Research Progress on the Chemical Composition and Intestinal Flora Regulation of Dietary Fiber from the Edible and Medicinal Plants
-
摘要: 膳食纤维作为一种新型营养素,可以通过调节肠道菌群结构在机体免疫、代谢等方面发挥重要作用。食药同源植物膳食纤维(Edible and medicinal dietary fiber,EMDF)同时兼具了中草药强身健体和膳食纤维促进肠道健康的双重作用,这使其具有不同于普通膳食纤维的性质特点。本文对EMDF研究进展进行总结,针对其化学组成、分布特点及肠道作用进行分析,重点就EMDF对肠道菌群结构的影响及其肠道作用机制进行综述,本研究为EMDF在功能性食品中的开发和利用提供了理论依据。Abstract: As a new nutrient, dietary fiber can play an important role in immunity and metabolism by regulating the structure of intestinal microflora. Edible and medicinal dietary fiber (EMDF) has the dual functions of strengthening the body with Chinese herbal medicine and promoting intestinal health with dietary fiber, which makes it different from ordinary dietary fiber. This paper summarizes the research progress of EMDF, analyzes its chemical composition, distribution characteristics and intestinal function, and focuses on the effect of EMDF on intestinal microflora structure and its action mechanism. This study would provide a theoretical basis for the development and utilization of EMDF in functional foods.
-
饮食是影响人类健康最主要的因素之一[1]。随着“健康中国2030”战略的提出,养生保健的生活方式越来越受到人们推崇,膳食纤维(Dietary fiber,DF)成为健康食品的标志性成分之一。大量研究证实,DF能够从菌-肠-器官轴角度对人体健康产生深远影响[2]。然而目前国内外对于天然膳食纤维的研究大多集中在谷物和水果中,对于富有特色的食药同源植物来源DF的研究相对较少[3]。食药同源植物膳食纤维(Edible and medicinal dietary fiber,EMDF)同时兼具了中草药强身健体和膳食纤维促进肠道健康的双重作用,具有十分重要的研究价值。本文总结了EMDF的化学组成、分布特点及肠道作用,重点就EMDF对肠道菌群结构的影响及其肠道作用机制进行综述,旨在为EMDF的开发和应用提供理论依据。
1. 膳食纤维化学成分及其主要功能
DF是不能被人体小肠内生酶水解的,具有十个或十个以上糖苷键的碳水化合物,包括天然存在的可食用碳水化合物、食品中提取的碳水化合物,以及人为合成的碳水化合物[4]。根据水溶性的差异,DF可分为水溶性膳食纤维(Soluble dietary fiber,SDF)和非水溶性膳食纤维(Insoluble dietary fiber,IDF)[5-6]。SDF可理解为不被人体小肠吸收但可在大肠中发酵的水溶性多糖类成分[7],包括果胶、抗性淀粉(Resistant starch,RS)、β-葡聚糖等。SDF具有降血脂、降血糖、抗衰老、抗辐射和预防结肠癌等生理功能[8],还能够调节肠道菌群结构、维持肠道微生态平衡[9-10]。IDF则是在小肠中不能消化,在结肠中也几乎不能被酵解的一类不溶于水的非淀粉多糖,包括纤维素、半纤维素、木质素。IDF具有促进肠道蠕动,促进粪便形成和有毒物质排出等作用[11]。食药同源指食物与药物来源一致,且具有成分同源性和理论同源性,许多食物既有食用性又有药用性,因此可用以养生保健及防病治病[3]。食物来源膳食纤维可长期食用,但不以治疗疾病为主要功效;药物来源膳食纤维长期食用存在一定的副作用,仅在限定剂量内食用安全。因此,EMDF除了具有安全、无毒等特点,还在营养调节、辅助治疗等方面发挥着重要的作用。
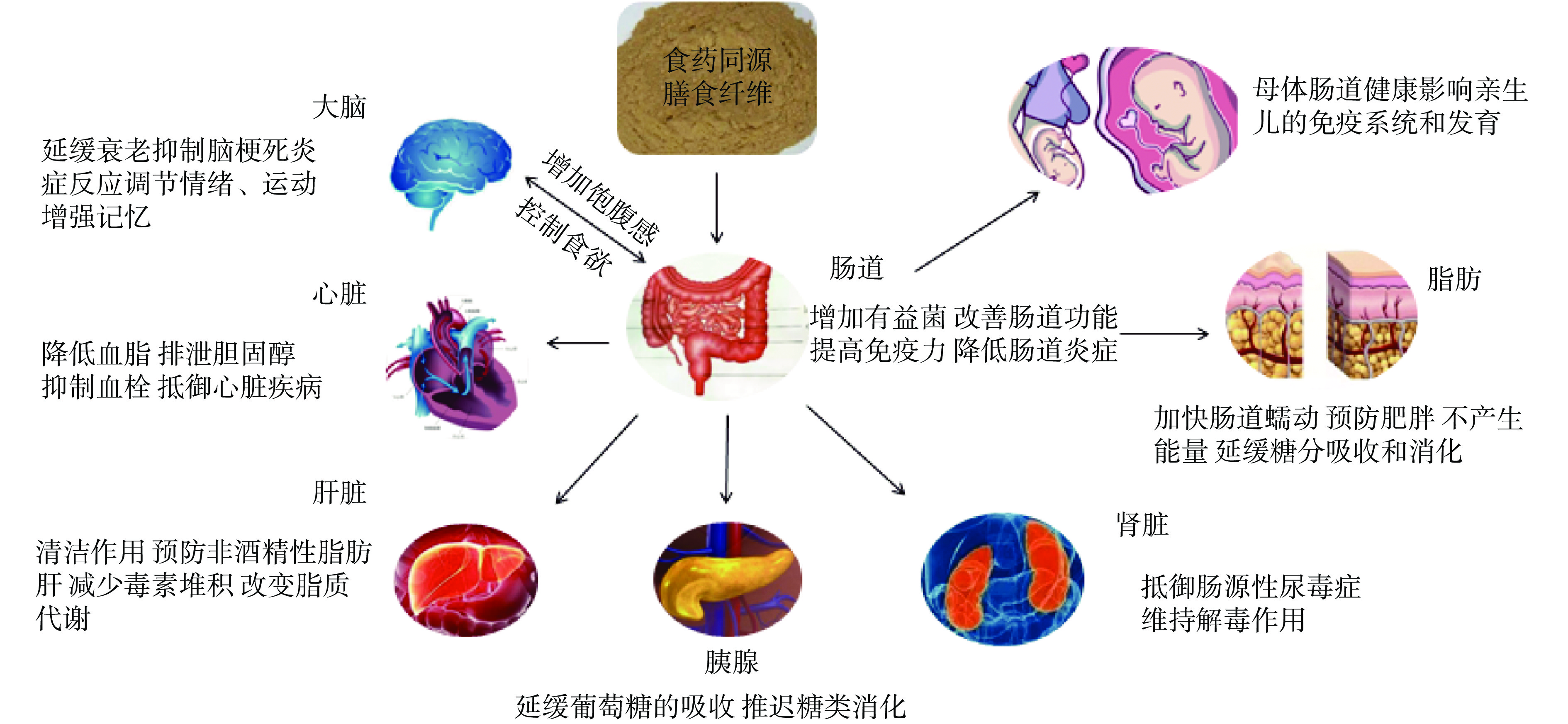
研究显示,EMDF化学成分多种多样,针对不同器官、组织发挥着不同的作用,在食品领域具有极高的研究价值和应用潜力。EMDF可通过菌-肠道-器官轴发挥功效,包括:作用于大脑实现对情绪的调控、抑制脑梗死相关炎症、增加饱腹感并控制食欲;作用于心脏,降低血糖血脂、抵御心脏疾病;作用于肝脏,改善脂质代谢、减少毒素堆积;作用于肠道,增加有益菌,改善肠道炎症、加速代谢,且由于母体肠道状态可影响新生儿的免疫和发育水平,进一步通过母体对胎儿产生影响。图1总结了EMDF对人体的不同起效器官及其作用方式,展示了EMDF的功效特征[1,10,12-14]。
2. EMDF成分
食药同源植物主要包含根、茎、叶、花、果实和种子几部分,其中除皂苷、黄酮、萜类等传统药用活性成分外,还含有极为丰富的EMDF成分[15]。EMDF不仅具有典型的肠道健康功效,同时还保留了部分药用活性,这样大大降低了过多摄入中草药可能存在的健康风险。DF有降血糖、抗衰老、调节免疫系统和消化系统、抗肠胃癌症、抗氧化等作用。但不同种类EMDF的化学成分和理化性质存在显著差异,对EMDF进行细致分类和详细研究具有重要意义。按照不同药用部位的特征,可将EMDF分为植株纤维素类、果实果胶类、块茎抗性淀粉类、真菌低聚糖类。表1中针对其来源、肠道菌群作用特点等方面进行了归纳。
表 1 典型食药同源植物膳食纤维调节肠道健康的作用Table 1. Regulation of dietary fiber from typical edible and medicinal homologous plant on intestinal health分类 典型食药同源植物 膳食纤维来源 肠道菌群 生理作用 参考文献 不溶性纤维素 刺梨(Rosa roxburghii) 刺梨果渣IDF 拟杆菌属、粪球菌属、SCFAs↑,厚壁菌、拟杆菌↓ 维持肠道稳态、增加肠道有益菌、促进肠道润肠通便 [16] 葛根 (Pueraria lobata) 葛根IDF 双歧杆菌和乳杆菌↑,肠杆菌、肠球菌、肠道pH↓ 肠道的润肠通便、抑制肥胖 [17] 果胶 山楂(Crataegus pinnatifida Bge.) 山楂果胶 耐酸有益菌如乳酸杆菌、双歧杆菌、SCFAs↑,肠杆菌、肠球菌、产气荚膜梭菌↓ 润肠通便、改善肠道菌群 [18] 枸杞(Lycium barbarum
L.)枸杞果胶 厚壁菌/拟杆菌比例↓,部分拟杆菌、SCFAs↑ 维持肠道菌群平衡,恢复肠道屏障,恢复肠道菌群、肠道屏障和抑制肝脏炎症,改善非酒精性脂肪性肝病 [19] 抗性淀粉 山药(Dioscorea oppositifolia L.) 山药抗性淀粉 拟杆菌、双歧杆菌、乳酸杆菌、瘤胃球菌、粪球菌属以及支原体菌科↑,厚壁菌门↓ 肠道菌群调节功能,促进益生菌生长及增强其活性 [20] 薏苡仁(Coix lacryma-jobi L.) 薏苡仁抗性淀粉 双歧杆菌和乳杆菌、SCFAs↑ 改善肠道菌群代谢、预防肠道疾病、提高机体免疫能力 [21] 低聚糖 黑木耳(Auricularia auricula) 黑木耳SDF 拟杆菌属和罗氏菌属水平,丙酸和丁酸含量↑,肠道内pH↓ 改善机体肠道微生态,具有益生元作用,理想的微生态调节剂 [8] 灵芝(Ganoderma Lucidum Karst) 灵芝多糖 肠道双歧杆菌、乳酸杆菌、SCFAs↑,肠杆菌、肠道pH↓ 调节肠道的微生态平衡,肠道动力机能,加强肠道的生物学屏障功能,维持肠道内环境的稳定性 [22] 人参(Panax ginseng C. A. Meyer) 人参SDF 厚壁菌门↑ ,乳酸杆菌菌属↑,拟杆菌门菌↓ 使肠道菌群结构向益生菌和纤维素降解菌增殖的方向改变 [23] 红枣(Ziziphus jujuba) 红枣多糖 厚壁菌、拟杆菌↓ 抑制癌变,修复DSS改变的肠道菌群结构,预防结肠癌 [24] 昆布(Laminaria Japonica) 昆布β-葡聚糖 乳酸杆菌、拟杆菌、双歧杆菌和乳杆菌、SCFAs↑,瘤胃球菌和幽门螺杆菌↓ 保持肠道菌群稳态平衡,改善机体肠道菌群的组成和比例,预防急性结肠炎 [25] 鱼腥草(Houttuynia cordata) 鱼腥草多糖 变形菌门、拟杆菌门↓ 调节肠道菌群紊乱,维持微生物稳态,恢复肠道菌群结构 [26] 注:SCFAs,短链脂肪酸。↑代表菌属丰度或pH、SCFAs水平上升;↓代表菌属丰度或pH、SCFAs水平下降。 2.1 不溶性膳食纤维
人参、西洋参以根入药食用,其中含有大量IDF。IDF是植物细胞壁的主要成分,是由葡萄糖组成的大分子多糖。研究发现,人参IDF含有蛋白质、氨基酸,不含脂肪,可作为高营养低热量的食品原料[27-28],此外还含有大量的钙、锌等微量元素,可作为一种微量元素补充剂。另外,人参IDF对葡萄糖、胆酸钠、亚硝酸盐等物质有显著吸附作用,是一种潜在的功能性食品成分[28]。黄芪是另一种以根部入药的食药同源植物。研究发现,黄芪IDF具有双向血糖调节作用,可使血液葡萄糖负荷水平显著下降,肝糖原含量显著下降,并能明显对抗肾上腺素引起的血糖升高[29]。盐地碱蓬IDF对于高脂血症有一定的缓解作用。动物实验表明,摄入盐地碱蓬IDF能够控制高血脂个体的体重增长,并实现降脂的目的[30]。黄精IDF能够促进肠道蠕动,缓解便秘,对于预防结肠癌有积极作用[31]。
2.2 果胶
山楂以果实入药食用,其主要膳食纤维成分为果胶。果胶是一类存在于植物细胞壁、由α-1,4糖苷键连接的酸性多糖[32]。据报道,山楂果胶可以降低血清和肝脏的总胆固醇和甘油三酯水平,提高高密度脂蛋白含量;它能显著促进便秘小鼠的小肠蠕动,增加排便量,改善肠道内环境;山楂果胶还有降血糖、预防胆结石的作用;它还可以降低肝脏、血液中的铅含量,这是机体排铅解毒的有效方式[18]。枸杞果胶能够在一定程度上修复肠道粘膜损伤,增加肠道菌群中有益菌的数量,通过维持肠道健康增强机体免疫功能[19]。除此之外,枸杞果胶还能够抑制肝脏炎症,改善非酒精性脂肪性肝病[33]。桑叶果胶具有肠道益生作用,可降低溃疡性结肠的病变,提高结肠内容物中短链脂肪酸(Short chain fatty acids,SCFAs)含量,增加肠道菌群多样性,维护肠道环境稳态[34]。
2.3 抗性淀粉
山药中主要的膳食纤维成分是RS。RS是由多个葡萄糖分子缩合而成,包括结晶区和无定形区的一类多糖[35]。RS结晶区主要是直链淀粉双螺旋相互叠加而构成,无定形区由无序排列的淀粉构成[35]。山药RS在调控肠道菌群组成及代谢产物生成方面具有独特的作用,它可以通过影响肠道菌群功能和宿主免疫系统稳态来抑制肠炎[35]。薏苡仁RS可以从增加肠道有益菌丰度、改善肠道pH等方面来调节肠道健康[21]。薏苡仁RS还可以加速肠道有毒物质的排出,促进肠上皮细胞的生长,调节与肠道菌群代谢相关的脂肪酸、氨基酸和糖物质代谢,进而改善机体代谢水平[21]。
2.4 β-葡聚糖
灵芝、黑木耳等以真菌子实体入药食用,其主要膳食纤维成分为β-葡聚糖。β-葡聚糖是一类在α-1,6键主链上连接α-1,4或α-1,6键侧链的葡聚糖[36]。灵芝SDF是一种酸性β-葡聚糖[14],含有一定量的糖醛酸和蛋白质,具有较强的持水性、持油性和溶胀性,以及一定的抗氧化能力,且抗氧化水平与其浓度成正相关[14]。灵芝β-葡聚糖能够降低促炎因子的表达,促进肿瘤细胞凋亡,还能够通过增加肠道SCFAs水平、降低肠道疾病活动指数来降低肠炎和结肠癌的患病风险;灵芝β-葡聚糖通过上调巨噬细胞IL-1的表达,增强肠道免疫力[37]。黑木耳SDF同样以β-葡聚糖为主,对葡萄糖和脂质代谢有调节作用,可以促进胰岛素分泌和葡萄糖的肠道吸收,延迟葡萄糖扩散,从而降低餐后血糖,在糖尿病预防等方面发挥重要作用[38]。燕麦β-葡聚糖可增加肠道中乳酸杆菌和双歧杆菌等有益菌的数量。研究发现,燕麦β-葡聚糖可在消化道中被完全发酵,从而在树突状细胞中下调了脂多糖(Lipopolysaccharide,LPS)诱导的细胞因子IL-12,上调了IL-10的水平,增强肠道免疫功能[36]。燕麦β-葡聚糖还能缓解LPS引起的非酒精性肝炎,增加小鼠血浆中胰高血糖素样肽-2(GLP-2)的含量,降低肠道通透性,从而减少LPS的肠吸收[39]。蘑菇β-葡聚糖能够产生对肠道有益的丙酸盐、丁酸盐等SCFAs[40]。这些SCFAs能够弱化组蛋白脱乙酰基酶(Histone deacetylase,HDAC)活性,干预IL-6、NO等促炎因子的表达,还可以通过HDAC抑制NF-κB信号通路中蛋白的乙酰化作用,从而抑制NF-κB通路活性,有效调节肠道健康[39]。
另有研究显示,肠道菌群代谢蛋白质产生三甲胺氮氧化物(Trimethylamine N-oxide,TMAO)。机体TMAO水平升高,可导致癌症、动脉硬化等多种疾病的发生。通过β-葡聚糖调节肠道菌群,可降低体内循环TMAO的浓度[41]。青稞β-葡聚糖被证实可以通过调节肠道菌群抑制TMAO产生,降低肠癌等疾病的患病风险[42]。
3. EMDF对肠道菌群的调节作用
3.1 SDF对肠道菌群的影响
SDF能够在肠道内被微生物分解、利用,产生以SCFAs为代表的代谢产物。SCFAs主要包括乙酸、丙酸、丁酸等短链脂肪酸产物,是肠上皮细胞提供能量的主要来源[43]。乙酸的主要合成途径是Wood-Ljungdahl通路,在结肠肠道厌氧条件下,丙酮酸在丙酮酸甲酸裂解酶作用下形成乙酰辅酶A(CoA)和甲酸,再由乙酰CoA经PTA-ACK途径生成乙酸。丙酸是通过琥珀酸途径、丙烯酸酯途径或丙二醇途径产生的[44]。丁酸则通过丁基辅酶A:乙酸辅酶A转移酶途径产生[44]。研究显示,患有结肠癌患者的粪便代谢物中乙酸、丙酸、丁酸等物质水平降低,而高膳食纤维饮食可使患者肠道中SCFAs水平显著增加[45]。可见,膳食纤维饮食的健康益处很大程度上与其肠道代谢产物SCFAs有关。
3.1.1 调节糖脂代谢
研究证实,血浆乙酸盐浓度的增加与血浆胰岛素水平负相关[46],后者改善由FFAR2机制介导的胰腺β细胞中的胰岛素反应,以实现血糖控制。乙酸盐还促进脂肪组织中脂质的分解,从而抑制游离脂肪酸运输到肝脏,降低脂肪肝的发病率[47]。丙酸通过PPAR-γ信号通路的腺苷单磷酸激活蛋白激酶调节肝脏中的脂质稳态[48],通过增降低甘油三酯的合成改善葡萄糖稳态[48]。研究发现,昆布SDF能够促使结肠产生SCFAs,降低血浆胆固醇、甘油三酯的水平,减轻高脂饮食引起的菌群失调[49];鹰嘴豆SDF可以降低总胆固醇、甘油三酯和低密度脂蛋白胆固醇水平,提高高密度脂蛋白胆固醇水平,提高高脂血症大鼠的SCFAs水平,通过改善肠道菌群的组成和增加有益菌的比例在提高能量稳态中发挥作用[50]。
3.1.2 调节激素
肠道中丙酸可以调节激素酪酪肽(PYY)和胰高血糖素样肽(GLP-1)的合成,进而调节人体食欲,影响肥胖和代谢性疾病的发生发展[51]。SCFAs通过FFAR2/3信号通路增加循环PYY浓度[48]。GLP-1增加胰岛素浓度,抑制胰腺分泌胰高血糖素,抑制胃排空,影响食欲和摄食量[52]。尽管有许多实验表明SDF可调节产生PYY与GLP-1从而调节体内激素,但EMDF的具体生理效应还需要在动物模型中进一步验证。
3.1.3 保护肠屏障,调节免疫
SCFAs还能够通过抑制组蛋白去乙酰酶活性,促进肠上皮细胞紧密连接来调节机体免疫功能,调控宿主新陈代谢[53]。炎性细胞表面的G蛋白偶联受体(G protein-coupled receptors,GPRs)能够调节转录因子的活性,影响炎性因子的合成和分泌。当SCFAs作用于GPRs时,能迅速激活包括有丝分裂原激活蛋白激酶、蛋白激酶C和ATF-2等转录因子,使其在中性白细胞、单核细胞以及脂肪细胞中大量表达,从而减少促炎因子和活性氧的分泌,最终达到抑制炎症的作用[52]。研究显示,EMDF可能通过类似机制作用于肠道免疫[53]。海藻SDF能够抑制免疫细胞在结肠中的聚集,保护肠上皮细胞的完整,减轻肠道炎症。同时,使肠道中髓过氧化物酶水平降低,从而减轻肠道炎症程度[54]。莲子抗性淀粉(LRS)通过促进SCFAs和琥珀酸等多种代谢物的产生来降低肠道pH,增加双歧杆菌、乳酸菌等肠道有益菌丰度[55]。此外,SCFA-LRS联合可抑制肠球菌等有害菌的增殖,进而调节肠道菌群结构、促进免疫调节和机体代谢[56]。
3.1.4 作用于肠-肺轴抗病毒
SCFAs作为肠道菌群的主要代谢产物可通过血液循环到达肺组织,诱导肺细胞产生和释放IFN-1,调控肺组织炎症应答。SCFAs作用于肺组织细胞表面的FFAR3受体,上调CD8+T淋巴细胞的代谢和病毒清除能力,另外补充膳食纤维也有双向调节免疫的作用,可减轻肺部损伤或感染[57]。目前新冠疫情的发展形势越发凸显出肠道健康对于机体的重要性。新型冠状病毒SARS-CoV-2可以感染肠道细胞并在其中复制。肠道中的ACE2受体是病毒侵入宿主细胞的关键,当感染SARS-CoV-2病毒时,病毒就会感染肠道细胞[58]。由此可见,由于肠-肺轴的存在,肺部健康与肠道健康的互作尤为关键[59]。随着关于肠道菌群治疗疾病机制的深入研究,利用肠道菌群辅助诊断、治疗和预后被认为是COVID-19治疗的一种有希望的手段[60]。而多种EMDF都被证实在抗病毒方面有显著疗效,这可能是未来EMDF研究的一个极为重要的方向[61]。
3.2 IDF对肠道菌群的影响
3.2.1 调节肠道菌群结构
研究证实,IDF可通过调节肠道代谢物种类和水平对肠道环境产生积极影响[62]。谷物、红枣、香蕉等食物中的IDF发酵缓慢,使其有足够时间在结肠末端产生SCFAs等代谢产物,进而促进双歧杆菌和乳杆菌等有益菌增殖,抑制肠杆菌、肠球菌等有害菌增殖。这些作用随着IDF浓度的增加而增强,对维持肠道代谢和肠道稳态具有重要意义[20,63]。
3.2.2 保护肠屏障,抑制肠道炎症
最新研究显示,IDF还可以通过维持宿主肠道的物理屏障和改变宿主的免疫因子来增强肠道健康,如肠道中的IDF含量不足,肠道菌群可能会开始降解宿主肠道黏液层,从而打破原有物理屏障[64]。海带IDF被发现在不改变结肠调节性T细胞(Treg)相关靶点的情况下,影响JAK-START途径中淋巴细胞(Th17)相关细胞因子及受体IL23R等因子的表达,减轻炎症性肠病的发生,增强肠道免疫水平[65]。以上研究显示,食药同源IDF可能是一种潜在的改善炎症性肠病的饮食成分。
3.2.3 增强饱腹感
IDF的另一个重要生理效应是增强饱腹感。IDF代谢产物SCFAs促进糖异生基因的表达,通过上调肝门静脉葡萄糖传感器促进饱腹感[44]。乙酸盐可以通过下丘脑信号增强饱腹感[66]。此外,IDF通过激活短链脂肪酸受体GPR41和GPR43,诱导结肠分泌PYY和GLP-1,并释放到体循环中[44]。一旦进入循环,它们可以通过中枢神经系统发出信号,发挥延迟胃排空的作用,从而延缓肠道蠕动,延长营养吸收能力[44]。有研究证实,黄精IDF可以通过促进GLP-1的分泌,增加饱腹感,间接调节肠道蠕动[63]。
4. 结语与展望
近年来,EMDF由于其来源广泛、安全健康等特点在功能性食品、药品等多个方面得到越来越广泛的应用。EMDF具有良好溶胀性和吸附性等理化性质,可通过增加肠道有益菌丰度和SCFAs浓度来调节肠道菌群结构,增强肠道健康,进而促进宿主能量代谢,增强机体免疫功能。目前对于EMDF的研究还存在一些问题:不同来源EMDF的提取方式不同,导致其含量和成分差异巨大,应建立不同类型EMDF成分谱;复杂的多糖结构造成EMDF理化性质各不相同,在应用时应根据其各自特点研制适合的产品;EMDF保留了微量药用成分,存在许多未知的功能活性。当作为保健食品食用时,应针对其健康功效及其与其他营养成分的协同作用进行更加全面的评价;EMDF与肠道菌群的互作机制尚未得到充分揭示,仍然需要大量实验数据的印证;目前对于EMDF的改性、与其他营养成分复配等研究十分匮乏,而这些研究对于EMDF产品品质的提升具有重要意义。因此,未来应致力于探索开发更多的EMDF资源,并对其体外理化性质和体内生理功效进行一系列的评价,深入研究EMDF通过菌-肠轴对机体各组织器官产生的健康影响,为以肠道菌群为作用靶点的EMDF功能性食品开发提供必要的理论依据。
-
表 1 典型食药同源植物膳食纤维调节肠道健康的作用
Table 1 Regulation of dietary fiber from typical edible and medicinal homologous plant on intestinal health
分类 典型食药同源植物 膳食纤维来源 肠道菌群 生理作用 参考文献 不溶性纤维素 刺梨(Rosa roxburghii) 刺梨果渣IDF 拟杆菌属、粪球菌属、SCFAs↑,厚壁菌、拟杆菌↓ 维持肠道稳态、增加肠道有益菌、促进肠道润肠通便 [16] 葛根 (Pueraria lobata) 葛根IDF 双歧杆菌和乳杆菌↑,肠杆菌、肠球菌、肠道pH↓ 肠道的润肠通便、抑制肥胖 [17] 果胶 山楂(Crataegus pinnatifida Bge.) 山楂果胶 耐酸有益菌如乳酸杆菌、双歧杆菌、SCFAs↑,肠杆菌、肠球菌、产气荚膜梭菌↓ 润肠通便、改善肠道菌群 [18] 枸杞(Lycium barbarum
L.)枸杞果胶 厚壁菌/拟杆菌比例↓,部分拟杆菌、SCFAs↑ 维持肠道菌群平衡,恢复肠道屏障,恢复肠道菌群、肠道屏障和抑制肝脏炎症,改善非酒精性脂肪性肝病 [19] 抗性淀粉 山药(Dioscorea oppositifolia L.) 山药抗性淀粉 拟杆菌、双歧杆菌、乳酸杆菌、瘤胃球菌、粪球菌属以及支原体菌科↑,厚壁菌门↓ 肠道菌群调节功能,促进益生菌生长及增强其活性 [20] 薏苡仁(Coix lacryma-jobi L.) 薏苡仁抗性淀粉 双歧杆菌和乳杆菌、SCFAs↑ 改善肠道菌群代谢、预防肠道疾病、提高机体免疫能力 [21] 低聚糖 黑木耳(Auricularia auricula) 黑木耳SDF 拟杆菌属和罗氏菌属水平,丙酸和丁酸含量↑,肠道内pH↓ 改善机体肠道微生态,具有益生元作用,理想的微生态调节剂 [8] 灵芝(Ganoderma Lucidum Karst) 灵芝多糖 肠道双歧杆菌、乳酸杆菌、SCFAs↑,肠杆菌、肠道pH↓ 调节肠道的微生态平衡,肠道动力机能,加强肠道的生物学屏障功能,维持肠道内环境的稳定性 [22] 人参(Panax ginseng C. A. Meyer) 人参SDF 厚壁菌门↑ ,乳酸杆菌菌属↑,拟杆菌门菌↓ 使肠道菌群结构向益生菌和纤维素降解菌增殖的方向改变 [23] 红枣(Ziziphus jujuba) 红枣多糖 厚壁菌、拟杆菌↓ 抑制癌变,修复DSS改变的肠道菌群结构,预防结肠癌 [24] 昆布(Laminaria Japonica) 昆布β-葡聚糖 乳酸杆菌、拟杆菌、双歧杆菌和乳杆菌、SCFAs↑,瘤胃球菌和幽门螺杆菌↓ 保持肠道菌群稳态平衡,改善机体肠道菌群的组成和比例,预防急性结肠炎 [25] 鱼腥草(Houttuynia cordata) 鱼腥草多糖 变形菌门、拟杆菌门↓ 调节肠道菌群紊乱,维持微生物稳态,恢复肠道菌群结构 [26] 注:SCFAs,短链脂肪酸。↑代表菌属丰度或pH、SCFAs水平上升;↓代表菌属丰度或pH、SCFAs水平下降。 -
[1] SHAH B R, LI B, SABBAH H A, et al. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations[J]. Trends in Food Science & Technology,2020,102(8):178−192.
[2] YANG H, SUN Y R, CAI R, et al. The impact of dietary fiber and probiotics in infectious diseases[J]. Microbial Pathogenesis,2020,140(3):1−18.
[3] 谢果珍, 唐雪阳, 梁雪娟, 等. 药食同源的源流内涵及定义[J]. 中国现代中药,2020,22(9):1423−1427, 1462. [XIE G Z, TANG X Y, LIANG X J, et al. The origination, connotation, and definition of “one root of medicine and food”[J]. Modern Traditional Medicine,2020,22(9):1423−1427, 1462. XIE G Z, TANG X Y, LIANG X J, et al. The origination, connotation, and definition of “one root of medicine and food”[J]. Modern Traditional Medicine, 2020, 22 (9): 1423−1427,1462.
[4] 徐守梁, 黄荣. 超声辅助酶法提取黑豆水溶性膳食纤维的工艺研究及理化性质分析[J]. 广东化工,2020,47(5):41−43. [XU S L, HUANG R. Ultrasonic assisted enzymatic extraction of water-soluble dietary fiber from black beans and analysis of its physical and chemical properties[J]. Guangdong Chemical Industry,2020,47(5):41−43. doi: 10.3969/j.issn.1007-1865.2020.05.017 XU S L, HUANG R. Ultrasonic assisted enzymatic extraction of water-soluble dietary fiber from black beans and analysis of its physical and chemical properties[J]. Guangdong Chemical Industry, 2020, 47 (5): 41-43. doi: 10.3969/j.issn.1007-1865.2020.05.017
[5] 钟菁华, 王中亮, 武涌, 等. 膳食纤维与肠道微生物互作调节食物过敏的研究进展[J]. 食品科学,2020,43(3):239−248. [ZHONG J H, WANG Z H, WU Y, et al. Dietary fiber regulates food allergies by interacting with the gut microbiota: A literature review[J]. Food Science,2020,43(3):239−248. ZHONG J H, WANG Z H, WU Y, et al. Dietary fiber regulates food allergies by interacting with the gut microbiota: A literature review[J]. Food Science, 2020, 43(3): 239−248.
[6] 蔡沙, 何建军, 施建斌, 等. 葛渣可溶性膳食纤维酶法制备工艺的研究[J]. 湖北农业科学, 2017, 56(24): 4863−4868,4874. CAI S, HE J J, SHI J B, et al. Studies on enzymatic preparation of soluble dietary fiber from Pueraria residues[J]. Hubei Agricultural Sciences, 2017, 56 (24): 4863−4868,4874.
[7] 刘世军, 高森, 唐志书, 等. 大枣膳食纤维的制备及其物化特性的研究[J]. 陕西农业科学,2016,62(10):72−73,89. [LIU S J, GAO S, TANG Z S, et al. Preparation and physicochemical properties of Ziziphus jujube Mill dietary fiber[J]. Shaanxi jputnal of Agricultural Sciences,2016,62(10):72−73,89. doi: 10.3969/j.issn.0488-5368.2016.10.025 LIU S J, GAO S, TANG Z S, et al. Preparation and physicochemical properties of Ziziphus jujube Mill dietary fiber[J]. Shaanxi jputnal of Agricultural Sciences, 2016, 62 (10): 72-73, 89. doi: 10.3969/j.issn.0488-5368.2016.10.025
[8] 郝敏, 李殿龙, 徐俊亭, 等. 黑木耳胞外多糖对小鼠肠道微生态及免疫调节的影响[J]. 中国食品学报,2021,21(3):63−70. [HAO M, LI D L, XU J T, et al. Effects of exopolysaccharides from Auricularia Auricula-judae on the intestinal microecology and immunomodulatory in mice[J]. Journal of Chinese Institute of Food Science and Technology,2021,21(3):63−70. HAO M, LI D L, XU J T, et al. Effects of exopolysaccharides from Auricularia Auricula-judae on the intestinal microecology and immunomodulatory in mice[J]. Journal of Chinese Institute of Food Science and Technology, 2021, 21 (3): 63-70.
[9] 李芳, 余梦瑶, 江南, 等. 灵芝抑制肿瘤微环境中Treg细胞功能的机制[J]. 四川大学学报(自然科学版),2021,58(2):199−205. [LI F, YU M Y, JIANG N, et al. Study on the mechanism of Ganoderma lucidum inhibiting Treg cell function in the mouse tumor microenvironment[J]. Journal of Sichuan University (Natural Science Edition),2021,58(2):199−205. LI F, YU M Y, JIANG N, et al. Study on the mechanism of Ganoderma lucidum inhibiting Treg cell function in the mouse tumor microenvironment[J]. Journal of Sichuan University(Natural Science Edition), 2021, 58(02): 199-205.
[10] 任多多, 江伟, 孙印石, 等. 果胶的分类、功能及其在食品工业中应用的研究进展[J]. 食品工业科技,2021,43(3):438−446. [REN D D, JIANG W, SUN Y S, et al. Research progress on the classification, function and application of pectin in food industry[J]. Science and Technology of Food Industry,2021,43(3):438−446. doi: 10.13386/j.issn1002-0306.2021020069 REN D D, JIANG W, SUN Y S, et al. Research progress on the classification, function and application of pectin in food industry[J]. Science and Technology of Food Industry, 2021, 43(3): 438−446. doi: 10.13386/j.issn1002-0306.2021020069
[11] 张颂. 杏鲍菇水不溶性膳食纤维对高脂小鼠模型的功效研究[D]. 哈尔滨: 黑龙江大学, 2020. ZHANG S. Study on the effect of insoluble dietary fiber of Pleurotus eryngii on high-fat mouse model[D]. Harbin: Heilongjiang University, 2020.
[12] 白颖慧, 鲁碧楠, 庞宗然. 5种药食同源维药防治2型糖尿病研究进展[J]. 天津中医药大学学报,2016,35(6):428−432. [BAI Y H, LU BI N, PANG Z R. The research progress of 5 kinds of medicine and food homologous medicine in the prevention and treatment of type 2 diabetes[J]. Journal of Tianjin University of Traditional Chinese Medicine,2016,35(6):428−432. BAI Y H, LU BI N, PANG Z R. The research progress of 5 kinds of medicine and food homologous medicine in the prevention and treatment of type 2 diabetes[J]. Journal of Tianjin University of Traditional Chinese Medicine, 2016, 35 (6): 428-432.
[13] 陈海红, 殷鹏飞, 殷军艺, 等. 黑灵芝水溶性膳食纤维的理化性质及抗氧化活性[J]. 食品工业科技,2016,37(8):116−119, 124. [CHEN H H, YIN P F, YIN J Y, et al. Physicochemical properties and antioxidant activity of water soluble dietary fiber from Ganoderma atrum[J]. Science and Technology of Food Industry,2016,37(8):116−119, 124. CHEN H H, YIN P F, YIN J Y, et al. Physicochemical properties and antioxidant activity of water soluble dietary fiber from Ganoderma atrum[J]. Science and Technology of Food Industry, 2016, 37 (8): 116-119,124.
[14] 华梅, 李珊珊, 曲迪, 等. 人参膳食纤维的营养成分和多糖结构及热稳定性研究[J]. 特产研究,2020,42(2):20−26. [HUA M, LI S H, QU D, et al. Study on nutritional ingredient, polysaccharide structure and thermal stability of ginseng dietary fiber[J]. Special Wild Economic Animal and Plant Research,2020,42(2):20−26. HUA M, LI S H, QU D, et al. Study on nutritional ingredient, polysaccharide structure and thermal stability of ginseng dietary fiber[J]. Special Wild Economic Animal and Plant Research, 2020, 42 (2): 20-26.
[15] 贾春伶, 王锦燕, 赵奎君, 等. 《本草纲目》草部药食同源药用植物的记载及启示[J]. 中国现代中药,2020,22(11):1769−1777. [JIA C L, WANG J Y, ZHAO K J, et al. Records of medicinal and plants in the wood section of compendium of Materia Medica relevation[J]. Modern Chinese Medicine,2020,22(11):1769−1777. JIA C L, WANG J Y, ZHAO K J, et al. Records of medicinal and plants in the wood section of compendium of Materia Medica relevation[J]. Modern Chinese Medicine, 2020, 22 (11): 1769-1777.
[16] 夏洁. 刺梨果渣水不溶性膳食纤维的制备、结构表征及其体外发酵特性研究[D]. 广州: 华南理工大学, 2020. XIA J. Study on extraction, structural characterization and in vitro fermentation characteristics of insoluble dietary fiber from Rosa roxburghii fruit[D]. Guangzhou: South China University of Technology, 2020.
[17] 陈融, 刘博, 陈凯, 等. 不同浓度的葛根多糖对小鼠肠道菌群的影响[J]. 中国畜牧杂志,2021,57(5):226−231,236. [CHEN R, LIU B, CHEN K, et al. Effects of different concentrations of Pueraria lobata polysaccharide on intestinal flora in mice[J]. Chinese Journal of Animal Husbandry Science,2021,57(5):226−231,236. CHEN R, LIU B, CHEN K, et al. Effects of different concentrations of Pueraria lobata polysaccharide on intestinal flora in mice[J]. Chinese Journal of Animal Husbandry Science, 2021, 57(5): 226-231, 236.
[18] 刘田, 崔同, 高哲, 等. 山楂膳食纤维的研究进展[J]. 食品研究与开发,2020,41(6):199−204. [LIU T, CUI T, GAO Z, et al. Recent advances in dietary fiber of hawthorn(Crataegus pinnatifida Bge)[J]. Food Research and Development,2020,41(6):199−204. LIU T, CUI T, GAO Z, et al. Recent advances in dietary fiber of hawthorn(Crataegus pinnatifida Bge)[J]. Food Research and Development, 2020, 41(6): 199-204.
[19] 王莹. 枸杞多糖的分离纯化及基于对肠道菌群调节的免疫作用机制研究[D]. 北京: 北京中医药大学, 2020. WANG Y. Isolation and purification of Lycium barbarum polysaccharides and Study on immune mechanism based on regulation of intestinal flora[D]. Beijing: Beijing University of Chinese Medicine, 2020.
[20] 李涛, 宋洪波, 安凤平, 等. 紫山药抗性淀粉调节高脂血症金黄地鼠脂质代谢[J]. 中国食品学报,2021,21(3):95−101. [LI TAO, SONG H B, AN FENG P, et al. The resistant starch from Purple yam regulates lipid metabolism in hyperlipidemic golden hamsters[J]. Journal of Chinese Institute of Food Science and Technology,2021,21(3):95−101. LI TAO, SONG H B, AN FENG P, et al. The resistant starch fromPurple yam regulates lipid metabolism in hyperlipidemic golden hamsters[J]. Journal of Chinese Institute of Food Science and Technology, 2021, 21 (3): 95-101.
[21] 包辰. 薏苡仁抗性淀粉结构特性及其对肠道菌群调节机制的研究[D]. 福州: 福建农林大学, 2017. BAO C. The structural characteristics of semen coicis (Coix lachryma-jibi) resistant starch and its mechanism in regulating intestinal flora[D]. Fuzhou: Fujian Agriculture and Forestry University, 2017.
[22] 丁翘. 基于肠道菌群探讨黑灵芝多糖对2型糖尿病大鼠的影响机制[D]. 南昌: 南昌大学, 2020. DING Q. The mechanism of polysaccharides from Ganoderma atrum on type 2 diabetic rats through gut microbiota[D]. Nanchang: Nanchang University, 2020.
[23] 华梅, 樊美玲, 李志满, 等. 人参水溶性膳食纤维对大鼠糖脂代谢、氧化应激和肠道健康的影响[J]. 食品科学,2021,42(13):127−135. [HUA M, FAN M L, LI Z M, et al. Effects of ginseng water-soluble dietary fiber on glucolipid metabolism, oxidative stress and intestinal health in rats[J]. Food Science,2021,42(13):127−135. doi: 10.7506/spkx1002-6630-20201013-119 HUA M, FAN M L, LI Z M, et al. Effects of ginseng water-soluble dietary fiber on glucolipid metabolism, oxidative stress and intestinal health in rats [J]. Food Science, 2021, 42(13): 127-135. doi: 10.7506/spkx1002-6630-20201013-119
[24] JI X L, HOU C Y, GAO Y G, et al. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model[J]. Food & Function,2020,11(1):163−173.
[25] 张宵, 刘杨, 滕博, 等. 基于肠道菌群的海藻多糖对部分疾病影响的研究进展[J]. 食品工业科技,2021,42(18):421−426. [ZHANG X, LIU Y, TENG B, et al. Research progress of the effects of seaweed polysaccharides on some diseases basedon intestinal flora[J]. Science and Technology of Food Industry,2021,42(18):421−426. doi: 10.13386/j.issn1002-0306.2020080239 ZHANG X, LIU Y, TENG B, et al. Research progress of the effects of seaweed polysaccharides on some diseases basedon intestinal flora[J]. Science and Technology of Food Industry, 2021, 42(18): 421−426. doi: 10.13386/j.issn1002-0306.2020080239
[26] 吴苹, 刘晋倩, 董晶, 等. 鱼腥草多糖对DSS诱导的小鼠结肠炎的改善作用[J]. 食品工业科技,2021,42(23):362−369. [WU P, LIU J Q, DONG J, et al. Improving effect of Houttuynia cordata polysaccharide on dextran sodium sulfate-induced ulcerative colitis[J]. Food Industry Science and Technology,2021,42(23):362−369. doi: 10.13386/j.issn1002-0306.2021030063 WU P, LIU J Q, DONG J, et al. Improving effect of Houttuynia cordata polysaccharide on dextran sodium sulfate-induced ulcerative colitis[J]. Food Industry Science and Technology, 2021, 42(23): 362−369. doi: 10.13386/j.issn1002-0306.2021030063
[27] 华梅, 樊美玲, 卢佳希, 董丽娜, 孙印石. 七种药食两用中药膳食纤维体外抗氧化及胆酸盐结合能力研究[J]. 食品工业科技,2021,42(11):314−320. [HUA M, FAN M L, LU J X, et al. Study on in vitro antioxidant and cholate binding capacity of seven dual-purpose traditional Chinese medicine dietary fibers[J]. Science and Technology of Food Industry,2021,42(11):314−320. HUA M, FAN M L, LU J X, et al. Study on in vitro antioxidant and cholate binding capacity of seven dual-purpose traditional Chinese medicine dietary fibers[J]. Science and Technology of Food Industry, 2021, 42(11): 314-320.
[28] HUA M, LU J, QU D, et al. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient[J]. Food Chemistry,2019,286(JUL.15):522−529.
[29] 尤良震, 林逸轩, 方朝晖, 等. 黄芪甲苷治疗糖尿病及其并发症药理作用研究进展[J]. 中国中药杂志,2017,42(24):4700−4706. [YOU L Z, LIN Y X, FANG Z H, et al. Pharmacological study of astragalus glucoside on diabetes melitus and its complications[J]. China Journal of Chinese Medicine Medica,2017,42(24):4700−4706. YOU L Z, LIN Y X, FANG Z H, et al. Pharmacological study of astragalus glucoside on diabetes melitus and its complications[J]. China Journal of Chinese Medicine Medica, 2017, 42 (24): 4700-4706.
[30] 阴龙飞. 盐地碱蓬膳食纤维制备及降脂作用研究[D]. 唐山: 华北理工大学, 2020. Yin L F. Study on preparation and lipid lowering effect of dietary fiber from Suaeda salsa[D]. Tangshan: North China University of Technology, 2020.
[31] 丁政宇, 张士凯, 何子杨, 等. 响应面优化黄精渣不溶性膳食纤维酶法提取工艺及其结构表征[J]. 食品工业科技, 2021, 42(20): 157−163 Optimization of enzymatic extraction process of insoluble dietary fiber from Polygonatum sibiricum residue by response surface methodology and its characterization[J]. Science and Technology Food Industry, 2021, 42(20): 157−163.
[32] 易建勇, 毕金峰, 刘璇, 等. 果胶结构域精细结构研究进展[J]. 食品科学,2020,41(7):292−299. [ YI J Y, BI J F, LIU X, et al. A review: Domain fine structure of pectic polysaccharides[J]. Food Science,2020,41(7):292−299. doi: 10.7506/spkx1002-6630-20190328-356 YI J Y, BI J F, LIU X, et al. A Review: Domain Fine Structure of Pectic Polysaccharides[J]. Food Science, 2020, 41(7): 292-299. doi: 10.7506/spkx1002-6630-20190328-356
[33] GAO L L, MA J M, FAN Y N, et al. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation[J]. International Journal of Biological Macromolecules,2021,183(7):1379−1392.
[34] 黄思雨. 绿茶、桑叶和臭黄荆叶细粉抗氧化、抗炎及肠道益生性探究[D]. 重庆: 西南大学, 2020. HUANG S Y. Study on anti-oxidation, anti-inflammatory and intestinal probiotic of fine powders of green tea, mulberry leaf and premna leaf[D]. Chongqing: Southwest University, 2020.
[35] 林炎, 王培鑫, 吕芳澜, 等. 抗性淀粉结构特性和肠道菌群调节功能的研究进展[J]. 食品科学,2020,41(11):222−232. [LIN Y, WANG P X, LÜ F L, et al. Recent advances in structural characteristics and intestinal flora-regulating function of resistant starch[J]. Food Science,2020,41(11):222−232. doi: 10.7506/spkx1002-6630-20190611-113 LIN Y, WANG P X, LÜ F L, et al. Recent advances in structural characteristics and intestinal flora-regulating function of resistant starch[J]. Food Science, 2020, 41 (11): 222-232. doi: 10.7506/spkx1002-6630-20190611-113
[36] SUCHECKA D, GROMADZKA-OSTROWSKA J, E ŻYłA, et al. Selected physiological activities and health promoting properties of cereal beta-glucans. A review[J]. Journal of Animal and Feed Sciences,2017,26(3):183−191.
[37] XIE J L, LIU Y X, CHEN B H, et al. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells[J]. Food & Nutrition Research,2019,63(2):1−10.
[38] 张燕燕, 刘新春, 王雪, 等. 黑木耳营养成分及生物活性研究进展[J]. 南方农业,2018,12(29):130−134. [ZHANG Y Y, LIU X C, WANG X, et al. Research progress on nutritional components and bioactivity of Auricularia auricula[J]. Southern China Agriculture,2018,12(29):130−134. ZHANG Y Y, LIU X C, WANG X, et al. Research progress on nutritional components and bioactivity of Auricularia auricula[J]. Southern China Agriculture, 2018, 12 (29): 130-134.
[39] JESENAK M, HRUBISKO M, MAJTAN J, et al. Anti-allergic effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections[J]. Phytotherapy Research,2014,28(3):471−474. doi: 10.1002/ptr.5020
[40] MITSOU E K, SAXAMI G, STAMOULOU E, et al. Effects of rich in β-glucans edible mushrooms on aging gut microbiota characteristics: An in vitro study[J]. Molecules,2020,25(12):2806. doi: 10.3390/molecules25122806
[41] 李丽娟, 师凤华, 谭木秀, 等. 膳食纤维调节肠道菌群的作用机制研究进展[J]. 中国现代医生,2020,58(36):188−192. [LI L J, SHI F H, TAN M X, et al. Progress in the mechanism of dietary fiber in regulating intestinal flora[J]. Modern Chinese Doctor,2020,58(36):188−192. LI L J, SHI F H, TAN M X, et al. Progress in the mechanism of dietary fiber in regulating intestinal flora[J]. Modern Chinese Doctor, 2020, 58(36): 188-192.
[42] 王梦倩, 孙 颖, 邵丹青, 等. 青稞的营养价值和功效作用研究现状[J]. 食品研究与开发,2020,41(23):206−211. [WANG M Q, SUN Y, SHAO D Q, et al. Research status of nutritional value and efficacy of highland barley[J]. Food Research and Development,2020,41(23):206−211. WANG M Q, SUN Y, SHAO D Q, et al. Research status of nutritional value and efficacy of highland barley[J]. Food Research and Development, 2020, 41(23): 206-211.
[43] KASSEM M, DEEHAN E C, JENS W, et al. The impact of dietary fiber on gut microbiota in host health and disease[J]. Cell Host & Microbe,2018,23(6):705−715.
[44] CRONIN P, JOYCE S A, O'TOOLE P W, et al. Dietary fibre modulates the gut microbiota[J]. Nutrients,2021,13(5):1655.
[45] BEST N V, ROLLE-KAMPCZYK U, SCHAAP F G, et al. Bile acids drive the newborn's gut microbiota maturation[J]. Nat Commun,2020(11):3692.
[46] LAYDEN B T, YALAMANCHI S K, WOLEVER T M, et al. Negative association of acetate with visceral adipose tissue and insulin levels[J]. Metabolic Syndrome and Obesity:Targets and Therapy,2012(5):49−55.
[47] CROUSE J R, GERSON C D, DECARLI L M, et al. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man[J]. Journal of Lipid Research,1968,9(4):509−512. doi: 10.1016/S0022-2275(20)42731-2
[48] DEN BESTEN G, BLEEKER A, GERDING A, et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation[J]. Diabetes,2015,64(7):2398−2408. doi: 10.2337/db14-1213
[49] 虞燕华, 夏恺徽, 吴家沁, 等. 藻类膳食纤维制备及功能进展[J]. 粮食与食品工业,2021,28(1):28−32. [YU Y H, XIA K H, WU J Q, et al. Advanced in preparation and function of algae dietary fiber[J]. Cereal & Food Industry,2021,28(1):28−32. doi: 10.3969/j.issn.1672-5026.2021.01.008 YU Y H, XIA K H, WU J Q, et al. Advanced in preparation and function of algae dietary fiber[J]. Cereal & Food Industry, 2021, 28(1): 28-32. doi: 10.3969/j.issn.1672-5026.2021.01.008
[50] HAN J, ZHANG R, MUHEYATI D, et al. The effect of chickpea dietary fiber on lipid metabolism and gut microbiota in high-fat diet-induced hyperlipidemia in rats[J]. Journal of Medicinal Food,2021,24(2):124−134. doi: 10.1089/jmf.2020.4800
[51] CHAMBERS ES, VIARDOT A, PSICHAS A, et al Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults[J]. Gut, 2015, 64(11): 1744–1754.
[52] JITENDRA K, KAVITA R, CHANDER D. Molecular link between dietary fibre, gut microbiota and health[J]. Molecular Biology Reports,2020,47:6229−6237. doi: 10.1007/s11033-020-05611-3
[53] TAN J, MCKENZIE C, POTAMITIS M, et al. The role of short-chain fatty acids in health and disease[J]. Advances in Immunology,2014,121:91−119. doi: 10.1016/B978-0-12-800100-4.00003-9
[54] 曹峻菡, 林鹏程, 王艳峰, 缪锦来, 解万翠. 海藻膳食纤维改善炎症性肠病(IBD)的作用机制研究进展[J]. 食品与机械,2021,37(6):1−7. [CAO J H, LIN P C, WANG Y F, et al. Research Progress on the mechanism of seaweed dietary fiber in improving inflammatory bowel disease(IBD)[J]. Food and Machinery,2021,37(6):1−7. doi: 10.13652/j.issn.1003-5788.2021.06.001 CAO J H, LIN P C, WANG Y F, et al. Research Progress on the mechanism of seaweed dietary fiber in improving inflammatory bowel disease(IBD)[J]. Food And Machinery, 2021, 37(6): 1-7. doi: 10.13652/j.issn.1003-5788.2021.06.001
[55] ZENG H L, HUANG C C, LIN S, et al. Lotus seed resistant starch regulates gut microbiota and increases short-chain fatty acids production and mineral absorption in mice[J]. Journal of Agricultural and Food Chemistry,2017,65(42):9217−9225. doi: 10.1021/acs.jafc.7b02860
[56] LI X, LEI S Z, LIU L, et al. Synergistic effect of lotus seed resistant starch and short-chain fatty acids on mice fecal microbiota in vitro[J]. International Journal of Biological Macromolecules,2021,183(7):2272−2281.
[57] TROMPETTE, AURÉLIEN, GOLLWITZER E S, et al. Dietary fiber confers protection against flu by shaping Ly6c patrolling monocyte hematopoiesis and CD8+T cell metabolism[J]. Immunity,2018,48(5):992−1005. doi: 10.1016/j.immuni.2018.04.022
[58] LAMERS M M, BEUMER J, VAART J, et al. SARS-CoV-2 productively infects human gut enterocytes[J]. Science,2020,369(6499):50−54.
[59] 彭远平, 葛兰, 黄从付. 肠-肺轴互作沟通机制及膳食纤维预防呼吸道病毒感染研究进展[J]. 中国微生态学杂志,2021,33(10):1228−1231,1237. [PENG Y P, GE L, HUANG C F. Gut lung axis interaction and communication mechanism and prevention of respiratory virus infection with dietary fiber: Research progress[J]. Chinese Journal of Microecology,2021,33(10):1228−1231,1237. PENG Y P, GE L, HUANG C F. Gut lung axis interaction and communication mechanism and prevention of respiratory virus infection with dietary fiber: research progress[J]. Chinese Journal of Microecology, 2021, 33(10): 1228-1231, 1237.
[60] REN Z G, WANG H Y, CUI G Y. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19[J]. Gut,2021,70(7):1253−1265. doi: 10.1136/gutjnl-2020-323826
[61] IWONA M-C, KUJAWOWICZ K, WITKOWSKA A M, et al. Beta-glucans from fungi: Biological and health-promoting potential in the COVID-19 pandemic erat[J]. Nutrients,2021,13(11):3960. doi: 10.3390/nu13113960
[62] 王津, 刘爽, 邹妍, 等. 膳食纤维和肠道微生物及相关疾病的研究进展[J]. 食品研究与开发,2020,41(11):201−207. [WANG J, LIU J, ZOU Y, et al. Research advances on the assdciations of dietary fiber with gut microbiota and related disease[J]. Food Research and Development,2020,41(11):201−207. WANG J, LIU J, ZOU Y, et al. Research advances on the assdciations of dietary fiber with gut microbiota and related disease[J]. Food Research and Development, 2020, 41(11): 201-207.
[63] 王晨, 钟赛意, 邹宇晓. 膳食纤维经肠道微生态途径调节脂质代谢作用的研究进展[J]. 食品科学,2019,40(3):338−347. [WANG C, ZHONG S Y, ZOU Y X. Dietary fiber regulates lipid metabolism through the gut microbiota: A literture review[J]. Food Science,2019,40(3):338−347. doi: 10.7506/spkx1002-6630-20171016-114 WANG C, ZHONG S Y, ZOU Y X. Dietary fiber regulates lipid metabolism through the gut microbiota: Aliterture review[J]. Food Science, 2019, 40 (3): 338-347. doi: 10.7506/spkx1002-6630-20171016-114
[64] 黄嫒. IDF对膳食能量摄入及肠道菌群的影响[D]. 成都: 西华大学, 2020. HUANG A. Effect of IDF on dietary energy intake and gut microbiota[D]. Chengdu: Xihua University, 2020.
[65] RYAN M T, O'SHEA C J, COLLINS C B, et al. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon[J]. Journal of Animal Science,2012,90(4):263−265.
[66] FROST G, SLEETH M L, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism[J]. Nat Commun,2014,29(5):3611.
-
期刊类型引用(3)
1. 乔常宏,陈翔宇,刘宝玲,罗琴,刘丁语,何振文,王晓虎,陈晶,张翩,黄元,白挨泉,王刚,蔡汝健. 多组学视角下中药抗菌机制研究进展. 中国畜牧兽医. 2025(01): 52-59 .  百度学术
百度学术
2. 蓝蔚青,赵家欣,谢晶. 香芹酚纳米乳液制备及其对腐败希瓦氏菌的抑制作用. 广东海洋大学学报. 2024(03): 128-135 .  百度学术
百度学术
3. 王亚哲,赵永强,王迪,陈胜军,于刚,冯阳,王悦齐,李春生. 多组学视角下植物精油抑菌机理的研究进展. 食品科学. 2024(17): 348-356 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:
 下载:
下载:



