the ionic liquid[J]. Journal of Molecular Liquids,2018,261(6):41−49.
[4] MAHDAVIMEHR M, MERATAN A A, GHOBEH M, et al. Inhibition of hewl fibril formation by taxifolin:Mechanism of action[J]. PLoS One,2017,12(11):e0187841. doi: 10.1371/journal.pone.0187841
[5] MURAMATSU D, UCHIYAMA H, KIDA H, et al. Cell cytotoxity and anti-glycation activity of taxifolin-rich extract from Japanese larch, Larix kaempferi[J]. Food Control,2019,5(7):e02047.
[6] 董潞娜, 曹浩, 张欣宇, 等. 二氢槲皮素的研究进展[J]. 生物技术进展,2020,10(3):8−35. [DONG L N, CAO H, ZHANG X Y, et al. Research progress on dihydroquercetin[J]. Current Biotechnology,2020,10(3):8−35.] DONG L N, CAO H, ZHANG X Y, et al. Research progress on dihydroquercetin[J]. Current Biotechnology, 2020, 10(3): 8−35.
[7] 周佳, 邱智东, 王雨辰, 等. 白屈菜红碱的研究进展[J]. 人参研究,2022,34(6):46−49. [ZHOU J, QIU Z D, WANG Y C, et al. Research progress of chelerythrine[J]. Ginseng Research,2022,34(6):46−49.] doi: 10.19403/j.cnki.1671-1521.2022.06.013 ZHOU J, QIU Z D, WANG Y C, et al. Research progress of chelerythrine[J]. Ginseng Research, 2022, 34(6): 46−49. doi: 10.19403/j.cnki.1671-1521.2022.06.013
[8] 韦庆慧. 白屈菜红碱及其衍生物对植物病原菌的抑制机理研究[D]. 哈尔滨:东北林业大学, 2020. [WEI Q H. Study on the inhibition me chanism of chel erythrine and its derivatives against plant patho gens[D]. Harbin:Northeast Forestry University, 2020.] WEI Q H. Study on the inhibition me chanism of chel erythrine and its derivatives against plant patho gens[D]. Harbin: Northeast Forestry University, 2020.
[9] 王佳奇, 宋明铭, 陈凯, 等. 响应面试验优化落叶松中二氢槲皮素纯化工艺[J]. 食品科学,2016,37(4):5−23. [WANG J Q, SONG M M, CHEN K, et al. Optimization of purification process for dihydroquercetin in larch roots by response surface methodology[J]. Food Science,2016,37(4):5−23.] doi: 10.7506/spkx1002-6630-201604004 WANG J Q, SONG M M, CHEN K, et al. Optimization of purification process for dihydroquercetin in larch roots by response surface methodology[J]. Food Science, 2016, 37(4): 5−23. doi: 10.7506/spkx1002-6630-201604004
[10] LUO S, XIE L, CHEN J, et al. Determination and pharmacokinetic profiles of four active components from Scrophularia ningpoensis hemsl. in rats[J]. Frontiers in Pharmacology,2021,11(6):12−26.
[11] PENG J S, KE S I, HAN N H. HPLC determination of bioactive flavonoids in hovenia dulcis fruit extracts[J]. Journal of Chromatographic Science,2016,6(13):23−36.
[12] 王筱瑜. 银杏落叶多糖的提取、分离纯化及其抑菌性的研究[D]. 锦州:锦州医科大学, 2019. [WANG X Y. Extraction, isolation, purification, and biological activities of polysaccharides from Ginkgo biloba L. leaves[D]. Jinzhou:Jinzhou Medical University, 2019.] WANG X Y. Extraction, isolation, purification, and biological activities of polysaccharides from Ginkgo biloba L. leaves[D]. Jinzhou: Jinzhou Medical University, 2019.
[13] 姜媛媛. 丹参药渣多糖的提取及其生物活性和安全性评价[D]. 雅安:四川农业大学, 2016. [Extraction, bioactivities and safety evaluation of polysaccharide isolated from Salvia miltiorrhiza Bunge residu[D]. Ya'an:Sichuan Agricultural University, 2016.] Extraction, bioactivities and safety evaluation of polysaccharide isolated from Salvia miltiorrhiza Bunge residu[D]. Ya'an: Sichuan Agricultural University, 2016.
[14] JING C, TAN W, MING Y. Surface physiological changes induced by lactic acid on pathogens in consideration of pKa and pH[J]. Food Control,2014,46(6):525−531.
[15] 胡梅. 鹿衔草水提物对粪肠球菌抑制作用及抑菌机理的研究[D]. 南宁:广西大学, 2018. [HU M. Study on the bacteriostatic action and mechanism of the aqueous extract of Herba pyrolae on enterococcus faecalis[D]. Nanning:Guangxi University, 2018.] HU M. Study on the bacteriostatic action and mechanism of the aqueous extract of Herba pyrolae on enterococcus faecalis[D]. Nanning: Guangxi University, 2018.
[16] METRYKA O, WASILKOWSKI D, MROZIK A. Insight into the antibacterial activity of selected metal nanoparticles and alterations within the antioxidant defence system in Escherichia coli, Bacillus cereus and Staphylococcus epidermidis[J]. International Journal of Molecular Sciences,2021,22(21):56−68.
[17] 吴海霞. 银杏种仁抑菌蛋白及其抑菌机制研究[D]. 南京:南京林业大学, 2014. [WU H X. Study on antimicrobial activity and mechanism of protein from Ginkgo biloba seeds[D]. Nanjing:Nanjing Forestry University, 2014.] WU H X. Study on antimicrobial activity and mechanism of protein from Ginkgo biloba seeds[D]. Nanjing: Nanjing Forestry University, 2014.
[18] HEGRENESS M, SHORESH N, DAMIAN D, et al. Accelerated evolution of resistance in multidrug environments[J]. Proceedings of the National Academy of Sciences of the United States of America,2008,105(37):77−86.
[19] NIU A, BIAN W P, FENG S L, et al. Role of manganese superoxide dismutase (Mn-SOD) against Cr(III)-induced toxicity in bacteria[J]. Journal of Hazardous Materials,2020,403(15):36−56.
[20] HOJABRI Z, ARAB M, DARABI N, et al. Evaluation of the commercial combined disk test and minimum inhibitory concentration (MIC) determination for detection of carbapenemase producers among Gram-negative Bacilli isolated in a region with high prevalence of blaOXA-48 and blaNDM[J]. International Microbiology,2019,22(1):81−89. doi: 10.1007/s10123-018-0030-1
[21] 刘文丛, 刘兴龙, 孟令全, 等. 二氢槲皮素的研究进展[J]. 吉林农业大学学报, 2018, 40(4):444-8. [LIU W C, LIU X L, MENG L Q, et al. Research progress of dihydroquercet[J]. Journal of Jilin Agricultural University, 2018, 40(4):444-448.] LIU W C, LIU X L, MENG L Q, et al. Research progress of dihydroquercet[J]. Journal of Jilin Agricultural University, 2018, 40(4): 444-448.
[22] AN V E, IING S V, IENG K O. Comparative analysis of morphology and optical properties of GaN layers on sapphire[J]. Journal of Surface Investigation X Ray Synchrotron & Neutron Techniques,2009,6(13):43−59.
[23] OU A T, OU M A, AN B S, et al. Antibacterial activity of Syzygium aromaticum seed:Studies on oxidative stress biomarkers and membrane permeability[J]. Microbial Pathogenesis,2016,95(15):208−15.
[24] AN A, SHENG A, DING A, et al. A facile one pot synthesis of novel pyrimidine derivatives of 1,5-benzodiazepines via domino reaction and their antibacterial evaluation[J]. Journal of Microbiological Methods,2008,163(5):53−69.
[25] GENNARO R, ZANETTI M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides[J]. Biopolymers,2015,55(1):31−49.
[26] AROKIYARAJ S, BHARANIDHARAN R, AGASTIAN P, et al. Chemical composition, antioxidant activity and antibacterial mechanism of action from Marsilea minuta leaf hexane:Methanol extract[J]. Chemistry Central Journal,2018,12(1):1−11. doi: 10.1186/s13065-017-0364-3
[27] ZHAO W, VICINI P, NOVICK S, et al. Detecting bliss synergy in in vivo combination studies with a tumor kinetic model[J]. Pharmaceutical Statistics,2019,39(5):67−79.
[28] 谭超, 周靖轩, 卢倩倩, 等. 紫草素与依布硒啉对金黄色葡萄球菌的协同作用[J]. 微生物学报, 2022, 62(3):1049−60. [TAN C, ZHOU J X, LU Q Q, et al. The synergistic effect of shikonin and ebselen against Staphylococcus aure[J]. Acta Microbiologica Sinica, 2022, 62(3):1049−1060.] TAN C, ZHOU J X, LU Q Q, et al. The synergistic effect of shikonin and ebselen against Staphylococcus aure[J]. Acta Microbiologica Sinica, 2022, 62(3): 1049−1060.
-
期刊类型引用(17)
1. 高思聪,黄文选,黄志超,缪铭. 食叶草蛋白的提取工艺及其性质研究. 食品与发酵工业. 2025(01): 189-198 .  百度学术
百度学术
2. 贺嫣然,王婧,许家强,夏昭昭,刘应蛟,董子舒,明良山,刘红宁,樊启猛. 补中益气方不同剂型中活性成分含量及其生物活性比较. 中成药. 2025(02): 357-364 .  百度学术
百度学术
3. 刘威,蔡卫佳,王昊,罗桂杰,刘旭. 食叶草研究进展. 中国农学通报. 2025(03): 36-41 .  百度学术
百度学术
4. 金敬红,张子昂,姚正颖,李楠楠. 食叶草降糖大米的研制及其对α-葡萄糖苷酶的抑制活性. 食品安全质量检测学报. 2025(06): 183-189 .  百度学术
百度学术
5. 何曾杨,李鑫垚,邹剑锋,孙梦姗,曾建国. 食叶草种子萌发特性研究. 湖南农业科学. 2025(02): 17-21 .  百度学术
百度学术
6. 龙伽雯,李跃辉. 新食品原料的审批及应用现状研究. 食品与发酵工业. 2025(08): 394-404 .  百度学术
百度学术
7. 刘威,王昊,蔡卫佳,罗桂杰,刘旭. 不同生育期食叶草营养物质变化规律及饲用价值分析. 中国奶牛. 2025(04): 47-52 .  百度学术
百度学术
8. 淳新月,任顺成. 食叶草蛋白的提取工艺优化及其氨基酸分析. 河南工业大学学报(自然科学版). 2024(01): 24-30+47 .  百度学术
百度学术
9. 吴瑞芳,刘瑶,赵玲玲,胥伟,郭丹郡,朱玲娇,易阳,王宏勋. 食叶草蛋白提取工艺的优化及功能特性分析. 武汉轻工大学学报. 2024(03): 66-75 .  百度学术
百度学术
10. 王龙霞,纵伟. 食叶草多酚超声提取工艺优化及抑制α-葡萄糖苷酶活性的研究. 食品与发酵科技. 2024(05): 61-66 .  百度学术
百度学术
11. 胡锦瑞,李鑫垚,凌浩,曾建国,刘秀斌. 食叶草不同部位营养成分分析. 饲料工业. 2024(21): 118-124 .  百度学术
百度学术
12. 何泽娟,操粮骏,王燕华,王冬钰,赵林芬,张乃明,谭超. 西垂茉莉的营养成分、抗氧化活性及代谢产物分析. 食品安全质量检测学报. 2024(21): 155-164 .  百度学术
百度学术
13. 王晓杰,刘抗,张丽,郑明明,周裔彬. 食叶草的营养价值及开发前景. 食品与机械. 2023(02): 164-169 .  百度学术
百度学术
14. 孟楠,秦令祥,曹源,高愿军. 超微冷冻前处理协同渗漉法提取食叶草黄酮工艺优化及其抗氧化、降血糖活性研究. 食品安全质量检测学报. 2023(13): 249-257 .  百度学术
百度学术
15. 胡高爽,刘紫洋,张亦琴,王晨宇,王君,王青华,郝建雄. 食叶草的营养成分及应用价值研究进展. 食品研究与开发. 2023(16): 208-212 .  百度学术
百度学术
16. 左梦微,任顺成,王凤成. 食叶草多酚类物质的提取、精制工艺研究. 河南工业大学学报(自然科学版). 2023(06): 36-43 .  百度学术
百度学术
17. 吴静,王珍珍,王晓宇,罗丹,蒋增良,沙如意,毛建卫,崔艳丽. 酿酒酵母发酵与自然发酵过程中霍山石斛酵素的代谢物及抗氧化变化. 中国生物工程杂志. 2022(11): 73-87 .  百度学术
百度学术
其他类型引用(5)





 下载:
下载:
 下载:
下载:



