Degradation of Imidacloprid,Acetamiprid and Triazophos in Aqueous Solution by Dielectric Barrier Discharge Low-Temperature Plasma
-
摘要: 为探究介质阻挡放电低温等离子体对吡虫啉、啶虫脒和三唑磷的降解作用,本研究构建水模拟体系,研究放电电压、时间、农药初始浓度和pH等因素对三种农药降解效果的影响,分析降解动力学,并在鉴定降解产物的基础上分析农药的降解途径。结果表明:低温等离子体能够有效降解水模拟体系中的三种农药残留;相同条件下,三种农药的降解率依次为:三唑磷>吡虫啉>啶虫脒;在本研究条件下,提高放电电压、延长放电时间、降低农药初始浓度有利于提高三种农药的降解率;碱性条件更有利于吡虫啉和啶虫脒的降解,而酸性条件更有利于三唑磷的降解;当放电电压为13.6 kV、时间5 min、农药浓度为1.9 mg/L时,吡虫啉、啶虫脒和三唑磷的降解率达到最大值,分别为62.5%(pH9.0)、42.4%(pH9.0)和94.5%(pH3.0);低温等离子体作用下三种农药的降解符合一级动力学模型(R2≥0.90);分别鉴定出吡虫啉和啶虫脒的降解产物各7种、5种;吡虫啉和啶虫脒降解产物的形成主要经历了分子中C-H、C-N等键的断裂和羟基自由基氧化取代的过程。Abstract: In order to explore the degradation of imidacloprid, acetamiprid and triazophos by dielectric barrier discharge low-temperature plasma, the aqueous solution was established to study the effects of the discharge voltage, discharge time, initial pesticide concentration and pH of solution on degradation. The degradation kinetics model was also analyzed and the degradation path was proposed based on the degradation products identification. The results showed that the low-temperature plasma technology could effectively degrade the three pesticide residues in the aqueous solution and triazophos was much more labile to low-temperature plasma treatment than imidacloprid and acetamiprid. Under the conditions of this study, the increase of discharge voltage and discharge time, and the decrease of initial pesticides concentration benefitted the increase of degradation rate. Alkaline conditions were conducive to the degradation of imidacloprid and acetamiprid, and acidic conditions benefitted the degradation of triazophos. The maximum degradations rates were 62.5% for imidacloprid (pH9.0), 42.4% for acetamiprid (pH9.0) and 94.5% for triazophos (pH3.0) after the treatment at 13.6 kV for 5 min at the initial concentration of 1.9 mg/L. The degradation kinetics of all pesticides were fitted to the first-order kinetics model well (R2≥0.90). Seven and five degradation products of imidacloprid and acetamiprid were identified respectively. The formation of imidacloprid and acetamiprid degradation products mainly experienced the breaking of C-H, C-N bonds and the oxidation and substitution of hydroxyl radicals.
-
Keywords:
- low-temperature plasma /
- imidacloprid /
- acetamiprid /
- triazophos /
- degradation products
-
农药是现代农业生产中重要的生产资料,能够有效地防治农作物的病虫害,提高农产品的产量和质量。吡虫啉(1-(6-氯-3-吡啶基)-N-硝基咪唑-2-亚胺)和啶虫脒((E)-N-[(6-氯-3-吡啶)甲基]-N'-氰基-N-甲基乙脒)是新烟碱类杀虫剂,能够作用于昆虫神经系统的烟碱乙酰胆碱受体,破坏正常的神经冲动传导,导致昆虫出现麻痹、痉挛而死亡;三唑磷(O,O-二乙基-O-(1-苯基-l,2,4-三唑-3-基)硫代磷酸酯)是一种高效、广谱性的有机磷杀虫、杀螨剂,对害虫具有较强的触杀、胃毒作用[1]。上述农药因具有广谱、高效和对人畜低毒等特点而在农业生产中被广泛地应用[2-4]。但值得注意的是,吡虫啉、啶虫脒和三唑磷对人体具有神经毒性[5-6]、生殖毒性[7]和遗传毒性[1]等,且它们在水中有较高的溶解度,长期使用不仅会污染水资源,破坏生态环境[8],更会给人类健康带来威胁[9-10]。因此,寻求一种能够有效降解上述农药的方法迫在眉睫。
低温等离子体技术是一种兼具高能电子辐射、湿式氧化、臭氧氧化、光化学氧化等多种氧化方式于一体的新型高级氧化技术[11]。根据低温等离子体产生方式的不同,可以分为辉光放电、电晕放电、射频放电以及介质阻挡放电等[12]。其中,介质阻挡放电具有结构简单、可在常温常压进行、易操作和成本低等优点,因此,其不仅适用于实验室研究,还能满足工业上的需求。目前在环境领域中被广泛应用于去除污染物如农残等[13],或者利用低温等离子体进行材料改性[14];在食品领域中,介质阻挡放电低温等离子体则被用来进行某些微生物的杀灭[15]和食品保鲜处理等[16]。但低温等离子体作用下吡虫啉、啶虫脒和三唑磷降解产物的鉴定及降解途径研究尚不完善。
本研究选择农业生产中的常用农药吡虫啉、啶虫脒和三唑磷为研究对象,建立水模拟体系,研究介质阻挡放电低温等离子体技术对上述农药的去除效果,并分析农药的降解动力学和降解产物,以期为低温等离子体技术降解水或果蔬汁等液态食品中农药残留的实际应用奠定基础。
1. 材料与方法
1.1 材料与仪器
吡虫啉、啶虫脒和三唑磷标准品(纯度≥98.0%) 德国Dr.Ehrenstorfer公司;乙腈 色谱纯,德国Merck Drugs & Biotechnology公司;实验用水 均为Milli-Q system超纯水(电阻率≥18.2 MΩ·cm),德国Merk-Millipore 公司;无水硫酸钠 分析纯,江苏强盛功能化学股份有限公司;氯化钠 分析纯,广州化学试剂厂;所有分离用有机溶剂 均为国产分析纯。
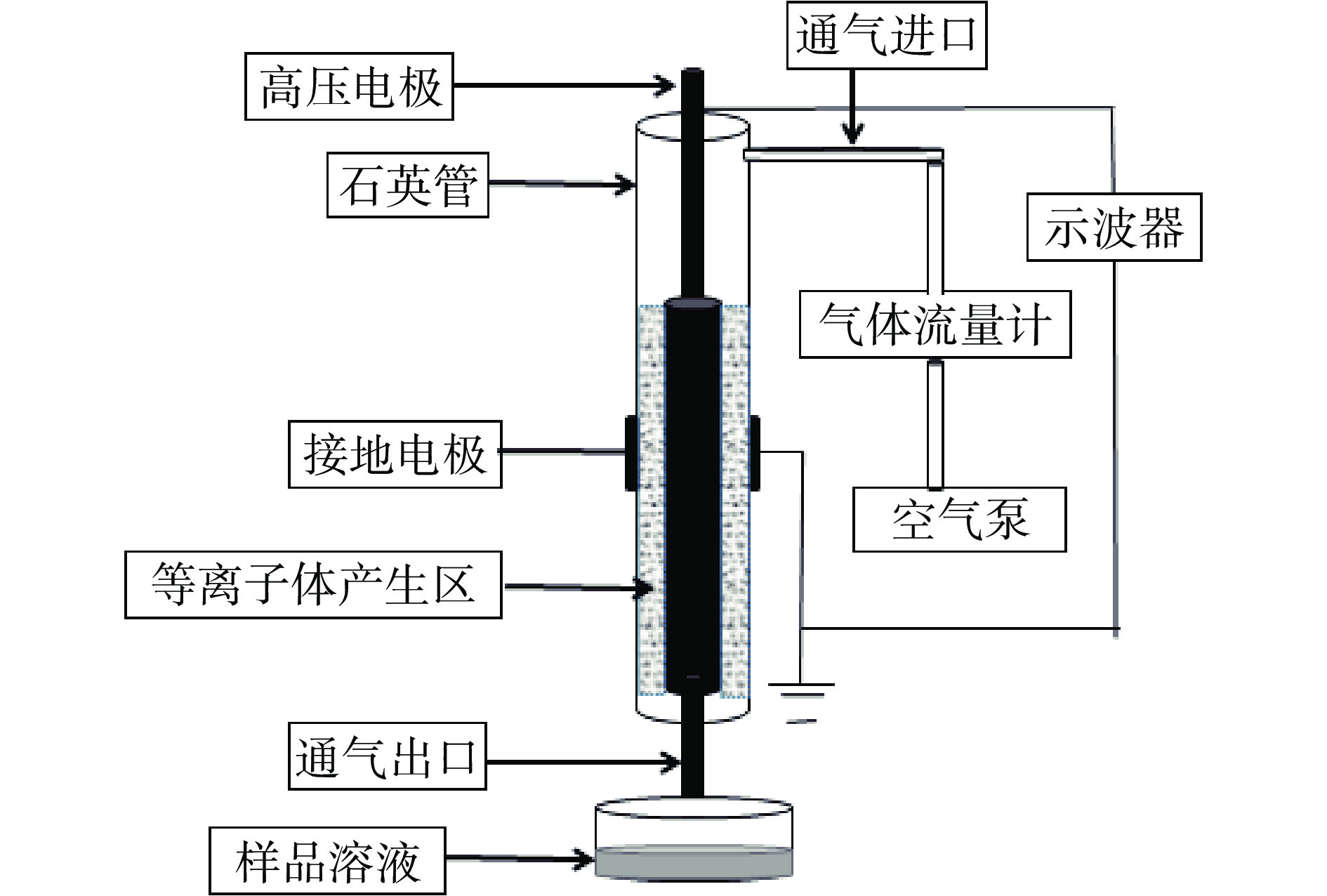
介质阻挡放电低温等离子体射流装置(如图1所示) 赣南师范大学低温等离子体技术研究所自制;CTP-2000K低温等离子体电源 南京苏曼电子有限公司;Tektronix TDS 2012 数字示波器、Tektronix P6015A高压探头、Tektronix P6021电流探头 泰克科技(中国)有限公司;OLF-2530无油空气压缩机 浙江盛源空压机制造有限公司;SHZ-D(Ⅲ)氮吹仪 天津市横奥科技发展有限公司;Agilent 1200高效液相色谱仪(High Performance Liquid Chromatography, HPLC)(配有二极管阵列检测器和B.04.02色谱工作站) 美国Agilent公司;QExactive plus超高分辨液质联用仪(High Performance Liquid Chromatography-Tandem Mass Spectrometry, HPLC-MS/MS)四极杆组合-静电场轨道阱(配有电喷雾离子源(Electrospray Ionization,ESI)及compound discoverer数据库检索软件) 美国Thermo公司。
1.2 实验方法
1.2.1 农药储备液的配制
1000 mg/L的吡虫啉、啶虫脒和三唑磷单标储备液:分别称取吡虫啉、啶虫脒和三唑磷标准品各100 mg(精确到0.0100 g),用乙腈溶解并定容至100 mL后,转移至棕色瓶中,置于−18 ℃冰箱中保存。农药标准工作液的配制:分别量取一定体积的农药单标储备液于10 mL容量瓶中,用乙腈定容,得到农药标准工作液,置于−18 ℃冰箱中保存[17]。
1.2.2 水模拟体系的构建
分别量取一定体积的吡虫啉、啶虫脒和三唑磷标准工作液于氮吹瓶中,氮气吹干后,用超纯水转移至容量瓶中定容,即获得三种农药的水模拟体系。探究不同pH对农药降解的影响时,分别用pH为3.0、7.0、9.0的水溶液(超纯水常温下呈弱酸性(pH为5.0左右),以0.1 mol/L HCl和NaOH溶液调节pH)将农药转移至容量瓶中定容。该体系用于研究放电电压等对低温等离子体降解农药的影响。
1.2.3 低温等离子体处理
在低温等离子体射流装置出气管口处放置100 mL烧杯,加入50 mL样品溶液,设置电源输出频率为8.87 kHz,空气流速为2 L/min,系统通电后开始计时,处理一定时间后关闭电源,处理后的样品在4 ℃下保存[18]。
1.2.3.1 放电电压对农药降解率的影响
本研究在预实验中发现,当放电电压高于13.6 kV时,低温等离子体射流装置会产生大量热量,使得低温等离子体产生后很快被分解,三种农药的降解率反而下降;而低于10.2 kV时,装置仅有微弱的放电现象,产生的低温等离子体少,农药几乎没有降解,因此,选择13.6和10.2 kV作为最高和最低放电电压。在放电时间为5 min、溶液初始pH5.0、吡虫啉、啶虫脒和三唑磷的初始浓度分别为1.9、1.9、1.5 mg/L(本研究中农药浓度均为实测浓度)的条件下,采用10.2、12.2、13.0、13.6 kV的放电电压对水溶液进行处理,研究放电电压对水模拟体系中三种农药降解效果的影响。
1.2.3.2 放电时间对农药降解率的影响
在放电电压为13.6 kV、吡虫啉、啶虫脒和三唑磷的初始浓度分别为1.9、1.9、1.5 mg/L的条件下,对水溶液(溶液初始pH5.0)处理1、2、3、4、5 min,考察放电时间对水模拟体系中三种农药降解效果的影响。
1.2.3.3 农药初始浓度对农药降解率的影响
在放电电压为13.6 kV、溶液初始pH5.0的条件下,对不同农药初始浓度(2.0~8.0 mg/L)的吡虫啉、啶虫脒和三唑磷水溶液处理1、2、3、4、5 min,研究农药初始浓度对降解率的影响。
1.2.3.4 溶液pH对农药降解率的影响
据文献报道[17],农药在不同pH下降解速率不同,因此本文分别探究pH为酸性、中性和碱性时对三种农药降解速率的影响。在放电电压为13.6 kV的条件下,分别对pH3.0、7.0、9.0时的吡虫啉、啶虫脒和三唑磷水溶液(初始浓度均为1.9 mg/L)进行处理,探究溶液pH对水模拟体系中三种农药降解率的影响。
1.2.4 吡虫啉和啶虫脒的检测
取一定体积的样品溶液,采用0.22 μm的水相滤膜过滤后转移至样品瓶中待测。HPLC的检测条件为:采用Agilent XDB-C18(5 μm×4.6 mm (i.d)×250 mm)色谱柱;流动相为超纯水(A)和乙腈(B);梯度洗脱条件为:20% B保持2 min,13 min内增加到35% B,14 min降至20% B,共14 min;紫外检测检测波长为:吡虫啉270 nm、啶虫脒245 nm;柱温40 ℃;进样体积10 μL;流速0.8 mL/min[17]。
1.2.5 三唑磷的检测
取10 mL的样品溶液至30 mL离心管中,加入10 mL乙酸乙酯和1 g氯化钠,涡旋1 min后静置,待溶液分层后取出有机相,用2×10 mL乙酸乙酯对水相萃取2次,合并有机相,在35 ℃水浴下氮吹至近干,加入2 mL乙腈,涡旋30 s,采用0.22 μm的有机滤膜过滤后转移至样品瓶中待测。HPLC检测条件:色谱柱为Agilent XDB-C18(5 μm×4.6 mm (i.d)×250 mm),柱温30 ℃;流动相为乙腈:水=85:15,等度洗脱10 min,流速1.0 mL/min;检测波长为245 nm;进样量10 μL。
1.2.6 吡虫啉和啶虫脒降解产物的鉴定
在13.6 kV的放电电压下分别处理pH5、浓度为1.9 mg/L的吡虫啉、啶虫脒水溶液10 min,采用HPLC-MS/MS分析降解产物。取10 mL样品溶液于50 mL离心管中,加入10 mL乙酸乙酯/环己烷(5/5,v/v)和1 g氯化钠,涡旋1 min;6000 r/min离心5 min,移取有机相,水相用2×10 mL乙酸乙酯/环己烷(5/5,v/v)提取两次,合并有机相,在35 ℃下氮气吹至近干;加入1 mL甲醇溶解,涡旋30 s,采用0.22 μm的有机滤膜过滤后转移至样品瓶中待HPLC-MS/MS检测。HPLC条件:色谱柱为Hypersil GOLD C18(1.9 μm×2.1 mm (i.d)×100 mm),流动相为0.1% 甲酸(A)和100%甲醇(B),柱温30 ℃。检测波长为:270 nm和245 nm;进样体积5 μL;流速0.2 mL/min;洗脱条件为:0~2 min,2% B;2~20 min,2%~100% B;20~24 min,100% B;24~25 min,100%~2% B;25~30 min,2% B。质谱条件:采用ESI源(正离子加负离子模式),喷雾电压:正离子3.5 kV/负离子3.0 kV;扫描模式:一级全扫描,范围:70~1500 m/z;二级扫描采集模式:TOP 6,DDA(Data Dependent Acquisition)-MS2,分辨率:一级扫描70000,二级扫描17500;离子源温度:320 ℃;鞘气(N2):40 arb,辅助气体(N2):10 arb;碰撞能量:40 eV。
1.2.7 农药降解率的计算
农药的降解率采用公式(1)进行计算。
农药降解率(%)=(C0−Ct)/C0×100 (1) 一级动力学模型能从总体上描述农药在环境中的变化和降解规律[19],其表达式如公式(2)。
Ct=C0e−kt (2) 将公式(2)两边取自然对数,可以得到公式(3):
ln(Ct/C0)=−kt (3) 以ln(Ct/C0)为纵坐标,以时间为横坐标做图,得到以k为斜率的直线方程。上述公式中:t表示低温等离子体处理样品溶液的时间(min);C0和Ct分别表示低温等离子体处理前、处理t时间后样品溶液中的农药浓度,mg/L;k表示一级速率常数。
1.3 数据处理
图表数据以平均值±标准偏差表示,每个实验重复3次,利用Excel 2016进行数据处理,采用SPPS 21.0进行方差分析和多重比较,显著性水平为P<0.05,采用Origin 2018画图。
2. 结果与分析
2.1 放电电压对水模拟体系中三种农药降解的影响
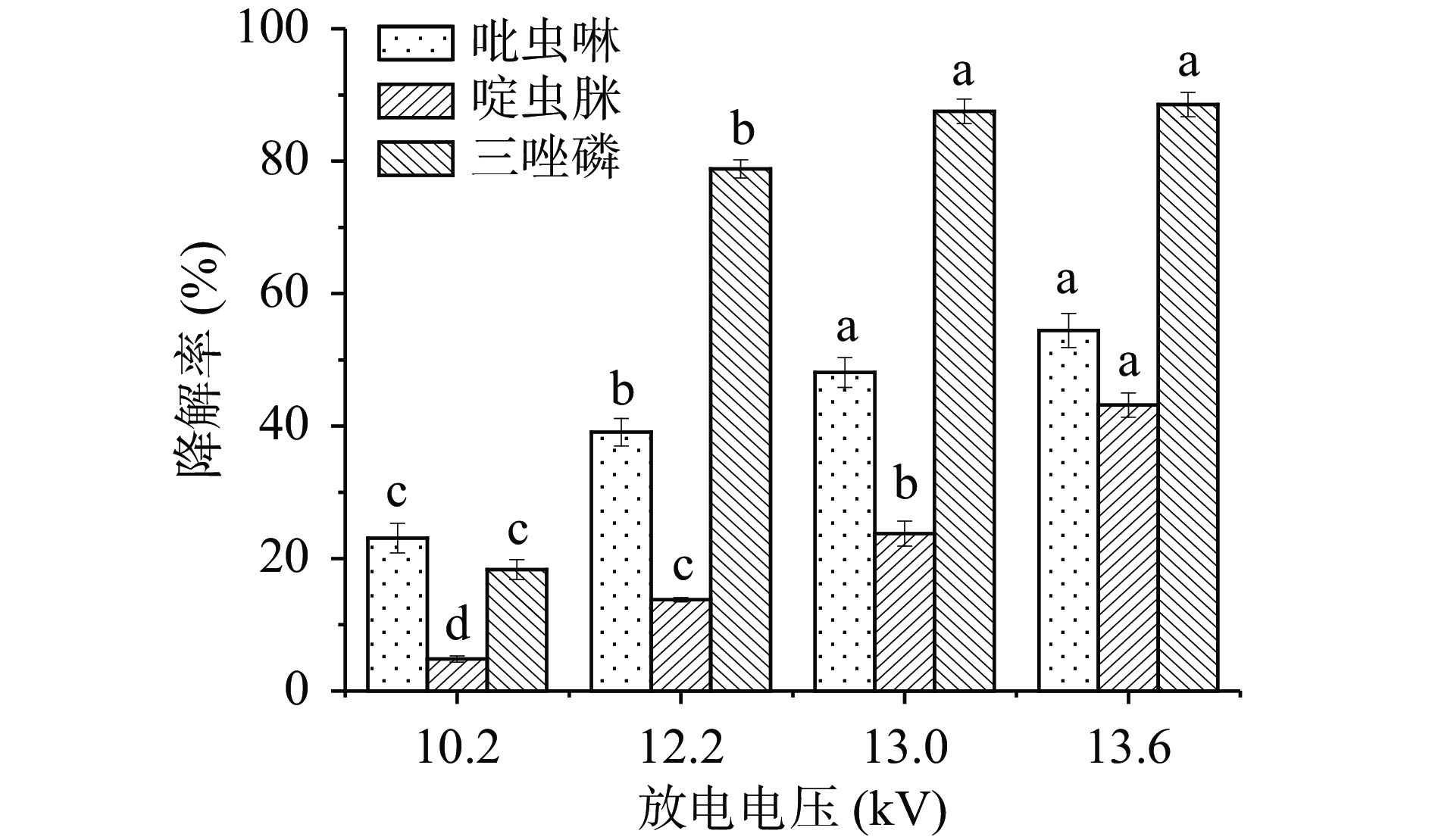
放电电压对水模拟体系中三种农药降解的影响如图2所示,放电电压对三种农药的降解率均有显著影响(P<0.05),且其降解率随着放电电压的增大而增大,当放电电压为13.6 kV时,吡虫啉、啶虫脒和三唑磷的降解率最高,分别为54.5%、43.2%和87.6%,比10.2 kV时分别增加了31.4%、38.4%和70.3%。增加放电电压能够提高农药降解率的原因可能是:在一定的放电电压范围内,当放电电压增加时,电场能量增加,使得单位时间内放电产生的臭氧和·OH等高能活性粒子的数量增加,并和更多的农药分子反应进而提高其降解率[20]。因此,为确保农药的降解效果,选择13.6 kV作为放电电压开展后续研究。
![]() 图 2 放电电压对吡虫啉、啶虫脒和三唑磷降解率的影响注:同种农药不同字母表示差异显著(P<0.05);图3同。Figure 2. The effect of discharge voltage on degradation of imidacloprid, acetamiprid and triazophos
图 2 放电电压对吡虫啉、啶虫脒和三唑磷降解率的影响注:同种农药不同字母表示差异显著(P<0.05);图3同。Figure 2. The effect of discharge voltage on degradation of imidacloprid, acetamiprid and triazophos2.2 放电时间对水模拟体系中三种农药降解的影响
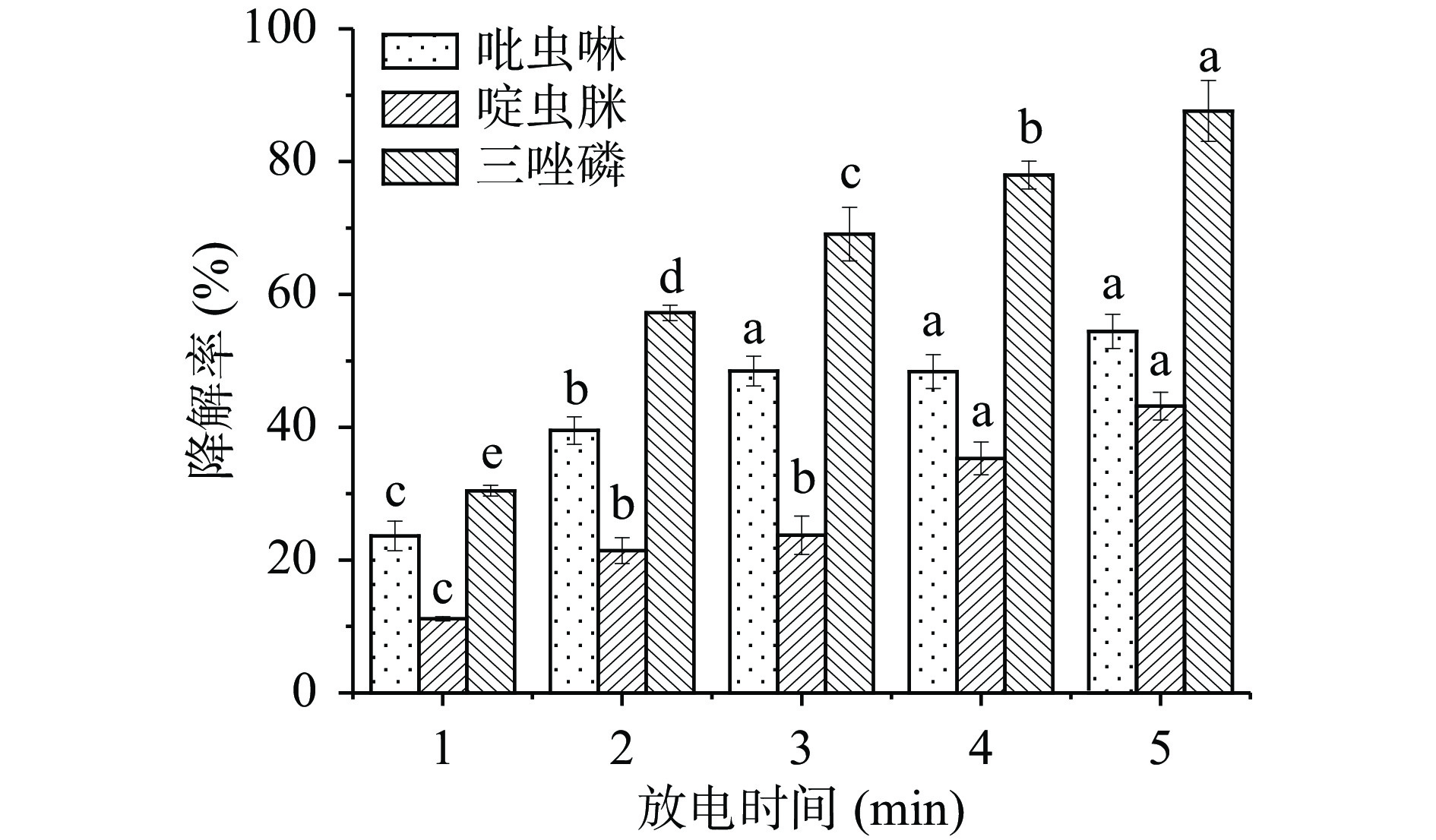
放电时间对水模拟体系中三种农药降解的影响如图3所示,方差分析结果表明,延长放电时间对三种农药的降解均具有显著促进作用(P<0.05),当放电时间为5.0 min时,吡虫啉、啶虫脒和三唑磷的降解率达到最大值,分别为54.5%、43.2%和87.6%。值得注意的是,当延长放电时间时,尽管三种农药的降解率持续提高,但其增幅下降,其原因可能是:在放电电压相同的情况下,反应体系中∙OH、O3等高能活性粒子的生成速率是一定的;在反应初期,农药浓度高,降解效率高;但随着反应时间的延长,目标农药浓度下降,同时可能有大量中间产物生成,这些中间产物也消耗了活性粒子,导致农药的降解效率下降[21]。
2.3 农药初始浓度对水模拟体系中三种农药降解的影响
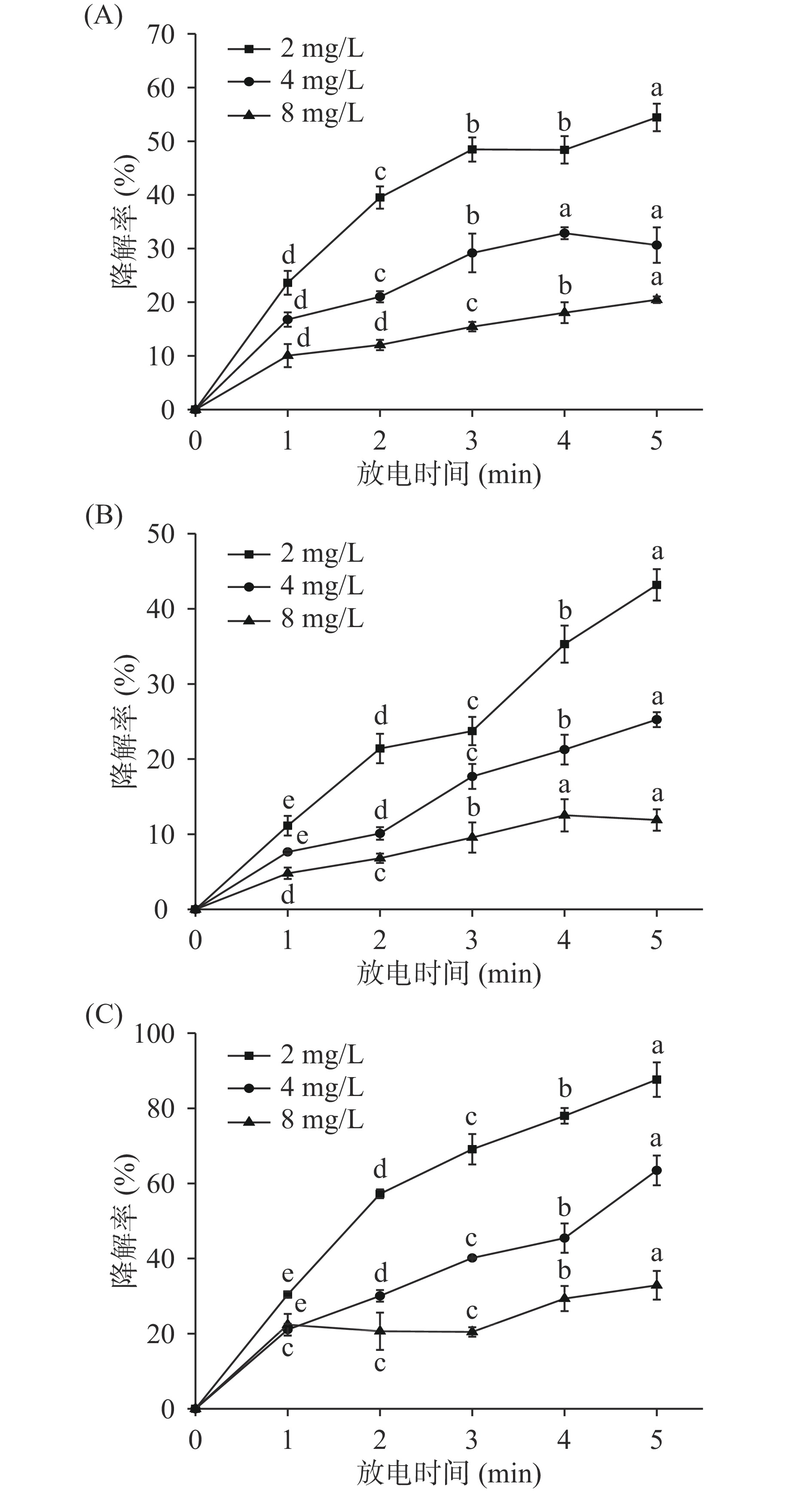
农药初始浓度对水模拟体系中三种农药降解的影响如图4所示,随着农药初始浓度的降低,农药的降解率显著提高(P<0.05)。在放电时间均为5.0 min的条件下,当农药初始浓度从8.0 mg/L降低至2.0 mg/L时,吡虫啉、啶虫脒和三唑磷的降解率分别增加了34.0%、31.3%和54.8%。这是因为当放电电压一定时,单位时间内电场中产生的∙OH、O3等高能活性粒子的数量是一定的,农药初始浓度越低,则农药分子与活性粒子的接触越充分,从而使得农药的降解率提高[22]。因此,在实际应用中,可适当降低农药的初始浓度以提高其降解率。
![]() 图 4 农药初始浓度对吡虫啉(A)、啶虫脒(B)和三唑磷(C)降解率的影响注:同浓度不同小写字母表示差异显著(P<0.05);图5同。Figure 4. The effect of initial concentration of pesticides on degradation of imidacloprid(A), acetamiprid(B) and triazophos(C)
图 4 农药初始浓度对吡虫啉(A)、啶虫脒(B)和三唑磷(C)降解率的影响注:同浓度不同小写字母表示差异显著(P<0.05);图5同。Figure 4. The effect of initial concentration of pesticides on degradation of imidacloprid(A), acetamiprid(B) and triazophos(C)2.4 溶液pH对水模拟体系中三种农药降解的影响
溶液pH对水模拟体系中三种农药降解的影响如图5所示,方差分析结果表明,溶液pH对三种农药的降解率有显著影响(P<0.05),且pH对各农药的影响不同:随着pH从3.0提高至9.0时,吡虫啉和啶虫脒的降解率显著增加,当pH为9.0、放电时间5.0 min时,二者的降解率最高,分别为62.5%、42.4%;另外,三唑磷的降解率在pH3.0时达到最高,在放电时间5.0 min时三唑磷的降解率高达94.5%,比pH为7.0和9.0时分别提高了8.1%和10.1%。在低温等离子体作用下,溶液pH对三种农药降解率的影响不同,这可能与农药的理化性质有关[23-24]。
2.5 降解动力学
采用一级动力学拟合不同农药初始浓度和pH下吡虫啉、啶虫脒和三唑磷的降解过程,所得参数如表1~2所示。从表1和表2可以看出,各条件下,方程的回归系数R²均大于0.90,说明在低温等离子体作用下,水模拟体系中三种农药的降解均符合一级动力学模型。
表 1 不同农药初始浓度下三种农药的一级动力学模型参数Table 1. Parameters calculated by first-order kinetics model of imidacloprid, acetamiprid and triazophos with different initial concentrations农药种类 农药初始浓度
(mg/L)回归方程 k t1/2
(min)R² 吡虫啉 2.0 ln(Ct/C0)=−0.1789t 0.1789 3.9 0.9672 4.0 ln(Ct/C0)=−0.0930t 0.0930 7.5 0.9490 8.0 ln(Ct/C0)=−0.0510t 0.0510 13.6 0.9687 啶虫脒 2.0 ln(Ct/C0)=−0.1147t 0.1147 6.0 0.9878 4.0 ln(Ct/C0)=−0.0598t 0.0598 11.6 0.9952 8.0 ln(Ct/C0)=−0.0304t 0.0304 22.8 0.9725 三唑磷 2.0 ln(Ct/C0)=−0.4016t 0.4016 1.7 0.9978 4.0 ln(Ct/C0)=−0.1810t 0.1810 3.8 0.9845 8.0 ln(Ct/C0)=−0.0870t 0.0870 8.0 0.9258 表 2 不同pH下三种农药的一级降解动力学参数Table 2. Parameters calculated by first-order kinetics model of imidacloprid, acetamiprid and triazophos in aqueous solution with different pH农药种类 pH 回归方程 k t1/2
(min)R² 吡虫啉 3.0 ln(Ct/C0)=−0.0900t 0.0900 7.7 0.9721 7.0 ln(Ct/C0)=−0.1306t 0.1306 5.3 0.9816 9.0 ln(Ct/C0)=−0.1898t 0.1898 3.7 0.9876 啶虫脒 3.0 ln(Ct/C0)=−0.0608t 0.0608 11.4 0.9970 7.0 ln(Ct/C0)=−0.0935t 0.0935 7.4 0.9663 9.0 ln(Ct/C0)=−0.1089t 0.1089 6.4 0.9981 三唑磷 3.0 ln(Ct/C0)=−0.5252t 0.5252 1.3 0.9897 7.0 ln(Ct/C0)=−0.3684t 0.3684 1.9 0.9878 9.0 ln(Ct/C0)=−0.3828t 0.3828 1.8 0.9952 在不同农药初始浓度下,三种农药的降解速率常数k值依次为:k2.0 mg/L>k4.0 mg/L>k8.0 mg/L;在不同pH溶液下,吡虫啉和啶虫脒:kpH9>kpH7>kpH3,三唑磷:kpH3>k pH9>k pH7;这与农药初始浓度和pH对吡虫啉、啶虫脒和三唑磷降解率的影响一致。值得注意的是,当处理条件相同时,k三唑磷>k吡虫啉>k啶虫脒,说明在水体系中三唑磷对低温等离子体的处理最为敏感。
2.6 降解产物及降解途径分析
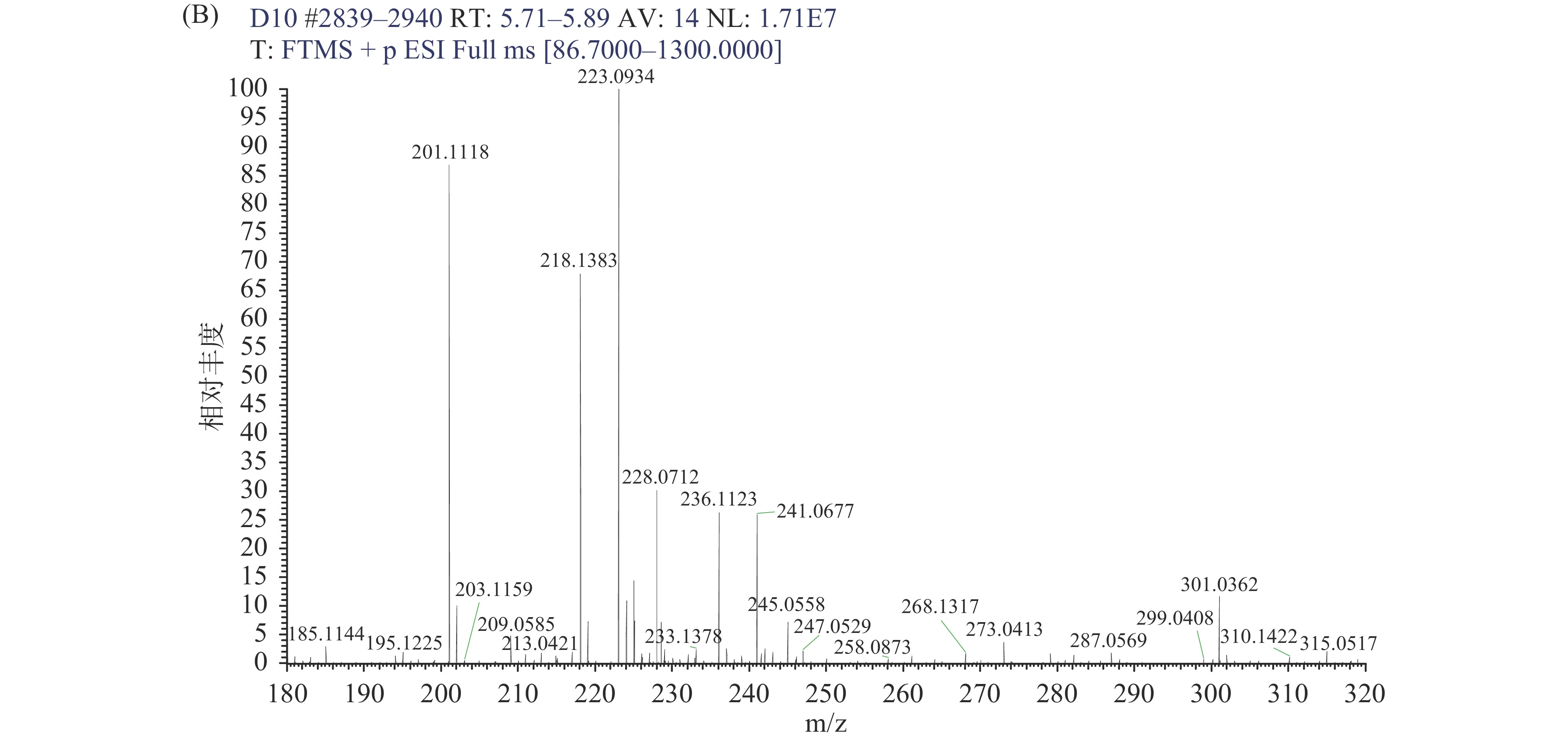
三种农药的标准溶液液相色谱图如图6、图7所示,低温等离子体处理吡虫啉和啶虫脒溶液前后结果如图8、图9所示。
对比图8A、B和图9A、B可知,经过低温等离子体处理后,吡虫啉、啶虫脒溶液中分别有7种、5种降解产物生成。采用compound discoverer数据库检索软件分析降解产物,获得的相对分子质量、分子结构等信息如表3、表4所示。
表 3 低温等离子体作用下吡虫啉的降解产物Table 3. Degradation products of imidacloprid after low-temperature plasma treatment化合物 Rt(min) 分子式 分子离子
质荷比(m/z)分子结构式 吡虫啉 5.78 C9H10ClN5O2 255.04 
P224 5.41 C9H9ClN4O 224.05 
P225 5.52 C9H8ClN3O2 225.02 
P240 5.94 C9H9ClN4O2 240.02 
P269 5.90 C9H8ClN5O3 269.02 
P271-A 5.41 C9H10ClN5O3 271.04 
P271-B 5.46 C9H10ClN5O3 271.04 
P287 5.49 C9H10ClN5O4 287.02  表 4 低温等离子体作用下啶虫脒的降解产物Table 4. Degradation products of acetamiprid after low-temperature plasma treatment
表 4 低温等离子体作用下啶虫脒的降解产物Table 4. Degradation products of acetamiprid after low-temperature plasma treatment化合物 Rt(min) 分子式 分子离子
质荷比(m/z)分子结构式 啶虫脒 6.13 C10H11ClN4 222.68 
P90 3.32 C3H10N2O 90.08 
P97 0.89 C4H7N3 97.06 
P141 5.07 C6H4ClNO 140.99 
P143 1.97 C6H6ClNO 143.01 
P157 5.86 C6H4ClNO2 156.99 
根据吡虫啉、啶虫脒降解产物的分子结构并结合文献信息,推测低温等离子体作用下吡虫啉、啶虫脒的降解途径分别如图10、图11所示。
吡虫啉含有吡啶环和咪唑环,以与吡啶环相连接的N为中心原子,N周围的饱和C为α-C原子,是吡虫啉降解的重要活性位点[25]。从图10可以看出,本研究发现了吡虫啉的两个降解产物P271-A和P271-B,即为与中心N原子连接的两个α-C原子发生羟基化反应而生成,且二者互为同分异构体,该过程在紫外/氯联用降解吡虫啉的研究中已得到证实[26];在羟基自由基作用下,P271-A和P271-B可进一步转化为P288,该产物在高锰酸钾氧化降解水体中吡虫啉的研究中也有报道[27]。咪唑环上的α-C原子可以发生羰基化生成P269,P269在羟基自由基的作用下易被氧化,一方面结合水分子后脱去过氧化氢自由基和·N2生成P226[26],另一方面在自由基作用下脱去·NO2生成P225;产物P225在自由基的作用下,连接吡啶环和咪唑环的α-C原子发生羟基化生成P241。上述降解途径表明,吡虫啉在低温等离子体作用下发生降解的部位主要在咪唑环上,而吡啶结构相对稳定[27];吡虫啉在自由基的攻击下主要经历了C-H、C-N和N-N键的断裂和H、N等原子被·OH的取代的过程。
从图11可以看出,啶虫脒在低温等离子体作用下的降解涉及氧化以及碳氮键的水解等过程。首先啶虫脒分子中与苯环连接的C-N键易被羟基自由基作用进而发生水解断裂,生成6-氯烟醇(P143)和P97[28];在羟基自由基的作用下,6-氯烟醇进一步氧化生成了6-氯烟醛(P141)和6-氯烟酸(P157),该过程已经在臭氧降解啶虫脒的研究中得到证实[29];同时,羟基自由基也会攻击P97的C-N键,使其断裂并脱去分子中的氰根生成P90。因此,啶虫脒的降解是分子中的C-N键断裂后,吡啶环结构降解产物不断被羟基氧化取代及碳氮链结构降解产物去饱和的过程。
3. 结论
低温等离子体技术可有效降解水模拟体系中的吡虫啉、啶虫脒和三唑磷,且三唑磷的低温等离子体降解效果优于吡虫啉和啶虫脒。
在本研究条件下,放电电压、放电时间、农药初始浓度和pH均会影响三种农药的降解率(P<0.05)。放电电压越大、放电时间越长、农药初始浓度越低,三种农药残留的降解率越高;碱性条件更利于吡虫啉和啶虫脒的降解,而酸性条件更利于三唑磷的降解。本实验得到三种农药的最大降解率分别为:吡虫啉62.5%、啶虫脒42.4%(放电电压13.6 kV,时间为5 min,pH9.0,农药初始浓度为1.9 mg/L),三唑磷94.5%(放电电压为13.6 kV,时间为5 min,pH3.0,农药初始浓度1.9 mg/L)。低温等离子体作用下,吡虫啉、啶虫脒和三唑磷的降解均符合一级动力学模型。
鉴定出吡虫啉的7种降解产物为C9H9ClN4O、C9H8ClN3O2、C9H9ClN4O2、C9H8ClN5O3、C9H10ClN5O3(含一个同分异构体)、C9H10ClN5O4,啶虫脒的5种降解产物为C3H10N2O、C4H7N3、C6H4ClNO、C6H6ClNO、C6H4ClNO2。初步提出了农药的降解途径,其中,吡虫啉的降解涉及C-H、C-N和N-N键的断裂和H、N等原子被·OH的取代等,啶虫脒的降解涉及分子中的C-N键断裂以及产物不断被羟基氧化取代与去饱和。本研究为低温等离子体技术降解水体系或液态食品中的以上三种农药及类似农药提供了一定的研究基础。
-
图 2 放电电压对吡虫啉、啶虫脒和三唑磷降解率的影响
注:同种农药不同字母表示差异显著(P<0.05);图3同。
Figure 2. The effect of discharge voltage on degradation of imidacloprid, acetamiprid and triazophos
图 4 农药初始浓度对吡虫啉(A)、啶虫脒(B)和三唑磷(C)降解率的影响
注:同浓度不同小写字母表示差异显著(P<0.05);图5同。
Figure 4. The effect of initial concentration of pesticides on degradation of imidacloprid(A), acetamiprid(B) and triazophos(C)
表 1 不同农药初始浓度下三种农药的一级动力学模型参数
Table 1 Parameters calculated by first-order kinetics model of imidacloprid, acetamiprid and triazophos with different initial concentrations
农药种类 农药初始浓度
(mg/L)回归方程 k t1/2
(min)R² 吡虫啉 2.0 ln(Ct/C0)=−0.1789t 0.1789 3.9 0.9672 4.0 ln(Ct/C0)=−0.0930t 0.0930 7.5 0.9490 8.0 ln(Ct/C0)=−0.0510t 0.0510 13.6 0.9687 啶虫脒 2.0 ln(Ct/C0)=−0.1147t 0.1147 6.0 0.9878 4.0 ln(Ct/C0)=−0.0598t 0.0598 11.6 0.9952 8.0 ln(Ct/C0)=−0.0304t 0.0304 22.8 0.9725 三唑磷 2.0 ln(Ct/C0)=−0.4016t 0.4016 1.7 0.9978 4.0 ln(Ct/C0)=−0.1810t 0.1810 3.8 0.9845 8.0 ln(Ct/C0)=−0.0870t 0.0870 8.0 0.9258 表 2 不同pH下三种农药的一级降解动力学参数
Table 2 Parameters calculated by first-order kinetics model of imidacloprid, acetamiprid and triazophos in aqueous solution with different pH
农药种类 pH 回归方程 k t1/2
(min)R² 吡虫啉 3.0 ln(Ct/C0)=−0.0900t 0.0900 7.7 0.9721 7.0 ln(Ct/C0)=−0.1306t 0.1306 5.3 0.9816 9.0 ln(Ct/C0)=−0.1898t 0.1898 3.7 0.9876 啶虫脒 3.0 ln(Ct/C0)=−0.0608t 0.0608 11.4 0.9970 7.0 ln(Ct/C0)=−0.0935t 0.0935 7.4 0.9663 9.0 ln(Ct/C0)=−0.1089t 0.1089 6.4 0.9981 三唑磷 3.0 ln(Ct/C0)=−0.5252t 0.5252 1.3 0.9897 7.0 ln(Ct/C0)=−0.3684t 0.3684 1.9 0.9878 9.0 ln(Ct/C0)=−0.3828t 0.3828 1.8 0.9952 表 3 低温等离子体作用下吡虫啉的降解产物
Table 3 Degradation products of imidacloprid after low-temperature plasma treatment
化合物 Rt(min) 分子式 分子离子
质荷比(m/z)分子结构式 吡虫啉 5.78 C9H10ClN5O2 255.04 
P224 5.41 C9H9ClN4O 224.05 
P225 5.52 C9H8ClN3O2 225.02 
P240 5.94 C9H9ClN4O2 240.02 
P269 5.90 C9H8ClN5O3 269.02 
P271-A 5.41 C9H10ClN5O3 271.04 
P271-B 5.46 C9H10ClN5O3 271.04 
P287 5.49 C9H10ClN5O4 287.02 
表 4 低温等离子体作用下啶虫脒的降解产物
Table 4 Degradation products of acetamiprid after low-temperature plasma treatment
化合物 Rt(min) 分子式 分子离子
质荷比(m/z)分子结构式 啶虫脒 6.13 C10H11ClN4 222.68 
P90 3.32 C3H10N2O 90.08 
P97 0.89 C4H7N3 97.06 
P141 5.07 C6H4ClNO 140.99 
P143 1.97 C6H6ClNO 143.01 
P157 5.86 C6H4ClNO2 156.99 
-
[1] YANG F W, LI Y X, REN F Z, et al. Toxicity, residue, degradation and detection methods of the insecticide triazophos[J]. Environmental Chemistry Letters,2019,17(4):1769−1785. doi: 10.1007/s10311-019-00910-z
[2] 王圣印, 刘永杰, 周仙红, 等. 新烟碱类杀虫剂吡虫啉的研究进展[J]. 江西农业学报,2012,24(3):76−79. [WANG S Y, LIU Y J, ZHOU X H, et al. Research progress in new neonicotinoid insecticide imidacloprid[J]. Acta Agriculturae Jiangxi,2012,24(3):76−79. doi: 10.3969/j.issn.1001-8581.2012.03.024 [3] 周育, 庾琴, 侯慧锋, 等. 新型烟碱类杀虫剂啶虫脒研究进展[J]. 植物保护,2006,32(3):16−20. [ZHOU Y, YU Q, HOU H F, et al. Progress in chloronicotinyl insecticide acetamiprid[J]. Plant Protection,2006,32(3):16−20. doi: 10.3969/j.issn.0529-1542.2006.03.005 [4] 刘长令. 世界农药大全[M]. 北京: 化学工业出版社, 2012: 244−256. LIU C L. World pesticide encyclopedia[M]. Beijing: Chemical Industry Press, 2012: 244−256.
[5] KLEIN M. Scientific opinion on the developmental neurotoxicity potential of acetamiprid and imidacloprid[J]. European Food Safety Authority Journal,2013,12(11):3471.
[6] ANADON A, ARES I, MARTINEZ M, et al. Neurotoxicity of neonicotinoids[J]. Advances in Neurotoxicology,2020,4:167−207.
[7] STIVAKTAKIS P D, KAVVALAKIS M P, TZATZARAKIS M N, et al. Long-term exposure of rabbits to imidaclorpid as quantified in blood induces genotoxic effect[J]. Chemosphere,2016,149:108−113. doi: 10.1016/j.chemosphere.2016.01.040
[8] 王未, 黄从建, 张满成, 等. 我国区域性水体农药污染现状研究分析[J]. 环境保护科学,2013,39(5):5−9. [WANG W, HUANG C J, ZHANG M C, et al. Study on status of regional water pollution by pesticides in China[J]. Environmental Protection Science,2013,39(5):5−9. doi: 10.3969/j.issn.1004-6216.2013.05.002 [9] 张俊, 王定勇. 蔬菜的农药污染现状及农药残留危害[J]. 农村经济与科技,2004,15(3):16−17. [ZHANG J, WANG D Y. Present situation of pesticide pollution in vegetables and harm of pesticide residues[J]. Rural Economy and Science-Technology,2004,15(3):16−17. doi: 10.3969/j.issn.1007-7103.2004.03.008 [10] SZPYRKA E, SŁOWIK-BOROWIEC M, MATYASZEK A, et al. Pesticide residues in raw agricultural products from the south-eastern region of Poland and the acute risk assessment[J]. Roczniki Panstwowego Zakadu Higieny,2016,67(3):237−245.
[11] GERRITY D, STANFORD B D, TRENHOLM R A, et al. An evaluation of a pilot-scale nonthermal plasma advanced oxidation process for trace organic compound degradation[J]. Water Research,2010,44(2):493−504. doi: 10.1016/j.watres.2009.09.029
[12] 孟月东, 钟少锋, 熊新阳. 低温等离子体技术应用研究进展[J]. 物理,2006,35(2):140−146. [MENG Y D, ZHONG S F, XIONG X Y. Advances in applied low-temperature plasma technology[J]. Physics,2006,35(2):140−146. doi: 10.3321/j.issn:0379-4148.2006.02.009 [13] 丛来欣, 黄明明, 章建浩, 等. 高压电场低温等离子体对马拉硫磷的降解效能及降解途径[J]. 食品工业科技,2020,41(21):37−42+47. [CONG L X, HUANG M M, ZHANG J H, et al. The degradation efficiency and pathway of malathion treated by high voltage electric field cold plasma[J]. Science and Technology of Food Industry,2020,41(21):37−42+47. [14] 陈桂芸, 赵爽, 何适, 等. 低温等离子体改性玉米醇溶蛋白基膜表面亲水性的研究[J]. 食品研究与开发, 2018, 39(20): 8−12. CHEN G Y, ZHAO S, HE S, et al. Effect of cold plasma on the structure and surface hydrophilicity of zein-based films[J]. Food Research and Development, 2018, 39(20): 8−12.
[15] 林向阳, 李雁晖, 黄彬红, 等. 介质阻挡放电等离子体(DBDP)对橙汁杀菌及钝化酶的影响[J]. 中国食品学报,2010,10(6):14−21. [LIN X Y, LI Y H, HUANG B H, et al. Effect of dielectric barrier discharge plasma(DBDP) on the sterilization and enzyme inactivation of orange juice[J]. Journal of Chinese Institute of Food Science and Technology,2010,10(6):14−21. doi: 10.3969/j.issn.1009-7848.2010.06.003 [16] 刘品, 陈静. 低温等离子体对南美白对虾防黑变及品质的研究[J]. 食品工业,2018,39(11):184−187. [LIU P, CHEN J. Study on prevention of blackening of penaeus vannamei by low temperature plasma[J]. The Food Industry,2018,39(11):184−187. [17] 李斌, 殷桃, 张媛媛, 等. 紫外光照射降解水中吡虫啉和啶虫脒的研究[J]. 现代食品科技,2014,30(5):145−149. [LI B, YIN T, ZHANG Y Y, et al. Degradation of imidacloprid and acetamiprid in aqueous solution by ultraviolet irradiation[J]. Modern Food Science and Technology,2014,30(5):145−149. [18] 李星, 王兴权, 杨洪宇, 等. 介质阻挡放电等离子体降解制药企业废水污染物的研究[J]. 电工电能新技术,2020,39(1):76−81. [LI X, WANG X Q, YANG H Y, et al. Research of degradation on wastewater pollutants discharged from pharmaceutical company by dielectric barrier discharge plasma[J]. Advanced Technology of Electrical Engineering and Energy,2020,39(1):76−81. [19] FREISSINET C, VAUCLIN M, ERLICH M. Comparison of first-order analysis and fuzzy set approach for the evaluation of imprecision in a pesticide groundwater pollution screening model[J]. Journal of Contaminant Hydrology,1999,37(1/2):21−43.
[20] 程虎, 叶齐政, 覃世勋, 等. 放电等离子体水处理中有机物的降解速率[J]. 高电压技术,2007,33(2):150−153,185. [CHENG H, YE Q Z, QIN S X, et al. Influential factors on degradation rate of organic contamination in water treatment by discharge plasma[J]. High Voltage Engineering,2007,33(2):150−153,185. doi: 10.3969/j.issn.1003-6520.2007.02.036 [21] 黄芳敏, 王红林, 严宗诚, 等. 介质阻挡放电等离子体对亚甲基蓝的降解[J]. 环境科学与技术,2010,33(2):35−38. [HUANG F M, WANG H L, YAN Z C, et al. Degradation of methylene blue by dielectric barrier discharge plasma[J]. Environmental Science and Technology,2010,33(2):35−38. doi: 10.3969/j.issn.1003-6504.2010.02.009 [22] 宋玲. 气相介质阻挡放电活性粒子喷射降解水中有机污染物的研究[D]. 大连: 大连理工大学, 2008. SONG L. Degradation of organic compounds in wastewater by active species sprayed in a gas phase dieleetric barrier discharge system[D]. Dalian: Dalian University of Technology, 2008.
[23] 司唤. 啶虫脒水解机理的理论研究[D]. 重庆: 重庆师范大学, 2016. SI H. Theoretical studies on the hydrolysis mechanism of acetamiprid[D]. Chongqing: Chongqing Normal University, 2016.
[24] 郑巍, 宣日成, 刘维屏. 新农药吡虫啉水解动力学和机理研究[J]. 环境科学学报,1999(1):103−106. [ZHENG W, XUAN R C, LIU W P. Kinetics and mechanism of pesticide imidacloprid hydrolysis[J]. Acta Scientiae Circumstantiae,1999(1):103−106. [25] LUKE T L, MOHAN H, MANOJ V M, et al. Reaction of sulphate radical anion(SO4-) with hydroxy-and methyl-substituted pyrimidines: A pulse radiolysis study[J]. Research on Chemical Intermediates,2003,29(4):379−391. doi: 10.1163/156856703765694327
[26] 邓永秀. UV/Chlorine降解水中吡虫啉和噻虫啉的研究[D]. 长沙: 湖南大学, 2018. DENG Y X. Degradation of IMD and THIA in water via UV/Chlorine process[D]. Changsha: Hunan University, 2018.
[27] 方倩囡. 淡水水体中农药残留的化学氧化降解及机理研究[D]. 北京: 中国农业科学院, 2019. FANG Q N. Degradation and mechanism of chemical oxidation of pesticide residues in freshwater bodies[D]. Beijing: Chinese Academy of Agricultural Sciences, 2019.
[28] LI S P, MA X L, JIANG Y Y, et al. Acetamiprid removal in wastewater by the low-temperature plasma using dielectric barrier discharge[J]. Ecotoxicology and Environmental Safety,2014,106:146−153. doi: 10.1016/j.ecoenv.2014.04.034
[29] 赵青花. 臭氧化降解水中噻虫嗪和啶虫脒的研究[D]. 泰安: 山东农业大学, 2016. ZHAO Q H. Degradation of thiamethoxam and acetamiprid in aqueous solution by ozonation[D]. Taian: Shandong Agricultural University, 2016.
-
期刊类型引用(2)
1. 付严昆,蔡语铮,冯志强,陈思如,王田林,宋莲军,李天歌. 核桃肽通过促进白色脂肪棕色化预防肥胖的作用. 食品工业科技. 2025(03): 376-385 .  本站查看
本站查看
2. 张文凯,洪滔,郑梓泓,李雨露,徐佳玲,刘志勇. 肝纯片对实验性肥胖高脂血症大鼠生理生化指标的影响. 江西科学. 2024(04): 698-703 .  百度学术
百度学术
其他类型引用(1)





 下载:
下载:










 下载:
下载:



