Compound Collagen Peptides Powder Improves Chronic Skin Damage Resulting from Ultraviolet Irradiation in Mice and the Mechanism
-
摘要: 目的:观察复方胶原蛋白肽粉对小鼠皮肤慢性紫外线损伤的改善作用,并探讨其作用机制。方法:ICR雌性小鼠随机分成正常组、模型组、复方胶原蛋白肽粉组、鱼胶原蛋白肽组。使用紫外光疗仪模拟日光照射小鼠背部皮肤建立皮肤慢性紫外线损伤模型。每周对小鼠皮肤表观进行评分,每3周拍照记录一次小鼠皮肤外观,9周后采用干燥法测定小鼠皮肤含水量;苏木精-伊红(HE)染色法观察小鼠皮肤组织病理形态学变化;采用FRAP法检测小鼠皮肤组织中总抗氧化能力(T-AOC)水平;酶联免疫吸附(ELISA)法检测皮肤组织中I型胶原蛋白(Col I)、弹性蛋白(ELN)、透明质酸(HA)、超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)、丙二醛(MDA) 、糖基化终末产物(AGEs)水平;采用蛋白免疫印迹法(Western blot)检测皮肤组织中转化生长因子β1(TGF-β1)、转化生长因子β受体2(TGF-βR2)、Smad2、Smad3、Smad7蛋白表达水平;采用实时荧光定量聚合酶链式反应(RT-qPCR)检测皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3、Smad7 mRNA表达水平。结果:与模型组比较,复方胶原蛋白肽粉组小鼠皮肤皱纹减少,无表皮损伤,宏观等级评分极显著降低(P<0.01),皮肤组织病变程度减轻;皮肤组织含水量极显著升高(P<0.01);皮肤组织T-AOC水平极显著升高(P<0.01);皮肤组织中Col I、ELN、HA、GSH-Px、SOD水平极显著升高(P<0.01),MDA、AGEs水平极显著降低(P<0.01);皮肤组织的TGF-β1、TGF-βR2、Smad2、Smad3蛋白及mRNA表达显著升高(P<0.05),Smad7蛋白及mRNA表达极显著降低(P<0.01)。结论:复方胶原蛋白肽粉可有效改善小鼠皮肤慢性紫外线损伤,其机制可能与减轻氧化应激反应,调控TGF-β1/Smads信号转导增加细胞外基质合成有关。Abstract: Objective: To study the effect and action mechanism of compound collagen peptides powder on chronic skin damage resulting from ultraviolet irradiation in mice. Methods: Female ICR mice were randomly divided into the following groups according to their weights: Normal, model, compound collagen peptides powder, fish collagen peptide. Mice in the model and treatment groups were irradiated on back skin using UV phototherapy apparatus to induce chronic skin damage. During the experiment, the skin appearance of mice was scored each week and photos were taken every 3 weeks. The skin water content was determined by drying method after 9 weeks. The histopathological changes of mice skin were observed using Hematoxylin-Eosin (HE) staining. The total antioxidant capacity (T-AOC) in skin was detected using fluorescene recovery after photobleaching (FRAP). The levels of type I collagen (Col I), elastin (ELN), hyaluronic acid (HA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA) and advanced glycation end products (AGEs) in the skin were examined using ELISAs. RT-PCR and Western blot analyses were performed to determine the mRNA and protein expression of transforming growth factor β1 (TGF-β1), transforming growth factor β receptor 2 (TGF-βR2), Smad2, Smad3 and Smad7. Results: Compared to the model group, mice in the compound collagen peptides powder group had less wrinkles, no epidermal damage and the skin scores were significantly decreased (P<0.01). The lesions of skin were improved. The water content of skin and T-AOC were significantly increased (P<0.01). The levels of Col I, ELN, HA, GSH-Px, SOD in the skin were significantly increased (P<0.01), whereas MDA and AGEs were significantly decreased (P<0.01). In the skin, the mRNA and protein expression of TGF-β1, TGF-βR2, Smad2, Smad3 were increased (P<0.05), whereas Smad7 was significantly decreased (P<0.01). Conclusion: The compound collagen peptides powder improved the chronic skin damage caused by ultraviolet irradiation. The action mechanism seemed to be related to reduction of oxidative stress, regulation and control signal transduction of TGF-β1/Smads pathway for increasing extracellular matrix synthesis.
-
日光中的紫外线(ultraviolet,UV)辐射是造成皮肤损伤的主要外因之一,反复的UV辐射易引起皮肤氧化应激、色素沉积、炎症反应、干燥、皱纹等慢性损伤[1-3],甚至会诱导皮肤癌的发生[4]。Col Ⅰ、ELN、HA作为细胞外基质(extracellular matrix,ECM)的主要成分,与皮肤的弹性、光泽、保湿性和张力等密切相关,过量的UV辐射会导致它们降解,皮肤逐渐变得松弛和干燥而呈现出的光老化状态是皮肤慢性紫外线损伤的重要表征[5]。TGF-β1是转化生长因子β(TGF-β)家族的重要成员,已经被证明可以刺激Ⅰ型胶原蛋白和其它ECM的合成,同时也能够抵抗ECM的降解[6],TGF-β1/Smads信号转导受到抑制是皮肤光老化重要的发病机制之一[7]。皮肤暴露于UV下会导致活性氧簇(reactive oxygen species,ROS)的快速生成和持续积累,ROS和抗氧化剂之间的不平衡导致氧化应激的发生,是诱导皮肤损伤,产生皱纹和病理变化的主要因素[8]。
复方胶原蛋白肽粉是江西伍方生物科技有限公司以胶原蛋白肽为主要原料,辅以针叶樱桃提取物、西兰花胚芽提取物、玫瑰花提取物、关山樱花提取物、壳寡糖及低聚果糖制成的一种复方制剂,富含维生素C、黄酮类、多酚和多糖类等天然抗氧化物。胶原蛋白肽作为目前研究广泛、疗效确切的功能性食品能够改善皮肤水和作用、弹性、粗糙度和密度,显著增加真皮层细胞外基质含量,从而有效减少皱纹[9-10],对光老化皮肤具有明显修复效果[11-12]。源自不同天然植物的有效成分如皂苷类、黄酮类、多酚类和多糖类等都能够通过清除自由基起到抗氧化、抗衰老作用[13-15]。复方胶原蛋白肽粉能否在调控TGF-β1/Smads通路增加ECM含量的同时,减轻氧化应激反应,从而改善皮肤慢性紫外线损伤,还有待深入探讨。故本研究旨在观察复方胶原蛋白肽粉以及单一鱼胶原蛋白肽对小鼠皮肤慢性UV损伤的改善效果,同时从调控小鼠皮肤中氧化水平和TGF-β1/Smads信号通路的角度,初步探讨复方胶原蛋白肽粉改善皮肤慢性紫外线损伤的重要机制,为复方类胶原蛋白肽产品的进一步开发和应用提供创新思路和理论依据。
1. 材料与方法
1.1 材料与仪器
ICR雌性小鼠 SPF级,(30±2)g,40只,购自湖南斯莱克景达实验动物有限公司,许可证号SCXK(湘)2019-0004。在江西中医药大学实验动物科技中心SPF级动物房适应性喂养3 d后开始实验。实验期间,小鼠自由摄食饮水,饲养室光照12 h,黑暗12 h,室温22~24 ℃,湿度恒定。本实验经江西中医药大学实验动物伦理委员会批准,批准文号JZLLSC20220757;复方胶原蛋白肽粉 江西伍方生物科技有限公司生产并提供(7.5 g/袋,批号20201206);鱼胶原蛋白肽 法国罗赛洛公司生产(10 g/袋,纯度96%,批号20200603),江西伍方生物科技有限公司提供;总抗氧化能力(T-AOC)生化试剂盒、超氧化物歧化酶(SOD)ELISA试剂盒、谷胱甘肽过氧化物酶(GSH-Px)ELISA试剂盒、丙二醛(MDA)ELISA试剂盒、I型胶原蛋白(Col Ⅰ)ELISA试剂盒、透明质酸(HA)ELISA试剂盒、弹性蛋白(ELN)ELISA试剂盒、晚期糖基化终末产物(AGEs)ELISA试剂盒 江苏酶免生物科技有限公司;总RNA提取试剂盒 北京天根生化科技有限公司;Real time PCR Master Mix (SYBR Green)试剂盒 日本TOYOBO公司;TGF-β1一抗 江苏亲科生物研究中心有限公司;GAPDH、SMAD7、SMAD3、SMAD2一抗 武汉三鹰生物技术有限公司。
SS-03AB型紫外线光疗仪 上海希格玛高技术有限公司;JRM2016型切片机、EG1150 HC型包埋机、DM2000 LED型荧光显微镜 德国Leica公司;光谱MAX190型酶标仪、CFX96TM型Real-Time PCR仪、ChemiDoc XRS+型凝胶成像分析系统 美国Bio-Rad公司。
1.2 实验方法
1.2.1 复方胶原蛋白肽粉的制备
以胶原蛋白肽为主要原料,针叶樱桃提取物、西兰花胚芽提取物、玫瑰花提取物、关山樱花提取物、壳寡糖及低聚果糖为辅料,混合制备成复方胶原蛋白肽粉,其中胶原蛋白肽含量为66.7%。
1.2.2 分组与给药
小鼠按体重随机分为:正常组、模型组、复方胶原蛋白肽粉组、鱼胶原蛋白肽阳性对照组,每组10只。适应性喂养3 d后,根据人和小鼠间按体表面积折算的等效剂量比值[16],设定给药剂量复方胶原蛋白肽粉组2.73 g/kg,鱼胶原蛋白肽组1.82 g/kg,两种受试品均为粉末状,配制溶液时使用超纯水混悬,正常组和模型组给予相应体积超纯水,每天灌胃一次,连续9周。
1.2.3 小鼠皮肤慢性紫外线损伤模型的建立
辐照前24 h,对所有小鼠进行背部皮肤脱毛处理,先用宠物剃毛器剃去小鼠背部较长毛发,然后均匀涂抹脱毛膏去除余下细短毛发,使其形成约4 cm×3 cm大小裸露区域。除正常组外,其余各组置于紫外光治疗仪下30 cm处,UVB强度0.45 mW/cm2,UVA强度6.7 mW/cm2,采用UVA联合UVB模拟日光进行5次/周照射,每日一次,照射剂量UVA:UVB=10:1,紫外照射结束后随即灌胃受试品,实验周期为9周。第一周紫外线的起始强度为最小红斑剂量(minimal erythemal dose,MED),照射剂量UVA为0.7 J/cm2,UVB为0.07 J/cm2,每周增加0.5个MED,整个实验周期UVA、UVB总照射剂量分别为94.5 J/cm2、9.45 J/cm2。当小鼠皮肤出现粗糙皱纹、局部损伤且有皮革样触感时,表明小鼠皮肤慢性紫外线损伤模型构建成功[17-18]。
1.2.4 样本采集
参考邓明高[19]的方法进行。末次给药24 h后,用化妆棉蘸取纯水清洗小鼠背部裸露皮肤后腹腔注射剂量为80 mg/kg的1%戊巴比妥钠,麻醉处死,分离背部中央皮肤,剪去皮下组织和粘连脂肪后使用4 ℃预冷PBS浸泡去除血液,滤纸吸干表面液体,将皮肤组织切分成三份,一份液氮速冻后转入−80 ℃冻存,一份放入4%多聚甲醛中固定,一份用于当日干燥法测定皮肤含水量。
1.2.5 小鼠皮肤状态变化记录及宏观等级评分
实验期间,每周最后一天利用宏观等级评分方法(见表1)对小鼠背部皮肤皱纹情况进行评分[20],每3周对小鼠背部裸露皮肤进行拍照记录一次,期间光照条件尽量保持一致。
表 1 皮肤皱纹情况评分标准Table 1. Evaluation standard of skin wrinkles级别 评分标准 0 正常皮肤细腻纹理 1 皮肤出现细磨痕 2 出现少量浅皱纹 3 出现较多浅皱纹 4 皮肤粗糙,有深皱纹产生 5 有较多粗糙皱纹产生 6 皱纹粗糙,出现皮肤局部损伤 1.2.6 皮肤含水量测定
参考路宁宁[21]的方法进行。先用电子天平称取部分干燥前的背部皮肤质量,标号并记录,于60 ℃烘箱中烘干处理6 h,相同方法再称取干燥后的背部皮肤质量,计算皮肤含水百分比。
皮肤含水量(%)=干燥前质量−干燥后质量干燥前质量×100 1.2.7 皮肤组织病理学观察
参考逯岩松[22]的方法进行。取4%多聚甲醛固定的小鼠背部皮肤组织,常规脱水、包埋,制备石蜡切片,HE染色后光学显微镜下观察皮肤组织形态学变化。
1.2.8 FRAP法检测皮肤组织中T-AOC水平
参考王天顺[23]方法。称取0.1 g皮肤组织,加入1 mL的80%乙醇进行匀浆,匀浆后转入2 mL离心管中;于60 ℃,200~300 W条件下超声提取30 min(间隔5 min振荡混匀一次),12000 r/min离心10 min,取上清,按试剂盒说明书,检测T-AOC水平。 1.2.9 ELISA检测皮肤组织中Col I、ELN、HA、SOD、GSH-Px、MDA、AGEs水平
参考王明月[24]方法。取适量皮肤组织,剪碎,加入4 ℃ 0.9% NaCl溶液(0.9%NaCl溶液的体积总量是皮肤质量的9倍)和适量蛋白裂解液,用组织匀浆机匀浆(频率90 Hz,冰上操作,每次10 s,间隔15 s,重复6次),匀浆后在3000 r /min、4 ℃下冷冻离心15 min,取适量上清液按试剂盒说明检测并计算皮肤组织中上述指标含量。
1.2.10 RT-qPCR检测皮肤组织中TGF-β1/Smads信号通路相关mRNA表达
参考谢璟[25]方法。称取0.1 g小鼠皮肤组织,加入1 mL裂解液后充分研磨匀浆提取组织总RNA,酶标仪检测其含量及纯度,OD260/280在1.9~2.2,说明RNA的纯度符合要求,根据逆转录试剂盒说明进行反转录后得到cDNA,以cDNA为模板进行PCR扩增,条件设置为95 ℃预变性60 s,95 ℃变性15 s,65 ℃退火15 s,72 ℃延伸60 s,共40个循环。结果以GAPDH作为内参,使用2−ΔΔCt表示最终实验数据,采用相对定量法进行分析后得到mRNA的表达水平。所有引物均由北京鼎国昌盛生物技术公司设计并合成,引物序列见表2。
表 2 引物序列Table 2. Primer sequence基因 序列(5'-3') 长度(bp) TGF-β1 上游AGGGCTACCATGCCAACTTC 168 下游CCACGTAGTAGACGATGGGC TGF-βR2 上游GTCGGATGTGGAAATGGAAGC 186 下游CAGGACTTCTGGTTGTCGCA Smad2 上游AATCATTGCAACAAGAGGCAGT 156 下游ATTCCCGTCCCCATCATCCT Smad3 上游CCGTGCGGAAACCCAAACTT 188 下游ACTCTGGAGAACTTGCCCG Smad7 上游ACAGAGGATCTTGTCCCCGA 102 下游CTGGCTGGCTGCGTCTC GAPDH 上游GGTGAAGGTCGGTGAACG 233 下游CTCGCTCCTGGAAGATGGTG 1.2.11 Western blot检测皮肤组织中TGF-β1/Smads信号通路相关蛋白表达
参考金媛媛[26]方法。称取小鼠皮肤组织约0.1 g,加入10倍体积量的RIPA组织裂解液,于4 ℃下消化1 h后匀浆,匀浆后13000 r/min、4 ℃冷冻离心40 min,取上清20 μL根据BCA法检测蛋白浓度。剩余上清液加入1/4体积量的5×蛋白上样缓冲液,100 ℃下煮15 min使蛋白变性,自然冷却、混匀后分装。蛋白上样量30 μg,加样经电泳、转膜、封闭后,用5%的BSA按1:10000比例稀释一抗4 ℃摇床孵育过夜,12 h后用5%的BSA按1:1000比例稀释对应二抗,室温孵育75 min,TBST清洗3次,每次10 min后,滴入ECL超敏显影液曝光成像,采用Image J 1.53a软件进行灰度值分析,以内参GAPDH计算蛋白相对灰度值。
1.3 数据处理
采用SPSS 21.0和GraphPad Prism software 8.1统计分析软件处理。计量数据采用平均数±标准差(¯x±s)表示,P<0.05表示差异有统计学意义。
2. 结果与分析
2.1 小鼠背部皮肤评价
2.1.1 小鼠背部皮肤外观变化
图1所示为各组小鼠在第0周、第3周、第6周及第9周最后一天时的背部裸露皮肤外观,正常组小鼠背部皮肤纹理细腻,光滑饱满;模型组小鼠背部皮肤逐渐增厚,产生较多粗糙皱纹,有些许色素沉着,最后伴有局部损伤出现;与模型组相比,复方胶原蛋白肽粉组和鱼胶原蛋白肽组小鼠皮肤外观均有不同程度的改善,其皮肤皱纹减少,表皮增厚减弱,色素沉着减轻,外观较为接近正常组;复方胶原蛋白肽粉组小鼠皮肤始终未出现粗大皱纹,细浅皱纹也较少,同时表皮增厚肉眼观察不明显,表明虽然鱼胶原蛋白肽组小鼠的皮肤损伤得到一定的改善,但没有复方胶原蛋白肽粉组效果显著。
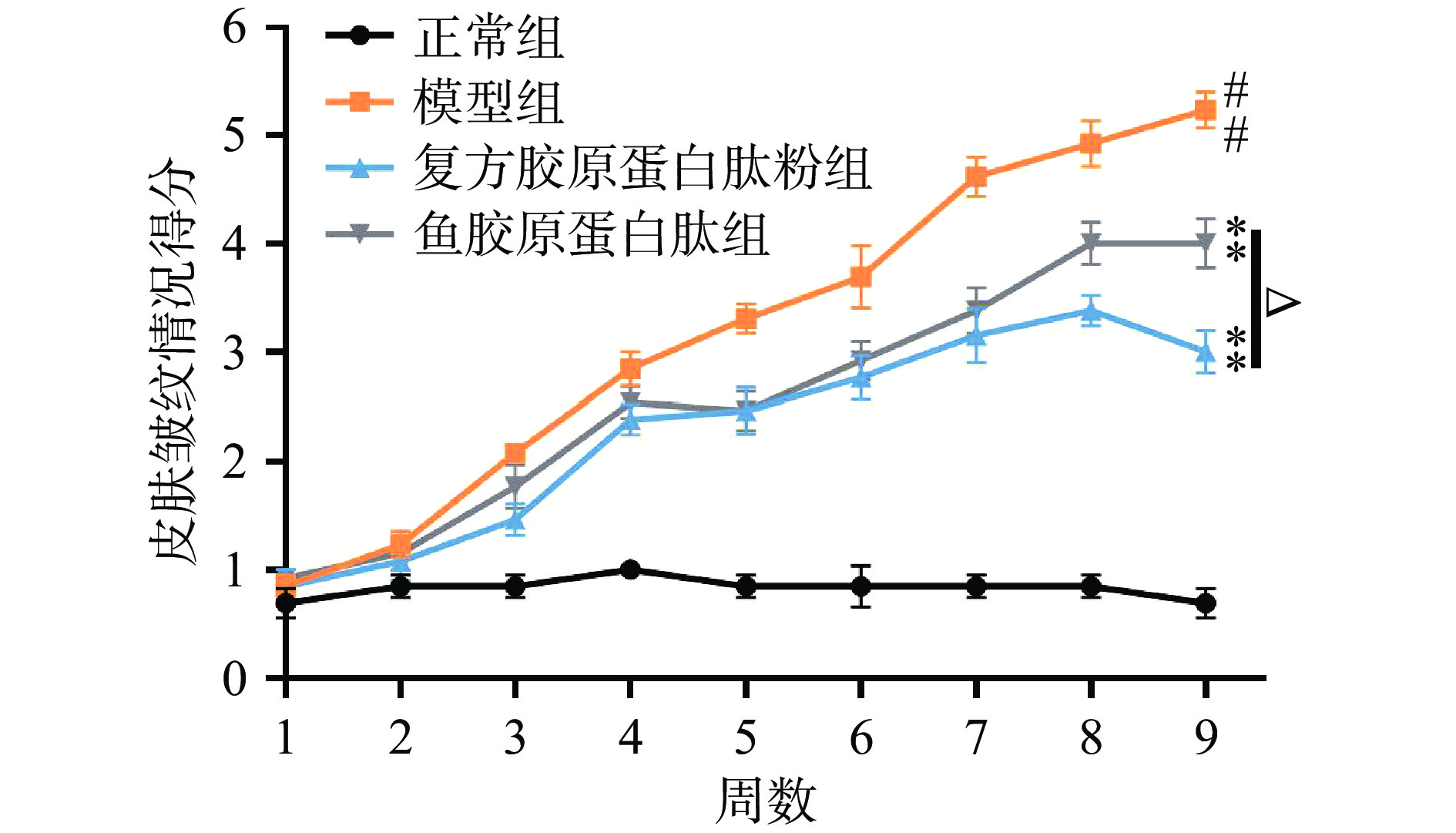
2.1.2 小鼠皮肤皱纹宏观等级评分
如图2所示,随着累积辐照时长的增加,模型组小鼠皮肤皱纹得分不断递增,说明其皱纹逐渐由少变多,由浅入深,从第7周开始,小鼠皮肤开始有深皱纹出现,亦有一只小鼠出现皮肤破损现象;复方胶原蛋白肽粉组和鱼胶原蛋白肽组小鼠得分均极显著低于模型组(P<0.01),表明复方胶原蛋白肽粉和鱼胶原蛋白肽可缓解紫外线辐射引起的皮肤皱纹;除第5周外,复方胶原蛋白肽粉组得分均低于鱼胶原蛋白肽组,第8周和第9周有显著性差异(P<0.05),表明复方胶原蛋白肽粉对皮肤皱纹的改善效果更加显著。
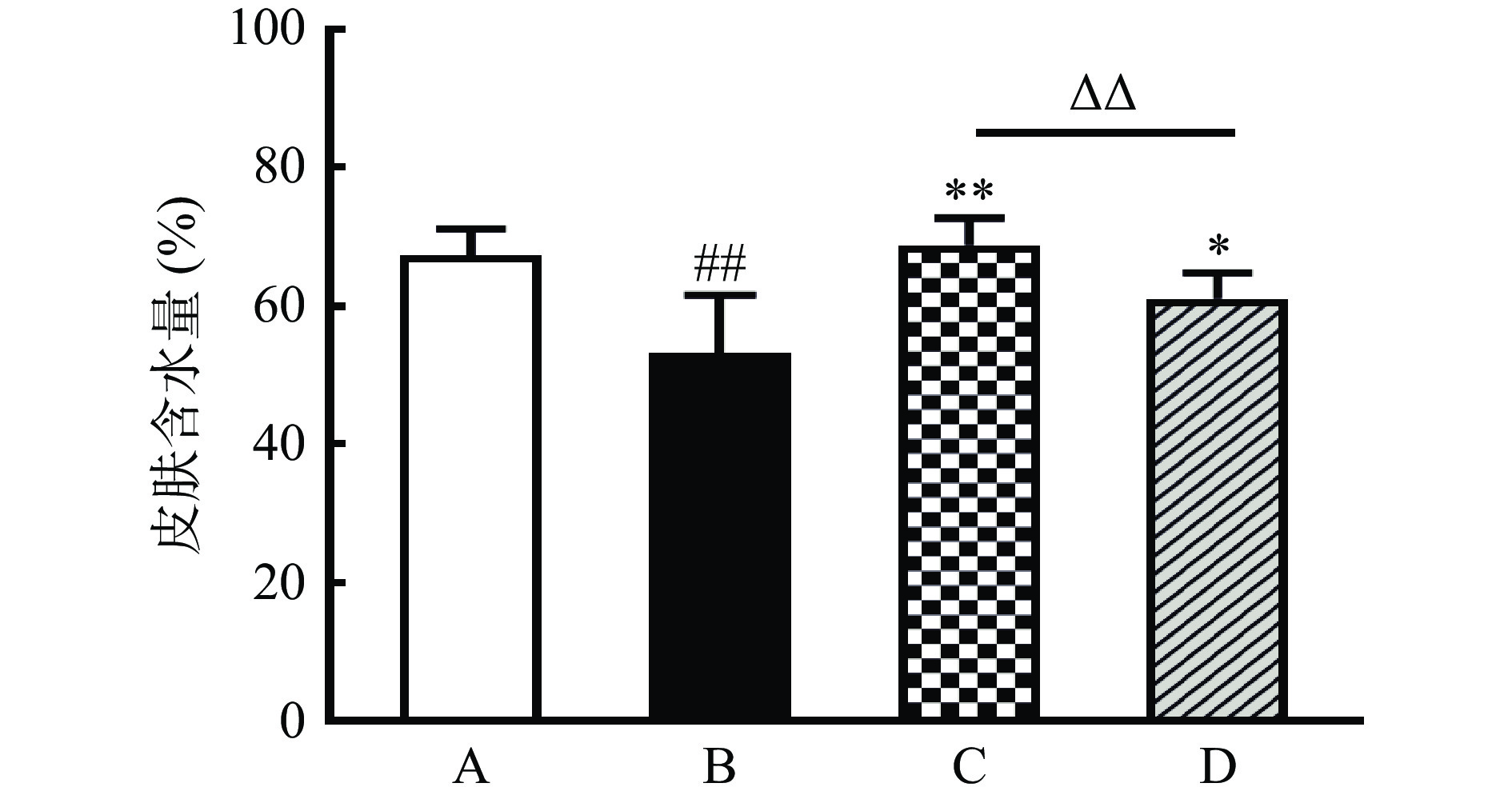
2.2 对小鼠皮肤含水量的影响
皮肤含水量是评估皮肤角质层屏障功能和健康状况的常用指标。皮肤暴露于紫外线会对皮肤屏障功能产生影响,真皮深层的水分通过表皮蒸发散失,从而造成皮肤含水量下降[27-28]。有研究表明,皮肤含水量与皮肤的光泽度、粗糙度、饱满度密切相关,皮肤含水量下降会造成皮肤暗沉、触感变硬、表面粗糙有细纹[29]。各组小鼠皮肤含水量如图3所示,与正常组比较,模型组小鼠皮肤含水量极显著降低(P<0.01),说明经过紫外线辐照后,小鼠皮肤角质层屏障功能受到严重破坏,导致背部皮肤水分大量散失;与模型组比较,复方胶原蛋白肽粉组和鱼胶原蛋白肽组小鼠皮肤含水量显著升高(P<0.05),表明复方胶原蛋白肽粉和鱼胶原蛋白肽都能够有效抑制紫外线辐照过程中小鼠背部皮肤的水分散失,对小鼠皮肤屏障具有保护作用;复方胶原蛋白肽粉组小鼠皮肤含水量极显著高于鱼胶原蛋白肽组(P<0.01),表明复方胶原蛋白肽粉对皮肤的保湿补水效果更佳。
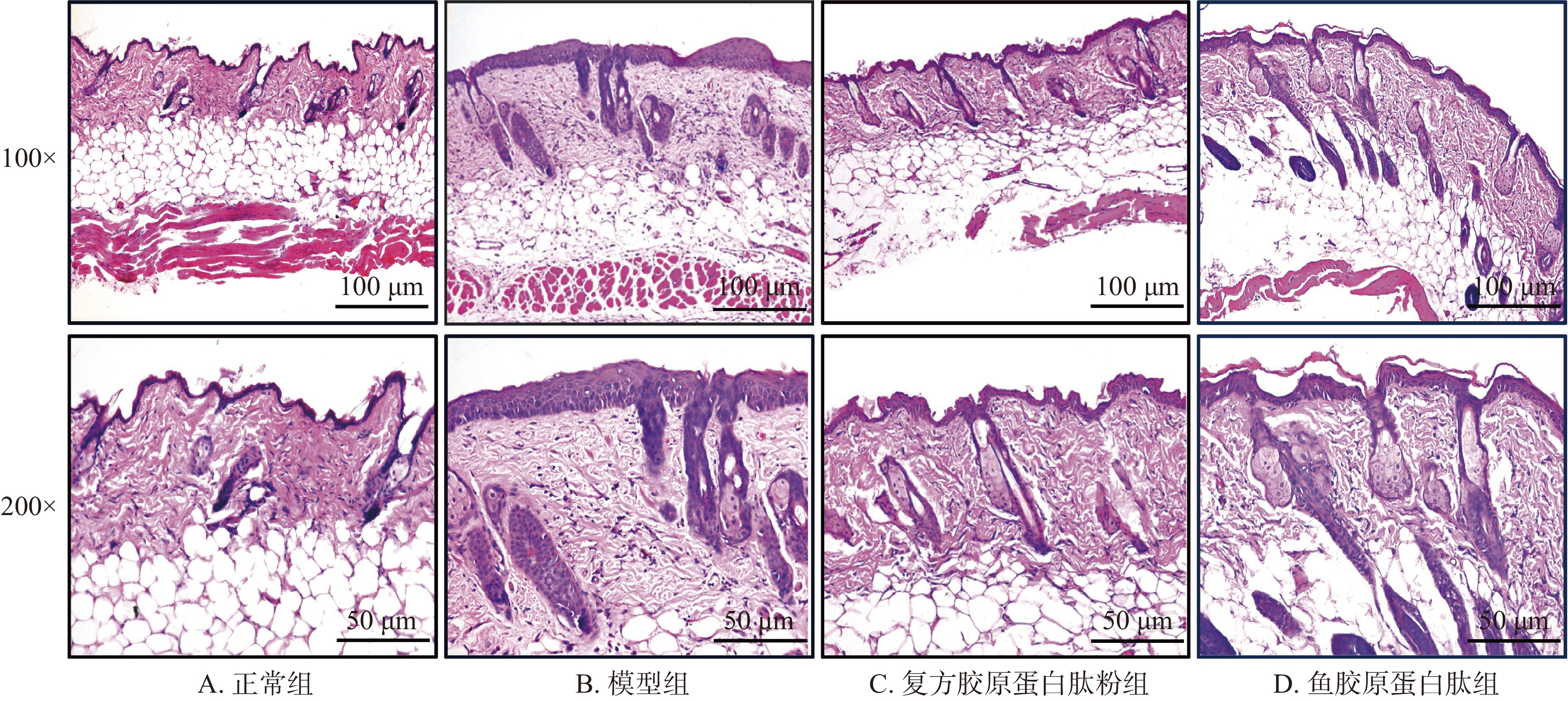
2.3 皮肤组织病理学改变
图4所示为紫外线辐照结束后,各组小鼠皮肤组织结构变化HE染色图。正常组小鼠表皮层厚度均匀且较薄,真皮-表皮连接(dermal-epidermal junction,EDJ)区结构呈波浪状起伏,有明显表皮突和真皮乳头,真皮层内胶原纤维束较粗大,且相互交织,排列紧密[30];模型组小鼠表皮层角化过度,较正常组显著增厚,EDJ区结构扁平,表皮突和真皮乳头不明显,真皮层内胶原纤维束较为纤细,排列分散,这与紫外线辐照引起的皮肤组织病理学改变特征相一致[31];与模型组相比,复方胶原蛋白肽粉组和鱼胶原蛋白肽组皮肤结构均有一定程度的改善,表皮层厚度减少,EDJ区结构再次呈现出波浪状,表皮突和真皮乳头数量增多,真皮层胶原纤维较为饱满,排列趋向整齐,说明复方胶原蛋白肽粉和鱼胶原蛋白肽均能有效改善紫外线辐照引起的皮肤组织病理学改变。其中复方胶原蛋白肽粉组改善效果更明显,较为接近正常组小鼠皮肤组织。
2.4 对皮肤组织中氧化指标水平的影响
皮肤暴露于紫外辐照环境中,极易导致活性氧簇(reactive oxygen species,ROS)过度蓄积[32]。抗氧化防御系统主要由SOD、GSH-Px为主的抗氧化酶组成[33],ROS诱导氧化应激时,SOD、GSH-Px等能够通过清除自由基,避免脂质过氧化和蛋白质糖基化的发生,起到抗氧化、抗衰老作用[34],同时氧化反应发生也能够降低抗氧化酶活性[35]。MDA、AGEs分别是膜脂质过氧化和蛋白质糖基化的产物,可以很好地反映氧化应激严重程度[36]。如表3所示,与正常组比较,模型组的T-AOC、SOD、GSH-Px水平均极显著降低(P<0.01),MDA、AGEs水平极显著升高(P<0.01),说明经紫外线辐照后,小鼠体内抗氧化酶活性降低,总抗氧化能力减弱,相反氧化损伤加重,小鼠模型构建成功;与模型组比较,复方胶原蛋白肽粉组和鱼胶原蛋白肽组T-AOC、SOD、GSH-Px水平均极显著升高(P<0.01),MDA、AGEs水平极显著降低(P<0.01),其中复方胶原蛋白肽粉组SOD、MDA、AGEs水平变化较鱼胶原蛋白肽组更加显著(P<0.05),表明复方胶原蛋白肽粉和鱼胶原蛋白肽均能有效缓解氧化应激反应,提高小鼠的总抗氧化能力,整体上复方胶原蛋白肽粉的抗氧化效果更佳。
表 3 各组小鼠皮肤组织中T-AOC、SOD、GSH-Px、MDA、AGEs水平的检测结果(n=8)Table 3. Levels of T-AOC, SOD, GSH-Px, MDA and AGEs in skin of mice in each group (n=8)组别 T-AOC(U/mL) SOD(ng/g) GSH-Px(ng/mL) MDA(nmol/g) AGEs(pg/g) 正常组 0.39±0.03 66.10±6.71 154.94±16.30 114.63±20.55 4686.75±465.57 模型组 0.23±0.04## 41.06±4.84## 94.13±25.67## 197.64±23.53## 5805.58±681.73## 复方胶原蛋白肽粉组 0.36±0.05** 67.78±7.46**△△ 123.63±11.57** 132.31±16.90**△△ 4208.78±361.36**△ 鱼胶原蛋白肽组 0.35±0.04** 58.52±4.69** 132.41±14.54** 166.07±17.17** 4772.76±469.90** 注:#与正常组比较,差异显著P<0.05;##与正常组比较,差异极显著P<0.01;*与模型组比较,差异显著P<0.05;**与模型组比较,差异极显著P<0.01;△与鱼胶原蛋白肽组比较,差异显著P<0.05;△△与鱼胶原蛋白肽组比较,差异极显著P<0.01;表4~表6同。 2.5 对皮肤组织中细胞外基质水平的影响
Col I、ELN、HA是真皮层重要的细胞外基质,也是评估一般功能性食品对皮肤营养补充效果的关键性指标[37]。Col I、ELN属结构蛋白,起支撑、填充作用,对维持皮肤的饱满度和弹性至关重要[38];HA是皮肤中的“保湿因子”,起滋润和营养作用,直接决定皮肤含水量和保湿能力[39]。有研究表明,经紫外线辐照后的皮肤中Col I、ELN、HA含量下降,导致真皮层营养流失,水合作用大大减弱,由此皮肤褶皱变形,从而加速衰老[40]。各组小鼠ECM水平如表4所示,与正常组比较,模型组小鼠皮肤组织中的Col I、ELN、HA水平均极显著降低(P<0.01),符合紫外线损伤小鼠皮肤细胞外基质含量变化特征;与模型组比较,复方胶原蛋白肽粉组和鱼胶原蛋白肽组小鼠皮肤组织中Col I、ELN、HA水平均显著升高(P<0.05),其中复方胶原蛋白肽粉组变化更为显著(P<0.05),表明复方胶原蛋白肽粉和鱼胶原蛋白肽均可有效抵抗紫外辐照诱导的小鼠真皮层细胞外基质流失,增加小鼠皮肤细胞外基质含量,其中复方胶原蛋白肽粉效果更加显著。
表 4 各组小鼠皮肤组织中Col I、ELN、HA水平的检测结果(n=8)Table 4. Levels of Col I, ELN and HA in skin of mice in each group (n=8)组别 Col I(ng/g) ELN(ng/g) HA(ng/g) 正常组 220.87±0.03 541.63±88.23 1655.13±147.52 模型组 167.85±21.86## 309.40±91.23## 1054.48±156.71## 复方胶原蛋白
肽粉组248.80±19.06**△△ 557.39±80.54**△ 1588.32±201.70**△△ 鱼胶原蛋白肽组 202.93±24.67** 450.45±74.39** 1240.20±89.71* 2.6 对TGF-β1/Smads通路相关mRNA表达水平的影响
TGF-β1/Smads信号通路在ECM的体内合成过程中扮演着重要的角色[41],TGF-β1信号受激后的主要下游靶点是多个Smad蛋白,当TGF-β1与细胞膜上相应受体结合形成复合物时,Smad2和Smad3蛋白被其受体磷酸化,随后与Smad4形成异二聚体复合物转移到细胞核中,激活相关启动子,增加胶原蛋白、弹性蛋白和透明质酸合成酶的表达[42-43],而Smad7作为负调控因子,能够阻滞Smad2/3活化,抑制TGF-β1/Smads的正向信号转导[34]。多项研究表明[36,41,44],UV辐照能够通过ROS介导TGF-β/Smad信号转导阻滞,与TGF-β配体和受体及下游Smad蛋白及其mRNA表达下调有关。表5所示为各组小鼠皮肤组织中TGF-β1/Smads通路相关mRNA表达水平,与正常组比较,模型组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3 mRNA表达水平均显著降低(P<0.05),Smad7 mRNA表达水平显著升高(P<0.05),表明经紫外线辐照后,小鼠皮肤中TGF-β/Smad信号转导受到抑制;与模型组比较,复方胶原蛋白肽粉组和鱼胶原蛋白肽组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3 mRNA表达水平均显著升高(P<0.05),Smad7 mRNA表达水平极显著降低(P<0.01),其中鱼胶原蛋白肽组的Smad2 mRNA、复方胶原蛋白肽粉组的Smad3 mRNA表达水平与模型组比较有上升趋势,但无统计学意义(P>0.05)。
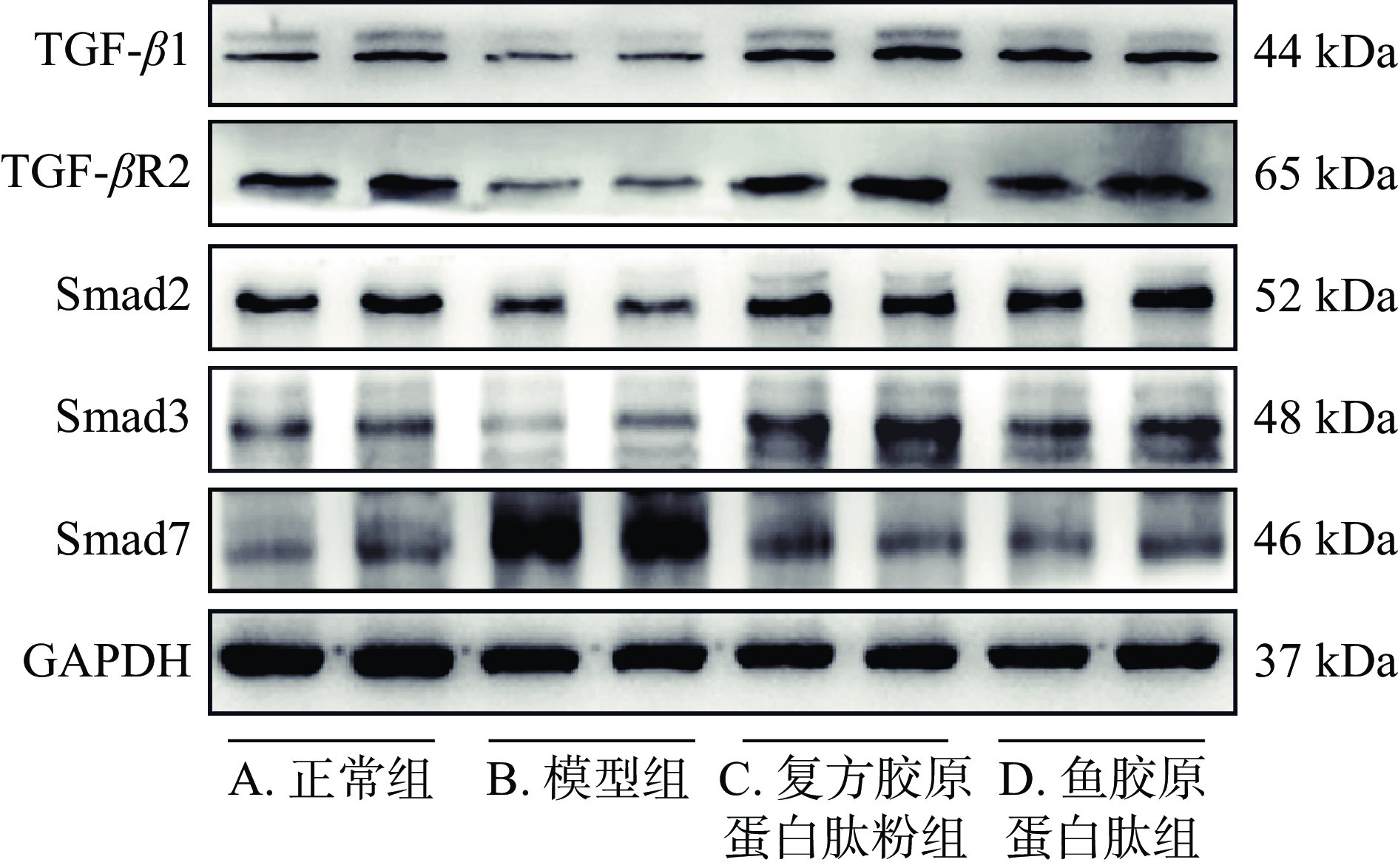
表 5 各组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3、Smad7 mRNA表达水平检测结果(n=6)Table 5. mRNA Expression of TGF-β1, TGF-βR2, Smad2, Smad3 and Smad7 in skin of mice in each group (n=6)组别 TGF-β1 mRNA TGF-βR2 mRNA Smad2 mRNA Smad3 mRNA Smad7 mRNA 正常组 1.14±0.58 1.02±0.19 1.13±0.53 1.03±0.28 1.60±1.32 模型组 0.47±0.11# 0.52±0.20## 0.22±0.04## 0.36±0.38## 3.94±1.70# 复方胶原蛋白肽粉组 1.81±1.16* 1.82±0.87** 0.77±0.54* 0.85±0.50 1.42±0.86** 鱼胶原蛋白肽组 1.10±0.40** 1.01±0.28** 0.57±0.37 0.99±0.42* 1.38±0.84** 2.7 对TGF-β1/Smads通路相关蛋白表达的影响
图5、表6所示为各组小鼠皮肤组织中TGF-β1/Smads通路相关蛋白表达水平,与正常组比较,模型组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3蛋白表达水平显著降低(P<0.05),Smad7蛋白表达水平极显著升高(P<0.01),表明经紫外线辐照后,小鼠皮肤中TGF-β/Smad信号转导受到抑制,与表5所示结果相一致;与模型组相比,复方胶原蛋白肽粉组和鱼胶原蛋白肽组小鼠皮肤组织TGF-β1、TGF-βR2、Smad2、Smad3蛋白表达水平极显著升高(P<0.01),Smad7蛋白表达水平极显著降低(P<0.01),其中复方胶原蛋白肽粉组的Smad3蛋白变化更为显著(P<0.01)。综合表5的数据显示,复方胶原蛋白肽粉和鱼胶原蛋白肽均可能是通过激活TGF-β1/Smads通路增加小鼠真皮层细胞外基质合成,从而有效改善皮肤慢性紫外线损伤。
表 6 各组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3、Smad7蛋白表达水平测定结果(n=4)Table 6. Protein expressions of TGF-β1, TGF-βR2, Smad2, Smad3 and Smad7 in skin of mice in each group (n=4)组别 TGF-β1/GAPDH TGF-βR2/GAPDH Smad2/GAPDH Smad3/GAPDH Smad7/GAPDH 正常组 1.22±0.16 0.81±0.13 0.99±0.14 0.91±0.16 0.82±0.11 模型组 0.88±0.07# 0.45±0.07## 0.69±0.07# 0.52±0.13# 1.11±0.06## 复方胶原蛋白肽粉组 1.37±0.17** 1.00±0.18** 1.11±0.08** 1.09±0.08**△△ 0.83±0.10** 鱼胶原蛋白肽组 1.13±0.07** 0.96±0.21** 1.10±0.16** 0.90±0.03** 0.68±0.12** 3. 结论
本实验以复方胶原蛋白肽粉为研究对象,通过建立小鼠皮肤慢性紫外线损伤模型,观察复方胶原蛋白肽粉对慢性紫外线损伤小鼠皮肤的改善作用,并初步探讨其作用机制。经复方胶原蛋白肽粉灌胃后,小鼠皮肤外观出现明显好转,皱纹情况得分显著降低,皮肤水分流失得到有效抑制,一定程度上改善了小鼠皮肤组织病理变化;小鼠皮肤中SOD、GSH-Px活性均有不同程度的提高,MDA、AGEs含量有所下降,有效抑制了体内氧化反应;增加了小鼠皮肤中Col I、ELN、HA含量,有效抵抗真皮层细胞外基质流失;同时TGF-β1、TGF-βR2、Smad2、Smad3蛋白及mRNA表达增强,激活皮肤中TGF-β1/Smads信号转导。
综上所述,复方胶原蛋白肽粉可有效改善小鼠皮肤慢性紫外线损伤,其机制可能与减轻氧化应激反应,同时调控TGF-β1/Smads信号转导增加细胞外基质合成有关。另一方面,通过和单一鱼胶原蛋白肽的疗效对比,发现复方胶原蛋白肽粉对皮肤慢性紫外线损伤的改善作用更加显著,表明胶原蛋白肽和多种植物有效成分结合后产生协同增效作用。本研究为复方类胶原蛋白肽产品的进一步开发和应用提供了创新思路与理论依据,但复方胶原蛋白肽粉改善皮肤慢性紫外线损伤的机制远不止于此,还有待于从动物实验向细胞实验和临床实验拓展,进一步明确其可调控的分子机制与途径,并根据实验数据逐步优化其配方选择。
-
表 1 皮肤皱纹情况评分标准
Table 1 Evaluation standard of skin wrinkles
级别 评分标准 0 正常皮肤细腻纹理 1 皮肤出现细磨痕 2 出现少量浅皱纹 3 出现较多浅皱纹 4 皮肤粗糙,有深皱纹产生 5 有较多粗糙皱纹产生 6 皱纹粗糙,出现皮肤局部损伤 表 2 引物序列
Table 2 Primer sequence
基因 序列(5'-3') 长度(bp) TGF-β1 上游AGGGCTACCATGCCAACTTC 168 下游CCACGTAGTAGACGATGGGC TGF-βR2 上游GTCGGATGTGGAAATGGAAGC 186 下游CAGGACTTCTGGTTGTCGCA Smad2 上游AATCATTGCAACAAGAGGCAGT 156 下游ATTCCCGTCCCCATCATCCT Smad3 上游CCGTGCGGAAACCCAAACTT 188 下游ACTCTGGAGAACTTGCCCG Smad7 上游ACAGAGGATCTTGTCCCCGA 102 下游CTGGCTGGCTGCGTCTC GAPDH 上游GGTGAAGGTCGGTGAACG 233 下游CTCGCTCCTGGAAGATGGTG 表 3 各组小鼠皮肤组织中T-AOC、SOD、GSH-Px、MDA、AGEs水平的检测结果(n=8)
Table 3 Levels of T-AOC, SOD, GSH-Px, MDA and AGEs in skin of mice in each group (n=8)
组别 T-AOC(U/mL) SOD(ng/g) GSH-Px(ng/mL) MDA(nmol/g) AGEs(pg/g) 正常组 0.39±0.03 66.10±6.71 154.94±16.30 114.63±20.55 4686.75±465.57 模型组 0.23±0.04## 41.06±4.84## 94.13±25.67## 197.64±23.53## 5805.58±681.73## 复方胶原蛋白肽粉组 0.36±0.05** 67.78±7.46**△△ 123.63±11.57** 132.31±16.90**△△ 4208.78±361.36**△ 鱼胶原蛋白肽组 0.35±0.04** 58.52±4.69** 132.41±14.54** 166.07±17.17** 4772.76±469.90** 注:#与正常组比较,差异显著P<0.05;##与正常组比较,差异极显著P<0.01;*与模型组比较,差异显著P<0.05;**与模型组比较,差异极显著P<0.01;△与鱼胶原蛋白肽组比较,差异显著P<0.05;△△与鱼胶原蛋白肽组比较,差异极显著P<0.01;表4~表6同。 表 4 各组小鼠皮肤组织中Col I、ELN、HA水平的检测结果(n=8)
Table 4 Levels of Col I, ELN and HA in skin of mice in each group (n=8)
组别 Col I(ng/g) ELN(ng/g) HA(ng/g) 正常组 220.87±0.03 541.63±88.23 1655.13±147.52 模型组 167.85±21.86## 309.40±91.23## 1054.48±156.71## 复方胶原蛋白
肽粉组248.80±19.06**△△ 557.39±80.54**△ 1588.32±201.70**△△ 鱼胶原蛋白肽组 202.93±24.67** 450.45±74.39** 1240.20±89.71* 表 5 各组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3、Smad7 mRNA表达水平检测结果(n=6)
Table 5 mRNA Expression of TGF-β1, TGF-βR2, Smad2, Smad3 and Smad7 in skin of mice in each group (n=6)
组别 TGF-β1 mRNA TGF-βR2 mRNA Smad2 mRNA Smad3 mRNA Smad7 mRNA 正常组 1.14±0.58 1.02±0.19 1.13±0.53 1.03±0.28 1.60±1.32 模型组 0.47±0.11# 0.52±0.20## 0.22±0.04## 0.36±0.38## 3.94±1.70# 复方胶原蛋白肽粉组 1.81±1.16* 1.82±0.87** 0.77±0.54* 0.85±0.50 1.42±0.86** 鱼胶原蛋白肽组 1.10±0.40** 1.01±0.28** 0.57±0.37 0.99±0.42* 1.38±0.84** 表 6 各组小鼠皮肤组织中TGF-β1、TGF-βR2、Smad2、Smad3、Smad7蛋白表达水平测定结果(n=4)
Table 6 Protein expressions of TGF-β1, TGF-βR2, Smad2, Smad3 and Smad7 in skin of mice in each group (n=4)
组别 TGF-β1/GAPDH TGF-βR2/GAPDH Smad2/GAPDH Smad3/GAPDH Smad7/GAPDH 正常组 1.22±0.16 0.81±0.13 0.99±0.14 0.91±0.16 0.82±0.11 模型组 0.88±0.07# 0.45±0.07## 0.69±0.07# 0.52±0.13# 1.11±0.06## 复方胶原蛋白肽粉组 1.37±0.17** 1.00±0.18** 1.11±0.08** 1.09±0.08**△△ 0.83±0.10** 鱼胶原蛋白肽组 1.13±0.07** 0.96±0.21** 1.10±0.16** 0.90±0.03** 0.68±0.12** -
[1] SOLANO F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources[J]. Molecules,2020,25(7):1537. doi: 10.3390/molecules25071537
[2] YARDMAN-FRANK J M, FISHER D E. Skin pigmentation and its control: From ultraviolet radiation to stem cells[J]. Experimental Dermatology,2021,30(4):560−571. doi: 10.1111/exd.14260
[3] ANSARY T M, HOSSAIN M, KAMIYA K, et al. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging[J]. International Journal of Molecular Sciences,2021,22(8):3974. doi: 10.3390/ijms22083974
[4] CADET J, DOUKI T. Formation of UV-induced DNA damage contributing to skin cancer development[J]. Photochemical & Photobiological Sciences,2018,17(12):1816−1841.
[5] LI L, HWANG E, NGO H T T, et al. Antiphotoaging effect of Prunus yeonesis blossom extract via inhibition of MAPK/AP‐1 and regulation of the TGF‐βI/Smad and Nrf2/ARE signaling pathways[J]. Photochemistry and Photobiology,2018,94(4):725−732. doi: 10.1111/php.12894
[6] XU M Y, PANG Q Q, XU S Q, et al. Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts[J]. Oncotarget,2018,9(3):3188. doi: 10.18632/oncotarget.23225
[7] ZHENG Y, XU Q F, CHEN H Y, et al. Inhibition of MMPs Cat G and downregulates the signaling of TGF-β/Smad in chronic photodamaged human fibroblasts[J]. Eur Rev Med Pharmacol Sci,2017,21(22):5160−5165.
[8] CHEN X, YANG C S, JIANG G, Research progress on skin photoaging and oxidative stress[J]. Postepy Dermatol Alergol, 2021, 38(6): 931-936.
[9] BOLKE L, SCHLIPPE G, GERß J, et al. A collagen supplement improves skin hydration, elasticity, roughness, and density: Results of a randomized, placebo-controlled, blind study[J]. Nutrients,2019,11(10):2494. doi: 10.3390/nu11102494
[10] PROKSCH E, SCHUNCK M, ZAGUE V, et al. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis[J]. Skin Pharmacology and Physiology,2014,27(3):113−119. doi: 10.1159/000355523
[11] OBA C, OHARA H, MORIFUJI M, et al. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice[J]. Photodermatology, Photoimmunology & Photomedicine,2013,29(4):204−211.
[12] XIE Z, WANG X G, YU S Y, et al. Antioxidant and functional properties of cowhide collagen peptides[J]. J Food Sci,2021,86(5):1802−1818. doi: 10.1111/1750-3841.15666
[13] 赵芷芊, 王敏, 张志清. 植物多糖的提取及抗氧化功效的研究进展[J]. 食品工业科技,2018,39(13):337−342. [ZHAO Z Q, WANG M, ZHANG Z Q. Research progress on extraction and antioxidant effect of plant polysaccharides[J]. Science and Technology of Food Industry,2018,39(13):337−342. doi: 10.13386/j.issn1002-0306.2018.13.062 ZHAO Z Q, WANG M, ZHANG Z Q. Research progress on extraction and antioxidant effect of plant polysaccharides[J]. Science and Technology of Food Industry, 2018, 39(13): 337-342. doi: 10.13386/j.issn1002-0306.2018.13.062
[14] MARIA C, BIRGIT W, GIORGIA B, et al. Plant extracts and natural compounds used against UVB-induced photoaging[J]. Biogerontology,2017,18(4):499−516. doi: 10.1007/s10522-017-9715-7
[15] 郑瑞生. 植物中抗氧化活性成分及其提取技术的研究[J]. 食品工业科技,2011,32(11):459−463, 467. [ZHENG R S. Study on antioxidant components and extraction technology in plants[J]. Science and Technology of Food Industry,2011,32(11):459−463, 467. ZHENG R S. Study on antioxidant components and extraction technology in plants[J]. Science and Technology of Food Industry, 2011, 32(11): 459-463, 467.
[16] 徐叔云, 卞如濂, 陈修. 药理实验方法学[M]. 第三版. 北京: 人民卫生出版社, 2002: 1698 XU S Y, BIAN R L, CHEN X. Methodology of pharmacological experiment[M]. The third edition. Beijing: People’s Medical Publishing House, 2002: 1698.
[17] 韩旭, 蒋靖. 慢性紫外线损伤小鼠模型皮肤角质形成细胞中CK1和CK10的表达研究[J]. 临床皮肤科杂志,2016,45(11):762−765. [HAN X, JIANG J. Expression of CK1 and CK10 in keratinocytes of mouse model with chronic ultraviolet radiation injury[J]. Journal of Clinical Dermatology,2016,45(11):762−765. HAN X, JIANG J. Expression of CK1 and CK10 in keratinocytes of mouse model with chronic ultraviolet radiation injury[J]. Journal of Clinical Dermatology, 2016, 45(11): 762-765.
[18] 王莹. Caspase-3、Bax、Bcl-2和Beclin-1在慢性紫外线损伤小鼠模型表皮角质形成细胞中的表达及意义[D]. 天津: 天津医科大学, 2012 WANG Y. The expression and significance of Caspase-3, Bax, Bcl-2 and Beclin-1 in epidermal keratinocytes of chronic ultraviolet injury mouse model[D]. Tianjin: Tianjin Medical Sciences University, 2012.
[19] 邓明高. 松茸提取物对UVB诱导的小鼠皮肤氧化应激和炎症的保护作用[D]. 广州: 广东工业大学, 2020 DENG M G. Protective effects of matsutake extract on UVB-induced oxidative stress and inflammation in mice skin[D]. Guangzhou: Guangdong University, 2020.
[20] BISSETT D L, CHATTERJEE R, HANNON D P. Photoprotective effect of topical anti-inflammatory agents against ultraviolet radiation-induced chronic skin damage in the hairless mouse[J]. Photodermatology, Photoimmunology & Photomedicine,1990,7(4):153−158.
[21] 路宁宁. 水母雪莲多糖对小鼠光老化皮肤含水量、AQP-3的影响研究[D]. 西宁: 青海大学, 2021 LU N N. Effect of Saussurea medusa polysaccharide on water content and AQP-3 of photoaging skin in mice[D]. Xining: Qinghai University, 2021.
[22] 逯岩松. 芍药苷对UVA诱导皮肤光损伤的防护作用及机制研究[D]. 沈阳: 中国医科大学, 2020 LU Y S. Protective effect and mechanism of paeoniflorin on skin photodamage induced by UVA[D]. Shenyang: China Medical University, 2020.
[23] 王天顺. 杭白菊、野菊花和神农香菊抗氧化损伤作用及有效成分研究[D]. 武汉: 湖北中医药大学, 2022 WANG T S. Study on antioxidative damage and effective components of Chrysanthemum morifolium, Chrysanthemum indicum and Chrysanthemum indicum[D]. Wuhan: Hubei University of Chinese Medicine, 2022.
[24] 王明月. 潞党参通过IL-15及其受体调控光老化小鼠皮肤炎症反应作用机制[D]. 沈阳: 辽宁中医药大学, 2020 WANG M Y. Mechanism of Ludangshen regulating skin inflammation in photoaging mice by IL-15 and its receptor[D]. Shenyang: Liaoning University of Chinese Medicine, 2020.
[25] 谢璟. 杨梅黄酮对紫外线诱导的皮肤光老化保护作用及潜在机制研究[D]. 济南: 山东大学, 2019 XIE J. Study on the protective effect and potential mechanism of myricetin on UV-induced skin photoaging[D]. Jinan: Shandong University, 2019.
[26] 金媛媛. 刺五加糖蛋白对紫外线引起的皮肤光老化的修复作用及机制研究[D]. 长春: 长春中医药大学, 2022 JIN Y Y. Study on the repair effect and mechanism of Acanthopanax senticosus glycoprotein on skin photoaging induced by ultraviolet radiation[D]. Changchun: Changchun University of Chinese Medicine, 2022.
[27] XIAO P, CHEN D. The effect of sun tan lotion on skin by using skin TEWL and skin water content measurements[J]. Sensors (Basel),2022,22(9):3595. doi: 10.3390/s22093595
[28] LIN Y C, CHEN Y C, HWANG B F, et al. Acute dermal effects of solar UV irradiation and efficacy of sunscreen use[J]. Environmental Pollutants and Bioavailability,2022,34(1):456−68. doi: 10.1080/26395940.2022.2128883
[29] KANG M K, KIM DONG Y, OH H, et al. Dietary collagen hydrolysates ameliorate furrowed and parched skin caused by photoaging in hairless mice[J]. Int J Mol Sci,2021,22(11):6137. doi: 10.3390/ijms22116137
[30] 于建伟, 杜芬, 陶宇, 等. 南极磷虾肽抗皮肤光老化作用的研究[J]. 食品工业科技,2021,42(20):372−376. [YU J W, DU F, TAO Y, et al. Study on anti-skin photoaging effect of antarctic krill peptide[J]. Science and Technology of Food Industry,2021,42(20):372−376. doi: 10.13386/j.issn1002-0306.2021030351 YU J W, DU F, TAO Y, et al. Study on anti-skin photoaging effect of antarctic krill peptide[J]. Science and Technology of Food Industry, 2021, 42(20): 372-376. doi: 10.13386/j.issn1002-0306.2021030351
[31] KHAN A, BAI H, KHAN A, et al. Neferine prevents ultraviolet radiation-induced skin photoaging[J]. Exp Ther Med,2020,19(5):3189−96.
[32] DE JAGER T L, COCKRELL A E, DU PLESSIS S S. Ultraviolet light induced generation of reactive oxygen species[J]. Ultraviolet Light in Human Health, Diseases and Environment,2017,996:15−23.
[33] WIRAGUNA A A G P, PANGKAHILA W, ASTAWA I N M. Antioxidant properties of topical Caulerpa sp. extract on UVB-induced photoaging in mice[J]. Dermatology Reports,2018,10(2):7597.
[34] ROCHA-GUZMÁN N E, SIMENTAL-MENDÍA L E, BARRAGÁN-ZÚÑIGA L J, et al. Effect of Buddleja scordioides K. leaves infusion on lipid peroxidation in mice with ultraviolet light-induced oxidative stress[J]. Medicinal Chemistry Research,2018,27(10):2379−2385. doi: 10.1007/s00044-018-2243-4
[35] BAVKAR L N, PATIL R S, ROOGE S B, et al. Acceleration of protein glycation by oxidative stress and comparative role of antioxidant and protein glycation inhibitor[J]. Mol Cell Biochem,2019,459(1):61−71.
[36] LYU J L, LIU Y J, WEN K C, et al. Protective effect of djulis (Chenopodium formosanum) extract against UV- and AGEs-induced skin aging via alleviating oxidative stress and collagen degradation[J]. Molecules,2022,27(7):2332. doi: 10.3390/molecules27072332
[37] GENG R, KANG S G, HUANG K, et al. Boosting the photoaged skin: The potential role of dietary components[J]. Nutrients,2021,13(5):1691. doi: 10.3390/nu13051691
[38] WANG J, QIU H, XU Y, et al. The biological effect of recombinant humanized collagen on damaged skin induced by UV-photoaging: An in vivo study[J]. Bioact Mater,2022,11:154−165. doi: 10.1016/j.bioactmat.2021.10.004
[39] PARK S-J, KIM D, LEE M, et al. GT collagen improves skin moisturization in UVB-irradiated HaCaT cells and SKH-I hairless mice[J]. J Med Food,2021,24:1313−1322. doi: 10.1089/jmf.2021.K.0089
[40] CHOWDHURY A, NOSOUDI N, KARAMCHED S, et al. Polyphenol treatments increase elastin and collagen deposition by human dermal fibroblasts; Implications to improve skin health[J]. J Dermatol Sci,2021,102(2):94−100. doi: 10.1016/j.jdermsci.2021.03.002
[41] FAN Y F, TAE-HYUN C, JEE-HYEOK C, et al. Hyaluronic acid-cross-linked filler stimulates collagen type 1 and elastic fiber synthesis in skin through the TGF-β/Smad signaling pathway in a nude mouse model[J]. J Plast Reconstr Aesthet Surg,2019,72(8):1355−1362. doi: 10.1016/j.bjps.2019.03.032
[42] POMATTO L C D, DAVIES J A. Adaptive homeostasis and the free radical theory of ageing[J]. Free Radic Biol Med,2018,124:420−30. doi: 10.1016/j.freeradbiomed.2018.06.016
[43] OH J H, KIM J, KARADENIZ F, et al. Santamarine shows anti-photoaging properties via inhibition of MAPK/AP-1 and stimulation of TGF-β/Smad signaling in UVA-irradiated HDFs[J]. Molecules,2021,26(12):3585. doi: 10.3390/molecules26123585
[44] PARK B, HWANG E, SEO S A, et al. Eucalyptus globulus extract protects against UVB-induced photoaging by enhancing collagen synthesis via regulation of TGF-β/Smad signals and attenuation of AP-1[J]. Archives of Biochemistry & Biophysics,2018,637:31−39.
-
期刊类型引用(0)
其他类型引用(2)





 下载:
下载:

 下载:
下载:



