Determination of Iodine in Food by Gold Nanoparticles Modified Electrode Cyclic Voltammetry
-
摘要: 为寻求一种快速、简便、灵敏的食品中碘的测定方法,利用循环伏安法(CV)构建金纳米粒子修饰电极检测碘离子(I-)体系。利用甲烷氧化菌素(Mb)原位还原纳米金(Mb@AuNPs),电沉积法制备自组装修饰电极。通过透射电子显微镜对Mb@AuNPs表征,CV考察碘离子的电化学行为。确定碘离子检测的优化条件为:电沉积扫描速率0.11 V/s、扫描圈数30圈、缓冲溶液浓度0.05 mol/L、缓冲溶液pH6.5。氧化峰电流与I-浓度在0.01~10.00 μmol/L范围内有良好的线性关系,R2为0.9992,检出限为2.88 nmol/L(S/N),定量限为9.60 nmol/L,该方法检测不同食品中碘含量的加标回收率为96.22%~103.57%。结果表明该修饰电极对I-的测定具有良好的精密度、稳定性和重现性,以及较好的抗干扰能力,符合测定方法要求,可用于实际样品中碘的测定。Abstract: In order to find a rapid, simple and sensitive method for determination of iodine in food, a gold nanoparticle modified electrode was constructed by cyclic voltammetry (CV) to detect iodine ion (I-). In situ reduction of gold nanoparticles (Mb@AuNPs) using methanobactin (Mb), and the self-assembled modified electrode was preparaed by electrodeposition. Mb@AuNPs was characterized by transmission electron microscope (TEM), the electrochemical behavior of iodine ion (I-) was investigated by CV. The optimized conditions for iodine ion detection were as follows: Electrodeposition scanning rate 0.11 V/s, number of scanning cycles 30, buffer concentration 0.05 mol/L, buffer pH 6.5. There was a good linear relationship between oxidation peak current and I- concentration in the range of 0.01~10.00 μmol/L, R2 was 0.9992. The detection limit was 2.88 nmol/L (S/N), and the quantitation limit was 9.60 nmol/L. The recoveries of iodine content in different foods were 96.22%~103.57%. The results show that the modified electrode has good precision, stability, reproducibility and anti-interference ability for the determination of I-, which meets the requirements of the determination method and can be used for the determination of I- in practical samples.
-
Keywords:
- methanobactin (Mb) /
- gold nanoparticle (AuNPs) /
- cyclic voltammetry /
- electrode /
- iodine ion /
- self-assemble
-
碘是维持人体正常生命活动必要的微量元素之一,在甲状腺功能和形态的维持方面具有重要作用[1],人们通过加碘食盐或一些海产品、酱类食品、乳制品等含碘食品摄入碘[2],但是,人体对碘的摄入量无论过多还是过少都是有害的[3]。碘缺乏与碘过量都会引起甲状腺肿大等疾病[4]。因此,通过对食品中碘含量的测定,控制人体碘的摄入量对人体健康十分重要。
目前,碘的测定方法主要有氧化还原滴定法[5]、荧光光谱法[6]、高效液相色谱法 [7]、分光光度法[8]、电化学方法[9-10]等。由于I-能够引起铜纳米粒子的荧光猝灭,WANG等[11]利用聚二甲基二烯丙基氯化铵(PDDA)完成了超灵敏、无标记的I-检测,该方法的检出限为0.45 μmol/L。SONG[12]根据I−诱导CQDs/AuNPs聚集,导致颜色和吸光度的变化,基于CQDs/AuNPs复合物,建立了一种检出限为2.3 μmol/L的I−的比色检测方法。在食品检测领域中电化学一直是近些年来的研究热点[13-14]。QIN等[15]利用银纳米结构的高表面积和快速电子转移速率提高了电极的响应信号,采用循环伏安法进行I-的定量研究,线性范围为50 μmol/L~20.2 mmol/L。TRÉSOR等[9]研究了I-在复合电极上的电化学还原行为,研制了一种银基固体碳糊电极,用于碘的灵敏测定,检出限为3.7×10−9 mol/L。上述荧光和比色的方法存在着检测成本偏高、灵敏度较差等缺点,电化学方法具有工艺流程简单、检测成本低、检测速率快、灵敏度高等优点。此外,纳米材料,尤其是金属纳米材料由于具有高比表面积、电催化作用并能够加快电子传质速率而被广泛的应用于电极表面的修饰[16-17],也为电极检测提供更好地分析性能。虽然已经展现了一定的优越性,但在食品中碘的测定上仍然存在着电极修饰过程复杂,稳定性较差等不足。
为了探究灵敏度更高,操作更简便,成本低的I-检测方法,本研究利用甲烷氧化菌素(Methanobactin, Mb)原位还原纳米金(Mb@AuNPs)制备修饰电极用于食品中I-的测定。其中,Mb是由甲烷氧化菌分泌的小肽,并具有类过氧化物酶活性[18],因此可加速I-在电极表面的氧化还原反应。此外,Mb可作为还原剂与稳定剂将Au(III)还原成Au(0),形成纳米金(AuNPs)。该反应过程中,形成的Mb@AuNPs十分稳定。因此,采用电沉积的方法将Mb@AuNPs自组装修饰在金电极表面,进而构建一种电极组装简便、稳定性好、灵敏度高、检出限低的食品中碘的测定方法。
1. 材料与方法
1.1 材料与仪器
甲基弯菌M. trichosporium OB3b 由清华大学提供,本实验室4 ℃保存;氯金酸(HAuCl4)溶液 阿拉丁试剂有限公司;甲烷氧化菌素(Mb) 实验室发酵培养[19];铁氰化钾(K3[Fe(CN)6])、亚铁氰化钾(K4[Fe(CN)6])、磷酸氢二钠(Na2HPO4)、磷酸二氢钠(NaH2PO4)、碘化钾(KI)、氯化钾(KCl) 天津市天力化学试剂有限公司;中盐食盐、海天黄豆酱、飞鹤奶粉 均由超市购买;实验中所用试剂 均为分析纯。
Pt 213铂电极、R0302氯化银电极 天津艾达试剂公司;CHI660A型电化学工作站、φ=3 mm金盘电极 上海辰华仪器公司;JEM-2100透射电子显微镜 上海化科实验器材有限公司;PB-10 pH计 赛丽朵思公司;HNY-100B恒温培养振荡器 天津市欧诺仪器仪表有限公司;R-20旋转蒸发仪 上海一恒科学仪器有限公司。
1.2 实验方法
1.2.1 Mb@AuNPs的制备
将实验室培养和纯化的Mb[20]与HAuCl4溶液以1:3的比例混合,Mb浓度经铬天青法[21]测定为0.26 mmol/L,混合后根据刘丰源[22]的优化条件在60 ℃水浴加热2 h颜色变为蓝紫色取出,放置于4 ℃环境中备用。采用透射电子显微镜分析Mb@AuNPs的表观形貌特征。
1.2.2 自组装电极的制备与表征
根据文献[23]进行裸电极的制备,将抛光的裸电极置于Mb@AuNPs溶液中,以0.1 V/s的扫描速率,在−0.6~0.4 V电位窗口,CV扫描30圈,将上述电极置于电解液中,并通过循环伏安法[24]在−0.2~0.6 V电位窗口范围内对其进行电化学性能表征,获得Mb@AuNPs自组装修饰电极。
1.2.3 Mb@AuNPs自组装修饰电极检测I-的条件优化
1.2.3.1 电沉积圈数对I-检测的影响
以0.1 V/s的扫描速率CV扫描10、20、30、40、50圈将Mb@AuNPs修饰于裸金电极表面,制备Mb@AuNPs自组装修饰电极,将上述制备好的电极分别置于0.02 mol/L的碘化钾(KI)溶液中,在pH7.0的0.1 mol/L磷酸钠盐PBS缓冲液与KI溶液3:1体积比混合的溶液中,再以0.1 V/s的扫描速率在电位窗口为0~0.7 V的范围内CV扫描2圈,得到I-在修饰电极表面的响应信号,以峰电流为标准选择电沉积圈数。
1.2.3.2 缓冲溶液浓度对I-检测的影响
以上述选择的电沉积圈数修饰Mb@AuNPs电极,置于0.02 mol/L的碘化钾(KI)溶液分别于pH7.0的0.02、0.05、0.1、0.15、0.2 mol/L PBS溶液中进行I-的测定,选择较优的PBS浓度。
1.2.3.3 缓冲溶液pH对I-检测的影响
选择上述最优电沉积圈数以及缓冲液浓度,将修饰电极置于pH6.0、6.5、7.0、7.5、8.0的缓冲溶液中进行I-检测,选择较优的体系pH。
1.2.3.4 扫描速率对I-检测的影响
在上述选定的修饰电极与检测体系条件下,以0.04、0.06、0.08、0.10、0.12 V/s的扫描速率在电位窗口为0~0.7 V的范围内CV扫描。根据峰电流的变化,优化检测条件,判断反应类型。
1.2.4 I-的电化学检测
配制浓度分别为0.01、0.1、1、2、5、10 μmol/L的KI溶液,将Mb@AuNPs自组装修饰电极分别置于不同浓度I-与pH7.0的0.1 mol/L磷酸盐PBS缓冲液3:1混合的溶液中,利用CV法,在电位窗口为0~0.7 V,0.1 V/s扫速条件下,进行I−的检测。建立循环伏安扫描的氧化峰电流(A)与检测I−浓度的标准曲线;测定空白加样数据20次,计算出检出限(LOD)和定量限(LOQ)。
1.2.5 抗干扰实验
利用电流-时间I-t曲线的方法,在缓冲溶液中加入两次0.001 mol/L KI溶液,随后加入KI溶液100倍浓度的钙离子(Ca2+)、钾离子(K+)、锌离子(Zn2+)、硫酸根离子(SO42)−、葡萄糖、氯化钠(NaCl)、溴离子(Br−),观察I-t曲线电流变化,考察其对检测结果的影响,评价自组装修饰电极抗干扰性能。
1.2.6 重复性、稳定性与重现性实验
在I-检测体系中连续CV扫描 8次;修饰电极在20 d内在I-检测体系中CV扫描;平行制备8根相同的修饰电极依次对I-检测,根据扫描结果确定方法的精密度、稳定性与重现性。
1.2.7 加标回收实验
依据文献[25-26]对加碘盐进行预处理:将10.0 g加碘盐与0.10 g抗坏血酸于棕色容量瓶中沸水定容至100 mL,使加碘盐中的碘酸根(IO3−)还原为I-;对其他食品进行的预处理:参考文献[27-28]方法,将粉碎黄豆酱、奶粉取0.5 g置于坩埚中,依次加入1.0 mL 1 mol/L的氢氧化钾(KOH)溶液,0.5 mL 1 mol/L硫代硫酸钠溶液与10 g/L抗坏血酸溶液,马弗炉温度550 ℃灰化40 min,冷却,加水溶解、过滤备用。
选择加碘盐、黄豆酱、奶粉作为实际样品,采用标准加入法,考察Mb@AuNPs自组装修饰电极在实际样品检测中的检测性能,于3 mL经预处理的样品中加入20 mg/kg的KI标准溶液,计算方法的加标回收率。
1.3 数据处理
所有实验均重复3次,利用SPSS 17.0软件采用Duncan’s 新复极差检验法进行显著性差异分析,显著性水平P<0.05,并运用Origin 2017绘制数据图。
2. 结果与分析
2.1 Mb@AuNPs表征
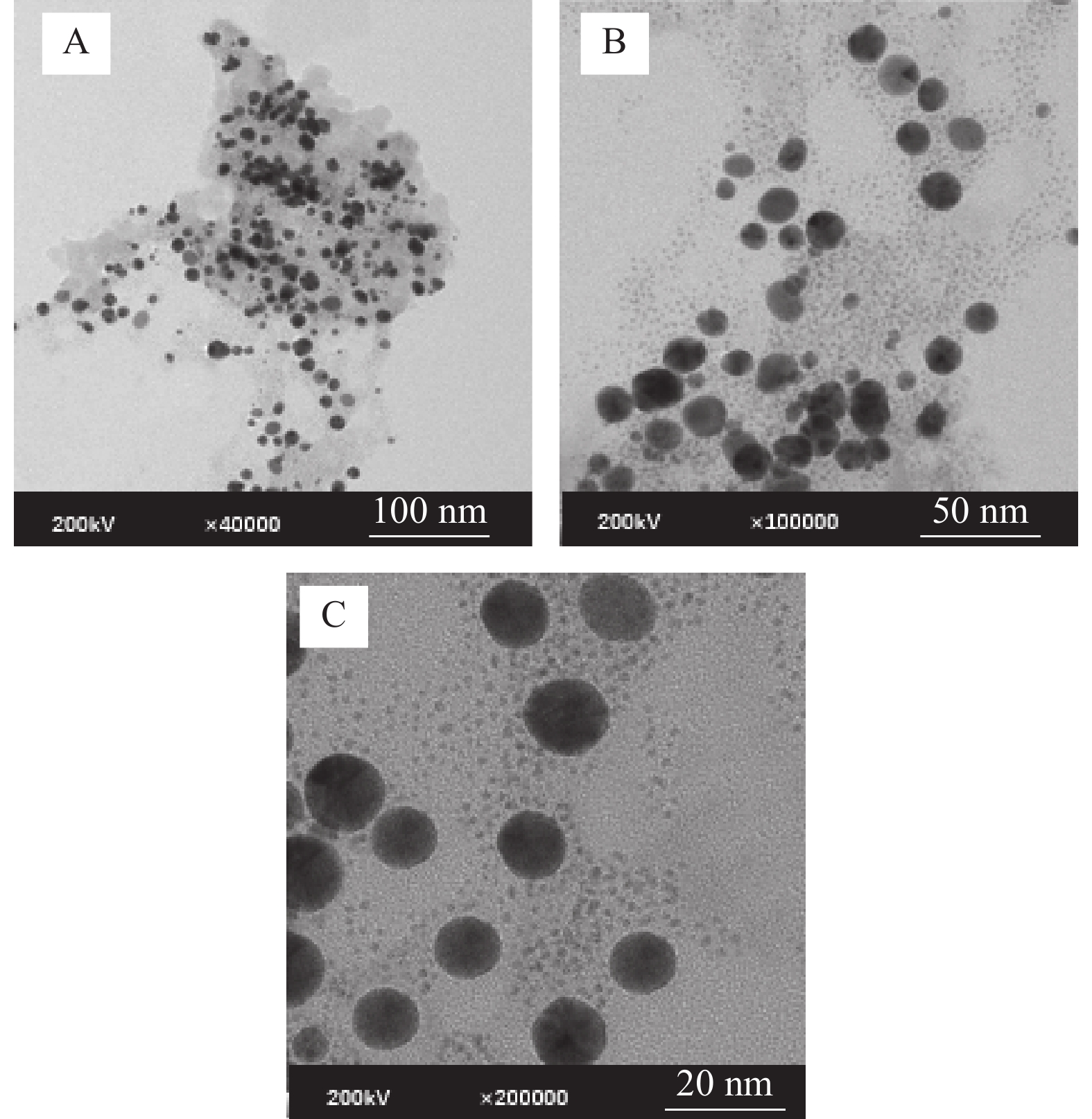
将制备好的待测样品滴加到带有碳膜的铜网上,自然冷却风干后利用透射电镜扫描,图1A~C为不同放大倍数下Mb原位还原AuNPs复合纳米粒子的透射电镜图谱,制备的Mb@AuNPs形状近似为圆球形,粒径多为10~15 nm,平均粒径为12.94 nm,分散性较好,Mb包覆在AuNPs表面,避免了AuNPs之间的聚集[29],使形成的Mb@AuNPs更稳定。
2.2 Mb@AuNPs自组装修饰电极表征
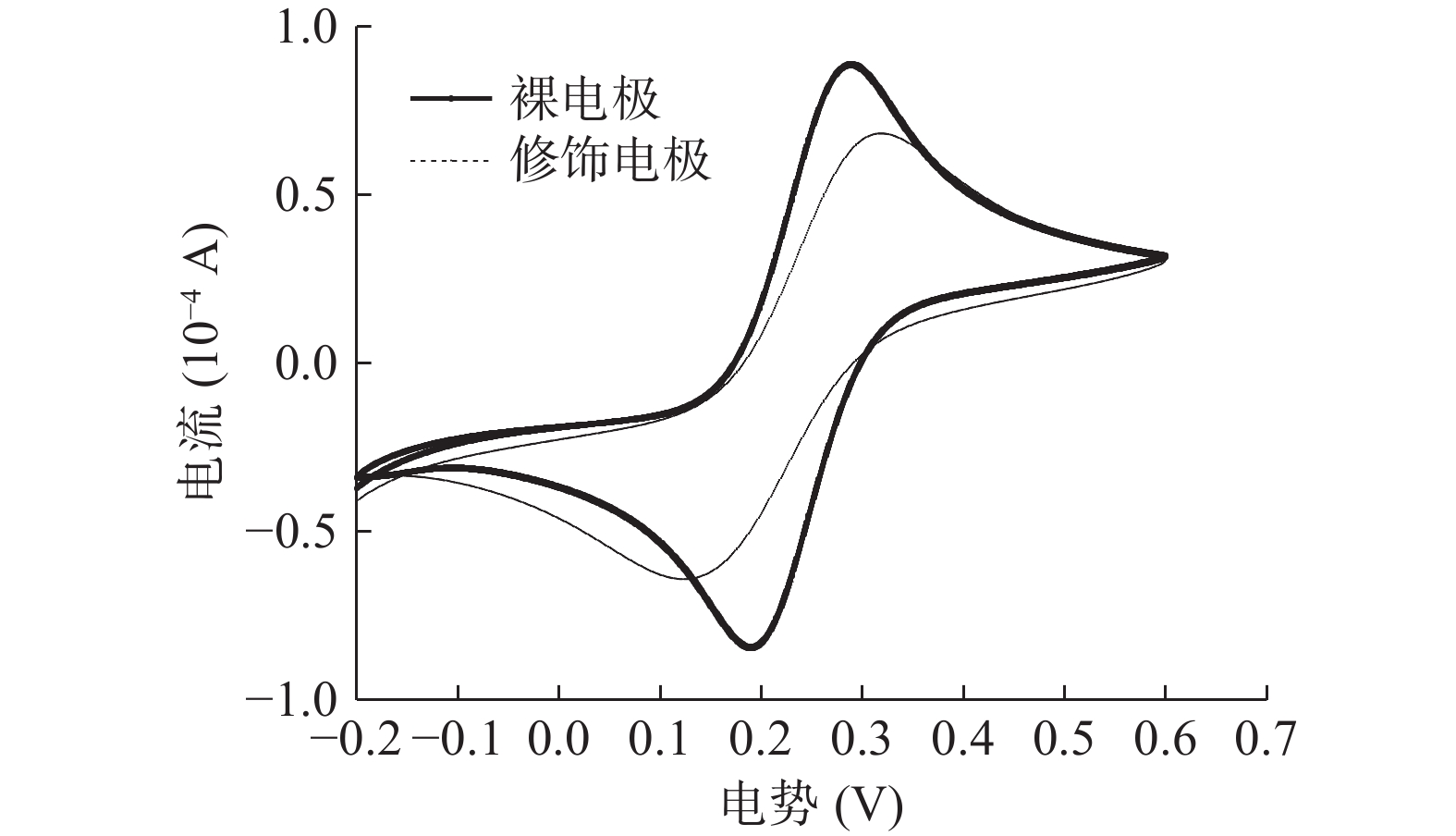
由图2可知,通过CV的方法对Mb@AuNPs自组装修饰电极进行表征,修饰后的电极与裸电极相比,氧化还原峰电流从0.888×10−4 A降低到0.634×10−4 A,说明Mb@AuNPs已成功修饰到了电极表面,这可能是由于Mb@AuNPs在电极表面形成了一层有序致密的自组装膜,Mb@AuNPs膜可阻碍电极表面与电解液间的电子传递,从而导致氧化还原峰电流的降低[30]。
2.3 Mb@AuNPs自组装修饰电极检测I−的优化条件
2.3.1 沉积圈数对修饰电极I-检测的影响
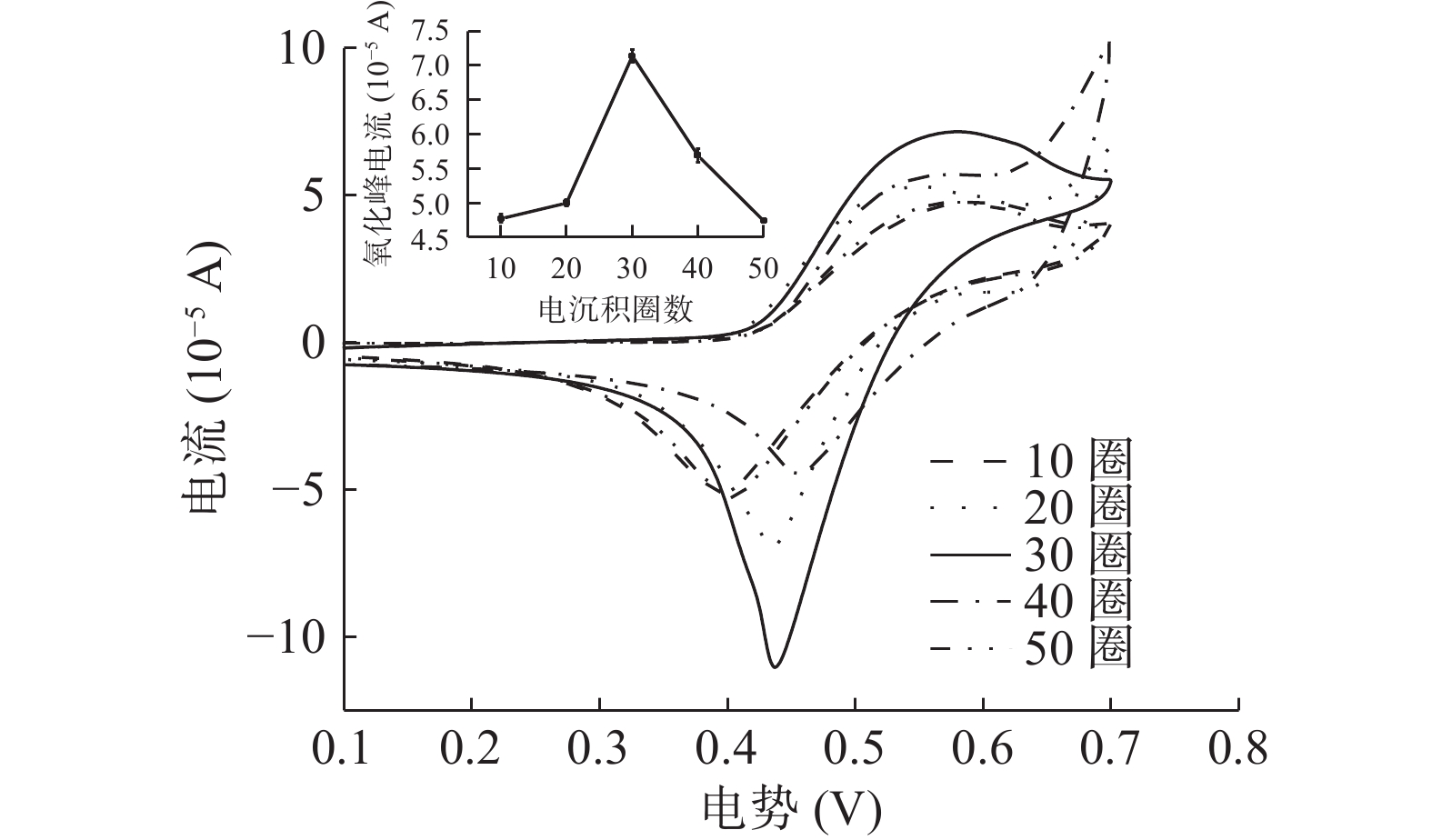
由图3可知,该CV图谱存在氧化还原峰电流,表明I−在电极表面的反应过程可逆。不同扫描圈数修饰电极检测I−的峰电流存在显著差异(P<0.05),扫描圈数10~50圈修饰电极检测I-氧化峰电流先增大后减小。扫描圈数10~30,氧化峰电流从4.77×10−5 A上升至7.14×10−5 A,扫描30圈后,电流响应下降,由7.14×10−5 A下降至4.74×10−5 A。随着扫描圈数的增加,Mb@AuNPs修饰到电极表面形成的膜更加致密,电子的阻碍作用增强,但是扫描圈数增加至30圈后,可能是由于沉积圈数的增加降低了电极的电化学活性表面积,这是由于大尺寸纳米粒子对电催化活性的负面影响所致[31]。因此,可以认为扫描圈数为30圈的修饰电极检测效果较好。
2.3.2 缓冲溶液PBS浓度对I-检测的影响
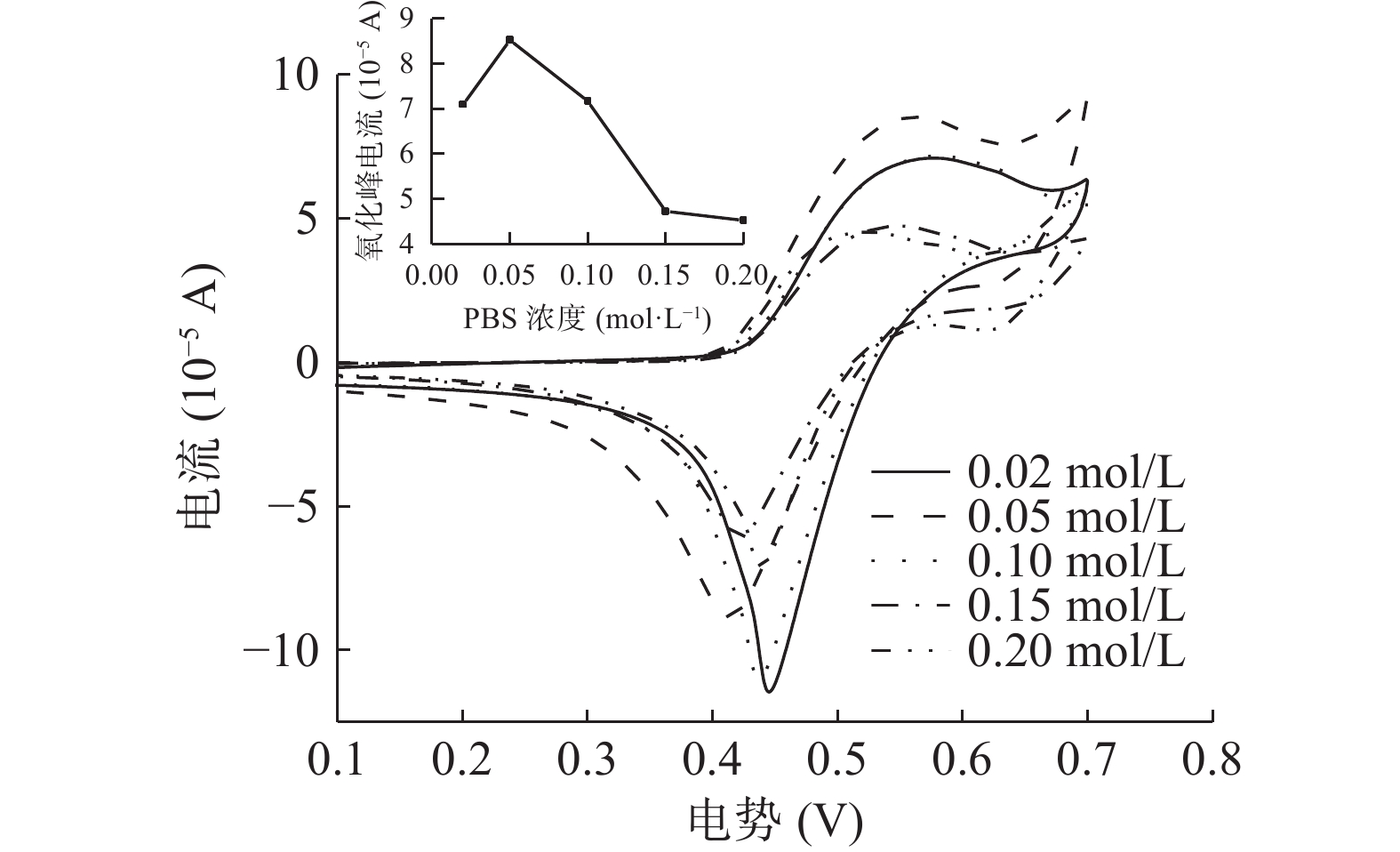
由图4可知,在检测体系中分别加入不同浓度的PBS缓冲溶液,PBS浓度为0.02~0.05 mol/L循环伏安曲线的氧化峰电流随PBS溶液浓度的增加而增大,从7.10×10−5 A上升到了8.44×10−5 A;PBS浓度为0.05~0.2 mol/L循环伏安曲线的氧化峰电流随PBS溶液浓度的增加而降低,从8.44×10−5 A降低到4.53×10−5 A;缓冲溶液PBS浓度为0.05 mol/L时,I−在修饰电极上呈现出了最优的电流响应。随着缓冲溶液浓度的升高缓冲容量会有一定程度的增大,进而促进电极表面的电子传递速率,增大氧化还原峰信号,但由于I−在电极表面的反应过程可逆,当PBS缓冲溶液的浓度超过一定的限度时,可能会对反应产生抑制作用而降低电流响应[32]。因此,可选择缓冲溶液浓度0.05 mol/L为检测体系的较优条件。
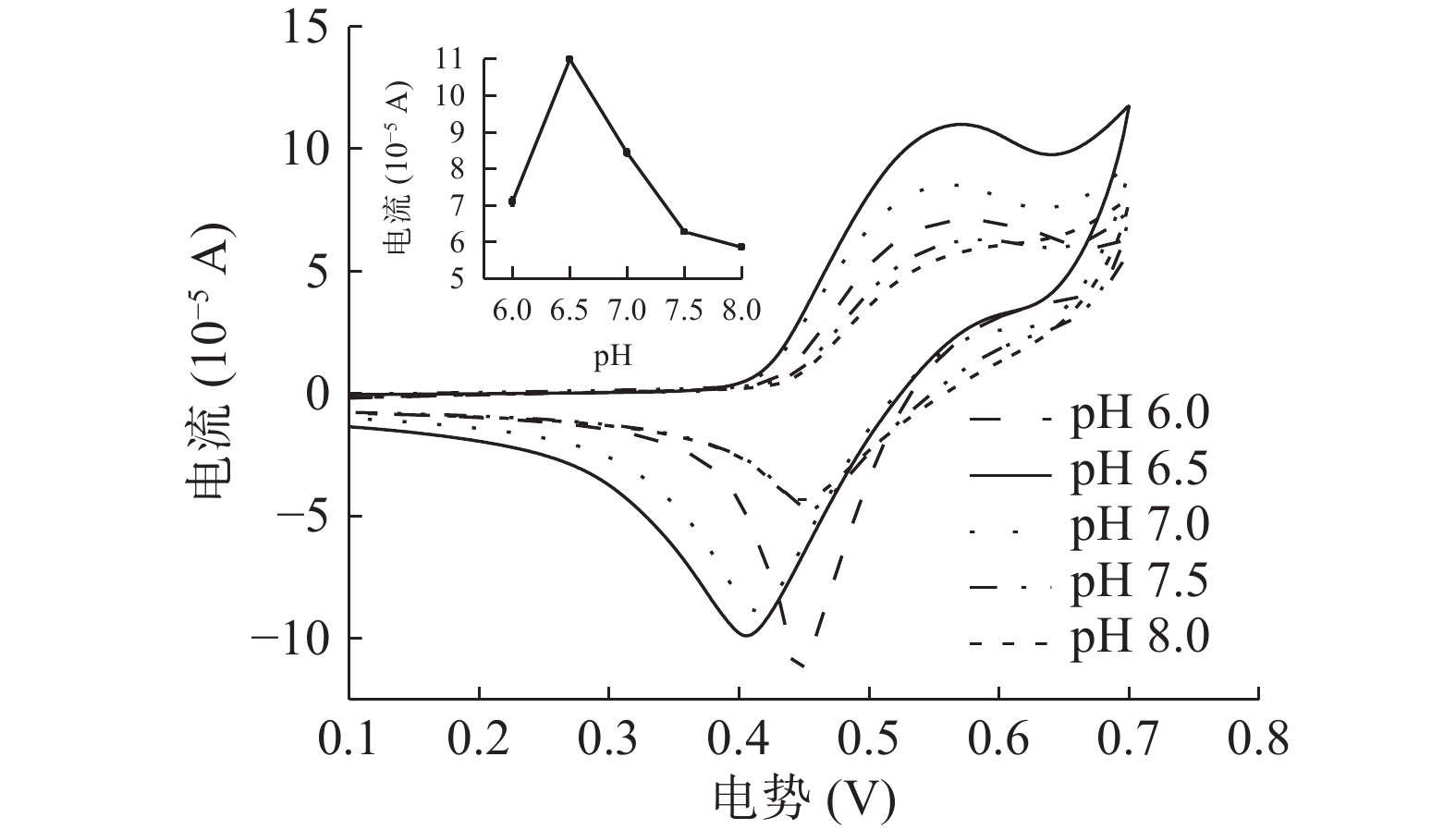
2.3.3 缓冲溶液pH对I-检测的影响
由图5可知,在检测体系中分别加入不同pH的PBS缓冲溶液。pH6.0~8.0的范围内,I−在修饰电极上的电信号响应先增大后减小,这表明修饰电极的电化学性质在很大程度上取决于检测体系的pH。如图5所示,氧化峰电流在pH6.0~6.5呈上升趋势,当pH为6.5时氧化峰电流值达到最大,并且当pH进一步加大时氧化峰电流减小。这可能是由于质子参与了I−在电极上的氧化还原反应[33],当pH>6.5时,由于缺少质子,导致I−在修饰电极上发生氧化反应的难度增加。因此,为了提高检测的灵敏度,可以选择pH6.5为检测体系缓冲溶液的较优条件。
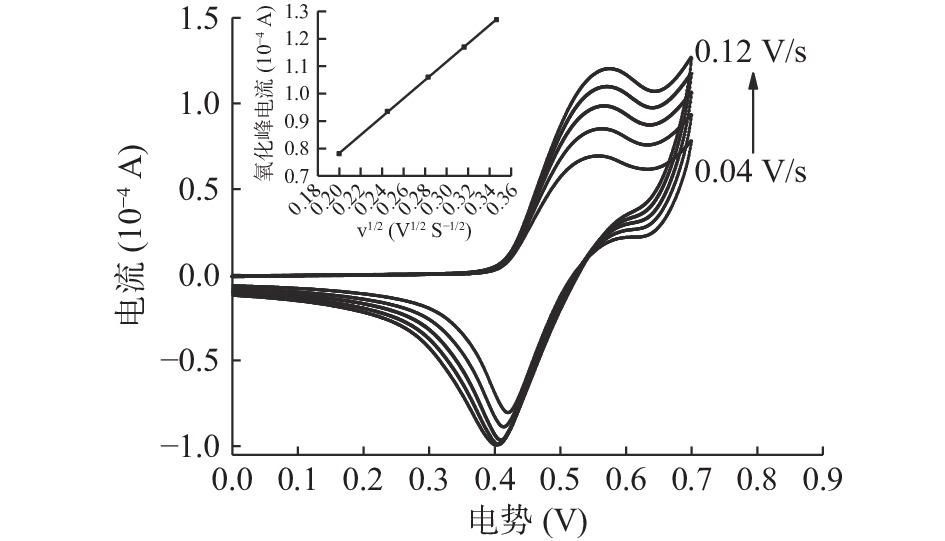
2.3.4 扫描速率对I-检测体系的影响
为了了解电极反应的控制步骤,利用CV研究了扫描速率对0.02 mol/L I−在修饰电极中催化氧化的影响,由图6可知,峰值电流Ip与扫描速率V之间的电位关系可以获得电化学信息,通过CV图谱可知,在0.04~0.12 V/s扫速范围内氧化峰电流与扫描速率成正比,在扫速增加的同时,电位增大,氧化峰电位右移。峰电流与扫描速率的平方根之间建立的线性方程为Ip=35.286v1/2−0.1369(V1/2·S−1/2)(R2=0.9996),线性关系良好,这些特征与JIGYASA等[34]的实验结果一致,表明电子在电极自组装膜表面的转移过程是控制扩散过程[33]。
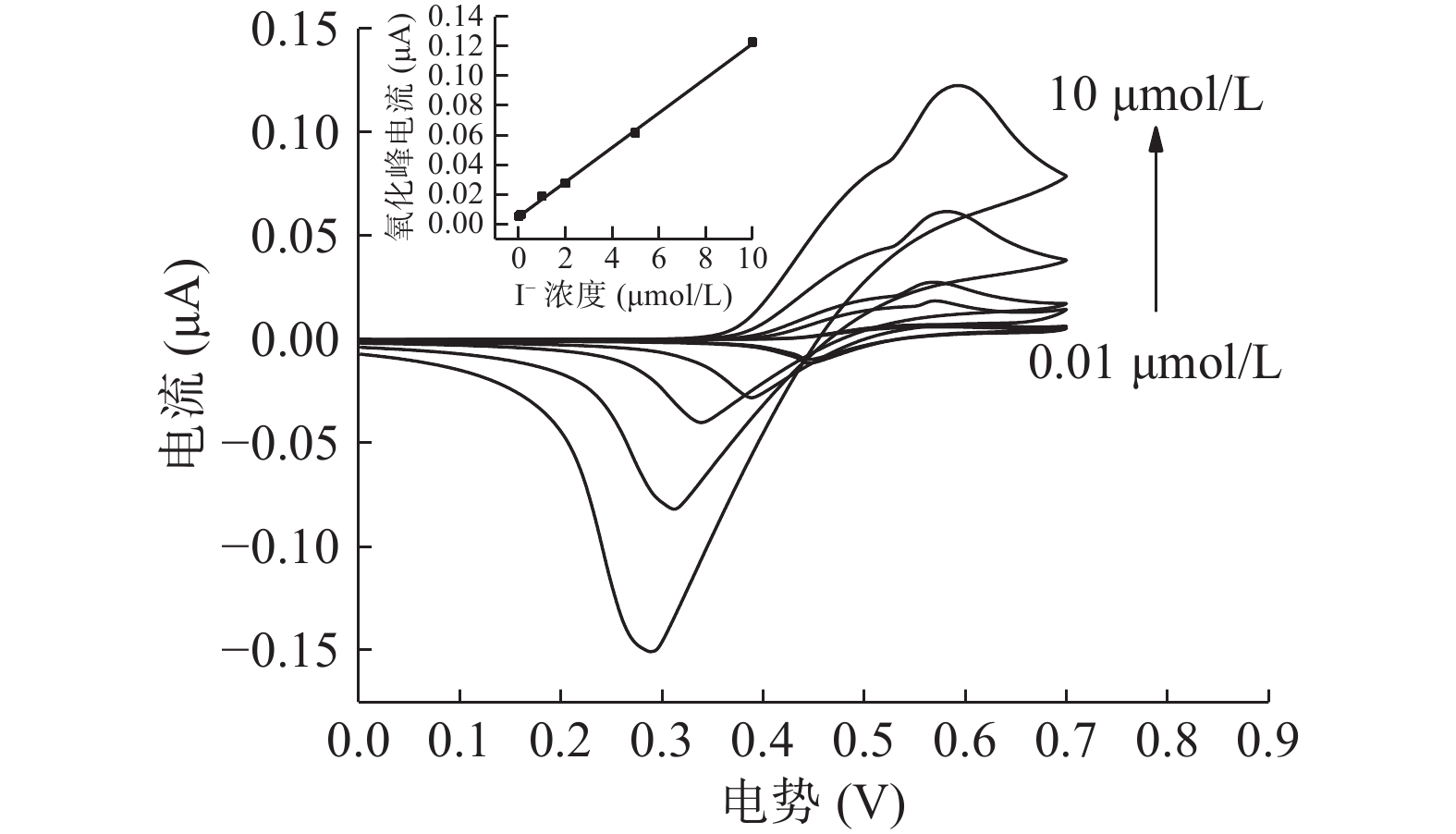
2.4 标准曲线与检出限
如图7所示,随着I-浓度的升高,氧化还原峰电流出现了明显的升高, I-浓度C与峰电流Ip之间存在明显的线性关系,利用Origin软件拟合可得到线性回归方程为Ip = 11.65C+0.00514,线性范围为0.01~10 μmol/L,决定系数 R2=0.9992,说明I-浓度与峰电流的线性关系良好。
计算可得该方法的检出限为2.88 nmol/L,定量限可达9.60 nmol/L,低于国标方法[35]与相关研究结果(如表1),且电极构建较简便。
表 1 Mb@AuNPs自组装修饰电极与其它电化学检测方法比较Table 1. Mb@AuNPs comparison of self-assembled decorated electrode with other electrochemical detection methods2.5 抗干扰性能
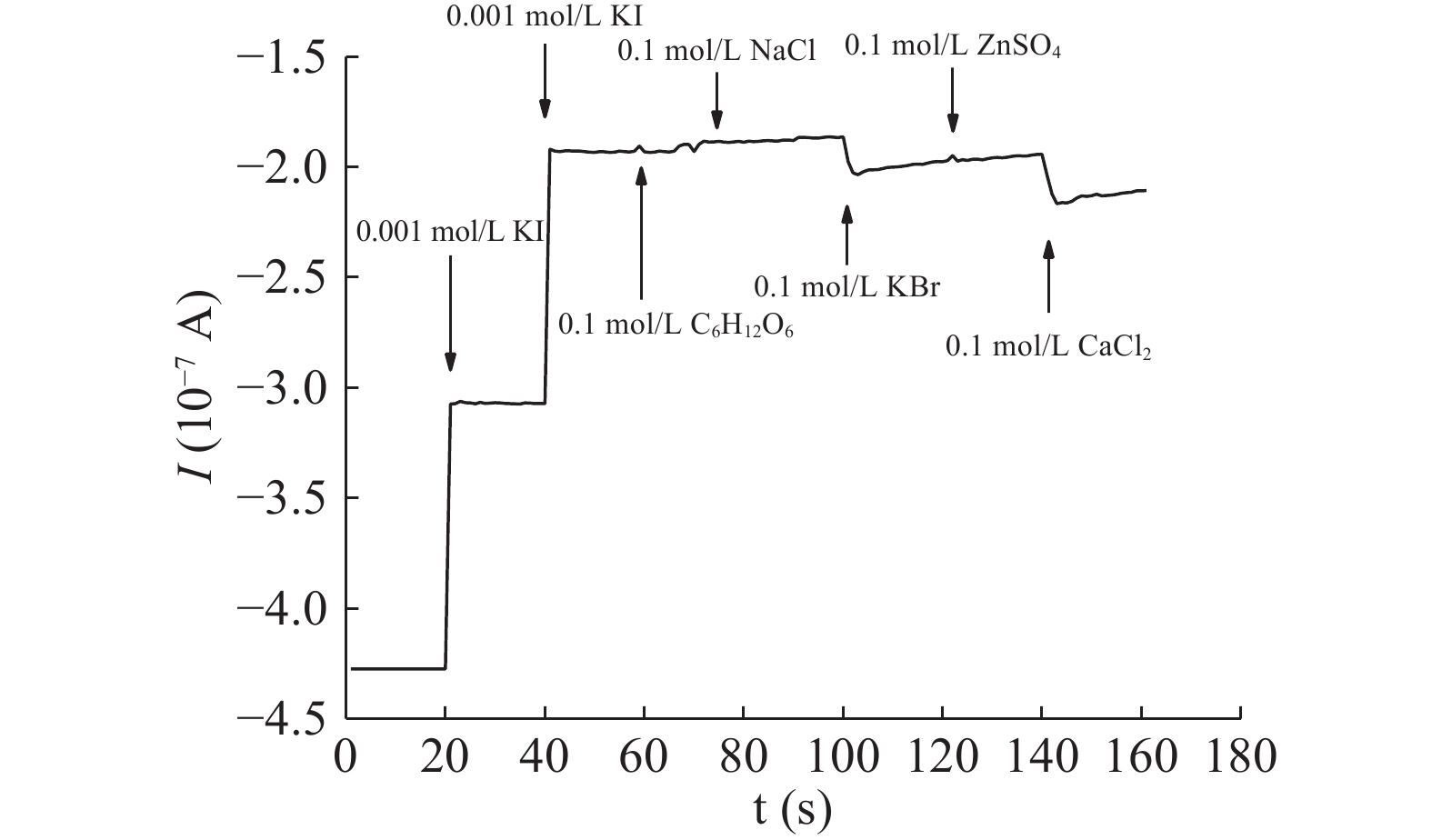
为探究该修饰电极检测I-的选择性,在检测体系中分别加入100倍浓度的干扰离子,研究其对检测结果的影响,结果如图8所示,在0.001 mol/L的KI溶液中加入100倍浓度的K+、Zn2+、SO42−、葡萄糖、NaCl等的检测结果与未加入相比,添加干扰物之后I-t曲线峰电流无明显变化,Ca2+、Br−对修饰电极有轻微的电流响应,但是对检测结果的无明显影响,可证明该修饰电极对Ca2+、K+、Zn2+、SO42−、葡萄糖、NaCl与Br−的催化性能,即上述物质对I-的检测结果影响较小,表明该电极在I-的检测上具有较强的抗干扰能力。
2.6 重复性、稳定性与重现性
用循环伏安法研究了Mb@AuNPs电极的稳定性、重现性和重复性。在1 mmol/L I-溶液中使用相同的修饰电极进行8次重复试验,氧化峰电流的相对标准偏差为2.06%,可证明该修饰电极的重复性良好。通过在20 d内测试该电极对I-的电化学响应,测定电极的稳定性,制备的电极电流响应约为初始电极响应的81%。对8根平行制备的电极进行分析,测定的电流响应标准偏差(RSD)为2.53%,可证实修饰电极的重现性。
2.7 加标回收率
对加碘盐、奶粉以及黄豆酱进行碘的电化学方法定量,进行加标回收实验,得到结果如表2所示。
表 2 检测方法的加标回收率Table 2. Recovery rate of standard addition of detection method实际样品 测定值
(mg/kg)加标量
(mg/kg)加标测定值
(mg/kg)回收率
(%)RSD
(%)加碘盐 29.67 20 47.79 96.22 2.83 奶粉 0.43 20 21.16 103.57 2.12 黄豆酱 33.81 20 52.92 98.35 4.95 于3 mL经预处理的样品中添加20 mg/kg I-标准样品进行分析并计算回收率,结果如表2,回收率为96.22%~103.57%,相对标准偏差RSD在2.12%~4.95%,加碘食盐、奶粉和黄豆酱中的碘含量符合国家标准添加量。表明该方法稳定可靠,可用于实际样品中碘含量的测定。
3. 结论
基于电沉积方法制备甲烷氧化菌素原位还原纳米金Mb@AuNPs自组装修饰电极,通过循环伏安法考察I-在修饰电极上的电化学行为,得到修饰电极检测体系的优化条件。在该条件下修饰电极对I-的检测性能良好,I-浓度与氧化峰电流间具有良好的线性关系,检出限与相关文献相比相对较低,且该电极具有良好的重现性、精密度和稳定性以及较强的抗干扰能力。可实现对实际食品样品的检测,且电极制备方法简单、易操作,可为食品中碘测定方法的改进提供依据。
-
表 1 Mb@AuNPs自组装修饰电极与其它电化学检测方法比较
Table 1 Mb@AuNPs comparison of self-assembled decorated electrode with other electrochemical detection methods
表 2 检测方法的加标回收率
Table 2 Recovery rate of standard addition of detection method
实际样品 测定值
(mg/kg)加标量
(mg/kg)加标测定值
(mg/kg)回收率
(%)RSD
(%)加碘盐 29.67 20 47.79 96.22 2.83 奶粉 0.43 20 21.16 103.57 2.12 黄豆酱 33.81 20 52.92 98.35 4.95 -
[1] OPAZO M C, CORONADO-ARRÁZOLA I, VALLEJOS O P, et al. The impact of the micronutrient iodine in health and diseases[J]. Critical Reviews in Food Science and Nutrition,2020,13(1):1−14.
[2] OYUNCHIMEG D, BYAMBATOGTOKH B, YAMADA C. Activities and achievements in the elimination of iodine deficiency disorders[J]. International Congress,2004,1267(1):127−130.
[3] MORSETH M S, AAKRE I, BARIKMO I, et al. High iodine content in local animal milk and risk of exceeding EFSA upper intake level for iodine among Saharawi women[J]. PloS One,2019,14(2):1−11.
[4] ZIMMERMANN M B. Iodine and the iodine deficiency disorders[J]. Present Knowledge in Nutrition,2020,25(1):429−441.
[5] NAMEKAR S B, LOKHANDE S P, JADHAV G R, et al. Estimation of Iodine content by iodometric titration and spectrophotometric evaluation method in commercially Available salts[J]. Indian Journal of Nutrition & Dietetics,2017,54(4):465−476.
[6] HOU Wenli, CHEN Yuan, LU Qiujun, et al. Silver ions enhanced AuNCs fluorescence as a turn-off nanoprobe for ultrasensitive detection of iodide[J]. Talanta,2018,180(17):144−149.
[7] JENNY N, LAWRENCE P, DONG S, et al. Simultaneous analysis of iodine and bromine species in infant formula using HPLC-ICP-MS[J]. Journal of AOAC International,2019,102(4):1199−1204. doi: 10.5740/jaoacint.18-0352
[8] ARAYA M, SAMANTHA G, SEBASTIÁN P. Iodine and iodate determination by a new spectrophotometric method using N, N-dimethyl-p-phenylenediamine, validated in veterinary supplements and table salt[J]. Analytical Methods,2020,12(2):205−211. doi: 10.1039/C9AY02249J
[9] TRÉSOR K M, STÉPHANIE P, MACOURS P, et al. Highly sensitive determination of iodide by ion chromatography with amperometric detection at a silver-based carbon paste electrode[J]. Talanta,2008,76(3):540−547. doi: 10.1016/j.talanta.2008.03.053
[10] ESPADA-BELLIDO E, BI Z, SALAÜN P, et al. Determination of iodide and total iodine in estuarine waters by cathodic stripping voltammetry using a vibrating silver amalgam microwire electrode[J]. Talanta,2017,174(17):165−170.
[11] NING Wang, LU Ga, JUN Ai. Synthesis of novel fluorescent copper nanomaterials and their application in detection of iodide ions and catalysis[J]. Analytical Methods,2018,11(1):44−48.
[12] SONG Juanjuan, ZHAO Li, WANG Yesheng, et al. Carbon quantum dots prepared with chitosan for synthesis of CQDs/AuNPs for iodine ions detection[J]. Nanomaterials,2018,8(12):1043−1053. doi: 10.3390/nano8121043
[13] LIU Xu, GAO Hui, SUN Dengming, et al. Determination of trace of iodide at a poly-l-methionine modified glassy carbon electrode[J]. Revue Roumaine De Chimie,2019,64(4):311−316. doi: 10.33224/rrch/2019.64.4.03
[14] TITRETIR S, ERDOGDU G, KARAGZLER A. Determination of iodide ions at poly(3-methylthiophene)-modified electrode by differential pulse stripping voltammetry[J]. Journal of Analytical Chemistry,2006,61(6):592−595. doi: 10.1134/S1061934806060141
[15] QIN Xia, WANG Huicai, MIAO Zhiying, et al. Synthesis of silver nanowires and their applications in the electrochemical detection of halide[J]. Talanta,2011,84(3):673−678. doi: 10.1016/j.talanta.2011.01.064
[16] MASATAKE Haruta A, MASAKAZU Daté B. Advances in the catalysis of Au nanoparticles[J]. Applied Catalysis A:General,2001,222(1-2):427−437. doi: 10.1016/S0926-860X(01)00847-X
[17] PENG Xiange, WAN Gengping, WU Lihong, et al. Peroxidase-like activity of Au@TiO2 yolk-shell nanostructure and its application for colorimetric detection of H2O2 and glucose[J]. Sensors & Actuators B Chemical,2018,257(10):166−177.
[18] 辛嘉英, 姜加良, 张帅, 等. 甲烷氧化菌素-铜配合物催化过氧化氢氧化对苯二酚[J]. 高等学校化学学报,2013,34(5):1233−1239. [XIN Jiaying, JIANG Jialiang, ZHANG Shuai, et al. Oxidation of hydroquinone by hydrogen peroxide catalyzed by methanogen copper complex[J]. Journal of chemistry of Colleges and Universities,2013,34(5):1233−1239. doi: 10.7503/cjcu20120793 [19] 张伟. 甲烷氧化菌素模拟SOD的光谱学与电化学研究[D]. 哈尔滨: 哈尔滨商业大学, 2018 ZHANG Wei. Spectroscopic and electrochemical study of methane oxidation toxin simulating SOD [D]. Harbin: Harbin University of Commerce, 2018.
[20] BEHLING L A, HARTSEL S C, LEWIS D E, et al. NMR, mass spectrometry and chemical evidence reveal a different chemical structure for methanobactin that contains oxazolone rings[J]. Journal of the American Chemical Society,2008,130(38):12604−12605. doi: 10.1021/ja804747d
[21] 陈林林, 韩可, 李伟, 等. 铬天青S法结合高效液相色谱法测定甲烷氧化菌素含量[J]. 食品工业科技,2019,40(13):148−153. [CHEN Linlin, HAN Ke, LI Wei, et al. Determination of methanotrophin by chrome azurol S method combined with high performance liquid chromatography[J]. Food Industry Science and Technology,2019,40(13):148−153. [22] 刘丰源. 微量铜的甲烷氧化菌素功能化纳米金检测研究[D]. 哈尔滨: 哈尔滨商业大学, 2020 LIU Fengyuan. Detection of trace copper by methanobactone functionalized gold nanoparticles [D]. Harbin: Harbin University of Commerce, 2020.
[23] 杨洋, 崔磊磊, 姚颖悟. 电沉积法制备二氧化铅电极的研究进展[J]. 电镀与精饰,2018,40(9):29−34. [YANG Yang, CUI Leilei, YAO Yingwu. Research progress of lead dioxide electrode prepared by electrodeposition[J]. Electroplating and Finishing,2018,40(9):29−34. doi: 10.3969/j.issn.1001-3849.2018.09.007 [24] EVANS D H, O'CONNELL K M, PETERSEN R A, et al. Cyclic voltammetry[J]. Journal of Chemical Education,1983,60(4):290−293. doi: 10.1021/ed060p290
[25] 杨彦丽, 林立, 寇琳娜. 电感耦合等离子体质谱-离子色谱法检测食盐中的碘[J]. 分析化学,2010,38(9):1381−1381. [YANG Yanli, LIN Li, KOU Linna. Determination of iodine in salt by inductively coupled plasma mass spectrometry ion chromatography[J]. Analytical Chemistry,2010,38(9):1381−1381. [26] MACHADO A, MESQUITA R B R, OLIVEIRA S, et al. Development of a robust, fast screening method for the potentiometric determination of iodide in urine and salt samples[J]. Talanta,2017,167(6):688−694.
[27] 陈光, 寇琳娜, 周谙非, 等. 离子色谱-安培检测器测定食品中的碘[J]. 食品科学,2010,31(18):292−294. [CHEN Guang, KOU Lina, ZHOU Congfei, et al. Determination of iodine in food by ion chromatography with amperometric detector[J]. Food Science,2010,31(18):292−294. [28] 周贤亚. 海带中碘含量的测定[J]. 广东化工,2018,45(13):276−276. [ZHOU Xianya. Determination of iodine content in kelp[J]. Guangdong Chemical Industry,2018,45(13):276−276. doi: 10.3969/j.issn.1007-1865.2018.13.130 [29] XIN Jiaying, CHENG Dandan, ZHANG Lanxuan, et al. Methanobactin-mediated one-step synthesis of gold nanoparticles[J]. International Journal of Molecular Sciences,2013,14(11):21676−21688. doi: 10.3390/ijms141121676
[30] 窦博鑫, 辛嘉英, 王振兴, 等. 甲烷氧化菌素功能化金纳米层层自组装修饰电极上过氧化氢的催化还原[J]. 分子催化,2017,31(6):534−543. [DOU Boxin, XIN Jiaying, WANG Zhenxing, et al. Catalytic reduction of hydrogen peroxide on layer by layer self-assembled gold electrode functionalized with methanobactone[J]. Molecular Catalysis,2017,31(6):534−543. [31] 赵燕荣. 基于电化学传感器的吗啡检测方法研究[D]. 苏州: 苏州大学, 2009: 21. ZHAO Yanrong. Study on detection method of morphine based on electrochemical sensor [D]. Suzhou : Suzhou University, 2009.
[32] 叶耘峰. 基于碳纳米金复合材料电化学检测BHA[J]. 食品工业,2020,41(11):293−296. [YE Yunfeng. Electrochemical detection of BHA based on carbon nano gold composite[J]. Food Industry,2020,41(11):293−296. [33] CHEN Huanyin, YANG Tao, LIU Faqian, et al. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite[J]. Sensors and Actuators,2019,286(18):401−407.
[34] JIGYASA, PRATIBHA, RAJPUT J K. Alkali metal (Na/K) doped graphitic carbon nitride (g-C3N4) for highly selective and sensitive electrochemical sensing of nitrite in water and food samples[J]. Journal of Electroanalytical Chemistry,2020,878(16):1572−1580.
[35] 中华人民共和国国家卫生健康委员会, 国家市场监督管理总局. 食品安全国家标准食品中碘的测定: GB 5009.267-2020 [S].北京: 中国标准出版社, 2020 National Health Commission of the People's Republic of China, State Administration of market supervision and administration. Determination of iodine in food safety national standard food: GB 5009.267-2020[S]. Beijing: China Standard Press, 2020.
[36] CINCY J, MILJA T E, PRATHISH K P. Fabrication of a flexible carbon cloth based solid contact iodide selective electrode[J]. Analytical Methods,2017,9(20):2947−2956. doi: 10.1039/C7AY00733G
[37] DANA V, NICOLETA P, GHEORGHE F C, et al. Potentiometric sensors for iodide and bromide based on Pt(II)-porphyrin[J]. Sensors,2018,18(7):2297−2314. doi: 10.3390/s18072297
[38] LAZAROVA Y, YANNA I, SHTEREV T, et al. Highly sensitive electrochemical detection of iodate based on glassy carbon electrode modified with iridium oxide[J]. Monatshefte Fur Chemie,2018,149(11):1955−1962. doi: 10.1007/s00706-018-2275-y
[39] 任蓉. 碘的电化学研究及测定[D].淮北: 淮北师范大学, 2020. REN Rong. Electrochemical study and determination of iodine [D]. Huaibei: Huaibei Normal University, 2020.
-
期刊类型引用(4)
1. 赵红,雷舒雯,赵春燕,罗会清,彭春秀,龚加顺. 加工方式和体外模拟消化对4种食用昆虫营养成分及抗氧化活性的影响. 粮食与油脂. 2025(01): 145-150 .  百度学术
百度学术
2. 王馨,吴孟仙,陈媛媛,王林峰,杨生玉,李星科. 杜仲叶固态混菌发酵工艺优化及其体外活性研究. 中国食品添加剂. 2025(03): 29-37 .  百度学术
百度学术
3. 王喜龙,张丽蓉,欧晓彬,雷琴珍,陈甜,黄秋玲. 响应面法优化紫苏叶中抑菌成分的提取工艺. 化学与生物工程. 2024(12): 36-43 .  百度学术
百度学术
4. 邱卫华,于晓慧. 响应面法优化紫苏叶中花色苷与多酚的同步高效提取工艺. 现代食品. 2024(21): 155-162 .  百度学术
百度学术
其他类型引用(2)





 下载:
下载:
 下载:
下载:



