Autolysis Process of Shrimp By-products and Identification of Potential Antifreeze Peptides
-
摘要: 为加强海洋副产物的高值化加工利用,本文以虾副产物为原料,在原始pH(7.81~7.83)与50 ℃水浴条件下进行自溶反应,将酶解产物于95 ℃水浴加热20 min使内源酶失活,研究了过程中酶解产物的多肽组成变化,并基于活性预测平台筛选得到抗冻肽序列,提供了一种高效率筛选活性肽的方法。自溶处理的0~3 h,酶解物的水解度(DH)和TCA可溶性肽含量急剧增加,分别达到49.8%和26.1 mg/g,随后增长速度变缓。在酶解0.5 h产物(A-0.5)中检出1301条肽段,酶解3 h产物(A-3)中检出197条;A-0.5中大于1600 Da的肽含量达到69.5%,而A-3中肽以600~1600 Da为主。基于活性预测平台Cryoprotect,筛选QVHPDTGISS、GYGCARPNYPGV、TTGEVCDSGDGVTH、EQICINFCNEK、DEYEESGPGIVH、DIDNDGFLDK和DIDNNGFLDK 7个序列为抗冻肽,并通过酶切模拟得到肽段的来源。Abstract: In order to strengthen the high-value processing and utilization of marine by-products, shrimp by-products were used in this study. The autolysis reaction was carried out in a water bath at 50 ℃ and pH(7.81~7.83), then the enzymatic hydrolysate was heated in water bath at 95 ℃ for 20 min to inactivate endogenous enzymes. This paper studied the changes in the polypeptide composition of the enzymatic hydrolysis products during the process, and screened the antifreeze peptide sequences based on the activity prediction platform, and provided a method for screening active peptides with high efficiency. When the autolysis treatment was 0~3 h, the content of degree of hydrolysis (DH) and TCA soluble peptides in the enzymatic hydrolyzate increased sharply, reaching 49.8% and 26.1 mg/g, respectively, and then the growth rate slowed down. 1301 peptides were detected in the 0.5 h enzymatic hydrolysis product (A-0.5), and 197 in the 3 h enzymatic hydrolysis product (A-3). The content of peptides greater than 1600 Da in A-0.5 reached 69.5%, while the peptides in A-3 were dominated by 600~1600 Da. Based on the activity prediction platform Cryoprotect, 7 sequences of QVHPDTGISS, GYGCARPNYPGV, TTGEVCDSGDGVTH, EQICINFCNEK, DEYEESGPGIVH, DIDNDGFLDK and DIDNNGFLDK were screened as antifreeze peptides, and the source of the peptides was obtained by enzyme cleavage simulation.
-
Keywords:
- autolysis /
- polypeptide compositions /
- activity prediction /
- antifreeze peptide /
- shrimp by-products
-
虾是重要的渔业资源,自2013年以来,我国虾养殖和产量呈逐年上升趋势,据统计,2020年虾总产量达630.73万吨,位居世界前列[1]。虾的副产物,头、壳和尾等部位,占整虾重量的50%~60%[2],因此在加工过程中会产生大量加工废弃物[3]。目前,仅有少部分用作养殖业饲料,绝大部分没有得到有效处理和利用,带来资源浪费与环境负担[4]。通过酶解副产物以回收其中的蛋白质资源是当前较流行的副产物高值化利用的方法[5-6]。酶解法需要添加外源性的酶,成本较高。与此同时,虾消化系统中内源性酶却并未得到有效利用,造成资源浪费。有报道指出,虾副产物的自溶产物具有抗氧化和抑菌活性,是活性肽的优良来源[7-8]。
抗冻肽(antifreeze peptide,AFP)是具有热滞活性和冰晶生长抑制能力的优秀水产制品抗冻剂[9]。从酶解产物中寻找抗冻肽一直是研究热点。其中Chen等[10]报道了罗非鱼鳞片水解物对嗜热链球菌(Streptococcus thermophilus)的低温保护作用;Du等[11]从鸡皮胶原中筛选出具有冰晶生长抑制作用的AFPs。一般来说,鉴定和表征AFPs的流程包括原料酶解、从水解物的复杂产物中分离纯化和定性分析等步骤[12],费力且低效。近年来,随着高通量质谱技术的发展,多肽组学(peptidomics)技术成为分析水解液中复杂多肽产物的有效方法[13]。此法被广泛应用于农产品副产物的高值化加工[14-15]。研究人员已成功从辣椒种子[16]和乳清[17]中制备得到具有抑菌、抗氧化和ACE抑制活性的肽。已有部分研究在虾副产物中提取油脂产物,但虾副产物也是蛋白质的良好来源,相关技术还未成熟,基于此,本文通过虾副产物的自溶反应,研究了自溶过程中酶解产物的多肽组成变化,利用活性预测平台从中筛选抗冻肽序列,旨在为海洋副产物的高值化利用提供新思路。
1. 材料与方法
1.1 材料与仪器
鹰爪虾 购于舟山国际水产城,冰盒保险运至实验室;三氯乙酸、邻苯二甲醛、丝氨酸标准品(98%)、四硼酸钠、二硫苏糖醇 均为国产分析纯,中国国药集团;A045-3型BCA蛋白定量试剂盒 南京建成生物科技有限公司。
Freeze Zone冷冻干燥机 美国Labconco公司;粉碎机 永康市红太阳机电有限公司;紫外灯 苏州国牛灯具有限公司;恒温水浴锅 上海力辰仪器科技有限公司;SORVALL LYNX 4000离心机、Evo-lution 60S紫外测定仪 美国Thermo公司;RE-3000型旋转蒸发仪 上海亚荣生化仪器公司;T18高速分散机 德国IKA集团。
1.2 实验方法
1.2.1 自溶产物制备
鹰爪虾流水解冻,采集虾头、外骨骼、腹足、虾尾等副产物,使用去离子水清洗。随后冻干至水分含量低于10%并粉碎成粉末。将副产物粉末与水按照1:3(w/w)的比例调配成匀浆。自溶处理前,为提高内源性蛋白酶活性,将匀浆倒入30 cm×20 cm的瓷盘中,匀浆厚度5 mm,随后至于253.7 nm紫外灯照射20 min(高度25 cm)[18-19]。自溶反应在原始pH(7.81~7.83)和50 ℃水浴条件下进行,分别自溶处理0.5、1、1.5、2、3、4、5、6 h,随后将酶解产物95 ℃水浴加热20 min用于对内源酶灭活。在10000 r/min、4 ℃条件下离心收集15 min,并用双层滤纸抽滤上层清液,得到最终自溶产物。冷冻干燥72 h即可得酶解物粉末,装入封口袋密封并置于−18 ℃保存。
1.2.2 TCA可溶性肽含量测定
参考Nikoo等[7]的方法测定不同处理时间的酶解物中TCA可溶性肽含量,并略作修改。1 mL自溶产物与9 mL 5% TCA溶液混合并振荡30 s,随后4 ℃,10000 r/min下离心10 min,收集上清。采用BCA蛋白定量试剂盒测定上清液中TCA可溶性肽含量。
1.2.3 水解度(DH)测定
采用OPA法[20-21]测定不同自溶处理时间酶解物的DH值,略作修改。取0.3 g自溶产物溶于去离子水,并定容至100 mL,制得样品溶液;取7.620 g四硼酸钠,200 mg SDS,160 mg OPA,176 mg DTT和4 mL乙醇溶于水中,并定容至150 mL,制得OPA溶液。400 mL样品溶液(Asample)、0.9516 mmol/L丝氨酸标准溶液(Astandard)和去离子水(Ablank)中分别加入3 mL OPA溶液,孵育2 min,随后于340 nm波长处测定吸光度。DH值(%)按照以下公式计算:
DH(%)=hhtot×100 (1) 式中:h=(serine-NH2-b)/a meqv/g protein;htot是每个蛋白质当量的肽键摩尔数;对于虾肉蛋白,htot=8,b=0.4,a=1[22]。
Serine-NH2表示丝氨酸氨基的毫摩尔数:
Serine-NH2=Asample−AblankAstandard−Ablank×0.9516meqv/L×0.1×100L/gproteinX×P 式中:X表示样品重量;P表示样品蛋白含量。
1.2.4 多肽组学分析
1.2.4.1 还原烷基化
在进行LC-MS/MS分析之前,样品需要先经过还原烷基化处理。将100 mL 20 mg/mL样品-ddH2O溶液与100 mL 20 mmol/L DTT溶液混匀,随后于56 ℃水浴中1 h。之后加入200 mL 100 mmol/L碘乙酰胺溶液并室温避光孵育40 min以完成烷基化。45 ℃真空旋蒸去除溶剂并用2~20 mL 0.1%甲酸溶解。
1.2.4.2 LC-MS/MS分析
液相色谱条件:进样量5 mL;色谱柱150 mm×15 cm C18-AQ反向纳米柱;流动相:A:0.1%甲酸水溶液、B:0.1%甲酸乙腈溶液;流速600 nL/min;梯度洗脱:0~3 min 4%~8%B相、3~89 min 8%~28% B相、89~109 min 28%~40% B相、109~110 min 40%~95% B相、110~120 min 95% B相。
质谱条件:一级质谱,Resolution:70000、AGCtarget:3e6、MaxinumIT:40 ms、Scanrange:300~1800 m/z;二级质谱,Resolution:17500、AGCtarget:1e5、MaxinumIT:60 ms、TopN:20、NCE/steppedNCE:27。
1.2.4.3 多肽序列分析
分析质谱数据并通过Max Quant(1.6.2.10)软件比对目标蛋白数据库。参数设定如下:固定修饰:carbamidomethylation(C);可变修饰:oxidation(M),Acetyl(Protein N-term);酶:Unspecific;遗漏酶切位点:2;一级质谱误差:20 ppm;二级质谱误差:20 ppm;肽段/碎片离子质量数:Monoisotopic;显著性阈值:0.01。选择其中高置信度的肽段进行基于UniProt蛋白库(https://www.uniprot.org/)的下游蛋白鉴定分析。
1.2.5 多肽理化性质预测
基于序列信息,通过ProtParam(https://web.expasy.org/protparam/)分析多肽的理化性质,包括序列长度、理论等电点、分子量、电荷以及不稳定指数;通过ProtScale(https://web.expasy.org/protscale/)计算总平均疏水指数(grand average of hydropathicity,GRAVY)。
1.2.6 多肽抗冻活性预测
使用抗冻活性预测平台Cryoprotect(http://codes.bio/cryoprotect/)进行活性预测。
1.2.7 同源模型建立
多肽的结构模型由I-TASSER(https://zhanggroup.org/I-TASSER/)构建;同源模型(Homology models)由SWISS-MODEL(https://swissmodel.expasy.org/)建立。
1.2.8 酶切模拟
通过模拟肽被水解释放的过程,可了解该肽的来源。蛋白水解模拟(胰蛋白酶和糜蛋白酶)由PeptideCutter(https://web.expasy.org/peptide_cutter/)构建。
1.3 数据处理
采用SPSS 21.0软件进行数据处理,ANOVA分析采用最小显著性差异法(LSD),显著性差异水平设定为P<0.05,并标注显著性差异。以上各组实验均进行3组平行测定。
2. 结果与分析
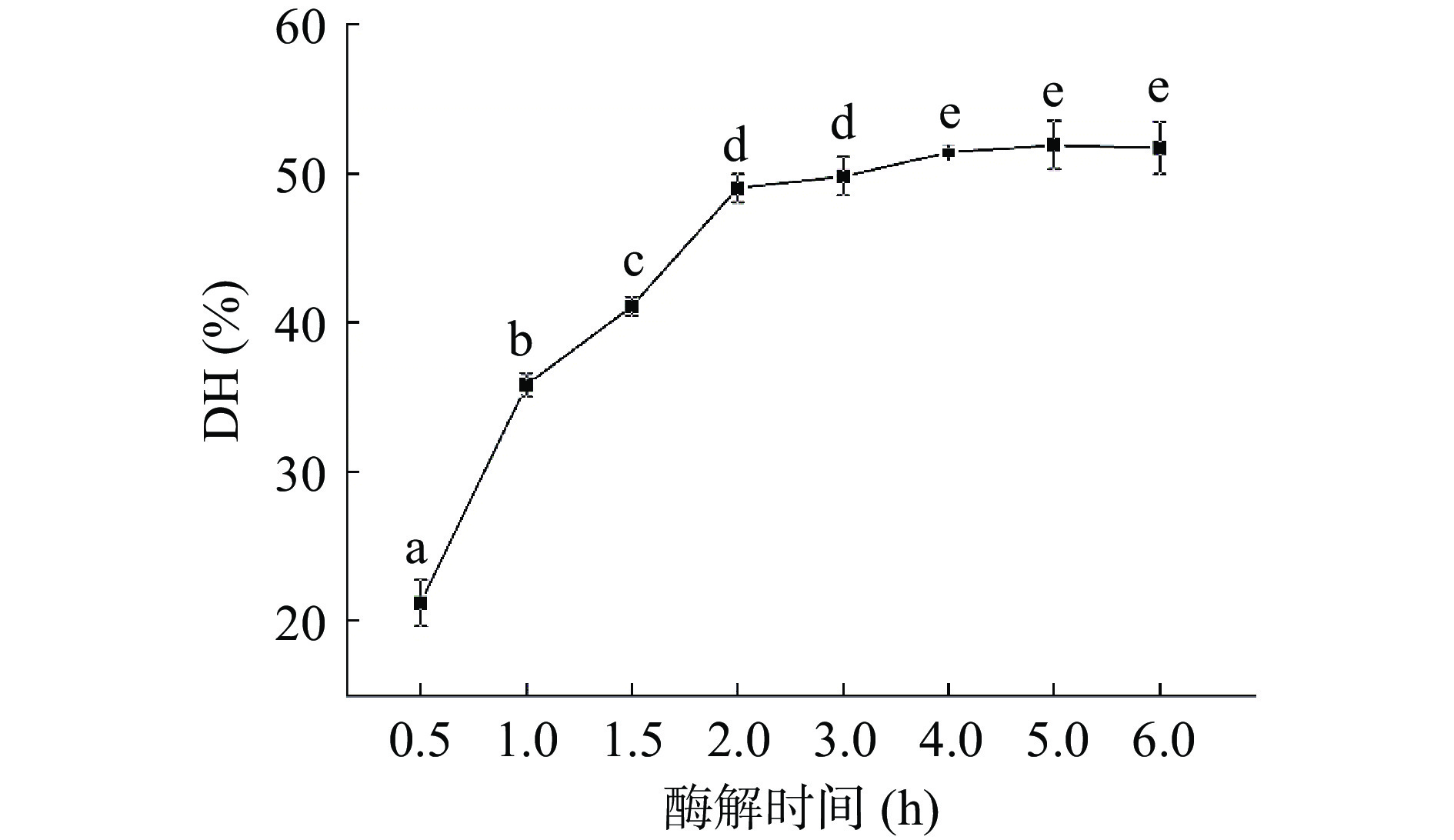
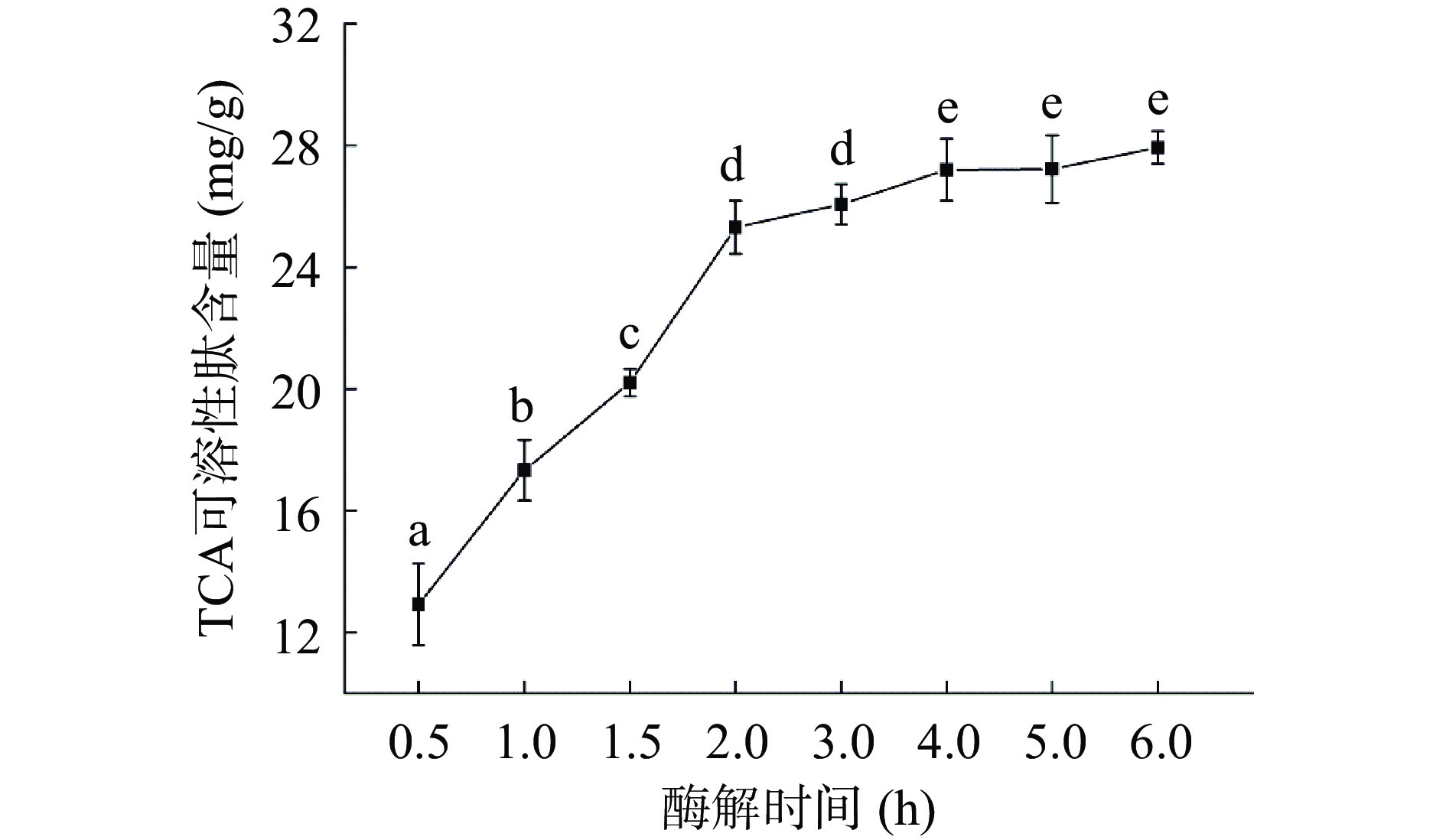
2.1 自溶过程中DH和TCA可溶性肽含量变化
不同自溶处理时间会显著影响酶解产物中多肽的组成,通常表现为DH和TCA可溶性肽含量的变化[23-24]。
如图1所示,酶解物的DH随酶解时间增加不断上升。最初0.5 h DH为21.2%,随着酶解时间的增加,DH不断上升,直至6 h达到51.7%。其中3 h后的增长明显变缓。Cao等[25]也报道虾头的自溶反应在最初的3 h基本结束,最终的DH为45%。如图2所示,类似的情况也在TCA可溶性含量变化中发现:肽含量在0~3 h迅猛增长而在3~6 h相对稳定。虾消化系统中含有内源性胰凝乳蛋白酶和胰蛋白酶,可对副产物中的蛋白质进行水解,从而产生短肽和游离氨基酸,这可能是DH和TCA可溶性肽含量增加的主要原因[22]。然而酶解过程中底物蛋白浓度的降低和酶的受热变性可能是增长减缓的原因之一[7]。
2.2 自溶过程中肽组分变化
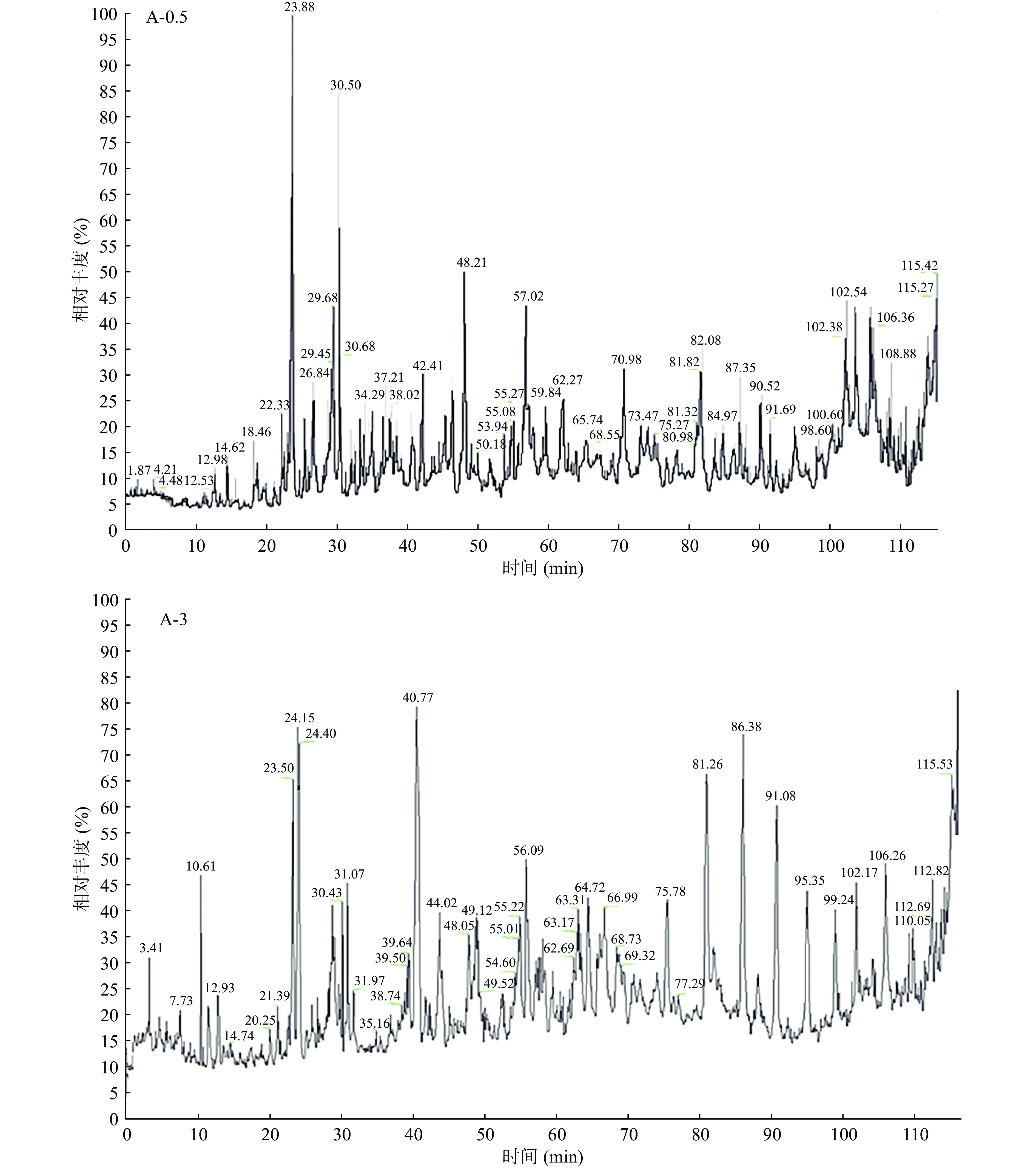
根据2.1中DH随自溶处理时间的变化趋势,选取自溶时间0.5 h(A-0.5)和3 h(A-3)的酶解产物作为LC-MS/MS分析对象,总离子流色谱如图3所示。
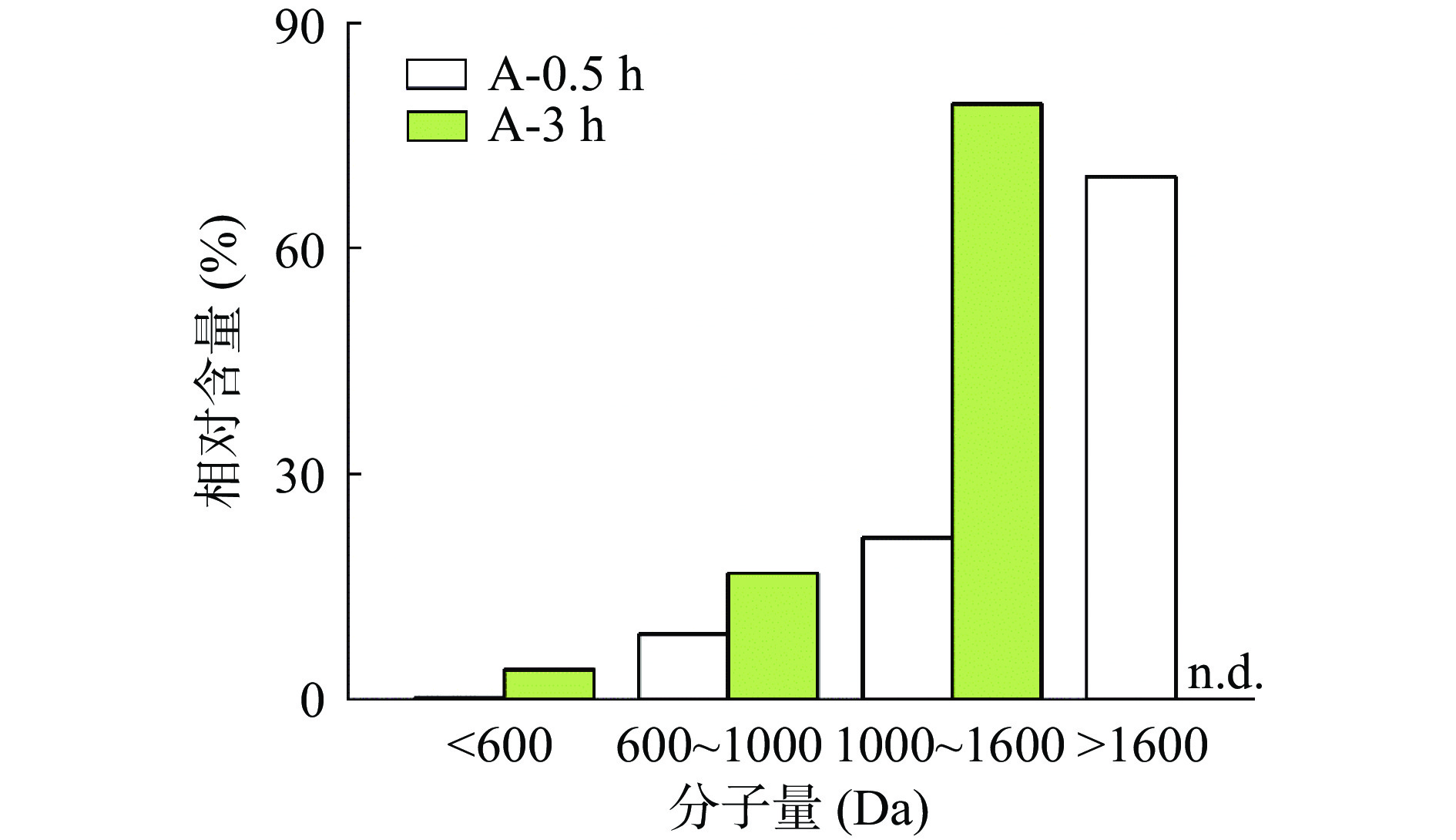
结果表明,两次分析均在120 min内完成,其中A-0.5中检出1301条肽段,A-3中检出197条。这说明,随着时间的增加,副产物蛋白和长链肽被不断酶解,产生短肽和游离氨基酸。直观表现为组分中肽分子量的变化。A-0.5中大于1600 Da的肽占据了绝大部分(69.5%)。而在A-3中未检出大于1600 Da的肽,1000~1600、600~1000和<600 Da的肽含量则明显增加,分别为79.2%、16.8%、4.0%(图4)。这表明,随着酶解的进行,长链肽会被水解,产生短链肽和游离氨基酸。通常来说,低分子量的短肽具有高生物活性的概率高于长链肽[26],因此,为获得含有更大比例低分子量短肽的酶解产物和防止进一步酶解发生,选择3 h的酶解产物进行抗冻肽筛选。
此外,通过对酶解物中肽来源的蛋白质进行研究,可明确副产物中的主要蛋白种类。如表1所示,A-3中肽主要来源于8类蛋白,包括血蓝蛋白、肌动蛋白、肌球蛋白重链、肌动蛋白轻链、胰蛋白酶、木糖异构酶、肌浆蛋白和其它。其中虾血蓝蛋白和肌动蛋白含量(39.61%和36.56%)明显高于其他6种蛋白,说明这两种蛋白很可能是鹰爪虾副产物中的主要蛋白。
表 1 酶解产物肽的源蛋白Table 1. Source protein of peptide obtained by enzymatic digestion源蛋白类型 含量(%) 血蓝蛋白 39.61 肌动蛋白 36.56 肌球蛋白轻链 6.6 肌球蛋白重链 5.12 胰蛋白酶 4.57 肌浆蛋白 1.66 木糖异构酶 5.26 其它 0.62 2.3 抗冻肽筛选与活性预测
为缩小筛选范围,从所获得的197条肽中选出26条具有高置信度的肽序列。肽段的序列长度、所带电荷、亲/疏水氨基酸组成都会影响肽的抗冻活性[27-28]。如表2所示,26条序列长度为7~14个氨基酸(amino acid,AA),这被认为是抗冻肽的合适长度[29]。肽段所带电荷取决于组成其AA侧链所带的电荷,如Asp、Glu、Lys、Arg、His,这些AA使得肽更亲水[30]。而含有更多极性的AA,如Ser、Thr、Cys、Tyr、Asn、Gln,肽具有更高的GRAVY值。此外,根据报道,不稳定系数(instability index)大于40的肽段通常拥有较低的体外稳定性[31],不能作为抗冻肽,因此排除在筛选范围外。
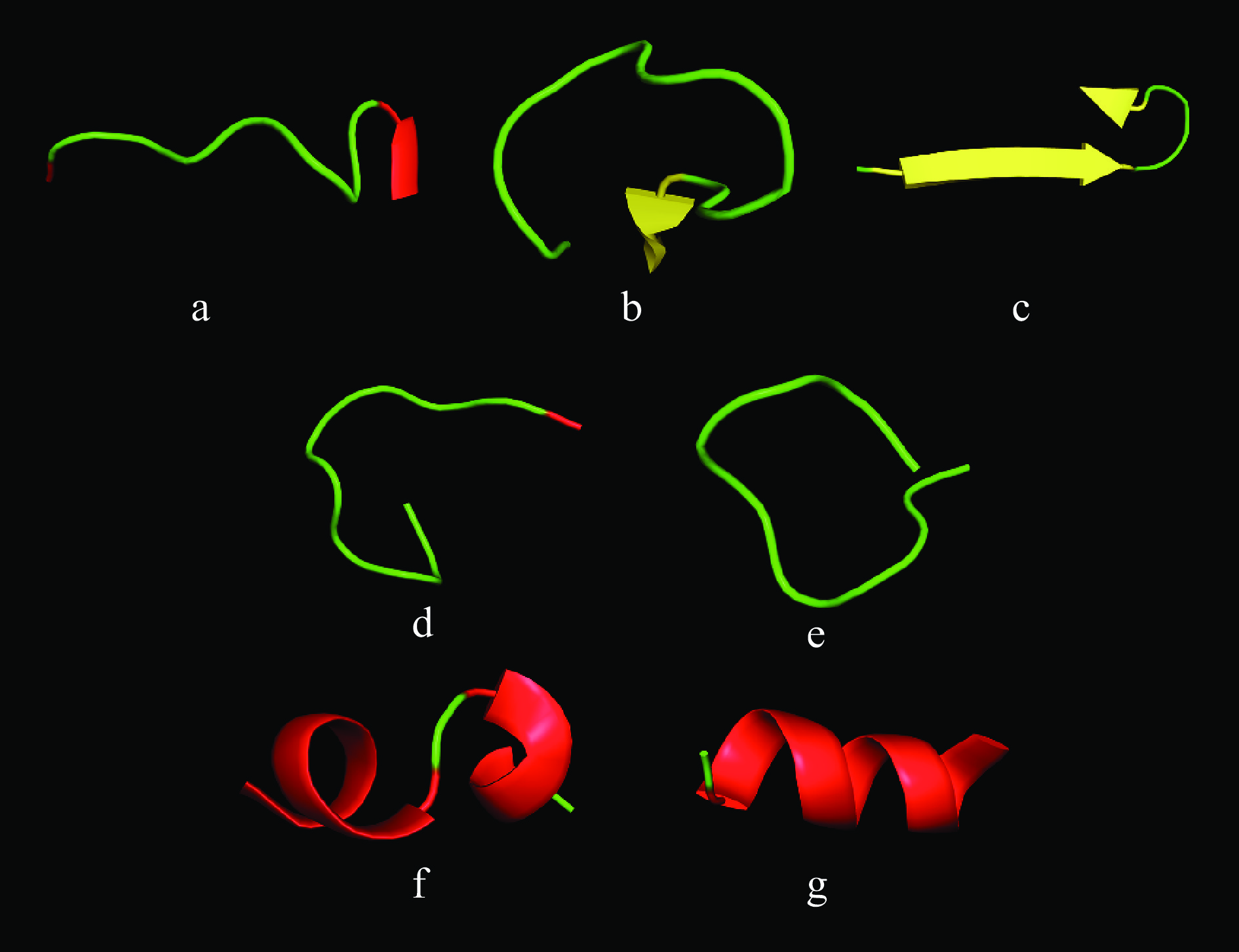
表 2 虾副产物肽段的理化性质检测Table 2. Physicochemical characterization detection of shrimp by-products peptides序号 序列 长度 理论等电点 分子量(Da) 电荷(负;正) 不稳定指数 亲水性 1 DEYEESGPGIVH 12 4.00 1331.36 4;0 31.95 −1.083 2 DIDNDGFLDK 10 3.77 1151.19 4;1 0.51 −1.070 3 DIDNNGFLDK 10 3.93 1150.21 3;1 −14.52 −1.070 4 GEDIGMNTH 9 4.35 937.03 2;0 12.48 −0.978 5 QVHPDTGISS 10 5.08 1040.10 1;0 −21.71 −0.580 6 GYGCARPNYPGV 12 8.20 1253.40 0;1 21.53 −0.542 7 DESGPGIVH 9 4.35 909.95 2;0 11.42 −0.522 8 ITIGNER 7 6.00 801.90 1;1 −3.56 −0.514 9 TTGEVCDSGDGVTH 14 4.02 1377.4 3;0 19.29 −0.493 10 SNHLDPVGEL 10 4.35 1080.16 2;0 12.33 −0.47 11 NHLDPVGEL 9 4.35 993.08 2;0 12.48 −0.433 12 EQICINFCNEK 11 4.53 1340.53 2;1 12.94 −0.418 13 ESGPGIVH 8 5.24 794.86 1;0 11.6 −0.150 14 EHVVLPPL 8 5.24 903.09 1;0 47.48(unstable) 0.762 15 EESGPGIVH 9 4.51 923.98 2;0 47.64(unstable) −0.522 16 DSFDPEADLS 10 3.37 1095.08 4;0 49.49(unstable) −0.880 17 DEEGCIPYEP 10 3.50 1151.21 4;0 50.44(unstable) −1.190 18 IEYRPDAGPEDTLV 14 3.92 1574.71 4;1 52.4(unstable) −0.700 19 YDLPPGVLEH 10 4.35 1139.21 2;0 63.66(unstable) −0.330 20 YGGQFPSRPDNV 12 5.84 1336.43 1;1 64.01(unstable) −1.175 21 GGQFPARPDN 10 5.84 1058.12 1;1 64.04(unstable) −1.440 22 YGGQFPSRPDN 11 5.84 1237.29 1;1 68.92(unstable) −1.664 23 GGQFPSRPDNVN 12 5.84 1287.35 1;1 71.08(unstable) −1.358 24 GGQFPSRPDNV 11 5.84 1173.25 1;1 76.64(unstable) −1.164 25 GGQFPSRPDN 10 5.84 1074.12 1;1 83.30(unstable) −1.700 26 SSESAVTVPDVPS 13 3.67 1274.35 2;0 85.79(unstable) 0.023 利用抗冻肽活性预测平台Cryoprotect对筛选所得序列进行活性预测,预测结果如表3所示。7个肽序列,QVHPDTGISS、GYGCARPNYPGV、TTGEVCDSGDGVTH、EQICINFCNEK、DEYEESGPGIVH、DIDNDGFLDK 和DIDNNGFLDK被认定为“AFP”。其二级结构如图5所示。基于活性预测结果的抗冻肽筛选简化了一般分离纯化和表征的程序,提高了效率。但以上7种肽抗冻活性仍需要进一步验证。
表 3 虾副产物抗冻活性预测结果Table 3. Prediction of antifreeze activity of shrimp by-products序号 序列 蛋白质鉴定 名称 位置 预测 1 GEDIGMNTH A0A059TGC6 Hemocyanin-like protein 516~524 Non-AFP 2 QVHPDTGISS A0A3R7PKU8 Histone H2B 262~271 AFP 3 GYGCARPNYPGV A0A6N0C4I7 Trypsin 239~250 AFP 4 DESGPGIVH A0A3R7SM13 Actin 1 137~150 Non-AFP 5 ITIGNER A0A3R7M3B1 Actin 2 604~610 Non-AFP 6 TTGEVCDSGDGVTH A0A3R7Q5M8 Actin 2 150~163 AFP 7 SNHLDPVGEL A9Q7C4 Hemocyanin-like protein 55~64 Non-AFP 8 NHLDPVGEL A9Q7C4 Hemocyanin-like protein 56~64 Non-AFP 9 EQICINFCNEK K4Q2S1 Myosin heavy chain type 2 475~485 AFP 10 ESGPGIVH A0A3R7M3B1 Actin 2 720~727 Non-AFP 11 DEYEESGPGIVH A0A3R7N633 Actin 1 361~372 AFP 12 DIDNDGFLDK P02635 Sarcoplasmic calcium-binding protein, beta chain 17~26 AFP 13 DIDNNGFLDK A0A3R7SPI4 Sarcoplasmic calcium-binding protein 3~12 AFP 2.4 酶切模拟
通过建立模型和酶切模拟可进一步了解7种肽所处源蛋白位置和酶解位点。如图6所示,7条序列分别来自于组蛋白H2B(A0A3R7PKU8)、胰蛋白酶(A0A6N0C4I7)、肌动蛋白2(A0A3R7Q5M8)、肌球蛋白重链type 2(K4Q2S1)、肌动蛋白1(A0A3R7N633)、肌浆钙结合蛋白β链(P02635)和肌浆钙结合蛋白(A0A3R7SPI4)水解所得。EQICINFCNEK位于K4Q2S1蛋白(SI为68.71%)的Glu,475–Lys,485,水解位点为:Phe,474–Glu,475(C端)(糜蛋白酶,80.8%)和Lys,485–Leu,486(C端)(胰蛋白酶,94.7%)。DEYEESGPGIVH的源蛋白A0A3R7N633拥有85.07%序列置信度(sequence identity),证明其具有良好的同源性。
选择虾副产物中主要的内源性蛋白酶、胰凝乳蛋白酶和胰蛋白酶作为水解酶,对7种肽的源蛋白进行酶切模拟,模拟的结果如表4所示。序列1:QVHPDTGISS是由A0A3R7PKU8在261,Lys的羧基端肽键被胰凝乳蛋白酶(100%概率)水解和272,Lys的氨基端肽键被胰凝乳蛋白酶(100%概率)水解所得;而序列3:TTGEVCDSGDGVTH的产生极有可能发生了二次酶解,即首次酶解产生了TTGEVCDSGDGVTHF,随后164,Phe的氨基端肽键被胰凝乳蛋白酶(72.6%概率)水解使得TTGEVCDSGDGVTH游离出来。同理可知其它序列的产生过程。
表 4 酶切模拟结果Table 4. Result of hydrolysis simulation序号 序列 蛋白质 酶切位点(酶:概率) 1 QVHPDTGISS A0A3R7PKU8 261(T:100%);272(T:100%) 2 GYGCARPNYPGV A0A6N0C4I7 238(C:87.1%);251(C:91.8%) 3 TTGEVCDSGDGVTH A0A3R7Q5M8 149(T:100%);163(C:6.3%);164(C:72.6%) 4 EQICINFCNEK K4Q2S1 474(C:80.8%);485(T:94.7%) 5 DEYEESGPGIVH A0A3R7N633 360(T:100%);372(C:34.3%);373(T:100%) 6 DIDNDGFLDK P02635 16(C:83.8%);26(T:63.1%) 7 DIDNNGFLDK A0A3R7SPI4 2(C:83.8%);12(T:63.1%、C:2.5%) 注:C:chymotrypsin,胰凝乳蛋白酶;T:trypsin,胰蛋白酶。 3. 结论
本文基于虾副产物的自溶反应研究了过程中酶解产物的多肽组成变化,并基于活性预测平台筛选得到7条抗冻肽序列。自溶处理的0~3 h,酶解物的DH和TCA可溶性肽含量急剧增加,分别达49.8%和26.1 mg/g,随后增长速度变缓。在A-0.5中检出1301条肽段,A-3中检出197条;A-0.5中大于1600 Da的肽含量达到69.5%,而A-3中肽以600~1600 Da为主。A-3中肽主要来源于血蓝蛋白、肌动蛋白、肌球蛋白重链、肌动蛋白轻链、胰蛋白酶、木糖异构酶、肌浆钙结合蛋白和其它8等类蛋白。基于活性预测平台Cryoprotect,筛选QVHPDTGISS、GYGCARPNYPGV、TTGEVCDSGDGVTH、EQICINFCNEK、DEYEESGPGIVH、DIDNDGFLDK 和DIDNNGFLDK7个序列为抗冻肽。通过酶切模拟可知7条序列分别来自于组蛋白H2B(A0A3R7PKU8)、胰蛋白酶(A0A6N0C4I7)、肌动蛋白2(A0A3R7Q5M8)、肌球蛋白重链type 2(K4Q2S1)、肌动蛋白1(A0A3R7N633)、肌浆钙结合蛋白β链(P02635)和肌浆钙结合蛋白(A0A3R7SPI4)的水解。抗冻肽对虾肉有显著抗冻效果,且抗冻活性与浓度正相关;两种抗冻肽拥有良好的冰亲和力,这有利于抗冻肽对冰晶起到吸附-抑制作用,这是一种高准确性的鉴定方法。但以此方法获取高纯度的活性肽需要消耗的成本较高,未来还需开发一种更简单、低经济的合成方法。
-
表 1 酶解产物肽的源蛋白
Table 1 Source protein of peptide obtained by enzymatic digestion
源蛋白类型 含量(%) 血蓝蛋白 39.61 肌动蛋白 36.56 肌球蛋白轻链 6.6 肌球蛋白重链 5.12 胰蛋白酶 4.57 肌浆蛋白 1.66 木糖异构酶 5.26 其它 0.62 表 2 虾副产物肽段的理化性质检测
Table 2 Physicochemical characterization detection of shrimp by-products peptides
序号 序列 长度 理论等电点 分子量(Da) 电荷(负;正) 不稳定指数 亲水性 1 DEYEESGPGIVH 12 4.00 1331.36 4;0 31.95 −1.083 2 DIDNDGFLDK 10 3.77 1151.19 4;1 0.51 −1.070 3 DIDNNGFLDK 10 3.93 1150.21 3;1 −14.52 −1.070 4 GEDIGMNTH 9 4.35 937.03 2;0 12.48 −0.978 5 QVHPDTGISS 10 5.08 1040.10 1;0 −21.71 −0.580 6 GYGCARPNYPGV 12 8.20 1253.40 0;1 21.53 −0.542 7 DESGPGIVH 9 4.35 909.95 2;0 11.42 −0.522 8 ITIGNER 7 6.00 801.90 1;1 −3.56 −0.514 9 TTGEVCDSGDGVTH 14 4.02 1377.4 3;0 19.29 −0.493 10 SNHLDPVGEL 10 4.35 1080.16 2;0 12.33 −0.47 11 NHLDPVGEL 9 4.35 993.08 2;0 12.48 −0.433 12 EQICINFCNEK 11 4.53 1340.53 2;1 12.94 −0.418 13 ESGPGIVH 8 5.24 794.86 1;0 11.6 −0.150 14 EHVVLPPL 8 5.24 903.09 1;0 47.48(unstable) 0.762 15 EESGPGIVH 9 4.51 923.98 2;0 47.64(unstable) −0.522 16 DSFDPEADLS 10 3.37 1095.08 4;0 49.49(unstable) −0.880 17 DEEGCIPYEP 10 3.50 1151.21 4;0 50.44(unstable) −1.190 18 IEYRPDAGPEDTLV 14 3.92 1574.71 4;1 52.4(unstable) −0.700 19 YDLPPGVLEH 10 4.35 1139.21 2;0 63.66(unstable) −0.330 20 YGGQFPSRPDNV 12 5.84 1336.43 1;1 64.01(unstable) −1.175 21 GGQFPARPDN 10 5.84 1058.12 1;1 64.04(unstable) −1.440 22 YGGQFPSRPDN 11 5.84 1237.29 1;1 68.92(unstable) −1.664 23 GGQFPSRPDNVN 12 5.84 1287.35 1;1 71.08(unstable) −1.358 24 GGQFPSRPDNV 11 5.84 1173.25 1;1 76.64(unstable) −1.164 25 GGQFPSRPDN 10 5.84 1074.12 1;1 83.30(unstable) −1.700 26 SSESAVTVPDVPS 13 3.67 1274.35 2;0 85.79(unstable) 0.023 表 3 虾副产物抗冻活性预测结果
Table 3 Prediction of antifreeze activity of shrimp by-products
序号 序列 蛋白质鉴定 名称 位置 预测 1 GEDIGMNTH A0A059TGC6 Hemocyanin-like protein 516~524 Non-AFP 2 QVHPDTGISS A0A3R7PKU8 Histone H2B 262~271 AFP 3 GYGCARPNYPGV A0A6N0C4I7 Trypsin 239~250 AFP 4 DESGPGIVH A0A3R7SM13 Actin 1 137~150 Non-AFP 5 ITIGNER A0A3R7M3B1 Actin 2 604~610 Non-AFP 6 TTGEVCDSGDGVTH A0A3R7Q5M8 Actin 2 150~163 AFP 7 SNHLDPVGEL A9Q7C4 Hemocyanin-like protein 55~64 Non-AFP 8 NHLDPVGEL A9Q7C4 Hemocyanin-like protein 56~64 Non-AFP 9 EQICINFCNEK K4Q2S1 Myosin heavy chain type 2 475~485 AFP 10 ESGPGIVH A0A3R7M3B1 Actin 2 720~727 Non-AFP 11 DEYEESGPGIVH A0A3R7N633 Actin 1 361~372 AFP 12 DIDNDGFLDK P02635 Sarcoplasmic calcium-binding protein, beta chain 17~26 AFP 13 DIDNNGFLDK A0A3R7SPI4 Sarcoplasmic calcium-binding protein 3~12 AFP 表 4 酶切模拟结果
Table 4 Result of hydrolysis simulation
序号 序列 蛋白质 酶切位点(酶:概率) 1 QVHPDTGISS A0A3R7PKU8 261(T:100%);272(T:100%) 2 GYGCARPNYPGV A0A6N0C4I7 238(C:87.1%);251(C:91.8%) 3 TTGEVCDSGDGVTH A0A3R7Q5M8 149(T:100%);163(C:6.3%);164(C:72.6%) 4 EQICINFCNEK K4Q2S1 474(C:80.8%);485(T:94.7%) 5 DEYEESGPGIVH A0A3R7N633 360(T:100%);372(C:34.3%);373(T:100%) 6 DIDNDGFLDK P02635 16(C:83.8%);26(T:63.1%) 7 DIDNNGFLDK A0A3R7SPI4 2(C:83.8%);12(T:63.1%、C:2.5%) 注:C:chymotrypsin,胰凝乳蛋白酶;T:trypsin,胰蛋白酶。 -
[1] 农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2021渔业统计年鉴[M]. 北京: 中国农业出版社, 2021 Fisheries and Fisheries Administration, Ministry of Agriculture and Rural Affairs, National Aquatic Technology Promotion Station, China Fisheries Society. 2021 Fisheries statistical yearbook[M]. Beijing: China Agricultural Press, 2021.
[2] ABDOLLAHI M, OLOFSSON E, ZHANG J N, et al. Minimizing lipid oxidation during pH-shift processing of fish by-products by cross-processing with lingonberry press cake, shrimp shells or brown seaweed[J]. Food Chemistry,2020,10:127078.
[3] 徐文思, 杨祺福, 张梦媛, 等. 两步酶解法制备小龙虾副产物多肽及其抗氧化性研究[J]. 食品研究与开发,2021,42(24):147−154. [XU W S, YANG Q F, ZHANG M Y, et al. Preparation of by-product polypeptides of crayfish by two-step enzymatic hydrolysis and their antioxidant activity[J]. Food Research and Development,2021,42(24):147−154. [4] DOAN C T, TRAN T N, WEN I H, et al. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by Paenibacillus sp[J]. Catalysts,2019,9(10):14.
[5] 唐志红, 余良, 贺晓丽, 等. 酶解小龙虾副产物蛋白制备α-葡萄糖苷酶抑制肽的研究[J]. 食品科技,2021,46(11):23−27. [TANG Z H, YU L, HE X L, et al. Study on preparation of α-glucosidase inhibitory peptide by enzymatic hydrolysis of crayfish by-product protein[J]. Food Technology,2021,46(11):23−27. doi: 10.3969/j.issn.1005-9989.2021.11.spkj202111004 [6] VICENTE F A, VENTURA S P M, PASSOS H, et al. Crustacean waste biorefinery as a sustainable cost-effective business model[J]. Chemical Engineering Journal,2022,442:13.
[7] NIKOO M, XU X, REGENSTEIN J M, et al. Autolysis of Pacific white shrimp (Litopenaeus vannamei) processing by-products: Enzymatic activities, lipid and protein oxidation, and antioxidant activity of hydrolysates[J]. Food Bioscience,2021:39.
[8] PEREIRA N D A, FANGIO M F, RODRIGUEZ Y E, et al. Characterization of liquid protein hydrolysates shrimp industry waste: Analysis of antioxidant and microbiological activity, and shelf life of final product[J]. J Food Process Pres,2021:e15526.
[9] 陈旭, 蔡茜茜, 汪少芸, 等. 抗冻肽的研究进展及其在食品工业的应用前景[J]. 食品科学,2019,40(17):331−337. [CHEN X, CAI Q Q, WANG S Y, et al. Research progress of antifreeze peptide and its application prospect in food industry[J]. Food Science,2019,40(17):331−337. [10] CHEN X, WU J H, LI L, et al. Cryoprotective activity and action mechanism of antifreeze peptides obtained from tilapia scales on Streptococcus thermophilus during cold stress[J]. J Agr Food Chem,2019,67(7):1918−1926. doi: 10.1021/acs.jafc.8b06514
[11] DU L H, BETTI M. Identification and evaluation of cryoprotective peptides from chicken collagen: Ice-growth inhibition activity compared to that of type I antifreeze proteins in sucrose model systems[J]. J Agr Food Chem,2016,64(25):5232−5240. doi: 10.1021/acs.jafc.6b01911
[12] CAO H, ZHENG X Z, LIU H, et al. Cryo-protective effect of ice-binding peptides derived from collagen hydrolysates on the frozen dough and its ice-binding mechanisms[J]. LWT-Food Science and Technology,2020,131:109678. doi: 10.1016/j.lwt.2020.109678
[13] DE CICCO M, MAMONE G, DI STASIO L, et al. Hidden “Di-gestome”: Current analytical approaches provide incomplete peptide inventories of food digests[J]. J Agr Food Chem,2019,67(27):7775−7782. doi: 10.1021/acs.jafc.9b02342
[14] MARTINI S, SOLIERI L, TAGLIAZUCCHI D. Peptidomics: New trends in food science[J]. Curr Opin Food Sci,2021,39:51−59. doi: 10.1016/j.cofs.2020.12.016
[15] MONARI S, FERRI M, RUSSO C, et al. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing[J]. PloS One,2019,14(12):e0226834. doi: 10.1371/journal.pone.0226834
[16] HOU X Y, LI S S, LUO Q Y, et al. Discovery and identification of antimicrobial peptides in Sichuan pepper (Zanthoxylum bungeanum Maxim) seeds by peptidomics and bioinformatics[J]. Appl Microbiol Biot,2019,103(5):2217−2228. doi: 10.1007/s00253-018-09593-y
[17] PESCUMA M, HÉBERT E M, HAERTLÉ T, et al. Lactobacillus delbrueckii subsp. bulgaricus CRL 454 cleaves allergenic peptides of β-lactoglobulin[J]. Food Chemistry,2015,170:407−414. doi: 10.1016/j.foodchem.2014.08.086
[18] CAO W H, TIAN S X, WANG H, et al. Release principle of peptides and amino acids during the autolysis of shrimp head from Litopenaeus vannamei after UV-C irradiation stress[J]. Food Science & Nutrition,2020,8(1):170−178.
[19] CAO W, TAN C, ZHAN X, et al. Ultraviolet irradiation and gradient temperature assisted autolysis for protein recovery from shrimp head waste[J]. Food Chemistry,2014,164:136−141. doi: 10.1016/j.foodchem.2014.05.042
[20] MOGHADAM M, SALAMI M, MOHAMMADIAN M, et al. Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis[J]. Food Bioscience,2020,36:7.
[21] 张建萍, 陈振家, 闫舟, 等. 不同蛋白酶水解小米分离蛋白工艺[J]. 食品工业,2019,40(12):123−127. [ZHANG J P, CHEN Z J, YAN Z, et al. Different protease hydrolysis process of millet protein isolate[J]. Food Industry,2019,40(12):123−127. [22] ROBERT M, ZATYLNY-GAUDIN C, FOURNIER V, et al. Transcriptomic and peptidomic analysis of protein hydrolysates from the white shrimp (L. vannamei)[J]. Journal of Biotechnology,2014,186:30−37. doi: 10.1016/j.jbiotec.2014.06.020
[23] 郑杰, 宋志远, 于笛, 等. 海参体壁自溶的响应面优化及其体外抗氧化活性研究[J]. 中国食品添加剂,2018(12):90−97. [ZHENG J, SONG Z Y, YU D, et al. Response surface optimization of sea cucumber body wall autolysis and its antioxidant activity in vitro[J]. Chinese Food Additives,2018(12):90−97. [24] 刘芳芳, 林婉玲, 李来好, 等. 海鲈鱼糜加工及凝胶形成过程中蛋白质的变化机理[J]. 食品科学,2020,41(14):15−22. [LIU F F, LIN W L, LI L H, et al. The mechanism of protein change during seabass surimi processing and gel formation[J]. Food Science,2020,41(14):15−22. [25] CAO W, ZHANG C, HONG P, et al. Response surface methodology for autolysis para meters optimization of shrimp head and amino acids released during autolysis[J]. Food Chemistry,2008,109(1):176−183. doi: 10.1016/j.foodchem.2007.11.080
[26] ZHANG M M, XIN X, WU H, et al. Debittering effect of partially purified proteases from soybean seedlings on soybean protein isolate hydrolysate produced by alcalase[J]. Food Chemistry,2021,362:130190. doi: 10.1016/j.foodchem.2021.130190
[27] 黄沐晨, 杨傅佳, 陈旭, 等. 海洋源生物活性肽的构效关系与作用机理研究进展[J]. 食品科学,2021,42(19):271−280. [HUANG M C, YANG B J, CHEN X, et al. Research progress on structure-activity relationship and mechanism of action of marine bioactive peptides[J]. Food Science,2021,42(19):271−280. [28] 刘含, 曹慧, 徐斐, 等. 不同分子质量胶原抗冻肽的抗冻性能及机理研究[J]. 食品与发酵工业,2021,47(11):104−110. [LIU H, CAO H, XU F, et al. Antifreeze properties and mechanism of collagen antifreeze peptides with different molecular weights[J]. Food and Fermentation Industry,2021,47(11):104−110. doi: 10.13995/j.cnki.11-1802/ts.025969 [29] PRATIWI R, MALIK A A, SCHADUANGRAT N, et al. CryoProtect: A web server for classifying antifreeze proteins from nonantifreeze proteins[J]. J Chem,2017(8):1−15.
[30] CHEN X, WU J H, CAI X X, et al. Production, structure-function relationships, mechanisms, and applications of antifreeze peptides[J]. Compr Rev Food Sci F,2021,20(1):542−562. doi: 10.1111/1541-4337.12655
[31] 齐诗哲. 乳源抗菌肽的制备及蛋白质组学分析[D]. 大连: 大连海洋大学, 2022 QI S Z. Preparation and proteomic analysis of milk derived antimicrobial peptides[D]. Dalian: Dalian Ocean University, 2022.
-
期刊类型引用(3)
1. 杨春晖,王文平,续丹丹,崔宇倩,鞠岩,许春艳,吕小婷. 不同原料酿造酱油功能成分及抗氧化活性比较. 食品工业科技. 2023(14): 318-325 .  本站查看
本站查看
2. 张荣,古丽吉合热·阿布拉. 新疆黑枸杞原花青素的提取及抗氧化活性研究. 食品工业. 2023(12): 27-31 .  百度学术
百度学术
3. 只德贤,张妮,李建颖. 微波超声协同提取白刺果原花青素工艺及抗氧化性研究. 食品工业科技. 2022(13): 171-179 .  本站查看
本站查看
其他类型引用(2)







 下载:
下载:

 下载:
下载:



