Research Progress on Antitumor Mechanism of 6-Shogaol
-
摘要: 癌症的发病率与死亡率逐年递增,严重威胁人类健康。6-姜烯酚是生姜中一种主要活性成分,因其具有安全、低毒的优势成为国内外学者关注的热点,6-姜烯酚具有抗炎、抗氧化、抗肿瘤等药理作用,尤其是其抗肿瘤活性日趋成为研究焦点。本文对6-姜烯酚的抗肿瘤作用机制进行综述,主要包括抑制细胞增殖、诱导细胞凋亡、阻滞细胞周期、抑制细胞的迁移和侵袭、影响肿瘤细胞自噬等,以期为6-姜烯酚抗肿瘤的进一步研究、开发和应用提供思路。Abstract: The incidence and mortality of cancer are increasing year by year, which seriously threatens human health. 6-Shogaol, as one of the main active components in ginger, has become the focus of attention in recent years due to its advantages of safety and low toxicity. Moreover, it has anti-inflammatory, antioxidant, anti-tumor and other pharmacological effects. Particularly, its anti-tumor activity has received increasing attention. This paper reviews the anti-tumor mechanism of 6-shogaol, including inhibition of cell proliferation, induction of cell apoptosis, arrest of cell cycle, inhibition of cell migration and invasion, effect of autophagy in tumor cells, etc., in order to provide ideas for further research, development and application of 6-shogaol in anti-tumor.
-
Keywords:
- 6-shogaol /
- anti-tumor /
- mechanism of action /
- research progress
-
最新癌症统计数据显示,全球癌症发病率和死亡率不断增加[1]。癌症不仅严重威胁患者的身体健康,还给人们带来沉重的心理和经济负担[2]。目前癌症最常见的治疗方法是化疗,虽能延长患者的生存时间,但其严重的副作用往往使患者预后不良,故寻找高效低毒的抗癌药物已成为全世界共同关注的焦点。药食同源的天然产物具有不良反应小、多靶点调控等优势,其抗肿瘤活性受到广大科研工作者的广泛关注。
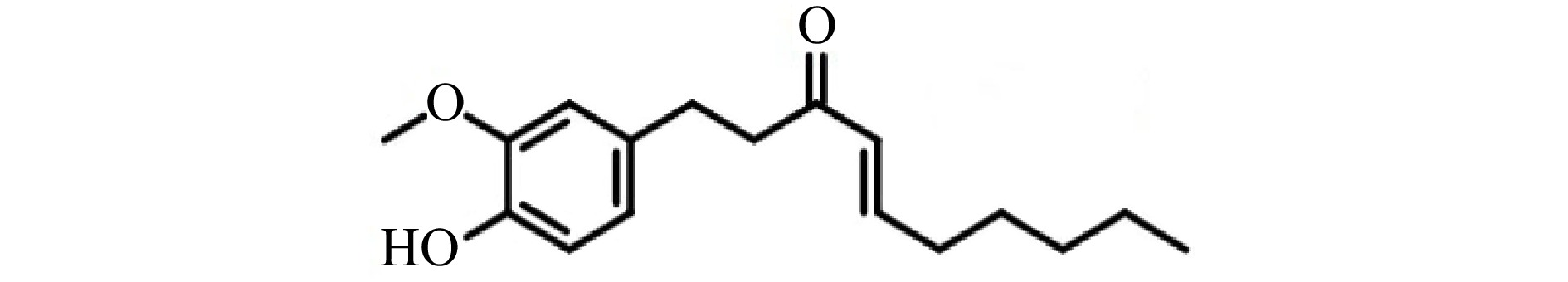
《本草纲目》中记载:姜(Zingiber officinale Rosc.)是姜科植物姜的鲜嫩茎,为多年生草本宿根植物,有发表、散寒、止呕、化痰等功效。生姜气味辛、微温、无毒,通神明,经五脏除风邪寒热,伤寒头痛鼻塞,去痰下气。生姜是最常用的具有辛辣味的调味料之一,发挥生物活性的主要物质是植物素,尤其是脱水产物姜烯酚[3-5],其中6-姜烯酚(6-shogaol)的生物活性最强[6]。6-姜烯酚的相对分子质量为276.37,分子式为C17H24O3,化学结构见图1。
6-姜烯酚具有多种生物活性,如抗炎、抗肿瘤(表1)、抗氧化应激等[5]。研究表明,6-姜烯酚能够抑制多种肿瘤细胞的增殖,对肝癌[7]、乳腺癌[8-9]、口腔癌[10]、肺癌[11]、胰腺癌[12]、黑色素瘤[13]、前列腺癌[14-16]、结直肠癌[11, 17-18]、胃癌[19]、头颈癌[20]、肉瘤[21]等都表现出良好的抗癌效果,重要的是,6-姜烯酚的抗肿瘤活性对癌细胞具有特异性,正常细胞不受影响[10, 17, 21]。6-姜烯酚主要通过抑制肿瘤细胞增殖、诱导细胞凋亡、阻滞细胞周期、抑制细胞迁移和侵袭、影响细胞自噬及与其他药物联合使用等途径发挥抗肿瘤活性。本文对6-姜烯酚的抗肿瘤机制进行总结,为6-姜烯酚的进一步研究及合理开发利用提供科学依据和理论基础。
表 1 6-姜烯酚的抗肿瘤作用及其机制Table 1. Antitumor effect and its mechanism of 6-shogaol药理作用 癌症类型 癌症细胞系或动物模型 作用机制 参考文献 抑制肿瘤细胞增殖 肝癌 Bel-7402 调控Wnt/β-Catenin信号通路 [7] HepG2 Bax、细胞色素C和Caspase3表达升高 [28] 乳腺癌 MCF-7和T47D 靶向Notch信号通路,抑制细胞自噬 [8] 口腔癌 YD-10B和Ca9-22 调控PI3K/AKT/mTOR信号通路 [10] OC2 显著增加Ca2+含量,影响内质网功能 [63] 8~10周龄的雄性金色叙利亚仓鼠 NF-κB和AP-1致癌信号来减轻炎症和细胞增殖 [64] 胰腺癌 Panc-1、AsPC-1、BxPC-3、CAPAN-2、CFPAC-1、MIAPaCa-2和SW1990 通过影响内源性线粒体功能,造成Caspase3释放 [12] 结肠癌 COLO 205 ROS水平升高,Bax、Fas、FasL表达上调,Bcl-2、Bcl-XL表达下调 [36] 脂肪肉瘤 SW872和93T449 调控STAT-3、AMPK信号通路,影响内质网功能 [21] 诱导肿瘤细胞凋亡 肝癌 Bel-7402、HepG2 调控Wnt/β-Catenin信号通路 [7] SMMC-7721 抑制elF-2α的磷酸化,影响内质网应激 [39] 前列腺癌 LNCaP、DU145和PC3 调控STAT3和 NF-κB信号通路 [16] 结肠癌 HCT-116、SW-480和HT-29 促进Bax、Caspase3和PARP1蛋白的表达,抑制Bcl-2的表达 [24, 26] 胃癌 BGC-823 调控EMT信号通路 [29] 淋巴癌 CCRF-CEM和Nalm-6 调控p53信号通路,影响ROS水平 [32] 宫颈癌 HeLa和SiHa 通过影响线粒体功能,进而影响ROS,
造成Caspase3释放[27, 54] 阻滞肿瘤细胞周期 前列腺癌 LNCaP、DU145和PC3 p21和p27蛋白表达明显升高,Cyclin D1蛋白表达下降 [16] 肺癌 H1650、H520和H1975 下调Cyclin D1和Cyclin D3,调控Akt [47] 宫颈癌 HeLa 显著下调Cyclin B1、PCNA表达,上调p21蛋白表达 [27] 结肠癌 HCT-116和SW480 上调p53蛋白和CDK抑制剂表达,下调cdc2蛋白表达 [46] 淋巴癌 CCRF-CEM和Nalm-6 调控p53信号通路,影响ROS水平 [32] 抑制肿瘤细胞迁移和侵袭 肝癌 Hep3B 调控NF-κB、MAPK信号通路 [51] 乳腺癌 MDA-MB231 调控Hedgehog/Gli1通路 [52] 胃癌 BGC-823 调控EMT信号通路 [29] 口腔癌 YD-10B和Ca9-22 调控Akt信号通路 [10] 宫颈癌 HeLa 与ECM信号通路相关蛋白表达水平有关 [54] 影响肿瘤细胞自噬 肺癌 A549 阻断Akt-mTOR信号通路,诱导细胞自噬 [65] 肝癌 Huh7 通过影响p53和ROS水平,导致自噬通量衰减 [59] 乳腺癌 MCF-7 抑制beclin-I和LC3-II的表达,抑制细胞自噬 [8] 结肠癌 HT29 形成大量自噬小体,Caspase3和Caspase7活性升高 [57] 1. 诱导细胞凋亡
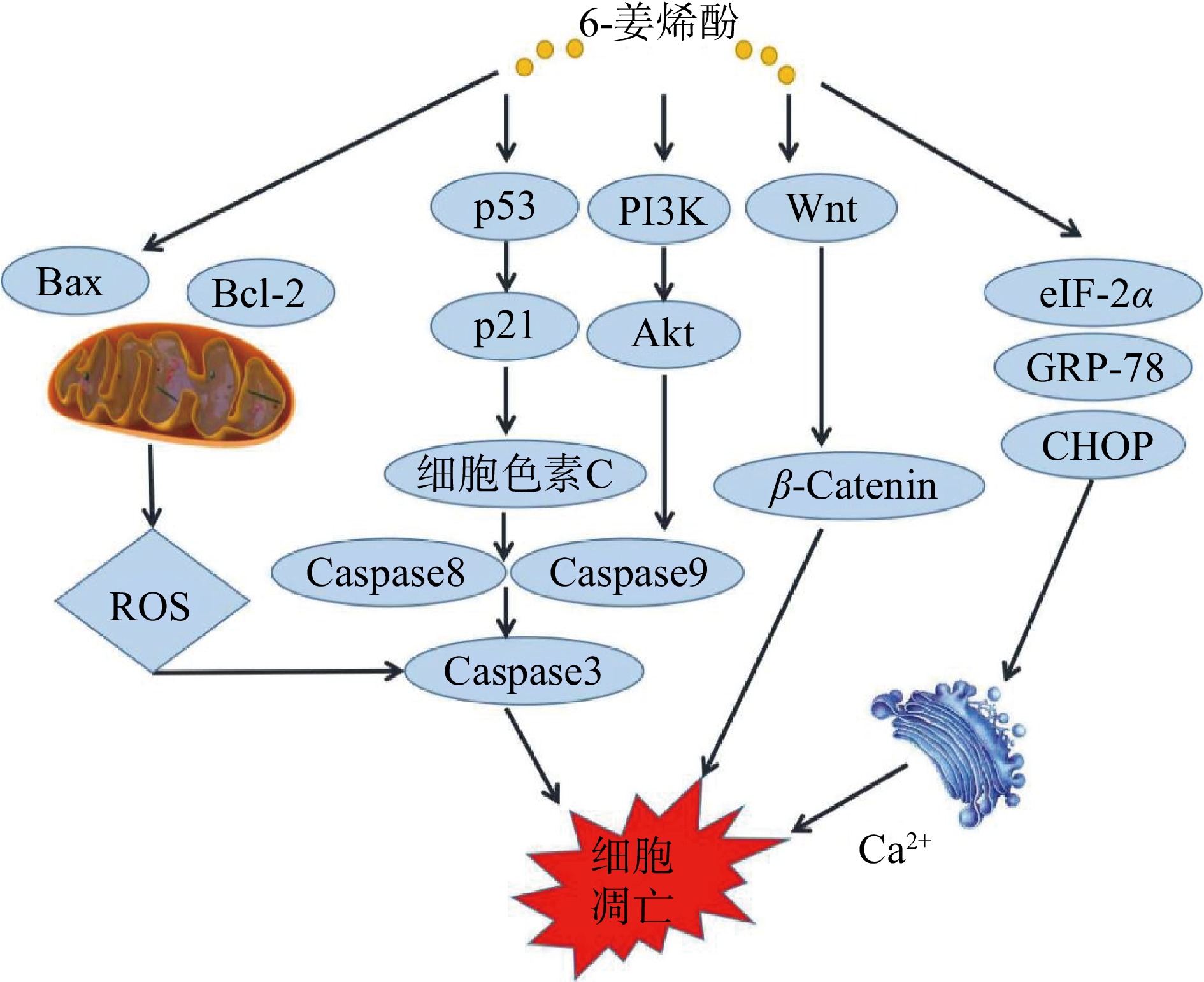
细胞凋亡是机体为维护内环境稳态,由基因控制的细胞自主发生的有序死亡,是机体清理衰老细胞自发进行的一个过程。线粒体途径、死亡受体和内质网途径是传统三大凋亡途径。目前已有研究表明,6-姜烯酚可通过内源性线粒体途径、内质网途径等诱导细胞凋亡[22]。作用机制见图2。
1.1 内源性线粒体途径
线粒体途径是细胞凋亡的研究重点,其主要通过改变线粒体膜通透性、释放细胞色素C、激活Caspase来诱导细胞凋亡。研究发现,6-姜烯酚在多种肿瘤细胞系及小鼠等动物模型中靶向参与线粒体死亡途径,从而启动凋亡。
1.1.1 抑制B淋巴细胞瘤-2(Bcl-2)家族
Bcl-2家族蛋白存在于线粒体外膜,其表达变化会改变线粒体通透性,降低线粒体膜电位,将可溶性蛋白质从线粒体膜间隙释放到细胞质或转移到细胞核,如细胞色素C的释放和线粒体向细胞核的易位,从而刺激含有半胱氨酸的天冬氨酸蛋白级联反应来诱导细胞凋亡[23]。研究发现,6-姜烯酚可不同程度地诱导结肠癌HCT116及HT29凋亡,促进Bax、Caspase3和PARP1蛋白水平表达,抑制Bcl-2蛋白水平表达,并增加Bax/Bcl-2比值比例[24-26]。Pei等[27]使用不同浓度的6-姜烯酚处理宫颈癌HeLa和SiHa细胞,发现6-姜烯酚可以诱导HeLa和SiHa细胞凋亡,并且呈现时间和浓度依赖性,使用Western blot 检测Bax、Caspase3、细胞色素C,发现其表达升高,而Bcl-2蛋白表达水平降低。Govindhan等[28]使用6-姜烯酚处理人肝癌HepG2细胞后发现,处理组细胞ROS水平增强,脂质过氧化导致线粒体膜电位改变,HepG2细胞DNA损伤增加,Bcl-2的表达下调,同时Bax、细胞色素C、Caspase-9和Caspase-3蛋白表达上调,同样的结果在人胃癌BGC-823细胞中得到证实[29-30]。以上结果表明,6-姜烯酚可以在多种肿瘤细胞中通过改变线粒体膜电位,诱导细胞凋亡。
1.1.2 激活肿瘤抑制因子p53
p53作为一种转录因子,能通过调节其下游靶基因表达的方式参与多种重要的生物学过程,如细胞周期阻滞、细胞凋亡等一系列抗增殖过程,并通过影响ROS的生成,促进细胞凋亡[31]。Dorcheh等[32]研究发现,6-姜烯酚通过靶向上调p53信号和促进ROS的生成,诱导急性淋巴母细胞白血病细胞(CCRF-CEM和Nalm-6)凋亡和细胞周期阻滞。Warin等[33]发现6-姜烯酚通过p53途径,阻滞结肠癌细胞周期在G2/M,促进细胞中细胞色素C的释放,提高Caspases-3和9的表达量,并且在异种移植裸鼠模型中,30 mg/kg剂量的6-姜烯酚能显著降低肿瘤负荷[18]。以上研究结果表明6-姜烯酚可通过调节p53信号分子促进肿瘤细胞凋亡、阻滞细胞周期和降低动物模型肿瘤负荷率。
1.1.3 增加活性氧(ROS)的产生
活性氧通常被认为是细胞代谢过程中氧化还原反应形成的产物,ROS与肿瘤密切相关,研究表明多种肿瘤细胞中ROS含量较正常细胞明显升高[34-35]。Pan等[36]使用6-姜烯酚处理人结肠癌COLO 205细胞,发现细胞ROS水平升高,Bax、Fas、FasL表达上调,Bcl-2、Bcl-XL表达下调,而抗氧化剂N-乙酰半胱氨酸(NAC)的加入使ROS产生减少,细胞活力恢复。该机制在人胰腺癌细胞Panc-1、AsPC-1、BxPC-3、CAPAN-2、CFPAC-1、MIAPaCa-2和SW1990[12],纤维肉瘤HT1080细胞[37],宫颈癌Hela和Siha细胞[27]中也得到了同样的证实。
1.2 内质网途径
内质网是细胞内重要的细胞器,可参与细胞内蛋白质的合成和折叠、细胞应激等活动,同时也是Ca2+的最主要储存库。当细胞内Ca2+稳态失衡、蛋白质出现未折叠和(或)错误折叠时,会引发内质网应激,eIF-2α的磷酸化可作为内质网氧化应激的标志[38]。6-姜烯酚可通过控制Caspase通路、抑制STAT3、激活AMPK、影响内质网应激,从而在人脂肪肉瘤细胞SW872中发挥促凋亡作用。6-姜烯酚作用于人脂肪肉瘤细胞SW872后,可上调内质网应激标志物GRP-78、eIF-2α、ATF4和CHOP在细胞中的表达和磷酸化[21],诱导内质网应激。在人肝癌SMMC-7721细胞中,Hu等[39]研究证明,6-姜烯酚可以抑制eIF-2α的磷酸化,并通过内质网应激发挥体内抗肿瘤活性,触发人肝癌SMMC-7721细胞的凋亡。6-姜烯酚也可通过内质网应激和内质网的扩张诱导乳腺癌MDA-MB-231细胞和肺癌A549细胞质空泡化,使细胞凋亡因子易位到细胞核,最终导致DNA碎裂[40]。上述研究表明,6-姜烯酚可以通过内质网途径影响脂肪肉瘤、肝癌、乳腺癌和肺癌细胞凋亡,从而发挥抗肿瘤作用。
2. 抑制肿瘤细胞增殖,诱导肿瘤细胞周期阻滞
细胞增殖是细胞生长和分化的重要组成部分,癌症的一个典型特征是异常的细胞增殖,因此,抑制肿瘤细胞增殖对于抗肿瘤具有重要作用[41-42]。人前列腺癌细胞LNCaP、DU145、PC3和小鼠前列腺癌细胞HMVP2细胞在6-姜烯酚的作用下,细胞中IL-6表达降低,STAT3被激活,TNF-α诱导的NF-κB活性被抑制。此外,6-姜烯酚还调控STAT3和NF-κB靶基因的表达,如Cyclin D1、生存素和cMyc,并调节趋化因子、细胞因子、细胞周期和凋亡调控基因(IL-7、CCL5、Bax、Bcl-2、p21和p27)的mRNA水平[16]。在小鼠肝癌移植肿瘤模型中,TLR4和Wnt/β-catenin表达上调,FOXO3a失活,而6-姜烯酚的作用逆转了肝癌细胞中TLR4、Wnt/β-catenin和FOXO3a的表达,减缓了小鼠肝癌移植肿瘤的生长[7]。mTOR在正常细胞和癌细胞的代谢和增殖中起着核心作用,研究发现6-姜烯酚可通过下调mTOR通路来抑制乳腺癌大鼠的肿瘤进展[43]。上述研究说明6-姜烯酚可通过诱导STAT3的激活,抑制TNF-α的活性,抑制细胞增殖,并减缓小鼠肝移植瘤的生长、抑制大鼠肿瘤进程。
细胞周期是细胞生命活动的基本过程,所有体细胞均通过细胞周期介导的有丝分裂过程来进行增殖。靶向研究肿瘤细胞周期,使肿瘤细胞周期阻滞,从而阻断肿瘤细胞增殖,是目前治疗肿瘤的一个研究热点。细胞周期涉及许多调节基因或蛋白,如细胞周期蛋白(Cyclin)、细胞周期蛋白依赖性激酶(CDK)和调控细胞周期各阶段的有丝分裂检查点(mitotic checkpoint proteins)[44-45],Cyclin和CDKs对细胞周期运行起着核心调控作用。研究发现,6-姜烯酚可引起Notch信号下调(Hes1和CyclinD1基因),诱导G2/M细胞周期阻滞,且呈时间依赖性[8]。Qi等[18]和Lee等[46]发现,用6-姜烯酚处理结肠癌细胞HCT-116和SW480后,细胞生长被阻滞在G2/M期,Western blot检测结果发现,6-姜烯酚显著上调p53和CDK抑制剂p21waf1/cip1的表达,下调cdc2蛋白的表达。Kim等[47]发现,6-姜烯酚诱导细胞周期阻滞(G1或G2/M),下调Cyclin D1和Cyclin D3蛋白的表达,并且通过直接调控Akt1/2来抑制非小细胞肺癌细胞H1650、H520和H1975的生长,导致细胞周期阻滞,该机制在人宫颈癌细胞HeLa[48]上得到同样证实。以上结果表明,6-姜烯酚阻滞细胞周期主要是在G2/M期,其调控机制主要与Cyclin和CDKs表达有关。作用机制见图3。
3. 抑制肿瘤细胞侵袭和迁移
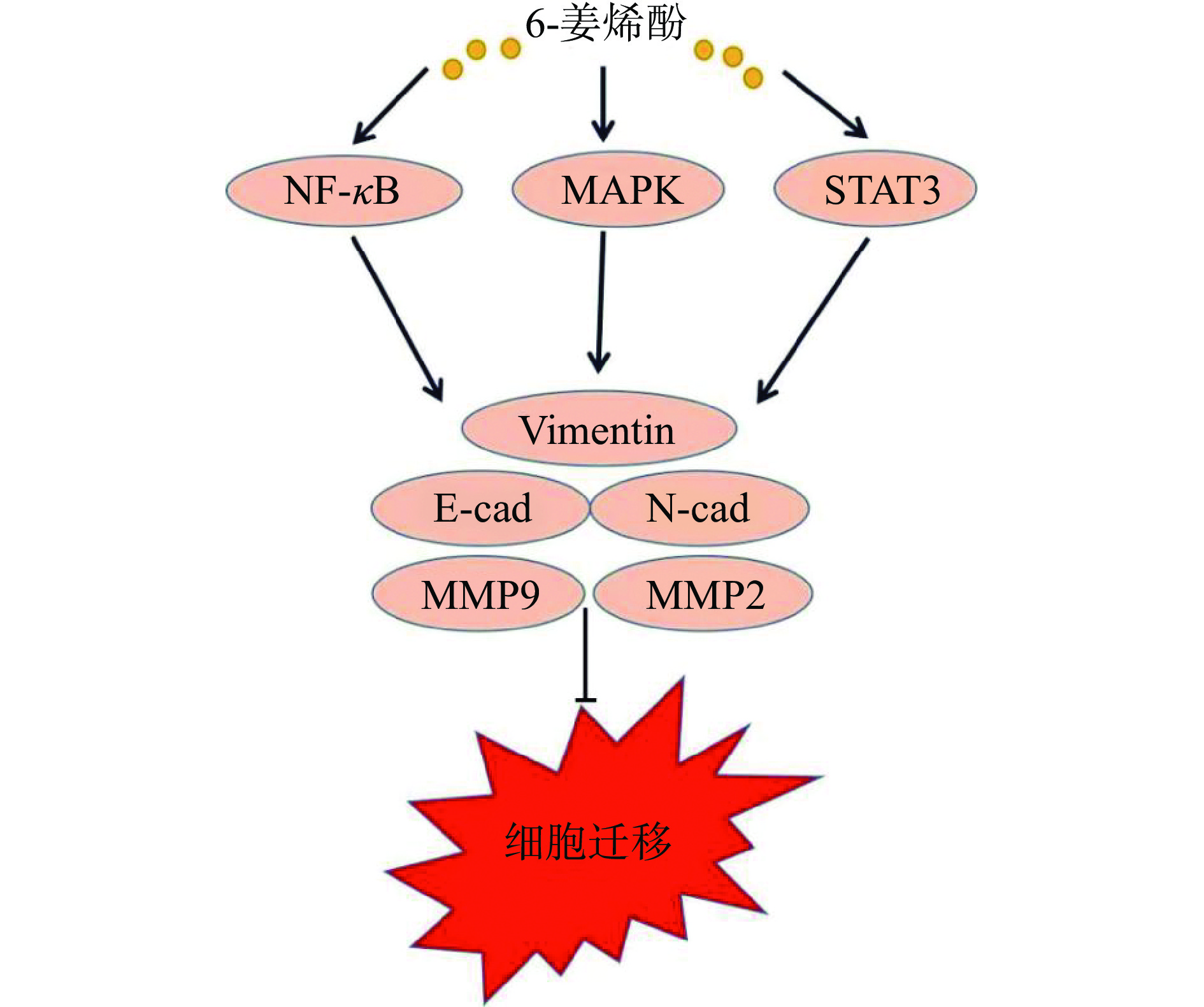
侵袭和转移是恶性肿瘤高复发率和高死亡率的重要原因,是肿瘤细胞的特征之一。目前临床上许多肿瘤患者预后不良均与肿瘤细胞的迁移性与侵袭性密切相关。上皮-间充质转换(epithelial-mesenchymal transition,EMT)被认为是转移的初始步骤之一,可使上皮肿瘤细胞具有更强的侵袭性和降解细胞外基质(extracellular matrix,ECM)的能力,其过程与E-cadherin(E-钙黏蛋白)和N-cadherin(N-钙黏蛋白)和Vimentin(波形蛋白)表达水平有关[49]。基质金属蛋白酶(matrix metalloproteinase,MMPs),尤其是MMP-2和MMP-9,可通过降解ECM参与肿瘤侵袭与转移[50]。Huang等[10]研究发现,6-姜烯酚通过上调E-cadherin,下调N-cadherin蛋白表达水平,抑制口腔鳞状细胞癌的迁移和侵袭。Weng等[51]研究发现,6-姜烯酚通过调控MAPK、NF-κB、STAT3、MMP-2/-9、uPA表达和PI3k/Akt信号通路,阻断血管生成等多种分子机制有效抑制肝癌侵袭转移。林嘉怡等[52]和Hong等[53]研究证明,人乳腺癌MDA-MB231细胞经6-姜烯酚处理后,其增殖及侵袭迁移能力减弱,MMP-2、MMP-9和Vimentin蛋白表达降低。同样的结果在人胃癌BGC-823细胞[29]和宫颈癌细胞HeLa[54]上也得到证实。作用机制见图4。
4. 影响肿瘤细胞自噬
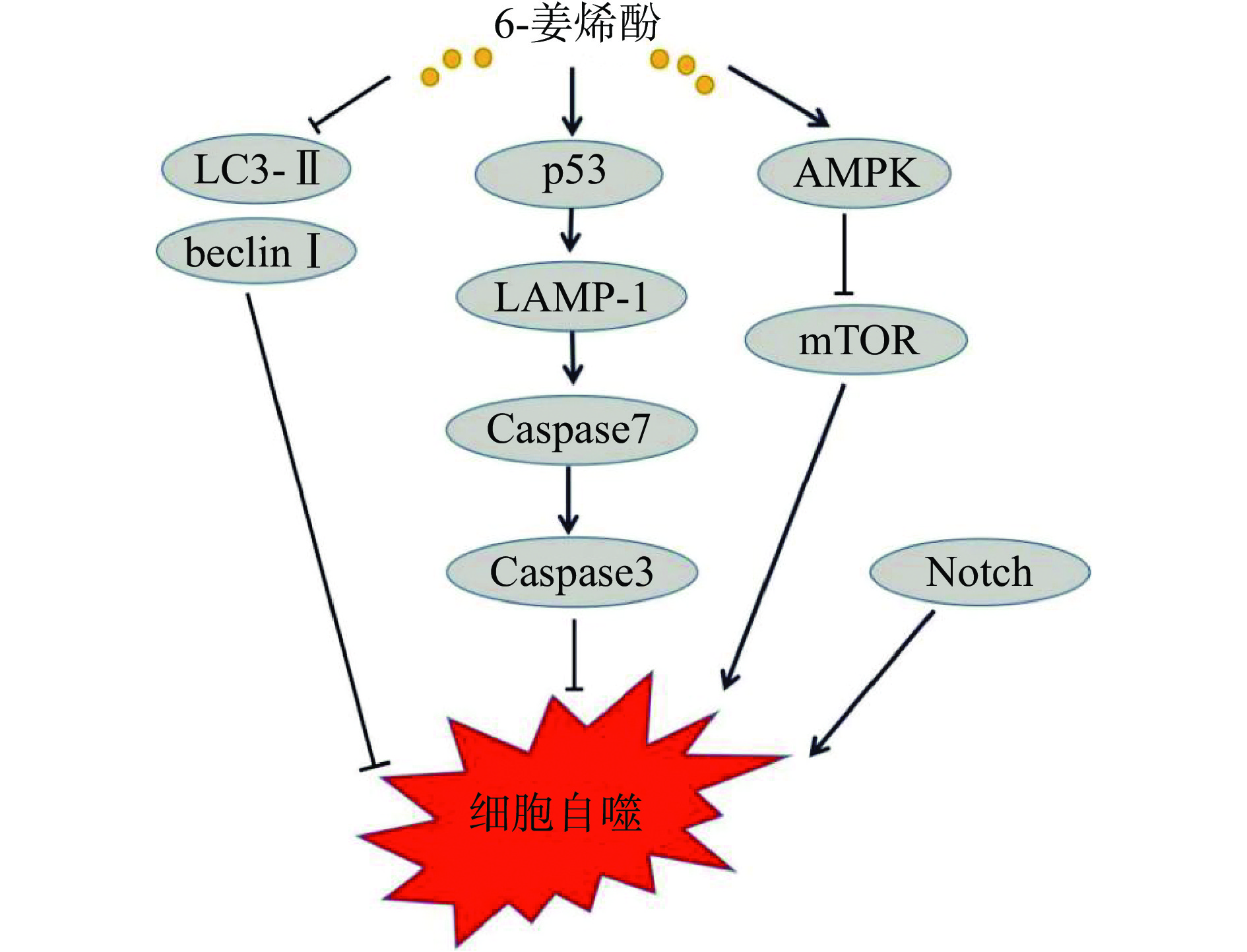
细胞自噬是细胞在自噬相关基因的调控下,利用溶酶体清除自身受损的细胞器及大分子物质的过程,是维持细胞内环境稳定的重要过程[55]。自噬对于肿瘤细胞存在双向效应,在肿瘤发生早期,自噬可抑制癌前细胞的持续生长,此时自噬发挥着抑癌的作用;但是在肿瘤细胞持续分裂增殖的过程中,癌细胞通过自噬对抗营养缺乏和缺氧,此时自噬过程的过度激活能使肿瘤细胞存活和生长,发挥促癌作用[56]。beclin-I和LC3-II是与自噬过程相关的重要基因,二者高表达将促进细胞自噬。Bawadood等[8]将乳腺癌MCF-7细胞用6-姜烯酚处理后,发现6-姜烯酚显著抑制beclin-I和LC3-II的mRNA水平,抑制自噬,从而抑制乳腺癌细胞MCF-7的生长。胰腺癌Panc-1细胞经6-姜烯酚处理后,细胞中LC3-II/LC3-I比值升高,SQSTM1/p62蛋白表达水平降低,细胞质的空泡化增强,自噬的正调节因子AMPK被激活,负调控自噬因子mTOR受抑制,触发了细胞自噬[12]。Li等[57]发现,6-姜烯酚可诱导结肠癌HT-29细胞的细胞周期G2/M阻滞、自噬和凋亡,HT-29细胞在细胞周期的每个阶段都形成了大量的自噬小体,酸性囊泡和LAMP-1(溶酶体相关膜蛋白1)的增加表明6-姜烯酚导致细胞自噬死亡、细胞收缩、Caspase-3/7活性升高。在乳腺癌细胞上,6-姜烯酚通过下调γ-分泌酶介导的Notch信号通路,引起自噬通量导致细胞死亡[58]。以上研究均表明6-姜烯酚可通过细胞自噬促进细胞凋亡,作用机制见图5。
5. 6-姜烯酚与其他化合物的协同抗癌活性
在癌症治疗过程中,化疗是最常见的治疗方式,但其具有一定的毒副作用和局限性,为了提高患者的生存质量,科研工作者一直都在努力解决此难题。目前,天然产物与化疗药物的联合使用,常常被认为可减轻患者病痛,达到协同抗癌的目的。Nazim等[59]研究发现,6-姜烯酚联合肿瘤坏死因子(TNF)相关凋亡诱导配体(TRAIL)具有减弱肿瘤细胞增殖,诱导肝癌细胞死亡的作用,提示TRAIL联合6-姜烯酚可能是治疗肝癌耐药的潜在方案。另有研究表明,6-姜烯酚与5-氟尿嘧啶联合使用,可抑制肝癌细胞活力,促进G0/G1细胞周期阻滞,并加速细胞凋亡,下调Akt/mTOR通路和细胞周期相关蛋白的表达水平,增强对5-氟尿嘧啶治疗肝癌的效果[60]。6-姜烯酚还可以选择性地协同增强甲氨蝶呤对恶性淋巴母细胞的细胞效应,促进恶性淋巴母细胞的凋亡[32]。在胰腺癌中,6-姜烯酚与吉西他滨联合使用,可调节TLR4/NF-κB信号通路,增强吉西他滨的抗肿瘤活性[61]。在卵巢癌中,6-姜烯酚与紫杉醇联合使用,可调控TLR4-MD2/MyD88和NF-κB信号通路,增强紫杉醇治疗卵巢癌的疗效[62]。以上研究均表明,6-姜烯酚与其他化合物联合使用,可提高药物的治疗效果,更好地发挥抗肿瘤作用。
6. 总结与展望
癌症严重危害人类健康,6-姜烯酚是一种从生姜中提取的天然产物,在肝癌、乳腺癌、结肠癌、胰腺癌、宫颈癌、口腔癌、前列腺癌、胃癌等多种恶性肿瘤细胞中都具有较好的抑制作用。6-姜烯酚发挥抗肿瘤活性的作用机制主要有(表1):通过调控Notch信号通路、Wnt/β-catenin信号通路、影响内质网功能,来抑制肿瘤细胞的增殖。通过p53、PI3K/Akt、MAPK等信号通路及内源性线粒体途径诱导肿瘤细胞发生细胞凋亡。影响细胞周期相关蛋白CDK6、p21、CyclinD1、CyclinD3表达,阻滞细胞周期;调控PI3K/Akt/mTOR、STAT3/MMP2、EMT、NF-κB等信号通路,下调迁移和侵袭相关分子MMP2、MMP9、N-cad、Vimentin等蛋白表达,抑制肿瘤细胞的迁移和侵袭。调控Akt-mTOR信号通路,阻断p53和ROS水平,抑制beclin-I和LC3-II的表达等影响肿瘤细胞自噬。
虽然6-姜烯酚的抗肿瘤作用报道较多,但目前仍有更广的研究前景:6-姜烯酚作用直接靶点的鉴定、成药工艺研究等方面缺乏深入的探索,今后可以应用基因的敲低或过表达技术,进行作用靶点的直接鉴定,并对6-姜烯酚的成药工艺进行进一步优化。6-姜烯酚的抗肿瘤分子机制之间具有关联性,通过不同分子之间的相互作用影响肿瘤细胞的增殖、迁移、侵袭和凋亡,其发挥抗肿瘤的机制一般由多条信号通路的共同参与,因此进一步研究6-姜烯酚发挥抗肿瘤作用的信号通路对6-姜烯酚的发展及应用有很大的推动作用。6-姜烯酚的治疗效果受到水溶性差、口服吸收差和代谢快的限制,今后可通过纳米材料或使用微胶囊技术将其包埋,提高治疗效果。目前对6-姜烯酚的抗肿瘤机制研究大多局限于体外细胞实验,异种移植的动物模型相对较少,因此需要进行更多的体内实验来进一步验证其抗肿瘤作用,同时开展临床试验评估其在人体不同肿瘤预防和治疗的有效性和安全性,从而使6-姜烯酚最大程度的发挥抗癌功效,提高患者生存质量。综上所述,6-姜烯酚具有重要的研究意义和潜在的临床应用价值。
-
表 1 6-姜烯酚的抗肿瘤作用及其机制
Table 1 Antitumor effect and its mechanism of 6-shogaol
药理作用 癌症类型 癌症细胞系或动物模型 作用机制 参考文献 抑制肿瘤细胞增殖 肝癌 Bel-7402 调控Wnt/β-Catenin信号通路 [7] HepG2 Bax、细胞色素C和Caspase3表达升高 [28] 乳腺癌 MCF-7和T47D 靶向Notch信号通路,抑制细胞自噬 [8] 口腔癌 YD-10B和Ca9-22 调控PI3K/AKT/mTOR信号通路 [10] OC2 显著增加Ca2+含量,影响内质网功能 [63] 8~10周龄的雄性金色叙利亚仓鼠 NF-κB和AP-1致癌信号来减轻炎症和细胞增殖 [64] 胰腺癌 Panc-1、AsPC-1、BxPC-3、CAPAN-2、CFPAC-1、MIAPaCa-2和SW1990 通过影响内源性线粒体功能,造成Caspase3释放 [12] 结肠癌 COLO 205 ROS水平升高,Bax、Fas、FasL表达上调,Bcl-2、Bcl-XL表达下调 [36] 脂肪肉瘤 SW872和93T449 调控STAT-3、AMPK信号通路,影响内质网功能 [21] 诱导肿瘤细胞凋亡 肝癌 Bel-7402、HepG2 调控Wnt/β-Catenin信号通路 [7] SMMC-7721 抑制elF-2α的磷酸化,影响内质网应激 [39] 前列腺癌 LNCaP、DU145和PC3 调控STAT3和 NF-κB信号通路 [16] 结肠癌 HCT-116、SW-480和HT-29 促进Bax、Caspase3和PARP1蛋白的表达,抑制Bcl-2的表达 [24, 26] 胃癌 BGC-823 调控EMT信号通路 [29] 淋巴癌 CCRF-CEM和Nalm-6 调控p53信号通路,影响ROS水平 [32] 宫颈癌 HeLa和SiHa 通过影响线粒体功能,进而影响ROS,
造成Caspase3释放[27, 54] 阻滞肿瘤细胞周期 前列腺癌 LNCaP、DU145和PC3 p21和p27蛋白表达明显升高,Cyclin D1蛋白表达下降 [16] 肺癌 H1650、H520和H1975 下调Cyclin D1和Cyclin D3,调控Akt [47] 宫颈癌 HeLa 显著下调Cyclin B1、PCNA表达,上调p21蛋白表达 [27] 结肠癌 HCT-116和SW480 上调p53蛋白和CDK抑制剂表达,下调cdc2蛋白表达 [46] 淋巴癌 CCRF-CEM和Nalm-6 调控p53信号通路,影响ROS水平 [32] 抑制肿瘤细胞迁移和侵袭 肝癌 Hep3B 调控NF-κB、MAPK信号通路 [51] 乳腺癌 MDA-MB231 调控Hedgehog/Gli1通路 [52] 胃癌 BGC-823 调控EMT信号通路 [29] 口腔癌 YD-10B和Ca9-22 调控Akt信号通路 [10] 宫颈癌 HeLa 与ECM信号通路相关蛋白表达水平有关 [54] 影响肿瘤细胞自噬 肺癌 A549 阻断Akt-mTOR信号通路,诱导细胞自噬 [65] 肝癌 Huh7 通过影响p53和ROS水平,导致自噬通量衰减 [59] 乳腺癌 MCF-7 抑制beclin-I和LC3-II的表达,抑制细胞自噬 [8] 结肠癌 HT29 形成大量自噬小体,Caspase3和Caspase7活性升高 [57] -
[1] FERLAY J, COLOMBET M, SOERJOMATARAM I, et al. Cancer statistics for the year 2020: An overview[J]. International Journal of Cancer, 2021.
[2] CAO W, CHEN H D, YU Y W, et al. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020[J]. Chinese Medical Journal (England),2021,134(7):783−791.
[3] MOHD Y Y A. Gingerol and its role in chronic diseases[J]. Advances in Experimental Medicine and Biology,2016,929:177−207.
[4] MARX W, RIED K, MCCARTHY A L, et al. Ginger-mechanism of action in chemotherapy-induced nausea and vomiting: A review[J]. Critical Reviews in Food Science and Nutrition,2017,57(1):141−146. doi: 10.1080/10408398.2013.865590
[5] SEMWAL R B, SEMWAL D K, COMBRINCK S, et al. Gingerols and shogaols: Important nutraceutical principles from ginger[J]. Phytochemistry,2015,117:554−568. doi: 10.1016/j.phytochem.2015.07.012
[6] KOU X, LI X Z, RAMIM T R, et al. Efficient dehydration of 6-gingerol to 6-shogaol catalyzed by an acidic ionic liquid under ultrasound irradiation[J]. Food Chemistry,2017,215:193−199. doi: 10.1016/j.foodchem.2016.07.106
[7] ZHANG Y, WANG J J, QU Y, et al. 6-Shogaol suppresses the progression of liver cancer via the inactivation of Wnt/Catenin signaling by regulating TLR4[J]. American Journal of Chinese Medicine,2021,49(8):2033−2048. doi: 10.1142/S0192415X21500968
[8] BAWADOOD A S, ABBASI F A, ANWAR F, et al. 6-Shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway[J]. Biomedicine & Pharmacothertpy,2020,128:110302.
[9] WU C H, HONG B, HO C T, et al. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene[J]. Journal of Agricultural and Food Chemistry,2015,63(9):2432−2441. doi: 10.1021/acs.jafc.5b00002
[10] HUANG H, KIM M K, KIM K R. Anticancer effects of 6-shogaol via the AKT signaling pathway in oral squamous cell carcinoma[J]. Journal of Applied Oral Science,2021,29:e209. doi: 10.1590/1678-7757-2021-0209
[11] ZHU Y D, WARIN R F, SOROKA D N, et al. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: Chemical synthesis and biological evaluation[J]. PLoS One,2013,8(1):e54677. doi: 10.1371/journal.pone.0054677
[12] AKIMOTO M, IZUKA M, KANEMATSU R, et al. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death[J]. PLoS One 2015, 10(5): e0126605.
[13] YAO C, OH J H, OH I G, et al. [6]-Shogaol inhibits melanogenesis in B16 mouse melanoma cells through activation of the ERK pathway[J]. Acta Pharmacologica Sinica,2013,34(2):289−294. doi: 10.1038/aps.2012.134
[14] LIU C M, KAO C, TSENG Y T, et al. Ginger phytochemicals inhibit cell growth and modulate drug resistance factors in docetaxel resistant prostate cancer cell[J]. Molecules,2017,22(9):1477. doi: 10.3390/molecules22091477
[15] BRAHMBHATT M, GUNDALA S R, ASIF G, et al. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation[J]. Nutrition and Cancer,2013,65(2):263−272. doi: 10.1080/01635581.2013.749925
[16] SAHA A, BLANDO J, SILVER E, et al. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling[J]. Cancer Prevention Research,2014,7(6):627−638. doi: 10.1158/1940-6207.CAPR-13-0420
[17] FU J S, CHEN H D, SOROKA D N, et al. Cysteine-conjugated metabolites of ginger components, shogaols, induce apoptosis through oxidative stress-mediated p53 pathway in human colon cancer cells[J]. Journal of Agricultural and Food Chemistry,2014,62(20):4632−4642. doi: 10.1021/jf501351r
[18] QI L W, ZHANG Z Y, ZHANG C F, et al. Anti-colon cancer effects of 6-shogaol through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk[J]. American Journal of Chinese Medicine,2015,43(4):743−756. doi: 10.1142/S0192415X15500469
[19] ISHIGORO K, ANDO T, MAEDA O, et al. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms[J]. Biochemical and Biophysical Research Communications,2007,362(1):218−223. doi: 10.1016/j.bbrc.2007.08.012
[20] KOTOWSKI U, KADLETZ L, SCHNEIDER S, et al. 6-Shogaol induces apoptosis and enhances radiosensitivity in head and neck squamous cell carcinoma cell lines[J]. Phytotherapy Research,2018,32(2):340−347. doi: 10.1002/ptr.5982
[21] YADAV A K, JANG B C. Anti-survival and pro-apoptotic effects of 6-Shogaol on SW872 human liposarcoma cells via control of the intrinsic caspase pathway, STAT-3, AMPK, and ER Stress[J]. Biomolecules,2020,10(10):1380. doi: 10.3390/biom10101380
[22] WONG R S. Apoptosis in cancer: From pathogenesis to treatment[J]. Journal of Experimental & Clinical Cancer Research,2011,30(1):87−87.
[23] AOUACHERIA A, BAGHDIGUIAN S, LAMB H M, et al. Connecting mitochondrial dynamics and life-or-death events via Bcl-2 family proteins[J]. Neurochemistry International,2017,109:141−161. doi: 10.1016/j.neuint.2017.04.009
[24] 王宇锋, 杨春, 陈超, 等. 6-姜烯酚诱导SW480凋亡及对APC基因表达的影响[J]. 现代食品科技,2018,34(2):14−19, 175. [WANG Y F, YANG C, CHEN C, et al. Effect of 6-shogaol on apoptosis of SW480 and APC gene expression[J]. Modern Food Technology,2018,34(2):14−19, 175. [25] 王宇锋, 刘旭, 陈超, 等. 6-姜烯酚对结直肠癌细胞中Keap1/Nrf2通路及下游基因表达的影响[J]. 宁夏医科大学学报,2017,39(10):1127−1132, 1241. [WANG Y F, LIU X, CHEN C, et al. Effect of 6-shogaol on the Keap1/Nrf2 pathway and downstream gene expression in colorectal cancer cells[J]. Journal of Ningxia Medical University,2017,39(10):1127−1132, 1241. doi: 10.16050/j.cnki.issn1674-6309.2017.10.004 [26] 王宇锋, 陈超, 杨春, 等. 6-姜烯酚诱导人结直肠癌细胞凋亡及与Bax、BCL2、Caspase3和PARP1基因表达的影响[J]. 现代食品科技,2017,33(11):7−15. [WANG Y F, CHEN C, YANG C, et al. Effects of hydrophenol inducing apoptosis and gene expression with Bax, BCL2, Caspase3, and PARP1 in human colorectal cancer cells[J]. Modern Food Technology,2017,33(11):7−15. doi: 10.13982/j.mfst.1673-9078.2017.11.002 [27] PEI X D, HE Z L, YAO H L, et al. 6-Shogaol from ginger shows anti-tumor effect in cervical carcinoma via PI3K/Akt/mTOR pathway[J]. European Journal of Nutrition,2021,60(5):2781−2793. doi: 10.1007/s00394-020-02440-9
[28] GOVINDHAN A, SURESH K, NAGAPPAN K. [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in Hep-2 cells[J]. Biomedecine & Pharmacotherapie,2016,82:226−236.
[29] 赵行宇, 张漠, 朱志华, 等. 6-姜烯酚对胃癌BGC-823细胞侵袭及迁移的影响及相关作用机制研究[J]. 上海中医药杂志,2020,54(10):82−86. [ZHAO X Y, ZHANG M, ZHU Z H, et al. Effects of 6-shogaol on invasion and migration of BGC-823 cells in gastric cancer[J]. Shanghai Journal of Traditional Chinese Medicine,2020,54(10):82−86. [30] 赵行宇, 侯以森, 刘雅范, 等. 6-姜烯酚诱导胃癌BGC-823细胞凋亡及其机制研究[J]. 上海中医药杂志,2018,52(2):84−88. [ZHAO X Y, HOU Y S, LIU Y F, et al. Apoptosis and its mechanism of 6-shogaol induced BGC-823 cells in gastric cancer[J]. Shanghai Journal of Traditional Chinese Medicine,2018,52(2):84−88. doi: 10.16305/j.1007-1334.2018.02.021 [31] LI L, MAO Y X, ZHAO L N, et al. P53 regulation of ammonia metabolism through urea cycle controls polyamine biosynthesis[J]. Nature,2019,567(7747):1−4.
[32] DORCHEH S N, RAHGOZAR S, TALEI D. 6-Shogaol induces apoptosis in acute lymphoblastic leukaemia cells by targeting p53 signalling pathway and generation of reactive oxygen species[J]. Journal of Cellular and Molecular Medicine,2021,25(13):6148−6160. doi: 10.1111/jcmm.16528
[33] WARIN R F, CHEN H D, SOROKA D N, et al. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2[J]. Journal of Agricultural & Food Chemistry,2014,62(6):1352−1362.
[34] ZROVO D B, FILBURN C R, KLOTZ L, et al. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release[J]. Physiological Reviews,2014,94(3):909. doi: 10.1152/physrev.00026.2013
[35] WU H M, LI Y M. In vitro antitumor activity of guttiferone-A in human breast cancer cells is mediated via apoptosis, mitochondrial mediated oxidative stress and reactive oxygen species production[J]. Journal of Buon,2017,22(6):1500−1504.
[36] PAN M H, HSIEH M C, KUO J M, et al. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression[J]. Molecular Nutrition & Food Research,2008,52(5):527−537.
[37] ROMERO A C, SEQUEDA L G, ANDRES A F, et al. Effect of 6-shogaol on the glucose uptake and survival of HT1080 fibrosarcoma cells[J]. Pharmaceuticals (Basel), 2019, 12(3): 131.
[38] 李帅, 张炳东. 细胞凋亡途径的研究进展[J]. 山东医药,2017,57(37):103−106. [LI S, ZHANG B D. Progress in studying the apoptotic pathways[J]. Shandong Medicine,2017,57(37):103−106. doi: 10.3969/j.issn.1002-266X.2017.37.036 [39] HU R, ZHOU P, PENG Y B, et al. 6-Shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress[J]. PLoS One,2012,7(6):e39664. doi: 10.1371/journal.pone.0039664
[40] NEDUNGADI D, BINOY A, VINOD V, et al. Ginger extract activates caspase independent paraptosis in cancer cells via ER stress, mitochondrial dysfunction, AIF translocation and DNA damage[J]. Nutrion Cancer,2021,73(1):147−159. doi: 10.1080/01635581.2019.1685113
[41] JARRETT A M, LIMA E, HORMUTH D, et al. Mathematical models of tumor cell proliferation: A review of the literature[J]. Expert Review of Anticancer Therapy,2018,18(12):1271−1286. doi: 10.1080/14737140.2018.1527689
[42] DÍAZ-CORÁNGUEZ M, LIU X, ANTONETTI D A. Tight junctions in cell proliferation[J]. International Journal of Molecular Science,2019,20(23):5972. doi: 10.3390/ijms20235972
[43] MATHIYAZHAGAN J, SIVA R, JAYARAJ R, et al. Preventive effect of combined zingiber officinale and terminalia chebula against DMBA-induced breast cancer rats via mTOR inhibition[J]. Nutrition Cancer,2022,74(2):687−696. doi: 10.1080/01635581.2021.1903948
[44] OTTO T, SICINSKI P. Cell cycle proteins as promising targets in cancer therapy[J]. Nature Reviews Cancer,2017,17(2):93−115. doi: 10.1038/nrc.2016.138
[45] WENZEL E S, SINGH A T K. Cell-cycle checkpoints and aneuploidy on the path to cancer, in vivo[J]. Vivo,2018,32(1):1−5.
[46] LEE K, WU K, YEN C, et al. 6-Shogaol antagonizes the adipocyte-conditioned medium-initiated 5-fluorouracil resistance in human colorectal cancer cells through controlling the SREBP1 level[J]. Life (Basel),2021,11(10):1067.
[47] KIM M, LEE M, OI N, et al. [6]-Shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2[J]. Carcinogenesis,2014,35(3):683−691. doi: 10.1093/carcin/bgt365
[48] LIU Q, PENG Y B, QI L W, et al. The cytotoxicity mechanism of 6-shogaol-treated HeLa human cervical cancer cells revealed by label-free shotgun proteomics and bioinformatics analysis[J]. Evidence-based Complementary and Alternative Medicine,2012,1(1):278652.
[49] CHEN T, YOU Y N, JIANG H, et al. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis[J]. Journal of Cellular Physiology,2017,232(12):3261−3272. doi: 10.1002/jcp.25797
[50] MARKOPOULOS G S, ROEPAKIA E, MARCU K B, et al. Epigenetic regulation of inflammatory cytokine-induced epithelial to mesenchymal cell transition and cancer stem cell generation[J]. Cells,2019,8(10):1143. doi: 10.3390/cells8101143
[51] WENG C, CHOU C P, HO C, et al. Molecular mechanism inhibiting human hepatocarcinoma cell invasion by 6-shogaol and 6-gingerol[J]. Molecular Nutrition & Food Research,2012,56(8):1304−1314.
[52] 林嘉怡, 柯乔丹, 吴锦如, 等. 6-姜烯酚介导Hedgehog/Gli1通路对三阴性乳腺癌细胞侵袭及迁移作用机制[J]. 中国药理学通报,2022,38(3):373−379. [LIN J Y, KE Q D, WU J R, et al. Mechanism of 6-gingerenol mediated Hedgehog/Gli1 pathway on invasion and migration of triple negative breast cancer cells[J]. China Pharmacology Bulletin,2022,38(3):373−379. [53] HONG B, WU C H, YEH C T, et al. Invadopodia-associated proteins blockade as a novel mechanism for 6-shogaol and pterostilbene to reduce breast cancer cell motility and invasion[J]. Molecular Nutrition & Food Research,2013,57(5):886−895.
[54] 赵行宇, 张漠, 朱志华, 等. 6-姜烯酚对HPV阳性及阴性宫颈癌细胞侵袭及迁移的作用及其机制[J]. 解放军医学杂志,2020,45(7):691−696. [ZHAO X Y, ZHANG M, ZHU Z H, et al. Effect and mechanism of 6-gingerol on invasion and migration of HPV-positive and negative cervical cancer cells[J]. Medical Journal of PLA,2020,45(7):691−696. doi: 10.11855/j.issn.0577-7402.2020.07.02 [55] ONORATI A V, DYCZYNSKI M, OJHA R, et al. Targeting autophagy in cancer[J]. Cancer,2018,124(16):3307−3318. doi: 10.1002/cncr.31335
[56] LI Y, YANG C, SHARO A T, et al. Autophagy pathway: Cellular and molecular mechanisms[J]. Taylor & Francis,2018,14(2):207−215.
[57] LI T Y, CHIANG B H. 6-Shogaol induces autophagic cell death then triggered apoptosis in colorectal adenocarcinoma HT-29 cells[J]. Biomedicine & Pharmacotherapy,2017,93:208−217.
[58] RAY A, VASUDEVAN S, SENGUPTA S. 6-Shogaol inhibits breast cancer cells and stem cell-like spheroids by modulation of Notch signaling pathway and induction of autophagic cell death[J]. PLoS One,2015,10(9):e0137614. doi: 10.1371/journal.pone.0137614
[59] NAZIM U M, PARK S Y. Attenuation of autophagy flux by 6-shogaol sensitizes human liver cancer cells to TRAIL-induced apoptosis via p53 and ROS[J]. International Journal of Molecular Medicine,2019,43(2):701−708.
[60] ZHANG Y, QU Y, CHEN Y Z. Influence of 6-shogaol potentiated on 5-fluorouracil treatment of liver cancer by promoting apoptosis and cell cycle arrest by regulating AKT/mTOR/MRP1 signalling[J]. Chinese Journal of Natural Medicines,2022,20(5):352−363. doi: 10.1016/S1875-5364(22)60174-2
[61] ZHOU L, QI L, JIANG L, et al. Antitumor activity of gemcitabine can be potentiated in pancreatic cancer through modulation of TLR4/NF-κB signaling by 6-shogaol[J]. The AAPS Journal,2014,16(2):246−257. doi: 10.1208/s12248-013-9558-3
[62] ZHU Y, ZHANG G, LI M, et al. Ultrasound-augmented phase transition nanobubbles for targeted treatment of paclitaxel-resistant cancer[J]. Bioconjug Chemistry,2020,31(8):2008−2020. doi: 10.1021/acs.bioconjchem.0c00364
[63] CHEN C Y, YANG Y H, KUO S Y. Effect of [6]-shogaol on cytosolic Ca2+ levels and proliferation in human oral cancer cells (OC2)[J]. Journal of Natural Products,2010,73(8):1370−1374. doi: 10.1021/np100213a
[64] ANNARMALAI G, SERESH K. [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis[J]. Biomedicine & Pharmacotherapy,2018,98:484−490.
[65] HUNG J, HSU Y L, LI C, et al. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells[J]. Journal of Agricultural and Food Chemistry,2009,57(20):9809−9816. doi: 10.1021/jf902315e
-
期刊类型引用(5)
1. 王共明,黄会,丁玉竹,薛敬林,舒志强,井月欣,矫春娜,张健. 海参粉超临界CO_2萃取脱脂工艺优化及对挥发性风味物质的影响. 食品工业科技. 2025(03): 241-248 .  本站查看
本站查看
2. 李正阳,刘畅,费靖淳,周浩,韩万鑫,潘一萍,祁艳霞,赵前程. 刺参自溶肽美拉德反应产物表征及抗氧化活性研究. 食品安全质量检测学报. 2025(04): 224-233 .  百度学术
百度学术
3. 赵影,田柬昕,钟碧銮,李萌,苏可珍. 海参深加工脱腥技术研究进展. 食品工业. 2024(03): 229-235 .  百度学术
百度学术
4. 王志龙,王禹,段静瑶,苏岩峰,喻佩. 海参制品腥味化合物形成与脱腥技术研究进展. 中国调味品. 2024(06): 206-212 .  百度学术
百度学术
5. 马慧,顾雪敏,李芳,梅洁,王梓棚,孔令明. 菌酶协同处理在食品加工中的研究进展及应用. 中国调味品. 2024(07): 208-213 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:

 下载:
下载:



