Inhibitory Effect of Tremella fuciformis Polysaccharide on Starch Digestive Enzymes and Its Action Mechanism
-
摘要: 目的:研究银耳多糖对胰α-淀粉酶和α-葡萄糖苷酶的抑制作用及机制。方法:以干银耳为原料,分别采用碱法提取、酶法脱蛋白和柱层析分离,得到总糖含量为92.45%的银耳多糖(Tremella fuciformis polysaccharide,TP),采用可见光分光光度法分析了TP对胰α-淀粉酶和α-葡萄糖苷酶的抑制作用,采用荧光光谱法和圆二色谱法表征了TP对该两种酶结构的影响。结果:TP能抑制该两种酶的活性,其对胰α-淀粉酶的抑制作用明显高于α-葡萄糖苷酶,对该两种酶的半抑制浓度(IC50)分别为7.6835和16.9306 mg/mL。TP通过与该两种淀粉消化酶发生相互作用抑制其活性。TP与胰α-淀粉酶相互作用明显,可静态猝灭此酶,改变其二级结构;TP与α-葡萄糖苷酶相互作用微弱,不能改变其二级结构。结论:TP通过与淀粉消化酶发生相互作用抑制其活性。Abstract: Objective: To investigate the inhibitory effect of Tremella fuciformis polysaccharide (TP) on pancreatic α-amylase and α-glucosidase and its action mechanism. Methods: TP with a total sugar content of 92.45% was obtained by alkaline extraction from dried Tremella fuciformis, enzymatic deproteinization and column chromatography separation in turn. The inhibitory effect of TP on pancreatic α-amylase and α-glucosidase was measured by visible spectrophotometry, and its action on the structures of these two enzymes was characterized by fluorescence spectrometry and circular dichroism. Results: TP could inhibit the activities of these two enzymes, and its inhibition on pancreatic α-amylase was significantly higher than that on α-glucosidase, and its half inhibitory concentrations (IC50) on these two enzymes were 7.6835 and 16.9306 mg/mL, respectively. TP inhibited the activity of the enzymes by interacting with them. TP interacted strongly with pancreatic α-amylase. It could statically quench pancreatic α-amylase and change its secondary structure. However, TP interacted weakly with α-glucosidase and could not change its secondary structure. Conclusion: TP inhibited the activity of starch-digestive enzymes by interacting with them.
-
Keywords:
- Tremella fuciformis polysaccharide /
- pancreatic α-amylase /
- α-glucosidase /
- inhibition /
- mechanism
-
多糖是银耳的主要活性成分,占其干重的60%~70%,具有抗衰老[1]、降血糖[2]、降血脂[3]、调节免疫[4]等作用,在食品和医药领域显示出良好的应用前景[5]。小肠内淀粉的消化是在两种淀粉消化酶,即α-淀粉酶和α-葡萄糖苷酶的催化下逐步水解成葡萄糖的过程,其中前者可催化淀粉水解成麦芽糖、α-极限糊精和少量葡萄糖,是淀粉水解的先决条件;后者可将麦芽糖和α-极限糊精进一步水解成为葡萄糖,是控制葡萄糖释放的关键酶[6]。因此,抑制淀粉消化酶就能减少食物中淀粉的消化,延缓餐后血糖的升高。研究表明,活性多糖可通过抑制α-淀粉酶和α-葡糖糖苷酶的活性降低淀粉消化速率[7-8]。
动物实验研究结果显示,银耳多糖(Tremella fuciformis polysaccharide, TP)具有降血糖作用。Cho等[9]研究表明银耳胞外多糖可显著降低小鼠的血糖水平,提高其口服葡萄糖耐量,激活PPAR-γ的表达;银耳胞外多糖可能通过调节PPAR-γ-介导的脂质代谢发挥明显的降血糖和增强胰岛素敏感性的作用。田春雨等[10]研究了银耳多糖对链脲佐菌素诱导的2型糖尿病大鼠空腹血糖的影响,发现该多糖可明显降低糖尿病大鼠的血糖水平,并能缓解糖尿病所引起的多食、多尿、体重减轻等症状。然而,目前有关银耳多糖的降血糖作用机制尚不明确,并且关于其延缓淀粉体外消化的研究也鲜见报道。基于此,本文中研究了银耳多糖对胰α-淀粉酶和α-葡萄糖苷酶的体外抑制作用,分析了其对该两种酶结构的影响和可能的抑制作用机理。研究结果既可丰富银耳多糖的降血糖作用理论,还能为其精细化利用提供有价值的参考。
1. 材料与方法
1.1 材料与仪器
干银耳 福建古田袋料栽培银耳,市售;葡萄糖系列标准品(分子量范围2.5~5348 kDa) 色谱纯,美国Sigma公司;牛血清蛋白 上海阿拉丁试剂有限公司;瓜尔胶 山东广浦生物科技有限公司;猪胰α-淀粉酶(>10 U/mg)、α-葡萄糖苷酶(50 U/mg) 上海源叶生物科技公司;DEAE纤维素-52、对硝基苯基-β-D-吡喃葡萄糖苷(PNPG) 北京索莱宝科技公司;其它化学试剂 均为国产分析纯。
UV6000PC型紫外可见分光光度计 上海元析仪器有限公司;ZWY-100H恒温培养振荡器 上海智城分析仪器制造有限公司;Synergy H1型全功能微孔板检测仪 美国Biotek公司;F-7000荧光分光光度计 日立高新技术公司;J-1500-150型圆二色光谱仪 日本JASCO公司。
1.2 实验方法
1.2.1 银耳多糖的制备
参考并适当修改Lu等[11]报道的碱法提取银耳多糖。将1.0 g干银耳放入80 mL的去离子水中,于室温下浸泡20 min后打浆,用0.1 mol/L NaOH溶液调整其pH至12.0,50 ℃下提取4 h,过滤后以8000 r/min离心15 min,收集上清液。在25 mL上清液中加入3%(w/v)木瓜蛋白酶,混匀后以1 mol/L HCl调节其pH至6.5,于55 ℃下酶解2 h,沸水浴10 min,冷却至室温后于8000 r/min离心20 min,将所得上清液依次进行透析、浓缩、醇沉和真空干燥,得到脱蛋白银耳多糖。将其配制成浓度为5 mg/mL溶液,取10 mL上样,采用DEAE-52柱层析对其进行纯化,先用去离子水洗脱,然后依次以0.1、0.2、0.3、0.4 mol/L的NaCl溶液洗脱,收集峰面积最大的洗脱液,将其合并后依次进行透析、真空浓缩、冷冻干燥,得到白色絮状的纯化银耳多糖(TP)。经测定,TP总糖含量为92.45%,蛋白质含量为0.22%。
1.2.2 TP对胰α-淀粉酶和α-葡萄糖苷酶抑制作用的测定
适当修改Ademiluyi等[12]的方法测定TP对α-淀粉酶活性的抑制作用。移取不同浓度TP溶液(1、2、4、6、8和10 mg/mL)100 μL,加入1%(w/v)马铃薯淀粉溶液100 μL,于25 ℃孵育10 min后加入0.5 mg/mL胰α-淀粉酶溶液100 μL,继续孵育10 min,振荡后加入3,5-二硝基水杨酸试剂200 μL,沸水浴5 min,冷却至室温后加入去离子水2 mL,混匀后测定反应液在540 nm处的吸光度。以阿卡波糖为阳性对照。按照以下公式计算样品对胰α-淀粉酶的抑制率。
将Li等[13]的方法适当修改后测定TP对α-葡萄糖苷酶活性的抑制作用。移取不同浓度TP溶液(1、2、4、6、8和10 mg/mL)40 μL,加入0.1 U/L α-葡萄糖苷酶溶液40 μL,混匀后于37 ℃孵育5 min,加入5 mmol/L PNPG溶液20 μL,混匀后继续孵育30 min,加入0.2 mol/L碳酸钠溶液50 μL,混匀后室温孵育5 min,测定反应液在405 nm处的吸光度。以阿卡波糖为阳性对照。按照以下公式计算样品对α-葡萄糖苷酶的抑制率。
样品对该两种酶的抑制率计算公式如下:
(1) 式中:A1为样品组吸光度;A2为样品背景组吸光度(以等体积0.1 mol/L、pH6.9磷酸盐缓冲液PBS替代酶液);A3为对照组吸光度(以等体积PBS替代样品液);A4为对照背景组吸光度(以等体积PBS替代样品和酶液)。
1.2.3 TP对胰α-淀粉酶和α-葡萄糖苷酶的荧光猝灭作用及其结合位点测定
参考并适当修改的Jin等[14]报道的方法测定TP对胰α-淀粉酶荧光猝灭作用。以20 mmol/L PBS(pH6.9)配制浓度为1 mg/mL的α-淀粉酶溶液,使用0.2 μm水系滤膜过滤后取3份2 mL酶液,分别将其与8 mL不同浓度TP溶液(2、4和8 mg/mL)混匀,得到混合液1。
参考并适当修改的Wang等[7]报道的方法测定TP对α-葡萄糖苷酶荧光猝灭作用。以20 mmol/L PBS(pH6.9)配制浓度为0.75 U/mL的α-葡萄糖苷酶溶液,将3份1.5 mL酶液分别与1.5 mL不同浓度TP溶液(2、4和8 mg/mL)混匀后于30 ℃下孵育10 min,得到混合液2。
设置激发波长为280 nm,激发和发射狭缝宽度为5 nm,分别记录上述2种混合液在300~400 nm范围的荧光光谱。
TP与酶之间的猝灭效率符合Stern-Volmer方程[15]:
(2) 式中:F0和F分别为酶与TP作用前后的荧光强度;[Q]为TP浓度(mol/L);Ksv为动态猝灭常数;Kq为双分子猝灭过程速率常数;τ0为无猝灭剂荧光物质的平均寿命,约为10−8 s。
TP与酶之间的结合位点数(n)通过以下公式计算:
(3) 式中:F0和F分别为酶与TP作用前后的荧光强度;Ka为结合常数;n为结合位点数;C为TP浓度。
根据方程以lg
1.2.4 TP对胰α-淀粉酶和α-葡萄糖苷酶二级结构的影响
参考并适当修改Wang等[7]报道的方法。分别吸取100 μL的5 mg/mL胰α-淀粉酶和1.4 U/mL α-葡萄糖苷酶溶液,加入100 μL不同浓度TP溶液(2、4和8 mg/mL),混匀后于37 ℃孵育15 min,得到待测样品。采用圆二色光谱仪在190~250 nm范围内对待测样品进行扫描,横坐标为波长(nm),纵坐标为圆二色性(mdeg),绘制色谱图谱,并计算两种酶的二级结构含量变化。
1.3 数据处理
实验结果以(
2. 结果与分析
2.1 TP对胰α-淀粉酶和α-葡萄糖苷酶的抑制作用
小肠中淀粉的消化先经由胰α-淀粉酶将其水解为麦芽糖、α-极限糊精和少量葡萄糖,然后麦芽糖和α-极限糊精进一步被小肠细胞分泌的α-葡萄糖苷酶水解成为葡萄糖。因此,胰α-淀粉酶是淀粉消化的先行条件,其活性高低可影响淀粉消化反应进程。阿卡波糖等α-葡萄糖苷酶抑制剂是临床用于辅助治疗糖尿病的一种有效药物,可通过减少小肠对消化产物葡萄糖的释放来降低患者的餐后血糖水平[16]。
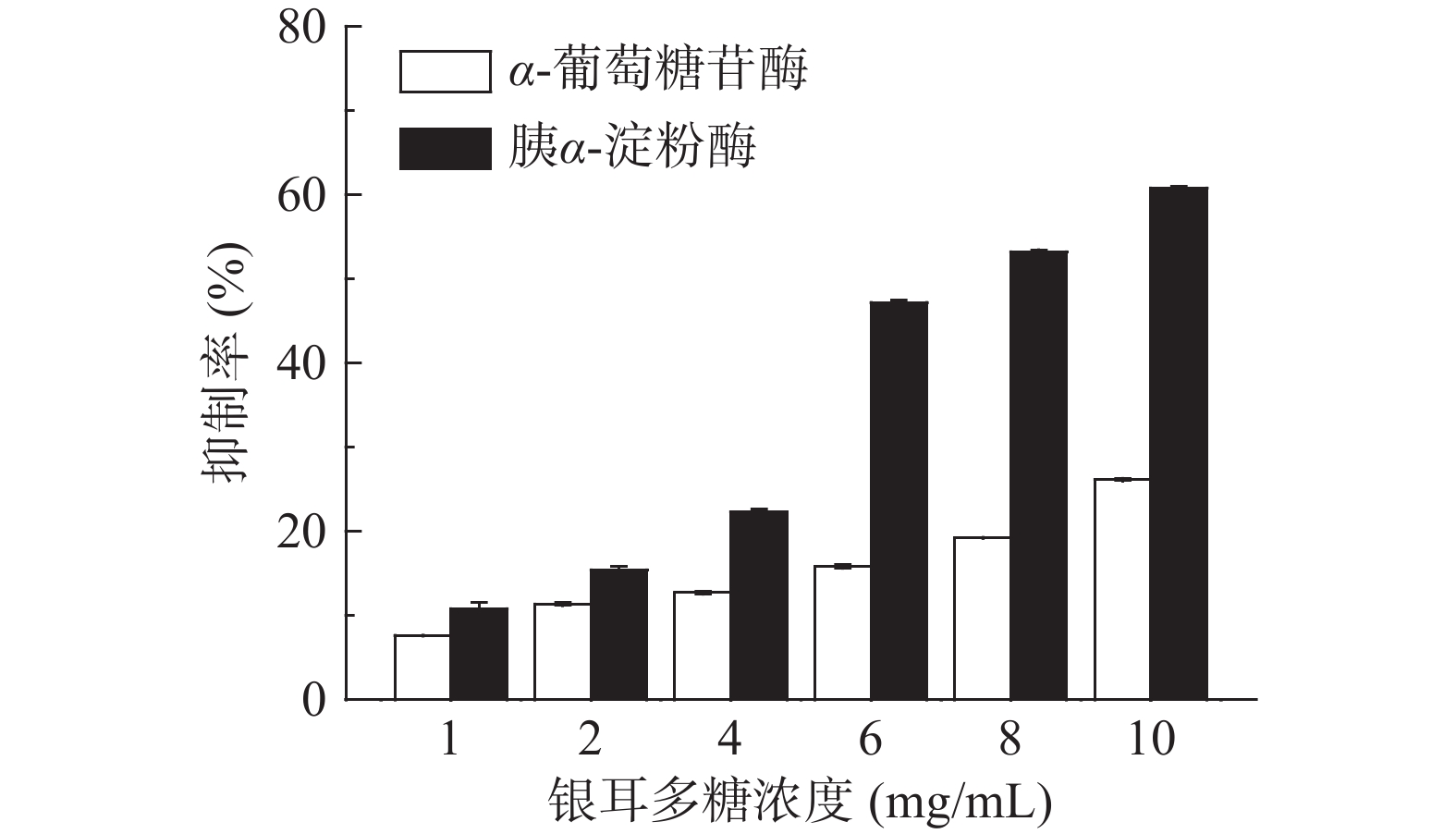
TP对胰α-淀粉酶和α-葡萄糖苷酶的抑制作用如图1所示。可以看出,随着TP浓度升高,其对该两种淀粉消化酶的抑制作用逐渐增大,尤其是对胰α-淀粉酶活性的抑制作用呈现出明显的剂量依赖关系。该结果与麦麸多糖[17]和番荔枝多糖[18]对胰α-淀粉酶和α-葡萄糖苷酶的抑制作用相似。本实验中提取的银耳多糖主要由甘露糖、岩藻糖、阿拉伯糖、木糖等单糖构成,并且其阿拉伯糖和木糖含量丰富,该两种单糖可较好抑制α-葡萄糖苷酶活性[19]。
将TP浓度与其对胰α-淀粉酶和α-葡萄糖苷酶抑制率进行拟合,得到拟合方程,计算其50%抑制作用浓度(IC50),结果见表1。可以看出,TP对胰α-淀粉酶的IC50为7.6835 mg/mL,略高于阿卡波糖(5.1870 mg/mL),但明显低于桑葚多糖[20](>19.31 mg/mL);对α-葡萄糖苷酶的IC50为16.9306 mg/mL,显著(P<0.05)高于阿卡波糖(0.2 μg/mL)。这说明TP是良好的胰α-淀粉酶抑制剂,其对胰α-淀粉酶的抑制作用明显强于α-葡萄糖苷酶。阿卡波糖对α-葡萄糖苷酶抑制率显著(P<0.05)高于TP,这可能与二者对该酶的结合位点不同有关。α-葡萄糖苷酶上具有与寡糖及双糖相结合的位点,阿卡波糖结构类似于寡糖,因此可竞争性地与α-葡萄糖苷酶上的结合位点结合而抑制其活性,是一种特异性葡萄糖苷酶抑制剂[21]。TP是一种多糖组分,其对α-葡萄糖苷酶的抑制作用位点不同于阿卡波糖。有关TP与该酶的具体结合位点还有待于进一步探讨。
表 1 TP对胰α-淀粉酶和α-葡萄糖苷酶半数抑制作用浓度(IC50)Table 1. Half maximal inhibition concentrations of TP on pancreatic α-amylase and α-glucosidase (IC50)样品 IC50(mg/mL) 胰α-淀粉酶 α-葡萄糖苷酶 TP 7.6835 16.9306 阿卡波糖 5.1870 0.0002 综上所述,TP可抑制胰α-淀粉酶和α-葡萄糖苷酶的活性,并且其对前者的抑制作用明显强于后者,这样就能有效抑制淀粉消化的起始速率。
2.2 TP对胰α-淀粉酶和α-葡萄糖苷酶的荧光猝灭作用
色氨酸、酪氨酸和苯丙氨酸的存在赋予淀粉消化酶内源荧光性,其中色氨酸与酪氨酸残基在280 nm波长时被激发,并且这两种残基所处的微环境还会影响其产生的荧光强度与位置[22]。
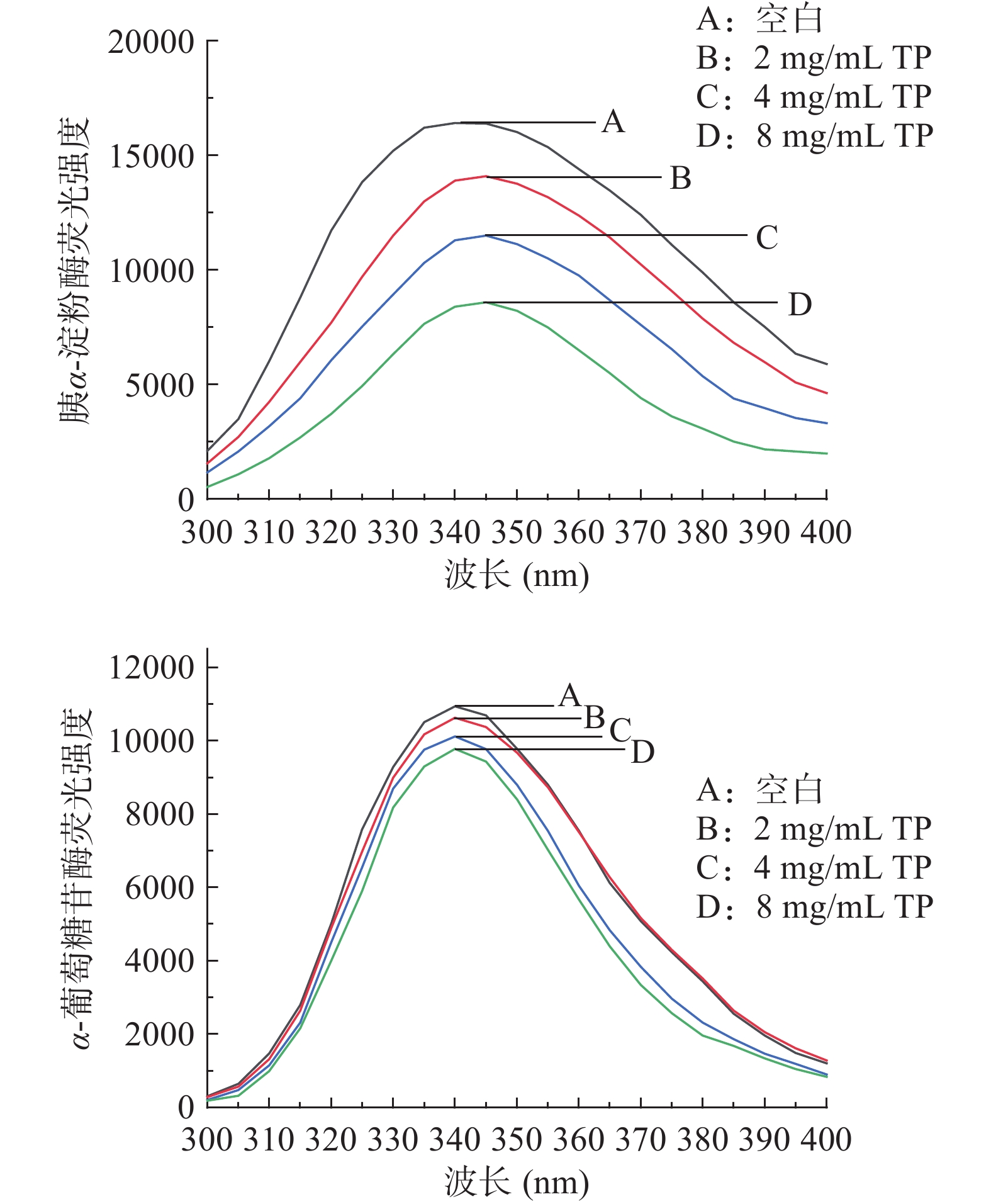
不同浓度TP对胰α-淀粉酶和α-葡萄糖苷酶内源荧光强度的影响如图2所示。在发射波长为300~400 nm时,胰α-淀粉酶和α-葡萄糖苷酶均具有较强的荧光强度,最大发射峰在340 nm附近。随着TP浓度增大,该两种酶的荧光强度逐渐降低,其中胰α-淀粉酶的变化更为显著,且发生红移,这与曾傲琼[23]关于条斑紫菜多糖的研究结果一致。说明胰α-淀粉酶和α-葡萄糖苷酶的荧光主要源于其中的色氨酸和酪氨酸残基,TP可与胰α-淀粉酶的色氨酸和酪氨酸残基发生静电引力或氢键作用,改变胰α-淀粉酶的结构,导致其活性降低,但其对α-葡萄糖苷酶的作用则较微弱[24]。TP与胰α-淀粉酶的相互作用引起酶中色氨酸或酪氨酸残基的极性变化,微环境由亲水性变为疏水性,导致荧光氨基酸残基发生去折叠和荧光猝灭[25]。此外,随着TP浓度增加,胰α-淀粉酶的荧光曲线发生了红移,说明TP对该酶的构象中其它部分也产生了影响。
2.3 TP对胰α-淀粉酶的荧光猝灭类型
荧光猝灭作用可分为动态猝灭和静态猝灭,其中动态猝灭是由于酶与猝灭剂因热运动相互碰撞而引起。假定TP对胰α-淀粉酶的荧光猝灭作用为动态猝灭,其猝灭常数Ksv为5.3094×1010 L/mol,双分子猝灭过程速率常数Kq为5.3094×1018 L/mol·s,远大于最大碰撞猝灭常数2.0×1010 L/mol·s。因此,TP对胰α-淀粉酶的荧光猝灭为静态猝灭,由此可推断TP与胰α-淀粉酶可能通过静电引力或氢键等非共价键产生相互作用并形成复合物,这可能与TP提高粘度、延缓或减少胰α-淀粉酶与底物淀粉的接触有关。
2.4 TP与胰α-淀粉酶结合位点数
静态猝灭是猝灭剂与荧光物质发生相互作用、形成基态的猝灭剂-荧光物质复合物的过程。通过公式计算出TP与胰α-淀粉酶结合位点数n=1.2334,接近1,表明TP与胰α-淀粉酶存在1个结合位点。由于胰α-淀粉酶分子中具有ASP197、GLU233和ASP300多个重要活性位点[26],因此关于二者具体结合位点尚待进一步研究。
2.5 TP对胰α-淀粉酶和α-葡萄糖苷酶二级结构的影响
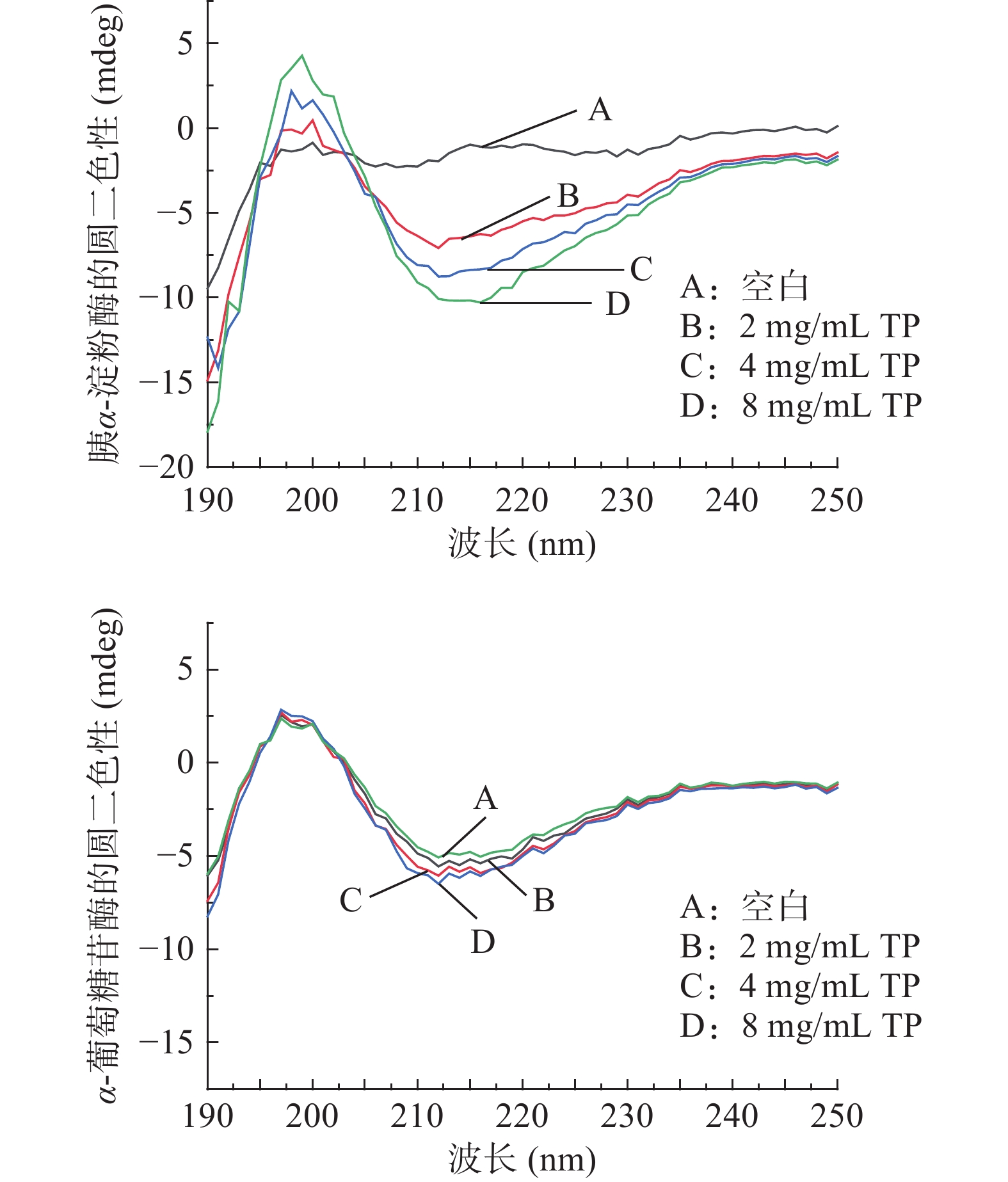
圆二色光谱是一种可定量的光谱技术,能灵敏反映蛋白质二级结构的变化,已被广泛用于蛋白质的构象研究[27]。不同浓度TP对胰α-淀粉酶和α-葡萄糖苷酶二级结构的影响如图3所示。可以看出,TP可导致两种消化酶的圆二色谱峰高和峰形发生变化。其中,胰α-淀粉酶组在200 nm处峰形明显高于空白组,在215 nm处峰形则明显低于空白组,而α-葡萄糖苷酶组峰形与空白组相比变化则不明显。随着TP浓度的增加,该两种酶在215 nm处的圆二信号强度增大,胰α-淀粉酶组变化明显,说明TP的添加对胰α-淀粉酶的二级结构产生了影响,而对α-葡萄糖苷酶二级结构影响不明显。
胰α-淀粉酶由496个氨基酸残基、1个钙离子、1个氯离子和3个水分子组成[28],其中100~168号氨基酸组成了8个α-螺旋和8个β-折叠交替出现的(β/α)8的结构模体,且该酶的活性中心位于α-螺旋结构中[29]。采用CDPro软件处理数据,通过SELCON3算法得到该酶的α-螺旋、β-折叠、β-转角和无规则卷曲结构的相对含量,结果见表2。可以看出,随TP浓度升高,胰α-淀粉酶中的α-螺旋和β-折叠比例增加,β-转角和无规则卷曲比例减少。α-螺旋比例逐渐增大,不利于酶活性中心形成,从而限制酶与底物的结合,使其进一步失去活性。这与条斑紫菜多糖[23]对胰α-淀粉酶二级结构影响的研究结果一致。α-螺旋和β-折叠中含有大量氢键,二者共同维持蛋白质二级结构的刚性。TP导致胰α-淀粉酶中的β-折叠增多,从而增强酶的刚性[30]。此外,β-转角和无规则卷曲比例减少则表明酶的柔性减弱。
表 2 TP对胰α-淀粉酶和α-葡萄糖苷酶二级结构的影响Table 2. Effects of TP on the secondary structures of pancreatic α-amylase and α-glucosidase淀粉消化酶 TP浓度
(mg/mL)α-螺旋
(%)β-折叠
(%)β-转角
(%)无规则卷曲
(%)胰α-淀粉酶 0 18.3 11.5 25.4 42.7 2 26.1 10.3 24.1 40.0 4 27.7 19.5 22.9 33.3 8 33.8 19.5 20.4 27.1 α-葡萄糖苷酶 0 25.7 26.4 21.6 28.5 2 25.8 25.9 21.3 28.0 4 26.1 25.4 20.6 28.1 8 26.0 25.4 20.2 28.4 由表2可知,加入TP后α-葡萄糖苷酶的各构象比例变化很小,说明TP对该酶构象影响不大。由此推测,TP与α-葡萄糖苷酶的相互作用微弱,对其二级结构影响极小。
3. 结论
TP能够抑制胰α-淀粉酶和α-葡萄糖苷酶的活性,且其对前者的抑制作用显著强于后者。TP与胰α-淀粉酶的结合位点数为1,二者之间发生了静态猝灭作用,这种猝灭作用改变了胰α-淀粉酶的二级结构,使其刚性增强、柔性减弱,结构变得更为紧密,影响了酶活性中心的形成,从而抑制了该酶的活性。TP与α-葡萄糖苷酶之间发生了微弱的相互作用,这种作用对该酶的二级结构影响甚微。TP可通过抑制淀粉消化酶的活性影响淀粉的消化,延缓葡萄糖的释放。本研究结果可为银耳多糖在降血糖保健食品和药品中的应用提供理论依据。
-
表 1 TP对胰α-淀粉酶和α-葡萄糖苷酶半数抑制作用浓度(IC50)
Table 1 Half maximal inhibition concentrations of TP on pancreatic α-amylase and α-glucosidase (IC50)
样品 IC50(mg/mL) 胰α-淀粉酶 α-葡萄糖苷酶 TP 7.6835 16.9306 阿卡波糖 5.1870 0.0002 表 2 TP对胰α-淀粉酶和α-葡萄糖苷酶二级结构的影响
Table 2 Effects of TP on the secondary structures of pancreatic α-amylase and α-glucosidase
淀粉消化酶 TP浓度
(mg/mL)α-螺旋
(%)β-折叠
(%)β-转角
(%)无规则卷曲
(%)胰α-淀粉酶 0 18.3 11.5 25.4 42.7 2 26.1 10.3 24.1 40.0 4 27.7 19.5 22.9 33.3 8 33.8 19.5 20.4 27.1 α-葡萄糖苷酶 0 25.7 26.4 21.6 28.5 2 25.8 25.9 21.3 28.0 4 26.1 25.4 20.6 28.1 8 26.0 25.4 20.2 28.4 -
[1] WEN L R, GAO Q, MA C W, et al. Effect of polysaccharides from Tremella fuciformison UV-induced photoaging[J]. Journal of Function Foods,2016,20:400−410. doi: 10.1016/j.jff.2015.11.014
[2] KIHO T, TSUJIMURA Y, SAKUSHIMA M, et al. Polysaccharides in fungi. XXXIII. Hypoglycemic activity of an acidic polysaccharide (AC) from Tremella fuciformis[J]. Journal of the Pharmaceutical Society of Japan,1994,114(5):308−315.
[3] CHEUNG P C K. The hypocholesterolemic effect of two edible mushrooms: Auricularia auricula (tree-ear) and Tremella fuciformis(white jelly-leaf) in hypercholesterolemic rats[J]. Nutrition Research,1996,16(10):1721−1725. doi: 10.1016/0271-5317(96)00191-1
[4] ZHOU Y, CHEN X, YI R, et al. Immunomodulatory effect of Tremella polysaccharides against cyclophosphamide-induced immunosuppression in mice[J]. Molecules,2018,23(2):239. doi: 10.3390/molecules23020239
[5] ZHANG Y K, ZHANG Q, LU J, et al. Physicochemical properties of Tremella fuciformis polysaccharide and its interactions with myofibrillar protein[J]. Bioactive Carbohydrates and Dietary Fibre,2017(11):18−25.
[6] QUEZADA C R, ROBAYO T C C, AO Z H, et al. Lumianl substrate “brake” on mucosal maltase-glycoamylase activity regulates total rate of starch digestion to glucose[J]. Journal of Pediatric Gastroenterology and Nutrition,2007,45(1):32−43. doi: 10.1097/MPG.0b013e31804216fc
[7] WANG L, CHEN C, ZHANG B, et al. Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its α-glucosidase inhibitory activity[J]. Food & Function,2018,9:3974−3985.
[8] ZHANG C, LI J, HU C, et al. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice[J]. Scientific Reports,2017,7(1):10847. doi: 10.1038/s41598-017-11457-w
[9] CHO E J, HWANG H J, KIM S W, et al. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice[J]. Applied Microbiology and Biotechnology,2007,75(6):1257−1265. doi: 10.1007/s00253-007-0972-2
[10] 田春雨, 薄海美, 李继安. 银耳多糖对实验性2型糖尿病大鼠血糖及血脂的影响[J]. 辽宁中医杂志,2011,38(5):986−987. [TIAN C Y, BO H M, LI J A. The influence of Tremella polysaccharide on blood glucose and serum lipoprotein in experimental type 2 diabetic rats[J]. Liaoning Journal of Traditional Chinese Medicine,2011,38(5):986−987. [11] LU A X, YU M E, SHEN M, et al. Preparation of the Auricularia auricular polysaccharides simulated hydrolysates and their hypoglycaemic effect[J]. International Journal of Biological Macromolecules,2018,106:1139−1145. doi: 10.1016/j.ijbiomac.2017.08.118
[12] ADEMILUYIA O, OBOH G. Soybean phenolic-rich extracts inhibit key enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro[J]. Experimental and Toxicologic Pathology,2013,65(3):305−309. doi: 10.1016/j.etp.2011.09.005
[13] LI D Q, ZHAO J, XIE J, et al. A novel sample preparation and on-line HPLC-DAD-MS/MS-BCD analysis for rapid screening and characterization of specific enzyme inhibitors in herbal extracts: Case study of α-glucosidase[J]. Journal of Pharmaceutical and Biomedical Analysis,2014,88:130−135. doi: 10.1016/j.jpba.2013.08.029
[14] JIN W P, WANG Z F, PENG D F, et al. Effect of pulsed electric field on assembly structure of α-amylase and pectin electrostatic complexes[J]. Food Hydrocolloids,2020,101:105547. doi: 10.1016/j.foodhyd.2019.105547
[15] KANDAGAL P B, ASHOKA S, SEETHARAMAPPA J, et al. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach[J]. Journal of Pharmaceutical and Biomedical Analysis,2006,41:393−399. doi: 10.1016/j.jpba.2005.11.037
[16] CHIASSON J L, JOSSE R G, GOMIS R, et al. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomized trial[J]. The Lancet,2002,359(9323):2072−2077. doi: 10.1016/S0140-6736(02)08905-5
[17] LV Q Q, CAO J J, LIU R, et al. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran[J]. Food Chemistry,2020,341:128218.
[18] GU S S, SUN H Q, ZHANG X L, et al. Structural characterization and inhibitions on α-glucosidase and α-amylase of alkali-extracted water-soluble polysaccharide from Annona squamosa residue[J]. International Journal of Biological Macromolecules,2021,166:730−740. doi: 10.1016/j.ijbiomac.2020.10.230
[19] WANG P, HOU C, ZHAO X, et al. Molecular characterization of water-extractable arabinoxylan from wheat bran and its effect on the heat-induced polymerization of gluten and steamed bread quality[J]. Food Hydrocolloids,2018,87:570−581.
[20] ZHANG J Q, CHAO L, QIANG H, et al. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures[J]. Food & Function,2019,10:584−588.
[21] 李雅珊. 靛玉红类α-葡萄糖苷酶抑制剂的活性与机理研究[D]. 天津: 天津科技大学, 2016. LI Y S. Study on α-glucosidase inhibitory activity and mechanism of indirubin derivatives[D]. Tianjin: Tianjin University of Science and Technology, 2016.
[22] MARK J W, JAMES M H, ANTHONY T M, et al. Streptavidin cooperative allosterism upon binding biotin observed by differential changes in intrinsic fluorescence[J]. Biochemistry and Biophysics Reports,2019,17:127−131. doi: 10.1016/j.bbrep.2018.12.011
[23] 曾傲琼. 条斑紫菜多糖抑制α-淀粉酶特性与降血糖作用[D]. 无锡: 江南大学, 2018. ZENG A Q. Study on the inhibiting characteristics and hypoglycemic effect of Porphyra yezoensis polysaccharide on α-amylase[D]. Wuxi: Jiangnan University, 2018.
[24] HUANG Y M, WU P, YING J, et al. Mechanistic study on inhibition of porcine pancreatic α-amylase using the flavonoids from dandelion[J]. Food Chemistry,2021,344:128610. doi: 10.1016/j.foodchem.2020.128610
[25] XU D, WANG Q, ZHANG W, et al. Inhibitory activities of caffeoylquinic acid derivatives from Ilex kudingcha C. J. Tseng on α-glucosidase from Saccharomy cerevisiae[J]. Journal of Agricultural and Food Chemistry,2015,63(14):3694−3704. doi: 10.1021/acs.jafc.5b00420
[26] BRAYER G D, SIDHU G, MAURUS R, et al. Subsite mapping of the human pancreatic α-amylase active site through structural, kinetic, and mutagenesis techniques[J]. Biochemistry,2000,39(16):4778−4791. doi: 10.1021/bi9921182
[27] KEIDERLING T A. Protein and peptide secondary structure and conformational determination with vibrational circular dichroism[J]. Current Opinion in Chemical Biology,2002,6(5):682−688. doi: 10.1016/S1367-5931(02)00369-1
[28] LARSON S B, GREENWOOD A, CASCIO D, et al. Refined molecular structure of pig pancreatic α-amylase at 2.1 Å resolution[J]. Journal of Molecular Biology,1994,235(5):1560−1584. doi: 10.1006/jmbi.1994.1107
[29] QIAN M, HASER R, PAYAN F. Structure and molecular model refinement of pig pancreatic α-amylase at 2.1 Å resolution[J]. Journal of Molecular Biology,1993,231(3):785−799. doi: 10.1006/jmbi.1993.1326
[30] FAN M C, LIAN W J, LI T T, et al. Characterization of promising natural blue pigment from Vaccinium bracteatum Thunb. leaves: Insights of the stability and the inhibition of α-amylase[J]. Food Chemistry,2020,326:126962. doi: 10.1016/j.foodchem.2020.126962
-
期刊类型引用(17)
1. 高思聪,黄文选,黄志超,缪铭. 食叶草蛋白的提取工艺及其性质研究. 食品与发酵工业. 2025(01): 189-198 .  百度学术
百度学术
2. 贺嫣然,王婧,许家强,夏昭昭,刘应蛟,董子舒,明良山,刘红宁,樊启猛. 补中益气方不同剂型中活性成分含量及其生物活性比较. 中成药. 2025(02): 357-364 .  百度学术
百度学术
3. 刘威,蔡卫佳,王昊,罗桂杰,刘旭. 食叶草研究进展. 中国农学通报. 2025(03): 36-41 .  百度学术
百度学术
4. 金敬红,张子昂,姚正颖,李楠楠. 食叶草降糖大米的研制及其对α-葡萄糖苷酶的抑制活性. 食品安全质量检测学报. 2025(06): 183-189 .  百度学术
百度学术
5. 何曾杨,李鑫垚,邹剑锋,孙梦姗,曾建国. 食叶草种子萌发特性研究. 湖南农业科学. 2025(02): 17-21 .  百度学术
百度学术
6. 龙伽雯,李跃辉. 新食品原料的审批及应用现状研究. 食品与发酵工业. 2025(08): 394-404 .  百度学术
百度学术
7. 刘威,王昊,蔡卫佳,罗桂杰,刘旭. 不同生育期食叶草营养物质变化规律及饲用价值分析. 中国奶牛. 2025(04): 47-52 .  百度学术
百度学术
8. 淳新月,任顺成. 食叶草蛋白的提取工艺优化及其氨基酸分析. 河南工业大学学报(自然科学版). 2024(01): 24-30+47 .  百度学术
百度学术
9. 吴瑞芳,刘瑶,赵玲玲,胥伟,郭丹郡,朱玲娇,易阳,王宏勋. 食叶草蛋白提取工艺的优化及功能特性分析. 武汉轻工大学学报. 2024(03): 66-75 .  百度学术
百度学术
10. 王龙霞,纵伟. 食叶草多酚超声提取工艺优化及抑制α-葡萄糖苷酶活性的研究. 食品与发酵科技. 2024(05): 61-66 .  百度学术
百度学术
11. 胡锦瑞,李鑫垚,凌浩,曾建国,刘秀斌. 食叶草不同部位营养成分分析. 饲料工业. 2024(21): 118-124 .  百度学术
百度学术
12. 何泽娟,操粮骏,王燕华,王冬钰,赵林芬,张乃明,谭超. 西垂茉莉的营养成分、抗氧化活性及代谢产物分析. 食品安全质量检测学报. 2024(21): 155-164 .  百度学术
百度学术
13. 王晓杰,刘抗,张丽,郑明明,周裔彬. 食叶草的营养价值及开发前景. 食品与机械. 2023(02): 164-169 .  百度学术
百度学术
14. 孟楠,秦令祥,曹源,高愿军. 超微冷冻前处理协同渗漉法提取食叶草黄酮工艺优化及其抗氧化、降血糖活性研究. 食品安全质量检测学报. 2023(13): 249-257 .  百度学术
百度学术
15. 胡高爽,刘紫洋,张亦琴,王晨宇,王君,王青华,郝建雄. 食叶草的营养成分及应用价值研究进展. 食品研究与开发. 2023(16): 208-212 .  百度学术
百度学术
16. 左梦微,任顺成,王凤成. 食叶草多酚类物质的提取、精制工艺研究. 河南工业大学学报(自然科学版). 2023(06): 36-43 .  百度学术
百度学术
17. 吴静,王珍珍,王晓宇,罗丹,蒋增良,沙如意,毛建卫,崔艳丽. 酿酒酵母发酵与自然发酵过程中霍山石斛酵素的代谢物及抗氧化变化. 中国生物工程杂志. 2022(11): 73-87 .  百度学术
百度学术
其他类型引用(5)










 下载:
下载:
 下载:
下载:



