Optimization of Polysaccharide Extraction Process from Tuber liyuanum and Analysis of Its Monosaccharide Composition
-
摘要: 目的:本文以梨园块菌Tuber liyuanum为实验材料,优化和确定其多糖的提取工艺,并对单糖组分进行分析。方法:在单因素实验的基础上,采用正交试验设计、优化和确定水提醇沉法提取多糖的最适工艺条件;通过1-苯基-3-甲基-5-吡唑啉酮(1-phenyl-3-menthy-5pyrazolone,PMP)柱前衍生化HPLC法分析梨园块菌单糖组分。结果:梨园块菌多糖的最佳提取工艺条件为提取温度90 ℃、提取时间60 min、料液比1:25 g/mL,在此条件下多糖得率为10.57% ± 0.31%。梨园块菌多糖主要由D-葡萄糖和少量的D-甘露糖、D-半乳糖组成,其物质的量之比为1:0.023:0.006。结论:采用水提醇沉法,在最佳提取工艺条件下能够获得较高的梨园块菌多糖得率,方法简单且稳定可行;使用柱前衍生化HPLC法测定梨园块菌多糖中的单糖组成,具有操作简便、可重复性和准确度高的优点,可为进一步研究梨园块菌多糖提供理论依据。
-
关键词:
- 梨园块菌 /
- 多糖 /
- 提取工艺 /
- 单糖组成 /
- 1-苯基-3-甲基-5-吡唑啉酮柱前衍生 /
- 高效液相色谱(HPLC)
Abstract: Objective: In this study, Tuber liyuanum was used as the experimental material to optimize and determine the extraction process of polysaccharides, and analyze the monosaccharide components. Methods: On the basis of single factor experiment, L9(34) orthogonal experiment was used to design, optimize and determine the optimum technological conditions for extracting polysaccharides by water extraction and alcohol precipitation, and the monosaccharide components of T. liyuanum were analyzed by 1-phenyl-3-methyl-5-pyridine pre-column derivatization HPLC with oxazolinone. Results: The optimum process conditions of extracting polysaccharides from T. liyuanum were as follows: Extraction temperature was 90 ℃, extraction time was 60 min, and solid-to-liquid ratio was 1:25 g/mL. Under these conditions, the yield of polysaccharides was 10.57% ± 0.31%. The polysaccharides of T. liyuanum were mainly composed of D-glucose and a small amount of D-mannose and D-galactose. And the ratio of its substances was 1:0.023:0.006. Conclusion: The water extraction and alcohol precipitation method could obtain a higher yield of T. liyuanum polysaccharides under the optimal extraction process conditions, which was simple, stable and feasible. The determination of monosaccharides composition in T. liyuanum polysaccharides by pre-column derivatization HPLC was simple, reproducible and accurate, which could provide a theoretical basis for further research on T. liyuanum polysaccharides in pear orchards. -
块菌Tuber,又称猪拱菌、无娘果、松毛茯苓等,在商业上常被称为松露,隶属子囊菌亚门盘菌目块菌科,是一类与树木共生和生长在地下的名贵野生食用菌,因其独特的香气,深受国际美食界的喜爱[1-4]。块菌在北半球有着较广的分布,在欧洲主要分布在地中海沿岸国家,在我国的分布以云南、四川、西藏等地为主[5]。块菌子实体含有丰富的多糖、氨基酸、多不饱和脂肪酸和蛋白质等营养成分,在块菌多糖的研究中,已有研究表明大多数块菌中的多糖主要组成成分是鼠李糖、半乳糖、葡萄糖、甘露糖,也有部分块菌中存在着特殊种类的多糖组成,如岩藻糖、阿拉伯糖等。在印度块菌(T. indicum)、夏块菌(T. aestivum)和棕红块菌(T. rufum)的子实体中多糖组成主要包括葡萄糖、甘露糖、半乳糖、鼠李糖、阿拉伯糖和岩藻糖等单糖[6-9]。目前,常用的多糖提取方法主要有水浴加热提取法、酶提取法、微波辅助法、超声波辅助法、碱提法和酸提法等,其中水提醇沉法已广泛应用于产业化且方法成熟,此法操作简单、成本低、能够最大程度减少多糖在提取过程中水解[10]。由于多糖类物质存在较好的生物活性,具有抗氧化、抗肿瘤、增强免疫力、降血糖和降血脂等功效[11-16],在食品、药品和化妆品行业中具有较好的应用前景。
在多糖分析中,由于多糖化合物种类较多、化学性质相似,经硫酸-苯酚、硫酸-蒽酮等显色反应后,使用紫外分光光度法测定其总多糖含量[17-19]。通常情况下,多糖需要进行酸水解以后使其糖苷键断裂,再通过色谱技术测定水解液中的单糖和含量。目前,常用的色谱分析方法包括薄层色谱法(TLC)、高效液相色谱法(HPLC)和气相色谱法(GC)等多种分析方法[20-22],因多糖本身存在挥发性和热稳定性较低等问题,在使用这些分析方法时都会存在着一定的不足之处[23]。例如,采用GC法检测时需要对其衍生化后才可进行,衍生化过程复杂且色谱峰容易出现异构化裂分,不适宜对糖醛酸和氨基糖的分析,适用范围不广泛。其中,HPLC法具有良好的稳定性以及可供选择的色谱柱类型较多,是单糖组成分析中应用最广泛的技术之一。但糖类物质无紫外吸收基团,不能直接使用HPLC-UV法进行定量分析,在对单糖进行衍生化后,可以采用HPLC-UV法进行分析且具有较高的灵敏度[24]。玄光善等[25]采用1-苯基-3-甲基-5-吡唑啉酮(1-phenyl-3-menthy-5-pyrazolone,PMP)柱前衍生化HPLC法,分析了桦褐孔菌Inonotus obliquus多糖的单糖组成,此法反应条件温和,分析快速、稳定,比柱前衍生化气相色谱法更为简便[26-27]。
本研究以梨园块菌Tuber liyuanum为实验材料,采用水提醇沉法提取多糖,对提取工艺进行优化,并建立多糖的单糖组成分析检测方法,为梨园块菌多糖的开发利用奠定基础。
1. 材料与方法
1.1 材料与仪器
新鲜样品 2020年7月采购于云南省昆明市盘龙区双龙乡,经中国科学院昆明植物研究所刘建伟,进行分子鉴定为梨园块菌Tuber liyuanum;单糖标准品D-葡萄糖(HPLC≥98%)、D-甘露糖(HPLC≥98%)、D-半乳糖(HPLC≥98%)、L-岩藻糖(HPLC≥98%) 北京索莱宝科技有限公司;D-无水葡萄糖、苯酚、氢氧化钠、盐酸、磷酸二氢钾 国药集团化学试剂有限公司;浓硫酸 重庆川东化工(集团)有限公司;无水乙醇 天津市大茂化学试剂厂;三氟乙酸(TFA)、1-苯基-3-甲基-5-吡唑啉酮(PMP) 上海麦克林生化科技有限公司;蒸馏水 实验室自制;所有试剂均为分析纯。
Agilent 1260型高效液相色谱仪 美国安捷伦公司;BUCHI R-100旋转蒸发仪 瑞士步琦有限公司;SHB-Ⅲ S循环水式多用真空泵 郑州市亚荣仪器有限公司;3H16RI智能高速冷冻离心机 湖南赫西仪器装备有限公司;SCIENTZ-10N冷冻干燥机 宁波新芝生物科技股份有限公司;ME 104E型万分之一电子天平 梅特勒-托利多仪器(上海)有限公司。
1.2 实验方法
1.2.1 样品预处理及多糖提取
将块菌样品洗净后,在40 ℃条件下风干至恒重后,粉碎,加入适量无水乙醇在45 ℃水浴中搅拌浸提3次,每次1 h,将浸泡过后的样品粉末晾干后备用。
多糖提取工艺:称取梨园块菌样品粉末,按比例加入蒸馏水进行加热回流提取,提取结束后收集上清液并趁热过滤,避免冷却后影响溶液过滤的速度;将滤液浓缩至小体积后,加入多糖浓缩液1/3体积的Sevage试剂(V三氯甲烷:V正丁醇=4:1)进行震荡、静置和分液等一系列操作去除蛋白,然后将4倍体积无水乙醇加入上清液中于4 ℃静置过夜,促使多糖充分析出,使用离心方式收集沉淀并冷冻干燥得到梨园块菌粗多糖。
1.2.2 多糖含量的测定
参照苯酚-硫酸法测定样品的总糖含量[28]。
1.2.2.1 葡萄糖标准曲线的绘制
精密称取干燥的无水葡萄糖标准品0.1003 g于100 mL容量瓶中,加蒸馏水定容至刻度,配成浓度为1.003 mg/mL的葡萄糖标准对照品溶液,备用。准确吸取葡萄糖标准对照品溶液1.0、2.0、3.0、4.0、5.0、6.0 mL分别定容至50 mL作稀释液。取各稀释液1.0 mL于试管中,加蒸馏水和5%苯酚试液各1.0 mL,摇匀,迅速加入5.0 mL浓硫酸,混匀后放置5 min置于沸水浴中加热20 min,冷却至室温在490 nm波长处测定其吸光度值A,以蒸馏水代替葡萄糖溶液参与反应的体系作空白对照。以浓度x为横坐标、吸光度值A为纵坐标,建立标准曲线回归方程:
1.2.2.2 供试品溶液的制备
配成浓度是1.0 mg/mL的粗多糖溶液,准确吸取1.0 mL于试管中,按照上述的方法测定供试品的吸光度值A,计算梨园块菌的多糖得率。
多糖得率(%)=(粗多糖质量×多糖含量/原料质量)×100
1.2.3 单因素实验
针对多糖提取工艺的研究,其中影响多糖得率最主要的因素包括提取料液比、提取时间和提取温度等,在本论文中分别选择不同的因素条件进行多糖得率的单因素实验考察。
1.2.3.1 提取料液比对多糖得率的影响
每次实验固定提取温度为90 ℃、提取时间为90 min,分别提取3次,考察不同料液比(1:10、1:15、1:20、1:25、1:30 g/mL)条件下对多糖得率的影响。
1.2.3.2 提取时间对多糖得率的影响
固定料液比为1:20 g/mL、提取温度为90 ℃,分别提取3次,考察不同提取时间(30、60、90、120、150 min)条件下对多糖得率的影响。
1.2.3.3 提取温度对多糖得率的影响
固定料液比为1:20 g/mL、提取时间为90 min,分别提取3次,考察不同提取温度(80、85、90、95、100 ℃)条件下对多糖得率的影响。
1.2.4 正交试验设计
在上述单因素实验的基础上,采用L9(34)的正交试验设计,试验的因素与水平见表1。
表 1 试验的因素水平Table 1. Factor and level of the experiment水平 因素 A-料液比(g/mL) B-提取时间(min) C-提取温度(℃) 1 1:20 30 85 2 1:25 60 90 3 1:30 90 95 1.2.5 单糖组成测定
样品制备:准确称取2 mg样品于5 mL安瓿瓶中,加入2 mol/L三氟乙酸1 mL氮气封口后110 ℃水解4 h,蒸干,用甲醇洗涤后蒸干,反复3次去除残留的三氟乙酸,再加1 mL蒸馏水溶解得2 mg/mL多糖水解液。取200 μL水解液与200 μL 0.6 mol/L的NaOH溶液,再加入0.5 mol/L的1-苯基-3-甲基-5-吡唑啉酮(PMP)甲醇溶液200 μL,混匀后在70 ℃水浴中反应30 min,冷却至室温加入0.6 mol/L的HCl溶液200 μL以中和NaOH。然后再加入1 mL三氯甲烷,旋涡振荡、离心,去三氯甲烷层,重复萃取3次除去多余的PMP,水相用0.45 μm微孔滤膜过滤,滤液稀释2倍用于HPLC分析。
色谱条件:采用Agilent 1260 型高效液相色谱系统分析,Agilent ZORBAX SB-C18色谱柱(4.6×150 mm,5 μm);流速为1.0 mL/min;柱温30 ℃;进样量20 μL;检测波长为250 nm。流动相A为0.02 mol/L的KH2PO4缓冲液,流动相B为乙腈,在0 min→10 min→30 min内变化乙腈的比例为15%→20%→25%进行梯度洗脱。
1.3 数据处理
每组试验平行重复3次,用SPSS 17.0软件对试验结果进行t-检验和LSD多重比较分析,结果以均值±标准差(Mean ± SD)表示,用Excel 2010和Origin软件进行作图。
2. 结果与分析
2.1 单因素实验结果
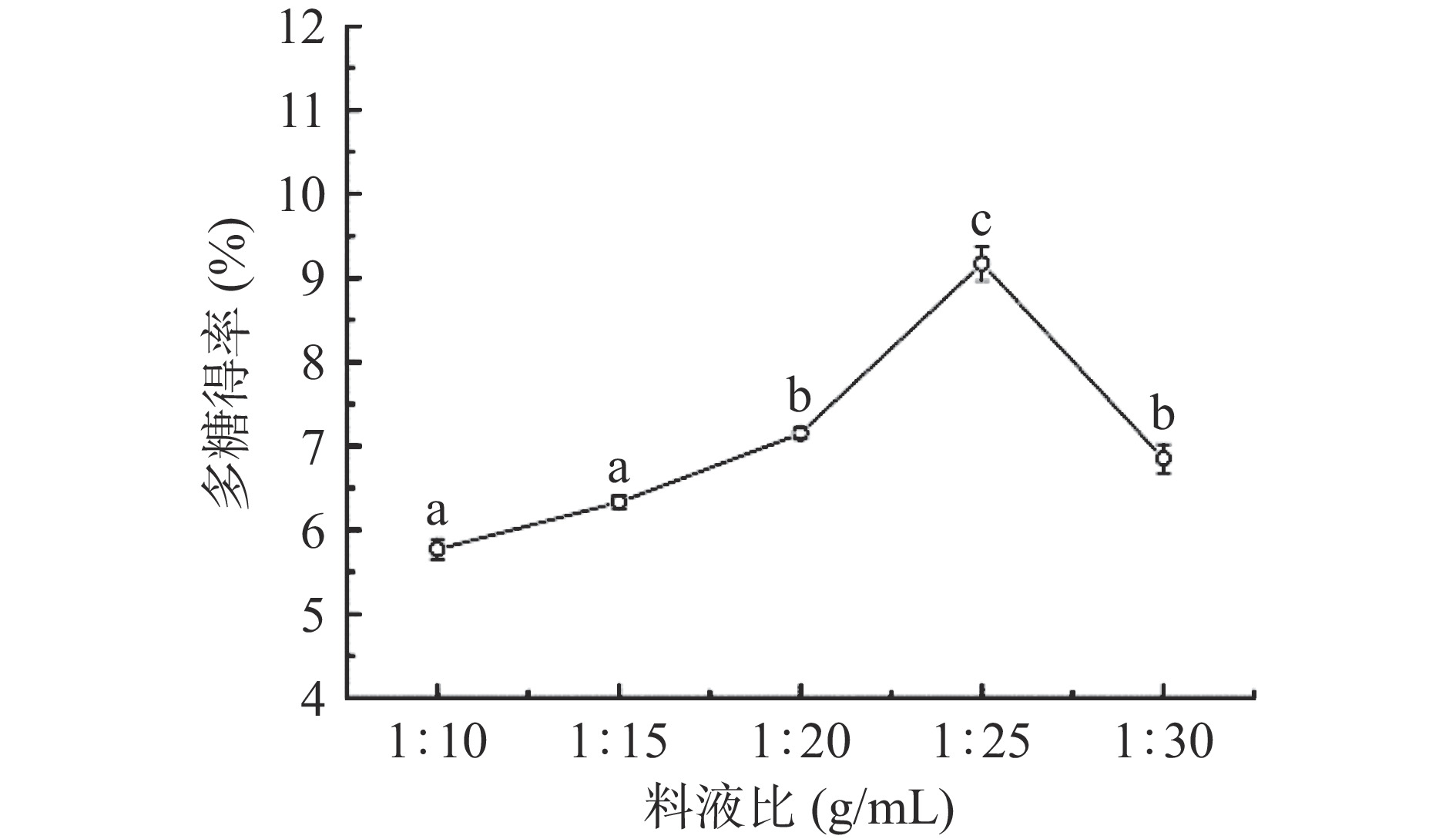
2.1.1 料液比对多糖得率的影响
由图1可知,当提取所用料液比在1:10~1:25 g/mL之间,随着料液比体积的增加,提取多糖的得率也随之增高,且在料液比为1:25 g/mL时,得率达到最大值9.17%;而后再提高料液比体积,多糖的得率并未提高,反而呈现出下降趋势。在较低料液比体积提取下,由于提取溶剂较少,多糖没有得到充分溶解,使得得率不高;此外,增加溶剂有助于提高多糖的溶出,但提高溶剂量会导致浓缩过程时间较长,可能会造成多糖的分解,从而影响多糖的得率[32]。因此,选择料液比为1:25(g/mL)作为后续试验优化的参数条件。
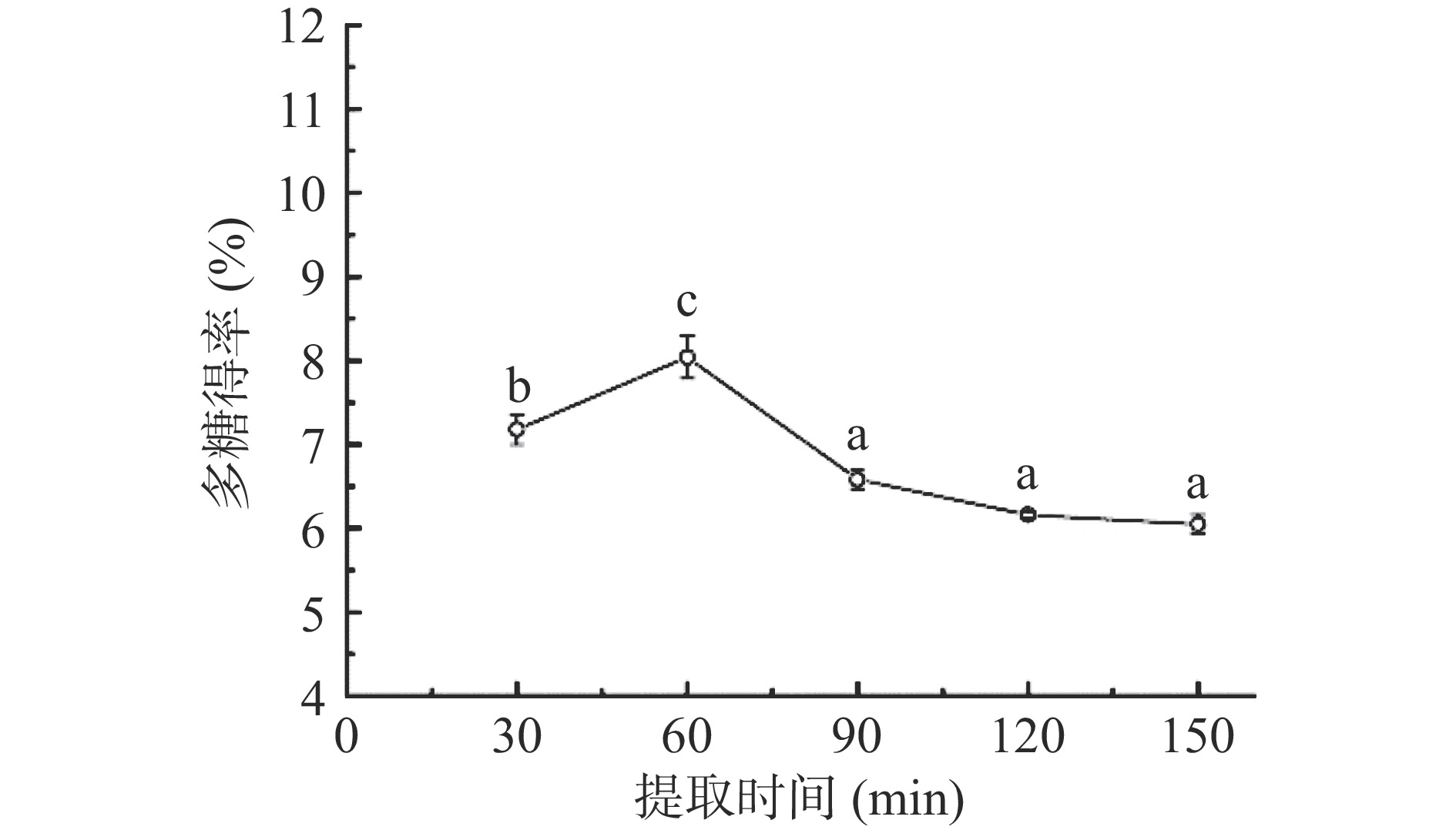
2.1.2 提取时间对多糖得率的影响
由图2可知,当提取时间在30~60 min之间,随着提取时间的延长,多糖的得率也随之增高,提取时间在60 min时,得率达到最大值8.04%;而后再延长提取时间,得率反而下降并趋于平稳。这可能是由于提取时间短,多糖提取不充分,导致得率低;提取时间长使多糖不断溶出,与此同时在长时间的高温加热下容易造成多糖结构的破坏和分解,也可能存在因多糖分子内部沸腾引起扩散通道堵塞的情况,使多糖难以溶出导致得率降低[33-34]。因此,选择提取时间为60 min作为后续试验优化的参数条件。
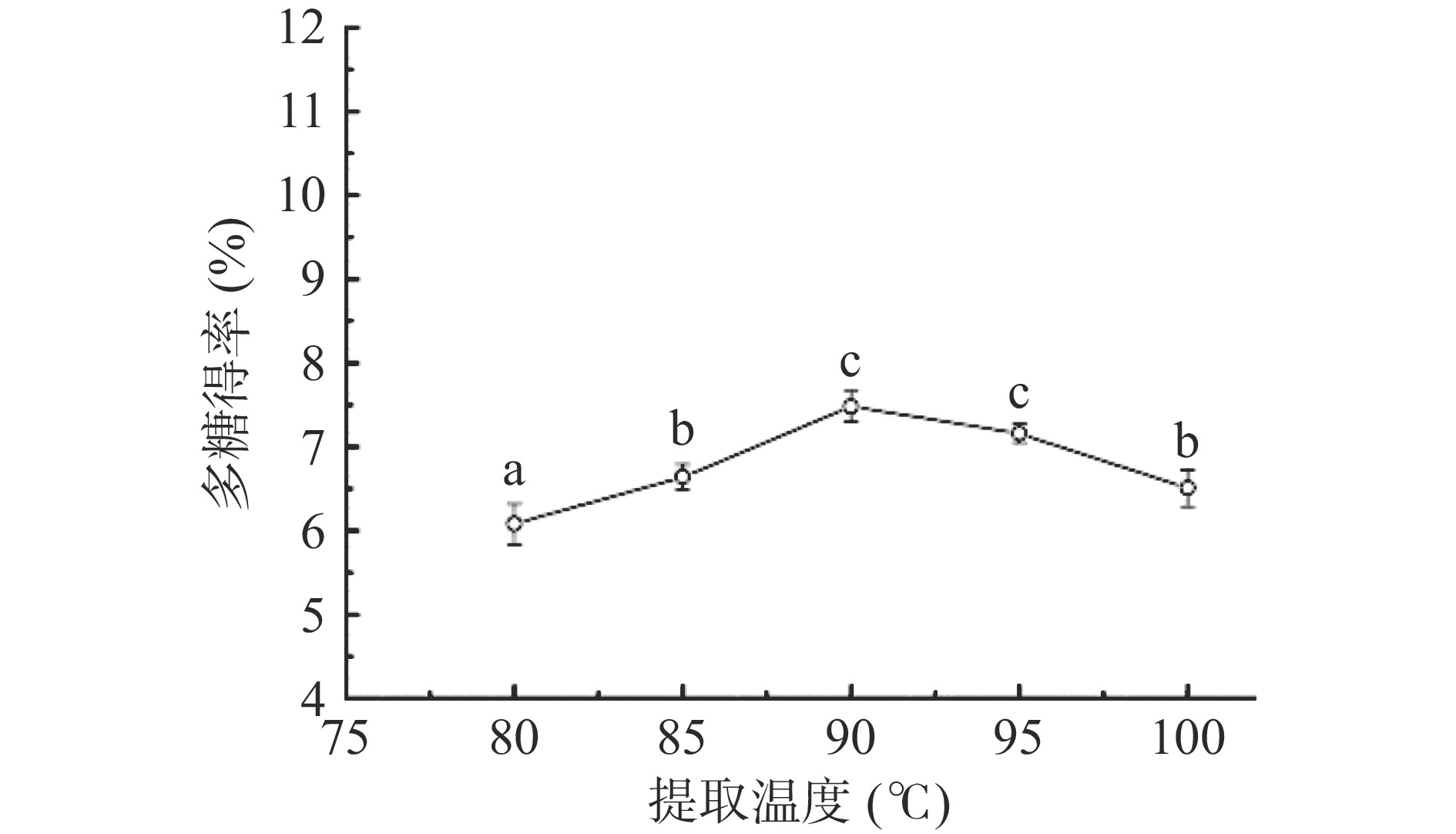
2.1.3 提取温度对多糖得率的影响
由图3可知,随着提取温度的升高,多糖的得率也随之提高,当提取温度达到90 ℃时,多糖的得率最大,为7.48%;之后进一步升高提取温度,得率出现下降。分析原因可能是在一定温度范围内随着温度升高,加快了分子间的运动,有助于水溶性多糖溶出[35]。但温度超过范围,会存在高温加速破坏多糖分子结构的情况,进而导致多糖得率的降低[36]。因此,可选择提取温度为90 ℃作为后续试验优化的参数条件。
2.2 多糖得率正交试验设计结果与分析
根据正交试验设计,考察料液比(g/mL)、提取时间(min)、提取温度(℃)等因素的相互作用对梨园块菌多糖得率的影响,进行试验设计结果的极差分析(表2)和对因素水平间进行方差分析(表3),结果如下所示。
表 2 梨园块菌多糖提取的L9(34)正交试验设计Table 2. L9(34) orthogonal design of polysaccharide extraction from T. liyuanum试验编号 A-料液比 B-提取时间 C-提取温度 得率(%) 1 1 1 1 8.26 2 1 2 2 9.83 3 1 3 3 8.64 4 2 1 2 9.11 5 2 2 3 9.89 6 2 3 1 8.42 7 3 1 3 8.67 8 3 2 1 7.95 9 3 3 2 8.51 K1 26.73 26.04 24.63 K2 27.42 27.67 27.45 K3 25.13 25.57 27.20 X1 8.91a 8.68b 8.21b X2 9.14a 9.22a 9.15a X3 8.38b 8.52b 9.07a R 0.76 0.70 0.94 注:表中不同上标小写字母a和b表示差异显著(P<0.01)。 表 3 因素水平间梨园块菌多糖得率的方差分析Table 3. Variance analysis of yield of polysaccharide from T. liyuanum差异来源 离差平方和 df 均方 F P A 0.000 2 0.000 10.413 0.001 B 0.000 2 0.000 9.165 0.001 C 0.000 2 0.000 18.385 0.000 误差 0.000 20 0.000 总计 0.001 26 根据表2和表3的结果分析可知,在方差分析中因素A、B、C之间对梨园块菌多糖得率具有极显著的差异影响(P<0.01)。通过正交试验设计的极差分析可得,在料液比、提取时间、提取温度等因素之间相互作用中,对梨园块菌多糖得率的影响程度次序按由大到小排列为:C(提取温度)>A(料液比)>B(提取时间)。综上所述,多糖提取的理论最佳工艺水平组合为A2B2C2,即料液比为1:25 g/mL、提取时间为60 min、提取温度为90 ℃。
2.3 试验验证
采用上述正交试验设计中所获得的理论优水平组合,对梨园块菌多糖提取试验验证,进行3次重复试验,测得多糖的平均得率为10.57% ± 0.31%。因此,该提取工艺条件具有较好的可行性,可供后续梨园块菌多糖提取研究作参考。
2.4 单糖组成分析
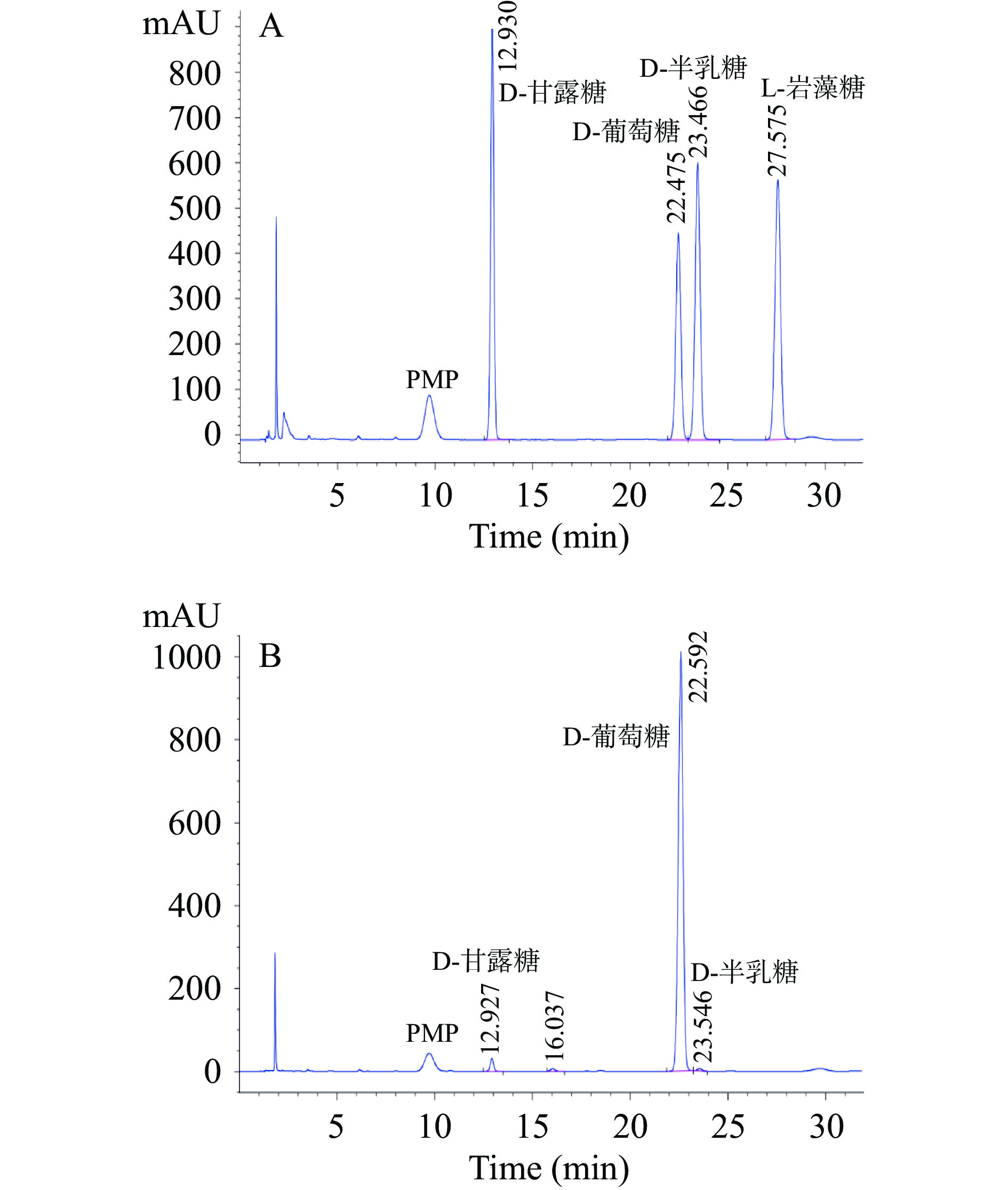
2.4.1 标准单糖的衍生化分析
在本项研究工作开展之前,本研究团队查阅了大量文献资料发现在块菌多糖研究中,主要是由鼠李糖、半乳糖、葡萄糖、甘露糖和少量的岩藻糖、阿拉伯糖等单糖组成。因此,在研究过程中经综合考虑后选择其中4个标准单糖作为标准对照进行初步研究分析与探讨。在这里,分别选择了D-甘露糖、L-岩藻糖、D-葡萄糖和D-半乳糖等标准品并进行精确称量,加水溶解配成浓度为1 mg/mL的混标储备液,再取混标储备液稀释2、4、8、16、32、64倍配成梯度工作液进行PMP衍生化处理和HPLC分析。以各单糖组分的浓度为横坐标(x)、峰面积为纵坐标(y)建立标准曲线,结果见表4,根据信噪比计算检测限(RSN=3)和定量限(RSN=10),回归方程相关系数均为0.99以上,表明各单糖衍生物浓度在3.906~250 μg/mL之间线性关系较好。
表 4 四种单糖组分的标准曲线、检测限和定量限Table 4. Calibration curves, limits of detection (LODs) and limits of quantitation (LOQs) for 4 different monosaccharides标准单糖 线性回归方程 相关系数 线性浓度范围(μg/mL) 检测限(μg/mL) 定量限(μg/mL) D-甘露糖 y=42.655x−396.257 0.9954 3.906~250 0.414 1.380 D-葡萄糖 y=32.479x−292.796 0.9922 0.751 2.504 D-半乳糖 y=44.166x−321.332 0.9959 0.449 1.497 L-岩藻糖 y=45.885x−366.053 0.9943 0.563 1.878 2.4.2 梨园块菌单糖组成测定结果
对工艺优化后提取的梨园块菌多糖样品进行单糖组成测定,经衍生化后进行HPLC分析,结果与标准单糖色谱图进行对比(图4),判断出多糖水解产物中单糖的种类。在色谱图16.037 min处出现一个无标准单糖对照的未知峰,由于在本项研究工作中只采用了4种标准单糖进行对照分析与初步探讨,可能存在标准单糖种类和数量选择不合适的问题,对此处出现的未知峰猜测可能是其他类型的单糖,针对这个问题也进行了相关文献的查阅,希望能够根据已报道的相关同类文献资料中出现的物质进行简单比较分析;遗憾的是同类相关文献仅查阅到有限的数量并且不能进行比较分析,无法推测该未知峰的单糖种类。因梨园块菌单糖组成的分析研究工作报道较少,可供参考的文献资料有限,本研究团队将在后续的工作中围绕这个未知峰进行探索分析和完善,以明确该未知峰的单糖种类。综上,与4种标准单糖对比分析后,可推断出梨园块菌多糖主要组成成分是D-葡萄糖,与少量的D-甘露糖、D-半乳糖等共同组成,其物质的量比为1:0.023:0.006。本实验的结果与李美凤等[37]的研究结果相似,在梨园块菌多糖中的葡萄糖含量最高,占其多糖组成的90%以上,其余由少量的甘露糖和半乳糖共同组成,同时也说明了梨园块菌多糖中的单糖组成分析检测结果准确,这一结果也得到其他相关研究[38-39]的证实。
3. 结论
采用水提醇沉方式提取梨园块菌多糖,以单因素条件筛选和正交试验相结合方式对其提取工艺进行优化,最终选择结果良好的工艺参数并确定:提取温度90 ℃、提取时间60 min和料液比1:25 g/mL,通过验证后在上述参数条件下,梨园块菌的多糖得率达10.57% ± 0.31%;对所得多糖样品进行单糖组成测定,经水解、PMP衍生化试剂反应和HPLC分析,结果显示梨园块菌多糖主要由D-葡萄糖以及少量的D-甘露糖、D-半乳糖等单糖组成,其物质的量比为1:0.023:0.006。以上结果可为梨园块菌多糖开发利用奠定基础。
-
表 1 试验的因素水平
Table 1 Factor and level of the experiment
水平 因素 A-料液比(g/mL) B-提取时间(min) C-提取温度(℃) 1 1:20 30 85 2 1:25 60 90 3 1:30 90 95 表 2 梨园块菌多糖提取的L9(34)正交试验设计
Table 2 L9(34) orthogonal design of polysaccharide extraction from T. liyuanum
试验编号 A-料液比 B-提取时间 C-提取温度 得率(%) 1 1 1 1 8.26 2 1 2 2 9.83 3 1 3 3 8.64 4 2 1 2 9.11 5 2 2 3 9.89 6 2 3 1 8.42 7 3 1 3 8.67 8 3 2 1 7.95 9 3 3 2 8.51 K1 26.73 26.04 24.63 K2 27.42 27.67 27.45 K3 25.13 25.57 27.20 X1 8.91a 8.68b 8.21b X2 9.14a 9.22a 9.15a X3 8.38b 8.52b 9.07a R 0.76 0.70 0.94 注:表中不同上标小写字母a和b表示差异显著(P<0.01)。 表 3 因素水平间梨园块菌多糖得率的方差分析
Table 3 Variance analysis of yield of polysaccharide from T. liyuanum
差异来源 离差平方和 df 均方 F P A 0.000 2 0.000 10.413 0.001 B 0.000 2 0.000 9.165 0.001 C 0.000 2 0.000 18.385 0.000 误差 0.000 20 0.000 总计 0.001 26 表 4 四种单糖组分的标准曲线、检测限和定量限
Table 4 Calibration curves, limits of detection (LODs) and limits of quantitation (LOQs) for 4 different monosaccharides
标准单糖 线性回归方程 相关系数 线性浓度范围(μg/mL) 检测限(μg/mL) 定量限(μg/mL) D-甘露糖 y=42.655x−396.257 0.9954 3.906~250 0.414 1.380 D-葡萄糖 y=32.479x−292.796 0.9922 0.751 2.504 D-半乳糖 y=44.166x−321.332 0.9959 0.449 1.497 L-岩藻糖 y=45.885x−366.053 0.9943 0.563 1.878 -
[1] LEE H, NAM K, ZAHRA Z, et al. Potentials of truffles in nutritional and medicinal applications: A review[J]. Fungal Biology and Biotechnology,2020,7(1):1−10. doi: 10.1186/s40694-019-0091-8
[2] LIU C J, LI Y. Evaluation of the volatile profile of Tuber liyuanum by HS-SPME with GC-MS[J]. Natural Product Research,2017,31(8):972−975. doi: 10.1080/14786419.2016.1253082
[3] 冯涛, 水梦竹, 宋诗清, 等. 云南新鲜松露和干制松露挥发性风味成分的对比分析[J]. 精细化工,2019,36(2):262−270. [FENG T, SHUI M Z, SONG S Q, et al. Comparative analysis of volatile flavor compounds of fresh truffles and dried truffles in Yunnan[J]. Fine Chemicals,2019,36(2):262−270. FENG T, SHUI M Z, SONG S Q, et al. Comparative Analysis of volatile flavor compounds of fresh truffles and dried truffles in Yunnan[J]. Fine Chemicals, 2019, 36(2): 262-270.
[4] LIU R S, LI D C, LI H M, et al. Evaluation of aroma active compounds in Tuber fruiting bodies by gas chromatography-olfactometry in combination with aroma reconstitution and omission test[J]. Applied Microbiology and Biotechnology,2012,94(2):353−363. doi: 10.1007/s00253-011-3837-7
[5] TEJEDOR-CALVO E, AMARA K, REIS F S, et al. Chemical composition and evaluation of antioxidant, antimicrobial and antiproliferative activities of Tuber and Terfezia truffles[J]. Food Research International,2021,140:110071. doi: 10.1016/j.foodres.2020.110071
[6] LUO Q, ZHANG J, YAN L, et al. Composition and antioxidant activity of water-soluble polysaccharides from Tuber indicum[J]. Journal of Medicinal Food,2011,14(12):1609−1616. doi: 10.1089/jmf.2011.1659
[7] BHOTMANGE D, WALLENIUS J H, SINGHAL R S, et al. Enzymatic extraction and characterization of polysaccharide from Tuber aestivum[J]. Bioactive Carbohydrates and Dietary Fibre,2017,10:1−9. doi: 10.1016/j.bcdf.2017.02.001
[8] PATTANAYAK M, SAMANTA S, MAITY P, et al. Polysaccharide of an edible truffle Tuber rufum: Structural studies and effects on human lymphocytes[J]. International Journal of Biological Macromolecules,2017,95:1037−1048. doi: 10.1016/j.ijbiomac.2016.10.092
[9] 李杨, 郭顺星, 陈娟. 珍稀名贵食药用真菌块菌属的化学成分及药理活性研究进展[J]. 菌物学报,2021,40(4):873−894. [LI Y, GUO S X, CHEN J. Research progress on chemical constituents and pharmacological effects of Tuber (Pezizales, Ascomycota)[J]. Mycosystema,2021,40(4):873−894. LI Y, GUO S X, CHEN J. Research progress on chemical constituents and pharmacological effects of Tuber (Pezizales, Ascomycota)[J]. Mycosystema, 2021, 40(4): 873-894.
[10] 张家晨, 秦良云, 刘怡, 等. 响应面法优化西藏金耳多糖提取工艺研究[J]. 食品研究与开发,2020,41(11):113−117. [ZHANG J C, QIN L Y, LIU Y, et al. Study on Optimization of polysaccharide extraction from Tremella aurantialba in Tibet by response surface methodology[J]. Food Research and Development,2020,41(11):113−117. ZHANG J C, QIN L Y, LIU Y, et al. Study on Optimization of Polysaccharide extraction from Tremella aurantialba in Tibet by response surface methodology[J]. Food Research and Development, 2020, 41(11): 113-117.
[11] 李晓勇, 范黎. 李氏块菌提取物抗氧化活性的研究[J]. 菌物学报,2015,34(3):465−472. [LI X Y, FAN L. Antioxidant activities of different extracts from Tuber liyuanum[J]. Mycosystema,2015,34(3):465−472. LI X Y, FAN L. Antioxidant activities of different extracts from Tuber liyuanum[J]. Mycosystema, 2015, 34(3): 465-472.
[12] 张存艳, 魏蔼玲, 岳茂林, 等. 不同干燥方式对松露多糖含量及其抗氧化活性的影响[J]. 食品工业,2020,41(12):214−218. [ZHANG C Y, WEI A L, YUE M L, et al. Effects of different drying methods on polysaccharide content and its antioxidant activity from truffle[J]. The Food Industry,2020,41(12):214−218. ZHANG C Y, WEI A L, YUE M L, et al. Effects of different drying methods on polysaccharide content and its antioxidant activity from truffle[J]. The Food Industry, 2020, 41(12): 214-218.
[13] LI X, ZHU Z, YE L, et al. Comparison of the partial structure and antioxidant activity of polysaccharides from two species of Chinese truffles[J]. Molecules,2020,25(18):4345. doi: 10.3390/molecules25184345
[14] MARATHE S J, HAMZI W, BASHEIN A M, et al. Anti-angiogenic and anti-inflammatory activity of the summer truffle (Tuber aestivum Vittad.) extracts and a correlation with the chemical constituents identified therein[J]. Food Research International,2020,137:109699. doi: 10.1016/j.foodres.2020.109699
[15] WU Z, MEENU M, XU B. Nutritional value and antioxidant activity of Chinese black truffle (Tuber indicum) grown in different geographical regions in China[J]. LWT-Food Science and Technology,2021,135:110226. doi: 10.1016/j.lwt.2020.110226
[16] 孔庆龙, 樊建, 赵天瑞. 块菌多糖提取工艺优化及粗多糖抗氧化性的测定[J]. 食品与发酵工业,2012,38(12):175−179. [KONG Q L, FAN J, ZHAO T R. Optimization of extraction technique of polysaccharides from truffles by response surface methodology and determination of crude polysaccharide antioxidant activity[J]. Food and Fermentation Industries,2012,38(12):175−179. KONG Q L, FAN J, ZHAO T R. Optimization of extraction technique of polysaccharides from truffles by response surface methodology and determination of crude polysaccharide antioxidant activity[J]. Food and Fermentation Industries, 2012, 38(12): 175-179.
[17] 韩苗苗, 张志阳, 孙一楠, 等. 柱前衍生化HPLC分析白玉菇多糖的单糖组成[J]. 食品科技,2013,34(10):286−288. [HAN M M, ZHANG Z Y, SUN Y N, et al. Analysis on monosaccharide composition of white Hypsizygus marmoreus polysaccharides by pre-column derivatization HPLC[J]. Food Science and Technology,2013,34(10):286−288. HAN M M, ZHANG Z Y, SUN Y N, et al. Analysis on monosaccharide composition of white Hypsizygus marmoreus polysaccharides by pre-column derivatization HPLC[J]. Food Science and Technology, 2013, 34(10): 286-288.
[18] 张媛媛, 张彬. 苯酚-硫酸法与蒽酮-硫酸法测定绿茶茶多糖的比较研究[J]. 食品科学,2016,37(4):158−163. [ZHANG Y Y, ZHANG B. Comparison of phenol-sulfuric acid and anthrone-sulfuric methods for determination of polysaccharide in green tea[J]. Food Science,2016,37(4):158−163. doi: 10.7506/spkx1002-6630-201604028 ZHANG Y Y, ZHANG B. Comparison of phenol-sulfuric acid and anthrone-sulfuric methods for determination of polysaccharide in green tea[J]. Food Science, 2016, 37(4): 158-163. doi: 10.7506/spkx1002-6630-201604028
[19] 刘晓涵, 陈永刚, 林励, 等. 蒽酮硫酸法与苯酚硫酸法测定枸杞子中多糖含量的比较[J]. 食品科技,2009,34(9):270−272. [LIU X H, CHEN Y G, LIN L, et al. Comparison of methods in determination of polysaccharide in Lycium barbarum L J]. Food Science and Technology,2009,34(9):270−272.
[20] MA X L, SONG F F, ZHANG H, et al. Compositional monosaccharide analysis of Morus nigra Linn by HPLC and HPCE quantitative determination and comparison of polysaccharide from Morus nigra Linn by HPCE and HPLC[J]. Current Pharmaceutical Analysis,2017,13(5):433−437.
[21] WANG X, ZHANG L, WU J, et al. Improvement of simultaneous determination of neutral monosaccharides and uronic acids by gas chromatography[J]. Food Chemistry,2017,220(1):198−207.
[22] HE Y L, ZHANG M, SHAN M, et al. Optimizing microwave-assisted hydrolysis conditions for monosaccharide composition analyses of different polysaccharides[J]. International Journal of Biological Macromolecules,2018,118(7):327−332.
[23] 赵非, 牛莉娟, 刘宗哲, 等. 高效液相法测定若羌红枣多糖的单糖组成[J]. 食品科技,2019,44(11):321−326. [ZHAO F, NIU L J, LIU Z Z, et al. Determination of monosaccharide composition in Ruoqiang jujube polysaccharide by high performance liquid chromatography[J]. Food Science and Technology,2019,44(11):321−326. ZHAO F, NIU L J, LIU Z Z, et al. Determination of monosaccharide composition in Ruoqiang jujube polysaccharide by high performance liquid chromatography[J]. Food Science and Technology, 2019, 44(11): 321-326.
[24] 符梦凡, 赵一帆, 阎卫东. 柱前衍生化HPLC法分析枸杞多糖中单糖组成[J]. 食品科学,2018,39(18):186−191. [FU M F, ZHAO Y F, YAN W D. Analysis of monosaccharide composition of polysaccharides from Lycium barbarum L. by HPLC with precolumn derivatization[J]. Food Science,2018,39(18):186−191. doi: 10.7506/spkx1002-6630-201818029 FU M F, ZHAO Y F, YAN W D. Analysis of Monosaccharide Composition of Polysaccharides from Lycium barbarum L. by HPLC with precolumn derivatization[J]. Food Science, 2018, 39(18): 186-191. doi: 10.7506/spkx1002-6630-201818029
[25] 玄光善, 李青, 王艳波. 桦褐孔菌多糖脱色方法及其成分分析[J]. 食品科学,2014,35(10):207−211. [XUAN G S, LI Q, WANG Y B. Decolorization and monosaccharide composition analysis of polysaccharides from Inonotus obliguus[J]. Food Science,2014,35(10):207−211. doi: 10.7506/spkx1002-6630-201410039 XUAN G S, LI Q, WANG Y B. Decolorization and monosaccharide composition analysis of polysaccharides from Inonotus obliguus[J]. Food Science, 2014, 35(10): 207-211. doi: 10.7506/spkx1002-6630-201410039
[26] BLACK I, HEISS C, AZADI P. Comprehensive monosaccharide composition analysis of insoluble polysaccharides by permethylation to produce methyl alditol derivatives for gas chromatography/mass spectrometry[J]. Analytical Chemistry,2019,91(21):13787−13793. doi: 10.1021/acs.analchem.9b03239
[27] 刘玉红, 张岱州, 任文静, 等. GC和HPLC分析北豆根多糖的单糖组成的方法比较[J]. 化学研究与应用,2020,32(8):1514−1518. [LIU Y H, ZHANG D Z, REN W J, et al. Comparison of GC and HPLC methods for analysis of monosaccharide composition of Rhizoma Menispermi polysaccharide[J]. Chemical Research and Application,2020,32(8):1514−1518. doi: 10.3969/j.issn.1004-1656.2020.08.031 LIU Y H, ZHANG D Z, REN W J, et al. Comparison of GC and HPLC methods for analysis of monosaccharide composition of Rhizoma Menispermi polysaccharide[J]. Chemical Research and Application, 2020, 32(8): 1514-1518. doi: 10.3969/j.issn.1004-1656.2020.08.031
[28] 周春元, 闫梅霞, 崔丽丽, 等. 云芝菌丝体多糖提取工艺优化[J]. 粮食与油脂,2020,33(12):95−97. [ZHOU C Y, YAN M X, CUI L L, et al. Optimization of extraction technique for mycelia polysaccharides from Coriolus versicolor[J]. Cereals & Oils,2020,33(12):95−97. doi: 10.3969/j.issn.1008-9578.2020.12.026 ZHOU C Y, YAN M X, CUI L L, et al. Optimization of extraction technique for mycelia polysaccharides from Coriolus versicolor[J]. Cereals & Oils, 2020, 33(12): 95-97. doi: 10.3969/j.issn.1008-9578.2020.12.026
[29] 周凌云, 鲁斌, 孙玉. 东方栓孔菌多糖对小鼠的降血糖和降血脂作用[J]. 南方医科大学学报,2020,40(8):1127−1133. [ZHOU L Y, LU B, SUN Y. Glucose-lowering effect of Trametes orientalis polysaccharides in hyperglycemic and hyperlipidemic mice[J]. J South Med Univ,2020,40(8):1127−1133. ZHOU L Y, LU B, SUN Y. Glucose-lowering effect of Trametes orientalis polysaccharides in hyperglycemic and hyperlipidemic mice[J]. J South Med Univ, 2020, 40(8): 1127-1133.
[30] 张倩男, 徐方敏, 连之娜, 等. 柱前衍生反相高效液相色谱法测定田菁种子水解液中的单糖[J]. 林产化学与工业,2020,40(3):31−38. [ZHANG Q N, XU F M, LIAN Z N, et al. Analysis of monosaccharide in Sesbania cannabina seed enzymatic hydrolysate by pre-column derivatization reversed-phase high performance liquid chromatography[J]. Chemistry and Industry of Forest Products,2020,40(3):31−38. doi: 10.3969/j.issn.0253-2417.2020.03.004 ZHANG Q N, XU F M, LIAN Z N, et al. Analysis of monosaccharide in Sesbania cannabina seed enzymatic hydrolysate by pre-column derivatization reversed-phase high performance liquid chromatography[J]. Chemistry and Industry of Forest Products, 2020, 40(3): 31-38. doi: 10.3969/j.issn.0253-2417.2020.03.004
[31] 周彦强, 吴光斌, 陈发河. PMP柱前衍生化HPLC法测定黄秋葵多糖的单糖组成[J]. 食品科学,2019,40(4):266−271. [ZHOU Y Q, WU G B, CHEN F H. Analysis of monosaccharide composition of polysaccharides from okra by pre-column derivatization high performance liquid chromatography[J]. Food Science,2019,40(4):266−271. doi: 10.7506/spkx1002-6630-20180130-426 ZHOU Y Q, WU G B, CHEN F H. Analysis of monosaccharide composition of polysaccharides from okra by pre-column derivatization high performance liquid chromatography[J]. Food Science, 2019, 40(4): 266-271. doi: 10.7506/spkx1002-6630-20180130-426
[32] 石子林, 李军乔, 王雅琼, 等. 密花香薷多糖提取工艺优化及抗氧化活性研究[J]. 食品科技,2020,45(2):231−236. [SHI Z L, LI J Q, WANG Y Q, et al. Optimization the extraction process and antioxidant activity of polysaccharide from Elsholtzia densa Benth[J]. Food Science and Technology,2020,45(2):231−236. SHI Z L, LI J Q, WANG Y Q, et al. Optimization the extraction process and antioxidant activity of polysaccharide from Elsholtzia densa Benth[J]. Food Science and Technology, 2020, 45(2): 231-236.
[33] ZHAO Z, XU X, YE Q, et al. Ultrasound extraction optimization of Acanthopanax senticosus polysaccharides and its antioxidant activity[J]. International Journal of Biological Macromolecules,2013,59:290−294. doi: 10.1016/j.ijbiomac.2013.04.067
[34] 孙思燕, 江宁, 戴竹青, 等. 荷叶离褶伞多糖提取工艺优化及抗氧化活性研究[J]. 食品工业科技,2020,41(9):155−167. [SUN S Y, JIANG N, DAI Z Q, et al. Study on the optimization of extraction technology and antioxidant activity of polysaccharides from Lyophyllum decastes(Fr.) Singer[J]. Science and Technology of Food Industry,2020,41(9):155−167. SUN S Y, JIANG N, DAI Z Q, et al. Study on the optimization of extraction technology and antioxidant activity of polysaccharides from Lyophyllum decastes( Fr. ) Singer[J]. Science and Technology of Food Industry, 2020, 41(9): 155-167.
[35] 宋姗姗, 杨艾华, 王微微, 等. 湄潭白茶多糖提取工艺优化及其抑菌活性研究[J]. 食品工业科技,2021,42(13):230−234. [SONG S S, YANG A H, WANG W W, et al. Optimization of extraction technology of polysaccharides from Meitan white tea and its antibacterial activity[J]. Science and Technology of Food Industry,2021,42(13):230−234. SONG S S, YANG A H, WANG W W, et al. Optimization of extraction technology of polysaccharides from Meitan white tea and its antibacterial activity[J]. Science and Technology of Food Industry, 2021, 42(13): 230-234.
[36] 江和栋, 牛仙, 万仁口, 等. 灵芝孢子多糖的提取工艺优化及单糖组成、抗氧化活性分析[J]. 中国食品学报,2021,21(4):159−167. [JIANG H D, NIU X, WAN R K, et al. Optimization of extraction and analysis of monosaccharide composition and antioxidant activity of Ganoderma lucidum spore polysaccharide[J]. Journal of Chinese Institute of Food Science and Technology,2021,21(4):159−167. JIANG H D, NIU X, WAN R K, et al. Optimization of extraction and analysis of monosaccharide composition and antioxidant activity of Ganoderma lucidum spore polysaccharide[J]. Journal of Chinese Institute of Food Science and Technology, 2021, 21(4): 159-167.
[37] 李美凤, 袁明昊, 邹仕赟, 等. 松露多糖的提取、分离纯化和结构鉴定[J]. 食品与发酵工业,2020,46(16):196−200. [LI M F, YUAN M H, ZOU S Y, et al. Extraction, separation, purification and structure identification of polysaccharides from truffles[J]. Food and Fermentation Industries,2020,46(16):196−200. LI M F, YUAN M H, ZOU S Y, et al. Extraction, separation, purification and structure identification of polysaccharides from truffles[J]. Food and Fermentation Industries, 2020, 46(16): 196-200.
[38] 王小花. 块菌及其发酵体系中多糖的分离纯化、生物活性及结构研究[D]. 福州: 福建农林大学, 2011. WANG X H. Isolation, bioactivities and structural characterization of polysaccharides from truffle fruiting-bodies and fermentation systems[D]. Fuzhou: Fujian Agriculture and Forestry University, 2011.
[39] 孔庆龙. 印度块菌多糖的分离纯化、结构表征及抗氧化性研究[D]. 昆明: 昆明理工大学, 2013. KONG Q L. Study on isolation, structure characterization and antioxidant activity of polysaccharides from Tuber indicum[D]. Kunming: Kunming University of Science and Technology, 2013.
-
期刊类型引用(3)
1. Dai Zhaoyang,Hu Liyun,Li Fuyan,Ai Yong,Zhang Wenyun,Zhang Bing. Study on Antibacterial Efficacy of Plant-Derived Compound Preservative and Its Product Application. China Detergent & Cosmetics. 2023(01): 52-59 .  必应学术
必应学术
2. 赵海桃,吴小杰,钟明旭,邱隽蒙,石统帅,符群. 细叶小檗不同生长部位生物碱抑菌活性研究. 北京林业大学学报. 2022(07): 126-134 .  百度学术
百度学术
3. 戴朝阳,胡丽云,李福艳,艾勇,张文云,张兵. 植物源复配防腐剂抑菌效能及其产品应用研究. 日用化学品科学. 2022(10): 11-16 .  百度学术
百度学术
其他类型引用(1)






 下载:
下载:
 下载:
下载:



