Effects of Paeoniflorin on Anxiety-like Behavior, Inflammatory Factors and Intestinal Microflora in Alcohol Withdrawal Rats
-
摘要: 目的:以长期低剂量饮酒后戒断构建酒精戒断大鼠模型,探讨芍药苷给药治疗对大鼠焦虑样行为、炎症因子及肠道微生物的影响。方法:将24只雄性SD大鼠(200±20 g)随机分为3组,每组8只,除对照组(NCG)外,其余两组均以酒精浓度5 g/kg, 25% v/v进行灌胃,1次/ d,均连续灌胃28 d后,戒断3 d,戒断期芍药苷治疗组(PAG)以50 mg/(kg·BW)给药剂量进行灌胃,模型组与对照组(MCG)以等量的蒸馏水灌胃,于实验末进行行为学测试,采集大鼠血清用于测定炎症因子白细胞介素-6(IL-6)、白细胞介素-1β(IL-1β)和肿瘤坏死因子-α(TNF-α),采集粪便样品用于高通量测序分析肠道微生物的变化。结果:高架十字迷宫(EPM)和旷场实验(OF)中,EPM实验开臂时间百分比(PTO)和OF自发移动总距离(TD)MCG组最低,芍药苷给药能够显著缓解大鼠酒精戒断焦虑样行为(P<0.05),同时芍药苷给药能够显著降低血清炎症因子IL-6、TNF-α、IL-1β水平(P<0.05),相关性分析表明大鼠焦样行为与炎症因子显著正相关(P<0.05);16S rDNA 测序结果表明芍药苷给药对酒精戒断大鼠肠道菌群Alpha 多样性影响不显著(P>0.05),但可以显著改变β多样性及微生物群落结构(P<0.05),芍药苷可以显著增加属水平肠道瘤胃菌属(Ruminococcaccae UCG-005)和霍尔德曼氏菌(Holde-manella)相对丰度(P<0.05),并且显著降低罗姆布茨菌(Romboutsia)、Candidatus Saccharimonas相对丰度。霍尔德曼氏菌(Holdemanella)与血清炎症因子IL-6显著负相关(P<0.05),Candidatus Saccharimonas与IL-6、IL-1β和TNF-α均显著正相关(P<0.05)。结论:芍药苷可以有效缓解酒精戒断大鼠焦虑样行为,降低血清炎症因子水平,改变大鼠肠道微生物群落结构,增加抗炎相关菌属相对丰度,降低致病菌的相对丰度。Abstract: Objective: To explore the effects of paeoniflorin on alcohol withdrawal induced anxiety-like behavior, inflammatory factors and intestinal microorganisms in a long-term low-dose drinking and then withdrawal rats model. Methods: 24 Sprague Dawley rats were randomly divided into 3 groups with 8 rats in each group. Except the control group (NCG), the rats were treated with alcohol (5 g/kg and 25% v/v) once a day by gavage for 28 days. Then 3 days of alcohol withdrawal . During the withdrawal period, the rats in the paeoniflorin treatment group (PAG) were gavaged with a dose of 50 mg/(kg·BW), and the model group (MCG) and control group (NCG) were gavaged with the same amount of distilled water. At the end of the experiment, behavioral test was carried out, and the rat serum was collected to determine the inflammatory factors interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). Fecal samples were collected for high-throughput sequencing to analyze the changes in the microbiota. Results: In the elevated plus maze (EPM) and open field experiment (OF), the percentage of open arm time (PTO) and the total distance of spontaneous movement (TD) were the lowest in the MCG group. Paeoniflorin could significantly alleviate the anxiety-like behavior induced by alcohol withdrawal in rats (P<0.05), and paeoniflorin could significantly reduce the level of serum inflammatory factors IL-6, TNF-α and IL-1β. The correlation analysis showed that there was a significant positive correlation between anxiety-like behavior and inflammatory factors (P<0.05). 16S rDNA sequencing showed that paeoniflorin had no significant effect on the alpha diversity of gut microbiota in alcohol withdrawal rats (P>0.05), whereas the paeoniflorin could significantly change the β diversity and microbial community structure (P<0.05). Paeoniflorin could significantly increase the relative abundance of Ruminococcaccae UCG-005 and Holdemanella (P<0.05), and reduce relative abundance of Romboutsia and Candidatus saccharimonas. There was a significant negative correlation between Holdemanella and serum inflammatory factor IL-6 (P<0.05). Candidatus saccharimonas was significantly positively correlate with IL-6, IL- 1β and TNF-α (P<0.05). Conclusion: Paeoniflorin could effectively alleviate the anxiety-like behavior induced by alcohol withdrawal in rats, reduce the level of serum inflammatory factors, change the structure of intestinal microbial community, increase the relative abundance of anti-inflammatory related bacteria and reduce the relative abundance of pathogenic bacteria.
-
Keywords:
- paeoniflorin /
- ethanol withdrawal /
- gut microbiota /
- inflammation /
- anxiety /
- rat
-
长期饮酒会对多个末端器官造成损害,可诱发大脑功能障碍,造成局部炎症产生,引起肠道黏膜损伤,增加肠道通透性,导致肠道菌群组成的变化[1]。酒精依赖患者在停止饮酒后会出现情绪和自主神经不稳定行为,其特征是焦虑、激动、谵妄等。酒精的使用、戒断和炎症之间的关系是复杂的,相互作用机制尚不明确。本课题组前期研究结果发现短期低剂量饮酒戒断后能够改变大鼠肠道微生物组成丰度及群落结构,且大鼠酒精戒断后的肠道微生物与大鼠焦虑样行为具有相关性[2]。Gorky等[3]分析认为酒精成瘾或戒断会增加致病菌的丰富度,诱导炎症信号和细胞因子的释放。 Lowe等[4]同样认为酒精摄入改变了肠道菌群,增加肠道通透性,诱发了小肠促炎细胞因子的表达。 在临床研究中发现,促炎细胞因子白介素-6 (IL-6)和肿瘤坏死因子-α (TNF-α)表达的改变与情感性症状相关[5]。研究酒精介导下的微生物-肠-脑轴间的相互作用,有助于深入了解酒精对机体的作用机制。
芍药(Paeonia lactiflora Pall.)为毛茛目毛莨科芍药亚科芍药属的多年生植物,品种主要包括白芍和赤芍[6],两者药用功效有所不同,但主要药用成分均为芍药苷。芍药苷是一种单萜类苷,在一些中药配方中被广泛用于治疗抑郁症[7]。近年来,芍药苷被报道的具有抗炎、抗氧化、免疫调节、保护神经、抗抑郁的作用[8-10],这些药理作用为芍药苷成为治疗溃疡性结肠炎、动脉粥样硬化、疼痛、抑郁等多种疾病的潜在药物奠定了基础。王欣等[11]研究发现芍药苷可以改变肠炎小鼠肠道菌群结构,修复肠屏障损伤、降低炎症反应。大量体外和体内研究表明,芍药苷具有良好的神经保护作用,能够维持离子浓度和离子通道的平衡,抑制神经炎症,调节自噬途径,预防线粒体功能障碍等[12-14]。但目前芍药苷在治疗焦虑,特别是能否通过改变肠道微生物群落结构缓解酒精戒断产生的焦虑样行为目前研究相对较少。
本研究以大鼠为研究对象,通过构建长期低剂量饮酒后戒断的大鼠动物模型,研究在酒精戒断后芍药苷给药治疗对大鼠焦虑样行为、炎症因子及肠道微生物的作用,为药食同源中药治疗酒精戒断提供新的研究方向。
1. 材料与方法
1.1 材料与仪器
芍药苷(纯度 > 95.0%) 上海麦克林生化科技有限公司;酒精 北京化工厂;快速粪便基因组DNA提取试剂盒 Qiagen;ELISA 检测试剂盒 南京建成生物科技有限公司;24只SPF级雄性SD大鼠 体重(200±20)g,购买及饲养由齐齐哈尔医学院实验动物中心提供,动物饲养许可证(SYXK(黑)2016-001)。
Infinite M Plex 多功能微孔检测酶标仪 瑞士Tecan;MS200 多管涡旋震荡仪 杭州瑞成仪器有限公司;Centrifuge 5424R小型高速冷冻离心机 德国艾本德股份公司。
1.2 实验方法
1.2.1 动物分组及酒精成瘾模型建立
实验动物大鼠(动审[2021]83号 QMU-AECC-2021-83)入组后,进行适应性培养1周,后将其随机分为对照组(NCG,n=8)和酒精处理组(n=16)。酒精处理组以25%乙醇(5 g/kg, 25% v/v)灌胃1次/d,对照组以等量的蒸馏水灌胃。均连续灌胃28 d后,酒精处理组随机分为两组:酒精处理模型组(MCG,n=8)和芍药苷治疗组(PAG,n=8)。酒精戒断3 d[15],戒断期治疗组每日灌胃一次,PAG给药剂量为50 mg/(kg·BW)[16],NCG组和MCG组以等量的蒸馏水灌胃,戒断3 d后进行行为学测试、血清和粪便样品采集。
1.2.2 行为学测定
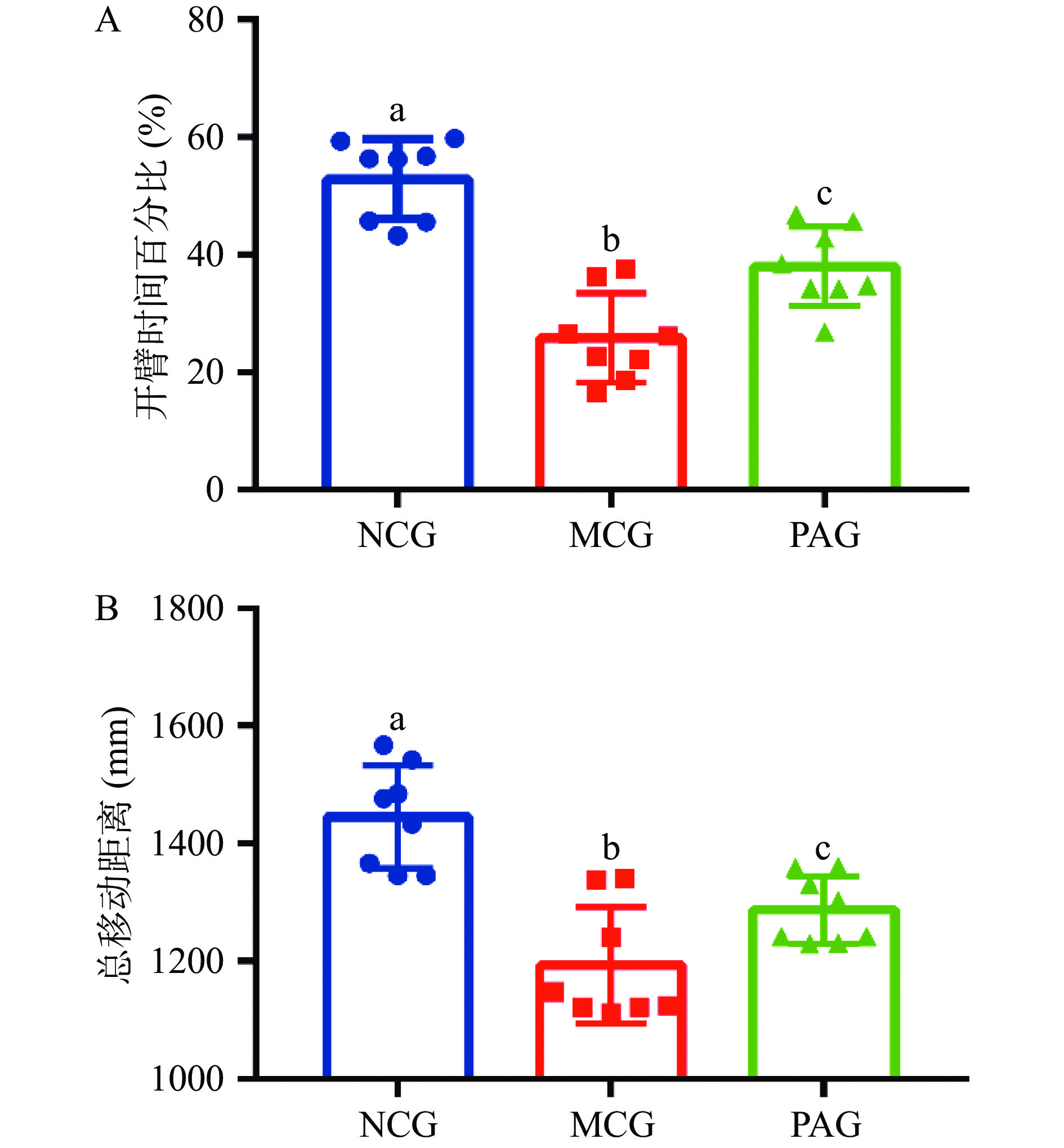
大鼠焦虑样行为采用高架十字迷宫(elevated plus-maze test,EPM)和旷场实验(open field test,OF)测定,其中EPM实验开臂时间百分比(percentage of time spent in open arms,PTO,%)=开臂滞留时间/(开臂滞留时间+闭臂滞留时间)×100,OF实验记录5 min 内大鼠的自发移动总距离(total distance,TD),具体实验方法参照本课题组前期研究方法[2]。
1.2.3 细胞因子测定
行为学测定结束后,收集血清,应用Elisa试剂盒测定大鼠血清中炎症因子IL-6、TNF-α、IL-1β测定过程严格按照说明书。
1.2.4 粪便基因组DNA提取及 16S rDNA 测序
实验结束,采用无菌操作采集大鼠粪便,取样后置于冻存盒中于−80 ℃保存。样品委托北京百迈克生物科技有限公司进行粪便微生物16S rDNA测序:参照试剂盒要求提取大鼠粪便基因组DNA,评价DNA提取质量及浓度,质量合格的DNA 样品构建细菌 16S rDNA V3+V4 区文库,使用Illumina HiSeq 测序平台进行测序。
1.3 数据处理
可操作性分类单元(operational taxonomic unit, OTU)的提取、细菌分类学注释、各分类水平下的物种丰度以及群落结构图、Beta多样性分析、随机森林分析和Spearman相关性分析焦虑相关参数,以及肠道微生物组成的相关性均使用百迈克云平台(www.biocloud.net)计算获得。使用Mothur软件,对各个样品的Alpha多样性指数(Shannon指数、Simpson指数、Chao1指数)进行评估,为比较样品间的多样性指数,分析时将样品所含序列数进行标准化。使用R语言工具进行主成分分析(principal componentanalysis,PCA)、非度量多维尺度分析(nonmetric multidimensional scaling,NMDS)和相似性分析(analysis of similarities,ANOSIM)。LDA值>4的物种为具有统计学差异的Biomarker。
2. 结果与分析
2.1 芍药苷干预酒精戒断大鼠焦虑样行为
相关研究表明酒精成瘾患者通常会伴随有精神神经病学的变化,动物实验和临床证据都表明酒精戒断时会出现焦虑、抑郁现象[15-16]。大鼠酒精戒断焦虑样行为研究结果表明,EPM实验中酒精戒断组模型组(MCG)大鼠PTO显著低于NCG组(P<0.05),在OF实验中,大鼠5 min内自发移动总距离(TD)MCG组显著低于NCG组(P<0.05),说明酒精戒断模型造模成功,大鼠出现焦虑样行为;芍药苷给药后,PAG组PTO值显著高于MCG组(P<0.05),结果见图1A; PAG组TD值显著高于MCG组(P<0.05),结果见图1B。说明芍药苷治疗能够有效缓解大鼠焦虑样行为。
2.2 芍药苷对大鼠血清炎症因子的影响
由表1可知,与NCG组对比,MCD组大鼠炎症因子IL-6、IL-1β和TNF-α水平显著升高(P<0.05),PAG组IL-6和TNF-α水平显著高于NCG组,IL-1β水平差异不显著(P>0.05);与MCD组对比,PAG组IL-6、IL-1β和TNF-α水平显著下降(P<0.05)。酒精会导致黏膜损伤,增加肠道通透性,导致内毒素和其他细菌产物转位进入门静脉,提高了血浆中肠源性细菌产物,刺激促炎介质的释放,如细胞因子TNF-α和IL-1β,对细胞产生伤害作用,导致炎症浸润[17],本结果说明长期饮酒戒断后大鼠血清炎症因子水平仍然较高,芍药苷给药治疗能够有效降低大鼠血清炎症因子水平。
表 1 芍药苷对大鼠血清炎症因子的影响Table 1. Effect of paeoniflorin on serum inflammatory factors in rats组别 IL-6 IL-1β TNF-α NCG 15.83±2.25a 59.46±9.74a 89.01±10.46a MCD 51.63±7.02b 100.04±13.22b 119.97±16.89b PAG 30.5±6.19c 78.49±11.40a 100.52±10.16c 注:同列不同小写字母表示差异显著,P<0.05。 Paerson相关性分析表明(表2),血清炎症因子IL-6、IL-1β和TNF-α与PTO值和TD值极显著负相关(P<0.01),PTO和TD值越低,大鼠焦虑样行为越显著,说明血清炎症因子水平与大鼠焦虑样行为显著正相关。
表 2 大鼠血清炎症因子与焦虑样行为指标相关性分析Table 2. Correlation analysis of serum inflammatory factors and anxiety-like behavior indexes in rats指标 TNF-α IL-6 IL-1β PTO −0.852** −0.704** −0.703** TD −0.778** −0.741** −0.493** 注:**代表极显著性,P<0.01。 2.3 芍药苷对大鼠肠道菌群多样性的影响
2.3.1 大鼠肠道菌群α多样性分析
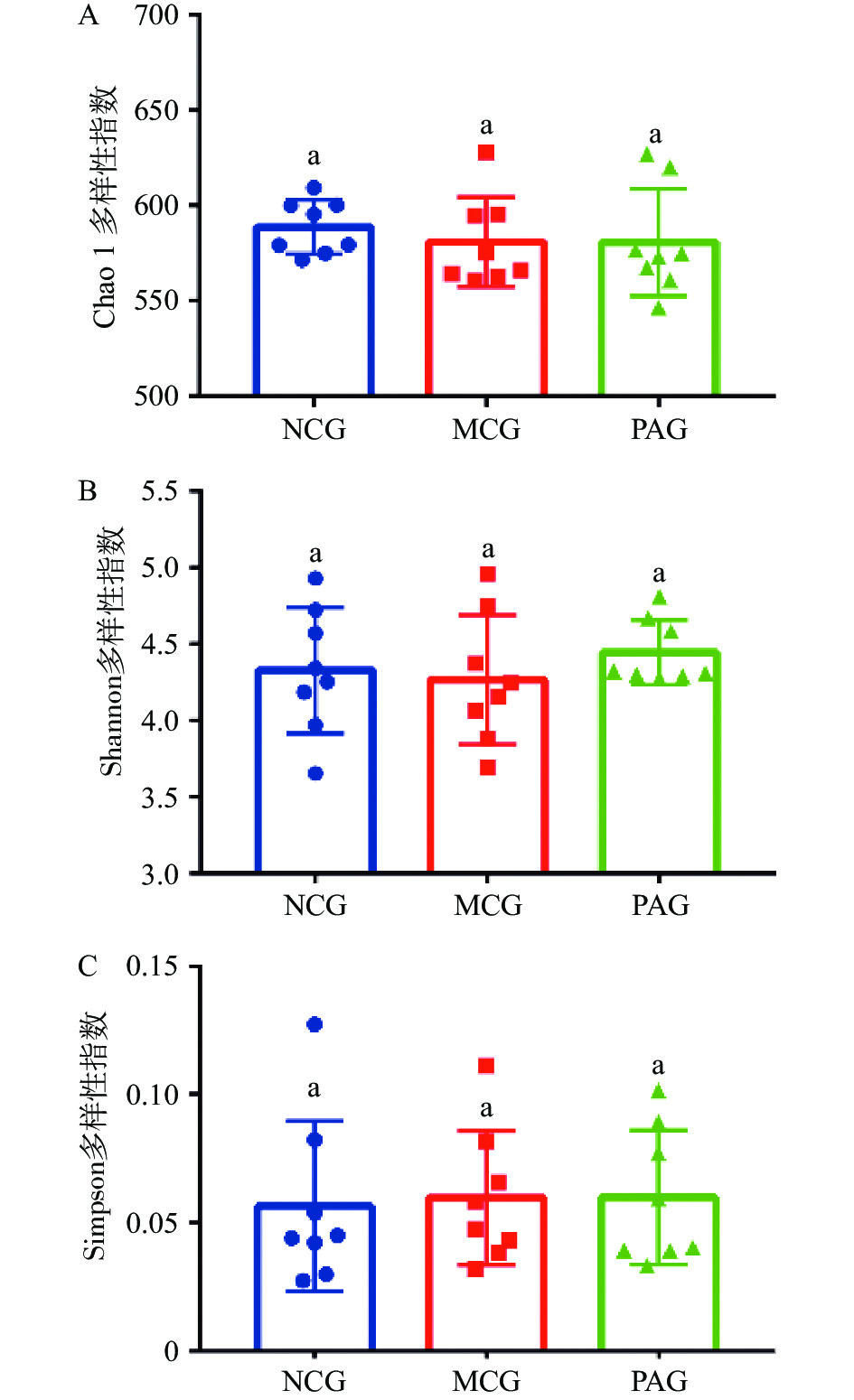
测序样本可操作性分类单元覆盖率均大于99%,采用Chao1、Shannon及Simpson指数评估物种的丰富度和多样性。由图2可见,Chao 1和Shannon指数分析表明,MCG组菌群多样性略低于NCG组,但差异不显著(P>0.05),PAG组Shannon指数高于MCG组,但差异也不显著(P>0.05);Simpson指数酒精戒断组(MCG和PAG)与NCG三组间差异不显著(P>0.05)。近期研究以及本课题组前期结果均表明长期饮酒和戒酒并没有明显改变微生物的多样性和丰富度[2,17],Fan等 [15]研究同样表明酒精戒断组与正常对照组比较,大鼠结肠肠道菌群Alpha多样性有所降低,但差异不显著。本研究结果表明酒精戒断后,芍药苷给药治疗对大鼠肠道菌Alpha多样性影响不显著, Yu等[18]通过连续给药14 d结果发现芍药苷对恢复抑郁症小鼠肠道菌群丰富度有一定作用,由于本研究给药时间仅3 d,可能是产生本研究结果的主要因素。
2.3.2 大鼠肠道菌群β多样性分析
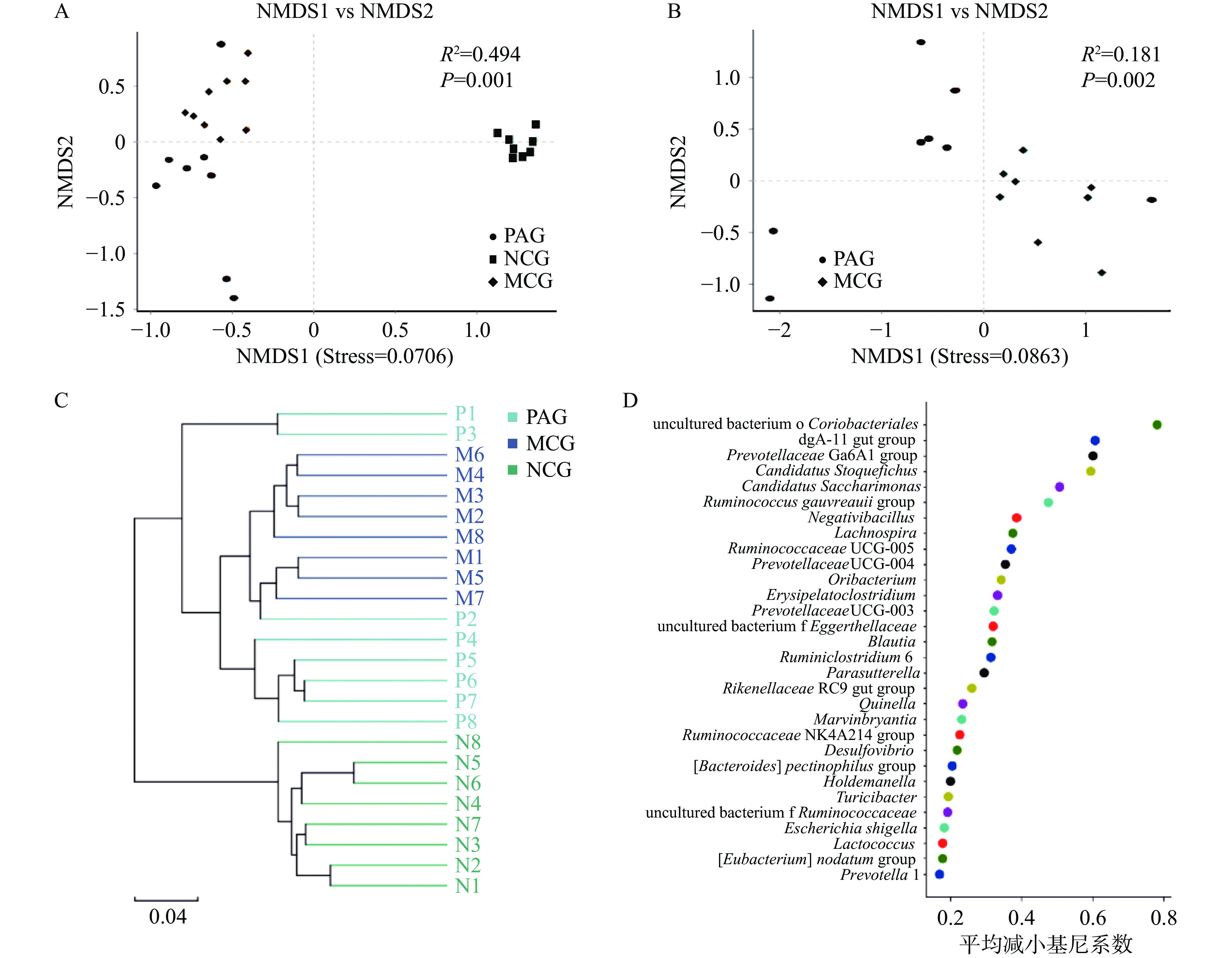
β多样性反映了不同组之间肠道微生物的群落结构差异,结果表明,NMDS分析中(图3A),三组样本中Stress=0.0706,小于0.2,表明分析具有较高的可靠性。三组处理样本分别聚集在不同区域,ANOSIM分析发现表明组间差异显著(R2=0.494, P<0.01);NCG组内样本相对集中,组间差异小,MCG组和PAG组内样本分布区域有部分重叠,通过进一步分析结果表明(图3B)MCG和PAG两组间群落结构差异显著(R2=0.181, P<0.01)。聚类分析结果表明(图3C),对照组与酒精戒断组首先聚为2支系,其中NCG组单独聚为一支,酒精戒断处理组样品聚为另外一支后,MCG与PAG之间聚为2个分支系,PAG组中P1与P3样品虽与同组其它样品分支较远,但与MCG组样品也存在明显差异。以上结果均说明酒精戒断组与对照组各样本间距离交远,MCG和PAG组间群落结构也存在显著差异。目前多项研究表明芍药苷可以显著改变肠道微生物群落结构[19-21],Chen等[20]研究表明芍药苷给药改变了肠道菌群的组成,改善了肥胖小鼠的肠道完整性。结合本研究结果说明芍药苷给药能够显著影响酒精戒断大鼠肠道微生物群落结构。
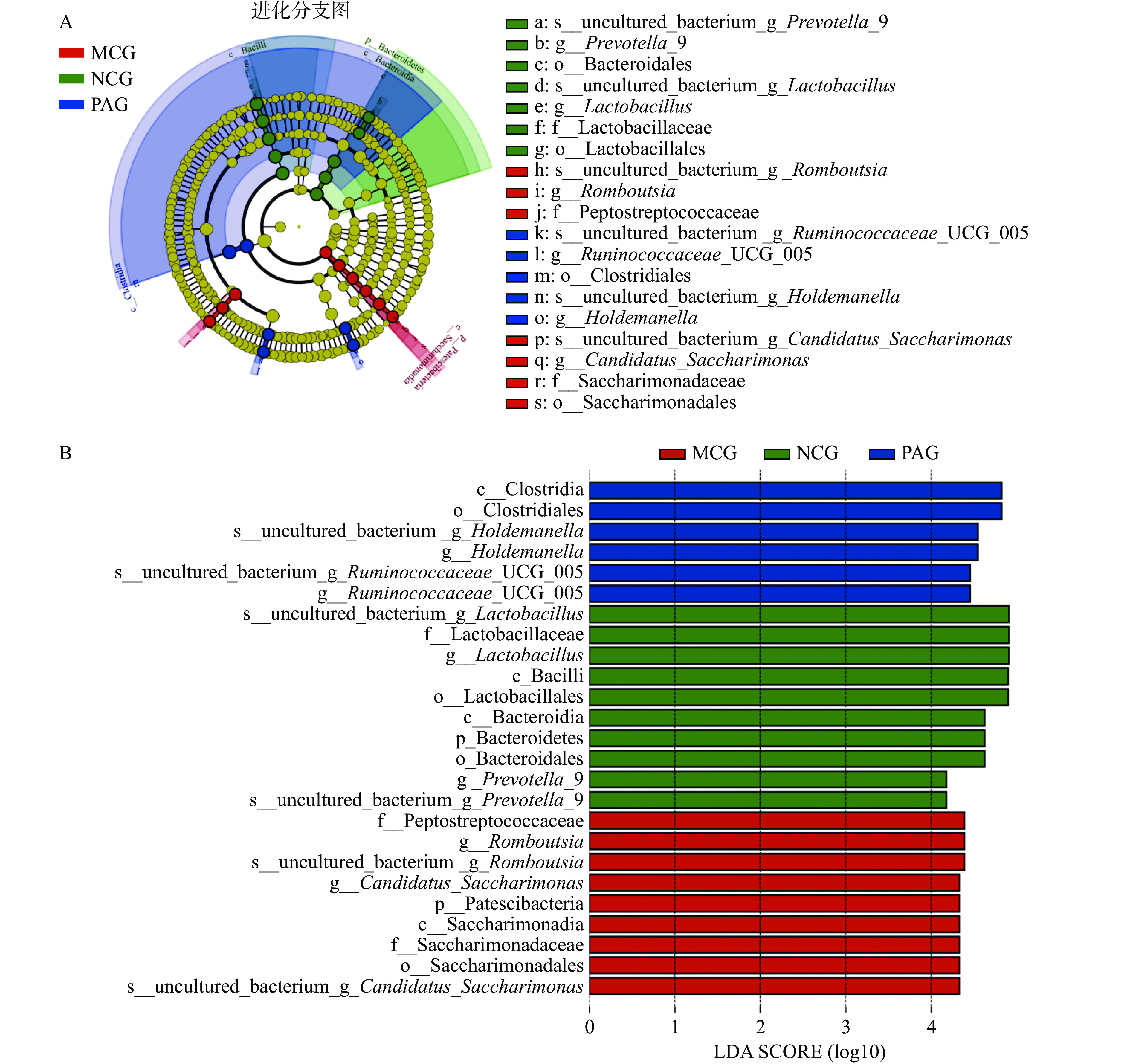
通过随机森林分析筛选出对样品组间差异具有重要影响的特征种属,基尼平均值(mean decrease gini)系数越大,表明去除该物种后,对样品组间分类的准确性影响越大,结果表明(图3D),红蝽菌目(uncultured_bacterium_o_Coriobacteriales)、dgA-11 gut_group、普雷沃氏菌(Prevotellaceae) Ga6A1 gro-up、Candidatus_Stoquefichus、Candidatus_Saccharimonas、瘤胃球菌属[Ruminococcus]_gauvreauii_group、Negativibacillus、毛螺菌属(Lachnospira)、瘤胃球菌 UCG-005(Ruminococcaceae UCG-005)、普雷沃氏菌 UCG-004(Prevotellaceae UCG-004)等种属在组间差异中贡献较大。
2.4 大鼠肠道微生物群落组成分析
2.4.1 大鼠肠道菌群不同分类水平组成分析
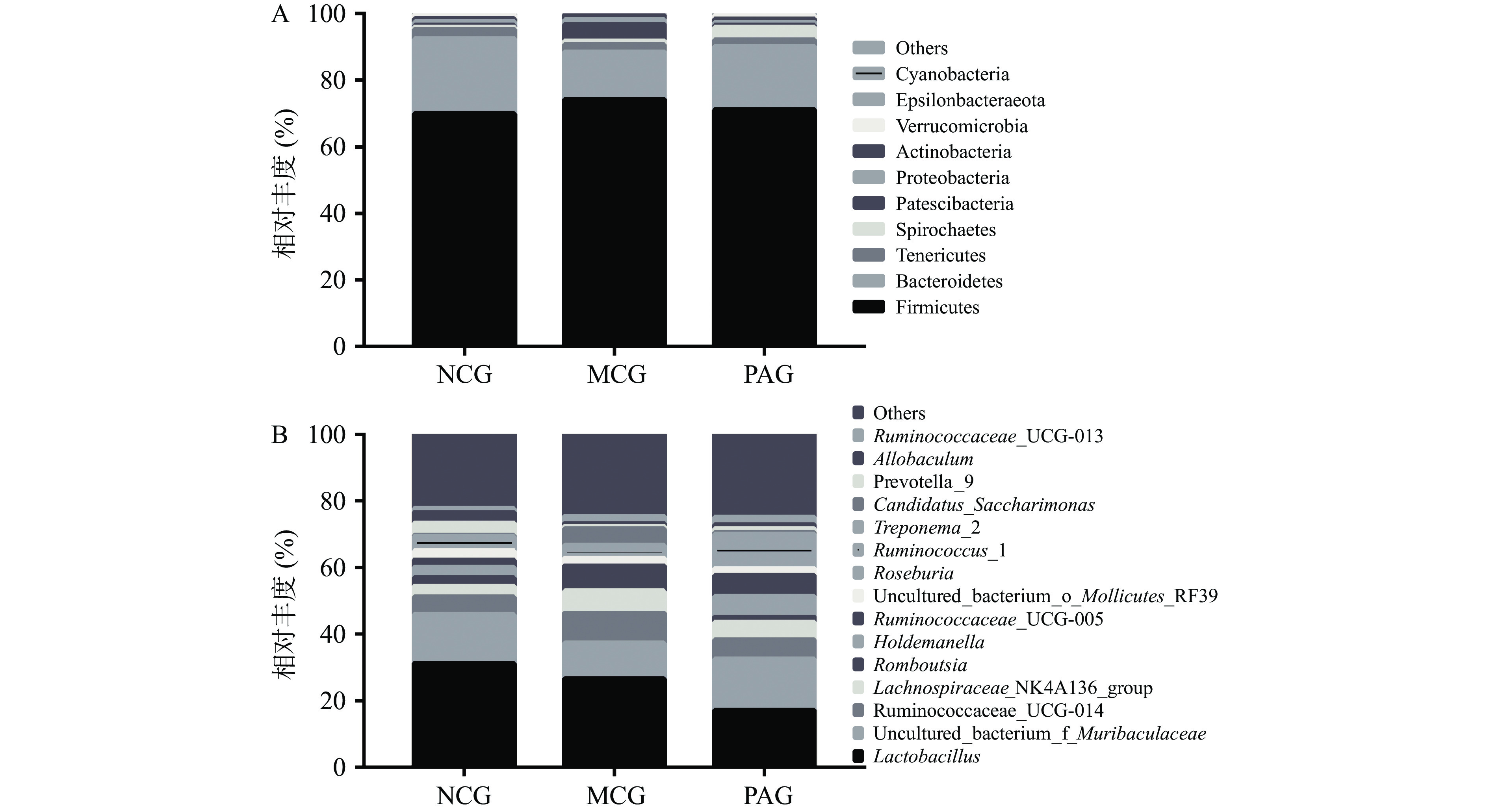
肠道98%的微生物属于四个细菌门之一,即厚壁菌门、拟杆菌门、变形菌门和放线菌门[22],细菌Phylum分类水平比较结果表明(图4A),三组处理厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidetes)是门水平上的主要菌门,二者相对丰度总量在NCG组、MCG组和PAG组中分别达到了92.45%、88.44%和90.14%;其中MCG组厚壁菌门(Firmicutes)、Patescibacteria、放线菌门(Actinobacteria)高于NCG组;拟杆菌门(Bacteroidetes)、软壁菌门(Tenericutes)相对丰度低于NCG组;PAG组拟杆菌门(Bacteroidetes)、螺旋体门(Spirochaetes)相对丰度高于MCG组。Bull等[23]基于肠道微生物组宏基因组分析的研究显示,在酒精喂养的小鼠中,拟杆菌门和厚壁菌门均出现下降,Yan等[24]发现相对丰富的与对照组相比,饮酒小鼠拟杆菌门增加,厚壁菌门减少。以上研究表明酒精摄入与肠道菌群的改变之间存在密切的关系,饮酒可以改变肠道菌群中主要群落的丰富度;本研究结果表明芍药苷给药治疗可以恢复部分菌群的丰富度。
物种丰度在前15的菌属中(图4B),MCG组乳酸菌属(Lactobacillus)、
uncultured bacterium_f_ Muribaculaceae、霍尔德曼氏菌属(Holdemanella)、瘤胃菌属(Ruminococcaccae UCG-005)、普氏菌属(Prevotella 9)和Allobaculum属相对丰度低于NCG组,瘤胃菌属(Ruminococcaccac UCG-014)、Lachnospiraccae NK4A136 group属、Romboutsia、Candidatus_Saccharimonas相对丰度高于NCG组;PAG组乳酸菌属(Lactobacillus)、Romboutsia属相对丰度小于MCG组, uncultured_bacterium_f_Muribaculaceae、霍尔德曼氏菌属(Holdemanella)、瘤胃菌属(Ruminococcaccae_UCG-005)、罗氏菌属(Roseburia)、密螺旋体属(Treponema)相对丰度高于MCG组(图4B)。有关研究发现,在酒精处理过的小鼠肠道内,乳酸杆菌的数量减少,并证实益生菌在预防增加肠道通透性、内毒素血症降低炎症损伤方面的有益作用[25]。 为进一步研究芍药苷对肠道菌群的影响,共筛选出21种差异显著菌属,结果显示(图5A~D),物种丰度在前15的菌属中,瘤胃菌属(Ruminococcaccae UCG-005)和霍尔德曼氏菌(Holdemanella)两个菌属相对丰度PAG组显著高于MCG组(P<0.05);罗姆布茨菌(Romboutsia)、Candidatus Saccharimonas相对丰度显著低于MCG组(P<0.05)。研究发现肠道中C18-3OH浓度增加可缓解DSS诱导的小鼠结肠炎,C18-3OH浓度增加与霍尔德曼氏菌(Holdemanella)的丰度增加相关[26];瘤胃球菌能够促进短链脂肪酸(SCFA)的产生,并且是将初级胆汁酸转化为次生胆汁酸的主要微生物,该菌的缺乏与溃疡性结肠炎密切相关[27]。结果表明,芍药苷给药可以增加与缓解肠炎相关菌属(Holdemanella和Ruminococcaccae UCG-005)的相对丰度。
2.4.2 大鼠肠道菌群差异微生物分析
LEfSe(Line Discriminant Analysis (LDA) Effect Size)能够在不同组间寻找具有统计学差异的生物标志物(Biomarker),图(6A~B)是从门到种水平下各组样本的进化分支图和LDA值分布柱状图,柱状图的长度代表差异物种的影响大小(即为 LDA Score),不同颜色表示不同分组的物种。结果分析表明,NCG组中对群落结构影响较大的物种有乳酸菌种(uncultured_bac-terium g Lactobacillus)、乳杆菌科(Lactobacillaceae)、乳杆菌属(Lactobacillus)、杆菌纲(Bacilli)、乳杆菌目(Lactobacillales)、拟杆菌纲(Bacteroidia)和拟杆菌门(Bacteroidetes)等;MCG组中对群落结构影响较大的物种有消化链球菌科(Peptostreptococcaceae)、罗姆布茨菌(Romboutsia)、Candidatus Saccharimonas、Patescibacteria等;PAG组中对群落结构影响较大的物种有梭菌纲(Clostridia)、梭菌目(Clostridiales)、uncultured_bacterium g Holdemanella菌种、霍尔德曼氏菌(Holdemanella)、瘤胃菌种(uncultured bacte-rium g Ruminococcaceae UCG-005)、瘤胃菌属(Rumi-nococcaceae UCG-005)等。在已有的研究中发现酒精依赖和戒断肠道菌群中乳酸菌、拟杆菌以及瘤胃球菌的丰度和组成发生显著变化,并且证实这些细菌对肠道屏障功能具有较大影响[28-30]。
2.5 肠道菌群相对丰度与炎症因子相关性分析
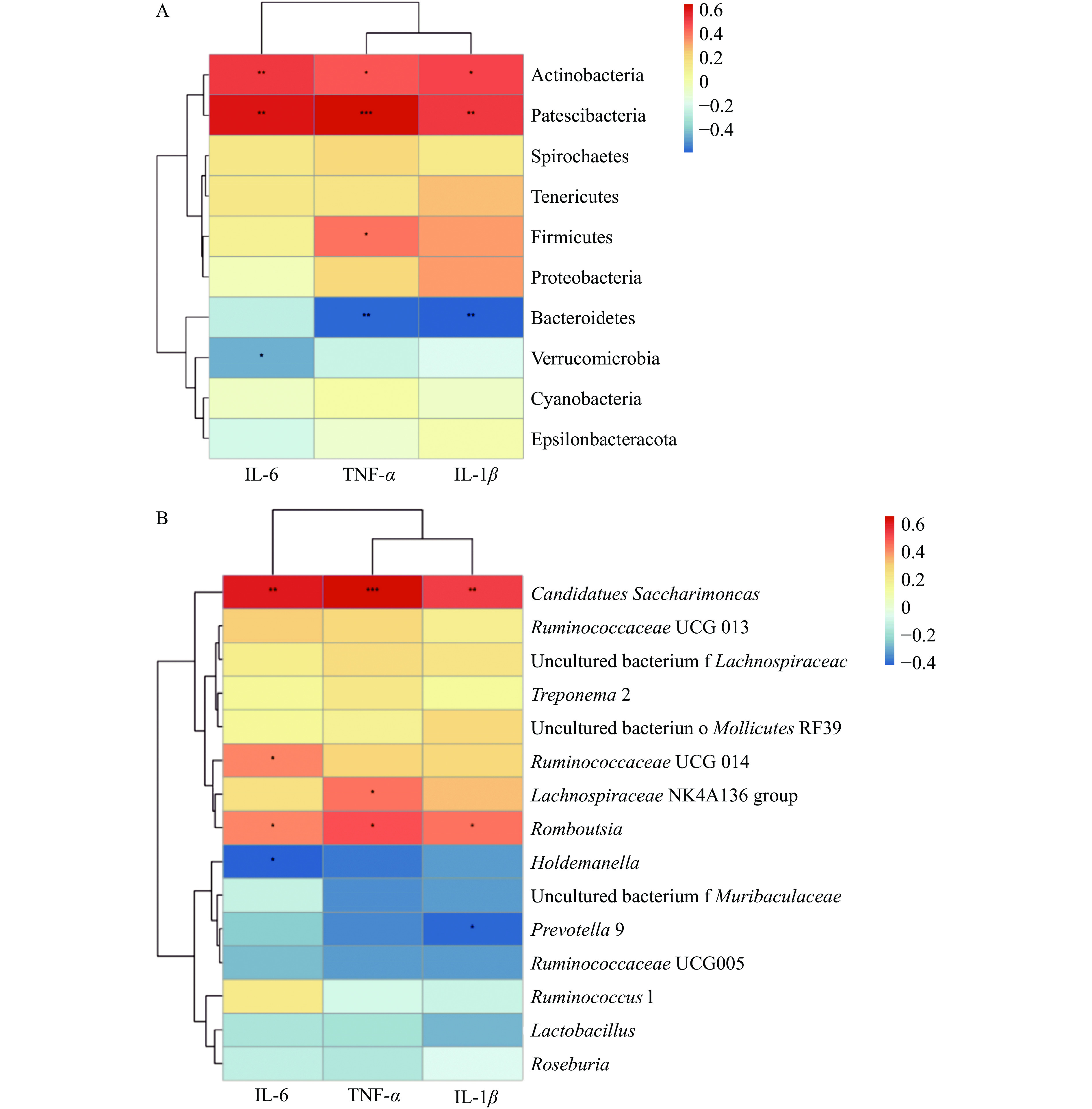
将炎症因子与微生物门水平丰富度前10和菌属丰富度前15物种数据进行Spearman相关性分析,如图7所示,在门水平上,放线菌门(Actinobacteria)、Patescibacteria与IL-6、IL-1β和TNF-α显著正相关(P<0.05),厚壁菌门(Firmicutes)与TNF-α显著正相关(P<0.05),拟杆菌门(Bacteroidetes)与IL-1β和TNF-α显著负相关(P<0.01),疣微菌门(Verrucomicrobia)与IL-6显著负相关(P<0.05)。
在属水平上,Candidatus Saccharimonas和罗姆布茨菌(Romboutsia)与IL-6、IL-1β和TNF-α显著正相关(P<0.05),瘤胃菌属(Ruminococcaceae UCG 0014)与IL-6显著正相关(P<0.05),毛螺菌属(Lachnospiraceae NK4A136 group)与TNF-α显著正相关(P<0.05);霍尔德曼氏菌(Holdemanella)与IL-6显著负相关(P<0.05),普氏菌属(Prevotella)与IL-1β显著负相关(P<0.05)。
3. 讨论与结论
芍药苷作为不良反应小、毒副作用较少的药食同源的一种免疫调节药,同时具备抗炎、抗氧化、脑保护和神经保护等作用。细胞因子是宿主对炎症和免疫系统反应的主要信号蛋白,包括白介素、趋化因子、生长因子、肿瘤坏死因子、干扰素和集落刺激因子。研究表明芍药苷降低炎症反应的作用方式涉及调节NF-κB通路,这可能是芍药苷抗炎的关键靶点之一。NF-κB调控的基因包括编码IL-2、IL-6、IL-8、IL-2受体TNF-α、干扰素-γ的基因(IFN-γ)和c-Myc[31-33]。同时研究发现长期饮酒患者血清中IL-6和TNF-α水平明显升高,焦虑、心理压力与外周血IL-6和TNF-α水平呈正相关[34-35]。本研究同样发现酒精戒断大鼠血清炎症因子水平与焦虑样行为正相关,芍药苷给药治疗可以显著缓解焦虑样行为,降低血清炎症因子水平。
肠道菌群在人类中枢神经系统疾病中也发挥着重要作用,影响神经系统的正常发育,参与了许多精神疾病的发病机制;肠道微生物同时可以调节焦虑、情绪、认知和疼痛[36]。在酒精依赖中存在肠-脑轴的作用关系,肠道微生物群可以改变肠道屏障,影响酒精依赖行为的严重程度,酒精的摄入会导致肠道细菌过度生长和失调,影响肠道菌群群落结构的改变[29]。慢性乙醇给药导致大鼠生态失调,小鼠瘤胃球菌科[37]水平下降, 厚壁菌门和拟杆菌门的增加[38]。Sophie等研究认为短期酒精戒断会改变肠道菌群的结构,特别是改变瘤胃球菌的丰富度 ,但是对物种的多样性影响较小。瘤胃菌属与缓解结肠炎密切相关,并且与炎症患者的焦虑正相关,而与记忆负相关[39] ,前期研究发现瘤胃菌属与 PTO、TD 显著负相关,表明该菌属也可能参与到中枢神经系统的调节。本研究结果发现芍药苷给药能够显著增加瘤胃菌属(Ruminococcaccae UCG-005),和缓解炎症反应相关的霍尔德曼氏菌(Holdemanella)的相对丰度。Yu等[18]同样发现芍药苷具有显著的抗抑郁活性,并且通过增加益生菌的丰度来缓解抑郁症状。LEfSe分析表明酒精戒断组Candidatus Saccharimonas和Patescibacteria对群落结构影响较大,其中Candidatus Saccharimonas属于条件性致病菌,而Patescibacteria在结肠炎小鼠模型相对丰度高于正常组[40],相关性分析同样表明Candidatus Saccharimonas与IL-6、IL-1β和TNF-α均显著正相关(P<0.05)。这进一步说明芍药苷可以通过降低肠道中致病菌的丰度,增加有益菌的丰度,缓解炎症反应,这也可能成为芍药苷缓解酒精戒断焦虑样行为的一种作用方式,使其成为治疗酒精戒断焦虑的潜在药物。
-
表 1 芍药苷对大鼠血清炎症因子的影响
Table 1 Effect of paeoniflorin on serum inflammatory factors in rats
组别 IL-6 IL-1β TNF-α NCG 15.83±2.25a 59.46±9.74a 89.01±10.46a MCD 51.63±7.02b 100.04±13.22b 119.97±16.89b PAG 30.5±6.19c 78.49±11.40a 100.52±10.16c 注:同列不同小写字母表示差异显著,P<0.05。 表 2 大鼠血清炎症因子与焦虑样行为指标相关性分析
Table 2 Correlation analysis of serum inflammatory factors and anxiety-like behavior indexes in rats
指标 TNF-α IL-6 IL-1β PTO −0.852** −0.704** −0.703** TD −0.778** −0.741** −0.493** 注:**代表极显著性,P<0.01。 -
[1] LECLERCQ S, CANI P D, NEYRINCK A M, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects[J]. Brain Behav Immun,2012,26:911−918. doi: 10.1016/j.bbi.2012.04.001
[2] 王小龙, 张春晶, 于海涛, 等. 短期低剂量饮酒后戒断改变雄性大鼠肠道微生物菌群并与焦虑样行为相关[J]. 中国生物化学与分子生物学报,2021,37(5):653−661. [WANG X L, ZHANG C J, YU H T, et al. The gut microbiota changed by short-term low-dose ethanol withdrawal and related to the anxiety like behavior in male rats[J]. Chinese Journal of Biochemistry and Molecular Biology,2021,37(5):653−661. WANG X L, ZHANG C J, YU H T, et al. The gut microbiota changed by short-term low-dose ethanol withdrawal and related to the anxiety like behavior in male Rats[J]. Chinese Journal of Biochemistry and Molecular Biology, 2021, 37(5): 653-661.
[3] GORKY J, SCHWABER J. The role of the gut-brain axis in alcohol use disorders[J]. Prog Neuropsychopharmacol Biol Psychiatry,2016,65:234−241. doi: 10.1016/j.pnpbp.2015.06.013
[4] LOWE P P, GYONGYOSI B, SATISHCHANDRANA A, et al. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression[J]. J Neuroinflammation,2018,15(1):298. doi: 10.1186/s12974-018-1328-9
[5] ZUNSZAIN P A, HEPGUL N, PARIANTE C M. Inflammation and depression[J]. Current Topics in Behavioral Neurosciences,2013,14:135−151.
[6] 车娅莉, 张文武, 尚立宏, 等. 芍药苷研究进展[J]. 中兽医医药杂志,2021,40(4):47−51. [CHE Y L, ZHANG W W, SHANG L H, et al. Research progress of paeoniflorin[J]. Journal of Traditional Chinese Veterinary Medicine,2021,40(4):47−51. CHE Y L, ZHANG S B, SHANG L H, et al. Research progress of paeoniflorin[J]. Journal of Traditional Chinese Veterinary Medicine, 2021, 40(4): 47-51.
[7] FENG S T, WANG X L, WANG Y T, et al. Efficacy of traditional chinese medicine combined with selective serotonin reuptake inhibitors on the treatment for Parkinson’s disease with depression: A systematic review and meta-analysis[J]. Am J Chin Med,2021,49:627−643. doi: 10.1142/S0192415X21500282
[8] GU X Y, CAI Z X, CAI M, et al. Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer's disease[J]. Mol Med Rep,2016,13(3):2247−2252. doi: 10.3892/mmr.2016.4805
[9] WANG X L, FENG S T, WANG Y T, et al. Paeoniflorin: A neuroprotective monoterpenoid glycoside with promising anti-depressive properties[J]. Phytomedicine,2021:153669.
[10] 李萍, 李艺杰, 薛玲, 等. 芍药苷对Bayk8644诱导大鼠抑郁焦虑样行为的改善作用及机制研究[J]. 实验动物与比较医学,2020,40(6):489−495. [LI P, LI Y J, XUE L, et al. Effect and mechanism of paeoniflorin on depression and anxiety behavior induced by Bayk8644 in rats[J]. Laboratory Animal and Comparative Medicine,2020,40(6):489−495. LI P, LI Y J, XUE L, et al. Effect and mechanism of paeoniflorin on depression and Anxiety behavior induced by bayk8644 in Rats[J]. Laboratory Animal and Comparative Medicine, 2020, 40(6): 489-495.
[11] 王欣, 朱敏, 董思晶, 等. 芍药苷对结肠炎小鼠肠道菌群及胆汁酸代谢的调节作用[J]. 药学学报,2021,56(7):1811−1819. [WANG X, ZHU M, DONG S J, et al. Paeoniflorin regulates gut microbiota and bile acids metabolism in colitis mice[J]. Acta Pharmaceutica Sinica,2021,56(7):1811−1819. WANG X, ZHU M, DONG S J, et al. Paeoniflorin regulates gut microbiota and bile acids metabolism in colitis mice[J]. Acta Pharmaceutica Sinica, 2021, 56(7): 1811-1819.
[12] CONG C, KLUWE L, LI S, et al. Paeoniflorin inhibits tributyltin chloride-induced apoptosis in hypothalamic neurons via inhibition of MKK4-JNK signaling pathway[J]. J Ethnopharmacol,2019,237:1−8. doi: 10.1016/j.jep.2019.03.030
[13] MAO Q Q, ZHONG X M, FENG C R, et al. Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca2+ antagonism[J]. Cell Mol Neurobiol,2010,30:1059−1066. doi: 10.1007/s10571-010-9537-5
[14] SONG C, WANG J, GAO D, et al. Paeoniflorin, the main active ingredient of Shuyu capsule, inhibits Cav1.2 and regulates calmodulin/calmodulin-dependent protein kinase II signalling[J]. BioMed Research International,2017:8459287.
[15] FAN Y, YAE Z, JIDONG W, et al. Comparison of microbial diversity and composition in jejunum and colon of the alcohol-dependent rats[J]. Journal of Microbiology and Biotechnology,2018,28(11):1993−1895.
[16] ASAI M, KAWASHIMA D, KATAGIRI K, et al. Protective effect of a molecular chaperone inducer, paeoniflorin, on the HCl- and ethanol-triggered gastric mucosal injury[J]. Life Sciences,2011,88(7-8):350−357. doi: 10.1016/j.lfs.2010.12.014
[17] XIAO H W, GE C, FENG G X, et al. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice[J]. Toxicol Lett,2018,287:23−30. doi: 10.1016/j.toxlet.2018.01.021
[18] YU J B, ZHAO Z X, PENG R, et al. Gut microbiota-based pharmacokinetics and the antidepressant mechanism of paeoniflorin[J]. Frontiers in Pharmacology,2019,10:268. doi: 10.3389/fphar.2019.00268
[19] PENG J, LU X, XIE K, et al. Dynamic alterations in the gut microbiota of collagen-induced arthritis rats following the prolonged administration of total glucosides of paeony[J]. Frontiers in Cellular and Infection Microbiology,2019,9:204. doi: 10.3389/fcimb.2019.00204
[20] CHEN, KAN J T, ZHENG N N, et al. A botanical dietary supplement from white peony and licorice attenuates nonalcoholic fatty liver disease by modulating gut microbiota and reducing inflammation[J]. Phytomedicine,2021,91(10):153693.
[21] XIA L A, XW A, SH A, et al. Paeoniflorin ameliorates experimental colitis by inhibiting gram-positive bacteria-dependent MDP-NOD2 pathway[J]. International Immunopharmacology,2021,90:107224. doi: 10.1016/j.intimp.2020.107224
[22] LOPETUSO LR, SCALDAFERRI F, PETITO V, et al. Commensal Clostridia: Leading players in the maintenance of gut homeostasis[J]. Gut Pathog,2013,5:23. doi: 10.1186/1757-4749-5-23
[23] BULL O L, FENG W, KIRPICH I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment[J]. PLoS ONE 2013, 8: e53028.
[24] YAN A W, FOUTS D E, BRANDL J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease[J]. Hepatology, 2011, 53: 96−105.
[25] NANJI A A, KHETTRY U, SADRZADEH S M. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease)[J]. Proc Soc Exp Biol Med, 1994, 205(3): 243–247.
[26] PUJO J, PETITFILS C, LE F P, et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis[J]. Gut, 2021, 70: 1088−1097.
[27] SINHA S R, HAILESELASSIE Y, NGUYEN L P, et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation[J]. Cell Host Microbe,2020,27(4):659−670. doi: 10.1016/j.chom.2020.01.021
[28] YANG F, WEI J, SHEN M, et al. Integrated analyses of the gut microbiota, intestinal permeability and serum metabolome phenotype in rats with alcohol withdrawal syndrome[J]. Applied and Environmental Microbiology,2021:AEM0083421.
[29] LECLERCQ S, STARKEL P, DELZENNE N M, TIMARY P. The gut microbiota: A new target in the management of alcohol dependence?[J]. Alcohol,2019,74:105−111. doi: 10.1016/j.alcohol.2018.03.005
[30] QAMAR N, CASTANO D, PATT C, et al. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact[J]. Behav Brain Res,2019,376:112196. doi: 10.1016/j.bbr.2019.112196
[31] CHEN J, ZHANG M, ZJU M, et al. Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1alpha/NF-kappaB signaling pathway[J]. Food Funct,2018,9:2386−2397. doi: 10.1039/C7FO01406F
[32] 杨山景, 封安杰, 孙越, 等. 白芍总苷的药理作用及机制研究进展[J]. 中国现代应用药学,2021,38(13):1627−1633. [YANG S J, FENG A J, SUN Y, et al. Research progress on mechanism and pharmacological activities of total glucosides of paeony[J]. Chin J Mod Appl Pharm,2021,38(13):1627−1633. YANG S J, FENG AJ, SUN Y, et al. Research progress on mechanism and pharmacological activities of total glucosides of paeony[J]. Chin J Mod Appl Pharm, 2021, 38(13): 1627-1633.
[33] 刘俊彤, 李轶聪, 董丽强, 等. 芍药苷对抗炎反应因子TNF-α 和IL6的作用研究[J]. 中西医结合心血管病电子杂志,2020,8(30):1−6. [LIU J T, LI Y C, DONG L Q, et al. Study on the effect of paeoniflorin on TNF-α and IL6[J]. Cardiovascular Disease Electronic Journal of integrated traditional Chinese and Western Medicine,2020,8(30):1−6. LIU J T, LI Y C, DONG L Q, et al. Study on the effect of paeoniflorin on TNF-a and IL6[J]. Cardiovascular Disease Electronic Journal of integrated traditional Chinese and Western Medicine, 2020, 8(30): 1, 6. `
[34] ANNEMARIE H, MARIUS K, RALF L, et al. TNF-α and IL-6 serum levels: Neurobiological markers of alcohol consumption in alcohol-dependent patients?[J]. Alcohol,2014,48(7):671−676. doi: 10.1016/j.alcohol.2014.08.003
[35] FUMAZ C R, GONZALEZ G M, BORRAS X, et al. Psychological stress is associated with high levels of IL-6 in HIV-1 infected individuals on effective combined antiretroviral treatment[J]. Brain, Behavior, and Immunity,2012,26(4):568−572. doi: 10.1016/j.bbi.2012.01.001
[36] MOHAJERI M H, BRUMMER R J, RASTALL R A, et al. The role of the microbiome for human health: From basic science to clinical applications[J]. Eur J Nutr,2018,57:1−14.
[37] BULL O L, FENG W, KIRPICH I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment[J]. PLoS ONE,2013,8(1):e53028. doi: 10.1371/journal.pone.0053028
[38] YAN A W, FOUTS D E, BRANDL J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease[J]. Hepatology,2011,53(1):96−105. doi: 10.1002/hep.24018
[39] BAJAJ J S, RIDLON J M, HYLEMON P B, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy[J]. Am J Physiol Gastrointest Liver Physiol,2012,302(1):G168−G175. doi: 10.1152/ajpgi.00190.2011
[40] 陈健, 张梁坤, 谷文超, 等. 半夏泻心汤对右旋葡聚糖硫酸钠诱导的溃疡性结肠炎小鼠肠道菌群的影响[J]. 中国中药杂志,2021,46(11):2871−2880. [CHEN J, ZHANG L S, GU W C, et al. Effect of banxia xiexin decoction on intestinal flora of mice with ulcerative colitis induced by dextran sodium sulfate[J]. China Journal of Chinese Materia Medica,2021,46(11):2871−2880. CHEN J, ZHANG L S, GU W C, et al. Effect of banxia xiexin decoction on intestinal flora of mice with ulcerative colitis induced by dextran sodium sulfate[J]. China Journal of Chinese Materia Medica, 2021, 46(11): 2871-2880.
-
期刊类型引用(11)
1. 刘影,庞富,陈佳鸿,陈炯葵,蔡烁仪. 本草清咽润喉糖的配方优化及抗氧化研究. 农产品加工. 2024(21): 41-46 .  百度学术
百度学术
2. 张敏君,段雪伟,王燕,杨慧文,刘冰,向文静,由天辉. 构树根皮活性成分乙醇提取工艺优化及其抗氧化活性分析. 食品工业科技. 2023(11): 196-203 .  本站查看
本站查看
3. 王蕙雯. 豫西自然发酵柿子醋抗氧化性研究. 江苏调味副食品. 2023(03): 20-23 .  百度学术
百度学术
4. 裴文清,吕泸楠,王靖宇,浦思琦,雷霜,王春丽. 木瓜皮多酚和黄酮提取工艺优化及酪氨酸酶与胰脂肪酶抑制活性研究. 食品工业科技. 2022(01): 188-195 .  本站查看
本站查看
5. 周新崇,易灿,刘进兵. 微波辅助提取崀山脐橙皮总黄酮及生物活性研究. 邵阳学院学报(自然科学版). 2022(02): 87-95 .  百度学术
百度学术
6. 张清月,董姝慧,李胤豪,赵艳丽,史彬林,闫素梅. 诺丽果不同提取物抗氧化能力的比较研究. 中国粮油学报. 2022(05): 144-150 .  百度学术
百度学术
7. 关随霞,王蕙雯,杨肖瑞,郭淑敏,张翅,张培杰,李道敏. 大青叶总黄酮提取工艺优化及抗氧化性研究. 中国食品添加剂. 2022(09): 138-144 .  百度学术
百度学术
8. 陈慧玲,刘芳,钟恒勤,王伟枫. 超声波辅助乙醇提取百香果皮黄酮的工艺优化及黄酮抗氧化性测定. 宁德师范学院学报(自然科学版). 2022(03): 280-287 .  百度学术
百度学术
9. 任海云,韩瑞,张磊. 基于Box-Behnken响应面法优化党参抗氧化活性组分提取工艺. 中医药信息. 2022(12): 5-10 .  百度学术
百度学术
10. 赵雨晴,王宝庆,徐汉,刘楠楠. 醉鱼草总黄酮的提取及抗氧化活性研究. 化学试剂. 2021(07): 979-985 .  百度学术
百度学术
11. 杨青青,龚吉军. 响应面法优化超声辅助葛根浸提工艺及浸提液抗氧化活性研究. 食品安全质量检测学报. 2021(13): 5409-5417 .  百度学术
百度学术
其他类型引用(3)





 下载:
下载:

 下载:
下载:



