Isolation, Identification and Biological Characteristics of Postharvest Pathogens of Yuluxiang Pear
-
摘要: 真菌侵染造成的腐烂是影响玉露香梨采后贮藏的主要因素。为明确导致玉露香梨采后腐烂的病原菌种类及其生物学特性,对其采后病原菌进行分离纯化,结合形态学和分子生物学特征确定其种类,并明确在不同温度、pH、碳源和氮源条件下病原菌的生长情况。结果表明:Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium是导致玉露香梨采后腐烂的主要病原菌。Alternaria alternata和Fusarium的最适生长温度为25~30 ℃,而Aspergillus和Penicillium polonicum最适生长温度分别为25~35 ℃和20~25 ℃;Alternaria alternata和Aspergillus的最适生长pH分别为6~10和7,而Penicillium polonicum和Fusarium最适生长pH则均为5~7;Alternaria alternata和Penicillium polonicum的最适生长碳源分别为葡萄糖和甘露醇,Aspergillus和Fusarium最适生长碳源为麦芽糖;Alternaria alternata、Aspergillus和Fusarium最适生长氮源为牛肉膏,而Penicillium polonicum最适生长氮源为蛋白胨。Abstract: The rot caused by fungal infection is the main factor affecting the postharvest storage of Yuluxiang pear. In order to clarify the species and biological characteristics of pathogenic bacteria that cause postharvest rot of Yuluxiang pear, the postharvest pathogenic bacteria were isolated and purified, combining morphological and molecular biological characteristics to determine its species, and to clarify the growth of pathogenic bacteria under different temperature, pH, carbon and nitrogen source conditions. The results showed that: Alternaria alternata, Aspergillus, Penicillium polonicum and Fusarium were the main pathogens causing postharvest rot of Yuluxiang pear. The optimum growth temperature of Alternaria alternata and Fusarium was 25~30 ℃, while the optimum growth temperature of Aspergillus and Penicillium polonicum were 25~35 ℃ and 20~25 ℃, respectively. The optimum growth pH of Alternaria alternata and Aspergillus were 6~10 and 7, respectively, while the optimum growth pH of Penicillium polonicum and Fusarium were both 5~7. The optimum growth carbon sources of Alternaria alternata and Penicillium polonicum are glucose and mannitol, respectively, the optimum growth carbon source of Aspergillus and Fusarium was maltose. The optimum growth nitrogen source of Alternaria alternata, Aspergillus and Fusarium was beef extract, and the optimum growth nitrogen source of Penicillium polonicum was peptone.
-
“玉露香”梨(Pyrus sinkiangensis‘Yuluxiang’)是以库尔勒香梨为母本,雪花梨为父本杂交育成的优质中熟梨新品种[1],富含多种糖类、矿物质及其它营养元素 [2]。但玉露香梨属于呼吸跃变型果实[3],水分含量高,采后贮运过程中易受到机械损伤,导致病原菌从伤口处侵入诱发病害。据统计,玉露香梨每年采后损失约20%~25%[4-5],而真菌侵染导致的腐烂是造成梨果采后损失重要因素之一[6-8]。有研究表明,引起梨果采后腐烂的病原微生物主要有Rhizopus、 Penicillium和Aspergillus等[9-11];牛佳佳等[12]分离到酥梨贮藏期间病原菌主要有Botryosphaeria、Alternaria、Fusarium 和Penicillium expansum,其中Penicillium expansum是生长最快和传染性较强的致病菌;对金刺梨黑斑病则主要是由Phomopsiscapsici真菌引起[13];而库尔勒香梨黑斑病则主要是由交链孢(Alternaria alternata)和细极链隔孢(Alternaria tenuissima)导致[14]。关于玉露香梨贮藏期间病原菌的研究少有报道,因此,本研究对玉露香梨贮藏期间病原菌进行分离鉴定,并初步研究其生物学特性,旨在为玉露香梨贮藏过程中病害的侵染规律和防治提供理论依据。
1. 材料与方法
1.1 材料与仪器
玉露香梨 在2020年9月份采于山西省临汾市隰县寨子乡,采后24 h内运回实验室。挑选大小均一、无病虫害、无机械损伤的果实,存放于0±1 ℃贮藏库中备用;Tris缓冲剂 北京索莱宝科技有限公司;葡糖糖 分析纯,天津市凯通化学试剂有限公司;琼脂粉 分析纯,北京奥博星生物技术有限责任公司;引物1、引物4 华大基因;PDA培养基(马铃薯200 g,蒸馏水1 L,葡萄糖20 g,琼脂18 g);PDB培养基(马铃薯200 g,蒸馏水1 L,葡萄糖20 g);查氏培养基(NaNO3 3 g,K2HPO4 1 g,MgSO4·7H2O 0.5 g,KCI 0.5 g;FeSO4·7H2O 0.01 g;蔗糖30 g,琼脂15 g,蒸馏水1 L)。
SW-CJ-1FD单人超净工作台 浙江苏净净化设备有限公司;DH电热恒温箱 天津市通利信达仪器厂;DYY-6C电泳仪 北京市六一仪器厂;WFH-202紫外透射分析仪 上海精科实业有限公司。
1.2 实验方法
1.2.1 病原菌的分离鉴定
1.2.1.1 病原菌的分离纯化
取自然发病梨果感染组织病健交界处的小块组织(约 5 mm×5 mm)放入75%的酒精中浸泡30 s,用无菌水冲洗3次,移入3%的次氯酸钠溶液浸泡3 min,再用无菌水清洗3次,取出后用滤纸吸干组织块,接入PDA平板中,28 ℃恒温培养。当培养出的菌落直径生长至1 cm时,挑取菌落边缘的菌丝转接入PDA培养基平板中,28 ℃恒温培养;重复上述操作2~3次得到纯化的病原菌,移入试管低温保存备用[15-16]。
1.2.1.2 病原菌的致病性检测
根据柯赫氏法则[17],选取健康大小、成熟度一致的玉露香梨,75%的酒精表面消毒后用无菌水冲洗,晾干后进行接种处理:在果肩部切下约5 mm深、5 mm直径的圆形伤口,用打孔器取PDA平板培养3~5 d的5 mm病原菌饼,接至伤口位置,28 ℃恒温培养,观察发病情况。以接PDA培养基为对照,重复3次。观察是否与自然发病的病原菌特征相一致。
1.2.1.3 病原菌的形态学观察
将纯化的病原菌培养5~7 d,观察菌落外观形态。待产孢后,挑取少量孢子或菌丝,在显微镜下观察菌株的组织结构:分生孢子及孢子梗的形状,有无分枝状态等。参照魏启超[18]和葛启新等[19]的方法,对病原菌进行初步鉴定。
1.2.1.4 病原菌的分子生物学鉴定
总DNA的提取:将纯化的病原菌接种到100 mL PDA培养基中,在室温下振荡培养7 d,然后对菌丝进行过滤和收集。液氮冷冻研磨成细粉,用CTAB法[20-21]提取纯化基因组DNA,将提取获得的DNA样品放于−60 ℃保存备用。
病原菌rDNA-ITS 扩增与序列分析[22-23]:按照上述方法提取真菌DNA后,采用真菌通用引物(ITS1:5'-TCCGTAGGTGAACCTGCGG-3'和ITS4:5'-TCCTCCGCTTATTGATATGC-3')扩增rDNA-ITS 区段。PCR反应体系为(50 μL):模板DNA 4 μL,引物1、引物4各2 μL,Premix Tap 25 μL,ddH2O 17 μL。PCR反应条件为:94 ℃预变性5 min;94 ℃变性55 s;50 ℃退火 50 s;72 ℃ 延伸 55 s;循环32次;72 ℃延伸10 min;4 ℃保存。PCR 产物用 1%琼脂糖凝胶电泳检测,并送去北京六合华大基因科技有限公司进行测序。
将所得rDNA-ITS区段序列在GenBank数据库中进行BLAST同源性检索,采用MEGA version 7.0.14软件对同源序列与目的序列进行比对,以Neighbor-Joining法构建系统发育树,分析亲缘关系。
1.2.2 生物学特性
1.2.2.1 最适温度的筛选
将直径5 mm的菌饼接入PDA培养基中央,分别置于5、10、15、20、25、30、35、40 ℃的恒温培养箱。培养5 d后用十字交叉法测量菌落直径,10 d后,用血球计数板测定产孢量,处理3 次重复[24]。
1.2.2.2 最适pH的筛选
用1 mol/L HCl和1 mol/L NaOH调配PDA培养基,使其pH分别为4、5、6、7、8、9、10、11,将菌饼接入不同pH培养基中央,28 ℃恒温培养[25],培养5 d后用十字交叉法测量菌落直径,10 d后,用血球计数板测定产孢量,处理3次重复。
1.2.2.3 最适碳源和氮源的筛选
以查氏培养基(Czapek)为基础培养基,按等量的碳源(麦芽糖、乳糖、葡萄糖、甘露醇、可溶性淀粉)替换Czapek培养基中的蔗糖,以缺碳作对照,研究不同碳源对菌丝体生长和产孢量的影响。同样,按等量氮源(蛋白胨、牛肉膏、甘氨酸、尿素、硝酸铵、硫酸铵)替换Czapek培养基中的硝酸钠,以缺氮作对照,28 ℃恒温培养[26],培养5 d后用十字交叉法测量菌落直径。10 d后,用血球计数板测定产孢量,处理3次重复。
1.3 数据处理
利用Excel 2007和SPSS 23.0软件对试验数据进行统计分析和Origin2021软件作图,并应用Duncan氏新复极差法进行差异显著性检验。
2. 结果与分析
2.1 病原菌的分离鉴定
2.1.1 病原菌的分离纯化
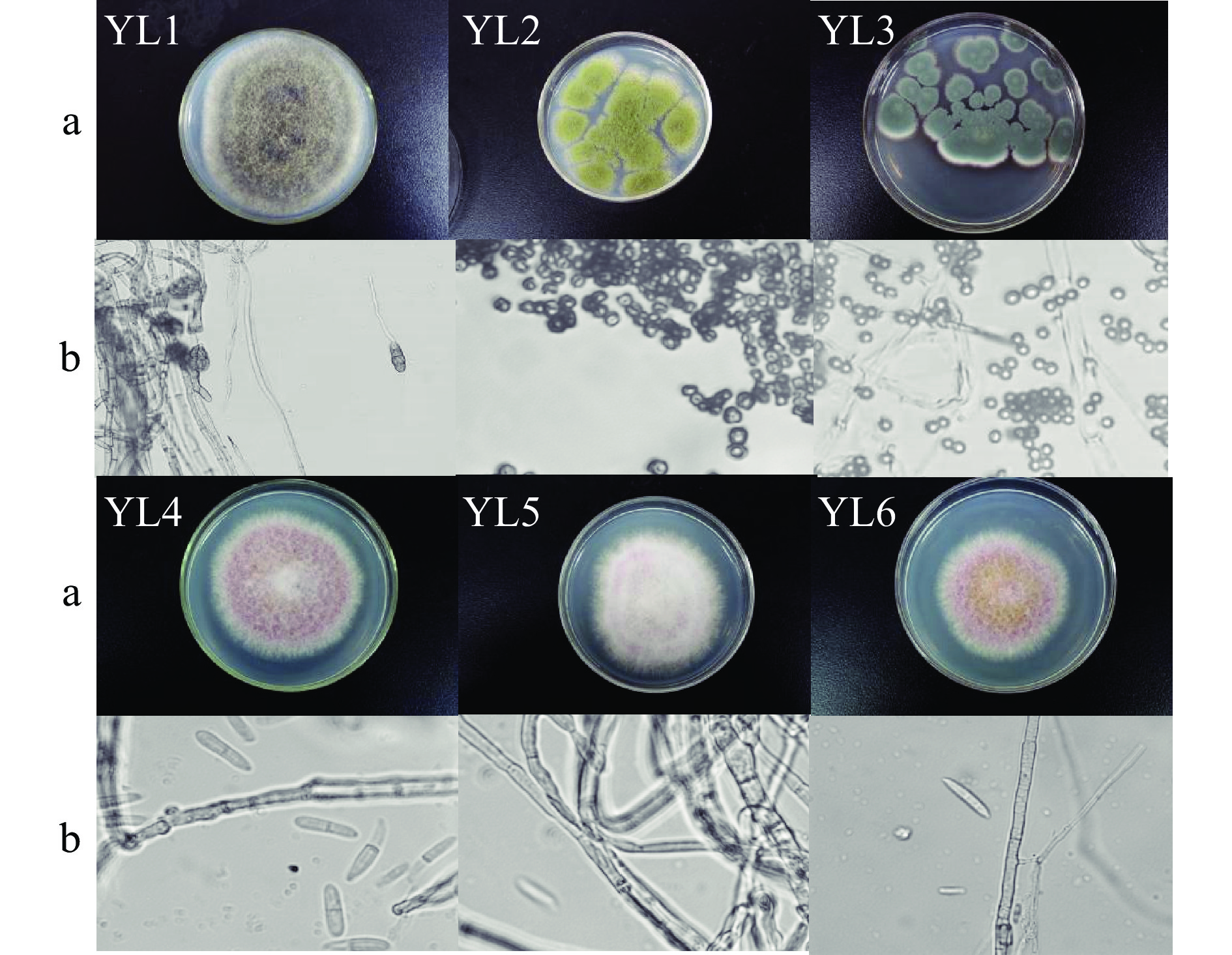
从玉露香梨病健交界处组织中共分离纯化出6种形态、颜色和生长速度差异较为明显的纯培养物,命名为YL1~6。YL1菌落初期为灰白色,后期为灰褐色;YL2菌落初期为黄白色,后期为黄绿色;YL3菌落初期为白色,后期长出许多灰蓝色孢子;YL4、YL5、YL6菌落颜色略有一些差异,菌丝体形态极为接近,可能为同一种病原菌。
2.1.2 病原菌致病性
纯化后的病原菌接种到梨果伤口3 d后,病斑从伤口处开始扩散,这表明所分离的菌株均具有致病性(图1),在玉露香梨果实上均表现出发病症状。YL1发病症状:近圆形病斑,初为褐色,后渐变为灰褐色,表面稍凹陷,四周黑褐色;YL2病果迅速软腐,贮藏时间长会流水,表面布满青绿色霉堆;YL3病果病斑近圆形,淡褐色,软腐,略凹陷;YL4、YL5和YL6发病症状相近,伤口中央有菌丝附着,略凹陷,轻微软腐。其发病症状与自然发病症状一致。对照不发病。对回接后的病原菌再次进行分离培养,经鉴定仍为最初接种的菌株。
2.1.3 病原菌的形态学鉴定分析
纯化的病原菌从菌落特征和显微形态观察研究发现(图2),病原菌YL1菌丝生长迅速、菌落均匀,有明显的基内菌丝,菌落逐渐从灰白色加深变为深灰色,菌丝绒状致密;显微镜观察分生孢子梗呈黄褐色,近椭圆形,具横隔,孢身中部的隔膜较粗,呈褐色;根据菌落、菌丝和孢子形态方面YL1同Alternaria较相似,与已报道的番莲果果腐病原菌Alternaria alternata[27]和奉新猕猴桃黑斑病病原菌Alternaria alternata[28]极为接近。
YL2菌落生长较快、结构疏松,呈黄绿色;显微镜下观察,菌丝体无色,分生孢子初为球形,后呈辐射型,无色;根据菌落、菌丝和孢子形态方面YL2同Aspergillus较为相似。
YL3菌落呈灰蓝色,密毡状,生长较为缓慢;显微镜下观察分生孢子链状着生,菌丝有分枝、分隔,有横膈膜;根据菌落、菌丝和孢子形态方面YL3同Penicillium较为相似,与已报道的柑橘采后腐烂病原菌Penicillium[29]较为相似。
YL4、YL5和YL6除PDA平板菌落颜色略有一些不同之外,菌丝和孢子形态极为接近。菌落突起白色绒毛状,浅粉色,菌丝白色质密;显微镜下观察小型分生孢子着生于单生瓶梗上,大型分生孢子无色、多胞,镰刀形,两端细胞稍尖;根据菌落、菌丝和孢子形态方面YL4 同Fusarium较为相似;YL4、YL5和YL6菌丝和孢子形态很接近,其形态特征与已报道的黄连根腐病镰刀菌(Fusarium oxysporum)[30]较为接近。
2.1.4 病原菌分子生物学鉴定结果
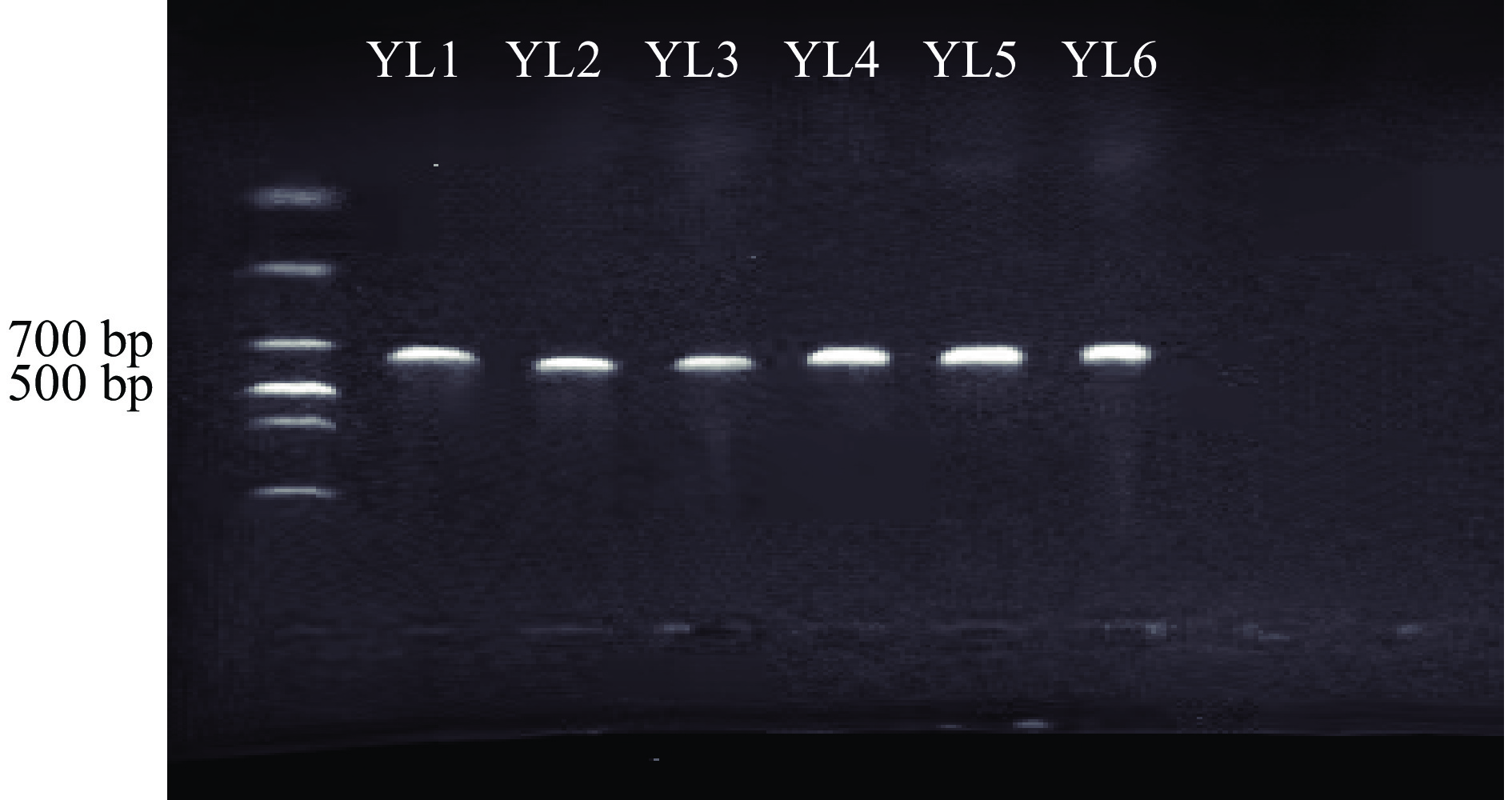
测序结果表明,菌株YL1、YL2、YL3、YL4、YL5、YL6的PCR扩增长度分别为500 bp左右(图3),符合真菌ITS区序列长度范围,表明ITS区通用引物成功的扩增了玉露香梨采后腐烂病原菌的ITS基因序列。构建系统发育树可知(图4~图7),YL1的ITS序列与Alternaria alternata的ITS序列(登录号:MZ951051.1、MZ298823.1、OL514181.1、MW474904.1、OM049821.1)紧密地聚在同一分枝,同源性达100%,结合形态学特征综合分析,病原菌YL1为Alternaria alternata ;YL2的ITS序列与Aspergillus sp.的ITS序列(登录号MT645617.1、MT645322.1、MT584825.1、MT497446.1、OK235450.1、OK217263.1、MW788477.1、NR171607.1、NR171606.1 )同源性达100%,结合形态学特征综合分析,病原菌YL2为Aspergillus sp.;YL3的ITS序列与 Penicillium polonicum的ITS序列(登录号MF979522.1、MT158684.1、MG228408.1 )同源性达100%,结合形态学特征综合分析,病原菌YL3为Penicillium polonicum;YL4~6的ITS序列与Fusarium sp.的ITS序列(登录号:MT482502.1、LC500064.1、OM017211.1、MW791922.1 、MT463390.1 、MK250655.1 、MK407333.1、AB470855.1、MK407347.1 、MK407211.1、MW067648.1)同源性达99%,结合形态学特征综合分析,病原菌YL4~6为Fusarium sp.。
2.2 生物学特性研究结果
2.2.1 最适温度
温度对病原菌Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium菌丝体生长和产孢量有显著(P<0.05)影响(图8)。该些菌株在10~35 ℃均可生长,不同温度下菌丝体生长和产孢量不同,不同病原菌的最适温度也不同。其中,Alternaria alternata和Fusarium最适生长温度为25~30 ℃,在此温度下,Alternaria alternata菌落直径达5.9和6.2 cm,产孢量达3.89和3.21 lg (CFU/mL),Fusarium菌落直径达6.2和6.1 cm,产孢量达4.39和3.48 lg (CFU/mL),显著(P<0.05)高于其余温度;Aspergillus最适温度为25 ~35 ℃,其菌落直径分别为5.23、6.11和6.21 cm,产孢量达7.9、6.5和6.8 lg (CFU/mL);Penicillium polonicum最适生长温度为20 ℃~25 ℃,菌落直径达3.6和2.7 cm,25 ℃时产孢量最高,可达8.4 lg (CFU/mL)。菌株Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium培养5和10 d后,在5 ℃条件下不生长且不产孢,Penicillium polonicum和Fusarium在温度≥35 ℃下生长缓慢,说明菌株不耐高温。而Alternaria alternata和Aspergillus在40 ℃仍可生长,说明菌株耐高温。四株菌株均在25 ℃时产孢量最高。因此,推测玉露香梨采后病原菌的发生多在25 ℃左右,在这个温度对应的季节之前应做好该病害的防控工作。
2.2.2 最适pH
pH对菌株的生长也有一定的影响(图9)。其中,Alternaria alternata最适生长pH在6~10,其菌落直径达6、6.1、6.4、6.3和5.7 cm,产孢量为5.3、4.8、4.5、4.3和4.1 lg (CFU/mL);Alternaria alternata在pH小于6时生长缓慢,说明该菌株在偏碱性环境中均可生长;Aspergillus最适生长pH为7,菌落直径达7.2 cm,产孢量为7.8 lg (CFU/mL),在pH大于7时生长逐渐下降,说明该菌株在偏中性环境中生长较好;Penicillium polonicum和Fusarium最适生长范围在5~7,Penicillium polonicum菌落直径分别为6.5、5.9和6.7 cm、Fusarium菌落直径为6.7、7和7.4 cm,Penicillium polonicum产孢量4.7、4.8和4.7 lg (CFU/mL)。Fusarium最适产孢量在7,产孢量为4.21 lg (CFU/mL),适合在偏酸中性环境中生长。
2.2.3 最适碳源
病原菌Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium可利用多种碳源(图10)其中,Alternaria alternata最适碳源为葡萄糖,菌落直径达6.5 cm、产孢量为5.42 lg (CFU/mL),显然葡萄糖对该菌株有促进生长的作用,碳源为果糖时生长较缓慢,菌落直径3.01 cm、产孢量为2.33 lg (CFU/mL);Aspergillus最适碳源为麦芽糖,直径达4.51 cm、产孢量4.2 lg (CFU/mL)。其次是甘露醇和乳糖,碳源为果糖和可溶性淀粉时生长较缓慢;Penicillium polonicum最适碳源为甘露醇,直径达4.5 cm、产孢量为6.52 lg (CFU/mL),碳源为葡萄糖和可溶性淀粉时生长较为缓慢,可见该菌对葡萄糖和可溶性淀粉的适应能力较差;Fusarium最适生长碳源为麦芽糖,直径6.75 cm,碳源是乳糖时产孢量最大,产孢量为4.31 lg (CFU/mL)。
2.2.4 最适氮源
病原菌Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium在6种供试氮源均可生长,同一菌株在不同氮源的生长不同,相同氮源培养基上各菌株生长和产孢也略有不同(图11),其中Alternaria alternata和Aspergillus最适生长氮源为牛肉膏,其菌落直径为6.8和6.52 cm,Alternaria alternata产孢量最大氮源为蛋白胨,达4.7 lg (CFU/mL),Penicillium polonicum最适生长氮源为蛋白胨,直径4.7 cm、产孢量4.75 lg (CFU/mL);Fusarium最适生长氮源为牛肉膏,菌落直径达7.8 cm,其次是硝酸铵7.3 cm,氮源是甘氨酸时产孢量最大,可达3.8 lg (CFU/mL)。当氮源为硫酸铵时,菌株Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium生长速度和产孢量明显低于CK值,由此可见,硫酸铵对该些病原菌有明显的抑制作用。根据这一研究结果可以考虑在玉露香梨种植过程中避免施加硝态类肥料,可适量施加硫酸铵以抑制病原菌生长,降低腐烂病的发生。
3. 结论
本研究通过分离鉴定得到玉露香梨采后病原菌主要有Alternaria alternata、Aspergillus、Penicillium polonicum和Fusarium。对玉露香梨采后腐烂病原菌生长温度、pH和最适碳氮源研究发现,Alternaria alternata最适生长温度为25~30 ℃,最适生长pH为6~10,最适生长碳氮源分别为葡萄糖和牛肉膏;Aspergillus最适生长温度为25~35 ℃,最适生长pH为6~10,最适生长碳氮源为麦芽糖和牛肉膏;Penicillium polonicum最适生长温度为20~25 ℃,最适生长pH为5~7,最适生长碳氮源为甘露醇和蛋白胨;Fusarium最适生长温度为25~30 ℃,最适生长pH为5~7,最适生长碳氮源为麦芽糖和牛肉膏。这一结论为探究玉露香梨贮期病原微生物多样性和采后贮藏特性提供理论基础。
-
-
[1] 马风丽, 杜艳民, 王阳, 等. 1-MCP 对‘玉露香’梨采后果实品质和叶绿素保持的影响[J]. 园艺学报,2019,46(12):2299−2308. [MA F L, DU Y M, WANG Y, et al. Effects of 1-Methylcyciopropene (1-MCP) on quality and chlorophyll maintenance of postharvest 'Yuluxiang' pear[J]. Acta Horticulturae Sinica,2019,46(12):2299−2308. MA F L, DU Y M, WANG Y, et al. Effects of 1-Methylcyciopropene (1-MCP) on quality and chlorophyll maintenance of postharvest 'Yuluxiang' pear[J]. Acta Horticulturae Sinica, 2019, 46(12): 2299-2308.
[2] STEFAN P, CHRISTIAN J S, SASKIA D M P, et al. Infection strategies deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifer as a function of tomato fruit ripening stage[J]. Frontiers in Plant Science,2019,10:223. doi: 10.3389/fpls.2019.00223
[3] STEFAN S, DANIJELA R, ZELJKO S, et al. Penicillium and talaromyces species as postharvest pathogens of pear fruit (Pyrus communis L.) in Serbia[J]. Plant Disease, 2021.
[4] LEGRAND N N G, QIAN X, YANG Q Y, et al. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections[J]. Comprehensive Reviews in Food Science and Food Safety, 2021.
[5] ANTONIOS Z, IOANNIS G, ATHANASIOS T, et al. Metagenomics analysis of fungal communities associated with postharvest diseases in pear fruits under the effect of management practices[J]. Archives of Microbiology, 2020.
[6] ABDULLAH Q, MAHMOUD A, AL-HARETHI A, et al. A peer-reviewed journal PSM microbiology isolation and identification of fungal post-harvest rot of some fruits in yemen[J]. Plant Pathology & Quarantine,2019,6(1):19−29.
[7] AL-HINDI R R, AL-NAJADA A R, MOHAMED S A. Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes[J]. African Journal of Microbiology Research,2011,5(4):443−448.
[8] YARADUA S S, YUSUF M, KANKARA S L. Isolation and identification of fungi from some selected vegetables in Kankara Local Governmnet Area, Katsina State[J]. UJMR,2018,3:76−80.
[9] BORECKA H. Fungi of the genus penicillium on apples and pears during the storage period[J]. Acta Agrobotanica,2015,30(2):213−227. doi: 10.5586/aa.1977.016
[10] VOLSCHENK Q, DU PLESSIS E M, DUVENAGE F J, et al. Effect of postharvest practices on the culturable filamentous fungi and yeast microbiota associated with the pear carpoplane[J]. Postharvest Biology and Technology,2016:118.
[11] BARKAI-GOLAN R, PASTER N. Mouldy fruits and vegetables as a source of mycotoxins: Part 1[J]. World Mycotoxin Journal,2008,1(2):147−159. doi: 10.3920/WMJ2008.x018
[12] 牛佳佳, 左倩倩, 梁慎, 等. 酥梨贮藏病害病原菌的分离鉴定及防治效果分析[J]. 保鲜与加工,2018,18(6):7−12. [NIU J, ZUO Q Q, LIANG S, et al. Isolation and identification of disease pathogenic bacteria of 'Suli' pear in storage period and analysis on the prevention effeciency[J]. Storage and Process,2018,18(6):7−12. doi: 10.3969/j.issn.1009-6221.2018.06.002 NIU J, ZUO Q Q, LIANG S, et al. Isolation and identification of disease pathogenic bacteria of ‘Suli’ pear in storage period and analysis on the prevention effeciency[J]. Storage and Process, 2018, 18(6): 7-12. doi: 10.3969/j.issn.1009-6221.2018.06.002
[13] 张秀伟, 张鹏, 李用奇. 金刺梨黑斑病病原菌的分离及鉴定[J]. 中国南方果树,2019,48(2):106−109. [ZHANG X W, ZHANG P, LI Y Q. Isolation and identification of the pathogen of black spot of golden thorn pear[J]. South China Fruit Tree,2019,48(2):106−109. ZHANG X W, ZHANG P, LI Y Q. Isolation and identification of the pathogen of black spot of golden thorn pear[J]. South China Fruit Tree, 2019, 48(2): 106-109.
[14] 任美佳, 张华平, 钟聪慧, 等. 库尔勒香梨黑斑病病原鉴定[J]. 石河子大学学报(自然科学版),2020,38(5):574−579. [REN M J, ZHANG H P, ZHONG C H, et al. Identification of the pathogens causing Korla pear black spot in alear[J]. Journal of Shihezi University Natural Science,2020,38(5):574−579. doi: 10.13880/j.cnki.65-1174/n.2020.23.021 REN M J, ZHANG H P, ZHONG C H, et al. Identification of the pathogens causing Korla pear black spot in alear [J]. Journal of Shihezi University Natural Science, 2020, 38(5): 574-579. doi: 10.13880/j.cnki.65-1174/n.2020.23.021
[15] 田艳丽, 胡旭东, 赵玉强, 等. 内蒙古马铃薯黑胫病病原菌的分离和鉴定[J]. 植物病理学报,2018,48(6):721−727. [TIAN N Y L, HU X D, ZHAO Y Q, et al. Isolation and identification of the pathogen causing potato blackleg in Inner Mongolia[J]. Acta Phytopathologyca Sinica,2018,48(6):721−727. TIAN N Y L, HU X D, ZHAO Y Q, et al. Isolation and identification of the pathogen causing potato blackleg in Inner Mongolia[J]. Acta Phytopathologyca Sinica, 2018, 48(6): 721-727.
[16] LI L, PAN H, CHEN M Y, et al. Isolation and identification of pathogenic fungi causing postharvest fruit rot of kiwifruit (Actinidia chinensis) in China[J]. Journal of Phytopathology, 2017, 165(11−12).
[17] 高振峰, 冯志宏, 赵佳. 红灯樱桃采后主要病原真菌的鉴定及Bacillus velezensis G-1天然产物广谱抑菌效果[J/OL]. 果树学报: 1−17 [2021-09-26]. https://doi.org/10.13925/j.cnki.gsxb.20210128. GAO Z F, FENG Z H, ZHAO J. Identification of main postharvest pathogenic fungi in Hongdeng cherry and the antifungal spectrum of Bacillus velezensis G-1 natural product [J/OL]. Journal of Fruit Science: 1−17[2021-09-26]. https://doi.org/10.13925/j.cnki.gsxb.20210128.
[18] 魏景超. 真菌鉴定手册[M]. 上海: 上海科学技术出版社, 1979 WEI J C. Handbook of fungi identification[M]. Shanghai: Shanghai Science and Technology Press, 1979.
[19] 葛起新, 陈育新, 徐同. 中国真菌志-拟盘多毛孢属[M]. 北京: 科学出版社, 2009 GE Q X, CHEN Y X, XU T. Chinese Fungi Chronicles-Phytochaetium[M]. Beijing: Science Press, 2009.
[20] 李焕宇, 付婷婷, 张云, 等. 5种方法提取真菌基因组DNA作为PCR模板效果的比较[J]. 中国农学通报,2017,33(16):28−35. [LI H Y, FU T T, ZHANG Y, et al. Effect comparison of five methods to extract fungal genomic DNA as PCR templates[J]. Chinese Agricultural Science Bulletin,2017,33(16):28−35. doi: 10.11924/j.issn.1000-6850.casb16060084 LI H Y, FU T T, ZHANG Y, et al. Effect comparison of five methods to extract fungal genomic DNA as PCR templates[J]. Chinese Agricultural Science Bulletin, 2017, 33(16): 28-35. doi: 10.11924/j.issn.1000-6850.casb16060084
[21] 张建航. 花生茎腐病病原菌鉴定与生物学特性及AP2/ERF的生物信息学分析[D]. 郑州: 河南农业大学, 2018 ZHANG J H. Pathogen identification and biological characteristics of peanut collar rot, bioinformatics analysis of AP2/ERF[D]. Zhengzhou: Henan Agricultural University, 2018.
[22] 周倩, 冯肖, 纪淑娟, 等. 蓝莓果实常温贮藏过程中表面病原真菌的分离与鉴定[J]. 中国食品学报,2020,20(2):271−279. [ZHOU Q, FENG X, JI S J, et al. Isolation and identification of surface pathogenic fungi for blueberry during storage at room temperature[J]. Journal Chinese Institute of Food Science and Technology,2020,20(2):271−279. doi: 10.16429/j.1009-7848.2020.02.033 ZHOU Q, FENG X, JI S J, et al. Isolation and identification of surface pathogenic fungi for blueberry during storage at room temperature[J]. Journal Chinese Institute of Food Science and Technology, 2020, 20(2): 271-279. doi: 10.16429/j.1009-7848.2020.02.033
[23] AL-NAJADA A R, GHERBAWY Y A. Molecular identification of spoilage fungi isolated from fruit and vegetables and their control with chitosan[J]. Food Biotechnology,2015,29(2):166−184. doi: 10.1080/08905436.2015.1027222
[24] 刘行风, 刘东, 陶磊, 等. 黑龙江省黄瓜链格孢叶斑病病原鉴定及生物学特性研究[J]. 中国蔬菜,2021(8):80−86. [LIU X F, LIU D, TAO L, et al. Pathogen identification and biological characteristics of cucumber alternaria leaf spot of in Heilongjiang Province[J]. Chinese Vegetables,2021(8):80−86. doi: 10.19928/j.cnki.1000-6346.2021.1034 LIU X F, LIU D, TAO L, et al. Pathogen identification and biological characteristics of cucumber alternaria leaf spot of in Heilongjiang Province[J]. Chinese Vegetables, 2021(8): 80-86. doi: 10.19928/j.cnki.1000-6346.2021.1034
[25] 张驰成. 热带牧草真菌病害调查、病原鉴定及基础生物学特性研究[D]. 海口: 海南大学, 2016 ZHANG C C. Survey, identification and biological characteristics determination of tropical forage fungal diseases [D]. Haikou: Hainan University, 2016.
[26] 王雪, 尹桥秀, 李冬雪, 等. 茶褐枯病病原菌(Colletotrichum camelliae)生物学特性研究[J]. 中国植保导刊,2019,39(6):5−11. [WANG X, YIN Q X, LI D X, et al. Biological characteristics of Colletotrichum camelliae causing tea brown blight disease[J]. China Plant Protection,2019,39(6):5−11. doi: 10.3969/j.issn.1672-6820.2019.06.001 WANG X, YIN Q X, LI D X, et al. Biological characteristics of Colletotrichum camelliae causing tea brown blight disease[J]. China Plant Protection, 2019, 39(6): 5-11. doi: 10.3969/j.issn.1672-6820.2019.06.001
[27] 马文娟, 梁向东, 刘月廉. 广西玉林西番莲果腐病病原菌的分离与鉴定[J]. 黑龙江农业科学,2021(10):43−45, 54. [MA W J, LIANG X D, LIU Y L. Isolation and identification of the pathogen of passion fruit rot in Yulin, Guangxi[J]. Heilongjiang Agricultural Sciences,2021(10):43−45, 54. MA W J, LIANG X D, LIU Y L. Isolation and identification of the pathogen of passion fruit rot in Yulin, Guangxi[J]. Heilongjiang Agricultural Sciences, 2021(10): 43-45, 54.
[28] 周英. 奉新猕猴桃3种真菌病害病原鉴定、生物学特性及药剂筛选研究[D]. 南昌: 江西农业大学, 2019 ZHOU Y. Identification, biological characteristics and fungicide screening of 3 fungal pathogens from kiwifruit in Fengxi County [D]. Nanchang: Jiangxi Agricultural University, 2019.
[29] 朱从一. 柑橘采后青霉属病原菌生物学特性及其系统发育[D]. 武汉: 华中农业大学, 2011 [ZHU C Y, The biological characteristics and molecular phylogeny analyses of Penicillium spp. that causing the mold fruit of citrus. [D]. Wuhan: Huazhong Agricultural University, 2011.
[30] 伍晓丽, 王钰, 刘飞, 等. 黄连根腐病镰刀菌属病原真菌鉴定[J]. 中国中药杂志,2020,45(6):1323−1328. [WU X L, WANG Y, LIU F, et al. Identification of the pythium pathogen responsible for root rot of Coptis chinensis[J]. China Journal of Chinese Materia Medica,2020,45(6):1323−1328. doi: 10.19540/j.cnki.cjcmm.20200112.102 [WU X L, WANG Y, LIU F, et al. Identification of the pythium pathogen responsible for root rot of Coptis chinensis[J]. China Journal of Chinese Materia Medica, 2020, 45(6): 1323- doi: 10.19540/j.cnki.cjcmm.20200112.102
-
期刊类型引用(5)
1. 尚学钰,美合日班,苏玲,王琦. 黑木耳可溶性膳食纤维功能特性和降脂活性研究. 食品工业科技. 2025(02): 112-121 .  本站查看
本站查看
2. 张潇予,王丹妮,柴欣,于卉娟,崔英,王跃飞. 补骨脂的质量特征解析及其在减毒工艺中的应用. 中草药. 2024(08): 2784-2791 .  百度学术
百度学术
3. 马力亚,李梅梅,黄玉卓,舒劲. 四神丸治疗溃疡性结肠炎的研究概况. 中医药临床杂志. 2024(05): 987-994 .  百度学术
百度学术
4. 徐波,陈天天,杜薛平,张海峰,陈伟,黄凯健,董大勇. 益肾化痰祛瘀方治疗绝经后骨质疏松症(肾虚血瘀型)的效果及对氧化应激的影响. 中医药学报. 2024(09): 60-63 .  百度学术
百度学术
5. 钟婉滢,苗建银,叶灏铎,马凤,胡一晨. 藜麦蛋白肽的酶解制备及体外降血脂与降尿酸活性研究. 食品工业科技. 2023(23): 156-166 .  本站查看
本站查看
其他类型引用(1)






 下载:
下载:








 下载:
下载:



