Simultaneous Determination of Lovastatin and Lovastatin Acid in Hongqu Fuling Tablets by on Quantitative Analysis of Multi—Components by Single Marker(QAMS)
-
摘要: 目的:建立适于红曲茯苓片质量控制的洛伐他汀和洛伐他汀酸的一测多评含量测定方法。方法:采用高效液相色谱法,以洛伐他汀为对照,建立与其开环产物洛伐他汀酸的校正因子关系,同时对产品中洛伐他汀和洛伐他汀酸两种成分同时进行定量检测的一测多评方法。结果:阴性样品无干扰,样品检测方法的专属性良好,洛伐他汀和洛伐他汀酸在相应的线性范围内线性关系良好,R2>0.9999,精密度和重复性RSD值均小于3%,加标回收率为在98.43%~103.18%之间。洛伐他汀对洛伐他汀酸的相对校正因子为0.9000;一测多评法测定三批红曲茯苓片中洛伐他汀酸的平均含量为0.3563 mg/g,外标法测定洛伐他汀酸的平均含量为0.3668 mg/g;洛伐他汀的平均含量为1.1113 mg/g,表明所构建的一测多评法可用于红曲茯苓片的多成分质量评价研究。结论:本研究建立的产品中洛伐他汀定量方法稳定准确,专属性强,为以红曲为原料的红曲茯苓片的质量控制和深度开发提供了科学依据。Abstract: Objective: To establish a quantitative analysis of multi-components by single marker (QAMS) method for the determination of lovastatin and lovastatin acid for the quality control of Hongqu Fuling tablets. Methods: Lovastatin as a control, the RP-HPLC method was used to establish the relationship between the correction factor of lovastatin and lovastatin acid, and to simultaneously quantify both lovastatin and lovastatin acid in the Hongqu Fuling tablets by QAMS. Results: The negative sample had no interference, the specificity of the sample detection method was good, the linear relationship between lovastatin and lovastatin acid was good within the corresponding linear range, R2>0.9999, the precision and repeatability RSD values were less than 3%, and the recovery was 98.43%~103.18%. The relative correction factor of lovastatin acid and lovastatin was 0.9000, and the repeatability was good. The average content of lovastatin acid was 0.3563 mg/g determined by QAMS, and the average content of lovastatin acid determined by external standard method was 0.3668 mg/g, and the average content of lovastatin was 1.1113 mg/g, which indicated that the constructed QAMS method could be used for the multi-component quality evaluation research of Hongqu Fuling Tablets. Conclusion: The QAMS method established in this study was stable, accurate and specific, and would provide a scientific basis for the quality control and in-depth development of Hongqu Fuling tablets.
-
红曲茯苓片是针对高脂血症并脾虚湿盛证的降血脂产品,由红曲、白术、茯苓、葛根、山楂五味药组成[1]。红曲为君药,是将紫色红曲霉菌菌株接种于稻米上经人工培养制成的一种食药双效发酵制品,具有良好的降胆固醇活性[2],临床用于高脂血症、动脉粥样硬化等症的治疗[3-4]。洛伐他汀类物质是其主要活性成分[5],包括闭环的内酯式洛伐他汀[6]和开环的酸式洛伐他汀酸[7],二者在体内同时存在,并相互转化。研究表明,洛伐他汀酸具有更强的生物学活性及安全性[8]。但目前在相关品种的现行质量标准中,仅以洛伐他汀作为唯一的指标成分[9],对洛伐他汀酸的测定关注不多。如果仅检测产品中洛伐他汀的含量,而忽略洛伐他汀酸的含量,就必然会影响到对含有红曲相关产品降血脂作用的物质基础的认识和研究。因此,非常有必要针对红曲茯苓片中两种形式的洛伐他汀同时进行含量测定。
目前,针对红曲及其制品中两种不同形式洛伐他汀含量测定的方法,包括了以洛伐他汀为对照,根据洛伐他汀酸及洛伐他汀的峰面积之和与洛伐他汀对照的峰面积比值进行测定的方法[10]以及以洛伐他汀和洛伐他汀酸为对照,在同一色谱条件下分别测定二者含量的方法[11]。但前者未考虑两者的响应因子差异带来的误差,后者受制于洛伐他汀酸对照品的化学稳定性较差,价格昂贵,不易获得和保存等问题,均限制了应用。同时,亦有文献报道可采用一测多评法以化学性质稳定、价廉易得的洛伐他汀为内标物[12],测定红曲及其制剂中两个成分的含量[9]。但仍存在如洛伐他汀酸标准品不能准确定量制备容易产生误差等问题。
因此,本研究旨在建立一个更加完善、简便、稳定地检测红曲茯苓片中洛伐他汀含量的一测多评的方法,降低检测成本和时间,提高方法的实用性,也为进一步以红曲为原料的降血脂产品的开发和中药复方产品研发评价体系的建立提供科学依据。
1. 材料与方法
1.1 材料与仪器
红曲茯苓片 中试样品,批号:210101、210102、210103;洛伐他汀对照品 HPLC≥98%,上海源叶生物科技有限公司,批号:AS0421LA14、Y18J11C116004;磷酸 色谱纯,上海阿拉丁生化科技股份有限公司,批号:K1912183;乙腈 色谱纯,上海星可离纯溶剂有限公司,批号:0114220309;纯化水 杭州娃哈哈集团有限公司;无水乙醇(99.7%)、氢氧化钠(95%,批号RH292944)、盐酸(95%) 分析纯,罗恩试剂。
FA2004型电子天平 万分之一,上海舜宇恒平科学仪器有限公司;FA305N型电子天平 十万分之一,上海菁海仪器有限公司;BT125D型电子天平 赛多利斯科学仪器(北京)有限公司;KQ250DE型数控超声波清洗器、KQ5200DE型数控超声波清洗器 昆山市超声仪器有限公司;FB20型实验室pH 计 奥豪斯仪器(常州)有限公司;TG16-WS型高速离心机 湖南湘鑫仪器仪表有限公司;Sorvall ST 8R型高速冷冻离心机 赛默飞世尔科技(中国)有限公司;LC-20AT型高效液相色谱仪 日本岛津株式会社;Haier BCD-258WBCS H型冰箱 青岛海尔股份有限公司。
1.2 实验方法
1.2.1 色谱条件
Dimonsil-C18(2)色谱柱(250 mm×4.6 mm,5 μm);以乙腈-0.1%磷酸水(65:35,V/V)为流动相等度洗脱,柱温为25 ℃,检测波长为238 nm,流速为1.0 mL/min,进样量为10 μL,理论塔板数以洛伐他汀计不低于4000[5,7]。
1.2.2 标准溶液制备
1.2.2.1 洛伐他汀和洛伐他汀酸标准储备溶液制备
精密称取洛伐他汀对照品16.00 mg,以75%乙醇定容至10 mL,配制浓度为1.600 mg/mL的洛伐他汀标准储备溶液。精密吸取储备液2.5 mL,置于5 mL容量瓶中,用0.2 mol/L氢氧化钠溶液定容至5 mL,于50 ℃下40 kHz超声转化1 h,放置至室温后再放置1 h,转入25 mL烧杯中,加入0.2 mol/L盐酸调节pH至中性(7.0~7.7),用体积分数75%乙醇定容至10 mL,制备浓度为400 µg/mL的洛伐他汀酸标准储备溶液。
1.2.2.2 洛伐他汀及洛伐他汀酸线性关系溶液制备
按洛伐他汀:洛伐他汀酸=1:1比例,分别精密吸取上述洛伐他汀和洛伐他汀酸标准储备溶液,制备总浓度为320、160、80、40、20、10 µg/mL的(洛伐他汀:洛伐他汀酸=1:1)。
1.2.2.3 混合标准溶液制备
按洛伐他汀:洛伐他汀酸=1:1比例,分别适量精密吸取上述洛伐他汀和洛伐他汀酸标准储备溶液,用75%乙醇制备浓度为40 µg/mL的洛伐他汀及40 µg/mL的洛伐他汀酸混合标准溶液。
1.2.3 供试品溶液制备方法考察
红曲茯苓片研细,精密称取粉末(过3号筛)1.2 g于塞锥形瓶中,加入50 mL 75%乙醇,密塞,称定重量,分别超声提取30、60 min,平行两份,放冷,再称定重量,用75%乙醇补足减失的重量,摇匀,以3500 r/min的转速离心10 min,取上清液,经0.45 µm微孔滤膜过滤,制备供试品溶液。
1.2.4 一测多评的方法学考察
1.2.4.1 专属性考察
按照红曲茯苓片的配方工艺称取白术、茯苓、葛根和山楂加水浸泡、煎煮、滤过、浓缩、干燥、粉碎得到干膏粉,与辅料经混合、制粒、总混、压片制备不含红曲的阴性样品,按1.2.3项下供试品溶液制备方法,制备阴性样品溶液,注入液相色谱仪,记录色谱图。
1.2.4.2 线性关系考察
分别精密吸取1.2.2项下线性关系溶液各10 µL进样,记录色谱图。以峰面积积分值为纵坐标(Y),标准品浓度为横坐标(X)进行线性回归,计算回归方程。
1.2.4.3 洛伐他汀酸校正因子的测定
取混合标准溶液按1.2.1项下色谱条件,连续进样测定3次,测定洛伐他汀和洛伐他汀酸峰面积,以洛伐他汀为参照物,计算洛伐他汀酸的相对校正因子(RCF)f=(As×Ci)/(Ai×Cs)(As为洛伐他汀对照溶液峰面积,Cs为洛伐他汀对照溶液浓度,Ai为洛伐他汀酸对照溶液峰面积,Ci为洛伐他汀酸对照溶液浓度A)。
1.2.4.4 精密度试验
吸取浓度为40 µg/mL的洛伐他汀标准溶液10 µL,按1.2.1项下色谱条件连续进样6次,记录色谱峰面积,并计算相RSD值。
1.2.4.5 稳定性试验
洛伐他汀酸具有不稳定性,按照1.2.3项下方法制备供试品溶液,分别在0、2、4、8、12、24 h对洛伐他汀和洛伐他汀酸含量进行了稳定性考察,计算洛伐他汀和洛伐他汀酸含量的RSD值。
1.2.4.6 重复性试验
按照1.2.3项下方法平行制备6份供试品溶液,进行HPLC测定,计算洛伐他汀的含量值及RSD值,并分别利用外标法和校正因子计算洛伐他汀酸含量和RSD值。
1.2.4.7 加标回收率试验
取样品(洛伐他汀含量以重复性试验平均值计)适量,共9份,精密称量,分别加入洛伐他汀储备液,使得加入洛伐他汀对照品的量与供试品中洛伐他汀的量之比约为0.8:1、1:1、1:1.2,按照1.2.3项下供试品溶液方法制备,每个比例的样品平行三次,进行色谱测定,计算加样回收率及RSD。
1.2.4.8 样品含量测定
按照1.2.3项下供试品溶液制备方法制备三批红曲茯苓片中供试品溶液,计算洛伐他汀的含量值,并分别采用外标法以及一测多评法计算洛伐他汀酸的含量。
2. 结果与分析
2.1 供试品溶液制备方法考察
按照1.2.3供试品溶液制备方法,考察样品经超声提取30、60 min后所洛伐他汀、洛伐他汀酸和总洛伐他汀含量,结果见表1,样品经超声提取30、60 min后所测得的洛伐他汀、洛伐他汀酸和总洛伐他汀含量均相差不大,从节约成本以及检测方法更加简便、快捷的因素,确定样品的超声提取时间为30 min。
表 1 供试品溶液制备方法考察结果Table 1. Results of preparation method of sample solution提取时间(min) 洛伐他汀含量(mg/g) 洛伐他汀酸含量(mg/g) 总洛伐他汀含量(mg/g) 30 1.0063 0.3223 1.3286 1.0175 0.3283 1.3458 60 1.0208 0.3266 1.3474 1.0205 0.3655 1.3860 2.2 一测多评的方法学考察结果
2.2.1 专属性试验
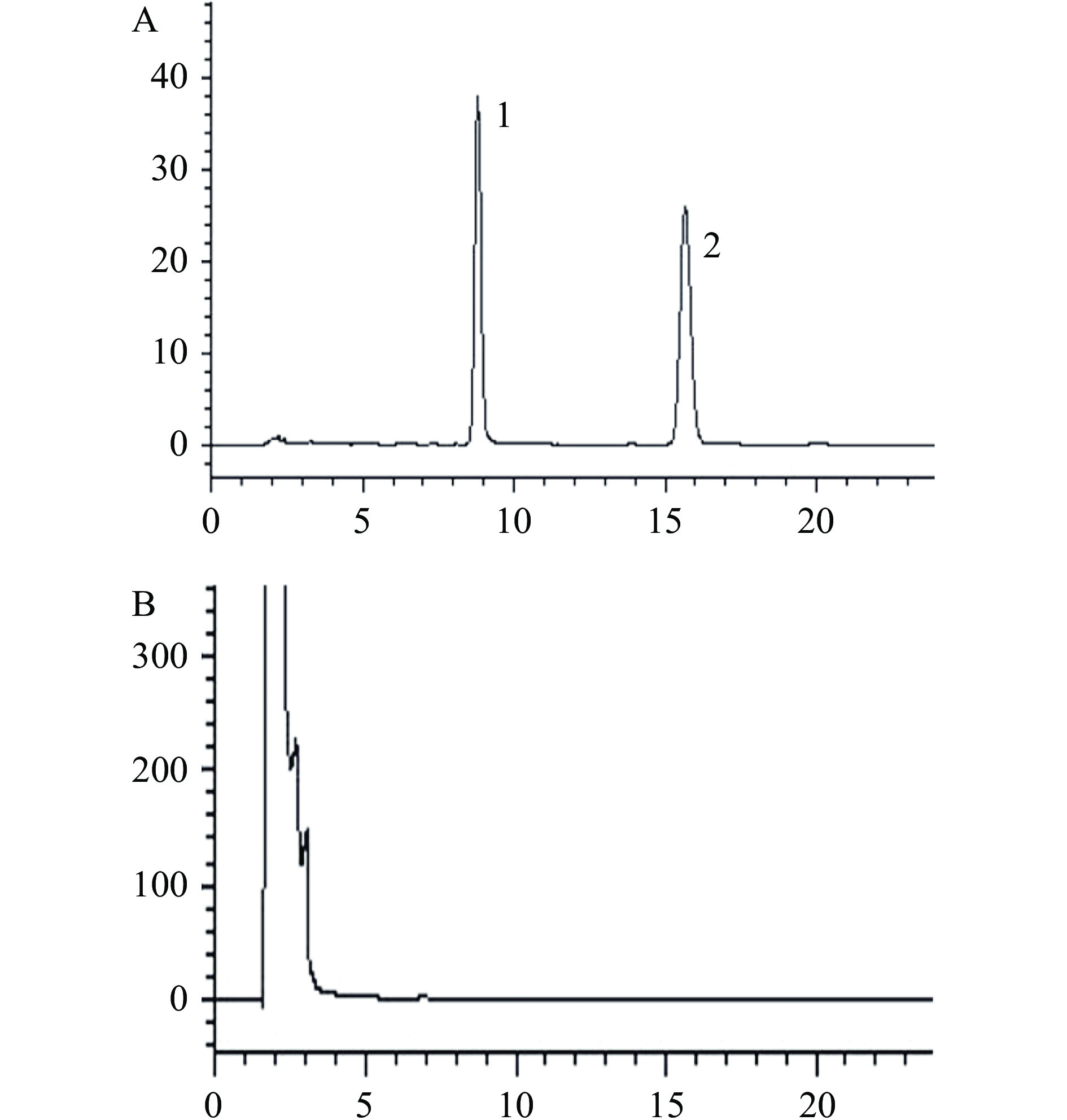
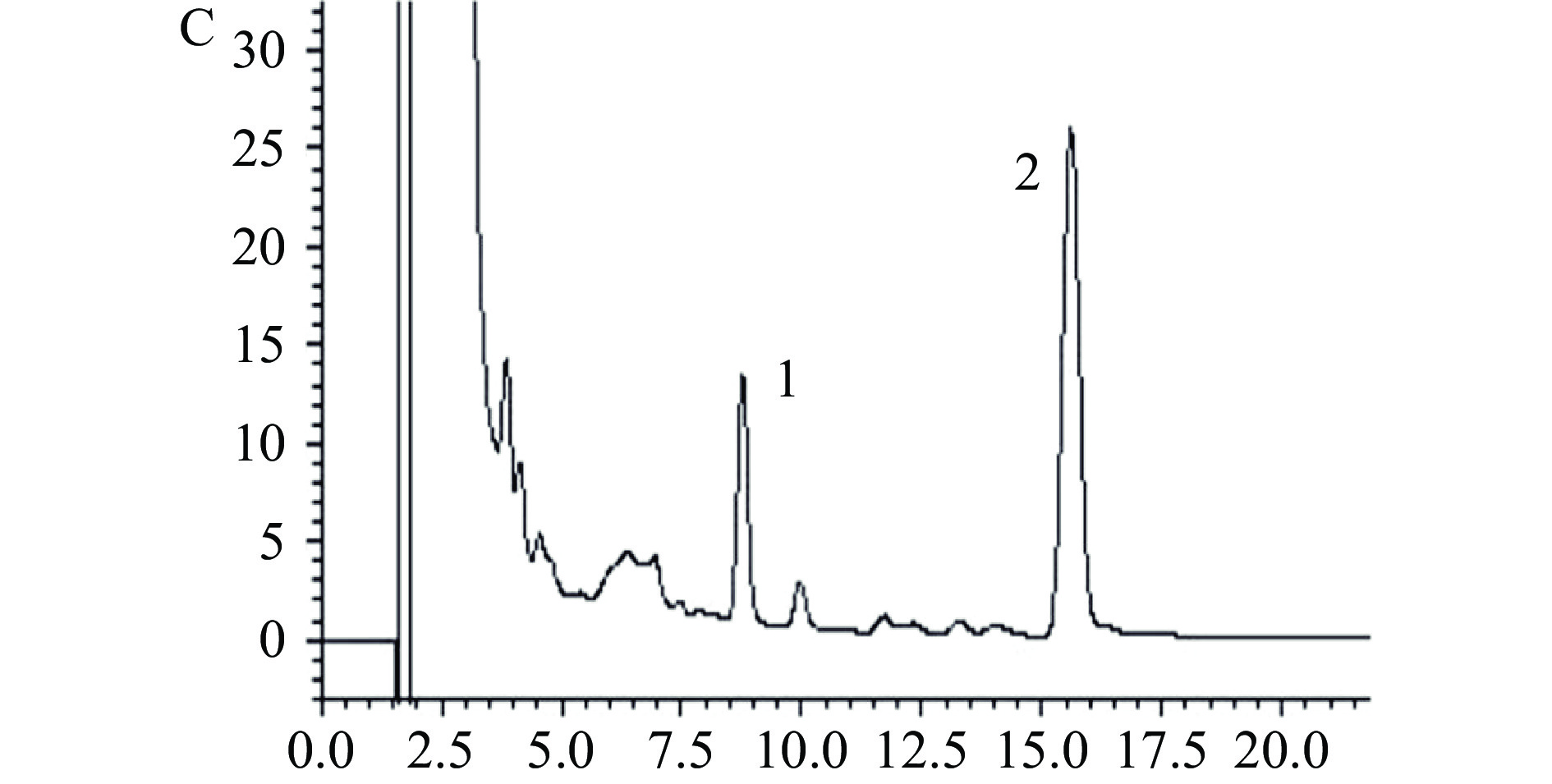
取混合标准溶液、供试品溶液及阴性样品溶液,按1.2.1项下色谱条件分别进样测定。结果如图1所示,洛伐他汀酸的保留时间为8.425 min,洛伐他汀保留时间为15.570 min,色谱峰峰形良好,分离度良好,不受其他杂质峰干扰。阴性样品在两个目标峰的出峰位置上均未出峰,供试品在相应位置则均有出峰,表明该方法的专属性高,可用于红曲茯苓片中洛伐他汀和洛伐他汀酸的的测定。
2.2.2 线性关系考察
按照1.2.4.2项下进行试验,结果表明,洛伐他汀和洛伐他汀酸在5~160 µg/mL范围内线性关系良好,其中洛伐他汀线性方程为Y=29138.34X+27264.74(R2=0.9999),洛伐他汀酸线性方程为Y=27011.87X+18408.09(R2=0.9999),表明在该浓度区间内具有良好的线性关系。
2.2.3 洛伐他汀酸校正因子的测定
按1.2.4.3项下进行试验,结果如表2所示,洛伐他汀对洛伐他汀酸的相对校正因子(RCF)的平均值为0.9000,RSD值分别为0.16%。
表 2 洛伐他汀酸的相对校正因子Table 2. Relative correction factors for lovastatin acid编号 1 2 3 As 567484 566411 566196 Ai 631425 629418 628090 RCF 0.8987 0.8999 0.9015 平均RCF 0.9000 RSD(%) 0.16 2.2.4 精密度考察
按1.2.4.4项下进行试验,洛伐他汀和洛伐他汀酸峰面积的RSD值分别为0.65%和0.29%,表明仪器精密度良好。
2.2.5 稳定性考察
按1.2.4.5项下进行试验,结果如表3,供试品溶液中洛伐他汀、洛伐他汀酸在24 h内的色谱峰峰面积的RSD值分别为1.12%、0.37%,表明供试品溶液在24 h内稳定性良好。
表 3 红曲茯苓片中洛伐他汀、洛伐他汀酸的稳定性试验结果Table 3. Stability results of lovastatin and lovastatin acid in Hongqu Fuling tablets时间(h) 0 2 4 6 8 12 24 洛伐他汀 含量(mg/g) 1.0122 1.0416 1.0418 1.0426 1.0436 1.0439 1.0433 平均含量(mg/g) 1.0384 RSD(%) 1.12 洛伐他汀酸 含量(mg/g) 0.3233 0.3244 0.3228 0.3218 0.3235 0.3253 0.3247 平均含量(mg/g) 0.3237 RSD(%) 0.37 2.2.6 重复性考察
按1.2.4.6项下进行试验,结果见表4,测得样品中洛伐他汀的平均含量值为1.0322 mg/g,RSD值为0.51%。外标法和校正因子法计算洛伐他汀酸含量值分别为0.3582和0.3562 mg/g,RSD值分别为0.61%和0.58%,表明该方法重复性良好。
表 4 重复性试验结果Table 4. Repeatability test results编号 1 2 3 4 5 6 取样量(g) 1.2001 1.2005 1.2004 1.2005 1.2002 1.2003 外标法计算 洛伐他汀含量(mg/g) 1.0259 1.0338 1.0352 1.0379 1.0253 1.0350 平均含量(mg/g) 1.0322 RSD(%) 0.51 洛伐他汀酸含量(mg/g) 0.3550 0.3570 0.3578 0.3610 0.3582 0.3603 平均含量(mg/g) 0.3582 RSD(%) 0.61 一测多评法计算 洛伐他汀酸含量(mg/g) 0.3532 0.3551 0.3559 0.3589 0.3562 0.3581 平均含量(mg/g) 0.3562 RSD(%) 0.58 2.2.7 加标回收率试验
按1.2.4.7项下进行试验,结果见表5,红曲茯苓片中洛伐他汀的加标回收率在98.43%~103.18%之间,RSD值为1.51%,表明各组成回收率良好。
表 5 洛伐他汀加标回收率试验结果Table 5. Results of recovery test of lovastatin编号 称样量
(g)样品中含量
(mg)对照品加入量(mg) 对照品加入量与
样品中含量之比实测含量
(mg)回收率
(%)平均回收率
(%)RSD (%) 1 0.6004 0.6197 0.500 0.8:1 1.1086 102.28 101.26 1.51 2 0.6003 0.6196 0.500 1.1107 101.82 3 0.6002 0.6195 0.500 1.1085 102.26 4 0.6003 0.6196 0.625 1:1 1.2347 101.61 5 0.6001 0.6194 0.625 1.2318 102.07 6 0.6002 0.6195 0.625 1.2253 103.18 7 0.6005 0.6198 0.750 1:1.2 1.3697 100.01 8 0.6003 0.6196 0.750 1.3718 99.71 9 0.6004 0.6197 0.750 1.3817 98.43 2.2.8 样品含量测定
三批样品检测结果见表6,测得洛伐他汀的含量为1.1113 mg/g,外标法测得洛伐他汀酸的平均含量为0.3668 mg/g,一测多评法测定洛伐他汀酸的平均含量为0.3563 mg/g,表明所构建的一测多评法可用于红曲茯苓片的多成分质量评价研究。
表 6 红曲茯苓片中洛伐他汀、洛伐他汀酸含量测定结果Table 6. Determination of lovastatin and lovastatin acid in Hongqu Fuling tablets批号 洛伐他汀(mg/g) 洛伐他汀酸(mg/g) 外标法计算 一测多评法计算 210101 1.0670 0.3186 0.3095 210102 1.0796 0.3875 0.3764 210103 1.1872 0.3942 0.3829 平均值 1.1113±0.066 0.3668±0.042 0.3563±0.041 3. 讨论与结论
洛伐他汀和洛伐他汀酸是目前市售的以红曲为原料的降血脂作用产品的主要活性成分[13-14],但对二者含量的检测方法仍有不足,这必然会影响到产品疗效。本研究采用一测多评和高效液相色谱法(HPLC)对洛伐他汀和洛伐他汀酸的混合标准溶液的相对校正因子(RCF)进行测定和确立[15-16],并对样品进行HPLC分析,利用已经得到的RCF对样品中的洛伐他汀、洛伐他汀酸进行定量分析,从而测定红曲茯苓片中洛伐他汀和洛伐他汀酸的含量。
本研究所建立的一测多评法同时测定红曲茯苓片中洛伐他汀和洛伐他汀酸的含量,不仅减小了洛伐他汀酸标准品不能准确定量制备而产生的误差;而且针对洛伐他汀酸在酸性条件下不稳定,随时间会转化为洛伐他汀等问题[17],通过采用碱处理配制供试液来进行调整解决,同时还对产品供试液的制备方法进行考察。在本研究中,洛伐他汀和洛伐他汀酸的分离度大于1.5;精密度RSD%为0.32%、稳定性RSD%为0.44%、0.36%及0.55%、重复性RSD%为0.58%及0.49%、加样回收率RSD%为1.04%,均符合《药典(2020版)》[18]的要求,说明建立的方法线性良好、专属性高、精密准确、稳定快速,可同时测定红曲茯苓片中洛伐他汀、洛伐他汀酸含量。通过该方法计算出红曲茯苓片中洛伐他汀的含量为1.1113 mg/g,洛伐他汀酸的含量为0.3563 mg/g,表明该方法不仅可以用于红曲茯苓片等以红曲为原料的产品中洛伐他汀和洛伐他汀酸的同时测定,并且可以进一步计算红曲茯苓片等产品中洛伐他汀和洛伐他汀酸的比例,从而更好的保证了产品功效。
综上所述,本研究建立的方法不仅可以用于红曲茯苓片中洛伐他汀及洛伐他汀酸的含量测定,还可用于其他含红曲的其他制剂,能够为以红曲为原料的降血脂产品的深度开发、中药复方产品研发评价体系的建立提供科学依据。
-
表 1 供试品溶液制备方法考察结果
Table 1 Results of preparation method of sample solution
提取时间(min) 洛伐他汀含量(mg/g) 洛伐他汀酸含量(mg/g) 总洛伐他汀含量(mg/g) 30 1.0063 0.3223 1.3286 1.0175 0.3283 1.3458 60 1.0208 0.3266 1.3474 1.0205 0.3655 1.3860 表 2 洛伐他汀酸的相对校正因子
Table 2 Relative correction factors for lovastatin acid
编号 1 2 3 As 567484 566411 566196 Ai 631425 629418 628090 RCF 0.8987 0.8999 0.9015 平均RCF 0.9000 RSD(%) 0.16 表 3 红曲茯苓片中洛伐他汀、洛伐他汀酸的稳定性试验结果
Table 3 Stability results of lovastatin and lovastatin acid in Hongqu Fuling tablets
时间(h) 0 2 4 6 8 12 24 洛伐他汀 含量(mg/g) 1.0122 1.0416 1.0418 1.0426 1.0436 1.0439 1.0433 平均含量(mg/g) 1.0384 RSD(%) 1.12 洛伐他汀酸 含量(mg/g) 0.3233 0.3244 0.3228 0.3218 0.3235 0.3253 0.3247 平均含量(mg/g) 0.3237 RSD(%) 0.37 表 4 重复性试验结果
Table 4 Repeatability test results
编号 1 2 3 4 5 6 取样量(g) 1.2001 1.2005 1.2004 1.2005 1.2002 1.2003 外标法计算 洛伐他汀含量(mg/g) 1.0259 1.0338 1.0352 1.0379 1.0253 1.0350 平均含量(mg/g) 1.0322 RSD(%) 0.51 洛伐他汀酸含量(mg/g) 0.3550 0.3570 0.3578 0.3610 0.3582 0.3603 平均含量(mg/g) 0.3582 RSD(%) 0.61 一测多评法计算 洛伐他汀酸含量(mg/g) 0.3532 0.3551 0.3559 0.3589 0.3562 0.3581 平均含量(mg/g) 0.3562 RSD(%) 0.58 表 5 洛伐他汀加标回收率试验结果
Table 5 Results of recovery test of lovastatin
编号 称样量
(g)样品中含量
(mg)对照品加入量(mg) 对照品加入量与
样品中含量之比实测含量
(mg)回收率
(%)平均回收率
(%)RSD (%) 1 0.6004 0.6197 0.500 0.8:1 1.1086 102.28 101.26 1.51 2 0.6003 0.6196 0.500 1.1107 101.82 3 0.6002 0.6195 0.500 1.1085 102.26 4 0.6003 0.6196 0.625 1:1 1.2347 101.61 5 0.6001 0.6194 0.625 1.2318 102.07 6 0.6002 0.6195 0.625 1.2253 103.18 7 0.6005 0.6198 0.750 1:1.2 1.3697 100.01 8 0.6003 0.6196 0.750 1.3718 99.71 9 0.6004 0.6197 0.750 1.3817 98.43 表 6 红曲茯苓片中洛伐他汀、洛伐他汀酸含量测定结果
Table 6 Determination of lovastatin and lovastatin acid in Hongqu Fuling tablets
批号 洛伐他汀(mg/g) 洛伐他汀酸(mg/g) 外标法计算 一测多评法计算 210101 1.0670 0.3186 0.3095 210102 1.0796 0.3875 0.3764 210103 1.1872 0.3942 0.3829 平均值 1.1113±0.066 0.3668±0.042 0.3563±0.041 -
[1] 刘金莲, 张建军, 刘曦, 等. 基于辨证保健理论探讨红曲茯苓片对脾虚湿盛型高脂血症大鼠的影响[J]. 环球中医药,2020,13(11):1837−1845. [LIU Jinlian, ZHANG Jianjun, LIU Xi, et al. Discussion on effects of Hongqu Fuling tablets on hyperlipidemia rats with overabundant dampness due to spleen deficiency based on the theory of syndrome differentiation and health care[J]. Global Traditional Chinese Medicine,2020,13(11):1837−1845. LIU Jinlian, ZHANG Jianjun, LIU Xi, et al. Discussion on effects of Hongqu Fuling tablets on hyperlipidemia rats with overabundant dampness due to spleen deficiency based on the theory of syndrome differentiation and health care [J]. Global Traditional Chinese Medicine, 2020, 13(11): 1837-1845.
[2] 刘轶. DFT探究红曲色素的光化学特性以及降胆固醇活性[D]. 新乡: 河南师范大学, 2016. LIU Yi. Det study on the photochemical features and the cholesterol lowering actives of monascus pigments[D]. Xinxiang: the Graduate School of Henan Normal University, 2016.
[3] JONES P, KAFONEK S, LAURORA I, et al. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study)[J]. Am J Cardiol,1998,81(5):582−587. doi: 10.1016/S0002-9149(97)00965-X
[4] FENG S S, LI W, HU Y J, et al. The biological activity and application of Monascus pigments: A mini review[J]. Int J Food Eng,2022,18(4):253−266. doi: 10.1515/ijfe-2021-0235
[5] LIANG J X, ZHANG Q Q, HUANG Y F, et al. Comprehensive chemical profiling of monascus-fermented rice product and screening of lipid-lowering compounds other than monacolins[J]. J Ethnopharmacol,2019,238:111879. doi: 10.1016/j.jep.2019.111879
[6] ZHANG Y R, CHEN Z T, WEN Q Y, et al. An overview on the biosynthesis and metabolic regulation of monacolin K/lovastatin[J]. Food & Function,2020,11(7):5738−5748.
[7] FENG Y, SHAO Y, ZHOU Y, et al. Effects of glycerol on pigments and monacolinKproduction by the high-monacolin K-producing but citrinin-free strain, Monascus pilosus MS-1[J]. Eur Food Res Technol,2015,240(3):635−643. doi: 10.1007/s00217-014-2365-y
[8] MA J, LI Y, YE Q, et al. Constituents of red yeast rice, a traditional Chinese food and medicine[J]. J Agric Food Chem,2000,48(11):5220−5225. doi: 10.1021/jf000338c
[9] 郝盛源, 王磊, 李红, 等. 一测多评法测定红曲及脂必妥片中洛伐他汀和洛伐他汀酸[J]. 中国实验方剂学杂志,2017,23(5):74−78. [HAO Shengyuan, WANG Lei, LI Hong, et al. Simultaneous determination of lovastatin and lovastatin acid in fermentum rubrum and zhibituo tablets by QAMS[J]. Chinese Journal of Experimental Traditional Medical Formulae,2017,23(5):74−78. HAO Sheng-yuan, WANG Lei, LI Hong, et all. Simultaneous determination of lovastatin and lovastatin acid in fermentum rubrum and zhibituo tablets by QAMS[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2017, 23(5): 74-78.
[10] 于文斌, 孟青青, 张松玲, 等. 高效液相法测定琦红丸中酸式及内酯洛伐他汀含量[J]. 长春中医药大学学报,2011,27(5):848−849. [YU Wenbin, MENG Qingqing, ZHANG songlin, et al. The content of acid form and lactone lovastatin in Qihong pill was determined by high performance liquid chromatography[J]. Journal of Changchun University of Traditional Chinese Medicine,2011,27(5):848−849. YU Wenbin, MENG Qingqing, ZHANG songlin, et al. The content of acid form and lactone lovastatin in Qihong pill was determined by high performance liquid chromatography[J]. Journal of Changchun University of Traditional Chinese Medicine, 2011, 27(5): 848-849.
[11] 杨艳, 郑仁锦, 华永有, 等. 高效液相色谱法同时测定保健红曲中酸式与内酯式洛伐他汀含量[J]. 中国卫生检验杂志,2014,24(12):1714−1717. [YANG Yan, ZHENG Renjin, HUA Yongyou, et al. High-performance liquid chromatographic analysis of lovastatin (lactone) and lovastatin acid in red yeast rice[J]. Chinese Journal of Health Laboratory Technology,2014,24(12):1714−1717. YANG Yan, ZHENG Renjin, HUA Yongyou, et al. High - performance liquid chromatographic analysis of lovastatin (lactone) and lovastatin acid in red yeast rice[J]. Chinese Journal of Health Laboratory Technology, 2014, 24(12): 1714-1717.
[12] 王云, 陈影, 黄琪, 等. 基于一测多评法研究黄芩酒炙前后12个黄酮类成分的含量变化[J]. 世界中医药,2022,17(9):1233−1239,1245. [WANG Yun, CHEN Ying, HUANG Qi, et al. Simultaneous determination of 12 flavonoids in crude and wine-processed radix scutellariae based on quantitative analysis of multi-components by single marker (qams)[J]. World Chinese Medicine,2022,17(9):1233−1239,1245. WANG Yun, CHEN Ying, HUANG Qi, et al. Simultaneous determination of 12 flavonoids in crude and wine - processed radix scutellariae based on quantitative analysis of multi - components by single marker (qams)[J]. World Chinese Medicine, 2022, 17(9): 1233-1239, 1245.
[13] 王玲, 吴军林, 吴清平. 红曲降血脂功能的研究及应用概况[J]. 食品工业科技,2014,35(8):387−389,393. [WANG Ling, WU Junlin, WU Qingping. Research and application progress of red yeast rice for lowering the blood chelosterol level[J]. Science and Technology of Food Industry,2014,35(8):387−389,393. WANG Ling, WU Jun-lin, WU Qing-ping. Research and application progress of red yeast rice for lowering the blood chelosterol level[J]. Science and Technology of Food Industry, 2014, 35(8): 387-389, 393.
[14] FUKAMI H, HIGA Y, HISANO T, et al. A review of red yeast rice, a traditional fermented food in Japan and east Asia: lts characteristic ingredients and application in the maint enance and improvement of health in lipid metabolism and the circulatory system[J]. Molecules,2021(266):1619.
[15] 王智民, 钱忠直, 张启伟, 等. 一测多评法建立的技术指南[J]. 中国中药杂志,2011,36(6):657−658. [WANG Zhimin, QIAN Zhongzhi, ZHANG Qiwei, et al. Technical guidelines for the establishment of a multiple-score one-test approach[J]. China Journal of Chinese Materia Medica,2011,36(6):657−658. WANG Zhimin, QIAN Zhongzhi, ZHANG Qiwei, et al. Technical guidelines for the establishment of a multiple-score one-test approach[J]. China Journal of Chinese Materia Medica, 2011, 36(6): 657-658.
[16] 于世林. 高效液相色谱方法及应用(第三版)[M]. 北京: 化学工业出版社, 2018. YU Shilin. High Performance liquid chromatography method and application[M]. Third Edition. Beijing: Chemical Industry Press, 2018.
[17] 王颖, 黄瑛, 龙敏. 一测多评法测定含红曲产品中的洛伐他汀和洛伐他汀酸[J]. 华西药学杂志,2020,35(6):664−667. [WANG Ying, HUANG Yin, LONG Min. Simultaneous determination of lovastatin and lovastatin acid in health foods containing red koji by QAMS[J]. West China Journal of Pharmaceutical Sciences,2020,35(6):664−667. WANG Ying, HUANG Yin, LONG Min. Simultaneous determination of lovastatin and lovastatin acid in health foods containing red koji by QAMS[J]. West China Journal of Pharmaceutical Sciences, 2020, 35(6): 664-667.
[18] 国家药典委员会. 中华人民共和国药典(2020): 二部[M]. 北京: 中国医药科技出版社, 2020: 1023. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China(2020): Part Ⅱ[M]. Beijing: China Medical Science Press, 2020: 1023.
-
期刊类型引用(11)
1. 刘小芳,郭向融,李爽,辛云,蒋永毅,冷凯良,姜维. 南极磷虾磷脂的组成及其抗氧化活性. 食品研究与开发. 2024(03): 16-21 .  百度学术
百度学术
2. 刘礼泉,尹晓晨,王蔚峰,胡余明. 蛋黄磷脂的毒理学安全性评价试验. 实用预防医学. 2024(08): 929-936 .  百度学术
百度学术
3. 宁一霏,毕添玺,陈奕蒙,王成成,姜晓明,王玉明,薛长湖,张恬恬. 膨腹海马脂质及其脂肪酸组成分析. 食品研究与开发. 2024(17): 178-183 .  百度学术
百度学术
4. 吕爽,刘倩,李红波,张小勇,徐丹,苏从毅,胡梁斌,莫海珍. 羊骨脂质的组成分析. 中国油脂. 2023(01): 32-36 .  百度学术
百度学术
5. 田笑,仝其根. 蛋黄油脂质组分的提取分离与脂肪酸组成分析. 中国油脂. 2023(06): 130-135 .  百度学术
百度学术
6. 邓胜国,黄艳,姜红宇,尹爱武,黄光文. 超声辅助提取黑芝麻油脚卵磷脂及其抗氧化活性研究. 湖南理工学院学报(自然科学版). 2023(03): 28-32 .  百度学术
百度学术
7. 兰天彤,宋亭喻,钱圣,张浩,刘景圣. 压力、超声物理场辅助构建蛋白-卵磷脂复合体系在递送系统中的研究进展. 食品科学. 2023(19): 220-229 .  百度学术
百度学术
8. 路婷婷,陈华,权囯栋,赵延红,李万宏,翁秀秀. 不同稀释液对绵羊精液4℃保存效果的影响. 中国畜牧杂志. 2023(12): 228-232 .  百度学术
百度学术
9. 王瑞,段旭林,张琦弦,吕远平,何强,迟原龙. 氨基柱高效液相色谱法检测大豆提取物中的磷脂酰胆碱. 中国测试. 2022(04): 68-72 .  百度学术
百度学术
10. 林艺璇,曾巧玲,张玲云,潘雅欣,张华丹,祝玉杰,杜艳瑜,梁鹏. 大黄鱼鱼卵磷脂对面团流变学和面包感官品质的影响. 食品安全质量检测学报. 2022(13): 4174-4179 .  百度学术
百度学术
11. 田笑,仝其根. 不同蛋黄油组分促进小鼠消化性胃溃疡愈合作用. 食品科学. 2022(21): 163-170 .  百度学术
百度学术
其他类型引用(10)





 下载:
下载:
 下载:
下载:



