Preparation and Adsorption Properties of Chitosan/Active Clay Composite as Sugar Juice Decolorization Agent
-
摘要: 以活性白土和壳聚糖制备壳聚糖/活性白土复合脱色剂,糖汁脱色效果作为评价指标,分别考察制备过程中反应温度、反应时间、壳聚糖添加量、壳聚糖分子量等因素对其脱色效果的影响,获得最佳条件为:反应温度为60 ℃,反应时间为7 h,壳聚糖添加量为2.0 g,壳聚糖分子量为200万。该复合物的糖汁脱色率最高可达96.2%,明显优于活性白土的56.7%和壳聚糖的42.2%。通过扫描电镜、红外光谱、X射线衍射和热失重分析仪对其进行结构表征,结果证明壳聚糖较均匀分布于活性白土的表面,形成了较为稳定的复合结构,并保持活性白土多孔隙结构,其热稳定性较壳聚糖的高。该复合物对糖汁中没食子酸的吸附实验表明,其吸附动力学符合准二级动力学模型,吸附速率受颗粒外扩散过程的控制,其吸附行为符合Langmuir等温吸附模型,为单分子层吸附。Abstract: Chitosan/activated clay composite was prepared with chitosan solution and activated clay. With the decolorization ratio of sugar juice as an index, the effects of reaction temperature, reaction time, chitosan dosage and molecular weight on the preparation of chitosan/activated clay composite were investigated. The optimum conditions were obtained as follows: temperature 60 ℃, reaction time 7 h, chitosan dosage 2.0 g and molecular weight 2,000,000. The decolorization rate of the composite was up to 96.2%, which was significantly better than 56.7% of activated clay and 42.2% of chitosan. The decolorization rate of the structure of the composite was characterized by scanning electron microscopy, infrared spectroscopy, X-ray diffraction and thermogravimetric analysis. The results showed that chitosan was evenly distributed on the surface of activated clay, forming a more stable composite structure and maintaining the porous structure of activated clay. The thermal stability of the composite was higher than that of chitosan. The adsorption experiment of gallic acid in sugar juice showed that the adsorption kinetics of the composite conformed to the quasi second order kinetic model, the adsorption rate was controlled by the diffusion process outside the particles, and the adsorption process conformed to the Langmuir isothermal adsorption model, which was monolayer adsorption.
-
色值是衡量制糖工艺效果的重要指标。甘蔗制糖的澄清工序是影响白糖色值的关键环节,其任务主要是将糖汁中的胶体、色素等非糖成份除去,从而提高蔗汁的澄清度[1-2]。蔗汁中的色素主要为酚类物质,其种类繁多(如没食子酸、单宁酸、天冬氨酸等等),该类物质易被空气氧化成醌类化合物,造成蔗汁颜色加深,且在酸性条件下与铁、氧气会生成深色的铁络合物,因此酚类物质的去除率直接影响白糖产品的质量。
目前国内绝大多数甘蔗糖厂采用亚硫酸法澄清工艺,该过程通过添加二氧化硫(SO2)、石灰乳和磷酸,形成亚硫酸钙沉淀和磷酸钙沉淀,同时糖汁中的胶体、色素被其吸附而除去[3-5],该方法具有流程短、成本低等优势,但也存在SO2逸出污染环境、SO2残留超标、高温运行副反应多等问题[6-7]。开发低硫、低温、无毒、高效的澄清技术,是未来甘蔗制糖行业的发展趋势[8-9]。
吸附法用于澄清脱色工艺具有操作简便、安全、设备简单等优点,但吸附剂的选择是关键。壳聚糖是一种含有很多羟基(-OH)和氨基(-NH2)基团的天然直链高聚物,有良好的吸附能力[10-11],且无毒,可以降解,是典型的环境友好材料。壳聚糖在甘蔗混合汁(酸性溶液)中带有正电荷的特性,具有络合、吸附作用,它们的复合物既可以吸附蔗汁中的单宁等多酚类物质,又可以吸附果胶质、多糖、蛋白质等大分子胶体粒子[12]。但由于壳聚糖密度小、易漂浮,难于与蔗汁中酚类等有色物质接触,影响了吸附效果和沉降过滤,且壳聚糖的价格相对较高,单独用于吸附澄清时成本偏高,因此常以壳聚糖为原料进行复合改性制备复合澄清剂[13],目前壳聚糖衍生物、活性炭负载壳聚糖复合物在糖汁澄清中的应用已有较多报道[14-16]。活性白土是由无机酸对天然粘土(主要是膨润土)进行酸性改性得到的一种粘土改性产品,作为无毒、无臭、无味的绿色环保化工原料,具有比表面积大、吸附能力强、性质稳定、可回收处理重复利用等优点,被广泛应用于食油脱色、环保等领域[17-20]。笔者在前期研究中将活性白土用于甘蔗汁澄清脱色的预处理[21],也得到较好的效果,但其脱色率相比壳聚糖/蒙脱土复合物还有一定的差距。由于活性白土对阴离子及有机物的吸附效果较差,限制了应用的范围,许多学者通过采用Ca(OH)2、长链烷基季铵盐、阴离子表面活性剂、硅烷偶联剂和有机胺等改性剂改善活性白土的吸附性能[22]。将活性白土与壳聚糖复合可得到具有稳定结构的活性白土/壳聚糖复合物,其作为吸附剂既能弥补壳聚糖或活性白土作为单一吸附剂的缺陷,又能够发挥活性白土与壳聚糖协同吸附性能,其吸附性能可大幅提高。壳聚糖/活性白土复合物在污水处理、重金属离子吸附、食品果汁澄清等领域中已有相关研究及应用[23-26]。徐启杰等[25]以硅烷偶联剂将壳聚糖与活性白土复合,制得活性白土/壳聚糖复合吸附剂,并探讨验证了所制备的吸附剂对西瓜汁、草莓汁和橙汁的吸附澄清效果。结果表明,以活性白土/壳聚糖复合吸附剂来澄清果汁,具有澄清度明显、澄清的时间短、操作简单等优点。该课题组还采用氯乙酸为交联剂将羧甲基壳聚糖与活性白土复合,研究发现活性白土/羧甲基壳聚糖复合材料对三种蔬菜汁具有较好的澄清作用,对三种蔬菜汁的澄清效果优于单一成分活性白土、壳聚糖和羧甲基壳聚糖[26]。
在制糖工业的糖汁澄清脱色方面,目前尚无活性白土/壳聚糖复合物吸附剂用于澄清甘蔗汁的相关研究报道。基于此,本研究首先用十六烷基三甲基溴化铵对活性白土进行改性,同时采用低浓度的乙酸溶解壳聚糖,再将十六烷基三甲基溴化铵改性后的活性白土与壳聚糖-乙酸溶液复合,制得壳聚糖/活性白土复合物,以不同制备条件所得的壳聚糖/活性白土作为吸附剂研究其对糖汁脱色率的影响规律,进一步对其结构进行表征分析,并研究其对糖汁中典型多酚类物质——没食子酸的吸附行为。为开发无硫、低温、高效的糖汁脱色剂提供理论依据。
1. 材料与方法
1.1 材料与仪器
活性白土 食品级,郑州森海环保有限公司;壳聚糖 脱乙酰度90%,深圳市中发源生物科技有限公司;粗制白砂糖 仅用石灰初步处理,脱色不完全,广西东亚糖业;冰乙酸、无水碳酸钠、蔗糖 西陇科学股份有限公司;十六烷基三甲基溴化铵 天津市光复精细化工研究所;没食子酸 天津市光复精细化工研究所;福林酚试剂 天津市大茂化学试剂厂;所用试剂 均为国产分析纯。
DF-101S型集热式恒温加热磁力搅拌器 巩义市予华仪器有限责任公司;电热恒温鼓DHG-9013A型风干燥箱 上海一恒科学仪器有限公司;BS224S型分析天平 北京赛多利斯仪器系统有限公司;2WAJ型阿贝折射仪 上海申光仪器仪表有限公司;T6型紫外分光光度计 北京普析通用仪器有限责任公司;78-1型磁力加热搅拌器 江苏金怡仪器科技有限公司;SLDC-0506型低温恒温槽 南京顺流仪器有限公司;PerkinEler型傅立叶红外光谱仪 苏州众艾有限公司;SSUPRA55型场发射扫描电镜 德国ZEIS公司;Rigaku Dmax-rA型X-射线衍射仪 日本理学公司;TGA 55型热重分析仪 美国TA公司。
1.2 实验方法
1.2.1 壳聚糖/活性白土复合物的制备及效果分析
1.2.1.1 壳聚糖/活性白土复合物的制备工艺
将20 g活性白土加入到200 mL蒸馏水中分散,并放入恒温加热磁力搅拌器中搅拌升温(设定水浴升温目标为60 ℃);升温期间称量2 g壳聚糖,量取100 mL配制好的1%(V/V)乙酸溶液,将称好的壳聚糖加入到1%乙酸溶液中溶解;再称取0.5 g十六烷基三甲基溴化铵溶解于20 mL蒸馏水中。当恒温加热磁力搅拌器的水浴温度升至60 ℃时,先向活性白土水溶液中缓慢滴加溶解好的十六烷基三甲基溴化铵溶液,再继续缓慢滴加已溶解好的壳聚糖-乙酸溶液。滴加完毕后,在60 ℃下恒温搅拌反应6 h。取出,静置过夜。次日60 ℃烘干,研磨,即得到壳聚糖/活性白土复合物,或壳聚糖/活性白土复合脱色剂。
1.2.1.2 壳聚糖/活性白土复合物制备的效果评价
以糖汁脱色效果作为评价指标,参照文献[27]对壳聚糖/活性白土复合物制备的效果进行评价,即:以粗制白砂糖配制质量浓度为15%、50 mL的糖汁,然后添加壳聚糖/活性白土0.5 g,并在吸附时间40 min、吸附温度50℃的条件下进行脱色实验,过滤,取滤液检测其色值,计算脱色率,得到壳聚糖/活性白土复合物的糖汁脱色效果。糖汁色值及脱色率的计算公式[28]如下:
IU=1000×Ab×c 式中:IU表示波长为560 nm处的糖色值;A表示波长为560 nm时测得样液的吸光度;b表示比色皿厚度,cm;c表示固溶物的修正浓度(20 ℃),g/mL;其中,c=折光锤度×20 ℃时的相应视密度/100。
脱色率的计算公式[19]如下:
D=IU前−IU后IU前×100 式中:D为脱色率,%;IU前表示原糖汁色值,即为处理前的糖汁色值;IU后为脱色处理后糖汁色值。
1.2.2 单因素实验
称取20 g活性白土,加入200 mL蒸馏水溶解分散,分别以温度(40、50、60、70、80 ℃);时间(4、5、6、7、8 h);壳聚糖添加量(1.0、1.5、2.0、2.5、3.0 g);壳聚糖分子量(0.3、5、30、200、300万)为影响因素,以糖汁脱色率为指标,参照文献[27]和文献[29]考察这四个因素对壳聚糖与活性白土复合改性效果的影响。
1.2.3 三种吸附材料糖汁脱色效果的对比实验
取活性白土、壳聚糖和壳聚糖/活性白土复合物(单因素最佳条件)三种吸附脱色剂各0.5 g,分别加入到15%、50 mL粗制白砂糖糖汁中,在温度50 ℃下搅拌吸附40 min,检测糖汁脱色效果,进一步计算、对比其脱色率。
1.2.4 壳聚糖/活性白土复合物的结构表征
为进一步了解活性白土/壳聚糖复合物在糖汁澄清方面的优势,及其在结构上与壳聚糖、活性白土的异同,本文以单因素最佳条件制得的壳聚糖/活性白土及其原料(壳聚糖、活性白土)样品进行如下结构表征分析。
1.2.4.1 电镜扫描(SEM)分析
对壳聚糖、活性白土、壳聚糖/活性白土样品喷金处理后,采用扫描电子显微镜对其进行表面形貌扫描,观测三者表面形貌特征。测试条件:电压25 kV,放大10000倍。
1.2.4.2 傅里叶红外光谱(FT-IR)分析
将壳聚糖、活性白土、壳聚糖/活性白土试样采用KBr压片,在4000~400 cm−1 范围内进行FT-IR扫描摄谱,以漫反射的方法进行,分别分析其主要成分及官能团。
1.2.4.3 X射线衍射(XRD)分析
采用X射线衍射仪对壳聚糖、活性白土、壳聚糖/活性白土进行晶型结构的分析。测试条件为CuK α 辐射(λ=0.154 nm),管电压40 kV,电流200 mA,扫描范围2θ=3~40°,扫描速度2°·min−1。
1.2.4.4 热重分析(TGA)分析
采用TGA-55热分析系统对壳聚糖、活性白土、壳聚糖/活性白土进行热失重分析。测试条件:空气气氛,程序升温加热至800 ℃并达到稳定,升温速率为10 ℃·min−1。
1.2.5 壳聚糖/活性白土复合物对糖汁中没食子酸的吸附性能研究
配制0.100 g/L没食子酸标准溶液:称量0.010 g没食子酸,用15%蔗糖溶液在小烧杯中溶解,移入100 mL容量瓶,以15%蔗糖溶液定容,静置待用。分别吸取不同体积的0.100 g/L的没食子酸−15%蔗糖溶液于50 mL容量瓶中,分别加入1.0 mL福林酚试剂,摇匀,静置5 min,加入5 mL 7.5%碳酸钠溶液,加入15%蔗糖溶液定容,避光静置30 min,在λ=765 nm处测其吸光度。以没食子酸溶液的浓度为X轴(横轴),其吸光度为Y轴(纵轴),拟合出线性回归方程为:N=109.62 M+0.0594,R2=0.9887。相关系数r=(R2)1/2=0.9943,显著水平取α=0.005,实验次数n=6。根据相关系数表,查得Rmin=0.811,r>Rmin,所建立的直线方程与实验数据点拟合得较好。
用移液枪移取10.0 mL没食子酸溶液,加入不同质量的壳聚糖/活性白土复合物(单因素最佳条件),在50 mL夹套恒温石英杯中5 ℃磁力搅拌吸附40 min,再静置20 min,取其上清液2.0 mL,测定其吸光度,按照上述回归的方程求得没食子酸溶液的浓度。
壳聚糖/活性白土对没食子酸的吸附效果以吸附量和吸附率表示,其计算公式为:
Qe=(C0−Ce)VW E(%)=C0−CeC0×100 式中:Qe为达到吸附平衡时吸附量,mg/g;E为吸附率;C0为没食子酸的初始浓度,mg/mL;Ce为吸附达到平衡后没食子酸的浓度,mg/mL;V为样品溶液体积,mL;W为壳聚糖/活性白土的质量,mg。
在获得相关吸附数据的基础上,进一步研究吸附过程的吸附动力学和吸附等温线,采用相关模型对其拟合。对于吸附动力学,采用准一级动力学模型和准二级动力学模型进行拟合。同时以Langmuir吸附模型、Freundlich吸附模型拟合其吸附等温线。
1.3 数据处理
材料制备及吸附性能测试的实验均平行进行3次,取其平均值作为测定结果,运用Microsoft Excel2016进行数据处理,Origin Pro9.1作图。
2. 结果与分析
2.1 单因素实验结果
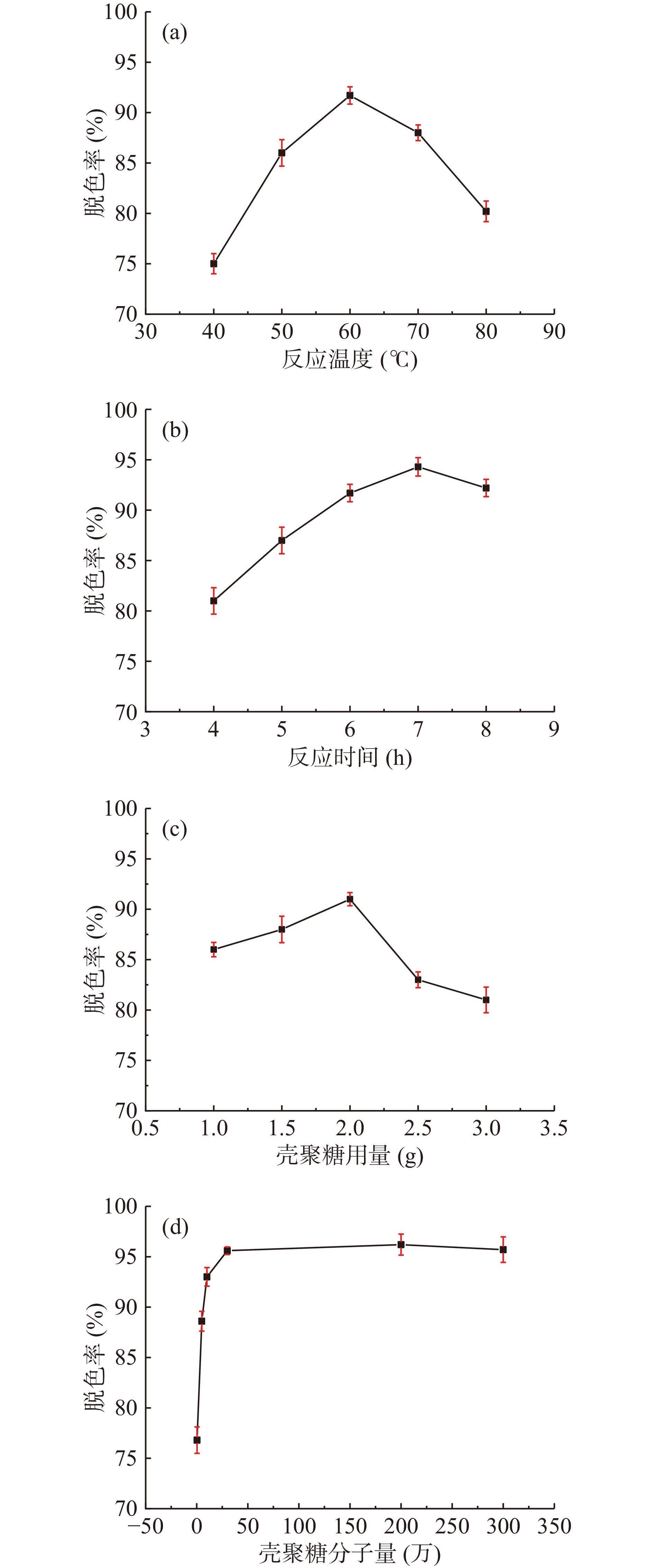
2.1.1 材料制备温度对脱色效果的影响
图1(a)可以看出,糖汁脱色率随着壳聚糖/活性白土制备温度的升高而逐渐升高,在60 ℃时达到最高脱色率,随后温度继续升高,脱色率下降幅度较大。这是因为温度较低时,体系黏度大,分子运动缓慢,不利于壳聚糖对活性白土进行改性反应;随着反应温度的升高,壳聚糖分子活动增强,壳聚糖分子链间的氢键作用减弱从而更容易负载到活性白土上,有利于壳聚糖与活性白土的复合,生成结构稳定、负载量较大的复合物,进而提高其对糖汁的脱色效果;但如果温度过高时,壳聚糖会发生降解反应,壳聚糖的负载量下降,脱色率随之降低,因此材料制备温度以60 ℃为宜。
2.1.2 材料制备时间对脱色效果的影响
图1(b)为制备时间对脱色效果的影响规律。由图可以看出,原糖脱色率随制备时间的升高逐渐升高,当反应时间为7 h时达到最高脱色率,但随着反应时间的延长,脱色率逐渐下降;造成这样种现象可能的原因是:壳聚糖与活性白土的复合是一个动态平衡过程;反应初始,由于壳聚糖分子量比较大,负载速度较慢,与活性白土的复合需要一定的时间,当制备时间为7 h时,壳聚糖的负载量最大,此时可认为反应已达平衡;当反应时间超过7 h之后,负载在活性白土表面的部分壳聚糖可能发生了脱附或降解等现象,导致壳聚糖负载量下降,脱色效果随之下降。
2.1.3 壳聚糖添加量对脱色效果的影响
图1(c)为反应壳聚糖添加量与脱色率的关系图。图中可清楚看出,糖汁脱色率随壳聚糖添加量为2.0 g时脱色率达到最大,说明在此用量时壳聚糖与活性白土的复合效果较好,壳聚糖的负载量较高;但随着壳聚糖的添加量继续增加,脱色率急速下降,造成这种现象可能原因为:添加壳聚糖量过多,体系黏度大,对复合反应不利,使壳聚糖在活性白土上的有效负载量降低。因此,壳聚糖的添加量以2.0 g为最佳。
2.1.4 壳聚糖分子量对脱色效果的影响
图1(d)为壳聚糖分子量对糖汁脱色效果的影响。图中可以看出,随着壳聚糖分子量的增大,糖汁的脱色率呈增加的趋势,当壳聚糖的分子量达到30万以上增加的趋势开始减缓,壳聚糖分子量到达200万时糖汁的脱色率达最高,再继续增加壳聚糖的分子量,脱色率趋于平缓。可能原因是壳聚糖的分子量增大有利于对改性后活性白土的负载,可形成稳定的复合物,但壳聚糖分子量的继续增大有可能不利于其附着在活性白土表面及进入层间,因而复合反应的壳聚糖分子量以200万为宜。
由单因素实验得到最佳的复合物制备条件为:温度60 ℃,时间7 h,壳聚糖添加量2.0 g,壳聚糖分子量200万。最高脱色率达96.2%。
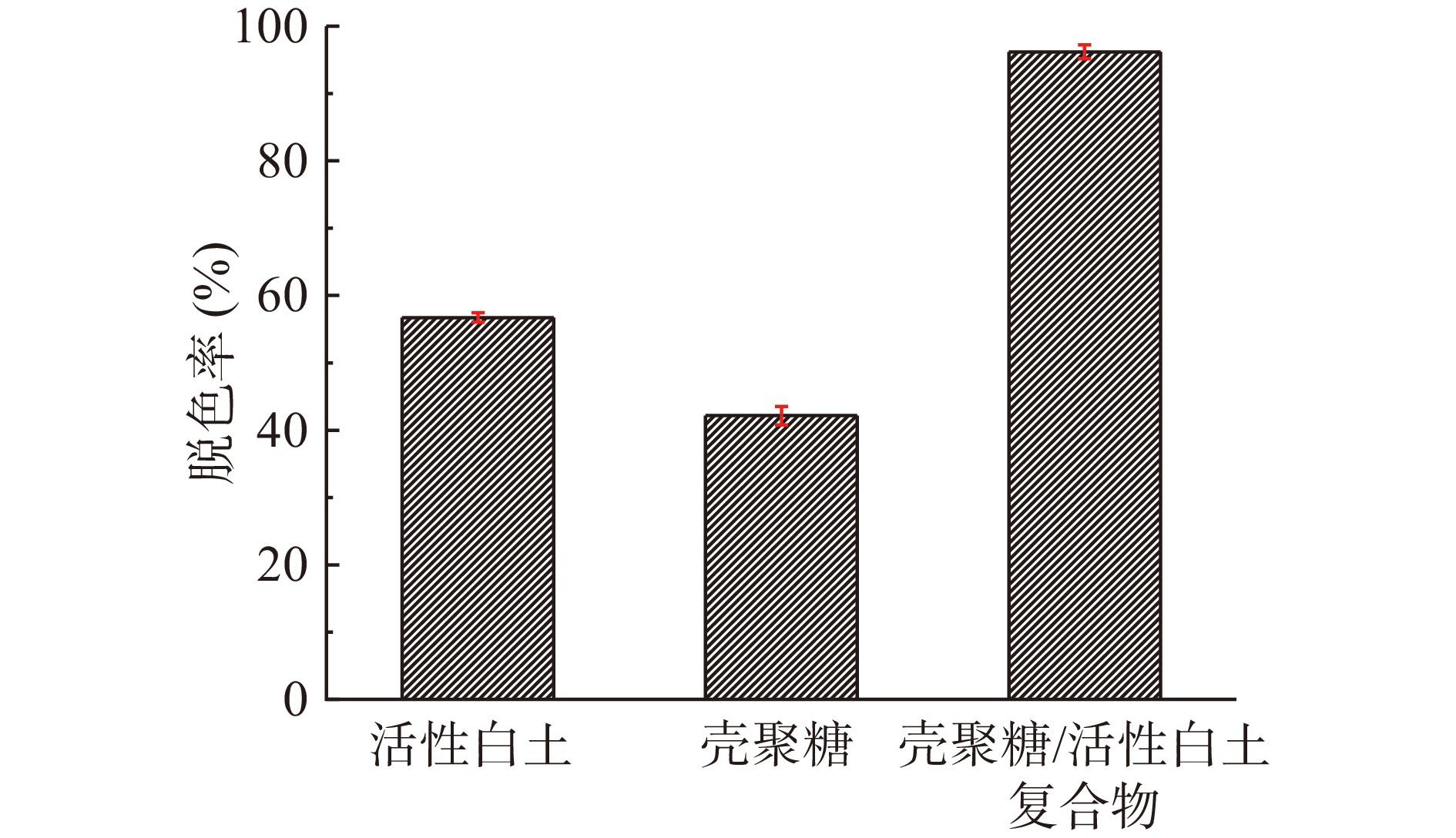
2.2 三种吸附材料糖汁脱色效果的对比
图2为活性白土、壳聚糖和壳聚糖/活性白土复合物三种吸附脱色剂在同一条件下的糖汁脱色效果。从图中的数据可知,活性白土、壳聚糖和壳聚糖/活性白土复合物的糖汁脱色率分别为56.72%、42.16%和94.23%,壳聚糖/活性白土复合物脱色效果明显优于活性白土、壳聚糖单一吸附剂,可能的原因是:a.由于壳聚糖的加入,可提高活性白土的有机物亲和性,利于糖汁有色物质有机小分子与其接触,提高了对糖汁中有色物质的吸附性能[23-25];b.壳聚糖本身就是一种很好的吸附剂,与活性白土复合后,能够发挥两种吸附剂的性能,从而达到更好的吸附效果[26,27,29]。这与其在果汁中的澄清效果及现象比较一致。
2.3 结构表征结果
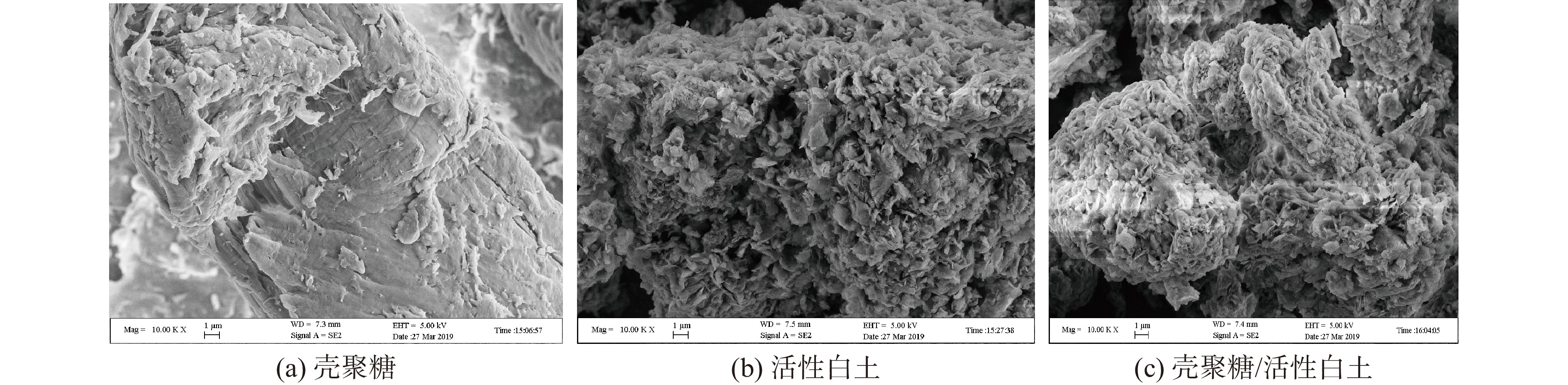
2.3.1 电镜扫描分析
图3分别为壳聚糖、活性白土和壳聚糖/活性白土的扫描电镜图。从图3中可以看出,壳聚糖固体的片状结构较为明显,片层堆叠,结构较致密。活性白土的表面及边缘比较粗糙,层间孔隙明显,大部分聚集成薄厚不一的团块,片体较厚,边部呈尖剌状、卷边状等。由于壳聚糖溶解于乙酸后再与活性白土复合,复合后大部分壳聚糖分子较均匀地分散于活性白土表面,使活性白土的表面变得较密实,但仍保持有较多的孔隙,非常有利于对糖汁中杂质颗粒的吸附。
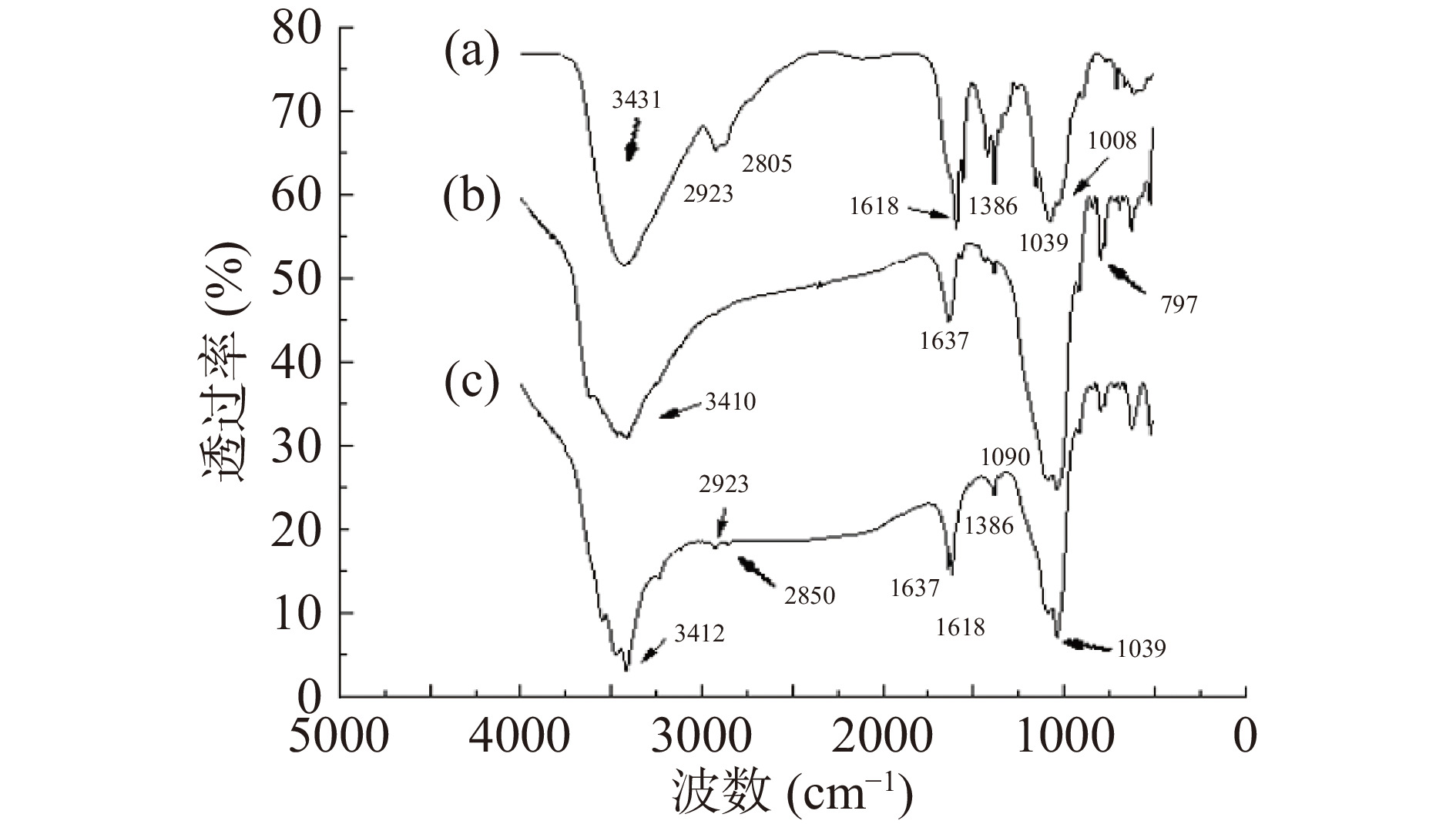
2.3.2 傅里叶红外光谱分析
图4分别为壳聚糖、活性白土和壳聚糖/活性白土三种材料的红外光谱图。从图4中可以看出,壳聚糖红外图谱中3431 cm−1处宽峰为壳聚糖的-OH和-NH2的吸收峰,1618 cm−1处是酰胺Ⅰ带吸收峰,2923和2850 cm−1处分别为C-H的两个振动吸收峰,1386和1405 cm−1处分别为-CH3和-CH2的变形振动吸收峰,1008 cm−1是一级醇羟基的吸收峰,1039 cm−1是二级醇羟基的吸收峰[30]。在活性白土的红外图谱中,3410 cm−1处为活性白土Si-OH-Al结构中-OH的伸缩振动,1637 cm−1为层间吸附水中H-O-H的弯曲振动,797 cm−1处为Si-O-Si的反对称伸缩振动,1090 cm−1处为Si-O-Si的反对称伸缩振动。在壳聚糖/活性白土复合物的红外光谱中,3412、1618和1039 cm−1处出现了壳聚糖的特征吸收峰,说明壳聚糖与活性白土形成了较为稳定的复合结构,壳聚糖的氨基基团成功负载至活性白土上,非常有利于与糖汁中带负电的果胶、单宁等杂质颗粒结合形成沉淀。活性白土晶格中Si-O-Si及Si-O-Al相关的在797、1090、3410 cm-1的振动峰没有发生明显的变化,因此壳聚糖对活性白土的复合改性后,所得壳聚糖/活性白土仍保持活性白土的结构。此外,由于十六烷基三甲基溴化铵的添加量比较少,可能其特征峰未显现或已被壳聚糖所掩盖(2923和2850 cm−1处C-H的振动吸收峰、1386和1405 cm−1处-CH3和-CH2变形振动吸收峰[31],均与壳聚糖的特征峰重合)。
2.3.3 X射线衍射分析
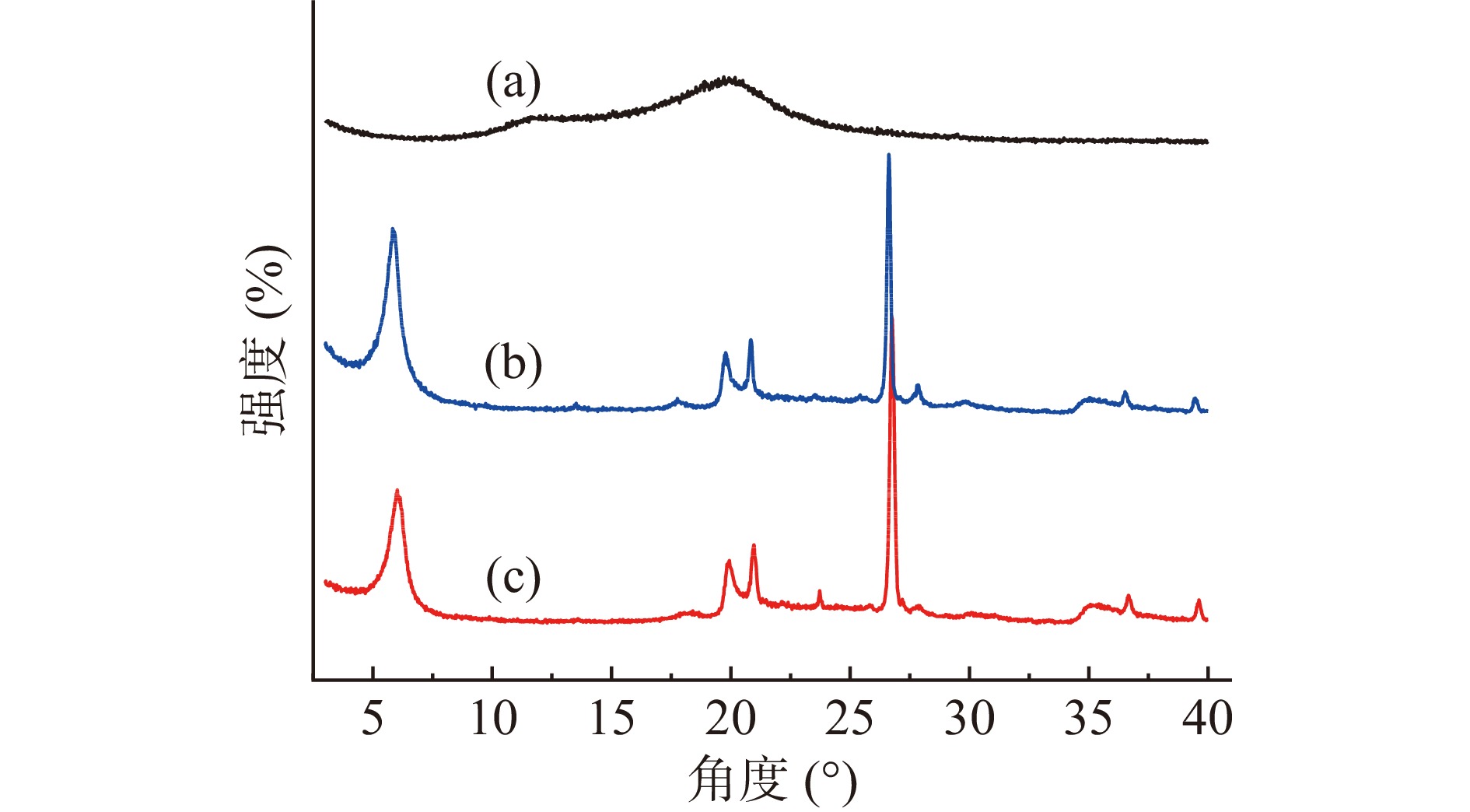
图5分别为壳聚糖、活性白土和壳聚糖/活性白土三种材料的X射线衍射图。由图5可以看出,壳聚糖在2θ=11.7°和19.9°处有两个特征峰[30];壳聚糖/活性白土复合物与活性白土的特征峰都极为相似,这说明壳聚糖/活性白土复合物仍保持活性白土的结构。活性白土的(d001)峰在2θ=5.893°处,根据Bragg方程计算蒙脱土的层间距为1.498 nm,而壳聚糖/活性白土复合物的(d001)峰在2θ=6.046°处出现,计算其层间距为1.460 nm,可见反应复合后活性白土的层间距并没有增大,说明进入活性白土层间的壳聚糖仅为少量(层间距未被撑大),大部分的壳聚糖均匀分散于活性白土的表面,形成复合吸附剂。这与扫描电镜、红外光谱的表征结果相一致,也与壳聚糖/蒙脱土复合物的表征结果较相似[27,29]。
2.3.4 热失重分析
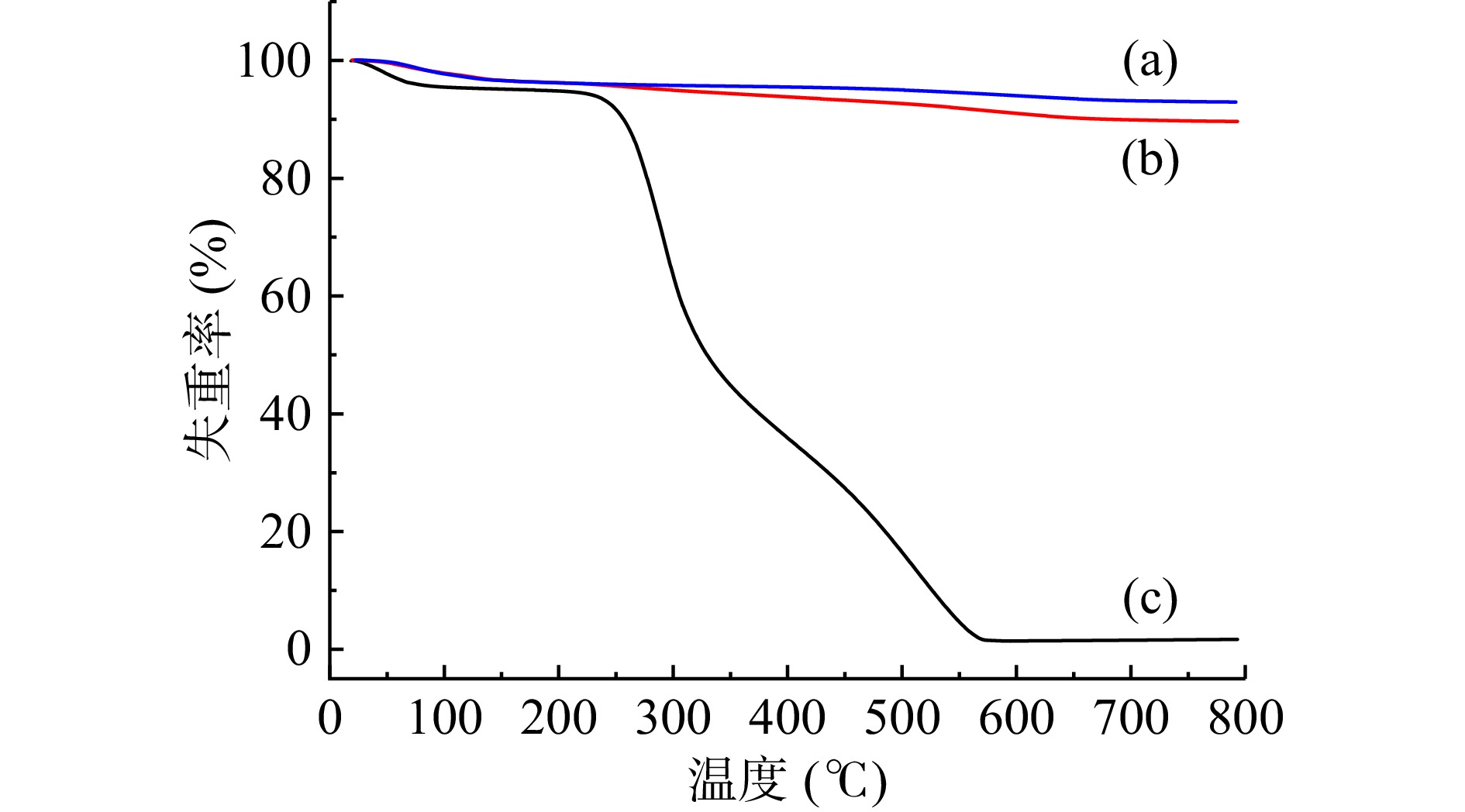
图6分别为壳聚糖、活性白土、壳聚糖/活性白土三种材料的热重分析曲线。从图中(a)线可以看出活性白土的失重量很小,仅在温度达到600 ℃后才有少量失重,200 ℃升至800 ℃时总失重率约为2.9863%,说明活性白土的热稳定性很好;从(c)线可以看出壳聚糖在105 ℃之前,出现轻微的失重现象,这说明失去了空腔内的结合水[22],在200~370 ℃、400~570 ℃两处温区失重率较大,主要为壳聚糖发生热分解,分子中的C-N、C-C化学键发生断裂,以小分子的形式放出,而发生严重的失重现象,达570 ℃时已基本上分解完全。从(b)线可以明显看出,复合脱色剂的失重率介于壳聚糖与活性白土之间,随着温度的升高,失重量逐渐增加,主要失重区间为500~700℃,为壳聚糖等有机高分子的烧失所致,说明壳聚糖已成功负载在活性白土上,且由于两者结合得比较牢固,热稳定性有所提高。由图还可知,壳聚糖/活性白土在200~800 ℃区间失重率为6.732%,按比例除去活性白土在此区间的失重量,计算出在200~800 ℃区间壳聚糖/活性白土失重率约为4.017%(因十六烷基三甲基溴化铵的添加量比较少,忽略其失重量),即为壳聚糖在活性白土上大致的负载量,此值小于理论计算值(约9.091%),说明在复合改性过程中壳聚糖发生降解、流失的量较大。
2.4 吸附性能研究
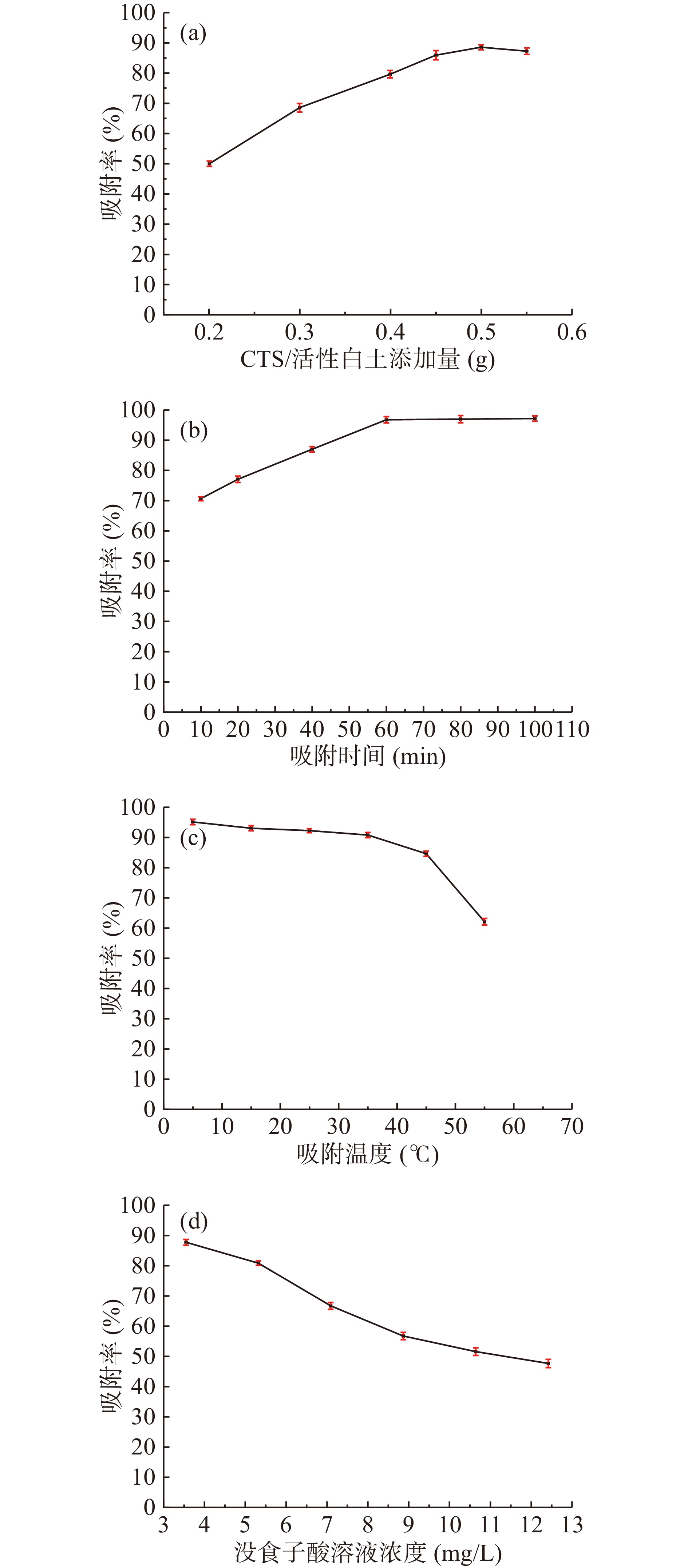
不同壳聚糖/活性白土添加量对糖汁中没食子酸的吸附曲线见图7(a),可知在复合物添加量小于0.3 g时,能够有效的吸附没食子酸。复合物用量超过0.3 g后,没食子酸溶液的吸附率总体上增大并且趋于平缓。不同时间内壳聚糖/活性白土复合物对糖汁中没食子酸溶液的吸附曲线见图7(b),可知复合物对没食子酸的吸附随着时间的不断增大,其中10 min内吸附效果最明显,超过10 min后吸附率不断增大并趋于平缓。不同温度下壳聚糖/活性白土复合物对糖汁中没食子酸的吸附曲线见图7(c),可以看出在5~45 ℃温度内,复合物对糖汁中没食子酸的吸附能力影响较小,超过45 ℃后,没食子酸溶液吸附率明显减小。不同浓度的没食子酸溶液对壳聚糖/活性白土复合物的吸附曲线如图7(d),可见随着没食子酸溶液浓度加大,复合物对其的吸附率逐渐减小,复合物的吸附能力达到饱和。
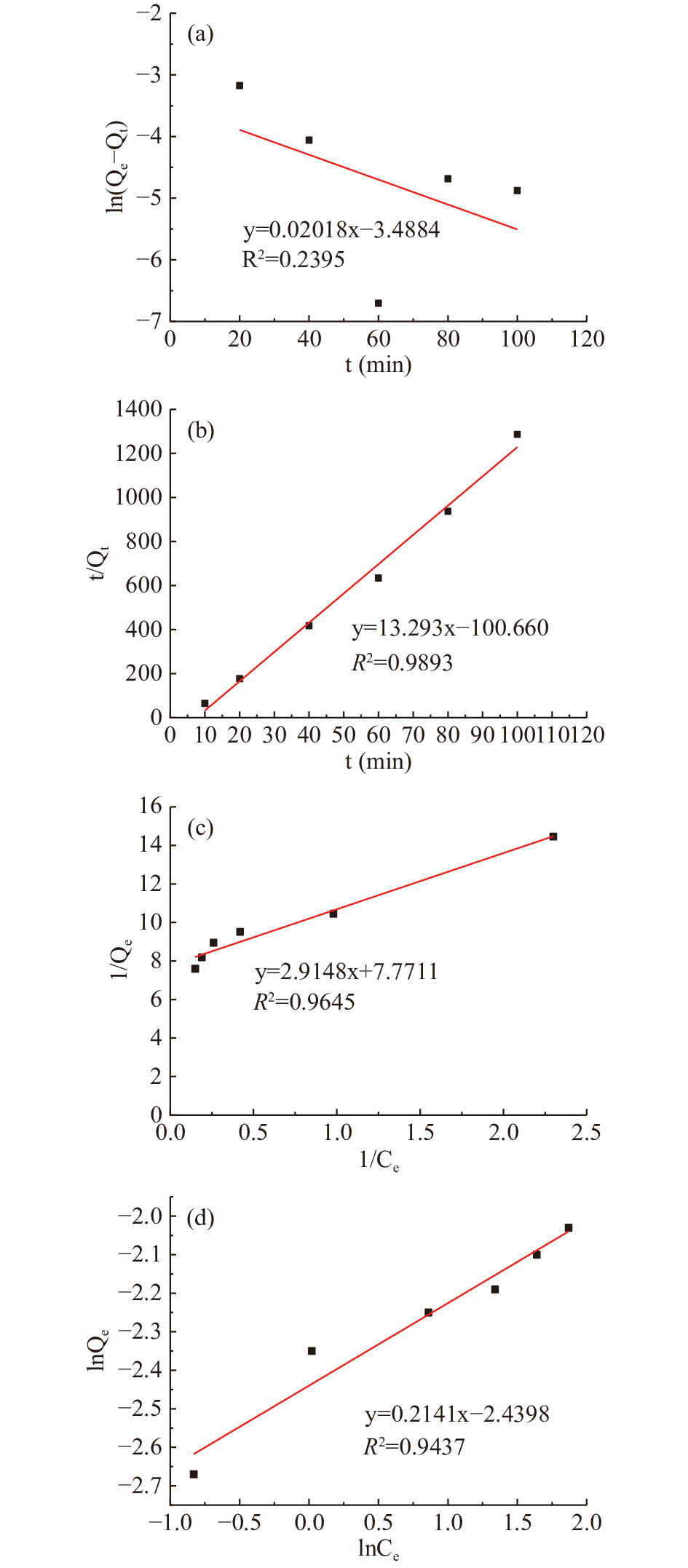
壳聚糖/活性白土复合物对糖汁中没食子酸的准一级、准二级动力学模型[9,32-34]的拟合曲线见图8(a)、(b)所示,吸附动力学方程如表1所示。从两种动力学吸附模型来看,该吸附过程是一个准二级吸附过程。
表 1 壳聚糖/活性白土复合物对糖汁中没食子酸的吸附动力学方程和等温吸附方程及其参数Table 1. Adsorption kinetic equation, isothermal adsorption equation and parameters of gallic acid in sugar juice by chitosan/activated clay composites模 型 方 程 R2 相关参数 动力学 准一级动力学模型 ln(Qe−Q−c)=−0.0202t−3.4884 0.2395 k1=0.0202 min−1
Qe=0.0305 mg/g准二级动力学模型 tQt=13.293t−100.660 0.9893 k2=164.4 g·min−1·mg−1
Qe=0.078 mg/g等温吸附 Langmuir模型 1Qe=2.91481Ce+7.7711 0.9645 1/Qm=7.7711
1/KL=0.3750Freundlich模型 lnQe=0.2141lnCe−2.4398 0.9437 1/n=0.2141
KF=0.08718根据吸附等温线的Langmuir和Freundlich吸附模型[19,32-35],可以做出壳聚糖/活性白土对糖汁中没食子酸溶液在5 ℃,复合物用量0.05 g,60 min内的吸附曲线,并进一步绘制其Langmuir吸附模型曲线和Freundlich吸附模型曲线,见图8(c)、图8(d),其等温吸附方程如表1所示。依据实验点的分布及R2的大小,可以看出Langmuir等温吸附模型相比于Freundlich模型能更好地表示该吸附系统,这表明吸附过程为单分子层吸附,还可以推测壳聚糖/壳聚糖复合物表面较为均匀,活性位点的吸附能力大致相等。与文献[9]所述磁性壳聚糖改性硅藻土对糖汁中没食子酸的吸附结果分析比较一致。
3. 结 论
以壳聚糖、活性白土为原料制得壳聚糖/活性白土复合物用于糖汁澄清脱色,其糖汁脱色率明显优于活性白土和壳聚糖。最佳制备条件为:壳聚糖添加量2.0 g、时间7 h、温度60 ℃、壳聚糖分子量为200万。结构表征结果表明:壳聚糖与活性白土复合后,大部分壳聚糖分子较均匀地分散于活性白土表面,在该复合物的红外光谱中出现了壳聚糖的特征吸收峰;其形成了较为稳定的复合结构,并保持活性白土多孔隙结构,热稳定性比壳聚糖有所提高。该复合物对糖汁中酚类物质——没食子酸的吸附反应的最佳条件是吸附温度5 ℃,吸附时间60 min,其吸附动力学符合准二级动力学模型,吸附速率受颗粒外扩散过程的控制,吸附过程符合Langmuir等温吸附模型,为单分子层吸附,其表面较为均匀,活性位点的吸附能力大致相等。由于甘蔗汁成份复杂,其中的非糖杂质很多,如绿原酸、咖啡酸、果胶、天冬氨酸等的吸附性能有待进一步研究。此外,从结构来看,本文所制备的壳聚糖/活性白土复合物,其中的壳聚糖主要是负载于活性白土表面,对于壳聚糖插层于活性白土层间复合材料的制备仍待后续的研究。壳聚糖/活性白土复合物具有澄清脱色效果好、操作简便等优点,在甘蔗汁无硫澄清的应用中具有良好的前景。
-
表 1 壳聚糖/活性白土复合物对糖汁中没食子酸的吸附动力学方程和等温吸附方程及其参数
Table 1 Adsorption kinetic equation, isothermal adsorption equation and parameters of gallic acid in sugar juice by chitosan/activated clay composites
模 型 方 程 R2 相关参数 动力学 准一级动力学模型 ln(Qe−Q−c)=−0.0202t−3.4884 0.2395 k1=0.0202 min−1
Qe=0.0305 mg/g准二级动力学模型 tQt=13.293t−100.660 0.9893 k2=164.4 g·min−1·mg−1
Qe=0.078 mg/g等温吸附 Langmuir模型 1Qe=2.91481Ce+7.7711 0.9645 1/Qm=7.7711
1/KL=0.3750Freundlich模型 lnQe=0.2141lnCe−2.4398 0.9437 1/n=0.2141
KF=0.08718 -
[1] HUGOT E. Handbook of cane sugar engineering[M]. Elsevier Publishing Company, 1960.
[2] 陈维均, 许斯欣. 甘蔗制糖原理与技术—蔗汁清净[M]. 北京: 中国轻工业出版社, 2001. CHEN Weijun, XU Sixin. Principle and technology of sugarcane sugar production-cane juice clean[M]. Beijing: China Light Industry Press, 2001.
[3] THAI C C D, DOHERTY W O S. Characterisation of sugarcane juice particles that influence the clarification process[J]. International Sugar Journal,2012,114(1366):719−724.
[4] 崔越, 李利军, 黄彩幸, 等. 糖汁亚硫酸法絮凝澄清过程中的Zeta电位[J]. 食品科学,2014,35(9):30−33. [CUI Yue, LI Lijun, HUANG Caixing, et al. Changes in zeta potential of sugar liquor during flocculation and clarification by culfurous acid method[J]. Food Science,2014,35(9):30−33. doi: 10.7506/spkx1002-6630-201409007 [5] SARANIN A. Technology of phosflotation of sugar melt[J]. Sugar Tech Rev,1972,2:1−72.
[6] 韦宇, 李岩, 卢家炯, 等. 甘蔗糖厂降低亚硫酸法产品残留量的初步研究[J]. 现代食品科技,2010,26(2):180−183. [WEI Yu, LI Yan, LU Jiajiong, et al. Primary study of residual sulfur content of sulfitation sugar mill product[J]. Modern Food Science & Technology,2010,26(2):180−183. [7] RODRIGUES R, SPERANDIO L C C, ANDRADE C M G. Investigation of color and turbidity in the clarification of sugarcane juice by ozone[J]. J Food Process Eng,2018,41:e12661. doi: 10.1111/jfpe.12661
[8] EGGLESTON G, LEGENDRE D, PONTIF K, et al. Improved control of sucrose losses and clarified juice turbidity with lime saccharate in hot lime clarification of sugarcane juice and other comparisons with milk of lime[J]. Journal of Food Processing and Preservation,2014,38(1):311−325. doi: 10.1111/j.1745-4549.2012.00779.x
[9] SONG X, CHAI Z, ZHU Y, et al. Preparation and characterization of magnetic chitosan-modified diatomite for the removal of gallic acid and caffeic acid from sugar solution[J]. Carbohydrate Polymers,2019,219:316−327. doi: 10.1016/j.carbpol.2019.04.043
[10] 曾德永, 张立钢, 赵玉红. 澄清剂对蓝靛果果汁活性成分和特性的影响[J]. 食品科学,2018,39(4):43−48. [ZENG Deyong, ZHANG Ligang, ZHAO Yuhong. Effects of clarifying agents on active Ingredients and characteristics of Haskap (Lonicera caerulea L.) juice[J]. Food Science,2018,39(4):43−48. doi: 10.7506/spkx1002-6630-201804008 [11] 谢捷, 刘小景, 朱兴一, 等. 壳聚糖澄清甜叶菊水提液及其澄清机理探讨[J]. 食品科学,2011,32(20):1−6. [XIE Jie, LIU Xiaojing, ZHU Xingyi, et al. Application of chitosan flocculation method in clarification of water extract from stevia rebaudiana bertoni leaves and flocculation mechanism analysis[J]. Food Science,2011,32(20):1−6. [12] OZGE TASTAN, TANER BAYSAL. Clarification of pomegranate juice with chitosan: Changes on quality characteristics during storage[J]. Food Chemistry,2015,180:211−218. doi: 10.1016/j.foodchem.2015.02.053
[13] 席超, 张赞, 闫振华, 等. 沸石负载壳聚糖对高酸苹果发酵酒的澄清工艺[J]. 食品科学,2010,31(22):164−169. [XI Chao, ZHANG Zan, YAN Zhenhua, et al. Effect of zeolite-loaded chitosan on the clarification of apple cider[J]. Food Chemistry,2010,31(22):164−169. [14] 何惠欢, 谢志荣, 刘继栋, 等. 羧甲基壳聚糖糖用澄清剂的制备及结构表征[J]. 食品工业科技,2015,36(6):117−121. [HE Huihuan, XIE Zhirong, LIU Jidong, et al. Preparation and structural characterization of carboxymethyl chitosan as a sugar cane juice clarifying agent[J]. Science and Technology of Food Industry,2015,36(6):117−121. [15] 任勤, 张佳欣, 雷财玉, 等. 壳聚糖及其衍生物在糖汁澄清过程中的研究进展[J]. 中国调味品,2017,42(4):159−162. [REN Qin, ZHANG Jiaxin, LEI Caiyu, et al. Research progress of chitosan and its derivative in the process of sugar juice clarification[J]. China Condiment,2017,42(4):159−162. doi: 10.3969/j.issn.1000-9973.2017.04.036 [16] 冯淑娟, 李利军, 夏兆博, 等. 活性炭负载壳聚糖的制备及其对糖汁清净性能研究[J]. 食品工业科技,2016,37(15):256−259. [FENG Shujuan, LI Lijun, XIA Zhaobo, et al. Preparation of activated carbon attached chitosan and its clarification performance for remelt syrup of brown granulated sugar[J]. Science and Technology of Food Industry,2016,37(15):256−259. [17] 张旭, 王玉琦, 张鲁, 等. 响应面法脱除玉米油中色素及蜡质工艺优化[J]. 食品科学,2017,38(6):248−252. [ZHANG xu, WANG Yuqi, ZHANG Lu, et al. Optimization of processing conditions for removal of pigment and wax from com oil using response surface methodology[J]. Food Science,2017,38(6):248−252. doi: 10.7506/spkx1002-6630-201706039 [18] 宋恭帅, 彭茜, 张蒙娜, 等. 5种脱色剂对粗鱼油挥发性风味物质及脂肪酸组成的影响[J]. 食品科学,2018,39(18):35−41. [SONG Gongshuai, PENG Xi, ZHANG Mengna, et al. Volatile flavor compounds and fatty acid profiles of crude fish oil decolorized with five decoloring agent[J]. Food Chemistry,2018,39(18):35−41. doi: 10.7506/spkx1002-6630-201818006 [19] S M SILVA, K A SAMPAIO, R CERIANI, et al. Adsorption of carotenes and phosphorus from palm oil onto acid activated bleaching earth: Equilibrium, kinetics and thermodynamics[J]. Journal of Food Engineering,2013,118(4):341−349. doi: 10.1016/j.jfoodeng.2013.04.026
[20] JIMOH K ADEWOLE, TAYE S KAZEEM, TAJUDEEN A OYEHAN. Tuning the chemistry of seawater with activated clay: An application in smart water enrichment for enhanced oil recovery[J]. Journal of Petroleum Exploration and Production Technology,2020,10:1−12.
[21] 廖耀文, 黄承都, 黄永春. 过氧化氢-维生素C体系协同壳聚糖/蒙脱土复合物对甘蔗汁的脱色研究[J]. 甘蔗糖业,2020,49(4):59−67. [LIAO Yaowen, HUANG Chengdu, HUANG Yongchun. Decolorization of sugarcane juice by hydrogen peroxide-vitamin C system with chitosan/montmorillonite composite[J]. Sugarcane and Canesugar,2020,49(4):59−67. [22] 高晓薇, 韦藤幼, 潘远凤, 等. 活性白土的化学改性及其作用机理研究[J]. 广西大学学报(自然科学版),2010,35(3):410−414,418. [GAO Xiaowei, WEI Tengyou, PAN Yuanfeng, et al. Study on chemical modification of activated clay and its mechanism[J]. Journal of Guangxi University(Nat Sci Ed),2010,35(3):410−414,418. [23] 潘庆才, 徐启杰, 张昕, 等. 活性白土/壳聚糖复合物的制备及其吸附性能研究[J]. 化工新型材料,2016,44(4):161−163. [PAN Qingcai, XU Qijie, ZHANG Xin, et al. Study on the preparation and absorption property of activated clay/chitosan composite[J]. New Chemical Materials,2016,44(4):161−163. [24] 徐启杰, 赵梦溪, 潘庆才, 等. 活性白土/壳聚糖复合物对多成分污染物的吸附行为研究[J]. 应用化工,2016,45(10):1908−1912. [XU Qijie, ZHAO Mengxi, PAN Qingcai, et al. Study on the adsorption behaviors of activated clay/chitosan composite on the multi-component pollutant[J]. Applied Chemical Industry,2016,45(10):1908−1912. [25] 徐启杰, 孙楠, 李伟, 潘庆才, 等. 活性白土/壳聚糖复合物吸附剂对果汁的澄清作用研究[J]. 化学试剂,2017,39(3):247−250,312. [XU Qijie, SUN Nan, LI Wei, et al. Clarified effect of activated clay/chitosan composite on juice[J]. Chemical Reagents,2017,39(3):247−250,312. [26] 徐启杰, 潘庆才, 耿电光, 等. 活性白土/羧甲基壳聚糖复合物对蔬菜汁的澄清作用[J]. 化学研究,2018,29(4):405−410. [XU Qijie, PAN Qingcai, GENG Dianguang, et al. Clarified effect of activated clay/carboxymethyl chitosan composite on vegetable juice[J]. Chemical Research,2018,29(4):405−410. [27] 黄承都, 黄永春, 任仙娥, 等. CTS/MMT糖用澄清剂的制备及其糖汁脱色工艺研究[J]. 中国调味品,2019,44(12):31−34,49. [HUANG Chengdu, HUANG Yongchun, REN Xian’e, et al. Research on preparation of CTS/MMT as sugarcane clarifying agent and decolorization process of sugar juice[J]. China Condiment,2019,44(12):31−34,49. doi: 10.3969/j.issn.1000-9973.2019.12.007 [28] 华南工学院, 等编. 制糖工业分析[M]. 北京: 轻工业出版社, 1981. South China Institute of Technology, et al. Analysis of sugar industry[M]. Beijing: Light Industry Press, 1981.
[29] 黄承都, 艾硕, 黄永春, 等. 壳聚糖/蒙脱土复合糖用澄清剂的制备工艺及优化[J]. 中国食品添加剂,2020,31(4):35−41. [HUANG Chengdu, AI Shuo, HUANG Yongchun, et al. Preparation technology and optimization of chitosan/montmorillonite composite as sugarcane clarifying agent[J]. China Food Additives,2020,31(4):35−41. [30] GRANT S, BLAIR H S, MCKAY G. Structural studies on chitosan and other chitin derivatives[J]. Macromolecular Chemistry and Physics,1989,190:2279−2286. doi: 10.1002/macp.1989.021900928
[31] 于海琴, 闫良国, 辛晓东, 等. CTMAB和PDMDAAC有机改性膨润土的制备及其表征[J]. 光谱学与光谱分析,2011(5):1393−1397. [YU Haiqin, YAN Liangguo, XIN Xiaodong, et al. Synthesis and characterization of CTMAB and PDMDAAC modified organobentonite[J]. Spectroscopy and Spectral Analysis,2011(5):1393−1397. doi: 10.3964/j.issn.1000-0593(2011)05-1393-05 [32] 田玉红, 徐想丽, 李利军, 等. 新生磷酸钙对没食子酸的吸附性能研究[J]. 食品工业科技,2014,35(8):126−130. [TIAN Yuhong, XU Xiangli, LI Lijun, et al. Adsorption characteristics of gallic acid on fresh calcium phosphate[J]. Science and Technology of Food Industry,2014,35(8):126−130. [33] 金科, 李振兴, 吴玟静, 等. 蛤蜊壳羟基磷灰石的制备及对Pb2+的吸附性能[J]. 食品科学,2013,34(13):39−44. [JIN Ke, LI Zhenxing, WU Wenjing, et al. Preparation of hydroxyapatite from clam shell and its adsorption capacity for Pb2+[J]. Food Science,2013,34(13):39−44. doi: 10.7506/spkx1002-6630-201313009 [34] 刘漫, 李和生, 徐祥浩. 乌贼墨黑色素对Pb2+的吸附特性研究[J]. 食品工业科技,2014,35(4):131−134,139. [LIU Man, LI Hesheng, XU Xianghao, et al. Study on adsorption characteristics of sepia melanin adsorbing Pb2+[J]. Science and Technology of Food Industry,2014,35(4):131−134,139. [35] 孙俊, 于雷, 胡坤雅, 等. 新型磁性纳米载体的制备及其对溶菌酶吸附特性研究[J]. 食品工业科技,2013,34(22):119−123. [SUN Jun, YU Lei, HU Kunya, et al. Study on preparation of the novel magnetic nanoparticles and their application to lysozyme adsorption[J]. Science and Technology of Food Industry,2013,34(22):119−123.









 下载:
下载:
 下载:
下载:
