Effect of Polygonatum sibiricum Extract in Improving the Organ Function of D-Galactose-induced Aging Mice and Its Mechanism
-
摘要: 目的:旨在探讨黄精提取物(Polygonatum sibiricum extract,PSE)对D -半乳糖(D-galactose,D -gal)致衰老小鼠重要脏器的保护作用及其潜在的分子机制。方法:将小鼠随机分为五组(n=10):对照组、D-gal(500 mg/kg)组、PSE低(0.5 g/kg)、中(1 g/kg)、高(2 g/kg)剂量组。测定小鼠器官指数(胸腺、脾脏、肝脏、肾脏);测定血清中肌酸激酶(CK)、肌酸激酶同工酶(CK-MB)、谷丙转氨酶(ALT)、谷草转氨酶(AST)、碱性磷酸酶(ALP)、尿酸(UA)、尿素(UREA)、肌酐(CREA)、尿素肌酐比值(BUN/Scr)、肾小球滤过率(eGFR)活性;HE及Masson染色观察各组小鼠皮肤、肝脏、肾脏、心脏、大脑、肺等器官病理学变化;用Real-time PCR法检测各组小鼠肝脏、肾脏、心脏、大脑等组织中p53、p16、p21、RB、HO-1、Nrf2及Keap1 mRNA的表达水平。结果:与D-gal组相比,PSE各剂量组胸腺、脾脏、肝脏和肾脏指数均升高(P<0.05);此外,在各治疗组中,PSE降低了(P<0.05)小鼠血清中CK、CK-MB、ALT、AST、ALP、UA、UREA、CREA和BUN/Scr水平,升高了eGFR(P<0.05)水平;HE及Masson染色发现,PSE能减轻D-gal对小鼠重要器官造成的病理损伤;与对照组比较,模型组肝、肾、心、脑中p53、p16、p21、RB、Keap1 mRNA表达升高(P<0.05),HO-1、Nrf2 mRNA表达降低(P<0.05),与D-gal组比较,PSE各治疗组小鼠肝脏、肾脏、心脏、大脑组织中p53、p16、p21、RB、Keap1 mRNA降低(P<0.05),HO-1、Nrf2 mRNA升高(P<0.05)。结论:PSE具有延缓衰老作用,其机制可能与其抑制p53/p21和p16-RB通路以及Keap1/Nrf2/HO-1通路有关。
-
关键词:
- 黄精提取物 /
- 抗衰老 /
- p53/p21信号通路 /
- p16-RB信号通路 /
- Keap1/Nrf2/HO-1信号通路
Abstract: Objective: The purpose of this study was to explore the protective effects of Polygonatum sibiricum extract (PSE) on the important organs of D-galactose (D-gal)-induced aging mice and its potential molecular mechanisms. Methods: The mice were randomly divided into five groups (n=10): control group, D-gal (500 mg/kg) group, low-PSE dose (0.5 g/kg) group, medium-PSE dose (1 g/kg) group, and high-PSE dose (2 g/kg) group. The organ indices (thymus, spleen, liver, and kidney) were detected. The levels of creatine kinase (CK), creatine kinase isoenzyme (CK-MB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), uric acid (UA), urea (UREA), creatinine (CREA), urea–creatinine ratio (BUN/Scr), and glomerular filtration rate (eGFR) in serum were measured. Pathological changes in the skin, liver, kidney, heart, brain, and lung of mice in different groups were analyzed by HE and Masson staining. The expressions of p53, p16, p21, RB, HO-1, Nrf2, and Keap1 mRNA in the liver, kidney, heart, and brain of each group of mice were detected by real-time PCR. Results: The thymus, spleen, liver, and kidney indices were increased in each PSE treatment group compared with those in the D-gal group (P<0.05). Moreover, in each treatment group, PSE decreased (P<0.05) the levels of CK, CK-MB, ALT, AST, ALP, UA, UREA, CREA, and BUN/Scr in mouse serum and increased the level of eGFR (P<0.05). HE and Masson staining showed that PSE could reduce the pathological damage caused by D-gal to the vital organs of mice. Compared with the blank group, the model group presented increased expression levels of p53, p16, p21, RB, and Keap1 mRNA in the liver, kidney, heart, and brain (P<0.05) and decreased HO-1 and Nrf2 mRNA (P<0.05). Compared with the D-gal group, mice in each PSE treatment group exhibited decreased mRNA levels of p53, p16, p21, RB, and Keap1 in the liver, kidney, heart, and brain tissues (P<0.05) and increased mRNA levels of HO-1 and Nrf2 (P<0.05). Conclusion: PSE has anti-aging effects, and its mechanism may be related to its inhibition of the p53/p21, p16-RB, and Keap1/Nrf2/HO-1 pathways. -
根据最新统计数据,预计到2050年,全球65岁以上老年人口数量将增加21.5%[1],达到15亿[2]。衰老是一种无法避免的自然现象,也是一个持续的生理过程,并且伴随着细胞、组织和器官的物质、形态改变和功能退化[3]。衰老会加剧许多与年龄有关的各种疾病的发生,包括神经系统退行性变、心血管疾病等。虽然衰老不可逆转,但可通过多种措施来延缓衰老,如食疗、中药干预、加强运动等途径[4-6]。进入21世纪后,随着全球老龄化日趋严重,由衰老和衰老相关疾病引起的健康问题日益突出。因此,尽快阐明衰老的机制,探寻有效的抗衰老药物,已成为刻不容缓的任务,有着重大的社会意义。
黄精是百合科植物多花黄精(Polygonatum cyrtonema Hua)、黄精(Polygonatum sibiricum Red.)或滇黄精(Polygonatum kingianum Coll.et Hemsl.)的干燥根茎,被收载于2020版中国药典。黄精是一种传统经典的药食两用植物,具有补气养阴、益肾、健脾、润肺的功效[7]。其口感好,具有甜味和香味,可长期大量食用。黄精作为药物或膳食补充剂的应用历史可以追溯到2000多年前,当时它被记录在汉末的《名医别录》一书中[8-9]。现代研究表明黄精具有多种活性成分,包括黄精多糖、薯蓣皂苷元、凝集素和高异黄烷酮等化合物[10-12]。现代药理学研究表明,黄精在抗菌、抗糖尿病、增强免疫力、抗疲劳、抗衰老等方面具有广泛的药理作用[13-15]。目前仅有的研究报道黄精具有延缓大脑衰老的作用。可通过清除自由基、减少Aβ的聚集、逆转突触结构改变、加速神经元的损伤修复与再生、改善学习记忆能力等来改善大脑衰老[16-19]。本课题组前期研究表明,黄精可通过提高端粒酶活性,降低细胞ROS水平,减轻细胞氧化损伤来保护衰老大鼠骨髓内皮祖细胞(endothelial progenitor cells, EPCs)的功能[20-21]。但未进行体内实验研究黄精对衰老小鼠器官功能的影响及机制。
因此,本研究利用D-gal诱导建立小鼠衰老模型,观察黄精提取物对衰老模型小鼠重要器官的保护作用,并探讨其抗衰老作用的分子机制,旨在为开发功能性食品和新型抗衰老及相关疾病的药物提供新思路,同时促进道地药材黄精的开发利用。
1. 材料与方法
1.1 材料与仪器
SPF级昆明小鼠50只 雌雄各半,8周龄,体重20±2 g,购自贵州医科大学实验动物中心(动物生产合格证号:SCXK(京)2019-0010),饲养于贵州医科大学组织工程与干细胞实验中心标准动物房:室温20~25 ℃,湿度30%~40%,光照明暗交替12 h,自由饮水,常规饲料喂养;黄精饮片(20062701) 贵州一品药业连锁有限公司;D-半乳糖(V900922-100 g) 美国Sigma公司;trizol试剂(15596026-100 mL) 美国Invitrogen公司;逆转录(RT)试剂盒、荧光定量PCR试剂盒(RR047A-100T, RR820A -200T) TaKaRa公司;PCR引物 Invtrogen公司。
Hc3016r 高速冷冻离心机 安徽中科中佳科学仪器有限公司;Gscan20 数字病理切片扫描仪 日本Hamamatsu有限公司;JC-FD200 金属恒温孵育器 德国Eppendorf公司;CFX96荧光定量PCR仪 美国Bio-Rad公司;TBAFX8 全自动生化分析仪 上海瑞美电脑科技有限公司。
1.2 实验方法
1.2.1 黄精提取物(PSE)的制备
取黄精饮片15 g,粉碎,过4号筛;用95%的乙醇提取三次;每次按1:10的比例,提取温度为80 ℃,提取时间为1 h;收集三次粗液,冷却后过滤,浓缩至浸膏,制备得到PSE[22]。计算PSE得率为23.48%,−20 ℃保存备用。用UHPLC-Q-Orbitrap-HRMS鉴定PSE成分为皂苷类。
1.2.2 分组、造模与治疗
50只小鼠随机分为正常组(Control组)、模型组(D-gal组)、PSE低剂量组(L-PSE组)、PSE中剂量组(M-PSE组)、PSE高剂量组(H-PSE组)。本次研究造模方法参照文献[23],模型组、PSE低、中、高剂量组小鼠颈背部皮下注射500 mg/ kg/d D-半乳糖构造衰老模型,连续8周。第4周开始灌胃相应剂量的PSE(低、中、高剂量组分别为0.5、1、2 g/kg),连续给药四周,正常组皮下注射等量的生理盐水作为对照,正常组与模型组灌胃生理盐水。(皮下注射生理盐水是与D-gal对照,而灌胃生理盐水是与给药组进行对照)。贵州医科大学动物伦理委员会审查并批准实验程序(NO:2001976)。
1.2.3 血清样本采集
小鼠禁食12 h,眼眶采血,3500 r/min离心15 min,吸取上清,−20 ℃保存备用。采用全自动血液生化分析仪测定肌酸激酶(CK)、肌酸激酶同工酶(CK-MB)、谷丙转氨酶(ALT)、谷草转氨酶(AST)、碱性磷酸酶(ALP)、尿酸(UA)、尿素(UREA)、肌酐(CREA)、尿素肌酐比值(BUN/Scr)、肾小球滤过率(eGFR)活性。
1.2.4 脏器指数的测定
小鼠禁食12 h,颈椎脱位处死小鼠,取出各组小鼠的肝、胸腺、脾脏、肾脏等组织,用生理盐水清洗干净脏器组织,称取脏器组织重量。
1.2.5 组织病理学和纤维化分析
取小鼠皮肤、肝脏、肾脏、心脏、大脑等组织进行HE和Masson染色,光学显微镜观察各组小鼠以上组织病理学和纤维化变化。
1.2.6 Realtime-PCR 检测大脑、肝脏、肾脏、心脏组织中p53、p16、p21、RB、HO-1、Nrf2、Keap1 mRNA表达水平
取血后,颈椎脱位处死小鼠,取心脏,肝脏和肾脏和全脑组织。参照说明书用Trizol法提取大脑、肝脏、肾脏、心脏总mRNA,用Takara逆转录试剂盒合成cDNA。合成后,按照TB Green Premix Ex Taq[TM]试剂盒说明书依次添加试剂,再置于PCR仪进行基因扩增。分析PCR反应中各组样本的Ct 值,并用2-△△Ct表示mRNA 相对表达水平。p53、p16、p21、RB、HO-1、Nrf2、Keap1及内参GAPDH序列见表1。
表 1 RT-PCR引物序列Table 1. Primer sequence of RT- PCR基因 正向引物(5’-3’) 反向引物(5’-3’) p53 TGGAAGGAAAATTTGTATCCCGA GTGGATGGTGGTATACTCAGAG p16 ACATCAAGACATCGTGCGATATT CCAGCGGTACACAAAGACCA p21 TGTCTTGCACTCTGGTGTCTG ATCTGCGCTTGGAGTGATAGA RB TCGATACGAGTACCAAGGTTGA ACACGTCCGTTCTAATTTGCTG HO-1 AAGCCGAGAATGCTGAGTTCA GCCGTGTAGATGGTACAAGGA Nrf2 GTGCTCCTATGCGTGAAT TACCTCTCCTGCGTATATCT Keap1 TGCCCCTGTGGTCAAAGTG GGTTCGGTTACCGTCCTGC GAPDH AAGGTCATCCATGACAACTTTGGC ACAGTCTTCTGGGTGGCAGTGAT 1.3 数据处理
所有数据均表示为平均值±SD。使用SPSS26.0进行统计分析。采用单因素方差分析(ANOVA)进行不同组间的比较,P<0.05为具有统计学意义。
2. 结果与分析
2.1 PSE对D-gal致衰老小鼠脏器指数的影响
免疫器官随着衰老会导致免疫器官指数的降低,这通常被用作评估衰老小鼠模型成功的标志。结果如表2所示,8周的D-gal治疗导致胸腺和脾脏指数降低(P<0.01,P<0.05),D-gal组小鼠胸腺和脾脏萎缩, 说明造模成功。给予低中高剂量的PSE治疗则有所恢复胸腺指数和脾脏指数,L-PSE, M-PSE, H-PSE胸腺指数极显著提高(P<0.01),L-PSE脾脏指数较模型组提高(P<0.05),M-PSE, H-PSE脾脏指数较模型组极显著提高(P<0.01),说明PSE能延缓免疫器官衰老。肝、肾脏器指数的变化可体现肝脏和肾脏的衰老情况,一般机体正常时脏器指数比较恒定。当机体受外界因素影响时,引起脏器受损,其质量发生改变,故脏器指数也随之而改变。肝、肾脏器指数减小,表明肝肾损伤及其他退行性改变[24-25]。与对照组相比, D-gal组的肝脏指数、肾脏指数极显著降低(P<0.01),说明连续8周的D-gal治疗对小鼠肝脏和肾脏造成了损伤。低中高剂量的PSE治疗能升高肝脏和肾脏指数,L-PSE、M-PSE、H-PSE肝脏指数与模型组比较有所提高(P<0.05),L-PSE, M-PSE, H-PSE肾脏指数与模型组比较极显著提高(P<0.01),说明PSE具有保护小鼠脏器损伤、延缓衰老的功能。
表 2 PSE对D-gal致衰老小鼠脏器指数的影响($\overline{{{\rm{X}}}}\pm {{\rm{SD}}}$,n=10)Table 2. Effect of PSE on organ index of aging mice induced by D-gal ($\overline{\rm{X}}\pm \rm{SD}$, n=10)组别 胸腺指数(mg/g) 脾脏指数(mg/g) 肝脏指数(mg/g) 肾脏指数(mg/g) Control 3.98±0.25 3.05±0.53 61.19±2.64 13.98±0.46 D-gal 1.97±0.57** 2.11±0.47* 51.97±3.05** 11.63±0.50** L-PSE 3.48±0.50## 2.92±0.26# 58.25±2.87# 15.46±0.82## M-PSE 3.46±0.37## 4.37±0.54## 58.43±4.32# 14.95±1.11## H-PSE 3.54±0.30## 3.89±0.55## 60.29±6.36# 16.33±0.42## 注:*与Control组相比差异显著(P<0.05);**与Control组相比差异极显著(P<0.01);#与D-gal组相比差异显著(P<0.05);##与D-gal组相比,差异极显著(P<0.01);表3~表4同。 2.2 PSE对D-gal致衰老小鼠生化指标的影响
分析PSE对衰老小鼠血清中心肌酶水平的影响,肌酸激酶(CK)、肌酸激酶同工酶(CK-MB)可反应心脏功能,是心肌坏死的指标[26]。CK是心肌组织损伤的敏感指标之一,在心肌损伤期间呈上升趋势,临床上常作为急性心肌梗死的辅助诊断指标[27]。CK主要分布于心肌细胞中,当心脏发生病理性损伤时,心肌细胞中的代谢酶会释放入血,血清中CK活性会迅速升高[28]。CK-MB是CK的一种同工酶,主要存在于心肌细胞中。其含量在常态下较低,然而当心肌细胞受到损伤后,由于细胞和亚细胞室的解体而泄漏到细胞外液中,其水平会立即升高。是心肌损伤以及缺血坏死的早期标志物,其水平越高则表示心肌损伤加重[29-30]。如表3,在模型组小鼠的血清中观察到D-gal处理导致CK、CK-MB 活性升高(P<0.01),分别提高了170.54%、67.23%, 说明D-gal治疗损害了心脏功能。在本次研究中,用不同剂量PSE治疗可抑制血清中CK、CK-MB活性升高(P<0.01,P<0.05),PSE低、中、高剂量组CK水平分别降低了66.67%、68.53%和68.70%;CK-MB水平分别下降42.44%、48.35%和49.71%,表明PSE对D-gal 诱导的心脏损伤具有保护作用。
表 3 PSE对D-gal致衰老小鼠血清生化指标的影响($\overline{\rm{X}}\pm \rm{SD}$,n=10)Table 3. Effects of PSE on serum biochemical indexes in aging mice induced by D-gal ($\overline{\rm{X}}\pm \rm{SD}$, n=10)组别 心脏 肝脏 CK(U/L) CK-MB(U/L) ALP(U/L) AST(U/L) ALT(U/L) Control 486.5±226 185.67±28.34 55.88±7.64 63.29±8.92 30.17±3.98 D-gal 1316.17±221.58** 310.50±45.55** 84.57±13.03** 88.57±10.76** 38.83±2.54** L-PSE 438.67±55.15## 178.71±57.86# 57.38±15.76# 68.71±19.54# 31.00±4.00# M-PSE 414.17±142.46## 160.38±23.45## 53.25±15.01## 67.43±12.83# 28.33±6.77# H-PSE 412±180.78## 156.14±18.943## 48.88±13.03## 56.43±10.29## 25.83±5.76## 血清中ALP、AST和ALT水平是评价肝损伤的重要指标,连续8周的D-gal治疗会导致肝功能障碍,引起血清中ALP、AST和ALT水平显著升高[31-32]。ALT和AST是催化氨基酸和酮酸之间氨基转移的酶,主要位于肝细胞。当肝功能受损,例如葡萄糖和脂质代谢异常时,细胞膜的通透性增加,它们会释放到血液中,导致血清中ALT和AST水平升高。因此,血清ALT和AST水平是评估肝功能的重要且敏感的生化指标,可提示早期肝损伤,其异常升高可引起肝细胞损伤和坏死[33-34]。ALP是一种酸性水解酶,广泛存在于人体各组织细胞中,包括肝细胞。当肝组织发生病变坏死时,病变周围组织中肝细胞合成过度释放引起ALP排泄障碍,导致血清中ALP含量增加。因此ALP表达增多,提示机体肝脏功能受损[35]。如表3所示,与Control组相比,D-gal 组小鼠血清中ALP、AST和ALT水平显著上升(P<0.01),分别升高了51.34%、39.95%、28.72%,表明D-gal诱导肝脏发生损伤。与模型组相比,PSE低中高剂量组均能显著降低小鼠血清中ALP、AST和ALT水平(P<0.01,P<0.05),L-PSE、M-PSE、H-PSE组的ALP 活性分别降低了32.16%、37.04% 和42.20%;AST活性分别降低了22.41%、23.87%和36.29%;ALT活性分别降低了20.17%、27.04% 和33.47%。结果表明PSE对肝脏具有一定的保护作用。
有文献报道,用D-gal治疗会导致肾脏损伤以及功能障碍,随后血清酶的活性或水平升高[36],UA、UREA、CREA、BUN/Scr、eGFR通常被认为是肾脏的关键损伤指标,其水平可以评估衰老肾脏的功能性和器质性损伤[37]。UREA是机体蛋白质代谢的最终产物,是由肾脏排泄,其含量的高低由蛋白质的分解代谢与肾脏的排泄功能决定。在机体稳定的情况下,UREA和肾小球滤过率(eGFR)功能成反比关系, UREA升高,则eGFR降低,提示肾小球滤过功能减退[38]。血清UA过度生成/或排泄不足导致血清UA过度积累可能会加重炎症反应,进而干扰肾功能[39]。CREA、BUN/Scr水平明显异常时,引起机体内含氮化合物的代谢和肾小球滤过功能的异常改变,进而导致肾功能异常[40]。如表4所示,本研究表明,与对照组相比,D-gal组小鼠的UA、UREA、CREA、BUN/Scr水平较高(P<0.01,P<0.05),eGFR水平较低(P<0.05),这与文献报道一致[41]。然而PSE灌胃治疗后小鼠血清中UA、UREA、CREA、BUN/Scr水平降低(P<0.01,P<0.05),eGFR水平升高(P<0.05),表明PSE对D-gal诱导的肾损伤具有保护作用。
表 4 PSE对D-gal致衰老小鼠血清生化指标的影响($\overline{\rm{X}}\pm \rm{SD}$,n=10)Table 4. Effects of PSE on serum biochemical indexes in aging mice induced by D-gal ($\overline{\rm{X}}\pm \rm{SD}$, n=10)组别 肾脏 UA(mg/dL) UREA(mg/dL) CREA(mg/dL) eGFR(mg/dL) BUN/Scr(mg/dL) Control 106.67±34.66 6.13±0.52 11.92±1.33 245.38±31.53 121.88±8.91 D-gal 167.5±17.52** 7.50±0.94** 13.90±0.37* 236.88±6.28* 150.50±18.85* L-PSE 124.33±15.77# 5.59±0.83## 10.95±1.04# 244.25±12.91# 123.88±13.68# M-PSE 116.67±16.39## 5.54±0.88## 10.77±1.50# 244.25±11.21# 120.63±13.31## H-PSE 112.67±10.03## 5.46±0.50## 10.63±1.20## 251.75±40.41# 119.63±25.40## 2.3 PSE对D-gal致衰老小鼠脏器组织病理和胶原纤维的影响
2.3.1 PSE对D-gal致衰老小鼠皮肤组织病理和胶原纤维的影响
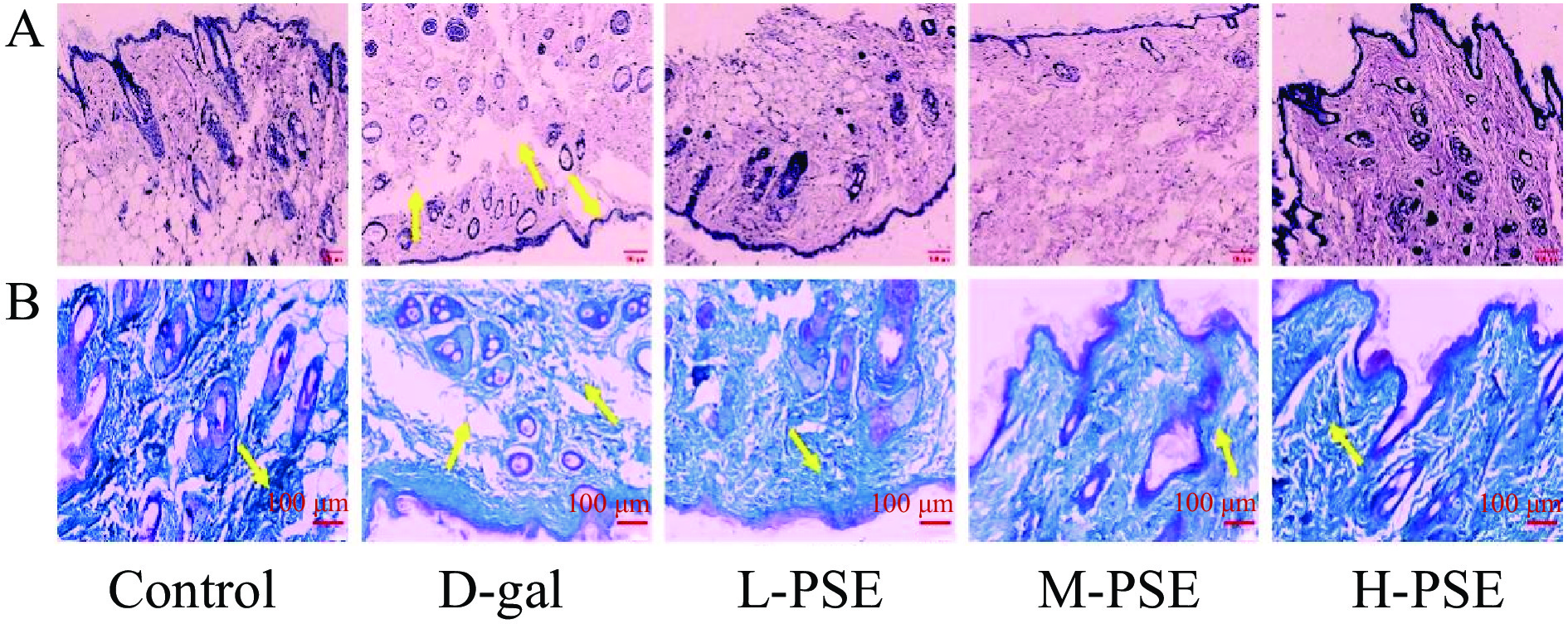
正常组小鼠皮肤结构正常完整,表皮厚度正常,皮脂腺副器官结构正常,胶原纤维束无明显断裂和溶解。D-gal 组的皮肤组织厚度不均匀,表皮缺失的部分明显更薄,纤维组织排列紊乱,有明显的碎裂。但是,在不同剂量PSE治疗的小鼠中,这些病理改变得到改善(图1A)。胶原纤维(主要是胶原蛋白)是真皮的重要组成部分,随着年龄的增长,胶原纤维的含量会显着下降[42]。Control组胶原纤维排列整齐且胶原沉积面积大,连续8周D-gal治疗导致胶原蛋白流失引起皮肤老化,不同剂量PSE治疗组胶原纤维排列整齐且胶原沉积面积较大(图1B)。说明PSE可减缓皮肤组织结构的破坏,延缓皮肤组织衰老的进程。
2.3.2 PSE对D-gal致衰老小鼠肝脏组织病理和胶原纤维的影响
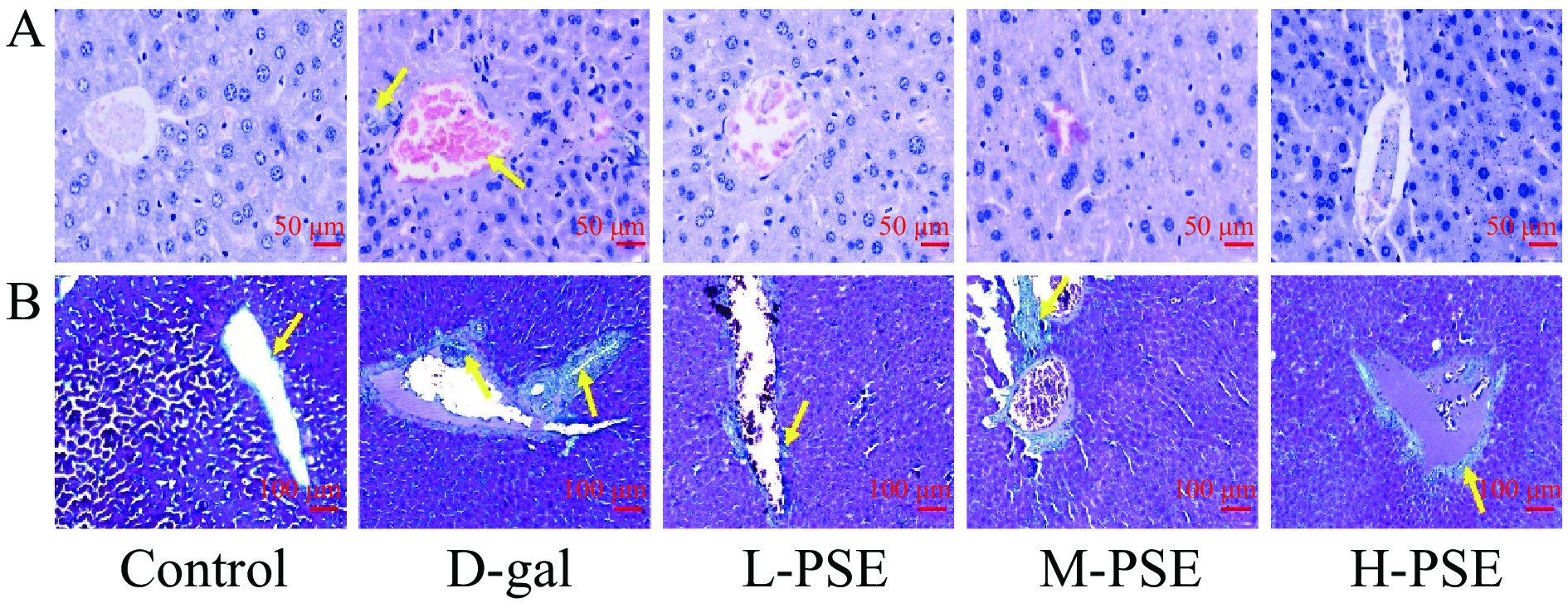
由结果(图2A)可知,小鼠在给予D-gal 8周后,其肝组织出现肝细胞变性和炎性细胞浸润等。采用不同剂量PSE治疗后,肝组织病理学改变得到恢复。此外,Masson染色显示D-gal组肝脏组织纤维组织增生增加,纤维间隔形成;采用不同剂量PSE治疗后,肝组织纤维组织增生减小(图2B)。说明PSE对D-半乳糖引起的衰老小鼠肝损伤有一定保护作用。
2.3.3 PSE对D-gal致衰老小鼠肾脏组织病理和胶原纤维的影响
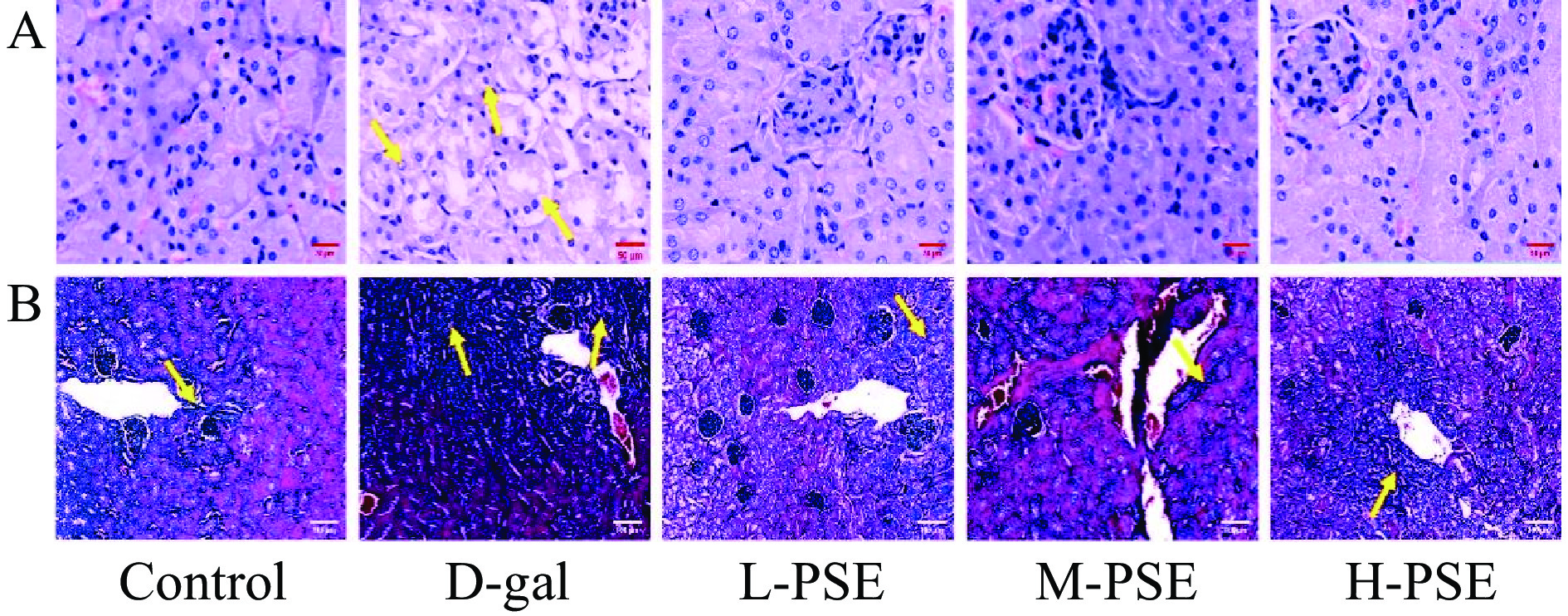
由结果(图3A)可知,Control组中,肾脏无任何病理损伤变化。注射D-gal可导致正常肾小球数量减少,肾小管硬化、萎缩、水肿、空泡变性和肾间质纤维。经过不同剂量PSE治疗,可减轻以上病理损伤。Control组肾脏组织胶原沉积面积较小;而衰老模型组肾脏组织可见大量胶原纤维,胶原沉积面积大。经过不同剂量PSE治疗,可减轻以上纤维化程度(图3B)。说明PSE对D-半乳糖引起的衰老小鼠肾损伤有一定保护作用。
2.3.4 PSE对D-gal致衰老小鼠心脏组织病理和胶原纤维的影响
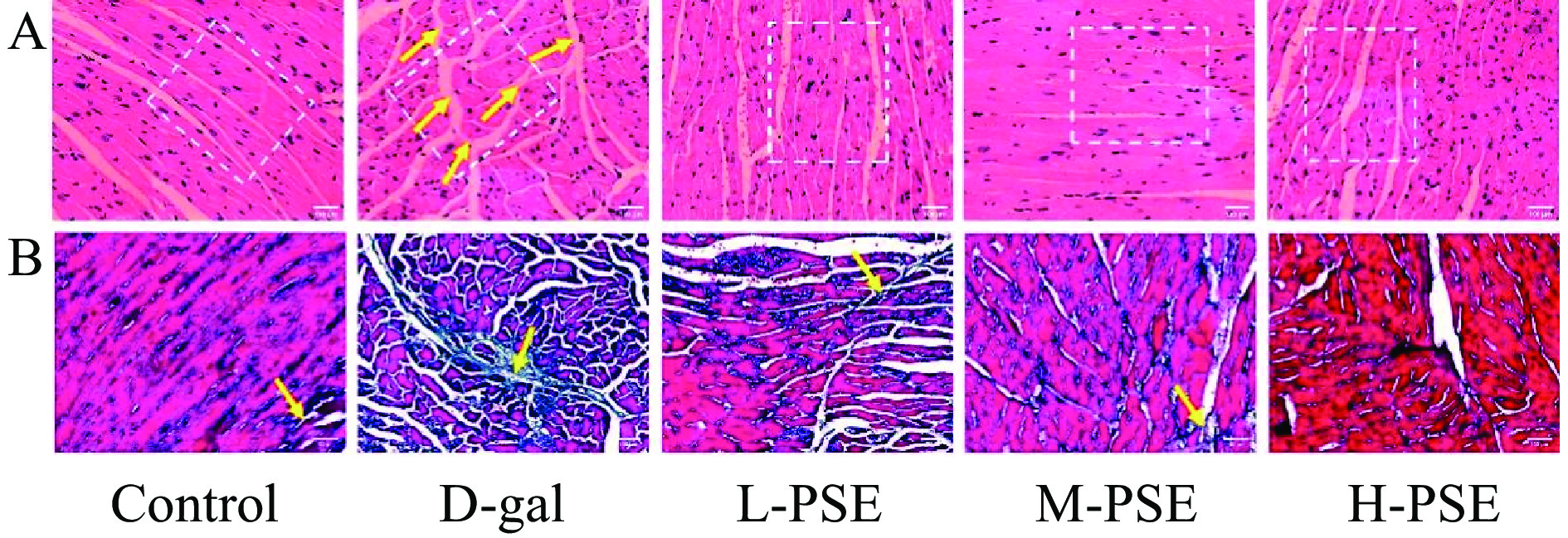
正常组心脏纤维排列整齐,无心脏纤维断裂。D-gal治疗组心肌组织损伤严重,PSE改善了心脏病理变化,表现为减少了肌纤维断裂,核大小不规则减少,核碎裂减少,心肌细胞收缩和溶解减少(图4A)。此外,Masson染色显示PSE能减少老化心脏的胶原沉积和纤维化(图4B)。说明PSE对D-半乳糖引起的衰老小鼠心脏损伤有一定保护作用。
2.3.5 PSE对D-gal致衰老小鼠肺组织病理和胶原纤维的影响
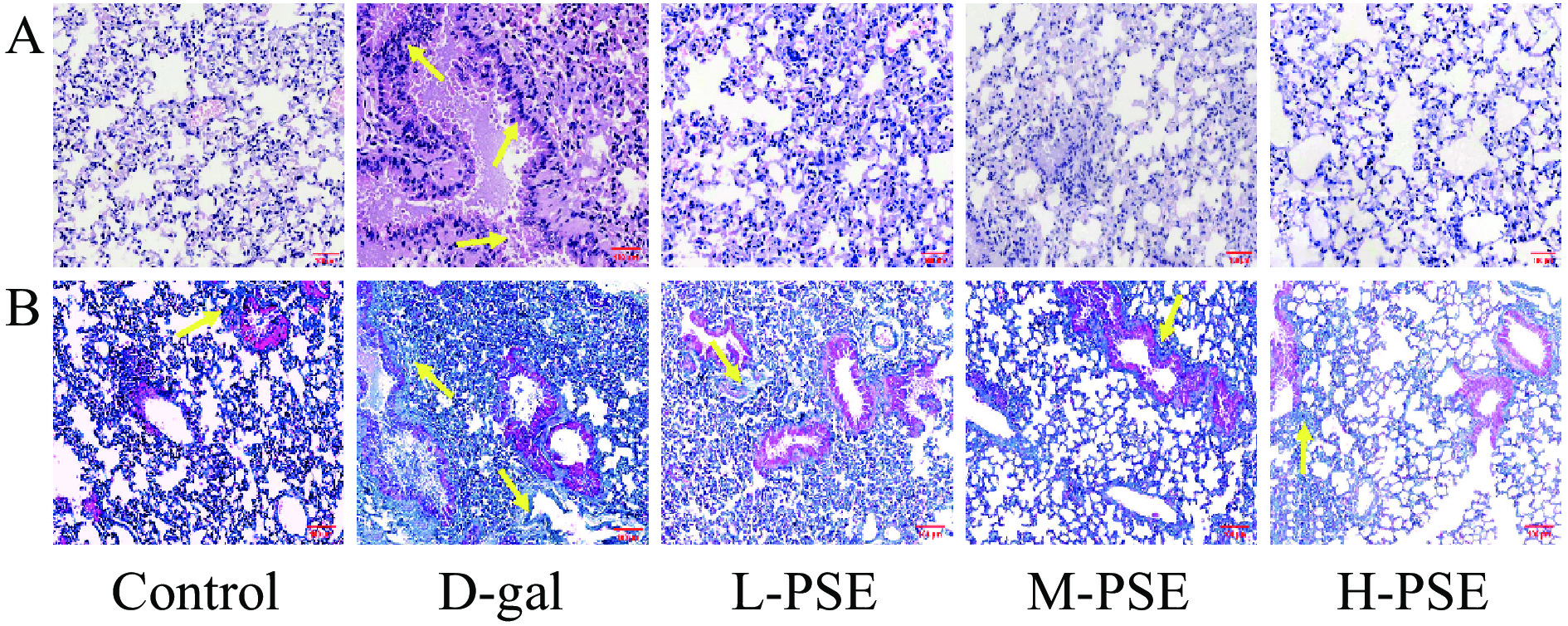
对照组肺泡结构正常。D-gal 注射8周后,HE 和 Masson 染色显示与对照组相比,D-gal治疗组肺泡壁明显增厚,肺泡间隔塌陷、炎症细胞浸润、肺结构丧失和胶原蛋白沉积过多,而PSE治疗减轻了D-gal引起的肺结构损伤和胶原蛋白沉积(图5)。说明PSE对D-半乳糖引起的衰老小鼠肺损伤有一定保护作用。
2.3.6 PSE对D-gal致衰老小鼠大脑组织病理的影响
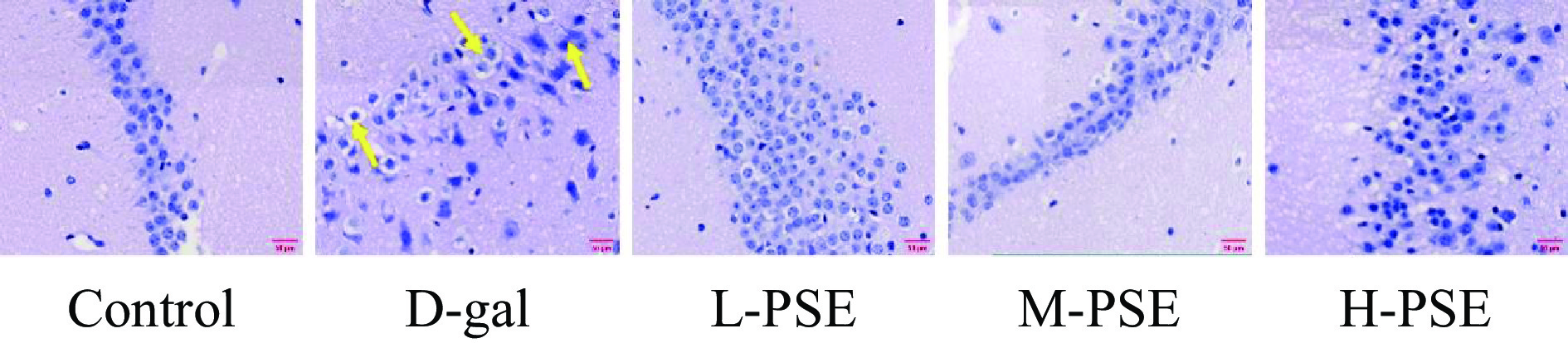
对照组椎体细胞形态正常,无明显病理改变。D-gal组锥体细胞染色浓密,胞浆稀少,呈不规则三角形,形状不规则,核深染,胞浆嗜酸性到空泡化。采用不同剂量PSE治疗后,少部分锥体细胞出现结构损伤,其余细胞保持正常细胞结构,细胞质均匀,核仁明显,空泡化少见(图6)。说明PSE对D-半乳糖引起的衰老小鼠脑损伤有一定保护作用。
2.4 PSE对D-gal致衰老小鼠重要脏器组织衰老相关基因的影响
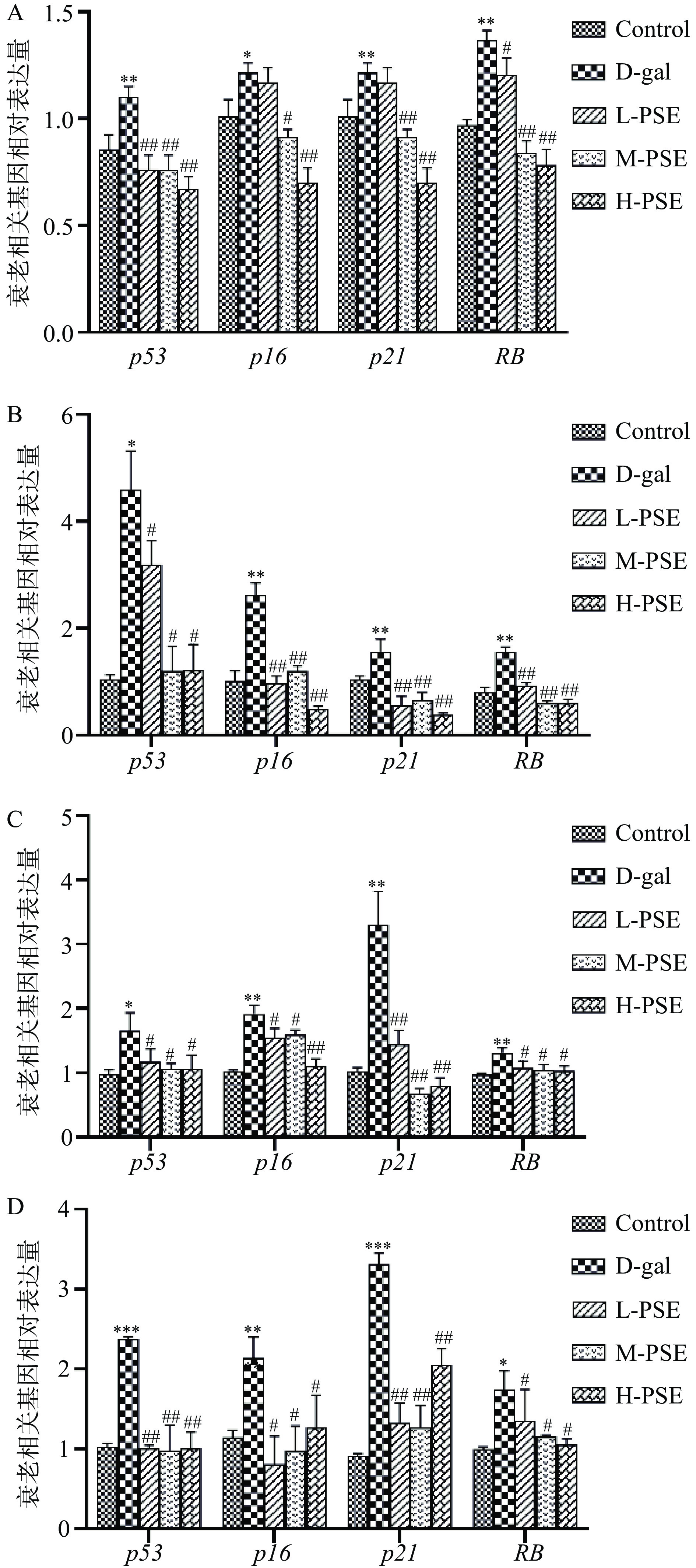
p53/p21和p16-RB两条信号通路与细胞和组织衰老关系密切。当机体发生氧化应激时,细胞受到刺激后诱导DNA损伤,使p53上调,活化的p53诱导细胞周期蛋白依赖性激酶抑制剂p21的上调,进而抑制细胞周期阻遏物(CDK6,CDK4,cyclinD1)的激活,导致去磷酸化和细胞周期抑制因子视网膜母细胞瘤(RB)的激活,这与细胞周期停滞引发的细胞衰老密切相关[43-44]。同时D-半乳糖引起氧化应激导致小鼠体内发生DNA损伤,从而使p16上调,被上调的p16与细胞周期阻遏物(CDK6,CDK4, cyclinD1)结合后去抑制RB的磷酸化,从而引起细胞衰老[45-46]。结果显示:与Control组相比,衰老模型组小鼠肝脏、大脑、肾脏、心脏组织中p53、p16、p21和RB mRNA均增加(P<0.05,P<0.01),不同剂量组PSE治疗均抑制了大脑、肝脏、肾脏、心脏组织中p53、p16、p21和RB水平的升高(图7),其中L-PSE组大脑组织中p16、p21无统计学意义(P>0.05),H-PSE组大脑组织中p53、p16、p21和RB极显著降低(P<0.01)(图7A)。而在肝脏、肾脏、心脏组织中PSE三个剂量组与模型组相比,p53、p16、p21和RB均具有统计学意义(P<0.05,P<0.01)。其原因有可能是PSE对不同的组织器官具有剂量差异。以上结果说明PSE可能通过抑制p53-p21和p16-RB信号通路的活化,进而发挥其抗衰老和多组织保护作用。
2.5 PSE对D-gal致衰老小鼠重要脏器组织氧化应激相关基因的影响
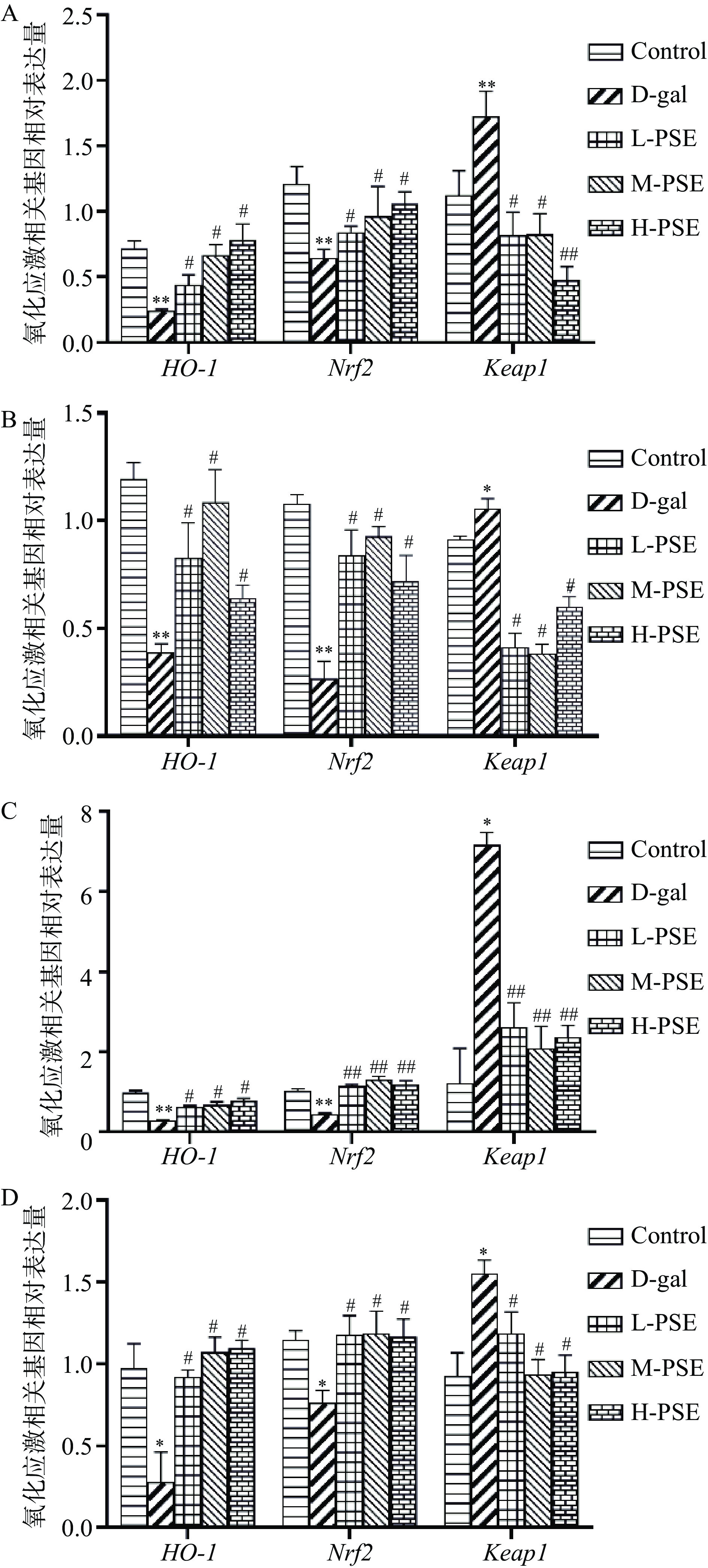
研究证明Keap1/Nrf2/HO-1是防止细胞氧化应激的重要抗氧化途径之一[47-48]。在正常细胞中,Nrf2 以二聚体的形式与Keap1结合并且处于低水平状态,从而使Nrf2发生泛素化并被其蛋白酶降解[49]。连续8周给小鼠皮下注射D-gal,使小鼠处于氧化应激状态下,导致磷酸化的Nrf2从Keap1中释放出来并转运至细胞核发挥其功能,最终刺激HO-1活性[50]。本研究结果表明,与Control组相比,D-gal组小鼠心脏组织中Nrf2和HO-1 mRNA降低(P<0.05),大脑、肝脏、肾脏组织中Nrf2和HO-1 mRNA显著降低(P<0.01),大脑组织中Keap1 mRNA显著升高(P<0.01),心脏、肝脏、肾脏组织中Keap1 mRNA升高(P<0.05)。与D-gal组相比,L-PSE、M-PSE、H-PSE组大脑、肝脏、心脏组织中Keap1 mRNA降低(P<0.05),Nrf2和HO-1 mRNA升高(P<0.05),L-PSE、M-PSE、H-PSE组肾脏组织中Keap1 mRNA显著降低(P<0.01),HO-1 mRNA升高(P<0.05),Nrf2 mRNA显著升高(P<0.01)(图8)。说明PSE可能通过激活Keap1-Nrf2-HO-1通路发挥抗氧化应激和多组织保护作用。
3. 结论
本研究利用D-gal诱导构建衰老小鼠模型来研究PSE抗衰老和对组织器官的保护作用。实验结果表明,PSE可以有效提高衰老模型小鼠肝、肾脏、胸腺、脾脏组织的脏器指数,还可以有效降低衰老小鼠血清中CK、CK-MB、ALP、AST、ALT、UA、UREA、CREA、BUN/Scr 活性,提高血清中eGFR 含量。同时,PSE使衰老小鼠的肾脏、肝脏、皮肤、肺、心脏和大脑等组织的细胞形态及结构功能得到明显改善。这些结果表明,PSE能够有效缓解和改善小鼠衰老。作用机制研究结果表明,PSE可通过降低衰老基因p53 、p21、p16、RB和Keap-1以及增加抗氧化因子Nrf2、HO-1的表达,减轻D-gal所致衰老小鼠多器官损伤,从而达到抗衰老和多组织保护作用。综上所述,本研究报道了PSE对D-gal诱导的多器官衰老和损伤的有益作用,从而为进一步开发抗衰老及改善多器官功能的药物和保健食品提供实验依据和新思路。但PSE中发挥抗衰老和多组织保护作用的具体物质基础还不明确,还需进一步深入研究。
-
表 1 RT-PCR引物序列
Table 1 Primer sequence of RT- PCR
基因 正向引物(5’-3’) 反向引物(5’-3’) p53 TGGAAGGAAAATTTGTATCCCGA GTGGATGGTGGTATACTCAGAG p16 ACATCAAGACATCGTGCGATATT CCAGCGGTACACAAAGACCA p21 TGTCTTGCACTCTGGTGTCTG ATCTGCGCTTGGAGTGATAGA RB TCGATACGAGTACCAAGGTTGA ACACGTCCGTTCTAATTTGCTG HO-1 AAGCCGAGAATGCTGAGTTCA GCCGTGTAGATGGTACAAGGA Nrf2 GTGCTCCTATGCGTGAAT TACCTCTCCTGCGTATATCT Keap1 TGCCCCTGTGGTCAAAGTG GGTTCGGTTACCGTCCTGC GAPDH AAGGTCATCCATGACAACTTTGGC ACAGTCTTCTGGGTGGCAGTGAT 表 2 PSE对D-gal致衰老小鼠脏器指数的影响($\overline{{{\rm{X}}}}\pm {{\rm{SD}}}$,n=10)
Table 2 Effect of PSE on organ index of aging mice induced by D-gal ($\overline{\rm{X}}\pm \rm{SD}$, n=10)
组别 胸腺指数(mg/g) 脾脏指数(mg/g) 肝脏指数(mg/g) 肾脏指数(mg/g) Control 3.98±0.25 3.05±0.53 61.19±2.64 13.98±0.46 D-gal 1.97±0.57** 2.11±0.47* 51.97±3.05** 11.63±0.50** L-PSE 3.48±0.50## 2.92±0.26# 58.25±2.87# 15.46±0.82## M-PSE 3.46±0.37## 4.37±0.54## 58.43±4.32# 14.95±1.11## H-PSE 3.54±0.30## 3.89±0.55## 60.29±6.36# 16.33±0.42## 注:*与Control组相比差异显著(P<0.05);**与Control组相比差异极显著(P<0.01);#与D-gal组相比差异显著(P<0.05);##与D-gal组相比,差异极显著(P<0.01);表3~表4同。 表 3 PSE对D-gal致衰老小鼠血清生化指标的影响($\overline{\rm{X}}\pm \rm{SD}$,n=10)
Table 3 Effects of PSE on serum biochemical indexes in aging mice induced by D-gal ($\overline{\rm{X}}\pm \rm{SD}$, n=10)
组别 心脏 肝脏 CK(U/L) CK-MB(U/L) ALP(U/L) AST(U/L) ALT(U/L) Control 486.5±226 185.67±28.34 55.88±7.64 63.29±8.92 30.17±3.98 D-gal 1316.17±221.58** 310.50±45.55** 84.57±13.03** 88.57±10.76** 38.83±2.54** L-PSE 438.67±55.15## 178.71±57.86# 57.38±15.76# 68.71±19.54# 31.00±4.00# M-PSE 414.17±142.46## 160.38±23.45## 53.25±15.01## 67.43±12.83# 28.33±6.77# H-PSE 412±180.78## 156.14±18.943## 48.88±13.03## 56.43±10.29## 25.83±5.76## 表 4 PSE对D-gal致衰老小鼠血清生化指标的影响($\overline{\rm{X}}\pm \rm{SD}$,n=10)
Table 4 Effects of PSE on serum biochemical indexes in aging mice induced by D-gal ($\overline{\rm{X}}\pm \rm{SD}$, n=10)
组别 肾脏 UA(mg/dL) UREA(mg/dL) CREA(mg/dL) eGFR(mg/dL) BUN/Scr(mg/dL) Control 106.67±34.66 6.13±0.52 11.92±1.33 245.38±31.53 121.88±8.91 D-gal 167.5±17.52** 7.50±0.94** 13.90±0.37* 236.88±6.28* 150.50±18.85* L-PSE 124.33±15.77# 5.59±0.83## 10.95±1.04# 244.25±12.91# 123.88±13.68# M-PSE 116.67±16.39## 5.54±0.88## 10.77±1.50# 244.25±11.21# 120.63±13.31## H-PSE 112.67±10.03## 5.46±0.50## 10.63±1.20## 251.75±40.41# 119.63±25.40## -
[1] 原新, 金牛. 世界人口负增长的趋势展望与影响应对[J]. 河北大学学报,2021,46(1):82−91. [YUAN Xin, JIN Niu. Prospects and countermeasures of the global negative population growth[J]. Journal of Hebei University,2021,46(1):82−91. YUAN Xin, JIN Niu. Prospects and countermeasures of the global negative population growth[J]. Journal of Hebei University, 2021, 46(1): 82-91.
[2] United Nations. World population ageing 2019: Highlights. New York: UN, 2019.
[3] FEEHAN J, TRIPODI N, APOSTOPOULOS V. The twilight of the immune system: The impact of immunosenescence in aging[J]. Maturitas,2021,147:17−13.
[4] 王培昌. 细胞衰老与衰老相关疾病[J]. 中华检验医学杂志,2021,44(1):7−11. [WANG Peichang. Cell aging and aging related diseases[J]. Chinese Journal of Laboratory Medicine,2021,44(1):7−11. doi: 10.3760/cma.j.cn114452-20201030-00809 WANG Peichang. Cell aging and aging related diseases[J]. Chinese Journal of Laboratory Medicine, 2021, 44(1): 7-11. doi: 10.3760/cma.j.cn114452-20201030-00809
[5] MA S, SUN S, GENG L, et al. Caloric restriction reprograms the single-cell transcriptional landscape of Rattus norvegicus aging[J]. Cell,2020,180(5):984−1001. doi: 10.1016/j.cell.2020.02.008
[6] BARARDO D, THORNTON D, THOPPIL H, et al. The drug age database of aging-related drugs[J]. Aging Cell,2017,16(3):594−597. doi: 10.1111/acel.12585
[7] 国家药典委员会. 中华人民共和国药典: 一部[M]. 北京: 中国医药科技出版社, 2020: 319 Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China: subdivision[M]. Beijing: China Medical Science and Technology Press, 2020: 319.
[8] 赵祺, 任仙樱, 姜程曦. 九华黄精本草考证[J]. 中草药,2018,49(17):4184−4188. [ZHAO Qi, REN Xianying, JIANG Chengxi. Herbal textual research of Jiuhua Polygonatum cyrtonema[J]. Chinese Traditional and Herbal Drugs,2018,49(17):4184−4188. ZHAO Qi, REN Xianying, JIANG Chengxi. Herbal textual research of Jiuhua Polygonatum cyrtonema[J]. Chinese Traditional and Herbal Drugs, 2018, 49(17): 4184-4188.
[9] REN H M, DENG Y L, ZHANG J L, et al. Research progress on processing history evolution, chemical components and pharmacological effects of Polygonati Rhizoma[J]. China journal of Chinese materia medica,2020,45(17):4163−4182.
[10] CHEN Z, LIU J, KONG X, et al. Characterization and Immunological Activities of Polysaccharides from Polygonatum sibiricum[J]. Biological and Pharmaceutical Bulletin,2020,43(6):959−967. doi: 10.1248/bpb.b19-00978
[11] ZHAO X, PATIL S, QIAN A, et al. Bioactive compounds of Polygonatum sibiricum-therapeutic effect and biological activity[J]. EndocrMetab Immune Disord Drug Targets,2022,22(1):26−37. doi: 10.2174/1871530321666210208221158
[12] 李亚霖, 周芳, 曾婷, 等. 药用黄精化学成分与活性研究进展[J]. 中医药导报,2019,25(5):86−89. [LI Yalin, ZHOU Fang, ZENG Ting, et al. Research progress on chemical components and activities of Huangjing[J]. Guiding Journal of Traditional Chinese Medicine,2019,25(5):86−89. LI Yalin, ZHOU Fang, ZENG Ting, et al. Research progress on chemical components and activities of Huangjing[J]. Guiding Journal of Traditional Chinese Medicine, 2019, 25(5): 86-89.
[13] ZHAO P, ZHAO C, LI X, et al. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology[J]. Journal of Ethnopharmacology,2018,214:274−291. doi: 10.1016/j.jep.2017.12.006
[14] SHU G, XU D, ZHAO J, et al. Protective effect of Polygonatum sibiricum polysaccharide on cyclophosphamide-induced immunosuppression in chickens[J]. Research in Veterinary Science,2021,135:96−105. doi: 10.1016/j.rvsc.2020.12.025
[15] LIU B, TANG Y, SONG Z, et al. Polygonatum sibiricum F. Delaroche polysaccharide ameliorates HFD-induced mouse obesity via regulation of lipid metabolism and inflammatory response[J]. Spandidos Publications,2021,24(1):501. doi: 10.3892/mmr.2021.12140
[16] 马凤巧, 张海艳. 黄精对衰老大鼠学习记忆能力的改善及机制[J]. 中国老年学杂志,2010,30(15):2191−2192. [MA Fengqiao, ZHANG Haiyan. Improvement and mechanism of Polygonatum sibiricum on learning and memory ability in aging rats[J]. Chinese Journal of Gerontology,2010,30(15):2191−2192. MA Fengqiao, ZHANG Haiyan. Improvement and mechanism of Polygonatum sibiricum on learning and memory ability in aging rats[J]. Chinese Journal of Gerontology, 2010, 30(15): 2191-2192.
[17] XIAOWEI C, WEI W, HONG G, et al. Review of Polygonatum sibiricum: A new natural cosmetic ingredient[J]. Pharmazie,2019,74(9):513−519.
[18] ZHANG H, CAO Y, CHEN L, et al. A polysaccharide from Polygonatum sibiricum attenuates amyloid-β-induced neurotoxicity in PC12 cells[J]. Carbohydrate Polymers,2015,117:879−886. doi: 10.1016/j.carbpol.2014.10.034
[19] CUI X, WANG S, CAO H, et al. A Review: The bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides[J]. Molecules,2018,23(5):1170. doi: 10.3390/molecules23051170
[20] 秦臻, 韦正新, 许键炜. 黄精对衰老大鼠内皮祖细胞DNA损伤检测点ATM/ATR通路的影响[J]. 中药新药与临床药理,2019,30(5):529−534. [QIN Zhen, WEI Zhengxin, XU Jianwei. The Effect of Polygonati rhizoma on DNA damage checkpoint ATM/ATR of endothelail progenitor cells in aged rats[J]. Traditional Chinese Drug Research and Clinical Pharmacology,2019,30(5):529−534. QIN Zhen, WEI Zhengxin, XU Jianwei. The Effect of Polygonati rhizoma on DNA damage checkpoint ATM/ATR of endothelail progenitor cells in aged rats[J]. Traditional Chinese Drug Research and Clinical Pharmacology, 2019, 30(5): 529-534.
[21] 秦臻, 韦正新, 宰青青, 等. 黄精降低活性氧水平促进衰老内皮祖细胞功能的研究[J]. 中国药理学通报,2019,35(1):123−127. [QIN Zhen, WEI Zhengxin, ZAI Qingqing et al. Effect of Rhizoma Polygonati on functional activity of endothelial progenitor cells to delay senescense via decrease of ROS[J]. Chinese Pharmacological Bulletin,2019,35(1):123−127. QIN Zhen, WEI Zhengxin, ZAI Qingqing et al. Effect of Rhizoma Polygonati on functional activity of endothelial progenitor cells to delay senescense via decrease of ROS[J]. Chinese Pharmacological Bulletin, 2019, 35(1): 123-127.
[22] REN Y, AI J, LIU X, et al. Anticoagulant active ingredients identification of total saponin extraction of different Panax medicinal plants based on grey relational analysis combined with UPLC-MS and molecular docking[J]. Ethnopharmacol,2020,260:112955. doi: 10.1016/j.jep.2020.112955
[23] DU H M, WANG Y J, LIU X, et al. Defective central immune tolerance induced by high-dose D-galactose resembles aging[J]. Biochemistry,2019,84(6):617−626.
[24] 刘阳, 许悦, 王语嫣, 等. 紫苏梗乙酸乙酯提取物对D-半乳糖衰老模型小鼠的抗衰老作用研究[J]. 中华中医药学刊,2022,40(9):52−55. [LIU Yang, XU Yue, WANG Yuyan, et al. Anti-senescence effect of ethyl acetate extract from Zisugeng (Caulis Perillae) on D-galactose aging model mice[J]. Chinese Archives of Traditional Chinese Medicine,2022,40(9):52−55. LIU Yang, XU Yue, WANG Yuyan, et al. Anti-senescence effect of ethyl acetate extract from Zisugeng (Caulis Perillae) on D-galactose aging model mice[J]. Chinese Archives of Traditional Chinese Medicine, 2022, 40(9): 52-55.
[25] KHAN S S, SINGER B D, VAUGHAN D E. Molecular and physiological manifestations and measurement of aging in humans[J]. Aging Cell,2017,16(4):624−633. doi: 10.1111/acel.12601
[26] MAYNARD S J, MENOWN I B, ADGEY A A. Troponin T or troponin I as cardiac markers in ischaemic heart disease[J]. Heart,2000,83(4):371−373. doi: 10.1136/heart.83.4.371
[27] 曾瑾, 崔凤娟, 王海珍, 等. 阿霉素诱导和腹主动脉缩窄术复制大鼠慢性心力衰竭模型的比较[J]. 皖南医学院学报,2014,33(3):189−192. [ZENG Jin, CUI Fengjuan, WANG Haizhen, et al. Comparison of the two heart failure models in rats by abdominal aorta constriction and intraperitoneal injection of adriamycin[J]. Journal of Wannan Medical College,2014,33(3):189−192. ZENG Jin, CUI Fengjuan, WANG Haizhen, et al. Comparison of the two heart failure models in rats by abdominal aorta constriction and intraperitoneal injection of adriamycin[J]. Journal of Wannan Medical College, 2014, 33(3): 189-192.
[28] SUN F, ZUO Y Z, GE J, et al. Transport stress induces heart damage in newly hatched chicks via blocking the cytoprotective heat shock response and augmenting nitric oxide production[J]. Poultry Science,2018,97(8):2638−2646. doi: 10.3382/ps/pey146
[29] 储莉, 刘伏元, 闻伟, 等. CK-MB、cTnI 联合Fib对老年慢性心力衰竭患者诊断和预后的价值[J]. 中国老年学杂志,2021,41(14):2927−2931. [CHU Li, LIU Fuyuan, WEN Wei, et al. Diagnostic and prognostic value of CK-MB, cTnI, and Fib in elderly patients with chronic heart failure[J]. Chinese Journal of Gerontology,2021,41(14):2927−2931. CHU Li, LIU Fuyuan, WEN Wei, et al. Diagnostic and prognostic value of CK-MB, cTnI, and Fib in elderly patients with chronic heart failure[J]. 2021, 41(14): 2927-2931.
[30] OJHA S, GOYAL S, SHARAM C, et al. Cardioprotective effect of lycopene against isoproterenol-induced myocardial infarction in rats[J]. Human and Experimental Toxicology,2013,32(5):492−503. doi: 10.1177/0960327112454890
[31] NYBLOM H, BERGGREN U, BALLDIN J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking[J]. Alcohol and Alcoholism,2004,39(4):336−339. doi: 10.1093/alcalc/agh074
[32] NYBLOM H, BJORNSSON E, SIMREN M, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC[J]. Liver International,2006,26(7):840−845. doi: 10.1111/j.1478-3231.2006.01304.x
[33] XIE C, JINGJING W, LII X, et al. Protective effect of SKLB010 against D-galactosamine/lipopolysaccha-ride-induced acute liver failure via nuclear factor-kappaB signaling pathway in macrophages[J]. International Immunopharmacology,2014,21:261−268. doi: 10.1016/j.intimp.2014.05.012
[34] CHEN X L, HAN Y D, WANG H. Relations of hepatic steatosis with liver functions, inflammations, glucolipid metabolism in chronic hepatitis B patients[J]. European Review for Medical and Pharmacological,2018,22:5640−5646.
[35] 宗艺, 王加. 血清5’NT、LAP、ALP与GGT检测在肝脏疾病中的临床价值[J]. 医学食疗与健康,2022,20(16):193−195. [ZONG Yi, WANG Jia. The clinical value of serum 5'NT, LAP, ALP, and GGT in liver disease[J]. Medical Diet and Health,2022,20(16):193−195. ZONG Yi, WANG Jia. The clinical value of serum 5'NT, LAP, ALP, and GGT in liver disease[J]. Medical Diet and Health, 2022, 20(16): 193-195.
[36] ZHANG Z F, LU J, ZHENG Y L, et al. Purple sweet potato color protects mouse liver against d-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3K/Akt pathway[J]. Food and Chemical Toxicology,2010,48(8-9):2500−2507. doi: 10.1016/j.fct.2010.06.023
[37] WANG A, XIAO Z, ZHOU L, et al. The protective effect of atractylenolide I on systemic inflammation in the mouse model of sepsis created by cecal ligation and puncture[J]. Pharmaceutical Biology,2016,54(1):146−150. doi: 10.3109/13880209.2015.1024330
[38] 李艳菊, 李琴山, 田硕, 等. 天冬总皂苷对D-半乳糖致衰老大鼠肾脏p53基因表达的影响[J]. 中国老年学杂志,2012,32(18):3961−3963. [LI Yanju, LI Qinshan, TIAN Shuo. The effects of total asparagine saponins on p53 gene expression in aging rats induced by D-galactose[J]. Chinese Journal of Gerontology,2012,32(18):3961−3963. LI Yanju, LI Qinshan, TIAN Shuo. The effects of total asparagine saponins on p53 gene expression in aging rats induced by D-galactose[J]. Chinese Journal of Gerontology, 2012, 32(18): 3961-3963.
[39] JUNG S W, KIM S M, KIM Y G, et al. Uric acid and inflammation in kidney disease[J]. American Journal of Physiology-Renal Physiology,2020,318:1327−1340. doi: 10.1152/ajprenal.00272.2019
[40] AMARA I, SAIAH A, TIMOUMI R, et al. Effect of di(2-ethylhexyl) phthalate on Nrf2-regulated glutathione homeostasis in mouse kidney[J]. Cell Stress Chaperones,2020,25(6):919−928. doi: 10.1007/s12192-020-01127-8
[41] GAGLIANO N, GRIZZI F, ANNONI G. Mechanisms of aging and liver functions[J]. Dig Dis,2007,25(2):118−123. doi: 10.1159/000099475
[42] PROKSCH E, SEGGER D, DEGWERT J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study[J]. Skin Pharmacology and Physiology,2014,27(1):47−55. doi: 10.1159/000351376
[43] MUNOZ-ESPIN D, SERRANO M. Cellular senescence: From physiology to pathology[J]. Nature Reviews Molecular Cell Biology,2014,15(7):482−496. doi: 10.1038/nrm3823
[44] ZHANG J, CAO M, DONG J, et al. ABRO1 suppresses tumourigenesis and regulates the DNA damage response by stabilizing p53[J]. Nature Communications,2014,5(1):5059. doi: 10.1038/ncomms6059
[45] DAVALOS A R, COPPE J P, CAMPISI J, et al. Senescent cells as a source of inflammatory factors for tumor progression[J]. Cancer and Metastasis Reviews,2010,29(2):273−283. doi: 10.1007/s10555-010-9220-9
[46] GIACINTI C, GIORDANO A. RB and cell cycle progression[J]. Oncogene,2006,25(38):5220−5227. doi: 10.1038/sj.onc.1209615
[47] DESHMUKH P, UNNI S, KRISHNAPPA G, et al. The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases[J]. Biophysical Reviews,2017,9(1):41−56. doi: 10.1007/s12551-016-0244-4
[48] YAMAZAKI H, TANJI K, WAKABAYASHI K, et al. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases[J]. Pathology International,2015,65(5):210−219. doi: 10.1111/pin.12261
[49] NGUYEN T, NIOI P, PICKETT C B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress[J]. Journal of Biological Chemistry,2009,284(20):13291−13295. doi: 10.1074/jbc.R900010200
[50] SHUKLA K, PAL P B, SONOWAL H, et al. Aldose reductase inhibitor protects against hyperglycemic stress by activating Nrf2-dependent antioxidant proteins[J]. Journal of Diabetes Research,2017,2017:6785852.
-
期刊类型引用(1)
1. 王聪颖,黄小强,徐玥玥,茹国华. 枸杞子多糖的提取纯化、体外抗氧化及其抗衰老作用. 食品工业科技. 2024(19): 1-8 .  本站查看
本站查看
其他类型引用(3)





 下载:
下载:
 下载:
下载:



