Preliminary Study of Reaction Conditions for the Synthesis of AA-2G by Cyclodextrin Glucosyltransferase
-
摘要: 为了探究重组菌株pET-28a(+)-cgt-T1/BL21(DE3)产生的环糊精葡萄糖基转移酶(Cyclodextrin glucosyltransferase,CGTase)催化合成2-O-α-D-吡喃葡萄糖基-L-抗坏血酸(Ascorbic acid 2-glucoside,AA-2G)的效果,用LB发酵培养基表达重组蛋白CGTase-T1,经亲和纯化柱纯化并浓缩后,测定其β-环化活性和歧化活性。以维生素C(Vitamin C,VC)和β-环糊精为底物,酶法合成AA-2G,并通过单因素优化实验,进一步探究了不同糖基供体、底物浓度、pH、温度、底物比例、蛋白浓度以及反应时间对AA-2G产量的影响,对酶合成AA-2G的动力学进行了分析。结果表明:此酶具有合成AA-2G的能力,未优化前AA-2G的产量为0.67 g/L。优化后,考虑到低成本和经济效益,选择可溶性淀粉和麦芽糊精为糖基供体,当糖基供体为可溶性淀粉时,底物浓度为70 g/L,反应pH4.5,反应温度37 ℃,底物比例3/3(VC/糖基供体),蛋白浓度5.0 mg/mL,反应时间为42 h时,该重组CGTase催化合成AA-2G的产量最高能达到12.68 g/L;当糖基供体为麦芽糊精时,底物浓度为30 g/L,反应pH5.0,反应温度37 ℃,底物比例4/2,蛋白浓度5.0 mg/mL,反应时间为30 h时,该重组CGTase催化合成AA-2G的产量最高能达到4.96 g/L。在最适条件下,AA-2G的产量分别是未优化反应条件下的18.93倍和7.40倍。经动力学分析发现可溶性淀粉作为糖基供体的催化效率高于麦芽糊精,因此,可溶性淀粉作为糖基供体比麦芽糊精更具有优势,合成得到AA-2G的产量更高。本研究成功将重组CGTase-T1用于AA-2G的合成,通过优化酶法合成的反应条件,使AA-2G的产量得到了大幅提高,为实现AA-2G的工业生产提供参考意义。
-
关键词:
- 环糊精葡萄糖基转移酶 /
- 2-O-α-D-吡喃葡糖基-L-抗坏血酸 /
- 生物酶法 /
- β-环化活性 /
- 歧化活性
Abstract: To investigate the effect of cyclodextrin glucosyltransferase (CGTase) produced by the recombinant strain pET-28a(+)-cgt-T1/BL21 (DE3) on the synthesis of 2-O-α-D-glucoside (AA-2G), the recombinant protein CGTase-T1 was expressed in the fermentation medium LB, purified by affinity chromatography and concentrated, followed by the determination of β-cyclization and disproportionation activity. AA-2G was synthesized using vitamin C and β-cyclodextrin as substrates. The effects of different glycan donors, substrate concentration, pH, temperature, substrate ratio, protein concentration and reaction time on AA-2G yield were further explored by single-factor optimization experiments, and the kinetics of CGTase were also analyzed. The results showed that CGTase could synthesize AA-2G, and the yield of AA-2G was 0.67 g/L before optimization of reaction conditions. Considering low cost and economic benefits, soluble starch and maltodextrin were selected as sugar donors in reaction condition optimization. When soluble starch was used as glycan donor, the yield of AA-2G catalyzed by the recombinant CGTase could reach up to 12.68 g/L under the condition as follows: The concentration of soluble starch at 70 g/L, the amount of VC: glycosyl at 3:3, CGTase-T1 used at 5.0 mg/mL, pH4.5 at 37 ℃ for 42 h reaction. While maltodextrin was used as glycan donor, the yield of AA-2G catalyzed by the recombinant CGTase could reach up to 4.96 g/L under the condition as follows: the concentration of soluble starch at 30 g/L, the amount of VC: glycosyl at 4:2, CGTase-T1 used at 5.0 mg/mL, pH5.0 at 37 ℃ for 30 h reaction. Under the optimal conditions, the yield of AA-2G was 18.93 times and 7.40 times higher than those of the unoptimized reaction conditions respectively. Through the kinetic analysis of CGTase-T1, it was found that the catalytic efficiency of soluble starch as sugar donor was higher than that of maltodextrin. Therefore, soluble starch as sugar donor was better than maltodextrin, resulting in a higher yield of AA-2G synthesis. In this study, the recombinant CGTase-T1 was successfully applied in the synthesis of AA-2G. By optimizing the reaction conditions of enzymatic catalysis, the output of AA-2G was greatly increased, providing a reference for the industrial production of AA-2G. -
维生素C(Vitamin C,VC)广泛存在于水果蔬菜中,是人类和少数生物体内不可或缺的营养元素。它能保护人体免受氧化剂伤害、预防疾病和治疗坏血病等[1]。但VC在氧气、pH、金属和热量中容易降解,难以长期保存[2],故限制了其他领域的应用。为改善VC的稳定性,许多VC衍生物被相继合成,如VC金属盐,VC脂类和VC糖苷类等[3]。其中VC糖苷类是对VC的C2、C3、C5和C6原子上进行糖基化修饰后获得的VC衍生物[4]。2-O-α-D-吡喃葡萄糖基-L-抗坏血酸(Ascorbic acid 2-glucoside,AA-2G)是VC的C2原子上连接了一个吡喃葡萄糖苷而形成的衍生物,在所有的VC糖苷类衍生物之中,以AA-2G的药理学研究最多,应用也十分广阔,因为AA-2G是最理想的VC衍生物,是VC最好的替代品[5],市场需求量较大。它除了具有与VC相同的活性外[6],还具有良好的稳定性和安全性,优异的抗氧化性,容易被人体吸收,可用于化妆品、医药、食品等领域[7],具有巨大的开发价值。
目前,AA-2G的合成以生物酶法为主,与化学法相比生物法更高效,反应条件温和,可用于连续生产,更适合工业化生产[8]。用于合成AA-2G的酶有α-葡萄糖苷酶[9]、环糊精葡萄糖基转移酶[10](Cyclodextrin glucosyltransferase,CGTase)、淀粉酶[11]、蔗糖磷酸化酶[12]和α-异麦芽糖基葡萄糖形成酶[13]。其中,CGTase是一种多功能酶,且作为一种工业用酶,因其具有底物特异性高和转化率高等优点,被认为是合成AA-2G最高效的酶,是催化合成AA-2G的酶中应用最广、研究最多的一种酶,已被广泛用于AA-2G工业生产[14]。其合成原理是将糖基供体的糖苷键转移至糖基受体的C2位点上生成中间产物AA-2Gn(n≤6),然后添加糖化酶水解混合物产生AA-2G和其他副产物[15]。CGTase能将多种糖基供体用于AA-2G的合成,但产量受糖基供体的影响较大[16]。目前CGTase催化合成AA-2G存在转化率低、酶产量低等问题,导致AA-2G的生产成本高,限制了其广泛应用[17]。所以优化AA-2G的合成是目前研究的热点,如利用分子半侣共表达[18],增加异源蛋白的可溶性表达,从而提高合成产量;余磊等[19]用全细胞催化方法生产AA-2G,产量达到了35.7 g/L;郭娇梅[20]对CGTase进行固定化后制备AA-2G,使酶可以重复利用并增强其稳定性;对CGTase进行分子改造[21-26],提高酶的热稳定性和对底物的特异性,提高合成产物的特异性和产量;为了提高AA-2G作为抗氧化剂的有效性,Violaine等[27]研究了AA-2G和辅助抗氧化剂的协同作用。此外,有研究表明环糊精为AA-2G合成的最优糖基供体[28],但由于环糊精价格昂贵导致生产成本过高,不适于大规模的工业生产,故选择成本低的糖基供体对AA-2G的生产具有重要意义。
目前国内缺少可用于AA-2G工业化生产的酶,市场上的两种商品化CGTase仅由国外提供,价格十分昂贵,特别是在化妆品领域中,AA-2G大部分来源于日本林原国际有限公司,占据垄断地位。因此需要挖掘和改造适合生产AA-2G的CGTase,本实验室以前期构建的重组表达菌株为研究对象,首次将其用于AA-2G的合成,探究其合成效果,同时,对合成AA-2G的反应条件进行单因素优化,选择合适且成本低的糖基供体,确定最适的合成条件,以提高AA-2G的产量,为寻找合适的工业用酶,加快工业化生产提供参考意义。
1. 材料与方法
1.1 材料与仪器
重组菌株pET-28a(+)-cgt-T1/BL21(DE3) 为本实验室构建保存[29];4,6-亚乙基-对硝基苯-α-D-麦芽七糖苷(4,6-Ethylidene-p-nitrobenzene-α-D-maltopeptide,EPS)、异丙基-β-D-硫代半乳糖糖苷(Isopropyl-beta-D-thiogalactoside,IPTG)、AA-2G和VC标准品 上海源叶生物科技有限公司;甲醇 色谱纯,美国TEDDIA公司;VC、可溶性淀粉和葡萄糖 国药集团化学试剂有限公司;考马斯亮蓝R250、酚酞 天津天力化学试剂有限公司;α-环糊精(Cyclodextrin,α-CD)、β-环糊精(Cyclodextrin,β-CD)、γ-环糊精(Cyclodextrin,γ-CD) 梯希爱化成工业发展有限公司;α-葡萄糖苷酶、糖化酶(100 U/mg)、牛血清白蛋白 北京索莱宝科技有限公司;硫酸卡那霉素(Kanamycin sulfate,Kana)、麦芽糊精、麦芽糖 上海麦克林生化科技有限公司;酵母提取物、胰蛋白胨 英国OXOID公司;所用无机试剂和有机溶剂均为国产分析纯;LB培养基:酵母提取物5.0 g,胰蛋白胨10.0 g,氯化钠10.0 g溶解到1 L去离子水,使用NaOH调节pH至7.0~7.2,121 ℃灭菌20 min。
TE3102S,TE124S电子分析天平 德国赛多利斯公司;MJ-54A高压灭菌锅 美国施都凯公司;TU1900紫外分光光度计 北京普析公司;IS-RDS3恒温摇床 美国Crystal公司;SPX-250B-Z生化培养箱 上海博迅实业有限公司;5417R超速冷冻离心机 德国Fritsch公司;SCIENTZ-IID超声波细胞粉碎机 宁波新芝生物科技股份有限公司;DYCZ-24DN双垂直电泳槽、DYY-8C型电泳仪电源 北京市六一仪器厂;Gel Doc EZ凝胶图像仪 美国BIO-RAD公司;Ni-NTA His Bind Resin亲和纯化柱 上海七海复泰生物科技有限公司;Millipore超滤离心管 上海羽令过滤器材有限公司;PB-10 pH计 德国赛多利斯公司;HH-4水浴锅 金坛市杰瑞尔电器有限公司;X-30R离心机 美国Beckman公司;Water e2695 HPLC高效液相仪 美国Water公司;Enspire酶标仪 铂金埃尔默企业管理(上海)有限公司。
1.2 实验方法
1.2.1 重组菌株诱导表达及粗酶纯化
在前期研究中确定了最佳诱导条件[29],把重组菌株接种至30 mL含终浓度为50 μg/mL Kana的LB液体培养基中,37 ℃,200 r/min过夜培养,然后按1%接种量转接至1 L含终浓度为50 μg/mL Kana的LB液体培养基中,当菌体浓度OD600达到0.80时,添加终浓度为0.30 mmol/L的IPTG溶液,25 ℃,200 r/min条件下,培养10 h。在4 ℃,10000 r/min离心5 min,用0.01 mol/L PBS缓冲液悬浮细胞,得到菌悬液,冰浴中超声破碎,参数设置75%功率(总功率500 W),工作3 s,间歇6 s,总时长35 min。4 ℃,10000 r/min离心15 min,收集上清,即为粗酶液。
使用Ni-NTA His Bind Resin亲和纯化柱,参照七海生物产品说明书对粗酶液进行纯化。然后将目的蛋白流出液置于超滤离心管中,4 ℃,5000 r/min,离心40 min,进行除盐和蛋白浓缩,得到纯化酶,于−20 ℃保存。用SDS-PAGE分析纯化酶,使用10%的分离胶和5%的浓缩胶,取适量纯化酶加入上样缓冲液,混匀,煮沸10 min,8000 r/min离心6 min,取上清液10 μL上样,5 μL Marker,80 V电泳25 min,调电压至120 V,电泳1 h左右,然后将凝胶用考马斯亮蓝R250染色液染色,再用脱色液脱色,最后用凝胶图像仪观察结果。采用Bradford法[30]测定纯化酶浓度,用于后续比酶活的测定。
1.2.2 纯化酶β-环化活性的测定
参考Tesfai等[31]的方法,对其稍加修改,测定其酶活与比酶活。取0.10 mL适当稀释的纯化酶液(0.01~0.03 mg/mL),加入1.00 mL的4%(w/v)淀粉溶液(使用pH6.0的10 mmol/L磷酸盐缓冲液配置),混匀,65 ℃水浴反应10 min,加入3.50 mL 30 mmol/L NaOH溶液终止反应,再向混合物中加入0.50 mL 0.02%(w/v)酚酞溶液,室温静置15 min,550 nm处测量吸光度。以蒸馏水为空白调零,灭活酶液为对照。一个酶活单位(U)的定义为上述条件下每分钟生成1 μmol β-环糊精的酶量。
1.2.3 纯化酶歧化活性的测定
参考Vanderveen等[32]的方法,对其稍加修改,测定其酶活与比酶活。取1.00 mL 6 mmol/L的EPS和10 mmol/L的麦芽糖(用10 mmol/L pH6.0磷酸盐缓冲液配制)的混合底物,65 ℃预热10 min后,加入0.10 mL适当稀释的纯化酶液(0.01~0.03 mg/mL),65 ℃反应10 min,取0.10 mL混合反应物,加入20 μL 1.20 mol/L的HCl溶液(4 ℃),60 ℃孵育10 min失活,再加入20 μL 1.20 mol/L的NaOH溶液中和后,添加60 μL α-葡萄糖苷酶(1 U,使用10 mmol/L pH7.0磷酸缓冲液溶解),37 ℃反应1 h,最后加入1.00 mL 1 mol/L Na2CO3溶液(调pH至8以上),401 nm测定吸光度。以蒸馏水为空白调零,灭活酶液为对照。一个酶活单位(U)的定义为上述条件下每分钟转化1 µmol EPS的酶量。
1.2.4 AA-2G的酶法合成
参考韩瑞枝[33]的方法,将纯化后的环糊精葡萄糖基转移酶用10 mmol/L乙酸-乙酸钠缓冲液(pH5.0)稀释到蛋白质浓度为1.00 mg/mL,以5%(w/v)的纯化酶液加入底物终浓度均为10 g/L的VC和β-CD(糖基供体)的等比例(VC/糖基供体)混合的底物中(底物溶液使用10 mmol/L pH5.0乙酸钠缓冲液配制,VC溶液使用20% NaOH溶液调pH至5.0),混合均匀,反应pH为5.0。将混合物置于37 ℃(反应温度)、黑暗、无氧的条件下恒温反应24 h(反应时间)。最后将10 U糖化酶(使用与配制底物相同的缓冲液进行配制)加入到反应混合物中,在60 ℃的条件下静置24 h,使中间体AA-2Gn(其中n是连接到L-抗坏血酸的糖基的数目)水解,最终得到AA-2G。
1.2.5 AA-2G的标准曲线及定性与定量检测
取5.00 mg AA-2G标准品用pH2.5的0.01 mmol/L磷酸缓冲液定容至5 mL容量瓶中,配制成1.00 g/L的标准品溶液。再用标准品溶液和pH2.5的0.01 mmol/L磷酸缓冲液配制浓度分别为0、0.025、0.05、0.10、0.20、0.40、0.60和0.80 g/L的AA-2G溶液。采用HPLC色谱法检测AA-2G[33],色谱柱:Inertsil ODS-3 C18柱(5 μm,250×4.60 mm);紫外检测波长:238 nm;流动相:2%的甲醇和98% 0.01 mmol/L磷酸缓冲液(85%磷酸调pH至2.5);流速:0.60 mL/min,进样量:10 μL;柱温:25 ℃。HPLC图谱中AA-2G的峰面积(uV·S)和浓度(g/L)成正比,以AA-2G的峰面积为纵坐标,AA-2G浓度为横坐标,绘制标准曲线。得到标准曲线公式:y=25901377x+24727,回归系数R2=0.9994,可信度较高。
将反应结束后的反应混合物适当稀释,8000 r/min,离心10 min,取上清液,并使用0.22 μm滤膜过滤,得到反应液样品,与AA-2G和VC的标准品同时进行HPLC检测,参数同上。根据得到的色谱图数据,对比标准品与反应液样品出峰时间是否一致,对AA-2G进行定性分析;将反应液样品AA-2G的峰面积代入标准曲线公式,得出AA-2G的浓度,对AA-2G进行定量分析。
1.2.6 AA-2G的酶法合成条件初探
各因素的实验水平设计均参考宋凯[34]的方法,对其稍加修改。
1.2.6.1 糖基供体对反应的影响
将AA-2G酶法合成的糖基供体β-CD分别更换为α-CD,β-CD,γ-CD,可溶性淀粉,麦芽糊精,麦芽糖和葡萄糖,其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量。
1.2.6.2 底物浓度对反应的影响
将AA-2G酶法合成的底物终浓度分别更换为10、30、50、70、90和110 g/L的VC和糖基供体(可溶性淀粉和麦芽糊精),其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量,计算AA-2G生成率。由于底物浓度不同,无法进行AA-2G含量的比较,故选用AA-2G生成率作为此优化因素的比较依据。
AA-2G生成率计算公式:
A(%)=ca×100 (1) 式中:A:不同底物浓度下AA-2G生成率,%;c:对应条件下生成AA-2G的含量,g/L;a:对应条件下底物VC的含量,g/L。
1.2.6.3 pH对反应的影响
在最适底物浓度下,将AA-2G酶法合成的反应pH分别更换为4.0、4.5、5.0、5.5、6.0(用10 mmol/L的乙酸-乙酸钠缓冲液配制底物溶液)6.0、6.5、7.0(用0.01 mmol/L磷酸缓冲液配制底物溶液),其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量。
1.2.6.4 温度对反应的影响
在最适底物浓度和pH下,将AA-2G酶法合成的反应温度分别更换为23、30、37、44和51 ℃,其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量。
1.2.6.5 底物比例对反应的影响
在最适pH和温度下,将AA-2G酶法合成的底物比例(VC/糖基供体)分别更换为1/5、2/4、3/3、4/2、5/1,其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量。
1.2.6.6 蛋白浓度对反应的影响
在最适pH,温度和底物比例下,将AA-2G酶法合成的蛋白浓度分别更换为1.0、5.0、10.0、15.0和20.0 mg/mL,其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量。
1.2.6.7 时间对反应的影响
在最适pH,温度,底物比例和蛋白浓度下,将AA-2G酶法合成的反应时间分别更换为0、6、12、18、24、30、36和42 h, 其他条件与1.2.4相同,反应产物使用HPLC色谱法检测AA-2G的含量。
1.2.7 AA-2G的酶法合成动力学分析
将糖基供体的不同底物浓度(2.0、4.0、8.0、16.0 g/L)进行固定,测定其不同浓度下VC(4.0、8.0、16.0、20.0、32.0 g/L)合成AA-2G的产量,在最适反应温度和pH下,反应6 h,根据下面的公式[33],计算出合成AA-2G的动力学参数。
乒乓反应机制:
V=Vmax×a×bKmA×b+KmB×a+a×b (2) 底物抑制反应机制:
V=Vmax×a×bKmB×a+KmA×b×1+bKiB+a×b (3) 式中:V:不同底物浓度下的反应速率(每毫克酶每小时催化生成AA-2G的量),g/L/mg/h;Vmax:反应的最大速率,g/L/mg/h;a:受体(VC)的浓度,g/L;b:糖基供体(麦芽糊精/可溶性淀粉)的浓度,g/L;KmA:VC的亲和常数;KmB,糖基供体的亲和常数;KiB:底物糖基供体的抑制常数。
1.3 数据处理
酶活性测定实验每个反应重复3次,单因素优化实验每个反应设置2个平行,数据以平均数±标准差(mean±SD)表示。利用Excel 2019进行数据处理和绘图,利用SPSS 19.0软件进行数据统计分析,显著性水平P<0.05。
2. 结果与分析
2.1 CGTase-T1的纯化
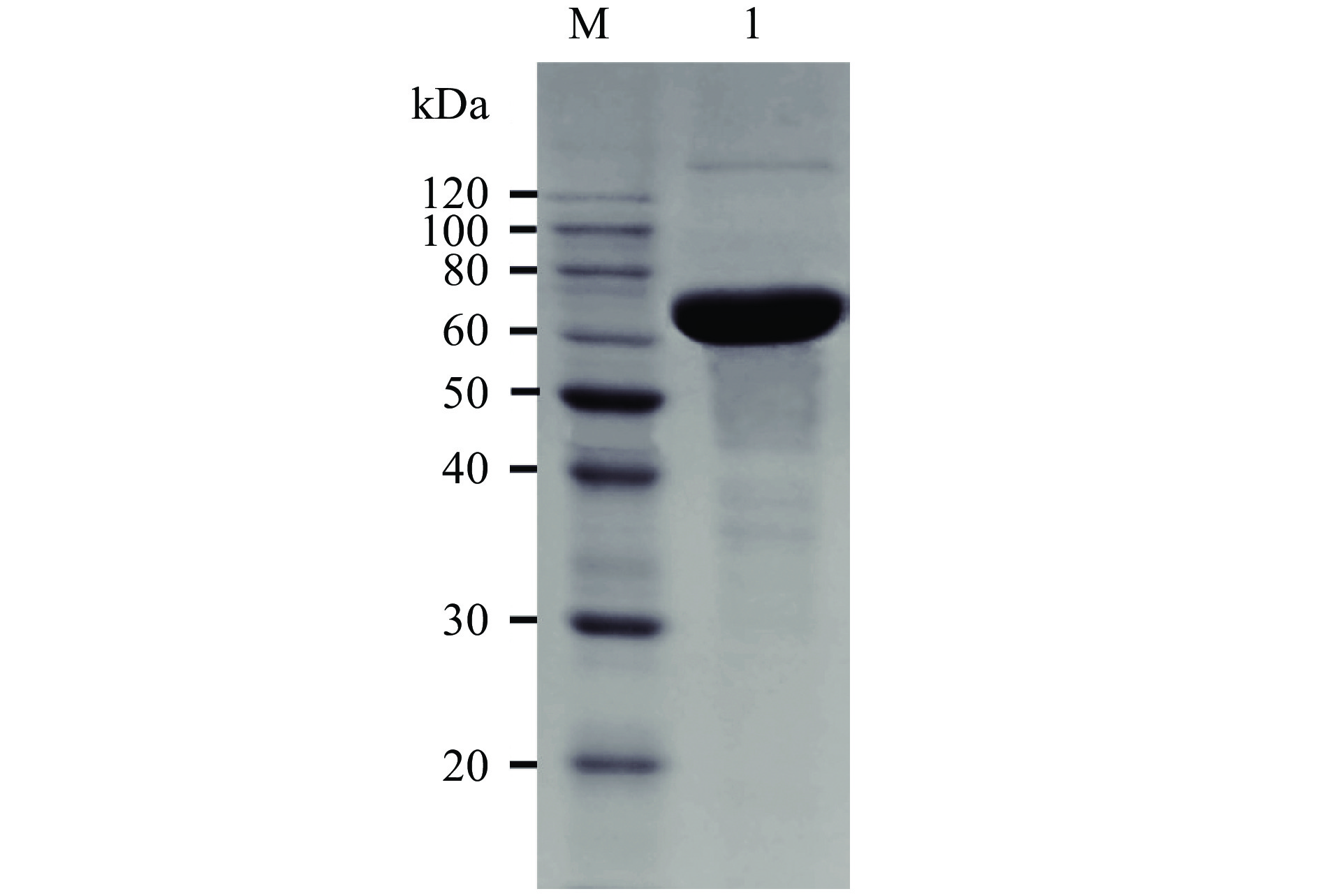
CGTase-T1经纯化和脱盐浓缩后,得到了分子量为75 kDa的单一条带(图1),与大多数CGTase的分子量相符[35],说明得到了纯化的环糊精葡萄糖基转移酶。
2.2 酶活性测定
分别测定了纯化酶的β-环化活性和歧化活性,测得纯化酶的浓度为7.368 mg/mL,经计算得出其酶活与比酶活。如表1所示,β-环化反应的酶活性最高,比酶活可达1169.40 U/mg。大多数的CGTase以环化反应为主活性,与本文结果相同,可能是由于CGTase更喜欢吸附淀粉分子内部的α-1,4链,然后转移新形成的寡聚糖的还原端到自身的非还原端,产生α-1,4环[36]。而且环化反应是CGTase的特征反应,是一种分子内转糖基反应,原理是将直链麦芽低聚糖上非还原末端的O-4或C-4转移到同一直链上还原末端的C-1或O-1上,形成6-8个糖基的环状结构[37]。
表 1 酶反应的环化和歧化反应的活性Table 1. Activity of cyclization and disproportionation reactions of enzymatic reactions酶活活性 酶活性(U) 比酶活(U/mg) β-环化反应 2.62±0.33 1169.40±5.88 歧化反应 1.74±0.28 189.60±0.42 2.3 AA-2G的定性与定量测定
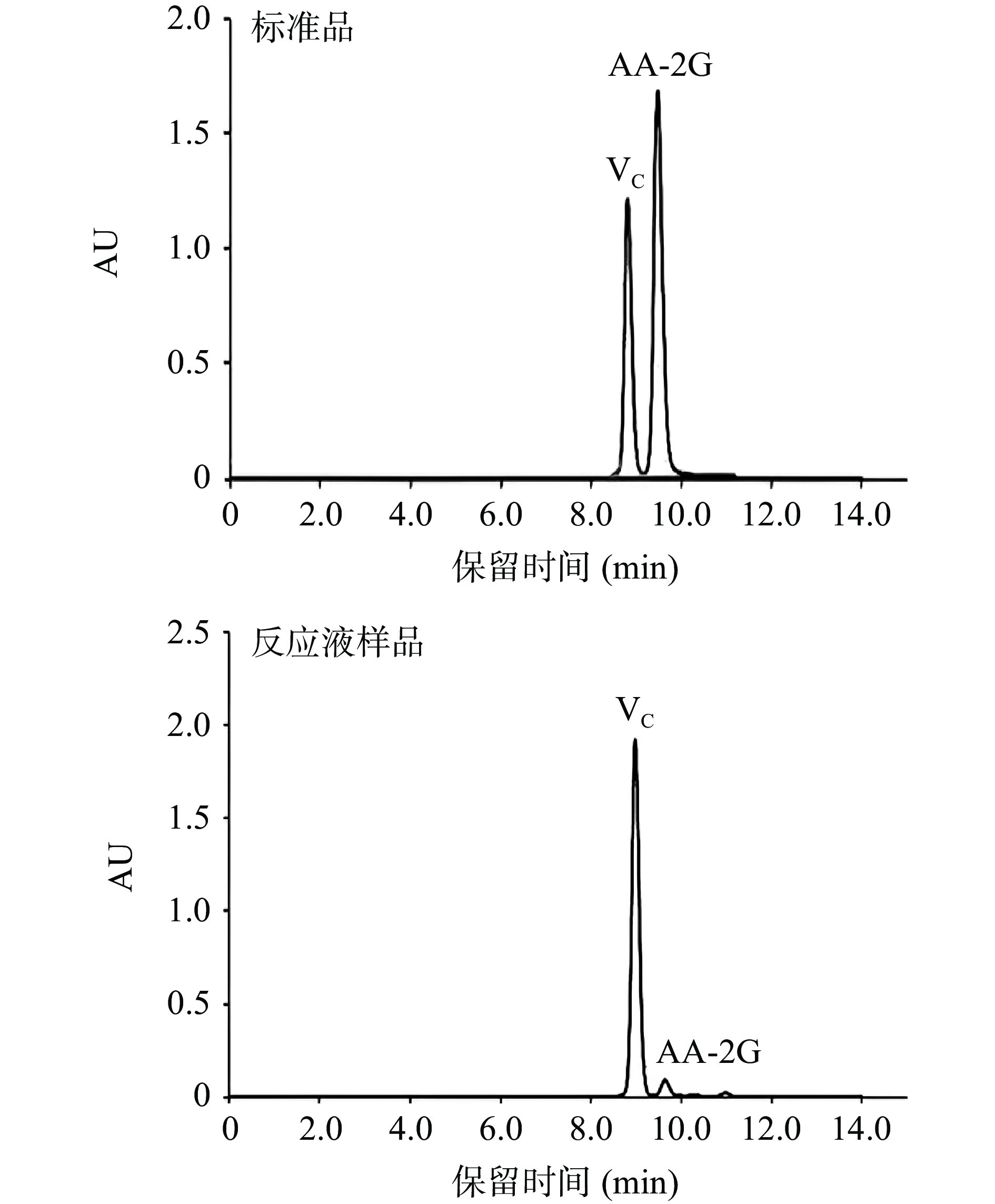
使用HPLC色谱法分析AA-2G的含量,由于VC与AA-2G的结构相似度较高,在238 nm处均有吸光度,由图2所示,反应样品AA-2G和VC的出峰时间分别为9.6 min和8.9 min,与标准品的出峰时间一致,表明CGTase-T1具有合成AA-2G的能力。根据AA-2G标准曲线,计算所得反应样品中AA-2G含量为0.67 g/L,合成产量较低,剩余大量的VC未被消耗,说明糖基受体VC未得到来自糖基供体的糖苷键,未生成大量中间体合成产物。据报道,经反应条件优化后,来源于Bacillus alkalophilus 7-12[38]、Paenibacillus sp.[39]、Bacillus sp. SK 13.002[40]、Bacillus agaradhaerens Y112[41]、Paenibacillus macerans 154[42]、Anaerobranca gottschalkii[43]、Bacillus circulans 251[43]和Thermoanaerobacter sp.[44]的CGTase合成产量分别为0.095、2.98、5.50、10.60、17.50、28.90、35.70和143.00 g/L,与大多用于合成AA-2G的CGTase相比,CGTase-T1的合成产量较低,所以对其反应条件进行优化,探究其合成AA-2G的潜力。
2.4 AA-2G酶法合成条件初探
2.4.1 糖基供体对反应的影响
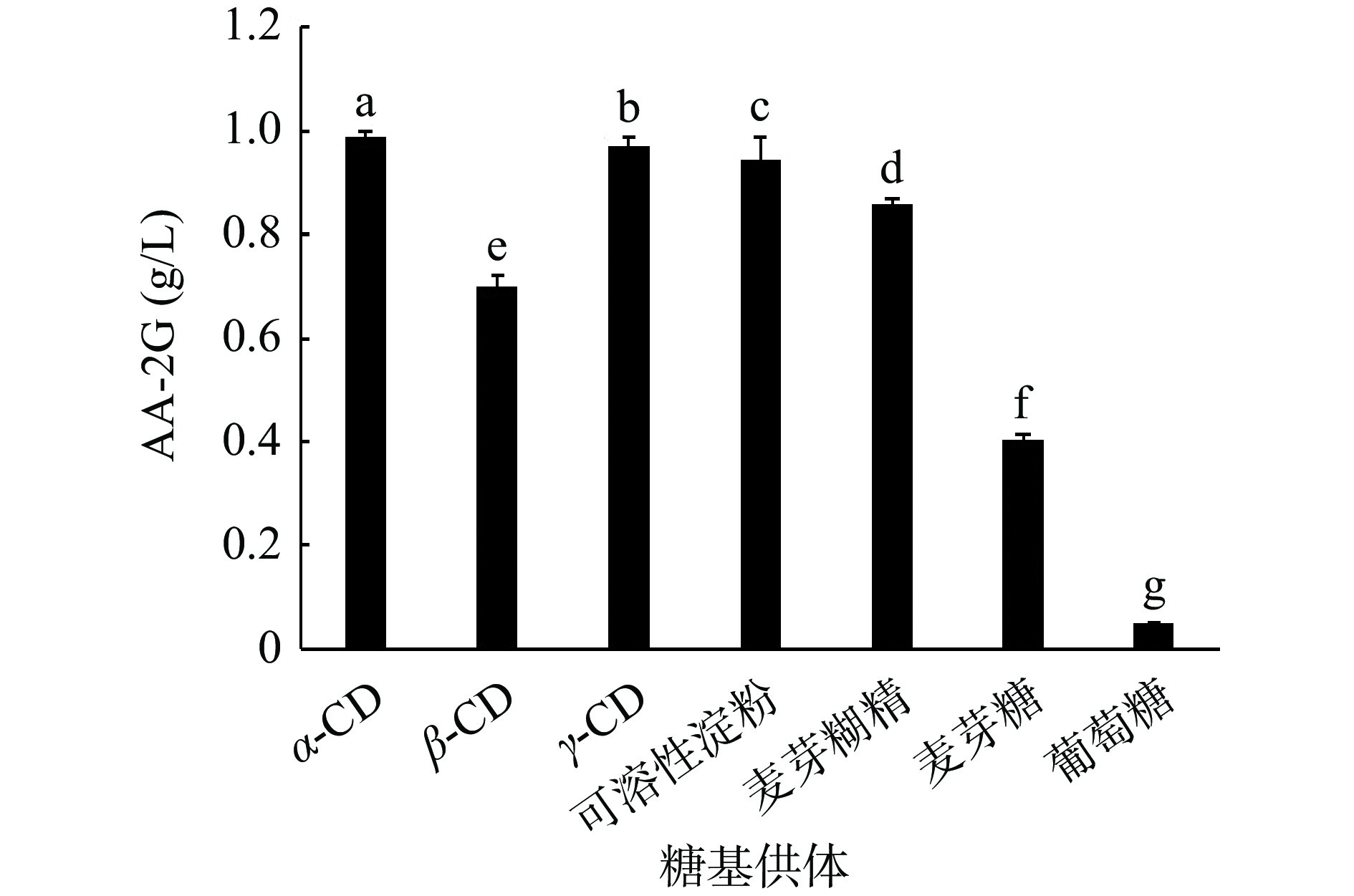
CGTase可与不同糖基供体生成AA-2G,故寻找合适的糖基供体可增加AA-2G的产量。由图3所示,最适糖基供体为α-CD,其次为γ-CD、可溶性淀粉和麦芽糊精。据报道,可用于合成AA-2G的大多数CGTase的最适糖基供体为α-CD,它与CGTase有着较高的专一性,致使其转化率高于其他糖基供体[45],而γ-CD虽然有较高的溶解度和较大的空腔能包接较大的分子[36],但当α-CD和γ-CD用于AA-2G合成时,会导致生产成本过高,不利于AA-2G的大规模工业生产。各组之间均存在显著性差异,根据结果,为降低生产成本,故选用价格便宜的可溶性淀粉和麦芽糊精作为糖基供体,用于后续实验。
2.4.2 底物浓度对反应的影响
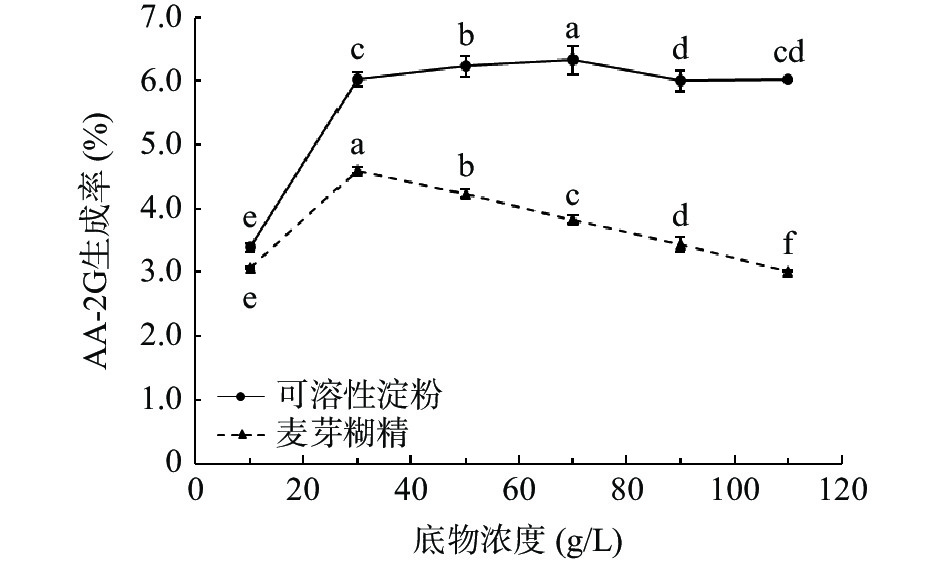
为考察底物浓度对AA-2G合成的影响,选用了不同底物浓度用于AA-2G的合成。由图4所示,当糖基供体为可溶性淀粉时,最适底物浓度为70 g/L,AA-2G的生成率最高;当糖基供体为麦芽糊精时,最适底物浓度为30 g/L,AA-2G的生成率最高。同时,发现在底物浓度过高,如麦芽糊精的浓度超过30 g/L时,会抑制AA-2G的合成,导致AA-2G生成率下降,可能与底物抑制反应机制有关[33]。各组之间只有可溶性淀粉作为糖基供体时,110 g/L组与30、90 g/L两组之间没有显著性差异,根据结果,最终选择可溶性淀粉反应的最适底物浓度为70 g/L,麦芽糊精反应的最适底物浓度为30 g/L。
2.4.3 pH对反应的影响
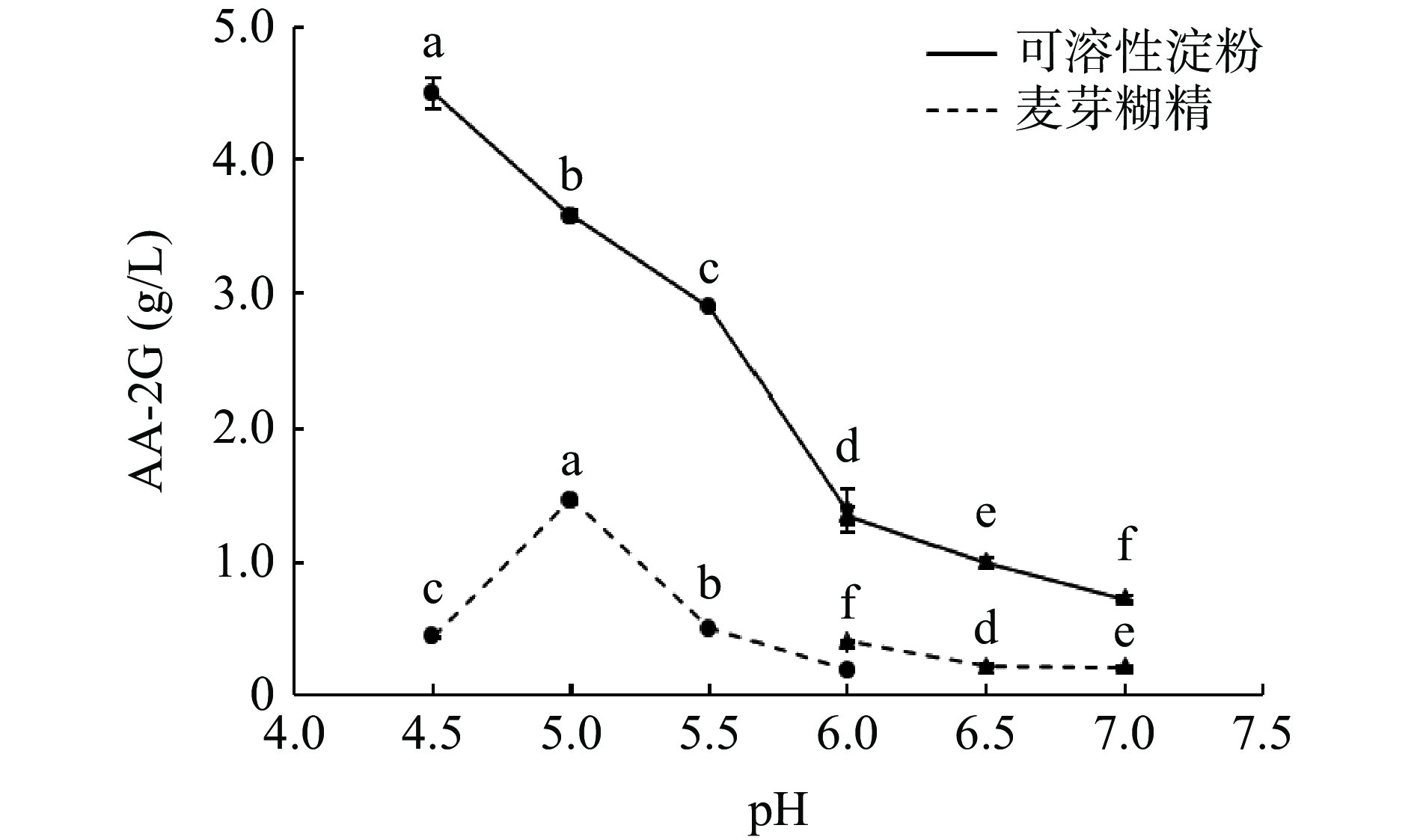
为考察pH对AA-2G合成的影响,选用了不同pH的缓冲液用于AA-2G的合成。由图5所示,当可溶性淀粉为糖基供体时,最适pH为4.5,当pH大于4.5之后,AA-2G的产量近乎直线下降;当麦芽糊精为糖基供体时,最适pH为5.0,当pH大于5.0之后,AA-2G的产量减少到与pH4.5时基本相同。结果表明,该酶是一种酸性CGTase,随着pH的增加,对AA-2G的合成影响增大,与宋凯[34]的研究结果相一致。可能是VC在高pH下不稳定[17],在不同pH条件下底物分子和酶分子的带电状态不同,从而影响酶和底物的结合,影响酶的稳定性[46],导致AA-2G的产量变低。各组之间均存在显著性差异,根据结果,最终选择可溶性淀粉的最适反应pH为4.5,麦芽糊精的最适反应pH为5.0。
2.4.4 温度对反应的影响
为考察温度对AA-2G合成的影响,选用了不同温度用于AA-2G的合成。由图6所示,当可溶性淀粉和麦芽糊精为糖基供体时,最适温度均为37 ℃。当温度低于37 ℃时,AA-2G产量随温度的上升而增加,而当温度高于37 ℃时,AA-2G的产量明显下降。可能是温度过低不利于CGTase的活性反应,而当温度过高时VC会降解[17],AA-2G在高温时也会容易分解[47],均导致AA-2G产量降低。而单丽媛等[48]以可溶性淀粉和VC为底物,其反应最适温度为15 ℃,相比而言,其最适温度较低,可能是由于酶的来源不同,在低温下其活性较高。各组之间均存在显著性差异,根据结果,最终选择可溶性淀粉和麦芽糊精的最适反应温度均为37 ℃。
2.4.5 底物比例对反应的影响
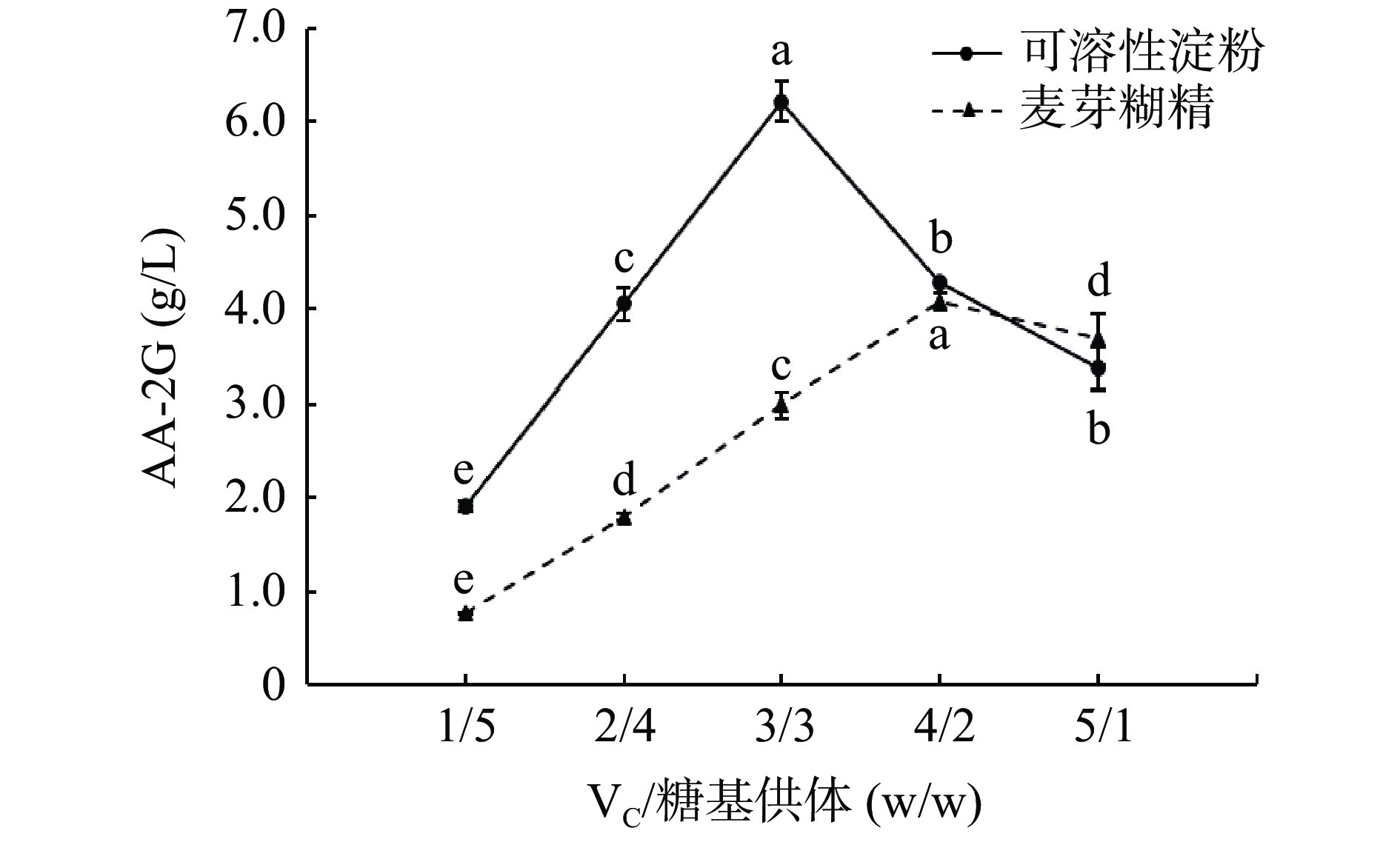
为考察底物比例对AA-2G合成的影响,选用了不同底物比例用于AA-2G的合成。由图7所示,当可溶性淀粉为糖基供体时,最适底物比例为3/3(VC/糖基供体,w/w),为等比比例;当麦芽糊精为糖基供体时,最适底物比例为4/2(VC/糖基供体,w/w),为倍比比例。结果表明,不同的糖基供体用于AA-2G合成时,它们的底物比例会有所差异,可能是因为酶对底物的特异性不同,且底物比例过高或过低都容易造成低含量底物供应不足,而不利于AA-2G的合成,与黄燕等[49]对底物比例优化的结果一致。各组之间均存在显著性差异,根据结果,最终选择可溶性淀粉反应的最适底物比例为3/3,麦芽糊精反应的最适底物比例为4/2。
2.4.6 蛋白浓度对反应的影响
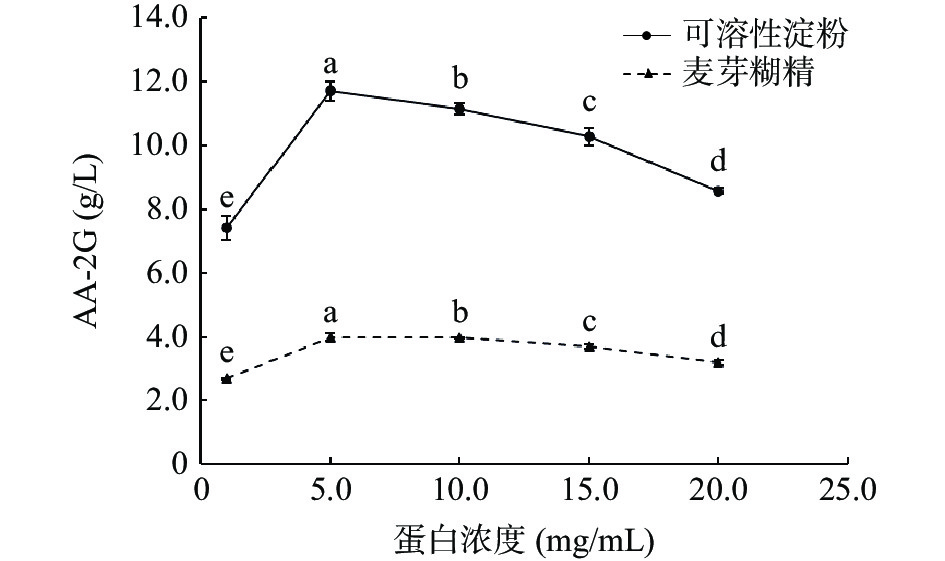
为考察蛋白浓度对AA-2G合成的影响,选用了不同蛋白浓度用于AA-2G的合成。由图8所示,当可溶性淀粉和麦芽糊精为糖基供体时,最适蛋白浓度均为5.0 mg/mL。当蛋白浓度超过5.0 mg/mL时,AA-2G的产量逐渐下降,表明过高的蛋白浓度不利于AA-2G的合成。随着加酶量的增加,AA-2G的产量并没有增加,可能是因为CGTase是一种多功能酶,可以催化4种反应,其中歧化反应和耦合反应可以降解糖基供体产生小分子糖,随着加酶量的增加,这2种反应强度增强,小分子糖增多,而小分子糖的存在会使得该酶的耦合反应加剧,使小分子糖无法与受体结合,从而降低AA-2G的产量[32]。各组之间均存在显著性差异,根据结果,最终选择可溶性淀粉和麦芽糊精反应的最适蛋白浓度均为5.0 mg/mL。
2.4.7 时间对反应的影响
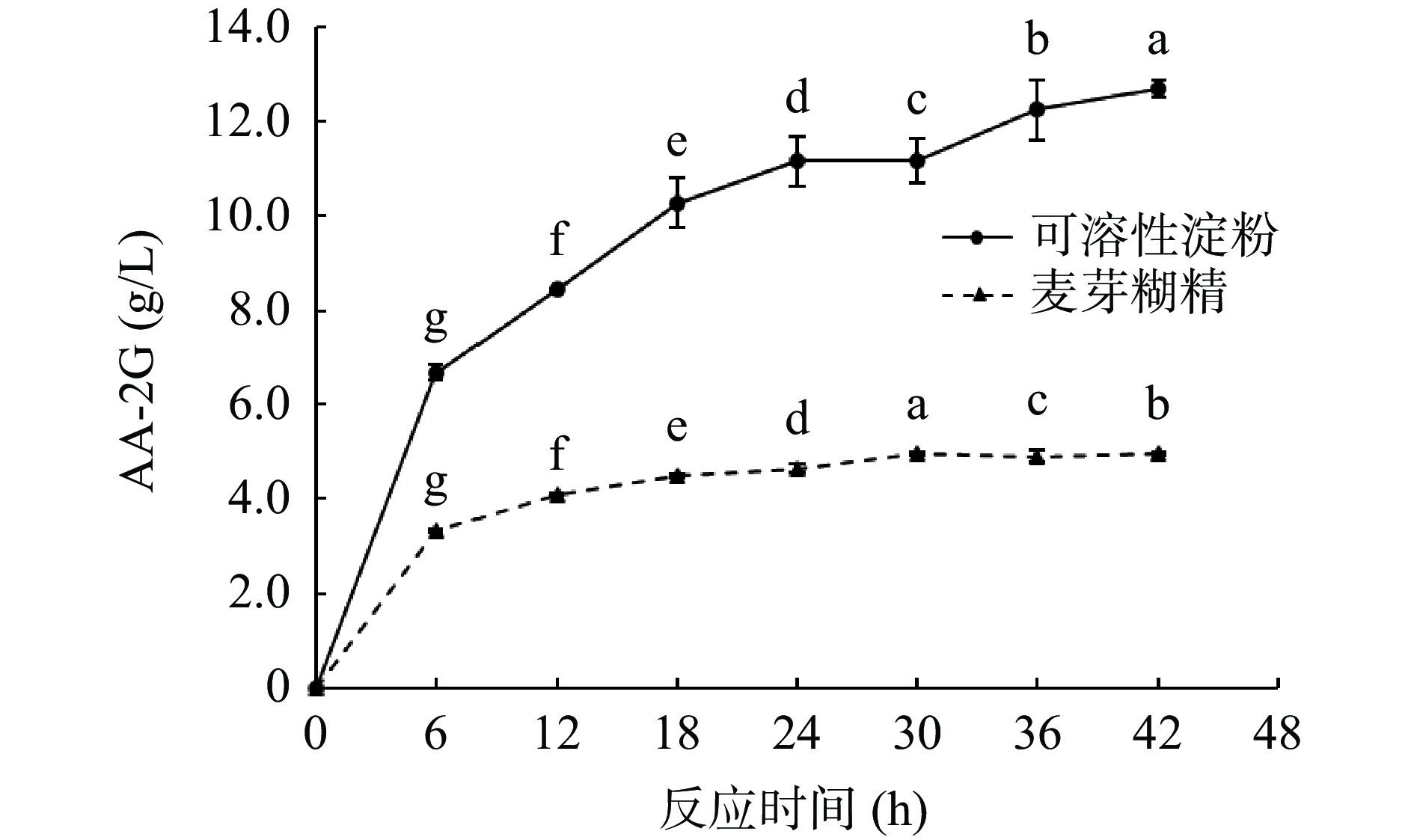
为考察时间对AA-2G合成的影响,选用了不同的反应时间合成AA-2G。由图9所示,AA-2G的产量随着反应时间的增加而逐渐增加。当可溶性淀粉为糖基供体时,反应42 h后AA-2G的产量达到最高(12.68 g/L);当麦芽糊精为糖基供体时,反应30 h后AA-2G的产量达到最高(4.96 g/L),它们分别是未优化反应条件产量的18.93倍和7.40倍。在反应初期处于消耗底物的快速反应期,6 h后开始逐渐变得平缓,反应后期随着底物和酶的消耗反应逐渐停止,可溶性淀粉作为糖基供体时,初始合成速度更快,合成产量更高,可能是由于不同糖基供体对酶的亲和力不同而影响了反应的快慢和合成的产量[45],而且较少的反应时间和较高的产量有利于降低生产成本,提高反应效率。各组之间均存在显著性差异,根据结果,最终选择可溶性淀粉的最适反应时间为42 h,麦芽糊精的最适反应时间为30 h。
2.5 AA-2G的酶法合成动力学分析
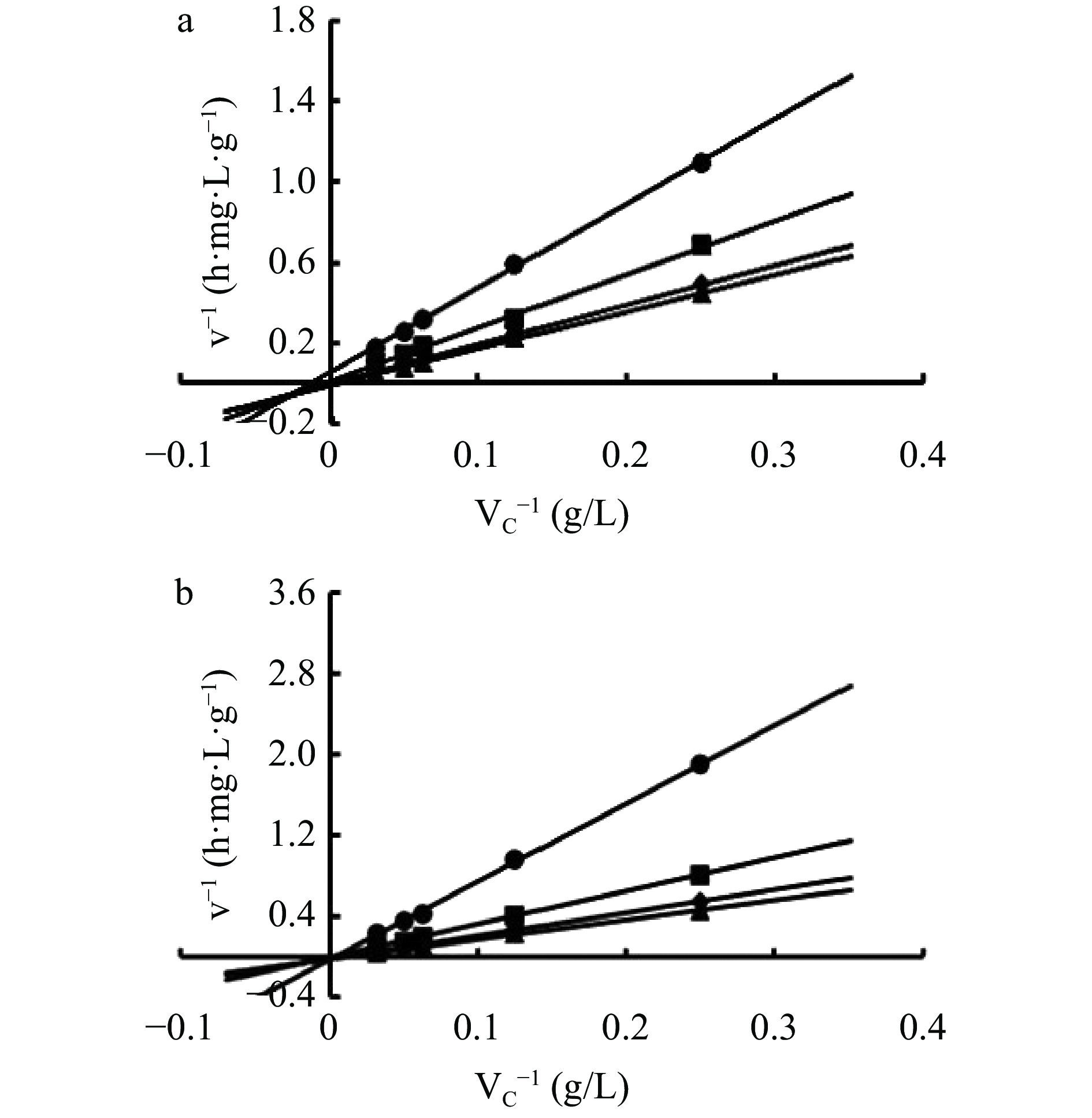
为考察CGTase-T1合成AA-2G的动力学,选用了不同浓度的底物用于AA-2G的合成。经数据拟合后,得到CGTase-T1合成AA-2G反应的Lineweaver-Burk曲线(图10)。线性拟合结果符合底物抑制反应机制,表明底物会对AA-2G的合成产生抑制作用。带入其公式中,得到酶法合成AA-2G的动力学参数,由表2所示,可溶性淀粉的Kcat值高于麦芽糊精,表明可溶性淀粉合成AA-2G反应中的催化效率较高,致使其合成AA-2G的产量高于麦芽糊精。而且可溶性淀粉的KmA高于麦芽糊精,表明在不同糖基供体合成时,酶对不同底物的特异性不同,会影响CGTase-T1与底物的亲和力,可能与底物作用力有关[50],故选用合适的糖基供体有利用AA-2G的产量的增加。
表 2 动力学参数Table 2. Kinetic parameters底物 Vmax(g/L/mg/h) Kcat(1/h) KmA(g/L) Kcat/KmA KmB(g/L) Kcat/KmB KiB(g/L) 麦芽糊精 49.26±0.01 4678.20±0.01 182.78±0.03 25.59±0.01 6.94±0.05 674.48±0.01 26.85±0.02 可溶性淀粉 102.04±0.02 9690.50±0.04 629.47±0.01 15.39±0.01 1.91±0.01 5051.42±0.01 19.56±0.01 注:A:VC;B:糖基供体(麦芽糊精或可溶性淀粉)。 3. 结论
本研究将异源表达的CGTase-T1用于合成AA-2G,经高效液相色谱法分析后,得到含量为0.67 g/L的AA-2G,合成量较少。为增加CGTase-T1合成AA-2G的产量,依次对糖基供体、底物浓度、反应pH、反应温度、底物比例、蛋白浓度和反应时间进行了单因素优化。当糖基供体为可溶性淀粉时,AA-2G最优合成条件是:底物浓度70 g/L,反应pH4.5,反应温度37 ℃,底物比例3/3(VC/糖基供体),蛋白浓度5.0 mg/mL,反应时间42 h;当糖基供体为麦芽糊精时,AA-2G最优合成条件是:底物浓度30 g/L,反应pH5.0,反应温度37 ℃,底物比例4/2,蛋白浓度5.0 mg/mL,反应时间30 h。在最适条件下,AA-2G的最高产量分别为12.68 g/L和4.96 g/L,是未优化反应条件下的18.93倍和7.40倍。同时,对其合成AA-2G的动力学分析进行分析,发现糖基供体可溶性淀粉的催化效率高于麦芽糊精,致使其AA-2G的产量较高,表明此酶对可溶性淀粉的特异性优于麦芽糊精,可溶性淀粉更适合作为此酶的糖基供体合成AA-2G。
CGTase是AA-2G工业生产中主要用酶,但生产成本和转化率限制了大规模AA-2G的工业生产,导致其价格昂贵。而本研究以可溶性淀粉和麦芽糊精作为AA-2G合成的底物,降低了生产成本,可为其AA-2G的工业化生产提供参考。但本研究仍存在蛋白可溶性表达量不足,产物特异性差异大、底物转化率低、合成产量不足等问题,后续可将CGTase-T1进行分子改造,改善酶的稳定性和底物特异性,进一步提高AA-2G的产量。
-
表 1 酶反应的环化和歧化反应的活性
Table 1 Activity of cyclization and disproportionation reactions of enzymatic reactions
酶活活性 酶活性(U) 比酶活(U/mg) β-环化反应 2.62±0.33 1169.40±5.88 歧化反应 1.74±0.28 189.60±0.42 表 2 动力学参数
Table 2 Kinetic parameters
底物 Vmax(g/L/mg/h) Kcat(1/h) KmA(g/L) Kcat/KmA KmB(g/L) Kcat/KmB KiB(g/L) 麦芽糊精 49.26±0.01 4678.20±0.01 182.78±0.03 25.59±0.01 6.94±0.05 674.48±0.01 26.85±0.02 可溶性淀粉 102.04±0.02 9690.50±0.04 629.47±0.01 15.39±0.01 1.91±0.01 5051.42±0.01 19.56±0.01 注:A:VC;B:糖基供体(麦芽糊精或可溶性淀粉)。 -
[1] 苏桂棋, 黄和林, 蒋娜, 等. 维生素C的作用及常见不良反应[J]. 世界最新医学信息文摘,2019,19(8):120−121, 125. [SU G Q, HUANG H L, JIANG N, et al. The role of vitamin C and common adverse reactions[J]. World Latest Medical Information Digest,2019,19(8):120−121, 125. doi: 10.19613/j.cnki.1671-3141.2019.08.056 SU G Q, HUANG H L, JIANG N, et al. The role of vitamin C and common adverse reactions[J]. World Latest Medical Information Digest, 2019, 19(8): 120-121, 125. doi: 10.19613/j.cnki.1671-3141.2019.08.056
[2] NAIDU K A. Vitamin C in human health and disease is still a mystery? An overview[J]. Nutrition Journal,2003,2(1):7. doi: 10.1186/1475-2891-2-7
[3] 饶建华, 韩璐, 张自萍. 维生素C糖苷类衍生物的研究进展[J]. 中国新药杂志,2012,21(7):761−766. [RAO J H, HAN L, ZHANG Z P. Research progress of vitamin C glycoside derivatives[J]. China Journal of New Drugs,2012,21(7):761−766. RAO J H, HAN L, ZHANG Z P. Research progress of vitamin C glycoside derivatives[J]. China Journal of New Drugs, 2012, 21(7): 761-766.
[4] HAN R, LIU L, LI J, et al. Functions, applications and production of 2-O-D-glucopyranosyl-L-ascorbic acid[J]. Applied Microbiology and Biotechnology,2012,95(2):313−320. doi: 10.1007/s00253-012-4150-9
[5] 李标, 唐双焱. 2-O-α-D-吡喃葡萄糖基抗坏血酸研究进展[J]. 广西科学,2017,24(1):48−53. [LI B, TANG S Y. Research progress of 2-O-α-D-glucopyranosyl-ascorbic acid[J]. Guangxi Science,2017,24(1):48−53. LI B, TANG S Y. Research progress of 2-O-α-D-glucopyranosyl-ascorbic acid[J]. Guangxi Science, 2017, 24(1): 48-53.
[6] WAKAMIYA H, SUZUKI E, YAMAMOTO I, et al. Vitamin C activity of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid in guinea pigs[J]. Journal of Nutritional Science & Vitaminology,1992,38(3):235−245.
[7] ZHANG W, HUANG Q, YANG R, et al. 2-O-D-glucopyranosyl-L-ascorbic acid: Properties, production, and potential application as a substitute for L-ascorbic acid[J]. Journal of Functional Foods,2021,82(7):104481.
[8] TOYODAONO Y, MAEDA M, NAKAO M, et al. 2-O-(beta-D-glucopyranosyl) ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit[J]. Journal of Agricultural and Food Chemistry,2004,52(7):2092−2096. doi: 10.1021/jf035445w
[9] YAMAMOTO I, MUTO N, NAGATA E, et al. Formation of a stable L-ascorbic acid alpha-glucoside by mammalian alpha-glucosidase-catalyzed transglucosylation[J]. Biochimica et Biophysica Acta,1990,1035(10):44−50.
[10] TANAKA M. Characterization of Bacillus stearothermophilus cyclodextrin glucanotransferase in ascorbic acid 2-O-alpha-glucoside formation[J]. Biochimica et Biophysica Acta,1991,1078(2):127−132. doi: 10.1016/0167-4838(91)99000-1
[11] LEE S, LIM J, LEE J H, et al. Ascorbic acid 2-glucoside stably promotes the primitiveness of embryonic and mesenchymal stem cells through ten-eleven translocation and cAMP-responsive element-binding protein-1-dependent mechanisms[J]. Antioxidants & Redox Signaling,2004,32(1):35−59.
[12] GUDIMINCHI R K, NIDETZKY P. Walking a fine line with sucrose phosphorylase: efficient single-step biocatalytic production of 1-ascorbic acid 2-glucoside from sucrose[J]. Chembiochem:a European Journal of Chemical Biology,2017,18(14):1387−1390. doi: 10.1002/cbic.201700215
[13] MUKAI K, TSUSAKI K, KUBOTA M, et al. Process for producing 2-O-alpha-D-glucopyranosyl-L-ascorbic acid: Europe, EP20030741243[P]. 2003-07-07.
[14] 黄敏, 裘娟萍. 生物转化法生产2-O-α-D-吡喃型葡萄糖基L-抗坏血酸的研究进展[J]. 工业微生物,2004(2):41−44. [HUANG M, QIU J P. Advances in research of preparing 2-O-α-D-glucopyranosyl-L-ascorbic acid by biological transformation[J]. Industrial Microbiology,2004(2):41−44. doi: 10.3969/j.issn.1001-6678.2004.02.010 HUANG M, QIU J P. Advances in research of preparing 2-O-α-D-glucopyranosyl-L-ascorbic acid by biological transformation[J]. Industrial Microbiology, 2004, (2): 41-44. doi: 10.3969/j.issn.1001-6678.2004.02.010
[15] ZHANG Z, LI J, LIU L, et al. Enzymatic transformation of 2-O-α-D-glucopyranosyl-L-ascorbic acid by α-cyclodextrin glucanotransferase from recombinant Escherichia coli[J]. Biotechnology & Bioprocess Engineering,2011,16(1):107−113.
[16] TAO X, SU L, WU J. Current studies on the enzymatic preparation 2-O-α-D-glucopyranosyl-L-ascorbic acid with cyclodextrin glycosyltransferase[J]. Critical Reviews in Biotechnology,2018,39(2):1−9.
[17] 陶秀梅. Bacillus stearothermophilus NO2环糊精葡萄糖基转移酶的分子改造及制备AA-2G的研究[D]. 无锡: 江南大学, 2020. TAO X M. Molecular modification of Bacillus stearothermophilus NO2 cyclodextrin glucosyltransferase and research on the preparation of AA-2G[D]. Wuxi: Jiangnan University, 2020.
[18] 李晓涵, 郝建华, 郭姣梅, 等. 环糊精葡萄糖基转移酶高效异源表达研究进展[J]. 微生物学通报,2020,47(2):615−622. [LI X H, HAO J H, GUO J M, et al. Advance in high-level heterologous expression of cyclodextrin glucosyltransferase[J]. Bulletin of Microbiology,2020,47(2):615−622. doi: 10.13344/j.microbiol.china.190295 LI X H, HAO J H, GUO J M, et al. Advance in high-level heterologous expression of cyclodextrin glucosyltransferase[J]. Bulletin of Microbiology, 2020, 47(2): 615-622. doi: 10.13344/j.microbiol.china.190295
[19] 余磊, 李骥璇, 王忆茗, 等. 蔗糖磷酸化酶全细胞催化AA-2G的条件优化[J]. 现代生物医学进展,2017,17(14):2601−2605. [YU L, LI J X, WANG Y M, et al. Optimization of whole-cell synthesis of AA-2G by sucrose phosphorylase[J]. Advances in Modern Biomedicine,2017,17(14):2601−2605. doi: 10.13241/j.cnki.pmb.2017.14.001 YU L, LI J X, WANG Y M, et al. Optimization of whole-cell synthesis of AA-2G by sucrose phosphorylase[J]. Advances in Modern Biomedicine, 2017, 17(14): 2601-2605. doi: 10.13241/j.cnki.pmb.2017.14.001
[20] 郭姣梅. 海洋环糊精葡萄糖基转移酶的固定化及其性能研究[D]. 上海: 上海海洋大学, 2020. GUO J M. Study on immobilization of marine cyclodextrin glucosyltransferase and its properties[D]. Shanghai: Shanghai Ocean University, 2020.
[21] 郑丹妮. Bacillus sp. FJAT-44876 γ-环糊精葡萄糖基转移酶的表征及热稳定性提升研究[D]. 无锡: 江南大学, 2020. ZHENG D N. Characterization and thermostability improved of γ-CGTase from Bacillus sp. FJAT-44876[D]. Wuxi: Jiangnan University, 2020.
[22] 王蕾. 环糊精葡萄糖基转移酶的产物特异性分子改造及发酵制备研究[D]. 无锡: 江南大学, 2018. WANG L. Product specificity engineering and fermentation of cyclodextrin glycosyltransferase[D]. Wuxi: Jiangnan University, 2018.
[23] 李晓涵, 郭姣梅, 宋凯等. Bacillus sp. Y112环糊精葡萄糖基转移酶位点R81定点突变提高产物特异性[J]. 食品科学,2021,42(10):133−138. [LI X H, GUO J M, SONG K, et al. Improvement of the product specificity of Bacillus sp. Y112 cyclodextrin glucosyltransferase by site-directed mutagenesis of arginine 81[J]. Food Science,2021,42(10):133−138. doi: 10.7506/spkx1002-6630-20200205-031 LI X H, GUO J M, SONG K, et al. Improvement of the product specificity of Bacillus sp. Y112 cyclodextrin glucosyltransferase by site-directed mutagenesis of arginine 81[J]. Food Science, 2021, 42(10): 133-138. doi: 10.7506/spkx1002-6630-20200205-031
[24] 左方圆. Bacillus stearothermophilus NO2环糊精葡萄糖基转移酶的分子改造及制备α-环糊精的研究[D]. 无锡: 江南大学, 2022. ZUO F Y. Molecular modification of Bacillus stearothermophilus NO2 cyclodextrin glucosyltransferase and preparation of α-cyclodextrin[D]. Wuxi: Jiangnan University, 2022.
[25] 柴宝成. 分子改造环糊精葡萄糖基转移酶合成糖基化染料木素[D]. 无锡: 江南大学, 2021. CHAI B C. Engineering of cyclodextrin glucosyltransferase for the synthesis of glycosylated genistein[D]. Wuxi: Jiangnan University, 2021.
[26] 花敬涵. β-环糊精糖基转移酶的催化机制及理性改造研究[D]. 合肥: 合肥工业大学, 2019. HUA J H. Catalytic mechanism and rational transformation of β-cyclodextrin glycosyltransferase[D]. Hefei: Hefei University of Technology, 2019.
[27] VIOLAINE, GERARD, EMEL, et al. Ascorbic acid derivatives as potential substitutes for ascorbic acid to reduce color degradation of drinks containing ascorbic acid and anthocyanins from natural extracts.[J]. Journal of Agricultural and Food Chemistry,2019,67(43):12061−12071. doi: 10.1021/acs.jafc.9b05049
[28] AGA H, YONEYAMA M, SAKAI S, et al. Synthesis of 2-O-α-D-glucopyranosyl 1-ascorbic acid by cyclomaltodextrin glucanotransferase from Bacillus stearothermophilus[J]. Journal of the Agricultural Chemical Society of Japan,1991,55(7):1751−17556.
[29] LIU Z, WU G, WU H. Molecular cloning, and optimized production and characterization of recombinant cyclodextrin glucanotransferase from Bacillus sp. T1[J]. 3 Biotech,2022,12(3):58. doi: 10.1007/s13205-022-03111-8
[30] BRADFORD M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Analytical biochemistry,1976,72:248−254. doi: 10.1016/0003-2697(76)90527-3
[31] TESFAI B T, WU D, CHEN S, et al. Effect of organic solvents on the yield and specificity of cyclodextrins by recombinant cyclodextrin glucanotransferase (CGTase) from Anaerobranca gottschalkii[J]. Journal of Inclusion Phenomena & Macrocyclic Chemistry,2013,77(1-4):147−153.
[32] VANDERVEEN B A, VANALEBEEK G J, UITDEHAAG J C, et al. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms[J]. European Journal of Biochemistry,2001,267(3):658−665.
[33] 韩瑞枝. 环糊精葡萄糖基转移酶的分子改造及合成糖基化L-抗坏血酸[D]. 无锡: 江南大学, 2013. HAN R Z. Molecular modification of cyclodextrin glucosyltransferase and synthesis of glycosylated L-ascorbic acid[D]. Wuxi: Jiangnan University, 2013.
[34] 宋凯. 海洋环糊精葡萄糖基转移酶的表达、酶学性质及AA-2G制备的研究[D]. 上海: 上海海洋大学, 2021. SONG K. Heterogeneous expression, enzyme properties and preparation of AA-2G of cyclodextrin glycosyltransferase[D]. Shanghai: Shanghai Ocean University, 2021.
[35] 张国超, 王岁楼, 吴晓宗. 环糊精葡萄糖转移酶的研究进展[J]. 生物学杂志,2008(2):55−58. [ZHANG G C, WANG S L, WU X Z. Research progress on cyclodextrin glucotransferase[J]. Journal of Biology,2008(2):55−58. doi: 10.3969/j.issn.2095-1736.2008.02.016 ZHANG G C, WANG S L, WU X Z. Research progress on cyclodextrin glucotransferase[J]. Journal of Biology, 2008(2): 55-58. doi: 10.3969/j.issn.2095-1736.2008.02.016
[36] 王月明, 孙万刚, 张斌等. 环糊精及环糊精葡萄糖基转移酶特性研究[J]. 安徽农业科学,2007(27):8430−8431, 8434. [WANG Y M, SUN W G, ZHANG B, et al. Study on the characteristics of cyclodextrin and cyclodextrin glucosyltransferase[J]. Anhui Agricultural Science,2007(27):8430−8431, 8434. doi: 10.3969/j.issn.0517-6611.2007.27.004 WANG Y M, SUN W G, ZHANG B, et al. Study on the characteristics of cyclodextrin and cyclodextrin glucosyltransferase[J]. Anhui Agricultural Science, 2007(27): 8430-8431+8434. doi: 10.3969/j.issn.0517-6611.2007.27.004
[37] 张国宁, 冯婧娴, 杨颖博等. 环糊精葡萄糖基转移酶在天然产物糖基化修饰中的应用[J]. 生物技术通报,2022,38(3):246−255. [ZHANG G N, FENG J X, YANG Y B, et al. Application of cyclodextrin glucosyltransferase in the glycosylation modification of natural products[J]. Biotechnology Bulletin,2022,38(3):246−255. doi: 10.13560/j.cnki.biotech.bull.1985.2021-0642 ZHANG G N, FENG J X, YANG Y B, et al. Application of cyclodextrin glucosyltransferase in the glycosylation modification of natural products[J]. Biotechnology Bulletin, 2022, 38(03): 246-255. doi: 10.13560/j.cnki.biotech.bull.1985.2021-0642
[38] 曹新志, 刘芳, 明红梅等. 酶法合成葡萄糖基-L-抗坏血酸的产酶条件及转化条件优化[J]. 农产品加工(学刊),2008(1):19−22, 28. [CAO X Z, LIU F, MING H M, et al. Optimization of enzyme production and transformation conditions for enzymatic synthesis of glucosyl-L-ascorbic acid[J]. Agricultural Products Processing (Journal),2008(1):19−22, 28. CAO X Z, LIU F, MING H M, et al. Optimization of enzyme production and transformation conditions for enzymatic synthesis of glucosyl-L-ascorbic acid [J]. Agricultural Products Processing (Journal), 2008(1): 19-22, 28.
[39] HONG K J, KYUNG M B, SUNG K K. Production of 2-O-α-D-glucopyranosyl-L-ascorbic acid using cyclodextrin glucanotransferase from Paenibacillus sp.[J]. Biotechnology Letters,2001,23(21):1793−1797. doi: 10.1023/A:1012413301061
[40] EIBAID A, MIAO M, BASHARI M, et al. Biosynthesis of 2-O-α-D-glucopyranosyl-L-ascorbic acid from maltodextrin catalyzed by cyclodextrin glucanotransferase from Bacillus sp. SK13.002[J]. Journal of Food and Nutrition Research,2014,2(4):193−197. doi: 10.12691/jfnr-2-4-10
[41] 黄立萍, 郝建华, 王伟, 等. 2-氧-α-D-葡萄糖基-L-抗坏血酸酶法合成工艺优化[J]. 食品与机械,2018,34(11):7. [HUANG L P, HAO J H, WANG W, et al. Optimization of enzymatic synthesis of 2-O-α-D-glucosyl-L-ascorbate acid[J]. Food and Machinery,2018,34(11):7. HUANG L P, HAO J H, WANG W, et al. Optimization of enzymatic synthesis of 2-O-α-D-glucosyl-L-ascorbate acid[J]. Food and Machinery, 2018, 34(11): 7.
[42] 邢琳, 张秀华, 钊倩倩, 等. α-环糊精葡萄糖基转移酶的高效表达及酶法制备AA-2G条件优化[J]. 中国生化药物杂志,2016,36(11):5−8. [XING L, ZHANG X H, ZHAO Q Q, et al. High-efficiency expression of α-cyclodextrin glucosyltransferase and optimization of conditions for enzymatic preparation of AA-2G[J]. China Biochemical Medicine,2016,36(11):5−8. XING L, ZHANG X H, ZHAO Q Q, et al. High-efficiency expression of α-cyclodextrin glucosyltransferase and optimization of conditions for enzymatic preparation of AA-2G[J]. China Biochemical Medicine, 2016, 36(11): 5-8 .
[43] CHEN S, XIONG Y, SU L, et al. Position 228 in Paenibacillus macerans cyclodextrin glycosyltransferase is critical for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis[J]. Journal of Biotechnology, 2017, 247.
[44] GUDIMINCHI R K, TOWNS A, VARALWAR S, et al. Enhanced synthesis of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid from alpha-cyclodextrin by a highly disproportionating CGTase[J]. Acs Catalysis,2016,6(3):1606−1615. doi: 10.1021/acscatal.5b02108
[45] 许乔艳, 韩瑞枝, 李江华等. 亚位点+1处突变提高软化类芽胞杆菌环糊精糖基转移酶底物麦芽糊精特异性[J]. 生物工程学报,2014,30(1):98−108. [XU Q Y, HAN R Z, LI J H, et al. Mutation at subsite+1 improves the specificity of maltodextrin as a substrate of cyclodextrin glycosyltransferase from Paenibacillus softening[J]. Chinese Journal of Biological Engineering,2014,30(1):98−108. doi: 10.13345/j.cjb.130401 XU Q Y, HAN R Z, LI J H, et al. Mutation at subsite+1 improves the specificity of maltodextrin as a substrate of cyclodextrin glycosyltransferase from Paenibacillus softening[J]. Chinese Journal of Biological Engineering, 2014, 30(1): 98-108. doi: 10.13345/j.cjb.130401
[46] 张兴荣, 李峰, 贺连智等. 高产β-环糊精葡萄糖基转移酶菌株的筛选、产酶条件优化及酶学性质研究[J]. 中国酿造,2020,39(11):85−91. [ZHANG X R, LI F, HE L Z, et al. Screening and enzyme production condition optimization of high-yield β-CGTase strain and enzymatic property[J]. China Brewing,2020,39(11):85−91. doi: 10.11882/j.issn.0254-5071.2020.11.017 ZHANG X R, LI F, HE L Z, et al. Screening and enzyme production condition optimization of high-yield β-CGTase strain and enzymatic property[J]. China Brewing, 2020, 39 (11): 85-91. doi: 10.11882/j.issn.0254-5071.2020.11.017
[47] 丛远华, 朱沁, 冯春波. 高含量烟酰胺与AA-2G复配体系的水溶液的稳定性研究[J]. 化学与生物工程,2019,36(11):52−57, 63. [CONG Y H, ZHU Q, FENG C B. Stability of aqueous solution of high content nicotinamide and AA2G mixed system[J]. Chemical and Biological Engineering,2019,36(11):52−57, 63. CONG Y H, ZHU Q, FENG C B. Stability of aqueous solution of high content nicotinamide and AA2G mixed system[J] Chemical and Biological Engineering, 2019, 36 (11): 52-57, 63.
[48] 单丽媛, 刘龙, 李江华等. 利用环糊精糖基转移酶转化合成AA-2G的研究[J]. 食品与生物技术学报,2018,37(1):27−32. [SHAN L Y, LIU L, LI J H, et al. Enzyme synthesis of AA-2G by cyclodextrin glycosyltransferase[J]. Journal of Food Science and Biotechnology,2018,37(1):27−32. doi: 10.3969/j.issn.1673-1689.2018.01.005 SHAN L Y, LIU L, LI J H, et al. Enzyme synthesis of AA-2G by cyclodextrin glycosyltransferase[J]. Journal of Food Science and Biotechnology, 2018, 37(1): 27-32. doi: 10.3969/j.issn.1673-1689.2018.01.005
[49] 黄燕, 杨玉路, 夏伟等. 重组β-环糊精葡萄糖基转移酶生产偶合糖的工艺优化[J]. 生物工程学报,2021,37(4):1415−1424. [HUANG Y, YANG Y L, XIA W, et al. Optimization of maltooligosyl fructofuranosidesproduction by recombinant β-cyclodextrin glycosyltransferase[J]. Chinese Journal Biotechnology,2021,37(4):1415−1424. HUANG Y, YANG Y L, XIA W, et al. Optimization of maltooligosyl fructofuranosidesproduction by recombinant β-cyclodextrin glycosyltransferase[J]. Chinese Journal Biotechnology, 2021, 37(4): 1415-1424.
[50] 柴宝成, 姜钰琳, 倪晔等. 环糊精葡萄糖基转移酶182位点定点改造催化合成糖基化染料木素[J]. 生物工程学报,2022,38(2):749−759. [CHAI B C, JIANG Y L, NI Y, et al. Engineering the 182 site of cyclodextrin glucosyltransferase for glycosylated genistein synthesis[J]. Chinese Journal of Biological Engineering,2022,38(2):749−759. CHAI B C, JIANG Y L, NI Y, et al. Engineering the 182 site of cyclodextrin glucosyltransferase for glycosylated genistein synthesis[J] Chinese Journal of Biological Engineering, 2022, 38 (2): 749-759
-
期刊类型引用(12)
1. 向敏,韩小苗,吴苏喜,李万元,彭邵锋. 油茶籽淀粉的结构与体外模拟消化特性研究. 中国油脂. 2025(01): 84-88 .  百度学术
百度学术
2. 张楚佳,贾健辉,高嫚,王泽冉,刘颖,窦博鑫,张娜. 3种物理方法制备抗性粳米淀粉的结构与物化特性. 中国食品学报. 2025(01): 193-207 .  百度学术
百度学术
3. 高雪丽,赵丹,李光辉,王永辉,何胜华,黄继红,张贺晴,郭卫芸. 添加抗性淀粉对红薯粉条理化性能及结构的影响. 食品工业科技. 2024(14): 114-120 .  本站查看
本站查看
4. 谢水琪,张晓桐,靳奇文,刘利军,孟祥晨. 乳酸菌发酵剂对杂粮面团及馒头品质的影响. 食品科学. 2023(02): 189-194 .  百度学术
百度学术
5. 付梓平,范昱,赖弟利,张凯旋,朱剑锋,李基光,周美亮,王俊珍. 脱支和反复湿热处理对苦荞抗性淀粉含量和理化特性的影响. 作物杂志. 2023(01): 52-57 .  百度学术
百度学术
6. 姬小惠,张炜晨,周伟,朱玉莲,刘洋金,杜双奎. 蕨根抗性淀粉微波法制备条件的优化. 食品研究与开发. 2023(07): 109-114 .  百度学术
百度学术
7. 李佳璇,谢丹,王海燕,阮婧华,唐东昕,范东生,辛加敏,查鑫. 酶法纯化何首乌抗性淀粉及其对大肠杆菌增殖效力的影响. 食品研究与开发. 2023(08): 9-16 .  百度学术
百度学术
8. 李翠茹,周康,彭买姣,袁嘉丽,吴月滢,谭周进. 天麻饼干的研制. 保鲜与加工. 2023(07): 50-56 .  百度学术
百度学术
9. 张雅琦,董莹,阮长青,张东杰,李志江. 高抗性淀粉奶白花芸豆的压热法制备工艺研究. 食品工业科技. 2023(18): 1-9 .  本站查看
本站查看
10. 孙心怡,龚盛祥,宗爱珍,徐同成,楚兴刚,林冬梅. 低升糖黑小麦粗粮馒头配方优化及品质分析. 粮食与油脂. 2023(10): 96-100+106 .  百度学术
百度学术
11. 刘静雪,高婷婷,李凤林,田兰英,张国旗. 挤出处理对淀粉性质的影响研究进展. 中国果菜. 2022(08): 30-33+55 .  百度学术
百度学术
12. 柴子淇,徐恩波,郑瑜雪,孔祥礼,孙崇德,刘东红,叶兴乾,田金虎. 压热处理对不同晶型淀粉结构及消化特性的影响. 食品与生物技术学报. 2022(11): 64-72 .  百度学术
百度学术
其他类型引用(8)





 下载:
下载:

 下载:
下载:



