Antioxidant and Antibacterial Activities of Different Extracted Parts from Branches and Leaves Extracts of the Peristrophe baphica (Spreng.) Bremek
-
摘要: 以红蓝草枝和叶为原料,探究其乙醇提取物和不同溶剂萃取部位的抗氧化及抑菌活性。采用Folin-Ciocalteu法和NaNO2-Al(NO3)3法分别测定乙醇提取物及各萃取部位总酚和黄酮含量;以清除DPPH•、ABTS+•和总还原力为指标评价各部位抗氧化能力并分析抗氧化作用与两种成分之间的相关性。同时,采用牛津杯法测定各部位对常见致病菌的抑菌活性。结果表明,红蓝草枝和叶的乙酸乙酯部位总酚含量最高,分别达79.76 mg/g和80.21 mg/g;枝的氯仿部位和叶的乙醇提取物黄酮含量最高,分别为95.88 mg/g和96.75 mg/g。相关性分析显示红蓝草的抗氧化能力与其酚类含量具有较强相关性。在试验浓度范围内,红蓝草不同组织部位以及不同极性溶剂影响抗氧化及抑菌效果,枝的抗氧化效果优于叶,叶的抑菌效果优于枝。以枝的乙酸乙酯部位,叶的乙酸乙酯和正丁醇部位抗氧化和抑菌效果更为明显,值得进一步研究与开发。Abstract: In this work, the ethanol extracts of the branches and leaves of Peristrophe baphica (Spreng.) Bremk (PB) as well as the different solvent extracted part of the ethanol extracts were prepared and their antioxidant and antibacterial activities were studied. The contents of total polyphenols and flavonoids of the extracts were determined by Folin-Ciocalteu and NaNO2-Al(NO3)3 method, respectively. The antioxidant activities were evaluated by DPPH radical scavenging ability, ABTS+ radical scavenging ability and total reducing power. The relation between the antioxidant activities and the ethanol extracts as well as the different solvent extracted parts was correlated. The antimicrobial activities of the extracts against several common pathogenic bacteria were determined by Oxford cup method. The results showed that the content of total polyphenols in ethyl acetate part were the highest for both branches and leaves, which was 79.76 mg/g and 80.21 mg/g, respectively. The highest content of flavonoids located in chloroform part of branches and the ethanol extract of leaves, which was 95.88 mg/g and 96.75 mg/g, respectively. The correlation analysis showed that the antioxidant activities of each part were strongly correlated with the contents of total polyphenols. In the test concentration range, different parts of tissue and different solvents had important influence on the antioxidant and antimicrobial activities. The antioxidant activities of branches were better than those of leaves, but the antibacterial activities of leaves were better than those of branches. The ethyl acetate part of branches, the ethyl acetate part and the n-butanol part of leaves showed better antioxidant and antimicrobial activities and could be used for further application.
-
Keywords:
- Peristrophe baphica (Spreng.) Bremk /
- branch /
- leaf /
- extracts /
- extracted part /
- antioxidant activity /
- antibacterial activity
-
近年来,从植物和天然药物中寻找安全有效的抗氧化和抑菌活性成分,开发并应用于食品相关领域备受关注[1-3]。植物中的酚类、黄酮、多糖和生物碱等化学成分具有良好的抗氧化活性和抑菌效果[4-5],Kherbache等[6]发现蜡菊提取物中酚酸的体外抗氧化活性优于对照物。贾睿等[7]研究表明,红豆皮多酚对食源性致病菌—李斯特菌(Listeria monocytogenes)和沙门氏菌(Salmonella)有抑制作用。不同提取溶剂影响提取物的组成及生物活性[8-9],而植物的不同组织部位也影响提取物的抗氧化及抑菌活性[10-11],赵岩等[12]发现肉苁蓉的根部抗氧化活性强于中部和顶部。因此,利用不同极性溶剂对植物不同组织部位的活性物质进行提取,并进行生物活性研究,有利于有效筛选植物活性部位、合理进行品质评价和提高综合利用水平。
红蓝草(Peristrophe baphica (Spreng.) Bremk,PB)为爵床科观音草属植物,别名观音草、红丝线、山蓝,主要分布在我国广西、广东、云南、海南等省区,全年可采,在壮族聚集区,其汁液为“五色糯米饭”天然染料之一。全草具有降血压、保肝护肝和止咳祛痰等药理作用[13],主要含有酚类、黄酮、生物碱、三萜、甾体及其苷类、挥发油等化学成分[14]。已有研究[15-16]表明红蓝草色素具有一定的还原能力和自由基清除能力,对大肠杆菌、金黄色葡萄球菌等具有一定的抑制作用[17]。韦正等[18]优化了红蓝草中总酚酸的提取,并测定其具有一定的抗氧化活性。目前红蓝草的研究主要集中在全草化学成分、部分药理作用和色素方面[19-22]。针对红蓝草不同组织部位(枝、叶)生物活性的研究较少。
本研究分别制备红蓝草枝和叶的乙醇提取物,利用不同极性溶剂进行萃取,测定不同萃取部位总酚和黄酮含量,采用多种体外抗氧化活性评价方法研究其抗氧化效果,分析成分含量与抗氧化作用的相关性,并对比不同萃取部位抑菌效果。以期为红蓝草资源综合开发利用提供实验依据和理论基础。
1. 材料与方法
1.1 材料与仪器
红蓝草购于广西,经广东食品药品职业学院中药基础教研室鉴定为细毛无白斑种红蓝草;1, 1-二苯基-2-三硝基苯肼(DPPH)、2, 2-联氮基-双-(3-乙基苯并噻唑啉-6-磺)二氨盐(ABTS)、芦丁(95%)、维生素C(VC) 上海阿拉丁生化科技股份有限公司;没食子酸 天津市大茂化学试剂厂;福林-酚 北京索莱宝科技有限公司;三氯化铁、三氯乙酸、铁氰化钾 上海凌峰化学试剂有限公司;沙氏葡萄糖琼脂培养基、营养琼脂 北京陆桥技术股份有限公司;乙醇、石油醚、氯仿、乙酸乙酯、正丁醇、二甲基亚砜(DMSO) 广州化学试剂厂;菌种:金黄色葡萄球菌(Staphylococcus aureus)、大肠埃希氏菌(Escherichia coli)、蜡样芽胞杆菌(Bacillus cereus)、奇异变形杆菌(Proteus mirabilis)、铜绿假单胞菌(Pseudomonas aeruginosa)、白色念珠菌(Monilia albican)、阪崎肠杆菌(Enterobacter sakazakii)、肺炎克雷伯氏菌(Klebsiella Trevisan) 广东环凯微生物科技有限公司。
TU-1810紫外可见分光光度计 北京普析通用仪器有限责任公司;RE-52AA型旋转蒸发仪 上海亚荣生化仪器厂;TW223L型SHIMADZU岛津电子天平 上海知世仪器设备有限公司;H1850R离心机 湘仪离心机仪器有限公司;数显HH-6系列恒温水浴锅 金坛市国旺实验仪器厂;隔水式恒温培养箱 上海一恒科技有限公司。
1.2 实验方法
1.2.1 不同极性萃取部位的制备
红蓝草样品洗净,55 ℃烘干8 h,将枝和叶分开粉碎,过40目筛。分别取红蓝草枝和叶碎粉末100 g,75%的乙醇以1:30(W:V)料液比在80℃热回流提取2 h,重复提取3次,合并粗提液,抽滤,将滤液于45 ℃真空浓缩至提取物无醇味,得到乙醇提取物浸膏(枝:32.4 g;叶:33.0 g)。取部分直接冻干,为乙醇提取物(简称醇提物,下同)。
分别取枝和叶的乙醇提取物浸膏30.0 g,用150 mL去离子水充分混悬后,依次用石油醚、氯仿、乙酸乙酯和正丁醇进行萃取,每种溶剂萃取4次,每次150 mL,各部位萃取液合并,剩下为水相部分,真空浓缩得到石油醚萃取部位(枝:2.15 g;叶:2.29 g)、氯仿萃取部位(枝:3.94 g;叶:4.04 g)、乙酸乙酯萃取部位(枝:5.49 g;叶:5.55 g)、正丁醇萃取部位(枝:4.35 g;叶:4.39 g)和水相部位(枝:11.08 g;叶:11.14 g),冷冻干燥后,置于−20℃密封保存,备用。
1.2.2 样品溶液的制备
精密称取各萃取部位,加入DMSO溶剂振荡助溶,抗氧化实验需配成10.0 mg/mL的样品溶液,抑菌实验需配成250.0 mg/mL的样品溶液,实验前稀释至适宜浓度。
1.2.3 总酚含量测定
参考本课题组的前期研究方法[23]。
标准曲线制备:准确称取10.0 mg没食子酸,用蒸馏水定容至100 mL容量瓶,即为0.10 mg/mL的标准工作溶液。依次吸取没食子酸标准工作溶液0、0.10、0.20、0.30、0.40、0.50 mL至10 mL容量瓶中,加入0.50 mL福林-酚试剂,5 min后向每个容量瓶中加入20%碳酸钠溶液1.50 mL,用蒸馏水定容至刻度。定容后的溶液转移至15 mL离心管中,75℃水浴10 min,室温放置2 h,于760 nm处测定吸光度。以没食子酸浓度为横坐标,吸光度为纵坐标,绘制标准曲线。
样品总酚含量测定:将不同萃取部位分别配制成0.50 mg/mL溶液,吸取1.00 mL溶液按上述方法平行测定3次。根据标准曲线及其回归方程计算不同萃取部位总酚含量(以没食子酸计,mg/g)。
1.2.4 总黄酮含量的测定
参考文献[24]方法,稍作改动。
芦丁标准曲线制备:精密称取芦丁对照品5.00 mg,并用60%乙醇水溶解定容至10 mL的容量瓶中,摇匀即得芦丁标准溶液(0.50 mg/mL)。依次移取0、0.20、0.40、0.60、0.80、1.00 mL芦丁标准溶液分别置于10 mL棕色容量瓶中,加入0.50 mL 5% 的NaNO2溶液,混匀静置6 min;加入0.50 mL 10%的 Al(NO3)3溶液,混匀静置6 min;再加入4.00 mL 4%的NaOH溶液,混匀后用60%乙醇水溶液定容至刻度,静置15 min。于510 nm处测定吸光度。以芦丁浓度为横坐标,吸光度为纵坐标,绘制标准曲线。
样品黄酮含量测定:将不同萃取部位配制成2.00 mg/mL溶液,吸取1.00 mL溶液按上述方法平行测定3次。根据标准曲线及其回归方程计算不同萃取部位黄酮含量(以芦丁计,mg/g)。
1.2.5 抗氧化活性评价
1.2.5.1 DPPH自由基清除能力的测定
参考本课题组的前期研究方法[23]。使用60%乙醇水溶液分别将红蓝草枝和叶醇提物及其不同萃取部位配制成2.00、1.00、0.50、0.25、0.125、0.063 mg/mL系列质量浓度溶液(叶的氯仿部位为1.00、0.50、0.25、0.125、0.063、0.031 mg/mL)。分别取样品溶液2.00 mL和0.20 mmol/L DPPH乙醇溶液2.00 mL,混匀,避光反应30 min,于517 nm处以无水乙醇为空白对照,测定吸光度(A1);以等量无水乙醇替换0.20 mmol/L DPPH乙醇溶液,同法测吸光度(A2);以等量60%乙醇水溶液替换样品溶液,同法测定吸光度(A0)。以VC为对照,平行测定3次。按公式(1)计算DPPH自由基清除率。
DPPH自由基清除率(%)=[1−(A1−A2)/A0]×100 (1) 1.2.5.2 ABTS+自由基清除能力测定
参考文献[25-26]方法,略有改动。将7.00 mmol/L ABTS溶液与2.45 mmol/L过硫酸钾溶液等体积混匀,室温避光静置12~16 h,得到ABTS储备液。使用前用60%乙醇水溶液稀释,使其吸光度值在734 nm波长达到0.70±0.02,此为ABTS工作液。使用60%乙醇水溶液分别将红蓝草枝和叶醇提物及其不同萃取部位配置成4.00、2.00、1.00、0.50、0.25、0.125 mg/mL系列质量浓度溶液。分别取0.10 mL样品溶液与3.90 mL ABTS工作液混匀,避光反应6 min后,于734 nm处以60%乙醇水溶液为空白对照,测定吸光度(A1);以等量60%乙醇水溶液替换ABTS工作液,同法测定吸光度(A2);以等量60%乙醇水溶液替换样品溶液,同法测定吸光度(A0)。以VC为对照,平行测定3次。按公式(2)计算ABTS+自由基清除率。
ABTS+自由基清除率(%)=[1−(A1−A2)/A0]×100 (2) 1.2.5.3 总还原力测定
参考本课题组的前期研究方法[23]。使用60%乙醇水溶液分别将红蓝草枝和叶醇提物及其不同萃取部位配制成4.00、2.00、1.00、0.50、0.25、0.125 mg/mL系列质量浓度的溶液。取1.00 mL不同质量浓度的溶液于离心管中,分别加入pH6.6磷酸盐缓冲液2.50 mL和1%铁氰化钾2.50 mL,混匀后50 ℃水浴反应20 min。取出冷却,加入10%的三氯乙酸2.50 mL终止反应。取2.50 mL上述溶液加入2.00 mL蒸馏水和0.50 mL三氯化铁,混匀,于700 nm处以蒸馏水为空白对照,测定吸光度(A1);以等量60%乙醇水溶液替换样品溶液,同法测定吸光度(A0);以等量蒸馏水替换反应试剂,同法测定吸光度(A2)。以VC为对照,平行测定3次。按公式(3)计算总还原力。
总还原力=A1−A0−A2 (3) 1.2.6 体外抑菌活性测定
抑菌圈测定采用牛津杯法,参考文献[27]方法并稍作修改。
样品液:使用蒸馏水对1.2.2中的样品液进行倍比稀释,使红蓝草枝和叶的醇提物和不同萃取部位的质量浓度分别为250.0、125.0、62.5 mg/mL,0.22 µm的过滤器除菌后使用。
菌悬浮液制备:分别将金黄色葡萄球菌、大肠埃希氏菌、蜡样芽胞杆菌、奇异变形杆菌、铜绿假单胞菌、白色念珠球菌、阪崎肠杆菌和肺炎克雷伯氏菌活化培养,制成1×107 CFU/mL的菌悬液,待用。
抑菌圈测定:每个平板加入15.0 mL营养琼脂,凝固后待水分干燥,取0.10 mL菌悬液在平板上均匀涂布,以平板未见水滴为准,立刻进行抑菌实验。把四环牛津杯轻放于平板上,并用压板轻轻压紧。将0.15 mL样品液加入到牛津杯中,36±1 ℃下培养24 h。观察牛津杯底周围有无抑菌圈,重复3次,测量抑菌圈直径。阴性对照为蒸馏水。
1.3 数据处理
各试验重复3次,结果以平均值±标准差(
ˉX ±SD)表示。采用SPSS 19.0、Origin 9.0和Excel 2019 软件进行数据处理与绘图,利用Pearson法分析抗氧化活性与总酚、黄酮含量的相关性。2. 结果与分析
2.1 各萃取部位中总酚、总黄酮含量分析
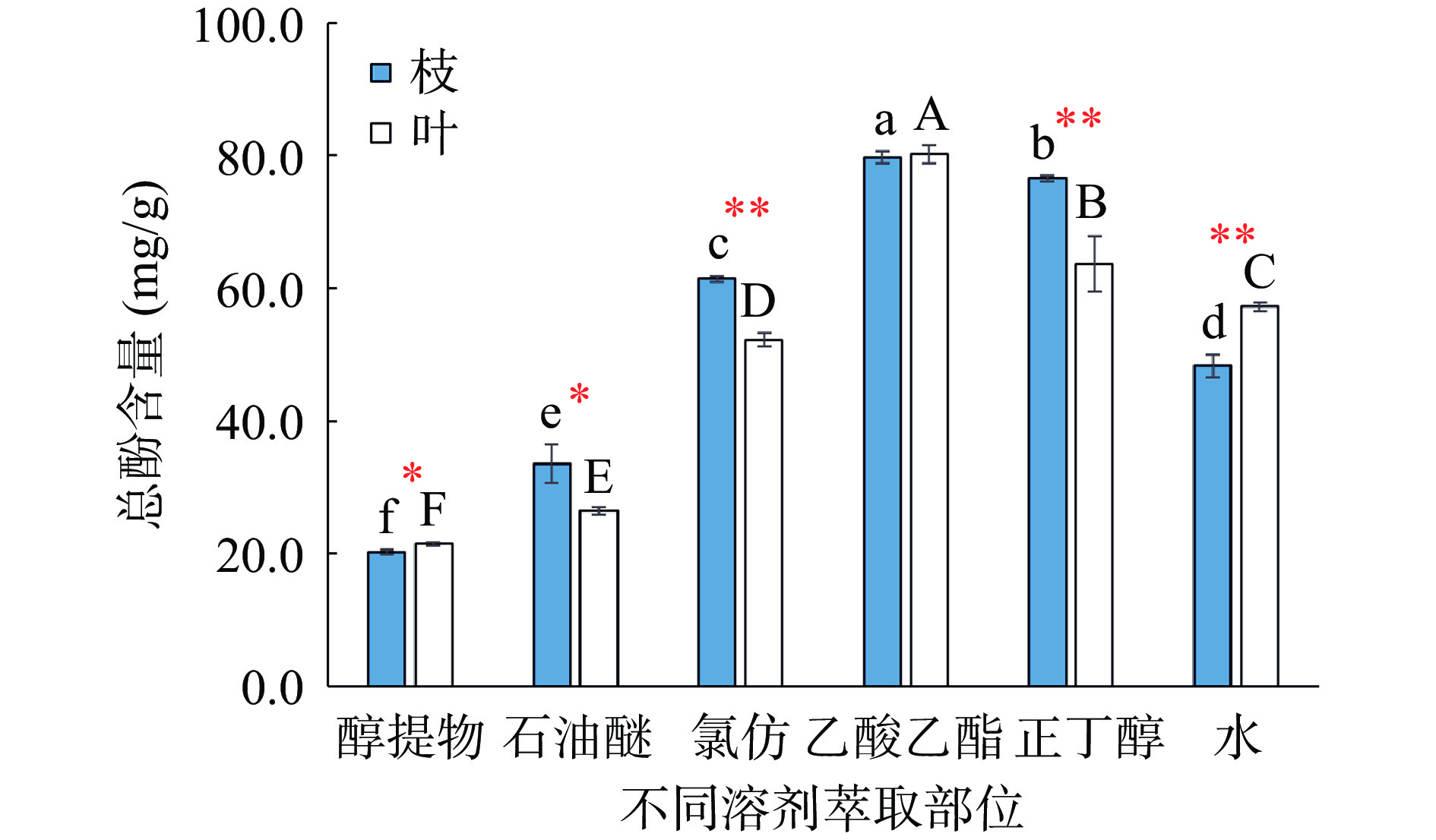
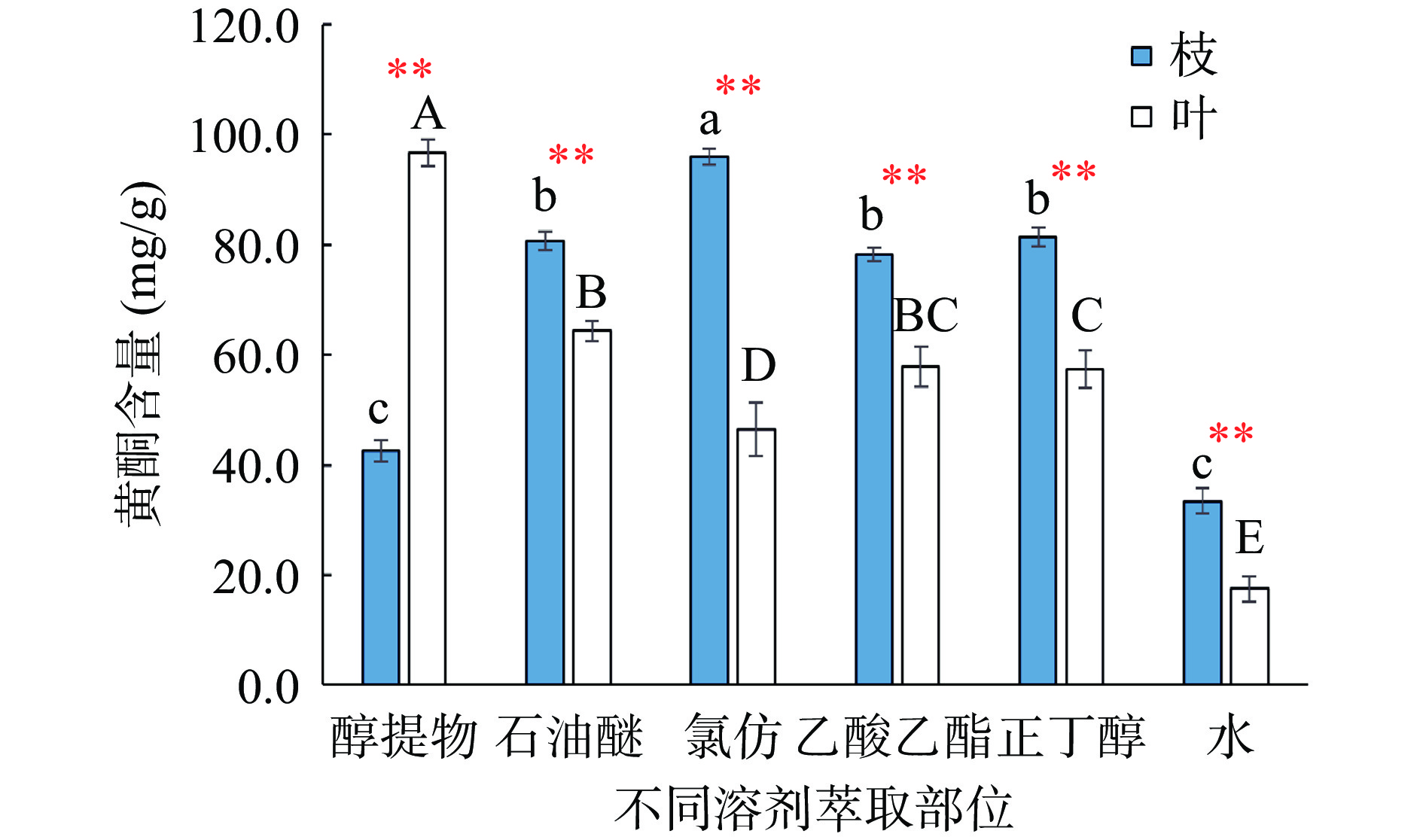
以质量浓度为横坐标(x),吸光度为纵坐标(y),得到没食子酸线性方程:y=118.82x+0.0048(R2=0.9998),线性范围为0.001~0.005 mg/mL;得到芦丁线性方程:y=12.089x−0.0035(R2=0.9998),线性范围为0.01~0.05 mg/mL。红蓝草枝和叶的醇提取物及其不同萃取部位均含总酚和黄酮,但含量有所差异。如图1所示,枝的总酚含量高低排序为:乙酸乙酯>正丁醇>氯仿>水>石油醚>醇提物;叶的总酚含量高低排序为:乙酸乙酯>正丁醇>水>氯仿>石油醚>醇提物。除乙酸乙酯部分外,枝和叶的不同溶剂萃取部位总酚含量差异显著(P<0.05),以乙酸乙酯部位总酚含量最高,分别达79.76 mg/g和80.21 mg/g,有研究[28-29]表明乙酸乙酯有利于植物中酚类物质溶解,而本实验结果类似。此外,氯仿和正丁醇两种溶剂中枝的总酚含量极显著高于叶子(P<0.01)。由图2可知,红蓝草枝中黄酮含量高低排序为:氯仿>正丁醇>石油醚>乙酸乙酯>醇提物>水,以氯仿部位黄酮含量最高,为95.88 mg/g。叶中黄酮含量高低排序为:醇提物>石油醚>乙酸乙酯、正丁醇>氯仿>水,以醇提物含量最高,为96.75 mg/g。不同溶剂萃取部位黄酮含量差异显著(P<0.05),但相同极性萃取部位枝的黄酮含量均高于叶子(醇提物除外)。
![]() 图 1 红蓝草枝和叶提取物不同萃取部位总酚含量(n=3)注:不同小写字母代表枝的不同溶剂萃取部位含量差异显著(P<0.05),不同大写字母代表叶的不同溶剂萃取部位含量差异显著(P<0.05);*表示同一溶剂不同组织部位含量差异显著(P<0.05),**表示同一溶剂不同组织部位含量差异极显著(P<0.01);图2同。Figure 1. Total phenol content in different extracted parts of PB branches and leaves extracts (n=3)
图 1 红蓝草枝和叶提取物不同萃取部位总酚含量(n=3)注:不同小写字母代表枝的不同溶剂萃取部位含量差异显著(P<0.05),不同大写字母代表叶的不同溶剂萃取部位含量差异显著(P<0.05);*表示同一溶剂不同组织部位含量差异显著(P<0.05),**表示同一溶剂不同组织部位含量差异极显著(P<0.01);图2同。Figure 1. Total phenol content in different extracted parts of PB branches and leaves extracts (n=3)2.2 体外抗氧化活性测定
2.2.1 对DPPH•清除作用
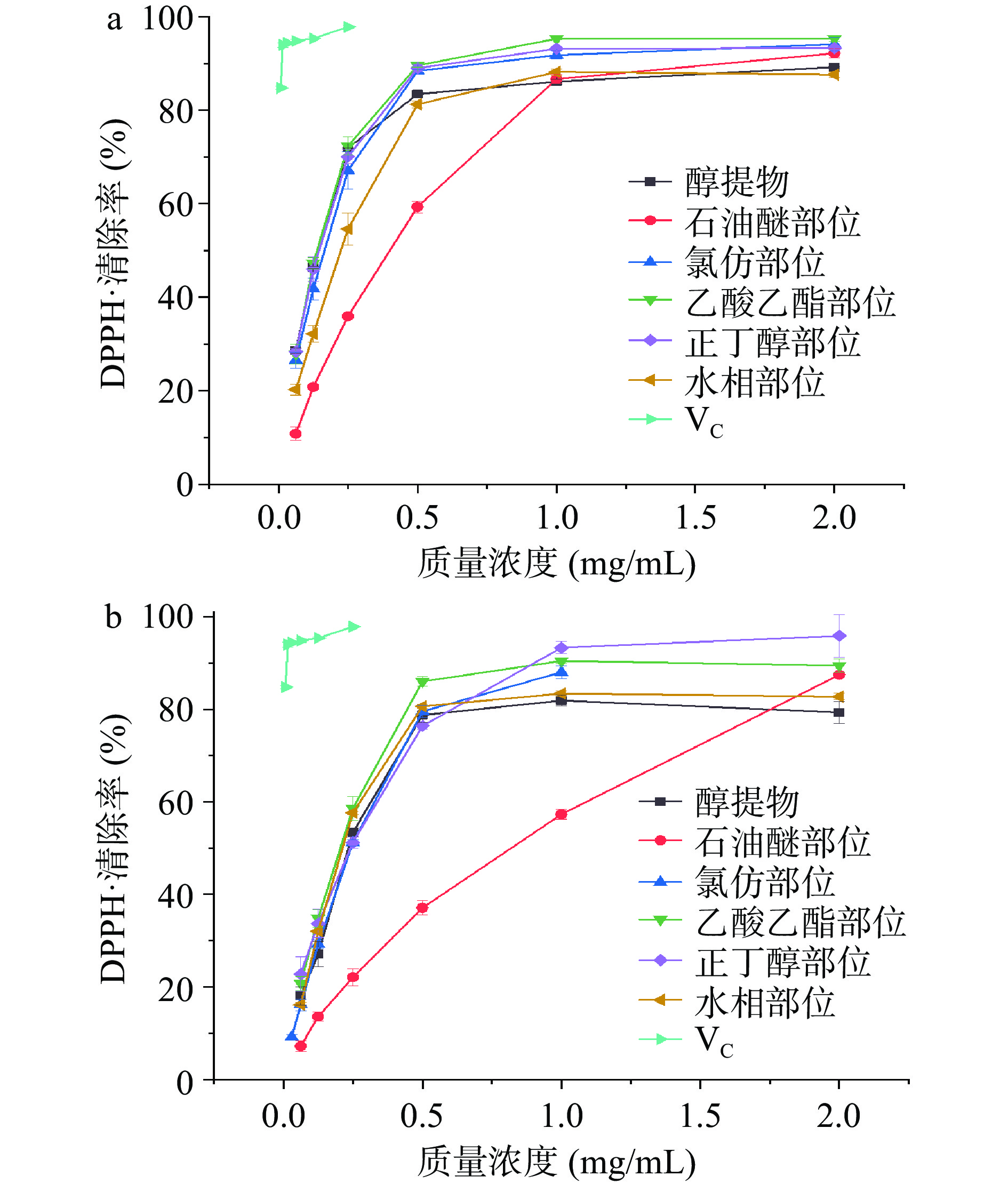
红蓝草枝和叶醇提物不同萃取部位对DPPH•清除能力如图3所示。在试验的质量浓度范围内,醇提物及各萃取部位的DPPH•清除能力随质量浓度的升高而增大。氯仿部位由于在2.00 mg/mL时本底较高,对清除效果的评价有影响,因此从1.00 mg/mL开始倍比稀释试验。在质量浓度为1.00 mg/mL时,枝的醇提物、石油醚、氯仿、乙酸乙酯、正丁醇和水相部位对DPPH•清除率分别为86.14%、86.67%、91.79%、95.31%、93.16%和88.19%,清除能力大小为:乙酸乙酯>正丁醇>氯仿>水>石油醚、醇提物,以乙酸乙酯部位最高,IC50值为0.16±0.01 mg/mL。叶的醇提物、石油醚、氯仿、乙酸乙酯、正丁醇和水部位的清除率分别为81.89%、57.29%、87.99%、90.42%、93.29%和83.40%,清除能力大小为:正丁醇>乙酸乙酯>氯仿>水>醇提物>石油醚,以正丁醇部位最高,IC50值为0.31±0.03 mg/mL。虽然对DPPH•清除效果不及VC,但红蓝草枝和叶的乙酸乙酯和正丁醇部位表现出良好的自由基清除能力,以IC50计,枝的DPPH•清除效果优于叶子。多酚结构中的酚羟基易被氧化,捕捉DPPH•能力更强[30]。乙酸乙酯和正丁醇部位中总酚含量较高,且自由基清除能力与样品质量浓度呈量效关系,显示萃取部位的自由基清除能力可能与总酚及其含量有关。
2.2.2 对ABTS+•的清除作用
以VC为对照,红蓝草枝和叶不同萃取部位对ABTS+•的清除能力如图4所示。红蓝草枝和叶各萃取部位对ABTS+•的清除能力不及VC,但在试验浓度范围内具有清除能力且呈现明显的量效关系。在质量浓度为4.00 mg/mL时,枝的醇提物、石油醚、氯仿、乙酸乙酯、正丁醇和水相部位对ABTS+•清除率分别为79.11%、49.45%、83.42%、95.22%、88.64%和71.80%,清除能力大小为:乙酸乙酯>正丁醇>氯仿>醇提物>水>石油醚,乙酸乙酯部位对ABTS+•清除率最高,IC50值为1.65±0.23 mg/mL。叶的醇提物、石油醚、氯仿、乙酸乙酯、正丁醇和水相部位对ABTS+•清除率分别为77.85%、42.45%、65.41%、86.32%、81.71%和93.18%,清除能力大小为:水>乙酸乙酯>正丁醇>醇提物>氯仿>石油醚,以极性强的水相部位ABTS+•清除能力最强,IC50值为3.00±0.26 mg/mL。这可能是水相部位中某些极性酚类物质在发挥作用[31]。李婧雯等[32]研究不同溶剂的蒲公英根提取物的抗氧化活性时也发现水相部位对ABTS+•清除能力最强。红蓝草枝和叶的不同萃取部位对ABTS+•清除强弱趋势不同,以IC50计,枝的ABTS+•清除效果优于叶子。可能是不同组织结构、不同极性溶剂萃取的酚类和黄酮的极性、结构和含量不同所引起的[33]。
2.2.3 总还原力
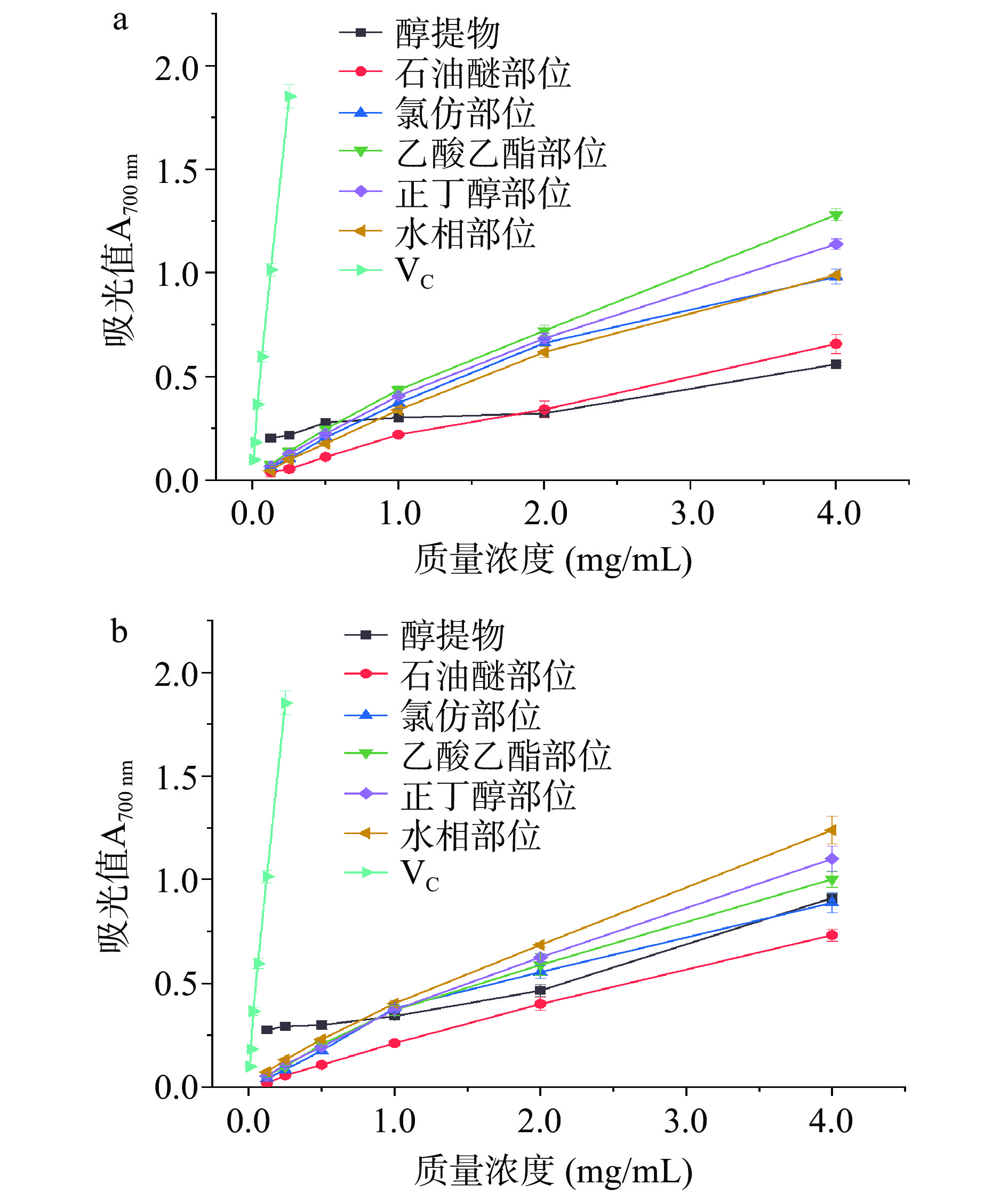
总还原能力是表征物质在氧化还原反应中给出电子而自身发生氧化的能力[34],吸光值越高,总还原力越强。以VC为对照,红蓝草枝和叶不同萃取部位总还原力如图5所示。当质量浓度低于2.00 mg/mL时,醇提物总还原力变化不大,这与韦正等[18]研究结果类似。其它萃取部位的总还原力随样品质量浓度的增加而增大。在质量浓度为4.00 mg/mL时,枝的醇提物和各萃取部位总还原力大小为:乙酸乙酯>正丁醇>水、氯仿>石油醚>醇提物,乙酸乙酯和正丁醇吸光值分别达到1.28±0.03和1.14±0.03;叶的醇提物各萃取部位总还原力大小为:水>正丁醇>乙酸乙酯>醇提物、氯仿>石油醚,水相和正丁醇部位的吸光值分别达到1.24±0.07和1.10±0.06。红蓝草中具有较强还原力的活性成分集中在极性强的水相部位、正丁醇部位和中等极性的乙酸乙酯部位。在相同的质量浓度下,枝和叶醇提物不同萃取物以石油醚部位总还原力最弱,可能是由于石油醚萃取的主要是脂类物质[35]。
2.2.4 抗氧化活性与成分含量相关性分析
以红蓝草各萃取部位质量浓度1.00 mg/mL测定DPPH•清除率,以4.00 mg/mL测定ABTS+•及总还原力。如表1所示,总酚对各部位的抗氧化活性贡献较大,枝的总酚含量与DPPH•清除能力和总还原力极显著相关(P<0.01)。黄酮含量对红蓝草的抗氧化活性无显著贡献,叶中黄酮含量与DPPH•清除能力、ABTS+•清除能力及总还原力呈负相关。植物中的多酚和黄酮具有优良的抗氧化效果,而红蓝草的抗氧化能力与其酚类含量具有较强相关性[36-37]。
表 1 体外抗氧化活性与成分含量相关系数Table 1. Correlation coefficient between antioxidant activity in vitro and component content体外抗氧化指标 枝 叶 总酚 黄酮 总酚 黄酮 DPPH•清除率 0.968** 0.569 0.696 −0.174 ABTS+•清除率 0.684 0.147 0.600 −0.312 总还原力 0.970** 0.342 0.639 −0.663 注:**双侧极显著相关(P<0.01)。 2.3 体外抑菌活性测定
通过抑菌圈直径大小可评价提取物的抑菌效果[38]。如表2所示,红蓝草醇提物和不同萃取部位在较高的质量浓度(62.5 mg/mL及以上)时才表现出抑菌效果。相同质量浓度下,针对同一种致病菌,叶的多个不同溶剂萃取部位比枝的抑菌效果好,抑菌圈显著大于枝(P<0.05)。其中,叶的醇提物和正丁醇部位抑菌谱最广,能抑制7种试验菌,包括4株革兰氏阴性菌、2株革兰氏阳性菌和1株真菌。醇提物和不同萃取部位含有多酚和黄酮类物质,这些活性物质可破坏细菌的细胞膜和细胞壁,使菌体生理功能丧失,从而抑制其生长[39]。然而,酚类和黄酮的组成及含量的差异,也会造成抑菌对象的不同[40]。红蓝草枝的乙酸乙酯部位对金黄色葡萄球菌、大肠埃希氏菌有明显抑制作用,在浓度为125.0 mg/mL时对两种致病菌的抑菌圈直径分别为14.02±0.55和16.18±0.42 mm,但叶的乙酸乙酯部位对大肠埃希氏菌无抑制作用;叶的正丁醇部位对金黄色葡萄球菌、蜡样芽胞杆菌和奇异变形杆菌均有较好的抑制效果,在浓度为125.0 mg/mL时对三种致病菌的抑菌圈直径分别为12.52±0.14、12.51±0.66和13.99±0.16 mm,但是对白色念珠菌和阪崎肠杆菌无抑制作用;此外,叶的石油醚部位和乙酸乙酯部位对金黄色葡萄球菌和奇异变形杆菌也具有抑制作用,但枝的石油醚部位和叶的水相部位在试验浓度范围内对8种试验菌株均无抑制作用。
表 2 红蓝草枝和叶提取物对不同菌株的抑制作用(n=3)Table 2. The inhibition effect of different extracted parts of PB branches and leaves extracts against different strains (n=3)试验菌株 样品质量
浓度
(mg/mL)抑菌直径(mm) 醇提物 石油醚部位 氯仿部位 乙酸乙酯部位 正丁醇部位 水相部位 枝 叶 叶 枝 叶 枝 叶 枝 叶 枝 金黄色葡萄球菌
(G+)62.5 − − 11.86±0.22a − − 10.68±0.34c 11.22±0.62b − 10.45±0.09c 9.21±0.20d 125 − 11.23±0.44e 13.32±0.85bc 9.04±0.32g 12.11±0.50d 14.02±0.55b 15.75±0.28a − 12.52±0.14cd 10.13±0.20f 250 13.19±0.30d 13.79±0.39d 15.34±0.78c 10.18±0.22f 13.27±0.42d 16.83±0.35b 18.75±0.63a − 16.22±0.51bc 12.15±0.29e 大肠埃希氏菌
(G−)62.5 − − − − − 13.18±0.38a − − − − 125 − − − 10.39±0.11b − 16.18±0.42a − − − − 250 − 11.86±0.48bc − 12.39±0.73b − 18.43±0.14a − − 11.41±0.13c − 蜡样芽胞杆菌
(G+)62.5 − − − − − − − − 9.49±0.20a − 125 − 10.00±0.42c − 11.18±0.16b − − − − 12.51±0.66a − 250 12.86±0.33b 13.92±0.51a − 13.05±0.34b − 11.91±0.41c 11.16±0.45d − 14.23±0.69a − 奇异变形杆菌
(G−)62.5 − 9.51±0.26b − − − − − − 10.70±0.32a − 125 9.44±0.31d 13.43±0.39b 9.23±0.33d − − − 12.09±0.18c − 13.99±0.16a − 250 10.99±0.28d 16.99±0.50a 12.89±0.27c − − − 15.78±0.48b − 17.15±0.25a 10.89±0.31d 铜绿假单胞菌
(G−)62.5 − − − − − − − − 9.10±0.53a − 125 − − − − − − − − 10.44±0.56a − 250 10.98±0.24b 11.10±0.68b − − − − − − 12.48±0.76a − 白色念珠菌
(真菌)62.5 − − − − − − − − − − 125 9.87±0.33a 9.25±0.20b − − − − − − − − 250 13.29±0.64a 11.27±0.46b − − − − − 13.23±0.47a − − 阪崎肠杆菌
(G−)62.5 − − − − − − − − − − 125 − 9.79±0.41a − − − − − − − − 250 − 13.08±0.60a − − − − − − − − 肺炎克雷伯氏菌
(G−)62.5 − − − − − − − − − − 125 − − − − − − − − − − 250 − − − − − − − − 11.99±0.17a 11.23±0.34b 蒸馏水 − − − − − − − − − − 注:牛津杯直径8 mm;“-”为未见抑菌圈;G+为革兰氏阳性菌,G−为革兰氏阴性菌。含不同小写字母代表不同部位在相同质量浓度对同一致病菌抑菌圈大小差异显著(P<0.05)。 3. 结论
本研究测定红蓝草枝和叶乙醇提取物及其不同溶剂萃取部位的总酚及黄酮含量。枝和叶的乙酸乙酯部位总酚含量较其它部位高,分别为79.76 mg/g和80.21 mg/g;枝的氯仿部位和叶的醇提物黄酮含量最高,分别为95.88 mg/g和96.75 mg/g。通过DPPH•、ABTS+•清除能力和总还原力的测定评价红蓝草枝和叶不同萃取部位的抗氧化活性。结果表明枝和叶的乙酸乙酯和正丁醇部位具有良好的DPPH•、ABTS+•清除能力;枝的乙酸乙酯部位、叶的水相部位还原能力高于其它部位。相关性分析显示各部位抗氧化活性与总酚含量具有较强的相关性。此外,枝和叶的醇提物及不同萃取部位对8种试验菌株具有抑制作用,叶的醇提物和正丁醇部位对7种试验菌株具有抑制作用,枝和叶的乙酸乙酯部位对金黄色葡萄球菌有明显的抑制作用。红蓝草不同组织部位及不同极性溶剂影响抗氧化及抑菌效果,枝的抗氧化效果优于叶,而叶的抑菌效果优于枝。以枝的乙酸乙酯部位,叶的乙酸乙酯和正丁醇部位抗氧化和抑菌效果更为明显。
综上,红蓝草枝和叶的乙酸乙酯部位、叶的正丁醇部位在较低质量浓度下具有较好的抗氧化和抑菌作用,拟作为下一步研究重点。分离有效活性成分,明确其化学组成和结构,分析构效关系,开发适用于食品及相关领域的安全有效的抗氧化剂、抑菌剂,提高红蓝草资源的综合利用水平。
-
图 1 红蓝草枝和叶提取物不同萃取部位总酚含量(n=3)
注:不同小写字母代表枝的不同溶剂萃取部位含量差异显著(P<0.05),不同大写字母代表叶的不同溶剂萃取部位含量差异显著(P<0.05);*表示同一溶剂不同组织部位含量差异显著(P<0.05),**表示同一溶剂不同组织部位含量差异极显著(P<0.01);图2同。
Figure 1. Total phenol content in different extracted parts of PB branches and leaves extracts (n=3)
表 1 体外抗氧化活性与成分含量相关系数
Table 1 Correlation coefficient between antioxidant activity in vitro and component content
体外抗氧化指标 枝 叶 总酚 黄酮 总酚 黄酮 DPPH•清除率 0.968** 0.569 0.696 −0.174 ABTS+•清除率 0.684 0.147 0.600 −0.312 总还原力 0.970** 0.342 0.639 −0.663 注:**双侧极显著相关(P<0.01)。 表 2 红蓝草枝和叶提取物对不同菌株的抑制作用(n=3)
Table 2 The inhibition effect of different extracted parts of PB branches and leaves extracts against different strains (n=3)
试验菌株 样品质量
浓度
(mg/mL)抑菌直径(mm) 醇提物 石油醚部位 氯仿部位 乙酸乙酯部位 正丁醇部位 水相部位 枝 叶 叶 枝 叶 枝 叶 枝 叶 枝 金黄色葡萄球菌
(G+)62.5 − − 11.86±0.22a − − 10.68±0.34c 11.22±0.62b − 10.45±0.09c 9.21±0.20d 125 − 11.23±0.44e 13.32±0.85bc 9.04±0.32g 12.11±0.50d 14.02±0.55b 15.75±0.28a − 12.52±0.14cd 10.13±0.20f 250 13.19±0.30d 13.79±0.39d 15.34±0.78c 10.18±0.22f 13.27±0.42d 16.83±0.35b 18.75±0.63a − 16.22±0.51bc 12.15±0.29e 大肠埃希氏菌
(G−)62.5 − − − − − 13.18±0.38a − − − − 125 − − − 10.39±0.11b − 16.18±0.42a − − − − 250 − 11.86±0.48bc − 12.39±0.73b − 18.43±0.14a − − 11.41±0.13c − 蜡样芽胞杆菌
(G+)62.5 − − − − − − − − 9.49±0.20a − 125 − 10.00±0.42c − 11.18±0.16b − − − − 12.51±0.66a − 250 12.86±0.33b 13.92±0.51a − 13.05±0.34b − 11.91±0.41c 11.16±0.45d − 14.23±0.69a − 奇异变形杆菌
(G−)62.5 − 9.51±0.26b − − − − − − 10.70±0.32a − 125 9.44±0.31d 13.43±0.39b 9.23±0.33d − − − 12.09±0.18c − 13.99±0.16a − 250 10.99±0.28d 16.99±0.50a 12.89±0.27c − − − 15.78±0.48b − 17.15±0.25a 10.89±0.31d 铜绿假单胞菌
(G−)62.5 − − − − − − − − 9.10±0.53a − 125 − − − − − − − − 10.44±0.56a − 250 10.98±0.24b 11.10±0.68b − − − − − − 12.48±0.76a − 白色念珠菌
(真菌)62.5 − − − − − − − − − − 125 9.87±0.33a 9.25±0.20b − − − − − − − − 250 13.29±0.64a 11.27±0.46b − − − − − 13.23±0.47a − − 阪崎肠杆菌
(G−)62.5 − − − − − − − − − − 125 − 9.79±0.41a − − − − − − − − 250 − 13.08±0.60a − − − − − − − − 肺炎克雷伯氏菌
(G−)62.5 − − − − − − − − − − 125 − − − − − − − − − − 250 − − − − − − − − 11.99±0.17a 11.23±0.34b 蒸馏水 − − − − − − − − − − 注:牛津杯直径8 mm;“-”为未见抑菌圈;G+为革兰氏阳性菌,G−为革兰氏阴性菌。含不同小写字母代表不同部位在相同质量浓度对同一致病菌抑菌圈大小差异显著(P<0.05)。 -
[1] LIM J Y, LEE C L, KIM G H, et al. Using lactic acid bacteria and packaging with grapefruit seed extract for controlling Listeria monocytogenes growth in fresh soft cheese[J]. Journal of Dairy Science,2020,103(10):8761−8770. doi: 10.3168/jds.2020-18349
[2] MARTINEZ L, JONGBERG S, ROS G, et al. Plant derived ingredients rich in nitrates or phenolics for protection of pork against protein oxidation[J]. Food Research International,2020,129:108789. doi: 10.1016/j.foodres.2019.108789
[3] RIAHI Z, PRIYADARSHI R, RHIM J W, et al. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications[J]. Food Hydrocolloids,2021,112:106314. doi: 10.1016/j.foodhyd.2020.106314
[4] SALEM M, EL-HEFNY M, ALI H M, et al. Plants-derived bioactives: Novel utilization as antimicrobial, antioxidant and phytoreducing agents for the biosynthesis of metallic nanoparticles[J]. Microbial Pathogenesis,2021,158(4):105107.
[5] ALONSO ESTEBAN J I, PINELA J, BARROS L, et al. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) seeds[J]. Industrial Crops & Products,2019,134:154−159.
[6] KHERBACHE A, SENATOR A, LAOUICHA S, et al. Phytochemical analysis, antioxidant and anti-inflammatory activities of Helichrysum stoechas (L.) Moench extracts[J]. Biocatalysis and Agricultural Biotechnology, 2020, 29(101826).
[7] 贾睿, 蔡丹, 葛思彤, 等. 红豆皮多酚提取物对两种致病菌的抑菌活性及作用机理[J]. 食品科学,2021,42(23):64−71. [JIA R, CAI D, GE S T, et al. Antibacterial activity and mechanism of polyphenol extracts from adzuki bean seed coat against two pathogens[J]. Food Science,2021,42(23):64−71. doi: 10.7506/spkx1002-6630-20201202-035 JIA R, CAI D, GE S T, et al. Antibacterial activity and mechanism of polyphenol extracts from adzuki bean seed coat against two pathogens[J]. Food Science, 2021, 42(23): 64-71. doi: 10.7506/spkx1002-6630-20201202-035
[8] BHAT M A , MALIK R A , PRAKASH P, et al. Preparation and evaluation of antibacterial potential of Pithecellobium dulce root extract against gram positive and gram negative bacteria[J]. Microbial Pathogenesis, 2018, 116: 49-53.
[9] RASHSI R, HOSSAIN M A, TOUBY S. Antioxidant and antibacterial activities of leaves crude extracts of Adenium obesum grown in Oman National Botanical Garden[J]. Advances in Biomarker Sciences and Technology,2021,3:8−14. doi: 10.1016/j.abst.2021.09.001
[10] PRABAKARAN M, KIM S H, SASIREKA A, et al. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera[J]. Food Bioscience,2018,26:23−29. doi: 10.1016/j.fbio.2018.09.003
[11] FARUQUE M O, ANKHI U R, KAMARUZZAMAN M, et al. Chemical composition and antimicrobial activity of Congeatomentosa, an ethnomedicinal plant from Bangladesh[J]. Industrial Crops and Products, 2019, 141(111745).
[12] 赵岩, 于鑫淼, 魏玉萍, 等. 青海管花肉苁蓉不同部位的功能性成分及其抗氧化活性[J]. 食品工业科技,2022,43(11):318−325. [ZHAO Y, YU X M, WEI Y P, et al. Bioactive components and antioxidant activities of different parts of Cistanche tubulosa in Qinghai Province[J]. Science and Technology of Food Industry,2022,43(11):318−325. ZHAO Y, YU X M, WEI Y P, et al. Bioactive components and antioxidant activities of different parts of Cistanche tubulosa in Qinghai Province[J]. Science and Technology of Food Industry, 2022, 43(11): 318-325.
[13] 韦正, 黄秀香, 赖红芳. 红蓝草的化学成分、药理活性及开发应用[J]. 天然产物研究与开发,2016,33(2):2035−2043. [WEI Z, HUANG X X, LAI H F. Review on chemical composition, pharmacological activity and application of Peristrophe baphica (Spreng) Bremek[J]. Natural Product Research and Development,2016,33(2):2035−2043. WEI Z, HUANG X X, LAI H F. Review on chemical composition, pharmacological activity and application of Peristrophe baphica (Spreng) Bremek[J]. Natural Product Research and Development, 2016, 33(2): 2035-2043.
[14] 蒋小华, 谢运昌, 梁靖, 等. 红丝线化学成分的研究[J]. 中成药,2017,39(11):2319−2321. [JIANG X H, XIE Y C, LIANG J, et al. Chemical constituents from Peristrophe baphica[J]. Chinese Traditional Patent Medicine,2017,39(11):2319−2321. JIANG X H, XIE Y C, LIANG J, et al. Chemical constituents from Peristrophe baphica[J]. Chinese Traditional Patent Medicine, 2017, 39(11): 2319-2321.
[15] 杨朝竣, 刘晓明, 晋兴华, 等. 山蓝中的色素成分鉴定及体外抗氧化活性研究[J]. 食品工业科技,2012,33(18):156−158. [YANG C J, LIU X M, JIN X H, et al. Identification and antioxidant activities of pigment in Shanlan[J]. Science and Technology of Food Industry,2012,33(18):156−158. YANG C J, LIU X M, JIN X H, et al. Identification and antioxidant activities of pigment in Shanlan[J]. Science and Technology of Food Industry, 2012, 33(18): 156-158.
[16] 祁百巍, 陈炼红. 响应面优化广西红蓝草红色素提取工艺及其特性研究[J]. 中国调味品,2021,46(5):151−160. [QI B W, CHEN L H. Study on the optimization of extraction process and characteristics of red pigment from Eccoilopus cotulifer by response surface method[J]. China Condiment,2021,46(5):151−160. QI B W, CHEN L H. Study on the optimization of extraction process and characteristics of red pigment from Eccoilopus cotulifer by response surface method[J]. China Condiment, 2021, 46(5): 151-160.
[17] 张贞发, 翁艳英, 彭金云, 等. 山蓝红色素的微波提取工艺及抑菌活性[J]. 食品工业科技,2016,37(16):312−314, 334. [ZHANG Z F, WENG Y Y, PENG J Y, et al. Microwave-assisted extraction and antibacterial activity of red pigment from Shanlan[J]. Science and Technology of Food Industry,2016,37(16):312−314, 334. ZHANG Z F, WENG Y Y, PENG J Y, et al. Microwave-assisted extraction and antibacterial activity of red pigment from Shanlan[J]. Science and Technology of Food Industry, 2016, 37(16): 312-314, 334.
[18] 韦正, 赖红芳, 彭丽娟, 等. 响应面优化红蓝草总酚酸的提取及其抗氧化活性研究[J]. 食品研究与开发,2020,41(18):84−90. [WEI Z, LAI H F, PENG L J, et al. Optimization of extraction process of total phenolic acids from the Peristrophe baphica (Spreng.) Bremek by response surface methodology and its antioxidation[J]. Food Research And Development,2020,41(18):84−90. WEI Z, LAI H F, PENG L J, et al. Optimization of extraction process of total phenolic acids from the Peristrophe baphica(Spreng. )Bremek by response surface methodology and its antioxidation[J]. Food Research And Development, 2020, 41(18): 84-90.
[19] 翁艳英, 苏秀芳. 红蓝草总黄酮的减压内部沸腾提取工艺优化[J]. 食品科技,2016,41(4):200−204. [WENG Y Y, SU X F. Optimization of the extraction for total flavonoids from Peristrophe roxburghiana by decompressing innner ebullition[J]. Food Science and Technology,2016,41(4):200−204. WENG Y Y, SU X F. Optimization of the extraction for total flavonoids from Peristrophe roxburghiana by decompressing innner ebullition[J]. Food Science and Technology, 2016, 41(4): 200-204.
[20] 徐玉琳, 马艺华, 谭文聪, 等. 红丝线草2种提取物的药效学研究[J]. 中药材,2011,34(10):1594−1597. [XU Y L, MA Y H, TAN W C, et al. Pharmacodynamic study on two kinds of extracts from Peristrophe baphica[J]. Journal of Chinese Medicinal Materials,2011,34(10):1594−1597. XU Y L, MA Y H, TAN W C, et al. Pharmacodynamic study on two kinds of extracts from Peristrophe baphica[J]. Journal of Chinese Medicinal Materials, 2011, 34(10): 1594-1597.
[21] 李云婷, 陈炼红, 王琳琳, 等. 响应面法优化紫兰草紫色素提取工艺及其稳定性研究[J]. 中国食品添加剂,2020,31(5):87−94. [LI Y T, CHEN L H, WANG L L, et al. Optimization of purple pigment extraction from purple grass by response surface and study of the pigment stability[J]. China Food Additives,2020,31(5):87−94. LI Y T, CHEN L H, WANG L L, et al. Optimization of purple pigment extraction from purple grass by response surface and study of the pigment stability[J]. China Food Additives, 2020, 31(05): 87-94.
[22] 弘子姗, 陆挎仙, 黄文豪, 等. 紫蓝草紫色素提取工艺优化及其对糯米染色的表观性能研究[J]. 食品与发酵工业,2021,47(20):180−187. [HONG Z S, LU K X, HUANG W H, et al. The optimization of extraction method of pigment from Peristrophe roxburghiana and its dyeing on glutinous rice[J]. Food and Fermentation Industries,2021,47(20):180−187. HONG Z S, LU K X, HUANG W H, et al. The optimization of extraction method of pigment from Peristrophe roxburghiana and its dyeing on glutinous rice[J]. Food and Fermentation Industries, 2021, 47(20): 180-187.
[23] 黄佳佳, 杨昭, 李燕杰, 等. 超声波辅助提取黑蒜多酚及体外抗氧化性探究[J]. 食品科技,2018,43(4):212−217. [HUANG J J, YANG Z, LI Y J, et al. Ultrasound-assisted extraction of black garlic polyphenols and study of its antioxidant activity[J]. Food Science and Technology,2018,43(4):212−217. HUANG J J, YANG Z, LI Y J, et al. Ultrasound-assisted extraction of black garlic polyphenols and study of its antioxidant activity[J]. Food Science and Technology, 2018, 43(4): 212-217.
[24] 李慧敏, 高月, 邵雪飞, 等. 柴胡不同部位总黄酮含量及抗氧化活性比较研究[J]. 中国食品添加剂,2022,33(4):211−217. [LI H M, GAO Y, SHAO X F, et al. Study on total flavonoids content and comparison of antioxidant activity in different parts of Bupleurum chinense DC. from different provenances[J]. China Food Additives,2022,33(4):211−217. LI H M, GAO Y, SHAO X F, et al. Study on total flavonoids content and comparison of antioxidant activity in different parts of Bupleurum chinense DC. from different provenances[J]. China Food Additives, 2022, 33(4): 211-217.
[25] 张明, 帅希祥, 杜丽清, 等. 澳洲坚果青皮多酚提取工艺优化及其抗氧化活性[J]. 食品工业科技,2017,38(22):195−199. [ZHANG M, SHUAI X X, DU L Q, et al. Optimization of extraction and antioxidant activity of polyphenols from macadamia green peel[J]. Science and Technology of Food Industry,2017,38(22):195−199. ZHANG M, SHUAI X X, DU L Q, et al. Optimization of extraction and antioxidant activity of polyphenols from macadamia green peel[J]. Science and Technology of Food Industry, 2017, 38(22): 195-199.
[26] 陈蓉, 徐俊, 陈华林, 等. 俄色果不同极性部位的抗氧化、降血糖活性[J]. 中成药,2021,43(9):2427−2433. [CHENR, XU J, CHEN H L, et al. Antioxidant and antihypoglycemic activities of different ese fruit extracts[J]. Chinese Traditional Patent Medicine,2021,43(9):2427−2433. CHENR, XU J, CHEN H L, et al. Antioxidant and antihypoglycemic activities of different ese fruit extracts[J]. Chinese Traditional Patent Medicine, 2021, 43(9): 2427-2433.
[27] 乔芊芊. 蛇莓提取物体外抑菌、抗氧化、抗炎活性的研究[D]. 北京: 中国农业科学院, 2021. QIAO Q Q. Study on antibacterial, antioxidant andanti-inflammatory activities of extracts from Duchesnea indica (Andr.) focke in vitro[D]. Beijing: Chinese Academy of Agricultural Sciences Thesis, 2021.
[28] 曾永芳, 廖华珍, 许海棠, 等. 瓶尔小草不同溶剂提取物的体外生物活性[J]. 食品工业科技,2021,42(22):363−368. [ZENG Y F, LIAO H Z, XU H T, et al. Study on biological activity of different solvent extracts from Ophiolossum vulgatum L. in vitro[J]. Science and Technology of Food Industry,2021,42(22):363−368. ZENG Y F, LIAO H Z, XU H T, et al. Study on biological activity of different solvent extracts from Ophiolossum vulgatum L. in vitro[J]. Science and Technology of Food Industry, 2021, 42(22): 363-368.
[29] 姚志仁, 李豫, 朱开梅, 等. 黄花倒水莲不同极性部位抗氧化和降血糖活性研究[J]. 食品工业科技,2020,41(7):55−59, 64. [YAO Z R, LI Y, ZHU K M, et al. Antioxidant and hypoglycemic activities of different parts partitioned from the ethanol extract of Polygala fallax Hemsl[J]. Science and Technology of Food Industry,2020,41(7):55−59, 64. YAO Z R, LI Y, ZHU K M, et al. Antioxidant and hypoglycemic activities of different parts partitioned from the ethanol extract of Polygala fallax Hemsl[J]. Science and Technology of Food Industry, 2020, 41(07): 55-59, 64.
[30] 戴鹂莹. 刺玫果水层萃取物的活性成分及其生物活性的研究[D]. 吉林: 吉林化工学院, 2022. DAI Y Y. Study on active components and biological activity of the aqueous extracts from fruits of Rosa davurica Pall[D]. Jilin: Jilin Institute of Chemical Technology, 2022.
[31] 喻艳, 逯海朋, 贾亚楠, 等. 桑椹中酚类物质极性分布及抗氧化活性评价[J]. 食品与发酵工业,2017,43(1):73−79. [YU Y, LU H P, JIA Y N, et al. Determination of mulberry total phenolic content and its antioxidant activity[J]. Food and Fermentation Industries,2017,43(1):73−79. YU Y, LU H P, JIA Y N, et al. Determination of mulberry total phenolic content and its antioxidant activity[J]. Food and Fermentation Industries, 2017, 43(1): 73-79.
[32] 李婧雯, 包怡红. 不同溶剂的蒲公英根提取物的抗氧化活性及降糖能力比较分析[J]. 现代食品科技,2020,36(5):64−72. [LI J W, BAO Y H. Comparative analysis of antioxidant and hypoglycemic capabilities of Taraxacum mongolicum root extracts in different solvents[J]. Modern Food Science and Technology,2020,36(5):64−72. LI J W, BAO Y H. Comparative analysis of antioxidant and hypoglycemic capabilities of Taraxacum mongolicum root extracts in different solvents[J]. Modern Food Science and Technology, 2020, 36(5): 64-72.
[33] 张勇, 黄思涵, 林大都, 等. 福建观音座莲叶提取物不同萃取部位成分含量及与抗氧化相关性分析[J]. 食品工业科技,2021,42(14):49−54. [ZHANG Y, HUANG S H, LIN D D, et al. Analysis of the content of components in different extracted parts of Angiopteris fokiensis Hieron leaf extracts and their correlation with antioxidant[J]. Science and Technology of Food Industry,2021,42(14):49−54. ZHANG Y, HUANG S H, LIN D D, et al. Analysis of the content of components in different extracted parts of Angiopteris fokiensis Hieron leaf extracts and their correlation with antioxidant[J]. Science and Technology of Food Industry, 2021, 42(14): 49-54.
[34] 曾维才, 石碧. 天然产物抗氧化活性的常见评价方法[J]. 化工进展,2013,32(6):1205−1213, 1247. [ZENG W C, SHI B. Common methods of antioxidant activity evaluation for natural products: A review[J]. Chemical Industry and Engineering Progress,2013,32(6):1205−1213, 1247. ZENG W C, SHI B. Common methods of antioxidant activity evaluation for natural products: A review[J]. Chemical Industry and Engineering Progress, 2013, 32(6): 1205-1213, 1247.
[35] 邹成梅, 厉莉, 史硕硕, 等. 苦丁茶提取物的分离纯化、鉴定及不同萃取部位活性分析[J]. 食品科学,2022,43(20):18−24. [ZOU C M, LI L, SHI S S, et al. Isolation, purification, identification and activity analysis of different extract parts of Ilex latifolia Thunb[J]. Food Science,2022,43(20):18−24. ZOU C M, LI L, SHI S S, et al. Isolation, purification, identification and activity analysis of different extract parts of Ilex latifolia Thunb [J]. Food Science, 2022, 43(20): 18-24.
[36] BARHE T A, TCHOUYA G F. Comparative study of the anti-oxidant activity of the total polyphenols extracted from Hibiscus sabdariffa L., Glycine max L. Merr., yellow tea and red wine through reaction with DPPH free radicals[J]. Arabian Journal of Chemistry,2016,9(1):1−8. doi: 10.1016/j.arabjc.2014.11.048
[37] SUN Y, DENG Z, LIU R, et al. A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products[J]. Food Chemistry,2020,325:126925. doi: 10.1016/j.foodchem.2020.126925
[38] 谭才邓, 朱美娟, 杜淑霞, 等. 抑菌试验中抑菌圈法的比较研究[J]. 食品工业,2016,37(11):122−125. [TAN C D, ZHU M J, DU S X, et al. Study on the inhibition zone method in antimicrobial test[J]. The Food Industry,2016,37(11):122−125. TAN C D, ZHU M J, DU S X, et al. Study on the inhibition zone method in antimicrobial test[J]. The Food Industry, 2016, 37(11): 122-125.
[39] 喜梅花, 候妤婕, 沈荷玉, 等. 电子束辐照预处理对核桃青皮活性物提取及其抑菌活性的影响[J]. 食品与发酵工业,2022,48(14):55−62. [XI M H, HOU Y J, SHEN H Y, et al. Effects of electron beam irradiation pretreatment on extraction and antibacterial activity of active compounds in green walnut husk[J]. Food and Fermentation Industries,2022,48(14):55−62. XI M H, HOU Y J, SHEN H Y, et al. Effects of electron beam irradiation pretreatment on extraction and antibacterial activity of active compounds in green walnut husk[J]. Food and Fermentation Industries, 2022, 48(14): 55-62.
[40] 强毅, 王政军, 陈克克, 等. 费菜多酚含量的测定及体外抗菌活性研究[J]. 食品工业科技,2013,34(5):53−56. [QIANG Y, WANG Z J, CHEN K K, et al. Determination of total polyphenols and antimicrobial activity of methanol extract from Sedum aizoon L[J]. Science and Technology of Food Industry,2013,34(5):53−56. doi: 10.13386/j.issn1002-0306.2013.05.043 QIANG Y, WANG Z J, CHEN K K, et al. Determination of total polyphenols and antimicrobial activity of methanol extract from Sedum aizoon L[J]. Science and Technology of Food Industry, 2013, 34(5): 53-56. doi: 10.13386/j.issn1002-0306.2013.05.043









 下载:
下载:

 下载:
下载:



