Effect of High Pressure Treatment on Papain Activity and Molecular Dynamics Simulation
-
摘要: 为研究超高压处理对木瓜蛋白酶活性的影响及分子机制,将木瓜蛋白酶溶液经0.1、200和600 MPa处理,测定其活性变化,并对木瓜蛋白酶在这3种压力下进行150 ns的分子模拟,分析RMSD(均方根误差)、RMSF(均方根涨落)、回旋半径、氢键、溶剂可及表面积、体积、二级结构和分子表面结构等的变化。结果显示,200 MPa对木瓜蛋白酶起到激活作用,而600 MPa条件下酶活性降低26.2%。分子模拟表明高压处理能减小酶蛋白结构的波动;减少蛋白体积,使蛋白结构更加致密;蛋白溶剂可及表面积降低,特别是疏水表面积;高压能破坏酶蛋白间的氢键,使蛋白中β-折叠增加,而α-螺旋则是在600 MPa明显减少。200 MPa下酶的激活可能与该压力下酶活性中心的结合口袋变大因而更易与目标蛋白质结合有关;而600 MPa下酶活性的部分抑制则与该压力下酶结构遭受较大破坏有关,如α-螺旋和氢键的破坏、疏水表面积和蛋白体积的减小。从分子机制上明确了高压下木瓜蛋白酶活性变化的机理。Abstract: To study the effect of ultrahigh pressure treatment on papain activity and the molecular mechanism, papain solutions were treated with 0.1, 200 and 600 MPa to determine the changes in activity. And molecular simulation of papain at these three pressures were performed for 150 ns, the changes of root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (Rg), hydrogen bonds, solvent accessible surface area, volume, secondary structure and molecular surface structure was evaluated. The results showed that 200 MPa had an activating effect on papain, while the enzyme activity was reduced by 26.2% at 600 MPa. Molecular simulation showed that high pressure treatment could reduce the fluctuation of protein structure, protein volume, and protein solvent accessible surface area, especially hydrophobic surface area, and make the protein structure more compact. High pressure could destroy the hydrogen bond between enzyme proteins and increase the content of β-sheet, while decrease the α-helix obviously at 600 MPa. The activation of the enzyme at 200 MPa may be related to the larger binding pocket in the active center of the enzyme at this pressure, which made it easier to bind to the target protein. The partial inhibition of enzyme activity under 600 MPa was related to the destruction of enzyme structure under this pressure, for example, the destruction of α-helix and hydrogen, the reduction of hydrophobic surface area and protein volume. The molecular mechanism of papain activity change under high pressure was clarified.
-
Keywords:
- high pressure /
- papain /
- molecular dynamics /
- enzyme activity
-
木瓜蛋白酶是番木瓜中含有的一种低特异性蛋白水解酶,广泛地存在于番木瓜的根、茎、叶和果实内。其活性中心含半胱氨酸,属于巯基蛋白酶,具有酶活高、热稳定性好、天然卫生安全等特点,可水解蛋白质和多肽中精氨酸和赖氨酸的羧基端,并能优先水解在肽键的N-端具有二个羧基的氨基酸或芳香L-氨基酸的肽键,因此在食品、医药、饲料、日化等行业得到广泛应用。木瓜蛋白酶是嫩肉粉的主要成分之一,能将肉中结缔组织、胶原蛋白、弹性蛋白等分解,进而破坏肉的结构,使肉的口感变得嫩滑,生成的小分子物质也更容易被人体吸收和利用[1]。
超高压是一种冷杀菌技术,在食品工业中应用越来越广泛,其主要是破坏食物中非共价键而对共价键几乎没有任何影响。因此其能在杀菌的同时对小分子风味、营养和功能物质得以完好保留。超高压在肉类加工中的应用也越来越多,除了杀菌,还能改善肉品嫩度、颜色、凝胶性和保水性[2-3]。超高压对食物进行处理时,不可避免会影响食物中酶的结构、性质和活性,进而影响到食品品质,相关的研究也非常多,如哈密瓜中相关酶的活性[4]、鲢鱼中脂肪氧合酶[5]、刺梨中多酚氧化酶[6]、芽孢皮层裂解酶[7]、猪肉中脂肪酶和脂肪氧合酶[8]等。一般认为在一定的压力范围内,部分酶存在激活作用,可能是压力促进溶酶体中部分酶的释放,或一定压力下酶的结构发生了有利于酶活增加的构象变化,或压力抑制了酶抑制剂[9-11],但超过一定压力后酶活会受到抑制,不同酶激活和抑制的压力分界点不一样,如肌肉中酸性磷脂酶[9]、鲑鱼中蛋白酶[10]、马铃薯中脂肪氧化酶[12]被激活的最大压力分别报道为500、300和400 MPa。就木瓜蛋白酶的高压处理而言,相关研究较少,刘平等[13]研究了600 MPa以内的压力对其活性的影响,发现200 MPa时酶被激活,随后随压力增加酶活被逐渐抑制。
近年来,光谱技术、波谱技术、量热技术等被广泛用于研究蛋白结构,但测得的只是静态结构,分子模拟现已成为研究蛋白质动态结构的一种有效手段,其是具有足够小的时间尺度和空间尺度的强大模拟技术[14]。一些学者采用分子模拟手段研究了酶蛋白结构的变化,如碱性蛋白酶[15]、胰蛋白酶[16]。高进等[1]模拟了木瓜蛋白酶在高压下的分子动力学变化规律,但只进行了10 ns的模拟,因此有必要进一步深入研究木瓜蛋白酶在高压下活性变化及其分子机理。
鉴于超高压技术和木瓜蛋白酶都广泛用于食品工业,为更好地促进木瓜蛋白酶在高压处理食品体系中的应用,并从分子机理上探究木瓜蛋白酶在高压下活性变化规律及机理,本研究在预试验基础上,采用200和600 MPa对木瓜蛋白酶进行处理,并进行150 ns的分子动力学模拟,也为高压加工食品中其他酶活性的变化奠定一定的理论参考。
1. 材料与方法
1.1 材料与仪器
木瓜蛋白酶(80万U/g) 北京索莱宝科技有限公司;pH7.4磷酸缓冲溶液 配制试剂均为分析纯。
HPP.L2-800/1型高压设备 天津华泰森淼生物工程技术股份有限公司;TP-1901紫外分光光度计 北京普析通用仪器有限责任公司。
1.2 实验方法
1.2.1 超高压处理
将木瓜蛋白酶溶于50 μmol/L磷酸钠缓冲溶液中(pH7.4),使木瓜蛋白酶最终质量浓度为2 mg/mL。再将木瓜蛋白酶溶液装入聚乙烯塑料袋中,每袋10 mL,真空包装后在超高压处理设备于200和600 MPa条件下处理10 min,对照组在常温下(0.1 MPa)放置10 min。处理结束后立即卸压并测定木瓜蛋白酶活性。
1.2.2 木瓜蛋白酶活性测定
木瓜蛋白酶活性用Folin-酚法进行测定[13],每组3个平行。酶活力计算如下:
式中:X为样品酶活力,U/g;V1为溶解木瓜蛋白酶所用容量瓶的体积,mL;A为木瓜蛋白酶样品稀释液的活力,可通过标准曲线算出,U/mL;V2为试管中反应液的总体积,mL;n为酶液稀释的倍数;10为样品反应时间,min;m为称取木瓜蛋白酶的质量,g。
1.2.3 分子动力学模拟
采用Gromacs(2019.6)软件[17],木瓜蛋白酶结构从RCSB网站下载(ID号:1 ppn),用PyMOL软件除去结晶水。模拟选用GROMOS54a7力场[18]、SPC水模型,蛋白离立方体盒子边缘最短距离为1 nm并使体系保持电中性。使用最速下降法对体系进行能量最小化后在NVT和NPT系综下分别进行400 ps的平衡,使温度达到300 K,压力达到预先设定的压力值(0.1、200或600 MPa)。最后进行150 ns的动力学模拟,模拟过程中使用LINCS算法约束所有键、PME计算静电作用,截断半径1.4 nm计算范德华相互作用。压浴方式选用Parrinello-Rahman,控压方式为Isotropic,控温方式为V-rescale。模拟步长为2 fs,每10 ps储存1次数据[14,19-20]。
模拟完成后,去除周期性边界,用Gromacs软件自带的命令分析不同压力处理时酶蛋白的均方根误差(RMSD)、均方根涨落(RMSF)、回旋半径(Rg)、二级结构、溶剂可及表面积、体积、氢键、活性中心残基间距离的变化。其中RMSD、RMSF、Rg、蛋白体积均取150 ns的数据,每0.1 ns提取1次,共1500个数据点,以分析整个模拟过程中蛋白结构的变化;而蛋白二级结构、可及表面积、活性中心残基间的距离变化只选取结构比较稳定的100到150 ns共500个数据点。蛋白表面结构提取不同压力模拟中100~150 ns的平均结构后用PyMOL软件绘制。
1.3 数据处理
酶活重复测定三次,用WPS软件计算标准差和作图;分子模拟部分的作图用Origin 8.0软件;分子模拟部分蛋白溶剂可及表面积、二级结构、酶活性中心氨基酸残基距离采用SPSS(V20版本)软件进行统计和计算。
2. 结果与分析
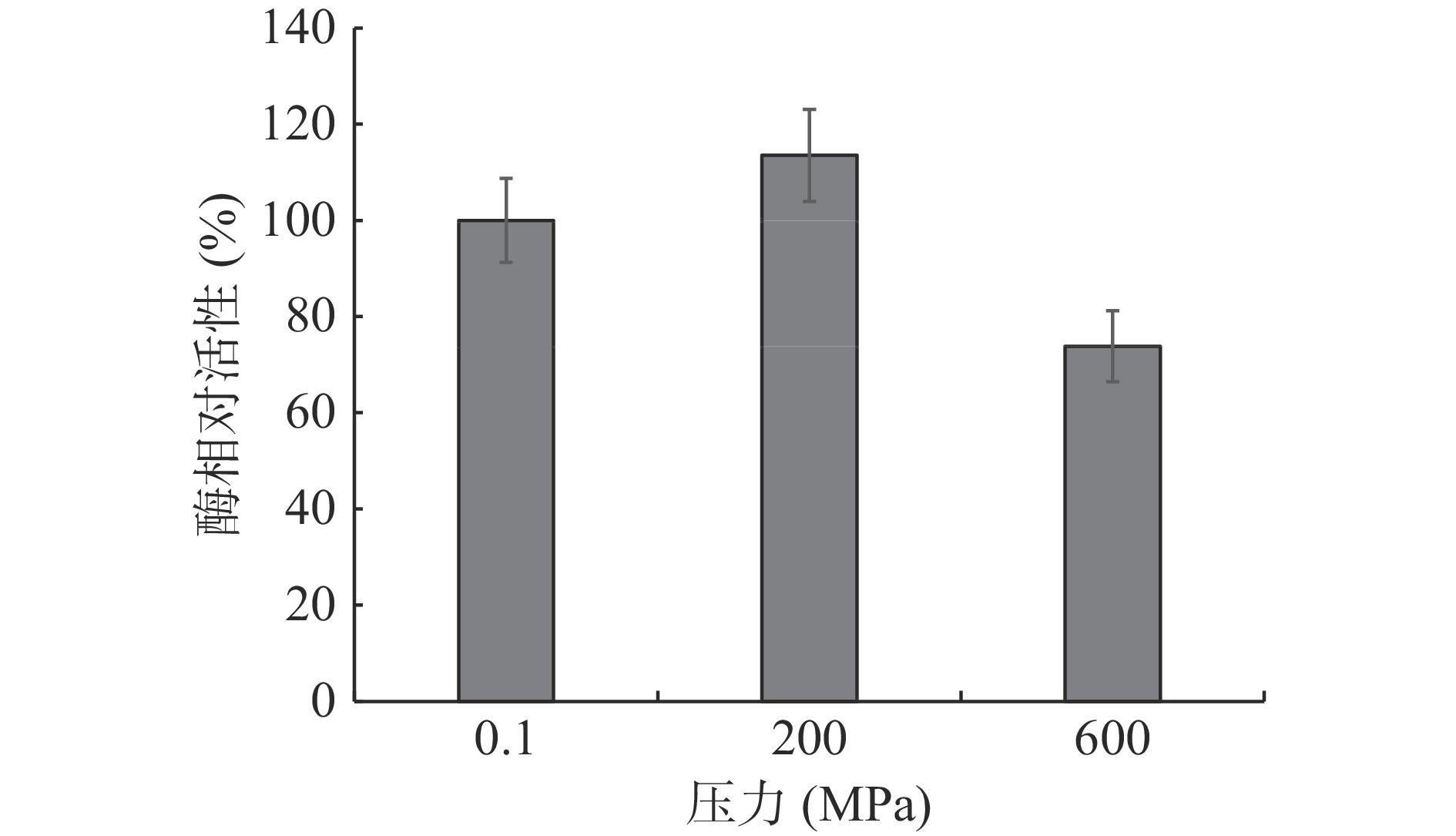
2.1 不同压力处理对木瓜蛋白酶活性的影响
如图1所示,200 MPa处理时酶活性增加了13.5%,而600 MPa处理下酶活性相比对照样降低了26.2%,这与刘平等[13]的报道是一致的,他们也发现木瓜蛋白酶200 MPa时被激活,随后随压力增加酶活被逐渐抑制。一般认为在不太高的压力下酶的激活可能是压力促进溶酶体中部分酶的释放,或一定压力下酶的结构发生了有利于酶活增加的构象变化,或压力抑制了酶抑制剂[9-11],当压力超过一定限值后,酶蛋白的结构破坏,酶活将逐渐被抑制。但不同酶压力激活的临界压力不一样,如肌肉中酸性磷脂酶[9]、鲑鱼中蛋白酶[10]、马铃薯中脂肪氧化酶[12]分别报道为500、300和400 MPa。压力对木瓜蛋白酶活性的影响机制将通过后面的分子动力学模拟进行探讨。因此,木瓜蛋白酶若经200 MPa左右的压力处理更能发挥其催化和嫩化的作用。
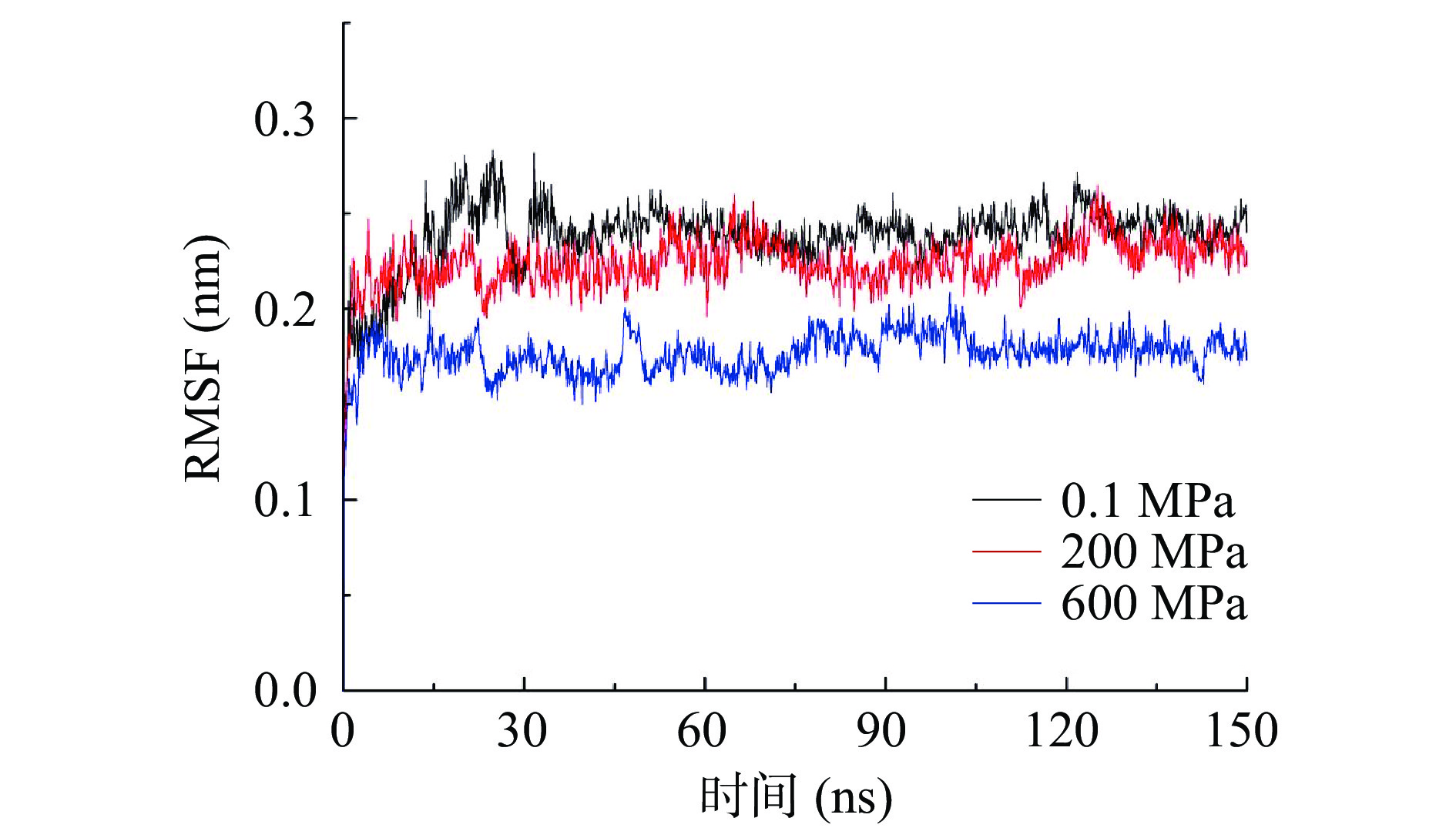
2.2 不同压力处理对酶蛋白RMSD的影响
均方根误差(RMSD)反映特定模拟时间蛋白结构与原始构象的平均偏差,是衡量研究体系是否稳定的重要指标。如图2所示,各组RMSD在整个模拟过程中均比较平稳,波动较小,特别是在30 ns后基本稳定在一恒定数值附近。总体上,随着压力增加,RMSD的数值不断减小,但200 MPa与对照组差别不大,说明压力能减小酶蛋白结构的波动。一些学者研究了高压下蛋白的结构波动,但结果并不一致,如Kurpiewska等[21]报道胰岛素在高压下结构波动更小,Hata等[22]也报道高压下蛋白的结构波动会减小,这都与本研究的结果一致;而孔庆新等研究高压下β-乳球蛋白的波动时,发现在200 MPa处理可以稳定蛋白结构,而500和800 MPa处理时蛋白结构的波动会大于常压[23]。
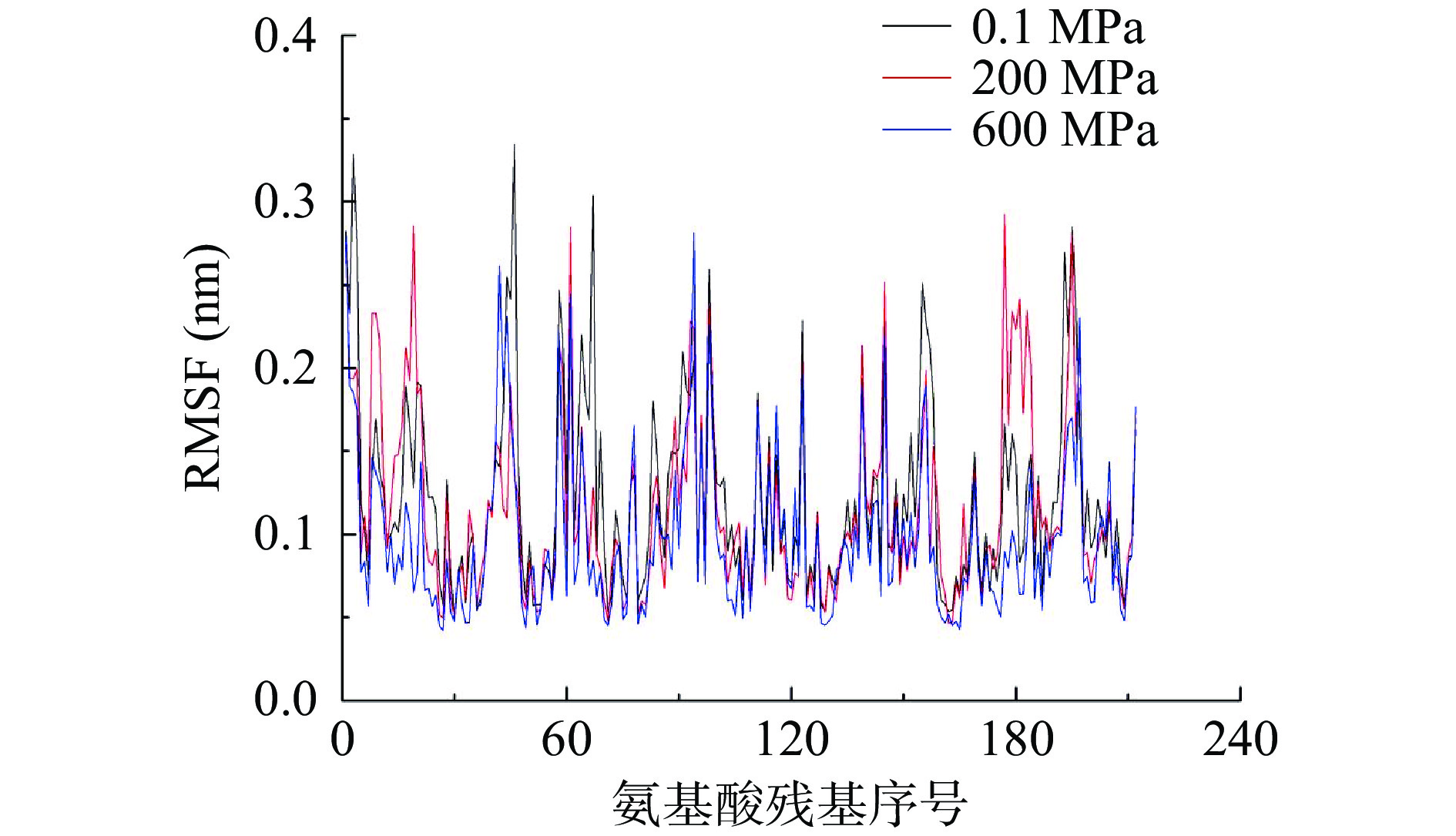
2.3 不同压力处理对酶蛋白RMSF的影响
均方根涨落(RMSF)是相比于平均构象,蛋白中每一个氨基酸残基的均方根位移。图3反映了不同压力下各残基的波动情况。可以看出,一方面,随压力的增加,残基的平均波动会减少,常压下,木瓜蛋白酶中平均残基波动为0.123 nm,而200和600 MPa条件下分别为0.117和0.098 nm;另一方面,活性中心的三个氨基酸残基(25、158和159号位的残基)[1]波动均较小,因此活性中心在高压条件下结构是比较稳定的。波动较大的主要是一些不规则结构中的氨基酸残基,而α-螺旋和β-折叠结构中的氨基酸波动均较小。
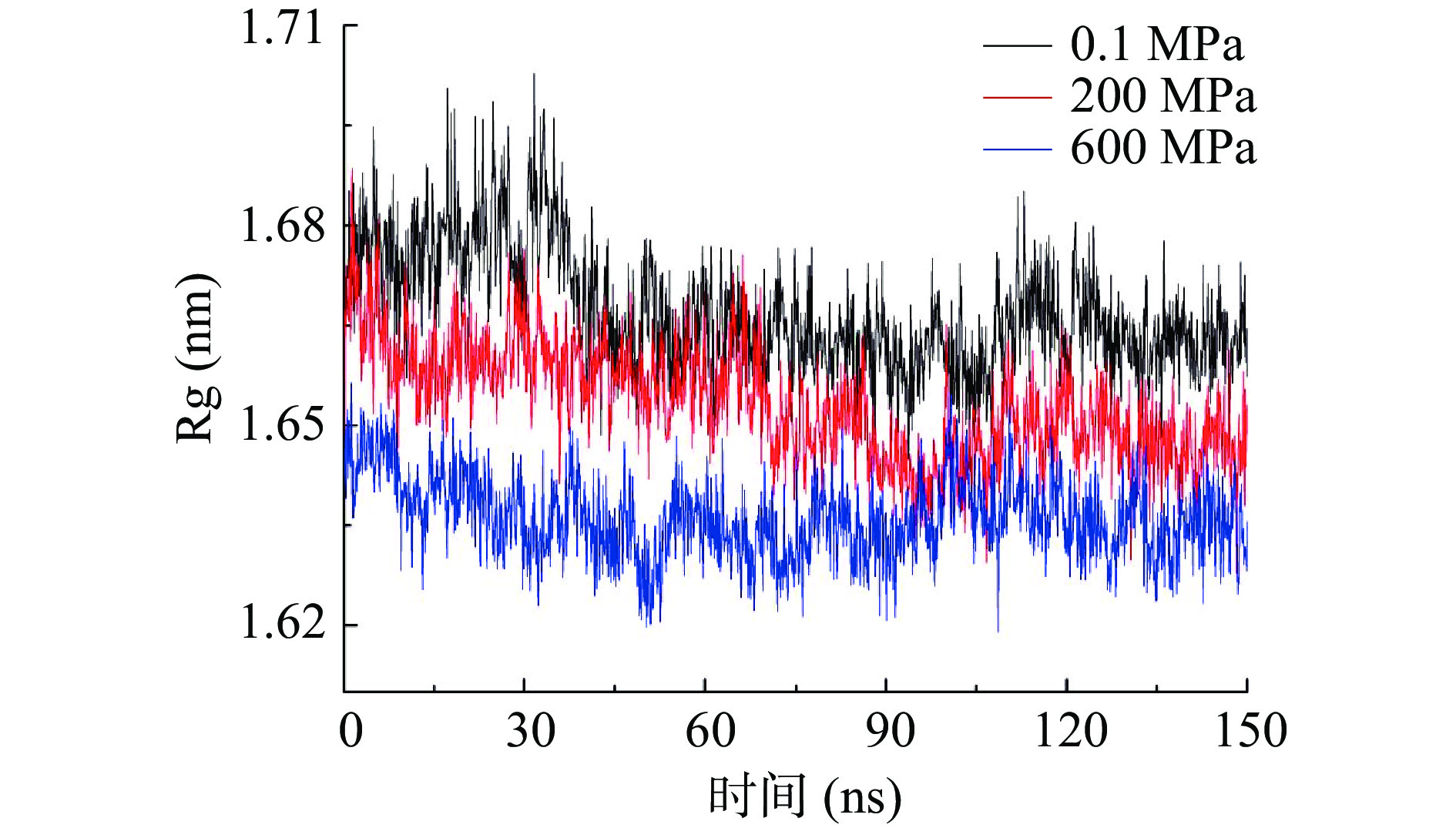
2.4 不同压力处理对酶蛋白回旋半径的影响
图4为不同压力对酶蛋白回旋半径(Rg)的影响,回旋半径越小说明蛋白结构越致密,反之结构越疏松。从图4可以看出,回旋半径均随模拟时间的增加而降低,基本在50 ns后保持稳定。模拟样品的回旋半径随压力的增加而减少,计算出模拟后50 ns的平均回旋半径,发现三个压力下分别为1.663、1.648、1.636 nm,说明施加的外源压力越大,木瓜蛋白酶越容易形成致密的结构。这与简清梅、Huang等[20,24]模拟高压对β-乳球蛋白回旋半径影响时得到的结论一致。这也与前面RMSD和RMSF分析的结果一致,说明蛋白结构越致密蛋白结构波动越小。
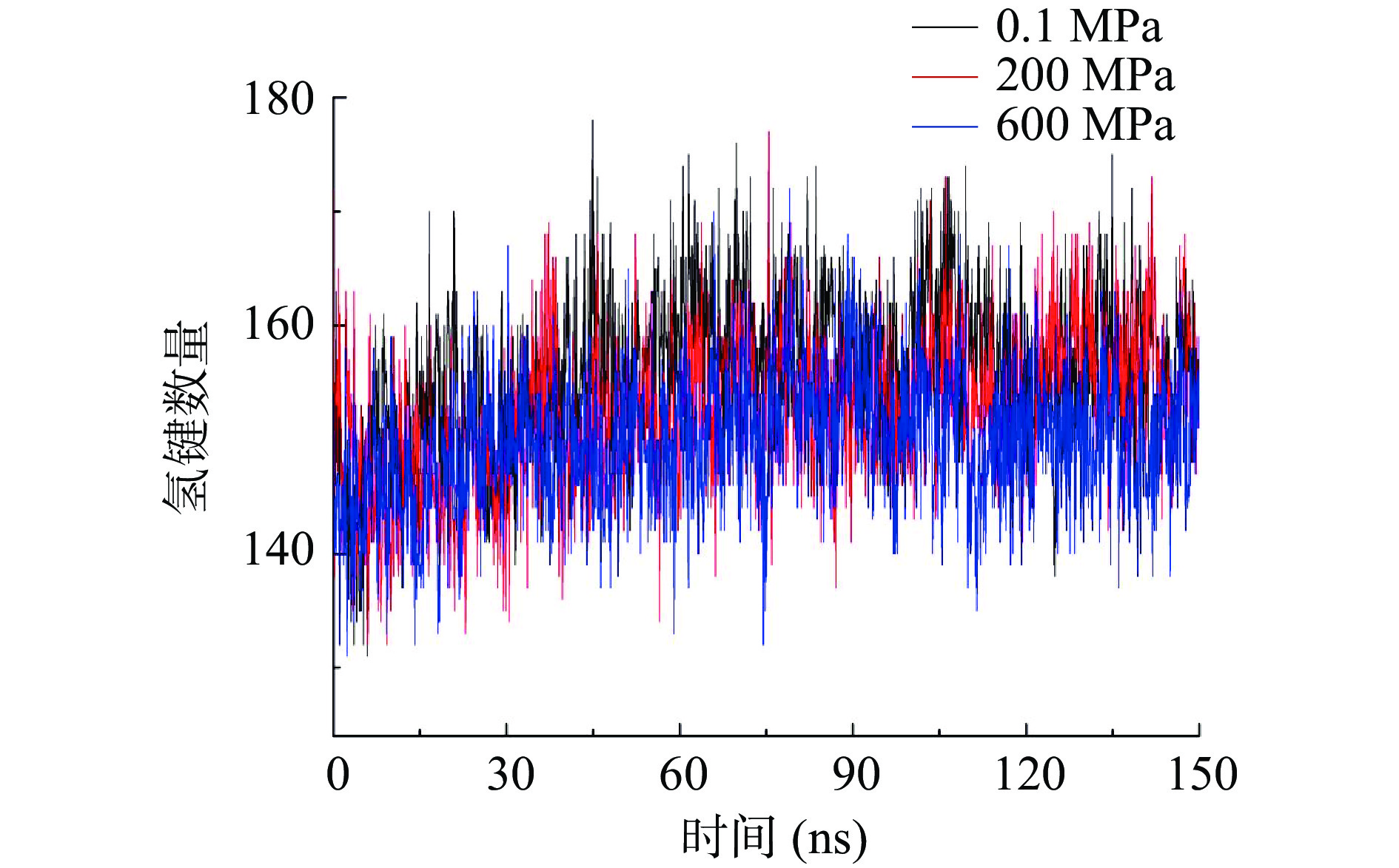
2.5 不同压力处理对酶蛋白间氢键的影响
氢键是稳定蛋白结构的一种重要非共价结合力,图5为不同压力处理条件下蛋白间氢键的变化规律,可以看出,随着压力的增加,蛋白间氢键逐渐减少,以模拟后50 ns进行计算,得到常压条件下蛋白间平均氢键个数为157.5个,当压力为200 MPa时氢键个数减少为155.5个,而当压力进一步增加到600 MPa时,氢键减少为150.5个,600 MPa时减少了7根氢键可能导致酶蛋白结构的部分破坏,这可能是该压力下酶活明显降低的重要原因之一,氢键是维持蛋白结构稳定特别是α-螺旋的重要因素。高压处理对酶蛋白间氢键的破坏与简清梅等[20]模拟高压条件下β-乳球蛋白时得到的结论一致,他们发现300 MPa处理时比常压条件下平均少了2根氢键,600 MPa比300 MPa又少了3根氢键。
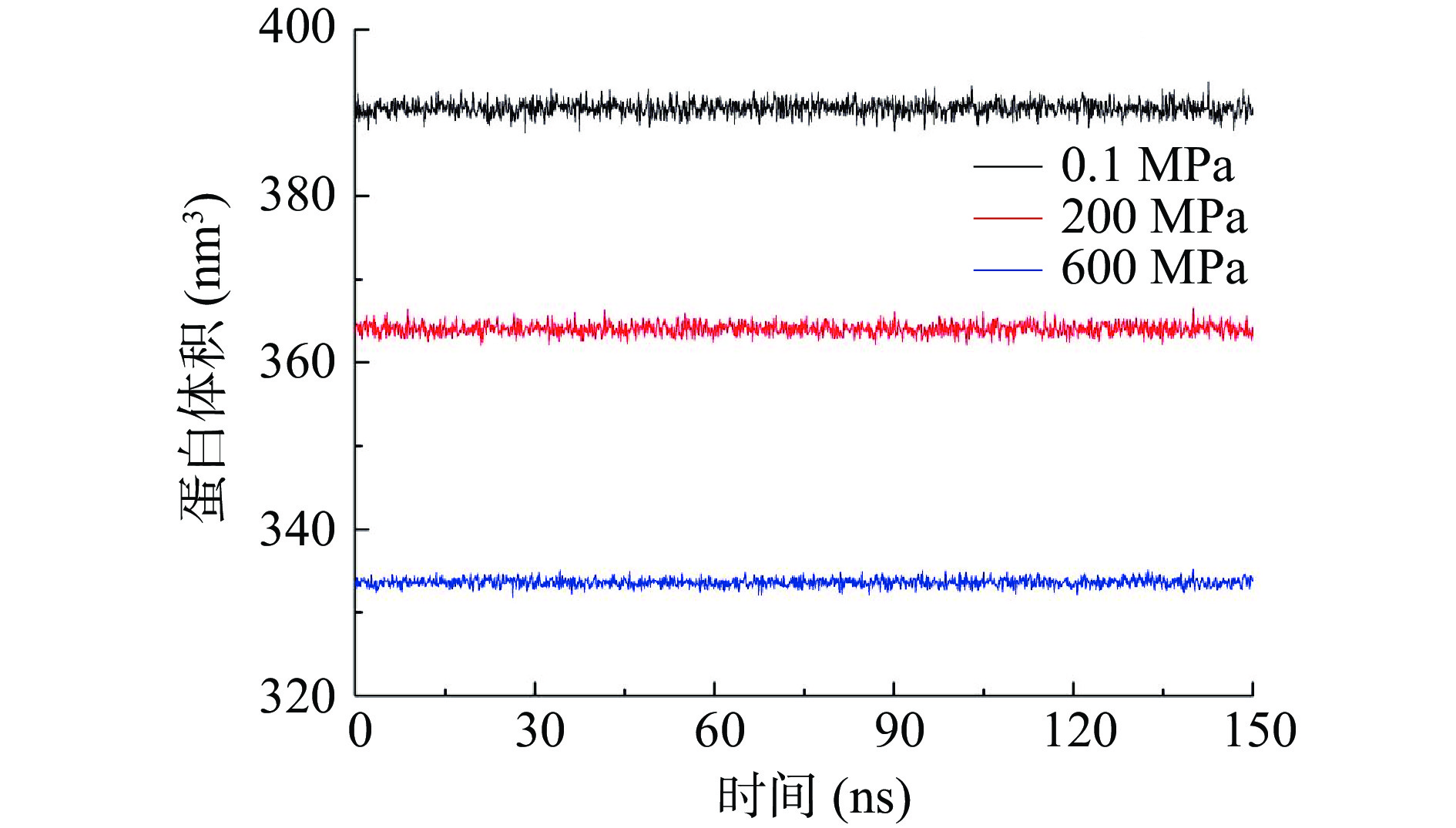
2.6 不同压力处理对酶蛋白体积的影响
如图6所示,各处理条件下酶蛋白体积均在模拟过程中保持恒定,超高压处理能显著降低酶蛋白的体积,且压力越大体积减少越明显。这与简清梅[20]和孔庆新[23]等得到的结论一致。根据Le Châtelier原理,高压下蛋白体积一般朝着减小的方向进行,主要是由于高压处理时水分子能够进入疏水腔,疏水腔变形后体积减小。
2.7 不同压力处理对酶蛋白溶剂可及表面积的影响
由表1可知,随着处理压力的增加,木瓜蛋白酶溶剂可及表面积明显减少,其中疏水和亲水表面积同时降低。溶剂可及表面积的减少可能与蛋白体积在高压下的减少有关,更多的疏水表面积减少是由于高压处理时水分子渗透进入酶蛋白分子内部使更多的疏水区域暴露在极性水溶液中[25-26]。简清梅[20]和孔庆新[23]也分别报道了高压条件下蛋白分子溶剂可及表面积的减小。在本研究中,亲水表面积在不同压力条件下总是大于疏水表面积,说明木瓜蛋白酶总体上在不同压力处理时均是水溶性的。因此,高压处理不会影响木瓜蛋白酶在亲水性食品体系中的使用。
表 1 不同处理压力对木瓜蛋白酶溶剂可及表面积的影响Table 1. Effect of different pressures on the solvent accessible surface area of papain处理压力(MPa) 总可及表面积(nm2) 疏水表面积(nm2) 亲水表面积(nm2) 0.1 103.46±1.79 40.88±1.13 62.59±1.55 200 101.27±2.17 39.98±1.02 61.29±1.79 600 99.09±1.85 37.57±1.10 61.53±1.47 注:数据为100~150 ns模拟中500个数据点的平均值。 2.8 不同压力处理对酶蛋白二级结构的影响
二级结构使用DSSP模块进行计算[27]。不同压力处理对木瓜蛋白酶二级结构的影响如表2所示,可以看出,200 MPa处理时,β-折叠的个数增加,而无规则卷曲明显减少,其它结构变化不大,因此200 MPa处理时蛋白的结构变得更加稳定和有规律;600 MPa处理时在200 MPa基础上无规则卷曲和α-螺旋含量明显降低,而弯曲和转角的含量增加,因此其结构相对破坏较大,这可能也与600 MPa时酶活性较大幅度的降低有关。邱春江[27]在研究鲢鱼中肌原纤维蛋白时也发现α-螺旋对压力十分敏感,在高压处理时其含量会降低,这可能是由高压条件下氢键部分被破坏引起的,因氢键对维持α-螺旋的稳定很重要。Chen等[28]的研究也发现400 MPa的压力处理会增加大豆蛋白中β-折叠的含量而减少α-螺旋的含量,这与本研究的结论是基本一致的。
表 2 不同处理压力对木瓜蛋白酶二级结构的影响Table 2. Effect of different pressures on the secondary structure of papain处理压力(MPa) 无规则卷曲 β-折叠 弯曲 转角 α-螺旋 0.1
200
60065.1±2.9
62.5±2.7
61.8±2.632.9±2.3
34.2±2.0
34.1±1.434.9±3.5
34.7±2.8
35.2±2.819.8±3.7
19.9±3.6
21.3±3.751.5±2.1
51.2±1.9
50.2±2.1注:数据为每种二级结构100~150 ns模拟中500个数据点的平均个数。 2.9 不同压力处理对酶蛋白活性中心残基间距离的影响
木瓜蛋白酶的活性中心主要是由25位的半胱氨酸、158位的天冬氨酸和159位的组氨酸残基构成[1],由表3可见,200 MPa处理时,25位的半胱氨酸与158、159两个残基的距离均增加,特别是与158号的天冬氨酸,而158和159位氨基酸间距离在200 MPa明显减少;600 MPa时,相比于对照样,25与158位残基的距离有所增加,另外两者相差不大。因此,推测200 MPa下酶的激活可能与活性中心氨基酸残基间距离变化有关,使目标蛋白更易与酶的活性中心接触从而引起催化作用;而600 MPa下酶活性的降低可能与蛋白结构的破坏有关,如α-螺旋的破坏等。高进[1]发现500和600 MPa处理对木瓜蛋白酶活性中心氨基酸残基之间的距离没有显著影响,但模拟时间较短(10 ns),也没有讨论158和159号氨基酸之间的距离变化。
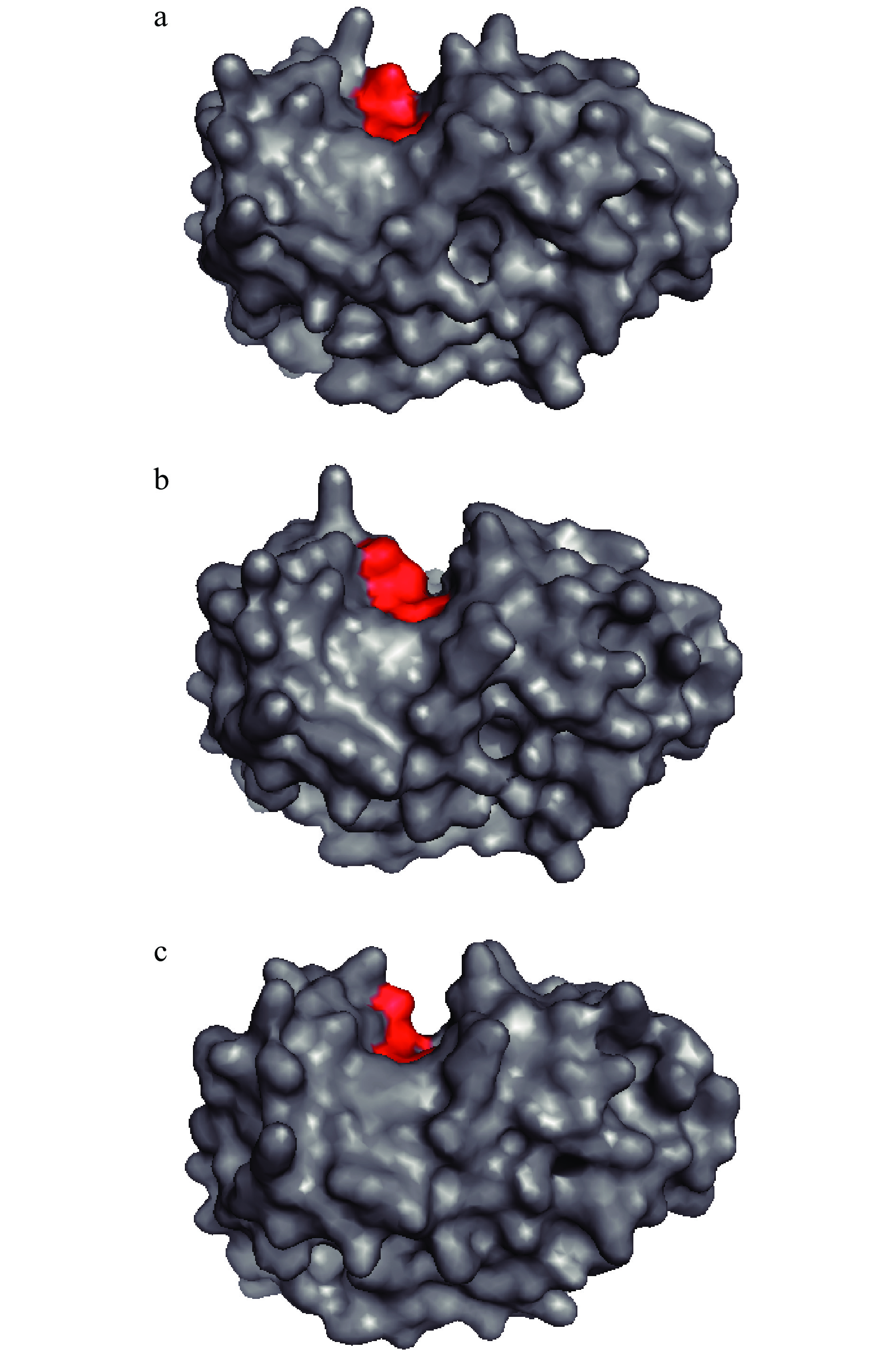
表 3 不同处理压力对木瓜蛋白酶活性中心残基间距离的影响(nm)Table 3. Effect of different pressures on the distance between residues in active center of papain (nm)处理压力(MPa) 25~158 25~159 158~159 0.1 0.816±0.050 0.538±0.024 0.575±0.032 200 0.919±0.034 0.550±0.029 0.506±0.014 600 0.846±0.039 0.548±0.017 0.570±0.024 注:数据为100~150 ns模拟过程中的平均距离。 2.10 不同压力处理对木瓜蛋白酶分子表面结构的影响
不同压力条件下酶分子表面结构如图7,其中红色为活性中心的三个氨基酸(25、158和159位的氨基酸)。可以看出,200 MPa的压力处理相比于常压时,活性中心的口袋处更大,更有利于被催化的蛋白与活性中心接触,这可能是该压力下酶活性有所增加的原因之一;从图7(c)可以看出600 MPa处理时活性口袋处明显变小了。另外,常压和200 MPa时,目标蛋白与活性中心的接触面比较平缓而宽阔,而600 MPa时,接触面相对比较陡且狭窄,更加不利于蛋白的结合,这两方面可能是600 MPa条件下酶活性明显降低的重要原因。
蛋白在高压作用下,体积会减少,结构会比原始构象更加扁平,因25号半胱氨酸与另外两个活性中心的氨基酸分别分布于活性中心的两侧,蛋白在200 MPa被压得更加扁平后,使两侧的距离更远(25号残基分别与158和159号残基的距离),而位于同侧的158和159号残基间距离更近,可能这种适度的结构变化更利于目标蛋白与酶活性中心的结合,也更利于酶发挥催化作用,使酶活性在200 MPa时有所增加。而600 MPa时酶的结构遭到较大破坏,如α-螺旋破坏、疏水表面积的进一步减小、蛋白体积的大幅度减小、氢键的破坏等,这些结构变化都会显著影响酶活性,使酶活在该压力下明显降低。
3. 结论
200 MPa处理使木瓜蛋白酶活性增加13.5%,而600 MPa下酶活性降低26.2%,分子模拟显示200 MPa下酶的激活与该压力下酶活性中心的结合口袋变大、以利于目标蛋白更好地结合于活性中心有关;而600 MPa下酶活性的降低则与该压力下酶结构破坏较大有关,如α-螺旋破坏、疏水表面积和蛋白体积的大幅度减小、氢键的破坏等。本研究从分子模拟角度论证了高压条件下木瓜蛋白酶活性变化的机理,为更好地促进木瓜蛋白酶在高压食品中的应用奠定了一定的理论基础。后续将从实验手段来验证高压下木瓜蛋白酶结构的变化,并模拟不同压力下酶结构变化后与蛋白底物分子的结合及催化机理。
-
表 1 不同处理压力对木瓜蛋白酶溶剂可及表面积的影响
Table 1 Effect of different pressures on the solvent accessible surface area of papain
处理压力(MPa) 总可及表面积(nm2) 疏水表面积(nm2) 亲水表面积(nm2) 0.1 103.46±1.79 40.88±1.13 62.59±1.55 200 101.27±2.17 39.98±1.02 61.29±1.79 600 99.09±1.85 37.57±1.10 61.53±1.47 注:数据为100~150 ns模拟中500个数据点的平均值。 表 2 不同处理压力对木瓜蛋白酶二级结构的影响
Table 2 Effect of different pressures on the secondary structure of papain
处理压力(MPa) 无规则卷曲 β-折叠 弯曲 转角 α-螺旋 0.1
200
60065.1±2.9
62.5±2.7
61.8±2.632.9±2.3
34.2±2.0
34.1±1.434.9±3.5
34.7±2.8
35.2±2.819.8±3.7
19.9±3.6
21.3±3.751.5±2.1
51.2±1.9
50.2±2.1注:数据为每种二级结构100~150 ns模拟中500个数据点的平均个数。 表 3 不同处理压力对木瓜蛋白酶活性中心残基间距离的影响(nm)
Table 3 Effect of different pressures on the distance between residues in active center of papain (nm)
处理压力(MPa) 25~158 25~159 158~159 0.1 0.816±0.050 0.538±0.024 0.575±0.032 200 0.919±0.034 0.550±0.029 0.506±0.014 600 0.846±0.039 0.548±0.017 0.570±0.024 注:数据为100~150 ns模拟过程中的平均距离。 -
[1] 高进. 木瓜蛋白酶加工过程中的性质及结构研究[D]. 天津: 天津科技大学, 2018. GAO Jin. Study on the properties and structure of panpain in processing[D]. Tianjin: Tianjin University of Science and Technology, 2018.
[2] HUANG Y, GAN Y, LI F, et al. Effects of high pressure in combination with thermal treatment on lipid hydrolysis and oxidation in pork[J]. LWT - Food Science and Technology,2015,63:136−143. doi: 10.1016/j.lwt.2015.03.103
[3] 甄宗圆, 李志杰, 粱迪, 等. 超高压技术在肉类杀菌及品质改善中的应用进展[J]. 现代食品科技,2021,37(8):350−356, 374. [ZHEN Zongyuan, LI Zhijie, LIANG Di, et al. Recent application of ultrahigh pressure for meat sterilization and quality improvement[J]. Modern Food Science & Technology,2021,37(8):350−356, 374. ZHEN Zongyuan, LI Zhijie, LIANG Di, et al. Recent application of ultrahigh pressure for meat sterilization and quality improvement[J]. Modern Food Science & Technology, 2021, 37(8): 350-356, 374.
[4] 侯思涵, 裴龙英, 陈计峦. 超高压处理对哈密瓜汁中酶活性与香气的影响[J]. 食品科学,2018,39(22):202−206. [HOU Sihan, PEI Longying, CHEN Jiluan. Effect of ultra-high pressure treatment on enzymatic activities and aroma compounds in Hami melon juice[J]. Food Science,2018,39(22):202−206. doi: 10.7506/spkx1002-6630-201822031 HOU Sihan, PEI Longying, CHEN Jiluan. Effect of ultra—high pressure treatment on enzymatic activities and aroma compounds in Hami melon juice[J]. Food Science, 2018, 39(22): 202-206. doi: 10.7506/spkx1002-6630-201822031
[5] 王帮国, 余振宇, 林琳, 等. 超声波、超高压对白鲢鱼肌肉脂肪氧合酶构象及酶活力的影响[J]. 食品科学,2018,39(3):169−175. [WANG Bangguo, YU Zhenyu, LIN Lin, et al. Effect of ultrasonic wave and ultra high pressure treatment on conformation and enzyme activity of lipoxygenase in silver carp muscle[J]. Food Science,2018,39(3):169−175. doi: 10.7506/spkx1002-6630-201803026 WANG Bangguo, YU Zhenyu, LIN Lin, et al. Effect of ultrasonic wave and ultra high pressure treatment on conformation and enzyme activity of lipoxygenase in silver carp muscle[J]. Food Science, 2018, 39(3): 169-175. doi: 10.7506/spkx1002-6630-201803026
[6] 尚海涛, 宜晓婷, 崔燕, 等. 超高压激活鲜榨梨汁多酚氧化酶的酶学特性[J]. 食品科学,2019,40(19):149−155. [SHANG Haitao, XUAN Xiaoting, CUI Yan, et al. Characterization of polyphenol oxidase from fresh pear juice after high pressure processing activation[J]. Food Science,2019,40(19):149−155. doi: 10.7506/spkx1002-6630-20180824-263 SHANG Haitao, XUAN Xiaoting, CUI Yan, et al. Characterization of polyphenol oxidase from fresh pear juice after high pressure processing activation[J]. Food Science, 2019, 40(19): 149-155. doi: 10.7506/spkx1002-6630-20180824-263
[7] 郭洪伟. 超高压、pH值对芽孢皮层裂解酶活力及二、三级结构影响的研究[D]. 银川: 宁夏大学, 2019. GUO Hongwei. Effects of ultra-high pressure and pH on the activity, the secondary and tertiary structure of spore's cortex lyase[D]. Yinchuan: Ningxia University, 2019.
[8] HUANG Y, WANG Y, WU Z, et al. Combined effects of high-pressure and thermal treatments on lipid oxidation and enzymes in pork[J]. Food Science and Biotechnology,2016,25(1):261−266. doi: 10.1007/s10068-016-0038-2
[9] HOMMA N, IKEUCHI Y, SUZUKI A. Effect of high pressure treatment on proteolytic system in meat[J]. Biotechnology Progress,1996,13:327−330.
[10] LAKSHMANAN R, PATTERSON MF, PIGGOTT JR. Effects of high-pressure processing on proteolytic enzymes and proteins in cold-smoked salmon during refrigerated storage[J]. Food Chemistry,2005,90:541−548. doi: 10.1016/j.foodchem.2004.05.015
[11] TEREFE N S, YANG Y H, KNOERZER K, et al. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree[J]. Innovative Food Science and Emerging Technologies,2010,11:52−60. doi: 10.1016/j.ifset.2009.08.009
[12] RODRIGO D, JOLIE R, VAY LOEY A, et al. Thermal and high pressure stability of tomato lipoxygenase and hydroperoxide lyase[J]. Journal of Food Engineering,2007,79:423−429. doi: 10.1016/j.jfoodeng.2006.02.005
[13] 刘平, 胡志和, 吴子健, 等. 超高压对木瓜蛋白酶构象及酶活力的影响[J]. 食品科学,2015,36(23):23−27. [LIU Ping, HU Zhihe, WU Zijian, et al. Effect of ultra high pressure treatment on the conformation and enzyme activity of papain[J]. Food Science,2015,36(23):23−27. doi: 10.7506/spkx1002-6630-201523005 LIU Ping, HU Zhihe, WU Zijian, et al. Effect of ultra high pressure treatment on the conformation and enzyme activity of papain[J]. Food Science, 2015, 36(23): 23-27. doi: 10.7506/spkx1002-6630-201523005
[14] 陈刚. 高压引发脂肪酶分子构象变化与催化行为关系的研究[D]. 无锡: 江南大学, 2017. CHEN Gang. Study on the correlation between the conformational chang and catalytic behavior of lipase induced by high pressure[D]. Wuxi: Jiangnan University, 2017.
[15] LI Y, ZHANG S, BAO Z, et al. Exploring the activation mechanism of alcalase activity with pulsed electric field treatment: Effects on enzyme activity, spatial conformation, molecular dynamics simulation and molecular docking parameters[J]. Innovative Food Science and Emerging Technologies,2022,76:102918. doi: 10.1016/j.ifset.2022.102918
[16] LI Y, YUAN Z, GAO Y, et al. Mechanism of trypsin activation by pulsed electric field treatment revealed based on chemical experiments and molecular dynamics simulations[J]. Food Chemistry, 2022, 394: 133477
[17] ABRAHAM M J, MURTOLA T, SCHULZ R, et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers[J]. SoftwareX,2015,s1−2:19−25.
[18] SCHMID N, EICHENBERGER A P, CHOUTKO A, et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7[J]. European Biophysics Journal with Biophysics Letters,2011,40(7):843−856. doi: 10.1007/s00249-011-0700-9
[19] 刘祥雨, 覃小丽, 钟金锋. 采用分子动力学模拟研究温度对乳球蛋白稳定性的影响[J]. 食品与发酵工业,2020,46(7):89−96. [LIU Xiangyu, QIN Xiaoli, ZHONG Jinfeng. Effect of temperature on the stability of lactoglobulin: A molecular dynamics simulation[J]. Food and Fermentation Industries,2020,46(7):89−96. LIU Xiangyu, QIN Xiaoli, ZHONG Jinfeng, et al. Effect of temperature on the stability of lactoglobulin: a molecular dynamics simulation[J]. . Food and Fermentation Industries, 2020, 46(7): 89-96.
[20] 简清梅, 索化夷, 张喜才, 等. 分子动力学模拟超高压结合热处理对β-乳球蛋白结构的影响[J]. 食品科学,2021,42(23):57−63. [JIAN Qingrnei, SUO Huayi, ZAHNG Xicai, et al. Effect of combined high pressure and thermal treatment on structure of β-lactoglobulin evaluated by molecular dynamics simulation[J]. Food Science,2021,42(23):57−63. doi: 10.7506/spkx1002-6630-20210427-378 JIAN Qingrnei, SUO Huayi, ZAHNG Xicai, et al. Effect of combined high pressure and thermal treatment on structure of β—lactoglobulin evaluated by molecular dynamics simulation[J]. Food Science, 2021, 42(23): 57-63. doi: 10.7506/spkx1002-6630-20210427-378
[21] KURPIEWSKA K, MIACZEWSKA A, LEWIŃSKI K. Insulin conformational changes under high pressure in structural studies and molecular dynamics simulations[J]. Journal of Molecular Structure,2020,1202:127251. doi: 10.1016/j.molstruc.2019.127251
[22] HATA H, NISHIYAMA M, KITAO A. Molecular dynamics simulation of proteins under high pressure: Structure, function and thermodynamics[J]. Biochimica et Biophysica Acta (BBA)-General Subjects,2019,1864(2):129395.
[23] 孔庆新, 罗丽梅, 黄业传, 等. 分子动力学探究高压对β-乳球蛋白与多酚结合的影响[J]. 食品与发酵工业,2022,48(3):107−114. [KONG Qingxin, LUO Limei, HUANG Yechuan, et al. The effect of high pressure on the combination of β-lactoglobulin and polyphenols using molecular dynamic model[J]. Food and Fermentation Industries,2022,48(3):107−114. KONG Qingxin, LUO Limei, HUANG Yechuan, et al. The effect of high pressure on the combination of β-lactoglobulin and polyphenols using molecular dynamic model[J]. Food and Fermentation Industries, 2022, 48(3) : 107 - 114.
[24] HUANG Y, ZHANG X, SUO H. Interaction between β-lactoglobulin and EGCG under high-pressure by molecular dynamics simulation[J]. PLoS One,2021,16(12):e0255866. doi: 10.1371/journal.pone.0255866
[25] KOLAKOWSKI P, DUMAY E, CHEFTEL J C. Effects of high pressure and low temperature on β-lactoglobulin unfolding and aggregation[J]. Food Hydrocolloids,2001,15:215−232. doi: 10.1016/S0268-005X(01)00017-0
[26] KABSCH W, SANDER C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features[J]. Biopolymers,1983,22(12):2577−2637. doi: 10.1002/bip.360221211
[27] 邱春江. 超高压对鲢鱼中关键酶与结构蛋白质构影响的研究[D]. 无锡: 江南大学, 2014. QIU Chunjiang. Effect of ultra-high pressure technology on key enzymes and structural proteins in silver carp processing[D]. Wuxi: Jiangnan University, 2014.
[28] CHEN G, WANG S, FENG B, et al. Interaction between soybean protein and tea polyphenols under high pressure[J]. Food Chemistry,2019,277:632−638. doi: 10.1016/j.foodchem.2018.11.024
-
期刊类型引用(11)
1. 苏敏,李红丽,白亚敏,黄大亮,刘元,吴彦蕾. 基于液相色谱-串联高分辨质谱技术的食品中污染物检测技术研究进展. 食品安全质量检测学报. 2025(04): 44-52 .  百度学术
百度学术
2. 张君. 我国南方部分地区蓝莓种植过程中农药残留检测结果分析. 河北农机. 2024(03): 136-138 .  百度学术
百度学术
3. 张申平,秦宇,顾颖娟. QuEChERS-超高效液相色谱-四极杆/静电场轨道阱质谱法测定牛羊乳及其乳粉中21种兽药. 乳业科学与技术. 2024(02): 24-29 .  百度学术
百度学术
4. 李红洲,国果,李博岩,梁桂娟,李志远. 超高效液相色谱-四极杆-飞行时间-高分辨质谱法分析6种李果实中的代谢物差异性. 食品安全质量检测学报. 2024(11): 63-73 .  百度学术
百度学术
5. 刘宇航,于寒冰,杨红菊,马啸,温雅君,孙志伟,习佳林,熊慧勤,肖志勇. 高效液相色谱-四极杆-飞行时间质谱法快速筛查蔬菜中124种药物与个人护理品残留量. 食品安全质量检测学报. 2024(16): 175-184 .  百度学术
百度学术
6. 朱春雨,吴移山,郑景娇. 高效液相色谱-串联质谱法测定鸡蛋中地克珠利、妥曲珠利及其代谢物残留量. 食品安全质量检测学报. 2024(16): 211-218 .  百度学术
百度学术
7. 肖泳,曾小明,李政,袁列江,邓航,王淑霞,潘照. 超高效液相色谱-四极杆/静电场轨道阱高分辨质谱法测定鸡蛋中94种农药残留. 食品与发酵工业. 2024(21): 333-340 .  百度学术
百度学术
8. 王颖怡,吴玉田,孟春杨,周贻兵,刘利亚. HPLC-MS/MS技术同时测定鸡蛋中5种抗球虫药. 食品工业. 2023(06): 291-294 .  百度学术
百度学术
9. 李晓慧,李建洪,王洪萍,金芬. 植物源性食品中化学性危害物质的色谱-质谱检测技术研究进展. 分析测试学报. 2023(10): 1357-1369 .  百度学术
百度学术
10. 周雪莼,胡婷婷,王佳慧,白静,杨颖,侯宇,张哲,张勋. 高效液相色谱-高分辨质谱法快速筛查动物源性药食同源产品中32种抗生素兽药残留. 吉林中医药. 2023(12): 1469-1474 .  百度学术
百度学术
11. 范轶欧,迟英欣,杨路平,焦燕妮. 高分辨质谱技术在环境和食品风险物质非靶向筛查检测中应用的研究进展. 预防医学论坛. 2023(12): 955-960 .  百度学术
百度学术
其他类型引用(0)






 下载:
下载:
 下载:
下载:



