Research Progress on the Effects of Bisphenol Compounds on Human Beings and Animals
-
摘要: 双酚类化合物(Bisphenol compounds,BPs)是合成高分子材料的重要原材料之一。目前,常见的双酚类化合物主要包括双酚A、双酚B、双酚F以及双酚AF等。现如今,双酚类化合物主要应用于塑料制品、食品包装、饮用水添加剂、海鲜等方面。大量的研究发现,双酚类化合物通过物理迁移、化学迁移、生物迁移途径侵入人类和动物体内,甚至在深海哺乳动物体内也检测到了该物质,从而影响了机体的生殖系统、神经系统、消化系统、心血管系统、行为、发育和代谢性疾病的发生发展。因此,双酚类化合物引起了更多的关注。因此,本文主要从BPs的分类、侵入机体的途径以及对机体的影响方面进行了综述,以期对BPs的进一步研究以及安全性和毒理学评价提供参考依据。Abstract: Bisphenol compounds (BPs) are one of the most significant raw materials, used in synthesizing high polymer materials. At present, common BPs mainly include bisphenol A, bisphenol B, bisphenol F, bisphenol AF and so on. Nowadays, BPs are principally applied in plastic products, the food packaging, drinking water additives, seafood and other fields. A large number of studies have found that BPs penetrate into human beings and animals by physical migration, chemical migration, biological migration and it even have been detected in deep-sea mammals, affecting the mechanisms of the reproductive system, central nervous system, digestive system, cardiovascular system, behavior, development, and metabolic diseases. Therefore, BPs have been attracting more attention. In the review, the classification, invasive pathway to foods of BPs, and its implication on human beings and animals are systematically reviewed, hoping to provide reference for the safety and toxicological evaluation of BPs.
-
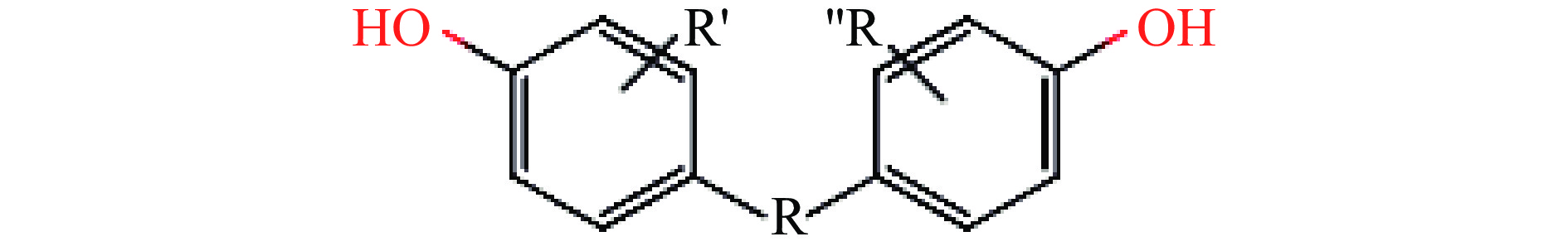
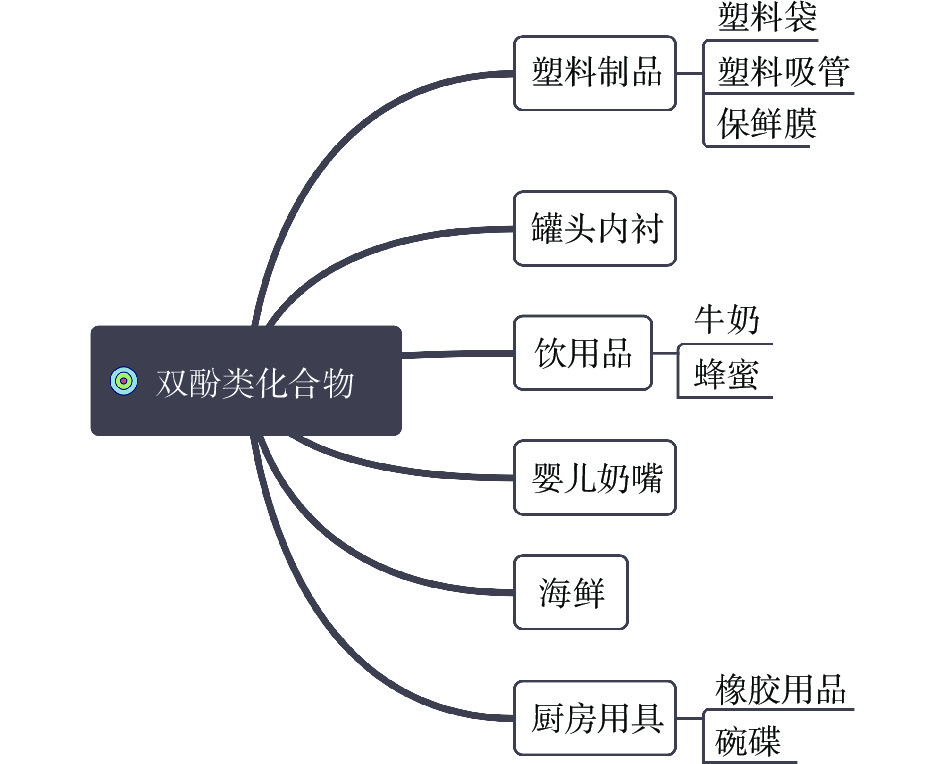
双酚类化合物(Bisphenol compounds,BPs)是一类常见的内分泌干扰物,这类化合物都具有由碳原子或硫原子将两个羟苯基相连的基本骨架[1-2](图1),是高分子材料合成的重要原料之一,主要用于农业、工业、医疗卫生等领域,极易暴露在日常生活环境中[3]。双酚类化合物主要用作生产聚碳酸酯、环氧树脂的中间体,可以用在食品包装中,如塑料水瓶、罐头包装、婴儿奶瓶、厨房用品,也存在于牛奶、蜂蜜等食品中。当在高温、酸碱、紫外条件下,双酚类化合物会通过这些材料进入到食品和水源中,从而被人体吸收,对机体产生影响。20世纪30年代,有研究发现BPA对小鼠生殖繁育有促进作用并暴露出雌激素作用[4-5],60年代后BPA被广泛应用于婴幼儿用品。美国毒理学报告指出,BPA对胎儿、婴幼儿的行为发育都有影响。在2008年,加拿大成为世界上第一个颁布BPA法规并禁止进口及销售含有BPA的婴儿食品容器的国家[6];随后欧盟及中国在2011年发布公告禁止BPA用于婴幼儿奶瓶[7],但是并没有严禁在其他用途中使用。据调查,BPA长期低浓度暴露会对机体的癌症方面[8]、生殖功能[9]、心血管系统[10]造成影响。由于双酚类化合物具有潜伏期长、蓄积等作用,不易被人体察觉,所以对双酚类化合物的管控尤为重要。双酚类化合物可以通过多种方式进入体内并产生影响,所以本文主要对其分类、侵入机体方式以及对机体的影响进行阐述。
1. BPs的分类及侵入方式
目前,常见的双酚类化合物主要包括双酚A(Bisphenol A,BPA)、双酚B(Bisphenol B,BPB)、双酚C(Bisphenol C,BPC)、双酚E(Bisphenol E,BPE)、双酚F(Bisphenol F,BPF)、双酚S(Bisphenol S,BPS)、双酚Z(Bisphenol Z,BPZ)、双酚P(Bisphenol P,BPP)、双酚AF(Bisphenol AF,BPAF)等[11]。其中,以双酚A使用最为广泛[12]。BPA为最典型的BPs,学名2,2-二(4-羟基苯基)丙烷,又称二酚基丙烷,化学式为C15H16O2,分子量228.286。在工业方面双酚A作为聚碳酸酯(Polycarbonate,PC)和环氧树脂(Phenolic epoxy resin,PE)以及其他中间产物的生产材料,在食品药品包装、牙科填充剂、罐头内衬、婴儿奶瓶等材料中都含有[13]。随着BPA的禁用,BPF、BPAF、BPS等其他BPs成为BPA的替代品[14]。
BPB与BPA具有很大的结构相似性,它与BPA的区别是中心碳原子上的一个乙基[15]。BPB现已进入市场逐渐替代BPA的作用,其年产量也逐步增加。BPB在农业、工业及生活用品中应用越来越广泛,现已在罐装食品、饮料、海鲜、牛奶等中都有检出[16-17]。研究发现BPB在人体血液及尿液中都被检测到,表明BPB可通过多种途径进入到人体中[18]。BPF是两个苯酚通过亚甲基连接形成的,含有BPF的环氧树脂比BPA具有更低的黏性及更强的耐受性,所以BPF的适用性更加广泛[19-20]。BPS是两个苯酚通过磺酰基连接形成的化合物,其酸性高于其他双酚类化合物,具有良好的耐光、耐高温、抗氧化的特质[21]。双酚F与双酚S作为双酚A的类似物,与双酚A相比结构相似,并且酸性更强且稳定性更高,被认为是双酚A的主要替代品[22]。BPAF是BPA氟化衍生物,具有较强的抗张强度,主要用作氟橡胶硫化促进剂和聚酯加工[23],广泛应用于高温复合材料、电子材料、透气膜和特殊聚合物的合成生产[24]。此外,双酚C、双酚E、双酚G、双酚M、双酚P、双酚Z、双酚AP、双酚BP、双酚PH、四溴双酚A和四氯双酚A等BPs在工业及农业上也有应用(图2,表1)。
表 1 双酚类化合物的结构与侵入方式Table 1. Structure and invasion pathways of BPs名称 分子式 分子结构 侵入方式 侵入途径 参考文献 双酚A(BPA)Bisphenol A C15H16O2 
物理迁移化学迁移生物迁移 皮肤和粘膜消化道呼吸道 [13] 双酚B(BPB)Bisphenol B C16H18O2 
[16−17] 双酚C(BPC)Bisphenol C C17H20O2 
[25] 双酚E(BPE)Bisphenol E C14H14O2 
[26] 双酚F(BPF)Bisphenol F C13H12O2 
[27] 双酚P(BPP)Bisphenol P C24H26O2 
[26] 双酚S(BPS)Bisphenol S C12H10O4S 
[28] 双酚Z(BPZ)Bisphenol Z C18H20O2 
[29] 双酚AF(BPAF)Bisphenol AF C15H10F6O2 
[23] 双酚AP(BPAP)Bisphenol AP C20H18O2 
[30] 四溴双酚A(TBBPA)Tetrabromo bisphenol A C15H12Br4O2 
[31] 四氯双酚A(TCBPA)Tetrachloro bisphenol A C15H12Cl4O2 
[32] 研究发现,双酚类化合物对机体产生影响的方式主要包括三种方式:物理迁移、化学迁移、生物迁移。物理迁移主要是由于在工业合成中环氧树脂和聚碳酸酯的反应不完全,双酚类化合物残留在中间体中向容器扩散引起的。而且,由于大多数聚碳酸酯产品都是重复使用的,这也加剧了BPs从食品包装进入人体的程度。化学迁移的主要来源是聚碳酸酯的降解,例如高温、沸水以及酒精消毒等方法都可以使容器表面的BPs释放出来。研究发现,BPA在酸性和醇溶液中易迁出,并随着浓度的增加,迁移量也增大;在高温环境中,时间越长,迁移的风险也越高。生物迁移是指通过皮肤接触进入人体,这种方式主要集中在以热敏为材料的制品中。例如购物小票,当人接触到热敏纸时,会导致其中的BPs残留在皮肤上进而被吸收进入人体。研究发现,双酚化合物在人体中残留时间最长,尽管大部分会通过代谢排出,但还是会有少部分残留在人体中并产生积累。我们在日常生活中经常会接触到含有双酚化合物的食品或用品,例如薯片、罐头、牛奶、塑料用品、厨具、婴幼儿用品等。在正常条件下BPs对人体不会产生毒性作用,所以应尽量避免对这些塑料、橡胶等制品进行高温蒸煮、沸水涮洗、酒精消毒等手段,并禁止在高温条件下储存。
2. BPs对生物机体的影响
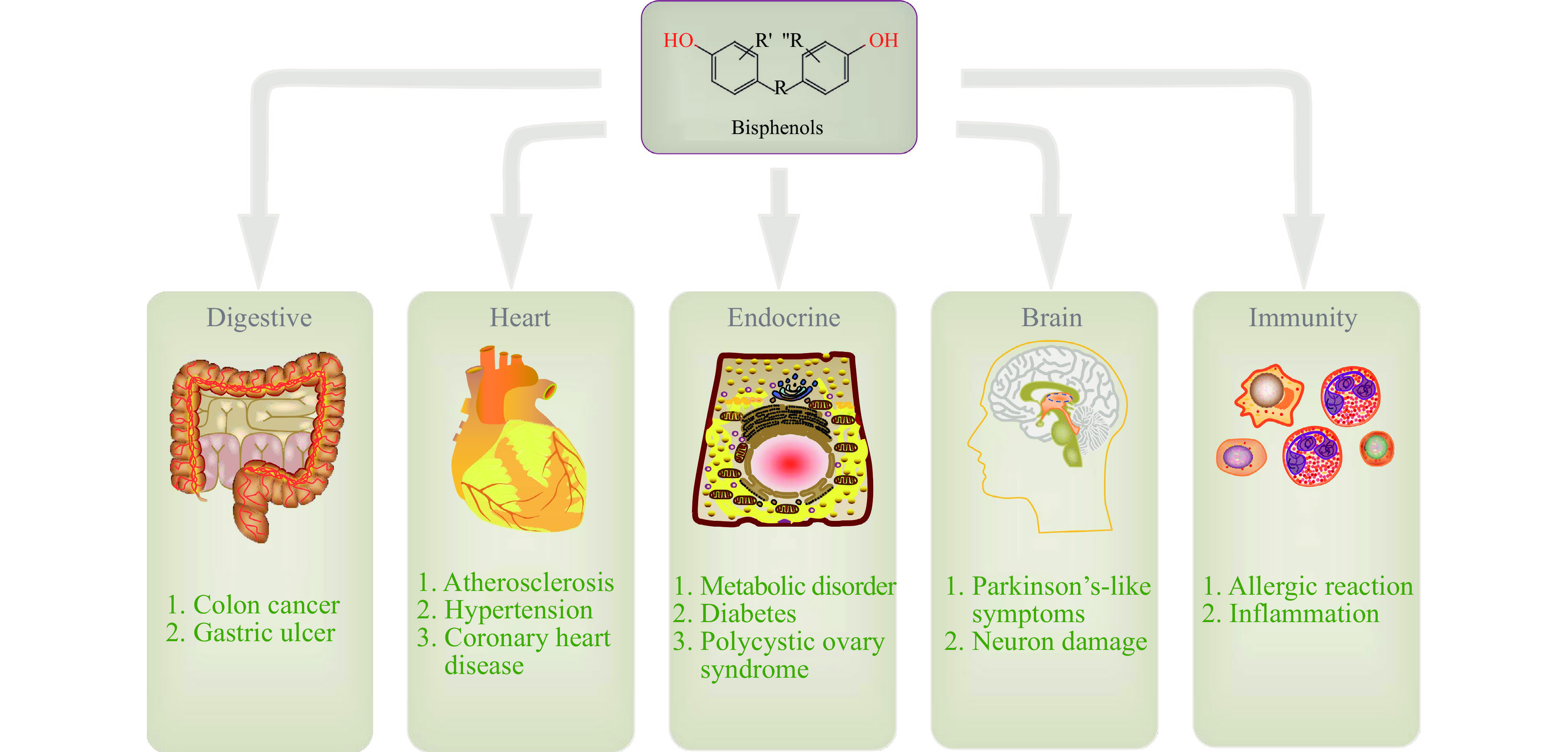
BPs是一种类雌激素作用的化学物质,它通过与雌激素受体(ERα、ERβ)结合影响生殖系统、神经免疫系统及心血管方面,调节机体生长发育和各种代谢,影响行为能力等(图3)。
2.1 BPs对生殖系统的影响
研究发现BPs对男性生殖及女性生殖系统可能会产生危害,并且伴随着致畸的危险,通过体内及体外研究证实了对生殖方面的影响。
2.1.1 BPs对雄性生殖系统的影响
BPs对雄性生殖系统影响较大,可以引起生殖障碍及发育异常,主要表现为男性雌性化、性腺发育不良、精子数目减少质量下降至无精、睾丸及附睾重量异常、性欲降低和不育症等[33]。
Bhandari等[34]研究发现,不同浓度的BPA暴露对不同发育阶段的青鳉生殖系统均具有一定的毒性作用,团队首先关注了高剂量BPA暴露对青鳉早期胚胎生殖发育的影响。他们首先在胚胎发育的前7 d将分化阶段的生殖细胞暴露于BPA(100 μg/L)中,在此浓度时F0或F1代青鳉没有产生明显的表型异常,但是会导致F2代的受精率显著降低、F3代的胚胎存活率降低。然后将受精8 h青鳉长期暴露于低浓度BPA(10 μg/L)中,通过RNA测序检查睾丸转录本的改变[35]。转录组学分析结果显示,BPA暴露下的睾丸与对照组有651个差异表达基因,并通过qRT-PCR实验验证了RNA测序的结果。两项实验结果表明BPA暴露对青鳉的生殖系统有一定的毒性作用。除此之外,Rezaee等[36]以成年NMRI小鼠的精子和睾丸为实验对象,暴露在BPA(8 mmol/L)中,发现BPA显著升高了睾丸线粒体中的活性氧(Reactive oxygen species,ROS)和丙二醛(Malondialdehyde,MDA)的水平,并降低了基质金属蛋白酶(Atrix metalloprotein,MMP)、超氧化物歧化酶(Superoxide Dismutase,SOD)和谷胱甘肽(Glutathione,GSH)的水平;BPA还严重损害了精子的活力。BPA的暴露还会导致小鼠前列腺重量增加[37],睾丸激素排泄和精子数量减少[38]。
2.1.2 BPs对雌性生殖系统的影响
BPs对雌性生殖系统的影响具体表现为青春期提前、子宫内膜异位、子宫内膜病变、月经周期异常、卵巢综合症、复发性流产和不孕不育等[39]。
将小鼠子宫内膜异位症(Endometriosis,EM)模型及人正常子宫内膜基质细胞进行BPA暴露,发现BPA可以升高小鼠子宫内膜中ERβ的表达,促进子宫内膜的病变;同时BPA还促进了WDR5的表达,激活ERβ的功能,促进了EM的病变发展[40]。除此之外,将怀孕小鼠暴露于BPA中会导致其后代性早熟,同时表现出生殖系统早衰[37](表2)。
表 2 BPs对生殖系统的影响Table 2. Effects of BPs on the reproductive system研究类型(模型) BPs 浓度 影响 参考文献 体外 牛卵母细胞和精子 BPA,BPS,BPF 0.05 mg/mL BPA处理:活性氧升高,重组人线粒体超氧化物歧化酶-2、谷胱甘肽过氧化物酶1、谷胱甘肽过氧化物酶4基因及蛋白表达降低;BPF、BPS处理:精子产量减少 [41] 体外 人类精子 BPA 0,10−3,10−2,10−1,10,103 nmol/L 精子活力、进行性运动和黄体酮诱导的顶体反应显着下降 [42] 体内 雄性大鼠 BPS 0.5,5,50 μg/L 精子活力、产量、附睾中精子数量减少;血浆睾酮、黄体生成素、卵泡刺激素浓度降低,雌二醇水平升高;睾丸中氧化应激水平升高 [43] 体内 成年雄性小鼠 BPA 5,50 mg/kg bw/day 生殖细胞比例异常、睾丸形态改变、精元干细胞功能特性丧失 [44] 体外 小鼠卵母细胞 BPA,BPF 5,25,50 μg/mL 纺锤体缩短,微管附着减少 [45] 体内 成年雄性大鼠 BPF 1,5,25,50 mg/kg/d 精子减少,睾丸激素分泌物水平降低 [46] 体内 雌性和雄性斑马鱼 BPB 0.001,0.01,0.1,1 mg/L 鱼卵数量减少,孵化率及存活率降低,生殖功能受损 [47] 体内 雌性和雄性斑马鱼 BPAF 0.05,0.25,1 mg/L 雄性斑马睾酮水平降低,鱼E2水平升高;雌性斑马鱼睾酮分泌增加 [48] 2.2 BPs对胚胎发育的影响
外源性物质在胚胎发育过程中极易产生干扰作用,关于BPs在胚胎发育过程中的毒性作用也有文献报道,具体表现为胚胎死亡率增加、产生畸变、心脏发育异常、诱导DNA损伤、行为功能受损等。
BPA对器官形成期的大鼠胚胎具有慢性毒性作用,并且呈现出明显的时间-效应和时间-反应关系[49]。研究发现,BPA暴露可以降低胚胎囊胚的发育率,并且随着浓度的增加,胚胎的死亡率也逐步增大[50]。裴新荣等[51]发现当双酚A的剂量≥60 mg/L时,会促进小鼠胚胎卵黄囊生长并且诱导血管分化不良、形态分化异常、生长迟缓;严重时会延缓心脏发育及产生心包积液等。
BPs对水生生物的胚胎发育也有一定的毒性作用。研究发现,当热带爪蟾胚胎暴露在BPA中后,导致ROS水平增加,引起DNA损伤和异常基因的表达,干扰胚胎发育的Nrf2信号通路,并引起畸胎[52]。BPA衍生物TBBPA对斑马鱼的胚胎发育也有抑制作用,可以引起孵化时间延长、死亡率增加、心率下降、心包水肿、躯干水肿和尾部畸形[53]。双酚S作为双酚A的替代品,发现其毒性不仅可以对胚胎发育产生影响,还发现其毒性对成鱼交配后的子代胚胎的发育产生毒性作用,具有可遗传性[54]。郭依晨等[55]发现受精6 h后的斑马鱼暴露双酚A后,斑马鱼存活率降低、畸形率逐渐升高;运动总量降低,对光暗刺激反应减弱;并且对DNA修复相关基因产生影响(表3)。
表 3 BPs对胚胎发育的影响Table 3. Effects of BPs on embryonic development2.3 BPs对神经系统的影响
研究发现,BPs可以对神经系统产生毒性作用,导致神经细胞受损,影响神经细胞的活动及其传导功能,从而造成生物机体的行为或功能异常紊乱。当斑马鱼幼鱼暴露在BPB中时,幼鱼的游动轨迹、平均速度及总距离都显著降低,表明BPB抑制了其运动神经元的发育;并且与神经元发育相关基因的转录也受到了抑制[60]。研究报道,BPA可以降低Na2神经细胞活力和轴突的生长,并且下调Bcl-2,上调HO-1,并激活了AMPK,从而导致神经元病变[61]。除此之外,通过研究发现双酚类物质可以通过诱导氧化应激产生神经变性。Sahoo等[62]通过BPA斑马鱼暴露实验发现,高浓度BPA(4 mg/L)暴露可能会诱发斑马鱼运动功能障碍,导致帕金森样表型,神经元受损,下调了帕金森相关靶标蛋白Nurr1和NeuN的表达,并且激活了细胞凋亡相关基因Caspase-3的表达;这些结果表明长期暴露于BPA可诱发神经的病变,并导致斑马鱼运动障碍和帕金森神经退行性疾病的发生发展。
2.4 BPs对免疫系统的影响
有研究发现,低剂量BPA暴露增强了卵白蛋白(Ovalbumin,OVA)诱导的炎症细胞浸润水平、Th2细胞因子和趋化因子及组织病理学的改变;此外OVA诱导的炎症细胞的分化在BPA暴露后增强;并且发现暴露于BPA后导致呼吸频率增加;结果显示,低剂量双酚A会破坏免疫系统并加重过敏反应[63]。Youn等的研究表明,从饮用水途径暴露BPA的小鼠体内分离出的T淋巴细胞产生的干扰素-γ数量增加,白介素-4数量减少[64],另一项体外研究则观察到BPA提高了小鼠T淋巴细胞中白介素-4和白介素-8的水平[65]。Sugita-Konishi[66]研究观察到BPA使感染大肠杆菌的小鼠体内的中性粒细胞活性降低并抑制了白介素-6的形成。同样,Goto等[67]注意到用BPA暴露后小鼠产生的淋巴细胞具有更高数量的免疫球蛋白。在对小鼠后代的研究发现,暴露于BPA的雌性小鼠后代其先天免疫力的调节能力变弱,更容易受到甲型流感病毒的感染[68]。
综上所述,双酚类化合物可以导致过敏反应加重、炎症因子分泌增加/减少、免疫系统功能紊乱,并且感染的风险加重。
2.5 BPs对心血管系统的影响
根据流行病学调查,BPA与高血压、冠心病、糖尿病及代谢性疾病都有关联,是诱发及促进心血管疾病的一项重要因素。Feiteiro等[69]通过研究雄性成年大鼠及其血管平滑肌细胞A7r5发现,BPA暴露阻断了A7r5细胞中的L型钙离子通道,并导致血管平滑肌松弛,这可能是引起心血管疾病的危险因素。Brown等[70]研究证明了BPA的代谢产物MBP在心脏瓣膜中的雌激素反应原件激活与心血管疾病发展之间的联系。将第三代纯合TG(ERE:GFP)斑马鱼暴露在BPA及其代谢产物MBP中,通过显微解剖心脏组织和转录组学分析显示,这两种化学物质都可以靶向构成钙化主动脉瓣疾病并引起细胞外基质(Extracellular matrix,ECM)的改变。组织病理学证实了高水平MBP暴露引起ECM胶原蛋白的缺乏和对心脏瓣膜结构完整性的影响,并且房室瓣结构缺陷与心血管功能受损。除此之外,BPS对心血管系统也有潜在的影响。BPS可能通过促进缺氧和CO2诱导组织中毒性的产生和抑制白细胞介导的免疫,对血液功能产生损伤;减少葡萄糖摄取和诱导糖原分解;对血细胞、心脏及血管壁组织造成损害;并且还可能通过增加总胆固醇、甘油三酯、低密度脂蛋白和极低密度脂蛋白浓度以及降低高密度脂蛋白水平,诱发心脏疾病的发生[71]。
2.6 BPs的致癌作用
有研究表明,双酚类化合物产生的雌激素作用可能会导致DNA损伤,引起癌基因及抑癌基因的变异,以及相关基因表达的发生改变[72],以致乳腺癌、甲状腺癌、卵巢癌等肿瘤的发生发展。当大鼠甲状腺癌细胞暴露在BPA中时,发现促进了癌细胞的生长,抑制抑癌基因PTEN的表达,激活AKT信号通路,上调c-MYC的表达[73]。李明等[3]发现BPAF、BPB、BPA、BPS、TCBPA、TBBPA都可以促进ERα阳性的乳腺癌细胞增殖,并且BPAF的活性最强。
2.7 BPs对机体的其他影响
有研究发现表明,双酚类化合物可以直接与雌激素受体作用,对糖脂和胰岛素代谢产生影响,引起糖尿病、脂肪肝、肝炎的发生。
当小鼠暴露于BPA中时均有肝脏脂肪堆积;并通过体外实验发现BPA产生了显著的葡萄糖代谢紊乱,并加重了高脂饮食(HFD)诱导的糖脂代谢紊乱,对糖脂代谢关键调控因子的表达产生了影响[74]。BPA暴露促使鼠源3T3-L1成纤维细胞分化为脂肪细胞,而且BPA和胰岛素的结合可以加速这一过程[75];并且BPA暴露后的3T3-L1脂肪细胞中的脂质以浓度和时间依赖的方式累积,同时参与脂质代谢的基因也呈上升趋势[76-77]。同时还发现BPA会通过引起氧化应激而对HepG2细胞产生损伤[78],Moon等[79]也指出BPA可以引起大鼠肝细胞炎性损伤。同样,在体外研究也发现BPA会产生ROS,并降低抗氧化基因的表达,从而引起肝毒性[80]。
2.8 BPs的作用机制
研究发现,双酚类化合物对机体产生影响是通过多种方式作用的。BPA可通过RGS4介导的BDNF/NTRK2信号转导对突触的形成和功能产生损伤,引起神经功能障碍[81]。同时研究发现,BPA可以通过Wnt/β-Catenin、TGF-β、ERRα信号通路调节神经干细胞NSC的增值和分化[82]。BPA还可以通过AMPK/mTOR信号通路诱导细胞凋亡[83];并作用于细胞周期影响纺锤体和减数分裂,进而导致卵母细胞发育异常[84]。(图4)
3. 结论与展望
双酚类化合物在日常生活中广泛存在,由于其潜伏期较长而不易发现。但大量证据表明,双酚类化合物即使在低剂量范围内对机体仍会产生生物学效应[85]。目前已有研究发现双酚类化合物与癌症、糖尿病、肥胖、神经免疫功能紊乱等疾病的发生发展有关,并且还有致不孕不育的风险[86-89]。这些疾病发生的机制很多,涉及核受体、膜受体、拮抗雄激素受体、干扰激素合成与代谢以及表观遗传失调等[90]。但是双酚类化合物对生物体的影响程度因种属、暴露剂量、染毒方式等不同而异,其作用范围、作用机制多样化,并且对机体的影响具有加和性。现在对双酚类化合物的研究主要集中在双酚A上,对其他双酚类化合物研究较少,并且研究模型不够全面,因此对双酚类化合物的毒性及其机制有待深入研究。
-
表 1 双酚类化合物的结构与侵入方式
Table 1 Structure and invasion pathways of BPs
名称 分子式 分子结构 侵入方式 侵入途径 参考文献 双酚A(BPA)Bisphenol A C15H16O2 
物理迁移化学迁移生物迁移 皮肤和粘膜消化道呼吸道 [13] 双酚B(BPB)Bisphenol B C16H18O2 
[16−17] 双酚C(BPC)Bisphenol C C17H20O2 
[25] 双酚E(BPE)Bisphenol E C14H14O2 
[26] 双酚F(BPF)Bisphenol F C13H12O2 
[27] 双酚P(BPP)Bisphenol P C24H26O2 
[26] 双酚S(BPS)Bisphenol S C12H10O4S 
[28] 双酚Z(BPZ)Bisphenol Z C18H20O2 
[29] 双酚AF(BPAF)Bisphenol AF C15H10F6O2 
[23] 双酚AP(BPAP)Bisphenol AP C20H18O2 
[30] 四溴双酚A(TBBPA)Tetrabromo bisphenol A C15H12Br4O2 
[31] 四氯双酚A(TCBPA)Tetrachloro bisphenol A C15H12Cl4O2 
[32] 表 2 BPs对生殖系统的影响
Table 2 Effects of BPs on the reproductive system
研究类型(模型) BPs 浓度 影响 参考文献 体外 牛卵母细胞和精子 BPA,BPS,BPF 0.05 mg/mL BPA处理:活性氧升高,重组人线粒体超氧化物歧化酶-2、谷胱甘肽过氧化物酶1、谷胱甘肽过氧化物酶4基因及蛋白表达降低;BPF、BPS处理:精子产量减少 [41] 体外 人类精子 BPA 0,10−3,10−2,10−1,10,103 nmol/L 精子活力、进行性运动和黄体酮诱导的顶体反应显着下降 [42] 体内 雄性大鼠 BPS 0.5,5,50 μg/L 精子活力、产量、附睾中精子数量减少;血浆睾酮、黄体生成素、卵泡刺激素浓度降低,雌二醇水平升高;睾丸中氧化应激水平升高 [43] 体内 成年雄性小鼠 BPA 5,50 mg/kg bw/day 生殖细胞比例异常、睾丸形态改变、精元干细胞功能特性丧失 [44] 体外 小鼠卵母细胞 BPA,BPF 5,25,50 μg/mL 纺锤体缩短,微管附着减少 [45] 体内 成年雄性大鼠 BPF 1,5,25,50 mg/kg/d 精子减少,睾丸激素分泌物水平降低 [46] 体内 雌性和雄性斑马鱼 BPB 0.001,0.01,0.1,1 mg/L 鱼卵数量减少,孵化率及存活率降低,生殖功能受损 [47] 体内 雌性和雄性斑马鱼 BPAF 0.05,0.25,1 mg/L 雄性斑马睾酮水平降低,鱼E2水平升高;雌性斑马鱼睾酮分泌增加 [48] 表 3 BPs对胚胎发育的影响
Table 3 Effects of BPs on embryonic development
-
[1] 刘洪媛, 金静, 郭崔崔, 等. 环境样品中双酚类化合物的固相萃取研究进展[J]. 色谱,2021,39(8):835−844. [LIU H Y, JIN J, GUO C C, et al. Research progress of solid phase extraction of bisphenols in environmental samples[J]. Chromatograph,2021,39(8):835−844. [2] 赵斌, 谭学蓉, 付瑜, 等. 基于QuEChERS的超高效液相色谱-串联质谱法快速测定果蔬中双酚类物质[J]. 分析化学,2022,50(5):810−821. [ZHAO B, TAN X R, FU Y, et al. Rapid determination of bisphenols in fruits and vegetables by ultra high-performance liquid chromatography-tandem mass spectrometry based on QuEChERS[J]. Analytical Chemistry,2022,50(5):810−821. [3] 李明, 高文晖, 余建龙, 等. 双酚类化合物促进乳腺癌细胞增殖活性的研究[J]. 毒理学杂志,2021,35(3):179−183,192. [LI M, GAO W H, YU J L, et al. Study on the effect of bisphenols on the proliferation of breast cancer cells[J]. Journal of Toxicology,2021,35(3):179−183,192. [4] LEE H R, JEUNG E B, CHO M H, et al. Molecular mechanism(s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors[J]. Journal of Cellular and Molecular Medicine,2013,17(1):1−11. doi: 10.1111/j.1582-4934.2012.01649.x
[5] COSTA E M, SPRITZER P M, HOHL A, et al. Effects of endocrine disruptors in the development of the female reproductive tract[J]. Arquivos Brasileiros de Endocrinologia e Metabologia,2014,58(2):153−161. doi: 10.1590/0004-2730000003031
[6] WILLHITE C C, DASTON G P. Bisphenol exposure, hazard and regulation[J]. Toxicology,2019,425:152243. doi: 10.1016/j.tox.2019.152243
[7] 吴皓, 孙东, 蔡卓平, 等. 双酚A的内分泌干扰效应研究进展[J]. 生态科学,2017,36(3):200−206. [WU H, SUN D, CAI Z P, et al. Research progress on endocrine disrupting effect of bisphenol A[J]. Ecological Science,2017,36(3):200−206. [8] WINKLER J, LIU P, PHONG K, et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome[J]. Proceedings of the National Academy of Sciences of the United States of America,2022,119(11):e2115308119. doi: 10.1073/pnas.2115308119
[9] REED J M, SPINELLI P, FALCONE S, et al. Evaluating the effects of BPA and TBBPA exposure on pregnancy loss and maternal-fetal immune cells in mice[J]. Environmental Health Perspectives,2022,130(3):37010. doi: 10.1289/EHP10640
[10] LIU J, LIAO M, HUANG R, et al. Perinatal combinational exposure to bisphenol a and a high-fat diet contributes to transgenerational dysregulation of cardiovascular and metabolic systems in mice[J]. Frontiers in Cell and Developmental Biology,2022,10:834346. doi: 10.3389/fcell.2022.834346
[11] CHEN D, KANNAN K, TAN H, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-a review[J]. Environmental Science & Technology,2016,50(11):5438−5453.
[12] VANDENBERG LN, HAUSER R, MARCUS M, et al. Human exposure to bisphenol A (BPA)[J]. Reproductive Toxicology,2007,24(2):139−77. doi: 10.1016/j.reprotox.2007.07.010
[13] ZINCKE T. Mittheilungen aus dem chemischen laboratorium der universitat marburg[J]. Justus Liebigs Annalen der Chemie,1905,343:75−99. doi: 10.1002/jlac.19053430106
[14] SABRY R, NGUYEN M, YOUNES S, et al. BPA and its analogs increase oxidative stress levels in in vitro cultured granulosa cells by altering anti-oxidant enzymes expression[J]. Molecular and Cellular Endocrinology,2022,545:111574. doi: 10.1016/j.mce.2022.111574
[15] SERRA H, BEAUSOLEIL C, HABERT R, et al. Evidence for bisphenol B endocrine properties: Scientific and regulatory perspectives[J]. Environmental Health Perspectives,2019,127(10):106001. doi: 10.1289/EHP5200
[16] CATENZA C J, FAROOQ A, SHUBEAR N S, et al. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues[J]. Chemosphere,2021,268:129273. doi: 10.1016/j.chemosphere.2020.129273
[17] WANG H, SONG S, SHAO M, et al. Determination of bisphenol analogues in food-contact plastics using diode array detector, charged aerosol detector and evaporative light-scattering detector[J]. Ecotoxicology and Environmental Safety,2019,186:109778. doi: 10.1016/j.ecoenv.2019.109778
[18] 杨倩, 刘建梅, 丁洁, 等. 双酚B对斑马鱼性别分化的影响及作用机制[J]. 生态毒理学报,2021,16(4):151−159. [YANG Q, LIU J M, DING J, et al. Effect and mechanism of bisphenol B on sex differentiation of zebrafish[J]. Journal of Ecotoxicology,2021,16(4):151−159. [19] LU S, YU Y, REN L, et al. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact[J]. The Science of the Total Environment,2018,21:389−1396.
[20] ROCHESTER J R, BOLDEN A L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol a substitutes[J]. Environmental Health Perspectives,2015,23(7):43−650.
[21] KURUTO-NIWA R, NOZAWA R, MIYAKOSHI T, et al. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system[J]. Environmental Toxicology and Pharmacology,2005,19(1):121−130. doi: 10.1016/j.etap.2004.05.009
[22] 李星, 王浩, 张文超, 等. 固相萃取-液相色谱-串联质谱法同时测定牛奶中双酚A、双酚F和双酚S[J]. 中国食品学报,2022,22(4):382−386. [LI X, WANG H, ZHANG W C, et al. Simultaneous determination of bisphenol A, bisphenol F and bisphenol S in milk by solid phase extraction-liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Food,2022,22(4):382−386. [23] MATSUSHIMA A, LIU X, OKADA H, et al. Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta[J]. Environmental Health Perspectives,2010,118(9):1267−1272. doi: 10.1289/ehp.0901819
[24] JI K, CHOI K. Endocrine disruption potentials of bisphenol A alternatives[J]. Korean Journal of Environmental Health Sciences,2013,39(1):1−18. doi: 10.5668/JEHS.2013.39.1.1
[25] 付国瑞, 鲁文嘉. 袋装液态乳中4种双酚类化合物的检测与分析[J]. 食品安全导刊,2018,197(6):153−156. [FU G R, LU W J. Detection and analysis of four bisphenols in bagged liquid milk[J]. Food Safety Guide,2018,197(6):153−156. [26] 黄苑, 张维, 王瑞国, 等. 双酚类化合物污染现状和内分泌干扰效应研究进展[J]. 生态毒理学报,2022,17(1):1−27. [HUANG Y, ZHANG W, WANG R G, et al. Research progress on pollution status and endocrine disrupting effects of bisphenols[J]. Journal of Ecotoxicology,2022,17(1):1−27. [27] LAUREL P. Potential designated chemicals: p,p'-Bisphenols and diglycidyl ethers of p,p'-bisphenols[C]//Biomonitoring California Scientific Guidance Panel Meeting. Sacramento: Office of Environmental Health Hazard Assessment, 2012: 3
[28] XUE J, KANNAN P, KUMOSANI T A, et al. Resin-based dental sealants as a source of human exposure to bisphenol analogues, bisphenol A diglycidyl ether, and its derivatives[J]. Environmental Research,2018,162:35−40. doi: 10.1016/j.envres.2017.12.011
[29] BECERRA V, ODERMATT J. Interferences in the direct quantification of bisphenol S in paper by means of thermochemolysis[J]. Journal of Chromatography A,2013,1275:70−77. doi: 10.1016/j.chroma.2012.12.034
[30] ZHANG L, FANG P, YANG L, et al. Rapid method for the separation and recovery of endocrine-disrupting compound bisphenol AP from wastewater[J]. Langmuir:The ACS Journal of Surfaces and Colloids,2013,29(12):3968−3975. doi: 10.1021/la304792m
[31] ZHANG J, SHI J, GE H, et al. Tiered ecological risk assessment of nonylphenol and tetrabromobisphenol A in the surface waters of China based on the augmented species sensitivity distribution models[J]. Ecotoxicology and Environmental Safety,2022,236:113446. doi: 10.1016/j.ecoenv.2022.113446
[32] YE G, CHEN Y, WANG H O, et al. Metabolomics approach reveals metabolic disorders and potential biomarkers associated with the developmental toxicity of tetrabromobisphenol A and tetrachlorobisphenol A[J]. Scientific Reports,2016,6:35257. doi: 10.1038/srep35257
[33] 杨丹, 李丹丹, 刘姗姗, 等. 双酚A对机体的影响及其作用机制[J]. 现代预防医学,2008(17):3280−3282,3287. [YANG D, LI D D, LIU S S, et al. Effect of bisphenol A on body and its mechanism[J]. Modern Preventive Medicine,2008(17):3280−3282,3287. doi: 10.3969/j.issn.1003-8507.2008.17.011 [34] BHANDARI R K, VOM-SAAL F S, TILLITT D E. Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes[J]. Scientific Reports,2015,5:9303. doi: 10.1038/srep09303
[35] BHANDARI R K, WANG X, SAAL F S V, et al. Transcriptome analysis of testis reveals the effects of developmental exposure to bisphenol a or 17α-ethinylestradiol in medaka (Oryzias latipes)[J]. Aquatic Toxicology,2020,225:105553. doi: 10.1016/j.aquatox.2020.105553
[36] REZAEE-TAZANGI F, ZEIDOONI L, RAFIEE Z, et al. Taurine effects on Bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility[J]. JBRA Assisted Reproduction,2020,24(4):428−435.
[37] MARKEY C M, COOMBS M A, SONNENSCHEIN C, et al. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs[J]. Evolution & Development,2003,5(1):67−75.
[38] RICHTER C A, BIRNBAUM L S, FARABOLLINI F, et al. In vivo effects of bisphenol A in laboratory rodent studies[J]. Reproductive Toxicology,2007,24(2):199−224.
[39] PIAZZA M J, URBANETZ A A. Environmental toxins and the impact of other endocrine disrupting chemicals in women's reproductive health[J]. JBRA Assisted Reproduction,2019,23(2):154−164.
[40] XUE W, YAO X, TING G, et al. BPA modulates the WDR5/TET2 complex to regulate ERβ expression in eutopic endometrium and drives the development of endometriosis[J]. Environmental Pollution,2021,268(Pt B):115748.
[41] NGUYEN M, SABRY R, DAVIS O S, et al. Effects of BPA, BPS, and BPF on oxidative stress and antioxidant enzyme expression in bovine oocytes and spermatozoa[J]. Genes,2022,13(1):142. doi: 10.3390/genes13010142
[42] LI N, KANG H, PENG Z, et al. Physiologically detectable bisphenol A impairs human sperm functions by reducing protein-tyrosine phosphorylation[J]. Ecotoxicology and Environmental Safety,2021,221:112418. doi: 10.1016/j.ecoenv.2021.112418
[43] ULLAH H, ULLAH F, REHMAN O, et al. Chronic exposure of bisphenol S (BPS) affect hypothalamic-pituitary-testicular activities in adult male rats: Possible in estrogenic mode of action[J]. Environmental Health and Preventive Medicine,2021,26(1):31. doi: 10.1186/s12199-021-00954-0
[44] KARMAKAR P C, AHN J S, KIM Y H, et al. Paternal Exposure to bisphenol-A transgenerationally impairs testis morphology, germ cell associations, and stemness properties of mouse spermatogonial stem cells[J]. International Journal of Molecular Sciences,2020,21(15):5408. doi: 10.3390/ijms21155408
[45] YANG L, BAUMANN C, DE-LA-FUENTE R, et al. Mechanisms underlying disruption of oocyte spindle stability by bisphenol compounds[J]. Reproduction,2020,159(4):383−396. doi: 10.1530/REP-19-0494
[46] ULLAH A, PIRZADA M, AFSAR T, et al. Effect of bisphenol F, an analog of bisphenol A, on the reproductive functions of male rats[J]. Environmental Health and Preventive Medicine,2019,24(1):41. doi: 10.1186/s12199-019-0797-5
[47] YANG Q, YANG X H, LIU J N, et al. Exposure to bisphenol b disrupts steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish[J]. Water Air & Soil Pollution,2017,228(3):112.1−112.12.
[48] YANG X, LIU Y, LI J, et al. Exposure to bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish[J]. Environmental Toxicology,2016,31(3):285−294. doi: 10.1002/tox.22043
[49] 谢军, 李小玲, 陈宏. 双酚A致体外培养大鼠胚胎发育毒性的时间-效应关系研究[J]. 实用预防医学,2010,17(8):1649−1651. [XIE J, LI X L, CHEN H, et al. Study on time-effect relationship of developmental toxicity of rat embryos induced by bisphenol A in vitro[J]. Practical Preventive Medicine,2010,17(8):1649−1651. doi: 10.3969/j.issn.1006-3110.2010.08.075 [50] 王连卿. 双酚A对小鼠早期胚胎体外发育的影响[D]. 哈尔滨: 东北农业大学, 2007 WANG L Q. Effect of bisphenol A on the development of early mouse embryos in vitro[D]. Harbin: Northeast Agricultural University, 2007.
[51] 裴新荣, 李勇, 龙鼎新, 等. 双酚A对小鼠早期胚胎发育毒性的体外实验研究[J]. 中国生育健康杂志,2003(1):34−37, 65. [PEI X R, LI Y, LONG D X, et al. Experimental study on the toxicity of bisphenol A on early embryonic development of mice in vitro[J]. Chinese Journal of Reproductive Health,2003(1):34−37, 65. [52] CHEN H, ZHONG K, ZHANG Y, et al. Bisphenol A interferes with redox balance and the Nrf2 signaling pathway in xenopus tropicalis during embryonic development[J]. Animals,2022,12(7):937. doi: 10.3390/ani12070937
[53] MCCORMICK J M, PAIVA M S, HÄGGBLOM M M, et al. Embryonic exposure to tetrabromobisphenol A and its metabolites, bisphenol A and tetrabromobisphenol A dimethyl ether disrupts normal zebrafish (Danio rerio) development and matrix metalloproteinase expression[J]. Aquatic toxicology,2010,100(3):255−262. doi: 10.1016/j.aquatox.2010.07.019
[54] 王英红, 徐虹, 孔庆鑫, 等. 双酚S急性暴露对斑马鱼胚胎及子代胚胎发育的毒性效应[J]. 中国卫生检验杂志,2019,29(1):32−36. [WANG Y H, XU H, KONG Q X, et al. Toxic effects of acute exposure to bisphenol S on embryo and offspring embryo development of zebrafish[J]. Chinese Journal of Health Inspection,2019,29(1):32−36. [55] 郭依晨, 韩静静, 严锐, 等. 双酚A胚胎期暴露对斑马鱼的发育毒性及运动行为的影响[J]. 毒理学杂志,2018,32(2):152−155. [GUO Y C, HAN J J, YAN R, et al. Effects of embryonic exposure to bisphenol A on developmental toxicity and exercise behavior of zebrafish[J]. Journal of Toxicology,2018,32(2):152−155. doi: 10.16421/j.cnki.1002-3127.2018.02.015 [56] 王宏烨, 曹诚, 刘益海, 等. 双酚AP对斑马鱼心脏发育毒性作用研究[J]. 环境科学与技术,2019,42(8):1−6. [WANG H B, CAO Y, LIU Y H, et al. Study on cardiac developmental toxicity of bisphenol AP on zebrafish[J]. Environmental science and technology,2019,42(8):1−6. [57] HARNETT K G, MOORE L G, CHIN A, et al. Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development[J]. Current research in Toxicology,2021,2:399−410. doi: 10.1016/j.crtox.2021.11.001
[58] ZHOU R, XIA M, ZHANG L, et al. Individual and combined effects of BPA, BPS and BPAF on the cardiomyocyte differentiation of embryonic stem cells[J]. Ecotoxicology and Environmental Safety,2021,220:112366. doi: 10.1016/j.ecoenv.2021.112366
[59] GE Y, REN F, CHEN L, et al. Bisphenol A exposure induces apoptosis and impairs early embryonic development in Xenopus laevis[J]. Environmental Pollution,2021,280:116901. doi: 10.1016/j.envpol.2021.116901
[60] YANG Q, ZHU Z, LIU Q, et al. Adverse effects of bisphenol B exposure on the thyroid and nervous system in early life stages of zebrafish[J]. Comparative Biochemistry and Physiology. Toxicology & Ppharmacology:CBP,2021,250:109167.
[61] LEE C T, HSIEH C F, WANG J Y. Bisphenol a Induces Autophagy Defects and AIF-Dependent Apoptosis via HO-1 and AMPK to Degenerate N2a Neurons[J]. International Journal of Molecular Sciences,2021,22(20):10948. doi: 10.3390/ijms222010948
[62] SAHOO P K, APARNA S, NAIK P K, et al. Bisphenol A exposure induces neurobehavioral deficits and neurodegeneration through induction of oxidative stress and activated caspase-3 expression in zebrafish brain[J]. Journal of Biochemical and Molecular Toxicology,2021,35(10):e22873.
[63] KOIKE E, YANAGISAWA R, WIN-SHWE T T, et al. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice[J]. International Journal of Immunopathology and Pharmacology,2018,32:1−14.
[64] YOUN J Y, PARK H Y, LEE J W, et al. Evaluation of the immune response following exposure of mice to bisphenol A: induction of Th1 cytokine and prolactin by BPA exposure in the mouse spleen cells[J]. Archives of Pharmacal Research,2002,25(6):946−953. doi: 10.1007/BF02977018
[65] LEE J, LIM K T. Plant-originated glycoprotein (36 kDa) suppresses interleukin-4 and -10 in bisphenol A-stimulated primary cultured mouse lymphocytes[J]. Drug and Chemical Toxicology,2010,33(4):421−429. doi: 10.3109/01480541003739229
[66] SUGITA-KONISHI Y, SHIMURA S, NISHIKAWA T, et al. Effect of Bisphenol A on non-specific immunodefenses against non-pathogenicEscherichia coli[J]. Toxicology Letters,2003,136(3):217−227. doi: 10.1016/S0378-4274(02)00388-0
[67] GOTO M, TAKANO-ISHIKAWA Y, ONO H, et al. Orally administered bisphenol A disturbed antigen specific immunoresponses in the naive condition[J]. Bioscience, Biotechnology, and Biochemistry,2007,71(9):2136−2143. doi: 10.1271/bbb.70004
[68] ROY A, BAUER S M, LAWRENCE B P. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection[J]. PLoS One,2012,7(6):e38448. doi: 10.1371/journal.pone.0038448
[69] FEITEIRO J, MARIANA M, GLÓRIA S, et al. Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle[J]. The Journal of Toxicological Sciences,2018,43(10):579−586. doi: 10.2131/jts.43.579
[70] BROWN A R, GREEN J M, MOREMAN J, et al. Cardiovascular effects and molecular mechanisms of bisphenol A and its metabolite MBP in zebrafish[J]. Environmental Science & Technology,2019,53(1):463−474.
[71] PAL S, SARKAR K, NATH P P, et al. Bisphenol S impairs blood functions and induces cardiovascular risks in rats[J]. Toxicology Reports,2017,4:560−565. doi: 10.1016/j.toxrep.2017.10.006
[72] ILOZUMBA M N, SHELVER W L, HONG C C, et al. Urinary concentrations of triclosan, bisphenol A, and brominated flame retardants and the association of triclosan with demographic characteristics and body fatness among women with newly diagnosed breast cancer[J]. International Journal of Environmental Research and Public Health,2022,19(8):4681. doi: 10.3390/ijerph19084681
[73] ZHANG X, GUO N, JIN H, et al. Bisphenol A drives di(2-ethylhexyl) phthalate promoting thyroid tumorigenesis via regulating HDAC6/PTEN and c-MYC signaling[J]. Journal of hazardous materials,2022,425:127911. doi: 10.1016/j.jhazmat.2021.127911
[74] 龙子, 樊隽澍, 吴光源, 等. 低剂量双酚A通过调控PPARγ致小鼠糖脂代谢紊乱的研究[J]. 癌变·畸变·突变,2020,32(04):245−255. [LONG Z, FAN J S, WU G Y, et al. Low-dose bisphenol A regulates glucose and lipid metabolism disorder induced by PPARγ in mice[J]. Cancerous Aberration Mutation,2020,32(04):245−255. [75] MASUNO H, KIDANI T, SEKIYA K, et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes[J]. Journal of Lipid Research,2002,43(5):676−684. doi: 10.1016/S0022-2275(20)30108-5
[76] WADA K, SAKAMOTO H, NISHIKAWA K, et al. Life style-related diseases of the digestive system: endocrine disruptors stimulate lipid accumulation in target cells related to metabolic syndrome[J]. Journal of Pharmacological Sciences,2007,105(2):133−137. doi: 10.1254/jphs.FM0070034
[77] SARGIS R M, JOHNSON D N, CHOUDHURY R A, et al. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation[J]. Obesity,2010,18(7):1283−1288. doi: 10.1038/oby.2009.419
[78] HUC L, LEMARIÉ A, GUÉRAUD F, et al. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells[J]. Toxicology in Vitro: An International Journal Published in Association with BIBRA,2012,26(5):709−717.
[79] MOON M K, KIM M J, JUNG I K, et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level[J]. Journal of Korean Medical Science,2012,27(6):644−652. doi: 10.3346/jkms.2012.27.6.644
[80] HASSAN Z K, ELOBEID M A, VIRK P, et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model[J]. Oxidative Medicine and Cellular longevity,2012,2012:194829.
[81] HYUN S A, KO M Y, JANG S, et al. Bisphenol-A impairs synaptic formation and function by RGS4-mediated negative regulation of BDNF/NTRK2 signaling in the cerebral cortex[J]. Disease Models & Mechanisms, 2022, 049177.
[82] DONG P, YE G, TU X, et al. Roles of ERRα and TGF-β signaling in stemness enhancement induced by 1 µM bisphenol A exposure via human neural stem cells[J]. Experimental and Therapeutic Medicine,2022,23(2):164.
[83] WU M, CONG Y, WANG K, et al. Bisphenol A impairs macrophages through inhibiting autophagy via AMPK/mTOR signaling pathway and inducing apoptosis[J]. Ecotoxicology and Environmental Safety,2022,234:113395. doi: 10.1016/j.ecoenv.2022.113395
[84] LOUP B, POUMEROL E, JOUNEAU L, et al. BPA disrupts meiosis I in oogonia by acting on pathways including cell cycle regulation, meiosis initiation and spindle assembly[J]. Reproductive Toxicology,2022,111:166−177. doi: 10.1016/j.reprotox.2022.06.001
[85] 杨静. 双酚A及其类似物生物效应及构效关系研究[D]. 黄石: 湖北师范大学, 2021 YANG J. Study on biological effects and structure-activity relationship of bisphenol A and its analogues[D]. Huangshi: Hubei normal University, 2021.
[86] KHAN N G, CORREIA J, ADIGA D, et al. A comprehensive review on the carcinogenic potential of bisphenol A:Clues and evidence[J]. Environmental Science and Pollution Research International, 2021, 28(16): 19643-19663.
[87] TUDURÍ E, MARROQUI L, DOS SANTOS R S, et al. Timing of exposure and Bisphenol-A: Implications for diabetes development[J]. Frontiers in Endocrinology,2018,9:648. doi: 10.3389/fendo.2018.00648
[88] VOM-SAAL F S, NAGEL S C, COE B L, et al. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity[J]. Molecular and Cellular Endocrinology,2012,354(1−2):74−84. doi: 10.1016/j.mce.2012.01.001
[89] SÁNCHEZ-GARRIDO M A, GARCÍA-GALIANO D, TENA-SEMPERE M. Early programming of reproductive health and fertility: Novel neuroendocrine mechanisms and implications in reproductive medicine[J]. Human Reproduction Update,2022,28(3):346−375. doi: 10.1093/humupd/dmac005
[90] ACCONCIA F, PALLOTTINI V, MARINO M. Molecular mechanisms of action of BPA[J]. Dose-response:A Publication of International Hormesis Society,2015,13(4):1−9.
-
期刊类型引用(8)
1. 甘杰,丰小阳,王国庆,蒋开年,熊芬,魏凤. 固相萃取液相色谱-三重四极杆质谱仪测定水中7种烷基酚类和双酚类化合物. 中国资源综合利用. 2025(02): 16-23 .  百度学术
百度学术
2. 吴刚,吴冰婵,阮凯,王力君,来燕芳,王忠才. 超高效液相色谱-串联质谱联用快速测定纺织品中多种双酚类化合物. 中国口岸科学技术. 2025(03): 54-62 .  百度学术
百度学术
3. 卢文婷,胡雄飞,陈志莲,张丽娜,杨秀鸿. 肉苁蓉提取物安全性的毒理学研究. 医学动物防制. 2024(10): 979-983+989 .  百度学术
百度学术
4. 徐金梅,谭智毅,麦晓霞,蒋中鸣,颜焯文,张子豪. 高效液相色谱紫外检测法测定纺织品中5种双酚类物质. 印染. 2023(08): 62-65 .  百度学术
百度学术
5. 孙颖,白林,任润涵,尚伟,翁云宣. 食品接触材料中双酚A分析方法的建立及验证. 中国塑料. 2023(11): 81-86 .  百度学术
百度学术
6. 董佳昱,田姗姗,钱丽,姜苏,唐云平. 富DHA-磷脂酰胆碱对双酚S致小鼠肝损伤的影响. 广东海洋大学学报. 2023(06): 138-145 .  百度学术
百度学术
7. 汪美凤,王娣,韦启信. 钴掺杂氮化碳传感器电化学检测牛奶中双酚A. 安庆师范大学学报(自然科学版). 2023(04): 90-95 .  百度学术
百度学术
8. Sha Wang. Literature review on waste management of online food delivery industry in China. Chinese Journal of Population, Resources and Environment. 2023(03): 197-202 .  必应学术
必应学术
其他类型引用(1)





 下载:
下载:

 下载:
下载:



