Research Progress on the Relationship between Short-chain Fatty Acids Metabolized by Intestinal Flora and Depression
-
摘要: 随着生物医学的快速发展,系列研究表明抑郁症与肠道微生物间存在密切联系,抑郁症患者通常伴随着肠道菌群种类、相对丰度及其代谢产物的改变。肠道菌群代谢产物短链脂肪酸(short-chain fatty acids,SCFA)是联系宿主和肠道菌群的重要中介物质,具有生物学效应,对宿主的生理功能产生影响。近年来,基于短链脂肪酸防治抑郁症的研究一直是该领域的研究热点,短链脂肪酸在肠道中的含量变化影响着抑郁症的发生发展。本文主要综述了SCFA对抑郁症的可能作用机制(调节下丘脑-垂体-肾上腺轴、影响色氨酸代谢以及减轻炎症反应)和外源干预抑郁症治疗的可能途径(食用益生菌、益生元和粪便菌群移植),以期为开发新型抑郁症治疗药物提供理论参考。
-
关键词:
- 抑郁症 /
- 肠道菌群代谢产物 /
- 短链脂肪酸 /
- 下丘脑-垂体-肾上腺轴 /
- 色氨酸
Abstract: With the rapid development of biomedicine, a series of studies have shown that there is a close relationship between depression and intestinal microorganisms. Patients with depression are usually accompanied by changes in the species, relative abundance and metabolites of intestinal microflora. Short-chain fatty acids, the metabolites of intestinal flora, are important mediators that connect the host and intestinal flora. They have biological effects and affect the physiological function of the host. In recent years, the prevention and treatment of depression based on short-chain fatty acids has been a hot topic in this field. The content of short-chain fatty acids in the intestinal tract affects the occurrence and development of depression. This article mainly reviews the possible mechanisms of SCFA on depression (regulating hypothalamus-pituitary-adrenal axis, affecting tryptophan metabolism and reducing inflammation) and the possible ways of exogenous intervention in the treatment of depression (edible probiotics, probiotics and fecal flora transplantation), in order to provide theoretical reference for the development of new depression treatment drugs. -
抑郁症是世界范围内的常见精神疾病,以显著而持久的思维缓慢、兴趣减退、情绪低落、消极悲观、躯体症状以及认知功能障碍为主要特征,严重者甚至产生自杀倾向,不仅给患者个人带来痛苦,还给家庭和社会造成沉重负担。抑郁症病因较为复杂,目前尚未完全阐明,一系列研究表明,它可能涉及神经生化、社会、遗传和心理等多种影响因素[1]。
数百万亿的肠道细菌居住在人体肠道中,共同构成肠道稳态系统,肠道菌群不仅与人体胃肠道健康密切相关,同时亦会影响中枢神经系统的生长发育和功能,因此,肠道菌群也常被称为“第二大脑”,在生理和病理条件下都可能对大脑的发育产生重大影响[2]。肠道菌群可通过自身及代谢产物对宿主机体产生影响,宿主机体也可通过神经、内分泌和免疫等调节肠道菌群,以维持肠道微生态的平衡,基于动物的诸多实验研究已证实微生物与其他精神疾病存在相互作用,这种肠道菌群与大脑之间的相互作用,常称为“微生物-肠-脑”(“Microbiota-Gut-Brain”,MGB)轴。而短链脂肪酸(Short-chain fatty acids,SCFA)是肠道菌群代谢产物,可以通过G蛋白偶联受体发挥中枢作用(如情绪状态和认知),是联系MGB轴的重要中介物质,可以直接或间接地参与MGB轴的功能性调节[3]。多项研究表明,食品的功能性成分如功能性多糖膳食纤维[4]、人体必需氨基酸色氨酸[5]以及微生态调节剂益生菌和益生元[6]等都会影响机体肠道菌群及其代谢产物,促进乳杆菌和双歧杆菌等有益菌丰度增加,并增加机体肠道内SCFA(如乙酸、丙酸和丁酸)的含量。研究发现,SCFA含量与抑郁症的病理机制密切相关[7]。因此,本文将通过重点阐述SCFA在抑郁症中的可能作用机制以及外源干预抑郁症治疗的可能途径,为基于肠道菌群为靶点治疗抑郁症提供新的思路。
1. SCFA
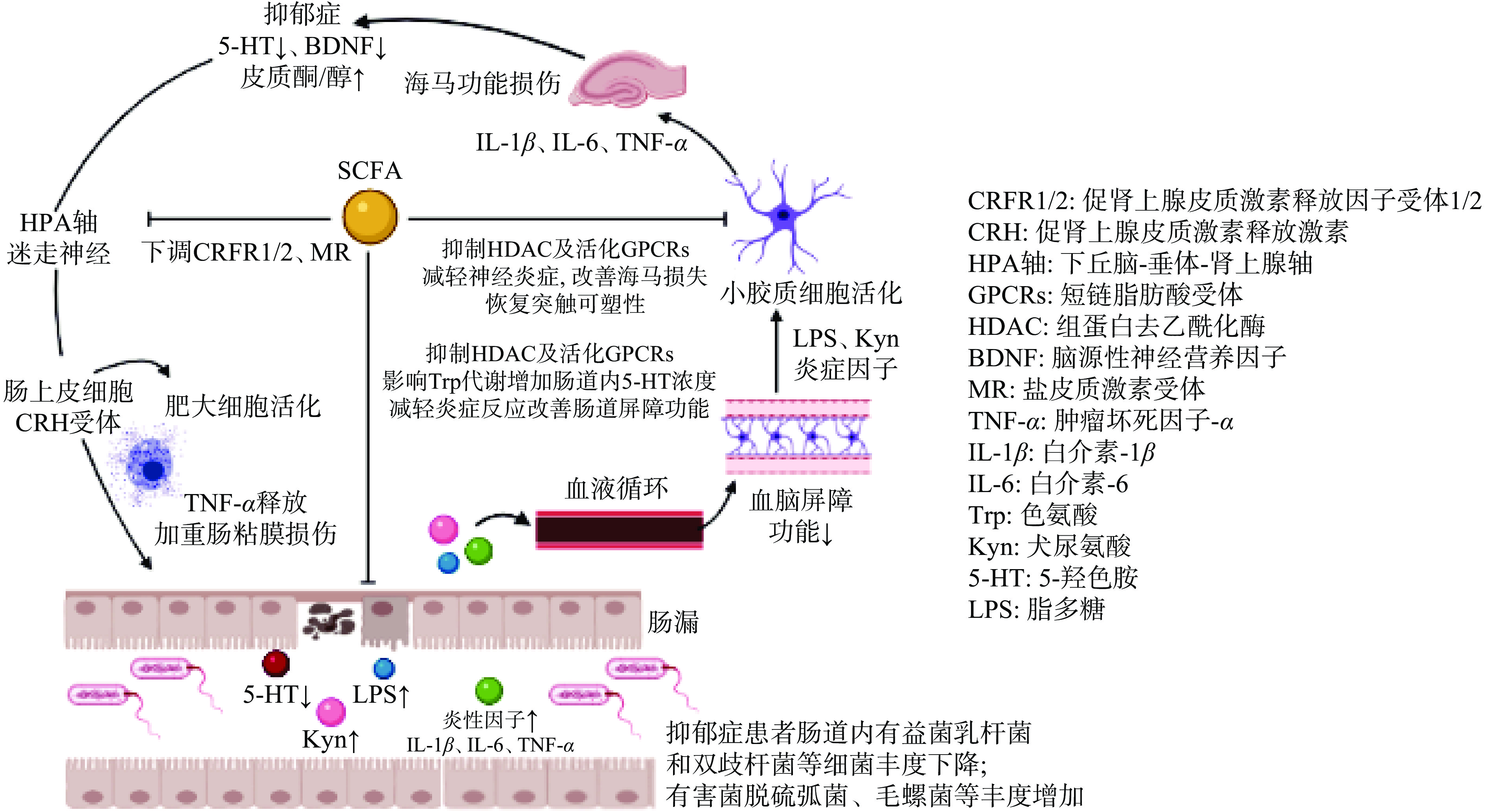
肠道菌群是位于人体胃肠道中复杂且庞大的细菌群落,研究表明抑郁症与肠道菌群组成的改变有关,慢性持续性压力诱导的动物抑郁行为通常伴随着肠道微生物多样性和相对丰度的改变[8]。对重度抑郁症(Major depressive disorder,MDD)患者和健康对照组的肠道菌群进行比较,以及基于抑郁动物模型的肠道菌群研究均表明,抑郁动物肠道菌群相对丰度发生了显著变化,如在接受抑郁粪便菌群移植(Fecal microbiota transplantation,FMT)的大鼠中双歧杆菌和乳杆菌等细菌的相对丰度降低,脱硫弧菌和毛螺菌等细菌相对丰度增加[9]。肠道菌群代谢产物SCFA是膳食纤维等耐消化物质在宿主体内经肠道微生物厌氧发酵的主要代谢产物[4],也可来自有机酸和氨基酸代谢[10],其主要产生部位在结肠,部分在肝脏,产生SCFA的菌主要包括厌氧类杆菌、真细菌、乳杆菌、链球菌和双歧杆菌等[11],通常在人体大肠中总浓度可达到50~200 mmol/L[12]。SCFA是弱酸,所以在肠道内大部分以游离阴离子形式存在,可在肠黏膜被吸收利用,或者通过多种转运蛋白运输至门静脉再由血液循环运输至其他器官,并可穿过血脑屏障发挥生理作用[13]。其中乙酸、丙酸和丁酸是SCFA中最重要且具有生物学效应的代表,比例约为 3:1:1[14],它们的来源、分布以及对宿主生理的潜在影响存在差异。乙酸被肠道上皮组织摄取后,在脂肪、肝脏和肌肉等组织中代谢并发挥调节作用,同时可为外周组织提供能量[15],且血浆中的乙酸盐可能是耐力运动中重要的能量底物[16],并有研究发现,乙酸可影响与抑郁症密切相关的中枢神经递质5-羟色胺(5-Hydrotryptamine,5-HT)的表达[17]。丙酸可增加肠源性调节性T细胞的量,还可以通过增加髓鞘再生而对中枢神经系统产生积极影响[18],研究显示,短期直肠内给予丙酸盐可以改善慢性不可预知温和应激(CUMS)模型大鼠的抑郁症状[19]。丁酸是结肠细胞的主要能量底物,它刺激结肠中钠和水的吸收,并对肠道细胞具有营养作用[20],研究显示,丁酸可增加中枢神经递质5-HT浓度,促进脑源性神经营养因子(BDNF)表达,同时可显著改善CUMS模型小鼠的抑郁样行为[21]。在一项对人体试验的研究中,通过气相色谱法测量了抑郁症患者与健康受试者粪便中SCFA的含量,发现抑郁症患者粪便中SCFA(如乙酸、丙酸和丁酸)含量相对偏低[7]。且动物试验的研究也表明抑郁组小鼠粪便中SCFA含量与正常对照组相比明显降低[22]。
2. SCFA影响抑郁症的可能机制
2.1 调节下丘脑-垂体-肾上腺轴
下丘脑-垂体-肾上腺(HPA)轴是神经内分泌系统的重要组成部分,其所释放的激素(如糖皮质激素类)可以激活交感神经系统,从而对行为的发育和调节产生作用,HPA轴影响去甲肾上腺素(NA/NE)的合成与释放,进而影响人体神经精神活动[23]。研究表明,肠道菌群可以影响HPA轴和行为的发育和调节[24],HPA轴与多种情绪和认知障碍的病理生理有关,其调节异常通常影响着抑郁症的发生和发展[25]。在对患有非精神病性重度抑郁症(NPMD)、精神病性重度抑郁症(PMD)和健康对照(HC)的患者HPA轴进行研究调查的结果显示,在所有受试者中,认知能力与较高的皮质醇呈负相关,并且PMD患者的皮质醇比NPMD和HC高[26],因此,较高水平的抑郁症和精神病可能表现出HPA轴过度活跃并随后出现皮质醇亢进。VAN等 [27]研究显示,向遭受心理压力的小鼠施用SCFA,下调了与HPA轴密切相关的促肾上腺皮质激素释放因子受体1(CRFR1)和CRFR2基因的表达;对小鼠下丘脑基因表达分析的结果显示,SCFA还下调了盐皮质激素受体(MR)基因的表达(如图1)。结果表明,SCFA可以减少编码HPA轴中涉及的蛋白质基因的表达从而减弱HPA轴响应。说明SCFA可能通过对HPA轴的作用从而对抑郁症产生重要影响。
2.2 影响色氨酸代谢
色氨酸(Tryptophan,Trp)是人体必需氨基酸,参与5-HT的生物合成,5-HT又名血清素,作为自体活性物质,大部分由肠嗜铬细胞合成并释放[28],仅少量的5-HT作为中枢神经递质,但它是维持大脑正常活动不可或缺的关键元素。多年来,关于抑郁症病理生理学的研究主要集中在5-HT和5-羟色胺能神经传递上,并认为抑郁症患者常伴随着大脑5-HT水平的低下[29]。在生理条件下,外周5-HT难以穿过血脑屏障,但是,外周Trp和5-HT的直接前体物质5-羟色氨酸(5-HTP)可以少部分的通过氨基酸转运体穿过血脑屏障,因此间接调节大脑5-HT的产生和功能,进而影响抑郁症的发生发展[30]。有研究表明用富含Trp的饮食补充可以对情绪和认知产生积极影响[31]。REIGSTAD等[5]在体外肠嗜铬细胞人体模型的研究中发现,肠道菌群产生的SCFA(乙酸和丁酸)以浓度依赖的方式显著影响了色氨酸羟化酶1(TPH1)的表达,而TPH1可催化Trp形成5-HTP,再经5-羟色氨酸脱羧酶催化成5-HT,因此SCFA可能通过对Trp代谢酶TPH1的作用影响中枢5-HT的形成,从而对中枢神经系统产生重要影响。另有研究利用GF小鼠(粪便SCFA含量可忽略)和TPH1基因敲除(KO)小鼠(肠粘膜5-HT缺乏)测试了SCFA在小鼠结肠运动中的作用,研究显示,丁酸可显著增加GF小鼠结肠迁移运动复合体(CMMC)频率和收缩百分比,然而在TPH1 KO小鼠中,丁酸不能增加CMMC频率和收缩百分比,这表明小鼠结肠运动可能需要肠粘膜5-HT的参与,且丁酸可改善异常性结肠运动[32](如图1)。这些结果表明来自人类和小鼠的肠道菌群可能通过SCFA对Trp代谢途径的作用影响中枢神经5-HT的生成,从而对抑郁症的发生发展产生重要影响。
2.3 减轻炎症反应
许多慢性炎症相关疾病通常伴随着肠道菌群失调,近年来,抑郁症与免疫反应之间的关系受到越来越多的关注,其中一个原因是MDD患者更常出现在患有慢性炎症性疾病(如类风湿性关节炎)的患者中[33]。炎症是与情感障碍相关的主要病理生理途径,抑郁症和炎症是相互促进、相互交织的两类疾病,对人体生理健康有着十分重要的影响[34]。有研究显示,MDD患者和健康对照组间促炎细胞因子存在差异,包括肿瘤坏死因子(TNF)、干扰素(IFNs)、白细胞介素(IL)、C反应蛋白(CRP)等,并指出较高的IL-6和CRP水平可预测后期抑郁症状的发展,同时抑郁症患者也可以观察到IL-6和CRP水平的变化[35],说明抑郁症与炎症间可能存在一种双向作用的联系。人体肠道菌群的营养缺乏会影响促炎性T细胞分化,并能促进器官特异性自身免疫[36]。SCFA通过维持肠道完整性和下调免疫炎症过程对肠道环境产生多种有益效果[37]。在基于转基因小鼠模型的研究中显示,肠道菌群代谢产物SCFA可通过激活肠道上皮细胞和免疫细胞中存在的G蛋白偶联受体(GPCRs)并抑制组蛋白去乙酰化酶(HDAC)从而在肠粘膜中发挥抗炎作用[38](如图1)。
SCFA作为GPCRs的激动剂,与之相关的G蛋白偶联受体主要有:G蛋白偶联受体41(GPR41/FFAR3)、G蛋白偶联受体43(GPR43/FFAR2)和G蛋白偶联受体109A(GPR109A/HCAR2)[39],这些受体介导SCFA通过丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)的信号通路,通过调节肠粘膜损伤和细胞凋亡等生理过程以及在人体肠道中对营养物质的吸收发挥重要作用。有研究发现GPCRs在动物肠道内的分泌细胞中高度表达,SCFA可通过GPCRs(如GPR41、GPR43和GPR109A)激活肠道上皮细胞控制免疫炎症反应[40]。说明SCFA可能会通过GPCRs介导的炎症反应对抑郁症产生影响。
SCFA(主要是丙酸和丁酸)是HDAC的有效抑制剂,选择性地抑制I类HDAC活性,可以直接通过细胞膜上的GPCRs进入细胞内抑制HDAC的表达,增加组蛋白的乙酰化,并通过下调促炎基因的表达从而抑制炎症过程的发生[41]。在一项与酒精戒断有关的抑郁症研究中,已通过几种行为学测试(如糖水偏好试验和强迫游泳试验等)证实在酒精戒断期间会诱发啮齿类动物的抑郁样行为,这种抑郁样行为可被辛二酰苯胺异羟肟酸(SAHA)(一种有效的HDAC抑制剂)所逆转。使用金免疫标记技术测量大鼠海马区组蛋白去乙酰化酶2(HDAC2)和组蛋白H3赖氨酸9乙酰化(H3K9ac)蛋白,结果显示,海马特定结构中的HDAC2增加和H3K9ac水平降低,并通过SAHA治疗可以恢复到正常水平[42]。说明SCFA可能通过HDAC途径调节炎症反应从而对抑郁症产生影响。
3. SCFA外源干预抑郁症的可能途径
SCFA广泛应用于胃肠道系统疾病,并通过在胃肠道屏障重构中的相互作用来发挥治疗效应。食用益生菌、益生元以及FMT都可影响肠道菌群及其代谢产物(如SCFA)的浓度,进而影响抑郁症的发生发展。
3.1 益生菌与益生元
益生菌是一种通过直接添加到人体肠道内,可改变肠道菌群相对丰度和多样性,具有调节肠道菌群平衡的外来菌。而益生元则是指一些不被宿主消化吸收却能够在人体内被肠道菌群利用、促进有益菌(包括益生菌)的生长和代谢,还能够抑制有害菌的繁殖,从而改善宿主健康的有机物质,包括抗性淀粉、非淀粉多糖和低聚糖等[43]。在一项随机临床试验研究中,将抑郁症患者随机分为益生菌组(长双歧杆菌和瑞士乳杆菌)、益生元组(低聚半乳糖)和安慰剂组(仅含赋形剂),结果显示,与安慰剂组相比,对患有MDD的受试者进行8周的益生菌治疗可显著降低贝克抑郁量表(BDI)评分,而益生元组BDI评分下降与安慰剂组相比并无显著差异[44]。LIU等[45]对34项对照临床试验进行了随机效应荟萃分析,益生菌的抗抑郁和抗焦虑作用得到了普遍支持,且用乳杆菌等益生菌单独试验对抑郁症的治疗作用没有多种益生菌合并(或益生菌合并应用益生元)治疗效果好。另有研究发现,益生菌和益生元可以通过调节肠道SCFA浓度来提高机体营养水平,如乳酸菌发酵产物乳酸可被某些细菌(如霍氏真杆菌)转化为不同的SCFA[6]。NAGPAL等[46]的研究也表明,补充乳酸菌和肠球菌可以改善机体营养不良,调节肠道菌群组成并增加SCFA产生(主要是丙酸和丁酸)。因此,食用相关的益生菌及益生元可能对治疗表现为肠道SCFA减少的有关疾病产生有益作用。然而,目前缺乏以临床抑郁症样本为特征的试验,因此,有必要对抑郁症样本进行额外的随机临床试验,以充分评估益生菌和益生元对抑郁症的治疗潜力。
3.2 粪便菌群移植
FMT是通过一系列操作处理、分离出健康机体的功能菌群,移植到患者肠腔中,重构肠菌稳态并促进胃肠动力恢复的方法。有研究显示将富含SCFA(主要是丁酸)的粪便菌群进行FMT可以调节宿主肠道菌群[47]。并且发现与源自健康对照个体的“健康微生物群”移植相比,直接用MDD患者衍生的“抑郁微生物群”对GF小鼠实施FMT可以导致小鼠的抑郁样行为[48]。HUANG等[49]研究也显示,FMT可以减轻抑郁相关症状。另有研究表明,FMT干预可增加小鼠肠道SCFA的产生,这主要与产SCFA菌的增加有关,如乳酸杆菌和丁酸梭菌含量的增加可以促进结肠中丁酸的生成,而丁酸可为结肠细胞提供能量并促进肠道屏障功能[50]。虽然诸多实验表明合理利用FMT对宿主机体是有益的,但目前对肠道菌群与宿主的相互作用并未完全阐明,粪便中可能存在一些危害人体生命健康的毒素或感染因子使FMT的应用产生很大的局限性,因此,需要更多的研究来降低FMT对机体的不良影响。
4. 总结与展望
肠道菌群代谢产物SCFA与抑郁症密切相关,SCFA可能通过HPA轴、色氨酸代谢以及炎症反应等多种作用机制参与抑郁症的发生与发展,外源干预(益生菌、益生元和FMT)可以调节肠道菌群相对丰度及其代谢产物SCFA的含量,进而改善抑郁症状,这可能为抑郁症患者提供一条新的治疗方法,并为未来预防与治疗抑郁症提供新思路、新靶点。但由于SCFA与宿主间相互作用以及抑郁症发病机制较为复杂,目前缺乏直接以SCFA为基础的有效治疗抑郁症的药物,因此,尚需加大以SCFA为基础的抑郁症样本进行随机临床试验,以充分评估SCFA对抑郁症的治疗潜力。
-
[1] ZHOU B, ZHU Z, RANSOM B R, et al. Oligodendrocyte lineage cells and depression[J]. Molecular Psychiatry,2020:1−15.
[2] SOCHOCKA M, DONSKOW-ŁYSONIEWSKA K, DINIZ B S, et al. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review[J]. Molecular Neurobiology,2019,56(3):1841−1851. doi: 10.1007/s12035-018-1188-4
[3] DINAN T G, CRYAN J F. The microbiome-gut-brain axis in health and disease[J]. Gastroenterology Clinics,2017,46(1):77−89. doi: 10.1016/j.gtc.2016.09.007
[4] KOH A, DE VADDER F, KOVATCHEVA-DATCHARY P, et al. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites[J]. Cell,2016,165(6):1332−1345. doi: 10.1016/j.cell.2016.05.041
[5] REIGSTAD C S, SALMONSON C E, RAINEY J F, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells[J]. The Faseb Journal,2015,29(4):1395−1403. doi: 10.1096/fj.14-259598
[6] MARKOWIAK-KOPEĆ P, ŚLIŻEWSKA K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome[J]. Nutrients,2020,12(4):1107. doi: 10.3390/nu12041107
[7] SKONIECZNA-ŻYDECKA K, GROCHANS E, MACIEJEWSKA D, et al. Faecal short chain fatty acids profile is changed in Polish depressive women[J]. Nutrients,2018,10(12):1939. doi: 10.3390/nu10121939
[8] KELLY J R, BORRE Y, O'BRIEN C, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat[J]. Journal of Psychiatric Research,2016,82:109−118. doi: 10.1016/j.jpsychires.2016.07.019
[9] WINTER G, HART R A, CHARLESWORTH R P G, et al. Gut microbiome and depression: What we know and what we need to know[J]. Reviews in the Neurosciences,2018,29(6):629−643. doi: 10.1515/revneuro-2017-0072
[10] NEIS E P J G, DEJONG C H C, RENSEN S S. The role of microbial amino acid metabolism in host metabolism[J]. Nutrients,2015,7(4):2930−2946. doi: 10.3390/nu7042930
[11] LAYDEN B T, ANGUEIRA A R, BRODSKY M, et al. Short chain fatty acids and their receptors: New metabolic targets[J]. Translational Research,2013,161(3):131−140. doi: 10.1016/j.trsl.2012.10.007
[12] LOUIS P, FLINT H J. Formation of propionate and butyrate by the human colonic microbiota[J]. Environmental Microbiology,2017,19(1):29−41. doi: 10.1111/1462-2920.13589
[13] FROST G, SLEETH M L, SAHURI-ARISOYLU M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism[J]. Nature Communications,2014,5(1):1−11.
[14] KAJI I, KARAKI S, KUWAHARA A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release[J]. Digestion,2014,89(1):31−36. doi: 10.1159/000356211
[15] GONZÁLEZ HERNÁNDEZ M A, CANFORA E E, JOCKEN J W E, et al. The short-chain fatty acid acetate in body weight control and insulin sensitivity[J]. Nutrients,2019,11(8):1943. doi: 10.3390/nu11081943
[16] OKAMOTO T, MORINO K, UGI S, et al. Microbiome potentiates endurance exercise through intestinal acetate production[J]. American Journal of Physiology-Endocrinology and Metabolism,2019,316(5):E956−E966. doi: 10.1152/ajpendo.00510.2018
[17] BHATTARAI Y, SCHMIDT B A, LINDEN D R, et al. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,2017,313(1):G80−G87. doi: 10.1152/ajpgi.00448.2016
[18] HIRSCHBERG S, GISEVIUS B, DUSCHA A, et al. Implications of diet and the gut microbiome in neuroinflammatory and neurodegenerative diseases[J]. International Journal of Molecular Sciences,2019,20(12):3109. doi: 10.3390/ijms20123109
[19] LI J, HOU L, WANG C, et al. Short term intrarectal administration of sodium propionate induces antidepressant-like effects in rats exposed to chronic unpredictable mild stress[J]. Frontiers in Psychiatry,2018,9:454. doi: 10.3389/fpsyt.2018.00454
[20] MANRIQUE V D, GONZÁLEZ S M E. Ácidos grasos de cadena corta (ácido butírico) y patologías intestinales[J]. Nutrición Hospitalaria,2017,34:58−61.
[21] SUN J, WANG F, HONG G, et al. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress[J]. Neuroscience Letters,2016,618:159−166. doi: 10.1016/j.neulet.2016.03.003
[22] WU M, TIAN T, MAO Q, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice[J]. Translational Psychiatry,2020,10(1):1−10. doi: 10.1038/s41398-019-0665-5
[23] SPENCER R L, DEAK T. A users guide to HPA axis research[J]. Physiology & Behavior,2017,178:43−65.
[24] SUDO N. Microbiome, HPA axis and production of endocrine hormones in the gut[M]//Microbial endocrinology: The microbiota-gut-brain axis in health and disease. Springer, New York, NY, 2014: 177−194.
[25] WOELFER M, KASTIES V, KAHLFUSS S, et al. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder[J]. Neuroscience,2019,403:93−110. doi: 10.1016/j.neuroscience.2018.03.034
[26] KELLER J, GOMEZ R, WILLIAMS G, et al. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition[J]. Molecular Psychiatry,2017,22(4):527−536. doi: 10.1038/mp.2016.120
[27] VAN DE WOUW M, BOEHME M, LYTE J M, et al. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations[J]. The Journal of physiology,2018,596(20):4923−4944. doi: 10.1113/JP276431
[28] ALCAINO C, KNUTSON K R, TREICHEL A J, et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release[J]. Proceedings of the National Academy of Sciences,2018,115(32):7632−7641. doi: 10.1073/pnas.1804938115
[29] DELL'OSSO L, CARMASSI C, MUCCI F, et al. Depression, serotonin and tryptophan[J]. Current Pharmaceutical Design,2016,22(8):949−954. doi: 10.2174/1381612822666151214104826
[30] AGUS A, PLANCHAIS J, SOKOL H. Gut microbiota regulation of tryptophan metabolism in health and disease[J]. Cell Host & Microbe,2018,23(6):716−724.
[31] STRASSER B, GOSTNER J M, FUCHS D. Mood, food, and cognition: Role of tryptophan and serotonin[J]. Current Opinion in Clinical Nutrition & Metabolic Care,2016,19(1):55−61.
[32] VINCENT A D, WANG X Y, PARSONS S P, et al. Abnormal absorptive colonic motor activity in germ-free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin[J]. American Journal of Physiology-Gastrointestinal and Liver Physiology,2018,315(5):G896−G907. doi: 10.1152/ajpgi.00237.2017
[33] JACOB L, ROCKEL T, KOSTEV K. Depression risk in patients with rheumatoid arthritis in the United Kingdom[J]. Rheumatology and Therapy,2017,4(1):195−200. doi: 10.1007/s40744-017-0058-2
[34] BAUER M E, TEIXEIRA A L. Inflammation in psychiatric disorders: What comes first?[J]. Annals of the New York Academy of Sciences,2019,1437(1):57−67. doi: 10.1111/nyas.13712
[35] BEUREL E, TOUPS M, NEMEROFF C B. The bidirectional relationship of depression and inflammation: Double trouble[J]. Neuron,2020,107(2):234−256. doi: 10.1016/j.neuron.2020.06.002
[36] WILCK N, MATUS M G, KEARNEY S M, et al. Salt-responsive gut commensal modulates TH 17 axis and disease[J]. Nature,2017,551(7682):585−589. doi: 10.1038/nature24628
[37] FARZI A, FRÖHLICH E E, HOLZER P. Gut microbiota and the neuroendocrine system[J]. Neurotherapeutics,2018,15(1):5−22. doi: 10.1007/s13311-017-0600-5
[38] VENEGAS D P, FUENTE M, LANDSKRON G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases[J]. Frontiers in Immunology,2019,10:277. doi: 10.3389/fimmu.2019.00277
[39] MIYAMOTO J, HASEGAWA S, KASUBUCHI M, et al. Nutritional signaling via free fatty acid receptors[J]. International Journal of Molecular Sciences,2016,17(4):450. doi: 10.3390/ijms17040450
[40] KIMURA I, ICHIMURA A, OHUE-KITANO R, et al. Free fatty acid receptors in health and disease[J]. Physiological Reviews,2020,100(1):171−210. doi: 10.1152/physrev.00041.2018
[41] SILVA L G, FERGUSON B S, AVILA A S, et al. Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells[J]. Journal of Animal Science,2018,96(12):5244−5252.
[42] CHEN W Y, ZHANG H, GATTA E, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) alleviates depression-like behavior and normalizes epigenetic changes in the hippocampus during ethanol withdrawal[J]. Alcohol,2019,78:79−87. doi: 10.1016/j.alcohol.2019.02.005
[43] TSAI Y L, LIN T L, CHANG C J, et al. Probiotics, prebiotics and amelioration of diseases[J]. Journal of Biomedical Science,2019,26(1):1−8. doi: 10.1186/s12929-018-0495-4
[44] KAZEMI A, NOORBALA A A, AZAM K, et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial[J]. Clinical Nutrition,2019,38(2):522−528. doi: 10.1016/j.clnu.2018.04.010
[45] LIU R T, WALSH R F L, SHEEHAN A E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials[J]. Neuroscience & Biobehavioral Reviews,2019,102:13−23.
[46] NAGPAL R, WANG S, AHMADI S, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome[J]. Scientific Reports,2018,8(1):1−15.
[47] CHEN R, XU Y, WU P, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota[J]. Pharmacological Research,2019,148:104403. doi: 10.1016/j.phrs.2019.104403
[48] ZHENG P, ZENG B, ZHOU C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism[J]. Molecular Psychiatry,2016,21(6):786−796. doi: 10.1038/mp.2016.44
[49] HUANG H L, CHEN H T, LUO Q L, et al. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota[J]. Journal of Digestive Diseases,2019,20(8):401−408. doi: 10.1111/1751-2980.12756
[50] ZHANG W, ZOU G, LI B, et al. Fecal microbiota transplantation (FMT) alleviates experimental colitis in mice by gut microbiota regulation[J]. Journal of Microbiology and Biotechnology,2020,30(8):1132−1141. doi: 10.4014/jmb.2002.02044
-
期刊类型引用(0)
其他类型引用(3)





 下载:
下载:
 下载:
下载:



