Effects of Different Lengths Sugar Chains on the Structure and Allergenicity of Allergic Epitopes 13KDLKGYGGVSLPEW26 in α-Lactalbumin
-
摘要: α-乳白蛋白的过敏表位是影响其致敏性强弱的分子基础,本文研究不同长度的糖链对其过敏表位结构和致敏性的影响。先合成α-乳白蛋白的IgE线性过敏表位(13KDLKGYGGVSLPEW26,13K-W26),选用葡萄糖、麦芽糖、麦芽三糖、麦芽五糖对其进行糖基化修饰,采用光谱、色谱分析其结构的变化;运用ELISA和细胞实验等方法来评价其IgE结合能力和KU812细胞释放组胺、白介素-4和白介素-6的能力。结果表明:不同长度的糖链与13K-W26发生糖基化反应后,降低其游离氨基含量,改变其荧光强度和紫外吸收强度,同时降低其IgE结合能力和KU812细胞中组胺、白介素-4和白介素-6的释放能力。因此,不同长度的糖链能改变13K-W26的结构,降低其致敏性,顺序为麦芽五糖>麦芽三糖>麦芽糖>葡萄糖。糖链可遮蔽α-乳白蛋白的线性表位13K-W26,糖链越长,遮蔽作用越强,致敏性消减效果越好。Abstract: The allergic epitopes of α-lactalbumin are the molecular basis for allergenicity. In this paper, the effects of different lengths sugar chains on the structure and allergenicity of allergic epitopes in α-lactalbumin were investigated. The IgE linear epitopes of 13KDLKGYGGVSLPEW26 (13K-W26) were synthesized and glycated with glucose, maltose, maltotriose and maltopentaose. Then the structural changes were analyzed by spectroscopy and chromatography. Cellular assays and ELISA were used to evaluate the histamine, interleukin-4 and interleukin-6 release ability of KU812 cells and the IgE binding ability. The results showed that 13K-W26 was glycated by different lengths sugar chains significantly decreased the free amino content and changed the fluorescence intensity and UV absorption intensity, and decreased the IgE binding ability and the release ability of histamine, interleukin-4 and interleukin-6 in KU812 cells. Therefore, different lengths of sugar chains can change the structure of 13K-W26, and reduce the allergenicity. The order was maltopentaose>maltotriose>maltose>glucose. In conclusion, the sugar chains could mask the linear epitopes 13K-W26 of α-lactalbumin, the sugar chains with longer length had stronger masking effects on epitopes, the better the effect of reducing the allergenicity.
-
Keywords:
- α-lactalbumin /
- IgE linear epitopes /
- glycation /
- allergenicity
-
牛乳中α-乳白蛋白(α-Lactalbumin, α-La)含有人体必需氨基酸,可在人乳短缺时作为替代补充婴幼儿营养,但其会引发较为严重的过敏反应,严重危害人体的安全,从而限制了其作为营养补给的使用[1-3]。α-La是一种球形蛋白,分子量约为14.4 kDa,主要有123个氨基酸组成,在其分子表面有着许多的过敏表位[4],包含线性表位和构象表位,其中IgE线性过敏表位有AA1-16、AA13-26、AA47-58、AA93-102等,13KDLKGYGGVSLPEW26作为α-乳白蛋白中的线性表位之一,与该蛋白中其他线性表位相比具有较强的IgE结合能力,能够被多数过敏患者的IgE抗体所识别[5-6]。且该线性表位上存在K13和K16两个糖基化位点[7],以该表位为例来研究糖链长度对线性过敏表位结构和致敏性的影响是一个较好的选择。
如何掩蔽或破坏这些过敏表位从而降低α-La致敏性是当前研究的热点和难点。热处理、超声处理、辐射技术及化学修饰等方式会破坏α-La的结构,从而降低其致敏性[8-9]。糖基化反应可用来掩蔽或破坏α-La的线性和构象表位,是目前较为常用的一种脱敏手段[10]。范金波等[11]发现α-La被不同长度的糖链修饰后,降低其抗原性,糖链的长度与抗原性的降低成正相关;卜单等[12]研究发现超声处理的α-La与不同还原糖进行糖基化反应后能够影响蛋白的结构和抗氧化活性;Zhang等[13]研究表明不同分子尺寸的糖与虾原肌球蛋白糖基化反应后可不同程度地降低其致敏性;芦晶等[14]用壳聚糖修饰乳清蛋白后,能显著改善其功能性质。陈文美等[15]研究发现通过半乳糖和焦磷酸钠先后对小清蛋白进行共价修饰,能够显著降低其致敏性。王亚婷[16]研究发现不同的二糖与卵清蛋白进行糖基化反应后,能够不同程度地改变其致敏性,且降低致敏性的程度与二糖的种类有关。这些研究表明不同的糖与蛋白质共价相互作用,能够改变蛋白质结构和功能特性,但是采用不同长度的糖链如麦芽糖、麦芽三糖和麦芽五糖这些以葡萄糖为单体的糖链对α-La分子中的过敏表位结构和致敏性的影响鲜有报道。
因此,本论文以α-La的IgE线性过敏表位13KDLKGYGGVSLPEW26为研究对象,选用葡萄糖、麦芽糖、麦芽三糖和麦芽五糖对其进行糖基化修饰,采用光谱、色谱等技术研究糖基化修饰前后13K-W26结构的变化;运用酶联免疫吸附试验(enzyme linked immunosorbent assay,ELISA)和KU812细胞实验研究糖基化修饰前后13K-W26致敏性的变化,对过敏蛋白线性表位结构和致敏性的研究对消减整体蛋白质的致敏性有着重要意义。
1. 材料与方法
1.1 材料和仪器
肽段13KDLKGYGGVSLPEW26(13K-W26) 购自生工生物工程上海股份有限公司;葡萄糖、麦芽糖 北京索莱宝科技有限公司;麦芽三糖、麦芽五糖 上海源叶生物科技有限公司;山羊抗人IgE-辣根过氧化物酶 美国Sigma公司;牛奶过敏患者血清(相关信息见表1) 美国Plasma Lab International公司;ELISA试剂盒 深圳欣博盛生物科技有限公司;其余常用试剂均为分析纯。
表 1 牛乳过敏患者信息Table 1. Information of milk allergic patients血清编号 IgE水平 年龄 性别 临床症状 PL 21902 16.4 kU·L−1 34 男 过敏性鼻炎、食物过敏 PL 27091 4.38 kU·L−1 29 女 过敏性鼻炎 PL 26655 11.6 kU·L−1 35 男 过敏性鼻炎 Synergy H1型酶标仪 美国伯腾仪器有限公司;F-7000型荧光光谱仪、U-2910型紫外可见光分光光度计 日本Hitachi公司;Nicolet 6700型傅里叶变换红外光谱仪 美国热电(尼高利)公司。
1.2 实验方法
1.2.1 样品制备
参照Liu等[17]方法,制备1.0 mg/mL的13K-W26溶液,称取等质量的葡萄糖、麦芽糖、麦芽三糖、麦芽五糖,分别溶于5 mL13K-W26溶液中,混均,冻干后,置于55 ℃和65%相对湿度的培养箱中糖基化反应2 h,将13K-W26经葡萄糖(Glucose, G)、麦芽糖(Maltose, M)、麦芽三糖(Maltotriose, MTS)和麦芽五糖(Maltopentaose, MPS)糖基化后的样品分别命名为13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS。
1.2.2 褐变度测定
参照Kim等[17-18]的方法并稍加修改,用酶标仪分别在294和420 nm处测定13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS、13K-W26-MPS(200 μL、1.0 mg/mL)样品的吸光度。
1.2.3 游离氨基测定
参照刘俊[19]的方法,用邻苯二甲醛法测定1 mg/mL 13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS中的游离氨基含量。
1.2.4 内源荧光及紫外吸收强度测定
参照张露等[20]的方法稍加修改,设定荧光光谱仪激发波长280 nm,扫描发射波长范围300~500 nm,测定13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的荧光强度。
参照刘俊等[21]的方法进行修改,采用紫外可见光光度计测定13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS、13K-W26-MPS的紫外吸收强度。
1.2.5 同步荧光光谱分析
采用荧光光谱仪测定13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的同步荧光强度,测定条件设定为:每种样品测定分别在Δλ=15 nm和Δλ=60 nm下各测一次。Δλ=15 nm时的激发波长为280 nm,发射波长范围为265~340 nm;Δλ=60 nm时的激发波长为310 nm,发射波长范围为250~340 nm。激发波长和发射波长的狭缝宽度都为5 nm。
1.2.6 红外光谱测定
采用傅里叶红外压片法测定13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的红外光谱,扫描范围为4000~400 cm–1。
1.2.7 IgE结合能力测定
参照朱一丹等[22]的方法且稍作调整。采用ELISA法来测定13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的IgE结合能力,牛乳过敏患者P1、P2和P3血清F77特异性IgE水平分别为16.4 、4.38 和11.6 kU·L−1。
1.2.8 人嗜碱性粒细胞(KU812)细胞脱颗粒实验
参考Appel等[23]报道的方法,培养KU812细胞,当细胞数量达到1×106个/mL时,将细胞按照每孔1 mL的量接种于24孔板中培养24 h,然后用牛奶过敏患者血清被动激活24 h,加入50 μg/孔13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS样品刺激4 h。采用ELISA法分析KU812细胞组胺(histamine,HIS)、白介素-4(interleukin-4,IL-4)及白介素-6(interleukin-6,IL-6)的释放情况,并参照生产商的说明进行分析。
1.3 数据处理
采用SPSS 24.0软件对于实验数据进行单因素方差分析,显著水平为P<0.05,所有数据作图均采用Orgin 2019软件进行作图,所有实验均重复三次。
2. 结果与分析
2.1 褐变度分析
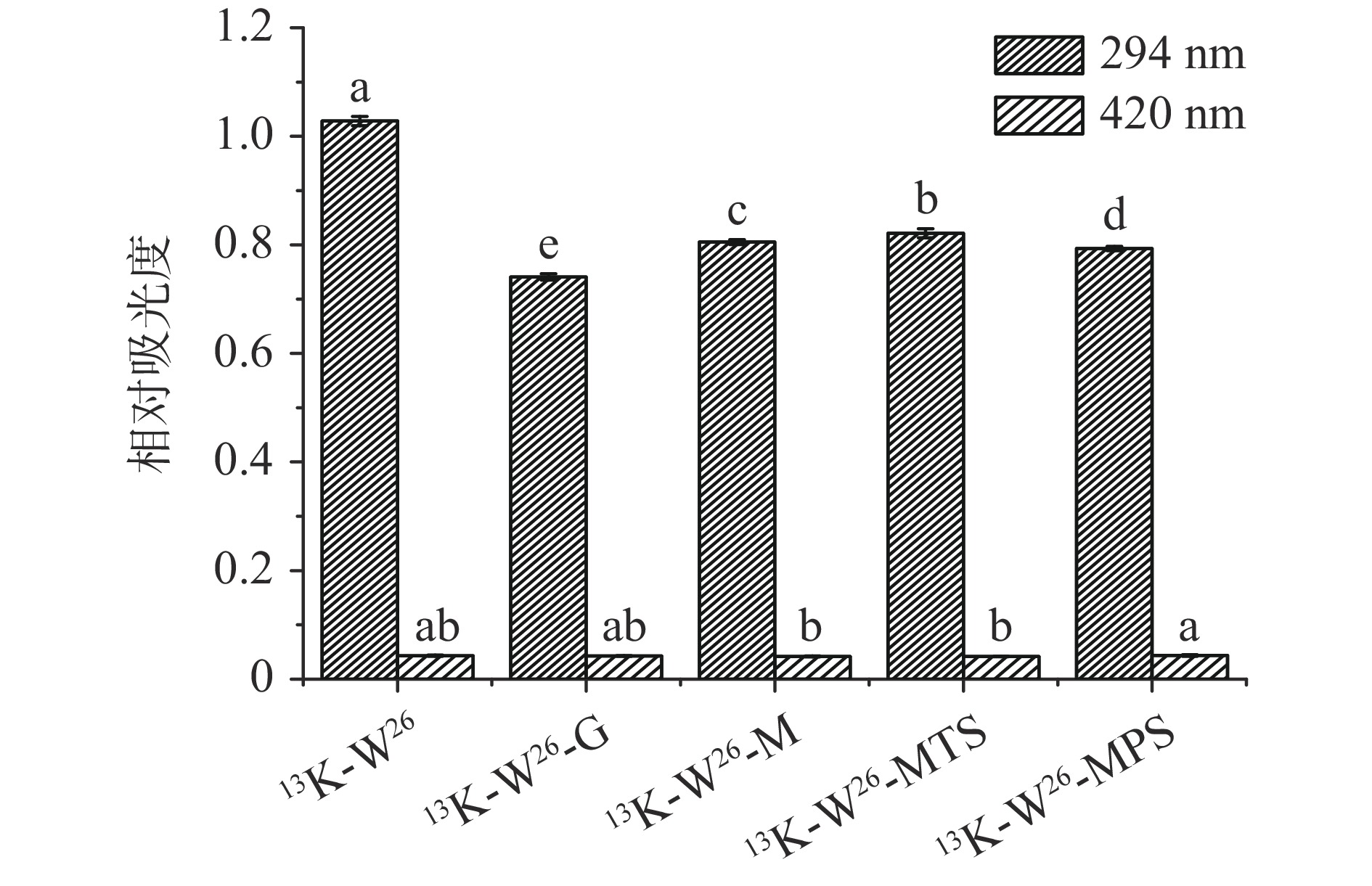
13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS在294和420 nm处的紫外吸光度如图1所示。13K-W26与不同长度的糖链糖基化反应后,A294显著降低(P<0.05),说明13K-W26与葡萄糖、麦芽糖、麦芽三糖和麦芽五糖发生美拉德反应,生成有颜色的化合物[24],且不同长度的糖链与13K-W26发生糖基化反应的速率不同。但糖基化样品的A420无显著性差异(P>0.05),可能是由于糖基化反应时间短,糖基化反应终产物的生成较少。实验表明,葡萄糖、麦芽糖、麦芽三糖和麦芽五糖与13K-W26发生糖基化反应的程度不同。
2.2 游离氨基
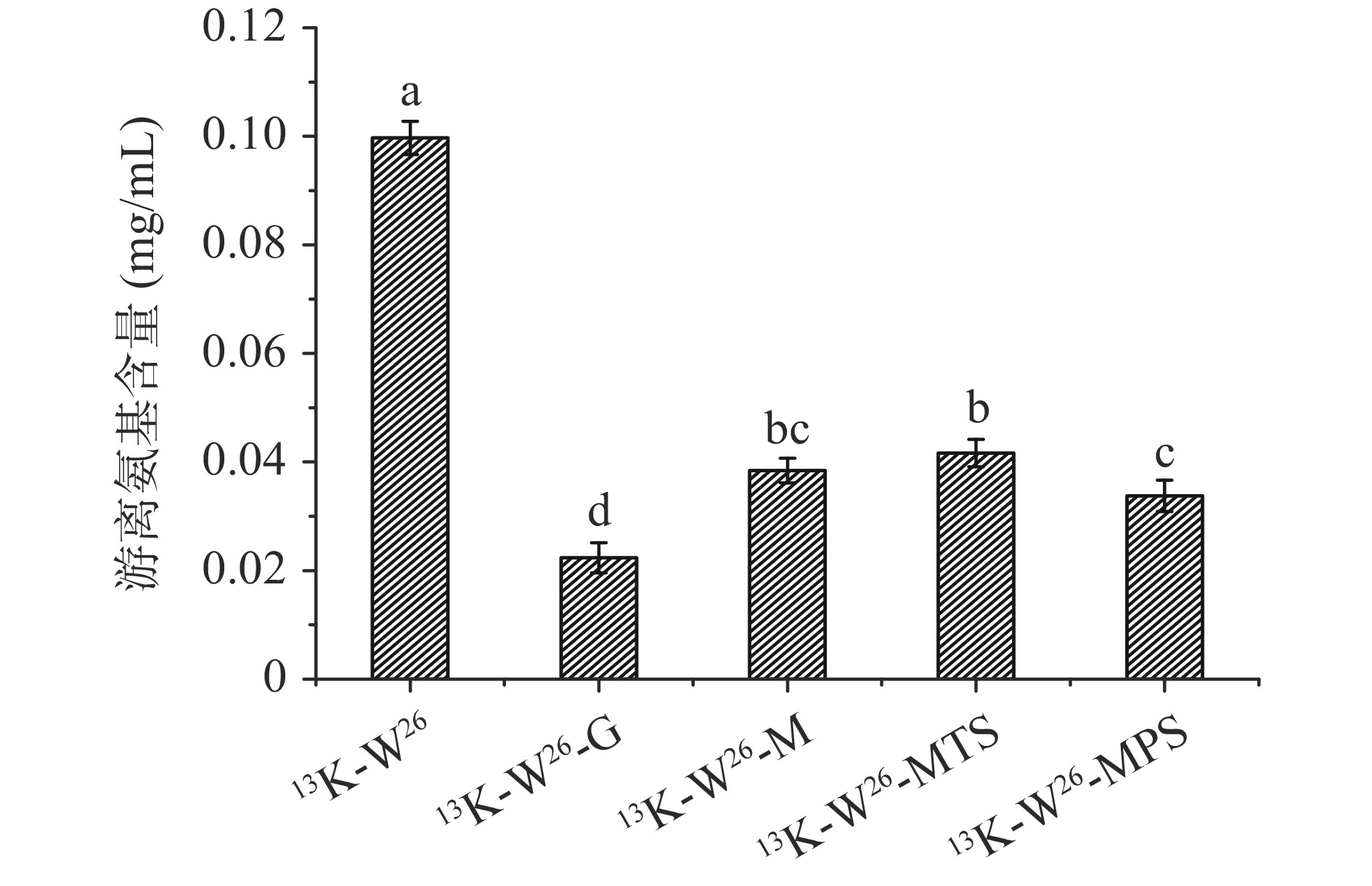
13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的游离氨基含量变化如图2所示。与13K-W26相比,13K-W26-G、13K-W26-M、13K-W26-MTS、13K-W26-MPS的游离氨基含量有着不同程度的显著降低(P<0.05),表明13K-W26与不同的糖发生了糖基化反应,降低其游离氨基的含量。13K-W26-G的游离氨基含量最低,其次分别是13K-W26-MPS、13K-W26-M和13K-W26-MTS,可能是由于不同长度的糖链与13K-W26反应时的空间位阻不同[11],导致其反应程度不同,反应程度为葡萄糖>麦芽五糖>麦芽糖>麦芽三糖。
2.3 内源荧光及紫外吸收强度
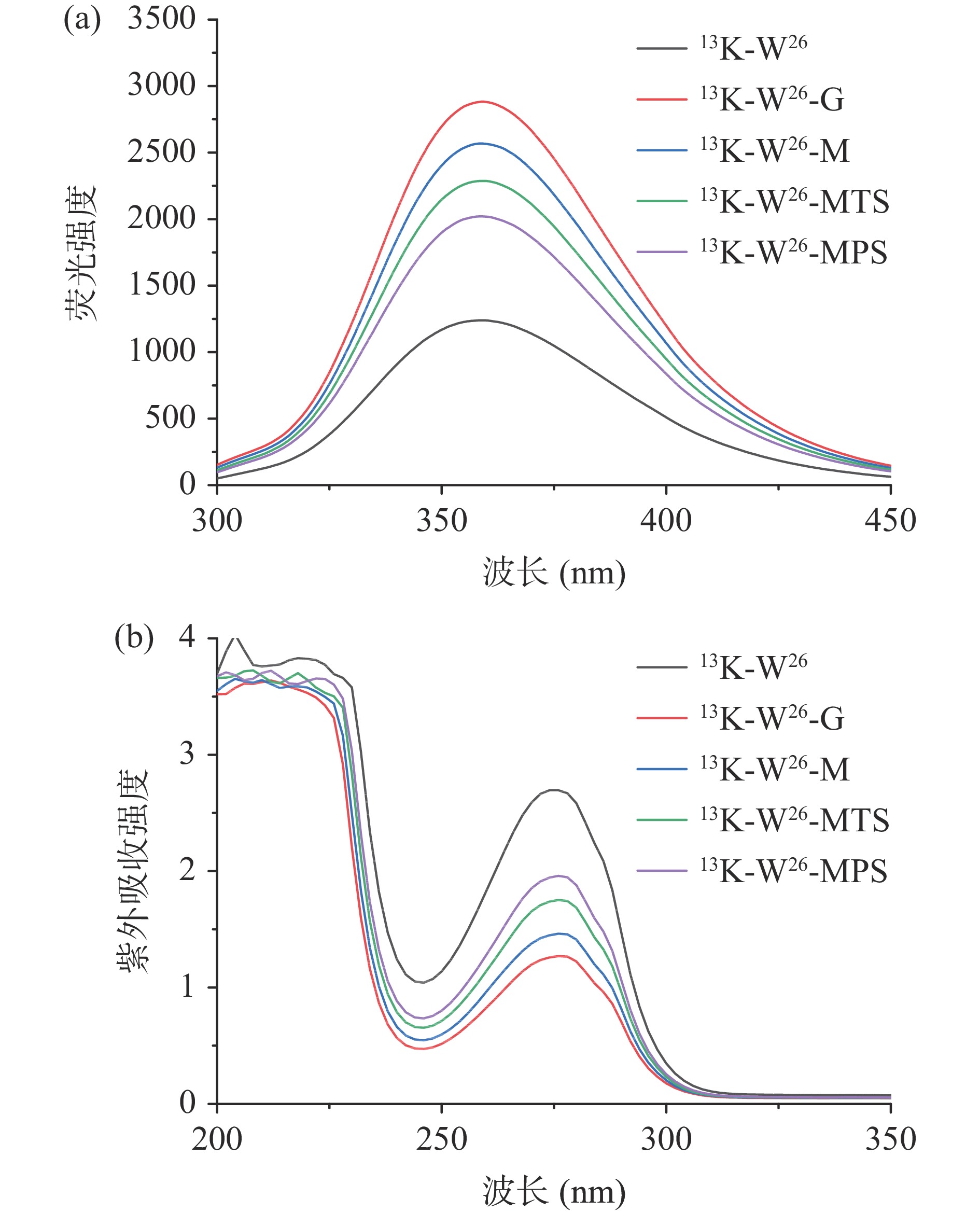
13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的内源荧光强度和紫外吸收强度的变化如图3所示。由图3(a)可以看出,在激发波长为280 nm时,13K-W26与不同长度的糖链发生糖基化后,其荧光强度逐渐增强,程度为13K-W26-G>13K-W26-M>13K-W26-MTS>13K-W26-MPS,这可能是由于13K-W26经不同长度的糖链修饰后,改变其微环境,导致色氨酸基团暴露[25],增强其荧光强度。由图3(b)可以看出13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS、13K-W26-MPS在280 nm处存在较强的吸收峰,与13K-W26相比,13K-W26-MPS、 13K-W26-MTS 、13K-W26-M、13K-W26-G的紫外吸收强度逐渐降低,主要是由于葡萄糖、麦芽糖、麦芽三糖和麦芽五糖与13K-W26糖基化反应后,改变其构象,不同程度地影响其紫外吸收强度[26]。实验结果显示,不同长度的糖链能影响13K-W26的构象结构。
2.4 同步荧光光谱
图4为13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS分别在Δλ为15 nm和Δλ为60 nm时的同步荧光光谱。对比图4a和图4b可知,13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS中Tyr的荧光强度出现降低,而其中Trp的荧光强度出现升高,其最大发射峰的波长均出现蓝移。这些结果表明,13K-W26与不同长度的糖链发生糖基化后,其构象发生变化,Tyr基团由于糖基化后存在糖的空间位阻而受到遮蔽,Trp基团被暴露出来,且两个基团的疏水性均增加。长度越长的糖链对13K-W26进行糖基化修饰后,Tyr的荧光强度降低地更少,说明对该过敏表位中的Tyr的遮蔽更少;而Trp荧光强度增加地更多,说明13K-W26与长度更长的糖链发生糖基化后,对其Trp所处结构位置的影响更大,使其被更多地暴露在更疏水的环境,以上表明经不同长度的糖链糖基化后13K-W26的构象结构发生改变[27],此结果与内源荧光强度结果一致。
2.5 红外光谱分析
图5为13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的红外光谱。13K-W26与不同长度的糖链进行糖基化反应后,发现在3600~3000 cm−1的吸收峰出现变化,该处主要反映酰胺A带的变化,可以发现13K-W26在3405.6和3077.8 cm−1有着吸收峰,而在13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS中3077.8 cm−1处吸收峰消失,而在3405.6 cm−1处的吸收峰值变大,这可能是糖接枝到13K-W26上,其羟基含量增加[28]。在13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的1000 cm−1处,与13K-W26相比出现较多新的吸收峰,这是因为13K-W26发生了糖基化反应,糖的O-H和C-C键的伸缩振动导致的[29],结合图1和图2实验结果,说明13K-W26分别与不同长度的糖链发生了糖基化反应。
2.6 特异性抗体IgE结合能力
通过ELISA法评价13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS与牛奶过敏患者血清的特异性IgE结合能力,结果如图6所示。13K-W26与不同长度的糖链发生糖基化修饰后,其特异性IgE结合能力显著降低(P<0.05),这可能是13K-W26与葡萄糖、麦芽糖、麦芽三糖和麦芽五糖发生糖基化反应后,赖氨酸被修饰,不同程度地掩盖线性表位,使得过敏表位部分无法被IgE抗体识别,从而降低13K-W26的IgE结合能力[30-31]。同一样品与不同患者血清的IgE结合能力存在一定差异,但总体而言,特异性IgE结合能力降低的趋势为13K-W26-MPS>13K-W26-MTS>13K-W26-M>13K-W26-G,其中13K-W26-MPS的特异性IgE结合能力下降程度最大,这可能是由于麦芽五糖与13K-W26糖基化反应后,麦芽五糖具有更长的糖链,能够更大程度地掩蔽其过敏表位。结果表明,不同长度的糖链与13K-W26发生糖基化反应后,糖链越长,降低致敏性的效果越好,顺序为麦芽五糖>麦芽三糖>麦芽糖>葡萄糖。
2.7 KU812细胞致敏性
如图7所示,13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS对KU812细胞HIS、IL-4、IL-6分泌能力的影响。与Control相比,13K-W26、13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS的HIS、IL-4、IL-6均有显著增加(P<0.05),这说明修饰前后的13K-W26均能引起KU812细胞分泌过敏相关的细胞因子。与13K-W26相比,13K-W26-G、13K-W26-M、13K-W26-MTS和13K-W26-MPS处理后的KU812细胞分泌的HIS、IL-4、IL-6的量出现不同程度的降低,可能的原因是糖基化修饰后的13K-W26诱导嗜碱性粒细胞脱颗粒的程度较弱,减弱了效应细胞分泌HIS、IL-4和IL-6的能力,而HIS、IL-4和IL-6的降低会减弱平滑肌收缩等病理变化,从而缓解过敏反应[32]。其中13K-W26-MPS具有最低的HIS、IL-4、IL-6含量,然后依次是13K-W26-MTS、3K-W26-M和13K-W26-G,这与图6的结果一致。这些结果表明经不同长度的糖链修饰的13K-W26可以减少KU812细胞的HIS、IL-4和IL-6的分泌,并降低其致敏性。
3. 结论
本文探究了不同长度的糖链(葡萄糖、麦芽糖、麦芽三糖和麦芽五糖)对α-La线性过敏表位13K-W26结构和致敏性的影响,结果表明这四种糖均能够改变其结构和降低其致敏性,反映在游离氨基含量的降低,荧光强度和紫外吸收强度的改变,IgE结合能力的降低以及KU812细胞中HIS、IL-4和IL-6含量的减少。其中麦芽五糖修饰的13K-W26具有最低的致敏能力,主要是由于麦芽五糖的糖链比葡萄糖、麦芽糖和麦芽三糖长,且有着更大的空间位阻和更强的电荷作用,与13K-W26发生糖基化反应后,可显著影响13K-W26的结构,降低其致敏性。综上所述,长度更长的糖链,对13K-W26结构和致敏性的影响越大,能够更好地降低其致敏性。本研究结果可为研发低致敏性乳制品提供一定的理论基础,但致敏性的评价不全面,需要后期开展更多的实验(如动物实验和临床实验等)来验证其效果。
-
表 1 牛乳过敏患者信息
Table 1 Information of milk allergic patients
血清编号 IgE水平 年龄 性别 临床症状 PL 21902 16.4 kU·L−1 34 男 过敏性鼻炎、食物过敏 PL 27091 4.38 kU·L−1 29 女 过敏性鼻炎 PL 26655 11.6 kU·L−1 35 男 过敏性鼻炎 -
[1] 罗永康, 沈小琴, 李朝慧. 牛乳蛋白过敏原改性的研究[J]. 中国乳品工业,2005,33(10):4−8. [LUO Y K, SHEN X Q, LI C H, et al. Studies on modification of milk protein allergens[J]. China Dairy Industry,2005,33(10):4−8. doi: 10.3969/j.issn.1001-2230.2005.10.001 LUO Y K, SHEN X Q, LI C H, et al. Studies on modification of milk protein allergens[J]. China Dairy Industry, 2005, 33(10): 4-8. doi: 10.3969/j.issn.1001-2230.2005.10.001
[2] 李晓彤, 徐兵洁, 张进, 等. 帕米尔牦牛乳蛋白分离及α-乳白蛋白纯化研究[J]. 食品与发酵工业,2022,48(14):188−194. [LI X T, XU B J, ZHANG J, et al. Protein isolation and purification of α-lactalbumin protein from pamir yak milk[J]. Food and Fermentation Industries,2022,48(14):188−194. LI X T, XU B J, ZHANG J, et al. Protein isolation and purification of α-lactalbumin protein from pamir yak milk[J]. Food and Fermentation Industries, 2022, 48(14): 188-194.
[3] 赵倩如, 朱丽英, 赵权宇, 等. 高效液相色谱法测定乳清蛋白中的α-乳白蛋白和β-乳球蛋白及其改性降敏[J]. 生物加工过程,2020,18(4):536−543. [ZHAO Q R, ZHU L Y, ZHANG Q Y, et al. Detection of α-lactalbumin and β-lactoglobulin using HPLC and optimization of enzymatic degradation[J]. Chinese Journal of Bioprocess Engineering,2020,18(4):536−543. ZHAO Q R, ZHU L Y, ZHANG Q Y, et al. Detection of α-lactalbumin and β-lactoglobulin using HPLC and optimization of enzymatic degradation[J]. Chinese Journal of Bioprocess Engineering, 2020, 18(4): 536-543.
[4] 丛艳君, 陈澍, 李晔, 等. 牛乳α-乳白蛋白IgE线性表位的关键氨基酸识别[J]. 食品科学,2017,38(11):1−5. [CONG Y J, CHEN S, LI Y, et al. Identification of the critical amino acids of IgE-binding epitopes in α-lactalbumin[J]. Food Science,2017,38(11):1−5. doi: 10.7506/spkx1002-6630-201711001 CONG Y J, CHEN S, LI Y, et al. Identification of the critical amino acids of IgE-binding epitopes in α-Lactalbumin[J]. Food Science, 2017, 38(11): 1-5. doi: 10.7506/spkx1002-6630-201711001
[5] JÄRVINEN K, CHATCHATEE P, BARDINA L, et al. IgE and IgG binding epitopes on α-lactalbumin and β-Lactoglobulin in cow's milk allergy[J]. International Archives of Allergy and Immunology,2001,126(2):111−118. doi: 10.1159/000049501
[6] 李欣, 陈红兵. 牛奶过敏原表位研究进展[J]. 食品科学,2006,27(11):592−598. [LI X, CHEN H B. Development of research on epitopes of milk allergens[J]. Food Science,2006,27(11):592−598. doi: 10.3321/j.issn:1002-6630.2006.11.148 LI X, CHEN H B. Development of research on epitopes of milk allergens[J]. Food Science, 2006, 27(11): 592-598. doi: 10.3321/j.issn:1002-6630.2006.11.148
[7] LIU J, TU Z C, LIU G X, et al. Ultrasonic pretreatment combined with dry-state glycation reduced the immunoglobulin E/immunoglobulin G-binding ability of alpha-lactalbumin revealed by high-resolution mass spectrometry[J]. Journal Of Agricultural And Food Chemistry,2018,66(22):5691−5698. doi: 10.1021/acs.jafc.8b00489
[8] 姜雪, 于鹏, 苗君莅, 等. 降低牛乳蛋白致敏性的改性方法研究进展[J]. 食品工业,2015,36(3):258−261. [JIANG X, YU P, MIAO J L. Advanced in research on the modification methods about reducing milk protein sensitization[J]. The Food Industry,2015,36(3):258−261. JIANG X, YU P, MIAO J L. Advanced in research on the modification methods about reducing milk protein sensitization[J]. The Food Industry, 2015, 36(3): 258-261.
[9] 吴佰林, 尤亮亮, 贾晓玲, 等. 牛奶过敏原主要检测技术和消减方法研究进展[J]. 食品工业,2021,42(12):406−410. [WU B L, YOU L L, JIA X L. Research progress on detection techniques and reduction methods of milk allergens[J]. The Food Industry,2021,42(12):406−410. WU B L, YOU L L, JIA X L. Research progress on detection techniques and reduction methods of milk allergens[J]. The Food Industry, 2021, 42(12): 406-410.
[10] 刘丽波, 孙迪, 李春, 等. 糖基化修饰牛乳清蛋白过敏原性研究进展[J]. 食品科学,2012,33(13):334−338. [LIU L B, SUN D, LI C. Research advances in allergenrity of glycosylated whey protein[J]. Food Science,2012,33(13):334−338. LIU L B, SUN D, LI C. Research advances in allergenrity of glycosylated whey protein[J]. Food Science, 2012, 33(13): 334-338.
[11] 范金波, 蔡茜彤, 侯宇, 等. 糖链长度对α-乳白蛋白-糖复合物抗原性的影响[J]. 食品与发酵工业,2014,40(10):27−31. [FAN J B, CAI Q T, HOU Y. Effect of sugar chain length on the antigenicity of alpha lactalbumin-glycoconjugates[J]. Food and Fermentation Industries,2014,40(10):27−31. FAN J B, CAI Q T, HOU Y. Effect of sugar chain length on the antigenicity of alpha lactalbumin-glycoconjugates[J]. Food and Fermentation Industries, 2014, 40(10): 27-31.
[12] 卜单, 涂宗财, 刘光宪, 等. 不同还原糖糖基化对超声波预处理α-乳白蛋白结构和抗氧化活性的影响[J/OL]. 食品科学: 1−10[2022-10-15]. http://kns.cnki.net/kcms/detail/11.2206.ts.20211220.1452.016.html BU D,TU Z C,LIU G X,et al.Effects of different reducing sugars glycation on structure and antioxidant activity of ultrasonic pretreatment α-lactalbumin[J].Food Science, 1−10. http://kns.cnki.net/kcms/detail/11.2206.ts.20211220.1452.016.html.
[13] ZHANG Z Y, LI X M, LI Z X, et al. Investigation of glycated shrimp tropomyosin as a hypoallergen for potential immunotherapy[J]. Food & Function,2021,12(6):2750−2759.
[14] 芦晶, 布冠好, 罗永康. 乳清蛋白-多糖的制备及功能特性的研究[J]. 中国乳品工业,2007,35(5):4−8,25. [LU J, BU G H, LUO Y K. Studies of forming whey protein-polysaccharides conjugates and it's functional properties[J]. China Dairy Industry,2007,35(5):4−8,25. doi: 10.3969/j.issn.1001-2230.2007.05.001 LU J, BU G H, LUO Y K. Studies of forming whey protein-polysaccharides conjugates and it's functional properties[J]. China Dairy Industry, 2007, 35(5): 4-8, 25. doi: 10.3969/j.issn.1001-2230.2007.05.001
[15] 陈文美, 周厚泽, 邵艳红, 等. 糖基化联合磷酸化降低鲢鱼小清蛋白致敏性的机制[J/OL]. 食品科学: 1-12.[2022-10-15]. http://kns.cnki.net/kcms/detail/11.2206.TS.20220621.1919.084.html CHEN W M,ZHOU H Z,SHAO Y H.Mechanism of glycation combined with phosphorylation to reduce sensitization of silver carp albumin[J]. Food Science, 1-12. http: //kns.cnki.net/kcms/detail/11.2206.TS.20220621.1919.084.html.
[16] 王亚婷. 不同二糖糖基化修饰在卵清蛋白改性中的应用研究[D]. 南昌: 南昌大学, 2021. WANG Y T. Study on the application of different disaccharides glycosylation modification in the modification of ovalbumin [D]. Nanchang: Nanchang University, 2021.
[17] LIU J, CHEN W M, SHAO Y H, et al. The mechanism of the reduction in allergenic reactivity of bovine alpha-lactalbumin induced by glycation, phosphorylation and acetylation[J]. Food Chemistry, 2020, 310.
[18] KIM J S, LEE Y S. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction[J]. Food Chemistry,2008,108(2):582−592. doi: 10.1016/j.foodchem.2007.11.014
[19] 刘俊. 超声波辅助糖基化修饰对蛋白质功能性质的影响及机制初探[D]. 南昌: 江西师范大学, 2018. LIU J. Effect and mechanism of ultrasound assisted glycation modification on protein functional properties[D]. Nanchang: Jiangxi Normal University, 2018.
[20] 张露, 徐亮, 涂宗财, 等. 基于荧光光谱技术的异槲皮素抑制晚期糖基化产物形成的机制研究[J]. 光谱学与光谱分析,2020,40(12):3755−3760. [ZHANG L, XU L, TU Z C. Mechanism of isoquercitrin inhibiting advanced glycation products formation based on fluorescence spectroscopy technique[J]. Spectroscopy and Spectral Analysis,2020,40(12):3755−3760. ZHANG L, XU L, TU Z C. Mechanism of isoquercitrin inhibiting advanced glycation products formation based on fluorescence spectroscopy technique[J]. Spectroscopy and Spectral Analysis, 2020, 40(12): 3755-3760.
[21] 刘俊, 王阳, 熊子豪, 等. 糖基化、磷酸化及乙酰化修饰对α-乳白蛋白致敏性和人肠道菌群的影响[J]. 食品与发酵工业,2021,47(17):91−97. [LIU J, WANG Y, XIONG Z H. Effects of glycation, phosphorylation and acetylation on allergenicity and human intestinal microbiota of α-lactalbumin[J]. Food and Fermentation Industries,2021,47(17):91−97. LIU J, WANG Y, XIONG Z H. Effects of glycation, phosphorylation and acetylation on allergenicity and human intestinal microbiota of α-lactalbumin[J]. Food and Fermentation Industries, 2021, 47(17): 91-97.
[22] 朱一丹, 谢国锦, 高岭, 等. 不同pH和离子强度条件下青鱼(Mylopharyngodon piceus)肌浆蛋白IgG/IgE结合能力的变化[J]. 食品与发酵工业,2020,46(14):34−39. [ZHU Y D, XIE G J, GAO L. Effect of different pH and ion strength on IgG/IgE binding capacity of sarcoplasmic protein of black carp (Mylopharyngodon piceus)[J]. Food and Fermentation Industries,2020,46(14):34−39. ZHU Y D, XIE G J, GAO L. Effect of different pH and ion strength on IgG/IgE binding capacity of sarcoplasmic protein of black carp (Mylopharyngodon piceus)[J]. Food and Fermentation Industries, 2020, 46(14): 34-39.
[23] APPEL K, MUNOZ E, NAVARRETE C, et al. Immunomodulatory and inhibitory effect of immulina (R), and immunloges (R) in the Ig-E mediated activation of RBL-2H3 cells. A new role in allergic inflammatory responses[J]. Plants-Basel,2018,7(1).
[24] HUANG X Q, TU Z C, XIAO H, et al. Characteristics and antioxidant activities of ovalbumin glycated with different saccharides under heat moisture treatment[J]. Food Research International,2012,48(2):866−872. doi: 10.1016/j.foodres.2012.06.036
[25] 张玥. 苦杏仁谷蛋白-糖复合物的制备及多级结构与功能特性的研究[D]. 乌鲁木齐: 新疆农业大学, 2021. ZHANG Y. Preparation of bitter almond gluten sugar complex and study on its multi-level structure and functional properties[D]. Urumqi: Xinjiang Agricultural University, 2021.
[26] 钟比真. 微波场中卵清蛋白糖基化反应不均匀性的研究[D]. 南昌: 南昌大学, 2021. ZHONG B Z. Study on heterogeneity of glycosylation of ovalbumin in microwave field[D]. Nanchang: Nanchang University, 2021.
[27] 任国艳, 孙贺, 樊金玲, 等. 荧光光谱法和分子对接模拟技术研究白藜芦醇与胃蛋白酶的相互作用[J]. 光谱学与光谱分析,2019,39(4):1103−1108. [REN G Y, SUN H, FAN J L. Study on interaction between resveratrol and pepsin by fluorescence spectroscopy and molecular modeling[J]. Spectroscopy and Spectral Analysis,2019,39(4):1103−1108. REN G Y, SUN H, FAN J L. Study on interaction between resveratrol and pepsin by fluorescence spectroscopy and molecular modeling[J]. Spectroscopy and Spectral Analysis, 2019, 39(4): 1103-1108.
[28] 闫雨洁. 超声结合糖基化反应改性卵白蛋白及其理化性质与乳化性能研究[D]. 南宁: 广西大学, 2021. YAN Y J. Study on the modification of ovalbumin by ultrasound combined with glycation reaction and its physical and chemical properties and emulsification performance[D]. Nanning: Guangxi University, 2021.
[29] 王楠, 章涵钰, 楼博, 等. 甲鱼抗氧化肽对非酶糖基化反应及其终产物的抑制作用[J]. 中国食品学报,2022,22(2):58−64. [WANG N, ZHANG H Y, LOU B. Inhibitory effect of antioxidant peptides of turtle on non-enzymatic gluco-sylation and its end products[J]. Journal of Chinese Institute of Food Science and Technology,2022,22(2):58−64. doi: 10.16429/j.1009-7848.2022.02.007 WANG N, ZHANG H Y, LOU B. Inhibitory effect of antioxidant peptides of turtle on non-enzymatic gluco-sylation and its end products[J]. Journal of Chinese Institute of Food Science and Technology, 2022, 22(2): 58-64. doi: 10.16429/j.1009-7848.2022.02.007
[30] 黄美佳. 钙离子对牛乳α-乳白蛋白结构与致敏性的影响[D]. 南昌: 南昌大学, 2018. HUANG M J. The effect of calcium ions on the structure and sensitization of α-bovine milk albumin [D]. Nanchang: Nanchang University, 2018.
[31] ARITA K, BABIKER E E, AZAKAMI H, et al. Effect of chemical and genetic attachment of polysaccharides to proteins on the production of IgG and IgE[J]. Journal Of Agricultural and Food Chemistry,2001,49(4):2030−2036. doi: 10.1021/jf001120t
[32] EIGENMANN P A. Mechanisms of food allergy[J]. Pediatric Allergy and Immunology,2009,20(1):5−11. doi: 10.1111/j.1399-3038.2008.00847.x
-
期刊类型引用(0)
其他类型引用(1)





 下载:
下载:




 下载:
下载:



