Research Progress on Regulation of Antioxidant Transcription in Reactive Oxygen Species Metabolism of Postharvest Fruits and Vegetables during Low Temperature Storage
-
摘要: 果蔬采后活性氧(reactive oxygen species,ROS)代谢失衡造成氧化胁迫,加速品质劣变,破坏其商业价值。文章以ROS代谢与果蔬贮藏品质的关系为切入点,探讨低温诱导抗氧化酶活性延缓果蔬采后品质劣变的作用;同时,从转录调控角度综述bHLH、WRKY及NAC等低温响应转录因子参与果蔬采后抗氧化作用,进而揭示低温延缓果蔬品质劣变的抗氧化转录调控作用的可能机制,旨在为开展果蔬采后品质生理生化及分子生物学等基础研究及进一步阐明果蔬采后成熟衰老和品质劣变机制提供新的理论依据。Abstract: Imbalance of active oxygen metabolism can lead to oxidative stress in postharvest fruits and vegetables, which could accelerate their quality deterioration, and ruin their commercial value. In this review, the relationship between the antioxidant effect and storage quality of fruits and vegetables has been taken as the breakthrough point, and the effect of antioxidant enzyme activities on delaying postharvest quality deterioration of fruits and vegetables induced by low temperature has been discussed. Moreover, antioxidant effects in postharvest fruits and vegetables have been further analyzed from the perspective of transcriptional regulation, to explore the role of low temperature responsive transcription factors like bHLH, WRKY and NAC in the regulation of antioxidant enzyme activities during low temperature storage, and finally to reveal the possible mechanism of antioxidant transcription regulation that low temperature could delay the quality deterioration of fruits and vegetables. Therefore, this article was suggested to provide a new theoretical basis for carrying out basic research on postharvest quality of fruits and vegetables including physiology, biochemistry and molecular biology, and further elucidating the mechanism of ripening, senescence and quality deterioration in postharvest fruit and vegetables.
-
据统计,近年来我国年产超过2.8亿吨水果和7.97亿吨蔬菜[1],然而因采后处理不当,每年约有25%~40%的果蔬在采收及贮运过程中被浪费,部分发展中国家的果蔬采后损失率甚至高达75%[2]。研究发现,果蔬采后损耗与ROS代谢失衡密切相关:在贮藏过程中,由于受到体内酶活、微生物侵染及环境温湿度波动等因素的影响,果蔬呼吸代谢等发生急剧变化,产生过量ROS[3],如1O2(单线态氧)、·OH(羟基自由基)、O2−·(超氧阴离子自由基)、ROO·(脂类过氧化物)及H2O2等。过量的ROS将毒害细胞膜系统,引发果蔬细胞内脂质、蛋白质及DNA等各种生物大分子的氧化损伤,促进果蔬组织的衰老,直接影响其采后贮藏寿命[4-5]。低温贮藏是目前果蔬采后最常用的物理保鲜技术[3, 6],有助于改善果蔬采后品质,增强体内抗氧化能力。其中,果蔬体内抗氧化水平与耐贮性之间存在正相关[7-9]。通过进一步的分子机制研究,证实果蔬采后贮藏品质及抗氧化作用受转录因子调控[10-12]。部分转录因子可识别并作用于抗氧化相关靶基因的启动子序列,如玉米ZmNAC84转录因子可直接结合体内SOD2(superoxide dismutase, 超氧化物歧化酶)的CACGTG基序,上调SOD2的表达,从而发挥抗氧化功能,提高植株抗旱性[13];葡萄VvMYB5b可激活参与类黄酮合成途径中关键酶基因ANS(anthocyanidin synthase,花青素合酶)、CHI(chalcone isomerase,查尔酮异构酶)、LAR1(leucoanthocyantin reducase,无色花青素还原酶)的启动子顺式作用元件[14],诱导其表达,促进花青素的合成,保护葡萄细胞膜上的UFA(unsaturated fatty acid,不饱和脂肪酸)免于氧化。
然而,目前低温下果蔬采后生理变化及抗氧化转录调控机理的研究仍缺乏深层次的认识。因此,揭示低温贮藏过程中果蔬体内转录因子介导的抗氧化转录调控机制,对延缓果蔬采后成熟衰老进程,改善果蔬采后贮藏品质具有重要指导意义。本文综述了果蔬采后品质劣变与ROS代谢及相关转录因子介导的抗氧化转录调控机制的研究进展,并对今后研究做出进一步的展望,以期为研究低温延长果蔬贮藏期的分子机制提供理论指导。
1. ROS代谢与果蔬采后贮藏品质
在自身代谢及外界胁迫(如干旱、高温及机械损伤等)的双重作用下,采后果蔬体内ROS代谢失衡,造成萎蔫、褐变及软化等现象,对外观品质带来负面影响,直接影响消费者的接受度,造成新鲜农产品的经济损失[15-16]。研究表明,皇冠梨在贮藏期间易发生果皮褐变,不利于其市场营销[17];而褐变也是缩短双孢蘑菇货架期的主要因素。双孢蘑菇采后细胞内ROS过度积累,造成蛋白质和脂质氧化损伤,加速菇帽褐变和组织腐烂[18]。其中,蛋白质羰基化用于衡量蛋白质氧化损伤程度。Minas等[19]研究发现,‘Hayward’ 猕猴桃在成熟过程中伴随体内蛋白质羰基化含量的上升,同时组织硬度下降,发生软化,导致商品价值降低;在番茄和苹果果实衰老过程中,均发生蛋白质羰基化[20-21]。其次,脂质氧化也是破坏细胞膜结构完整性的主要原因。在常温贮藏过程中,荔枝细胞质的氧化酶以质体膜结构为媒介与液泡中的酚类底物发生反应,导致果皮色泽变暗,同时伴随持续失水现象,造成荔枝膜完整性降低、体内花青素降解,最终促进果皮褐变进程,果实失去商品价值[22-23]。可见,ROS的过度积累是果蔬褐变的诱因。此外,ROS含量还与果蔬组织木质化密切相关。研究发现,杏鲍菇木质化影响其贮藏寿命;杏鲍菇采后会发生两次木质化,第一次木质化期间由于机械损伤等原因产生大量O2−·及H2O2,进而再由ROS介导二次木质化,破坏细胞膜完整性,并造成杏鲍菇出现黄化、腐烂现象[24]。其中,黄化也是菜薹出现衰老的主要症状之一。研究表明,在菜薹采后衰老过程中,体内ROS不断蓄积,氧化还原态失衡,对脂质膜结构造成氧化损伤,加速叶片黄化[25]。因此,ROS代谢水平影响果蔬外观质量。
果蔬富含多酚、维生素和有机酸等营养物质,是人体健康所需基本元素和矿物质的主要来源[26]。然而,果蔬采后成熟衰老过程中,光合作用几乎停滞,呼吸作用、乙烯信号转导等成为主要的生理代谢活动[27]。ROS代谢失衡不仅破坏果蔬外观品质,还会增强果蔬的呼吸速率,损耗维生素C、可溶性糖等营养物质。研究表明,甜玉米在常温环境中呼吸速率可达405 mg CO2 kg−1·h−1[28],最多只能存放2 d。其中,可溶性固形物是甜玉米的重要品质指标,主要成分为可溶性糖。由于采后甜玉米的蔗糖供给链被割断,可溶性糖含量不再继续增加,在30 ℃的条件下,初始糖分损失可达60%[29]。芒果是一种典型的呼吸跃变型水果,在常温贮藏过程中,表现出较高的呼吸速率及代谢活性,体内O2−·及H2O2过量生成,ROS代谢出现紊乱,多糖类物质、有机酸及黄酮类化合物被不断消耗,促进细胞壁和淀粉的降解,细胞膜完整性降低,导致果实衰老进程加速[30]。甜樱桃果实采后也由于高蒸腾速率和呼吸速率,体内O2−·及H2O2大量富集,无法对ROS进行清除平衡,导致可溶性糖、可滴定酸、酚类物质及类胡萝卜素等营养成分损耗严重,贮藏品质下降[31]。‘Newhall’ 脐橙同样富含各类维生素及酚类化合物,然而在采后自身呼吸代谢的作用下,ROS随着贮藏时间的延长不断积累,脐橙体内糖类物质及有机酸等营养物质发生降解,并伴随着氧化造成脐橙风味恶化[32]。可见,避免果蔬采后营养品质劣变,需维持正常的体内ROS代谢水平。
因此,果蔬采后贮藏过程由于ROS代谢失衡,不仅容易造成果蔬组织发生褐变、木质化、黄化等外观品质劣变,而且能诱发果蔬采后呼吸速率上升,加速可溶性糖、维生素、有机酸等营养品质劣变,最终降低果蔬的商业价值,严重制约果蔬市场推广。
2. 低温诱导果蔬采后抗氧化酶活性
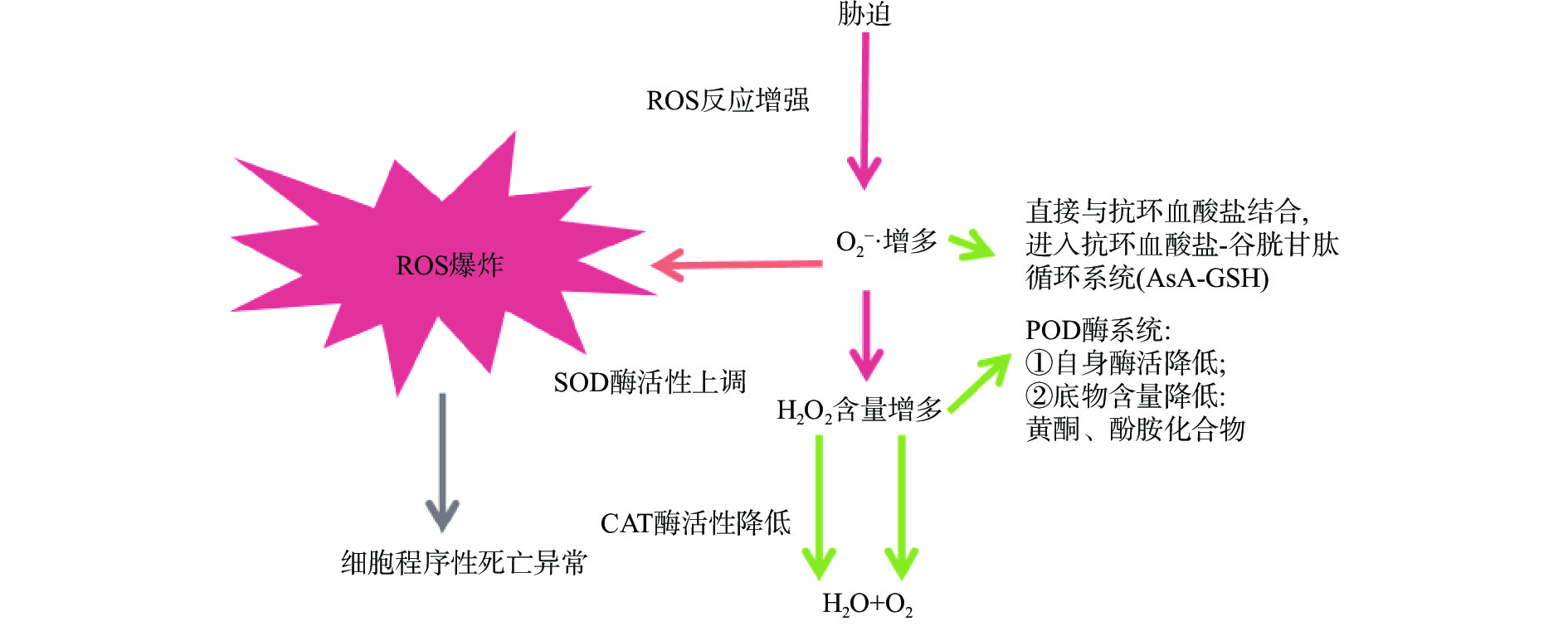
在低温环境下,果蔬可诱导自身应激系统进行适应性的调节反应,一方面通过降低自身呼吸速率,抑制细胞膜质过氧化,延缓营养成分消耗,另一方面则激活清除ROS的保护机制,减少膜脂过氧化,保护果蔬细胞免受低温带来的氧化应激[33-35],减缓果蔬的腐烂速率,从而维持采后贮藏品质[36]。植物体内有效清除ROS的保护机制分为非酶保护和酶保护两类系统(图1)[37],在延缓果蔬采后品质劣变中发挥积极作用:非酶保护主要通过抗坏血酸-谷胱甘肽(AsA-GSH)系统平衡ROS;酶保护系统包括SOD、CAT(catalase,过氧化氢酶)、APX(aseorbate peroxidase,抗坏血酸过氧化物酶)、POD(peroxisome,过氧化物酶)、GR(glutathione reductase,谷胱甘肽还原酶)等,是果蔬细胞中清除ROS的主要酶类,发挥抗氧化作用。其中,SOD是植物抗氧化的第一道防线,能清除细胞中多余的O2−·,将其歧化为H2O2和O2;CAT能降低细胞应激、自由基的积累和凋亡率,与POD协作清除过多的H2O2;APX与GR构成果蔬体内AsA-GSH循环中的关键酶,也可清除H2O2[38-39]。
目前,抗氧化作用是果蔬采后贮藏保鲜研究的热点问题之一[40]。研究表明,马铃薯于低温贮藏环境中,4 ℃较12 ℃的贮藏效果更显著,其体内可维持更高的还原糖含量及抗氧化活性,并且APX与CAT抗氧化酶活性的响应更加强烈[41],而APX、CAT活性的增强能防止马铃薯在低温贮藏期间产生H2O2。可见,适宜的低温处理在诱导马铃薯贮藏过程中的抗氧化活性发挥正向调控作用。Sang等[42]研究发现,冬枣果品质量与抗氧化酶之间存在显著正相关性。与常温(25 ℃)对照相比,低温(0 ℃)贮藏可有效抑制冬枣采后呼吸速率、失重率及腐烂率等的上升,显著延缓可滴定酸和维生素C含量的下降,并增强抗氧化酶活性,包括SOD、APX及GR活性均受到上调。其中,SOD能将有害的O2−·转化为H2O2,而APX和GR构成的AsA-GSH循环对H2O2进行清除,由此推测,低温下这三者构成了一个完整的抗氧化防御体系,协同平衡ROS水平,维持冬枣采后品质。桃果实采后迅速成熟衰老,出现脱水、干瘪、软化、风味丧失、可溶性固形物和抗坏血酸含量下降及腐烂等现象[43]。An等[36]研究表明,“霞晖8号”桃果实的常温(25 ℃)对照组及低温(4 °C)处理组的贮藏期分别为8和20 d;流式细胞检测、转录组和蛋白组数据分析表明,低温能抑制SP(serine protease,丝氨酸酪蛋白酶)及LOX(lipoxygenase,脂氧合酶)等细胞凋亡相关酶类的活性,而增强SOD、POD和CAT等抗氧化酶活性。研究显示,ROS及其衍生物会诱发细胞凋亡[44];低温可能通过促进 ROS 清除酶 SOD、POD 和 CAT的活性进而抑制细胞凋亡相关酶类的生成,最终延长桃果实采后寿命。因此,低温贮藏能增强果蔬抗氧化能力,降低自由基含量,抑制细胞凋亡,从而延缓果蔬的成熟衰老;低温贮藏下抗氧化酶活性的提高有助于维持果蔬的贮藏品质。
此外,低温结合其他保鲜技术,能进一步改善果蔬的贮藏品质,提高贮藏性。Ge等[45]研究表明,经ASM(苯甲酸-S-甲基)结合4 ℃低温冷藏条件处理蓝莓后,能有效维持蓝莓中SOD、APX、CAT及GR抗氧化酶的活性,抑制H2O2的生成,提高总酚、黄酮和花青素的含量。可见,ASM结合低温处理可维持蓝莓果实中ROS平衡及抗氧化能力,延缓衰老。低温结合涂膜处理也能提高抗氧化酶活性,增强自由基清除能力,并抑制PPO(polyphenol oxidase, 多酚氧化酶)的表达,有效抑制骏枣腐烂;在贮藏后期,骏枣仍能保持其高浓度的总酚、类黄酮及抗环血酸[46]。由此推测,低温结合涂膜处理可能是通过诱导抗氧化酶活性及抗氧化物质水平的增强,作用于ROS,从而抑制骏枣褐变,维持外观品质,延长贮藏期。
但是,不适宜的低温可能导致果蔬发生冷害,影响其贮藏品质。研究表明,低温结合茉莉酸甲酯处理,可促进甜椒POD、CAT、APX抗氧化酶的表达,有效维持甜椒品质,减轻冷害[47],说明茉莉酸甲酯联合低温处理在减少甜椒发生冷害过程中,抗氧化酶活性与甜椒商品价值呈正相关。在黄瓜低温(7~10 ℃)贮藏前进行PsCA(冷驯化),可减缓黄瓜冷害并保持采后品质[48];与对照相比,PsCA处理能够通过降低ROS累积(O2−·及H2O2)和维持细胞膜完整性来改善黄瓜采后质量;而且PsCA处理组中SOD、POD、APX及CAT的基因表达量均显著上调,并且增强AsA和GSH含量。可见,PsCA处理能够作为黄瓜采后保鲜的有效方法,是通过诱导抗氧化酶的活性,从而避免低温氧化胁迫。此外,甘薯 4 ℃ 下贮藏 28 d后转入 10 ℃,能够有效增强体内 SOD、CAT 及 APX酶活性,抑制ROS含量累积,采后品质得到改善,进而延长其贮藏期[49]。因此,冷锻炼可通过增强果蔬体内抗氧化能力,提高果蔬低温耐受性。
由此可见,低温能诱导果蔬抗氧化酶活性的增强,减缓或避免其在贮藏过程中遭受氧化胁迫;抗氧化作用有助于延缓果蔬采后成熟衰老进程,从而维持贮藏品质,延长贮藏期。
3. 转录因子调控果蔬体内抗氧化水平
转录因子是一类响应特定条件(低温、激素、干旱及盐胁迫等因素)而差异表达的蛋白质,如bHLH、WRKY及NAC等,参与植物生长发育、防御反应等过程[50-52],在果蔬抗氧化相关酶基因表达的调控过程中发挥着重要的调节作用。它们与靶基因启动子的特定DNA元件结合,激活或抑制靶基因的特异性转录表达[53]。随着模式植物(如番茄、柑橘及拟南芥等)转录因子功能研究的深入开展,果蔬成熟衰老过程中的转录调控机制已成为当前采后生物学研究的重点工作之一[54]。
bHLH转录因子是植物转录因子中最大的家族之一,而低温敏感性是制约甜橙产业可持续发展的主要因素。Geng等[55]实验表明,在甜橙冷应激反应中,大量CsbHLHs转录因子被诱导;其中,CsbHLH18的表达量最高,与三叶橙bHLH基因的核苷酸序列同源性>98%。利用三叶橙进行RNAi沉默CsbHLH18后,三叶橙对低温的耐受性降低,体内清除ROS的抗氧化酶SOD、POD及CAT活性也受到抑制,造成ROS含量的累积;然而,CsbHLH18的过表达则增强了转基因烟草的耐寒性,且体内抗氧化酶活性及转录水平均显著上升,抑制ROS的产生。可见,CsbHLH18在抗寒中发挥积极作用,CsbHLH18介导的冷耐受性可能是由于通过直接结合甜橙体内抗氧化酶基因启动子序列或间接调控其它可直接结合抗氧化酶基因启动子的转录因子的表达水平进而抑制甜橙ROS的累积;其中,由SOD将细胞中多余的O2−·歧化为H2O2,CAT再和POD共同作用于H2O2,从而维持采后甜橙的商品品质。ICE1也属于bHLH转录因子家族成员之一。据报道,番茄转录因子SlICE1的表达量与番茄果实的采后品质呈正相关,可提高其耐寒性[56]。研究进一步表明,过表达SlICE1能够促进番茄果实体内β-胡萝卜素、番茄红素、ASA及GSH的含量积累,番茄果实抗氧化活性得到增强,有效抑制ROS累积;而且转基因SlICE1番茄果实内糖分含量高于野生型番茄果实[57]。其中,β-胡萝卜素及番茄红素能够通过能量传递机制淬灭1O2,抑制自由基引发的脂质过氧化,清除巨噬细胞呼吸所爆发的ROS自由基,保持细胞正常代谢[58];ASA-GSH循环系统可有效清除自由基,参与体内多种氧化还原反应。由此推测,低温作为促进番茄果实SlICE1表达的诱因,SlICE1再通过直接或间接结合抗氧化剂β-胡萝卜素、番茄红素及AsA-GSH循环中相关酶类的启动子基序,激活其表达,最终诱导番茄果实抗氧化能力,提高番茄果实的采后品质。
此外,在低温胁迫过程中,橄榄果实中CaWRKY21的表达水平相比对照得到明显上升,且果实品质未发生明显改变,果实T-AOC(total antioxidant capacity,总抗氧化能力)也得到显著提升[59]。可见,低温可能通过上调CaWRKY21的转录水平,激活橄榄体内抗氧化相关酶的表达,进而增强果实T-AOC,延缓橄榄采后的品质劣变。
低温、高温、高盐、干旱等环境条件是影响苹果的地理分布和产量的主要限制因素。研究发现,苹果MbNAC29的表达可受到非生物胁迫诱导,而且拟南芥MbNAC29转基因植株在低温胁迫(−4 ℃)和盐胁迫(200 mmol/L NaCl)下表现出更高的抗性,存活率亦高于野生型,其体内脯氨酸的含量以及ROS清除抗氧化酶SOD、POD和CAT活性均提高,且MDA(malonaldehyde,丙二醛)含量则得到有效控制[60]。其中,脯氨酸能够清除高浓度的活性氧,保护膜和蛋白质免受无机离子和极端温度的不利影响[61];而MDA含量则代表膜脂过氧化产物对细胞膜造成的氧化损伤程度。可见,苹果转录因子MbNAC29可能通过作用于ROS清除抗氧化酶及脯氨酸相关合成酶启动子的顺式作用元件,上调其表达,维持细胞膜通透性,并在应对冷、盐胁迫中发挥重要作用,最终提高其抗性。
研究表明,GRAS亚家族DELLA蛋白可增加ROS清除酶基因的表达水平提高果蔬非生物胁迫的抗性,从而降低ROS水平,延缓细胞死亡并促进其耐受性[62]。其中,番茄转录因子SlGRAS4受低温诱导,且过表达SlGRAS4能够促进番茄叶片及果实的耐冷性,而RNAi沉默SlGRAS4番茄植株体内的MDA含量却不断累积;DLR(dual-luciferase reporter,双荧光素酶报告实验)进一步证实番茄SlGRAS4可直接结合番茄体内GST(Solyc02g094180)、POD(Solyc07g056480)和APX(Solyc02g083620)等抗氧化酶的启动子序列[63]。由此推测,番茄冷响应SIGRAS4转录因子通过直接结合GST、POD及APX抗氧化酶的顺式作用元件,上调番茄体内抗氧化能力,发挥ROS清除功能,降低过氧化物的水平,赋予番茄果实耐寒性。因此,bHLH、WRKY、NAC及GRAS等转录因子在果蔬采后抗氧化作用中发挥积极的调控作用;低温能通过调控抗氧化基因的表达来提高果蔬耐冷性。
但是,不同转录因子家族成员在果蔬采后贮藏过程中的调控作用存在差异性。在菜心采后衰老期间,转录因子BrNAC055与ROS代谢途径中呼吸爆发氧化酶BrRBOB、BrRbohC-like及叶绿素降解代谢基因BrNYC1、BrNYE1具有相似的表达模式;通过EMSA(electrophoretic mobility shift assay,电泳迁移率转移分析)和DLR实验进一步表明,BrNAC055可直接结合BrRBOB、BrRbohC-like、BrNYC1及BrNYE1的启动子序列,进而诱导叶绿素降解代谢基因BrNYC1、BrNYE1表达的上调,加速菜心衰老[64]。Yan等[65]通过qRT-PCR(实时荧光定量PCR)发现油菜BnaNAC87在衰老叶片中高度表达;组织学染色实验发现,BnaNAC87的表达显著诱导了ROS生成(RBOB)、细胞死亡(VPE1a、ZEN1)、叶片衰老(WRKY6、ZAT12)等相关基因;EMSA分析进一步确认BnaNAC87直接与ZEN1、ZAT12、HIN1和PR5基因启动子中含NACR的片段进行结合。因此,油菜NAC87是作为ROS代谢和细胞死亡的正调控因子。此外,柑橘同源结构域亮氨酸拉链I(HZ-ZIP I)的转录因子CsHB5与果实衰老密切相关[38]。CsHB5在柑橘愈伤组织中的过表达显著了增加ABA(abscisic acid,脱落酸)和H2O2的含量,而RNAi沉默CsHB5则下调了ABA相关基因的表达;在番茄过表达CsHB5还导致暗诱导叶片衰老变黄进程提前,且果实中ABA和H2O2水平也显著升高,但番茄红素含量降低。对过表达CsHB5的柑橘愈伤组织和转基因番茄开展转录组数据分析后发现,CsHB5参与叶绿素降解、营养合成与运输,以及ABA和ROS信号转导等过程。进一步研究表明,CsHB5可结合BCH1(ABA生物合成基因β-胡萝卜素羟化酶1)和NCED2(9-顺式环氧类胡萝卜素双加氧酶2)启动子序列,激活其转录。因此,CsHB5通过直接控制ABA的积累参与柑橘衰老过程。
由此可见,转录因子在果蔬采后抗氧化过程中具有不同调控作用;目前有关果蔬低温贮藏过程中的抗氧化转录调控作用机制仍有待进一步深入研究,特别是参与延缓果蔬低温贮藏品质劣变转录因子的筛选。
4. 结论与展望
综上所述,低温通过酶促和非酶促抗氧化作用平衡果蔬体内ROS代谢水平,在维持果蔬采后贮藏品质过程中发挥重要作用;其中,低温响应转录因子介导抗氧化转录调控作用参与果蔬成熟衰老过程,能减少氧化胁迫,最终有利于避免果蔬贮藏外观和营养品质劣变。然而,目前大部分研究是通过转基因植物的异源表达,进而分析转录因子的转录水平以及抗氧化相关酶基因的表达量,间接阐明抗氧化转录调控作用与果蔬贮藏品质的关系,而有关转录因子对果蔬体内抗氧化关键酶基因的直接调控作用研究(转录因子结合启动子调控元件)仍不多见。深入研究ROS代谢水平与低温延缓果蔬品质劣变的关系,需探究抗氧化系统在果蔬低温贮藏过程中应对氧化胁迫的防御作用,更需阐明转录因子介导抗氧化防御系统在果蔬应对生物和非生物应激反应中的调控作用。因此,今后可通过以下几个方面研究低温延缓果蔬采后品质劣变的机制:一是进一步利用番茄、柑橘等模式果蔬的转基因体系,筛选氧化胁迫敏感型突变体,重点关注果蔬低温贮藏抗氧化转录调控机制;二是以低温贮藏作为切入点,结合转录组、蛋白组、代谢组等多组学技术及实时荧光定量PCR,深度挖掘低温响应转录因子家族成员和抗氧化酶关键靶基因;三是利用酵母单双杂交实验、染色质免疫共沉淀测序、EMSA及DLR等分子生物学实验方法,明确低温响应转录因子对抗氧化酶靶基因的调控方式(直接或间接)及其调控通路。从抗氧化转录调控作用角度揭示果蔬成熟衰老品质劣变的机理,为果蔬保鲜技术提供理论基础,有助于研发改进果蔬采后品质的保鲜技术。
-
[1] 胡筱, 潘浪, 丁胜华, 等. 1-MCP作用机理及其在果蔬贮藏保鲜中的应用研究进展[J]. 食品工业科技,2019,40(8):304−309, 316. [HU X, PAN L, DING S H, et al. Research progress on the mechanism of action of 1-MCP and its application in postharvest fruits and vegetables storage[J]. Science and Technology of Food Industry,2019,40(8):304−309, 316. doi: 10.13386/j.issn1002-0306.2019.08.051 [2] SHIPMAN E N, YU J W, ZHOU J Q, et al. Can gene editing reduce postharvest waste and loss of fruit, vegetables, and ornamentals?[J]. Horticulture Research,2021,8(1):51−71. doi: 10.1038/s41438-021-00488-0
[3] JI L, PANG J, LI S, et al. Application of new physical storage technology in fruit and vegetable industry[J]. African Journal of Biotechnology,2012,11(25):6718−6722.
[4] KIM M J, PARK E J. Feature analysis of different in vitro antioxidant capacity assays and their application to fruit and vegetable samples[J]. Journal of the Korean Society of Food Science & Nutrition,2011,40(7):1053−1062.
[5] FANG Y J, WAKISAKA M. A review on the modified atmosphere preservation of fruits and vegetables with cutting-edge technologies[J]. Agriculture-Basel,2021,11(10):992. doi: 10.3390/agriculture11100992
[6] PATRAS A, TIWARI B K, BRUNTON N P. Influence of blanching and low temperature preservation strategies on antioxidant activity and phytochemical content of carrots, green beans and broccoli[J]. LWT-Food Science and Technology,2011,44(1):299−306. doi: 10.1016/j.lwt.2010.06.019
[7] ZHAO H D, WANG B G, CUI K B, et al. Improving postharvest quality and antioxidant capacity of sweet cherry fruit by storage at near-freezing temperature[J]. Scientia Horticulturae,2019,246:68−78. doi: 10.1016/j.scienta.2018.10.054
[8] LIU D K, XU C C, GUO C X, et al. Sub-zero temperature preservation of fruits and vegetables: A review[J]. Journal of Food Engineering,2019,275:109881.
[9] NASSARAWA S S, ABDELSHAFY A M, XU Y Q, et al. Effect of light-emitting diodes (LEDs) on the quality of fruits and vegetables during postharvest period: A review[J]. Food and Bioprocess Technology,2021,14(3):388−414. doi: 10.1007/s11947-020-02534-6
[10] HUANG G H, QU Y, LI T, et al. Comparative transcriptome analysis of Actinidia arguta fruits reveals the involvement of various transcription factors in ripening[J]. Horticultural Plant Journal,2018,4(1):35−42. doi: 10.1016/j.hpj.2018.01.002
[11] LUPI A C D, LIRA B S, GRAMEGNA G, et al. Solanum lycopersicum GOLDEN 2-LIKE 2 transcription factor affects fruit quality in a light- and auxin-dependent manner[J]. Plos One,2019,14(2):e0212224. doi: 10.1371/journal.pone.0212224
[12] XIE X B, LI S, ZHANG R F, et al. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples[J]. Plant, Cell & Environment,2012,35(11):1884−1897.
[13] HAN T, YAN J W, XIANG Y, et al. Phosphorylation of ZmNAC84 at Ser-113 enhances the drought tolerance by directly modulating ZmSOD2 expression in maize[J]. Biochemical and Biophysical Research Communications,2021,567:86−91. doi: 10.1016/j.bbrc.2021.06.026
[14] DELUC L, BOGS J, WALKER A R, et al. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries[J]. Plant Physiology,2008,147(4):2041−2053. doi: 10.1104/pp.108.118919
[15] XU D Y, SHI M, JIA B, et al. Effect of ozone on the activity of antioxidant and chlorophyll-degrading enzymes during postharvest storage of coriander (Coriandrum sativum L.)[J]. Journal of Food Processing and Preservation,2019,43(8):e14020.
[16] 张丹丹, 屈红霞, 段学武, 等. 热带果蔬采后冷害研究进展[J]. 热带作物学报,2020,41(10):2062−2079. [ZHANG D D, QU H X, DUAN X W, et al. Advances in postharvest chilling injury of tropical fruit and vegetable[J]. Chinese Journal of Tropical Crops,2020,41(10):2062−2079. doi: 10.3969/j.issn.1000-2561.2020.10.013 [17] CHEN Q M, WANG Q G, FU M R. Postharvest low temperature conditioning reduces peel browning and improves fruit quality in storage and subsequent shelf life of Huangguan pear[J]. Food & Nutrition Sciences,2015,6:1351−1361.
[18] SHEKARI A, HASSANI R N, AGHDAM M S, et al. The effects of melatonin treatment on cap browning and biochemical attributes of Agaricus bisporus during low temperature storage[J]. Food Chemistry,2021,348:129074. doi: 10.1016/j.foodchem.2021.129074
[19] MINAS I S, TANOU G, BELGHAZI M, et al. Physiological and proteomic approaches to address the active role of ozone in kiwifruit post-harvest ripening[J]. Journal of Experimental Botany,2012,63(7):2449−2464. doi: 10.1093/jxb/err418
[20] LOPEZ-VIDAL O, CAMEJO D, RIVERA-CABRERA F, et al. Mitochondrial ascorbate-glutathione cycle and proteomic analysis of carbonylated proteins during tomato (Solanum lycopersicum) fruit ripening[J]. Food Chemistry,2016,194:1064−1072. doi: 10.1016/j.foodchem.2015.08.055
[21] QIN G Z, WANG Q, LIU J, et al. Proteomic analysis of changes in mitochondrial protein expression during fruit senescence[J]. Proteomics,2009,9(17):4241−4253. doi: 10.1002/pmic.200900133
[22] SHAFIQUE M, KHAN A S, MALIK A U, et al. Exogenous application of oxalic acid delays pericarp browning and maintain fruit quality of litchi cv. "Gola"[J]. Journal of Food Biochemistry,2016,40(2):170−179. doi: 10.1111/jfbc.12207
[23] LIU J L, SUN J H, PAN Y G, et al. Endogenous melatonin generation plays a positive role in chilling tolerance in relation to redox homeostasis in litchi fruit during refrigeration[J]. Postharvest Biology and Technology,2021,178:111554. doi: 10.1016/j.postharvbio.2021.111554
[24] WANG Y, MO Y L, LI D Q, et al. The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence[J]. Food Chemistry,2019,301:125224. doi: 10.1016/j.foodchem.2019.125224
[25] WANG Y Q, ZHANG L J, ZHU S J. 1-Methylcyclopropene (1-MCP)-induced protein expression associated with changes in Tsai Tai (Brassica chinensis) leaves during low temperature storage[J]. Postharvest Biology and Technology,2014,87:120−125. doi: 10.1016/j.postharvbio.2013.08.016
[26] MAHAJAN P V, CALEB O J, SINGH Z, et al. Postharvest treatments of fresh produce[J]. Philosophical Transactions of the Royal Society of London a Mathematical Physical & Engineering Sciences,2014,372(2017):20130309.
[27] KERCH G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review[J]. Trends in Food Science & Technology,2015,46(2):159−166.
[28] LIU H, LI D L, XU W C, et al. Application of passive modified atmosphere packaging in the preservation of sweet corns at ambient temperature[J]. LWT-Food Science and Technology,2021,136:110295. doi: 10.1016/j.lwt.2020.110295
[29] 李云峰, 范競升, 陈冰琳, 等. 3个甜玉米品种在不同储藏条件下可溶性固形物含量及挥发性风味成分变化[J]. 华南农业大学学报,2021,42(3):33−44. [LI Y F, FAN J S, CHEN B L, et al. Changes of soluble solid contents and volatile flavor components of three sweet corn cultivars under different storage conditions[J]. Journal of South China Agricultural University,2021,42(3):33−44. doi: 10.7671/j.issn.1001-411X.202007003 [30] LI J, FU Y L, YAN J Q, et al. Forced air precooling enhanced storage quality by activating the antioxidant system of mango fruits[J]. Journal of Food Quality,2019,2019:1606058.
[31] ZHAO H D, LIU B D, ZHANG W L, et al. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage[J]. Postharvest Biology and Technology,2019,147:113−122. doi: 10.1016/j.postharvbio.2018.09.013
[32] MA Q L, LIN X, WEI Q J, et al. Melatonin treatment delays postharvest senescence and maintains theorganoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity[J]. Scientia Horticulturae,2021,286:110236. doi: 10.1016/j.scienta.2021.110236
[33] ZHAO Y Y, CHEN J X, TAO X Y, et al. The possible role of BAX and BI-1 genes in chilling-induced cell death in cucumber fruit[J]. Acta Physiologiae Plantarum,2014,36(6):1345−1351. doi: 10.1007/s11738-014-1513-0
[34] MEITHA K, PRAMESTI Y, SUHANDONO S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits[J]. International Journal of Food Science,2020,2020:8817778.
[35] YANG H Q, WU F H, CHENG J Y. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response[J]. Food Chemistry,2011,127(3):1237−1242. doi: 10.1016/j.foodchem.2011.02.011
[36] AN X J, XU Y, JIANG L, et al. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8)[J]. Scientia Horticulturae,2018,245:178−184.
[37] 陈琳. 植物代谢通路及KEGG使用指南[M]. 武汉: 迈维代谢出版社, 2022: 117 CHEN L. Metabolic pathways of plant and and guidelines for the use of KEGG [M]. Wuhan: Metware Press, 2022: 117.
[38] ZHANG Y, ZHANG Y Z, SUN Q, et al. Citrus transcription factor CsHB5 regulates abscisic acid biosynthetic genes and promotes senescence[J]. The Plant Journal,2021,108(1):151−168. doi: 10.1111/tpj.15431
[39] RADYUK M S, DOMANSKAYA I N, SHCHERBAKOV R A, et al. Effect of low above-zero temperature on the content of low-molecular antioxidants and activities of antioxidant enzymes in green barley leaves[J]. Russian Journal of Plant Physiology,2009,56(2):175−180. doi: 10.1134/S1021443709020058
[40] 马亚丹, 张翠翠, 李林杰, 等. 荧光和发光二极管辐射技术调控果蔬采后抗氧化活性及其机制研究进展[J]. 食品科学,2019,40(5):276−281. [MA Y D, ZHANG C C, LI L J, et al. Progress in understanding the regulatory effect and mechanism of fluorescent light and light-emitting diode irradiation on antioxidant properties of fruits and vegetables during postharvest storage[J]. Food Science,2019,40(5):276−281. [41] BANDANA, SINGH B, SHARMA V, et al. Influence of low and elevated temperature on antioxidant enzymes and quality of potato during storage[J]. International Journal of Tropical Agriculture,2018,35(4):810−817.
[42] SANG Y Y, YANG W T, LIU Y X, et al. Influences of low temperature on the postharvest quality and antioxidant capacity of winter jujube (Zizyphus jujuba Mill. cv. Dongzao)[J]. LWT-Food Science and Technology,2022,154:112876. doi: 10.1016/j.lwt.2021.112876
[43] JIANG L, ZHANG L, SHI Y, et al. Proteomic analysis of peach fruit during ripening upon post-harvest heat combined with 1-MCP treatment[J]. Journal of Proteomics,2014,98:31−43. doi: 10.1016/j.jprot.2013.11.019
[44] 周春丽, 钟贤武, 苏虎, 等. 一氧化氮对果蔬采后保鲜机理的研究进展[J]. 湖北农业科学,2011,50(10):1954−1957. [ZHOU C L, ZHONG X W, SU H, et al. Study on progress of fresh preservation mechanism of nitric oxide on vegetables and fruits[J]. Hubei Agricultural Sciences,2011,50(10):1954−1957. doi: 10.3969/j.issn.0439-8114.2011.10.004 [45] GE Y H, TANG Q, LI C Y, et al. Acibenzolar-S-methyl treatment enhances antioxidant ability and phenylpropanoid pathway of blueberries during low temperature storage[J]. LWT-Food Sciencence and Technology,2019,110:48−53. doi: 10.1016/j.lwt.2019.04.069
[46] YU Y, GUO W B, LIU Y X, et al. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao)[J]. Scientia Horticulturae,2021,288:110372. doi: 10.1016/j.scienta.2021.110372
[47] WANG Y X, GAO L P, WANG Q, et al. Low temperature conditioning combined with methyl jasmonate can reduce chilling injury in bell pepper[J]. Scientia Horticulturae,2019,243:434−439. doi: 10.1016/j.scienta.2018.08.031
[48] WANG B, ZHU S J. Pre-storage cold acclimation maintained quality of cold-stored cucumber through differentially and orderly activating ROS scavengers[J]. Postharvest Biology and Technology,2017,129:1−8. doi: 10.1016/j.postharvbio.2017.03.001
[49] LI X, YANG H Q, LU G Q. Low-temperature conditioning combined with cold storage inducing rapid sweetening of sweetpotato tuberous roots (Ipomoea batatas (L.) Lam) while inhibiting chilling injury[J]. Postharvest Biology and Technology,2018,142:1−9. doi: 10.1016/j.postharvbio.2018.04.002
[50] WANG H Y, WANG H L, SHAO H B, et al. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology[J]. Frontiers in Plant Science,2016,7:67.
[51] LU M, SUN Q P, ZHANG D F, et al. Identification of 7 stress-related NAC transcription factor members in maize (Zea mays L.) and characterization of the expression pattern of these genes[J]. Biochemical & Biophysical Research Communications,2015,426(2):144−150.
[52] LIAN X Y, ZHAO X Y, ZHAO Q, et al. Mddreb2a in apple is involved in the regulation of multiple abiotic stress responses[J]. Horticultural Plant Journal,2021,7(3):197−208. doi: 10.1016/j.hpj.2021.03.006
[53] YAMASAKI K, KIGAWA T, SEKI M, et al. DNA-binding domains of plant-specific transcription factors: Structure, function, and evolution[J]. Trends in Plant Science,2013,18(5):267−276. doi: 10.1016/j.tplants.2012.09.001
[54] 范中奇, 邝健飞, 陆旺金, 等. 转录因子调控果实成熟和衰老机制研究进展[J]. 园艺学报,2015,42(9):1649−1663. [FAN Z Q, KUANG J F, LU W J, et al. Advances in research of the mechanism of transcription factors involving in regulating fruit ripening and senescence[J]. Acta Horticulturae Sinica,2015,42(9):1649−1663. doi: 10.16420/j.issn.0513-353x.2015-0356 [55] GENG J J, LIU J H. The transcription factor CsbHLH18 of sweet orange (Citrus sinensis) functions in modulation of cold tolerance and reactive oxygen species homeostasis by regulating the antioxidant gene[J]. Journal of Experimental Botany,2018,69(10):2677−2692. doi: 10.1093/jxb/ery065
[56] MIURA K, SHIBA H, OHTA M, et al. SlICE1 encoding a MYC-type transcription factor controls cold tolerance in tomato, Solanum lycopersicum[J]. Plant Biotechnology,2012,29(3):253−260. doi: 10.5511/plantbiotechnology.12.0303a
[57] MIURA K, SATO A, SHIBA H, et al. Accumulation of antioxidants and antioxidant activity in tomato, Solanum lycopersicum, are enhanced by the transcription factor SlICE1[J]. Plant Biotechnology,2012,29(3):261−269. doi: 10.5511/plantbiotechnology.12.0303b
[58] 金思, 马空军. 超声波辅助提取类胡萝卜素研究进展[J]. 食品研究与开发,2017,38(9):192−197. [JIN S, MA K J. Research progress in ultrasound-assisted extraction of carotenoids[J]. Food Research and Development,2017,38(9):192−197. doi: 10.3969/j.issn.1005-6521.2017.09.044 [59] 赖瑞联, 冯新, 程春振, 等. 橄榄WRKY转录因子的克隆及其在低温胁迫下的表达分析[J]. 果树学报,2018,35(12):1455−1466. [LAI R L, FENG X, CHENG C Z, et al. Cloning of WRKY transcription factors and their expression analysis under low temperature stress in Canarium album (Lour.) Raeusch[J]. Journal of Fruit Science,2018,35(12):1455−1466. doi: 10.13925/j.cnki.gsxb.20180190 [60] HAN D G, DU M, ZHOU Z Y, et al. An NAC transcription factor gene from Malus baccata, MbNAC29, increases cold and high salinity tolerance in Arabidopsis[J]. In Vitro Cellular & Developmental Biology-Plant,2020,56(5):588−599.
[61] BANERJEE A, SAMANTA S, ROYCHOUDHURY A. Spermine ameliorates prolonged fluoride toxicity in soil-grown rice seedlings by activating the antioxidant machinery and glyoxalase system[J]. Ecotoxicology and Environmental Safety,2020,189:109737. doi: 10.1016/j.ecoenv.2019.109737
[62] ACHARD P, RENOU J P, BERTHOME R, et al. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species[J]. Current Biology,2008,18(9):656−660. doi: 10.1016/j.cub.2008.04.034
[63] LIU Y D, SHI Y, ZHU N, et al. SlGRAS4 mediates a novel regulatory pathway promoting chilling tolerance in tomato[J]. Plant Biotechnology Journal,2020,18(7):1620−1633. doi: 10.1111/pbi.13328
[64] FAN Z Q, TAN X L, CHEN J W, et al. BrNAC055, a novel transcriptional activator, regulates leaf senescence in Chinese flowering cabbage by modulating reactive oxygen species production and chlorophyll degradation[J]. Journal of Agricultural and Food Chemistry,2018,66(36):9399−9408. doi: 10.1021/acs.jafc.8b02309
[65] YAN J L, TONG T T, LI X, et al. A novel NAC-type transcription factor, NAC87, from oilseed rape modulates reactive oxygen species accumulation and cell death[J]. Plant and Cell Physiology,2018,59(2):290−303. doi: 10.1093/pcp/pcx184
-
期刊类型引用(2)
1. 文华英,王傅玉,张玉红. 青稞蕨麻酵素发酵工艺优化及其品质评价. 中国酿造. 2024(02): 199-205 .  百度学术
百度学术
2. 董平,徐向波,周奎,曹娜娜,吴华昌,邓静. 沙米面包配方优化及其品质研究. 食品工业科技. 2024(14): 155-164 .  本站查看
本站查看
其他类型引用(3)





 下载:
下载:
 下载:
下载:



