Effect of Processing Methods on Protein Oxidation of Beef
-
摘要: 为了研究热加工过程中蛋白质氧化的变化规律,以牛腱子和牛肩为研究对象,对其进行不同温度(40、50、60、70、80、90、100 ℃,保温时间30 min)以及70 kPa高压下保温不同时间(15、20、25、30、35 min)的热处理,通过分析羰基含量、巯基含量、蛋白粒径、表面疏水性、SDS-PAGE电泳以及蛋白二级结构等的变化,结果表明:温度可以导致蛋白羰基含量增加,巯基含量先升高后降低,蛋白粒径增大,蛋白表面疏水性先增大后减小;高压导致蛋白羰基含量增加,巯基含量减少,蛋白粒径先增大后减少,蛋白疏水性逐渐增加。另外,不同热处理下肌原纤维蛋白发生了明显的降解聚集,出现大量小分子蛋白质。红外研究发现在热加工过程中,肌原纤维蛋白的二级结构不断转变,促使α-螺旋、β-折叠和无规则卷曲之间的转换。可知,温度和压力都能促进牛肉蛋白的氧化并能够改变牛肉蛋白的化学作用力及二级结构。本研究为低温牛肉产品的工业化调控提供理论依据。Abstract: In order to study the changes of protein oxidation during heat processing, cattle tendon and shoulder were treated at different temperatures (40, 50, 60, 70, 80, 90, 100 ℃, holding time 30 min) and at 70 kPa high pressure for different times (15, 20, 25, 30, 35 min). The changes of carbonyl content, sulfhydryl content, protein particle size, surface hydrophobicity, SDS-PAGE electrophoresis and protein secondary structure were analyzed. The results showed that temperature could lead to the increasing of protein carbonyl content, sulfhydryl content first increased and then decreased, protein particle size increased, protein surface hydrophobicity first increased and then decreased. High pressure resulted in the increasing of protein carbonyl content, the decreasing of protein sulfhydryl content, the increasing of protein particle size and the increasing of protein hydrophobicity. In addition, myofibrillar proteins were degraded and aggregated obviously under different heat treatments, and a large number of small molecular proteins appeared. Infrared studies showed that the secondary structure of myofibrillar protein changes during heat processing, which urged the formation of the secondary structure of myofibrillar protein α-spiral β-the conversion between folding and irregular curling. It was concluded that temperature and pressure could promote the oxidation of beef protein and change the chemical force and secondary structure of beef protein. This study would provide a theoretical basis for the industrial regulation of low-temperature beef products.
-
Keywords:
- protein oxidation /
- processing methods /
- beef /
- high pressure treatment
-
牛肉中蛋白质是人类能量和必需氨基酸的重要来源。加热温度是影响牛肉性能和微生物灭活阶段的关键因素之一。高压能有效消除致病菌和食品腐败菌,提高食品的安全性并延长保质期[1]。蛋白质在热处理过程中会经历结构修饰如氧化、聚集、降解和变性。蛋白质氧化涉及许多其他精确的化学修饰,如色氨酸损失、羰基化、羧化和交联的形成[2-4]。
近几年,国内外学者针对肉类在加热及高压过程中蛋白质氧化测定的部分指标研究较广。曹莹莹[5]研究发现随着压力增加,肌球蛋白的疏水基团和巯基含量逐渐增加。Wan等[6]研究结论得出,温度从80 ℃升高到90 ℃时,水溶性胶原蛋白含量增加,对牛肉制品的风味、口感、嫩度和总体偏好无不良影响。Pang等[7]发现蒸煮过程中肌球蛋白和肌动蛋白含量的变化相似,说明肌原纤维蛋白的变性在蒸煮损失中起着关键作用。结果表明,肉类的蒸煮温度和蛋白质变性之间有很强的相关性,说明加热温度引起了肉类蛋白质的变化。Scholliers等[8]研究发现在加热过程中,牛肉蛋白凝胶特性与疏水相互作用的形成有关,加热后肉蛋白表面疏水性的增加证实了这一点。Han等[9]在20~74 ℃条件下测定了蛋白质的构象和化学键,结果表明,在55 ℃以上,表面疏水性显著增强,游离巯基含量降低。Edyta等[10]研究发现在压力处理下会导致肉中蛋白质的变性和聚集现象。Stéphanie等[11]发现当压力超过300 MPa时,蛋白质氧化产物增加,结构发生不可逆变化。热处理对牛肉蛋白质氧化、结构和性能的影响国内外报道较少,不同部位的牛肉品质不同,适宜的加工方法也不同。
因此,本文研究对热加工过程中不同温度和70 kPa高压下不同保压时间对牛肉中羰基含量、巯基含量、蛋白粒径、表面疏水性,十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate - polyacrylamide gel electrophoresis,SDS-PAGE)和蛋白二级结构进行分析,以探究蛋白质氧化在热加工过程中的变化规律,为选择合适的原料,实现牛肉价值最大化,以及生产出不同加工方法下质优价廉的标准化产品提供理论依据。
1. 材料与方法
1.1 材料与仪器
牛腱子、牛肩 锦州新玛特超市;浓盐酸、磷酸氢二钠、氯化钠、磷酸二氢钠、盐酸胍、氯化镁、甲醇、溴化钾、冰醋酸、乙醇、甘油、尿素、考马斯亮蓝染色液 唐山三鼎化工有限公司;溴酚蓝、Tris、β-巯基乙醇、乙酸乙酯、2,4-二硝基苯肼、三氯乙酸、乙二胺四乙酸(EDTA)、2-硝基苯甲酸(DTNB)、十二烷基硫酸钠(SDS) 烟台健硕化工有限公司;所有试剂均为分析纯。
JD-3电子天平 沈阳龙腾电子有限公司;KH20R高速冷冻离心机 湖南凯达科学仪器有限公司;CH6数字显示温度计 上海亚度电子科技有限公司;JJ-2高速匀浆机 常州润华电器有限公司;HH-4D恒温水浴锅 上海比朗仪器制造有限公司;U-T6紫外可见分光光度计 屹谱仪器制造有限公司;DYCZ-25D型双垂直电泳 济南好来宝医疗器材有限公司;PHSJ-5型pH计 上海仪电科学仪器股份有限公司;NKT-N9纳米粒度仪 山东耐克特分析仪器有限公司;Great20傅立叶红外光谱仪 中科瑞捷科技有限公司;FD-1A-50冷冻干燥机 上海争巧科学仪器有限公司;高压锅 美的公司。
1.2 实验方法
1.2.1 样品预处理
清洗牛肉,然后去除脂肪和筋膜,切成3.0 cm×4.0 cm×5.0 cm的长方体,放在4 ℃的冰箱备用。处理牛肉分别采用两种加工方式。
水浴加热组:水浴锅温度设为 40、50、60、70、80、90、100 ℃,加热30 min。
高压保温组:压力为70 kPa,温度为110 ℃,保压时间设为15、20、25、30、35 min。
1.2.2 肌原纤维蛋白的提取
参考Zhong等[12]方法稍加修改。将样品冷却至室温,称取10 g,加入20 mmol/L磷酸盐缓冲液(含1 mmol/L EDTA,0.1 mol/L NaCl和pH7.0)定容至100mL,混合,摇匀,匀浆后在6000 r/min和4 ℃下离心10 min,取沉淀,加入50 mL 20 mmol/L磷酸盐缓冲液,在上述条件下离心,重复3次,然后加入25 mmol/L磷酸盐缓冲液(含0.6 mol/L NaCl,pH7.0),使沉淀均匀。用四层纱布过滤,滤液为肌原纤维蛋白溶液。以牛血清蛋白为标准,在280 nm处测定吸光度,用双缩脲法测蛋白浓度(mg/mL)。经考察,回归方程y=0.0501x+0.0022,R2=0.9998。
1.2.3 羰基含量的测定
参考Marco等[13]方法稍加修改。取0.1 mL蛋白溶液和0.5 mL二硝基苯肼(DNPH)(溶剂为2 mol/L HCl),空白样品不加DNPH,暗室静置1 h(每20 min摇动1次),加5 mL 20%三氯乙酸,摇匀后,在10000 r/min,15 min条件下4 ℃离心,弃上清。沉淀用1 mL乙酸乙酯-乙醇溶液(1:1)洗涤3次。加入1 mL 6 mol·L−1盐酸胍溶液,在37 ℃水浴20 min,然后10000 r/min下离心5 min。在370 nm波长处测定吸光度,羰基含量通过22000 L/(mol·cm)的摩尔吸收率计算。
式中:A370为370 nm下的吸光度106为摩尔基础单位;C为测得的蛋白浓度(mg/mL)。
1.2.4 巯基含量的测定
参考Yang等[14]方法稍加修改。取0.1 mL蛋白溶液、1 mL 50 mmol·L−1 Tris-HCl缓冲液(含有1mmol·L−1 EDTA、6mol·L−1盐酸胍,pH8.3)以及10 μL 10mmol·L−1DTNB,混合摇匀,室温下静置25 min,在412 nm处测定吸光度。巯基含量由13600 L/(mol·cm)的摩尔吸收率计算。
式中:A412为412 nm下的吸光度;106 为摩尔基础单位;C为测得的蛋白浓度(mg/mL)。
1.2.5 粒径的测定
参考Zhang等[15]方法稍加修改。利用纳米粒度仪测定蛋白质粒径。以去离子水为分散剂,粒子的折射率为1.414,吸收率为0.001。
1.2.6 表面疏水性的测定
参考Zhu等[16]方法稍加修改。取1 mL蛋白溶液,加入40 μL溴酚蓝溶液(1 mg/mL),振荡10 min,4 ℃下2000 r/min离心15 min,取上清稀释10倍,在595 nm处测定吸光度。空白对照为20 mmol/L磷酸盐缓冲液。表面疏水性计算公式如下:
式中:A空白为空白组的吸光度;A样品为样品组的吸光度。
1.2.7 SDS-PAGE凝胶电泳
参考Shi等[17]方法稍加修改。电泳采用10%分离胶和5%浓缩胶。每个时期的上样液量为5 μL,初始电泳电压为75 V,样品进入分离胶后,升压至100 V,电泳结束后剥离胶片,考马斯亮蓝染色30 min,再用脱色液脱色3次。
1.2.8 傅里叶变换红外光谱测定
参考Kun等[18]方法稍加修改。蛋白样品在−20 ℃冷冻5 h,然后在冷冻干燥机中干燥12 h,取1 mg样品放入研钵再和100 mg溴化钾充分研磨成细粉。在模具内使分布均匀,将模具水平放置在模座上,加压至10 t/cm2,保持1 min,用傅里叶红外光谱仪进行分析,条件为:光谱范围:500~4000 cm−1,分辨率:4 cm−1,扫描积累32次。
1.3 数据处理
采用Office 2019进行数据处理及图表绘制,计算标准差。
2. 结果与分析
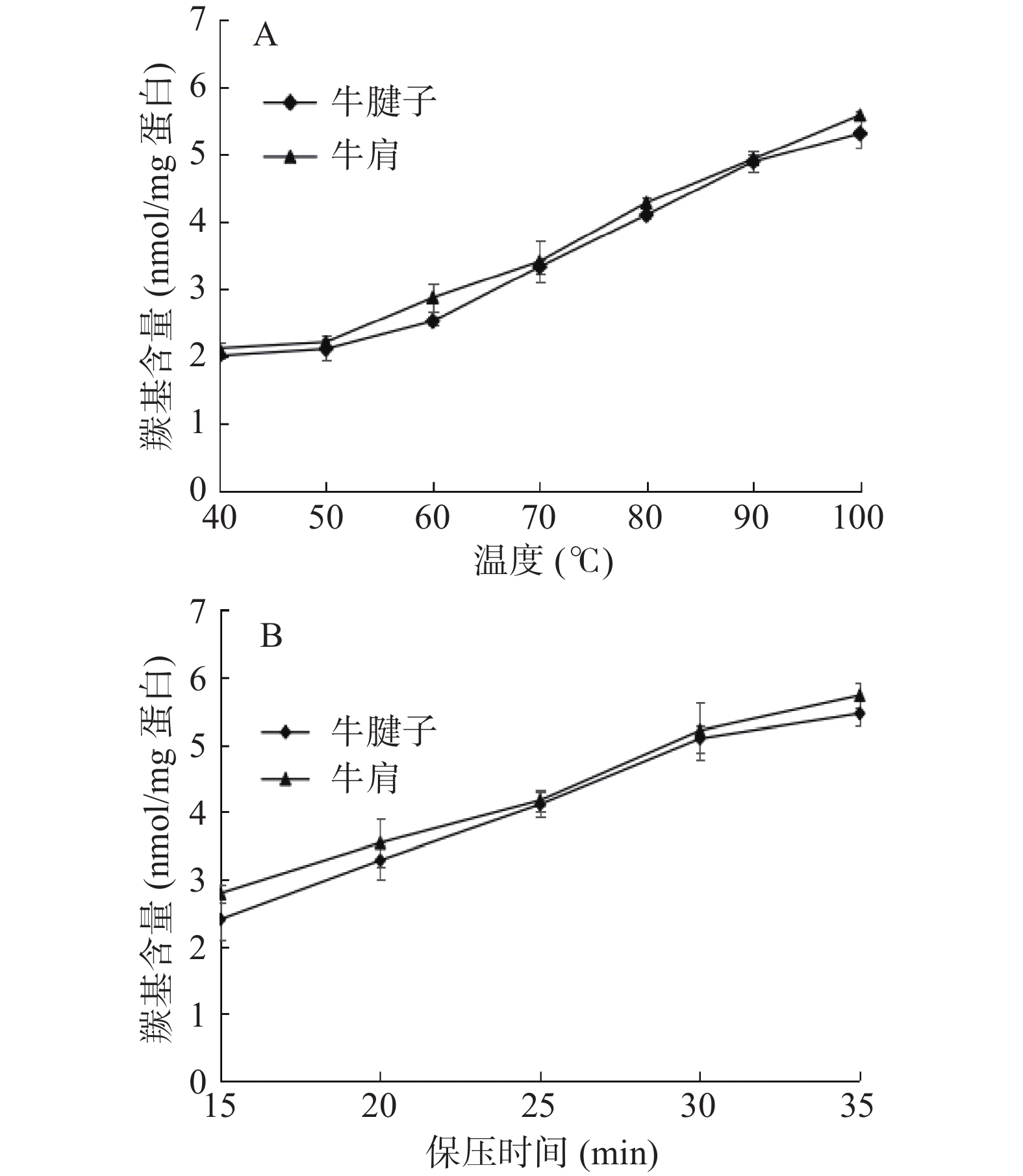
2.1 不同加热方式对蛋白羰基含量的影响
羰基的形成是蛋白质氧化的一个明显标志,反映了蛋白氧化损伤的程度。由图1可见,随着温度的升高和保压时间的延长,蛋白质羰基含量逐渐增加。在蒸煮和高压加热过程中,蛋白质结构发生变化,大部分肌浆蛋白、肌球蛋白和肌动蛋白变性,细胞膜结构遭到破坏,导致铁等过渡金属离子释放。活性氧和无氧自由基是由催化剂或前体产生的,包括一些血红素、过渡金属离子和各种氧化酶。这些自由基攻击蛋白质,催化蛋白质氧化,导致羰基含量增加[19]。羰基值的增加也可能是脂质氧化的产物。肽骨架裂解成短肽和蛋白质也可以与还原糖反应生成羰基。热处理后的牛肉肌原纤维蛋白发生蛋白质氧化反应,由于水浴持续加热时间较长,更容易诱导肉中脂肪氧化和蛋白质羰基的形成。但高压下升温速度快,可能导致脂肪氧化和羰基氧化水平低于水浴加热。牛腱子的羰基含量较牛肩低,是由于牛腱子脂肪含量较牛肩低,所以氧化程度较低。Xiong等[20]发现超高压与热处理相结合时,猪肉脂肪氧化程度增加,这可能是蛋白质氧化的诱因之一。
2.2 不同加热方式对蛋白巯基含量的影响
肌原纤维蛋白中存在大量巯基,氧化条件下易转化为二硫键,造成巯基含量下降。所以,巯基对维持蛋白质的结构稳定性起着重要作用。
由图2A可知,随着温度的升高,巯基含量先升高后降低。热加工过程影响肌动球蛋白的解离程度,40~60 ℃可以导致肌动蛋白解离,60 ℃是促进肌动蛋白解离的最佳温度,因此60 ℃时巯基含量最高[21]。当温度继续升高时,肌动球蛋白构象改变,巯基被氧化成二硫键,使巯基含量降低。从图2B可以看出,巯基含量随着保压时间的增加而减少。这表明肌原纤维蛋白在高压处理下打开,巯基通过二硫键聚集。因为在高压处理下巯基暴露在分子表面,与空气中的氧充分结合形成二硫键致使巯基减少[22]。万红兵等[23]发现,在牛排的油炸过程中,热处理对巯基和二硫键的含量有显著影响,牛肉蛋白中巯基含量先升高后降低。
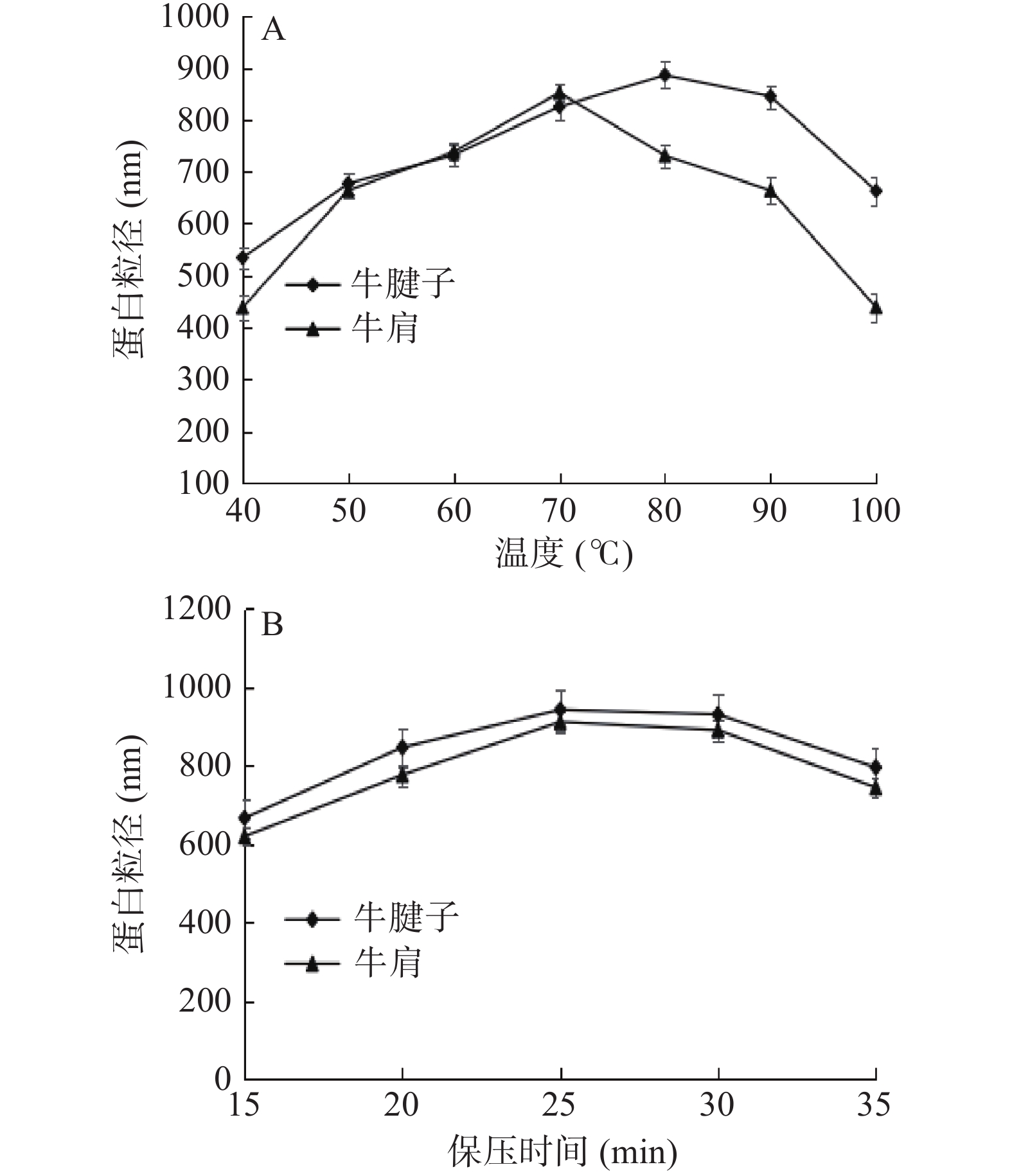
2.3 不同加热方式对蛋白粒径的影响
粒径是反应蛋白聚集状态的一个指标,在一定程度上可以反映反应蛋白在降解过程中的状态。
由图3可知,随着温度的升高和保压时间的延长,蛋白质的粒径逐渐增大。在80 ℃、保压25 min时,牛腱子的粒径最大,而在70 ℃、保压25 min时,牛肩的粒径最大;继续加热,蛋白质的粒径逐渐减小,这进一步说明在热加工过程中,蛋白质在加热的作用下首先形成聚集现象。另一方面,然后持续加热,蛋白质变性,使其中一些不耐热的化学键发生断裂,减弱了交联作用,结果蛋白质聚集体被部分破坏,因此粒径变小[24]。在高压过程中,当时间在15~25 min时,蛋白质进一步变性,蛋白质膨胀程度增加,变性蛋白通过分子内或分子间的相互作用增强了蛋白质的聚集程度,形成的聚集物尺寸增大,导致蛋白质粒径增大;随着时间的进一步增加,蛋白质的聚集率增加,打破了蛋白质变性率和聚集率之间的平衡,形成的聚集体无序,不能形成较大的聚集体,或者形成的聚集体不稳定,不能保持原来的形态,导致粒径减小[25]。
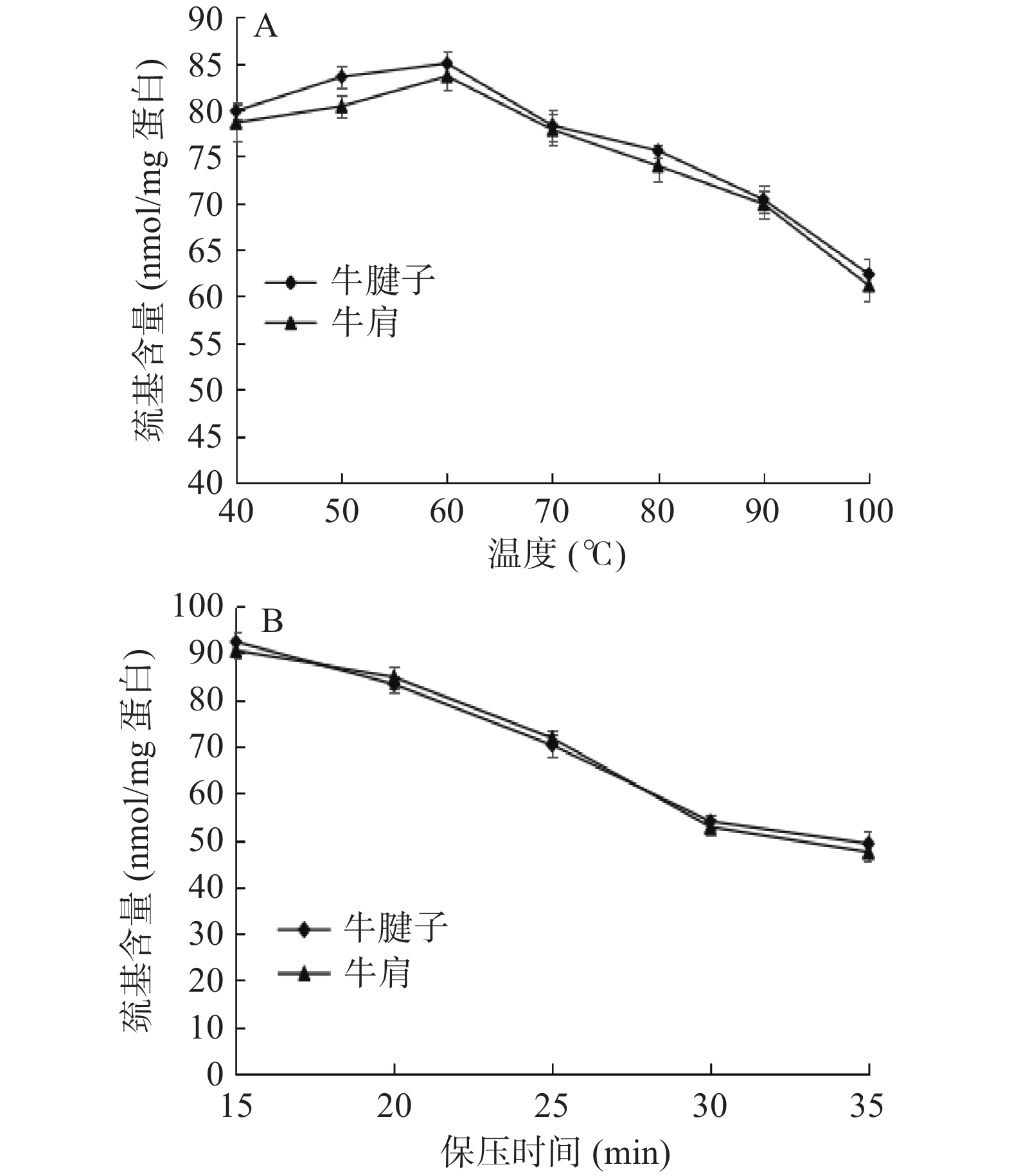
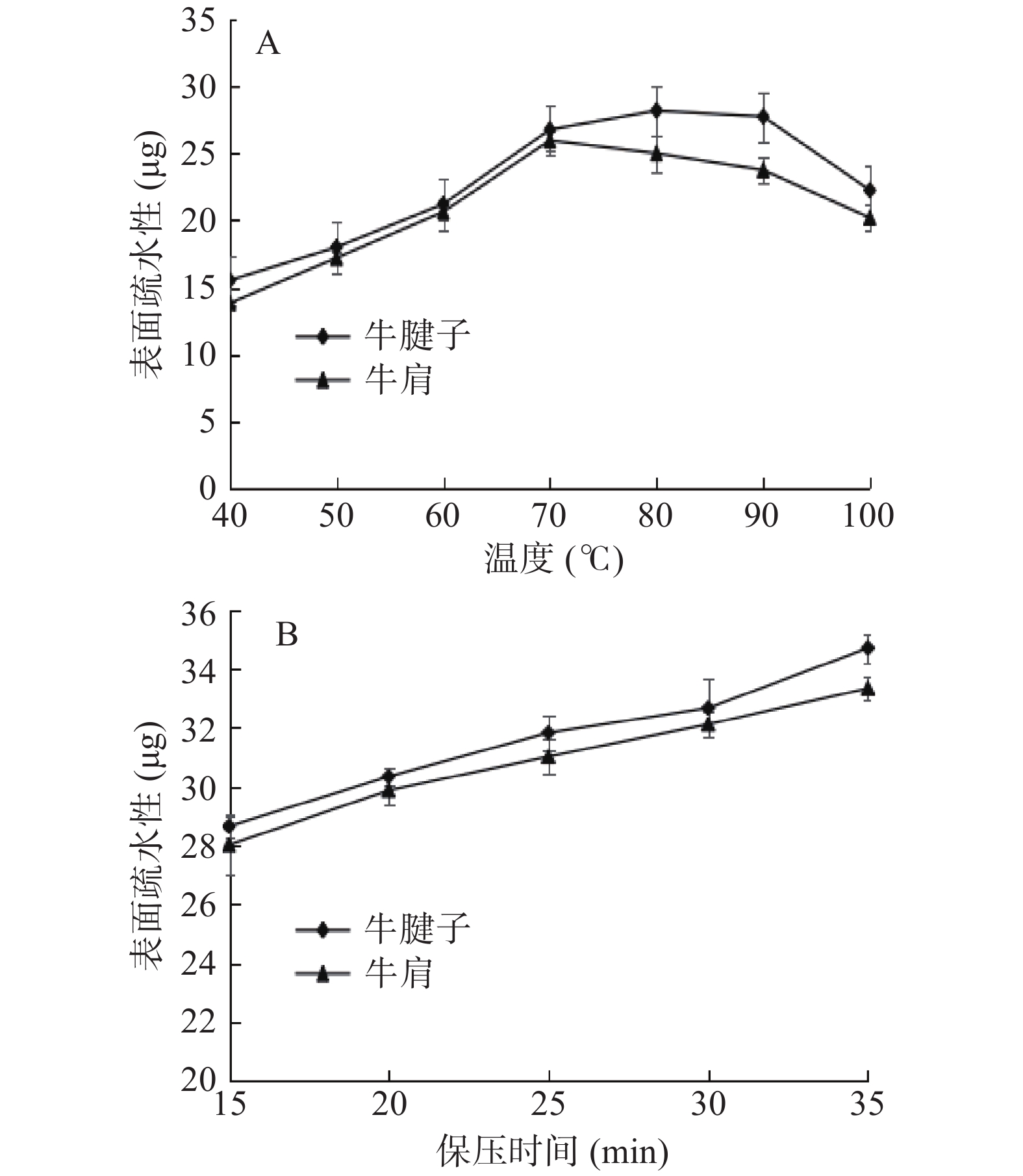
2.4 不同加热方式对蛋白表面疏水性的影响
蛋白质的表面疏水性反映其结构稳定性,并影响蛋白凝胶形成。疏水相互作用对蛋白凝胶的形成和蛋白结构的稳定性起着非常重要的作用。
由图4A得出,随着温度的升高,蛋白质的表面疏水性逐渐增加,在80 ℃时牛腱子达到最大值,在70 ℃时牛肩达到最大值,然后随温度升高而降低。其原因可能是随着温度的升高,蛋白质疏水侧链的暴露程度增加,使蛋白质的水溶性环境变为疏水性环境,导致表面疏水性增加。70~80 ℃后肌球蛋白变性,肌原纤维蛋白聚集体与侧链之间发生反应,产生沉淀[26-27]。随着蛋白质聚集度的增加,少量疏水基团被再次包埋,疏水基团的结合降低了表面疏水性。从图4B可以看出,蛋白质的疏水性随着保压时间的增加而增加。压力使肌原纤维蛋白展开,暴露出蛋白质的疏水残基,同时压力可诱导蛋白质表面出现特殊氨基酸残基,导致疏水性增加。表面疏水性变化也可能是因为蛋白质的降解。
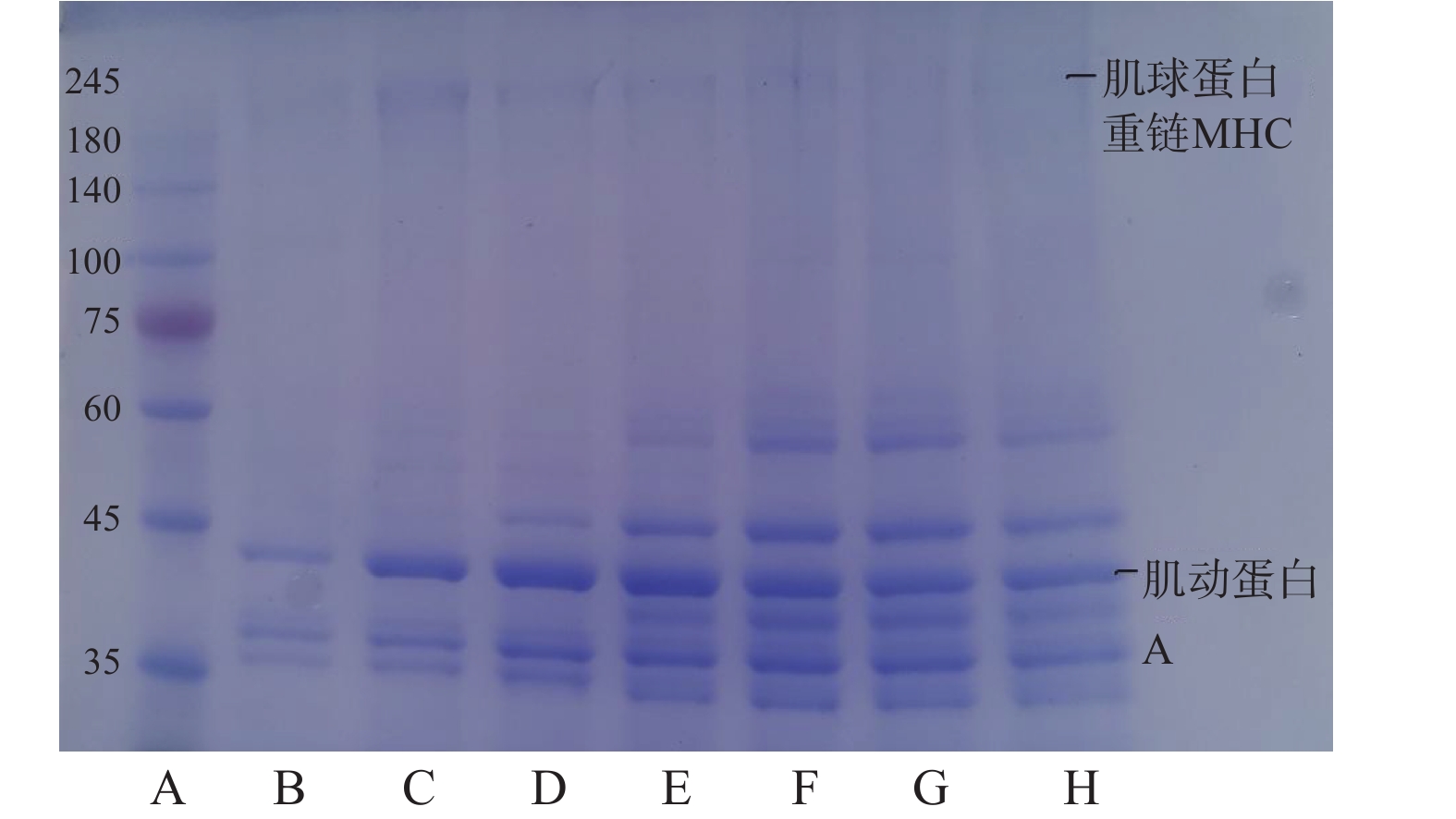
2.5 SDS-PAGE凝胶电泳结果
肌原纤维蛋白主要由副肌球蛋白(100 kDa)、肌钙蛋白(75 kDa)、肌动蛋白(43 kDa)、肌球蛋白重链(200 kDa)等组成。
肌原纤维蛋白经高温高压后进一步降解,造成其变性。如图5所示,随着温度升高,牛腱子中的肌原纤维蛋白出现降解和聚集,导致许多电泳条带在245~75 kDa范围内明显模糊,甚至消失。随着温度的升高,200 kDa处的肌球蛋白重链带颜色逐渐变浅,在50~80 ℃时可以看到条带密度的明显变浅,这可能是由于高温加热,导致蛋白质之间产生了相互作用,在90~100 ℃温度下条带基本消失,表明温度过高使得蛋白质的变性和聚集速度加快,肌球蛋白重链结构被破坏,使其分解,说明肌球蛋白重链具有不耐热性,这可能是由于某些蛋白质随着温度的升高而变性所致,高温使肌球蛋白重链分解,形成分子量小的肌球蛋白轻链,肌动蛋白也分解成原肌球蛋白,造成条带颜色变浅。另外,由于肌球蛋白重链在加热后聚集成大分子,不能进入电泳条带,因此在凝胶浓缩液的上界面无法观察到大分子蛋白形成的条带,使蛋白条带颜色变浅[28-29]。而在40 ℃时43 kDa处的肌动蛋白略有降解,但随温度的升高,没有观察到肌动蛋白条带的显著差异,可能是肌动蛋白通过非共价键与肌球蛋白相互作用,如疏水相互作用。也可能是肌动蛋白聚合导致变性,形成聚集体,氢键、疏水相互作用、二硫键和由内源转化酶诱导的ɛ-(γ-谷氨酰)赖氨酸键是形成蛋白质聚集体的主要化学键。进一步说明肌球蛋白的热稳定性没有肌动蛋白比高[30]。此外,35~45 kDa范围内的蛋白质条带数量随温度升高急剧增加,这表明在此期间也发生了蛋白水解降解。
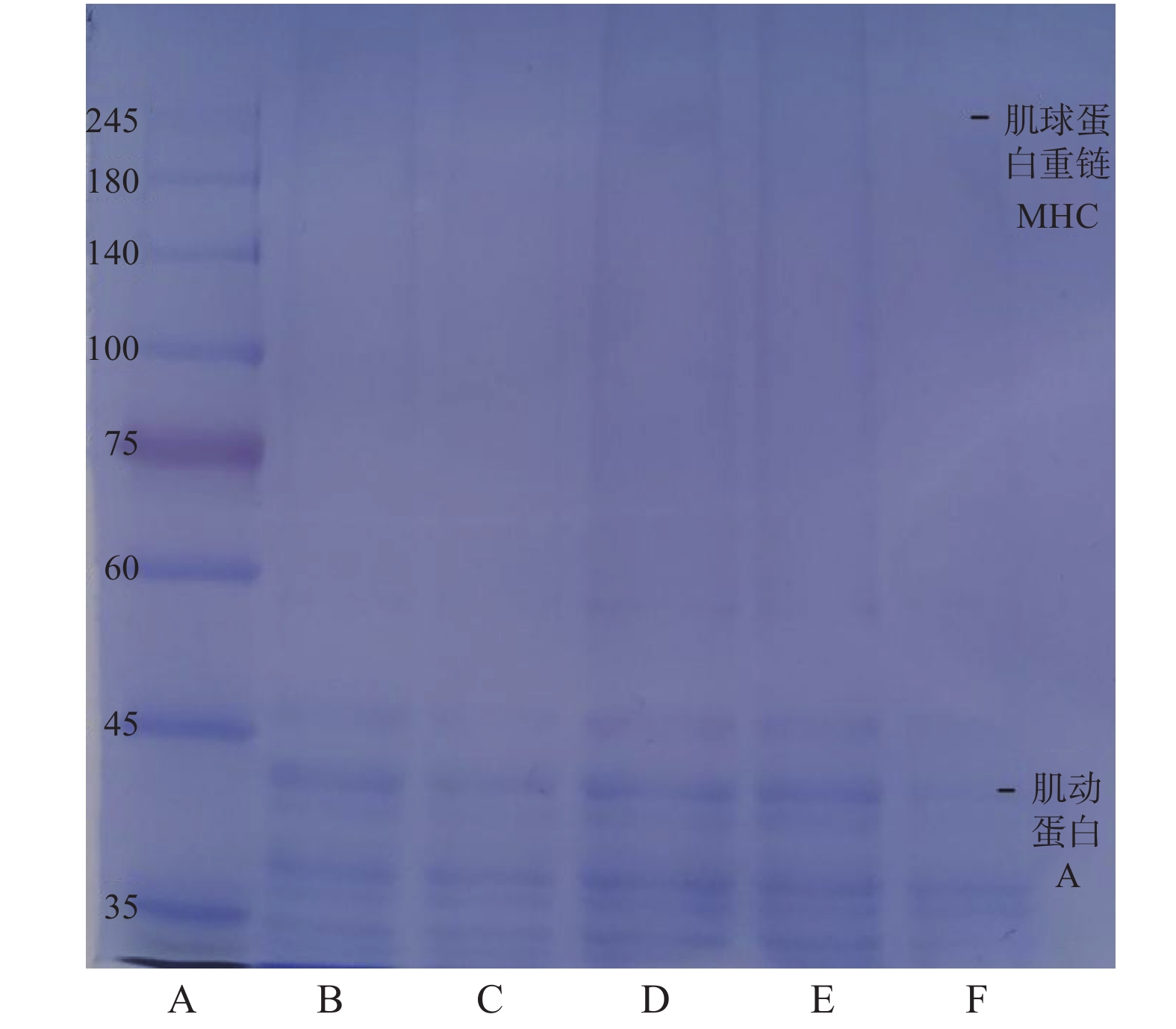
如图6所示,随着保压时间的延长,肌球蛋白重链条带颜色变浅甚至消失,可能是肌球蛋白重链对压力不稳定,高压导致这些蛋白质降解,抑制肌球蛋白的交联,从而使肌原纤维蛋白解聚作用,溶解度降低。而肌动蛋白的条带一直存在,在15 min时略有降解,随后颜色加深,在35 min时条带颜减弱,表明肌动蛋白在高压加热过程中部分分解。Zhang等[31]的电泳结果表明,鲢鱼鱼糜凝胶在加热过程中MHC带的强度随着加热温度和时间的增加而急剧下降,而25~35 kDa范围内的蛋白质条带数量急剧增加。
不同热处理引起蛋白质降解的差异与传热介质有关,不同于蒸煮加热过程中由外到内的传热方式,高压使极性分子在加热过程中通过摩擦产生热量,而高压加热速率较快,因此结构破坏最为严重,造成蛋白质降解严重。然而,SDS-PAGE结果显示,在蒸煮过程中,蛋白质的降解相对温和。
2.6 不同加热方式对肌原纤维蛋白二级结构的影响
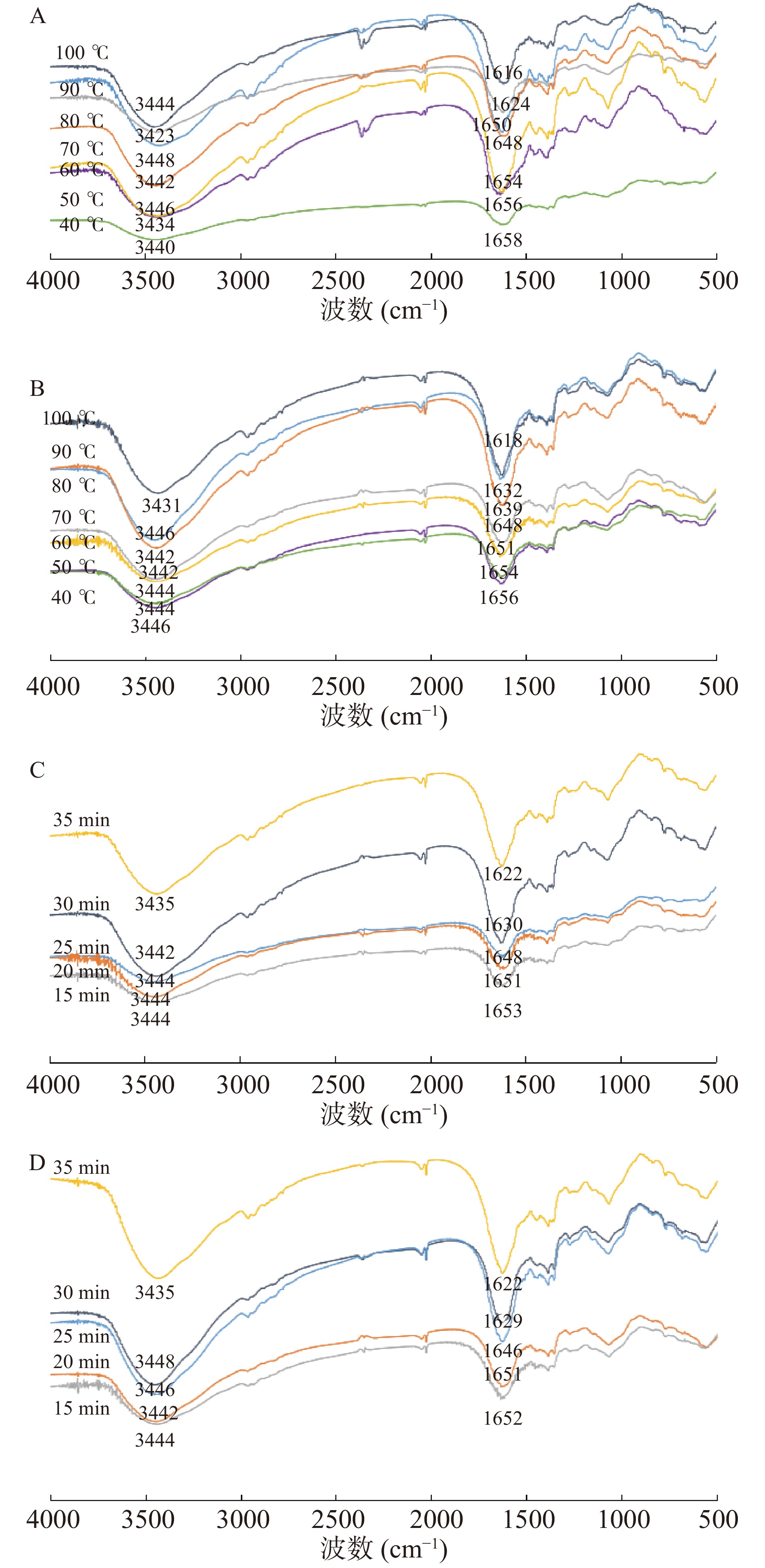
常见的蛋白质二级结构可分为α-螺旋、β-折叠、β-螺旋和无规卷曲。在高温高压作用下,蛋白质二级结构发生变化,造成不可逆转变。蛋白质红外光谱主要集中在1600~1700 cm−1的酞胺Ⅰ带,这是蛋白质二级结构最敏感的区间,常用来分析蛋白质二级结构[32]。
由图7可知,牛肉肌原纤维的酰胺A带由3448 cm−1向3423 cm−1移动,这可能是酰胺A带的N-H伸缩振动和氢键缔合的形成,改变了位移,说明氢键产生变化。不同温度处理组和不同保压时间组的蛋白酰胺Ⅰ带的吸收峰形状相似,但两个波数位置不同,说明传统水浴加热肌球蛋白的二级结构不同于高压肌球蛋白。随着温度的增加和保压时间的延长,不同温度下牛腱子酰胺Ⅰ带从1658 cm−1变为1616 cm−1,牛肩酰胺Ⅰ带从1656 cm−1变为1618 cm−1,而高压下牛腱子酰胺Ⅰ带从1653 cm−1变为1622 cm−1,牛肩酰胺Ⅰ带从1652 cm−1变为1622 cm−1,说明加热后牛肉肌原纤维的α-螺旋结构先转变为无规则卷曲结构,再转化为β-折叠。加热导致蛋白质变性,从而破坏羰基和氨基之间的氢键,α-螺旋分裂,可能向无规则卷曲和β-折叠转化。在加热后期,水分子随着二级结构的膨胀而迁移,疏水基团和巯基的暴露提供了较大的空间。此时,蛋白质逐渐聚合,β-折叠结构开始分解,分子间的疏水作用形成无规则卷曲[33]。可以看出,随着热处理温度的升高和保压时间的延长,酰胺Ⅰ带的峰值位置向低的波数移动,这反映了蛋白质在热处理过程中的变性和蛋白质从α-螺旋转化为无规则卷曲和β-折叠。
3. 结论
随着温度的升高,蛋白羰基含量逐渐增加,巯基含量呈现先升高后降低的变化趋势,蛋白粒径增大,蛋白表面疏水性先增大后减小。随着保压时间的延长,蛋白羰基含量增加,巯基含量减少,蛋白粒径先增大后减少,蛋白疏水性逐渐增加。SDS-PAGE研究发现,随着温度升高,肌球蛋白重链发生了降解明显,而肌动蛋白加热后开始略有降解,随着温度升高没有明显的变化趋势;在高压条件下,肌球蛋白重链条带逐渐减弱甚至消失,而在不同保压时间肌动蛋白条带无明显变化。与高压相比,在蒸煮过程中,蛋白质的降解相对温和。红外结果表明,随着温度的升高和保压时间的延长,酰胺Ⅰ带的峰值位置向低的波数移动,反映蛋白质在热处理过程中的变性和蛋白质从α-螺旋转变为无规则卷曲和β-折叠。本文为不同部位的牛肉精深加工精确工艺参数的设定提供了有力的理论依据。
-
-
[1] 郑海波. 高压下加热处理对鸡肉糜凝胶品质的影响及机制研究[D]. 南京: 南京农业大学, 2018. ZHENG H B. Effect of heat treatment on gel quality of chicken meat paste under high pressure and its mechanism [D]. Nanjing: Nanjing Agricultural University, 2018.
[2] 伏慧慧, 马雪莲, 普莉雯, 等. 干腌牛肉加工过程中蛋白质变化对品质的影响[J]. 食品与发酵工业,2021,47(9):223−230. [FU H H, MA X L, PU L W, et al. Effect of protein change on quality of dry cured beef during processing[J]. Food and Fermentation Industry,2021,47(9):223−230. [3] RIMI M, MASAHIRO A, RIEKO H, et al. Rapid inspection method for investigating the heat processing conditions employed for chicken meat using Raman spectroscopy[J]. Journal of Bioscience and Bioengineering, 2020, 129(6).
[4] 吴倩蓉, 朱宁, 陈松, 等. 加工工艺对酱牛肉中蛋白质降解及风味物质的影响[J]. 食品科学,2021,42(12):76−84. [WU Q R, ZHU N, CHEN S, et al. Effect of processing technology on protein degradation and flavor compounds in sauced beef[J]. Food Science,2021,42(12):76−84. doi: 10.7506/spkx1002-6630-20200518-206 [5] 曹莹莹. 超高压结合热处理对肌球蛋白凝胶特性的影响研究[D]. 南京: 南京农业大学, 2012. CAO Y Y. Effect of ultra high pressure combined with heat treatment on myosin gel properties [D]. Nanjing: Nanjing Agricultural University, 2012.
[6] WAN Hongbing, LI Haipeng, LEI Yuanhua, et al. Influence of stewing conditions on tenderness and protein structure in beef[J]. Journal of Food Processing and Preservation, 2021, 45(3): e15208.
[7] PANG B, YU X, BOWKER B, et al. Effect of meat temperature on moisture loss, water properties, and protein profiles of broiler pectoralis major with the woody breast condition[J]. Poultry Science,2021,100(2):1283−1290.
[8] SCHOLLIERS J, STEEN L, FRAEYE I. Gelation of a combination of insect and pork proteins as affected by heating temperature and insect: Meat ratio[J]. Food Research International,2020:137.
[9] HAN Z Y, ZHANG J L, ZHENG J Y, et al. The study of protein conformation and hydration characteristics of meat batters at various phase transition temperatures combined with low-field nuclear magnetic resonance and fourier transform infrared spectroscopy[J]. Food Chemistry,2019:280.
[10] EDYTA M, MARTA W, ROMAN P, et al. The effect of high pressure at subzero temperature on proteins solubility, drip loss and texture of fish (cod and salmon) and mammal’s (pork and beef) meat[J]. Food Science and Technology International,2014,20(5):383−395. doi: 10.1177/1082013213488901
[11] STÉPHANIE J, MARIE L, MOHAMED G. Modifications of ultrastructure and myofibrillar proteins of post-rigor beef treated by high pressure[J]. LWT-Food Science and Technology,2000,33(4):313−319. doi: 10.1006/fstl.2000.0654
[12] ZHONG H, MENG J C, JUN H C, et al. Effects of microwave and water bath heating on the interactions between myofibrillar protein from beef and ketone flavour compounds[J]. International Journal of Food Science & Technology, 2019, 54(5): 1787-1793.
[13] MARCO C, MÓNICA F, ANTONIO M, et al. Effect of high pressure treatment on colour, microbial and chemical characteristics of dry cured loin[J]. Meat Science,2008,80(4):1174−1181. doi: 10.1016/j.meatsci.2008.05.011
[14] YANG K, WANG L M, GUO J J et al. Structural changes induced by direct current magnetic field improve water holding capacity of pork myofibrillar protein gels[J]. Food Chemistry,2021:345.
[15] ZHANG C, LIU H T, XIA X F, et al. Effect of ultrasound-assisted immersion thawing on emulsifying and gelling properties of chicken myofibrillar protein[J]. LWT,2021,142(3):111016.
[16] ZHU M M, ZHANG J, PENG Z Y, et al. Fluctuated low temperature combined with high-humidity thawing to retain the physicochemical properties and structure of myofibrillar proteins from porcine longissimus dorsi[J]. LWT,2021,142:111001.
[17] SHI H B, ZHANG X X, CHEN X, et al. How ultrasound combined with potassium alginate marination tenderizes old chicken breast meat: possible mechanisms from tissue to protein[J]. Food Chemistry,2020:328.
[18] KUN Y, YUAN H Z, GUO J J, et al. Low frequency magnetic field plus high pH promote the quality of pork myofibrillar protein gel: a novel study combined with low field NMR and raman spectroscopy[J]. Food Chemistry,2020:326.
[19] 杨慧娟. 超高压处理改善低脂低盐猪肉糜制品乳化凝胶特性研究[D]. 南京: 南京农业大学, 2017. YANG H J. Ultra high pressure treatment to improve the emulsifying properties of low fat and low salt pork products [D]. Nanjing: Nanjing Agricultural University, 2017.
[20] XIONG Y L, PARK D K, OOIZUMI T. Variation in the cross-linking pattern of porcine myofibrillar protein exposed to three oxidative environments[J]. Journal of Agricultural and Food Chemistry,2009,57(1):153−159. doi: 10.1021/jf8024453
[21] 韦婕妤. 热加工方式对羊肉制品蛋白质氧化及体外模拟消化性的影响研究[D]. 成都: 西南民族大学, 2019. WEI J Y. Effect of heat treatment on protein oxidation and in vitro digestibility of mutton products [D]. Chengdu: Southwest University For nationalities, 2019.
[22] 刘海梅. 鲢鱼糜凝胶及形成机理的研究[D]. 武汉: 华中农业大学, 2007. LIU H M. Study on the gel and formation mechanism of silver carp surimi [D]. Wuhan: Huazhong Agricultural University, 2007.
[23] 万红兵, 李海鹏, 雷元华, 等. 烹饪熟度对肌原纤维蛋白结构特性和氧化特性的影响[J]. 食品科学,2021,42(13):17−25. [WAN H B, LI H P, LEI Y H, et al. Effects of cooking maturity on structural and oxidative properties of myofibrillar protein[J]. Food Science,2021,42(13):17−25. doi: 10.7506/spkx1002-6630-20200717-229 [24] 游佳伟. 不同部位牛肉烤制加工适宜性研究[D]. 锦州: 渤海大学, 2020. YOU J W. Study on the processing suitability of different parts of beef [D]. Jinzhou: Bohai University, 2020.
[25] ILHAM C, PHILIPPE G, VÉRONIQUE S L. Technical note: a simplified procedure for myofibril hydrophobicity determination[J]. Meat Science,2006,74(4):681−683. doi: 10.1016/j.meatsci.2006.05.019
[26] WANG Z F, HE Z F, ZHANG D, et al. The effect of linalool, limonene and sabinene on the thermal stability and structure of rabbit meat myofibrillar protein under malondialdehyde-induced oxidative stress[J]. LWT,2021:148.
[27] Kang Z L, Zhang X H, Li X, et al. The effects of sodium chloride on proteins aggregation, conformation and gel properties of pork myofibrillar protein running head: Relationship aggregation, conformation and gel properties[J]. Journal of Food Science and Technology,2021,58(6):1−7.
[28] NIU Y H, CHEN J H, FAN Y L, et al. Effect of flavonoids from Lycium barbarum leaves on the oxidation of myofibrillar proteins in minced mutton during chilled storage[J]. Journal of Food Science, 2021, 86(5): 1766-1777.
[29] XIAO H M, WU Y F, LIU J, et al. Potential mechanism of different gelation properties of white and red muscle fibre from crocodile (Crocodylus siamensis) meat: Study of myofibrillar protein[J]. LWT,2021:143.
[30] QIAN J, WANG C, ZHUANG H, et al. Evaluation of meat-quality and myofibrillar protein of chicken drumsticks treated with plasma-activated lactic acid as a novel sanitizer[J]. LWT,2021:138.
[31] ZHANG L T, LI Q, SHI J, et al. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: effects of different washing solutions[J]. Food Hydrocolloids,2018,75:116−124. doi: 10.1016/j.foodhyd.2017.09.007
[32] 蔡梦洁. 恒功率微波处理牛肉肌原纤维蛋白对风味物质吸附能力研究[D]. 广州: 华南理工大学, 2019. CAI M J. Adsorption capacity of beef myofibrillar protein treated by constant power microwave on flavor compounds [D]. Guangzhou: South China University of technology, 2019.
[33] 孙佳悦, 钱方, 姜淑娟, 等. 基于红外光谱分析热处理对牛乳蛋白质二级结构的影响[J]. 食品科学,2017,38(23):82−86. [SUN J Y, QIAN F, JIANG S J, et al. Effect of heat treatment on secondary structure of milk protein based on infrared spectroscopy[J]. Food Science,2017,38(23):82−86. doi: 10.7506/spkx1002-6630-201723014 -
期刊类型引用(1)
1. 孙晓飞,万超,宋大贺,贾赟. 4种鳕鱼多重PCR检测方法建立. 现代食品科技. 2021(08): 302-307+349 .  百度学术
百度学术
其他类型引用(3)








 下载:
下载:
 下载:
下载:



