Research Progress of Fish Drug Residues Detection in Aquatic Products
-
摘要: 孔雀石绿和结晶紫、氯霉素、硝基呋喃类药物曾被广泛用于抑制微生物生长、预防或治疗细菌性疾病。在养殖过程中,仍存在非法使用鱼药的现象。为了防止受污染的产品在食物链中流通,事先实施分析是一项重要的水产品安全保护措施。本文综述了水产品中孔雀石绿和结晶紫、氯霉素、硝基呋喃类药物及其代谢物残留的检测方法,介绍了色谱法、表面增强拉曼光谱、免疫分析法及电化学传感器在该领域的应用,对前处理和验证结果进行了归纳,并讨论了每种方法的优缺点,旨在为监测残留物提供新的思路。具体而言,基于大型仪器的方法检测限低,但固有的缺陷限制了其发展。随着新型纳米材料的发展,表面增强拉曼光谱、免疫分析法、电化学传感器必将成为评估水产品中鱼药残留的有效工具。Abstract: Malachite green, crystal violet, chloramphenicol and nitrofurans have been widely used to inhibit the growth of microorganisms and to prevent or treat the emergence and spread of bacterial diseases. The above three types of drugs are carcinogenic, teratogenic and mutagenic, which have been banned in many countries. However, due to their advantages of low cost and significant effect, they are still used illegally in the aquaculture process. In order to prevent contaminated products circulating in the food chain and endangering consumer health. Advance analysis of aquatic products is an essential safety protection measure. This paper reviews the analytical methods concerning malachite green, crystal violet, chloramphenicol, nitrofuran and their metabolites residues in aquatic products, describes the application of chromatography, surface-enhanced Raman spectroscopy, enzyme-linked immunosorbent assay, immu-nochromatography assay, chemiluminescence enzyme immunoassay and electrochemical sensors in this field, and systematically summarizes the sample pretreatment procedures, chromatographic conditions and validation results, and discusses the advantages and disadvantages of each method. The aim of this work is to provide new ideas for monitoring residues and to present a thorough overview of current trends in this field. To be specific, high performance liquid chromatography and liquid chromatography tandem-mass spectrometry have low detection limits, yet they require cumbersome pretreatment procedures, expensive instruments and professional labor, which limit the development of this technique. With the development of new nanomaterials, surface-enhance Raman spectroscopy, immunoassay and electrochemical sensors have excellent prospects in assessing fish drug residues, which do not require large instruments, are easy to operate, and can be used for rapid detection in the field, and they will certainly become the effective tools for monitoring illegal fish drugs in aquatic products.
-
Keywords:
- malachite green (MG) /
- crystal violet (CV) /
- chloramphenicol (CAP) /
- nitrofurans /
- residual analysis
-
水产品是人们摄取优质蛋白质、脂肪、维生素和微量元素的重要来源[1]。水产品需求的增加和水产养殖业的快速发展导致集约化生产方式的实施[2],在养殖过程中,常加入抗菌药物以防止鱼类疾病的出现和传播。然而,鱼药的不当使用可能导致细菌耐药性的产生[3],残留于机体并通过食物链转移到人类身上,对行业发展和消费者健康造成潜在危害[4]。
我国是世界上最大的水产品生产国和贸易国,水产品安全与我国国家利益和国际市场竞争力息息相关。为了保障水产品的食用安全,迫切需要建立一种灵敏、可靠的检测手段,在食用之前监测鱼药的残留情况。已有研究者针对牛奶或其他动物源食品中的单一药物残留进行论述,少数是关于水产品的,鲜有同时概述多种鱼药的报道。本文以方法分类,通过整理和比较近年来发表的最新文献,概述了检测水产品中三类违禁药物的代表性工作。介绍了国内外水产品中孔雀石绿和结晶紫、氯霉素、硝基呋喃类药物的样品预处理及检测方法,指出了该领域的未来发展方向,可为新方法的建立或采用提供参考依据。与其他有关综述相比,本文的报道范围更广,可以让读者对鱼药残留的分析方法有更加完整的了解。
1. 孔雀石绿及结晶紫
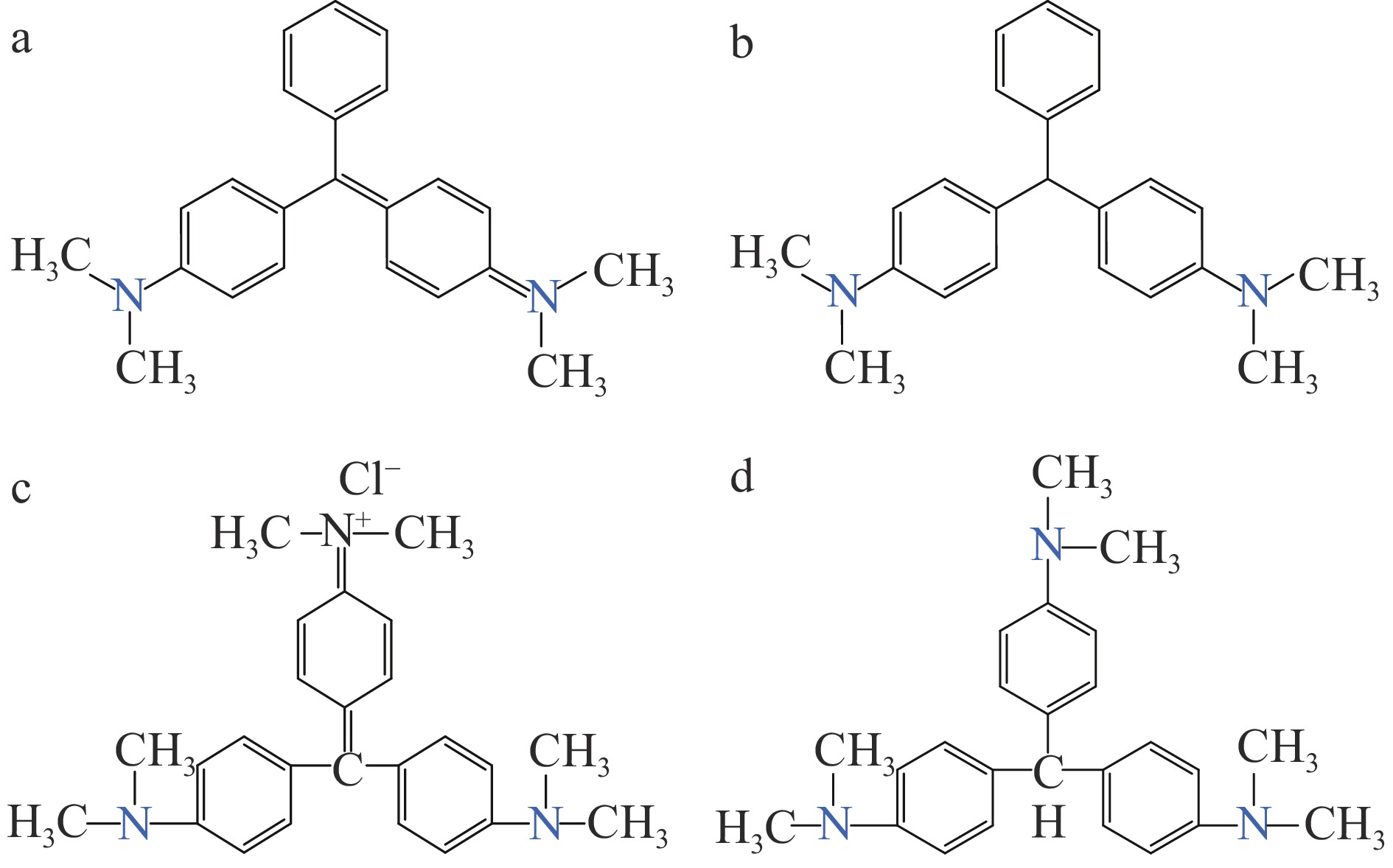
孔雀石绿(malachite green,MG)和结晶紫(crystal violet,CV)属于三芳基甲烷类工业染料。它们可有效防止原生动物感染,在水产养殖业中常被用作局部抗菌剂、防腐剂、杀寄生虫剂[5-6]。进入动物组织后易还原为无色孔雀石绿(leucomalachite green,LMG)和无色结晶紫(leucocrystal violet,LCV),其结构如图1所示。据报道,孔雀石绿及其关联化合物具有强毒性、高残留和三致效应[6-7],可在机体内长时间残留,损害生物体的健康。
因此,MG已被严格限制使用[8],CV被列入欧盟REACH法规第七批候选物质清单[9]。欧盟法案2002/675/EC规定动物源食品中母体药物及其无色形式总限量为2.0 μg/kg[10];美国FDA和我国农业部[11-12]规定水产品中MG和CV监管检测的最低灵敏度为1.0 μg/kg。加拿大和日本明确规定禁止在养殖过程中使用孔雀石绿和结晶紫。但因其低成本、高效易得,仍存在被养殖者非法使用的现象[13]。
在过去几十年中,人们已开发出多种用于识别、检测水产品中孔雀石绿和结晶紫的方法,主要包括高效液相色谱法、表面增强拉曼散射光谱、免疫学法及电化学传感器。
1.1 高效液相色谱法
1.1.1 前处理方法
我国水产品种类繁多、化学成分复杂,兽药残留可检测的水平含量较低。且孔雀石绿和结晶紫在水产品中主要以代谢物的形式存在,具有很好的亲脂性,脂肪含量较高,不利于目标分析物的提取和净化。必须通过分离、提取、萃取等方法对待检水产品进行前处理,以减少或消除油脂和基质干扰,提高分析的准确率[14],有效保护色谱柱和质谱仪。
孔雀石绿、结晶紫及其代谢物检测的前处理主要包括两个步骤:一是用乙腈和不同试剂提取目标物[15];二是纯化提取物,可利用的技术包括QuEChERS技术[14]、微波辅助萃取[16]、固相萃取(磁性、分子印迹)[17-19]、免疫亲和柱[20]和快速滤过型净化柱[21]等。最常用的有固相萃取和液液萃取,液液萃取可有效排除极性干扰物质,但需消耗大量溶剂,且常用的二氯甲烷对人体健康有害;固相萃取提取效率高,但存在耗时、昂贵、繁琐的不足。水产品基质复杂,经过合适的预处理后,基于化合物及其代谢物在色谱柱上有不同保留时间的原理进行分析。常与高灵敏度检测器结合使用,如紫外检测器、荧光检测器及质谱仪等。
1.1.2 检测分析
紫外检测器主要利用MG、CV及其代谢物的吸收波长差异进行定量。MG和CV在紫外-可见区的最大吸收波长分别为618和588 nm[17],而LMG和LCV无吸收峰。在分析前,需用氧化剂将LMG转化为MG[22]。常用氧化剂包括PbO2柱后衍生、电化学氧化及DDQ柱前氧化[23]。在此基础上,Liu等[18]以磁性多孔有机笼为固相萃取吸附剂,结合高效液相色谱法实现了水产品中三苯甲烷阳离子染料的多残留检测,简化了样品预处理的步骤、减少了从大量样品中浓缩痕量目标物所需的吸附剂、洗脱剂和时间。将来,学者们可多加关注有良好吸附能力的多孔纳米材料,开发可纯化提取物的不同吸附剂,扩展其在样品制备中的应用。
MG、CV没有荧光性质,LMG、LCV的入射波长和发射波长分别为265、360 nm。需利用硼氢化钾将母体还原为代谢物,再通过荧光检测器测定[12]。学者们在国标基础上不断改良、优化[24-25],剖析样品制备、净化及检测环节遇到的问题,提高了该法的灵敏度。但该法仍存在着通用性差,只适用于能产生荧光的物质的不足。为了提高分析效率和方法灵敏度,Mitrowska等[26]开发了同时检测MG、LMG的方案:连接紫外检测器和荧光检测器,前者用于分析MG(λ=618 nm),后者用于分析LMG(λex=265 nm,λem=360 nm)。该法省略了氧化柱的使用,不仅缩短了检测时间,也提高了环境安全性,同样适用于CV和LCV的情形。
串联质谱法结合了液相色谱和质谱的优点,覆盖面广、鉴别能力强[15],是鱼药残留分析的常用确证手段。样品经过提取、富集、净化后,利用电离子喷雾技术产生用于MG、CV分析的离子,在正离子、多反应监测扫描模式下分析,常用的质谱仪包括三重四级杆质谱[27]和高分辨率飞行质谱[28]。表1显示了该领域的最新进展。可以看出,色谱法应用范围广、高通量能力优越,但因基质、检测器以及前处理等条件的不同,所得检出限差别较大。总的来说,高效液相色谱串联质谱法检出限较低、具有更高的准确性和灵敏度。但也存在着需要复杂的样品制备、大型精密仪器和专业人员,无法满足现场快速检测的不足。
表 1 测定水产品中孔雀石绿和结晶紫的色谱方法Table 1. Chromatographic methods for the determination of malachite green and crystal violet in aquatic products分析物 检测器 基质 样品前处理 色谱柱 检出限
(μg/kg)MG、LMG、CV、LCV[14] 二极管阵列
检测器鳕鱼 QuEChERS
a. NaCl和MgSO4盐析;
b. d-SPE固相萃取净化XCharge C18 MG:3.2
LMG:24.1
CV:1.9
LCV:23.4MG、CV[16] 串联质谱 养殖鱼、河鱼和海鱼 微波辅助萃取、分散-液液微萃取结合固相纯化 BEH Phenyl柱
(50 mm×2.1 mm,1.7 μm)MG:4.54×10−6
CV:6.02×10−6MG、CV[17] 二极管阵列、荧光检测器、三重四级杆质谱仪 斑点叉尾鱼 McIlvaine-乙腈缓冲溶液提取、SPE筒吸附净化 a. ODS-3 C18
(150 mm×4.6 mm,3 μm)
b. Oasis MCX SPE
(150 mg/6 mL)MG:0.38
LMG:0.26
CV:0.26
LCV:0.09MG、CV、RB、BG、VPBO[18] 紫外-可见
检测器鲤鱼、鲶鱼、草鱼 乙腈提取、石油醚脱脂、磁性多孔有机笼富集、吸附 TC-C18
(250 mm×4.6 mm)0.67~8.00 MG、LMG、CV、LCV[19] 二极管阵列 草鱼、虾、甲壳类 乙腈-醋酸铵提取、分子印迹固相萃取 Symmetry C18
(150 mm×4.6 mm,5 μm)MG:0.13~0.14
LMG:0.12~0.14
CV:0.11~0.13
LCV:0.12~0.14MG、LMG、CV、LCV[20] 紫外、荧光
检测器鱼肌肉 乙腈-醋酸铵提取、二氯甲烷、免疫亲和柱萃取净化 Cloversil-C18
(250 mm×4.6 mm,5 μm)MG:0.15
LMG:0.18
CV:0.10
LCV:0.14MG、LMG、CV、LCV[21] 三重四级杆
质谱仪草鱼、罗非鱼、鲢鱼 乙腈提取、冷冻除脂、快速滤过型净化柱净化 BEH C18
(50 mm×2.1 mm,1.7 μm)均为0.05 MG、LMG、CV、LCV[27] 三重四级杆
质谱仪虹鳟鱼 乙腈(含1%乙酸)提取 Inertsil ODS-4 C18
(50 mm×2.1 mm,3 μm)MG:0.43
LMG:0.24
CV:0.33
LCV:0.28MG、LMG、CV[28] 高分辨率飞行质谱仪 虾、鲑鱼、鲭鱼、
小龙虾乙腈-水提取、无需纯化 AcclaimTM 120 C18
(150 mm×2.1 mm,2.2 μm)MG:0.01~0.02
LMG:0.10~0.30
CV:0.011.2 表面增强拉曼光谱
表面增强拉曼光谱(surface-enhanced Raman spectroscopy,SERS)是一种光学增强现象,基于电磁增强或化学增强机制,增强吸附于金属颗粒粗糙表面的振动光谱[29-30],可产生高达1014的拉曼增强因子,随着合适基底的发展,其已成为一种有用的痕量分析技术。
研究表明,以各种形状的银纳米颗粒[31]、金纳米棒[32]及其复合材料[33-37]作为SERS基底,可实现孔雀石绿和结晶紫的无损检测。不同基底检测限的比较如表2所示。贵金属纳米颗粒可增强待测物的拉曼散射信号,Zhang等[31]将SERS与等离激元Ag底物相结合,利用循环伏安法使部分银纳米线转化为纳米棒和纳米颗粒,提高了检测灵敏度。不仅如此,该法的检测可通过简单地擦拭表面来实现,适合现场快速检测。贵金属纳米结构与氧化石墨烯具有协同效应,Zhang的团队[34-36]制备了许多由氧化石墨烯和等离激元Au或Ag杂化物组成的固体SERS基底,可快速检测鱼类样品中的MG和CV。
表 2 孔雀石绿和结晶紫检测中几种SERS基底的检测限Table 2. Detection limits of some SERS substrates for malachite green and crystal violet detectionSERS基底 检出限 优点 参考文献 银纳米线膜 CV:5.1×10−11 mol·L−1
MG:0.00693 ng易变形、可用于现场检测 [31] 一次性Au/Ag纸基 MG:4.3×10−9 mol·L−1
CV:8.1×10−8 mol·L−1基底制备简单、重现性和稳定性好; [33] 二氧化钛-银-氧化石墨烯
(TiO2-Ag-GO)MG:10−7 mol·L−1
CV:10−9 mol·L−1可同时检测多种化合物;
氧化石墨烯和等离子银的修饰可提高基底检测灵敏度、
重复性、稳定性[34] AgNPs/Cu复合材料 MG:0.94×10−7 mol·L−1
CV:1.14×10−9 mol·L−1检测限低、信号分布均匀、稳定性好;
操作简单、可用于实际样品的检测[35] 铜板-氧化石墨烯修饰银纳米粒子(GO/AgNPs/Cu) MG:10−8 mol·L−1
CV:10−8 mol·L−1再现性好、线性浓度宽;
快速、准确、可用于现场测定;
AgNPs的粗糙表面提供了更多热点[36] 金纳米棒-单宁酸薄膜涂层(GNR/TA) MG:0.07 μg·L−1
CV:0.03 μg·L−1低成本、一次性现场应用;
重复性好、高性能的原位检测[37] SERS信号强度受各种因素干扰,且活性底物的集成极大限制了其在食品监测中的应用。今后,需要更多的研究来确定在复杂的样品基质中获得可重复结果的最佳条件。此外,与传统的刚性基底(硅、玻璃和金属)相比,柔性纸基的成本效益和时间效益高、对样品采集的要求低,为提高检测效率,应继续开发此类基底。
1.3 免疫学法
免疫学法特异性高,具有更高的样品吞吐量,能满足现场快速检测的要求。其中,酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)最为常用,磁性微球、量子点等纳米材料更是促进了该法发展,可以极大限度地减少基质干扰,并提高方法准确性。
ELISA以酶为标记物,结合了酶的高效催化、信号放大与抗原抗体的特异性作用。当所需的酶标抗体起作用时,其可将抗原抗体相互作用转化为色度变化,可根据有色物质的深浅进行分析[38]。半抗原设计是成功制备针对小分子目标物的抗体的关键步骤,MG、CV为小分子物质,无免疫原性。通常将其与蛋白载体偶联成免疫抗原,与卵清蛋白偶联为包被抗原,再通过杂交瘤技术产生抗目标物的单克隆抗体,基于此构建直接或间接竞争ELISA。如表3所示,Shen等[39]合成了几种异源涂层半抗原,获得了针对MG、CV的多克隆抗体,首次开发出测定MG、CV的间接ELISA。其中,MG和CV的半抑制浓度IC50分别为1.61和1.34 ng/mL。研究表明,单克隆抗体较多克隆抗体具有更好的特异性和稳定性,并提供更少的批间变异。Xu等[40]以阳离子化牛血清白蛋白(cBSA)为载体蛋白,利用单克隆抗体偶联与cBSA结合的半抗原。值得注意的是,该研究利用DDQ将初级代谢物氧化为母体物质,开发了定量鱼肉组织中总MG、CV的直接ELISA,提高了检测效率。
表 3 基于免疫学法对孔雀石绿和结晶紫的检测Table 3. Detection of malachite green and crystal violet based on immunological method量子点具有激发光谱宽、光稳定性好、发射光谱窄等特点,基于分析物对量子点的荧光猝灭,Chen等[41]以量子点为荧光供体,利用乙腈提取后,加入正己烷和中性氧化铝处理以消除基质效应,结合免疫亲和试验柱和荧光共振能量转移,实现了MG、CV的可视化检测,与色谱法的结果高度相关。磁性纳米微球具有磁分离性能,可高效富集和纯化样品、减少干扰并提高信号灵敏度,陈义元[42]基于竞争性抑制原理,根据反应体系荧光强度的变化,实现了MG、CV的快速分析,显著提高了方法检出限。
未来,应不断优化、改进样品前处理条件,利用氧化剂(如DDQ、电化学氧化)将代谢物氧化为母体物质,致力于实现四者的同时检测,反映化合物在动物体内残留的真实水平。
1.4 电化学传感器
电化学传感器是对目标物敏感并将其浓度转换为可被测量的电信号,从而进行分析的装置[43-44],可小型化、响应速度快[45]。电化学发光法是一种通过反应将电化学能量转换为辐射能量来发出可测量的发光信号的方法[46]。基于对三联吡啶钌-三丙胺(Ru(bpy)32+-TPA)的猝灭效应[47],该法对MG和CV的检出限分别为1.0×10−10 和1.1×10−10 mol/L[48],已应用于实际水样检测[49-51],相信其未来也可用于水产品中的MG和CV检测。
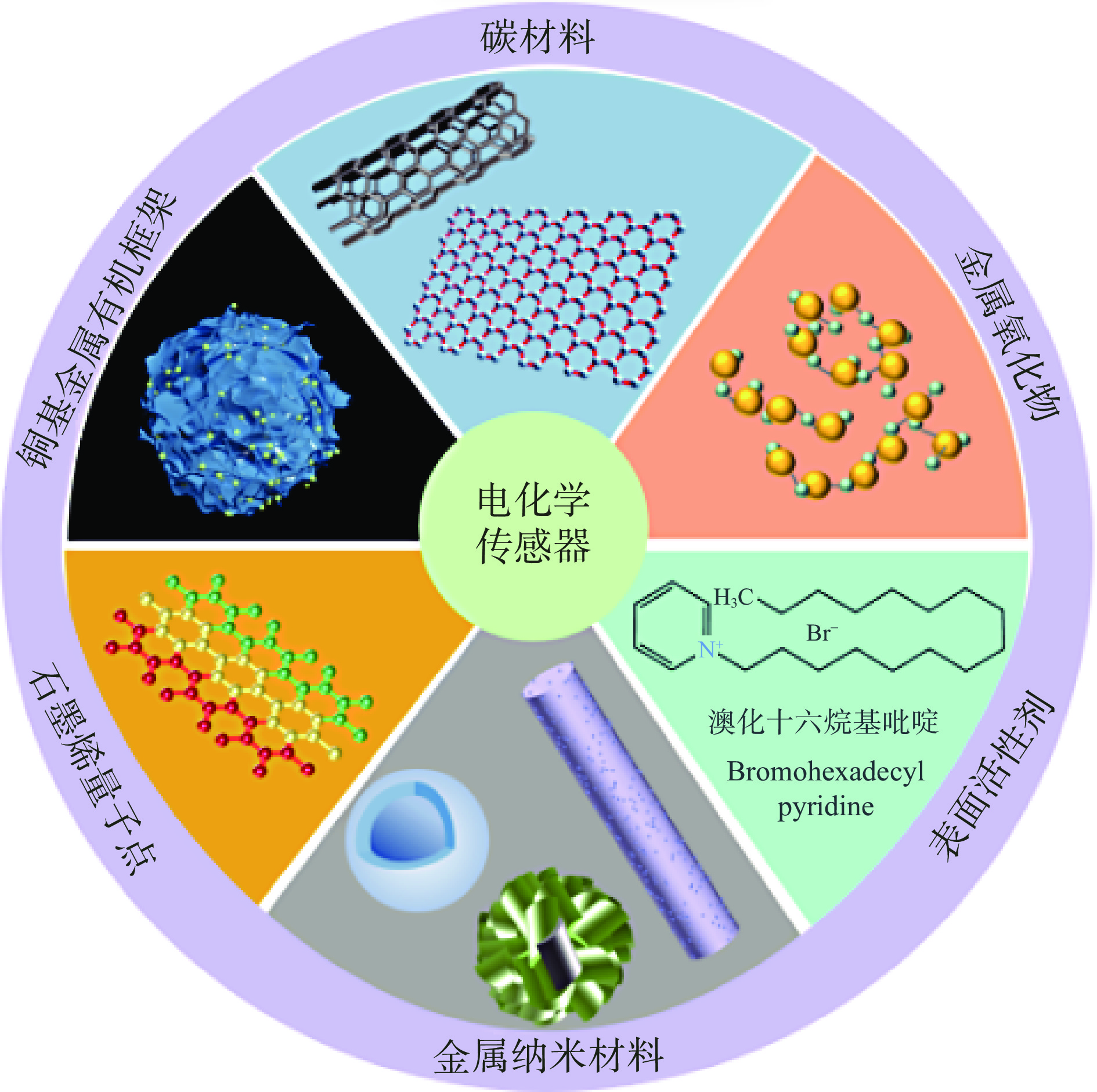
基于电化学传感器分析水产养殖业中MG残留的研究已十分成熟,常用修饰材料如图2所示。少数是关于CV残留的报道,且前期的研究大多集中在水体样品中[52-53]。金属有机框架是一类高度有序的晶体材料,孔隙丰富、结构可调、比表面积大[54]。其中,铜基金属有机框架可显著提高MG的氧化信号和检测灵敏度[55]。Zhou等[56]将银修饰的铜基金属有机框架修饰在玻碳电极上,方法的检出限为2.2×10−9 mol/L。溴化十六烷基吡啶属于阳离子表面活性剂,可提高目标物的电氧化性能。Deng等[57]制备了十六烷基溴化吡啶修饰乙炔黑糊电极,基于线性扫描伏安法测定鱼中的MG,检测限为4.0×10−9 mol/L,样品回收率高。分子印迹聚合物可克服非目标物质的干扰、对MG进行选择性地识别和吸附[58]。以分子印迹聚合物作为受体,开发的电化学传感器已用于鲤鱼[59]、草鱼[60]中MG残留的检测。碳纳米管是一种新型纳米材料,具有独特的结构和电子特性,已在电化学领域得到广泛应用。Yi等[61]利用多壁碳纳米管修饰玻碳电极测定鱼类中的MG,检测限为6.0×10−9 mol/L。此外,还常使用复合修饰材料制备工作电极。石墨烯是由杂化碳原子构成的二维蜂窝网络[62],具有良好的导电性和较强吸附能力。小于100 nm的石墨烯片为石墨烯量子点。Wang等[63]制备了基于纳米颗粒/石墨烯量子点-二硫化钨纳米复合材料的无标记电化学适配体,检测限为3.38×10−9 mol/L。用于检测刺鱼样品的MG,线性范围宽。Luo等[64]在玻碳电极表面修饰多壁碳纳米管-聚乙烯亚胺复合材料。与多壁碳纳米管/玻碳电极相比,在复合改性电极上获得的MG峰值电流大大增强。在最佳条件下,检测限为2.58×10−9 mol/L。
相较其他方法来说,电化学传感器制备简单、抗干扰能力强。随着修饰材料的不断丰富,孔雀石绿的残留分析较为完善,而针对结晶紫的报道仍处于起步阶段。未来应不断优化电极材料,开发成本低、可重复利用的修饰电极,积极探索检测水产品中结晶紫残留的应用,如基于新型共价有机框架材料构建吸附剂[65],高效富集样品中的待测物,提高方法的灵敏度。
2. 氯霉素
氯霉素(chloramphenicol,CAP)是一种高效广谱抗生素,通过使核糖体变性来抑制菌体蛋白的合成[66],广泛用于畜牧业疾病的治疗和预防[67-68]。然而,其毒性作用严重,例如可导致再生障碍性贫血、灰婴综合征及粒细胞缺乏症等[69],低浓度的残留还会诱发致病菌的耐药性[70]。因此,欧盟、日本、美国、韩国均已禁止在养殖过程中使用CAP[71]。欧盟指定CAP最大残留限量为0.3 μg/kg,新西兰规定食品中CAP的最大残留限量为0.15 μg/kg,它在我国也属于违禁药物,判定限量值为0.1 μg/kg。
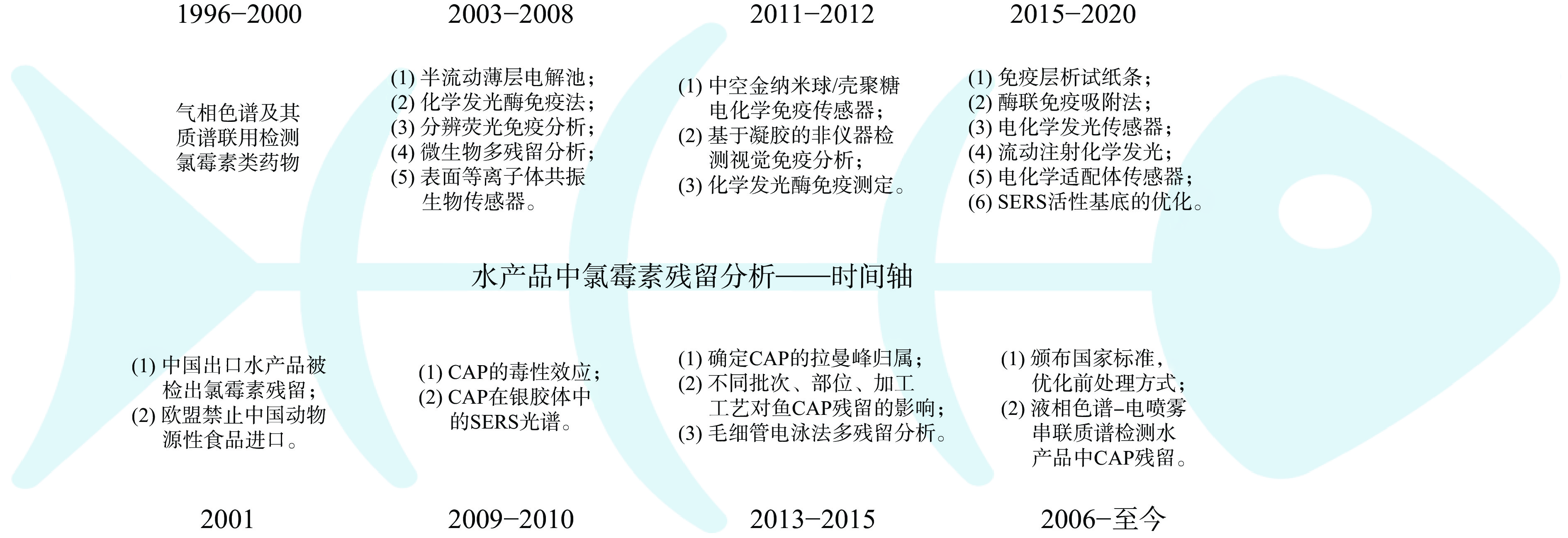
关注水产品中氯霉素的残留程度是保护消费者安全的重要措施。在过去二十几年中,该领域的研究取得了长足的进展,本文按时间顺序梳理了有关代表性工作(图3)。常用的监测方法包括液相色谱串联质谱法、表面增强拉曼光谱、酶联免疫吸附法、免疫层析法、化学发光酶免疫分析及测流免疫分析法。
2.1 液相色谱串联质谱法
氯霉素具有羟基、氯原子等极性基团,不易气化[72],运用气相色谱及气质联用前需进行复杂的衍生化处理,在痕量水平上不可重复[73],限制了使用。而液相色谱串联质谱法(LC-MS/MS)无需衍生化,分离能力强,是常用的确证方法(表4)。利用该法检测CAP时,通常利用乙酸乙酯提取样品,乙腈-水在C18柱上进行梯度洗脱以实现分离[74],以氘化氯霉素(d5-CAP)为内标,采用负电喷雾电离、多反应监测扫描进行分析。
表 4 水产品中氯霉素的不同检测方法的比较Table 4. Comparison of detection methods for detection of chloramphenicols in aquatic products基质及参考文献 方法 前处理 检测限(μg/kg) 线性范围或IC50(μg/kg) 虾、鳗鱼和比目鱼[76] LC-MS/MS 改进QuEChERS
(使用伯仲胺和MgSO4纯化样品)LOD:0.005~3.100
LOQ:0.02~10.4− 虾[77] LC-ESI-MS/MS 乙酸乙酯提取、液-液萃取 CCα:0.06
CCβ:0.100.1~2.0 尼罗鱼、对虾[78] LC-MS/MS 乙腈-氯仿提取、液-液萃取 CCα:0.04
CCβ:0.060.1~1.0 对虾[79] LC-MS/MS McIlvaine提取、免疫亲和柱净化 LOD:0.05 − 鱼肉[80] HPLC-MS/MS 乙酸乙酯提取、石墨烯固相吸附 LOD:0.036 0.5~100 虾[90] BSAS-direct Hap coated ELISA 乙酸乙酯提取、异辛烷-氯仿溶解 LOD:0.2 IC50=10.5 鲈鱼、金枪鱼[91] ELISA 缓冲液提取、正己烷脱脂 CCα:0.025
CCβ:0.25− 虾[92] ELISA 研磨均质、加乙酸乙酯、氨水和无水硫酸钠后离心 LOD:0.06 IC50=0.46 鲫鱼[94] GICA 甲醇、PBS缓冲液均质、滤纸过滤 LOD:0.5 − 虾[96] TRFIA 丙酮/二氯甲烷(1:1)提取、氮气蒸发 LOD:0.008 0.008~100 虾[99] ic-CLEIA 乙酸乙酯均质、正己烷溶解 LOD:0.01 0.03~23.70 鲢鱼[100] LFA − LOD:0.36 IC50=12.94 注:1.BSAS-direct Hap coated ELISA即生物素-链霉亲和素系统直接包被酶联免疫吸附法;2.“−”代表原文中无相关数据。 传统的预处理和清理程序比较耗时、有机溶剂成本较高且劳动密集。QuEChERS是近些年发展起来的技术,学者们也在不断尝试,使样品前处理向着绿色、清洁的方向改进。2019年,Chen等[75]利用LC-MS/MS优化了样品吞吐量,使用QuEChERS提取、净化分析物,建立了高效定量鳟鱼、鲑鱼和虾中的21种违禁药物的方法。2022年,Jung等[76]改进了QuEChERS,在反向分析柱上分离目标物,开发了可定量和验证虾、鳗鱼和比目鱼等动物源性食品中氯霉素类药物的LC-MS/MS,灵敏度高。
2.2 表面增强拉曼光谱
近年来,国内外研究人员不断探究和改进基底的制备方法,相继将该法应用于水产品中CAP的检测,已取得了一定的成果[77-80]。Si等[81]记录了CAP在银胶体中的SERS光谱。吉薇[82]通过密度泛函理论确定了CAP主要拉曼峰的归属:1102、1344及1596 cm−1。也有学者以胶体金粒子[83]、银纳米线[84]及金纳米粒子-氧化石墨烯复合材料[85]为基底,实验结果显示CAP的检测限均为0.1 μg/mL,并应用于罗非鱼和对虾等实际样品分析中。
Li等[86]利用活性花状银纳米粒子研发出灵敏度可达到皮克(pg)水平的柔性纸基SERS传感器。Hassan等[87]合成了空心金/银纳米花,CAP在其表面吸附时产生很强的SERS信号,在0.0001~1000 μg/mL范围内线性良好,有望推广到水产品中CAP的无标记和超灵敏检测。说明SERS技术能够快速、准确地识别CAP,增强因子大。未来还需要开发更简单、高效的预处理方法,并将其与SERS结合。Yu等[88]制备了既可作为固相萃取材料,又可充当SERS活性基底的磁性复合材料(Fe3O4@GO@Ag),可高效地从样品基质中富集和分离目标物,并通过纤维改性提高性能。为今后提供了新的发展方向。
2.3 免疫法
2.3.1 酶联免疫吸附法
基于抗原抗体的特异性作用,ELISA因其高通量、特异性强的特点,被广泛用于鱼类中CAP的残留检测,我国农业部已规定分析鱼和虾中氯霉素残留的相关步骤[89]。该法的关键在于制备特异性单克隆抗体,分别以CAP-BSA和CAP-OVA作为免疫原和包被抗原。后来的学者[90-92]也做了相关研究,如表4所示。2020年,Ji等[38]制备了用酶和抗体标记的双功能介孔硅纳米球,该复合物具有优异的酶负载效率,可用于吸附载体蛋白、放大色度信号,提高ELISA的灵敏度。
2.3.2 免疫层析技术
根据标记物的不同,免疫层析技术可分为胶体金免疫层析(GICA)及时间分辨荧光免疫分析(TRFIA)。胶体金免疫层析是以胶体金为示踪标记物,基于竞争性抑制原理,以固相膜为载体的一种新型的快速检测技术[93]。Zhou等[94]以硝酸纤维素膜为载体、CAP多克隆抗体为标记蛋白,将CAP抗体固定在结合垫上,建立了一种快速测定水产品中CAP残留量的GICA。铕螯合物是常用的荧光剂,荧光强度好、稳定性强、灵敏度高[95]。通常利用铕标记山羊抗兔抗体,以建立测定食品中CAP含量的间接竞争性TRFIA[96],用于筛选大量样品。
2.3.3 其它方法
化学发光酶免疫分析(CLEIA)是一种将化学发光与免疫反应相结合的新型技术,可根据发光强度测定目标化合物含量[97-98]。通常使用过氧化物酶标记抗体,鲁米诺作为底物。间接竞争性CLEIA已被开发用于检测虾中的CAP残留[99]。此外,基于毛细管力的作用,样品和试剂分别移动到测试区和控制区,并与检测抗体反应,测流免疫分析法(LFA)可于10~20 min内完成测试。2021年,Pan等[100]开发了基于与金属纳米粒子耦合的SERS的LFA条带,实现了水产品中氯霉素类药物的同时分析。
2.4 不同样品基质中氯霉素检测方法的比较
色谱法已发展为一种有效的验证方法,但费时费力、有机溶剂消耗大,不适合现场检测。与色谱法相比,免疫学法省去了样品预浓缩和清理步骤,快速、方便,可用于快速筛选。但ELISA存在非靶分析物和抗体之间非特异性结合的可能性,容易产生基质效应。且生物抗体需要免疫动物、制备周期长,蛋白质、脂肪可能影响抗体与CAP的结合,易出现假阳性[101]。GICA制作成本低、分析用时短。但准确度不够高,主要用于视觉评估和定性分析,以检测阈值水平的污染。为了快速识别CAP残留物,开发更简单、灵敏的分析方法至关重要。值得注意的是,SERS可快速获得化合物的特征信息,灵敏度较高且几乎不需要样品制备,在食品安全检测方面具有潜在应用,未来应朝着开发各种稳定性好的活性基底方向发展。
3. 硝基呋喃类药物及其代谢物
硝基呋喃是一类广谱抗菌剂,包括呋喃它酮、呋喃西林、呋喃唑酮和呋喃妥因,特征结构为5-硝基呋喃(表5)。其常被用作治疗药物、饲料添加剂和生长促进剂,且价格低、抑菌效能好[93]。其中,母体药物以非活性形式给药,在体内只能存在几个小时,通过正常代谢过程转化为活性形式的药物。代谢物可与组织蛋白质紧密结合,形成有毒且稳定的加合物[102-103],引起“三致”作用。欧盟、日本、美国、韩国和中国均颁发了硝基呋喃类抗生素的使用禁令,规定其在所有食品动物中不得被检出[71,104]。我国硝基呋喃代谢物的检出判定限量值为1.0 μg/kg,基于液相色谱-串联质谱法的检出限均为0.5 μg/kg[105]。
表 5 四种硝基呋喃及其代谢物的结构Table 5. Structures of four kinds of nitrofurans and their metabolites母体药物 结构式 代谢物 结构式 呋喃它酮
(FTD)
5-吗啉甲基-3-氨基-2-唑烷基酮
(AMOZ)
呋喃西林
(NFZ)
氨基脲
(SEM)
呋喃唑酮
(FZD)
3-氨基-2-噁唑烷酮
(AOZ)
呋喃妥因
(NFT)
1-氨基-2-内酰脲
(AHD)
2021年,国家产地水产品兽药残留监测合格率为99.9%,仍有硝基呋喃代谢物的检出。在水产品流入市场前,应开发性能良好的检测方法,用于检测其在动物体内的残留程度。
3.1 液相色谱法
硝基呋喃代谢物可在动物体内稳定存在,通常作为判断是否滥用药物的标志[106]。步骤如下:通过酸水解和衍生化试剂衍生释放硝基呋喃的完整侧链,提取、浓缩、净化,在电喷雾正离子源多反应监测模式下测定。如表6所示,常用的衍生剂包括2-萘甲醛[107]、2-羟基-1-萘甲醛[108]、4-苯甲醛[109]、2-硝基苯甲醛[110]和4-氯甲酸苄酯[111]。
表 6 硝基呋喃及其代谢物残留的检测方法Table 6. Methods for the determination of nitrofurans and their metabolites基质及参考文献 目标物 预处理 检测技术 检出限(μg/kg) 淡水虾、凡纳滨对虾和
斑节对虾[107]AHD、AOZ、SEM、AMOZ 0.2 mol/L HCl调节pH、2-萘甲醛衍生、乙酸乙酯萃取、氮气流蒸发、在乙腈-水中溶解、己烷除杂 LC-DAD;
ChromSpher 5 C18
(250 mm×4.6 mm,5 μm)AHD:0.2039
AOZ:0.1610
SEM:0.2032
AMOZ:0.2697对虾[108] AHD、AOZ、SEM、AMOZ 酸水解、2-羟基-1-萘甲醛衍生、磷酸二氢钠溶液、乙酸乙酯萃取 HPLC-FLD;YMC-Pack Polymer C18(250 mm×4.6 mm,6 μm) AHD:0.26
AOZ:0.20
SEM:0.23
AMOZ:0.24对虾[109] AHD、AOZ、SEM、AMOZ 生理盐水-乙酸乙酯均质、混合酸溶液水解、微波辅助衍生(4-苯甲醛) HPLC-FLD;
Eclipse XDB-C18AHD:0.44
AOZ:0.52
SEM:0.40
AMOZ:0.56虾[110] AHD、AOZ、SEM、AMOZ 0.2 mol/L HCl水解、2-硝基苯甲醛衍生、乙酸乙酯萃取 LC-IDMS/MS
Symmetry C18
(150 mm×2.1 mm,3.5 μm)CCα及CCβ:
AHD:0.16、0.16
AOZ:0.08、0.13
SEM:0.36、0.85
AMOZ:0.20、0.29草鱼、南美白对虾[114] AMOZ 酸水解、2-NBA衍生、过夜孵育、乙酸乙酯提取、己烷除脂、PBS稀释 ELISA
0.01对虾[115] AOZ 2-NBA衍生、乙酸乙酯提取、己烷洗涤 ELISA 0.1 鱼、虾[116] AHD 盐酸水解、2-NBA衍生、乙酸乙酯提取、己烷除杂 ELISA
0.12~0.13鱼、虾[117] SEM 加入水、盐酸和衍生化试剂;孵育;磷酸氢二钠和氢氧化钠调节pH;乙酸乙酯提取、正己烷除杂 ic-ELISA 0.03 鱼[118] AHD、AOZ、SEM、AMOZ 搅拌机均质、加入盐酸和4-NBA进行水解和衍生;调节pH;乙酸乙酯提取、正己烷除杂 ICT(免疫层析) AHD:0.75
AOZ:0.5
SEM:0.75
AMOZ:0.75鱼[119] AHD、AOZ、SEM、AMOZ 盐酸水解、2-NBA衍生、加入磷酸氢二钠和氢氧化钠、乙酸乙酯提取、己烷-PBS溶解衍生化合物 M-EICA
(多重免疫色谱分析)
AHD:0.2
AOZ:0.1
SEM:0.15
AMOZ:0.25小龙虾[124] NFZ 乙腈-水提取、超声30 min、于pH6.0的
柠檬酸缓冲溶液中测定功能化多酸-石墨烯修饰电极[Ru-PMo12/PDDA-GO]3
循环伏安法、计时安培法0.08952 μmol/L 虾、螃蟹、鳗鱼肌肉[129] AOZ 加水和盐酸水解、磷酸氢二钠调节pH、
乙酸乙酯提取、甲醇-PBS溶解残留物共价固定抗AOZ抗体-自组装单层修饰金电极;
循环伏安法、电化学阻抗谱20.0 虾[130] AMOZ 同上 共价固定抗AMOZ抗体-自组装单层修饰金电极;
循环伏安法、电化学阻抗谱1.0 虾、螃蟹[131] AHD 同上 共价固定抗AHD抗体-玻碳电极;电化学阻抗谱 2.0 虾[132] SEM 盐酸水解、磷酸氢二钾调节pH、
乙酸乙酯提取、正己烷萃取分子印迹-羧基化单壁碳纳米管-壳聚糖-玻碳电极
(MIP/SWNTs-COOH/CS);
循环伏安法、差分脉冲伏安0.025 鱼类[133] FTD 三氯乙酸分散鱼肉、超声30 min、过滤、
乙醇稀释氧化锌-氧化锌钴双金属纳米异质结构-玻碳电极
ZnO-ZnCo2O4NH/GCE;
循环伏安法、电化学阻抗谱、微分脉冲伏安法1.46、34.1 早期常使用液相色谱法和光电二极管阵列(DAD)或荧光检测器(FLD)分析硝基呋喃类药物。但因吸收波长的限制,可能不足以同时识别所有分析物。近年来,常利用稳定同位素标记代谢物,以此作为内标物,联用液相色谱同位素稀释与串联质谱(LC-IDMS/MS),以减小基质效应、提高分析结果的可靠性。2019年,Oye等[112]以AOZ-D4、AMOZ-D5、AHD-13C3及SEM-15N213C为内标,利用盐酸水解、2-硝基苯甲醛衍生,将该法用于海鲜中硝基呋喃代谢物的识别与检测,并首次报告了AHD的非组织结合假阳性,效果优异。
3.2 免疫法
为了寻找一种便携式和高通量筛选方法,免疫学法已被广泛关注。硝基呋喃类代谢物为小分子化合物,无法产生特异性抗体、引发免疫反应。基于ELISA进行分析时,通常在酸性条件下释放与蛋白质结合的代谢物,利用2-硝基苯甲醛衍生化,以增加分子量,制备免疫半抗原[113]。将其与载体蛋白偶联产生特异性抗体[114],再基于免疫法检测衍生物浓度,最后转换为硝基呋喃代谢物浓度。Cooper等[115]首次利用ELISA检测对虾组织中AOZ残留。Jiang等[116]、Shen等[114]、徐冬梅等[117]开发了测定水产品中AMOZ、AHD、SEM的间接竞争ELISA,检出限均小于0.3 μg/kg(表6)。
与液相色谱法相比,ELISA成本低、前处理步骤简单,但灵敏度和准确度不够好,且无法同时测定四种主要的硝基呋喃代谢物。基于以上局限性,其它方法应运而生。2017年,Wang等[118]开发了一基于抗体的复合条带,允许同时检测鱼类样品中四种硝基呋喃代谢物。反应迅速,15 min内可得到结果。2019年,Dong等[119]建立了复合铕纳米颗粒免疫层析法,可在10 min内完成4种化合物的高效检测。说明免疫学方法与新型纳米材料相结合,可拓展方法应用领域。此外,还出现了基于分子印迹技术的仿生ELISA法[120],该法克服了传统ELISA需要免疫动物、周期长、不能在室温下储存,且获得的生物抗体不稳定的局限性。
3.3 电化学传感器
作为一种重现性好、响应速度快的方法,该法在分析水产品违禁鱼药方面拥有巨大潜力,得到了越来越多关注。近年来,已有将电化学传感器应用于检测水产品中硝基呋喃类残留的报道。具体情况如表6所示。多金属氧酸盐是一类由前过渡金属通过氧连接而形成的纳米级无机金属-氧簇化合物[121-122],接受电子能力强、具有优异的氧化还原性质[123]。2019年,Cai等[124]首次将功能化的多金属氧酸盐和石墨烯修饰电极相结合,实现了呋喃西林的灵敏检测,并运用于小龙虾样品中。
已有众多学者合成新型纳米材料,如硼掺杂碳氮化物包裹三维花状氧化镍、六方纳米板状磷酸锆、羧基多壁碳纳米管、纳米金/石墨烯,分别用于呋喃妥因[125]、呋喃唑酮[126]、呋喃西林及其代谢物[127-128]的电化学检测中。说明今后应多多关注具有优异电子转移能力和导电性的新型材料,从样品基质出发,扩大传感器平台的应用范围。如何通过改进修饰电极的协同效应来提高电化学传感器的灵敏度和分析性能,也将成为今后研究的重点。
4. 结语
水产养殖是一个快速增长的行业,违禁鱼药的不当使用可能会影响水产品出口和人类健康。开发可有效识别、分离和量化低浓度水平鱼药的分析方法十分必要。水产品中鱼药残留分析包括样品前处理及检测两部分。传统的预处理步骤繁琐、费时,有机溶剂消耗量大。现已发展出一些替代程序,如QuEChERS、免疫亲和柱、分子印迹和磁性固相萃取等,朝着环保、省时、高效的方向发展。在检测方面:仪器分析法是首选手段,可提供高灵敏度和特异性,并实现几种药物的同时检测。但受到仪器昂贵、前处理繁杂、劳动力密集等限制。孔雀石绿和结晶紫、氯霉素及硝基呋喃类药物均为小分子化合物,为了节省成本、提高检测效率,可基于竞争性抑制原理,通过免疫法进行大批量初筛,再利用色谱-质谱联用技术进行确证,避免假阳性现象的出现。相较其他方法来说,电化学传感器制备简单、不需要大型仪器、抗干扰能力强。但在结晶紫、氯霉素和硝基呋喃类药物的残留分析方面仍处于起步阶段,今后,应不断优化电极材料,提高方法的灵敏度,开发成本低、可重复利用的修饰电极,拓宽该法在违禁药物检测领域应用的可能性。
未来,应不断简化样品前处理步骤、清除杂质的干扰,深入了解新型多孔纳米材料的结构和性能(如量子点、碳材料、多金属氧酸盐及金属纳米粒子),发挥其在样品富集、表面增强拉曼光谱集成、修饰电极、电化学催化方面的协同作用。继续探寻廉价、简便、高效的检测技术,以满足不同层次的检测需求,保障水产品安全。
-
表 1 测定水产品中孔雀石绿和结晶紫的色谱方法
Table 1 Chromatographic methods for the determination of malachite green and crystal violet in aquatic products
分析物 检测器 基质 样品前处理 色谱柱 检出限
(μg/kg)MG、LMG、CV、LCV[14] 二极管阵列
检测器鳕鱼 QuEChERS
a. NaCl和MgSO4盐析;
b. d-SPE固相萃取净化XCharge C18 MG:3.2
LMG:24.1
CV:1.9
LCV:23.4MG、CV[16] 串联质谱 养殖鱼、河鱼和海鱼 微波辅助萃取、分散-液液微萃取结合固相纯化 BEH Phenyl柱
(50 mm×2.1 mm,1.7 μm)MG:4.54×10−6
CV:6.02×10−6MG、CV[17] 二极管阵列、荧光检测器、三重四级杆质谱仪 斑点叉尾鱼 McIlvaine-乙腈缓冲溶液提取、SPE筒吸附净化 a. ODS-3 C18
(150 mm×4.6 mm,3 μm)
b. Oasis MCX SPE
(150 mg/6 mL)MG:0.38
LMG:0.26
CV:0.26
LCV:0.09MG、CV、RB、BG、VPBO[18] 紫外-可见
检测器鲤鱼、鲶鱼、草鱼 乙腈提取、石油醚脱脂、磁性多孔有机笼富集、吸附 TC-C18
(250 mm×4.6 mm)0.67~8.00 MG、LMG、CV、LCV[19] 二极管阵列 草鱼、虾、甲壳类 乙腈-醋酸铵提取、分子印迹固相萃取 Symmetry C18
(150 mm×4.6 mm,5 μm)MG:0.13~0.14
LMG:0.12~0.14
CV:0.11~0.13
LCV:0.12~0.14MG、LMG、CV、LCV[20] 紫外、荧光
检测器鱼肌肉 乙腈-醋酸铵提取、二氯甲烷、免疫亲和柱萃取净化 Cloversil-C18
(250 mm×4.6 mm,5 μm)MG:0.15
LMG:0.18
CV:0.10
LCV:0.14MG、LMG、CV、LCV[21] 三重四级杆
质谱仪草鱼、罗非鱼、鲢鱼 乙腈提取、冷冻除脂、快速滤过型净化柱净化 BEH C18
(50 mm×2.1 mm,1.7 μm)均为0.05 MG、LMG、CV、LCV[27] 三重四级杆
质谱仪虹鳟鱼 乙腈(含1%乙酸)提取 Inertsil ODS-4 C18
(50 mm×2.1 mm,3 μm)MG:0.43
LMG:0.24
CV:0.33
LCV:0.28MG、LMG、CV[28] 高分辨率飞行质谱仪 虾、鲑鱼、鲭鱼、
小龙虾乙腈-水提取、无需纯化 AcclaimTM 120 C18
(150 mm×2.1 mm,2.2 μm)MG:0.01~0.02
LMG:0.10~0.30
CV:0.01表 2 孔雀石绿和结晶紫检测中几种SERS基底的检测限
Table 2 Detection limits of some SERS substrates for malachite green and crystal violet detection
SERS基底 检出限 优点 参考文献 银纳米线膜 CV:5.1×10−11 mol·L−1
MG:0.00693 ng易变形、可用于现场检测 [31] 一次性Au/Ag纸基 MG:4.3×10−9 mol·L−1
CV:8.1×10−8 mol·L−1基底制备简单、重现性和稳定性好; [33] 二氧化钛-银-氧化石墨烯
(TiO2-Ag-GO)MG:10−7 mol·L−1
CV:10−9 mol·L−1可同时检测多种化合物;
氧化石墨烯和等离子银的修饰可提高基底检测灵敏度、
重复性、稳定性[34] AgNPs/Cu复合材料 MG:0.94×10−7 mol·L−1
CV:1.14×10−9 mol·L−1检测限低、信号分布均匀、稳定性好;
操作简单、可用于实际样品的检测[35] 铜板-氧化石墨烯修饰银纳米粒子(GO/AgNPs/Cu) MG:10−8 mol·L−1
CV:10−8 mol·L−1再现性好、线性浓度宽;
快速、准确、可用于现场测定;
AgNPs的粗糙表面提供了更多热点[36] 金纳米棒-单宁酸薄膜涂层(GNR/TA) MG:0.07 μg·L−1
CV:0.03 μg·L−1低成本、一次性现场应用;
重复性好、高性能的原位检测[37] 表 3 基于免疫学法对孔雀石绿和结晶紫的检测
Table 3 Detection of malachite green and crystal violet based on immunological method
表 4 水产品中氯霉素的不同检测方法的比较
Table 4 Comparison of detection methods for detection of chloramphenicols in aquatic products
基质及参考文献 方法 前处理 检测限(μg/kg) 线性范围或IC50(μg/kg) 虾、鳗鱼和比目鱼[76] LC-MS/MS 改进QuEChERS
(使用伯仲胺和MgSO4纯化样品)LOD:0.005~3.100
LOQ:0.02~10.4− 虾[77] LC-ESI-MS/MS 乙酸乙酯提取、液-液萃取 CCα:0.06
CCβ:0.100.1~2.0 尼罗鱼、对虾[78] LC-MS/MS 乙腈-氯仿提取、液-液萃取 CCα:0.04
CCβ:0.060.1~1.0 对虾[79] LC-MS/MS McIlvaine提取、免疫亲和柱净化 LOD:0.05 − 鱼肉[80] HPLC-MS/MS 乙酸乙酯提取、石墨烯固相吸附 LOD:0.036 0.5~100 虾[90] BSAS-direct Hap coated ELISA 乙酸乙酯提取、异辛烷-氯仿溶解 LOD:0.2 IC50=10.5 鲈鱼、金枪鱼[91] ELISA 缓冲液提取、正己烷脱脂 CCα:0.025
CCβ:0.25− 虾[92] ELISA 研磨均质、加乙酸乙酯、氨水和无水硫酸钠后离心 LOD:0.06 IC50=0.46 鲫鱼[94] GICA 甲醇、PBS缓冲液均质、滤纸过滤 LOD:0.5 − 虾[96] TRFIA 丙酮/二氯甲烷(1:1)提取、氮气蒸发 LOD:0.008 0.008~100 虾[99] ic-CLEIA 乙酸乙酯均质、正己烷溶解 LOD:0.01 0.03~23.70 鲢鱼[100] LFA − LOD:0.36 IC50=12.94 注:1.BSAS-direct Hap coated ELISA即生物素-链霉亲和素系统直接包被酶联免疫吸附法;2.“−”代表原文中无相关数据。 表 5 四种硝基呋喃及其代谢物的结构
Table 5 Structures of four kinds of nitrofurans and their metabolites
母体药物 结构式 代谢物 结构式 呋喃它酮
(FTD)
5-吗啉甲基-3-氨基-2-唑烷基酮
(AMOZ)
呋喃西林
(NFZ)
氨基脲
(SEM)
呋喃唑酮
(FZD)
3-氨基-2-噁唑烷酮
(AOZ)
呋喃妥因
(NFT)
1-氨基-2-内酰脲
(AHD)
表 6 硝基呋喃及其代谢物残留的检测方法
Table 6 Methods for the determination of nitrofurans and their metabolites
基质及参考文献 目标物 预处理 检测技术 检出限(μg/kg) 淡水虾、凡纳滨对虾和
斑节对虾[107]AHD、AOZ、SEM、AMOZ 0.2 mol/L HCl调节pH、2-萘甲醛衍生、乙酸乙酯萃取、氮气流蒸发、在乙腈-水中溶解、己烷除杂 LC-DAD;
ChromSpher 5 C18
(250 mm×4.6 mm,5 μm)AHD:0.2039
AOZ:0.1610
SEM:0.2032
AMOZ:0.2697对虾[108] AHD、AOZ、SEM、AMOZ 酸水解、2-羟基-1-萘甲醛衍生、磷酸二氢钠溶液、乙酸乙酯萃取 HPLC-FLD;YMC-Pack Polymer C18(250 mm×4.6 mm,6 μm) AHD:0.26
AOZ:0.20
SEM:0.23
AMOZ:0.24对虾[109] AHD、AOZ、SEM、AMOZ 生理盐水-乙酸乙酯均质、混合酸溶液水解、微波辅助衍生(4-苯甲醛) HPLC-FLD;
Eclipse XDB-C18AHD:0.44
AOZ:0.52
SEM:0.40
AMOZ:0.56虾[110] AHD、AOZ、SEM、AMOZ 0.2 mol/L HCl水解、2-硝基苯甲醛衍生、乙酸乙酯萃取 LC-IDMS/MS
Symmetry C18
(150 mm×2.1 mm,3.5 μm)CCα及CCβ:
AHD:0.16、0.16
AOZ:0.08、0.13
SEM:0.36、0.85
AMOZ:0.20、0.29草鱼、南美白对虾[114] AMOZ 酸水解、2-NBA衍生、过夜孵育、乙酸乙酯提取、己烷除脂、PBS稀释 ELISA
0.01对虾[115] AOZ 2-NBA衍生、乙酸乙酯提取、己烷洗涤 ELISA 0.1 鱼、虾[116] AHD 盐酸水解、2-NBA衍生、乙酸乙酯提取、己烷除杂 ELISA
0.12~0.13鱼、虾[117] SEM 加入水、盐酸和衍生化试剂;孵育;磷酸氢二钠和氢氧化钠调节pH;乙酸乙酯提取、正己烷除杂 ic-ELISA 0.03 鱼[118] AHD、AOZ、SEM、AMOZ 搅拌机均质、加入盐酸和4-NBA进行水解和衍生;调节pH;乙酸乙酯提取、正己烷除杂 ICT(免疫层析) AHD:0.75
AOZ:0.5
SEM:0.75
AMOZ:0.75鱼[119] AHD、AOZ、SEM、AMOZ 盐酸水解、2-NBA衍生、加入磷酸氢二钠和氢氧化钠、乙酸乙酯提取、己烷-PBS溶解衍生化合物 M-EICA
(多重免疫色谱分析)
AHD:0.2
AOZ:0.1
SEM:0.15
AMOZ:0.25小龙虾[124] NFZ 乙腈-水提取、超声30 min、于pH6.0的
柠檬酸缓冲溶液中测定功能化多酸-石墨烯修饰电极[Ru-PMo12/PDDA-GO]3
循环伏安法、计时安培法0.08952 μmol/L 虾、螃蟹、鳗鱼肌肉[129] AOZ 加水和盐酸水解、磷酸氢二钠调节pH、
乙酸乙酯提取、甲醇-PBS溶解残留物共价固定抗AOZ抗体-自组装单层修饰金电极;
循环伏安法、电化学阻抗谱20.0 虾[130] AMOZ 同上 共价固定抗AMOZ抗体-自组装单层修饰金电极;
循环伏安法、电化学阻抗谱1.0 虾、螃蟹[131] AHD 同上 共价固定抗AHD抗体-玻碳电极;电化学阻抗谱 2.0 虾[132] SEM 盐酸水解、磷酸氢二钾调节pH、
乙酸乙酯提取、正己烷萃取分子印迹-羧基化单壁碳纳米管-壳聚糖-玻碳电极
(MIP/SWNTs-COOH/CS);
循环伏安法、差分脉冲伏安0.025 鱼类[133] FTD 三氯乙酸分散鱼肉、超声30 min、过滤、
乙醇稀释氧化锌-氧化锌钴双金属纳米异质结构-玻碳电极
ZnO-ZnCo2O4NH/GCE;
循环伏安法、电化学阻抗谱、微分脉冲伏安法1.46、34.1 -
[1] WANG X Y, CHEN Y L, XIAO X H, et al. Recent advances in sample preparation technologies for analysis of harmful substances in aquatic products[J]. Chromatography,2021,39(1):34−45.
[2] REVERTER M, BONTEMPS N, LECCHINI D, et al. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives[J]. Aquaculture,2014,433:50−61. doi: 10.1016/j.aquaculture.2014.05.048
[3] CHEN J M, SUN R X, PAN C G, et al. Antibiotics and food safety in aquaculture[J]. Journal of Agricultural and Food Chemistry,2020,68(43):11908−11919. doi: 10.1021/acs.jafc.0c03996
[4] ZHANG Y, HU Y, DENG R J, et al. Engineering multivalence aptamer probes for amplified and label-free detection of antibiotics in aquatic products[J]. Journal of Agricultural and Food Chemistry,2020,68(8):2554−2561. doi: 10.1021/acs.jafc.0c00141
[5] SRIVASTAVA S, SINHA R, ROY D. Toxicological effects of malachite green[J]. Aquatic Toxicology,2004,66(3):319−329. doi: 10.1016/j.aquatox.2003.09.008
[6] ZHAO J, WEI D Q, YANG Y L. Magnetic solid-phase extraction for determination of the total malachite green, gentian violet and leucomalachite green, leucogentian violet in aquaculture water by high-performance liquid chromatography with fluorescence detection[J]. Journal of Separation Science,2016,39(12):2347−2355.
[7] CULP S J, MELICK P W, TROTTER R W, et al. Carcinogenicity of malachite green chloride and leucomalachite green in B6C3F1 mice and F344 rats[J]. Food and Chemical Toxicology,2006,44(8):1204−1212. doi: 10.1016/j.fct.2006.01.016
[8] 食品动物禁用的兽药及其它化合物清单[J]. 中国兽药杂志, 2012, 46(S1): 48 List of veterinary drugs and other compounds prohibited for food animals[J]. China Journal of Veterinary Medicine, 2012, 46(S1): 48.
[9] 王建平, 吴岚, 司芳, 等. 对欧盟拟修订REACH法规附件XVII的解读[J]. 印染,2016,42(20):46−51, 59. [WANG J P, WU L, SI F, et al. An interpretation of the proposed amendments to annex XVII of the REACH regulations[J]. Dyeing and Finishing,2016,42(20):46−51, 59. [10] 高海岗. 孔雀石绿纳米抗体的制备及其免疫层析检测方法的建立与应用[D]. 扬州: 扬州大学, 2021 GAO H G. Preparation of malachite green nanobody and establishment and application of immuno-chromatography detection method[D]. Yangzhou: Yangzhou University, 2021.
[11] 国家标准化管理委员会. GB/T 19857-2005 水产品中孔雀石绿和结晶紫残留量的测定[S]. 北京: 中国标准出版社, 2005: 1−6 Standardization Administration. GB/T 19857-2005 Determination of malachite green and crystal violet residues in aquatic product[S]. Beijing: Standards Press of China, 2005: 1−6.
[12] 国家标准化管理委员会. GB/T 20361-2006 水产品中孔雀石绿和结晶紫残留量的测定 高效液相色谱荧光检测法[S]. 北京: 中国标准出版社, 2006: 1−3 Standardization Administration. GB/T 20361-2006 Determination of malachite green and crystalline violet residues in aquatic products high performance liquid chromatography fluorescence detection method[S]. Beijing: Standards Press of China, 2006: 1−3.
[13] 农业农村部. 2021年国家产地水产品兽药残留监测抽检合格率为99.9%[J]. 水产科技情报,2022,49(1):58. [Ministry of Agriculture and Rural Affairs of the People’s Republic of China. The sampling rate of veterinary drug residue monitoring in aquatic products of orgin in 2021 is 99.9%[J]. Fisheries Science and Technology Information,2022,49(1):58. [14] ZHU C Y, WEI J, DONG X F, et al. Fast analysis of malachite green, leucomalachite green, crystal violet and leucocrystal violet in fish tissue based on a modified QuEChERS procedure[J]. Chinese Journal of Chromatography,2014,32(4):419−425. doi: 10.3724/SP.J.1123.2014.01016
[15] NEBOT C, LGLESIAS A, BARREIRO R, et al. A simple and rapid method for the identification and quantification of malachite green and its metabolite in hake by HPLC-MS/MS[J]. Food Control,2013,31(1):102−107. doi: 10.1016/j.foodcont.2012.09.020
[16] HUANG P T, ZHAO P, DAI X P, et al. Trace determination of antibacterial pharmaceuticals in fishes by microwave-assisted extraction and solid-phase purification combined with dispersive liquid-liquid microextraction followed by ultra-high performance liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences,2016,1011:136−144. doi: 10.1016/j.jchromb.2015.12.059
[17] CHEN G Y, MIAO S. HPLC determination and MS confirmation of malachite green, gentian violet, and their leuco metabolite residues in channel catfish muscle[J]. Journal of Agricultural and Food Chemistry,2010,58(12):7109−7114. doi: 10.1021/jf9043925
[18] LIU J Y, ZHAO Q Y, CAO W Q, et al. Simple synthesis of magnetic porous organic cages for adsorption of triphenylmethane dyes in aquatic products[J]. Microchemical Journal,2020,158:105275. doi: 10.1016/j.microc.2020.105275
[19] LONG C Y, MAI Z B, YANG Y F, et al. Determination of multi-residue for malachite green, gentian violet and their metabolites in aquatic products by high-performance liquid chromatography coupled with molecularly imprinted solid-phase extraction[J]. Journal of Chromatography A,2009,1216(12):2275−2281. doi: 10.1016/j.chroma.2009.01.047
[20] XIE J, PENG T, CHEN D D, et al. Determination of malachite green, crystal violet and their leuco-metabolites in fish by HPLC-VIS detection after immunoaffinity column clean-up[J]. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences,2013,913−914:123−128. doi: 10.1016/j.jchromb.2012.12.002
[21] 高晓敏, 王琚钢, 马智玲, 等. 快速滤过型净化法结合超高效液相色谱-串联质谱同时检测水产品中孔雀石绿和结晶紫残留量[J]. 食品与发酵工业,2021,47(24):249−255. [GAO X M, WANG J G, MA Z L, et al. Simultaneous determination of malachite green and crystal violet residues in aquatic products by multi-plug filtration cleanup method combined with ultra-performance liquid chromatography-tandem mass spectrometry[J]. Food and Fermentation Industries,2021,47(24):249−255. doi: 10.13995/j.cnki.11-1802/ts.027068 [22] VALLE L, DIAZ C, ZANOCCO A L, et al. Determination of the sum of malachite green and leucomalachite green in salmon muscle by liquid chromatography-atmospheric pressure chemical ionisation-mass spectrometry[J]. Journal of Chromatography A,2005,1067(1-2):101−105. doi: 10.1016/j.chroma.2004.10.049
[23] LEE J B, KIM H Y, JANG Y M, et al. Determination of malachite green and crystal violet in processed fish products[J]. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure and Risk Assessment,2010,27(7):953−961.
[24] 杨贤庆, 孙满义, 李来好, 等. 水产品中孔雀石绿高效液相荧光检测法的改良研究[J]. 食品科学,2008(8):526−529. [YANG X Q, SUN M Y, LI L H, et al. Improvement on determination of malachite green in fishery products by HPLC with fluorescence detector[J]. Food Science,2008(8):526−529. doi: 10.3321/j.issn:1002-6630.2008.08.125 [25] 吴蓓琦, 刘畅, 李萍, 等. 水产品中孔雀石绿残留检测方法的优化[J]. 江苏农业科学,2016,44(7):333−335. [WU P Q, LIU C, LI P, et al. Optimization of a method for the determination of malachite green residues in aquatic products[J]. Jiangsu Agricultural Sciences,2016,44(7):333−335. doi: 10.15889/j.issn.1002-1302.2016.07.098 [26] MITROWSKA K, POSYNIAK A, ZMUDZKI J. Determination of malachite green and leucomalachite green in carp muscle by liquid chromatography with visible and fluorescence detection[J]. Journal of Chromatography A,2005,1089(1-2):187−192. doi: 10.1016/j.chroma.2005.07.004
[27] KAPLAN M, OLGUN E O, KARAOGLU O. A rapid and simple method for simultaneous determination of triphenylmethane dye residues in rainbow trouts by liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography A,2014,1349:37−43. doi: 10.1016/j.chroma.2014.04.091
[28] AMELIN V G, KOROTKOV A I, ANDORALOV A M. Simultaneous determination of dyes of different classes in aquaculture products and spice using HPLC-high-resolution quadrupole time-of-flight mass spectrometry[J]. Journal of Analytical Chemistry,2017,72(2):183−190. doi: 10.1134/S1061934817020034
[29] CRAIG A P, FRANCA A S, IRUDAYARAJ J. Surface-enhanced raman spectroscopy applied to food safety[J]. Annual Review of Food Science and Technology,2013,4:369−380. doi: 10.1146/annurev-food-022811-101227
[30] 赵瑞. 表面增强拉曼光谱技术在兽药检测方面的研究与应用[D]. 长春: 长春师范大学, 2021 ZHAO R. Research and application of surface-enhanced raman spectroscopy in veterinary drug detection[D]. Changchun: Changchun Normal University, 2021.
[31] ZHANG R, LAI Y C, ZHAN J H. Enhancing the activity of silver nanowire membranes by electrochemical cyclic voltammetry as highly sensitive flexible SERS substrate for on-site analysis[J]. Nanomaterials,2021,11(3):672. doi: 10.3390/nano11030672
[32] YANG N, YOU T T, GAO Y K, et al. Fabrication of a flexible gold nanorod polymer metafilm via a phase transfer method as a SERS substrate for detecting food contaminants[J]. Journal of Agricultural and Food Chemistry,2018,66(26):6889−6896. doi: 10.1021/acs.jafc.8b01702
[33] YANG G H, FANG X J, JIA Q, et al. Fabrication of paper-based SERS substrates by spraying silver and gold nanoparticles for SERS determination of malachite green, methylene blue, and crystal violet in fish[J]. Microchimica Acta,2020,187(5):310. doi: 10.1007/s00604-020-04262-2
[34] ZHANG M F, SUN H R, CHEN X, et al. The influences of graphene oxide (GO) and plasmonic Ag nanoparticles modification on the SERS sensing performance of TiO2 nanosheet arrays[J]. Journal of Alloys and Compounds,2021,864:158189. doi: 10.1016/j.jallcom.2020.158189
[35] ZHANG M F, YANG J, WANG Y R, et al. Plasmon-coupled 3D porous hotspot architecture for super-sensitive quantitative SERS sensing of toxic substances on real sample surfaces[J]. Physical Chemistry Chemical Physics,2019,21(35):19288−19297. doi: 10.1039/C9CP03058A
[36] ZHANG M F, CHEN Z X, WANG Z E, et al. Graphene oxide coated popcorn-like Ag nanoparticles for reliable sensitive surface-enhanced raman scattering detection of drug residues[J]. Journal of Materials Research,2019,34(17):2935−2943. doi: 10.1557/jmr.2019.78
[37] KIM S, JOO J H, KIM W, et al. A facile, portable surface-enhanced raman spectroscopy sensing platform for on-site chemometrics of toxic chemicals[J]. Sensors and Actuators B-Chemical,2021,343:130102. doi: 10.1016/j.snb.2021.130102
[38] JI H X, XIA C X, XU J, et al. A highly sensitive immunoassay of pesticide and veterinary drug residues in food by tandem conjugation of bi-functional mesoporous silica nanospheres[J]. Analyst,2020,145(6):2226−2232. doi: 10.1039/C9AN02430A
[39] SHEN Y D, DENG X F, SUN Y M. Simultaneous determination of malachite green, brilliant green and crystal violet in grass carp tissues by a broad-specificity indirect competitive enzyme-linked immunosorbent assay[J]. Analytica Chimica Acta,2011,707(1):148−154.
[40] XU H Y, CHEN X L, GUO L, et al. Monoclonal antibody-based enzyme-linked immunosorbent assay for detection of total malachite green and crystal violet residues in fishery products[J]. International Journal of Environmental Analytical Chemistry,2013,93(9):959−969. doi: 10.1080/03067319.2012.672982
[41] CHEN Y Y, DING M L, LI J Q, et al. Fluorescence quenching immunoaffinity test column with quantum dots as fluorescence donors for the quick detection of malachite green and crystal violet in aquatic products[J]. Food Analytical Methods,2018,11(12):3362−3370. doi: 10.1007/s12161-018-1312-0
[42] 陈义元. 孔雀石绿和结晶紫的免疫检测新方法研究[D]. 天津: 天津科技大学, 2018 CHEN Y Y. New immunoassay method for malachite green and crystal violet[D]. Tianjin: Tianjin University of Science and Technology, 2018.
[43] 张爽. 基于纳米复合材料的电化学传感器的构建及应用[D]. 烟台: 烟台大学, 2019 ZHANG S. Construction and application of electronchemical sensors based on nanocomposites[D]. Yantai: Yantai University, 2019.
[44] CESEWSKI E, JOHNSON B N. Electrochemical biosensors for pathogen detection[J]. Biosensors and Bioelectronics,2020,159:112214. doi: 10.1016/j.bios.2020.112214
[45] CAI S X, CHEN X W, LIU J, et al. Highly efficient detection of tricaine methanesulfonate based on the nanoporous gold electrochemical sensor[J]. Materials Letters,2021,301:130286. doi: 10.1016/j.matlet.2021.130286
[46] LIU Z Y, QI W J, XU G B. Recent advances in electrochemiluminescence[J]. Chemistry Society Reviews,2015,44(10):3117−3142. doi: 10.1039/C5CS00086F
[47] WANG S, HAO T T, GUO Z Y. Development of electrochemiluminescent inhibition method for determination of gentian violet in aquatic water[J]. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy,2012,89:25−29. doi: 10.1016/j.saa.2011.12.012
[48] HUANG B M, ZHOU X B, LU X Q. Quenching of the electrochemiluminescence of Ru(bpy)32+/TPA by malachite green and crystal violet[J]. Talanta,2013,106:174−180. doi: 10.1016/j.talanta.2012.12.025
[49] LIU F Y, YANG X, ZHAO Y Q, et al. Detection of malachite green and leucomalachite green based on electrochemiluminescence of mono- and bimetallic ruthenium tris-bipyridyl complexes at an Au electrode[J]. Analytical Methods,2013,5(3):660−665. doi: 10.1039/C2AY26010G
[50] SHAO J T, ZHAO Y M, LIU F Y, et al. Determination of malachite green and leucomalachite green based on electrochemiluminescence of Ru(bpy)32+ at graphene oxide modified glassy carbon electrodes[J]. Rsc Advances,2015,5(19):14547−14552. doi: 10.1039/C4RA09915J
[51] CHEN X H, SHAN X L, LAN Q F, et al. Electrochemiluminescence quenching sensor of a carboxylic carbon nanotubes modified glassy carbon electrode for detecting crystal violet based on nitrogen-doped graphene quantum dots@peroxydisulfate system[J]. Analytical Sciences,2019,35(8):929−934. doi: 10.2116/analsci.19P090
[52] 谷孝磊. 海洋渔业环境中几种禁限用渔药的电化学检测研究[D]. 青岛: 青岛科技大学, 2014 GU X L. Study on the electrochemical detection of several banned and restricted fishery drugs in marine fishery environment[D]. Qingdao: Qingdao University of Science and Technology, 2014.
[53] 刘文文. 基于导电碳黑糊电极的电化学传感器对渔业环境中残留药物的检测应用[D]. 青岛: 中国海洋大学, 2015 LIU W W. Application of electrochemical sensors based on conductive carbon black paste electrode for residual drugs in fishery environment[D]. Qingdao: Ocean University of China, 2015.
[54] XU Y X, LI Q, XUE H G, et al. Metal-organic frameworks for direct electrochemical applications[J]. Coordination Chemistry Reviews,2018,376:292−318. doi: 10.1016/j.ccr.2018.08.010
[55] LI C, WU K. Cu-BTC frameworks based electrochemical sensor for hazardous malachite green in aquaculture[J]. Analytica Chimica Acta,2021,1162:338473. doi: 10.1016/j.aca.2021.338473
[56] ZHOU Y, LI X, PAN Z, et al. Determination of malachite green in fish by a modified MOF-based electrochemical sensor[J]. Food Analytical Methods,2019,12(5):1246−1254. doi: 10.1007/s12161-019-01459-x
[57] DENG P H, FENG J X, WEI Y P, et al. Fast and ultrasensitive trace malachite green detection in aquaculture and fisheries by using hexadecylpyridinium bromide modified electrochemical sensor[J]. Journal of Food Composition and Analysis,2021,102:104003. doi: 10.1016/j.jfca.2021.104003
[58] 邵可满, 傅桂瑜, 陈素艳, 等. 稀土配合物分子印迹荧光探针的制备及检测孔雀石绿的残留[J]. 光谱学与光谱分析,2022,42(3):808−813. [SHAO K M, FU G Y, CHEN S Y, et al. Preparation of molecularly imprinted fluorescent probe for rare earth complex and determination of malachite green residue[J]. Spectroscopy and Spectral Analysis,2022,42(3):808−813. doi: 10.3964/j.issn.1000-0593(2022)03-0808-06 [59] 陈宏, 靖玉, 孙思阳, 等. 分子印迹电化学传感器检测水产品中孔雀石绿[J]. 化学研究与应用,2020,32(2):208−213. [CHEN H, JING Y, SUN S Y, et al. Determination of malachite green in aquatic products by electrochemical sensor based on molecularly imprinted polymer[J]. Chemical Research and Application,2020,32(2):208−213. doi: 10.3969/j.issn.1004-1656.2020.02.006 [60] WEI S L, WU J Y, HUANG X J, et al. Preparation and application of malachite green electrochemical sensor based on molecularly imprinted polymer[J]. Chinese Journal of Analytical Chemistry,2020,48(1):145−152.
[61] YI H C, QU W Y, HUANG W S. Electrochemical determination of malachite green using a multi-wall carbon nanotube modified glassy carbon electrode[J]. Microchemica Acta,2008,160(1−2):291−296. doi: 10.1007/s00604-007-0814-z
[62] NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science,2004,306(5696):666−669. doi: 10.1126/science.1102896
[63] WANG Q Q, QIN X F, GENG L P, et al. Label-free electrochemical aptasensor for sensitive detection of malachite green based on Au nanoparticle/graphene quantum dots/tungsten disulfide nanocomposites[J]. Nanomaterials,2019,9(2):229. doi: 10.3390/nano9020229
[64] LUO Y Z, LI Z Y. A sensitive electrochemical sensor manufactured from multi-wall carbon nanotubes-polyethylenimine nanocomposite for malachite green detection[J]. Journal of Alloys and Compounds,2021,897:163216.
[65] 刘继超. 新型共价有机框架材料的构建及其在动物源性食品污染物检测中的应用[D]. 西安: 陕西科技大学, 2021 LIU J C. Construction of novel covalent organic framework materials and their application in the determination of animal-derived food contaminants[D]. Xian: Shaanxi University of Science & Technology, 2021.
[66] VOLKOV I L, SEEFELDT A C, JOHANSSON M. Tracking of single tRNAs for translation kinetics measurements in chloramphenicol treated bacteria[J]. Methods,2019,162:23−30.
[67] PENG B, LU Y, LUO J, et al. Visible light-activated self-powered photoelectrochemical aptasensor for ultrasensitive chloramphenicol detection based on DFT-proved Z-scheme Ag2CrO4/g-C3N4/graphene oxide[J]. Journal of Hazardous Materials,2020,401:123395.
[68] SAMSONVOA J V, CANNAVAN A, ELLIOTT C T. A critical review of screening methods for the detection of chloramphenicol, thiamphenicol, and florfenicol residues in foodstuffs[J]. Critical Reviews in Analytical Chemistry,2012,42(1):50−78. doi: 10.1080/10408347.2012.629951
[69] AMELINE A, TAQUET M C, TERRADE J E, et al. Identification of chloramphenicol in human hair leading to a diagnosis of factitious disorder[J]. Clinical Toxicology,2020,58(9):926−930. doi: 10.1080/15563650.2019.1708375
[70] ZHANG Z, ZHOU H, JIANG C, et al. Molecularly imprinted polymer functionalized flower-like BiOBr microspheres for photoelectrochemical sensing of chloramphenicol[J]. Electrochimica Acta,2020,344:136161. doi: 10.1016/j.electacta.2020.136161
[71] 朱文嘉, 王联珠, 郭莹莹, 等. 国内外鱼类产品兽药残留限量标准对比分析[J]. 水产科技情报,2013,40(5):225−231. [ZHU W J, WANG L Z, GUO Y Y, et al. Comparative analysis of veterinary drug residue limit standards for fish products at home and abroad[J]. Fisheries Science and Technology Information,2013,40(5):225−231. doi: 10.3969/j.issn.1001-1994.2013.05.001 [72] 刘畅. 氯霉素和二甲基异茨醇单克隆抗体的制备及酶联免疫吸附分析方法的建立[D]. 苏州: 苏州大学, 2019 LIU C. Preparation of monoclonal antibodies against chloramphenicol and 2-methylisoborneol and establishment of enzyme-linked immunosorbent assays[D]. Suzhou: Suzhou University, 2019.
[73] GIKAS E, KORMALI P, TSIPI D, et al. Development of a rapid and sensitive SPE-LC-ESI MS/MS method for the determination of chloramphenicol in seafood[J]. Journal of Agricultural and Food Chemistry,2004,52(5):1025−1030. doi: 10.1021/jf030485l
[74] BARRETO F, RIBEIRO C, HOFF R B, et al. Determination of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in poultry, swine, bovine and fish by liquid chromatography-tandem mass spectrometry[J]. Journal of Chromatography A,2016,1449:48−53. doi: 10.1016/j.chroma.2016.04.024
[75] CHEN D M, DELMAS J M, HURTAUD-PESSEL D, et al. Development of a multi-class method to determine nitroimidazoles, nitrofurans, pharmacologically active dyes and chloramphenicol in aquaculture products by liquid chromatography-tandem mass spectrometry[J]. Food Chemistry,2020,311:125924. doi: 10.1016/j.foodchem.2019.125924
[76] JUNG H N, PARK D, CHOI Y J, et al. Simultaneous quantification of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in animal and aquaculture products using liquid chromatography-tandem mass spectrometry[J]. Frontiers in Nutrition,2022,8:812803. doi: 10.3389/fnut.2021.812803
[77] TYAGI A, VERNEKAR P, KARUNASAGAR I, et al. Determination of chloramphenicol in shrimp by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS-MS)[J]. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure and Assessment,2008,25(4):432−437.
[78] BARRETO F, RIBEIRO C, HOFF R B, et al. Determination and confirmation of chloramphenicol in honey, fish and prawns by liquid chromatography-tandem mass spectrometry with minimum sample preparation: validation according to 2002/657/EC directive[J]. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure and Risk Assessment,2012,29(4):550−558.
[79] MACKIE J, MARLEY E, DONNELLY C. Immunoaffinity column cleanup with LC/MS/MS for the determination of chloramphenicol in honey and orawns: single-laboratory validation[J]. Journal of Aoac International,2013,96(4):910−916. doi: 10.5740/jaoacint.12-320
[80] WU J B, CHEN L Y, MAO P P, et al. Determination of chloramphenicol in aquatic products by graphene-based SPE coupled with HPLC-MS/MS[J]. Journal of Separation Science,2012,35(24):3586−3592. doi: 10.1002/jssc.201200617
[81] SI M Z, KANG Y P, ZHAGN Z G. Surface-enhanced raman scattering (SERS) spectra of chloramphenicol in Ag colloids prepared by microwave heating method[J]. Journal of Raman Spectroscopy,2009,40(9):1319−1323. doi: 10.1002/jrs.2286
[82] 吉薇. 表面增强拉曼光谱测定氯霉素及其琥珀酸钠的研究[D]. 无锡: 江南大学, 2013 JI W. Studies on the detection of chloramphenicol and its sodium succinate by surface-enhanced raman scattering[D]. Wuxi: Jiangnan University, 2013.
[83] JI W, YAO W R. Rapid surface enhanced raman scattering detection method for chloramphenicol residues[J]. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy,2015,144:125−130. doi: 10.1016/j.saa.2015.02.029
[84] SONG J, HUANG Y, FAN Y, et al. Detection of prohibited fish drugs using silver nanowires as substrate for surface-enhanced raman scattering[J]. Nanomaterials,2016,6(9):175. doi: 10.3390/nano6090175
[85] 赵燕. 氧化石墨烯复合基底的制备及其在食品安全检测中的应用[D]. 上海: 上海海洋大学, 2019 ZHAO Y. Fabrication of graphene oxide hybrid substrate and application in food safety detection[D]. Shanghai: Shanghai Ocean University, 2019.
[86] LI H H, GENG W H, HASSAN M M, et al. Rapid detection of chloramphenicol in food using SERS flexible sensor coupled artificial intelligent tools[J]. Food Control,2022,128:108186.
[87] HASSAN M M, HE P H, ZAREEF M, et al. Rapid detection and prediction of chloramphenicol in food employing label-free HAu/Ag NFs-SERS sensor coupled multivariate calibration[J]. Food Chemistry, 2022, 374: 131765.
[88] YU S H, LIU Z G, LI H W, et al. Combination of a graphene SERS substrate and magnetic solid phase micro-extraction used for the rapid detection of trace illegal additives[J]. Analyst,2018,143(4):883−890. doi: 10.1039/C7AN01547J
[89] 农业部. 农业部1025号公告-26-2008 动物源食品中氯霉素残留检测 酶联免疫吸附法[S]北京: 中国标准出版社, 2008: 1−4 Ministry of Agriculture of The PRC. Ministry of agriculture announcement No.1025-26-2008 determination of chloramphenicol in edible animal tissues by immunoassay[S] Beijing: Standards Press of China, 2008: 1−4.
[90] SAI N, CHEN Y P, LIU N, et al. A sensitive immunoassay based on direct hapten coated format and biotin-streptavidin system for the detection of chloramphenicol[J]. Talanta,2010,82(4):1113−1121. doi: 10.1016/j.talanta.2010.06.018
[91] CONTI G O, COPAT C, WANG Z H, et al. Determination of illegal antimicrobials in aquaculture feed and fish: An ELISA study[J]. Food Control,2015,50:937−941. doi: 10.1016/j.foodcont.2014.10.050
[92] CHANG L, DENG D, DI X, et al. Development of a monoclonal antibody based-ELISA for the detection of chloramphenicol in shrimp, feed and milk samples and validation by LC-MS/MS coupled with immunoaffinity clean-up[J]. Analytical Methods,2019,11(4):507−516. doi: 10.1039/C8AY02284D
[93] 夏菲, 刘秀英, 高雪, 等. 免疫层析技术在检测食品中硝基呋喃类药物的应用[J]. 中国食品学报,2021,21(11):397−409. [XIA F, LIU X Y, GAO X, et al. Application of immunochromatography in the detection of nitrofurans in food[J]. Journal of Chinese Institute of Food Science and Technology,2021,21(11):397−409. doi: 10.16429/j.1009-7848.2021.11.043 [94] ZHOU C N, ZHANG X Y, HUANG X X, et al. Rapid detection of chloramphenicol residues in aquatic products using colloidal gold immunochromatographic assay[J]. Sensors,2014,14(11):21872−21888. doi: 10.3390/s141121872
[95] SUN Y W, CHAGN Y, JIN Y F, et al. Study of synthesis and spectral property of europium cryptate[J]. Spectroscopy and Spectral Analysis,2018,38(7):2189−2193.
[96] ZHOU B, ZHANG J, FAN J, et al. A new sensitive method for the detection of chloramphenicol in food using time-resolved fluoroimmunoassay[J]. European Food Research and Technology,2015,240(3):619−625. doi: 10.1007/s00217-014-2363-0
[97] FANG Q, WANG L, HUA X, et al. An enzyme-linked chemiluminescent immunoassay developed for detection of Butocarboxim from agricultural products based on monoclonal antibody[J]. Food Chemistry,2015,166:372−379. doi: 10.1016/j.foodchem.2014.06.060
[98] AHMED S, NING J N, PENG D P, et al. Current advances in immunoassays for the detection of antibiotics residues: A review[J]. Food and Agricultural Immunology,2020,31(1):268−290. doi: 10.1080/09540105.2019.1707171
[99] XU C L, PENG C F, HAO K, et al. Chemiluminescence enzyme immunoassay (CLEIA) for the determination of chloramphenicol residues in aquatic tissues[J]. Luminescence,2006,21(2):126−128. doi: 10.1002/bio.892
[100] PAN Y, FEI D W, LIU P H, et al. Surface-enhanced raman scattering-based lateral flow immunoassay for the detection of chloramphenicol antibiotics using Au@Ag nanoparticles[J]. Food Analytical Methods,2021,14(12):2642−2650. doi: 10.1007/s12161-021-02091-4
[101] JIANG W, WANG Z, BEIER R C, et al. Simultaneous determination of 13 fluoroquinolone and 22 sulfonamide residues in milk by a dual-colorimetric enzyme-linked immunosorbent assay[J]. Analytical Chemistry,2013,85(4):1995−1999. doi: 10.1021/ac303606h
[102] DIBLIKOVA I, COPPER K M, KENNEDY D G, et al. Monoclonal antibody-based ELISA for the quantification of nitrofuran metabolite 3-amino-2-oxazolidinone in tissues using a simplified sample preparation[J]. Analytica Chimica Acta,2005,540(2):285−292. doi: 10.1016/j.aca.2005.03.039
[103] FAN W L, YANG S W, GAO W X, et al. Highly sensitive bromide aided SERS detection of furazolidone and 3-Amino-2-oxazolidinone residual in aquaculture products[J]. Microchemical Journal,2021,169(4):106532.
[104] MCCRACKEN R J, SPENCE D E, FLOYD S D, et al. Evaluation of the residues of furazolidone and its metabolite, 3-amino-2-oxazolidinone (AOZ), in eggs[J]. Food Addit Contam,2001,18(11):954−959. doi: 10.1080/02652030110050375
[105] 国家卫生健康委员会. GB 31656.13-2021 食品安全国家标准 水产品中硝基呋喃类代谢物多残留的测定 液相色谱-串联质谱法[S]. 北京: 中国农业出版社, 2021: 1−3 National Health Commission. GB 31656.13-2021 Food safety national standards Determination of multi-residues of nitrofuran metabolites in aquatic products Liquid chromatography-tandem mass spectrometry[S]. Beijing: China Agriculture Press, 2021: 1−3.
[106] 苏丽红. 纳米信号探针用于呋喃唑酮免疫层析检测方法的研究[D]. 咸阳: 西北农林科技大学, 2021 SU L H. Study on lateral flow immunoassay for detection of furazolidone based on nano-signal probe[D]. Xianyang: Northwest A & F University, 2021.
[107] CHUMANEE S, SUTTHIVAIYAKIT S, SUTTHIVAIYAKIT P, et al. New reagent for trace determination of protein-bound metabolites of nitrofurans in shrimp using liquid chromatography with diode array detector[J].Journal of Agricultural & Food Chemistry, 2009, 57(5): 1752-1759.
[108] DU N N, CHEN M M, SHEGN L Q, et al. Determination of nitrofuran metabolites in shrimp by high performance liquid chromatography with fluorescence detection and liquid chromatography-tandem mass spectrometry using a new derivatization reagent[J]. Journal of Chromatography A,2014,1327:90−96. doi: 10.1016/j.chroma.2013.12.065
[109] LUO X Z, YU Y X, KONG X J, et al. Rapid microwave assisted derivatization of nitrofuran metabolites for analysis in shrimp by high performance liquid chromatography-fluorescence detector[J]. Microchemical Journal,2019,150:104189. doi: 10.1016/j.microc.2019.104189
[110] DOUNY C, WIDART J, DEPAUW E, et al. Development of an analytical method to detect metabolites of nitrofurans: application to the study of furazolidone elimination in vietnamese black tiger shrimp (Penaeus monodon)[J]. Aquaculture,2013,376:54−58.
[111] YU Y X, LI N, JIN Q Q, et al. Novel fluorescence labeling reagent 4-(carbazole-9-yl)-benzyl chloroformate and its application in the determination of nitrofuran metabolites compounds in foodstuffs by high performance liquid chromatography with fluorescence detection[J]. Microchemical Journal,2019,145:9−17. doi: 10.1016/j.microc.2018.10.021
[112] OYE B E, COUILLARD F D, VALDERSNES S. Complete validation according to current international criteria of a confirmatory quantitative method for the determination of nitrofuran metabolites in seafood by liquid chromatography isotope dilution tandem mass spectrometry[J]. Food Chemistry,2019,300:125175. doi: 10.1016/j.foodchem.2019.125175
[113] CONNEELY A, NUGENT A, OKEEFFE M. Use of solid phase extraction for the isolation and clean-up of a derivatised furazolidone metabolite from animal tissues[J]. Analyst,2002,127(6):705−709. doi: 10.1039/b203058f
[114] SHEN Y D, XU Z L, ZHANG S W, et al. Development of a monoclonal antibody-based competitive indirect enzyme-linked immunosorbent assay for furaltadone metabolite AMOZ in fish and shrimp samples[J]. Agricultural and Food Chemistry,2012,66(4):10991−10997.
[115] COOPER K M, ELLIOTT C T, KENNEDY D G. Detection of 3-amino-2-oxazolidinone (AOZ), a tissue-bound metabolite of the nitrofuran furazolidone, in prawn tissue by enzyme immunoassay[J]. Food Additives and Contaminaminants Part A-Chemistry Analysis Control Exposure and Risk Assessment,2004,21(9):841−848.
[116] JIANG W X, LUO P J, WNAG X, et al. Development of an enzyme-linked immunosorbent assay for the detection of nitrofurantoin metabolite, 1-amino-hydantoin, in animal tissues[J]. Food Control,2012,23(1):20−25. doi: 10.1016/j.foodcont.2011.05.014
[117] 徐冬梅, 李亚英, 李玉静. 呋喃西林代谢物酶联免疫吸附测定方法的建立及性能测定[J]. 食品研究与开发,2020,41(6):164−168. [XU D M, LI Y Y, LI Y J. Establishment of enzyme-linked immunosorbent assay for determination of furacillin metabolites and its properties[J]. Food Research and Development,2020,41(6):164−168. doi: 10.12161/j.issn.1005-6521.2020.06.028 [118] WANG Q, LIU Y C, WANG M Y, et al. A multiplex immunochromatographic test using gold nanoparticles for the rapid and simultaneous detection of four nitrofuran metabolites in fish samples[J]. Analytical and Bioanalytical Chemistry,2018,410(1):223−233. doi: 10.1007/s00216-017-0714-y
[119] DONG X, GAO Y Q, ZHAGN X, et al. Multiplex europium (III) nanoparticles immunochromatographic assay method for the detection of four nitrofuran metabolites in fish sample[J]. Microchemical Journal,2019,150:104207. doi: 10.1016/j.microc.2019.104207
[120] YU W L, LIU M X, LIU R B, et al. Development of biomimetic enzyme-linked immunosorbent assay based on molecular imprinting technique for semicarbazide detection[J]. Food and Agricultural Immunology,2019,31(1):17−32.
[121] CHEN L Y, LUQUE R, LI Y W. Controllable design of tunable nanostructures inside metal-organic frameworks[J]. Chemical Society Reviews,2017,46(15):4616−4630.
[122] GUMEROVA N, ROMPEL A. Synthesis, structures and applications of electron-rich polyoxometalates[J]. Nature Reviews Chemistry,2018,2(2):0122.
[123] 齐雯. 含钒多金属氧酸盐抗乳腺癌MCF-7细胞作用研究[D]. 长春: 吉林大学, 2016 QI W. Research on the antitumor effect of polyoxometalates on human breast cancer MCF-7 cells[D]. Changchun: Jilin University, 2016.
[124] CAI S X, JIAO T H, WANG L, et al. Electrochemical sensing of nitrofurazone on Ru(bpy)32+ functionalized polyoxometalate combined with graphene modified electrode[J]. Food Chemistry,2022,378:132084. doi: 10.1016/j.foodchem.2022.132084
[125] KOKULNATHAN T, WANG T J. Synthesis and characterization of 3D flower-like nickel oxide entrapped on boron doped carbon nitride nanocomposite: An efficient catalyst for the electrochemical detection of nitrofurantoin[J]. Composites Part B-Engineering,2019,174:106914. doi: 10.1016/j.compositesb.2019.106914
[126] KOKULNATHAN T, WANG T J, KUMAR E A, et al. Development of an electrochemical platform based on nanoplate-like zirconium phosphate for the detection of furazolidone[J]. Acs Applied Nano Materials,2020,3(5):4522−4529. doi: 10.1021/acsanm.0c00594
[127] HE B S, DNOG X Z. Powder microelectrode embedded with carboxyl multi-walled carbon nanotubes for sensitive and quantitative detection of nitrofuran residues[J]. Analytical Methods,2018,10(11):1372−1378. doi: 10.1039/C8AY00289D
[128] HE B S, LIU H. Electrochemical determination of nitrofuran residues at gold nanoparticles/graphene modified thin film gold electrode[J]. Microchemical Journal,2019,150:104108. doi: 10.1016/j.microc.2019.104108
[129] YANG G J, JIN W J, WU L P, et al. Development of an impedimetric immunosensor for the determination of 3-amino-2-oxazolidone residue in food samples[J]. Analytica Chimica Acta,2011,706(1):120−127. doi: 10.1016/j.aca.2011.08.018
[130] JIN W J, YANG G J, WU L P, et al. Detecting 5-morpholino-3-amino-2-oxazolidone residue in food with label-free electrochemical impedimetric immunosensor[J]. Food Control,2011,22(10):1609−1616. doi: 10.1016/j.foodcont.2011.03.017
[131] JIN W J, YANG G J, SHAO H X, et al. A label-free impedimetric immunosensor for detection of 1-aminohydantoin residue in food samples based on sol-gel embedding antibody[J]. Food Control,2014,39:185−191. doi: 10.1016/j.foodcont.2013.11.001
[132] YU W L, TANG Y W, SANG Y X, et al. Preparation of a carboxylated single-walled carbon-nanotube-chitosan functional layer and its application to a molecularly imprinted electrochemical sensor to quantify semicarbazide[J]. Food Chemistry,2021,333:127524.
[133] AMALRAJ A J J, MURTHY U N, WANG S F. Ultrasensitive electrochemical detection of an antibiotic drug furaltadone in fish tissue with a ZnO-ZnCo2O4 self-assembled nano-heterostructure as an electrode material[J]. Microchemical Journal,2021,169:106566. doi: 10.1016/j.microc.2021.106566
-
期刊类型引用(9)
1. 李杰,马丹丹. 以正磷酸盐为酯化剂制备磷酸酯玉米淀粉及其对Ni~(2+)的吸附性能. 印染助剂. 2025(02): 36-41 .  百度学术
百度学术
2. 安鸿雁,刘馨远,赵国兴,李义. 蜡质玉米淀粉乙酰化的工艺研究. 当代化工. 2023(02): 477-480 .  百度学术
百度学术
3. 孔刘娟,段元良,栾庆民,王欣彤,袁世英,李新华,刘开昌,李克文. 食用变性淀粉研究进展. 精细与专用化学品. 2023(03): 24-29 .  百度学术
百度学术
4. 西芳,刘馨远,曹国强. 蜡质玉米淀粉制备乙酰化二淀粉磷酸酯的工艺优化. 广州化工. 2023(08): 80-82+104 .  百度学术
百度学术
5. 肖志刚,邵晨,杨柳,杨庆余,朱旻鹏,杨宏黎. 淀粉改性方法的研究现状及进展. 农产品加工. 2020(03): 81-84+88 .  百度学术
百度学术
6. 爨珊珊,石海信,王爱荣,欧春兰,耿昊天,王小丽. 两性淀粉制备与应用的机理及研究进展. 化工技术与开发. 2019(02): 11-16+33 .  百度学术
百度学术
7. 刘丹,邓利玲,郑连姬,钟耕. 高取代度磷酸魔芋葡甘聚糖酯的制备. 食品与发酵工业. 2019(08): 149-155 .  百度学术
百度学术
8. 沈艳琴,杨树,武海良,王忠梁. 浆纱用中低温水溶性淀粉浆料的研究进展. 纺织学报. 2019(06): 143-152 .  百度学术
百度学术
9. 冯琳,黄祖强,孙宁钊,胡华宇,张燕娟,玉琼广. 三聚磷酸钠对醋酸酯淀粉反应效率的影响及工艺优化. 中国食品添加剂. 2018(12): 149-155 .  百度学术
百度学术
其他类型引用(6)





 下载:
下载:
 下载:
下载:



